Abstract
Antibody-drug conjugates (ADCs) are a promising class of cancer biopharmaceuticals that exploit the specificity of a monoclonal antibody (mAb) to selectively deliver highly cytotoxic small molecules to targeted cancer cells, leading to an enhanced therapeutic index through increased antitumor activity and decreased off-target toxicity. ADCs hold great promise for the treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer after the approval and tremendous success of trastuzumab emtansine and trastuzumab deruxtecan, representing a turning point in both HER2-positive breast cancer treatment and ADC technology. Additionally and importantly, a total of 29 ADC candidates are now being investigated in different stages of clinical development for the treatment of HER2-positive breast cancer. The purpose of this review is to provide an insight into the ADC field in cancer treatment and present a comprehensive overview of ADCs approved or under clinical investigation for the treatment of HER2-positive breast cancer.
Keywords: antibody-drug conjugates, ADCs, immunoconjugates, human epidermal growth factor receptor 2, HER2, targeted therapy, HER2-postive breast cancer
Graphical abstract
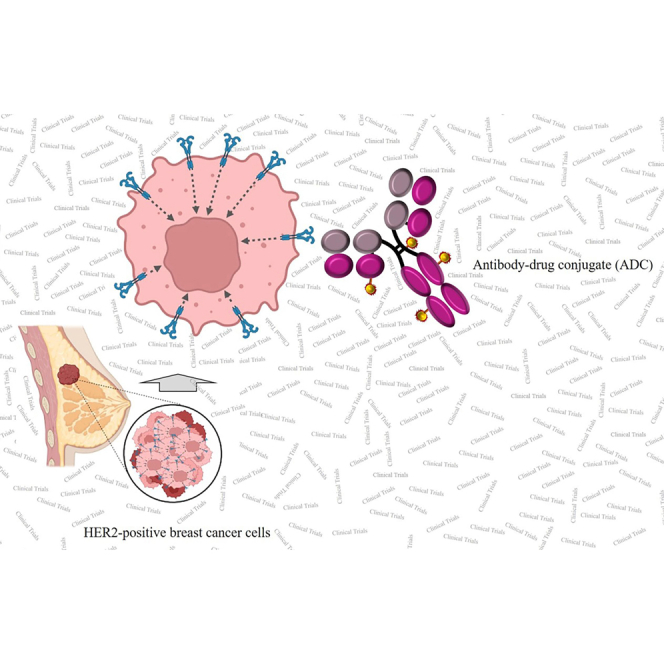
There are currently a total of 29 ADCs in different stages of clinical trials for the treatment of HER2-positive breast cancer, some of which, including A166, ALT-P7, SYD985, BDC-1001, RC48, ARX788, PF-06804103, Ujvira, and DX126-262, have shown promising results.
Introduction
Breast cancer is the most common reason for cancer-related mortality among women worldwide,1,2 surpassing lung cancer and ranking first with an estimated 2.3 million new cases (11.7%).3 The incidence and mortality rates of breast cancer vary across countries, with the standardized age incidence ranging from the highest, 112.3 per 100,000 population to the lowest, 35.8 per 100,000 population in Belgium and Iran, respectively.4 Accumulating documents have suggested that 30% of women will develop breast cancer during their lifetime, 15% of whom will die.5,6
Approximately 15%–20% of the women suffering from breast cancer amplify or overexpress human epidermal growth factor receptor 2 (HER2; a transmembrane receptor of tyrosine kinases and a member of the epidermal growth factor receptor [EGFR] family), known as HER2-positive breast cancer. Besides breast cancer,7 HER2 was found to be overexpressed in different types of cancers, including lung,8 gastric,9 and ovarian10 cancers. HER2 overexpression triggers a variety of downstream pathways, leading to increased proliferation of cancer cells.11,12 HER2 is closely associated with enhanced malignancy, poor prognosis, and resistance to chemotherapeutic agents.13 On the other hand, normal adult cells have no considerable HER2 expression and therefore are less sensitive to anti-HER2 therapy,14 making HER2 a valuable and rational therapeutic target for the treatment of various HER2-positive cancers, including HER2-positive breast cancer.
There are a variety of anti-HER2 therapies currently used for the treatment of HER2-positive breast cancer, including monoclonal antibodies (mAbs) such as trastuzumab, pertuzumab, and margetuximab, tyrosine kinase inhibitors (TKIs) such as lapatinib, neratinib, and tucatinib, and antibody-drug conjugates (ADCs) such as trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd).15 mAbs and TKIs have demonstrated significant clinical benefits for patients with early-stage and metastatic HER2-positive breast cancers. Nevertheless, a large proportion of patients have shown therapeutic resistance to mAbs and TKIs, as either single agents or in combination with chemotherapies in the metastatic setting.16 Nonetheless, mAbs and TKIs are outside the scope of this study and have been widely covered elsewhere.17 ADCs, as a promising class of anticancer therapeutic agents, have opened a new avenue for the treatment of cancers, particularly HER2-positive breast cancer.
This review provides a brief overview of ADCs, presents the current state of the ADC field in HER2-positive breast cancer, summarizes the two approved ADCs (T-DM1 and T-DXd), and focuses mainly on the ADCs under clinical development for the treatment of HER2-positive breast cancer.
Antibody-drug conjugates
ADCs are an evolving class of therapeutic agents intended to selectively deliver cytotoxic small molecules to targeted cancer cells. An ADC is composed of an antigen-specific mAb conjugated to a potent cytotoxic agent through a linker, resulting in an enhanced therapeutic index through increased antitumor activity and decreased off-target toxicity.
Anatomy of ADCs
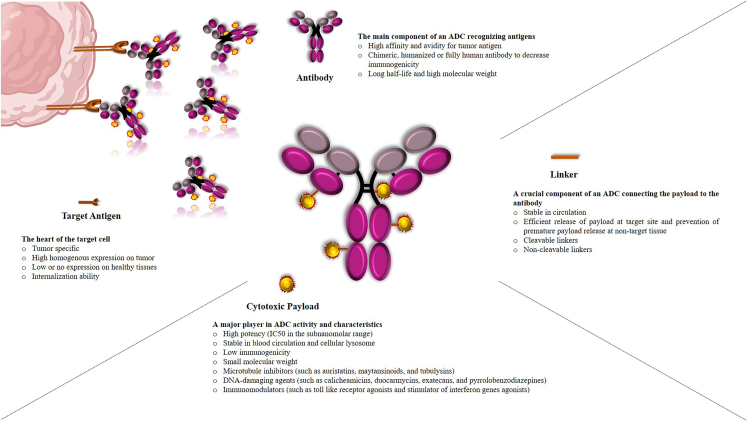
ADCs are composed of four key components, including (1) a target antigen, (2) an antibody construct, (3) a cytotoxic agent (generally known as a payload), and (4) a linker (Figure 1). Successful development of ADCs needs to meet stringent selection criteria for each ADC component to achieve the expected pharmacokinetics (PK), pharmacodynamics (PD), and safety profile.
Figure 1.
Schematic representation of an antibody-drug conjugate
Target antigen
An ideal antigen needs to fulfill several requirements, including localization on the cell surface to allow ADC binding, high and homogeneous expression on cancer cells or differentially increased expression on tumor cells as compared with normal cells, the ability to be internalized upon ADC binding where the drug can be released, minimal ADC recycling to the cell surface, and increased delivery of the internalized antigen/ADC to the lysosome.
Antibody
Antibodies, as an important moiety playing a key role in ADC selectivity, require crucial attributes to guarantee ADC success, including high specificity for the tumor antigen, high affinity binding to the target antigen for effective uptake into target cells, minimally immunogenic profile, and ideal PK characteristics comprising longer half-life with slower plasma clearance. Of note, immunoglobulin G (IgG), especially IgG1, is the most common antibody subclass used in the ADC architecture.
Payload
The cytotoxic small molecules, as expected, play a key role in ADC activity and characteristics. The optimal payload needs to have the following characteristics: high potency (in the sub-nanomolar range) because of the restricted number of payloads localized to solid tumors (noting that the number of payloads that each antibody can deliver is defined as drug-to-antibody ratio [DAR]), the presence of an appropriate functional group for conjugation to antibodies via an appropriate linker, stability under physiological conditions (in the blood circulation and cellular lysosomes), resistance to multidrug resistance protein 1 (MDR1), relative hydrophobic properties, and low immunogenicity. The payloads currently being used in ADCs normally fall into three main categories, namely tubulin inhibitors (such as auristatins, maytansinoids, and tubulysins), DNA-damaging agents (such as calicheamicins, duocarmycins, exatecans, and pyrrolobenzodiazepines [PBDs]), and immunomodulators (such as Toll-like receptor [TLR] agonists and stimulator of interferon genes [STING] agonists). Tubulin inhibitors (including tubulin polymerization promoters and tubulin polymerization inhibitors) exert their anticancer activity through tubulin binding, microtubule destabilization, and G2/M phase cell-cycle arrest, while DNA-damaging agents mediate their activity either by DNA minor groove binding followed by DNA strand scission, alkylation or crosslinking, or by binding to the topoisomerase I (TOP1) and DNA complex and preventing DNA religation followed by DNA damage, which result in apoptosis. On the other hand, immunomodulators, known as immune-stimulating antibody conjugates (ISACs), take advantage of the specificity of antibody-navigated targeting and the potential of small-molecule-based modulation of the innate and adaptive immune systems.
Linker
The linker moiety, as a crucial component of the ADC complex, is used to conjugate the payload to the antibody, playing a key role in accurate release of the cytotoxic drug at tumor sites. An ideal linker needs to have the key characteristics of sufficient stability in the blood while allowing quick release of the active free drug inside tumor cells. Linker formats currently being used in the ADC architecture can be broadly classified into two groups, cleavable and non-cleavable linkers. Cleavable linkers, including acid-labile linkers (such as hydrazine groups), protease-cleavable linkers (including valine-alanine [va], phenylalanine-lysine, and valine-citrulline [vc] dipeptides), and disulfide linkers are tailored to be stable in the blood/plasma but become unstable and cleaved in different intracellular situations such as the low pH environment in lysosomes, the presence of proteases inside cancer cell lysosomes, and high glutathione concentrations, respectively. Cleavable linkers, although representing the main class of linkers used in ADCs, are less stable in the systemic circulation. Alternatively, non-cleavable linkers are highly stable and release the payloads following complete ADC complex internalization and, consequently, proteolytic degradation of the entire mAb components by the lysosomes.
Bioconjugation
Bioconjugation, the attachment of a drug-linker moiety to a mAb, is a crucial factor strongly influencing the heterogeneity, DAR, therapeutic potential, and the ultimate success of an ADC. An ideal bioconjugation strategy should cause no changes in the integrity of the antibody, the biological activity of the payload, the binding capacity of the antibody to the antigen, the native forms of antibody and payload components, and, if present, the effector functions of the antibody. Payloads are usually attached to the antibody through amino- or thiol-specific linkers that react with lysine (through acylation of reachable lysine side-chain amines) or cysteine (through alkylation of reduced interchain disulfides or genetically engineered cysteines) residues on the antibody surface, commonly suffering from heterogeneity. Heterogeneous ADCs may consist of substantial amounts of unconjugated antibodies that compete with conjugated antibodies for antigen binding, leading to inhibition or decrease of ADC activity. However, conjugation through cysteines leads to decreased ADC heterogeneity when compared with lysine conjugation, due to fewer possible conjugation sites. The optimal DAR in most ADCs ranges from 2 to 8 drugs per antibody, which depends on various factors. To improve the pharmacological characteristics of existing and future ADCs, novel site-specific conjugation strategies are currently being developed to synthesize more homogeneous ADCs. These approaches can be categorized into three different classes: engineered amino acids, enzyme-mediated approach, and linker modification. Engineered amino acid approaches include engineered cysteines and engineered non-natural amino acids, enzyme-mediated approaches include transglutaminase, formylglycine-generating enzyme (fge), sortase A (SrtA), glycosyltransferases, and endoglycosidase, and linker-based approaches include hydrophilic linkers, bis-alkylating linkers, next-generation maleimides (ngms), dibromopyridazinediones, and dibromomaleimides, which have been reviewed in detail elsewhere.18,19,20,21,22
Mechanism of action of ADCs
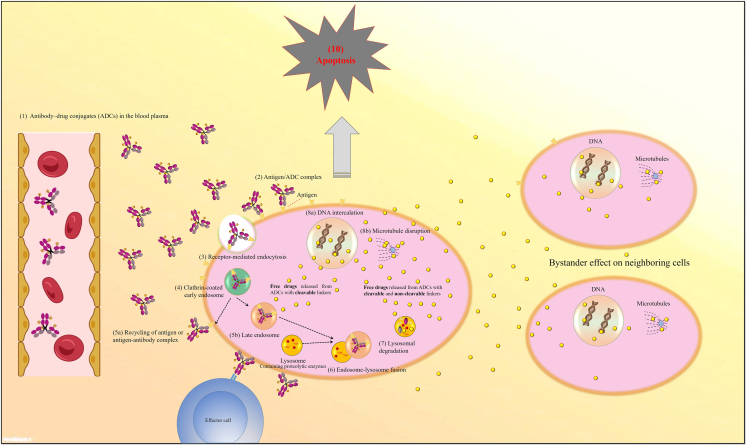
ADCs are designed to eradicate cancer cells in a target-dependent manner. For this purpose, ADCs are intravenously injected directly into the blood circulation to be safe from gastric acid and prevent mAb backbone degradation by proteolytic enzymes. After binding of the ADC to the cancer cell surface antigen, the antigen-ADC complex is generally internalized via receptor-mediated endocytosis. After formation of early endosomes and then trafficking to late endosomes and lysosomes, free payloads are released into the cell either by linker cleavage or ADC degradation, resulting in cell death and apoptosis. The mechanism of action of cell death can vary based on the class of payloads used, as mentioned above. Neighboring cancer cells may also be killed when ADCs are designed to stimulate the release of the payloads into the extracellular space or once free drugs are released into the tumor environment through the dying cell, in a process known as the bystander effect (Figure 2).19,20,21,22,23
Figure 2.
Mechanism of action of antibody-drug conjugates
Coming-of-age of ADCs in cancer therapeutics
The evolution of ADC development, based on drug compositions and technology characteristics, is generally divided into three generations. In the early stage, ADCs were mostly made up of mouse-original or chimeric/humanized antibodies conjugated, via random lysines, to conventional chemotherapy drugs, including calicheamicin, duocarmycin, or doxorubicin, through an unstable linker, resulting in ADCs with an uncontrollable DAR of 0–8.0. Gemtuzumab ozogamicin and inotuzumab ozogamicin are representative examples of the first generation of ADCs. Early generations of ADCs suffered from heterogeneity, negligible potency, lack of efficacy, narrow therapeutic index, off-target toxicity as premature drug loss, and high immunogenicity, leading to little success in clinical trials. Subsequent efforts to increase ADC efficiency resulted in the optimization of ADC components, such as antibody structure, payload diversification, and conjugation chemistries, resulting in the advent of second-generation ADCs. In the second generation, ADCs consisted of humanized antibodies conjugated, via random lysines or reduced interchain cysteines, to more potent payloads, such as auristatins or mytansinoids, through cleavable or non-cleavable linkers with improved stability, resulting in ADCs with an average DAR of 4.0–8.0. Brentuximab vedotin and ado-trastuzumab emtansine are representative examples of second-generation ADCs. Although showing improved targeting ability, more potency, and lower immunogenicity, the second-generation ADCs suffered from heterogeneity, fast clearance for high DARs, off-target toxicity as premature drug loss, and drug resistance. In the third generation, ADCs are composed largely of human antibodies site-specifically conjugated to highly potent payloads, such as PBDs or tubulysin as well as novel payloads such as immunomodulators, through linkers stable in circulation with precise control drug release into tumor sites, resulting in ADCs with an average DAR of 2.0–4.0. Polatuzumab vedotin, enfortumab vedotin, and fam-trastuzumab deruxtecan are representative examples of the third generation of ADCs, taking advantage of higher efficacy even in cancer cells with low antigen, improved DAR along with improved stability and PK/PD, more potent payloads, and less off-target toxicity.24
Gemtuzumab ozogamicin was the first ADC approved in 2000 by the United States Food and Drug Administration (FDA) for the treatment of patients with CD33-positive relapsed acute myeloid leukemia (AML). Gemtuzumab ozogamicin, though withdrawn from the market owing to toxicity concerns, was the beginning of a new era accelerating the translation of ADCs from ideas to routine clinical workflow, as documented by approved ADCs and a wide variety of ADCs progressing to clinical trials in the last 20 years. It is, of course, important to note that gemtuzumab ozogamicin was later reintroduced to the market in 2017 after receiving FDA approval for the treatment of adults with newly diagnosed CD33-positive AML and adults and children 2 years and older with relapsed or refractory CD33-positive AML.
By February 2023, 12 ADCs have been approved in oncology indications by the FDA: six for hematologic malignancies, namely gemtuzumab ozogamicin (Mylotarg), brentuximab vedotin (Adcetris), inotuzumab ozogamicin (Besponsa), moxetumomab pasudotox (Lumoxiti), polatuzumab vedotin (Polivy), and loncastuximab tesirine (Zynlonta); and six for solid tumors, namely ado-trastuzumab emtansine (Kadcyla), enfortumab vedotin (Padcev), fam-trastuzumab deruxtecan (Enhertu), sacituzumab govitecan (Trodelvy), tisotumab vedotin (Tivdak), and mirvetuximab soravtansine-gynx (Elahere). In addition, two ADCs, cetuximab sarotalocan (Akalux) and disitamab vedotin (Aidixi), have been approved by Japan’s Pharmaceuticals and Medical Devices Agency and China’s National Medical Products Administration (NMPA), respectively. Unfortunately, belantamab mafodotin-blmf (Blenrep), although granted accelerated approval in August 2020 by the FDA, was then withdrawn from the market on November 22, 2022, following the request of FDA based on the previously announced outcome of the DREAMM-3 phase III confirmatory trial.
In addition to these approved ADCs, more than 140 ADC candidates are currently in different stages of clinical development or have progressed to clinical trials for a variety of solid and hematologic cancers, sparking industry-wide interest in this modality.19,20,21,22,24,25,26
Antibody-drug conjugates for HER2-positive breast cancer
Breast cancer played a leading role in the evolution of ADCs because of the fact that three out of the 12 FDA-approved ADCs, including HER2-targeting T-DM1, HER2-targeting T-DXd, and TROP2-targeting sacituzumab govitecan (SG), are used for the treatment of breast cancer. The approval and early achievement of T-DM1 in the HER2-positive metastatic setting confirmed the effectiveness of the targeted delivery of chemotherapeutics through the ADC modality in solid tumors, especially in breast cancer, resulting in myriad hopes, later dashed by the approval of T-DXd.27 Importantly, most ADCs under development against breast cancer target the HER2 antigen thanks to its long history as a certified therapeutic target, the presence of trastuzumab as a backbone for ADC synthesis, a higher incidence of HER2-positive breast cancer, and the important role of HER2 testing as part of the standard of care.
FDA-approved antibody-drug conjugates for the treatment of HER2-positive breast cancer
The two FDA-approved ADCs against HER2-positive breast cancer, T-DM1 and T-DXd, will be discussed in the following sections. Table 1 indicates the main characteristics of the ADCs approved for the treatment of HER2-positive breast cancer.
Table 1.
Antibody-drug conjugates approved for the treatment of HER2-positive breast cancer as of February 2023
| ADC | Trade name | Target antigen | Antibody | Payload | Linker | Average DAR | Developer | Approved date | Approved indications |
|---|---|---|---|---|---|---|---|---|---|
| Trastuzumab emtansine (ado-trastuzumab emtansine; T-DM1) | Kadcyla | HER2 | humanized IgG1 (trastuzumab) | DM1 | non-cleavable (SMCC) | 3.5 | Genentech | February 2013 | Patients with HER2-positive metastatic breast cancer who have received prior treatment with trastuzumab and a taxane, separately or in combination |
| May 2019 | The adjuvant treatment of patients with HER2-positive early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment | ||||||||
| Trastuzumab deruxtecan (fam-trastuzumab deruxtecan-nxki; T-DXd; DS8201a) | Enhertu | HER2 | humanized IgG1 (MAAL-9001) | DXd | cleavable (maleimide GGFG peptide) | 7.0–8.0 | Daiichi Sankyo | December 2019 | adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting (accelerated approval) |
| May 2022 | adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within 6 months of completing therapy |
HER2, human epidermal growth factor receptor 2; ADC, antibody-drug conjugate; DAR, drug-to-antibody ratio; SMCC, succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate; GGFG, glycine-glycine-phenylalanine-glycine.
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1; ado-trastuzumab emtansine; T-MCC-DM1; Kadcyla) is the first-in-class anti-HER2 ADC approved in 2013 for the treatment of patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxane (separately or in combination), and in 2019 for the adjuvant treatment of patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment.28
T-DM1 consists of trastuzumab (Herceptin, a humanized anti-HER2 IgG1 mAb approved for the treatment of HER2-positive breast cancer) conjugated through random lysins to N(2ʹ)-deacetyl-N(2ʹ)-(3-mercapto-1-oxopropyl)-maytansine (DM1, a highly potent derivative of the naturally occurring maytansinoid toxin which inhibits tubulin polymerization and induces death in proliferating cells) via a stable non-cleavable thioether linker succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate (SMCC; MCC after conjugation), showing an average DAR of 3.5.29,30 Of note, T-DM1 preserves all the cytotoxic functions of trastuzumab, including antibody-dependent cell-mediated cytotoxicity (ADCC) and signaling inhibition.31 T-DM1, after binding to HER2, undergoes receptor-mediated internalization, trafficking from the endosomes to the lysosomes and subsequent proteolytic degradation in the lysosome, leading to release of the active T-DM1 catabolite (Lys-MCC-DM1). This metabolite is transported from the lysosomal lumen to the cytosol, where it inhibits tubulin polymerization and causes apoptosis in target cells.32,33 Nonetheless, Lys-SMCC-DM1 exhibits weak membrane permeability with a minimal bystander effect.34
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-DXd; fam-trastuzumab deruxtecan; DS-8201a; DS-8201; Enhertu) is an anti-HER2 ADC approved in 2022 for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy.28
T-DXd consists of a humanized anti-HER2 IgG1 mAb (MAAL-9001, with the same amino acid sequence as trastuzumab except that lysine residues at the heavy-chain C terminus remain in MAAL-9001) conjugated through reduced interchain disulfides to approximately 7.0–8.0 molecules of deruxtecan (MAAA 1162a). Deruxtecan is an ADC drug-linker conjugate containing an exatecan (DX-8951, a water-soluble hexacyclic analog of camptothecin with a potent DNA TOP1 inhibitor activity) derivative DXd (MAAA-1181a or MAAA-1181) and an enzymatically cleavable tetrapeptide-based (maleimide glycine-glycine-phenylalanine-glycine [GGFG] peptide) linker.35,36,37 T-DXd, after binding to HER2, is internalized and trafficked intracellularly to lysosomes; the linker is selectively cleaved by lysosomal cathepsins such as cathepsins B (which are upregulated in tumor cells), and the released DXd eventually induces double-strand DNA damage and cell apoptosis through binding to the TOP1-DNA complex.38 Importantly, DXd was confirmed to be about ten times more potent than SN-38 (a payload used in SG).36
More importantly, T-DXd has been demonstrated to have not only potential clinical activity in tumors with different HER2 expression levels, even in HER2-heterogeneous and -low tumors, but also a bystander effect in surrounding cells because of the membrane-permeable nature of the payload used. Preclinical studies demonstrated that T-DXd possesses more antitumor activity than T-DM1. A higher payload delivery (DAR: 7.0–8.0), the cleavable GGFG peptide linker, and higher membrane permeability leading to a bystander effect and lower affinity for efflux transporters MDR1 of DXd may explain the antitumor potential of T-DXd in T-DM1-resistant tumors.36,39,40,41 At the same time, there are currently more than 100 clinical trials under way to assess the use of the two aforementioned approved ADCs, as monotherapy or in combination with other therapeutic agents. Table 2 summarizes the most important ongoing/completed phase III or ongoing phase IV clinical trials with the two approved ADCs.
Table 2.
Ongoing/completed phase III or ongoing phase IV clinical trials evaluating trastuzumab emtansine or trastuzumab deruxtecan in patients with HER2-positive breast cancer as of February 2023
| ADC | Treatment arms | Sponsor | Indications | Phase (status) | NCT identifier |
|---|---|---|---|---|---|
| Trastuzumab emtansine (ado-trastuzumab emtansine; T-DM1) | T-DXd versus T-DM1 | Daiichi Sankyo | high-risk patients with residual invasive breast cancer following neoadjuvant therapy | III (recruiting) | NCT04622319 |
| T-DXd versus T-DM1 | Daiichi Sankyo | patients with HER2-positive, unresectable, and/or metastatic breast cancer subjects previously treated with trastuzumab and taxane | III (active, not recruiting) | NCT03529110 | |
| Tucatinib in combination with T-DM1 versus placebo in combination with T-DM1 | Seagen | patients with unresectable locally advanced or metastatic HER2-positive breast cancer | III (recruiting) | NCT03975647 | |
| T-DM1 | Hoffmann-La Roche | patients with HER2-positive locally advanced or metastatic breast cancer patients who have received prior anti-HER2 and chemotherapy-based treatment | III (completed) | NCT01702571 | |
| T-DM1 versus T-DM1 and tucatinib | Alliance for Clinical Trials in Oncology | patients with high-risk HER2-positive breast cancer | III (recruiting) | NCT04457596 | |
| T-DM1 versus TPC | Hoffmann-La Roche | patients with HER2-positive metastatic breast cancer who have received at least two prior regimens of HER2-directed therapy | III (completed) | NCT01419197 | |
| T-DM1 versus taxane (docetaxel or paclitaxel) | Hoffmann-La Roche | patients with previously treated locally advanced or metastatic HER2-positive gastric cancer, including adenocarcinoma of the gastroesophageal junction | II/III (terminated) | NCT01641939 | |
| T-DM1 in combination with atezolizumab or placebo | Hoffmann-La Roche | patients with HER2-positive and PD-L1-positive locally advanced or metastatic breast cancer who have received prior trastuzumab (±pertuzumab)-based and taxane-based therapy | III (recruiting) | NCT04740918 | |
| T-DM1 versus lapatinib plus capecitabine | Hoffmann-La Roche | patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy | III (active, not recruiting) | NCT03084939 | |
| T-DM1 in combination with pertuzumab or T-DM1 in combination with pertuzumab-placebo (blinded for pertuzumab), versus the combination of trastuzumab plus taxane, as first-line treatment | Hoffmann-La Roche | patients with HER2-positive progressive or recurrent locally advanced or metastatic breast cancer | III (completed) | NCT01120184 | |
| T-DM1 plus pertuzumab versus chemotherapy plus trastuzumab and pertuzumab | Hoffmann-La Roche | patients with HER2-positive breast cancer | III (completed) | NCT02131064 | |
| T-DM1 versus capecitabine in combination with lapatinib | Hoffmann-La Roche | patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy | III (completed) | NCT00829166 | |
| T-DM1 versus the combination of trastuzumab plus docetaxel as first-line treatment | Hoffmann-La Roche | patients with HER2-positive progressive or recurrent locally advanced or metastatic breast cancer | III (terminated) | NCT02144012 | |
| adjuvant atezolizumab or placebo and T-DM1 | Hoffmann-La Roche | patients with HER2-positive breast cancer at high risk of recurrence following preoperative therapy | III (recruiting) | NCT04873362 | |
| T-DM1 versus trastuzumab as adjuvant therapy | Hoffmann-La Roche | patients with HER2-positive primary breast cancer who have residual tumor present pathologically in the breast or axillary lymph nodes following preoperative therapy | III (active, not recruiting) | NCT01772472 | |
| T-DM1 plus pertuzumab following anthracyclines versus trastuzumab plus pertuzumab plus a taxane following anthracyclines | Hoffmann-La Roche | patients with operable HER2-positive primary breast cancer | III (completed) | NCT01966471 | |
| T-DM1 versus capecitabine | University of Virginia | patients with high-risk breast cancer | II/III (not yet recruiting) | NCT05288777 | |
| combination of docetaxel, trastuzumab, and pertuzumab (arm A) or T-DM1 (arm B) | Thomas Hatschek | patients with HER2-positive breast cancer | II/III (active, not recruiting) | NCT02568839 | |
| Trastuzumab deruxtecan (fam-trastuzumab deruxtecan-nxki; T-DXd; DS8201a) | T-DXd versus T-DM1 | Daiichi Sankyo | high-risk patients with residual invasive breast cancer following neoadjuvant therapy | III (recruiting) | NCT04622319 |
| T-DXd with or without pertuzumab versus taxane, trastuzumab, and pertuzumab | AstraZeneca | patients with HER2-positive, first-line metastatic breast cancer | III (recruiting) | NCT04784715 | |
| T-DXd versus investigator’s choice chemotherapy | AstraZeneca | patients with HER2-low, hormone receptor-positive breast cancer patients whose disease has progressed on endocrine therapy in the metastatic setting | III (recruiting) | NCT04494425 | |
| T-DXd monotherapy or T-DXd in sequence with THP versus standard treatment (ddAC-THP) | AstraZeneca | patients with high-risk HER2-positive early-stage breast cancer | III (recruiting) | NCT05113251 | |
| T-DXd | AstraZeneca | patients with or without baseline brain metastasis with previously treated advanced/metastatic HER2-positive breast cancer | IIIb/IV (recruiting) | NCT04739761 | |
| T-DXd | AstraZeneca | patients with unresectable, locally advanced, or metastatic NSCLC harboring HER2 exon 19 or 20 mutations | III (recruiting) | NCT05048797 | |
| T-DXd versus T-DM1 | Daiichi Sankyo | patients with HER2-positive, unresectable, and/or metastatic breast cancer previously treated with trastuzumab and taxane | III (active, not recruiting) | NCT03529110 | |
| T-DXd versus ramucirumab and paclitaxel | Daiichi Sankyo | patients with HER2-positive gastric or GEJ adenocarcinoma who have progressed on or after a trastuzumab-containing regimen and have not received any additional systemic therapy | III (recruiting) | NCT04704934 |
HER2, human epidermal growth factor receptor 2; ADC, antibody-drug conjugate; HR, hormonal receptor; AC, Adriamycin + cyclophosphamide; THP, Taxol + Herceptin + pertuzumab; NSCLC, non-small cell lung cancer; TPC, treatment of physician’s choice; GEJ, gastroesophageal junction.
Antibody-drug conjugates for the treatment of HER2-positive breast cancer in clinical pipelines
The success of T-DM1 and T-DXd has not only revolutionized the treatment of HER2-positive breast cancer but also re-energized the ADC field for the development of novel ADCs. In light of this, a great number of new anti-HER2 ADC candidates are being investigated for the treatment of HER2-positive breast cancer, with varying degrees of clinical success, as discussed below. Table 3 presents all of the anti-HER2 ADCs under investigation for the treatment of HER2-positive breast cancer in various stages of clinical trials (as of February 2023).
Table 3.
Antibody-drug conjugates under development for patients with HER2-positive breast cancer in clinical trials as of February 2023
| ADC | Antibody | Payload | Linker | Average DAR | Sponsor | Indications | Phase (status) | NCT identifier |
|---|---|---|---|---|---|---|---|---|
| A166 | humanized IgG1 (trastuzumab) | duostatin-5 (Duo-5; MMAF derivative) | cleavable (valine-citrulline) | 2.0 | Fudan University | HER2-positive patients with refractory unresectable locally advanced or metastatic breast cancer who have failed previous ADC drug therapy | II (not yet recruiting) | NCT05346328 |
| Klus Pharma | patients with relapsed/refractory cancers expressing the HER2 antigen or having amplified the her2 gene, including HER2-positive breast cancer | I/II (active, not recruiting) | NCT03602079 | |||||
| Sichuan Kelun Pharmaceutical Research Institute | patients with unresectable, locally advanced or metastatic HER2-expressing solid tumors, including breast cancer | I (recruiting) | NCT05311397 | |||||
| – | patients with HER2-expressing locally advanced or metastatic solid tumors, including HER2-positive breast cancer | I | CTR20181301 | |||||
| LCB14-0110 (FS-1502) | trastuzumab | MMAF | cleavable (β-glucuronide) | 2.0 | Shanghai Fosun Pharmaceutical Industrial Development | patients with HER2-expressing advanced malignant solid tumors (phase Ia) and patients with metastatic HER2-positive breast cancer (an expanded cohort; phase Ib) to evaluate the ADC in patients with metastatic, HER2-positive breast cancer | I (recruiting) | NCT03944499 |
| ALT-P7 | trastuzumab biobetter (HM2) | MMAE | cleavable | 2.0 | Alteogen | patients with HER2-positive metastatic breast cancer who have progressed on previous trastuzumab-based therapy | I (completed) | NCT03281824 |
| ADCT-502 | an engineered version of trastuzumab | tesirine (a PBD-based dimer (SG3249)) | cleavable (valine-alanine) | 1.7 | ADC Therapeutics | patients with advanced solid tumors, including HER2-positive breast cancer | I (terminated) | NCT03125200 |
| BAT8001 | trastuzumab biosimilar (BAT0606) | a maytansine derivative | non-cleavable (6-maleimidocaproic acid) | – | Bio-Thera Solutions | patients with HER2-positive advanced breast cancer | III (unknown) | NCT04185649 |
| Bio-Thera Solutions | patients with HER2-positive advanced solid tumors (BAT8001 in combination with BAT1306) | I/IIa (unknown) | NCT04151329 | |||||
| Bio-Thera Solutions | patients with HER2-positive solid tumors (breast cancer or gastric cancer) | I (unknown) | NCT04189211 | |||||
| trastuzumab duocarmazine (SYD985) | humanized IgG1 (with the same amino acid sequence as trastuzumab) | seco-DUBA (the duocarmycin prodrug) | cleavable (valine-citrulline) | 2.8 | Byondis | patients with HER2-positive locally advanced or metastatic breast cancer (SYD985 versus TPC) | III (active, not recruiting) | NCT03262935 |
| Byondis | patients with HER2-expressing locally advanced or metastatic solid tumors (the single arm part of the trial) and patients with locally advanced or metastatic HER2-positive breast cancer (the comparative part following primary favorable safety and efficacy) | I/II (active, not recruiting) | NCT04983238 | |||||
| QuantumLeap Healthcare Collaborative | patients with breast cancer (I-SPY TRIAL; I-SPY will compare the efficacy of novel drugs in combination with standard chemotherapy with the efficacy of standard therapy alone) | II (recruiting) | NCT01042379 | |||||
| QuantumLeap Healthcare Collaborative | patients with certain HER-positive advanced solid tumors or HER2-low breast cancer and patients with HER2-positive or -low breast cancer | I/Ib (recruiting) | NCT04602117 | |||||
| Byondis | patients with locally advanced or metastatic solid tumors, including breast cancer | I (completed) | NCT02277717 | |||||
| Byondis | patients with locally advanced or metastatic HER2-expressing solid tumors of any origin (part 1) and patients with advanced or metastatic breast, ovarian or endometrial cancers (part 2) (SYD985 in combination with niraparib) | I (active, not recruiting) | NCT04235101 | |||||
| SBT6050 | anti-HER2 IgG | TLR8 agonist | – | – | Silverback Therapeutics | patients with advanced HER2-expressing solid tumors (as monotherapy or in combination with PD-1 inhibitors, including pembrolizumab or cemiplimab) | I/Ib (active, not recruiting) | NCT04460456 |
| Silverback Therapeutics | patients with pretreated unresectable locally advanced and/or metastatic HER2-expressing or HER2-amplified cancers, including HER2-positive breast cancer (SBT6050 in combination with other HER2-directed therapies, including T-DXd, tucatinib, trastuzumab, and capecitabine) | I/II (terminated) | NCT05091528 | |||||
| BDC-1001 | humanized IgG1 (trastuzumab biosimilar) | TLR7/TLR8 agonist | non-cleavable | – | Bolt Biotherapeutics | patients with advanced HER2-expressing solid tumors (as monotherapy or in combination with nivolumab) | I/II (recruiting) | NCT04278144 |
| MEDI4276 | humanized biparatopic antibody (trastuzumab & 39S) | AZ13599185 (a tubulysin derivative) | cleavable (peptide-based maleimidocaproyl) | 4.0 | MedImmune | patients with HER2-expressing breast or gastric cancers | I/II (completed) | NCT02576548 |
| RC48-ADC (disitamab vedotin; Aidixi) | humanized IgG1 (hertuzumab) | MMAE | cleavable (mc-val-cit-PABC) | 4.0 | RemeGen | patients with locally advanced or metastatic breast cancer with HER2-low expression | III (recruiting) | NCT04400695 |
| RemeGen | patients with HER2-positive metastatic breast cancer with or without liver metastases | II/III (recruiting) | NCT03500380 | |||||
| Cancer Institute and Hospital, Chinese Academy of Medical Sciences | patients with HER2-expression metastatic breast cancer with abnormal activation of the PAM (PI3K/Akt/mTOR) pathway | II (recruiting) | NCT05331326 | |||||
| Xijing Hospital | patients with HER2-positive breast cancer | II (not yet recruiting) | NCT05134519 | |||||
| Second Affiliated Hospital of Soochow University | patients with HER2-positive solid tumors (RC48-ADC in combination with hypofractionated radiotherapy, PD-1/PD-L1 inhibitor sequential GM-CSF and IL-2) | II (not yet recruiting) | NCT05115500 | |||||
| RemeGen | patients with advanced breast cancer with HER2-positive or -low expression | Ib (active, not recruiting) | NCT03052634 | |||||
| RemeGen | patients with advanced malignant solid tumors with HER2-positive | I (completed) | NCT02881138 | |||||
| RemeGen | patients with HER2-positive malignant in advanced malignant solid tumors | I (completed) | NCT02881190 | |||||
| Peking University | patients with HER2-positive advanced malignant solid tumors (JS001 in combination with RC48-ADC) | I (unknown) | NCT04280341 | |||||
| ARX788 | humanized mAb incorporated with pAF (a non-natural amino acid) | MMAF | non-cleavable (hydroxylamine-PEG4) | 1.9 | Caigang Liu | patients with HER2-positive breast cancer (ARX788 in combination with pyrotinib maleate versus TCBHP [trastuzumab plus pertuzumab with docetaxel and carboplatin]) | II/III (recruiting) | NCT05426486 |
| – | Metastatic breast cancer/gastric cancer | II/III | CTR20201708 | |||||
| Fudan University | patients with unresectable and/or metastatic breast cancer with HER2-low expression | II (recruiting) | NCT05018676 | |||||
| Fudan University | patients with HER2-positive, metastatic breast cancer whose disease is resistant or refractory to TKI | II (recruiting) | NCT05018702 | |||||
| Shengjing Hospital | patients with stage II-III HER2-positive breast cancer who have poor outcomes after treatment with trastuzumab and pertuzumab (pyrotinib maleate in combination with ARX788) | II (recruiting) | NCT04983121 | |||||
| Ambrx | patients with HER2-positive, metastatic breast cancer whose disease is resistant or refractory to T-DM1, and/or T-DXd, and/or tucatinib-containing regimens | II (active, not recruiting) | NCT04829604 | |||||
| Ambrx | patients with HER2-mutated or HER2-amplified/overexpressed locally advanced or metastatic solid tumor cancers, including breast cancer, whose prior standard of care therapies have failed | II (withdrawn) | NCT05041972 | |||||
| Ambrx | patients with advanced cancer, including breast cancer, whose HER2 test results are ISH-positive or IHC 3+, based on safety, tolerability, PK findings, and antitumor activity | I (active, not recruiting) | NCT03255070 | |||||
| – | HER2-positive breast cancer | I | CTR20171162 | |||||
| – | HER2-positive gastric and breast cancer | I | CTR20190639 | |||||
| Zhejiang Medicine | patients with metastatic cancers with HER2 test result that is ISH-positive or IHC 3 + or 2+, including breast cancer | I (terminated) | NCT02512237 | |||||
| MRG002 | humanized IgG1 (sugar-modified trastuzumab) | MMAE | cleavable (valine-citrulline) | 3.8 | Shanghai Miracogen | patients with HER2-positive breast cancer with liver metastases | II (recruiting) | NCT05263869 |
| Shanghai Miracogen | patients with HER2-positive advanced solid tumors | I (recruiting) | NCT04941339 | |||||
| Shanghai Miracogen | patients with HER2-positive unresectable locally advanced or metastatic breast cancer | II (recruiting) | NCT04924699 | |||||
| Shanghai Miracogen | patients with HER2-low locally advanced or metastatic breast cancer | II (recruiting) | NCT04742153 | |||||
| Shanghai Miracogen | patients with HER2-expressed advanced malignant solid tumors (MRG002 in combination with HX008) | I/II (recruiting) | NCT05338957 | |||||
| – | patients with HER2-positive advanced solid tumors, including breast cancer | I | CTR20181778 | |||||
| DP303c | humanized IgG1 (DP001) | MMAE | cleavable (NH2-PEG3-val-cit) | 2.0 | CSPC ZhongQi Pharmaceutical Technology | patients with HER2-positive unresectable locally advanced, relapsed, or metastatic breast cancer | II (not yet recruiting) | NCT05334810 |
| CSPC ZhongQi Pharmaceutical Technology | patients with HER2-positive advanced solid tumors, including breast cancer | I (unknown) | NCT04146610 | |||||
| XMT-1522 (TAK-522) | human IgG1 (HT-19) | AF-HPA | a biodegradable hydrophilic polymer | 12.0 | Mersana Therapeutics | patients with advanced breast cancer and either a HER2 IHC score of at least 1 + using a validated IHC assay or with evidence of HER2 amplification (patients with HER2-positive [by IHC or amplification] gastric cancer or NSCLC may also be eligible for participation in dose escalation) | Ib (completed) | NCT02952729 |
| XMT-2056 | human IgG1 (HT-19) | STING agonist | – | – | Mersana Therapeutics | patients with advanced/recurrent HER2-expressing solid tumors, including HER2-positive and HER2-low breast cancer | I (recruiting) | NCT05514717 |
| PF-06804103 | humanized IgG1 | PF-06380101 (Aur0101; auristatin-0101) | cleavable (valine-citrulline) | 4.0 | Pfizer | patients with HER2-positive and -negative breast and gastric cancer (as monotherapy or in combination with letrozole and palbociclib) | I (completed) | NCT03284723 |
| DHES0815A (RG6148) | humanized IgG1 (hu7C2) | PBD-MA | cleavable (disulfide) | 2.0 | Genentech | patients with advanced and/or metastatic HER2-positive breast cancer for whom established treatment has proven ineffective or intolerable or is unavailable | I (completed) | NCT03451162 |
| Zanidatamab zovodotin (ZW49) | humanized, bispecific IgG1 (Zanidatamab or ZW25) | N-acyl sulfonamide auristatin | cleavable | – | Zymeworks | patients with locally advanced (unresectable) or metastatic HER2-expressing cancers, including breast cancer | I (recruiting) | NCT03821233 |
| MM-302 | scFv (F5) | liposomal doxorubicin | PEG-DSPE | – | Pamela Munster | patients with advanced HER2-positive cancer with new or progressive brain metastases (64Cu-MM-302 and unlabeled MM-302 in combination with trastuzumab) | early I (withdrawn) | NCT02735798 |
| Merrimack Pharmaceuticals | patients with advanced HER2-positive breast cancer (MM-302 with trastuzumab or trastuzumab plus cyclophosphamide) | I (unknown) | NCT01304797 | |||||
| Merrimack Pharmaceuticals | patients with locally advanced/metastatic HER2-positive breast cancer (MM-302 plus trastuzumab versus the CPC plus trastuzumab) | II/III (terminated) | NCT02213744 | |||||
| GQ1001 | Anti-HER2 mAb | DM1 | – | – | GeneQuantum Healthcare (Suzhou) | patients with HER2-positive advanced solid tumors, including HER2-positive breast cancer | I (recruiting) | NCT04450732 |
| B003 | humanized IgG | DM1 | non-cleavable (SMCC) | – | Shanghai Pharmaceuticals Holding | patients with HER2-positive recurrent or metastatic breast cancer | I (active, not recruiting) | NCT03953833 |
| BB-1701 | anti-HER2 IgG | – | – | – | Bliss Biopharmaceutical (Hangzhou) | patients with locally advanced/metastatic HER2-expressing solid tumors, including breast cancer | I (recruiting) | NCT04257110 |
| SHR-A1811 | anti-HER2 IgG | – | – | – | Jiangsu HengRui Medicine | patients with HER2-positive, unresectable and/or metastatic breast cancer previously treated with trastuzumab and taxane (SHR-A1811 versus pyrotinib in combination with capecitabine) | III (recruiting) | NCT05424835 |
| Suzhou Suncadia Biopharmaceuticals | patients with HER2-expressing advanced solid tumors (SHR-A1811 in combination with fluzoparib) | Ib/II (enrolling by invitation) | NCT05349409 | |||||
| Jiangsu HengRui Medicine | patients with HER2-positive non-resectable or metastatic breast cancer (SHR-A1811 in combination with pyrrolidone or patrozumab or SHR-1316 or albumin paclitaxel) | Ib/II (not yet recruiting) | NCT05353361 | |||||
| Jiangsu HengRui Medicine | patients with HER2-expressing or mutated advanced malignant solid tumors | I (recruiting) | NCT04446260 | |||||
| BICON-02 | trastuzumab | – | – | – | Biointegrator | patients with HER2-positive metastatic breast cancer, previously treated with trastuzumab | I (terminated) | NCT03062007 |
| TAA013 | trastuzumab | DM1 | non-cleavable (SMCC) | – | – | patients with relapsed HER2-positive breast cancer | I | CTR20181642 |
| – | patients with locally advanced or metastatic HER2-positive breast cancer (TAA013 versus lapatinib in combination with capecitabine) | III | CTR20200806 | |||||
| UJVIRA (ZRC-3256)a | trastuzumab | DM1 | non-cleavable (SMCC) | 3.5 | Cadila Healthcare | patients with HER2-positive metastatic breast cancer | III | CTRI/2018/07/014881 |
| SHR-A1201a | trastuzumab | DM1 | non-cleavable (SMCC) | 3.5 | Jiangsu Hengrui Pharmaceuticals | patients with metastatic breast cancer | I | CTR20191558 |
| DX126-262 | humanized IgG1 (DX-CHO9) | Tub114 (a tubulysin derivative) | – | 3.5–3.8 | – | patients with HER2-positive advanced breast and/or gastric cancer | I | CTR20191224 |
| HS630 | humanized mAb | DM1 | – | – | Zhejiang Hisun Pharmaceutical/Beijing Mabworks Biotech | patients with HER2-positive advanced breast cancer | I | CTR20181755 |
HER2, human epidermal growth factor receptor 2; ADC, antibody-drug conjugate; NSCLC, non-small cell lung cancer; DAR, drug-to-antibody ratio; MMAF, monomethyl auristatin F; MMAE, monomethyl auristatin E; PBD, pyrrolobenzodiazepine; seco-DUBA, seco-duocarmycin-hydroxybenzamide-azaindole; TLR, Toll-like receptor; mc-val-cit-PABC, maleimidocaproyl-valyl-citrullinyl-p-aminobenzyloxycarbonyl; pAF, para-acetyl phenylalanine; PEG, polyethylene glycol; AF-HPA, auristatin F-hydroxypropylamide; PBD-MA, pyrrolo[2,1-c][1,4]benzodiazepine monoamide; scFv, single-chain fragment variable; PEG-DSPE, poly(ethylene glycol)-distearoylphosphatidylethanolamine; SMCC, succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate; CPC, chemotherapy of physician’s choice; TPC, treatment of physician’s choice; TKI, tyrosine kinase inhibitors; ISH, in situ hybridization; IHC, immunohistochemistry; PK, pharmacokinetics; STING, the stimulator of interferon genes protein; –, unknown, undisclosed, or unavailable.
ADCs are the biosimilar of T-DM1; the linker structure, the payload, and DAR are speculated from T-DM1.
A166
A166 is composed of trastuzumab site-specifically conjugated to monomethyl auristatin F (MMAF, a highly potent synthetic auristatin derivative with the ability to inhibit cellular proliferation by disrupting tubulin polymerization)-derived payload duostatin-5 (Duo-5, a novel highly potent anti-microtubule auristatin), via a stable protease-cleavable vc linker, showing a homogeneous ADC with an average DAR of 2.0.42,43,44,45 Upon binding of A166 to HER2 and antigen/ADC internalization, the linker is enzymatically cleaved into the tumor cell and free payload is released, binds to tubulin, and inhibits polymerization, resulting in G2/M phase arrest and tumor cell apoptosis.
A166 is now under investigation in various clinical trials against HER2-positive solid tumors, including breast cancer, showing a promising antitumor potential, an acceptable safety profile, and good tolerability in early clinical studies.
LCB14-0110
LCB14-0110 (FS-1502; IKS014) is a novel prenyltransferase-mediated, site-specific ADC composed of trastuzumab site-specifically conjugated to MMAF through a proprietary β-glucuronide linker. LCB14-0110 was synthesized using a novel site-specific conjugation method through prenyltransferase-mediated conjugation of a ketone-functionalized isoprenoid to trastuzumab, with a C-terminus-appended CaaX motif, followed by payload conjugation via an oxime ligation reaction, yielding a DAR of 2.0.46,47,48
LCB14-0110 displayed good homogeneity with a defined DAR, an appropriate physiochemical profile, high stability during preclinical evaluation, in vitro dose-dependent specific cytotoxicity against HER2-positive cell lines, complete regression in breast cancer xenografts with moderate HER2 expression, potent antitumor activity in gastric cancer xenografts, encouraging PK in rat and monkey, and excellent tolerability in cynomolgus monkeys. The preclinical evidence warranted further investigation of LCB14-0110 for HER2-positive cancers.48,49 LCB14-0110 is currently being evaluated in a phase I clinical trial in China (NCT03944499).
ALT-P7
ALT-P7 (HM2-MMAE; HM2-drug conjugate) is a novel anti-HER2 ADC in which two molecules of monomethyl auristatin E (MMAE, a highly potent synthetic auristatin derivative and microtubule-disrupting agent) are conjugated site-specifically to a cysteine-containing peptide motif of the trastuzumab variant (trastuzumab biobetter HM2).50 ALT-P7 is developed by the NexMab ADC technology, a site-specific antibody-drug conjugation approach, by using ligand-protected cysteine-containing motifs. Upon binding of ALT-P7 to HER2 and antigen/ADC internalization, MMAE is released, binds to tubulin, and inhibits the tubulin polymerization, leading to G2/M phase arrest and apoptosis.51
ALT-P7 has great potential to treat refractory tumors and is a candidate ADC for HER2-positive gastric and breast cancers. ALT-P7 was investigated in a phase I clinical trial in patients with HER2-positive advanced breast cancer who have progressed on previous trastuzumab-based therapy (NCT03281824). This completed phase I clinical trial displayed promising results, including an acceptable safety and tolerability profile, which guarantees further investigation in a phase II clinical trial.46
ADCT-502
ADCT-502 is composed of an engineered version of trastuzumab conjugated site-specifically to tesirine (a PBD-based dimer [SG3249] with the ability to crosslink in the minor groove of DNA) via a cathepsin B-cleavable va linker, with an average DAR of 1.7.52,53,54,55 Upon binding of ADCT-502 to HER2 and antigen/ADC internalization, the linker is cleaved and the PBD moiety is released. PBD dimer SG3199, which is a released warhead component of tesirine (SG3249), induces interstrand crosslinks in the minor groove of DNA, resulting in G2/M cell-cycle arrest and cell death.53,56,57
ADCT-502 demonstrated potent in vitro cytotoxicity and bystander effect in various HER2-positive and -negative cell lines, respectively.55 More importantly, ADCT-502 exhibited strong and durable antitumor activity in HER2-positive, but not in HER2-negative, mouse xenografts. The ADC also showed superior in vivo antitumor activity when compared with T-DM1 in numerous tumor xenografts, including those with low HER2 levels.57 The safety, tolerability, PK, and antitumor activity of ADCT-502 were evaluated in patients with HER2-positive advanced solid tumors (NCT03125200). However, further development of ADCT502 was terminated because of poor efficacy and/or safety concerns.58
BAT8001
BAT8001 consists of a trastuzumab biosimilar (BAT0606) covalently conjugated to a drug linker, batansine (a derivative of maytansine linked to a novel non-cleavable linker, 6-maleimidocaproic acid, by a stable amide bond).59 Upon binding of BAT8001 to HER2 and antigen/ADC internalization, the linker is degraded in the lysosomes and the active metabolite is released. Thereafter, the maytansine derivative binds to tubulin and disrupts microtubule assembly, resulting in prevention of tumor cell proliferation and induction of apoptosis in HER2-expressing tumor cells.60,61
Preclinical studies demonstrated strong inhibition activity of BAT8001 in both cell-line xenografts (CLXs) and patient-derived xenografts (PDXs). Importantly, the novel non-cleavable linker used in BAT8001 was found to be more stable than that used in T-DM1, as documented by improved toxicity profile of BAT8001.62 BAT8001 is being evaluated in phase I, I/II, and III clinical trials in various tumor types, including metastatic breast cancer. Results from NCT04189211 showed satisfactory safety profiles and encouraging antitumor activity of BAT8001 in patients with HER2-positive locally advanced or metastatic breast cancer.59 However, BAT8001 was terminated because of failing to meet the endpoint events in a phase III clinical trial.63
Trastuzumab duocarmazine
Trastuzumab duocarmazine (trastuzumab vc-seco-DUBA; SYD985) is composed of a humanized anti-HER2 IgG1 mAb, with the same amino acid sequence as trastuzumab, conjugated to a highly potent DNA-alkylating payload, duocarmycin seco-duocarmycin-hydroxybenzamide-azaindole (seco-DUBA, a duocarmycin prodrug) through a protease-cleavable vc linker (valine-citrulline-seco-duocarmycin-hydroxybenzamide-azaindole; vc-seco-DUBA) with an average DAR of 2.8.64,65 Following binding of SYD985 to HER2 and antigen/ADC internalization, the linker is proteolytically cleaved in the lysosome and the active payload (DUBA) is released into the cells. In detail, the hydrolyzation of SYD985 leads to the delivery of para-aminobenzyl alcohol (PAB)-dimethyl ethylenediamine (DMEDA)-seco-DUBA, followed by sequential self-elimination of PAB, DMEDA, and chlorine hydride (HCL) to release active DUBA. The free DUBA subsequently binds to the minor groove of DNA in an AT-rich region, where it irreversibly alkylates DNA, leading to induced DNA damage in both dividing and non-dividing cells and eventual cell death.63,64 In addition, the free DUBA is cell permeable and can lead to a bystander effect, therefore providing a wide therapeutic window.66,67
Preclinical studies showed the in vitro stability of SYD985 in human and cynomolgus monkey, but not in mouse, plasma, and in vivo, promising antitumor activity in low- to high-HER2-expressing solid tumors as well as in HER2-positive metastatic breast cancer PDX models.65,68 SYD985 is now being tested in a variety of phase I, II, and III clinical trials, demonstrating potential antitumor activity in HER2-positive breast cancer.69 In the TULIP trial (a phase III clinical trial; NCT03262935), SYD985 demonstrated significantly improved progression-free survival in comparison with standard physician’s choice in patients with pretreated HER2-positive locally advanced or metastatic breast cancer, presumably providing a potential treatment option for patients with HER2-positive locally advanced or metastatic breast cancer.70
SBT6050
SBT6050 is the first novel class of targeted immuno-oncology agents (a novel ImmunoTAC therapeutic) composed of an anti-HER2 mAb (specifically binding to the HER2 subdomain II, the pertuzumab epitope) conjugated to a potent and highly specific TLR8 agonist payload, allowing for activation of human myeloid cells only in tumors with moderate to high HER2 expression.71,72 Because of TLR8 expression in myeloid cell types frequently present in human tumors, TLR8 agonism can trigger a wide range of antitumor immune mechanisms, such as pathways involved in the innate and adaptive immune response.
SBT6050 was found to potently activate human myeloid cells in the presence of HER2-expressing tumor cells, in turn driving an innate immune response for direct tumor killing and induction of a T cell response.71 In preclinical studies, SBT6050 demonstrated to potently induce a broad range of antitumor immune mechanisms, including pro-inflammatory cytokine and chemokine production, inflammasome activation, and indirect activation of T and natural killer cells. Preclinical studies also supported its combinations with checkpoint inhibitors and trastuzumab to further enhance antitumor activity.72 In addition, SBT6050 demonstrated single-agent efficacy in multiple mouse tumor models without peripheral cytokine production.71 SBT6050 is currently being studied in a clinical trial (NCT04460456) in patients with advanced or metastatic HER2-expressing or -amplified solid tumors, including HER2-positive breast cancer, as monotherapy or in combination with other anti-HER2 or immune therapies. A phase I/II clinical study (NCT05091528), evaluating the safety and preliminary activity of SBT6050 in combination with other HER2-directed therapies in HER2-positive cancers (including HER2-positive breast cancer), was terminated because of sponsor decision based on strategic realignment.
BDC-1001
BDC-1001 is a new HER2-targeting Boltbody ISAC consisting of a trastuzumab biosimilar conjugated to a TLR7/TLR8 agonist via a non-cleavable linker.73 BDC-1001 exerts its antitumor activity through induction of strong immune stimulation within the tumor environment, including the activation of the innate immune system, direct tumor cell killing by antibody-mediated effector functions such as ADCC, localized phagocytosis, and killing of HER2-positive tumor cells by activated myeloid antigen-presenting cells, as well as a durable adaptive immune response.74,75
Preclinical studies showed the potent and durable immune-mediated antitumor effect of BDC-1001 in xenograft models resistant to anti-HER2 treatments.76 BDC-1001 is currently being investigated in a phase I/II clinical trial in patients with advanced HER2-expressing solid tumors (NCT04278144), demonstrating a suitable tolerability and safety profile.74
MEDI4276
MEDI4276 is composed of a biparatopic (also known as bispecific) tetravalent humanized anti-HER2 mAb, through introduction of two cysteine residues per heavy chain (S239C and S442C), conjugated site-specifically to a potent microtubule inhibitor AZ13599185 (a tubulysin derivative with the ability to prevent microtubule polymerization during mitosis) through a protease-cleavable peptide-based maleimidocaproyl linker, with an average DAR of 4.0. MEDI4276 was constructed from 39S (a human IgG1 mAb binding to a HER2 epitope distinct from that of trastuzumab) by genetically linking the single-chain variable fragment (scFv) of trastuzumab to the amino terminus of the 39S heavy chain. This leads to the binding of the mAb moiety of MEDI4276 to two distinct epitopes on subdomains II and IV of the HER2 ectodomain.77,78 The biparatopic nature of the mAb component leads to increased MEDI4276 internalization, deeper inhibition of cancer cell proliferation, and induced cell death following linker cleavage and payload release. In addition, MEDI4276 was found to eradicate a heterogeneous tumor cell population containing neighboring HER2-positive and -negative tumor cells through an effective bystander effect.79
In vitro and in vivo studies showed enhanced cellular internalization and potential activity of MEDI4276 in T-DM1- and trastuzumab-resistant HER2-positive breast cancer cells.79 The encouraging activity in preclinical models supported further development of MEDI4276. A phase I/II clinical trial evaluated the safety, PK, immunogenicity, and antitumor activity of MEDI4276 on HER2-expressing advanced solid tumors, including breast and gastric cancers (NCT02576548). However, the clinical trial of MEDI4276, although displaying clinical activity, has been discontinued because of intolerable toxicity.63,80
Disitamab vedotin
Disitamab vedotin (RC48-ADC; RC48; AIDIXI) is composed of an anti-HER2 humanized IgG1 mAb (hertuzumab, with a higher affinity to HER2 than trastuzumab and greater ADCC activity in vitro) covalently conjugated to four molecules of MMAE through a protease-cleavable maleimidocaproyl-valyl-citrullinyl-p-aminobenzyloxycarbonyl (mc-val-cit-PABC) linker.81 After binding of RC48-ADC to HER2 and antigen/ADC internalization, the dipeptide vc linker is cleaved and MMAE is released into the cytosol, leading to induced apoptosis, as mentioned above.51
In vitro and in vivo studies confirmed potent antitumor activity of RC48-ADC in not only HER2-overexpressing but also trastuzumab- and lapatinib-resistant xenograft tumor models. Importantly, promising results were obtained in a mouse model where the combination of RC48-ADC and programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) immune checkpoint inhibition could significantly increase tumor suppression and antitumor immunity.81,82 RC48-ADC is currently being evaluated in several phase I, II, and III clinical trials as monotherapy or in combination with other anti-HER2 therapies, conventional chemotherapeutics, or immune checkpoint inhibitors in patients with multiple solid tumor types, including HER2-positive breast cancer, demonstrating robust antitumor activity. Importantly, favorable effects of this ADC were documented in different settings for the treatment of patients with advanced solid tumors and breast cancer.
In June 2021, the NMPA granted conditional approval of disitamab vedotin (Aidixi) for the treatment of patients with HER2-overexpressing locally advanced or metastatic gastric cancer (including gastroesophageal junction adenocarcinoma) who have received at least two systemic chemotherapy regimens.83
ARX788
ARX788 is a next-generation, site-specific ADC composed of a humanized anti-HER2 mAb site-specifically conjugated to a highly potent tubulin inhibitor amberstatin drug linker (AS269, containing a short, non-cleavable hydroxylamine-PEG4 linker attached to the N terminus of MMAF) using an Ambrx non-natural amino acid incorporation technology platform. The payload was conjugated site-specifically to a para-acetyl phenylalanine (pAF), a non-natural amino acid incorporated into a predetermined position (Ala114) on the heavy chain of the mAb, resulting in a highly stable and homogeneous ADC with an average DAR of 1.9.84 The active payload released after ADC lysosomal degradation has the pAF residue and cannot traverse the plasma membrane of neighboring cells.63
ARX788 proved in vitro and in vivo antitumor activity against HER2-positive ovarian, gastric, and breast cancer cell lines as well as against xenograft models.85 Importantly, ARX788 exhibited the ability to induce rapid tumor regression in a trastuzumab-resistant breast cancer xenograft, significantly more effective than T-DM1 at equivalent doses.85,86 There are currently various phase I, II, and III clinical trials to evaluate the role of ARX788 (either as monotherapy or in combination with other therapeutic agents) in HER2-positive breast cancer, showing good tolerability and clearance profiles.63,87
MRG002
MRG002 is a new vcMMAE-based ADC consisting of sugar-modified trastuzumab conjugated to MMAE via a protease-cleavable vc linker, with an average DAR of 3.8.88,89,90
Preclinical studies indicated potent in vitro cytotoxicity, similar antigen binding affinity, but much reduced ADCC activity compared with trastuzumab, potent antitumor activities, and a favorable toxicity profile in breast and gastric PDX models with high- and medium-to-low HER2 expression, as well as superior potency than trastuzumab and T-DM1 in mouse xenograft models. Furthermore, a combination of MRG002 with anti-PD-1 antibodies was demonstrated to significantly increase antitumor activity.88 MRG002 is now being tested in a variety of phase I and II clinical trials for safety, tolerability, PK, and preliminary antitumor activity in patients with HER2-positive or -low solid tumors, including breast cancer.89 In a phase II study (NCT04742153), MRG002 demonstrated promising efficacy and acceptable tolerability in patients with HER2-low breast cancer.90
DP303c
DP303c is a new third-generation site-specific HER2-targeting ADC consisting of a humanized anti-HER2 IgG1 mAb (DP001) conjugated to MMAE through an enzyme-based cleavable peptide linker (using the linker drug LND1002, a derivative of MMAE with an amine-PEG [NH2-PEG3-val-cit] linker), showing a steady and homogeneous ADC with an average DAR of 2.0. MMAE molecules were attached site-specifically through transamidation to glutamine residue 295 in the antibody heavy-chain constant region.91 Upon binding of DP303c to HER2 and antigen/ADC internalization, the cytotoxic agent is released and induces tumor cell apoptosis.
In vitro and in vivo studies exhibited remarkable antitumor activity of DP303c as compared with T-DM1 in a variety of HER2-positive cancer cells and cell-line-derived xenograft models, particularly in the HER2-low expressing cells. DP303c also displayed high homogeneity, high serum stability, good PK profile, high safety, and good tolerability profiles.91 DP303c is currently being investigated in clinical trials for the treatment of HER2-positive advanced solid tumors, including HER2-positive breast cancer.
XMT-1522
XMT-1522 (TAK-522) is a Dolaflexin ADC consisting of a human anti-HER2 IgG1 mAb (HT-19, which binds to domain IV of HER2) conjugated to auristatin F-hydroxypropylamide (AF-HPA) through a cysteine linkage using a biodegradable hydrophilic polymer, enabling high AF-HPA loadings.92 XMT-1522 is developed using a Dolaflexin platform, an innovative ADC technology with the ability to circumvent limitations of most ADC platforms through two strategic properties, including a higher DAR and a new auristatin with a controlled bystander effect.93,94,95 The Dolaflexin platform, as a biodegradable polymer-based conjugation approach, makes XMT-1522 with a high average DAR of 12.0 (a range of 10–15) with no aggregation or detrimental effect on PK.96 Upon binding of XMT-1522 to a unique epitope of HER2 and antigen/ADC internalization, the linker is cleaved and the payload is released; thereafter, the auristatin-derived molecules bind to tubulin and inhibit tubulin polymerization, leading to G2/M phase arrest and induced apoptosis in HER2-expressing tumor cells. The high auristatin molecules conjugated to HT-19 enable XMT-1522 to efficiently eradicate tumors with relatively low HER2 expression (as few as 22,000 copies of HER2 per cell), leading to increased therapeutic potential and favorable PK profiles.94
There is preclinical evidence demonstrating the promising in vitro and in vivo antitumor activity of XMT-1522 in T-DM1-resistant HER2-positive breast cancer and gastric cancer and in T-DM1-resistant xenograft models.92 XMT-1522, as the first Dolaflexin-based ADC with a high DAR, was evaluated in a phase Ib dose-escalation trial (NCT02952729) in patients with HER2-positive advanced breast cancer, gastric cancer, and non-small cell lung cancer (NSCLC), showing a favorable safety and efficacy profile.97 However, XMT-1522 was abandoned after an unsatisfactory risk-to-benefit ratio in a phase I study in patients with breast cancer.
XMT-2056
XMT-2056, an anti-HER2 Immunosynthen STING agonist ADC, is composed of HT-19 conjugated to a payload consisting of an STING agonist with potential immunoactivating and antineoplastic activities. XMT-2056 was developed via conjugation of Immunosynthen, a platform that uses a novel STING-agonist payload specifically designed for ADCs. Upon administration of XMT-2056, while the antibody moiety binds to a novel epitope of HER2 (which does not compete for binding with either trastuzumab or pertuzumab), the STING agonist targets and binds to STING on immune cells in the tumor microenvironment (TME), allowing for specific activation of the STING pathway in the TME. This, in turn, results in the production of pro-inflammatory cytokines including interferons, increases the cross-presentation of tumor-associated antigens by dendritic cells, and induces a cytotoxic T lymphocyte-mediated immune response against tumor cells.
In preclinical studies, XMT-2056 demonstrated more than 100-fold increased potency compared with the free STING-agonist payload, potent in vivo target- and dose-dependent antitumor activity in gastric and breast cancer models with varying HER2 expression levels, in vivo efficacy after a single intravenous dose with no major effect on systemic cytokines, favorable PK after repeat doses, ability to activate the STING pathway in both tumor-resident immune cells and tumor cells, and a favorable safety profile in non-human primates, as well as increased antitumor activity when combined with a variety of approved agents (including trastuzumab, pertuzumab, trastuzumab deruxtecan, or an anti-PD-1 agent).98,99 Together, these data supported both the potential of XMT-2056 as a monotherapy and in combination with other HER2-targeted agents as well as checkpoint inhibitors, and the clinical development of XMT-2056. The multicenter phase I open-label trial (NCT05514717) is under way to evaluate the safety, tolerability, preliminary antitumor effect, and PK of XMT-2056 in previously treated patients with advanced/recurrent HER2-expressing solid tumors, including HER2-postive and -low breast cancer. Importantly, the FDA has granted an orphan drug designation to XMT-2056 for the treatment of patients with gastric cancers, according to Mersana Therapeutics.100
PF-06804103
PF-06804103 consists of a humanized anti-HER2 IgG1 mAb (a trastuzumab-derived antibody) conjugated site-specifically through specific cysteines to a novel potent microtubule inhibitor auristatin derivative Aur0101 (PF-06380101 or auristatin-0101; an analog of dolastatin 10) via a protease-cleavable vc linker,101,102 showing a homogeneous ADC with a fixed DAR of 4.0. Upon binding of PF-06804103 to HER2 and antigen/ADC internalization, the linker is cleaved and the released Aur0101 binds to tubulin and inhibits tubulin polymerization, leading to G2/M phase arrest and apoptosis.
PF-06804103 showed an enhanced antitumor activity in HER2-low breast, gastric, and lung tumor models, CLXs, and PDXs, including those with low and heterogeneous HER2 expression, highlighting its ability to overcome in vitro- and in vivo-acquired T-DM1 resistance. Of note, PF-06804103 showed an enhanced safety profile by greater stability, increased pharmacokinetic parameters, and decreased off-target toxicities.102,103 PF-06804103 was examined in a phase I clinical trial in patients with HER2-positive metastatic breast or gastric cancers as monotherapy or in combination with letrozole and palbociclib (NCT03284723), showing manageable toxicity and promising antitumor activity.
DHES0815A
DHES0815A (RG6148; hu7C2-disulfide-PBD-MA) is composed of a humanized THIOMAB IgG1 mAb (hu7C2, which binds to domain 1 of the HER2 extracellular domain) conjugated to a DNA mono-alkylating agent pyrrolo[2,1-c][1,4]benzodiazepine monoamide (PBD-MA) via a disulfide linker, with an average DAR of 2.0.104 Following binding of DHES0815A to HER2 (at a distinct epitope from binding sites of trastuzumab and pertuzumab), antigen/ADC internalization, and lysosome-mediated cleavage, the payload PBD-MA is released and binds to and crosslinks specific sites of DNA through its imine groups, leading to DNA strand breaks, cell-cycle arrest, and cell death in HER2-expressing tumor cells.105
In vitro and in vivo studies confirmed the increased growth inhibition and potential antitumor activity of DHES0815A in HER2-overexpressing uterine serous carcinoma cell lines and xenografts, respectively. DHES0815A was unable to induce a significant bystander effect in HER2-negative tumors.105 DHES0815A was investigated in a phase I trial assessing the safety, tolerability, and PK in patients with HER2-positive advanced breast cancer (NCT03451162). Despite some antitumor activities, DHES0815A development was discontinued because of safety concerns and the narrow therapeutic window.106
Zanidatamab zovodotin
Zanidatamab zovodotin (ZW49), a novel bispecific anti-HER2 ADC developed using Zymeworks’ proprietary Azymetric and ZymeLink platforms, is composed of a humanized IgG1-like bispecific antibody, zanidatamab (also known as ZW25, which targets HER2 extracellular domains ECD2 and ECD4, pertuzumab and trastuzumab binding domains, respectively), through interchain disulfides, conjugated to a new auristatin derivative, N-acyl sulfonamide auristatin, through a protease-cleavable linker.107 After binding of ZW49 to HER2 and antigen/ADC internalization, the payload is released inside the cell and induces cancer cell death.108
In preclinical studies, ZW49 demonstrated more rapid internalization into HER2-expressing cells as compared with a monospecific trastuzumab-ADC, potent antitumor activity in HER2-high-expressing breast cancer cell lines and PDX models, and tumor regressions at exposure levels well tolerated in non-human primates.109 ZW49 is currently being evaluated in a phase I clinical trial in patients with locally advanced (unresectable) or metastatic HER2-expressing cancers (NCT03821233).
MM-302
MM-302, a PEGylated antibody-liposomal doxorubicin conjugate, is a novel anti-HER2 ADC (better known as antibody-conjugated nanoparticles [ACNPs], which take advantage of the potential of both antibody conjugation and nanotechnology) composed of an anti-HER2 scFv (F5, binding a different domain of HER2 compared with trastuzumab) conjugated to liposomal doxorubicin, also known as Caelyx or Myocet (doxorubicin encapsulated by a liposome) via poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE).110 Liposomal doxorubicin, used in MM-302, contains the chemotherapy drug doxorubicin encapsulated within a fatty covering liposome, which is used for the treatment of breast and ovarian cancer, myeloma, and HIV-related Kaposi’s sarcoma. MM-302 consists of a liposome encapsulating nearly 20,000 doxorubicin molecules in its core and 45 anti-HER2 scFv antibodies conjugated to its surface, allowing for targeted delivery of high-dose doxorubicin to tumor cells.111
Preclinical studies revealed greater antitumor activity of MM-302 as compared with both doxorubicin and PEGylated liposomal doxorubicin. Additionally, the combination of MM-302 and trastuzumab was found to be synergistic, showing enhanced antitumor activity in HER2-overexpressing xenograft models of breast and gastric cancer.112 In addition, cyclophosphamide pretreatment was found to increase the delivery and antitumor activity of MM-302.113 Promising results in preclinical studies led to evaluation of MM-302 in three clinical trials; however, disappointing results were obtained. One of the trials (NCT02735798) was withdrawn because the sponsor chose not to fund the trial. A phase II/III clinical study (NCT02213744), despite encouraging results in a phase I trial,114 was terminated owing to no clinical benefit of MM-302 plus trastuzumab in patients with refractory HER2-positive advanced/metastatic breast cancer.110 However, a phase I clinical study (NCT01304797) revealed a convenient safety profile and encouraging clinical activity of MM302, either as monotherapy or in combination with trastuzumab or trastuzumab and cyclophosphamide, in patients with HER2-positive advanced breast cancer. This clinical study is currently completed, and further clinical studies are under discussion.114
GQ1001
GQ1001 consists of an anti-HER2 mAb conjugated site-specifically to DM1 via an intelligent ligase-dependent conjugation (iLDC) system.115 Upon binding of GQ1001 to HER2 and antigen/ADC internalization, DM1 is released, binds to tubulin, and interferes with microtubule assembly and disassembly dynamics, resulting in the prevention of tumor cell proliferation and induction of apoptosis in HER2-expressing tumor cells. GQ1001 is being studied in a phase I clinical trial (NCT04450732) for patients with HER2-positive advanced solid tumors, including HER2-positive breast cancer.
B003
B003 consists of a recombinant humanized anti-HER2 mAb conjugated to DM1 via SMCC. Upon binding of B003 to HER2 and antigen/ADC internalization, the DM1 moiety is released, binds to tubulin, and disrupts microtubule assembly/disassembly dynamics, inhibiting cell division and proliferation in HER2-expressing tumor cells.63 B003 is currently under investigation in a phase I clinical trial in patients with HER2-positive recurrent or metastatic breast cancer.
BB-1701
BB-1701 consists of an anti-HER2 mAb conjugated to an as yet undisclosed payload. BB-1701 is currently being tested in a phase I clinical trial in patients with locally advanced/metastatic HER2-expressing solid tumors, including HER2-positive breast cancer.116
SHR-A1811
SHR-A1811 is composed of an ani-HER2 mAb conjugated to an as yet undisclosed payload. Upon binding of SHR-A1811 to HER2 and antigen/ADC internalization, the payload exerts its cytotoxic activity through inhibition of tumor cell proliferation and induction of apoptosis in HER2-expressing tumor cells. SHR-A1811 is currently being evaluated in several clinical trials in patients with HER2-expressing advanced solid tumors, including HER2-positive breast cancer.117
BICON-02
BICON-02 is a trastuzumab-based ADC. A phase I clinical trial (NCT03062007) evaluated safety, tolerability, and PK of multiple doses of BICON-02 in patients with HER2-positive metastatic breast cancer, previously treated with trastuzumab. The study was terminated because of the sponsor’s decision.118
TAA013
TAA013 consists of trastuzumab conjugated to DM1 through SMCC. Phase I and III studies of TAA013 were conducted in patients with HER2-positive breast cancer. The phase I study showed TAA013 safety and tolerability with efficacy in heavily pretreated HER2-positive breast cancer patients.119 The phase III study of TAA013 is in progress, comparing the efficacy and safety of TAA013 with lapatinib combined with capecitabine in patients with unresectable locally advanced or metastatic HER2-positive breast cancer.
Ujvira
Ujvira (ZRC-3256) is the first Drugs Controller General of India (DCGI)-approved biosimilar of Kadcyla, consisting of trastuzumab conjugated to DM1 via SMCC with an average DAR of 3.5 (because Ujvira is the Kadcyla biosimilar, the DAR is speculated from T-DM1).
In a study, Chiradoni Thungappa et al. compared the efficacy, safety, PK, and immunogenicity of Ujvira with those of T-DM1 in patients with HER2-positive metastatic breast cancer. Their findings revealed biosimilarity (efficacy, safety, PK, and immunogenicity) between Ujvira and Kadcyla. Thus, Ujvira proved itself as a cost-effective treatment alternative for Indian patients with HER2-positive metastatic breast cancer.120 Most importantly, Ujvira was launched by Zydus Cadila in India in 2021 for the treatment of both early and HER2-positive advanced breast cancer.121
SHR-A1201
SHR-A1201 is a biosimilar of Kadcyla, consisting of trastuzumab conjugated to DM1 via SMCC, with an average DAR of 3.5 (because SHR-A1201 is the Kadcyla biosimilar, the DAR is speculated from T-DM1). SHR-A1201 is currently being studied in a phase I clinical trial in patients with metastatic breast cancer.
DX126-262
DX126-262 consists of a recombinant humanized anti-HER2 IgG1 mAb (DX-CHO9) conjugated to Tub114, a derivative of tubulysins, through a cysteine thiol linkage, with an average ADR of 3.5–3.8.122 DX126-262 is currently undergoing a phase I clinical trial evaluating the safety, tolerability, and PK of DX126-262 in patients with HER2-positive advanced breast cancer and/or gastric cancer. Phased reports showed obvious tumor-killing effects, good effectiveness data, low toxicity, and good tolerability of DX126-262.
HS630
HS630 consists of a recombinant humanized anti-HER2 mAb conjugated to DM1. The toxicology study of HS630 was evaluated in Sprague-Dawley rats. HS630, at a certain dose, was found to be able to induce neurotoxicity, but the symptoms gradually recovered with the prolongation of administration time.123 HS630 was investigated in a study (CTR20181755) to evaluate the safety, tolerability, PK, immunogenicity, and efficacy of HS630 in single/multiple administration in patients with HER2-positive advanced breast cancer.
Antibody-drug conjugates in preclinical development against HER2-positive breast cancer
There are also additional new ADCs with potential antitumor activity in preclinical studies, underlining their potential clinical development in the not so distant future for patients with HER2-positive solid tumors, including HER2-positive breast cancer. In this section, we highlight some of such candidates.
GB251
GB251 is an anti-HER2 ADC consisting of GB221 (trastuzumab biosimilar) conjugated to MMAE via an innovative linker. Preclinical studies revealed the in vitro and in vivo inhibitory effects of GB251 against a variety of HER2-positive tumor cells and tumor-bearing mouse models at significantly lower doses than T-DM1. A phase Ia clinical trial is planned to be conducted for the evaluation of the safety, tolerability, PK/PD, and immunogenicity of GB251 in HER2-positive metastatic breast cancer patients.124
MI130004
MI130004 is composed of trastuzumab conjugated, via cysteine residues, to PM050489 (a marine compound that binds to β-tubulin at a new site and impaired tubulin polymerization, leading to mitotic aberrations, G2/M phase cell-cycle arrest, and cell death) through a non-cleavable linker, with an average DAR of 2.0. MI130004 showed extraordinary in vitro and in vivo antitumor activity against breast, ovary, and gastric cancers in a variety of HER2-positive cell lines and xenografts. Importantly, breast, gastric, and ovarian xenografts showed a significant decrease in tumor growth together with improved animal survival.125
CAT-01-106
CAT-01-106 is a trastuzumab-based ADC consisting of C-terminally aldehyde-tagged trastuzumab (CAT-01) conjugated site-specifically to the maytansine-based, non-cleavable linker payload (RED-106, with unique advantages such as excellent tolerability and efficacy providing a wide therapeutic window and resistance to the drug efflux pump, P-glycoprotein/MDR1), with an average DAR of 1.8. In a preclinical study, CAT-01-106 showed superior in vivo efficacy (including greater antitumor efficacy and considerable survival advantage) at equal payload dosing, good tolerability in efficacious dose ranges in both rats and cynomolgus monkeys, and improved PK with longer ADC exposure in the circulation, as compared with T-DM1. The data suggested that CAT-01-106 might be sufficiently tolerable to enable clinical dosing at trastuzumab-equivalent exposure levels and potentially improve patient outcomes.126
Conclusions and future perspectives
ADCs, as a smart anticancer approach, have gained tremendous attention over the past decades owing to targeted delivery of highly cytotoxic drugs to the tumors while sparing healthy cells. It is expected that ADCs will rapidly morph into remarkably superior replacements for the current anticancer therapeutics, particularly in breast cancer. Increasing rates of ADC approval by the FDA to treat solid tumors as well as progress in the development of innovative ADCs capable of acting in multiple malignancies resulted in their conquering the anticancer drug market. ADCs approved for the treatment of HER2-positive breast cancer, including T-DM1 and T-DXd, have brought tremendous clinical benefits to patients with advanced breast cancer, leading to a growing number of ADCs being developed for HER2-positive breast cancer. Considering growing trends of research and development in ADC technology, it is predicted that more ADCs will be granted approval in the near future in the form of new ADCs or label expansions of those that already exist.
A total of 29 HER2-directed ADCs are currently being investigated in clinical trials for HER2-positive breast cancer. These ADCs showed a broad range of diversity regarding ADC structure, including the mAbs, linkers, and payloads used, bioconjugation process, and optimization approaches (Table 3). Figure 3 also shows chemical structures of disclosed linker-drug moieties used in ADCs approved and under clinical trials for the treatment of HER2-positive breast cancer.
Figure 3.
Chemical structures of disclosed drug-linker moieties used in antibody-drug conjugates against HER2-positive breast cancer
The majority of ADCs currently under clinical investigation for the treatment of HER2-positive breast cancer use humanized IgG1 with a minimally immunogenic profile, ranging from biparatopic antibody (with the ability to simultaneously target two distinct antigens, theoretically making ADCs with improved specificity and better internalization) to THIOMAB (with engineered cysteine residues making homogeneous ADCs with defined and site-specific payloads) and scfv (with the ability to rapid penetration to tumors as compared with whole mAbs). Trastuzumab, or trastuzumab derivatives, was the most popular mAb, used in approximately 60% of the disclosed ADCs under clinical investigation against HER2-positive breast cancer (Figure 4A). This is because of the fact that not only is the biology of trastuzumab clinically well established but there is also still room for improvement in trastuzumab-based therapies.
Figure 4.
Antibody, payload, and linker types
Types of antibody (A), payload (B), and linker (C) used in antibody-drug conjugates under development for patients with HER2-positive breast cancer in clinical trials. mAb, monoclonal antibody.
The ADCs used in clinical trials against HER2-positive breast cancer bear different classes of payloads. More than 65% of the ADCs were found to carry microtubule inhibitors (such as auristatins, maytansinoids, and tubulysins), highlighting a central role of this class of payloads in ADCs under clinical studies. Approximately 14% and 10% of the ADCs utilize DNA-damaging agents (such as PBDs, duocarmycins, and doxorubicins) and immunomodulators (such as TLR agonists), respectively (Figure 4B). Most ADCs in the clinical trials (more than 90%) have an average DAR of 2–4; it seems that ADCs containing more potent payloads typically have a lower DAR of around 2, while those with less potent payloads appear to have a higher DAR value.
The cleavable linkers (particularly vc) are the most commonly used linkers in the ADCs under clinical investigation against HER2-positive breast cancer (Figure 4C). The unique characteristics of cleavable linkers, which make them a suitable fit for ADC development particularly for solid tumors, include potentials to release payloads in a more controlled, efficient, and faster manner while the ADC remains relatively stable in circulation to be cleaved in the TME (bystander effect), which makes them more effective when dealing with large solid tumors impermeable to large antibodies and tumors with non-internalizing or low-expression antigens, as well as having compatibility with a wide variety of payloads and utilizing a diversity of mechanisms of action.
The ADCs used in clinical trials against HER2-positive breast cancer use versatile and newly developed conjugation platforms, including prenyltransferase-mediated conjugation (LCB14-0110), NexMab ADC technology (ALT-P7), ImmunoTAC therapeutic (SBT6050), Ambrx’s non-natural amino acid incorporation technology platform (ARX788), Dolaflexin platform (XMT-1522), Immunosynthen platform (XMT-2056), ZymeLink platform (ZW49), and iLDC system (GQ1001). ADCs with the ZymeLink platform were reported to be more hydrophilic with more enhanced exposure and tolerability. The Dolaflexin platform with unique properties, including a higher DAR and controlled bystander effect, seems to be an attractive option for future studies, especially for resistant tumors.
Among ADCs under clinical investigation against HER2-positive breast cancer, A166, ALT-P7, SYD985, BDC-1001, RC48, ARX788, PF-06804103, Ujvira, and DX126-262 showed promising results and greater potency in patients with HER2-positive breast cancer. More importantly, Ujvira is the first DCGI-approved Kadcyla biosimilar launched for treating both early and advanced HER2-positive metastatic breast cancer patients in India. However, six of the ADCs, namely XMT-1522, ADCT-502, MEDI4276, MM-302, BAT8001, and DHES0815A, failed in clinical settings mainly because of a lack of efficacy or poor quality of efficacy.
Despite advancements in the treatments of patients with HER2-positive advanced breast cancers, resistance to ADCs, especially in T-DM1, is one of the main reasons for the lack of efficacy, remaining one of the major challenges. Such resistance may be developed after responding to the treatment (acquired resistance) or have existed from the beginning (de novo resistance).127
Several mechanisms of resistance to ADCs have been described, including antibody-mediated resistance (such as decreased HER2 expression, alterations in HER2 internalization, and reduced binding), impaired drug trafficking (such as compromised endocytosis, especially aberrant caveolae-mediated endocytosis), disrupted lysosomal function (such as lysosomal alkalization and impaired lysosomal proteolytic enzyme activity), and payload-related resistance (such as improved expression and activity of plasma membrane drug efflux pumps, and interrupting drug deposition and altered targets of payloads).115,127
Innovative development strategies and combination therapies, as well as analysis of predictive biomarkers for optimal therapy selection may help overcome or prevent resistance to ADCs.127 A combination therapy of ADCs with other therapeutic agents seems to be a potentially effective strategy for improving treatment outcomes, greater potency, and overcoming ADC resistance. Clinical trials combining ADCs and immune checkpoint inhibitors (such as anti-PD1 or anti-PD-L1 antibodies) demonstrated a synergistic rationale for ADCs and promising outcomes without significant new findings in toxicities. Other possible solutions in the anti-HER2 ADC resistance scenario include selection of small binding moieties while retaining high binding specificity to the tumor antigen, biparatopic antibodies capable of recognizing different binding epitopes of HER2 ECD, redirecting of HER2 internalization and recycling, more stable linkers to mitigate off-target toxicity with favorable PK properties, use of payloads with unique modalities that balance cell-killing potency, development of ADCs with different payloads containing multiple mechanisms of action, development of ADCs with a more potent payload or a higher DAR value, and advances in anti-HER2 ADC development in the preclinical stage.115,128 Of note, a variety of pharmaceutical companies are now screening new types of small-molecule drugs with higher efficiency. Most importantly, it seems that developing payloads with superior drug-like properties, including solubility, permeability, metabolic stability, and transporter substrate profile, could improve ADC performance and efficacy.26 In addition, most patients treated with ADCs suffer from certain side effects including hematotoxicity, nephrotoxicity, and lung or gastrointestinal reactions.129 The progress of ADCs with more stable linkers can be suggested to mitigate such side effects.
Overall, ADCs are likely to continue to be the fastest-growing drug class, more likely becoming the first-line treatment option for patients with cancers, including HER2-positive breast cancer, in the not so distant future. It is highly expected that novel HER2-targeting ADCs, whether monotherapy or in combination with other anticancer agents in development, will lead to breakthrough therapies and perhaps even ultimate cures for patients with HER2-positive advanced breast cancer. Importantly, recently emerging data (June 1, 2022) in a clinical trial (NCT03734029), showing the clinical benefit of T-DXd in patients with HER2-low, unresectable, and/or metastatic breast cancer which shocked the scientific community with its scientific importance,130,131 will shed light on the future development of ADCs for the treatment of patients with HER2-low breast cancer.
Acknowledgments
We would like to thank the authors and researchers whose valuable studies led to writing the manuscript. The authors also express special thanks to Mr. Saleh Javaher for his valuable comments and critical suggestions on study design. The authors received no financial support for the research, authorship, and publication of this manuscript.
Author contributions
M.A.-A. and H.S. conceived and designed the study. F.D., S.A.S., M.H.A., and N.M. also helped and participated in the study design. Z.N., F.D., Y.M., A.H.M., S.A.S., M.H.A., N.B., A.J., A.M., S.J., T.G., and Z.A. researched and collected the data, and prepared the original draft. F.D. and N.M. retrieved data from the clinical trials (https://clinicaltrials.gov). M.A.-A. and F.D. drew the tables. S.A.S., Z.N., F.D., and H.S. drew the figures. H.D. prepared the first draft of the breast cancer section and conducted the referencing process. M.A.A. and H.S. reviewed and edited the manuscript, and provided detailed feedback. All the authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Noushin Mashatan, Email: n.mashatan1@uni.brighton.ac.uk.
Hosein Shahsavarani, Email: hosein.shahsavarani@gmail.com.
Meghdad Abdollahpour-Alitappeh, Email: abdollahpour1983@yahoo.com.
References
- 1.Murray C.J., Lopez A.D. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Key T.J., Verkasalo P.K., Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Lei S., Zheng R., Zhang S., Wang S., Chen R., Sun K., Zeng H., Zhou J., Wei W. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021;41:1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muley H., Fadó R., Rodríguez-Rodríguez R., Casals N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020;177:113959. doi: 10.1016/j.bcp.2020.113959. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 7.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/s0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 8.Li B.T., Ross D.S., Aisner D.L., Chaft J.E., Hsu M., Kako S.L., Kris M.G., Varella-Garcia M., Arcila M.E. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J. Thorac. Oncol. 2016;11:414–419. doi: 10.1016/j.jtho.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerson J.N., Skariah S., Denlinger C.S., Astsaturov I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin. Investig. Drugs. 2017;26:531–540. doi: 10.1080/13543784.2017.1315406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang J., Dong L., Wang L., Wang L., Zhang J., Chen F., Zhang X., Huang M., Li S., Ma W., et al. HER2-targeted antibody drug conjugates for ovarian cancer therapy. Eur. J. Pharm. Sci. 2016;93:274–286. doi: 10.1016/j.ejps.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Vu T., Claret F.X. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdollahpour-Alitappeh M., Lotfinia M., Bagheri N., Sineh Sepehr K., Habibi-Anbouhi M., Kobarfard F., Balalaie S., Foroumadi A., Abbaszadeh-Goudarzi G., Abbaszadeh-Goudarzi K., Abolhassani M. Trastuzumab-monomethyl auristatin E conjugate exhibits potent cytotoxic activity in vitro against HER2-positive human breast cancer. J. Cell. Physiol. 2019;234:2693–2704. doi: 10.1002/jcp.27085. [DOI] [PubMed] [Google Scholar]
- 13.Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011;102:1–8. doi: 10.1111/j.1349-7006.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 14.Valabrega G., Montemurro F., Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 15.Wynn C.S., Tang S.C. Anti-HER2 therapy in metastatic breast cancer: many choices and future directions. Cancer Metastasis Rev. 2022;41:193–209. doi: 10.1007/s10555-022-10021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett J.T., Arteaga C.L. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biol. Ther. 2011;11:793–800. doi: 10.4161/cbt.11.9.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen D.L., Andersson M., Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat. Rev. 2009;35:121–136. doi: 10.1016/j.ctrv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Jackson D.Y. Processes for constructing homogeneous antibody drug conjugates. Org. Process. Res. Dev. 2016;20:852–866. [Google Scholar]
- 19.Fu Z., Li S., Han S., Shi C., Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct. Target. Ther. 2022;7:93. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panowski S., Bhakta S., Raab H., Polakis P., Junutula J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdollahpour-Alitappeh M., Lotfinia M., Gharibi T., Mardaneh J., Farhadihosseinabadi B., Larki P., Faghfourian B., Sepehr K.S., Abbaszadeh-Goudarzi K., Abbaszadeh-Goudarzi G., et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J. Cell. Physiol. 2019;234:5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 22.Yaghoubi S., Karimi M.H., Lotfinia M., Gharibi T., Mahi-Birjand M., Kavi E., Hosseini F., Sineh Sepehr K., Khatami M., Bagheri N., Abdollahpour-Alitappeh M. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J. Cell. Physiol. 2020;235:31–64. doi: 10.1002/jcp.28967. [DOI] [PubMed] [Google Scholar]
- 23.Conilh L., Sadilkova L., Viricel W., Dumontet C. Payload diversification: a key step in the development of antibody-drug conjugates. J. Hematol. Oncol. 2023;16:3. doi: 10.1186/s13045-022-01397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coats S., Williams M., Kebble B., Dixit R., Tseng L., Yao N.S., Tice D.A., Soria J.C. Antibody-drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin. Cancer Res. 2019;25:5441–5448. doi: 10.1158/1078-0432.ccr-19-0272. [DOI] [PubMed] [Google Scholar]
- 25.Chari R.V.J. Expanding the reach of antibody-drug conjugates. ACS Med. Chem. Lett. 2016;7:974–976. doi: 10.1021/acsmedchemlett.6b00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo R., Rich J.R. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell. 2022;40:1255–1263. doi: 10.1016/j.ccell.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Xie H., Adjei A.A. Antibody-drug conjugates for the therapy of thoracic malignancies. J. Thorac. Oncol. 2019;14:358–376. doi: 10.1016/j.jtho.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 28.FDA Drugs. 2022. https://www.fda.gov
- 29.Amiri-Kordestani L., Blumenthal G.M., Xu Q.C., Zhang L., Tang S.W., Ha L., Weinberg W.C., Chi B., Candau-Chacon R., Hughes P., et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 2014;20:4436–4441. doi: 10.1158/1078-0432.ccr-14-0012. [DOI] [PubMed] [Google Scholar]
- 30.Krop I.E., Kim S.B., González-Martín A., LoRusso P.M., Ferrero J.M., Smitt M., Yu R., Leung A.C.F., Wildiers H., TH3RESA study collaborators Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–699. doi: 10.1016/s1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 31.Drago J.Z., Modi S., Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021;18:327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damelin M. Springer; 2018. Innovations for Next-Generation Antibody-Drug Conjugates. [Google Scholar]
- 33.Manthri S., Singal S., Youssef B., Chakraborty K. Long-time response with ado-trastuzumab emtansine in a recurrent metastatic breast cancer. Cureus. 2019;11:e6036. doi: 10.7759/cureus.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter F.W., Barker H.R., Lipert B., Rothé F., Gebhart G., Piccart-Gebhart M.J., Sotiriou C., Jamieson S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer. 2020;122:603–612. doi: 10.1038/s41416-019-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogitani Y., Hagihara K., Oitate M., Naito H., Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N., Soma M., Okamoto H., Oitate M., Arakawa S., et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.ccr-15-2822. [DOI] [PubMed] [Google Scholar]
- 37.PMDA Report on the deliberation results. 2019. https://www.pmda.go.jp/files/000238706.pdf
- 38.Cardillo T.M., Govindan S.V., Sharkey R.M., Trisal P., Goldenberg D.M. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 2011;17:3157–3169. doi: 10.1158/1078-0432.ccr-10-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferraro E., Drago J.Z., Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 2021;23:84. doi: 10.1186/s13058-021-01459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doi T., Iwata H., Tsurutani J., Takahashi S., Park H., Redfern C.H., Shitara K., Shimizu C., Taniguchi H., Iwasa T. American Society of Clinical Oncology; 2017. Single Agent Activity of DS-8201a, a HER2-Targeting Antibody-Drug Conjugate, in Heavily Pretreated HER2 Expressing Solid Tumors. [Google Scholar]
- 41.Modi S., Tsurutani J., Takahashi S., Iwata H., Park H., Redfern C.H., Doi T., Li B., Iwasa T., Taira S. Safety and efficacy results from a phase 1 study of DS-8201a in patients with HER2 expressing breast cancers [abstract] Cancer Res. 2018 Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5-9; San Antonio, TX. Philadelphia (PA): AACR 78, Abstract nr PD3-07. [Google Scholar]
- 42.Liu Y., Lian W., Zhao X., Qi W., Xu J., Xiao L., Qing Y., Xue T., Wang J. A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are HER2-positive or HER2-amplified who did not respond or stopped responding to approved therapies. Am. Soc. Clin. Oncol. 2020;38:1049. [Google Scholar]
- 43.Hu X., Zhang J., Liu R., Gao S., Qing Y., Yi S., Yuan J., Chen H., Fan B., Zheng H. Phase I study of A166 in patients with HER2-expressing locally advanced or metastatic solid tumors [abstract] Cancer Res. 2020 Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10-14; San Antonio, TX. Philadelphia (PA): AACR 80, Abstract nr P1-18-16. [Google Scholar]
- 44.Lopez D.M., Barve M., Wang J., Bullock A.J., Pectasides E., Vaishampayan U., Spira A.I., Ulahannan S., Patnaik A., Sanborn R.E., et al. Abstract B005: A phase I study of A166, a novel anti-HER2 antibody-drug conjugate (ADC), in patients with locally advanced/metastatic solid tumors. Mol. Cancer Ther. 2019;18:B005. [Google Scholar]
- 45.Yu J., Fang T., Yun C., Liu X., Cai X. Antibody-drug conjugates targeting the human epidermal growth factor receptor family in cancers. Front. Mol. Biosci. 2022;9:847835. doi: 10.3389/fmolb.2022.847835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park Y.H., Ahn H.K., Kim J.-Y., Ahn J.S., Im Y.-H., Kim S.-H., Lee S., Chung H.-S., Park S.J. American Society of Clinical Oncology; 2020. First-in-human Phase I Study of ALT-P7, a HER2-Targeting Antibody-Drug Conjugate in Patients with HER2-Positive Advanced Breast Cancer. [Google Scholar]
- 47.Lee J.J., Choi H.J., Yun M., Kang Y., Jung J.E., Ryu Y., Kim T.Y., Cha Y.J., Cho H.S., Min J.J., et al. Enzymatic prenylation and oxime ligation for the synthesis of stable and homogeneous protein-drug conjugates for targeted therapy. Angew. Chem. Int. Ed. Engl. 2015;54:12020–12024. doi: 10.1002/anie.201505964. [DOI] [PubMed] [Google Scholar]
- 48.Lee B.I., Park M.H., Byeon J.J., Shin S.H., Choi J., Park Y., Park Y.H., Chae J., Shin Y.G. Quantification of an antibody-conjugated drug in fat plasma by an affinity capture LC-MS/MS method for a novel prenyl transferase-mediated site-specific antibody-drug conjugate. Molecules. 2020;25:1515. doi: 10.3390/molecules25071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deckert, J., Thirlway, J., Park, Y.-H., Song, H.Y., Chung, C.-W., Wang, X., Zhang, Z., and Lutz., R.J. (2022 Apr 8-13). IKS014, a HER2-targeting antibody drug conjugate incorporating novel bioconjugation and tumor-selective linker technology with improved in vivo efficacy and tolerability [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. Cancer Research 82 (Philadelphia (PA): AACR; Cancer Res 2022;82(12_Suppl):Abstract Nr 1753.).
- 50.NCI HM2/MMAE antibody-drug conjugate ALT-P7. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/hm2-mmae-antibody-drug-conjugate-alt-p7?redirect=true
- 51.Doronina S.O., Toki B.E., Torgov M.Y., Mendelsohn B.A., Cerveny C.G., Chace D.F., DeBlanc R.L., Gearing R.P., Bovee T.D., Siegall C.B., et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 52.Hartley J.A., Flynn M.J., Bingham J.P., Corbett S., Reinert H., Tiberghien A., Masterson L.A., Antonow D., Adams L., Chowdhury S., et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci. Rep. 2018;8:10479–10510. doi: 10.1038/s41598-018-28533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal S., Sau S., Iyer A.K., Dixit A., Kashaw S.K. Multiple strategies for the treatment of invasive breast carcinoma: a comprehensive prospective. Drug Discov. Today. 2022;27:585–611. doi: 10.1016/j.drudis.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Trail P.A., Dubowchik G.M., Lowinger T.B. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol. Ther. 2018;181:126–142. doi: 10.1016/j.pharmthera.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Zammarchi F., Reinert H.W., Janghra N., Corbett S., Mellinas-Gomez M., Chowdhury S., Arora N., Tyrer P., Bertelli F., Williams D.G. Mechanistic and benchmarking studies of ADCT-502, a pyrrolobenzodiazepine (PBD) dimer-containing antibody-drug conjugate (ADC) targeting HER2-expressing solid tumors [abstract] Cancer Res. 2017 doi: 10.1158/1538-7445.AM2017-1152. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR 77, Abstract nr 52. [DOI] [Google Scholar]
- 56.Tiberghien A.C., Levy J.N., Masterson L.A., Patel N.V., Adams L.R., Corbett S., Williams D.G., Hartley J.A., Howard P.W. Design and synthesis of tesirine, a clinical antibody-drug conjugate pyrrolobenzodiazepine dimer payload. ACS Med. Chem. Lett. 2016;7:983–987. doi: 10.1021/acsmedchemlett.6b00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zammarchi F., Chivers S., Williams D.G., Adams L., Mellinas-Gomez M., Tyrer P., Corbett S., D'Hooge F., Dissanayake S., Sims S., et al. ADCT-502, a novel pyrrolobenzodiazepine (PBD)-based antibody–drug conjugate (ADC) targeting low HER2-expressing solid cancers. Eur. J. Cancer. 2016;69:S28. [Google Scholar]
- 58.Businesswire ADC-Therapeutics-Announces-the-Termination-of-its-ADCT-502-Program-Targeting-HER2-Expressing-Solid-Tumors. 2018. https://www.businesswire.com/news/home/20180425005853/en/ADC-Therapeutics-Announces-the-Termination-of-its-ADCT-502-Program-Targeting-HER2-Expressing-Solid-Tumors
- 59.Hong R., Xia W., Wang L., Lee K., Lu Q., Jiang K., Li S., Yu J., Wei J., Tang W., et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: an open-label, dose-escalation, phase I study. Cancer Commun. 2021;41:171–182. doi: 10.1002/cac2.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson H.K., Park P.U., Widdison W.C., Kovtun Y.V., Garrett L.M., Hoffman K., Lutz R.J., Goldmacher V.S., Blättler W.A. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66:4426–4433. doi: 10.1158/0008-5472.can-05-4489. [DOI] [PubMed] [Google Scholar]
- 61.Austin C.D., De Mazière A.M., Pisacane P.I., van Dijk S.M., Eigenbrot C., Sliwkowski M.X., Klumperman J., Scheller R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell. 2004;15:5268–5282. doi: 10.1091/mbc.e04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang W., Deng X., Ou Z., Gan J., Dong Q., Tan B., Lu L., Chen B., Bao C., Li S. BAT8001, a potent anti-HER2 antibody-drug conjugate with a novel stable linker for the treatment of HER2-positive breast cancer [abstract] Cancer Res. 2019 Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4-8; San Antonio, TX. Philadelphia (PA): AACR 79, Abstract nr P6-17-39. [Google Scholar]
- 63.Zhang X., Huang A.C., Chen F., Chen H., Li L., Kong N., Luo W., Fang J. Novel development strategies and challenges for anti-Her2 antibody-drug conjugates. Antib. Ther. 2022;5:18–29. doi: 10.1093/abt/tbac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elgersma R.C., Coumans R.G.E., Huijbregts T., Menge W.M.P.B., Joosten J.A.F., Spijker H.J., de Groot F.M.H., van der Lee M.M.C., Ubink R., van den Dobbelsteen D.J., et al. Design, synthesis, and evaluation of linker-duocarmycin payloads: toward selection of HER2-targeting antibody-drug conjugate SYD985. Mol. Pharm. 2015;12:1813–1835. doi: 10.1021/mp500781a. [DOI] [PubMed] [Google Scholar]
- 65.Dokter W., Ubink R., van der Lee M., van der Vleuten M., van Achterberg T., Jacobs D., Loosveld E., van den Dobbelsteen D., Egging D., Mattaar E., et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol. Cancer Ther. 2014;13:2618–2629. doi: 10.1158/1535-7163.mct-14-0040-t. [DOI] [PubMed] [Google Scholar]
- 66.Eiger D., Agostinetto E., Saúde-Conde R., de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers. 2021;13:1015. doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staudacher A.H., Brown M.P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer. 2017;117:1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Lee M.M.C., Groothuis P.G., Ubink R., van der Vleuten M.A.J., van Achterberg T.A., Loosveld E.M., Damming D., Jacobs D.C.H., Rouwette M., Egging D.F., et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 2015;14:692–703. doi: 10.1158/1535-7163.mct-14-0881-t. [DOI] [PubMed] [Google Scholar]
- 69.Banerji U., van Herpen C.M.L., Saura C., Thistlethwaite F., Lord S., Moreno V., Macpherson I.R., Boni V., Rolfo C., de Vries E.G.E., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/s1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 70.Saura Manich C., O'Shaughnessy J., Aftimos P.G., van den Tweel E., Oesterholt M., Escrivá-de-Romaní S., Quenel Tueux N., Tan T.J., Lim J.S., Ladoire S., et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021;32:S1288. doi: 10.1016/j.annonc.2021.08.2088. [DOI] [Google Scholar]
- 71.Metz H., Childs M., Brevik J., Winship D., Brender T., Comeau M., Moyes K., Chang J., Adamo J., Setter B., et al. SBT6050, a HER2-directed TLR8 therapeutic, as a systemically administered, tumor-targeted human myeloid cell agonist. J. Clin. Oncol. 2020;38:3110. [Google Scholar]
- 72.Emens L., Beeram M., Hamilton E., Piha-Paul S., Odegard V., Hamke S., Hunder N., Klempner S. BMJ Specialist Journals; 2020. 317 A Phase 1/1b Study of SBT6050, a HER2-Directed Monoclonal Antibody Conjugated to a Toll-like Receptor 8 Agonist, in Subjects with Advanced HER2-Expressing Solid Tumors. [Google Scholar]
- 73.Sharma M., Carvajal R., Hanna G., Kang Y., Lee J., Lee K., Li B., Moore K., Pegram M., Rasco D., et al. 164P Preliminary results from a phase I/II study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. Ann. Oncol. 2021;32:S1453–S1454. [Google Scholar]
- 74.Sharma M., Carvajal R.D., Hanna G.J., Li B.T., Moore K.N., Pegram M.D., Rasco D.W., Spira A.I., Alonso M., Fang L., et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J. Clin. Oncol. 2021;39:2549. [Google Scholar]
- 75.Dumbrava E.I., Sharma M.R., Carvajal R.D., Catenacci D., Emens L.A., Gadgeel S.M., Hanna G.J., Juric D., Kang Y.-K., Lee J. Phase 1/2 study of a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), BDC-1001, as a single agent and in combination with an immune checkpoint inhibitor in patients with advanced HER2-expressing solid tumors [abstract] Cancer Res. 2021 Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR 81, Abstract nr OT-03-02. [Google Scholar]
- 76.Ackerman S.E., Pearson C.I., Gregorio J.D., Gonzalez J.C., Kenkel J.A., Hartmann F.J., Luo A., Ho P.Y., LeBlanc H., Blum L.K., et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer. 2021;2:18–33. doi: 10.1038/s43018-020-00136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faria M., Peay M., Lam B., Ma E., Yuan M., Waldron M., Mylott W.R., Jr., Liang M., Rosenbaum A.I. Multiplex LC-MS/MS assays for clinical bioanalysis of MEDI4276, an antibody-drug conjugate of tubulysin analogue attached via cleavable linker to a biparatopic humanized antibody against HER-2. Antibodies. 2019;8:11. doi: 10.3390/antib8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pegram M.D., Hamilton E.P., Tan A.R., Storniolo A.M., Balic K., Rosenbaum A.I., Liang M., He P., Marshall S., Scheuber A., et al. First-in-Human, phase 1 dose-escalation study of biparatopic anti-HER2 antibody-drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol. Cancer Ther. 2021;20:1442–1453. doi: 10.1158/1535-7163.mct-20-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J.Y., Perry S.R., Muniz-Medina V., Wang X., Wetzel L.K., Rebelatto M.C., Masson Hinrichs M.J., Bezabeh B.Z., Fleming R.L., Dimasi N., et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer cell. 2019;35:948–949. doi: 10.1016/j.ccell.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Pegram M., Hamilton E., Tan A., Storniolo A., Elgeioushi N., Marshall S., Abdullah S., Patel M. Phase 1 study of bispecific HER2 antibody-drug conjugate MEDI4276 in patients with advanced HER2-positive breast or gastric cancer. Ann. Oncol. 2018;29:iii8. [Google Scholar]
- 81.Yao X., Jiang J., Wang X., Huang C., Li D., Xie K., Xu Q., Li H., Li Z., Lou L., Fang J. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res. Treat. 2015;153:123–133. doi: 10.1007/s10549-015-3503-3. [DOI] [PubMed] [Google Scholar]
- 82.Li H., Yu C., Jiang J., Huang C., Yao X., Xu Q., Yu F., Lou L., Fang J. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol. Ther. 2016;17:346–354. doi: 10.1080/15384047.2016.1139248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deeks E.D. Disitamab vedotin: first approval. Drugs. 2021;81:1929–1935. doi: 10.1007/s40265-021-01614-x. [DOI] [PubMed] [Google Scholar]
- 84.Skidmore L., Sakamuri S., Knudsen N.A., Hewet A.G., Milutinovic S., Barkho W., Biroc S.L., Kirtley J., Marsden R., Storey K., et al. ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol. Cancer Ther. 2020;19:1833–1843. doi: 10.1158/1535-7163.mct-19-1004. [DOI] [PubMed] [Google Scholar]
- 85.Humphreys R.C., Kirtely J., Hewit A., Biroc S., Knudsen N., Skidmore L., Wahl A. Site specific conjugation of ARX-788, an antibody drug conjugate (ADC) targeting HER2, generates a potent and stable targeted therapeutic for multiple cancers [abstract] Cancer Res. 2015 doi: 10.1158/1538-7445.AM2015-1639. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA. Philadelphia (PA): AACR 75, Abstract nr 639. [DOI] [Google Scholar]
- 86.Nagaraja Shastri P., Zhu J., Skidmore L., Liang X., Ji Y., Gu Y., Tian F., Yao S., Xia G. Nonclinical development of next-generation site-specific HER2-targeting antibody-drug conjugate (ARX788) for breast cancer treatment. Mol. Cancer Ther. 2020;19:1822–1832. doi: 10.1158/1535-7163.mct-19-0692. [DOI] [PubMed] [Google Scholar]
- 87.Hu X., Zhang J., Ji D., Xia G., Ji Y., Xiong G., Liang X. Abstract P1-18-16: a phase 1 study of ARX788, a HER2-targeting antibody-drug conjugate, in patients with metastatic HER2-positive breast cancer. Cancer Res. 2020;80:P1-18-P16–P11-18-16. [Google Scholar]
- 88.Li H., Zhang X., Xu Z., Li L., Liu W., Dai Z., Zhao Z., Xiao L., Li H., Hu C. Preclinical evaluation of MRG002, a novel HER2-targeting antibody-drug conjugate with potent antitumor activity against HER2-positive solid tumors. Antib. Ther. 2021;4:175–184. doi: 10.1093/abt/tbab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J., Guo Y., Xue J., Peng W., Ge X., Zhao W., Dai C., Xue L., Tang W., Hu C. American Society of Clinical Oncology; 2020. First-in-human Phase I Study of Anti-HER2 ADC MRG002 in Patients with Relapsed/refractory Solid Tumors. [Google Scholar]
- 90.Jiang Z., Sun T., Wang X., Liu Q., Yan M., Tong Z., Geng C., Tang J., Yin Y., Yu G., et al. A multiple center, open-label, single-arm, phase II clinical trial of MRG002, an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer. J. Clin. Oncol. 2022;40:1102. doi: 10.1200/JCO.2022.40.16_suppl.1102. [DOI] [Google Scholar]
- 91.Hui X., Yuan C., Cao W., Ge W., Zhang D., Dan M., Zhao Q., Liu B., Yao B. An innovative site-specific anti-HER2 antibody-drug conjugate with high homogeneity and improved therapeutic index. Onco. Targets Ther. 2022;15:331–343. doi: 10.2147/OTT.S357326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Joncour V., Martins A., Puhka M., Isola J., Salmikangas M., Laakkonen P., Joensuu H., Barok M. A novel anti-HER2 antibody-drug conjugate XMT-1522 for HER2-positive breast and gastric cancers resistant to trastuzumab emtansine. Mol. Cancer Ther. 2019;18:1721–1730. doi: 10.1158/1535-7163.mct-19-0207. [DOI] [PubMed] [Google Scholar]
- 93.Yurkovetskiy A.V., Bodyak N.D., Yin M., Thomas J.D., Clardy S.M., Conlon P.R., Stevenson C.A., Uttard A., Qin L., Gumerov D.R., et al. Dolaflexin: a novel antibody-drug conjugate platform featuring high drug loading and a controlled bystander effect. Mol. Cancer Ther. 2021;20:885–895. doi: 10.1158/1535-7163.mct-20-0166. [DOI] [PubMed] [Google Scholar]
- 94.Bergstrom D., Bodyak N., Park P., Yurkovetskiy A., DeVit M., Yin M., Poling L., Thomas J., Gumerov D., Xiao D. XMT-1522 induces tumor regressions in pre-clinical models representing HER2-positive and HER2 low-expressing breast cancer [abstract] Cancer Res. 2016 Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2015 Dec 8-12; San Antonio, TX. Philadelphia (PA): AACR 76, Abstract nr P4-14-28. [Google Scholar]
- 95.Soleyman-Jahi S., Zendehdel K., Sadeghi F., Afshari Z., Barati T., Mohammadnezhad S., Ghasemi S., Amanpour S. Inhibitory effects of aprotinin on survival and local invasion of human breast cancer cell lines [abstract] Cancer Res. 2017 doi: 10.1158/1538-7445.AM2017-1154. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR 77, Abstract nr 4. [DOI] [Google Scholar]
- 96.Bergstrom D.A., Bodyak N., Yurkovetskiy A., Park P.U., DeVit M., Yin M., Poling L., Thomas J.D., Gumerov D., Xiao D. A novel, highly potent HER2-targeted antibody-drug conjugate (ADC) for the treatment of low HER2-expressing tumors and combination with trastuzumab-based regimens in HER2-driven tumors [abstract] Cancer Res. 2015 doi: 10.1158/1538-7445.AM2015-LB-1231. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA. Philadelphia (PA): AACR 75, Abstract nr LB-231. [DOI] [Google Scholar]
- 97.Hamilton E.P., Barve M.A., Bardia A., Beeram M., Bendell J.C., Mosher R., Hailman E., Bergstrom D.A., Burris H.A., Soliman H.H. American Society of Clinical Oncology; 2018. Phase 1 Dose Escalation of XMT-1522, a Novel HER2-Targeting Antibody-Drug Conjugate (ADC), in Patients (Pts) with HER2-Expressing Breast, Lung and Gastric Tumors. [Google Scholar]
- 98.Duvall J.R., Bukhalid R.A., Cetinbas N.M., Catcott K.C., Kelly S., Avocetien K., Bentley K.W., Bradley S., Clardy S., Scott D., et al. XMT-2056, a well-tolerated, Immunosynthen-based STING-agonist antibody-drug conjugate which induces anti-tumor immune activity [abstract] Cancer Res. 2021;81 [Google Scholar]
- 99.J.R. Duvall, R.A. Bukhalid, N.M. Cetinbas, K.C. Catcott, Kelly L., K.W. Bentley, S. Clark, S. Clardy, S.D. Collins, A. Dirksen, et al. (2022 Apr 8-13). XMT-2056, a HER2-targeted Immunosynthen STING-agonist antibody-drug conjugate, binds a novel epitope of HER2 and shows increased anti-tumor activity in combination with trastuzumab and pertuzumab [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. 82.
- 100.Therapeutics M. Mersana therapeutics announces initiation of phase 1 trial of XMT-2056 in HER2-expressing tumors. 2023. https://ir.mersana.com/news-releases/news-release-details/mersana-therapeutics-announces-initiation-phase-1-trial-xmt-2056
- 101.Meric-Bernstam F., Calvo E., Moreno V., Chung H.C., Park Y.H., Bang Y.-J., Rosen L.S., Mita M.M., Garrido-Laguna I., Leung A.C. American Society of Clinical Oncology; 2020. A Phase I Dose Escalation Study Evaluating the Safety and Tolerability of a Novel Anti-HER2 Antibody-Drug Conjugate (PF-06804103) in Patients with HER2-Positive Solid Tumors. [Google Scholar]
- 102.Graziani E.I., Sung M., Ma D., Narayanan B., Marquette K., Puthenveetil S., Tumey L.N., Bikker J., Casavant J., Bennett E.M., et al. PF-06804103, A site-specific anti-HER2 antibody-drug conjugate for the treatment of HER2-expressing breast, gastric, and lung cancers. Mol. Cancer Ther. 2020;19:2068–2078. doi: 10.1158/1535-7163.mct-20-0237. [DOI] [PubMed] [Google Scholar]
- 103.Betts A., Clark T., Jasper P., Tolsma J., van der Graaf P.H., Graziani E.I., Rosfjord E., Sung M., Ma D., Barletta F. Use of translational modeling and simulation for quantitative comparison of PF-06804103, a new generation HER2 ADC, with Trastuzumab-DM1. J. Pharmacokinet. Pharmacodyn. 2020;47:513–526. doi: 10.1007/s10928-020-09702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.NCI anti-HER2/PBD-MA antibody-drug conjugate DHES0815A. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/anti-her2-pbd-ma-antibody-drug-conjugate-dhes0815a?redirect=true
- 105.Tymon-Rosario J., Bonazzoli E., Bellone S., Manzano A., Pelligra S., Guglielmi A., Gnutti B., Nagarkatti N., Zeybek B., Manara P., et al. DHES0815A, a novel antibody-drug conjugate targeting HER2/neu, is highly active against uterine serous carcinomas in vitro and in vivo. Gynecol. Oncol. 2021;163:334–341. doi: 10.1016/j.ygyno.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krop I., Hamilton E., Jung K.H., Modi S., Kalinsky K.M., Phillips G., Shi R., Monemi S., Mamounas M., Saad O. A phase I dose-escalation study of DHES0815A, a HER2-targeting antibody-drug conjugate with a DNA monoalkylator payload, in patients with HER2-positive breast cancer [abstract] Cancer Res. 2022 Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7-10; San Antonio, TX. Philadelphia (PA): AACR 82, Abstract nr P2-13-25. [Google Scholar]
- 107.Kunte S., Abraham J., Montero A.J. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126:4278–4288. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 108.MSKCC A. Phase I study of ZW49 in inoperable or metastatic HER2-positive cancers. https://www.mskcc.org/cancer-care/clinical-trials/19-148
- 109.Hamblett K., Barnscher S., Davies R., Hammond P., Hernandez A., Wickman G., Fung V., Ding T., Garnett G., Galey A. ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers [abstract] Cancer Res. 2019 Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4-8; San Antonio, TX. Philadelphia (PA): AACR 79, Abstract nr P6-17-13. [Google Scholar]
- 110.Miller K., Cortes J., Hurvitz S.A., Krop I.E., Tripathy D., Verma S., Riahi K., Reynolds J.G., Wickham T.J., Molnar I., Yardley D.A. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC cancer. 2016;16:352. doi: 10.1186/s12885-016-2385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynolds J.G., Geretti E., Hendriks B.S., Lee H., Leonard S.C., Klinz S.G., Noble C.O., Lücker P.B., Zandstra P.W., Drummond D.C., et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol. Appl. Pharmacol. 2012;262:1–10. doi: 10.1016/j.taap.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 112.Espelin C.W., Leonard S.C., Geretti E., Wickham T.J., Hendriks B.S. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016;76:1517–1527. doi: 10.1158/0008-5472.can-15-1518. [DOI] [PubMed] [Google Scholar]
- 113.Geretti E., Leonard S.C., Dumont N., Lee H., Zheng J., De Souza R., Gaddy D.F., Espelin C.W., Jaffray D.A., Moyo V., et al. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302) Mol. Cancer Ther. 2015;14:2060–2071. doi: 10.1158/1535-7163.mct-15-0314. [DOI] [PubMed] [Google Scholar]
- 114.Munster P., Krop I.E., LoRusso P., Ma C., Siegel B.A., Shields A.F., Molnár I., Wickham T.J., Reynolds J., Campbell K., et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody-liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br. J. Cancer. 2018;119:1086–1093. doi: 10.1038/s41416-018-0235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Díaz-Rodríguez E., Gandullo-Sánchez L., Ocaña A., Pandiella A. Novel ADCs and strategies to overcome resistance to anti-HER2 ADCs. Cancers (Basel) 2021;14 doi: 10.3390/cancers14010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.A first-in-human study of multiple doses of BB-1701 in subjects with locally advanced/metastatic HER2 expressing solid tumors. https://clinicaltrials.gov/ct2/show/NCT04257110
- 117.Ib/II phase study of SHR-a1811 injection in HER2 positive breast cancer. https://clinicaltrials.gov/ct2/show/NCT05353361
- 118.Open-label study of safety, tolerability and pharmacokinetics of multiple doses of BI-CON-02 in patients with HER2-positive metastatic breast cancer, previously treated with trastuzumab. https://clinicaltrials.gov/ct2/show/NCT03062007
- 119.Liu J., Yin Y., Wu H., Li W., Huang X., Li X. TAA013 a trastuzumab antibody drug conjugate phase I dose escalation study in recurrent her2 positive breast cancer [abstract] Cancer Res. 2021 Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR 81, Abstract nr PS10-51. [Google Scholar]
- 120.Chiradoni Thungappa S., Maksud T., Raut N., Nagarkar R., Batra U., Kumar S., Parmar D., Study Investigator Group Comparison of the efficacy, safety, pharmacokinetic and immunogenicity of UJVIRA (ZRC-3256, trastuzumab emtansine) with the Kadcyla (trastuzumab emtansine) in the treatment of HER2-positive metastatic breast cancer: a randomized, open-label, multicenter study in India. Clin. Breast Cancer. 2022;22:300–307. doi: 10.1016/j.clbc.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 121.DEVELOPMENT B. https://www.biosimilardevelopment.com
- 122.Li, H., and Li, H. A narrative review of the current landscape and future perspectives of HER2-targeting antibody drug conjugates for advanced breast cancer.Transl. Breast Cancer Res.,2, p.29
- 123.Qian-qian L.I., Zhang Y., Xue-peng X.U., Wang C., Dan M., Wang X., Huo Y., Wang H.-B., San-Long W. Safety evaluation of antibody-drug-conjugates(ADC)HS630 on the central nervous system of rats. Chin. J. Pharm. Anal. 2018;38:1189–1195. [Google Scholar]
- 124.CMBI A biopharmaceutical company focusing on oncology and autoimmune therapies. 2020. https://www.cmbi.com/upload/202011/20201123840980.pdf
- 125.Avilés P., Domínguez J.M., Guillén M.J., Muñoz-Alonso M.J., Mateo C., Rodriguez-Acebes R., Molina-Guijarro J.M., Francesch A., Martínez-Leal J.F., Munt S., et al. MI130004, a novel antibody-drug conjugate combining trastuzumab with a molecule of marine origin, shows outstanding in vivo activity against HER2-expressing tumors. Mol. Cancer Ther. 2018;17:786–794. doi: 10.1158/1535-7163.mct-17-0795. [DOI] [PubMed] [Google Scholar]
- 126.Barfield R.M., Kim Y.C., Chuprakov S., Zhang F., Bauzon M., Ogunkoya A.O., Yeo D., Hickle C., Pegram M.D., Rabuka D., Drake P.M. A novel HER2-targeted antibody-drug conjugate offers the possibility of clinical dosing at trastuzumab-equivalent exposure levels. Mol. Cancer Ther. 2020;19:1866–1874. doi: 10.1158/1535-7163.MCT-20-0190. [DOI] [PubMed] [Google Scholar]
- 127.Chen Y.F., Xu Y.Y., Shao Z.M., Yu K.D. Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer Commun. 2023;43:297–337. doi: 10.1002/cac2.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barroso-Sousa R., Tolaney S.M. Clinical development of new antibody-drug conjugates in breast cancer: to infinity and beyond. BioDrugs. 2021;35:159–174. doi: 10.1007/s40259-021-00472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hackshaw M.D., Danysh H.E., Singh J., Ritchey M.E., Ladner A., Taitt C., Camidge D.R., Iwata H., Powell C.A. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 2020;183:23–39. doi: 10.1007/s10549-020-05754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.OncLive. https://www.onclive.com/view/antibody-drug-conjugates-may-represent-the-future-of-her2-low-breast-cancer-treatment
- 131.Zhang H., Karakas C., Tyburski H., Turner B.M., Peng Y., Wang X., Katerji H., Schiffhauer L., Hicks D.G. HER2-low breast cancers: current insights and future directions. Semin. Diagn. Pathol. 2022;39:305–312. doi: 10.1053/j.semdp.2022.07.003. [DOI] [PubMed] [Google Scholar]