Abstract
OBP-301 is an oncolytic adenovirus modified to replicate within cancer cells and lyse them. This open-label, non-comparative, phase I dose-escalation trial aimed to assess its safety and optimal dosage in 20 patients with advanced hepatocellular carcinoma. Good tolerance was shown with a maximum tolerated dose of 6 × 1012 viral particles. The most common treatment-emergent adverse events were influenza-like illness, pyrexia, fatigue, decreased platelet count, abdominal distension, and anemia. Cohorts 4 and 5 had approximately 50% higher levels of CD8+ T cells in the peripheral blood after injection. The best target response occurred in 14 patients, 4 of whom had progressive disease. Multiple intratumoral injections of OBP-301 were well tolerated in patients with advanced hepatocellular carcinoma. The stable disease rate for the injected tumors was greater than the overall response rate, even with no obvious tumor response. OBP-301 might have a greater impact on local response as histological examination revealed that the presence of OBP-301 was consistent with the necrotic area at the injection site. Increased infiltration of CD8+ T cells and <1% PD-L1 expression were observed in tumors after injection. Improved antitumor efficacy might be achieved in future studies via viral injection with volume adjustment and in combination with other immuno-therapeutics.
Keywords: OBP-301, suratodenoturav, oncolytic adenovirus, advanced hepatocellular carcinoma, telomerase reverse transcriptase gene, hTERT promoter, tumor microenvironment, clinical and translational
Graphical abstract
Heo and colleagues find that the stable disease rate for injected tumors was greater than overall response rate, and OBP-301 was well tolerated. OBP-301 injection modulates the tumor microenvironment. Despite multiple prior HCC therapies, patients had acceptable ECOG performance statuses and liver function.
Introduction
Hepatocellular carcinoma (HCC) is a major type of primary liver cancer and the fifth most common cancer worldwide.1,2 As it is highly refractory to conventional chemotherapy and radiotherapy, the associated mortality rate is increasing.3,4 HCC is endemic to Asia and a leading cause of cancer-related deaths there. All currently available treatments have limitations and the median overall survival (OS) time is approximately 7 months for patients with advanced HCC.5 Furthermore, if HCC is localized, the risk of death is high.
The first approved systemic therapy for HCC was sorafenib, a multikinase inhibitor that increases survival time but only by 3 months.6,7 Several other drugs have slightly increased survival time, but the mean-time in advanced HCC remains <1 year. With the recently approved combination treatment of atezolizumab and bevacizumab, the median survival time has increased to 19.2 months; however, no long-lasting antitumor effects after treatment cessation have been reported.8,9 Therefore, factors, such as chronic inflammation, immune evasion, immunosuppressive environment, and T cell exhaustion contributing to disease growth and progression and characterizing HCC must be considered when developing a novel therapy.10,11 Studies have indicated an oncolytic virus that selectively replicates in cancer cells to be clinically effective.12 As adenoviruses readily infect liver tissues, undergo selective replication in cancer cells, cause tumor cell lysis, and are easily genetically modified, they are most suitable for HCC applications.13,14,15
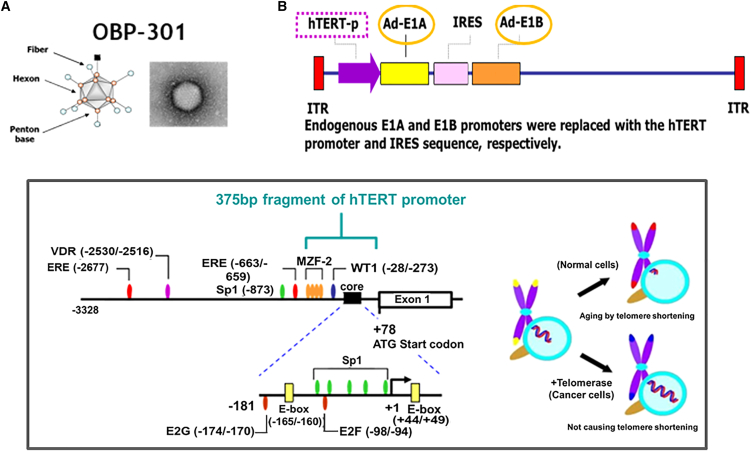
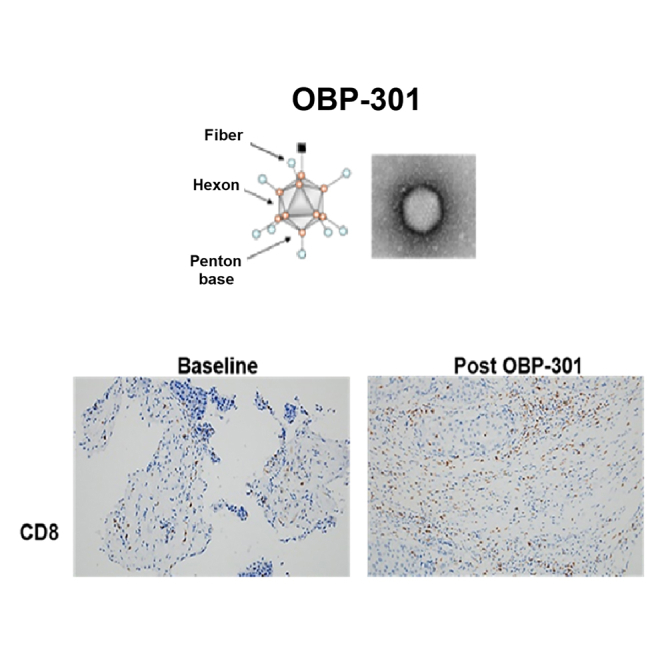
Recent clinical studies using adenoviruses have focused on the use of cancer-specific promoters to control the expression of viral genes necessary for replication.16,17 The human telomerase reverse transcriptase gene (hTERT) encodes the catalytic protein subunit of telomerase, which functions in the maintenance of telomere length. This gene typically has high expression in cancer cells, but little to no expression in healthy or differentiated cells. Therefore, the hTERT promoter can be utilized as a molecular switch for the selective expression of target genes in tumor cells. OBP-301 (suratadenoturev) is a recently developed attenuated type 5 adenovirus (Ad5), in which an hTERT promoter is used to increase expression of adenovirus early in regions associated with an internal ribosome entry site (IRES) sequence (Figure 1).18,19,20 This construct causes tumor-specific viral replication and lytic death of a variety of cancer cells. The virus OBP-301, which has the construct, replicates better in tissues having high levels of hTERT promoter-activating elements such as cancer. In normal tissues hTERT transcriptional activity is not observed basically. In other words, OBP-301 replicates and lyses the normal healthy cells hardly, thereby selectively causing cancer cell lysis. Although OBP-301 is replication competent, it replicates only in cells with an activated hTERT promoter. In vitro experiments have confirmed that OBP-301 selectively infects and lyses cancer cells,18 and animal studies have indicated that its intratumoral (i.t.) injection leads to antitumor activity, with no significant toxicity and distant viral uptake in contralateral, non-injected tumors.18
Figure 1.
Structures of hTERT promoter-driven oncolytic adenovirus, OBP-301
(A and B) OBP-301 (Suratadenoturev) is a recently developed attenuated type 5 adenovirus (Ad5) in which a promoter of hTERT is used to increase the expression of adenovirus early region 1A (E1A) and early region 1B (E1B), which are associated with an internal ribosome entry site (IRES) sequence. hTERT, human telomerase reverse transcriptase; ITR, inverted terminal repeat.
OBP-301 may kill tumor cells in vivo through several mechanisms. First, it may induce direct cell lysis owing to viral replication. Second, based on animal studies showing induction of acute and chronic inflammatory infiltration with local production of tumor necrosis factor and interleukin-1 and -6 during the acute phase,21 OBP-301 may induce local cytokine production with direct cytopathic effects and increase immune effector cell recruitment into the tumor. Third, OBP-301 may augment tumor antigen presentation and enhance tumor antigenicity, culminating in cytotoxic T cells with improved tumor cell recognition and cytotoxicity. A previous phase I clinical trial has assessed the effectiveness of locoregional administration of OBP-301 for advanced head and neck cancer and metastatic melanoma and confirmed its safety.22 However, no studies have evaluated OBP-301 for HCC. Therefore, in this study, we performed a phase I dose-escalation study of OBP-301 to assess its safety, response, and pharmacodynamics, focusing on the effects of single and multiple doses in patients with refractory advanced HCC.
Results
Patient profile
Baseline clinical and demographic characteristics were similar among the five cohorts (Table 1). Overall, 18 patients were male, 6 were Chinese, and 14 were Korean, with a median age of 59.39 years (48.4–65.9) and a median body mass index of 22.84 kg/m2 (17.5–27.4). The median time from the initial diagnosis of HCC was 3.24 years (0.2–8.3). The Eastern Cooperative Oncology Group (ECOG) performance status was 0 (10 patients), 1 (nine patients), or 2 (one patient). Overall, 13 patients had stage C cancer according to the Barcelona Clinic Liver Cancer (BCLC) system, all had Child-Pugh class A (5 or 6 points), and 17 had a Child-Pugh score of 5 points. In addition, 19 patients were hepatitis B positive and one was hepatitis C positive. All patients had received previous treatment (transcatheter arterial chemoembolization [TACE] and/or sorafenib). The median size of the largest target tumor was 2.85 cm (1.8–8.1) at screening. Eight patients had previously received more than five therapies (Table S1). Nineteen patients had previously undergone a non-surgical procedure, with radiotherapy being the most common (N = 17), and 17 had previously undergone a surgical procedure.
Table 1.
Baseline characteristics of enrolled patients who received OBP-301 for hepatocellular carcinoma
| Cohort 1 N = 3 |
Cohort 2 N = 3 |
Cohort 3 N = 3 |
Cohort 4 N = 3 |
Cohort 5 N = 8 |
Total N = 20 |
|
|---|---|---|---|---|---|---|
| Age at enrollment (years) | 61.34 (49.6–62.8) | 55.23 (48.4–61.9) | 53.15 (50.6–63.2) | 59.10 (50.0–62.2) | 60.69 (57.1–65.9) | 59.39 (48.4–65.9) |
| Duration since HCC diagnosis (years) | 3.73 (3.0–7.3) | 1.19 (0.2–1.4) | 6.00 (2.3–8.3) | 3.12 (1.5–3.4) | 4.97 (1.5–7.6) | 3.24 (0.2–8.3) |
| Gender | ||||||
| Male | 3 (100.0) | 2 (66.7) | 3 (100.0) | 2 (66.7) | 8 (100.0) | 18 (90.0) |
| Female | 0 | 1 (33.3) | 0 | 1 (33.3) | 0 | 2 (10.0) |
| Ethnicity | ||||||
| Chinese | 2 (66.7) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (12.5) | 6 (30.0) |
| Korean | 1 (33.3) | 2 (66.7) | 2 (66.7) | 2 (66.7) | 7 (87.5) | 14 (70.0) |
| Barcelona Clinic Liver Cancer stage | ||||||
| Stage B | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 1 (12.5) | 7 (35.0) |
| Stage C | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 7 (87.5) | 13 (65.0) |
| ECOG performance score | ||||||
| 0 | 2 (66.7) | 3 (100.0) | 2 (66.7) | 1 (33.3) | 2 (25.0) | 10 (50.0) |
| 1 | 1 (33.3) | 0 | 1 (33.3) | 2 (66.7) | 5 (62.5) | 9 (45.0) |
| 2 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (5.0) |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Child-Pugh status | ||||||
| 5 | 3 (100.0) | 3 (100.0) | 2 (66.7) | 2 (66.7) | 7 (87.5) | 17 (85.0) |
| 6 | 0 | 0 | 1 (33.3) | 1 (33.3) | 1 (12.5) | 3 (15.0) |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alpha-fetoprotein (ng/mL) | ||||||
| ≥400 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 5 (62.5) | 10 (50.0) |
| <400 | 2 (66.7) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 3 (37.5) | 10 (50.0) |
| Hepatitis B and hepatitis C screening | ||||||
| HBV-positive | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 7 (87.5) | 19 (95.0) |
| HCV-positive | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (5.0) |
| Previous treatment (TACE and/or sorafenib) | ||||||
| Yes | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 8 (100.0) | 20 (100.0) |
| No | 0 | 0 | 0 | 0 | 0 | 0 |
| Size of largest target tumor (cm) | 2.00 (1.8–3.0) | 4.00 (2.4–8.1) | 2.30 (2.2–2.7) | 2.50 (2.4–3.3) | 4.45 (2.1–7.6) | 2.85 (1.8–8.1) |
| Total cumulative dose (×1010 VP/tumor) | 1.00 (1.0–1.0) | 10 (10.0–10.0) | 100 (100–100) | 300 (300–300) | 600 (200–600) | |
Numbers indicate median (range) or N (% of patients).
Patient disposition
A total of 27 patients were initially screened, of whom 20 eligible patients were ultimately enrolled (Figure 2). Each of the four single-dose cohorts included three patients, and the multiple-dose cohort included eight. Most (18) completed the study treatment; two in cohort 5 did not complete the treatment due to adverse events (AEs) or other reasons (bacterial pneumonia). For the single-injection cohort, all patients received OBP-301 according to protocol. Six patients in cohort 5 received three doses of OBP-301; one patient in cohort 5 experienced dose delays due to increased total bilirubin levels.
Figure 2.
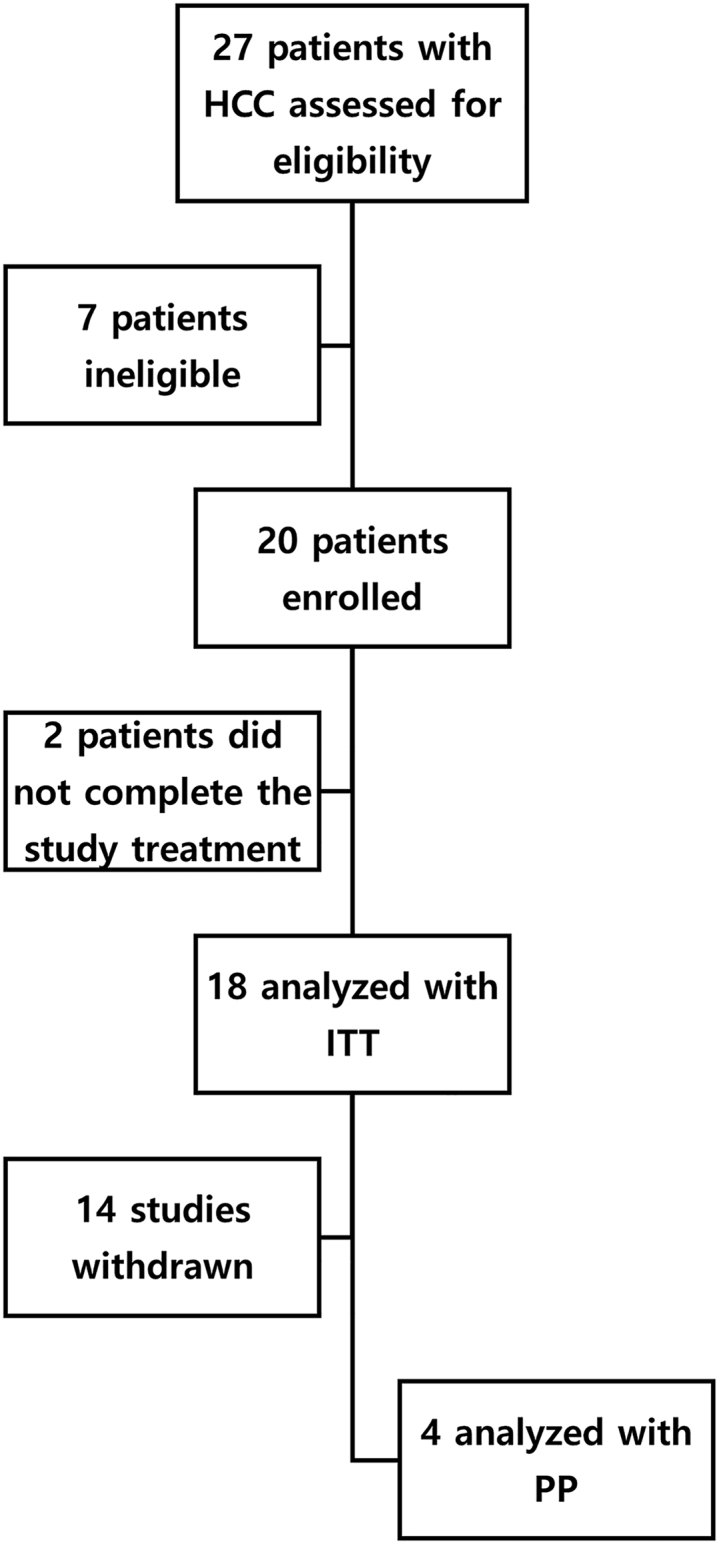
Assessment, enrollment, and analysis of enrolled patients
Initially, 27 patients were screened, and 20 eligible patients were ultimately enrolled. The four single-dose cohorts each had three patients and the multiple-dose cohort had eight patients. Most patients (18) completed the study treatment; 2 patients in cohort 5 did not complete treatment due to adverse events or other reasons.
Safety assessment
A dose-limiting toxicity (DLT) (grade 3 on day 57) of an increased blood bilirubin level occurred in one patient from cohort 5 after three doses had been administered; three patients were additionally enrolled and a similar DLT did not occur in this cohort. Except for DLT, two serious AEs (SAEs) occurred and were considered related to OBP-301 (decreased neutrophil count and fatigue). The neutrophil levels decreased to 690/mm3 1 day after 3 × 1012 viral particle (VP) injection in the participants but resolved to 3,730/mm3 3 days after SAE onset and there were no sequelae. Grade 2 fatigue was reported 3 days after the first OBP-301 injection (2 × 1012 VPs) in cohort 5, and symptoms of fatigue resolved 1 week after SAE onset.
We considered the maximum tolerated dose (MTD) to be greater than 6 × 1012 VPs/patient and the maximum feasible dose (MFD) to be greater than 3 × 1012 VPs/patient. Thus, OBP-301 appeared to be safe and well tolerated in these patients. A total of 44 treatment-emergent AEs (TEAEs) were reported in 13 patients (Tables S1 and S2), the most frequently reported being influenza-like illness (6 [30.0%] participants) (5 (62.5%) out of 6 from cohort 5), followed by pyrexia (3 [15.0%] participants) and fatigue, decreased platelet count, abdominal distension, and anemia (2 [10.0%] participants each) (Table S3).
We observed no relationship between the severity of toxicity and increasing doses, but the frequency of TEAEs related to OBP-301 was higher in cohorts 4 and 5 than in the others.
Efficacy assessment
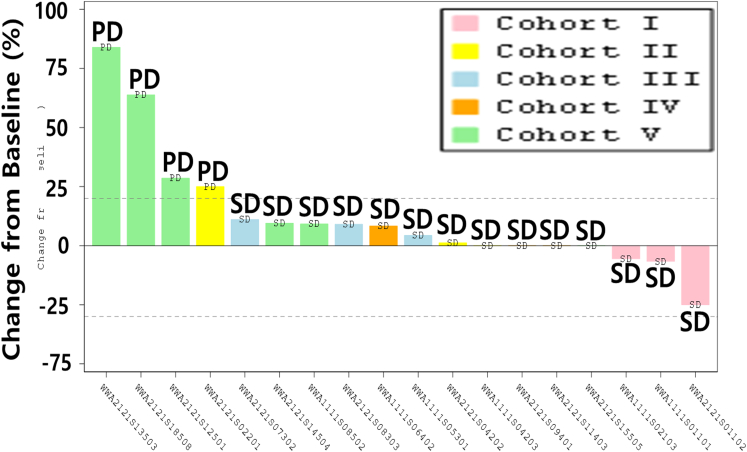
In our analysis of local and overall tumor responses (Tables S5 and S6), none of the patients achieved confirmed complete remission (CR) or partial remission (PR). However, the best local response occurred in 14 patients with stable disease (SD) and 4 with progressive disease (PD). SD was confirmed in seven patients (95% CI for disease control rate: 17.30, 64.25%). Overall, the mean (standard deviation, SD) time to disease control was 5.83 weeks (2.245). For targeted lesions, 14 patients had confirmed SD (95% CI for disease control rate: 52.36, 93.59). The overall mean (SD) time to disease control was 5.55 weeks (2.237) (Figure 3).
Figure 3.
Best local responses of individual patients (intention-to-treat analysis)
The best local response occurred in 14 patients with SD and 4 patients with PD. There were 7 patients with confirmed SD (95% confidence interval for disease control rate: 17.30, 64.25%). Overall, the mean (SD) time to disease control was 5.83 weeks (2.245). For targeted lesions, overall 14 patients had confirmed SD (95% confidence interval for disease control rate: 52.36, 93.59). The overall mean (SD) time to disease control was 5.55 weeks (2.237). The change in the longest diameter of the target lesion injected with OBP-301 before and after administration was measured. Tumor response is determined according to the international mRECIST guideline. Local responses of the same best value in multiple.
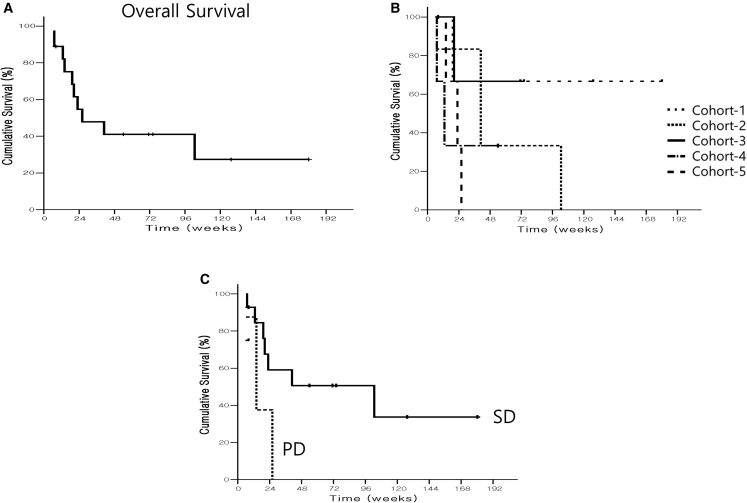
All efficacy analyses were performed using the intention-to-treat (ITT) and per-protocol (PP) analysis sets. Overall, 18 patients showed progression, 10 died with progression, and no patients were censored. The median times to progression (TTP) were 19.10 (cohort 1), 4.10 (cohort 2), 8.10 (cohort 3), 4.30 (cohort 4), and 8.10 weeks (cohort 5). The median TTP was 8.10 weeks. Analysis of the ITT population indicated that all evaluated patients showed progression. The median Kaplan-Meier estimate of TTP was greater in cohort 1 than in the other cohorts. The results of the Kaplan-Meier analysis of the PP analysis set were similar. Ten patients died and eight were censored. The median OS values are presented in Table S7 and Figure 4. There were no notable changes in efficacy after increasing doses.
Figure 4.
Cumulative survival (%) of OBP-301-injected patients
Kaplan-Meier curve of surviving patients. (A) Overall survival (OS) of all patients who completed the treatment protocols. The overall median OS was 26.00 weeks. (B) Survival curve according to groups. The median OS from the Kaplan-Meier analysis was 40.86 weeks (cohort 2), 12.86 weeks (cohort 4), and 23.00 weeks (cohort 5). (C) Survival curve according to the local response. SD, 102.6 weeks; PD, 14.1 weeks.
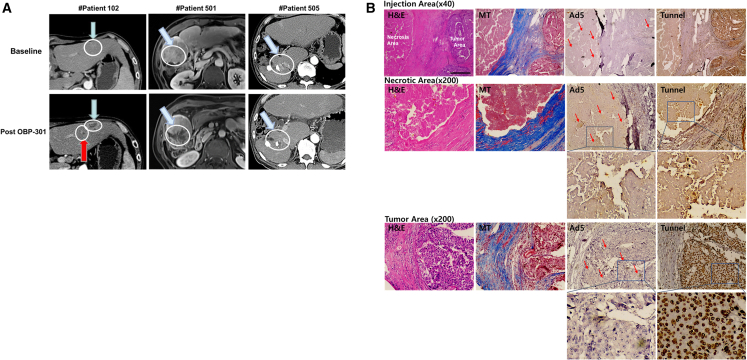
Figure 5 shows the CT results of three representative patients. Patient no. 102 (49-year-old male) was heavily treated 14 times with TACEs, radiation therapy, and sorafenib for portal tumor thrombosis and lung metastasis, but refused second-line targeted therapy because of sorafenib-induced TEAEs. After injection of OBP-301, the lesion exhibited definite necrosis (Figure 5A, left). Patient no. 501 was a 57-year-old male with portal tumor thrombosis who received 13 treatments with TACEs, radiation therapy, and sorafenib. The patient participated in this study because he did not want second-line targeted therapy. After three injections of OBP-301, significant tumor necrosis was observed (Figure 5A, middle panel); the patient later underwent palliative surgery, the findings of which indicated no malignant cells at the injection site (Figure 5B). Patient no. 505 was a 60-year-old male with multiple lymph node metastases who had received 14 treatments with TACEs and 2 with radiation treatments prior to this study; he participated owing to poor general condition and disinterest in additional targeted therapies. The results indicated a clear necrotic area in half of the lipiodolized tumor 5 days after the third OBP-301 injection (Figure 5A, right). Moreover, the local necrotic area matched the injection sites precisely, suggesting that increasing the injection volume is beneficial (Figure 5B, first column). Connective tissues stained with Masson’s trichrome (Figure 5B, second column) may hinder OBP-301 dissemination as Ad5 staining spots could be seen in areas other than the fibrotic area (Ad5 in Figure 5B, third column). However, we also observed an apoptotic area in the tumor region (tunnel in Figure 5B, last column).
Figure 5.
Representative computed tomography results of tumors in three patients who had target tumor responses (RECIST criteria)
(A) The white circle indicates tumor regions before and after the administration of OBP-301. Blue arrow: target lesion. Red arrow: bystander effect. (B) Histological assessment of the circled area of patient no. 501 after OBP-301 injection. H&E staining showed that necrotic cell death was found along the viral injection area. OBP-301 promoted apoptotic cell death. H&E, hematoxylin and eosin; MT, Masson trichrome; Ad5, adenovirus type 5; Tunnel, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Virus dissemination and adenovirus antibody assessment
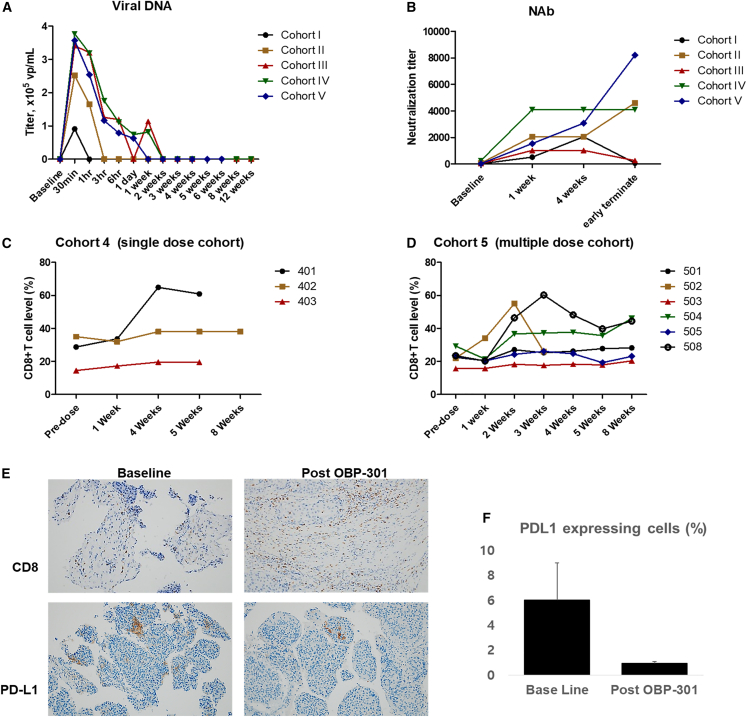
The systemic dissemination of OBP-301 was evaluated by collecting plasma and urine samples at different times and performing qPCR analysis with primers specific for the OBP-301 E1A and IRES regions. At baseline, OBP-301 DNA was undetectable in blood and urine. After administration, OBP-301 DNA was detectable in blood at 30 min and undetectable at 24 h in most participants. OBP-301 DNA was still positive in 3 patients at 24 h and in 2 patients at 7 days. All test results were negative for all patients 14 days after administration. Although some patients had positive blood and negative urine samples, only small amounts of viral DNA were detectable 24 h after OBP-301 injection (Figure 6A; Table S8).
Figure 6.
Pharmacokinetics and immunological evaluations of patients at baseline and different times after OBP-301 injection(s)
(A) Quantitation of OBP-301 viral DNA in plasma samples of different cohorts at different times. Note that viral shedding was greater in patients who received higher doses. (B) Neutralizing anti-adenovirus antibody (NAb) titers in plasma samples of different cohorts at different times. Note that all patients had increased NAb titers compared with that at baseline, and there was no correlation between absolute titer and dose of OBP-301. (C) The CD8+ T cell level (%) of peripheral blood samples collected from patients in cohort 4 at different time points. (D) The CD8+ T cell level (%) of peripheral blood samples collected from patients in cohort 5 at different time points. (E) Representative immunostaining for CD8 and PD-L1. Note that OBP-301 promoted CD8+ T cell recruitment. (F) PD-L1 expressing cells (%).
To identify possible systemic immune-activating events following OBP-301 treatment, we used a functional assay (OBP-301-infected human embryonic kidney 293 [HEK293] cells) to measure the neutralizing anti-adenovirus antibody (NAb) titer in patients. We identified increased blocking activity with increasing concentrations of pre- and post-treatment plasma (Figure 6B; Table S9). The baseline results also showed pre-existing anti-Ad5 antibodies, and the results of subsequent tests remained positive. After OBP-301 administration, the median level of anti-Ad5 antibodies was 32 times greater at 7 days post-treatment than at baseline, and these levels tended to continue increasing in most patients. The increasing NAb titer that occurred after the OBP-301 injection and subsequent viral DNA clearance may have been the reason that we observed no apparent effect of the OBP-301 dose on efficacy.
Immune phenotype assessment
We also analyzed immune phenotypes of peripheral blood samples collected at different time points. The mean number of CD8+ cells increased by 56.3% at 4 weeks after OBP-301 injection in cohort 4 and by 45.2% at 2 weeks after the first OBP-301 injection in cohort 5; however, the levels of CD8+ cells in these cohorts remained relatively constant thereafter (Figures 6C and 7D). In cohort 5, the mean number of CD4+ cells tended to show a slight decline (13.9%) 2 weeks after the first OBP-301 injection. However, in cohort 4, the mean number of CD4+ cells remained unchanged after 4 weeks. There was also great variability in the numbers of Treg cells and myeloid-derived suppressor cells (MDSC) after the OBP-301 injection (Table S10). Although the mean of natural killer cells decreased slightly, and that of B cells increased slightly after OBP-301 injection, a conclusion could not be made because of the huge standard deviation. It is impossible to observe the change in Tregs and MDSC after the OBP-301 injection because of great variability.
The histological assessment showed that CD8+ increased in tumor or necrotic areas after OBP-301 injection, suggesting that OBP-301 enhances antitumor immunity in the tumor microenvironment (TME, Figure 6E, upper panel). PD-L1 expression showed a slight difference between needle biopsy before (1%) and resected tumors after i.t. injection of OBP-301 (below 1%) (Figure 6E, bottom panel; Figure 6F). Since the PD-L1 levels decreased after virus administration, we anticipate that combined therapy with an immune check point inhibitor (ICI) may synergize the viral effect.
Discussion
We conducted an open-label, non-comparative, dose-escalating study of patients from two countries to assess the efficacy and safety of OBP-301 for advanced HCC. Overall, our results indicated that OBP-301 is a safe and well-tolerated treatment in patients with advanced HCC, consistent with the findings of previous studies.22,23 The most commonly occurring TEAEs had been reported previously or were consistent with the AEs reported for other adenovirus-based oncolytic virus treatments.22,23,24,25 They did not last more than 1 week. All influenza-like illnesses in the patients were ameliorated within 1 week. Our analysis of blood samples indicated clinically significant abnormalities in white blood cell (WBC) counts, platelet count, activated partial thromboplastin time (APTT), albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase. These findings are comparable with the safety results of a previous phase I study of OBP-301.22 In addition, all 20 patients reported higher visual analog scale pain scores after treatment than at baseline, which is consistent with the expected side effects of injection site pain after treatment with an adenovirus-based oncolytic virus.22,23,24,25
The efficacy of OBP-301 in terms of tumor response, time to progression, and OS was less than that of other second-line systemic chemotherapies such as regorafenib, cabozantinib, ramucirumab, and pembrolizumab.26,27,28 However, 19 of our patients had received prior non-surgical treatment for HCC and 8 received more than 5 previous treatments. Most received OBP-301 as a final treatment, and there were no available treatments for patients who had disease progression after treatment with this local therapy. In this disease, the OS of patients generally worsened after repeated failure of systemic treatments due to poor ECOG performance status and liver function and increased tumor burden. Despite multiple prior HCC therapies, our patients generally had acceptable ECOG performance status and liver function.
Our use of a 1–3 mL injection volume and increase in NAb and viral DNA clearance after OBP-301 treatment may have caused the lack of efficacy. Delivery in patients and dose-dependent responses were limited, possibly because of the increased level of NAbs during multiple i.t. injections. Importantly, histological examination revealed that the presence of OBP-301 was consistent with the necrotic area at the injection site. Increased infiltration of CD8+ T cells and less than 1% PD-L1 expression in tumors and peripheral areas after OBP-301 injection were observed. CD8+ T cells are read-outs of antitumor activity and also of antiviral immunity, which can be induced by the oncolytic virus, OBP-301, through multiple mechanisms: (1) selective viral replication in cancers causing direct oncolysis,29,30,31 (2) indirect effects of cancer cell death (either apoptosis-like or necrosis-like),32,33,34 (3) systemic activation of antitumor (and antiviral) immunity recruits corresponding immune cells into the TME.35,36,37,38,39 PD-L1 expression may be related to potential malignant invasiveness or metastasis of HCC.40 CD8+ immune cell infiltrates were associated with good prognosis and also associated with improved responses to chemotherapy and immunotherapy.41,42 Therefore, PD-L1low and CD8high expression after OBP-301 (Figure 6E) suggests that OBP-301 injection modulates the immunosuppressive TME and is more likely to improve the response to anti-PD-1/PD-L1 ICI therapy. More importantly, the bystander effect observed in patient no. 102 (Figure 5A) indicates the impact of using OBP-301 for the treatment of advanced HCC. Therefore, antitumor efficacy may be improved using a larger injection volume, which may allow OBP-301 to spread throughout the tumor, and combining OBP-301 with a systemic treatment such as a tyrosine kinase inhibitor, an immune checkpoint inhibitor, or an inhibitor of vascular endothelial growth factor receptor.
Our analysis of all safety-related events indicated that OBP-301 was well tolerated in patients with advanced HCC. There were no meaningful differences in the incidence of TEAEs between single and multiple doses. Only a small number of patients from the single escalating dose cohort entered the response follow-up phase, and none of the patients experienced CR or PR. However, the SD (assessed as the best local response) was 78%, which was higher than the 39% SD assessed as the overall response. Although we could not demonstrate an obvious antitumor activity of OBP-301, our results confirmed that OBP-301 improved local control in patients with advanced HCC.
Materials and methods
Patients
This was an open-label, multicenter, non-comparative, dose-escalating phase I trial of 20 patients with HCC in Korea and Taiwan. All patients had at least one unresectable, injectable solid tumor of the liver that had progressed after failure, intolerance, or ineligibility for sorafenib; stage B or C according to the BCLC system; and normal hematological and organ functions based on measurements of ALT (<2.5× upper normal limit [UNL]), AST (<2.5× UNL), WBCs ≥3,000/μL), serum creatinine (≤1.5× UNL), and APTT (<1.5× UNL). None of the patients had received chemotherapy during the 3 weeks preceding or tumor radiotherapy or any other investigational or antineoplastic agent during the 4 weeks preceding. Additional exclusion criteria included a history of bleeding, uncontrolled diabetes, active or chronic infection, acute viral infection syndrome within the preceding 2 weeks, concomitant hematological malignancy, active rheumatoid arthritis or any other autoimmune disease, receipt of long-term systemic immunosuppressive medication, receipt of organ transplant, prior participation in research that entailed the administration of an adenovirus vector, or receipt of any immune-related blood products during the 3 preceding months. All enrolled patients provided written informed consent. This study was performed in accordance with the International Council for Harmonization Good Clinical Practices and the Declaration of Helsinki. The institutional review board approved the study protocol. All decisions regarding dose escalation and major safety assessments were based on safety parameters including AEs, laboratory data, electrocardiography results, body weight, vital signs, MTD/MFD, and DLT.
Materials
OBP-301 preparation
OBP-301 was prepared as described previously18,19 at Introgen Therapeutics (Houston, TX) and maintained in a buffer (20 mM Tris [pH 8.0]) using aseptic techniques in a biocontainment level 2 ISO class 5 biosafety cabinet. The concentration was 1 × 1012 VPs per mL, and a 2 mL recoverable volume was added to 5 mL glass vials. OBP-301 was stored at −60°C or lower.
Dose selection
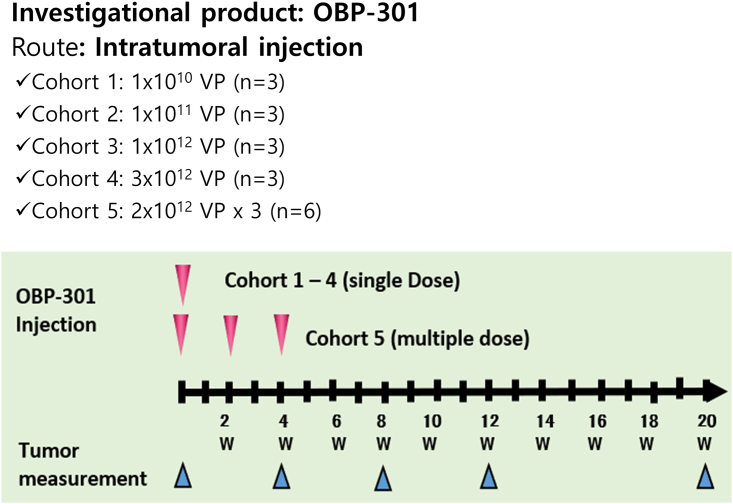
Previous studies on xenograft tumor-bearing mice and cotton rats have examined the effects of OBP-301. Systemic toxicity studies in these animals indicated no OBP-301-related effects following intramuscular (i.m.) injections. Conservatively, the cotton rat model tolerated a dose of 1 × 1010 VPs/kg (i.m. injection). Thus, a starting dose of 1010 VPs for humans was considered very conservative. Studies on HBxTg HCC animals have shown a dose of 1.3 × 1011 VPs/kg to be safe for injection into mouse livers, but a dose greater than 1012 VPs/kg may increase the risk of liver failure. Based on body weight, the human starting dose of 1010 VPs is 910 times lower than the dose administered to HBxTg mice assuming typical body weights. In addition to animal studies, a phase I clinical study examined the effect of OBP-301 on solid tumors,22 reporting that three single doses ranging from 1010 to 1012 VPs did not cause DLT. Further examination of the weekly doses of 5 × 1012 VPs also indicated that there were no DLTs. Therefore, the starting dose selected for this study was 1010 VPs. This was 100 times less than the maximum dose of the single-dose regimen (1 × 1012 VPs) and 500 times lower than the multiple-dose regimen (5 × 1012 VPs). Therefore, we selected the total doses injected between 1 × 1010 VPs/patient (single injection in cohort 1) and 6 × 1012 VPs/tumor (2 × 1012 VPs/patient × 3 times in cohort 5).
Treatment
Each patient received OBP-301 treatment within 14 days and was followed up for up to 12 weeks after the last injection. Each patient returned for weekly follow-up visits during the first month after the last injection and every 4 weeks until the end of the follow-up period of each phase. OBP-301 was administered by i.t. injection under ultrasound guidance, and patients received a dose-escalating regimen (Figure 7). If one DLT was observed in the first three patients in any cohort, an additional three patients were enrolled to evaluate the safety and tolerability of OBP-301 administration. For i.t. injections, the tumor lesion was measured and its shortest dimension was ensured to be at least 1 cm (≥2 cm for cohorts 4 and 5, and the alternative multiple-dose cohort) and considered suitable for repeat measurements (based on Response Evaluation Criteria in Solid Tumors version 1.1) and injection at the investigator’s discretion.
Figure 7.
Dose-escalating regimen of OBP-301
OBP-301 was administered by intratumoral injection using ultrasound guidance. Each patient received an OBP-301 treatment within 14 days and automatically entered the follow-up period for up to 12 weeks after the last injection. Each patient returned for follow-up visits each week during the first month after the last injection, and then every 4 weeks until the end of the follow-up period of each phase.
Efficacy and safety measurements
The patients were monitored for toxicity, viral distribution, antibody production, and tumor response (Tables S11 and S12). To determine the incidence of dose-limiting toxicities and identify the maximum tolerated dose, patients were monitored according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Safety parameters relative to baseline were used as primary endpoints, and MTD, MFD, and DLT as secondary endpoints. DLT was defined as one or both of the following events within 28 days of the last injection likely related to OBP-301: (1) CTCAE grade 4 hematologic toxicity and (2) grade 3 or higher non-hematologic toxicity. Tumor assessment was performed during response follow-up until progression. An ad hoc efficacy analysis was performed to assess the data. Indicator lesions were measured serially using endoscopy or radiographic scanning, and biopsies were performed for histological and immunohistochemical analyses. The objective response rate, OS, disease-free survival, and pharmacokinetic parameters were measured. The National Cancer Institute’s Response Evaluation Criteria in Solid Tumors were used, and a censoring rule was implemented as described in Table S3.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue samples cut at 4 μm were deparaffinized in xylene and rehydrated using a graded ethanol series. Samples were boiled in citrate buffer or EDTA buffer for 5 min in a microwave oven for antigen retrieval. After blocking, the samples were incubated with primary Abs for 1 h at room temperature or overnight at 4°C and then with peroxidase-conjugated secondary Ab for 30 min at room temperature. The samples were stained with 3,3-diaminobenzidine for signal generation, counterstained with Mayer’s hematoxylin, and then dehydrated and mounted. The following primary Abs were used, anti-Ad5 (cat. no. MA5-13643, Thermo Fisher Scientific, Waltham, MA), anti-CD8 (eBioscience, San Diego, CA), and anti-PD-L1 (SP142, Atezolizumab, Roche Diagnostics, Rotkreuz, Switzerland). Individual IgG isotypes were used as negative control.
Detection of viral DNA
Plasma and urine samples were collected for measurement of OBP-301 titers using a vector-specific DNA PCR assay, and viral DNA was quantified using real-time qPCR. First, DNA was extracted from plasma samples using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) and urine samples using the QIAamp Viral RNA Mini Kit. The extracted DNA was 10-fold diluted and examined with IRES and E1A primer sets, respectively, using SYBR Green (Thermo Fisher Scientific) based on triplicate experiments. The threshold for plasma was 2 × 104 VPs/mL and that for urine was 5 × 104 VPs/mL.
Primer sequences:
IRES forward: 5′-GAT TTT CCA TAT TGC CG-3′
IRES reverse: 5′-TTC ACG ACA TTC AAC AGA CC-3′
E1A forward: 5′-CCT GTG TCT AGA GAA TGC AA-3′
E1A reverse: 5′-ACA GCT CAA GTC CAA AGG TT-3′
Anti-adenovirus antibodies
NAbs were identified based on the inhibition of adenovirus-mediated cytolysis of cultured HEK293 cells, and the titer in plasma samples was determined based on the extent to which they blocked adenoviral infection in these cells. In brief, 2-fold serially diluted patient plasma samples were added to HEK293 cells infected with OBP-301. Microscopic examination was then performed to determine the percentage of cells that were lysed in the presence of patient plasma samples after infection. The titer of a sample was defined as the highest dilution of plasma that had a blocking effect, with 60% or more of the cells intact and attached as a monolayer. The presence of NAbs against Ad5 was determined before and throughout the study period. Blood samples were tested at baseline (day 1, pre-dose) and on days 7, 28, and 84 in the single-injection cohorts and at baseline (day 1, pre-dose) and days 7, 13 (before second dose), 21 (7 days after second dose), 27 (before third dose), 35 (7 days after third dose), 56, and 112 in the multiple-injection cohorts.
Data availability
The data of this study are available from the corresponding authors on reasonable request.
Acknowledgments
This work was supported by the Medigen Biotechnology Corporation, Oncolys Biopharma Inc., and a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. NRF-2020R1A2C2012316; 2021R1A2C2003908). Clinical trial number: NCT02293850.
Author contributions
J.H., S.C., Y.U., and P.-J.C. were responsible for the study design. J.H. and P.-J.C. were responsible for the management, coordination, and conduct of the trial. J.H., J.-D.L., C.W.K., H.Y.W., I.S., T.-H.S., Z.-Z.L., and P.-J.C. were responsible for patient accruals, trial conduct, and data collection. J.H. and S.Y.Y. were responsible for analyzing the biological samples. S.C. and Y.U. wrote the statistical analysis plan, which included the design of an expanded biomarker analysis. J.H., H.Y.W., S.Y.Y., and P.-J.C. had full access to and verified the data. J.H., H.Y.W., and S.Y.Y. analyzed the data and produced the results and figures. Y.U. supplied materials. All authors had access to and interpreted the data, and approved the draft and final versions of the manuscript. The corresponding authors are responsible for the final decision to submit for publication.
Declaration of interests
Y.U. is the president and CEO of Oncolys BioPharma, Inc., the manufacturer of OBP-301 (Telomelysin). P.-J.C. received consulting fees from Medigene. All remaining authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.04.006.
Contributor Information
Jeong Heo, Email: jheo@pusan.ac.kr.
Pei-Jer Chen, Email: peijerchen@ntu.edu.tw.
Supplemental information
References
- 1.Rawla P., Sunkara T., Muralidharan P., Raj J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018;22:141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn K.A., London W.T. The global epidemiology of hepatocellular carcinoma: present and future. Clin. Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J., Li L., Guo B., Liu D., Shi J., Wu C., Chen J., Zhang X., Wu J. Mechanisms of resistance to chemotherapy and radiotherapy in hepatocellular carcinoma. Transl. Cancer Res. 2018;7:765–781. [Google Scholar]
- 5.Giannini E.G., Farinati F., Ciccarese F., Pecorelli A., Rapaccini G.L., Di Marco M., Benvegnù L., Caturelli E., Zoli M., Borzio F., et al. Italian Liver Cancer ITA.LI.CA group Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61:184–190. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 8.Santoro A., Rimassa L., Borbath I., Daniele B., Salvagni S., Van Laethem J.L., Van Vlierberghe H., Trojan J., Kolligs F.T., Weiss A., et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 9.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Lim H.Y., Kudo M., Breder V.V., Merle P., et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J. Clin. Oncol. 2021;39:267. doi: 10.1200/JCO.2021.39.3_suppl.267. [DOI] [Google Scholar]
- 10.Pardee A.D., Butterfield L.H. Immunotherapy of hepatocellular carcinoma. OncoImmunology. 2012;1:48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo S.Y., Badrinath N., Woo H.Y., Heo J. Oncolytic virus-based immunotherapies for hepatocellular carcinoma. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/5198798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badrinath N., Heo J., Yoo S.Y. Viruses as nanomedicine for cancer. Int. J. Nanomedicine. 2016;11:4835–4847. doi: 10.2147/ijn.S116447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 2009;19:165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.-G., Huang P.-P., Zhang R., Ma B.-Y., Zhou X.-M., Sun Y.-F. Targeting adeno-associated virus and adenoviral gene therapy for hepatocellular carcinoma. World J. Gastroenterol. 2016;22:326–337. doi: 10.3748/wjg.v22.i1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Yu D.-C., Chen Y., Amin P., Zhang H., Nguyen N., Henderson D.R. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61:6428–6436. [PubMed] [Google Scholar]
- 16.Yang Z.R., Wang H.F., Zhao J., Peng Y.Y., Wang J., Guinn B.A., Huang L.Q. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther. 2007;14:599–615. doi: 10.1038/sj.cgt.7701054. [DOI] [PubMed] [Google Scholar]
- 17.Montaño-Samaniego M., Bravo-Estupiñan D.M., Méndez-Guerrero O., Alarcón-Hernández E., Ibáñez-Hernández M. Strategies for targeting gene therapy in cancer cells with tumor-specific promoters. Front. Oncol. 2020;10:605380. doi: 10.3389/fonc.2020.605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawashima T., Kagawa S., Kobayashi N., Shirakiya Y., Umeoka T., Teraishi F., Taki M., Kyo S., Tanaka N., Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.CCR-1075-3. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto Y., Watanabe Y., Shirakiya Y., Uno F., Kagawa S., Kawamura H., Nagai K., Tanaka N., Kumon H., Urata Y., Fujiwara T. Establishment of biological and pharmacokinetic assays of telomerase-specific replication-selective adenovirus. Cancer Sci. 2008;99:385–390. doi: 10.1111/j.1349-7006.2007.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara T., Urata Y., Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr. Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- 21.Inc, O.B. Environmental Protection Authority: New Zealand Government; 2016. To Obtain Approval to Release New Organisms. [Google Scholar]
- 22.Nemunaitis J., Tong A.W., Nemunaitis M., Senzer N., Phadke A.P., Bedell C., Adams N., Zhang Y.-A., Maples P.B., Chen S., et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirakawa Y., Tazawa H., Tanabe S., Kanaya N., Noma K., Koujima T., Kashima H., Kato T., Kuroda S., Kikuchi S., et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer. 2021;153:98–108. doi: 10.1016/j.ejca.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Brenner A.J., Peters K.B., Vredenburgh J., Bokstein F., Blumenthal D.T., Yust-Katz S., Peretz I., Oberman B., Freedman L.S., Ellingson B.M., et al. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro. Oncol. 2020;22:694–704. doi: 10.1093/neuonc/noz231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machiels J.P., Salazar R., Rottey S., Duran I., Dirix L., Geboes K., Wilkinson-Blanc C., Pover G., Alvis S., Champion B., et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE) J. Immunother. Cancer. 2019;7:20. doi: 10.1186/s40425-019-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouattour M., Mehta N., He A.R., Cohen E.I., Nault J.C. Systemic treatment for advanced hepatocellular carcinoma. Liver Cancer. 2019;8:341–358. doi: 10.1159/000496439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda M., Mitsunaga S., Ohno I., Hashimoto Y., Takahashi H., Watanabe K., Umemoto K., Okusaka T. Systemic chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Diseases. 2015;3:360–381. doi: 10.3390/diseases3040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marino D., Zichi C., Audisio M., Sperti E., Di Maio M. Second-line treatment options in hepatocellular carcinoma. Drugs Context. 2019;8:212577. doi: 10.7573/dic.212577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seegers S.L., Frasier C., Greene S., Nesmelova I.V., Grdzelishvili V.Z. Experimental evolution generates novel oncolytic vesicular stomatitis viruses with improved replication in virus-resistant pancreatic cancer cells. J. Virol. 2020;94 doi: 10.1128/JVI.01643-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altomonte J., Marozin S., Schmid R.M., Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol. Ther. 2010;18:275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollmann G., Rogulin V., Simon I., Rose J.K., van den Pol A.N. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 2010;84:1563–1573. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T.C., Hwang T., Park B.H., Bell J., Kirn D.H. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 33.Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H., et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.Can-12-2687. [DOI] [PubMed] [Google Scholar]
- 34.Jeong S.N., Yoo S.Y. Novel oncolytic virus armed with cancer suicide gene and normal vasculogenic gene for improved anti-tumor activity. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue T., Byrne T., Inoue M., Tait M.E., Wall P., Wang A., Dermyer M.R., Laklai H., Binder J.J., Lees C., et al. Oncolytic vaccinia virus gene modification and cytokine expression effects on tumor infection, immune response, and killing. Mol. Cancer Ther. 2021;20:1481–1494. doi: 10.1158/1535-7163.Mct-20-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu B., Tian L., Chen J., Wang J., Ma R., Dong W., Li A., Zhang J., Antonio Chiocca E., Kaur B., et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat. Commun. 2021;12:5908. doi: 10.1038/s41467-021-26003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uche I.K., Kousoulas K.G., Rider P.J.F. The effect of herpes simplex virus-type-1 (HSV-1) oncolytic immunotherapy on the tumor microenvironment. Viruses. 2021;13 doi: 10.3390/v13071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boagni D.A., Ravirala D., Zhang S.X. Current strategies in engaging oncolytic viruses with antitumor immunity. Mol. Ther. Oncolytics. 2021;22:98–113. doi: 10.1016/j.omto.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman M.M., McFadden G. Oncolytic viruses: newest frontier for cancer immunotherapy. Cancers. 2021;13:5452. doi: 10.3390/cancers13215452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan K.W., Chacko A.-M., Chew V. PD-1 expression and its significance in tumour microenvironment of hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019;4:51. doi: 10.21037/tgh.2019.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danilova L., Wang H., Sunshine J., Kaunitz G.J., Cottrell T.R., Xu H., Esandrio J., Anders R.A., Cope L., Pardoll D.M., et al. Associatation of PD-1/PD-L axis expression with cyolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc. Natl. Acad. Sci. USA. 2016;113:E7769–E7777. doi: 10.1073/pnas.1607836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H., Chen L., Pardoll D.M., Topalian S.L., Anders R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available from the corresponding authors on reasonable request.