Abstract
Conventional influenza vaccines focus on hemagglutinin (HA). However, antibody responses to neuraminidase (NA) have been established as an independent correlate of protection. Here, we introduced the ectodomain of NA into DNA vaccines that, as translated dimeric vaccine proteins, target antigen-presenting cells (APCs). The targeting was mediated by an single-chain variable fragment specific for major histocompatibility complex (MHC) class II, which is genetically linked to NA via a dimerization motif. A single immunization of BALB/c mice elicited strong and long-lasting NA-specific antibodies that inhibited NA enzymatic activity and reduced viral replication. Vaccine-induced NA immunity completely protected against a homologous influenza virus and out-competed NA immunity induced by a conventional inactivated virus vaccine. The protection was mainly mediated by antibodies, although NA-specific T cells also contributed. APC-targeting and antigen bivalency were crucial for vaccine efficacy. The APC-targeted vaccine was potent at low doses of DNA, indicating a dose-sparing effect. Similar results were obtained with NA vaccines that targeted different surface molecules on dendritic cells. Interestingly, the protective efficacy of NA as antigen compared favorably with HA and therefore ought to receive more attention in influenza vaccine research.
Keywords: DNA vaccine, antigen-presenting cells, APC targeting, influenza, hemagglutinin, neuraminidase, antibody responses, neuraminidase inhibition, T cell responses, antigen bivalency
Graphical abstract

Werninghaus et al. show that a DNA vaccine encoding neuraminidase targeted to antigen-presenting cells induced neuraminidase immunity superior to inactivated virus. Vaccine-induced antibodies efficiently inhibited neuraminidase enzymatic activity and viral replication. The antibody-mediated protection showed a dose-sparing effect that out-competed hemagglutinin. These findings could be important for future influenza vaccine design.
Introduction
The two most immunogenic antigens of influenza viruses are the envelope glycoproteins hemagglutinin (HA) and neuraminidase (NA).1 HA is the most abundant protein on the viral surface, while NA comprises only around 10%–20% of the amount of HA.2 HA and NA are essential in the viral life cycle. HA facilitates cellular entry by binding to sialic acid (SA)-linked cell surface molecules, whereas NA enzymatically cleaves SA residues, which enables access to host cells by cleaving decoy receptors in the mucus and facilitates efficient release of newly synthetized virions.3,4
Natural infection with influenza virus induces anti-HA and anti-NA antibodies.5 Traditionally, titers of neutralizing HA-specific antibodies have been used as a correlate of protection.6 However, NA inhibition (NAI) titers have recently been established as an independent correlate of protection.7,8,9 NA-specific antibodies can inhibit NA activity and virus replication,5 resulting in protection from clinically overt disease.9,10,11,12 For these reasons, use of NA in influenza vaccines has gained increasing attention.13,14,15,16
Conventional influenza vaccines are standardized based on their HA content and induction of HA-specific neutralizing antibody titers.14 The quantity and quality of NA are neither standardized nor monitored in conventional vaccine compositions, resulting in varying NA content and immunogenicity.17,18,19 In fact, conventional influenza vaccines only induce around 30% seroconversion toward NA.13,20,21 It is therefore an interesting approach to develop new vaccine formats that induce potent NA-specific responses. Multiple NA vaccines, such as inactivated vaccines,22 recombinant (rec.) NAs,23,24,25,26,27,28,29 virus-like particles,30,31,32 viral vectors,33 DNA,34,35 or mRNA,36 have already demonstrated immunity and protection against influenza viruses in animal models.16
DNA vaccines are attractive because they are safe and rapid to produce, provide sequence flexibility, and are stable with no need for a cold chain.37 However, DNA vaccines have been shown to have poor immunogenicity in humans38 and usually require optimized DNA vaccine formulations or delivery methods.39 A strategy to enhance immunogenicity of protein vaccines is to conjugate antigens to antibodies specific for antigen-presenting cells (APCs).40,41 Extending this strategy to DNA vaccines, we previously designed a DNA vaccine platform genetically linking a targeting unit, dimerization unit, and antigenic unit that together encode dimeric APC-targeted vaccine molecules, named Vaccibodies.42,43 After intramuscular (i.m.) DNA vaccination followed by electroporation (EP), the vaccine proteins are assembled and secreted by transfected muscle cells,44,45,46 which then bind cell-surface molecules on APCs for delivery of antigens.44 In the draining lymph nodes (dLNs), internalized and processed antigenic peptides are loaded onto major histocompatibility complex (MHC) molecules for presentation to T cells. Moreover, vaccine proteins temporarily bound to the surface of APCs are recognized by B cell receptors (BCRs) in APC-B cell synapses.46,47,48,49
Vaccibodies encode homodimeric fusion proteins with two identical arms dimerized by a CH3 domain of human immunoglobulin G3 (IgG3; hereafter called homodimers) containing two identical (bivalent) antigens.42,43 Vaccines that target influenza HA to APCs result in increased HA-specific antibody and T cell responses.50,51 A novel heterodimeric APC-targeted DNA vaccine platform is similarly efficient to elicit protective HA immunity.45 These heterodimeric vaccine proteins contain a modified acid/base (A/B) leucine zipper motif, enabling combination of two different polypeptide chains (hereafter called A/B heterodimers) with two identical (bivalent) antigens or combining two different (monovalent) antigens in one vaccine molecule.45,46
Here, we introduced NA as an antigen in the novel heterodimeric APC-targeted DNA vaccines. A single DNA immunization with an MHC class II-targeted vaccine elicited strong and long-lasting NA-specific antibody responses and NA-reactive T cells and provided complete protection against homologous influenza infection mediated by NA-inhibiting antibodies. We even found that the NA antibody responses were superior compared with a conventional inactivated virus vaccine. Similar results were obtained by specifically targeting dendritic cells (DCs). Thus, NA is a promising antigen in the APC-targeted DNA vaccine platform.
Results
NA in MHC class II-targeted heterodimeric DNA vaccines
To evaluate the protective potential of NA as an antigen in an APC-targeted DNA vaccine setting, we designed plasmids that express NA and/or HA as heterodimeric A/B vaccine proteins45 (heterodimers) (Figure 1A). The heterodimeric vaccine proteins consist of two different polypeptide chains that associate via a shortened hinge from human IgG3 (exon 1), followed by a modified Jun/Fos leucine zipper enriched for either acidic (for the A chain) or basic (for the B chain) amino acids.45 For APC targeting we used a single-chain variable fragment (scFv) specific for mouse MHC class II (I-Ed) because this targeting unit binds a broad variety of different APCs and has been shown to efficiently induce antibody responses.52 As a non-targeted control, we used a scFv specific for the hapten 4-hydroxy-3-iodo-5-nitrophenylacetic acid (NIP), which is not present in mice. To allow secretion of the vaccine proteins, a truncated NA (amino acids [aa] 36–454) was used that lacked intracellular and transmembrane regions, in analogy to previous experiments employing HA.45,50 NA and HA were from influenza H1N1 A/Puerto Rico/8/34/Mount Sinai (PR8). NA and HA were expressed with A and B cassette plasmids (Figure 1B), with the corresponding polypeptide chains associating to form heterodimeric vaccine proteins (Figures 1A and 1B, arrow). A and B vaccine plasmids were combined to construct heterodimers either including two identical (bivalent) antigens (e.g., NA/NA or HA/HA) or combining two different antigens with one (monovalent) copy of each (e.g., NA/HA). The nomenclature of the vaccine molecules is indicated in Figure 1A, top. Briefly, the targeting unit is mentioned first, followed by the dimerization unit, and finally the antigen expressed on either the A or B arm, separated by a slash (e.g., scFvαMHCII-A/B-NA/HA).
Figure 1.
In vitro characterization of MHC class II-targeted heterodimeric DNA vaccines containing NA and/or HA
(A) MHC class II-targeted heterodimeric acid/base (A/B) vaccine proteins consist of A and B chains, each having a targeting, dimerization, and antigenic unit. The targeting unit is either an scFv that binds MHC class II on APCs (scFvαMHCII, green) or a non-targeted control that binds the hapten NIP (scFvαNIP, white). The targeting units are genetically linked to a shortened hinge from human IgG3 (exon h1), allowing covalent disulfide bond formation between the A and B chains (black lines). The hinge is followed by a duplicated A (red) or B (blue) heterodimerization unit based on a modified Jun/Fos leucine zipper motif enriched for acidic or basic amino acids, respectively. The A and B chains are linked to antigenic units composed of NA (dark blue) or HA (orange) derived from influenza H1N1 PR8 virus. The heterodimeric vaccine proteins are named as indicated. The targeting unit is mentioned first, followed by the dimerization unit and finally the antigen on the A and the B arm, in that order, separated by a slash. (B) As an example, the vaccine plasmids encoding the two monomers for the scFvαMHCII-A/B-NA/HA vaccine protein are shown (arrow). The plasmids contain a CMV promoter (pCMV) and a leader sequence (L, gray boxes). Short linkers between units are shown as black boxes. Restriction enzyme sites are indicated. (C–F) HEK293E cells were transiently co-transfected with the indicated A and B plasmids. (C and D) Vaccine proteins in supernatants were analyzed by ELISA. Antibodies used for coating and detection are indicated. Mean of technical triplicates ± SD; results are representative of two independent experiments. (E) Western blot analysis. Heterodimeric vaccine proteins were detected with anti-A/B mAb under non-reducing conditions. Some supernatants were concentrated or diluted to ensure comparable vaccine protein loads. Putative heterodimers are indicated by an asterisk and depicted on the right side. Results are representative of three independent experiments. (F) Binding of heterodimeric vaccine proteins to BALB/c splenocytes was assessed with anti-A/B mAb by flow cytometry. CD14−CD49b−Ly-6G− single cells were gated as DCs (CD11c+), B cells (CD19+), or T cells (CD3+). Results are representative of technical triplicates for each sample.
To evaluate efficiency of translation into assembled vaccine proteins, HEK293E cells were co-transfected with A and B plasmids in vitro. The presence of anticipated heterodimeric vaccine proteins in the supernatant was analyzed by ELISA. The non-targeted (scFvαNIP) vaccines containing NA and/or HA were efficiently secreted as captured by NIP-BSA (bound by the targeting unit) and detected with a monoclonal antibody (mAb) that requires assembly of the A/B heterodimerization motif for its binding,53 thus confirming formation of heterodimers (Figure 1C). Expression of NA and/or HA in the MHC class II-targeted heterodimeric vaccines was confirmed with an anti-A/B mAb in combination with either NA-specific (Figure 1D, left) or HA-specific (Figure 1D, center) antibodies. Finally, NA/HA heterodimers were detected with a combination of NA- and HA-specific antibodies (Figure 1D, right). Notably, the antigen-monovalent NA/HA and HA/NA were consistently detected at lower levels than the antigen-bivalent HA/HA or NA/NA (Figures 1C and 1D). Because NA/HA consistently showed higher expression in vitro, only this antigen-monovalent variant was used in the following. Accessibility to NA epitopes detected by a C terminus-specific polyclonal antibody (pAb)54 did not seem to be obstructed when NA was paired with HA in the NA/HA construct compared with a heterodimer combining NA on one arm and a STOP codon instead of an antigen on the other arm (NA/STOP; Figure S1A, left). Detection of several HA epitopes (Sa, Sb, Cb, and stem) with different anti-HA mAbs55 similarly indicated that, although the stem domain was detected less efficiently, HA head epitopes were equally accessible in the NA/HA construct compared with a STOP/HA heterodimer (Figure S1A, right).
Western blot analysis with antibodies against the A/B dimerization unit (Figure 1E), NA, or HA (Figure S1B) showed heterodimeric vaccine proteins with bivalent antigen at the expected sizes, confirming correct assembly of the different heterodimers. NA/HA gave lower signals despite 10-fold up-concentration of supernatant and appeared to form aggregates of higher molecular weight (Figures 1E and S1B). However, when running the samples under reducing conditions, single A- and B-chain monomers were readily detected at the expected molecular weights (Figure S1B). The up-concentrated NA/HA also showed bands of smaller sizes (Figures 1E and S1B), which might represent cleavage products of the vaccine proteins released from lysed cells.
The functionality of the targeting unit was assessed by flow cytometry. MHC class II-targeted vaccine molecules, either bivalent for NA (Figure 1F, top) or HA (Figure 1F, bottom), bound BALB/c CD11c+ DCs and CD19+ B cells but not CD3+ T cells. The NIP-specific vaccine proteins failed to bind any of these cell populations, as expected for the non-targeted control.
These results indicate that DNA plasmids encoding the heterodimer proteins were correctly expressed and secreted by transfected cells and that the vaccine proteins were functional in targeting APCs in vitro.
A single DNA vaccination with MHC class II-targeted NA heterodimers efficiently induces antibody responses in mice
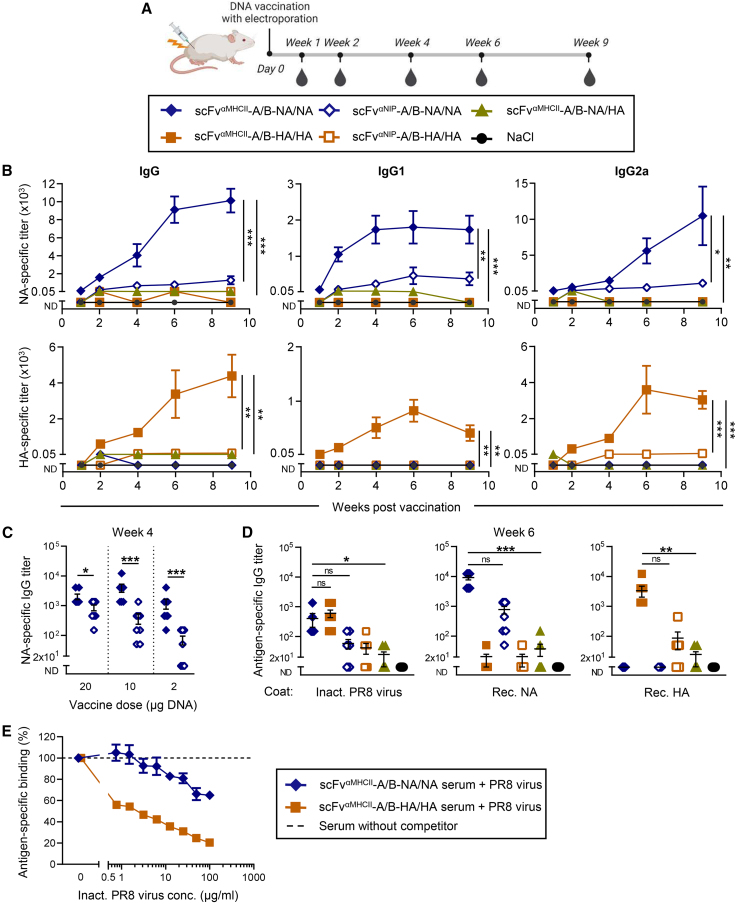
To assess vaccine-induced NA-specific immune responses, BALB/c mice were vaccinated once i.m. with 100 μg total of A and B plasmids encoding MHC class II-targeted NA heterodimers, immediately followed by EP. At the same dose, MHC class II-targeted HA has been shown previously to induce protective responses.45 Blood was collected several weeks after immunization to assess vaccine-induced antibody titers (Figure 2A). Both vaccines with antigen-bivalent NA or antigen-bivalent HA showed potent antigen-specific responses peaking at week 4 post immunization (Figure S2A). While MHC class II-targeting significantly increased HA-specific antibodies, NA appeared to be highly immunogenic because the non-targeted NA vaccine was as efficient as MHC class II-targeted NA. This suggested that vaccine dose titration was needed to reveal differences between the vaccine groups. A single DNA immunization with a lower vaccine dose (10 μg total) of MHC class II-targeted antigen-bivalent NA induced high levels of NA-specific IgG antibodies (Figure 2B, top) comparable with and even slightly higher than that seen with bivalent HA (Figure 2B, bottom). Induction of NA-specific antibodies started as early as 7 days post vaccination, reached plateau levels at approximately 6 weeks, and were sustained until week 9. The NA-specific antibody responses contained IgG1 and IgG2a isotypes. At this low dose, the non-targeted controls induced significantly lower levels of antibodies, showing that MHC class II-targeting was important for enhanced responses to bivalent NA (Figure 2B). Stepwise de-escalation of the DNA vaccine confirmed the enhancing effect of MHC class II-targeting for NA (Figure 2C). The antibody responses to bivalent NA were dose dependent, with doses as low as 2 μg DNA of MHC class II-targeted bivalent NA still eliciting high levels of IgG antibodies (Figure 2C). Overall, the antibody responses to the MHC class II-targeted NA/NA vaccine resembled those seen previously with the HA/HA equivalent (Figures 2B and S2A).45 Importantly, the vaccines containing bivalent NA/NA and HA/HA were clearly more immunogenic than vaccines combining monovalent NA and HA in single vaccine proteins (Figure 2B). The enhancing effect of antigen bivalency on antibody levels was seen across DNA doses, with a 50-fold higher DNA dose of the NA/HA vaccine resulting in even lower responses against NA and HA as the corresponding antigen-bivalent vaccines (Figure S2B). The bivalency effect can therefore not solely be explained by low secretion levels of NA/HA and the fact that vaccines with bivalent antigen express twice as much antigen as versions with monovalent antigen. Bivalent antigen in MHC class II-targeted vaccines has been shown previously to increase BCR cross-linking and activation of B cells in APC-B cell synapses.46
Figure 2.
MHC class II-targeted NA-bivalent heterodimers efficiently induce antibody responses after a single DNA vaccination
(A) BALB/c mice were vaccinated once i.m. with 5 μg of each A and B plasmids (10 μg DNA in total) encoding the indicated heterodimeric vaccine proteins (box), followed immediately by EP. Blood was sampled at the indicated time points. (B) NA-specific (top) and HA-specific (bottom) IgG (left), IgG1 (center), and IgG2a (right) titers were measured by ELISA at the indicated time points after immunization. Statistical analysis compared targeted vs. non-targeted and antigen-bivalent vs. monovalent groups. (C) BALB/c mice were immunized once i.m./EP with different amounts of the indicated DNA vaccines. Serum was analyzed for NA-specific IgG responses 4 weeks post vaccination. (D) IgG responses specific for inactivated H1N1 PR8 virus (left), rec. NA protein (center), or rec. HA protein (right) in sera harvested 6 weeks post vaccination from mice immunized once with 5 μg/plasmid (10 μg total) were analyzed in ELISA. Mean ± SEM of n = 8 mice/group. ND, not detected; ns, not significant. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; two-way ANOVA (B), two-tailed Mann-Whitney test (C), or Kruskal-Wallis multiple-comparisons test with Dunn’s correction (D). (E) A standard dilution of pooled sera from 5 mice/group vaccinated 4 weeks earlier (10 μg total DNA) were incubated with serial dilutions of inactivated influenza H1N1 PR8 virus. NA- or HA-specific IgG was measured in ELISA. Mean ± SD of technical triplicates.
Importantly, the NA-specific serum antibodies bound to formalin-inactivated H1N1 PR8 virus in ELISA (Figure 2D). Further, inactivated H1N1 PR8 virus inhibited binding of anti-NA antibodies to rec. NA in ELISA (Figure 2E). Together, this indicates that antibodies elicited by the MHC class II-specific NA/NA vaccine bind NA on the surface of inactivated virus, suggesting that the NA antibodies could protect vaccinated mice against infection with influenza virus. Similar findings were reached for anti-HA antibodies elicited by immunization with MHC class II-targeted HA/HA (Figures 2D and 2E), confirming earlier findings.45 It should be noted that NA-specific antibodies were inhibited by virus to a lesser extent than HA-specific antibodies (Figure 2E), which is consistent with the lower abundance of NA on the viral surface compared with HA.2
The MHC class II-targeted heterodimeric DNA vaccine elicits early NA-specific T cell responses
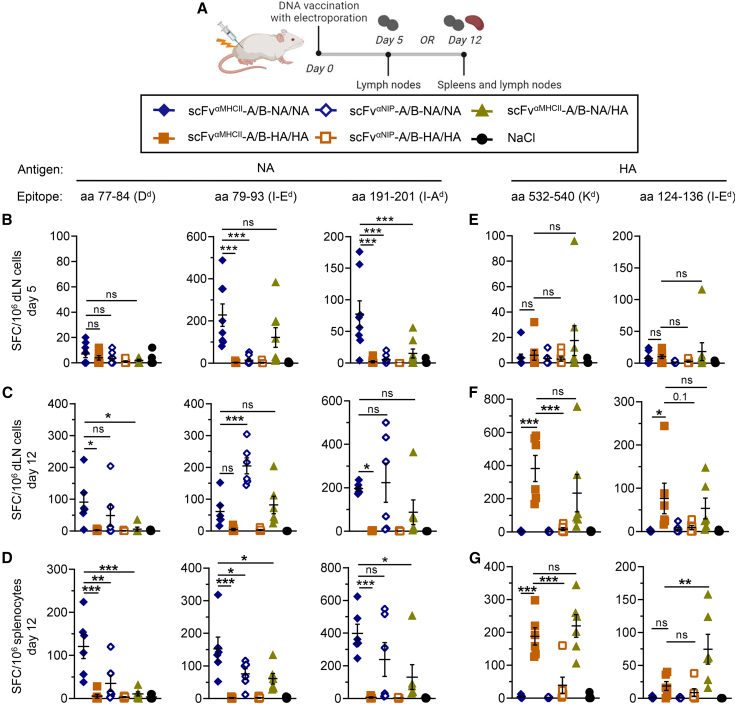
To investigate whether MHC class II-targeted NA and HA vaccines were able to stimulate T cell responses, BALB/c mice were vaccinated once with a total of 10 μg of A and B plasmids. Single-cell suspensions were generated from dLNs on days 5 and 12 or from spleens on day 12 after vaccination (Figure 3A). Cells were re-stimulated with previously defined MHC class I- or MHC class II-restricted peptides from NAPR856,57 and HAPR8.58,59 The MHC class II-targeted vaccine with bivalent NA induced antigen-reactive interferon γ (IFNγ)-secreting cells, suggesting elicitation of MHC class I- and MHC class II-restricted T cell responses (Figures 3B–3D). Targeting NA to MHC class II significantly increased the number of peptide-reactive IFNγ-secreting cells on day 5 in the dLNs compared with the non-targeted control (Figure 3B). The MHC class II-targeting effect was lost on day 12 in dLNs (Figure 3C), potentially because of early NA-specific T cells induced by targeted vaccines already egressing from the dLNs sometime after day 5. This hypothesis fits well with the increased T cell responses seen for the MHC class II-targeted vaccine in the spleen on day 12 (Figure 3D). By comparison, MHC class II-targeted bivalent HA induced delayed IFNγ responses compared with NA, which were low on day 5 in the dLNs (Figure 3E) but significant enhanced on day 12 compared with non-targeted controls in the dLNs (Figure 3F) and in the spleen (Figure 3G). Immunization with a 10-fold higher DNA dose (100 μg total) generally enhanced T cell responses, and the MHC class II-targeting effect was observed for HA but not for the NA vaccine at this dose (Figure S2C), as seen for the antibody responses.
Figure 3.
NA-specific T cell responses are detected early after immunization with MHC class II-targeted heterodimeric DNA vaccines
(A) BALB/c mice were vaccinated once i.m./EP with 5 μg of each A and B plasmids (10 μg total) encoding the indicated heterodimeric A/B vaccines (box). Draining lymph nodes (dLNs) and/or spleens were harvested as shown. (B–G) IFNγ-secreting spot-forming cells (SFCs) reactive to MHC class I-restricted (Dd, Kd) or MHC class II-restricted (I-Ed, I-Ad) peptides from NAPR8 (B–D) or HAPR8 (E–G) were measured by ELISpot on single-cell suspensions from dLNs on day 5 (B and E) or day 12 (C and F) or from spleens on day 12 (D and G). n = 6 mice/group; individual mice and mean ± SEM are indicated. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; multiple-comparisons one-way ANOVA with Tukey’s correction.
The MHC class II-targeted vaccine that expressed monovalent NA and HA induced IFNγ responses against both antigens, demonstrating their immunogenicity in the antigen-monovalent format. Antigen bivalency has been shown to be less important for T cell responses,46 and we did not observe a consistent difference between MHC-targeted antigen-bivalent NA/NA or HA/HA and the antigen-monovalent NA/HA vaccine in terms of induction of IFNγ-secreting cells (Figures 3B–3G and S2C). There was a tendency for vaccines with bivalent antigen to induce stronger responses, but the difference was predominantly seen for low doses of NA in the spleen and probably reflects the fact that vaccines with bivalent antigen express twice as much antigen, which has been observed previously.46
The MHC class II-targeted NA vaccine efficiently protects against homologous influenza virus
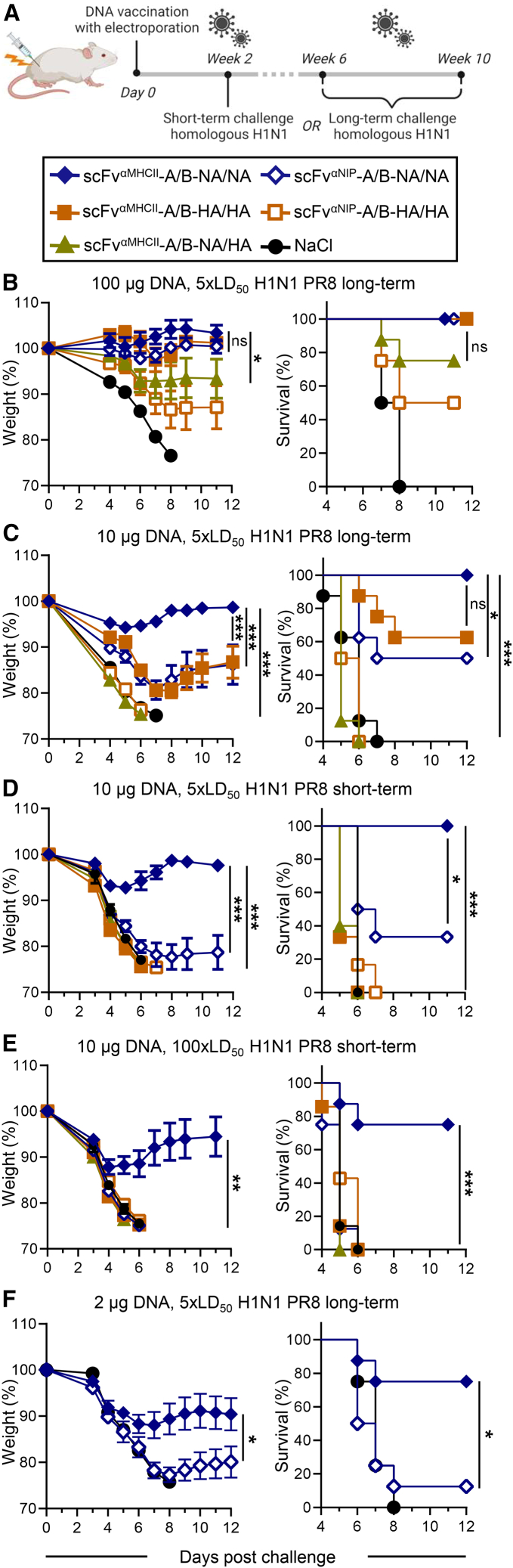
To investigate whether the vaccine-induced NA immune responses provide protection from influenza infection, vaccinated mice were challenged with a homologous influenza H1N1 PR8 virus. The challenges were performed either shortly (week 2) after vaccination, when MHC class II-targeted bivalent HA has shown complete protection,45 or long after (weeks 6–10) immunization (Figure 4A), when antibody levels had reached a stable plateau phase (Figures 2B and S2A). MHC class II-targeted bivalent NA completely protected (100% survival) against an influenza challenge 10 weeks after a single immunization with a high DNA dose (100 μg total), as did MHC class II-targeted HA (Figure 4B), as shown previously.45 With a 10-fold lower DNA dose (10 μg), targeted vaccines with bivalent NA still completely protected mice challenged 9 weeks later while the non-targeted version did not, demonstrating the enhanced protective effect of MHC class II targeting (Figure 4C). Interestingly, at this dose, mice vaccinated with MHC class II-targeted bivalent NA showed significantly better protection than HA, indicating a superior vaccine efficacy of NA. Mice vaccinated with the low dose (10 μg DNA) of MHC class II-targeted bivalent NA were already fully protected 2 weeks after vaccination when challenged with a viral dose of 5× lethal dose 50 (LD50) (Figure 4D) and considerably protected (75% survival) from challenge with an increased viral dose of 100× LD50 (Figure 4E). Under those conditions, all HA-vaccinated groups succumbed to the infection (Figures 4D and 4E). Finally, when further de-escalating the vaccine dose to 2 μg total DNA, MHC class II-targeted bivalent NA was still able to provide partial protection at 5× LD50 (75% survival; Figure 4F), showing the potent vaccine efficacy of the APC-targeted NA DNA vaccine. We observed that NA bivalency (Figures 4B–4E) and MHC class II targeting (Figures 4C–4F) clearly contributed to protection. Interestingly, the results indicate that NA/NA vaccines generally induced better protection than HA/HA in these experiments (Figures 4C–4E).
Figure 4.
A single DNA immunization with MHC class II-targeted NA/NA vaccines protects against homologous influenza virus challenge
(A) BALB/c mice were immunized once i.m./EP and challenged with homologous influenza A H1N1 PR8 virus at week 2 (short term) or between weeks 6 and 10 (long term) after vaccination. (B–F) 50 μg of each A and B plasmids (total 100 μg, B), 5 μg/plasmid (10 μg total, C–E), or 1 μg/plasmid (2 μg total, F) of the indicated DNA vaccines (box) were used. Mice were challenged with a lethal dose of 5× LD50 (B–D and F) or 100× LD50 (E) at week 9 (B), week 10 (C), week 2 (D and E), or week 6 (F). Weight (left, mean ± SEM) and survival (right) were monitored up to 12 days post challenge. n = 8 mice/group (B, C, E, and F) or n = 6 mice/group (D). Dead mice were assigned a weight of 75%. ∗p ≤ 0.05, ∗∗∗p ≤ 0.001; two-way ANOVA (weight curve) or Mantel-Cox test (survival). Statistical analysis compared MHC class II-targeted NA/NA versus HA/HA as well as targeted versus non-targeted and antigen-bivalent versus -monovalent NA.
The MHC class II-targeted NA-bivalent DNA vaccine induces superior NA-specific immunity compared with conventional inactivated virus
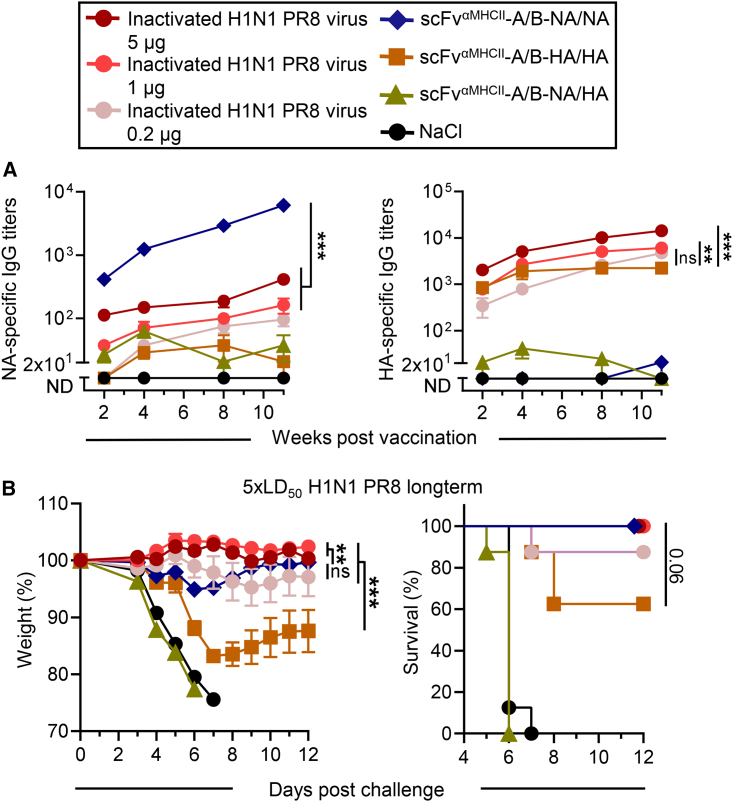
When comparing the DNA vaccine (10 μg total) encoding MHC class II-targeted heterodimers with antigen-bivalent NA with immunization with strain-matched formalin-inactivated H1N1 PR8 influenza virus, significantly higher NA-specific antibody titers were induced with scFvαMHCII-A/B-NA/NA (Figure 5A, left). This observation was independent of the vaccine dose of inactivated virus ranging from 0.2–5 μg. The same 10-μg DNA vaccine dose of MHC class II-targeted bivalent HA elicited HA-specific responses more similar to the inactivated virus vaccine, comparable with the lowest 0.2-μg dose (Figure 5A, right). After a lethal challenge with homologous influenza virus, MHC class II-targeted NA protected as well as the inactivated virus vaccine, even though slightly more initial weight loss was observed compared with the higher inactivated virus doses (Figure 5B). This demonstrates that even the low-dose DNA vaccine encoding MHC class II-targeted bivalent antigen, which induced comparable HA-specific responses, showed superior NA-specific antibodies and similarly potent protection compared with a conventional inactivated virus vaccine that contains HA and NA antigens.
Figure 5.
MHC class II-targeted NA DNA vaccine induces superior NA-specific antibody responses compared with conventional inactivated virus after one vaccination
(A, B) BALB/c mice were immunized once i.m. with 0.2, 1, or 5 μg of formalin-inactivated H1N1 PR8 virus or with 5 μg/plasmid (10 μg total) of the indicated DNA vaccines, followed by EP (box). (A) NA-specific (left) and HA-specific (right) titers were measured by ELISA at the indicated time points after immunization. Mean ± SEM is shown. (B) Mice were challenged with 5× LD50 H1N1 PR8 virus 11 week post vaccination. Weight (left, mean ± SEM) and survival (right) were monitored up to 12 days post challenge. Dead mice were assigned a weight of 75%; n = 8 mice/group. Statistical analysis compared the different doses of inactivated virus with DNA vaccination with MHC class II-targeted NA and/or HA. ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; two-way ANOVA (IgG titers, weight curve) or Mantel-Cox test (survival).
Vaccine-induced protection is mainly mediated by NA-specific antibodies
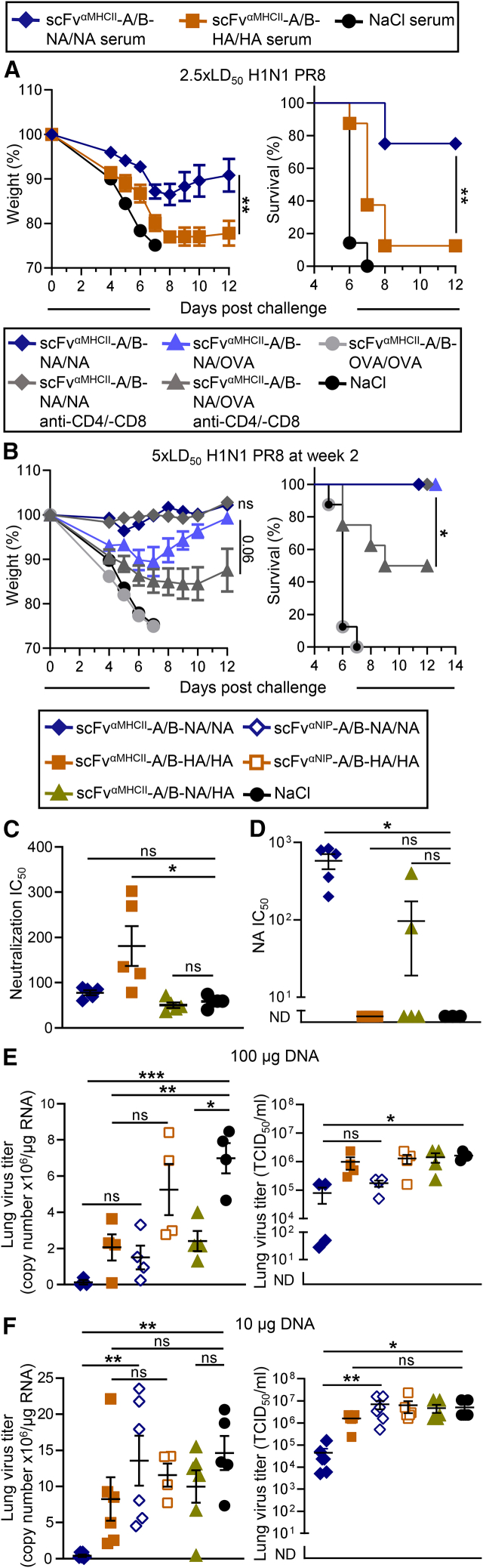
To investigate the protective role of antibodies, pooled serum from mice vaccinated once with a high DNA dose (100 μg total) was harvested 5 weeks after immunization and transferred intraperitoneally (i.p.) to naive mice, followed by a challenge with homologous influenza virus the subsequent day. Sera from mice immunized with bivalent NA or HA induced 75% and 10% survival, respectively (Figure 6A). This demonstrates that antibodies elicited by the MHC class II-targeted NA/NA DNA vaccine protect against influenza infection. Moreover, anti-NA antibodies had a superior protective effect compared with anti-HA antibodies.
Figure 6.
Vaccine-induced NA-specific antibodies mediate protection against homologous influenza infection, inhibit NA enzymatic activity, and block viral replication
(A–D) BALB/c mice were immunized once i.m./EP with 50 μg each of A and B plasmids (100 μg total) as indicated. (A) Pooled sera (350 μL) from mice 5 weeks after vaccination were transferred i.p. to naive BALB/c mice. One day after transfer, mice were challenged with 2.5× LD50 H1N1 PR8 virus. (B) 2 weeks after vaccination with the indicated DNA vaccines, mice were challenged with 5× LD50 H1N1 PR8 virus. Starting on day 12 after vaccination, mice were treated with anti-CD4 and anti-CD8 mAbs or isotype-matched control mAbs every second day. n = 8 mice/group; weight (left, mean ± SEM) and survival (right) were monitored up to 12 days post challenge, and dead mice were assigned a weight of 75% (A and B). (C) Neutralization of influenza A H1N1 PR8 virus by serum from vaccinated mice (box) obtained 4 weeks after immunization was measured by microneutralization assay and is shown as IC50. (D) The same sera were tested for ability to inhibit NA enzyme activity by ELLA, using a reassorted influenza A H6N1 virus containing NA from H1N1 PR8. IC50 is indicated. (E and F) BALB/c mice were immunized once i.m./EP with 50 μg of each A and B plasmids (100 μg total, E) or 5 μg of each A and B plasmids (10 μg total, F) as indicated. Mice were challenged with homologous H1N1 PR8 virus 6 weeks after vaccination, and lungs were harvested on day 5 post challenge. Viral loads were determined by qRT-PCR (left), showing gene copy number per microgram total tissue RNA, and by measuring in vitro cell culture infectivity (right), presented as 50% tissue culture infectious dose (TCID50) per milliliter of lung homogenates. Individual mice and mean ± SEM are shown; n = 4–6 mice/group (C–F). ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; two-way ANOVA (weight curve), Mantel-Cox test (survival), Kruskal-Wallis multiple-comparisons test with Dunn’s correction (C and D, TCID50), or multiple-comparisons one-way ANOVA with Tukey’s correction (copy number).
Because the MHC class II-targeted NA/NA vaccines induced high amounts of IFNγ-secreting cells (Figures 3B–3D), NA-specific T cells might additionally contribute to protection. We therefore performed T cell depletion in BALB/c mice vaccinated with MHC class II-targeting NA/NA DNA. Because we have shown previously that T cells are required for MHC class II-targeted vaccines to elicit antibodies,50 CD4- and CD8-depleting mAbs were first administered 2 days before challenge and repeatedly injected every other day until the end of the experiment. Successful T cell depletion was confirmed by flow cytometry (Figure S3). Mice immunized with MHC class II-targeted NA/NA and later T cell depleted were still completely protected from influenza infection (Figure 6B). This is not so surprising because anti-NA antibodies induced by such vaccination could be expected to be sufficient for complete protection (Figures 6A and S2A). We further tested whether NA-specific T cells could provide protection in mice immunized with monovalent antigen, where low amounts of protective antibodies were induced. Vaccines were constructed where MHC class II-targeted vaccine proteins expressed NA (or HA) on one arm and ovalbumin (OVA) on the other (which is irrelevant for protection against influenza virus) (Figures S4A and S4B). These vaccines with monovalent antigen induced few anti-NA (or anti-HA) antibodies (Figure S4C) and provided only partial protection with considerable weight loss (Figure S4D), as expected based on results from NA/HA and previous data.46,50 T cell depletion in such vaccinated mice significantly reduced protection, indicating that NA-specific T cells may confer some protection in the relative absence of protective antibodies (Figure 6B).
Vaccine-induced NA immunity inhibits NA enzymatic activity and reduces viral replication
The efficient NA-specific antibody responses in mice immunized with MHC class II-targeted NA/NA (Figures 4B and 4C) suggested testing for possible effector functions of anti-NA antibodies induced after immunization. Sera from mice vaccinated with a high DNA dose (100 μg) of MHC class II-targeted NA/NA did not neutralize influenza H1N1 PR8 virus in a microneutralization assay, whereas sera from mice vaccinated with MHC class II-targeted HA/HA did (Figure 6C). However, and importantly, vaccine-induced anti-NA serum antibodies inhibited enzyme activity of NA, as measured by enzyme-linked lectin assay (ELLA; Figure 6D), suggesting that viral replication could be inhibited by vaccine-induced anti-NA antibodies. This was confirmed by analyzing the viral loads in lung tissue of immunized mice harvested 5 days after a lethal influenza challenge when immunized with either a high (100 μg; Figure 6E) or a low DNA dose (10 μg; Figure 6F). The MHC class II-targeted bivalent NA vaccine significantly reduced the viral load at the high and the low vaccine dose, as shown by decreased viral genome copy numbers (Figure 6F, left) and reduced infectious viral particles (Figure 6F, right). The MHC class II-targeted bivalent HA demonstrated significantly lower viral loads by qPCR at the high DNA dose, although a trend for reduction of viral replication was seen at both vaccine doses. There was a tendency, however not significant, that antigen bivalency contributed to a reduction of viral load, while MHC class II-targeting was especially beneficial at the lower vaccine dose, which is consistent with findings on antibody levels and protection (Figures 2B, S2A, and 4).
NA Vaccibodies that target various cell-surface molecules on APCs induce efficient antibodies and protection against homologous influenza virus
We have previously shown that MHC class II-targeting is highly efficient in inducing antibody responses.52 However, targeting vaccines to different cell-surface molecules on different types of APCs can influence the phenotype of elicited T cell and antibody responses.51,52,60,61 All professional APCs, including B cells, macrophages, and DCs, express MHC class II molecules and are therefore targeted by scFvαMHCII (Figure 1F).46,60,62 To specifically target DCs, a cell type considered an especially important APC, we used either scFv specific for mouse CD11c (scFvαCD11c) present on cDC1 and cDC263 or the chemokine Xcl1, which binds the Xcr1 chemokine receptor on cDC1.61 Finally, another chemokine, MIP-1α, was used that binds to CCR1, CCR3, and CCR5 expressed on DCs and macrophages.42,52 The ectodomain of NA was cloned into plasmids with the mentioned targeting units and a previously described homodimerization unit consisting of a shortened hinge (exons 1 and 4) and CH3 from human IgG3, forming APC-targeted homodimeric Vaccibodies upon translation (Figure 7A).42,43 scFvαNIP was included as a non-targeted control, as well as a plasmid encoding the NA ectodomain alone.
Figure 7.
Vaccibodies targeting different APCs induce NA-specific immune responses and protection
(A) Vaccibodies are homodimers composed of two identical monomers, each having a targeting unit, a homodimerization unit, and an antigenic unit. The targeting units were scFvs binding to MHC class II (scFvαMHCII, green) or CD11c (scFvαCD11c, blue) or the chemokines mouse MIP-1α (light blue triangle) or mouse Xcl1 (Xcl1, green triangle). The targeting units are genetically linked to a homodimerization unit consisting of a shortened hinge (h1 and h4), ensuring covalent disulfide bonds between the two monomers (black lines), and CH3 from human IgG3. The antigen was NAPR8. scFvαNIP was used as a non-targeted control (white). A plasmid encoding NA alone was also included. The names of the vaccines are indicated above. (B and C) HEK293E cells were transiently transfected with vaccine plasmids as indicated. (B) Vaccine proteins in supernatants were analyzed by ELISA. Antibodies used for coating and detection are indicated. Mean of technical triplicates ± SD are shown; results are representative of two independent experiments. (C) In western blot analysis, Vaccibody proteins were detected with anti-NA pAb under non-reducing conditions. Putative homodimers are indicated by an asterisk and are schematically depicted on the right. Monomers and dimers of NA alone are indicated by a triangle. Results are representative of two independent experiments. (D–F) BALB/c mice were immunized once i.m./EP with 10 μg of plasmids as indicated. n = 4 mice/group. (D) NA-specific IgG responses in vaccinated mice were measured by ELISA at the indicated time points. Mean titers ± SEM are shown. (E) IgG1/IgG2a ratios of anti-NA serum antibodies at week 5. Individual mice and mean ± SEM are indicated. (F) Mice were challenged with 5× LD50 H1N1 PR8 virus 5 weeks after vaccination. Weight (left, mean ± SEM) and survival (right) were monitored for 12 days. Dead mice were assigned a weight of 75%. ∗p ≤ 0.05, ∗∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001; two-way ANOVA (D, left, and F, weight curve), Kruskal-Wallis multiple-comparisons test with Dunn’s correction (D, right, and E), or Mantel Cox (F, survival). Statistical analysis compared NA with different targeting units with non-targeted control and NA alone (D) or across different targeting units (E). Non-significant values are not indicated.
In vitro analysis confirmed that the vaccine constructs were successfully expressed, secreted, and assembled as vaccine proteins of the expected sizes after transient transfection of HEK293E cells (Figures 7B and 7C). Transfection of the ectodomain NA alone resulted in secretion of NA monomers and dimers but apparently not higher orders (Figure 7C). A single vaccination with 10 μg DNA encoding APC-targeted NA Vaccibodies induced high levels of anti-NA antibodies detectable as early as day 7 and increasing until week 5 (Figure 7D, left). MHC class II targeting was the most efficient, while all APC-targeted NA vaccines induced enhanced responses compared with the ectodomain NA alone. However, so did the NIP-targeted vaccine. Nevertheless, a noticeable enhancing effect of targeting NA to MHC class II and CD11c compared with the non-targeted control could be observed early after vaccination (Figure 7D, right). While MHC class II and CD11c targeting induced a balanced IgG1/IgG2a ratio, the chemokines MIP-1α and especially Xcl1 induced significantly more IgG2a-focused antibody responses 5 weeks after vaccination (Figure 7E). This suggests binding of MIP-1α and Xcl1 to Th1-skewing ACPs such as cDC1s and macrophages, whereas anti-MHC class II and anti-CD11c likely target several different types of APCs that together induce a balanced response, similar to previous results with other antigens.52,60 Importantly, mice vaccinated with the various APC-targeted NA Vaccibodies were protected against a viral challenge with homologous virus, while mice immunized with the ectodomain of NA alone succumbed to the infection (Figure 7F). These results highlight the potential of NA as antigen in vaccines targeting APCs and suggest that multiple targeting strategies can be used to induce protective immune responses.
Discussion
Potent NA immunity could provide an additional layer of protection against influenza. Novel vaccine approaches that induce strong and reliable responses toward NA are therefore an attractive goal. We constructed DNA vaccines that encode antigen-bivalent NA vaccine proteins that target surface molecules on APCs via an scFv specific for MHC class II. A single immunization induced better NA-specific immune responses in BALB/c mice than a conventional inactivated virus vaccine and provided complete protection against a lethal challenge with homologous influenza virus. Robust NA-specific responses were obtained with the original APC-targeted homodimeric as well as the novel APC-targeted heterodimeric A/B vaccines, with 4/4 different APC-specific targeting units tested. Interestingly, immune responses and protection elicited by the MHC class II-targeted bivalent NA were more potent than those elicited by an identical vaccine that carried bivalent HA. These results, and previous results of others,16,34,35,36 suggest that NA should receive increased attention in influenza vaccine development.
Antibody levels, T cells, and protection induced by the APC-targeted NA vaccine were consistent with previous studies on targeting influenza HA to APCs.45,50,64,65 Strikingly, titration of vaccine doses showed that APC-targeted NA was significantly more immunogenic and protective than HA. An earlier study using DNA vaccination and EP likewise found significantly better protection and a reduction of viral titers with NA compared with HA.34 NA also out-competed multiple different influenza antigens (stem HA, matrix protein 2, and nucleoprotein) in a mRNA vaccine approach.36 In addition, preexisting NI antibodies correlated with lower disease severity and virus shedding compared with HI antibodies in a controlled human influenza challenge.8 Together, this demonstrates that NA could provide an advantage over vaccination with HA and should be strongly considered in future influenza vaccine research.
After DNA vaccination and translation into protein, the secretion of APC-targeted vaccine molecules allows targeting the antigen to ACPs by binding cell-surface molecules. To avoid problems with secretion, we used a truncated version of NA where the transmembrane and cytosolic part had been removed. Such ectodomain NA alone was secreted by in vitro-transfected cells as monomers and dimers. The low immunogenicity and protection induced by ectodomain NA by itself (without APC targeting or the dimerization domain) here contrasts results of others with full-length NA delivered as DNA.34,35 A possible explanation for these differences is that the ectodomain NA used in our experiments is secreted but does not target APCs and thus elicits poor responses, while full-length NA used by others34,35 should be tethered as tetramers in the cell membrane of transfected cells.
The present results show that immunogenicity of ectodomain NA was increased by APC targeting and antigen bivalency. Targeting to APCs most likely increases uptake of secreted vaccine proteins by APCs and stimulation of T and B cells. It should be emphasized that the enhanced efficiency of targeting NA to MHC class II was revealed at early immune responses and low doses of DNA. This implies that APC targeting may represent a dose-sparing strategy, which could reduce the cost of vaccines and stretch the vaccine supply during pandemics.
There are many types of APCs, which appear to differ in the type of immune response they elicit. For example, in mice, while antigen presentation by cDC1 primarily induces Th1/CD8/IgG2a-focused responses, cDC2 elicits Th2/IgG1-dominant responses.66 We have observed previously that targeting vaccine proteins to different types of APCs could steer the phenotype of immune responses in a desired direction.52,60 In mice, MIP-1α targeting cDC1s and macrophages and Xcl1 targeting cDC1s induced Th1/CD8/IgG2a-polarized responses,52,60,61,67 while scFvαMHCII and scFvαCD11c, which target a wider range of APCs, elicited a mixed IgG1/IgG2a-type response.45,50,52 For NA, NA-inhibiting antibodies are mostly correlated with protection against virus, but mouse IgG2a-mediated cellular effector functions like antibody-dependent cellular cytotoxicity (ADCC) have been suggested to be an alternative protective mechanism.68,69 Moreover, for broadly protective anti-NA70 and anti-HA stem71 responses, mouse IgG2a is suggested to be the most efficient isotype. Consistent with previous findings,52,60 we here found that anti-NA isotypes could similarly be steered using MIP-1α, Xcl1, scFvαMHCII, or scFvαCD11c as targeting units, demonstrating that our vaccine technology allows influencing biological effector functions.72
We recently demonstrated that antigen bivalency in the APC-targeted vaccine increases B cell responses, ranging from early signaling to long-lived plasma cells and antibody production.46 APC-targeted vaccines can generate APC-B cell synapses in vitro, and we hypothesized that antigen bivalency could increase BCR cross-linking in such synapses and, consequently, B cell activation.46 The ability of APC-targeted bivalent NA/NA to enhance antibodies may be explained by this mechanism.
NA-specific antibodies can reduce severe disease in animal models but usually do not provide sterilizing immunity.24,25,30,32,73,74,75,76 This is because NA-specific antibodies do not target major steps in viral entry into host cells.5 Accordingly, we found that vaccine-induced anti-NA antibodies did not neutralize virus but instead inhibited NA enzyme activity and reduced viral titers. Similar to our findings, Chen et al.34 showed that NA-specific antibodies induced by a DNA vaccine inhibited viral replication to below the pathogenic threshold. NA enzyme-inhibiting antibodies prevent virus from being released from infected cells,5 thus inhibiting the influenza replication cycle.3,4 Additionally, other mechanisms could have contributed to reduced viral loads and protection. NA-inhibiting antibodies could mediate retention of incoming virions in the mucus before initial infection of host cells,77 and anti-NA antibodies with cellular effector functions such as ADCC have been described to aid in protection, as mentioned above.69,70,78 Fc-mediated effector functions such as ADCC or complement activation are mediated by IgG2a in mice, but whether the IgG2a elicited by the MHC class II-targeted NA DNA vaccine plays a protective role via ADCC or complement activation needs to be further investigated.
Enzymatic activity of NA requires an intact, native conformation of NA tetramers.79 It has therefore been suggested that tetrameric NA is needed for a vaccine to induce antibodies that inhibit NA.80,81 The MHC class II-specific NA/NA vaccine protein contains two ectodomain NA monomers that can hardly engage in building tetramers. Nevertheless, this vaccine induced antibodies that displayed NAI activity requiring binding to functional, tetrameric NA.79 The ability of ectodomain NA monomers to induce NA inhibitory antibodies could be of great importance for the design of future NA vaccines.
Protection elicited by the MHC class II-targeted NA vaccines was mainly provided by antibodies because T cells were not essential when sufficient levels of anti-NA antibody were induced. However, NA-specific T cells were clearly elicited by immunization and contributed to protection where levels of anti-NA antibodies were reduced. It has been demonstrated previously that cytotoxic T lymphocyte responses against NA can aid clearance of infected cells.82,83 The cytotoxic capability of NA-reactive T cells in our model remains to be investigated.
MHC class II-targeted NA was demonstrated to be more potent in inducing NA-specific humoral responses and to be similarly potent in providing protection against homologous virus as a conventional formalin-inactivated virus vaccine. Previously, MHC class II-targeted HA Vaccibodies have demonstrated comparable or even better immune responses and protection than conventional adjuvanted Pandemrix or non-adjuvanted, tetravalent inactivated virus vaccines.84 Intravirionic antigenic competition85,86 and poor preservation of NA epitopes21 have been suggested to cause poor induction of NA-specific responses by inactivated virus vaccines. This suggests that the APC-targeted antigen-bivalent DNA vaccine format could provide an advantage for elicitation of NA-specific responses over conventional influenza virus vaccines.
A desired feature of a universal influenza vaccine is to elicit broadly protective immune responses, ideally against all influenza strains. Pre-existing NA-specific immunity, either because of natural infection12,87 or vaccination,7,88,89,90 contributes to broadened protection against heterologous influenza strains. Therefore, it would be favorable for a vaccine to (1) induce potent responses against HA and NA (and possibly further antigens), and (2) elicit cross-reactive immune responses against a variety of HA or NA subtypes. Broader protection may be obtained by including NA and HA in the vaccine. Unfortunately, in this study, the MHC class II-targeted vaccine combining monovalent NA and HA induced few antibodies and little protection because bivalent antigen is needed to induce protective antibodies, as described previously46 and discussed above. Moreover, low secretion levels presumably also contributed. Immunodominance of HA over NA on the viral surface has been suggested to dampen NA-specific responses, likely because of the higher abundance of HA over NA- or HA-specific imprinting.68,86,91 This can be resolved when vaccines contain dissociated HA and NA and/or equal amounts of both antigens;85,91 therefore, it is unlikely that immunodominance of HA over NA played a role for the NA/HA heterodimers in this case. An improved vaccine design that enables potent responses against both antigens could be beneficial for influenza vaccination, as shown successfully by others.28,89,92,93,94
In summary, here we demonstrated that APC-targeted DNA vaccines with bivalent ectodomain NA elicited NA-specific antibodies, T cells, and protection against homologous influenza virus that were superior to HA as antigen. Comparison with an inactivated virus vaccine showed that this vaccine format induces equally potent protection and might provide an advantage over conventional vaccines for eliciting efficient NA immunity. APC targeting and antigen bivalency contributed to the strong vaccine responses, enabling a dose-sparing effect. NA is an understudied influenza vaccine antigen that deserves increased attention. Strategies to combine HA and NA and to induce broadly protective immune responses with the APC-targeted DNA vaccine platform should be explored further in the future.
Materials and methods
Mice, cell lines, and influenza viruses
6- to 8-week-old female BALB/c mice were from Janvier Labs (Le Genest Saint Isle, France). Animal experiments were reviewed and approved by the Norwegian Food Safety Authority (Mattilsynet, FOTS 19542 and 29069) and carried out in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the Norwegian National Institute of Health. HEK293E and Madin-Darby canine kidney (MDCK) cells were purchased from the ATCC (Manassas, VA, USA). Cells were cultured in RPMI 1460 medium (Invitrogen) and DMEM (Invitrogen), respectively, supplemented with 10% heat-inactivated fetal calf serum (Life Technologies), 24 mg/L gentamicin (Sanofi-Aventis Norge), 50 μM monothioglycerol (Sigma), 1 mM sodium pyruvate, and 0.1 mM non-essential amino acids (Lonza). Influenza viruses from strain H1N1 PR8 were used for animal challenge and microneutralization experiments (VR-95 and VR-1469, respectively; ATCC). A reassorted H6N1 influenza virus A/teal/Hong Kong/W 312/1997 with NA from influenza H1N1 PR8 was used for ELLA, kindly provided by Richard Webby (St. Jude Children’s Research Hospital, Memphis, TN, USA).
Cloning of DNA vaccine plasmids
Vaccine constructs were cloned in a pLNOH2 vector containing a human cytomegalovirus (CMV) promoter and the VH-leader sequence from the VH gene of the B1-8 mAb.95 For dimerization, a heterodimerization unit consisting of a hinge from exon 1 of human IgG3 and two consecutive and modified Jun/Fos leucine zipper motifs enriched for either acidic or basic amino acids (A/B heterodimers),45 or a homodimerization unit comprising hinge exons 1 and 4 and CH3 from human IgG3 (CH3 Vaccibodies/homodimers) were used as described previously.43 Targeting units upstream of the dimerization unit were scFvs of anti-I-Ed (scFvαMHCII, from mouse mAb 14-4-4S) and anti-CD11c (scFvαCD11c, N418; ATCC)52 and the chemokines mouse MIP-1α (GenBank: NM_011337.2)42 and mouse Xcl1 (GenPept: NP_032536.1).61 A scFv against NIP (scFvαNIP, from mouse mAb B1-8)43 was used as a non-targeting control. As antigenic units, the ectodomain of HAPR8 (aa 18–540)50 or OVA60 was used. The ectodomain of NAPR8 (aa 36–454) flanked with unique Sfil restriction sites (GenScript, GenPept: NP_040981) was subcloned into pLNOH2 vectors with various targeting and dimerization units. Moreover, NA alone was cloned into the pLNOH2 vector. The NA sequence was amplified by PCR with the following primers to introduce a 5′ BsmI and a 3′ BamHI restriction site: 5′-ACA GGT GTG CAT TCC CAT TCA ATT CAA ACT GGA-3′ and 5′-AGT GGA TCC TCA TCA CTT GTC AAT GCT GAA-3′ (restriction sites are underlined, and the STOP codon is shown in bold). The PCR product was then subcloned into the BsmI and BamHI restriction sites of the pLNOH2 vector. To produce rec. NA protein for serum ELISAs, the NA antigenic unit was subcloned into a Vaccibody with scFv against 4-ethoxymethylene-2-phenyl-2-oxazoline-5-one (phOx; scFvαphOx)96 as a targeting unit.
ELISA for analysis of vaccine proteins produced in vitro
7.5 × 105 HEK293E cells were seeded out in Costar 6-well plates (Corning) and transiently transfected the day after with 2.5 μg of each A and B vaccine plasmids or 5 μg of Vaccibody plasmids using 10 μg polyethylenimine (Polysciences). Supernatant was harvested after 3 days. For ELISA, Costar 96-well plates (Corning) were coated with NIP-BSA (2.5 μg/mL, Biosearch Technologies), anti-A/B (1 μg/mL, 2H11,53 kindly provided by Ellis Reinherz, Harvard Medical School, Boston, MA USA), anti-HAPR8 (1 μg/mL, H36-4-52,97 a kind gift from Siegfried Weiss, Medizinische Hochschule Hannover, Hannover, Germany), or the anti-human IgG3 CH3 domain (1 μg/mL, A57H, Invitrogen). After blocking (1% BSA in PBS), supernatants harvested from transfected cells were incubated in triplicates of 3-fold serial dilutions in ELISA buffer (0.1% BSA, 0.2% Tween 20 in PBS). Vaccine proteins were detected with biotinylated anti-A/B (1 μg/mL), anti-NA pAb (1:2,000, carboxy terminal specific,54 a kind gift from Jonathan Yewdell, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD, USA), biotinylated anti-HAPR8 (1 μg/mL), or anti-OVA (1 μg/mL, 23744-5, Polysciences). Anti-NA pAb and anti-OVA were followed with anti-rabbit IgG-alkaline phosphatase (ALP; 1:2,000, Sigma) and biotinylated antibodies with streptavidin-ALP (1:3,000, Southern Biotech). For detection of specific HAPR8 epitopes, an anti-HA stem (0.33 μg/mL, 2, Sino Biological) and the following anti-HAPR8 mAbs were used: H36-4-52 (Sb specific), Y8-1A6 (10 μg/mL, Sa specific,55 a kind gift from Jonathan Yewdell), and H17-L7 (10 μg/mL, Cb specific,55 a kind gift from Jonathan Yewdell). These were followed by a biotinylated anti-κ light chain (1 μg/mL, 187.1, ATCC). ELISAs were developed with 1 mg/mL phosphatase substrate (Sigma) in diethanolamine substrate buffer and optical density 405 (OD405) was measured using an EnVision 2104 multilabel reader with EnVision Manager 1.12 software (PerkinElmer).
Western blotting
HEK293E cells were transiently transfected, and supernatant was harvested as described above, except that the medium was replaced with FreeStyle 293 serum-free medium (Life Technologies). Some supernatants were concentrated 10-fold using Vivaspin 20 spin columns with 10-kDa cut off (Sartorius). Samples were run on Bolt 4%–12% Bis-Tris Plus gels (Invitrogen) and transferred to polyvinylidene fluoride (PVDF) membranes using the iBlot2 gel transfer system with a PVDF transfer stack (Invitrogen). After blocking with 2% membrane blocking agent (GE Healthcare), the membranes were incubated with biotinylated anti-A/B (0.33 μg/mL), anti-NA pAb (1:5000), or biotinylated anti-HAPR8 (0.33 μg/mL, H36-4-52). Vaccine proteins were detected with streptavidin-horseradish peroxidase (HRP) (1:5,000, Southern Biotech) or anti-rabbit IgG-peroxidase (1:5,000, A6154, Sigma) and developed with SuperSignal West Pico PLUS chemiluminescence substrate (Thermo Fisher Scientific). Signals were visualized with a G:BOX Chemi XX6 system (Syngene).
Flow cytometry analysis of MHC class II targeting
Spleens from BALB/c mice were dissociated with gentleMACS C tubes (Miltenyi Biotec), treated with Tris-buffered ammonium chloride, and filtered through 70-μm nylon strainers. Splenocytes were blocked with 50% heat-inactivated rat serum and stained with 2 μg/mL anti-CD14 fluorescein isothiocyanate (FITC; rmC5-3, BD Biosciences), anti-CD49b FITC (DX5, eBioscience), anti-Ly-6G FITC (1A8, Tonbo Biosciences), anti-CD3 Violet Fluor 450 (17A2, Tonbo Biosciences), anti-CD11c APC-Cyanine7 (N418, Tonbo Biosciences), and anti-CD19 APC (1D3, Tonbo Biosciences). Stained splenocytes were incubated with up-concentrated supernatant from transiently transfected HEK293E cells in triplicates. Bound vaccine proteins were detected with 2 μg/mL biotinylated anti-A/B followed by 2 μg/mL streptavidin-PE (BioLegend). Cells were fixed with 2% paraformaldehyde and analyzed using an Attune NxT flow cytometer (Thermo Fisher Scientific) and FlowJo software v.10.6.1 (BD Biosciences).
In vivo DNA vaccination of BALB/c mice
DNA vaccine plasmids were prepared with Endofree Mega Prep Kits (QIAGEN) and dissolved in 0.9% NaCl (B. Braun). BALB/c mice were anesthetized i.p. with 0.2 mL tiletamine/zolazepam mix (Zoletil Forte, 250 mg/mL, Virbac), xylazine (Rompun, 20 mg/mL, Bayer Animal Health), and fentanyl (50 μg/mL, Actavis). 2–100 μg of DNA in a total volume of 100 μL was injected as 50 μL in the quadriceps femoris muscle on each side. The i.m. injection was immediately followed by EP at the injection site using an Elgen 1000 needle electroporator (Inovio Biomedical). Pulses from needle electrodes inserted i.m. were delivered as 5 × 60 ms at 50 V/400 mA with 200-ms delay.52 0.2–5 μg of formalin-inactivated H1N1 PR8 virus (10100782, Charles River Laboratories) was injected i.m. (50 μL each side, 100 μL total).
Serum ELISA for detection of antigen-specific antibodies
Sera were obtained from vaccinated BALB/c mice by puncture of the saphenous vein and two consecutive centrifugations of the collected blood sample. Plates were coated with 0.5 μg/mL rec. HAPR8 (11684-V08H, Sino Biological), 1.25 μg/mL formalin-inactivated H1N1 PR8 virus (10100782, Charles River Laboratories), or rec. NA. For rec. NA, a scFvαphOx-CH3-NA fusion protein was produced in HEK293E cells and added to ELISA plates pre-coated with phOx-BSA (1 μg/mL, Sigma). The scFvαphOx unit binds the phOx-BSA coat, ensuring that NA is in solution and accessible for binding. Saturated loading with scFvαphOx-CH3-NA was verified with biotinylated anti-human IgG (Fc specific, HP6017, Sigma). Serum from individual mice was added in 3-fold serial dilutions in ELISA buffer (starting dilution 1:50). Antigen-specific antibodies were detected with anti-mouse IgG-ALP (1:5,000, A2429, Sigma), biotinylated anti-mouse IgG1[a] (10.9, BD Biosciences), or biotinylated anti-mouse IgG2a[a] (8.3, BD Biosciences). Otherwise, the ELISAs were performed as described above. The antibody titer was defined as the highest dilution of a serum sample with OD values greater than (2 × mean) of NaCl-vaccinated mice. Samples with titers of less than 50 were not detected (ND) and assigned an endpoint titer of 50/3 (starting dilution/dilution factor) = 16.6.
Inhibition ELISA
Plates were coated with rec. HA or NA antigen as described above. A 2-fold serial dilution of a competitor was added in the presence of a standard dilution of serum samples. Inactivated influenza H1N1 PR8 virus was used as a competitor. Serum pools from each vaccine group were diluted to an antigen-specific total IgG signal with an OD405 value of approximately 1, which was defined as the standard dilution. Each sample was run in duplicates. Bound antigen-specific serum antibodies were detected with anti-mouse IgG-ALP (1:5,000) as described above for ELISA. Inhibition was defined as percent binding compared with serum only (assigned 100%). Competitor only served as background.
Microneutralization assay
Viral 50% tissue culture infectious dose (TCID50) was determined by the Reed-Muench method.98 Serum was mixed 1:3 with receptor-destroying enzyme (1 mU/mL receptor-destroying enzyme [RDE], cholera filtrate, C8772, Sigma), incubated at 37°C overnight, and heat-inactivated at 56°C for 60 min. 2-fold serial dilutions of RDE-treated serum samples (starting dilution 1:10) were incubated with 100× TCID50 of H1N1 PR8 virus on a 96-well plate in virus diluent (DMEM supplemented with 48 mg/L gentamicin, 100 μM monothioglycerol, 2 mM sodium pyruvate, 0.2 mM non-essential amino acids, 1% bovine albumin fraction V, 0.02 M HEPES, and 1 μg/mL TPCK-trypsin) for 2 h at 37°C and 5% CO2. Virus without serum was used as a positive control (virus control [VC]) and virus diluent only as a negative control (cell control [CC]). After incubation, 2 × 105 MDCK cells were added per well and incubated for 18 h at 37°C, 5% CO2. After washing the cell layer once with PBS and fixation with 80% acetone, viral proteins were detected with biotinylated anti-nucleoprotein mAb (1 μg/mL, H16-L10-4R5, ATCC), followed by streptavidin-ALP (1:3,000). Signals were developed with phosphate substrate as described before. The 50% inhibitory concentration (IC50) was determined by non-linear regression as the reciprocal dilution giving 50% neutralization (cutoff = [average OD VC + average OD CC] / 2).
ELLA
96-well Nunc MaxiSorp plates were coated with 25 μg/mL fetuin (Sigma) in 1× coating solution (KPL). Heat-inactivated serum samples (56°C, 60 min) were serial diluted 2-fold (starting dilution 1:10) in sample buffer (0.5% Tween 20 in PBS with 0.9 mM CaCl2, 0.5 mM MgCl2, Sigma). A standard dilution of reassorted H6N1 virus (NAPR8) was pre-determined as the 90% maximal effective concentration and added to each well. Plates were incubated for 18 h at 37°C and 5% CO2. Virus without serum served as VC and sample buffer only as negative control. After incubation, 1 μg/mL peanut agglutinin-HRP (Sigma) was added. Signals were developed with tetramethylbenzidine solution (Sigma) for 5 min, stopped with 1 M H2SO4, and read at OD450nm. The NAI titer (IC50) was determined for each serum sample using a non-linear regression. The reciprocal dilution giving 50% inhibition of enzymatic activity was calculated as follows: cutoff = (mean OD VC – mean OD negative control)/2 + mean OD negative control.
IFNγ ELISpot assay
T cell ELISpot was performed with a Mouse IFNγ ELISpotPLUS kit (Mabtech) according to the instructions of the manufacturer. In brief, single-cell suspensions of splenocytes and iliac dLNs were prepared as described above. Pre-coated plates were blocked with complete cell culture medium. 5 × 105 splenocytes or 2.5 × 105 LN cells were seeded per well. Cells were re-stimulated with 2 μg/mL NAPR8 or HAPR8 peptides for 20 h at 37°C and 5% CO2. For NAPR8 the peptides aa 79–93 IRGWAIYSKDNSIRI (MHC class II [I-Ed] restricted),56 aa 191–201 LKYNGIITETI (MHC class II [I-Ad] restricted), or aa 77–84 CPIRGWAI (MHC class I [Dd] restricted)57 and for HAPR8 the peptides aa 124–136 SVSSFERFEIFPK (MHC class II [I-Ed] restricted)59 or aa 532–540 IYSTVASSL (MHC class I [Kd] restricted)58 were used (GeneScript). Spots were counted using an ImmunoSpot CORE cell counter (Cellular Technology). Wells with unstimulated cells served as unspecific background, and values were subtracted from cell counts.
Influenza virus challenge
Mice were challenged with influenza H1N1 PR8 virus (VR-95, ATCC). Anesthesia was applied as described above, and a lethal dose of virus (2.5–100× LD50 total) was administered in 10 μL of PBS into each nostril. For analysis of disease severity, body weight was measured. The humane endpoint was set as 20% weight loss of initial body weight, according to the guidelines of the Norwegian Food Safety Authority (Mattilsynet).
Viral titer determination
Lungs of vaccinated mice were harvested on day 5 post challenge. Half a lung was snap frozen in liquid nitrogen for analyzing the presence of functional virus in tissue culture, while the other half was preserved in RNAlater stabilization solution (Thermo Fisher Scientific) for qRT-PCR analysis. Viral RNA was purified with a QIAamp Viral RNA Mini Kit (QIAGEN) and treated on-column with DNase I (QIAGEN). qRT-PCR was carried out in triplicates using a SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher Scientific) with 0.2 μM primers 5ʹ-GAC CRA TCY TGT CAC CTC TGA C-3′ and 5ʹ-AGG GCA TTY TGG ACA AAK CGT CTA-3′ and 0.1 μM probe 5ʹ-[6FAM]-TGC AGT CCT CGC TCA CTG GGC ACG-[MBGEQ]-3ʹ (Eurofins Genomics) on a One Step Plus machine (Applied Biosystems) with the following program: 50°C (15 min), 95°C (2 min), followed by 45 cycles of 95°C (15 s) and 60°C (30 s). The copy number of viral RNA was determined with quantitative genomic RNA from H1N1 PR8 (VR-95DQ, ATCC) and shown per microgram total tissue RNA. Snap-frozen lungs were homogenized with GentleMACS M tubes (Miltenyi Biotec). A 10-fold dilution series of lung homogenates was incubated with 5 × 104 MDCK cells/well on a 96-well plate in TPCK-trypsin-supplemented virus diluent (described above) for 72 h at 37°C and 5% CO2. Viral TCID50 was determined by the Reed-Muench method98 and shown per milliliter of homogenate.
Serum transfer
Serum from immunized BALB/c mice was collected by terminal heart puncture 5 weeks post vaccination. Equal volumes of serum from individual mice were pooled within each group and transferred to naive BALB/c mice by i.p. injection of 350 μL serum. Mice were challenged 1 day post serum transfer with 2.5 × LD50 H1N1 PR8 virus as described above.
T cell depletion
Immunized BALB/c mice were challenged with influenza virus 2 weeks post vaccination as described above. Two days before infection, 100 μg of anti-CD8α (53–6.7, Bio X Cell) and anti-CD4 (GK1.5, Bio X Cell) mAbs or isotype-matched controls (Y13-238, ATCC; LTF-2, Bio X Cell) were injected i.p. in 400 μL PBS. This treatment was repeated every second day until the end of the experiment. On the day of termination, spleens were harvested from 4 mice/group to control the depletion of T cells. Splenocytes were prepared and analyzed by flow analysis as described above after staining with 2 μg/mL anti-CD3 Violet Fluor 450, anti-CD4 PerCP-Cyanine5.5 (GK1.5, Tonbo Biosciences), and anti-CD8α Alexa Fluor 700 (53–6.7, BioLegend).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 (GraphPad, La Jolla, CA, USA). Significant differences in antibody responses at specific time points were calculated using a two-tailed Mann-Whitney test or Kruskal-Wallis multiple-comparisons test (Dunn’s correction) or over time with a two-way ANOVA. T cell responses and gene copy numbers were tested with a multiple-comparisons one-way ANOVA (Tukey’s correction) and TCID50 and IC50 with a Kruskal-Wallis multiple-comparisons test (Dunn’s correction). Flow cytometry data were analyzed with an unpaired Student’s t test. Weight curves after viral infection were examined using a two-way ANOVA. Survival was compared by Mantel-Cox test. Values of p < 0.05 were considered statistically significant.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data are available from the corresponding author, R.B., upon reasonable request.
Acknowledgments
We kindly thank Jonathan Yewdell for providing us with the anti-NA pAb and the anti-HA mAbs and Richard Webby for the reassorted H6N1 virus. We also gratefully acknowledge Kristina Randjelovic and Peter Hofgaard for expert technical assistance. The study was funded by grants from South-Eastern Norway Regional Health Authority, Norway, projects 2019127 and 2020046 and innovation grants in 2020 and 2021 (to R.B.) and a grant from The Research Council of Norway, project 300049 (to B.B).
Author contributions
B.B. and R.B. conceived the idea of including NA in the APC-targeted DNA vaccine platform. R.B. supervised the project. D.M.H., B.B., and R.B. designed the study, and I.C.W., D.M.H., E.F., B.B., and R.B. designed and planned the experiments. I.C.W. and D.M.H. performed the experiments and analyzed results. I.C.W., B.B., and R.B. wrote the paper. All authors contributed comments and corrections to the manuscript before submission.
Declaration of interests
Oslo University Hospital filed a patent application (62/555,305 “Vaccine molecules”) on A/B heterodimeric vaccine molecules. R.B. and B.B. are inventors of this patent. B.B. is head of the scientific panel of Nykode AS (former Vaccibody AS) and holds shares in the company.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.03.012.
Contributor Information
Ina Charlotta Werninghaus, Email: ina.werninghaus@medisin.uio.no.
Ranveig Braathen, Email: ranveig.braathen@medisin.uio.no.
Supplemental information
References
- 1.Altman M.O., Angeletti D., Yewdell J.W. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunol. 2018;31:142–149. doi: 10.1089/vim.2017.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchinson E.C., Charles P.D., Hester S.S., Thomas B., Trudgian D., Martínez-Alonso M., Fodor E. Conserved and host-specific features of influenza virion architecture. Nat. Commun. 2014;5:4816. doi: 10.1038/ncomms5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dou D., Revol R., Östbye H., Wang H., Daniels R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018;9:1581. doi: 10.3389/fimmu.2018.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017;18:1541. doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019;19:383–397. doi: 10.1038/s41577-019-0143-6. [DOI] [PubMed] [Google Scholar]
- 6.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 7.Monto A.S., Petrie J.G., Cross R.T., Johnson E., Liu M., Zhong W., Levine M., Katz J.M., Ohmit S.E. Antibody to influenza virus neuraminidase: an independent correlate of protection. J. Infect. Dis. 2015;212:1191–1199. doi: 10.1093/infdis/jiv195. [DOI] [PubMed] [Google Scholar]
- 8.Memoli M.J., Shaw P.A., Han A., Czajkowski L., Reed S., Athota R., Bristol T., Fargis S., Risos K., Powers J.H., et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio. 2016;7:e004177-16. doi: 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch R.B., Atmar R.L., Franco L.M., Quarles J.M., Wells J., Arden N., Niño D., Belmont J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013;207:974–981. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy B.R., Kasel J.A., Chanock R.M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 11.Couch R.B., Kasel J.A., Gerin J.L., Schulman J.L., Kilbourne E.D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 1974;129:411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 12.Monto A.S., Kendal A.P. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973;1:623–625. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- 13.Krammer F., Fouchier R.A.M., Eichelberger M.C., Webby R.J., Shaw-Saliba K., Wan H., Wilson P.C., Compans R.W., Skountzou I., Monto A.S. NAction! how can neuraminidase-based immunity contribute to better influenza virus vaccines? MBio. 2018;9:e02332-17. doi: 10.1128/mBio.02332-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlbold T.J., Krammer F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses. 2014;6:2465–2494. doi: 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichelberger M.C., Monto A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J. Infect. Dis. 2019;219:75–80. doi: 10.1093/infdis/jiz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajendran M., Krammer F., McMahon M. The human antibody response to the influenza virus neuraminidase following infection or vaccination. Vaccines. 2021;9:846. doi: 10.3390/vaccines9080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanimoto T., Nakatsu R., Fuke I., Ishikawa T., Ishibashi M., Yamanishi K., Takahashi M., Tamura S.I. Estimation of the neuraminidase content of influenza viruses and split-product vaccines by immunochromatography. Vaccine. 2005;23:4598–4609. doi: 10.1016/j.vaccine.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Kendal A.P., Bozeman F.M., Ennis F.A. Further studies of the neuraminidase content of inactivated influenza vaccines and the neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. Infect. Immun. 1980;29:966–971. doi: 10.1128/iai.29.3.966-971.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendal A.P., Noble G.R., Dowdle W.R. Neuraminidase content of influenza vaccines and neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. J. Infect. Dis. 1977;136:S415–S424. doi: 10.1093/infdis/136.supplement_3.s415. [DOI] [PubMed] [Google Scholar]
- 20.Couch R.B., Atmar R.L., Keitel W.A., Quarles J.M., Wells J., Arden N., Niño D. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine. 2012;31:190–195. doi: 10.1016/j.vaccine.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y.Q., Wohlbold T.J., Zheng N.Y., Huang M., Huang Y., Neu K.E., Lee J., Wan H., Rojas K.T., Kirkpatrick E., et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell. 2018;173:417–429.e10. doi: 10.1016/j.cell.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broecker F., Zheng A., Suntronwong N., Sun W., Bailey M.J., Krammer F., Palese P. Extending the stalk enhances immunogenicity of the influenza virus neuraminidase. J. Virol. 2019;93:e00840-19. doi: 10.1128/JVI.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilbourne E.D., Couch R.B., Kasel J.A., Keitel W.A., Cate T.R., Quarles J.H., Grajower B., Pokorny B.A., Johansson B.E. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine. 1995;13:1799–1803. doi: 10.1016/0264-410x(95)00127-m. [DOI] [PubMed] [Google Scholar]
- 24.Wohlbold T.J., Nachbagauer R., Xu H., Tan G.S., Hirsh A., Brokstad K.A., Cox R.J., Palese P., Krammer F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio. 2015;6 doi: 10.1128/mBio.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W.-C., Lin C.-Y., Tsou Y.-T., Jan J.-T., Wu S.-C. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza A viruses. J. Virol. 2015;89:7224–7234. doi: 10.1128/JVI.00585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Job E.R., Ysenbaert T., Smet A., Christopoulou I., Strugnell T., Oloo E.O., Oomen R.P., Kleanthous H., Vogel T.U., Saelens X. Broadened immunity against influenza by vaccination with computationally designed influenza virus N1 neuraminidase constructs. NPJ Vaccin. 2018;3:55. doi: 10.1038/s41541-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brett I.C., Johansson B.E. Immunization against influenza A virus: comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology. 2005;339:273–280. doi: 10.1016/j.virol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Johansson B.E., Brett I.C. Recombinant influenza B virus HA and NA antigens administered in equivalent amounts are immunogenically equivalent and induce equivalent homotypic and broader heterovariant protection in mice than conventional and live influenza vaccines. Hum. Vaccin. 2008;4:420–424. doi: 10.4161/hv.4.6.6201. [DOI] [PubMed] [Google Scholar]
- 29.McMahon M., Kirkpatrick E., Stadlbauer D., Strohmeier S., Bouvier N.M., Krammer F. Mucosal immunity against neuraminidase prevents influenza B virus transmission in Guinea pigs. MBio. 2019;10:e00560-19. doi: 10.1128/mBio.00560-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith G.E., Sun X., Bai Y., Liu Y.V., Massare M.J., Pearce M.B., Belser J.A., Maines T.R., Creager H.M., Glenn G.M., et al. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology. 2017;509:90–97. doi: 10.1016/j.virol.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K.H., Lee Y.T., Park S., Jung Y.J., Lee Y., Ko E.J., Kim Y.J., Li X., Kang S.M. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology. 2019;535:179–188. doi: 10.1016/j.virol.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Easterbrook J.D., Schwartzman L.M., Gao J., Kash J.C., Morens D.M., Couzens L., Wan H., Eichelberger M.C., Taubenberger J.K. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology. 2012;432:39–44. doi: 10.1016/j.virol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooney A.J., Gabbard J.D., Li Z., Dlugolenski D.A., Johnson S.K., Tripp R.A., He B., Tompkins S.M. Vaccination with recombinant parainfluenza virus 5 expressing neuraminidase protects against homologous and heterologous influenza virus challenge. J. Virol. 2017;91:e01579-17. doi: 10.1128/JVI.01579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Fang F., Li X., Chang H., Chen Z. Protection against influenza virus infection in BALB/c mice immunized with a single dose of neuraminidase-expressing DNAs by electroporation. Vaccine. 2005;23:4322–4328. doi: 10.1016/j.vaccine.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 35.Sandbulte M.R., Jimenez G.S., Boon A.C.M., Smith L.R., Treanor J.J., Webby R.J. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. Plos Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freyn A.W., Ramos da Silva J., Rosado V.C., Bliss C.M., Pine M., Mui B.L., Tam Y.K., Madden T.D., de Souza Ferreira L.C., Weissman D., et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 2020;28:1569–1584. doi: 10.1016/j.ymthe.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M.A. DNA vaccines: an historical perspective and view to the future. Immunol. Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 38.Hobernik D., Bros M. DNA vaccines—how far from clinical use? Int. J. Mol. Sci. 2018;19:3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eusébio D., Neves A.R., Costa D., Biswas S., Alves G., Cui Z., Sousa Â. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today. 2021;26:2575–2592. doi: 10.1016/j.drudis.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura H., Berzofsky J.A. Enhancement of antigenic potency in vitro and immunogenicity in vivo by coupling the antigen to anti-immunoglobulin. J. Immunol. 1986;136:58–65. [PubMed] [Google Scholar]
- 41.Snider D.P., Segal D.M. Targeted antigen presentation using crosslinked antibody heteroaggregates. J. Immunol. 1987;139:1609–1616. [PubMed] [Google Scholar]
- 42.Fredriksen A.B., Bogen B. Chemokine-idiotype fusion DNA vaccines are potentiated by bivalency and xenogeneic sequences. Blood. 2007;110:1797–1805. doi: 10.1182/blood-2006-06-032938. [DOI] [PubMed] [Google Scholar]
- 43.Fredriksen A.B., Sandlie I., Bogen B. DNA vaccines increase immunogenicity of idiotypic tumor antigen by targeting novel fusion proteins to antigen-presenting cells. Mol. Ther. 2006;13:776–785. doi: 10.1016/j.ymthe.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Løvås T.O., Bruusgaard J.C., Øynebråten I., Gundersen K., Bogen B. DNA vaccines: MHC II-targeted vaccine protein produced by transfected muscle fibres induces a local inflammatory cell infiltrate in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braathen R., Spång H.C.L., Hinke D.M., Blazevski J., Bobic S., Fossum E., Bogen B. A DNA vaccine that encodes an antigen-presenting cell-specific heterodimeric protein protects against cancer and influenza. Mol. Ther. Methods Clin. Dev. 2020;17:378–392. doi: 10.1016/j.omtm.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinke D.M., Andersen T.K., Gopalakrishnan R.P., Skullerud L.M., Werninghaus I.C., Grødeland G., Fossum E., Braathen R., Bogen B. Antigen bivalency of antigen-presenting cell-targeted vaccines increases B cell responses. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110901. [DOI] [PubMed] [Google Scholar]
- 47.Fredriksen A.B., Sandlie I., Bogen B. Targeted DNA vaccines for enhanced induction of idiotype-specific B and T cells. Front. Oncol. 2012;2:154. doi: 10.3389/fonc.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudjonsson A., Lysén A., Balan S., Sundvold-Gjerstad V., Arnold-Schrauf C., Richter L., Bækkevold E.S., Dalod M., Bogen B., Fossum E. Targeting influenza virus hemagglutinin to Xcr1 + dendritic cells in the absence of receptor-mediated endocytosis enhances protective antibody responses. J. Immunol. 2017;198:2785–2795. doi: 10.4049/jimmunol.1601881. [DOI] [PubMed] [Google Scholar]
- 49.Gudjonsson A., Andersen T.K., Sundvold-Gjerstad V., Bogen B., Fossum E. Endocytosis deficient murine XCL1-fusion vaccine enhances protective antibody responses in mice. Front. Immunol. 2019;10:1086. doi: 10.3389/fimmu.2019.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grødeland G., Mjaaland S., Roux K.H., Fredriksen A.B., Bogen B. DNA vaccine that targets hemagglutinin to MHC class II molecules rapidly induces antibody-mediated protection against influenza. J. Immunol. 2013;191:3221–3231. doi: 10.4049/jimmunol.1300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambert L., Kinnear E., McDonald J.U., Grodeland G., Bogen B., Stubsrud E., Lindeberg M.M., Fredriksen A.B., Tregoning J.S. DNA vaccines encoding antigen targeted to MHC class II induce influenza-specific CD8+ T cell responses, enabling faster resolution of influenza disease. Front. Immunol. 2016;7:321. doi: 10.3389/fimmu.2016.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braathen R., Spång H.C.L., Lindeberg M.M., Fossum E., Grødeland G., Fredriksen A.B., Bogen B. The magnitude and IgG subclass of antibodies elicited by targeted DNA vaccines are influenced by specificity for APC surface molecules. ImmunoHorizons. 2018;2:38–53. doi: 10.4049/immunohorizons.1700038. [DOI] [PubMed] [Google Scholar]
- 53.Chang H.C., Bao Z., Yao Y., Tse A.G., Goyarts E.C., Madsen M., Kawasaki E., Brauer P.P., Sacchettini J.C., Nathenson S.G., et al. A general method for facilitating heterodimeric pairing between two proteins: application to expression of α and β T-cell receptor extracellular segments. Proc. Natl. Acad. Sci. USA. 1994;91:11408–11412. doi: 10.1073/pnas.91.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolan B.P., Li L., Takeda K., Bennink J.R., Yewdell J.W. Defective ribosomal products are the major source of antigenic peptides endogenously generated from influenza A Virus Neuraminidase. J. Immunol. 2010;184:1419–1424. doi: 10.4049/jimmunol.0901907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerhard W., Yewdell J., Frankel M.E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290:713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 56.Hackett C.J., Horowitz D., Wysocka M., Eisenlohr L.C. Immunogenic peptides of influenza virus subtype N1 neuraminidase identify a T-cell determinant used in class II major histocompatibility complex-restricted responses to infectious virus. J. Virol. 1991;65:672–676. doi: 10.1128/jvi.65.2.672-676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wysocka M., Eisenlohr L.C., Otvos L., Horowitz D., Yewdell J.W., Bennink J.R., Hackett C.J. Identification of overlapping class I and class II H-2d-restricted T cell determinants of influenza virus N1 neuraminidase that require infectious virus for presentation. Virology. 1994;201:86–94. doi: 10.1006/viro.1994.1268. [DOI] [PubMed] [Google Scholar]
- 58.Tamura M., Kuwano K., Kurane I., Ennis F.A. Definition of amino acid residues on the epitope responsible for recognition by influenza A virus H1-specific, H2-specific, and H1- and H2-cross-reactive murine cytotoxic T-lymphocyte clones. J. Virol. 1998;72:9404–9406. doi: 10.1128/jvi.72.11.9404-9406.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackett C.J., Dietzschold B., Gerhard W., Ghrist B., Knorr R., Gillessen D., Melchers F. Influenza virus site recognized by a murine helper T cell specific for H1 strains. Localization to a nine amino acid sequence in the hemagglutinin molecule. J. Exp. Med. 1983;158:294–302. doi: 10.1084/jem.158.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grødeland G., Mjaaland S., Tunheim G., Fredriksen A.B., Bogen B. The specificity of targeted vaccines for APC surface molecules influences the immune response phenotype. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fossum E., Grødeland G., Terhorst D., Tveita A.A., Vikse E., Mjaaland S., Henri S., Malissen B., Bogen B. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8 + T-cell responses against influenza virus. Eur. J. Immunol. 2015;45:624–635. doi: 10.1002/eji.201445080. [DOI] [PubMed] [Google Scholar]
- 62.Andersen T.K., Huszthy P.C., Gopalakrishnan R.P., Jacobsen J.T., Fauskanger M., Tveita A.A., Grødeland G., Bogen B. Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules. npj Vaccin. 2019;4:9–13. doi: 10.1038/s41541-019-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilliams M., Ginhoux F., Jakubzick C., Naik S.H., Onai N., Schraml B.U., Segura E., Tussiwand R., Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grødeland G., Bogen B. Efficient vaccine against pandemic influenza: combining DNA vaccination and targeted delivery to MHC class II molecules. Expert Rev. Vaccin. 2015;14:805–814. doi: 10.1586/14760584.2015.1029919. [DOI] [PubMed] [Google Scholar]
- 65.Andersen T.K., Zhou F., Cox R., Bogen B., Grødeland G. A DNA vaccine that targets hemagglutinin to antigen-presenting cells protects mice against H7 influenza. J. Virol. 2017;91:e01340-17. doi: 10.1128/JVI.01340-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moser M., Murphy K.M. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 67.Lysén A., Braathen R., Gudjonsson A., Tesfaye D.Y., Bogen B., Fossum E. Dendritic cell targeted Ccl3- and Xcl1-fusion DNA vaccines differ in induced immune responses and optimal delivery site. Sci. Rep. 2019;9:1820. doi: 10.1038/s41598-018-38080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krammer F., Palese P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 69.Wohlbold T.J., Podolsky K.A., Chromikova V., Kirkpatrick E., Falconieri V., Meade P., Amanat F., Tan J., Tenoever B.R., Tan G.S., et al. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat. Microbiol. 2017;2:1415–1424. doi: 10.1038/s41564-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiLillo D.J., Palese P., Wilson P.C., Ravetch J.V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 2016;126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dilillo D.J., Tan G.S., Palese P., Ravetch J.V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcR interactions for protection against influenza virus in vivo. Nat. Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruhns P., Jönsson F. Mouse and human FcR effector functions. Immunol. Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 73.Skarlupka A.L., Bebin-Blackwell A.-G., Sumner S.F., Ross T.M. Universal influenza virus neuraminidase vaccine elicits protective immune responses against human seasonal and pre-pandemic strains. J. Virol. 2021;95 doi: 10.1128/JVI.00759-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deroo T., Jou W.M., Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14:561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- 75.Johansson B.E., Grajower B., Kilbourne E.D. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine. 1993;11:1037–1039. doi: 10.1016/0264-410x(93)90130-p. [DOI] [PubMed] [Google Scholar]
- 76.Schulman J.L., Khakpour M., Kilbourne E.D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J. Virol. 1968;2:778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang X., Steukers L., Forier K., Xiong R., Braeckmans K., Van Reeth K., Nauwynck H. A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Job E.R., Ysenbaert T., Smet A., Van Hecke A., Meuris L., Kleanthous H., Saelens X., Vogel T.U. Fcγ receptors contribute to the antiviral properties of influenza virus neuraminidase-specific antibodies. MBio. 2019;10:e01667-19. doi: 10.1128/mBio.01667-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bucher D.J., Kilbourne E.D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J. Virol. 1972;10:60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker N.J., Gandhi S.S. Effect of Ca++ on the stability of influenza virus neuraminidase. Arch. Virol. 1976;52:7–18. doi: 10.1007/BF01317860. [DOI] [PubMed] [Google Scholar]
- 81.Sultana I., Gao J., Markoff L., Eichelberger M.C. Influenza neuraminidase-inhibiting antibodies are induced in the presence of zanamivir. Vaccine. 2011;29:2601–2606. doi: 10.1016/j.vaccine.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 82.Oh S., Belz G.T., Eichelberger M.C. Viral neuraminidase treatment of dendritic cells enhances antigen-specific CD8+ T cell proliferation, but does not account for the CD4+ T cell independence of the CD8+ T cell response during influenza virus infection. Virology. 2001;286:403–411. doi: 10.1006/viro.2001.0992. [DOI] [PubMed] [Google Scholar]
- 83.Stitz L., Huang R.T., Hengartner H., Rott R., Zinkernagel R.M. Cytotoxic T cell lysis of target cells fused with liposomes containing influenza virus haemagglutinin and neuraminidase. J. Gen. Virol. 1985;66(Pt 6):1333–1339. doi: 10.1099/0022-1317-66-6-1333. [DOI] [PubMed] [Google Scholar]
- 84.Andersen T.K., Bodin J., Oftung F., Bogen B., Mjaaland S., Grødeland G. Pandemic preparedness against influenza: DNA vaccine for rapid relief. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.747032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johansson B.E., Kilbourne E.D. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J. Virol. 1993;67:5721–5723. doi: 10.1128/jvi.67.10.5721-5723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johansson B.E., Moran T.M., Kilbourne E.D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl. Acad. Sci. USA. 1987;84:6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rockman S., Brown L.E., Barr I.G., Gilbertson B., Lowther S., Kachurin A., Kachurina O., Klippel J., Bodle J., Pearse M., Middleton D. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J. Virol. 2013;87:3053–3061. doi: 10.1128/JVI.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johansson B.E., Matthews J.T., Kilbourne E.D. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine. 1998;16:1009–1015. doi: 10.1016/s0264-410x(97)00279-x. [DOI] [PubMed] [Google Scholar]
- 89.Johansson B.E. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17:2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- 90.Fritz R., Sabarth N., Kiermayr S., Hohenadl C., Howard M.K., Ilk R., Kistner O., Ehrlich H.J., Barrett P.N., Kreil T.R. A vero cell–derived whole-virus H5N1 vaccine effectively induces neuraminidase-inhibiting antibodies. J. Infect. Dis. 2012;205:28–34. doi: 10.1093/infdis/jir711. [DOI] [PubMed] [Google Scholar]
- 91.Kosik I., Yewdell J.W. Influenza hemagglutinin and neuraminidase: Yin–Yang proteins coevolving to thwart immunity. Viruses. 2019;11:346. doi: 10.3390/v11040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Z., Matsuo K., Asanuma H., Takahashi H., Iwasaki T., Suzuki Y., Aizawa C., Kurata T., Tamura S. Enhanced protection against a lethal influenza virus challenge by immunization with both hemagglutinin- and neuraminidase-expressing DNAs. Vaccine. 1999;17:653–659. doi: 10.1016/s0264-410x(98)00247-3. [DOI] [PubMed] [Google Scholar]
- 93.Johansson B.E., Pokorny B.A., Tiso V.A. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine. 2002;20:1670–1674. doi: 10.1016/s0264-410x(01)00490-x. [DOI] [PubMed] [Google Scholar]
- 94.Bosch B.J., Bodewes R., de Vries R.P., Kreijtz J.H.C.M., Bartelink W., van Amerongen G., Rimmelzwaan G.F., de Haan C.A.M., Osterhaus A.D.M.E., Rottier P.J.M. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 2010;84:10366–10374. doi: 10.1128/JVI.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norderhaug L., Olafsen T., Michaelsen T.E., Sandlie I. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J. Immunol. Methods. 1997;204:77–87. doi: 10.1016/s0022-1759(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 96.Reiersen H., Løbersli I., Løset G.Å., Hvattum E., Simonsen B., Stacy J.E., McGregor D., Fitzgerald K., Welschof M., Brekke O.H., Marvik O.J. Covalent antibody display--an in vitro antibody-DNA library selection system. Nucleic Acids Res. 2005;33:e10. doi: 10.1093/nar/gni010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staudt L.M., Gerhard W. Generation of antibody diversity in the immune response of balb/c mice to influenza virus hemagglutinin: I. Significant variation in repertoire expression between individual mice. J. Exp. Med. 1983;157:687–704. doi: 10.1084/jem.157.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reed L.J., Muench H. A simmple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data are available from the corresponding author, R.B., upon reasonable request.