Abstract
CAR T cells recognizing CD19 effectively treat relapsed and refractory B-ALL and DLBCL. However, CD19 loss is a frequent cause of relapse. Simultaneously targeting a second antigen, CD22, may decrease antigen escape, but is challenging: its density is approximately 10-fold less than CD19, and its large structure may hamper immune synapse formation. The characteristics of the optimal CD22 CAR are underexplored. We generated 12 distinct CD22 antibodies and tested CARs derived from them to identify a CAR based on the novel 9A8 antibody, which was sensitive to low CD22 density and lacked tonic signaling. We found no correlation between affinity or membrane proximity of recognition epitope within Ig domains 3–6 of CD22 with CART function. The optimal strategy for CD19/CD22 CART co-targeting is undetermined. Co-administration of CD19 and CD22 CARs is costly; single CARs targeting CD19 and CD22 are challenging to construct. The co-expression of two CARs has previously been achieved using bicistronic vectors. Here, we generated a dual CART product by co-transduction with 9A8-41BBζ and CAT-41BBζ (obe-cel), the previously described CD19 CAR. CAT/9A8 CART eliminated single- and double-positive target cells in vitro and eliminated CD19- tumors in vivo. CAT/9A8 CART is being tested in a phase I clinical study (NCT02443831).
Keywords: Chimeric antigen receptor (CAR) T cell therapy, CD19 CAR, CD22 CAR, B-ALL, dual targeting, CAR screening, binder screening, CAR sensitivity
Graphical abstract
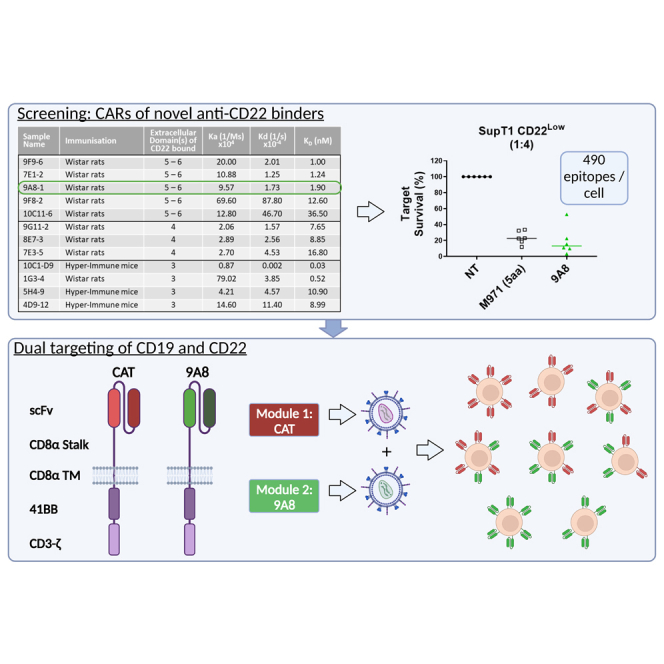
Pule et al. designed a high-sensitivity CD22 CAR after generating 12 novel CD22 antibodies. The candidate, 9A8, was co-transduced with CAT, a clinically relevant CD19 CAR, for a dual CART product. The efficiency of the CAT/9A8 CART product in vitro and in vivo led to an ongoing phase I trial (NCT02443831).
Introduction
CD19-directed chimeric antigen receptor (CAR) T cell therapy has shown remarkable success in the treatment of refractory B cell malignancies.1,2,3 The emergence of CD19 negative tumor escape is a frequent cause of relapse with a reported incidence between 25% and 70%.2,4,5,6 One strategy to prevent CD19-negative tumor escape is the simultaneous targeting of CD19 and a second B lineage antigen.
CD22 is expressed early in B cell ontogeny and continues to be expressed until differentiation into plasma cells.7,8 CD22 is expressed by B cell acute lymphoblastic leukemia (B-ALL),9,10,11,12 with 50%–100% of adult B-ALL13,14,15 and 90% pediatric B-ALL malignant cells being positive.16,17 CD22 is also expressed in other B cell malignancies such as mantle cell lymphoma,18 follicular lymphoma, and diffuse large B cell lymphoma (DLBCL).19 Further, clinical experience demonstrates efficacy of CD22 CAR in B-ALL and DLBCL.20,21
However, CD22 CAR design faces two challenges: first, CD22 density is modest, with a median density of 2,839–3,470 molecules per cell,12 which is further decreased after CAR targeting.20 Second, CD22 has a large, rigid, heavily glycosylated ectodomain22 comprising seven immunoglobular domains.23 The size and rigidity of the ectodomain can compromise immune synapse formation and hence impede CAR function.24
Binder requirements for optimal CD22 CAR function are not well established. Most pre-clinical and clinical exploration of CD22 CAR function has been performed using CARs derived from the human phage library antibody M971.25 To increase this experience, we developed a library of novel CD22 CARs, each based on a unique binder, of which 12 were interrogated for in vitro efficacy and sensitivity, allowing for the selection of an optimal CD22 CAR.
The optimal strategy for CAR targeting of two antigens has not been established. Clinical studies using a two-CAR approach against CD19 and CD22 have been successful, but require the manufacture of two products.26 Alternative approaches using single CARs that express two scFv are technically challenging, with recent disappointing clinical data.27
We have previously described CAT-41BBζ (obe-cel), a CD19 CAR designed to improve engraftment and decrease toxicity.28,29 We next explored a co-transduction approach to co-express CAT-41BBζ with the newly developed CD22 CAR. This approach allowed the generation of a CD19/CD22-specific CAR T product with activity against both antigens in vitro and in vivo.
Results
Screening novel CD22-targeting CARs for efficacy against low-density targets
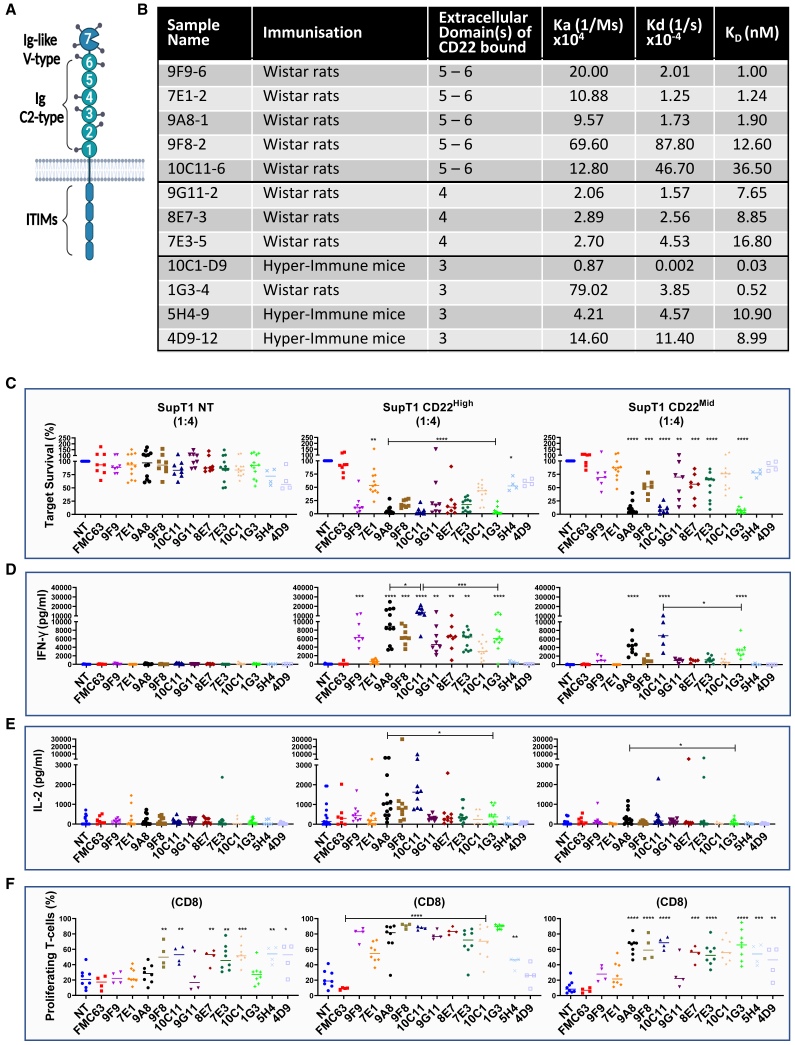
CD22 has a tall, bulky ectodomain (Figure 1A). We discovered a set of novel CD22-specific antibodies, which were derived from hybridomas from immunized Abveris DiversimAb mice and Wistar rats.30,31 Only antibodies binding the proximal 3–6 Ig domains of CD22 were characterized further. Five antibodies bound domains 5–6, three bound domain 4, and four bound domain 3. The affinity ranged between 28 pM and 36.5 nM (Figure S1B). The biophysical properties and identification of the domain recognized are summarized in Figure 1B. These antibodies were next formatted into CARs. To avoid the requirement for optimization of scFv orientation and linker lengths, a Fab 41BB-ζ CAR format was chosen for screening (Figure S1A).32
Figure 1.
Screening of novel binders recognizing CD22
(A) CD22 is a large protein bearing an ectodomain of seven Ig domains. The C2 type Ig domains are labeled 1–6, with domain 6 being the most proximal to-the-membrane domain. (B) Wistar rats or hyper-immune Abverimice were immunized against CD22 ectodomain to obtain novel binders recognizing against CD22. The biophysical properties of the binders, such as the Ig domain recognized and kinetics, are shown in (B). (C) All binders were incorporated into a retroviral cassette into a Fab CAR format and screened against the non-specific SupT1 NT cell line, or SupT1 cells engineered to express high- or mid-CD22 density. CAR T cells were challenged with 5 × 104 SupT1 NT, CD22High, or CD22Mid for 72 h at a 1:4 E:T ratio. We measured the secretion of IFN-γ (D) and IL-2 (E) by ELISA. The supernatant was harvested from a co-culture of 5 × 104 target cells with CAR T cells at 1:4, 72 h after the assay execution. (F) The proliferation of CAR T cells was validated by labeling the T cells with CTV and challenging them with 5 × 104 SupT1 NT, CD22High and CD22Mid at a 1:1 ratio. The CTV dilution was measured on day 4, and the percentage of proliferation for each CAR condition was normalized to the equivalent condition in SupT1 NT for CD8 cells. One-way ANOVA was used to measure the statistical significance of the novel CARs compared to the negative FMC63 control. Comparisons between two CARs were carried out using Student's t-test (n = 8–12).
Functional screening was performed using primary human T cells transduced to express the CD22 CARs. Our interest was focused on identifying CARs that are functional against low antigen density and high disease burden. Hence, we engineered SupT1 cells to express the pathological density of CD22 (3,594 copies per cell) as target cells (SupT1 CD22Mid). SupT1 target cells engineered to express CD22 (6,603 molecules/cell) were also used as targets (SupT1 CD22High) (Figure S2).
The level of expression for the 12 CD22 CARs was comparable as determined by flow cytometric analysis through labeling with soluble CD22 (sCD22). The majority of the CD22 CARs triggered lysis of SupT1 CD22High targets (Figure 1C), however only three (9A8, 10C11, and 1G3) eliminated SupT1 CD22Mid. The same three CARs triggered IFN-γ release against SupT1 CD22Mid (Figure 1D), whereas only 9A8 produced modest levels of IL-2 against the mid-density target (Figure 1E). Seven CARs led to the non-specific proliferation of CD8 T cells. 9A8 and 1G3 did not proliferate in response to SupT1 NT (Non-Transduced), but proliferated against SupT1 CD22Mid (Figures 1F and S3B). Hence, 9A8 and 1G3 were taken forward for further characterization.
Lack of correlation between antibody biophysical characteristics and CAR function
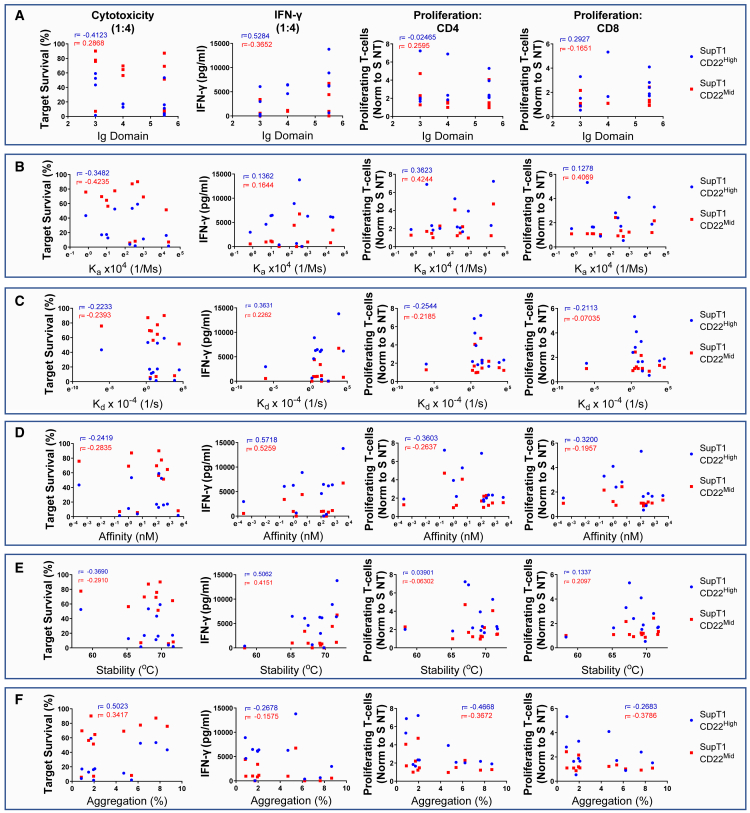
We attempted to find a correlation between binding on- and off-rates, binder stability, or the identity of membrane domain (3–6) recognized. Therefore, the CAR cytotoxicity (effector to target ratio (E:T = 1:4), cytokine production (E:T = 1:4), and proliferative capacity (E:T = 1:1) against the SupT1 CD22High and CD22Mid were correlated with the biophysical properties of the binders (Figures 2A–2E).
Figure 2.
Parameters affecting CAR efficacy
To determine the effect of the binder biophysical properties to the CAR efficacy, we calculated two-dimensional correlation plots of Ig domain (A), association rate (Ka) (B), dissociation rate (Kd) (C), affinity (KD) (D), binder stability (°C) (E), and aggregation (F) to the CAR efficacy. For the CAR efficacy, we introduced the cytotoxicity of the novel aCD22 binders against SupT1 CD22High (blue) or CD22Mid (red) in 1:4 ratio. The r values represent Pearson correlation coefficients (n = 8–12).
As shown in Figure 2A, the cytotoxic function of CART did not correlate with the CD22 Ig domain recognized by the CARs, as two of the most sensitive CARs bind between the fifth and sixth Ig domains, whereas another sensitive CAR binds the third Ig domain. Similarly, efficacy failed to correlate with the affinity, with the three most efficacious binders bearing an affinity of 0.52 nM, 1.9 nM, and 36.5 nM, respectively (Figure 2D). Further, neither the association, dissociation, nor the antibody stability were correlated with lytic capacity (Figures 2B and 2C). However, the stability range of the antibodies was limited with 11 of 12 binders falling in the 67.0–71.7°C window (Figure 2E).
There was no correlation observed between the different binders and the aforementioned parameters. Comparison of 7E1 and 9A8 is illustrative; both bore very similar biophysical properties and additionally bound overlapping epitopes (Figure S1C), while their binding distance from the membrane, affinity, and stability were matched. Despite almost having no differences in their biophysical properties, their efficacy against mid-density targets was markedly different with a median target cell survival of 87.24% and 5.62% for 7E1 and 9A8, respectively. The conventional screening methods, such as three-dimensional affinity based on surface plasmon resonance, failed to predict the disparity in the function of 7E1 and 9A8. Crystallography of 7E1 and 9A8 was attempted to explore if binding angle could explain functional differences; however, this failed because of the high CD22 glycosylation.
9A8-CAR is sensitive against low-density CD22 targets
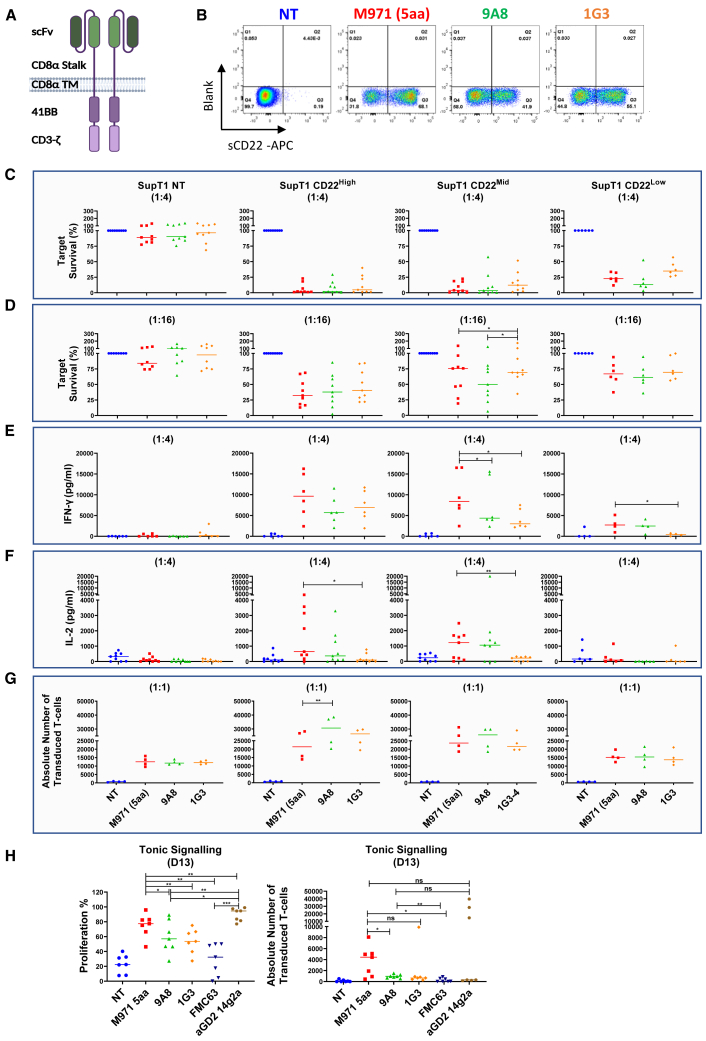
After the screening of the CD22 CARs, two candidates were selected (9A8 and 1G3) for further study. Both 9A8 and 1G3 folded as scFv as shown by efficient binding to sCD22 compared with Fab (Figure S4A), so a standard scFv architecture was used for further study.33 9A8 and 1G3 CAR-transduced T cells were labeled with recombinant sCD22 (Figure 3B). At this juncture, M971 CAR was also introduced as a reference given the experience with this CAR in the clinic.
Figure 3.
Comparison of novel CD22 binding CARs to M971 in a lentiviral platform
(A) The best aCD22 CAR binders, 9A8 and 1G3, were transferred into a lentiviral EF1a promoter platform in an scFv format. (B) The expression of the transgenes on T cells was analyzed by flow cytometry. Specifically, T cells were labeled with biotinylated sCD22, and subsequently labeled with streptavidin-APC and Sytox Blue viability dye. A representative donor is shown in (B). The CAR cytotoxicity was measured against 1 × 105 SupT1 CD22High and CD22Mid target cells at 72 h and an E:T ratio of 1:4 (C) or 1:16 (D). An additional target was introduced, SupT1 CD22Low engineered to express approximately 490 molecules/cell. The cytokine secretion was measured by ELISA at 72 h for 1:4 E:T. The cytokines measured were IFN-γ (E) and IL-2 (F). (G) We investigated the proliferative capacity of the CARs in a 1:1 co-culture measured by the T cell expansion by day 4. (H) CAR T cells were labeled with CTV and challenged in a starvation assay without antigen stimulus or IL-2 for 13 days to measure tonic signaling. The FMC63 and aGD2 CARs were introduced as tonic signaling negative and positive controls. The statistical significance was validated by Student’s t-test. (n = 6–9).
Next, we carried out co-cultures to investigate the cytotoxic capacity of CARs against SupT1 CD22High and CD22Mid. While CD22 target density on B-ALL is described as 2,839–3,470 molecules per cell,12 Fry et al.20 have described the downregulation of CD22 at the presentation of relapse. To test CART function against a lower antigen density platform, an additional SupT1 was engineered (SupT1 CD22Low), where transgenic CD22 expression was constrained by a preceding inefficient stop codon so that expression was approximately 490 molecules per cell (Figure S2).34
All CARs tested were functional against CD22High, CD22Mid, and CD22Low SupT1 tumor cells (Figures 3C and 3D). There was a trend of 9A8 displaying a higher sensitivity against the CD22Low targets at an E:T ratio of 1:4, but despite the six donors screened, the trend did not reach significance (Figure 3C). The overall cytotoxic capacity decreased at the suboptimal ratio of 1:16 (Figure 3D), although there was a trend of 9A8 being superior against CD22Mid-expressing targets. The IL-2 secretion showed no significant difference between M971 and 9A8, whereas the 1G3 IL-2-secreting function was decreased compared with M971 (Figure 3F).
The cytolytic activity of 9A8 CAR was also compared with that of LT22, another clinically proven CD22 CAR, against CD22Mid and CD22Low targets (Figure S3G).26 9A8 was superior to LT22 CAR, with higher sensitivity against both mid- and low-density target cells at a low E:T ratio.
Next, we compared the proliferative capacity of the three CARs against the SupT1 target cells (1:1). Figure 3G depicts the absolute number of transduced CART recovered after day 4 of co-culture. The proliferative capacity of 9A8 was superior to M971 when challenged with CD22High- and CD22Mid-expressing target cells.
Chronic basal signaling of CART in the absence of ligand, known as tonic signaling, may bestow a deleterious effect on CART efficacy and persistence.35,36 To interrogate the 9A8 and 1G3 CARs for tonic signaling, we investigated their capacity for autonomous proliferation in the absence of antigen (Figure 3H). For comparison, we introduced the anti-CD19 FMC63-CAR as a negative control, whereas anti-GD2 14g2a was used as a positive control for tonic signaling, as it has been previously reported.36 The T cells were labeled with CellTrace Violet and cultured in the absence of cytokines and antigenic stimulus. The exhaustion profile was comparable for M971, 9A8, 1G3, and FMC63 CARs (Figure S4E). However, the autonomous proliferation of M971 was significantly higher than the negative control FMC63, as well as 9A8 and 1G3. Both novel CD22 CARs were comparable with FMC63 in their level of tonic signaling.
To maximize the resolution of functional discrepancies, we set up a re-stimulation assay, wherein the T cells were sequentially stimulated with target cells expressing low levels of CD22 (SupT1 CD22Mid and CD22Low) (Figure S4F). M971 and 1G3 failed to retain the cytolytic capacity against SupT1 CD22Low by Stim 3, whereas 9A8 eliminated 50.4% of CD22Low cells.
An in vivo comparison of 9A8 with M971 was carried out in a Nalm-6 model. No significant differences were observed in tumor burden as measured by bioluminescence imaging (BLI) (Figures S5C and S5D) or flow cytometry (Figure S5E).
Of the 12 novel binders, we chose 9A8 as the binder for further study based on superior cytotoxicity against SupT1 CD22Low expressing targets, as well as increased IL-2 secretion and proliferation (Figure 3).
Co-transduction with a CD19/aCD22 dual CAR targeting
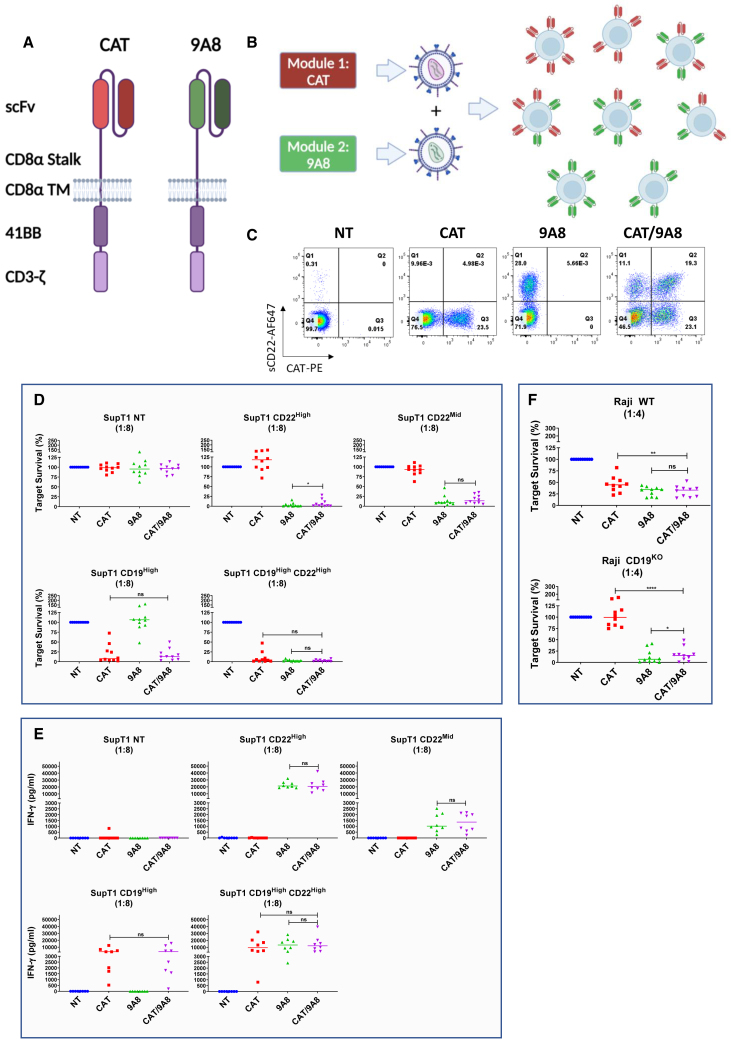
A key application for CD22 CARs is combination with CD19 CARs to prevent antigen escape.2,4,5 We next explored co-targeting CD22 and CD19 using 9A8. For CD19 targeting we used CAT-41BB-Z, a clinically proven CD19 CAR (Figure 4A).2,29 To avoid the loss of stability or loss of expression, we used co-transduction as a strategy for co-expression (Figure 4B).
Figure 4.
In vitro validation of CAT/9A8
(A) CAT and 9A8 were expressed on a separate lentiviral pCCL vectors driven by a PGK and EF1α promoter, respectively. (B) Lentiviral vectors produced transiently in 293T were mixed 1:1 to transduce primary T cells (MOI = 2.5 + 2.5). The CAT/9A8 product constitutes the expression of both CAT and 9A8 CARs at a stochastic mix. (C) The CAR expression was measured by the labeling of T cells with anti-CAT idiotype and sCD22 antigen as shown in x- and y axes, respectively. The mix of the vectors leads to a profile of three transduced populations of SingleCAT, Single9A8 and double-positive. We cultured 5 × 104 SupT1 cells engineered to express CD22High, CD22Mid, CD19High, or CD19HighCD22High with CAR T cells at a ratio of 1:8 for 72 h. At the endpoint of this assay, the target cell lysis (D) and IFN-γ release (E) were assessed. (F) The efficacy of the CAT/9A8 product was also tested against the physiological Raji WT cells and Raji CD19KO, which was implemented to simulate CD19 antigen escape. Student's t-test was utilized to determine statistical significance (n = 10).
CAT/9A8 CAR T cells were generated by double transduction with two separate lentiviral vectors at a multiplicity of infection (MOI) of 2.5/2.5. CAT and 9A8 CART were generated by single transduction with an MOI of 2.5. Labeling showed distinct single and double CAR-positive populations in CAT/9A8 CAR T cells as shown in Figure 4C. The median fluorescence intensity of the CAT or 9A8 CAR in the co-transduced product was comparable with the single CARs. The CAT/9A8 CAR comprises three transduced populations at a ratio of 1:2.1:1.7 for Single9A8, or SingleCAT or double-positive populations. The expression of the CAT or 9A8 CAR in the co-transduced product was comparable to the single CARs (Figures S6A and S6B). An anti-idiotype against CAT was used to label the CART for flow cytometry.
The effectors were challenged against SupT1 NT, SupT1 CD19High, CD22High, CD22Mid, and CD19HighCD22High. The cytotoxic and cytokine secretion capacity of the double transduced CAT/9A8 was comparable with the single CARs as shown in Figures 4D and 4E. Finally, all CARTs were tested against SupT1 CD22Mid, wherein CAT/9A8 was not inferior to 9A8. Notably, the magnitude of cytokine response was not increased with double-positive targets (Figures 4E and S6D–S6F).
Additionally, CAT, 9A8, and CAT/9A8 CARTs were tested in co-cultures with Raji and Raji CD19KO cells (Figure 4F). As expected, 9A8 and CAT/9A8 lysed both Raji wild type (WT) and the CD19KO. However, CAT was unable to eliminate Raji CD19KO. CAT/9A8 was comparable to the 9A8 single transduction against both Raji WT and CD19KO.
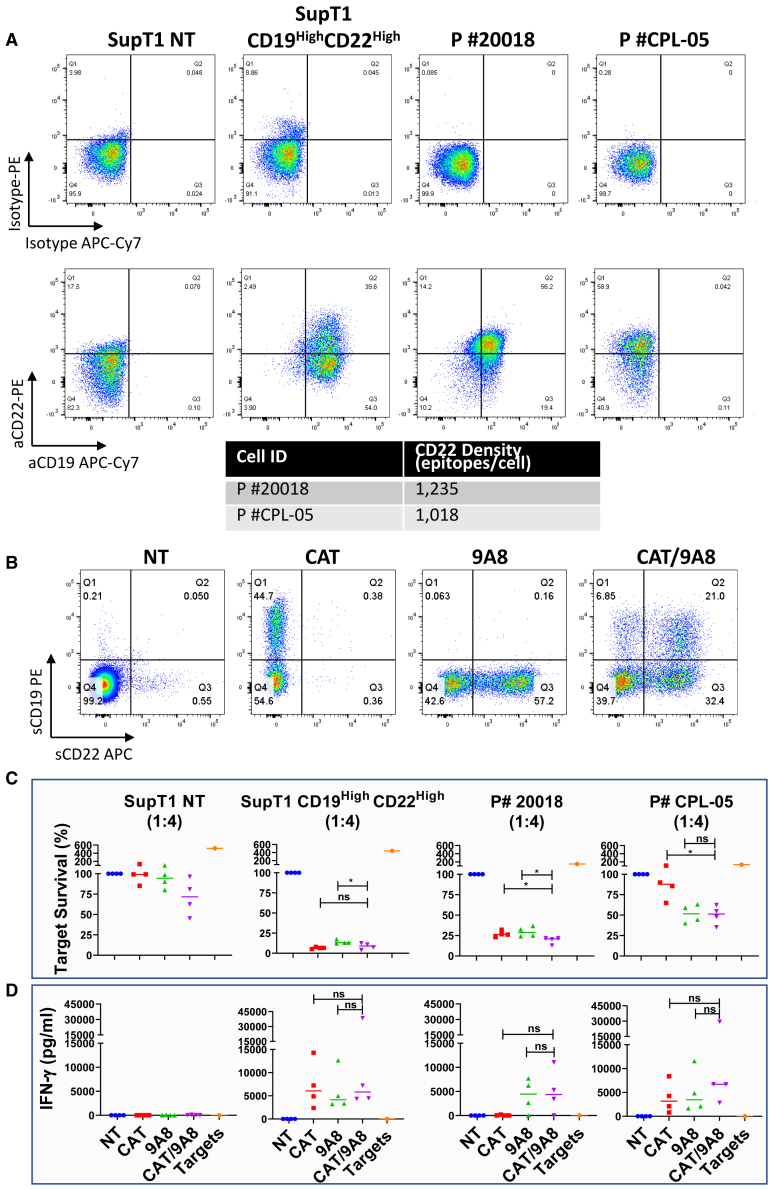
We next explored cytotoxicity against primary B-ALL samples (Figure 5). Although CAT efficiently eliminated the patient sample #20018, which was positive for both CD19 and CD22, it failed to eliminate the CD19– sample (P #CPL-05) (Figure 5C). In contrast, both 9A8 and CAT/9A8 conditions lysed P #CPL-05.
Figure 5.
Cytotoxic efficacy of CAT/9A8 against primary B-ALL patient cells
(A) The expression of CD22 and CD19 antigens was validated by flow cytometry and CD22 density was calculated by using Quantibrite beads. (B) PBMCs were transduced with either the single CAR constructs or co-transduced with the CAT/9A8 product at an MOI of 2.5 + 2.5. We validated the transduction yield by labeling the T cells with soluble antigens. (C) The cytotoxic efficacy of the CARs against the primary B-ALL samples was measured by co-culture and subsequent target cell enumeration at 48 h after co-culture (E:T = 1:4). SupT1 NT and CD19HighCD22High constituted a negative and positive lysis control, respectively. (D) IFN-γ production was measured by ELISA at 48 h, E:T ratio 1:4. Statistical significance was determined by Student's t-test (n = 4).
The cytotoxicity and cytokine secretion of CAT/9A8 in vitro was comparable with that of the single CARs, not compromised by the absence of CD19 or low CD22 expression, both of which constitute antigen escape mechanisms contributing to relapse in clinical studies.
CAT/9A8 is effective against a CD19 antigen escape xenograft model
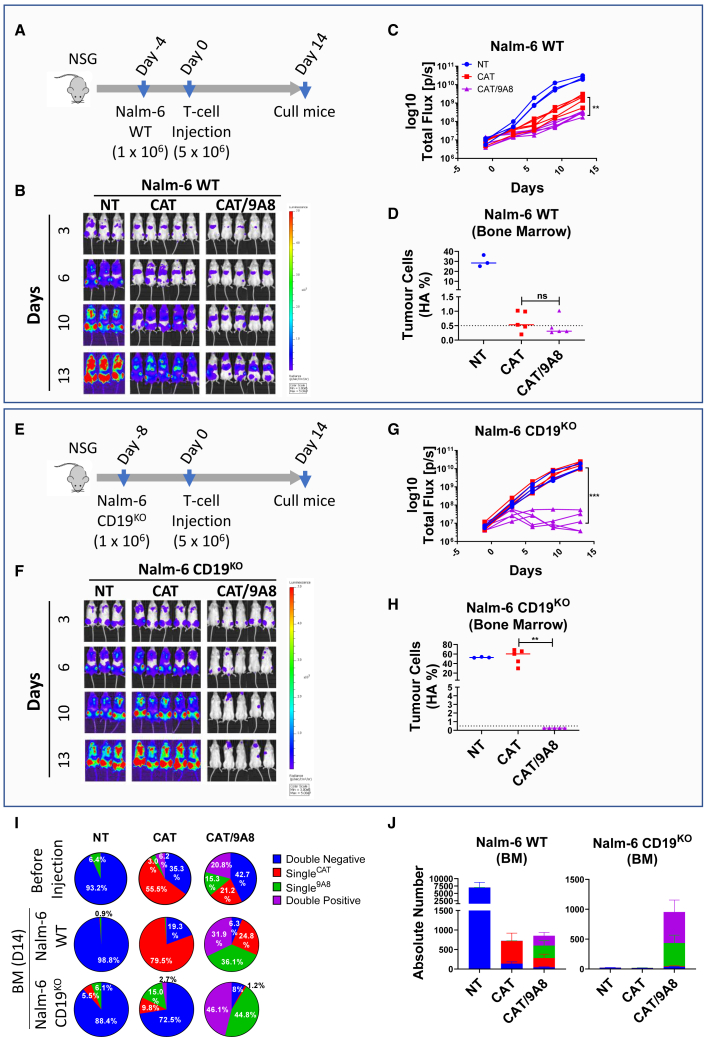
We next tested CAT/9A8 CAR T cells in an in vivo model of B-ALL. HA-tagged Luciferase-expressing NALM-6 cells were engrafted in NSG mice. At a dose of CAR T cells established to sub-optimally eradicate disease, we compared the efficacy of CAT versus CAT/9A8 T cells over the course of 14 days. Tumor eradication was monitored by BLI (Figures 6B and 6C) or HA-tag+ tumor cells in the bone marrow (Figure 6D). BLI imaging showed that CAT/9A8 (3.14xe8) was more efficacious at eliminating the tumor burden compared with CAT (21.3xe8).
Figure 6.
In vivo validation of CAT/9A8
(A) The Nalm-6 WT stress model was performed on NSG mice, where 106 cells were intravenously injected on day −4 and 5 × 106 CAR-expressing T cells were injected on day 0 intravenously. (B) The Nalm-6 tumor line was engineered to express HA-tag, as well as luciferase that was used to estimate tumor burden by BLI. (C) This graph illustrates the luciferase levels detected in (B). The presence of tumor cells in the bone marrow was evaluated by flow cytometry detecting the HA-tag on the tumor cells (D). (E) The CD19 antigen escape was simulated by knocking out CD19 in Nalm-6 cells. One million CD19KO Nalm-6 cells were injected on day −8 and 5 × 106 transduced T cells were injected on day 0. The tumor burden was measured by BLI to detect luciferase on mice over the course of 13 days. (H) The percentage of HA-expressing tumor cells was also enumerated in the bone marrow compartment by flow cytometry. (I) The expression of each CAR subpopulation in each condition was validated in vitro before the injection into mice, as well as in the bone marrow of mice sacrificed on day 14. The percentages shown for BM at day 14 in graph (I) are based on the cell number events shown in the graph in (J). Statistical analyses were performed by Student's t-test (n = 5 mice, T cells derived from 1 donor).
To simulate CD19 antigen escape, we genetically edited Nalm-6 cells with CRISPR/Cas9 to knockout CD19 (Figure S7). CAT and CAT/9A8 tumor eliminating efficacy was tested in a Nalm-6 CD19KO model in NSG mice. CAT CAR T cells failed to eradicate the tumors. In contrast, CAT/9A8 CAR T cells, ablated the CD19– tumors as shown by BLI and flow cytometry (Figures 6F–6H).
CAT/9A8 CART comprise three transduced populations: SingleCAT, Single9A8, and double-positive. The proportion of each population is shown in Figure 6I (and Figure S8). All three transduced populations were equally present before and 14 days after injection for Nalm-6 WT. In the absence of CD19 antigen, the SingleCAT population did not persist, whereas the expression of Single9A8 and double-positive were 1:1 at 44.8% and 46.1%, respectively.
The efficacy of CAT/9A8 was validated in vivo showing it is non-inferior to CAT CARTs in a Nalm-6 stress model. Additionally, CAT/9A8 was effective in a model of CD19-negative escape based on Nalm-6 CD19KO tumor cells.
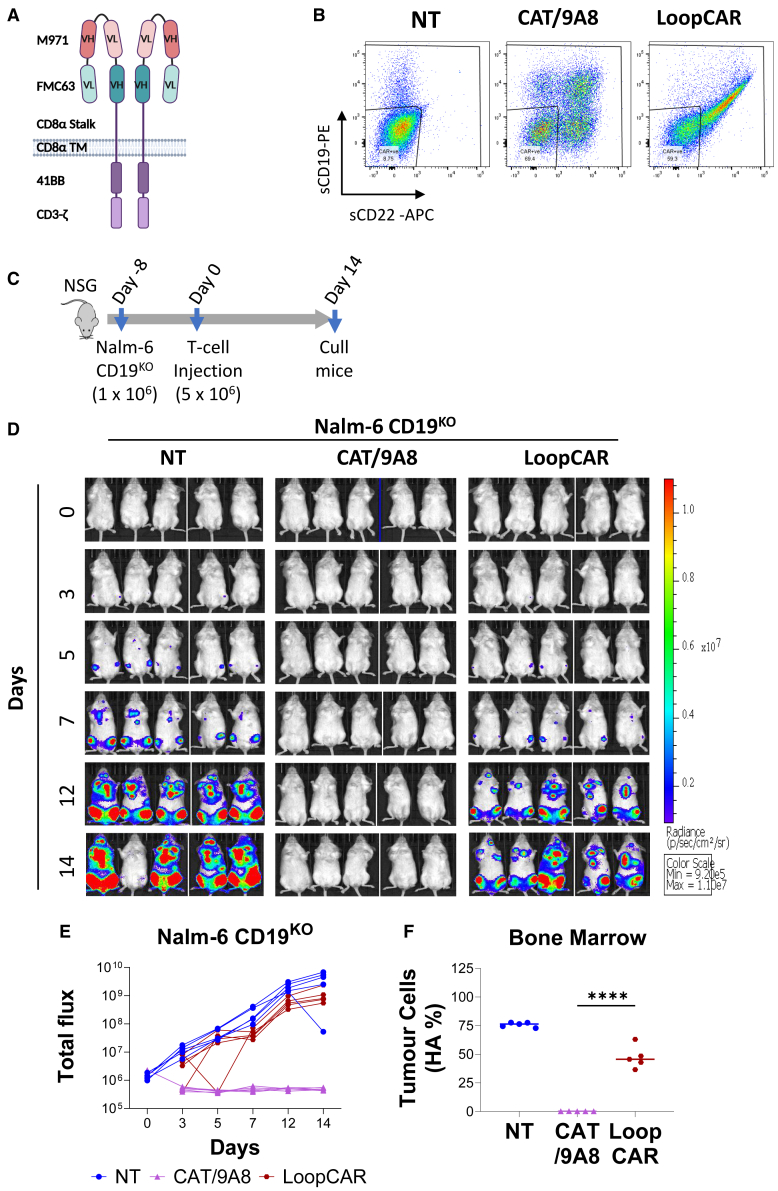
A comparison of CAT/9A8 was also done with the LoopCAR27,37 using Nalm-6 CD19KO. The LoopCAR failed to eliminate the CD19- tumors in contrast to CAT/9A8 (Figure 7).
Figure 7.
In vivo comparison of CAT/9A8 with LoopCAR
A Schematic representation of the LoopCAR structure is shown in (A). (B) T cells bearing the LoopCAR transgene or double-transduced with CAT/9A8 were labeled with sCD19 and sCD22 to determine the transduction efficiency prior to injecting into NSG mice. (C) Nalm-6 CD19KO were used to simulate an antigen escape model. One million Nalm-6 CD19KO were inoculated in mice and CAR T cells were introduced on day 7. The tumor burden is shown by flux (D and E), whereas tumor burden in bone marrow was also measured on day 14 by labeling for the HA marker (F). (n = 5 mice, T cells derived from 1 donor).
Discussion
Autologous CAR T cells targeting CD19 have had considerable success in the adoptive therapy of several B-lineage malignancies.2,29,38,39,40,41,42,43 CD19 loss is a well described mechanism of tumor escape after CD19 CAR T cell therapy.44 Loss of CD19 surface expression is found in 30%–90% of relapsed B-ALL.45 CD19 loss is less studied after CD19 CAR therapy in DLBCL, but is reported in 28%–62% of relapses.46,47 A strategy to reduce target antigen escape is to target a second B lineage antigen simultaneously. CD22 is an attractive option for such a second antigen, given its expression across B cell ontogeny and consequent broad expression by B cell malignancies.
CD22 has been successfully targeted using an antibody drug conjugate.48 However, CD22 is a challenging CAR target: its antigen density is 10-fold lower than CD1912,49 and can down-modulate upon CAR targeting.20 In addition, it is a tall, bulky, and heavily glycosylated protein that would be expected to resist effective approximation of T cells or target cells, preventing effective immune synapse formation.50,51 Despite this, CD22 CAR T cells have shown efficacy against both B-ALL and DLBCL.20,21 Notably, the receptor used in the majority of CD22 CAR T cell studies is based on the human naive phage library derived binder M971.25
The rules for designing a potent CAR, of particular importance to challenging targets such as CD22, seem to be established. For instance, the findings regarding the correlation between the biophysical properties of CARs and their performance weigh heavily in favor of high affinity binding domains,52,53,54,55,56,57 with rare contradictory reports.28,58 Similarly, several reports indicate that CARs targeting epitopes proximal to the membrane bear superior cytotoxic capacity,12,59,60 wherein a many-fold density increase was required for membrane-distal epitopes to stimulate a response comparable to membrane-proximal ones.61
One possible criticism of the above work is that relatively few reports test a large set of binding domains. Through two immunization campaigns in rats or hyper-immune mice, we obtained 12 novel and unique antibodies with an affinity range of 28 pM–36.5 nM. Those 12 antibodies were tested in CAR format in vitro. Analyses showed no correlation between the biophysical properties and in vitro CAR performance. This observation contrasts with the published literature, the majority of which reports a positive correlation between affinity and CAR cytotoxicity.52,53,54,55,56,57 Similarly, we observed no correlation between CAR function and epitope distance from the membrane. However, our study was limited between the third and sixth membrane proximal Ig domains and a threshold where efficient exclusion of CD45 is achievable may exist.51
7E1 and 9A8 constitute a matched pair and are illustrative since their biophysical properties are almost identical, and they bind overlapping epitopes. Nevertheless, 9A8 was the most sensitive CAR and 7E1 one of the least efficacious, with a 15.5-fold lower target cell killing. One possible explanation is a difference in the binding angle of the binder latching onto CD22, thus affecting the formation of the synapse. Crystallography failed, likely because of the high glycosylation of CD22. Further analysis will be required to elucidate the underlying mechanism explaining the differential function between these two CARs.
9A8- and 1G3-based CARs were further characterized and compared with the M971 CAR. In starvation assays, 9A8 showed no tonic signaling, whereas M971 showed non-antigen-specific proliferation. M971-based CARs are known to require an unusually short linker between variable fragment heavy chain/variable fragment light chain, which may promote concatemerization, allowing high sensitivity at the cost of tonic signaling.62 Further comparisons revealed a trend of 9A8 CART proliferating more than M971 and 1G3 CAR T cells, while showing minimal tonic signaling. Hence, we chose 9A8 for subsequent studies.
Several strategies can be used to simultaneously target CD19 and CD22 CARTs. The simplest approach is the sequential administration of anti-CD19 and anti-CD22 CAR T cells, which has been shown in clinical studies to decrease antigen escape.63,64 For example, Pan et al.63 demonstrated that the sequential approach is safe and achieved a 1-year leukemia-free survival rate of 79.5%. The limitation of this strategy is doubling of manufacture cost. Alternatively, dual targeting can be achieved. One approach to dual targeting is a single CAR that targets both CD19 and CD22 by bearing two antigen-binding domains. This approach is technically challenging and clinical testing of bi-specific CARs has been disappointing.27,65,66
A compromise between these two approaches is to co-express two separate CARs in a single T cell. The simplest way to achieve this is by use of a bicistronic cassette.26 One report of bicistronic CD19/CD22 CAR cassette showed poor CAR T cell persistence, precluding a complete assessment of effects on antigen escape. We have previously described and clinically tested CAT-41BBζ obecabtagene autoleucel (obe-cel), a fast binding off-rate CAR designed to decrease toxicity and improve persistence.28,29 It has been reported that bicistronic cassettes can decrease transgene expression.67 In the present study, we explored co-transduction as means of co-expression. This approach decreases the risk of perturbation of expression of either receptor.
The efficacy of the double transduced product was tested in vitro showing the activity of CAT/9A8 being comparable with the single CAR controls. We tested the cytotoxic and cytokine secretion capacity of CAT/9A8 against Raji and SupT1 target cells, including CD22Mid-expressing SupT1. CAT/9A8 consistently performed comparably with the single CARs.
An in vitro cytotoxicity assay against CD19KO Raji (1:4) showed slightly better cytotoxicity with 9A8 CAR T cell effectors in comparison with CAT/9A8. In these experiments, equal numbers of transduced T cells were used; however, not all of the CAT/9A8 CAR T cells express the 9A8 CAR and this lower 9A8 CAR transduction efficiency may explain the decreased cytotoxicity.
In vivo studies showed the efficacy of CAT/9A8 against a Nalm-6 tumor. In a Nalm6 CD19KO model, imitating CD19 antigen escape, CAT/9A8 eliminated the tumor burden. CAT/9A8 proved superior in a CD19 escape in vivo model to CD19/CD22 loop CAR.
CD22 CAR targeting is more challenging than CD19; for instance, in clinical studies relapse has been observed despite CD22 CAR T cell persistence.20 Notably, in cytotoxicity assays against primary B-ALL blasts, CAT/9A8 and 9A8 cytotoxicity was reduced against CD19–/CD22+ B-ALL compared with a double-positive patient sample. This may hint at an inherent biological resistance of CD22 as a target.
Strategies that allow the co-expression of two CARs afford the freedom of using different costimulatory domains tailored to each antigen.26,66 In fact, Shalabi et al.66 showed the benefit of mixing co-stimulatory domains using CD19-CD28ζ and CD22-41BBζ CARs. This is worthy of future exploration.
In summary, we identified a novel CD22 targeting CAR. The process of obtaining the novel antibody revealed a lack of correlation between binder biophysical properties and the CAR function. Functional selection allowed identification of CARs sensitive to very low antigen density. A co-transduction approach was explored with 9A8-41BBζ and CAT-41BBζ, resulting in a CAR T cell product effective at targeting both CD19 and CD22. Autologous T cells co-transduced with CAT/9A8 are currently being clinically tested in children with relapsed or refractory B-ALL (NCT02443831).
Materials and methods
Immunization campaign
Five mice (DiversimAb) were immunized with recombinant human CD22 (1968-SL, Sino Biologicals), according to proprietary protocol (Abveris). Three Wistar rats were genetically vaccinated with a truncated form of human CD22 encoding the four membrane proximal domains, according to proprietary protocol (Aldevron). Sera from immunized animals at intervals were screened for seroconversion against recombinant human CD22 via ELISA. Animals showing polyclonal reactivity against the target protein were selected for hybridoma generation.
Cell lines
HEK-293T (ATCC; ATCCCRL-11268) were cultured in Iscove’s modified Dulbecco’s medium (12-726F, Lonza) supplemented with 10% FBS (Labtech) and 2 mM GlutaMAX (35050061, Invitrogen). SupT1 (ECACC; 95013123), NALM6 (DSMZ; ACC 128) and Raji (ATCC, ATCC CCL-86) lines were cultured in complete RPMI (RPMI-1640, Lonza) supplemented with 10% FBS and 2 mM GlutaMAX.
Flow cytometry
Flow cytometry was performed using MACSQuant 10 and X flow cytometers (Miltenyi Biotec). Labeling was carried out at room temperature for 10 min with antibodies diluted in Cell Staining Buffer (420201, BioLegend). The antibodies used were:
aCD22 CAR was detected by sCD22 labeling (SI2-H82F8, Acro) and streptavidin-APC (405207, BioLegend); the cytotoxicity panel comprised aCD3-PeCy7 (344816, BioLegend), aCD2-PE (300208, BioLegend), aCD8-APC Cy7 (301016, BioLegend), and counting beads (C36950, Invitrogen); for the in vivo models we used aCD45-BV421 (304032, BioLegend), aHA-AF488 (901509, BioLegend), aCD22-PE (302506, BioLegend), 7AAD (420404, BioLegend). The CAT CAR was labeled with an anti-idiotype.
Retroviral and lentiviral production
HEK-293T cells (1.5 × 106) were transiently transfected with an RD114 envelope expression plasmid (RDF, a gift from M. Collins, University College London), and a Gag-pol expression plasmid (PeqPam-env, a gift from E. Vanin, Baylor College of Medicine), and transgene expressed in an SFG vector plasmid at a ratio of 1:1.5:1.5 (total DNA = 12.5 μg). The transfection was carried out with GeneJuice (70967-4, Millipore), according to the manufacturer’s guidelines.
The lentiviral transfection differed in the plasmid composition wherein Gag-pol (plasmid #12251, Addgene, Waterdown, MA), pRSV-Rev (#244772, Addgene), VSV-G envelope (plasmid #12259 Addgene), and pCCL vector were introduced in a ratio of 2:1:1:4 (total DNA = 12.5 uμ). The lentiviral transgenes were driven by an EF1α promoter.
Cytotoxicity assay
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient sedimentation via Ficoll. The source of PBMCs was buffy coats purchased from NHSBT (NC07). Isolated PBMCs were activated with aCD3 (130-093-387, Miltenyi Biotec)/aCD28 antibodies (130-093-375, Miltenyi Biotec), 50 U IL-2 (Z00368-1, 2BScientific Limited) added at 24 h, and engineered by retroviral and lentiviral vectors at 48h. 0.7 × 106 PBMCs were plated on Retronectin coated 6-well plates (T100B, Takara Clonetech) and span at RT, 1,000 × g for 40 min.
The cytotoxicity readout was based on flow cytometry and enumerating the surviving target cells after a 72-h co-culture. At 96 h after transduction, CART were co-cultured with the targets at ratios of 1:2, 1:4, and 1:8. Target number was stable at 0.5 × 105 or 1 × 105 target cells, while T cells differed depending on the effector to target (E:T) ratio. Targets cells were enumerated 72 h after co-culture as alive (7AAD−) and CD3−CD2−CD8–. Target cell survival percentage was calculated by normalizing the number of viable target cells to that recovered from co-cultures carried out with NT T cells.
Cytotoxicity of primary cells
The primary B-ALL cells were acquired from UCL Institute of Child Health cell bank (P #CPL-05) or purchased from the Fred Hutch Hematopoietic Diseases Repository (P #20018). The B-ALL samples were thawed in gradual volume of PBS plus 1% FCS, enumerated, and co-cultured with CTV labeled CAR-expressing PBMCs for 48 h at a 1:4 ratio. The assay was carried out as described above.
Cytokine measurements
The cytokine concentrations were determined by ELISA on co-culture supernatants harvested at 72 h. We used IFN-γ and IL-2 BioLegend Deluxe kits (431806 and 430106, BioLegend) according to the manufacturer’s instructions.
Proliferation assay and tonic signaling
PBMCs were labeled with CellTrace Violet (CTV) according to manufacturer’s instructions (C34557, ThermoFisher Scientific) and cultured with 0.5 × 105 target cells at a 1:1 E:T ratio for 96 h. The proliferation was determined as CD3+ sCD22+ transduced T cell number and percentage of CTV% decrease.
The tonic signaling constituted a starvation assay of 0.5 × 105 CTV-labeled T cells cultured for 13 days in the absence of targets or IL-2. The proliferation was calculated as described above.
Xenograft NSG model
All animal studies were performed under a United Kingdom Home Office-approved project license and all experiments were carried out according to the relevant regulatory standards. NSG mice (female, aged 6–10 weeks) were obtained from Charles River Laboratories and raised under pathogen-free conditions.
We inoculated 1 × 106 Nalm6 WT or CD19KO cells, engineered with an HA-luciferase cassette, intravenously into NSG mice on days −8 and −4, respectively. Tumor engraftment was measured by bioluminescent imaging using the IVIS spectrum system (PerkinElmer) after intra-luciferin peritoneal injection. The mice were randomized on day −1. On day 0, 5 × 106 CAR T cells were injected intravenously, and subsequently the mice were culled on day 14.
Statistical analyses
The data are visualized as median and analyses were performed in GraphPad Prism version 8.2. For a direct comparison of two conditions, we performed two-tailed paired t-test. For multiple comparisons with a single condition, we performed one-way ANOVA with Dunnett’s post-test. Significance of findings are defined as follows: NS, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Schematic cartoons
The cartoons were created in BioRender.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
M.P. is supported by the University College London Hospital Biomedical Research Centre. M.P. receives salary contribution and owns stock in Autolus Ltd. E.K., S.T., S.O., and S.C. are employees of Autolus Ltd. M.P., E.K., S.P.C., S.O., S.T., B.M., and M.F. are inventors on patent WO2019/220109. M.P. is an inventor on WO2016/139487. Both patents are related to this manuscript. The primary B-ALL cells were purchased from the Fred Hutch Hematopoietic Diseases Repository or gifted from Persis Amrolia’s group at UCL Child Health Institute.
Author contributions
M.P. conceived the project. S.O., B.M., and M.F. generated and characterized the binders. E.K. designed and performed the in vitro and in vivo experiments, analyzed the data and wrote the manuscript. T.G., W.H., S.M., E.C., J.T., I.G., and S.S. performed the in vitro experiments. L.S. and M.Robson carried out the in vivo work. J.S. carried out the knock-out experiments for obtaining CD19- cell lines. M.P. and S.C. reviewed the data and wrote the manuscript.
Declaration of interests
Financial conflict of interest as all authors have Autolus shares. (E.K., M.F., T.G., A.B., R.J., M.Robson, J.T., M.E.K., M.Righi, J.S., I.G., S.T., and M.P. are currently Autolus employees).
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.03.020.
Supplemental information
References
- 1.Park J.H., Geyer M.B., Brentjens R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghorashian S., Pule M., Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br. J. Haematol. 2015;169:463–478. doi: 10.1111/bjh.13340. [DOI] [PubMed] [Google Scholar]
- 3.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotillo E., Barrett D.M., Black K.L., Bagashev A., Oldridge D., Wu G., Sussman R., Lanauze C., Ruella M., Gazzara M.R., et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner R., Wu D., Cherian S., Fang M., Hanafi L.-A., Finney O., Smithers H., Jensen M.C., Riddell S.R., Maloney D.G., Turtle C.J. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalabi H., Kraft I.L., Wang H.-W., Yuan C.M., Yates B., Delbrook C., Zimbelman J.D., Giller R., Stetler-Stevenson M., Jaffe E.S., et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103:e215–e218. doi: 10.3324/haematol.2017.183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyron-Quiroz J.E., Partida-Sánchez S., Donís-Hernández R., Sandoval-Montes C., Santos-Argumedo L. Expression and function of CD22, a B-cell restricted molecule. Scand. J. Immunol. 2002;55:343–351. doi: 10.1046/j.1365-3083.2002.01063.x. [DOI] [PubMed] [Google Scholar]
- 8.Stoddart A., Ray R.J., Paige C.J. Analysis of murine CD22 during B cell development: CD22 is expressed on B cell progenitors prior to IgM. Int. Immunol. 1997;9:1571–1579. doi: 10.1093/intimm/9.10.1571. [DOI] [PubMed] [Google Scholar]
- 9.Boué D.R., LeBien T.W. Expression and structure of CD22 in acute leukemia. Blood. 1988;71:1480–1486. [PubMed] [Google Scholar]
- 10.Engel P., Wagner N., Miller A.S., Tedder T.F. Identification of the ligand-binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J. Exp. Med. 1995;181:1581–1586. doi: 10.1084/jem.181.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J., Fan G., Zhong Y., Gatter K., Braziel R., Gross G., Bakke A. Diagnostic usefulness of aberrant CD22 expression in differentiating neoplastic cells of B-Cell chronic lymphoproliferative disorders from admixed benign B cells in four-color multiparameter flow cytometry. Am. J. Clin. Pathol. 2005;123:826–832. doi: 10.1309/KPXN-VR7X-4AME-NBBE. [DOI] [PubMed] [Google Scholar]
- 12.Haso W., Lee D.W., Shah N.N., Stetler-Stevenson M., Yuan C.M., Pastan I.H., Dimitrov D.S., Morgan R.A., FitzGerald D.J., Barrett D.M., et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevallier P., Robillard N., Houille G., Ayari S., Guillaume T., Delaunay J., Harousseau J.-L., Avet-Loiseau H., Mohty M., Garand R. Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases. Leukemia. 2009;23:806–807. doi: 10.1038/leu.2008.303. [DOI] [PubMed] [Google Scholar]
- 14.Piccaluga P.P., Arpinati M., Candoni A., Laterza C., Paolini S., Gazzola A., Sabattini E., Visani G., Pileri S.A. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk. Lymphoma. 2011;52:325–327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H., Thomas D., Jorgensen J., Kebriaei P., Jabbour E., Rytting M., York S., Ravandi F., Garris R., Kwari M., et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119:2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raetz E.A., Cairo M.S., Borowitz M.J., Blaney S.M., Krailo M.D., Leil T.A., Reid J.M., Goldenberg D.M., Wegener W.A., Carroll W.L., et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J. Clin. Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwamoto S., Deguchi T., Ohta H., Kiyokawa N., Tsurusawa M., Yamada T., Takase K., Fujimoto J., Hanada R., Hori H., et al. Flow cytometric analysis of de novo acute lymphoblastic leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int. J. Hematol. 2011;94:185–192. doi: 10.1007/s12185-011-0900-1. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Wang S.A., Sun T. Composite mantle cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma. Cytometry B Clin. Cytom. 2018;94:148–150. doi: 10.1002/cyto.b.21512. [DOI] [PubMed] [Google Scholar]
- 19.Olejniczak S.H., Stewart C.C., Donohue K., Czuczman M.S. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol. Invest. 2006;35:93–114. doi: 10.1080/08820130500496878. [DOI] [PubMed] [Google Scholar]
- 20.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah N.N., Highfill S.L., Shalabi H., Yates B., Jin J., Wolters P.L., Ombrello A., Steinberg S.M., Martin S., Delbrook C., et al. CD4/CD8 T-Cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 2020;38:1938–1950. doi: 10.1200/JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ereño-Orbea J., Sicard T., Cui H., Mazhab-Jafari M.T., Benlekbir S., Guarné A., Rubinstein J.L., Julien J.-P. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun. 2017;8:764. doi: 10.1038/s41467-017-00836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson G.L., Fox C.H., Fauci A.S., Kehrl J.H. cDNA cloning of the B cell membrane protein CD22: a mediator of B-B cell interactions. J. Exp. Med. 1991;173:137–146. doi: 10.1084/jem.173.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong R., Libby K.A., Blaeschke F., Fuchs W., Marson A., Vale R.D., Su X. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR) EMBO J. 2020;39:e104730. doi: 10.15252/embj.2020104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X., Ho M., Zhu Z., Pastan I., Dimitrov D.S. Identification and characterization of fully human anti-CD22 monoclonal antibodies. MAbs. 2009;1:297–303. doi: 10.4161/mabs.1.3.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordoba S., Onuoha S., Thomas S., Pignataro D.S., Hough R., Ghorashian S., Vora A., Bonney D., Veys P., Rao K., et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegel J.Y., Patel S., Muffly L., Hossain N.M., Oak J., Baird J.H., Frank M.J., Shiraz P., Sahaf B., Craig J., et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghorashian S., Kramer A.M., Onuoha S., Wright G., Bartram J., Richardson R., Albon S.J., Casanovas-Company J., Castro F., Popova B., et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 29.Roddie C., Dias J., O’Reilly M.A., Abbasian M., Cadinanos-Garai A., Vispute K., Bosshard-Carter L., Mitsikakou M., Mehra V., Roddy H., et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J. Clin. Oncol. 2021;39:3352–3363. doi: 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen T.E., Abdullah R., Boucher J., Brousseau A.S., Dasuri N.K., Ditto N.T., Doucette A.M., Emery C., Gabriel J., Greamo B., et al. Accelerated antibody discovery targeting the SARS-CoV-2 spike protein for COVID-19 therapeutic potential. Antib. Ther. 2021;4:185–196. doi: 10.1093/abt/tbab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oettgen H.F., Binaghi R.A., Benacerraf B. The effect of various hormones on the serum level of anaphylactic antibody in the rat. Int. Arch. Allergy Appl. Immunol. 1966;29:131–139. doi: 10.1159/000229694. [DOI] [PubMed] [Google Scholar]
- 32.Nolan K.F., Yun C.-O., Akamatsu Y., Murphy J.C., Leung S.-O., Beecham E.J., Junghans R.P. Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin. Cancer Res. 1999;5:3928–3941. [PubMed] [Google Scholar]
- 33.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.-H., Geiger T.L., Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 34.Sillibourne J.E., Agliardi G., Righi M., Smetanova K., Rowley G., Speller S., Dolor A., Lamb K., Allen C., Karattil R., et al. A compact and simple method of achieving differential transgene expression by exploiting translational readthrough. Biotechniques. 2022;72:143–154. doi: 10.2144/btn-2021-0079. [DOI] [PubMed] [Google Scholar]
- 35.Frigault M.J., Lee J., Basil M.C., Carpenito C., Motohashi S., Scholler J., Kawalekar O.U., Guedan S., McGettigan S.E., Posey A.D., et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin H., Ramakrishna S., Nguyen S., Fountaine T.J., Ponduri A., Stetler-Stevenson M., Yuan C.M., Haso W., Shern J.F., Shah N.N., Fry T.J. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol. Ther. Oncolytics. 2018;11:127–137. doi: 10.1016/j.omto.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., et al. CD19-Targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grupp S.A., Maude S.L., Shaw P.A., Aplenc R., Barrett D.M., Callahan C., Lacey S.F., Levine B.L., Melenhorst J.J., Motley L., et al. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) Blood. 2015;126:681. [Google Scholar]
- 41.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlando E.J., Han X., Tribouley C., Wood P.A., Leary R.J., Riester M., Levine J.E., Qayed M., Grupp S.A., Boyer M., et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018;24:1504–1506. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 45.Majzner R.G., Mackall C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 46.Plaks V., Rossi J.M., Chou J., Wang L., Poddar S., Han G., Wang Z., Kuang S.-Q., Chu F., Davis R.E., et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138:1081–1085. doi: 10.1182/blood.2021010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oak J., Spiegel J.Y., Sahaf B., Natkunam Y., Long S.R., Hossain N., Mackall C.L., Kong K.A., Miklos D.B. Target antigen downregulation and other mechanisms of failure after axicabtagene ciloleucel (CAR19) therapy. Blood. 2018;132:4656. doi: 10.1182/blood-2018-99-120206. [DOI] [Google Scholar]
- 48.DiJoseph J.F., Popplewell A., Tickle S., Ladyman H., Lawson A., Kunz A., Khandke K., Armellino D.C., Boghaert E.R., Hamann P., et al. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol. Cancer Immunol. Immunother. 2005;54:11–24. doi: 10.1007/s00262-004-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majzner R.G., Rietberg S.P., Sotillo E., Dong R., Vachharajani V.T., Labanieh L., Myklebust J.H., Kadapakkam M., Weber E.W., Tousley A.M., et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anton van der Merwe P., Davis S.J., Shaw A.S., Dustin M.L. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Q., Zhang X., Tu L., Cao J., Hinrichs C.S., Su X. Size-dependent activation of CAR-T cells. Sci. Immunol. 2022;7:eabl3995. doi: 10.1126/sciimmunol.abl3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chames P., Willemsen R.A., Rojas G., Dieckmann D., Rem L., Schuler G., Bolhuis R.L., Hoogenboom H.R. TCR-like human antibodies expressed on human CTLs mediate antibody affinity-dependent cytolytic activity. J. Immunol. 2002;169:1110–1118. doi: 10.4049/jimmunol.169.2.1110. [DOI] [PubMed] [Google Scholar]
- 53.Chmielewski M., Hombach A., Heuser C., Adams G.P., Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 54.Hudecek M., Lupo-Stanghellini M.-T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caruso H.G., Hurton L.V., Najjar A., Rushworth D., Ang S., Olivares S., Mi T., Switzer K., Singh H., Huls H., et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X., Jiang S., Fang C., Yang S., Olalere D., Pequignot E.C., Cogdill A.P., Li N., Ramones M., Granda B., et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynn R.C., Feng Y., Schutsky K., Poussin M., Kalota A., Dimitrov D.S., Powell D.J., Jr. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turatti F., Figini M., Balladore E., Alberti P., Casalini P., Marks J.D., Canevari S., Mezzanzanica D. Redirected activity of human antitumor chimeric immune receptors is governed by antigen and receptor expression levels and affinity of interaction. J. Immunother. 2007;30:684–693. doi: 10.1097/CJI.0b013e3180de5d90. [DOI] [PubMed] [Google Scholar]
- 59.Hombach A.A., Schildgen V., Heuser C., Finnern R., Gilham D.E., Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J. Immunol. 2007;178:4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 60.Wilkie S., Picco G., Foster J., Davies D.M., Julien S., Cooper L., Arif S., Mather S.J., Taylor-Papadimitriou J., Burchell J.M., Maher J. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J. Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 61.James S.E., Greenberg P.D., Jensen M.C., Lin Y., Wang J., Till B.G., Raubitschek A.A., Forman S.J., Press O.W. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh N., Frey N.V., Engels B., Barrett D.M., Shestova O., Ravikumar P., Cummins K.D., Lee Y.G., Pajarillo R., Chun I., et al. Antigen-independent activation enhances the efficacy of 41BB co-stimulated CD22 CAR T cells. Nat. Med. 2021;27:842–850. doi: 10.1038/s41591-021-01326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan J., Zuo S., Deng B., Xu X., Li C., Zheng Q., Ling Z., Song W., Xu J., Duan J., et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135:387–391. doi: 10.1182/blood.2019003293. [DOI] [PubMed] [Google Scholar]
- 64.Wang N., Hu X., Cao W., Li C., Xiao Y., Cao Y., Gu C., Zhang S., Chen L., Cheng J., et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135:17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 65.Hossain N., Sahaf B., Abramian M., Spiegel J.Y., Kong K., Kim S., Mavroukakis S., Oak J., Natkunam Y., Meyer E.H., et al. Phase I experience with a Bi-specific CAR targeting CD19 and CD22 in adults with B-cell malignancies. Blood. 2018;132:490. doi: 10.1182/blood-2018-99-110142. [DOI] [Google Scholar]
- 66.Shalabi H., Qin H., Su A., Yates B., Wolters P.L., Steinberg S.M., Ligon J.A., Silbert S., DéDé K., Benzaoui M., et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140:451–463. doi: 10.1182/blood.2022015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar M., Keller B., Makalou N., Sutton R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.