Influenza A viruses pose an ongoing threat due to their role in seasonal epidemics and the potential for the emergence of novel viruses from the animal reservoir.1 Extensive efforts are being made to develop vaccines that elicit protection against multiple influenza virus antigens, as well as universal vaccines that confer broad protection against antigenically distinct seasonal and/or pandemic viruses. In order to achieve long-lived and cross-protective immunity, it is considered that combining distinct vaccine platforms in heterologous prime:boost regimens may be required.2
In this issue of Molecular Therapy, Werninghaus and colleagues evaluate the potential of a novel plasmid DNA-based vaccination approach in which the encoded antigen is targeted to antigen-presenting cells (APCs) by fusion to a single chain variable fragment (scFv) recognizing MHC II.3 Building upon the authors’ prior success in engineering a similar vaccine against influenza virus hemagglutinin (HA),4 in the current study, the authors selected influenza virus neuraminidase (NA) as their target antigen. Through a series of in vivo immunogenicity and efficacy experiments in mice, they demonstrated that their vaccine could elicit NA-specific humoral and cellular immune responses and confer protection against homologous influenza virus challenge. As such, this study expands our current understanding of NA-based immunity in the context of an innovative DNA vaccine platform, and it further solidifies NA as a leading target in influenza vaccine development.
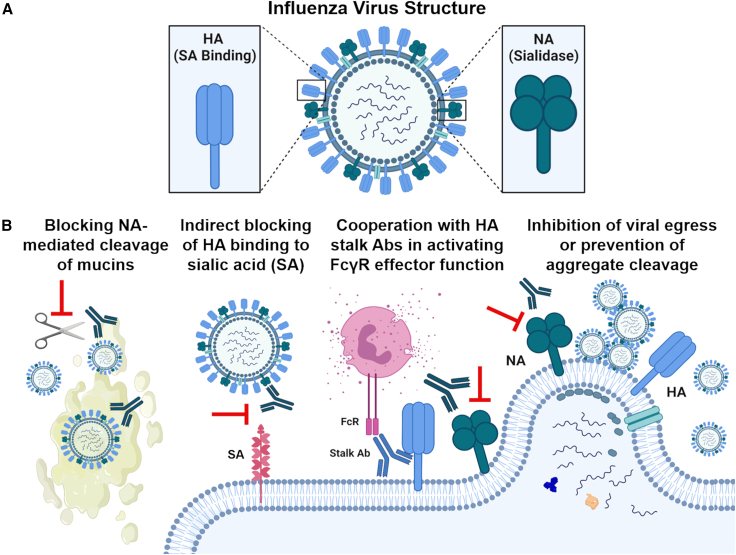
To date, influenza vaccines have largely focused on the major surface glycoprotein, trimeric HA. However in recent years, NA, the second surface glycoprotein, has reemerged as a promising target for universal influenza virus vaccines (Figure 1).5 NA is a tetrameric protein important in the virus life cycle; its sialidase activity is important for releasing virus trapped by natural defense proteins on mucosal surfaces, in addition to releasing nascent viruses from infected cells.6 Anti-NA antibodies (Abs) can protect through several mechanisms, including inhibiting the enzymatic activity of NA. Importantly, NA-specific Abs have been shown to limit disease severity in animal models, can elicit sterilizing protection in vivo,7 and have been associated with reducing viral shedding and symptomatic infection in human challenge.8 Notably, NA activity is the target of licensed antiviral drugs oseltamivir (Tamiflu) and zanamivir (Relenza). Although the therapeutic effects resulting from the inhibition of NA activity validate NA as a drug target, NA has been under-investigated as a vaccine antigen relative to HA.
Figure 1.
Mechanisms of action of NA-specific antibodies
(A) Current influenza virus vaccine development is focused on trimeric hemagglutinin (HA), the major viral surface glycoprotein important for virus binding to the viral entry receptor, sialic acid (SA). However, tetrameric neuraminidase (NA), the second viral surface glycoprotein, important for releasing virus from SA through its sialidase activity, is reemerging as a potential target. (B) Anti-NA Abs act to antagonize the virus by (1) blocking NA-mediated enzymatic cleavage of virus from mucins in the airway, (2) indirectly blocking HA binding to SA through steric hindrance, (3) acting synergistically with HA stalk Abs to activate FcγR effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), and (4) blocking NA enzymatic activity at the point of viral egress to prevent cleavage of viral aggregates or inhibit viral release and onward spread within the host. Figure created with BioRender.com.
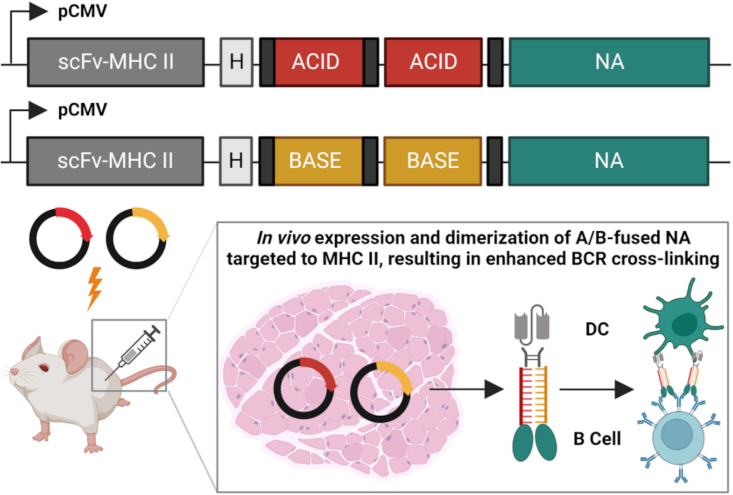
To assess the potential of NA using an innovative approach, Werninghaus et al. engineered DNA plasmids as three-component fusion constructs: (1) an APC-targeting unit (i.e., svFv-MHC II), (2) a dimerization unit containing either the acid or base (A/B) component of a modified Jun/Fos leucine zipper motif, and (3) a dimeric antigen unit encoding the ectodomain of N1 or H1 glycoproteins from H1N1 virus A/Puerto Rico/8/34/Mount Sinai (i.e., NA/NA, HA/HA, or NA/HA). The authors reasoned that by targeting vaccine antigen to professional APCs expressing MHC II using scFvMHCII, they could enhance immune recognition of the encoded NA or HA. To confirm the importance of targeting to MHC II, they also constructed control fusion constructs that were identical in design, with the exception that the “targeting unit” was swapped for an irrelevant scFv targeting the hapten NIP (scFvαNIP). In their vaccine approach, dimerization of the A/B unit is achieved following co-immunization with separate plasmids encoding the “A” fusion construct and the “B” fusion construct, resulting in heterodimerization of the expressed A/B units in vivo to create secreted dimeric antigen fusions (Figure 2).
Figure 2.
Novel dimeric NA-based APC-targeting DNA vaccine elicits protective anti-NA antibodies in vivo
Vaccine DNA plasmids were engineered as three-component fusion constructs encoding (1) an APC-targeting unit (svFv-MHC II), (2) a dimerization unit containing either the acid or base (A/B) component of a modified Jun/Fos leucine zipper motif, and (3) a dimeric antigen unit encoding the ectodomain of N1 from H1N1 virus A/Puerto Rico/8/34/Mount Sinai. Following immunization of mice, in vivo expression and dimerization of A/B-fused NA targeted to MHC II resulted in the induction of NA-based, Ab-mediated protection against homologous virus through a mechanism considered to enhance B cell receptor (BCR) cross-linking. Figure created with BioRender.com.
To characterize vaccine-induced immune responses against NA, the authors immunized BALB/c mice with a single dose of plasmids encoding the MHC II-targeted A/B NA, followed by electroporation. Following immunization, antigen-specific Ab (ELISA) and T cell responses (IFN-γ ELISpot) were detected. Additionally, NA-specific Abs were found to bind to formalin-inactivated homologous H1N1 virus (although it is important to note that the process of inactivation may be disruptive to NA quality). Interestingly, NA-specific immune responses induced by the MHC II-targeted dimeric NA vaccine (i.e., NA/NA) were elevated compared with the same vaccine encoding dimeric HA (i.e., HA/HA). Vaccines carrying one copy each of NA and HA (i.e., NA/HA), as well as vaccines lacking MHC II targeting, were poorly immunogenic compared with the MHC II-targeted dimeric NA vaccine. Thus, APC targeting and antigen bivalency (i.e., dimeric fusion) were shown to be important for the immunogenicity of NA.
After determining the immunogenicity of the MHC II-targeted bivalent NA vaccine in vivo, the authors sought to evaluate the protective efficacy of these immune responses. Mice immunized with a single dose of plasmid DNA vaccine were subsequently challenged with a homologous influenza H1N1 virus (PR8 strain). At a high plasmid vaccine dose (100 μg), the MHC II-targeted dimeric NA and dimeric HA vaccines and the dimeric NA vaccine lacking MHC II targeting were all completely protective. However, upon vaccine dose de-escalation, even at increased viral challenge doses, the MHC II-targeted dimeric NA vaccine was significantly more protective when compared with the other vaccine constructs.
It is well established that the quantity and quality of NA in conventional IIV formulations can vary between lots and is not currently standardized.9 Nonetheless, comparisons of new influenza vaccine strategies to the current standard of care, IIV, are useful.10 The scFvMHC II-A/B-NA/NA construct elicited higher levels of NA-specific Abs than a matched IIV and resulted in comparable protection following homologous challenge. Vaccine-induced protection for scFvMHC II-A/B-NA/NA was found to be primarily mediated by NA-specific antibodies, as sera from immunized mice passively transferred to naive mice, conferring significant protection against homologous virus challenge.
Finally, the authors sought to elucidate the mechanism of protection of the vaccine-induced NA-specific Abs. Typically, NA-specific Abs do not completely inhibit virus entry and are therefore not always capable of completely neutralizing the virus in vitro. Alternatively, NA-specific Abs can act during later stages of the virus life cycle, often through the inhibition of the enzymatic activity of NA, preventing viral budding from infected cells and subsequent spread (Figure 1). Werninghaus et al. found that although sera from mice vaccinated with MHC II-targeted dimeric NA did not neutralize homologous virus in an immunostaining-based microneutralization assay, vaccine-induced anti-NA serum Abs inhibited the enzymatic activity of NA in an enzyme-linked lectin assay. Furthermore, vaccination with the MHC II-targeted dimeric NA vaccine appeared to reduce viral load in the lung after homologous challenge. Therefore, as observed for the induction of immune responses, MHC II targeting and the use of dimeric antigen were also shown to be important for vaccine efficacy.
Next-generation influenza vaccines are urgently needed to provide broad protection against emerging influenza viruses. Although this study evaluated an innovative vaccine approach, protection was limited to a homologous challenge virus. Furthermore, current thinking in the field is that correctly folded, antigenically authentic tetrameric NA is required for optimal enzymatic activity and may be preferred for eliciting broad immunity.9 However, in this study, the vaccine platform did not express tetrameric NA (the antigen was dimeric). Despite this, the vaccine induced Abs that could inhibit the enzymatic activity of NA and could confer homologous in vivo protection in mice. Therefore, these findings highlight the importance of continuing to evaluate distinct vaccine platforms or antigen formulations to improve our understanding of how anti-NA Abs with various functional activities contribute to in vivo protection.
Acknowledgements
The Coughlan lab is funded by National Institute of Allergy and Infectious Diseases (NIAID) grant (R01AI148369), and by contract funding from NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs: 75N93019C00055).
Declaration of interests
The authors do not have any interests to disclose.
References
- 1.Kerstetter L.J., Buckley S., Bliss C.M., Coughlan L. Adenoviral vectors as vaccines for emerging avian influenza viruses. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.607333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlan L., Palese P. Overcoming barriers in the path to a universal influenza virus vaccine. Cell Host Microbe. 2018;24:18–24. doi: 10.1016/j.chom.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Werninghaus I.C., Hinke D.M., Fossum E., Bogen B., Braathen R. Neuraminidase delivered as an APC-targeted DNA vaccine induces protective antibodies against influenza. Mol. Ther. 2023 doi: 10.1016/j.ymthe.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinke D.M., Andersen T.K., Gopalakrishnan R.P., Skullerud L.M., Werninghaus I.C., Grødeland G., Fossum E., Braathen R., Bogen B. Antigen bivalency of antigen-presenting cell-targeted vaccines increases B cell responses. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110901. [DOI] [PubMed] [Google Scholar]
- 5.Wohlbold T.J., Krammer F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses. 2014;6:2465–2494. doi: 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., García-Sastre A. Influenza. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadlbauer D., Zhu X., McMahon M., Turner J.S., Wohlbold T.J., Schmitz A.J., Strohmeier S., Yu W., Nachbagauer R., Mudd P.A., et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science. 2019;366:499–504. doi: 10.1126/science.aay0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy B.R., Kasel J.A., Chanock R.M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 9.Krammer F., Fouchier R.A.M., Eichelberger M.C., Webby R.J., Shaw-Saliba K., Wan H., Wilson P.C., Compans R.W., Skountzou I., Monto A.S. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio. 2018;9 doi: 10.1128/mBio.02332-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss C.M., Freyn A.W., Caniels T.G., Leyva-Grado V.H., Nachbagauer R., Sun W., Tan G.S., Gillespie V.L., McMahon M., Krammer F., et al. A single-shot adenoviral vaccine provides hemagglutinin stalk-mediated protection against heterosubtypic influenza challenge in mice. Mol. Ther. 2022;30:2024–2047. doi: 10.1016/j.ymthe.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]