Abstract
Lung cancer causes the most cancer-related deaths worldwide. In recent years, molecular and immunohistochemical techniques have rapidly developed, further inaugurating an era of personalized medicine for lung cancer. The rare subset of lung cancers accounts for approximately 10%, each displaying distinct clinical characteristics. Treatments for rare lung cancers are mainly based on evidence from common counterparts, which may lead to unsolid clinical benefits considering intertumoral heterogeneity. The increasing knowledge of molecular profiling of rare lung cancers has made targeting genetic alterations and immune checkpoints a powerful strategy. Additionally, cellular therapy has emerged as a promising way to target tumor cells. In this review, we first discuss the current status of targeted therapy and preclinical models for rare lung cancers, as well as provide mutational profiles by integrating the results of existing cohorts. Finally, we point out the challenges and future directions for developing targeted agents for rare lung cancer.
Keywords: lung neoplasm, rare cancers, molecular targeted therapy, immune checkpoint inhibitors, preclinical model
Graphical abstract

Jianxin Xue and colleagues first briefly described rare lung cancers, then summarized the clinical efficacy of FDA-approved targeted agents. Furthermore, they discussed potential targets, with comprehensive mutational profile provided, and the research model status. They finally highlighted the challenges and prospects in developing targeted therapy for rare lung cancers.
Introduction
Lung cancer is the leading cause of cancer-related death in the United States and worldwide, representing approximately one in five cancer-related deaths.1,2 Non-small cell lung cancer (NSCLC) shares the largest proportion of 84.3% of all lung cancers, followed by small cell lung cancer (SCLC), accounting for 12.5%.3 NSCLC is further histologically subgrouped into common types, including adenocarcinoma (50.4%) and squamous carcinoma (22.6%), and rare types, e.g., large cell carcinoma, adenosquamous carcinoma, and sarcomatoid carcinoma. According to the Surveillance of Rare Cancers in Europe (RARECARE) project, rare cancers occur in fewer than six per 100,000 people.4 Each rare histological subtype of NSCLC meets not only the threshold of rare cancers but also the percentage frequency that is considered rare (5% cutoff) (Table 1).3,4,5,6,7,8,9,10,11 Although rare individually, these rare subtypes in total represent approximately one-tenth of all primary lung cancers (Figure 1A)3,5,6,7,8,9,10,11 and may affect over 200,000 people per year,2 thus causing an inescapable disease burden.
Table 1.
Incidence and proportion of histologic subtypes of lung cancers
| Cancer type | Incidence (/100,000) | Proportion (%) | References | |
|---|---|---|---|---|
| SCLC | 5.9 | 12.5 | Howlader et al.3 | |
| NSCLC | LUAD | 24.1 | 50.4 | Howlader et al.3 |
| LUSC | 10.9 | 22.6 | Howlader et al.3 | |
| LCC | NA | 3.2a | Chan et al.5 | |
| LASC | 0.3 | 0.4–4.0 | Gatta et al.4; de Jong et al.6; Li et al.7 | |
| pLEC | NA | 0.4–0.7 | Hu et al.8 | |
| pNMC | NA | 0.6 | Xie et al.9 | |
| PSC | 0.2 | 0.5 | Gatta et al.4; Steuer et al.10 | |
| pSGT | 0.1 | 0.1–0.2 | Gatta et al.4; Molina et al.11 | |
The proportion column represents the percentage of total lung cancer. NSCLC, non-small cell lung cancer; LASC, lung adenosquamous carcinoma; LCC, large cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NA, not available; pLEC, pulmonary lymphoepithelial carcinoma; pNMC, pulmonary nuclear protein of the testis midline carcinoma; PSC, pulmonary sarcomatoid carcinoma; pSGT, pulmonary salivary gland-type tumors; SCLC, small cell lung cancer.
Proportion of NSCLC.
Figure 1.
Overview of histological subtypes of all lung cancers and mutation profile of major subtypes of NSCLC
(A) Histological subtypes of all lung cancers.3,5,6,7,8,9,10,11 Since the proportion of LCC in all lung cancers is not available, we estimated that LCC accounts for 2.7% of all lung cancers based on its fraction in NSCLC. LASC, lung adenosquamous carcinoma; LCC, large cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; pLEC, pulmonary lymphoepithelial carcinoma; pNMC, pulmonary nuclear protein of the testis midline carcinoma; PSC, pulmonary sarcomatoid carcinoma; pSGT, pulmonary salivary gland-type tumors; SCLC, small cell lung cancer.
(B) The frequency of driver mutations in LUAD and LUSC. Data were collected from the LUNGevity Foundation (https://www.lungevity.org/).
Although surgery serves as the optimal treatment for lung cancer, over half of the patients (54% of NSCLC, 75% of SCLC) have distant metastasis at the time of diagnosis, and the 5-year survival rate is disappointing (26.3% in NSCLC, 6.7% in SCLC).3 Targeting SCLC is always challenging since RB1 and TP53 deficiency mainly serve as its driver mutations. In recent decades, with driver mutations detected in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (Figure 1B), an increasing number of targeted agents have emerged as more effective and endurable treatment options in lung cancers.12,13 In terms of rare lung cancers, management often follows the guidelines for common NSCLCs in the absence of standard treatments, which may lead to an unsatisfactory clinical response. Fortunately, booming genetic sequencing and immunohistochemical techniques have revealed the distinct molecular landscape of many rare lung cancers, enabling more precise molecular classification and novel therapeutic strategies. For example, Pécuchet et al.14 classified pulmonary sarcomatoid carcinoma (PSC) into two clusters (Csig4 and Csig2-3-13) based on mutational signatures and found that greater PD-L1 expression was associated with Csig4, while MET mutations and other targets were identified more frequently in Csig2-3-13. Despite some promising results with targeted therapy for specific subtypes of rare lung cancers, such as mesenchymal-epithelial transition factor (MET) inhibitors for PSC,15 the value of targeted therapy in many rare lung cancers remains unclear. Further research is needed to fully understand the potential benefits and limitations of this approach for these patients.
In this review, after briefly describing the clinical characteristics of rare lung cancers involved, we summarize the clinical efficacy of U.S. Food and Drug Administration (FDA)-approved targeted agents. Then we explore the potential to target other molecules and tumor cells. Furthermore, the preclinical model status is also presented, considering its important role in validating oncogenic mechanisms and drug screening. Finally, we point out the challenges and prospects of developing targeted therapy for rare lung cancers.
Histopathological and epidemiological traits
Large cell carcinoma
Large cell carcinoma (LCC), found in approximately 3.2% of NSCLC,5 can be diagnosed only on resected tumors after ruling out any clear morphologic or immunohistochemical differentiation toward small cell carcinoma, adenocarcinoma, or squamous cell carcinoma. Surgery could bring significant benefits to patients with LCC, while chemotherapy or radiotherapy showed limited efficacy. Exceeding 70% of LCC cases are identified in stage III/IV, and distant metastasis proneness also indicates a worse prognosis than other NSCLCs.16
Lung adenosquamous carcinoma
Lung adenosquamous carcinoma (LASC), accounting for 0.4%–4.0%4,6,7 of all lung cancers, is defined as a mixed-type tumor containing components of LUAD and LUSC, with each comprising at least 10% of the tumor. According to the proportion of the two components, LASC can be divided into three groups: adeno-predominant ASC (LUAD >60%), squamous-predominant ASC (LUSC >60%), and structure-balanced ASC (each component is between 40% and 60%).7 LASC is more prevalent in elderly men with a smoking history,7 and it has more invasive characteristics and a poorer prognosis than LUAD and LUSC.17
Pulmonary lymphoepithelial carcinoma
Pulmonary lymphoepithelial carcinoma (pulmonary LEC), occurring in less than 1% of all lung cancers,8 is closely associated with Epstein-Barr virus (EBV) infection and is now classified as a subtype of LUSC.18 The pathology describes a strong infiltration of abundant lymphocytic and plasma cells in the stroma; meanwhile, the intratumoral amyloid deposition and squamous differentiation can be found in pulmonary LEC.19 Most affected individuals are Asian with a mean age between 51 and 57 years old, and no obvious gender predilection is observed.19,20 Approximately half of patients are diagnosed at an early stage with the opportunity to receive surgery; therefore, pulmonary LEC has a higher 5-year survival rate than other subtypes of NSCLC.20 However, it is worth exploring more precise treatments considering its distinct molecular landscape.
Pulmonary nuclear protein of the testis midline carcinoma
Pulmonary nuclear protein of the testis (NUT) midline carcinoma (pulmonary NMC), which accounts for only 0.6% of lung malignancies,9 is a poorly differentiated and aggressive cancer defined by NUT rearrangement. The histopathological characteristics mainly refer to poorly differentiated cells with focal squamous differentiation, along with positive immunohistochemistry staining of NUT expression.9,21 Pulmonary NMC is prone to affect young adults with a mean age of 42–48 years old.9,22 No significant benefit has been shown in most cases, with advanced pulmonary NMC treated with chemotherapy or chemoradiotherapy.9,23 Pulmonary NMC has an extremely poor prognosis, with a median overall survival of 2.2–2.75 months.21,22
Pulmonary sarcomatoid carcinoma
PSC, representing 0.5% of all lung cancers,10 is a heterogeneous group of tumors that comprises five subsets, including pleomorphic carcinoma (PC), spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma. PC is the most common subtype, mainly composed of epithelial carcinoma and at least 10% sarcomatoid component (spindled or giant cells).24 Characterized by high invasiveness, 48.0%–69.2% of patients with PSC are diagnosed at stage IV, and its resistance to conventional systemic therapy may explain the inferior survival outcome.10,25,26
Pulmonary salivary gland-type tumor
Pulmonary salivary gland-type tumor (pulmonary SGT), accounting for only 0.1%–0.2% of all lung cancers, represents a distinct and heterogeneous group of NSCLCs, including mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (ACC), and four rarer subtypes. Pulmonary MEC consists of mucocytes, squamous cells, and intermediate cells, while pulmonary ACC is mainly composed of epithelial and myoepithelial cells and frequently exhibits submucosal spread and perineural invasion27,28 Patients with pulmonary SGT are distributed over a wide range of ages, mainly in middle age, and show no overt gender tendency.11,28,29,30 Most pulmonary SGTs are considered low-grade tumors characterized by weak invasiveness and long survival. However, pulmonary MEC seems to have higher mortality rates than pulmonary ACC, with the recurrence rate on the contrary.28
Efficacy of FDA-approved targeted therapies
Rare lung cancers have no standard treatments and have scarcely been enrolled in clinical trials of targeted therapies (Table 2),31,32,33,34,35,36 mainly due to the rarity of a single disease. Given that rare lung cancers are categorized as NSCLC, detecting biomarkers with confirmed benefits from matched targeted therapy for this indication may be more feasible in clinical practice.
Table 2.
Clinical trials of FDA-approved targeted agents with rare lung cancers enrolled
| Target | FDA-approved therapy | Clinical trial (phase) | Comparator | Treatment line | Rare lung cancers enrolled (n) | ORR | mPFS (mo) | mOS (mo) |
|---|---|---|---|---|---|---|---|---|
| EGFR | Osimertinib | FLAURA (III)31 | Erlotinib or Gefitinib | First-line | LASC (3), LCCa (5) | 80% | 18.9 | 38.6 |
| Mobocertinib | NCT02716116 (I/II)32 | No | Second-line | LCCa (1) | 28% | 7.3 | 24.0 | |
| MET | Capmatinib | GEOMETRY mono-1 (II)33 | No | Beyond first-line | LCCa (1) | 44% (68%)b | 5.4 | NA |
| Tepotinib | VISION (II)34 | No | First-line and beyond | PSC (1) | 46% | 8.5 | 17.1 | |
| KRAS | Sotorasib | CodeBreaK 100 (I)35 | No | Beyond first-line | LCCa (3) | 32% | 6.3 | 12.5 |
| BRAF | Dabrafenib plus Trametinib | BRF113928 (II)36 | No | First-line | LASC (1), LCCa (1) | 64% | 10.8 | 17.3 |
mOS, median overall survival; mPFS, median progression-free survival; ORR, objective response rate.
Not able to confirm exact histological subtypes due to the changing definition of LCC.
Indicates data for treatment on naive patients.
FDA-approved targets for NSCLC include sensitizing epidermal growth factor receptor (EGFR), mesenchymal-epithelial transition factor exon 14 (METex14) skipping mutations, kristen rat sarcoma 2 viral oncogene homolog (KRAS) point mutation, vascular endothelial growth factor (VEGF) mutations, BRAF V600E point mutations, anaplastic lymphoma kinase (ALK) fusions, ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) gene fusions, neurotrophin tyrosine kinase (NTRK) gene fusions, RET rearrangements, and immune checkpoints.
Synopsis of targets frequently reported in rare lung cancers
Targets that have been more frequently reported in rare lung cancers include EGFR, MET, KRAS, VEGF, and immune checkpoints (Figure 2A).
Figure 2.
Overview of reported targets in rare lung cancers
(A) A schematic tree model that displays targets covered (left) and not yet covered (right) by FDA-approved biomarkers of each rare lung cancer.
(B) Potential targets and simplified signaling pathways of rare lung cancers. In pLEC, EBV-infected tumor cells can upregulate EBNA1 expression to maintain EBV episome and facilitate oncogenesis. Additionally, TRAF3, a negative regulator of the NF-κB pathway, can be eliminated by the LMP1 protein, which is encoded by EBV, further promoting the NF-κB activity and MHC-I expression. Birinapant can mimic the effect of TRAF3 deficiency and enhance the response to immunotherapy. In pNMC, BET enables the phosphorylation of RNA Pol II to induce oncogene transcription. In PSC, TGFβ could downregulate the transcriptional factor OVOL2 and further activate EMT-drivers (ZEB1 and TWIST) to induce transcription of DDR2 and NCAD. In pACC, the MYB-NFIB fusion may be regulated by AKT-dependent IGF1R signaling.
The EGFR gene encodes a transmembrane protein of the HER/erbB family of receptor tyrosine kinases (RTKs).37 Mutated EGFR could trigger a cascade of intracellular signaling resulting in increased proliferation, metastasis, angiogenesis, and inhibition of tumor apoptosis, thus further leading to a poor clinical outcome. Multiple generations of EGFR tyrosine kinase inhibitors (TKIs) have been developed and approved by the FDA, showing considerable benefits to patients with NSCLC.13
MET alterations can display as an exon 14 skipping mutation, gene copy number amplification, protein overexpression, MET gene fusions, etc. Specifically, the METex14 skipping mutation is the major event that results in excessive tyrosine kinase signaling. Over the past few years, great progress has been made in developing agents targeting METex14 skipping mutations, including type I (e.g., capmatinib and savolitinib) and type II (e.g., cabozantinib and glesatinib) inhibitors based on different binding modes. Novel drugs, including glumetinib and vebreltinib, have been granted as “orphan drugs” by the FDA, and both are undergoing phase II clinical trials (NCT04270591 and NCT03175224, respectively). MET amplification, generally considered a mechanism of resistance to EGFR-TKIs and ALK inhibitors, has received growing attention in recent years.
The KRAS gene, the most frequently mutated subset of the RAS gene family (including HRAS, KRAS, and NRAS), encodes the KRAS protein, which mediates the proliferation, apoptosis, and differentiation of tumor cells. A common mutation in G12C has enabled the development of targeted agents in recent years, with sotorasib (AMG510) and adagrasib (MRTX849) approved by the FDA for treating NSCLC with the KRAS G12C mutation.
The VEGF gene, located on chromosome 6, encodes a VEGF protein that plays a key role in angiogenesis and endothelial cell differentiation by interacting with the tyrosine kinase receptor VEGFR. Bevacizumab and ramucirumab, monoclonal antibodies (mAbs) against VEGF and VEGFR, respectively, have been approved by the FDA for NSCLC. Multitargeted TKIs, such as anlotinib and apatinib, are under evaluation in clinical trials. In contrast to precise targeted therapies, antiangiogenic agents can be applied without certain VEGF gene mutations given that angiogenesis is one of the hallmarks of cancer.
Immune checkpoints refer to a group of molecules that transmit inhibitory signals on T cells, such as PD-1, CTLA-4, TIM3, LAG3, and TIGIT.38 Immune checkpoint blockade has proven to be effective in stimulating antitumor immunity, and the most successful clinical practice would be inhibiting the PD-1/PD-L1 axis and CTLA-4, with several immune checkpoint inhibitors (ICIs) approved for NSCLC. Moreover, a phase II, open-label study aimed to explore the antitumor activity and safety profile of atezolizumab in pretreated advanced NSCLC patients with rare histological subtypes (Table 3).
Table 3.
Clinical trials related to rare lung cancers without reported results
| Cancer type | Target(s) | Drug | Combination | Clinical trial (phase) | Treatment line | Status |
|---|---|---|---|---|---|---|
| LASC | EGFR | Almonertinib | No | NCT04354961 (II) | First-line | Not yet recruiting |
| PSC | PD-1 | Camrelizumab | Famitinib | NCT04888429 (II) | NA | Not yet recruiting |
| PD-1 | Toripalimab | Bevacizumab; Nab-paclitaxel; Carboplatin | NCT04725448 (I) | First-line | Recruiting | |
| NMCa | BRD4-BD1/2 | BI 894999 | No | NCT02516553 (Ia/Ib) | NA | Completed |
| HDAC and PI3K | CUDC-907 | No | NCT02307240 (I) | NA | Completed | |
| BET and CBP/p300 | EP31670 | No | NCT05488548 (I) | NA | Not yet recruiting | |
| NSCLC with rare histology | PD-L1 | Atezolizumab | No | NCT03976518 (II) | Beyond first-line | Recruiting |
NA, not available.
Midline carcinoma with NUT rearrangement regardless of the primary site.
Large cell carcinoma
KRAS mutations, mainly G12C mutations, were detected in 12.0%–31.6% of LCCs5,39,40,41, and approximately 52% of LCCs were altered in the RTK/RAS/RAF pathway. A single-group, phase 2 clinical trial investigated the antitumor activity of sotorasib as a subsequent therapy in NSCLC patients who had previously received platinum-based chemotherapy with or without immunotherapy, including three LCC patients.35 The objective response rate (ORR) was 37.1%, and the median progression-free survival (PFS) was 6.8 months; however, the response of LCC patients remains unclear since the results were not analyzed by histological type.
The PD-L1 expression rates were 44.4%–80.0% for LCC patients (≥1%), of whom 27.8%–40.0% had ≥50% tumor cell staining.5,42 A phase III clinical trial of pembrolizumab plus chemotherapy included five patients with LCC.43 Although not analyzed by histological subtype, inhibiting PD-L1 may be beneficial for LCC patients.
Lung adenosquamous carcinoma
EGFR is frequently mutated in LASC with a rate ranging from 11.0% to 55.4%, and the East Asian population (21.9%–55.4%) presents a higher frequency than the Western population (11%–13%).44,45,46,47,48,49,50,51,52 In addition, EGFR mutations are more common in young female patients and nonsmokers.49,52,53,54 Although displayed as a biphasic tumor, no significant deviation was found in major mutated genes between the LUAD and LUSC components of LASC.47,51,53 EGFR was considered one of the truncating genetic alterations of LASC. Therefore, EGFR-TKIs are likely to be promising therapeutic options. Song et al.55 retrospectively analyzed 49 Chinese LASC patients treated with gefitinib or erlotinib and demonstrated a significantly longer median PFS of EGFR-mutant cases than that of the wild-type group (8.7 versus 2.1 months). Comparable outcomes of first-generation EGFR-TKIs in patients harboring EGFR-mutant LASC were also reported by several retrospective analyses, with a median PFS from 9.3 to 12 months and an ORR ranging from 33.3% to 56.6%.49,51,56 However, acquired resistance to these agents inevitably develops. In the cohort of Hu et al.,49 eight patients (42.1%) who developed an EGFR T790M mutation after progression on the initial treatment showed a considerable response to osimertinib, a third-generation EGFR-TKI, with a median PFS of 10.2 months. This promising efficacy of osimertinib against resistance to first-generation TKIs, approximately half of which was attributed to the T790M mutation, has also been presented in another cohort.51 At present, a randomized, phase II clinical trial (NCT04354961) is ongoing to assess almonertinib (a third-generation EGFR-TKI) versus chemotherapy as first-line treatment in patients with LASC.
PD-L1 expression was detected in 39.2%–70.6% of patients with LASC50,57,58 and was higher in the squamous component than in the glandular component,57,59 while the efficacy of ICIs in LASC patients has rarely been reported. A retrospective study demonstrated a promising response to ICI-based therapy, with an ORR of 28%, median PFS of 6.0 months, and overall survival (OS) of 24.7 months, without a significant difference between the mono-ICI and chemo-ICI groups.60
Other targets, such as ROS1 fusion and EML4-ALK fusion, have also been identified.52,61,62,63,64 Wang et al.52 found eight out of 124 cases harboring ALK fusion, including six cases with EML4-ALK fusion, and there were four cases with ROS1 fusions. A patient with EML4-ALK rearrangement achieved partial response from neoadjuvant ceritinib treatment after failing neoadjuvant chemotherapy,64 suggesting a potential application of neoadjuvant TKI therapy in LASC patients with targetable alterations. Additionally, Cheng et al.63 provided the first case showing a response to second-line crizotinib in an LASC patient harboring CD74-ROS1 fusion, with a 4-month PFS achieved.
Pulmonary lymphoepithelial carcinoma
Pulmonary LEC lacks actionable driver genes such as EGFR, KRAS, ALK, ROS1, and BRAF.65,66,67,68 Unfortunately, even in those with sensitizing EGFR mutations, receiving EGFR-TKIs barely resulted in a PFS of 1 month,20,69,70 thus suggesting other driving forces of tumorigenesis.
The high PD-L1-positive rate ranging from 61.7% to 91.5%65,68,71,72 and abundant infiltration of cytotoxic T lymphocytes and plasma cells in the stroma indicate the potential value of immunotherapy. Xiao et al.73 conducted the first retrospective study on first-line immunotherapy with or without chemotherapy in patients with pulmonary LEC, demonstrating that immunotherapy had better efficacy than chemotherapy (11.0-month median PFS versus 6.9-month median PFS) and that chemoimmunotherapy might be the optimal first-line treatment.
Pulmonary NUT midline carcinoma
A recent targeted next-generation sequencing (NGS) study showed that pulmonary NMC rarely has mutations in classic oncogenes (e.g., EGFR, KRAS, BRAF) and SNVs were the dominant mutation type.74 These results suggest that somatic mutations are largely passenger events and that the efficacy of inhibiting these targets has seldom been reported in pulmonary NMC.
The PD-L1 expression status and efficacy of ICIs were mainly presented by case reports, and taken together, second-line ICIs are likely to further prolong survival.75 In addition, the combination of bromodomain and extraterminal (BET) domain inhibitors (BETis) and ICIs might cause synergistic effects in pulmonary NMC as well, considering that BETis could regulate the expression of PD-L1 in ovarian cancer and B-cell lymphoma.76,77
Pulmonary sarcomatoid carcinoma
EGFR mutations were found in 5.0%–22.6% of patients with PSC.78,79,80,81,82,83,84,85 Liu et al.83 reckoned EGFR-sensitizing mutations as one of the trunk mutations in PSC despite high intratumor heterogeneity (ITH). Of note, Chinese patients with PSC were more likely to harbor EGFR-sensitizing mutations,82,83 whereas most Caucasian counterparts possessed rare EGFR mutations (e.g., exon 2, 18, or 20), suggesting an ethnic difference.78,86,87 The efficacy of EGFR-TKIs in PSC patients has rarely been reported due to the relatively low and discrepant mutation rates in different cohorts. Zou et al. reported that a patient with advanced PSC received erlotinib once the EGFR L858R mutation was confirmed in metastasis, further leading to a PFS of 6 months.88
METex14 skipping mutations were detected in 2.0%–31.8% of PSC, which was more frequent than that of other NSCLC subtypes.78,79,80,81,82,83,84,85,86,89,90,91 Several retrospective studies of patients with METex14-positive lung cancer, including PSC patients, reported a partial response to first-line crizotinib, a multitargeted TKI covering MET.92,93 In addition, an open-label, phase 2 clinical trial of tepotinib, a highly selective MET TKI, enrolled two PSC patients out of 152 in total, with an ORR of 46% and a median duration of response of 11.1 months.34 However, the aforementioned results were not analyzed by histology. Savolitinib, a type Ib c-MET inhibitor, was explored in a multicenter, single-arm, open-label, phase 2 study that enrolled 70 METex14-positive NSCLC patients, including 25 PSC patients. Savolitinib showed promising antitumor activity with an ORR of 40.0% and a median PFS of 5.5 months in patients with PSC,15 thus making it approved for treating NSCLC in China. In a METex14-positive PSC case, acquired resistance to savolitinib developed after achieving a PFS of 36 weeks, which may be attributed to the emergence of EGFR, KRAS, and FGFR1 amplification.94
MET amplification has been less frequently reported in PSC, with a varying frequency from 4.8% to 13.6%.91,95,96 A PSC patient concurrently harboring the EGFR exon 21 L858R mutation and MET amplification remained stable for 9.7 months after receiving combination therapy with a first-generation EGFR-TKI (gefitinib) and MET inhibitor (crizotinib).97 In 2020, He et al.98 reported the first case in which afatinib, a second-generation EGFR inhibitor, combined with crizotinib resulted in a 4-month PFS on a PSC harboring a rare EGFR mutation and secondary MET amplification. At present, a clinical trial (NCT05015608) is designed to assess the efficacy of savolitinib combined with osimertinib versus chemotherapy in treating patients with locally advanced or metastatic NSCLC with MET amplification after failure of first-line EGFR inhibitor therapy. Therefore, more research focusing on the interaction between these mutations is warranted, and multitargeted therapy may solve the deficiency related to monotherapy.
KRAS mutations were identified in 14.6%–39.0% of PSC patients,78,80,81,82,83,84,85,86,89,90,99,100 while neither retrospective studies nor prospective studies are available. Notably, KRAS mutations were reported to have a positive relationship with TMB and PD-L1 expression (p = 0.031),101 and KRAS-mutated cases had a higher response rate of pembrolizumab.100
VEGF alterations were detected in 5.6% of patients with PSC.82 In another cohort of PC, the major subtype of PSC, many cases (15/75) showed VEGF overexpression, which was significantly associated with a worse prognosis.102 Similarly, Vieira et al.103 reckoned blood vessel invasion as one of the main histological characteristics of PSC. It has been reported that reciprocal regulation between immune reprogramming and vascular normalization could improve the curative effect. For example, a patient with advanced PSC showed a partial response to the third-line treatment of anlotinib plus nivolumab.104 In another patient with advanced PSC but negative PD-L1 expression, combination therapy with atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) resulted in marked shrinkage of the tumor, and continued shrinkage was observed during maintenance therapy with atezolizumab and bevacizumab.105 Other combinations, including anlotinib plus tislelizumab106 or sintilizumab,107 have also been reported to be effective.
Positive PD-L1 expression is commonly seen in PSC (45.0%–90.2%).84,100,101,103,108,109 Many retrospective studies109,110,111,112,113,114 and scattered case reports105,115,116,117,118,119,120 have confirmed the benefit of immunotherapy for PSC patients, and high tumoral PD-L1 expression seems to be associated with a better response to immunotherapy. For instance, Lee et al.111 reported that 49 patients with pulmonary PCs who received ICI monotherapy, mainly as second-line treatment, achieved an ORR of 49.0% and a median PFS of 7.2 months, with a positive relationship between PD-L1 expression and OS (p = 0.001). In another cohort, considerable efficacy of second-line ICIs was confirmed regardless of PD-L1 status.110 Increasing research has integrated immunotherapy into the first-line treatment of NSCLC. In PSC patients, Qian et al.113 demonstrated that first-line ICIs, mainly combined with antiangiogenic therapy, achieved a partial response rate of 57.1% and a median PFS of 9.2 months. Considering the synergistic effect, the combination of ICIs plus antiangiogenic therapies is being evaluated in two ongoing clinical trials (Table 3). Recently, a real-world study revealed that first-line combination therapy with ICIs and chemotherapy resulted in a higher ORR of 73.8% and a median PFS of 10.3 months.121 Based on the encouraging results of first-line immunotherapy, a clinical trial (ChiCTR2000031478) has been launched to test first-line camrelizumab plus chemotherapy in patients with advanced PSC. In addition, chemoimmunotherapy is likely to be effective in PSC patients who have failed EGFR-TKIs or MET-TKIs.121 Gu et al.122 reported a marked response of 15-month PFS to nivolumab in combination with chemotherapy after acquired crizotinib resistance in a METex14-positive PSC patient. Furthermore, synchronously inhibiting PD-1/PD-L1 and CTLA-4 has displayed promising antitumor effects in the first-line treatment of advanced NSCLC (NCT02542293). Similarly, a nonrandomized, open-label, phase II clinical trial assessed the effect of durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) in recurrent or metastatic PSC, with a 5.9-month median PFS and 15.4-month OS achieved.123
Other alterations, including AKT1-E17K mutation, BRAF V600E mutation, ALK fusion, RET fusion, and NTRK mutation, were identified in a few cases.80,82,124,125,126 Wu et al.125 reported the first case of PSC harboring KIF5B-RET gene fusion that achieved partial response after treatment with pralsetinib, a selective RET inhibitor.
Pulmonary salivary gland-type tumor
In pulmonary MEC, featuring the Mastermind Like Transcriptional Coactivator 2 (MAML2) rearrangement, EGFR mutations were less frequently observed but showed greater frequency in Asian populations.127 The antitumor activity of EGFR-TKIs was shown in pulmonary MEC patients with the EGFR L858R mutation.128 Interestingly, for cases without sensitizing EGFR mutations, in vitro data have revealed that the MAML2 rearrangement may predict the antitumor activity of gefitinib in NSCLC cell lines.129 Correspondingly, a partial response to EGFR-TKIs has been demonstrated in EGFR wild-type pulmonary MEC.130 In pulmonary ACC, no mutations were identified in the BRAF, PIK3CA, ALK, DDR2, and PDGFRA genes,131,132 while several cases harboring EGFR mutations displayed a favorable response to EGFR-TKIs, such as achieving a prolonged PFS (19 months) or disease control.133,134,135
Potential targets
The booming genetic detection technology has facilitated the exploration of genomic profiles in rare lung cancers, which may further deepen the understanding of oncogenic mechanisms and lead to the discovery of potential targets. First, pulmonary LEC and pulmonary NMC are largely driven by specific molecules other than the aforementioned FDA-approved genetic alterations. Second, targeting molecules or signaling pathways that play a subsidiary role in tumorigenesis may be valuable in combination with other treatments. Therefore, the following potential targets are worth discussing, although matched targeted agents have been less explored in rare lung cancers (Figure 2B).
Pulmonary lymphoepithelial carcinoma
EBV protein
In contrast to other NSCLCs, EBV plays a pivotal role in the carcinogenesis of pulmonary LEC. Recently, Wu et al.136 provided the first overview of the EBV genomic atlas derived from pulmonary LEC, which strongly resembled that of NPC. Over half (22/32) of the alterations associated with pulmonary LEC risk were located in the EBV-encoded small RNA (EBER) regions and could affect the pathogenicity of EBV by altering the secondary structure of EBER2. Other variations identified around the genes BILF2, BWRF1, BARF1, and BALF5 might be associated with the malignant transformation ability of the pulmonary epithelium of EBV. Notably, virus-host integration rarely occurred in pulmonary LECs (5.1%), with only four integration breakpoints located within cellular genes, and these events might promote carcinogenesis. Additionally, the apolipoprotein B mRNA editing enzyme catalytic polypeptide-like (APOBEC) mutational signature may occur in response to EBV infection and facilitate the oncogenesis of pulmonary LEC.66,68 Most T cell epitope changes were distributed in the regions of latent genes (e.g., LMPs and EBNAs).
Based on the genetic similarity in pulmonary LEC and NPC, a treatment developed in NPC may also bring considerable benefits to pulmonary LEC patients. For example, inhibiting the DNA-binding ability of EBNA1 protein was able to suppress EBV-positive tumor growth in xenograft models.137 EBNA1-targeting agents (e.g., VK-2019) have entered a clinical trial (NCT04925544) of patients with EBV-positive NPC. Cytotoxic T lymphocytes targeting EBV antigens, such as latent membrane proteins (LMPs), are an alternative approach in NPC and might further benefit other EBV-related malignancies, such as pulmonary LEC.138
NF-κB signaling pathway
The transcription factor nuclear factor-κB (NF-κB), a transcription factor of the Rel family, is selectively activated by two signaling pathways, including canonical and noncanonical pathways that mediate innate and adaptive immunity.139 Aberrations in NF-κB signaling influence the genes involved in cell proliferation, metastasis, and suppression of apoptosis and further promote oncogenesis.140
The NF-κB signaling pathway was activated in 13.2–30% of pulmonary LECs.66,67,141 Inactivation of multiple negative regulators of the NF-κB pathway was identified, e.g., TRAF3, NFKBIA, CYLD, NLRC5, and TNFAIP3, and TRAF3 loss was considered a core factor of NF-κB dysregulation. The aforementioned LMPs may also serve as an elementary activator of the NF-κB pathway. Specifically, TRAF3 is a gene encoding a key transduction protein of the TNF family and regulates the expression of MHC-I through the NF-κB signaling pathway. Although no agents targeting TRAF3 are currently available, drugs that can mimic the knockout effect of TRAF3, such as birinapant, were proven to enhance the response to ICIs in a melanoma mouse model.142
Pulmonary NUT midline carcinoma
Epigenetic regulators
Pulmonary NMC is mainly driven by BRD3/4-NUT fusions.9,143 Specifically, BRD4, a serine kinase of the BET family, binds to chromatin via its bromodomains, and NUT recruits p300/CBP to drive the transcription of oncogenes such as MYC, thus further blocking differentiation in NMC cells. NUT also fuses with rarer counterpart genes, e.g., NSD3, ZNF532, or CHRM5, and eventually, these genes tend to interact with BRD4, thus leading to comparable tumorigenic effects.22,74,144,145 Accordingly, BET inhibitors (BETi) are supposed to antagonize the effects of NUT fusion-induced MYC upregulation. Stathis et al.144 provided the first clinical evidence of the BETi birabresib (MK-8628/OTX015) against NMC. Of the four patients who received birabresib (a synthetic small molecule targeting BRD2, BRD3, and BRD4), two with pulmonary NMC achieved notably long survival (19 and 18 months, respectively). Subsequently, another NMC cohort receiving birabresib showed a disease control rate of 75% (6/8), and they determined the recommended phase II dose (RP2D) of 80 mg once daily since all patients achieving PR were reported in this group.146 However, considering the importance of BET proteins in fundamental transcription mechanisms, BETis could inevitably cause dose-limiting toxicities (DLTs), e.g., thrombocytopenia and neutropenia,147 thus prompting the development of selective BETis to both minimize side effects and maintain efficacy. For example, selective BETis, including molibresib/GSK525762148 and RO6870810/TEN-10,149 showed considerable efficacy and prolonged survival of patients with pulmonary NMC. Other BET inhibition strategies are being evaluated in several ongoing clinical trials for NMC (Table 2). Additionally, histone deacetylases (HDACs) are crucial for BRD4 binding to promoters; thus, inhibiting HDACs may undermine this modification.
PI3K/AKT signaling pathway
As one of the most altered pathways in cancer, the phosphatidylinositol 3-kinase (PI3K)/AKT pathway controls various biological processes, including survival, metabolism, and metastasis. The PI3K/AKT signaling pathway plays an important role in the drug resistance of lung cancer, and corresponding inhibitors, such as MK-2206, enhance TKI activity in erlotinib-resistant NSCLC.150 To date, many agents targeting the PI3K/AKT signaling pathway have been developed.151
Somatic mutations result in the dysfunction of many pathways, especially the PI3K/AKT signaling pathway. Xie et al.9 found that 60% (6/10) of pulmonary NMC had nonsynonymous mutations in the PI3K/AKT pathway, e.g., PIK3CA, PIK3R1, PTEN, and ERBB4. A previous Kinome small interfering RNA screening also identified PIK3CA as a positive hit.23 CUDC-907, a dual HDAC/PI3K inhibitor, was evaluated in a phase I clinical trial that enrolled NMC patients (NCT02307240).
Pulmonary sarcomatoid carcinoma
Epithelial-mesenchymal transition
Among these rare lung cancers, PSC cells are likely derived from epithelial-mesenchymal transition (EMT), a process by which epithelial cells lose their cell polarity and intercellular adhesion and gain invasive properties to transition into mesenchymal cells. The differentially expressed genes between the epithelial and sarcomatoid components of PSCs were enriched in pathways related to EMT.82 Similarly, a previous analysis validated the important role of transforming growth factor (TGF)β-mediated EMT in PSCs and demonstrated OVOL2 repression as a specific feature during EMT.90 In addition, concurrent ALK and MET amplification seemed to be synergistic in promoting EMT by recruiting the SRC and FAK pathways in PSCs.152 In the preclinical mouse model of PSC through combined deletion of TP53 and PTEN, the EMT process was confirmed by both genomic and transcriptome profiles. Inhibiting EMT may impede sarcomatoid-like cell proliferation and revert EMT-mediated resistance to EGFR inhibitors. Manzotti et al.90 provided preliminary evidence that the sarcomatoid-like H1299 cell line was sensitive to dasatinib, a wide-spectrum TKI that could suppress EMT.
Protection of telomeres 1
The Protection of Telomeres 1 (POT1) gene, a member of the telombin family, encodes a protein involved in telomere maintenance. POT1 mutations were identified in 28.0%–33.3% of patients with PSC,15,81 which was 6.7 times higher than that of other NSCLCs.81 Approximately 83% of POT1 mutations occurred in the OB1/OB2 domain with DNA-binding activity. Dysfunction of the OB1/OB2 domain of POT1 may cause abnormal ATR activation, implying the therapeutic activity of ATR kinase inhibitors, such as AZD6738153 or VX-970.154
Pulmonary salivary gland-type tumor
Research on MEC revealed the NOTCH and EGFR signaling pathways induced by MAML2 rearrangement and demonstrated enhanced anti-MEC activity by targeting these two pathways.155 Unfortunately, the mechanism of MAML2 rearrangement in pulmonary MEC remains unclear.
Approximately half of the pulmonary ACCs harbor the t(6; 9) (q22-23; p23-24) chromosomal translocation with MYB-NFIB (nuclear factor IB) gene fusion,156,157 which encodes a transcriptional regulator that is difficult to target. Intriguingly, in ACC, MYB-NFIB fusion is regulated by AKT-dependent IGF1R signaling, and in vitro, data show that pharmacological inhibition of IGF1R reduces cell proliferation.158 In a cohort with seven cases of pulmonary ACC, three cases had genetic alterations in the PI3K pathway.159 Therefore, inhibiting IGF/PI3K/AKT signaling may be a therapeutic opportunity for pulmonary ACC.
Targeting tumor cells
Chimeric antigen receptor (CAR)-T cell (CAR-T) therapy is a novel method against tumor cells that involves transferring genetically engineered T cells for tumor antigens. CAR-T therapy for solid tumors is growing vigorously, such as targeting EGFR, ERBB2, c-MET, PD-L1, and B7H3 for lung cancer.160
In rare lung cancers, EGFR mutation is relatively common in LASC and PSC as mentioned above. EGFR CAR-T cells have been proven to inhibit tumor growth in phase I clinical trial for advanced relapsed/refractory NSCLC, achieving a partial response (2/11) and stable disease (5/11).161 Another phase I study demonstrated a median PFS of 7.13 months and a median OS of 15.63 months.162 Unfortunately, no rare lung cancer has been enrolled in the aforementioned studies. At present, an early phase I clinical trial aims to evaluate the efficacy of EGFR/B7H3 CAR-T on lung cancer (NCT05341492). Additionally, c-MET overexpression caused by either MET mutation or amplification was detected in 42.9% of LASC, 17.0%–40.9% of PSC, and 15.6% of LCC.91,95,96 Preclinical studies revealed that c-MET CAR-T cells were able to kill NSCLC cells both in vitro and in vivo.163,164 The high PD-L1 expression in LCC, LASC, pulmonary lymphoepithelial carcinoma (pLEC), and PSC may suggest the response to PD-L1-CAR-T cells. A recent study demonstrated robust efficacy of PD-L1-CAR-T cells on PD-L1 high xenograft NSCLC tumors and that irradiation could improve the efficacy against PD-L1 low NSCLC patients.165
Preclinical model
Preclinical research models, including cell lines, patient-derived organoid (PDO), and mouse models, will facilitate the discovery of tumorigenic mechanisms and drug targets. Cell lines display a high success rate, high repeatability, and low cost, while these homogeneous cancer cells fail to form a tumor microenvironment similar to that of patient-derived tumor. Compared with cell lines, PDO is likely to be an ideal in vitro model since they largely retain the histology and genetic characteristics and require less time for establishment than patient-derived xenograft (PDX).166 Mouse models mainly refer to the genetically engineered mouse model (GEMM) and PDX. GEMM has major superiority of carrying genetic variations in core pathways, and thus could partially recapitulate clinical phenotype. In comparison, PDX is reported to better retain the heterogeneity of the original tumor, while the application of both GEMM and PDX has been hampered by long latency and incomplete penetrance. To date, progress has been made in preclinical models for rare lung cancers.
Lung adenosquamous carcinoma
The morphological heterogeneity of LASC makes it an ideal model to explore the transdifferentiation and clonal evolution of NSCLC. Several LASC cell lines are available (see Table S1). Additionally, given a higher STK11/LKB1 mutation rate in LASC (22%) than in ADC (15%), Zhang et al.167 developed an LASC mouse model through concomitant KRAS activation and LKB1 deletion. Several studies have confirmed the tumorigenesis ability of KRAS/LKB1 in promoting LASC.168,169 A recent whole exome sequencing study of LASC also demonstrated that SOX2 amplification and loss of STK11/LKB1 may facilitate adenosquamous transdifferentiation (AST) from LUAD to LUSC.170 However, no drug has been tested in KRAS/LKB1 comutated mice. Additionally, a few PDXs of LASC are available but have not been utilized for testing treatment response (Table 4).171,172,173,174
Table 4.
PDX models for rare lung cancers
| Publication | Tissue collect | Mouse strain | Engraftment rate | Rare lung cancer (n) |
|---|---|---|---|---|
| Fichtner et al.177 | surgical resection | NOD/SCID | 24.5%a | PSC (4), LCC (1) |
| Wang et al.172 | surgical resection | NOD/SCID | 28.8%a | LASC (1), PSC (5) |
| John et al.178 | surgical resection | NOD/SCID | PSC 100% (1/1); LCC 60% (3/5) | PSC (1), LCC (3) |
| Sun et al.173 | biopsy, surgical resection |
NOD/SCID | NA | LASC (1), PSC (2), LCC (1) |
| Woo et al.171 | biopsy, pleural effusion, surgical resection | NSG | 38.0%a | LASC (2), PSC (1) |
| Kita et al.181 | surgical resection | NSG, SHO | LCC 50% (1/2) | LCC (1) |
| Fang et al.174 | surgical resection | BALB/c nude | NA | LASC (3), PSC (2), LCC (2) |
| Nakajima et al.182 | EBUS-guided biopsy | NSG | LCC 100% (1/1) | LCC (1) |
NOD, non-obese diabetic; NSG, NOD-SCID gamma; SCID, severely compromised immune deficient; SHO, SCID Hairless Outbred; NA, not available.
Total engraftment rate.
Pulmonary sarcomatoid carcinoma
At present, few cell lines have been established for PSC (see Table S1). Moreover, Lázaro et al.175 developed the first mouse model of PSC through simultaneous deletion of PTEN and TP53, despite the long latency periods and incomplete penetrance. This model of PSC displayed both genetic similarity with LUAD and specific enrichment in hallmark genes of EMT. In addition, several PDOs176 and PDXs (Table 4)171,172,173,174,177,178 of PSC have been established, whereas drug sensitivity testing has been scarcely conducted to date.
Large cell carcinoma
Multiple cell lines are available for research on LCC (see Table S1). By investigating the aggressiveness mechanism of LCC, Gabasa et al.179 found an in vitro model based on the coculture of LCC cell lines with tumor-associated fibroblasts practical for testing potential therapies, such as senolytic and senostatic drugs.180 Moreover, PDXs of LCC have been reported in several studies (Table 4),173,174,177,178,181,182 while the accurate pathological type in each remains uncertain owing to the changing definition of LCC.
Challenges and prospects
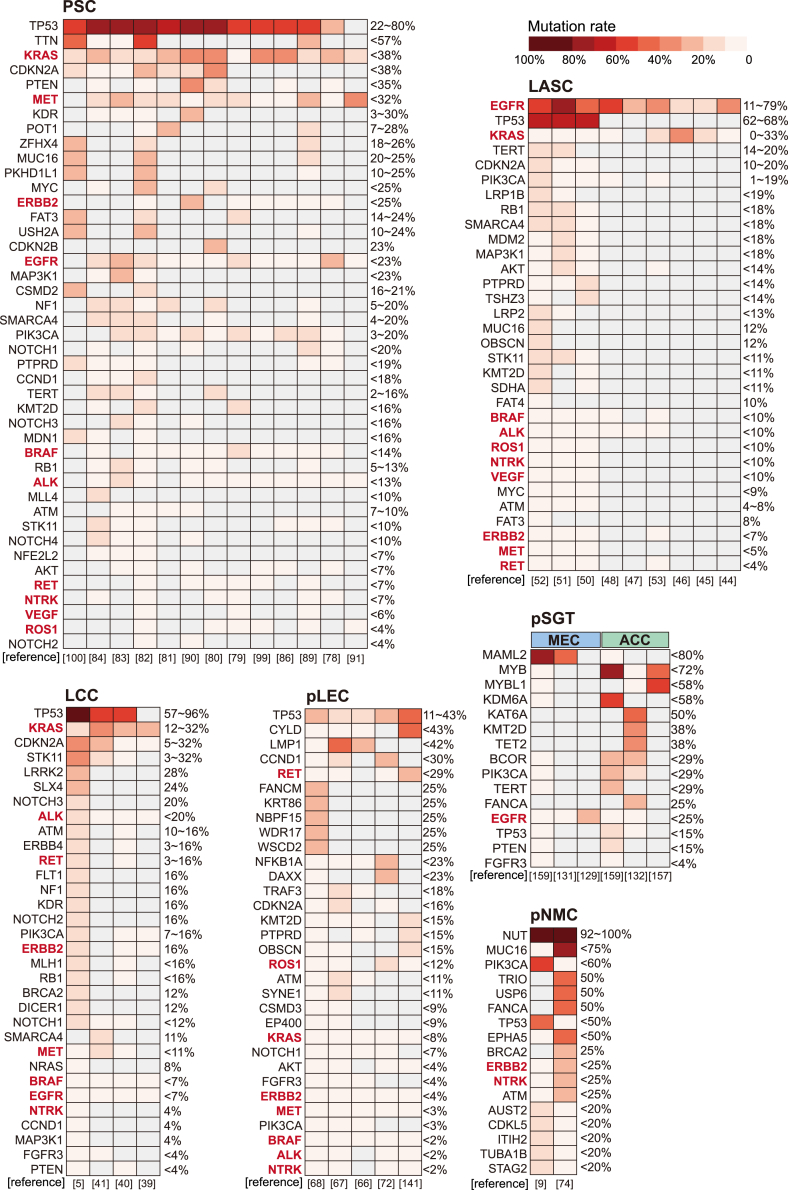
For decades, targeted therapy for rare lung cancers relies heavily on research on common subtypes. Fortunately, with the help of advanced sequencing technology, targeted therapy has been increasingly explored and shown promising efficacy in patients with rare lung cancers (Figure 2), and a comprehensive mutation profile based on data from other studies have been visualized to help explore novel therapeutic opportunities (Figure 3), with the detailed information provided (see Table S2). More importantly, what to address and how to realize are key issues to discuss. Specifically, each rare lung cancer goes in a different direction for developing targeted therapy in the future, while notably, the common characteristic, that is, the rarity, has raised problems and solutions in common.
Figure 3.
Summary of reported mutation rates of rare lung cancers
Mutation rates are presented in descending order. Each column represents a cohort that has received genetic detection and a corresponding reference is provided below each column. Genes with a reported mutation in at least one case are included. Gray checks represent genes that were not detected or not available. Percentages represent the mutation rates. Targets approved by FDA are highlighted in bold and red font.
Prospects of each rare lung cancer
Large cell carcinoma
Targetable mutations are rarely detected in LCC, while notably, the value of KRAS inhibitors and immunotherapy warrants further validation. Generally, LCC is regarded as a “basket” of lung cancers without clear immunohistochemistry features, while several studies have revealed a similar mutation pattern between LCC and LUAD.5,40,41 Therefore, comprehensive molecular subtyping of this entity is expected for further categorization and more personalized management of LCC.
Lung adenosquamous carcinoma
Routine test of EGFR mutations is recommended and the efficacy of EGFR-TKIs is worth further confirmation, especially in East Asia. Inhibiting the PD-1/PD-L1 axis displays therapeutic potential in LASC patients with high PD-L1 expression, which is expected to be verified in larger studies. Additionally, developing CAR-T therapy targeting MET overexpression may be interesting for exploration.
Pulmonary lymphoepithelial carcinoma
Based on the genetic similarity between pLEC and NPC, targeting EBNA1 and PD-L1 can be valuable and needs further research for patients with pLEC. Digging out the role of the NF-κB signaling pathway in pLEC would be interesting, especially exploiting TRAF3 deficiency to enhance the efficacy of ICIs.
Pulmonary NUT midline carcinoma
Since pNMC is unlikely to benefit from traditional targeted therapies for lung cancers, developing agents targeting BRD3/4-NUT fusions for pNMC is urgently needed to improve the prognosis.
Pulmonary sarcomatoid carcinoma
The efficacy of EGFR-TKIs has been witnessed in several retrospective studies, while prospective evidence in larger cohorts is needed, especially in Chinese patients. MET alterations can be targeted in PSC patients, but resistance to MET inhibitors inevitably occurs. To overcome resistance, the combination of targeted agents or the development of a bispecific antibody, such as amivantamab against both EGFR and MET, is worth further exploration. Developing CAR-T cells targeting MET overexpression may be suitable for patients who are not eligible for current anti-MET small-molecule agents. Additionally, the synergistic effect of ICIs and antiangiogenic therapies should be fully exploited. Notably, the role of POT1 mutation in PSC tumorigenesis needs to be clarified in the future.
Pulmonary salivary gland-type tumor
Pulmonary SGT lacks classic driver mutations of lung cancers and rarely responds to current targeted therapy for lung cancers. Mechanistic findings of SGT, as well as corresponding targeted therapies, need to be further validated in pulmonary SGT.
Common issues and solutions for rare lung cancer
Common issues lie in different rare lung cancers, including (1) the lack of comprehensive analysis of oncogenic mechanisms, further misleading clinical practice; (2) poorly developed preclinical models for mechanistic research and targeted agent screening; (3) unsolid clinical evidence, such as retrospective studies, case reports, and analyses according to the Surveillance, Epidemiology, and End Results (SEER) registry; and (4) the lack of available samples and datasets to promote basic research and clinical practice. Correspondingly, more efforts should be made in the following aspects.
Uncover oncogenic mechanisms
Integrating multidimensional information, including genomics, transcriptomics, epigenomics, proteomics, and metabolomics can reveal the interaction networks. NGS has been broadly used in research on rare lung cancers, while single-cell sequencing can serve as a complementary tool to enable unbiased analyses of ITH and the immune microenvironment at single-cell resolution.183 To date, single-cell RNA-sequencing has been conducted in a few rare lung cancers, such as PSC and pulmonary LEC. Exploiting single-cell analysis of rare lung cancers can provide novel molecular insights. Moreover, proteins serve as the actual executors of pathological processes and the bridge between genotype and phenotype, thus highlighting the role of proteomics. Traditional proteomics with mass spectrometry as its core is moving toward in-depth single-cell and single-molecule sequencing to complement single-cell genomics and transcriptomics in the near future.184,185 With increasing molecular regulatory networks identified, investigation based on diverse preclinical models is indispensable for validating oncogenes and elucidating the relationship between genotypes and phenotypes.
Develop preclinical model
Conducting high-throughput CRISPR screening in cell lines of rare lung cancers will facilitate the identification of drug targets and clarification of resistance mechanisms. For example, the p53 protein has gradually dismissed the “undruggable” idea, with many small molecule drugs under investigation.186 TP53 alterations were observed in over half of the patients with LASC, PSC, LCC, and pulmonary NMC, thus making it an appealing target for rare lung cancers. PDO is currently an optimal in vitro model for evaluating drug response. Employing more efficient frontier technology, such as using microwell array chips for constructing lung cancer organoids,187 may alleviate the problem of scarce clinical samples. Utilizing in vivo models, such as GEMM and PDX, to test the drugs screened by in vitro models is more cost-effective in rare lung cancers. The generation of PDO and PDX relies heavily on clinical tissue samples; therefore, there is an urgent need to establish biobanks for rare lung cancers.
Innovate clinical research
First, more cases with rare subtypes need to be enrolled in clinical trials for lung cancer, with detailed results of each subtype presented. Second, traditional clinical trials can be improved by alternative strategies, such as multicenter recruitment, factorial designs, and adaptive designs (e.g., Bayesian approaches to optimize the low number of enrollments, altering outcome measurements).188,189 On this basis, a greater understanding of cancer biology may broaden the inclusion criteria and promote novel clinical trial designs, such as basket trials and umbrella trials. A classic example is the NCI-MATCH trial, the largest basket trial thus far, which allows patients to receive treatment based on genetic changes regardless of cancer type.190 Third, if prospective trials are still impeded by the scarcity of patients, real-world data should be considered a valuable resource to serve as a prespecified external control. Improving electronic patient records is expected to allow large-scale and multicenter observational studies to be conducted.
Strengthen resource integration
First, optimize clinical registration to provide data on pathology, management, and specimens of rare lung cancers, which will contribute to frontier research, such as establishing preclinical models and conducting multiomics analysis. Of note, collecting samples capturing first diagnosis, relapse, and metastasis may be of great help for profiling the evolutional atlas of rare lung cancers. Second, a platform with shared basic and clinical resources should be launched to provide valuable information for making better clinical decisions and assist with multicenter cooperation, as well as better patient enrollment for clinical trials.
Conclusion
Taken together, progress has been made in developing targeted therapy for rare lung cancers, while there is much room for improvement. At present, the general principle of developing targeted therapy for rare lung cancers can be described as “seek common ground while reserve differences,” that is, when we notice the efficacy of current targeted therapies, we should also keep an eye on whether there are genetic or oncogenic characteristics of rare lung cancers distinct from common subtypes to reach a more personalized therapy. Notably, the clear correlation between genetic alterations and histological characteristics still needs further research, such as the comparative study of the genomic landscape or mechanism validation. In the future, rare lung cancers may be redefined according to molecular features or a more comprehensive therapy-oriented diagnosis system, thus achieving more personalized therapy.
Acknowledgments
This work was supported by the National Clinical Research Center for Geriatrics (Z2021JC001) and the Outstanding Youth Talent Foundation for Science and Technology of Sichuan Province (2022JDJQ0056).
Author contributions
C.W. and X.Y. contributed equally to this work. X.Y. collected the information. C.W. and X.Y. conducted the literature review and wrote the original manuscript. C.W. processed the tables and figures. J.X. conceived the idea for this manuscript. All authors approved the final version.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.05.007.
Supplemental information
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, N.A., Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, et al (eds). SEER Cancer Statistics Review, 1975-2017. https://seer.cancer.gov/csr/1975_2017/.
- 4.Gatta G., Capocaccia R., Botta L., Mallone S., De Angelis R., Ardanaz E., Comber H., Dimitrova N., Leinonen M.K., Siesling S., et al. Burden and centralized treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18:1022–1039. doi: 10.1016/S1470-2045(17)30445-X. [DOI] [PubMed] [Google Scholar]
- 5.Chan A.W., Chau S.L., Tong J.H., Chow C., Kwan J.S.H., Chung L.Y., Lung R.W., Tong C.Y., Tin E.K., Law P.P., et al. The landscape of actionable molecular alterations in immunomarker-defined large-cell carcinoma of the lung. J. Thorac. Oncol. 2019;14:1213–1222. doi: 10.1016/j.jtho.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 6.de Jong W.K., Schaapveld M., Blaauwgeers J.L.G., Groen H.J.M. Pulmonary tumours in The Netherlands: focus on temporal trends in histology and stage and on rare tumours. Thorax. 2008;63:1096–1102. doi: 10.1136/thx.2007.095067. [DOI] [PubMed] [Google Scholar]
- 7.Li C., Lu H. Adenosquamous carcinoma of the lung. Onco. Targets Ther. 2018;11:4829–4835. doi: 10.2147/OTT.S164574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y., Ren S., Liu Y., Han W., Liu W. Pulmonary lymphoepithelioma-like carcinoma: a mini-review. Onco. Targets Ther. 2020;13:3921–3929. doi: 10.2147/OTT.S241337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie M., Fu X., Wang W. Clinicopathological and molecular characterizations of pulmonary NUT midline carcinoma. Cancer Med. 2021;10:5757–5764. doi: 10.1002/cam4.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steuer C.E., Behera M., Liu Y., Fu C., Gillespie T.W., Saba N.F., Shin D.M., Pillai R.N., Pakkala S., Owonikoko T.K., et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin. Lung Cancer. 2017;18:286–292. doi: 10.1016/j.cllc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Molina J.R., Aubry M.C., Lewis J.E., Wampfler J.A., Williams B.A., Midthun D.E., Yang P., Cassivi S.D. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110:2253–2259. doi: 10.1002/cncr.23048. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F., Qiao M., Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell. Mol. Immunol. 2021;18:279–293. doi: 10.1038/s41423-020-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majeed U., Manochakian R., Zhao Y., Lou Y. Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. J. Hematol. Oncol. 2021;14:108. doi: 10.1186/s13045-021-01121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pécuchet N., Vieira T., Rabbe N., Antoine M., Blons H., Cadranel J., Laurent-Puig P., Wislez M. Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann. Oncol. 2017;28:1597–1604. doi: 10.1093/annonc/mdx162. [DOI] [PubMed] [Google Scholar]
- 15.Lu S., Fang J., Li X., Cao L., Zhou J., Guo Q., Liang Z., Cheng Y., Jiang L., Yang N., et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021;9:1154–1164. doi: 10.1016/S2213-2600(21)00084-9. [DOI] [PubMed] [Google Scholar]
- 16.Xiaochuan L., Jiangyong Y., Ping Z., Xiaonan W., Lin L. Clinical characteristics and prognosis of pulmonary large cell carcinoma: a population-based retrospective study using SEER data. Thorac. Cancer. 2020;11:1522–1532. doi: 10.1111/1759-7714.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mordant P., Grand B., Cazes A., Foucault C., Dujon A., Le Pimpec Barthes F., Riquet M. Adenosquamous carcinoma of the lung: surgical management, pathologic characteristics, and prognostic implications. Ann. Thorac. Surg. 2013;95:1189–1195. doi: 10.1016/j.athoracsur.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson A.G., Tsao M.S., Beasley M.B., Borczuk A.C., Brambilla E., Cooper W.A., Dacic S., Jain D., Kerr K.M., Lantuejoul S., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J. Thorac. Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Sathirareuangchai S., Hirata K. Pulmonary lymphoepithelioma-like carcinoma. Arch. Pathol. Lab. Med. 2019;143:1027–1030. doi: 10.5858/arpa.2018-0149-RS. [DOI] [PubMed] [Google Scholar]
- 20.Qin Y., Gao G., Xie X., Zhu Z., Guan W., Lin X., Xie Z., Ming O., Chen R., Zhong N., et al. Clinical features and prognosis of pulmonary lymphoepithelioma-like carcinoma: summary of eighty-five cases. Clin. Lung Cancer. 2019;20:e329–e337. doi: 10.1016/j.cllc.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Kong W., Liang W. NUT midline carcinoma: a rare solid tumour characterized by chromosome rearrangement. Evid. Based. Complement. Alternat. Med. 2022;2022:3369895. doi: 10.1155/2022/3369895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X.H., Wang L.Q., Qin Y.Y., Lin X.Q., Xie Z.H., Liu M., Zhang J.X., Ouyang M., Liu J., Gu Y.Y., et al. Clinical features, treatment, and survival outcome of primary pulmonary NUT midline carcinoma. Orphanet J. Rare Dis. 2020;15:183. doi: 10.1186/s13023-020-01449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung M., Kim S., Lee J.K., Yoon S.O., Park H.S., Hong S.W., Park W.S., Kim J.E., Kim J., Keam B., et al. Clinicopathological and preclinical findings of NUT carcinoma: a multicenter study. Oncologist. 2019;24:e740–e748. doi: 10.1634/theoncologist.2018-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Wu D., Liu H., Chen J. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920950207. 1758835920950207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sim J.K., Chung S.M., Choi J.H., Oh J.Y., Lee S.H., Kim J.H., Min K.H., Hur G.Y., Shim J.J., Kang K.H., et al. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J. Intern. Med. 2018;33:737–744. doi: 10.3904/kjim.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira T., Girard N., Ung M., Monnet I., Cazes A., Bonnette P., Duruisseaux M., Mazieres J., Antoine M., Cadranel J., Wislez M. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J. Thorac. Oncol. 2013;8:1574–1577. doi: 10.1097/01.JTO.0000437008.00554.90. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Gilani S., Xu H., Cai G. Salivary gland-type tumors of the lung. Arch. Pathol. Lab. Med. 2021;145:1379–1386. doi: 10.5858/arpa.2021-0093-RA. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Liu X., Gu Y., Zhang S. Clinical, laboratory, pathological, and radiological characteristics and prognosis of patients with pulmonary salivary gland-type tumors. J. Cancer Res. Clin. Oncol. 2022 doi: 10.1007/s00432-022-04295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang D.Y., Yoon Y.S., Kim H.K., Choi Y.S., Kim K., Shim Y.M., Kim J. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer. 2011;72:250–254. doi: 10.1016/j.lungcan.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Garg P.K., Sharma G., Rai S., Jakhetiya A. Primary salivary gland-type tumors of the lung: a systematic review and pooled analysis. Lung India. 2019;36:118–122. doi: 10.4103/lungindia.lungindia_284_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 32.Zhou C., Ramalingam S.S., Kim T.M., Kim S.W., Yang J.C.H., Riely G.J., Mekhail T., Nguyen D., Garcia Campelo M.R., Felip E., et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021;7:e214761. doi: 10.1001/jamaoncol.2021.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf J., Seto T., Han J.Y., Reguart N., Garon E.B., Groen H.J.M., Tan D.S.W., Hida T., de Jonge M., Orlov S.V., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N. Engl. J. Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 34.Paik P.K., Felip E., Veillon R., Sakai H., Cortot A.B., Garassino M.C., Mazieres J., Viteri S., Senellart H., Van Meerbeeck J., et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N. Engl. J. Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planchard D., Smit E.F., Groen H.J.M., Mazieres J., Besse B., Helland Å., Giannone V., D'Amelio A.M., Jr., Zhang P., Mookerjee B., Johnson B.E. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 37.da Cunha Santos G., Shepherd F.A., Tsao M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 38.He X., Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rekhtman N., Tafe L.J., Chaft J.E., Wang L., Arcila M.E., Colanta A., Moreira A.L., Zakowski M.F., Travis W.D., Sima C.S., et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod. Pathol. 2013;26:511–522. doi: 10.1038/modpathol.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelosi G., Fabbri A., Papotti M., Rossi G., Cavazza A., Righi L., Tamborini E., Perrone F., Settanni G., Busico A., et al. Dissecting pulmonary large-cell carcinoma by targeted next generation sequencing of several cancer genes pushes genotypic-phenotypic correlations to emerge. J. Thorac. Oncol. 2015;10:1560–1569. doi: 10.1097/JTO.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 41.Harms A., Endris V., Winter H., Kriegsmann M., Stenzinger A., Schirmacher P., Warth A., Kazdal D. Molecular dissection of large cell carcinomas of the lung with null immunophenotype. Pathology. 2018;50:530–535. doi: 10.1016/j.pathol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Wang F., Lu J.B., Wu X.Y., Feng Y.F., Shao Q., An X., Wang H.Y. Clinical genetic features and related survival implications in patients with surgically resected large-cell lung cancer. Cancer Manag. Res. 2019;11:5489–5499. doi: 10.2147/CMAR.S200263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 44.Jia X.L., Chen G. EGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lung. Lung Cancer. 2011;74:396–400. doi: 10.1016/j.lungcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Tochigi N., Dacic S., Nikiforova M., Cieply K.M., Yousem S.A. Adenosquamous carcinoma of the lung: a microdissection study of KRAS and EGFR mutational and amplification status in a western patient population. Am. J. Clin. Pathol. 2011;135:783–789. doi: 10.1309/AJCP08IQZAOGYLFL. [DOI] [PubMed] [Google Scholar]
- 46.Shu C., Cheng H., Wang A., Mansukhani M.M., Powell C.A., Halmos B., Borczuk A.C. Thymidylate synthase expression and molecular alterations in adenosquamous carcinoma of the lung. Mod. Pathol. 2013;26:239–246. doi: 10.1038/modpathol.2012.158. [DOI] [PubMed] [Google Scholar]
- 47.Morodomi Y., Okamoto T., Takenoyama M., Takada K., Katsura M., Suzuki Y., Fujishita T., Kitahara H., Shimamatsu S., Kohno M., et al. Clinical significance of detecting somatic gene mutations in surgically resected adenosquamous cell carcinoma of the lung in Japanese patients. Ann. Surg. Oncol. 2015;22:2593–2598. doi: 10.1245/s10434-014-4218-0. [DOI] [PubMed] [Google Scholar]
- 48.Shi X., Wu H., Lu J., Duan H., Liu X., Liang Z. Screening for major driver oncogene alterations in adenosquamous lung carcinoma using PCR coupled with next-generation and Sanger sequencing methods. Sci. Rep. 2016;6:22297. doi: 10.1038/srep22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu M., Zhang B., Xu J., Wang S., Zhao Y., Zhang L., Han B. Clinical outcomes of different generations of EGFR tyrosine kinase inhibitors in advanced lung adenosquamous carcinoma. Mol. Diagn. Ther. 2019;23:773–779. doi: 10.1007/s40291-019-00425-x. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y., Zhang Y., Yuan Y., Wang J., Liu K., Yu B., Xie L., Ou-Yang C., Wu L., Ye X. The comprehensive analyses of genomic variations and assessment of TMB and PD-L1 expression in Chinese lung adenosquamous carcinoma. Front. Genet. 2020;11:609405. doi: 10.3389/fgene.2020.609405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin G., Li C., Li P.S., Fang W.Z., Xu H.P., Gong Y.H., Zhu Z.F., Hu Y., Liang W.H., Chu Q., et al. Genomic origin and EGFR-TKI treatments of pulmonary adenosquamous carcinoma. Ann. Oncol. 2020;31:517–524. doi: 10.1016/j.annonc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Wang H., Liu J., Zhu S., Miao K., Li Z., Qi X., Huang L., Guo L., Wang Y., Cai Y., Lin Y. Comprehensive analyses of genomic features and mutational signatures in adenosquamous carcinoma of the lung. Front. Oncol. 2022;12:945843. doi: 10.3389/fonc.2022.945843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang R., Pan Y., Li C., Zhang H., Garfield D., Li Y., Ye T., Hu H., Luo X., Li H., et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J. Thorac. Oncol. 2014;9:760–768. doi: 10.1097/JTO.0b013e3182a406d1. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y., Dong Y., Zhao R., Zhang B., Wang S., Zhang L., Hu M., He Q., Zhang W., Han B. Expression profiling of driver genes in female never-smokers with non-adenocarcinoma non-small-cell lung cancer in China. Clin. Lung Cancer. 2020;21:e355–e362. doi: 10.1016/j.cllc.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Song Z., Lin B., Shao L., Zhang Y. Therapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinoma. J. Chin. Med. Assoc. 2013;76:481–485. doi: 10.1016/j.jcma.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Fan L., Yang H., Yao F., Zhao Y., Gu H., Han K., Zhao H. Clinical outcomes of epidermal growth factor receptor tyrosine kinase inhibitors in recurrent adenosquamous carcinoma of the lung after resection. Onco. Targets Ther. 2017;10:239–245. doi: 10.2147/OTT.S114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi X., Wu S., Sun J., Liu Y., Zeng X., Liang Z. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Sci. Rep. 2017;7:46209. doi: 10.1038/srep46209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C.C., Huang K.T., Chang H.C., Tseng C.C., Lai C.H., Lan J., Liu T.T., Huang C.C., Lin M.C. Comprehensive analysis of PD-L1 in non-small cell lung cancer with emphasis on survival benefit, impact of driver mutation and histological types, and archival tissue. Thorac. Cancer. 2022;13:38–47. doi: 10.1111/1759-7714.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hlaing A.M., Furusato B., Udo E., Kitamura Y., Souda M., Masutani M., Fukuoka J. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem. Biophys. Res. Commun. 2018;503:2764–2769. doi: 10.1016/j.bbrc.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Zheng X., Li P., Wang H., Hu J., Wu L., Wang Z., Guo H., Wu F., Zhong W., et al. Heterogeneity of tumor immune microenvironment and real-world analysis of immunotherapy efficacy in lung adenosquamous carcinoma. Front. Immunol. 2022;13:944812. doi: 10.3389/fimmu.2022.944812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaft J.E., Rekhtman N., Ladanyi M., Riely G.J. ALK-rearranged lung cancer: adenosquamous lung cancer masquerading as pure squamous carcinoma. J. Thorac. Oncol. 2012;7:768–769. doi: 10.1097/JTO.0b013e31824c9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu V.W., Upadhyay D., Schrock A.B., Gowen K., Ali S.M., Ou S.H.I. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer. 2016;97:48–50. doi: 10.1016/j.lungcan.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Y., Yang J., Wang D., Yan D. ROS1 fusion lung adenosquamous carcinoma patient with short-term clinical benefit after crizotinib treatment: a case report. Ann. Transl. Med. 2022;10:157. doi: 10.21037/atm-21-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mai S., Wang Y., Wang X., Yang W., Gao H., Xu Z., Xu L., Xu L., Ou Q., Chen H., Wang Z. Neoadjuvant ceritinib treatment in ALK-rearranged locally advanced adenosquamous carcinoma: a case report. Thorac. Cancer. 2022;13:2275–2278. doi: 10.1111/1759-7714.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang Y.L., Yang C.Y., Lin M.W., Wu C.T., Yang P.C. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: a potential rationale for immunotherapy. Lung Cancer. 2015;88:254–259. doi: 10.1016/j.lungcan.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Hong S., Liu D., Luo S., Fang W., Zhan J., Fu S., Zhang Y., Wu X., Zhou H., Chen X., et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat. Commun. 2019;10:3108. doi: 10.1038/s41467-019-10902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chau S.L., Tong J.H.M., Chow C., Kwan J.S.H., Lung R.W.M., Chung L.Y., Tin E.K.Y., Wong S.S.Y., Cheung A.H.K., Lau R.W.H., et al. Distinct molecular landscape of epstein-barr virus associated pulmonary lymphoepithelioma-like carcinoma revealed by genomic sequencing. Cancers (Basel) 2020;12:2065. doi: 10.3390/cancers12082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen B., Zhang Y., Dai S., Zhou P., Luo W., Wang Z., Chen X., Cheng P., Zheng G., Ren J., et al. Molecular characteristics of primary pulmonary lymphoepithelioma-like carcinoma based on integrated genomic analyses. Signal. Transduct. Target. Ther. 2021;6:6. doi: 10.1038/s41392-020-00382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L., Lin Y., Cai Q., Long H., Zhang Y., Rong T., Ma G., Liang Y. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J. Thorac. Dis. 2015;7:1556–1562. doi: 10.3978/j.issn.2072-1439.2015.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin K., Feng H.B., Li L.L., Chen Y., Xie Z., Lv Z.Y., Guo W.B., Lu D.X., Yang X.N., Yan W.Q., et al. Low frequency of mutation of epidermal growth factor receptor (EGFR) and arrangement of anaplastic lymphoma kinase (ALK) in primary pulmonary lymphoepithelioma-like carcinoma. Thorac. Cancer. 2020;11:346–352. doi: 10.1111/1759-7714.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Q., Wang W., Zhou P., Fu Y., Zhang Y., Shao Y.W., Jiang L. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol. Res. Pract. 2020;216:153043. doi: 10.1016/j.prp.2020.153043. [DOI] [PubMed] [Google Scholar]
- 72.Xie Z., Liu L., Lin X., Xie X., Gu Y., Liu M., Zhang J., Ouyang M., Lizaso A., Zhang H., et al. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod. Pathol. 2020;33:626–638. doi: 10.1038/s41379-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Y., He J., Luo S., Dong M., Li W., Liu G., Chen H., Yang X., Huang S. Comparison of immunotherapy, chemotherapy, and chemoimmunotherapy in advanced pulmonary lymphoepithelioma-like carcinoma A retrospective study. Front. Oncol. 2022;12:820302. doi: 10.3389/fonc.2022.820302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M., Yang J., Lv L., Li Y., Tang Y., Liu W., Wang W., Jiang L. Comprehensive genetic profiling of six pulmonary nuclear protein in testis carcinomas with a novel micropapillary histological subtype in two cases. Hum. Pathol. 2021;115:56–66. doi: 10.1016/j.humpath.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Li X., Shi H., Zhang W., Bai C., He M., Ta N., Huang H., Ning Y., Fang C., Qin H., Dong Y. Immunotherapy and targeting the tumor microenvironment: current place and new insights in primary pulmonary NUT carcinoma. Front. Oncol. 2021;11:690115. doi: 10.3389/fonc.2021.690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu H., Bengsch F., Svoronos N., Rutkowski M.R., Bitler B.G., Allegrezza M.J., Yokoyama Y., Kossenkov A.V., Bradner J.E., Conejo-Garcia J.R., Zhang R. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–2837. doi: 10.1016/j.celrep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hogg S.J., Vervoort S.J., Deswal S., Ott C.J., Li J., Cluse L.A., Beavis P.A., Darcy P.K., Martin B.P., Spencer A., et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cel Rep. 2017;18:2162–2174. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fallet V., Saffroy R., Girard N., Mazieres J., Lantuejoul S., Vieira T., Rouquette I., Thivolet-Bejui F., Ung M., Poulot V., et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta Panel: exploring therapeutic targets. Ann. Oncol. 2015;26:1748–1753. doi: 10.1093/annonc/mdv232. [DOI] [PubMed] [Google Scholar]
- 79.Li X., Wang D., Zhao Q., Ren D., Ren F., Chen G., Liu H., Chen J. Clinical significance and next-generation sequencing of Chinese pulmonary sarcomatoid carcinoma. Sci. Rep. 2017;7:3947. doi: 10.1038/s41598-017-04296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schrock A.B., Li S.D., Frampton G.M., Suh J., Braun E., Mehra R., Buck S.C., Bufill J.A., Peled N., Karim N.A., et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J. Thorac. Oncol. 2017;12:932–942. doi: 10.1016/j.jtho.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Shen E., Xiu J., Bentley R., López G.Y., Walsh K.M. Frequent mutations of POT1 distinguish pulmonary sarcomatoid carcinoma from other lung cancer histologies. Clin. Lung Cancer. 2020;21:e523–e527. doi: 10.1016/j.cllc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Yang Z., Xu J., Li L., Li R., Wang Y., Tian Y., Guo W., Wang Z., Tan F., Ying J., et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat. Commun. 2020;11:4878. doi: 10.1038/s41467-020-18702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X., Wang F., Xu C., Chen X., Hou X., Li Q., Li P., Xie Z., Liu Y., Chang L., et al. Genomic origin and intratumor heterogeneity revealed by sequencing on carcinomatous and sarcomatous components of pulmonary sarcomatoid carcinoma. Oncogene. 2021;40:821–832. doi: 10.1038/s41388-020-01573-9. [DOI] [PubMed] [Google Scholar]
- 84.Zhou F., Huang Y., Cai W., Li J., Su C., Ren S., Wu C., Zhou C. The genomic and immunologic profiles of pure pulmonary sarcomatoid carcinoma in Chinese patients. Lung Cancer. 2021;153:66–72. doi: 10.1016/j.lungcan.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z., Tian H., Li L., Li C., Xu J., Bie F., Chen Y., Tian Y., Bai G., Peng Y., et al. PSC subtyping based on TTF-1 and p40 expression reveals distinct molecular characteristics and therapeutic strategies. Int. J. Cancer. 2022;151:717–729. doi: 10.1002/ijc.34137. [DOI] [PubMed] [Google Scholar]
- 86.Lococo F., Gandolfi G., Rossi G., Pinto C., Rapicetta C., Cavazza A., Cesario A., Galeone C., Paci M., Ciarrocchi A. Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma. J. Thorac. Oncol. 2016;11:1282–1292. doi: 10.1016/j.jtho.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 87.Mehrad M., Roy S., LaFramboise W.A., Petrosko P., Miller C., Incharoen P., Dacic S. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology. 2018;73:207–214. doi: 10.1111/his.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou F., Xie G., Ma J.A., Zhou D.A., Jiang Y.I., Zheng J.Y. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: a case report. Oncol. Lett. 2015;9:2239–2243. doi: 10.3892/ol.2015.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X., Jia Y., Stoopler M.B., Shen Y., Cheng H., Chen J., Mansukhani M., Koul S., Halmos B., Borczuk A.C. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J. Clin. Oncol. 2016;34:794–802. doi: 10.1200/JCO.2015.62.0674. [DOI] [PubMed] [Google Scholar]
- 90.Manzotti G., Torricelli F., Benedetta D., Lococo F., Sancisi V., Rossi G., Piana S., Ciarrocchi A. An epithelial-to-mesenchymal transcriptional switch triggers evolution of pulmonary sarcomatoid carcinoma (PSC) and identifies dasatinib as new therapeutic option. Clin. Cancer Res. 2019;25:2348–2360. doi: 10.1158/1078-0432.CCR-18-2364. [DOI] [PubMed] [Google Scholar]
- 91.Tong J.H., Yeung S.F., Chan A.W.H., Chung L.Y., Chau S.L., Lung R.W.M., Tong C.Y., Chow C., Tin E.K.Y., Yu Y.H., et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin. Cancer Res. 2016;22:3048–3056. doi: 10.1158/1078-0432.CCR-15-2061. [DOI] [PubMed] [Google Scholar]
- 92.Yang H., Zhou Z., Lin L., Yang M., Li C., Li Z., Yu X., Lizaso A., Han-Zhang H., Li B., et al. Characterization of MET exon 14 alteration and association with clinical outcomes of crizotinib in Chinese lung cancers. Lung Cancer. 2020;148:113–121. doi: 10.1016/j.lungcan.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 93.Wong S.K., Alex D., Bosdet I., Hughesman C., Karsan A., Yip S., Ho C. MET exon 14 skipping mutation positive non-small cell lung cancer: response to systemic therapy. Lung Cancer. 2021;154:142–145. doi: 10.1016/j.lungcan.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 94.Han S., Fang J., Lu S., Wang L., Li J., Cheng M., Ren Y., Su W. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco. Targets Ther. 2019;12:7323–7328. doi: 10.2147/OTT.S210365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mignard X., Ruppert A.M., Antoine M., Vasseur J., Girard N., Mazières J., Moro-Sibilot D., Fallet V., Rabbe N., Thivolet-Bejui F., et al. c-MET overexpression as a poor predictor of MET amplifications or exon 14 mutations in lung sarcomatoid carcinomas. J. Thorac. Oncol. 2018;13:1962–1967. doi: 10.1016/j.jtho.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Liu X.W., Chen X.R., Rong Y.M., Lyu N., Xu C.W., Wang F., Sun W.Y., Fang S.G., Yuan J.P., Wang H.J., et al. MET exon 14 skipping mutation, amplification and overexpression in pulmonary sarcomatoid carcinoma: a multi-center study. Transl. Oncol. 2020;13:100868. doi: 10.1016/j.tranon.2020.100868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X., Cao J., Du W., Zhang W., Cao S. Response to gefitinib/crizotinib combination in a pulmonary sarcomatoid carcinoma patient harboring concurrent EGFR mutation and MET amplification. Clin. Case Rep. 2021;9:e04487. doi: 10.1002/ccr3.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He Q., Shi X., Zhu H., Yan J., Yu X.M. A case treated with Crizotinib after secondary MET amplification of A double Rare L747S and G719S EGFR mutation Pulmonary Sarcomatoid Carcinoma. Ann. Oncol. 2020;31:544–546. doi: 10.1016/j.annonc.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 99.Terra S.B., Jang J.S., Bi L., Kipp B.R., Jen J., Yi E.S., Boland J.M. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod. Pathol. 2016;29:824–831. doi: 10.1038/modpathol.2016.89. [DOI] [PubMed] [Google Scholar]
- 100.Zhang C., Li Z., Zhang Y., Zhao C., Wang H., Lin J., Liu C., Wang X., Wang H. Genomic variations and immune-related features of TMB, PD-L1 expression and CD8(+) T cell infiltration in Chinese pulmonary sarcomatoid carcinoma. Int. J. Gen. Med. 2022;15:4209–4220. doi: 10.2147/IJGM.S357659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lococo F., Torricelli F., Rossi G., Alifano M., Damotte D., Rapicetta C., Tamagnini I., Cavazza A., Piana S., Galeone C., et al. Inter-relationship between PD-L1 expression and clinic-pathological features and driver gene mutations in pulmonary sarcomatoid carcinomas. Lung Cancer. 2017;113:93–101. doi: 10.1016/j.lungcan.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Tsubata Y., Sutani A., Okimoto T., Matsuura M., Murakami I., Usuda R., Okumichi T., Kakegawa S.I., Togashi K., Kosaka S., et al. Tumor angiogenesis in 75 cases of pleomorphic carcinoma of the lung. Anticancer Res. 2012;32:3331–3337. [PubMed] [Google Scholar]
- 103.Vieira T., Antoine M., Hamard C., Fallet V., Duruisseaux M., Rabbe N., Rodenas A., Cadranel J., Wislez M. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer. 2016;98:51–58. doi: 10.1016/j.lungcan.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 104.Jin C., Yang B. Dramatic response of pulmonary sarcomatoid carcinoma to nivolumab combined with anlotinib: a case report. Case Rep. Oncol. 2020;13:601–605. doi: 10.1159/000507568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawatari K., Izumi M., Sone R., Hattori T., Sugimoto A., Eguchi Y., Mamoto T. A case of PD-L1 negative advanced pulmonary sarcomatoid carcinoma effectively treated with atezolizumab, carboplatin, paclitaxel, and bevacizumab. Respir. Med. Case Rep. 2022;36:101579. doi: 10.1016/j.rmcr.2022.101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y.F., Zhao X.F., Tian Y., Xiao X.Y., Yan C.Y., Shen H. Case Report: pulmonary sarcomatoid carcinoma complicating TP53 mutation treated successfully with Tislelizumab combined with Anlotinib-a case report. Front. Genet. 2022;13:949989. doi: 10.3389/fgene.2022.949989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piao M.N., Ma X.T., Tankere P., Liam C.K., Li J.L., Wang J.P. Anlotinib combined with chemotherapy and immunotherapy for advanced pulmonary sarcomatoid cancer: a case report and literature review. Ann. Transl. Med. 2022;10:1030. doi: 10.21037/atm-22-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim S., Kim M.Y., Koh J., Go H., Lee D.S., Jeon Y.K., Chung D.H. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur. J. Cancer. 2015;51:2698–2707. doi: 10.1016/j.ejca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 109.Babacan N.A., Pina I.B., Signorelli D., Prelaj A., Garassino M.C., Tanvetyanon T. Relationship between programmed death receptor-ligand 1 expression and response to checkpoint inhibitor immunotherapy in pulmonary sarcomatoid carcinoma: a pooled analysis. Clin. Lung Cancer. 2020;21:e456–e463. doi: 10.1016/j.cllc.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 110.Domblides C., Leroy K., Monnet I., Mazières J., Barlesi F., Gounant V., Baldacci S., Mennecier B., Toffart A.C., Audigier-Valette C., et al. Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma. J. Thorac. Oncol. 2020;15:860–866. doi: 10.1016/j.jtho.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Lee J., Choi Y.L., Jung H.A., Lee S.H., Ahn J.S., Ahn M.J., Park K., Sun J.M. Outstanding clinical efficacy of PD-1/PD-L1 inhibitors for pulmonary pleomorphic carcinoma. Eur. J. Cancer. 2020;132:150–158. doi: 10.1016/j.ejca.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 112.Manglaviti S., Brambilla M., Signorelli D., Ferrara R., Lo Russo G., Proto C., Galli G., De Toma A., Occhipinti M., Viscardi G., et al. Immune-checkpoint inhibitors in advanced non-small cell lung cancer with uncommon histology. Clin. Lung Cancer. 2022;23:e17–e28. doi: 10.1016/j.cllc.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Qian X., Wang Y., Liu F., Yuan Y., Fang C., Zhang X., Yuan S., Chen R., Yu B., Wang T., et al. The efficacy and safety analysis of first-line immune checkpoint inhibitors in pulmonary sarcomatoid carcinoma. Front. Immunol. 2022;13:956982. doi: 10.3389/fimmu.2022.956982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei J.W., Hao Y., Xiang J., Pu X.X., Wang L.P., Jiang Z.S., Wu J.X., Wang Q., Xu C.W., Wang W.X., Song Z.B. The prognostic impact of immune checkpoint inhibitors for the treatment of pulmonary sarcomatoid carcinoma: a multicenter retrospective study. Neoplasma. 2022;69:1437–1444. doi: 10.4149/neo_2022_220617N644. [DOI] [PubMed] [Google Scholar]
- 115.Chen P., Yu M., Zhang J.L., Chen W.Y., Zhu L., Song Y., Jiang C.Y., Zhang S. Significant benefits of pembrolizumab in treating refractory advanced pulmonary sarcomatoid carcinoma: a case report. World J. Clin. Cases. 2020;8:2876–2884. doi: 10.12998/wjcc.v8.i13.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cimpeanu E., Ahmed J., Zafar W., DeMarinis A., Bardarov S.S., Salman S., Bloomfield D. Pembrolizumab - emerging treatment of pulmonary sarcomatoid carcinoma: a case report. World J. Clin. Cases. 2020;8:97–102. doi: 10.12998/wjcc.v8.i1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishino K., Kunimasa K., Kimura M., Inoue T., Tamiya M., Kuhara H., Kumagai T. Favorable response to pembrolizumab after durvalumab failure in a stage III sarcomatoid carcinoma of the lung: a case report. BMC Pharmacol. Toxicol. 2020;21:26. doi: 10.1186/s40360-020-00404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiao Y., Liu M., Luo N., Guo H., Li J. Successful treatment of advanced pulmonary sarcomatoid carcinoma with the PD-1 inhibitor toripalimab: a case report. Oral Oncol. 2021;112:104992. doi: 10.1016/j.oraloncology.2020.104992. [DOI] [PubMed] [Google Scholar]
- 119.Taniguchi H., Takemoto S., Ozasa M., Honda N., Suyama T., Umeyama Y., Dotsu Y., Nakao T., Tomohito K., Gyotoku H., et al. Remarkable response to pembrolizumab with platinum-doublet in PD-L1-low pulmonary sarcomatoid carcinoma: a case report. Thorac. Cancer. 2021;12:1126–1130. doi: 10.1111/1759-7714.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu L., Tao N.N., Liang B., Li D.W., Li H.C., Su L.L. Use of PD-1 inhibitor tislelizumab in the treatment of advanced pulmonary sarcomatoid carcinoma: a case report. Thorac. Cancer. 2022;13:502–505. doi: 10.1111/1759-7714.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou F., Guo H., Zhou X., Xie H., Tian T., Zhao W., Gao G., Xiong A., Wang L., Li W., et al. Immune checkpoint inhibitors plus chemotherapy in patients with locally advanced or metastatic pulmonary sarcomatoid carcinoma: a multicentric real-world study. Ther. Adv. Med. Oncol. 2022;14 doi: 10.1177/17588359221136759. 17588359221136759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu L., Wei X., Zhang Z., Heng W. Treatment response to immunotherapy after crizotinib resistance in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Clin. Med. Insights. Oncol. 2022;16 doi: 10.1177/11795549211067185. 11795549211067185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim M., Keam B., Ock C.Y., Kim S.H., Kim Y.J., Lim S.M., Kim J.S., Kim T.M., Hong S.H., Ahn M.S., et al. Phase II study of durvalumab and tremelimumab in pulmonary sarcomatoid carcinoma: KCSG-LU16-07. Thorac. Cancer. 2020;11:3482–3489. doi: 10.1111/1759-7714.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ge J., Yao B., Huang J., Wu X., Bao H., Ou Q., Shao Y.W., Chen J. Molecular genetic characterization reveals linear tumor evolution in a pulmonary sarcomatoid carcinomas patient with a novel PHF20-NTRK1 fusion: a case report. BMC Cancer. 2019;19:592. doi: 10.1186/s12885-019-5780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Y., Yan Z., Pan J., Chang X., Huang B., Luo D., Meng R., Shi H., Fan J., Nie X. Partial response to pralsetinib in an advanced pulmonary sarcomatoid carcinoma patient harboring a KIF5B-RET rearrangement: a case report. World J. Surg. Oncol. 2022;20:386. doi: 10.1186/s12957-022-02848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang X., Li Q., Xu B., Hu S., Wang Q., Li Y., Zong Y., Zhang S., Li C. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int. J. Clin. Oncol. 2019;24:1061–1068. doi: 10.1007/s10147-019-01454-6. [DOI] [PubMed] [Google Scholar]
- 127.Huo Z., Wu H., Li J., Li S., Wu S., Liu Y., Luo Y., Cao J., Zeng X., Liang Z. Primary pulmonary mucoepidermoid carcinoma: histopathological and moleculargenetic studies of 26 cases. PLoS One. 2015;10:e0143169. doi: 10.1371/journal.pone.0143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han S.W., Kim H.P., Jeon Y.K., Oh D.Y., Lee S.H., Kim D.W., Im S.A., Chung D.H., Heo D.S., Bang Y.J., Kim T.Y. Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung Cancer. 2008;61:30–34. doi: 10.1016/j.lungcan.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 129.Yu Y., Song Z., Gao H., Zhu L., Lu S., Zhang J., Luo Q. EGFR L861Q mutation is a frequent feature of pulmonary mucoepidermoid carcinoma. J. Cancer Res. Clin. Oncol. 2012;138:1421–1425. doi: 10.1007/s00432-012-1211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee K.W.C., Chan A.B.W., Lo A.W.I., Lam K.C. Erlotinib in metastatic bronchopulmonary mucoepidermoid carcinoma. J. Thorac. Oncol. 2011;6:2140–2141. doi: 10.1097/JTO.0b013e318237746a. [DOI] [PubMed] [Google Scholar]
- 131.Huo Z., Wu H., Li S., Liang Z. Molecular genetic studies on EGFR, KRAS, BRAF, ALK, PIK3CA, PDGFRA, and DDR2 in primary pulmonary adenoid cystic carcinoma. Diagn. Pathol. 2015;10:161. doi: 10.1186/s13000-015-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M., Zhao B.R., Liu S.Q., An J., Deng P.B., Han-Zhang H., Ye J.Y., Mao X.R., Chuai S.K., Hu C.P. Mutational landscape and clonal diversity of pulmonary adenoid cystic carcinoma. Cancer Biol. Ther. 2018;19:898–903. doi: 10.1080/15384047.2018.1480296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song Z., Wu W., Zhang Y. Effective treatment with icotinib in primary adenoid cystic carcinoma of the lung with liver metastasis. J. Thorac. Oncol. 2014;9:e67–e69. doi: 10.1097/JTO.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 134.Fujita M., Matsumoto T., Hirano R., Uchino J., Hirota T., Yamaguchi E., Kubo A., Yokoi T., Nabeshima K., Watanabe K. Adenoid cystic carcinoma of the lung with an EGFR mutation. Intern. Med. 2016;55:1621–1624. doi: 10.2169/internalmedicine.55.6592. [DOI] [PubMed] [Google Scholar]
- 135.Mendes M.A., Barroso A., Campainha S. EGFR-variant adenoid cystic carcinoma of the lung. J. Thorac. Oncol. 2018;13:e178–e181. doi: 10.1016/j.jtho.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 136.Wu Y.X., Zhang W.L., Wang T.M., Liao Y., Zhang Y.J., Xiao R.W., Jia Y.J., Wu Z.Y., Deng C.M., Yang D.W., et al. Genomic landscapes of epstein-barr virus in pulmonary lymphoepithelioma-like carcinoma. J. Virol. 2022;96:e0169321. doi: 10.1128/JVI.01693-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin Z., Fu S., Zhou Y., Zhang X., Chen C., He L.N., Li H., Wang Y., Chen T., Zhang L., Hong S. First-line platinum-based chemotherapy and survival outcomes in locally advanced or metastatic pulmonary lymphoepithelioma-like carcinoma. Lung Cancer. 2019;137:100–107. doi: 10.1016/j.lungcan.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 138.Chia W.K., Teo M., Wang W.W., Lee B., Ang S.F., Tai W.M., Chee C.L., Ng J., Kan R., Lim W.T., et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol. Ther. 2014;22:132–139. doi: 10.1038/mt.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baldwin A.S., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 140.Baldwin A.S. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol. Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 141.Fan Y., Shan Q., Gong J., Qin J., Lu H. Molecular and clinical characteristics of primary pulmonary lymphoepithelioma-like carcinoma. Front. Mol. Biosci. 2021;8:736940. doi: 10.3389/fmolb.2021.736940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gu S.S., Zhang W., Wang X., Jiang P., Traugh N., Li Z., Meyer C., Stewig B., Xie Y., Bu X., et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11:1524–1541. doi: 10.1158/2159-8290.CD-20-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riess J.W., Rahman S., Kian W., Edgerly C., Heilmann A.M., Madison R., Ramkissoon S.H., Klaitman S.S., Chung J.H., Trabucco S.E., et al. Genomic profiling of solid tumors harboring BRD4-NUT and response to immune checkpoint inhibitors. Transl. Oncol. 2021;14:101184. doi: 10.1016/j.tranon.2021.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stathis A., Zucca E., Bekradda M., Gomez-Roca C., Delord J.P., de La Motte Rouge T., Uro-Coste E., de Braud F., Pelosi G., French C.A. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alekseyenko A.A., Walsh E.M., Zee B.M., Pakozdi T., Hsi P., Lemieux M.E., Dal Cin P., Ince T.A., Kharchenko P.V., Kuroda M.I., French C.A. Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc. Natl. Acad. Sci. USA. 2017;114:E4184–E4192. doi: 10.1073/pnas.1702086114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lewin J., Soria J.C., Stathis A., Delord J.P., Peters S., Awada A., Aftimos P.G., Bekradda M., Rezai K., Zeng Z., et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J. Clin. Oncol. 2018;36:3007–3014. doi: 10.1200/JCO.2018.78.2292. [DOI] [PubMed] [Google Scholar]
- 147.Sarnik J., Popławski T., Tokarz P. BET proteins as attractive targets for cancer therapeutics. Int. J. Mol. Sci. 2021;22:11102. doi: 10.3390/ijms222011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piha-Paul S.A., Hann C.L., French C.A., Cousin S., Braña I., Cassier P.A., Moreno V., de Bono J.S., Harward S.D., Ferron-Brady G., et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 2020;4:pkz093. doi: 10.1093/jncics/pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shapiro G.I., LoRusso P., Dowlati A., T Do K., Jacobson C.A., Vaishampayan U., Weise A., Caimi P.F., Eder J.P., French C.A., et al. A Phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br. J. Cancer. 2021;124:744–753. doi: 10.1038/s41416-020-01180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lara P.N., Jr., Longmate J., Mack P.C., Kelly K., Socinski M.A., Salgia R., Gitlitz B., Li T., Koczywas M., Reckamp K.L., Gandara D.R. Phase II study of the AKT inhibitor MK-2206 plus erlotinib in patients with advanced non-small cell lung cancer who previously progressed on erlotinib. Clin. Cancer Res. 2015;21:4321–4326. doi: 10.1158/1078-0432.CCR-14-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y., Sun M.M., Zhang G.G., Yang J., Chen K.S., Xu W.W., Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal. Transduct. Target. Ther. 2021;6:425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pelosi G., Gasparini P., Conte D., Fabbri A., Perrone F., Tamborini E., Pupa S.M., Ciravolo V., Caserini R., Rossi G., et al. Synergistic activation upon MET and ALK coamplification sustains targeted therapy in sarcomatoid carcinoma, a deadly subtype of lung cancer. J. Thorac. Oncol. 2016;11:718–728. doi: 10.1016/j.jtho.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 153.Wilson Z., Odedra R., Wallez Y., Wijnhoven P.W.G., Hughes A.M., Gerrard J., Jones G.N., Bargh-Dawson H., Brown E., Young L.A., et al. ATR inhibitor AZD6738 (ceralasertib) exerts antitumor activity as a monotherapy and in combination with chemotherapy and the PARP inhibitor olaparib. Cancer Res. 2022;82:1140–1152. doi: 10.1158/0008-5472.CAN-21-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gorecki L., Andrs M., Rezacova M., Korabecny J. Discovery of ATR kinase inhibitor berzosertib (VX-970, M6620): clinical candidate for cancer therapy. Pharmacol. Ther. 2020;210:107518. doi: 10.1016/j.pharmthera.2020.107518. [DOI] [PubMed] [Google Scholar]
- 155.Ni W., Chen Z., Zhou X., Yang R., Yu M., Lu J., Kaye F.J., Wu L. Targeting Notch and EGFR signaling in human mucoepidermoid carcinoma. Signal. Transduct. Target. Ther. 2021;6:27. doi: 10.1038/s41392-020-00388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roden A.C., Greipp P.T., Knutson D.L., Kloft-Nelson S.M., Jenkins S.M., Marks R.S., Aubry M.C., García J.J. Histopathologic and cytogenetic features of pulmonary adenoid cystic carcinoma. J. Thorac. Oncol. 2015;10:1570–1575. doi: 10.1097/JTO.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 157.Pei J., Flieder D.B., Patchefsky A., Talarchek J.N., Cooper H.S., Testa J.R., Wei S. Detecting MYB and MYBL1 fusion genes in tracheobronchial adenoid cystic carcinoma by targeted RNA-sequencing. Mod. Pathol. 2019;32:1416–1420. doi: 10.1038/s41379-019-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andersson M.K., Afshari M.K., Andrén Y., Wick M.J., Stenman G. Targeting the oncogenic transcriptional regulator MYB in adenoid cystic carcinoma by inhibition of IGF1R/AKT signaling. J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx017. [DOI] [PubMed] [Google Scholar]
- 159.Wang F., Xi S.Y., Hao W.W., Yang X.H., Deng L., Xu Y.X., Wu X.Y., Zeng L., Guo K.H., Wang H.Y. Mutational landscape of primary pulmonary salivary gland-type tumors through targeted next-generation sequencing. Lung Cancer. 2021;160:1–7. doi: 10.1016/j.lungcan.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 160.Qu C., Zhang H., Cao H., Tang L., Mo H., Liu F., Zhang L., Yi Z., Long L., Yan L., et al. Tumor buster - where will the CAR-T cell therapy 'missile' go? Mol. Cancer. 2022;21:201. doi: 10.1186/s12943-022-01669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng K., Guo Y., Dai H., Wang Y., Li X., Jia H., Han W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China. Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y., Zhang Z., Ding Y., Fang Y., Wang P., Chu W., Jin Z., Yang X., Wang J., Lou J., Qian Q. Phase I clinical trial of EGFR-specific CAR-T cells generated by the piggyBac transposon system in advanced relapsed/refractory non-small cell lung cancer patients. J. Cancer Res. Clin. Oncol. 2021;147:3725–3734. doi: 10.1007/s00432-021-03613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Min J., Long C., Zhang L., Duan J., Fan H., Chu F., Li Z. c-Met specific CAR-T cells as a targeted therapy for non-small cell lung cancer cell A549. Bioengineered. 2022;13:9216–9232. doi: 10.1080/21655979.2022.2058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiriaco C., Donini C., Cortese M., Ughetto S., Modica C., Martinelli I., Proment A., Vitali L., Fontani L., Casucci M., et al. Efficacy of CAR-T immunotherapy in MET overexpressing tumors not eligible for anti-MET targeted therapy. J. Exp. Clin. Cancer Res. 2022;41:309. doi: 10.1186/s13046-022-02479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu M., Wang X., Li W., Yu X., Flores-Villanueva P., Xu-Monette Z.Y., Li L., Zhang M., Young K.H., Ma X., Li Y. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis. 2020;9:72. doi: 10.1038/s41389-020-00257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao H., Korn J.M., Ferretti S., Monahan J.E., Wang Y., Singh M., Zhang C., Schnell C., Yang G., Zhang Y., et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 167.Zhang H., Fillmore Brainson C., Koyama S., Redig A.J., Chen T., Li S., Gupta M., Garcia-de-Alba C., Paschini M., Herter-Sprie G.S., et al. Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2. Nat. Commun. 2017;8:14922. doi: 10.1038/ncomms14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nagaraj A.S., Lahtela J., Hemmes A., Pellinen T., Blom S., Devlin J.R., Salmenkivi K., Kallioniemi O., Mäyränpää M.I., Närhi K., Verschuren E.W. Cell of origin links histotype spectrum to immune microenvironment diversity in non-small-cell lung cancer driven by mutant kras and loss of Lkb1. Cel Rep. 2017;18:673–684. doi: 10.1016/j.celrep.2016.12.059. [DOI] [PubMed] [Google Scholar]
- 169.Närhi K., Nagaraj A.S., Parri E., Turkki R., van Duijn P.W., Hemmes A., Lahtela J., Uotinen V., Mäyränpää M.I., Salmenkivi K., et al. Spatial aspects of oncogenic signalling determine the response to combination therapy in slice explants from Kras-driven lung tumours. J. Pathol. 2018;245:101–113. doi: 10.1002/path.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krause A., Roma L., Lorber T., Habicht J., Lardinois D., De Filippo M.R., Prince S.S., Piscuoglio S., Ng C., Bubendorf L. Deciphering the clonal relationship between glandular and squamous components in adenosquamous carcinoma of the lung using whole exome sequencing. Lung Cancer. 2020;150:132–138. doi: 10.1016/j.lungcan.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 171.Woo X.Y., Srivastava A., Mack P.C., Graber J.H., Sanderson B.J., Lloyd M.W., Chen M., Domanskyi S., Gandour-Edwards R., Tsai R.A., et al. A genomically and clinically annotated patient-derived xenograft resource for preclinical research in non-small cell lung cancer. Cancer Res. 2022;82:4126–4138. doi: 10.1158/0008-5472.CAN-22-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang D., Pham N.A., Tong J., Sakashita S., Allo G., Kim L., Yanagawa N., Raghavan V., Wei Y., To C., et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int. J. Cancer. 2017;140:662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 173.Sun H., Cao S., Mashl R.J., Mo C.K., Zaccaria S., Wendl M.C., Davies S.R., Bailey M.H., Primeau T.M., Hoog J., et al. Comprehensive characterization of 536 patient-derived xenograft models prioritizes candidates for targeted treatment. Nat. Commun. 2021;12:5086. doi: 10.1038/s41467-021-25177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fang D.D., Zhang B., Gu Q., Lira M., Xu Q., Sun H., Qian M., Sheng W., Ozeck M., Wang Z., et al. HIP1-ALK, a novel ALK fusion variant that responds to crizotinib. J. Thorac. Oncol. 2014;9:285–294. doi: 10.1097/JTO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 175.Lázaro S., Lorz C., Enguita A.B., Seller I., Paramio J.M., Santos M. Pten and p53 loss in the mouse lung causes adenocarcinoma and sarcomatoid carcinoma. Cancers (Basel) 2022;14:3671. doi: 10.3390/cancers14153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim M., Mun H., Sung C.O., Cho E.J., Jeon H.J., Chun S.M., Jung D.J., Shin T.H., Jeong G.S., Kim D.K., et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019;10:3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fichtner I., Rolff J., Soong R., Hoffmann J., Hammer S., Sommer A., Becker M., Merk J. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 2008;14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 178.John T., Kohler D., Pintilie M., Yanagawa N., Pham N.A., Li M., Panchal D., Hui F., Meng F., Shepherd F.A., Tsao M.S. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin. Cancer Res. 2011;17:134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 179.Gabasa M., Radisky E.S., Ikemori R., Bertolini G., Arshakyan M., Hockla A., Duch P., Rondinone O., Llorente A., Maqueda M., et al. MMP1 drives tumor progression in large cell carcinoma of the lung through fibroblast senescence. Cancer Lett. 2021;507:1–12. doi: 10.1016/j.canlet.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Short S., Fielder E., Miwa S., von Zglinicki T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine. 2019;41:683–692. doi: 10.1016/j.ebiom.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kita K., Fukuda K., Takahashi H., Tanimoto A., Nishiyama A., Arai S., Takeuchi S., Yamashita K., Ohtsubo K., Otani S., et al. Patient-derived xenograft models of non-small cell lung cancer for evaluating targeted drug sensitivity and resistance. Cancer Sci. 2019;110:3215–3224. doi: 10.1111/cas.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nakajima T., Geddie W., Anayama T., Ko H.M., da Cunha Santos G., Boerner S., Wang T., Wang Y.H., Li M., Pham N.A., et al. Patient-derived tumor xenograft models established from samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Lung Cancer. 2015;89:110–114. doi: 10.1016/j.lungcan.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 183.Lei Y., Tang R., Xu J., Wang W., Zhang B., Liu J., Yu X., Shi S. Applications of single-cell sequencing in cancer research: progress and perspectives. J. Hematol. Oncol. 2021;14:91. doi: 10.1186/s13045-021-01105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alfaro J.A., Bohländer P., Dai M., Filius M., Howard C.J., van Kooten X.F., Ohayon S., Pomorski A., Schmid S., Aksimentiev A., et al. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods. 2021;18:604–617. doi: 10.1038/s41592-021-01143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui M., Cheng C., Zhang L. High-throughput proteomics: a methodological mini-review. Lab. Invest. 2022;102:1170–1181. doi: 10.1038/s41374-022-00830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hassin O., Oren M. Drugging p53 in cancer: one protein, many targets. Nat. Rev. Drug Discov. 2023;22:127–144. doi: 10.1038/s41573-022-00571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu Y., Sui X., Song F., Li Y., Li K., Chen Z., Yang F., Chen X., Zhang Y., Wang X., et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 2021;12:2581. doi: 10.1038/s41467-021-22676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pallmann P., Bedding A.W., Choodari-Oskooei B., Dimairo M., Flight L., Hampson L.V., Holmes J., Mander A.P., Odondi L., Sydes M.R., et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018;16:29. doi: 10.1186/s12916-018-1017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ursino M., Stallard N. Bayesian approaches for confirmatory trials in rare diseases: opportunities and challenges. Int. J. Environ. Res. Public Health. 2021;18:1022. doi: 10.3390/ijerph18031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Middleton G., Robbins H., Andre F., Swanton C. A state-of-the-art review of stratified medicine in cancer: towards a future precision medicine strategy in cancer. Ann. Oncol. 2022;33:143–157. doi: 10.1016/j.annonc.2021.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.