Abstract
Background
Electrocardiograms (ECGs) are used by physicians to record, monitor, and diagnose the electrical activity of the heart. Recent technological advances have allowed ECG devices to move out of the clinic and into the home environment. There is a great variety of mobile ECG devices with the capabilities to be used in home environments.
Objective
This scoping review aimed to provide a comprehensive overview of the current landscape of mobile ECG devices, including the technology used, intended clinical use, and available clinical evidence.
Methods
We conducted a scoping review to identify studies concerning mobile ECG devices in the electronic database PubMed. Secondarily, an internet search was performed to identify other ECG devices available in the market. We summarized the devices’ technical information and usability characteristics based on manufacturer data such as datasheets and user manuals. For each device, we searched for clinical evidence on the capabilities to record heart disorders by performing individual searches in PubMed and ClinicalTrials.gov, as well as the Food and Drug Administration (FDA) 510(k) Premarket Notification and De Novo databases.
Results
From the PubMed database and internet search, we identified 58 ECG devices with available manufacturer information. Technical characteristics such as shape, number of electrodes, and signal processing influence the capabilities of the devices to record cardiac disorders. Of the 58 devices, only 26 (45%) had clinical evidence available regarding their ability to detect heart disorders such as rhythm disorders, more specifically atrial fibrillation.
Conclusions
ECG devices available in the market are mainly intended to be used for the detection of arrhythmias. No devices are intended to be used for the detection of other cardiac disorders. Technical and design characteristics influence the intended use of the devices and use environments. For mobile ECG devices to be intended to detect other cardiac disorders, challenges regarding signal processing and sensor characteristics should be solved to increase their detection capabilities. Devices recently released include the use of other sensors on ECG devices to increase their detection capabilities.
Keywords: electrocardiogram; mobile ECG; home use ECG; wearables; medical devices; ECG clinical validation, ECG technical characteristics
Introduction
Background
Cardiovascular diseases are the leading cause of mortality, accounting for approximately 31% of all deaths worldwide [1]. The leading contributors to cardiovascular death are ischemic heart disease, ischemic stroke, hemorrhagic stroke, hypertensive heart disease (which ultimately results in heart failure), cardiomyopathy, rheumatic heart disease, and atrial fibrillation (AF) [2]. To perform cardiovascular assessments, physicians require diagnostic tools such as the electrocardiogram (ECG) [3].
The ECG records the electrical signals generated by the heart’s electrical activity; the electrical currents arise owing to potential differences that spread to the surface of the body when cardiac impulses pass through the heart [4]. The traditional 12-lead ECG is recorded via electrodes placed on the limbs and chest wall [3]. The ECG is a tool used in the everyday practice of clinical medicine, with >300 million ECGs obtained annually [5].
The 12-lead ECG is the clinical gold standard and is reminiscent of the original recordings by Einthoven, which refers to the placement of 3 limb electrodes, from which 2 leads are measured, and other 4 leads are calculated, allowing 6 limb leads and creating a view of the heart in the vertical plane [6]. In addition, the 6 precordial leads (V1-V6) provide a view of the horizontal plane of the heart, using the Wilson central terminal as a reference [3]. Technological advances such as the miniaturization of electronic components, innovations in sensor technologies, and progress in mobile and communication technology have allowed innovations in mobile health devices. New technologies allow general practitioners or ambulance staff to record ECGs as routine in chest pain, whereas patients can perform self-monitoring at home. Thus, the ECG is moving from the clinical to the domestic environment [7].
Previous review studies on mobile ECG devices have focused primarily on wearable sensors that can be used in and outside of the clinic, the technological taxonomy of ECG devices, an analysis of single- and 3-lead devices, adhesive ECG patch devices, and devices focused only on diagnosing rhythm disorders and conduction system diseases; the studies’ secondary focus has been on the future of ECG technologies, including the necessary steps for integration in clinical infrastructures [7-13]. The published reviews have shown that a majority of mobile ECG devices are focused on screening for AF or other rhythm disorders [7,8,11,13]. The reviews partly cover the potential applications of mobile ECG devices. There is a gap in how the available devices in the market can be selected for use based on device characteristics, purpose, and clinical evidence.
Objectives
This review aimed to provide an overview of the mobile ECG devices that are available in the market, including the technology used, their intended clinical use, and the published clinical evidence used for validation. In this review, we defined the gaps and pitfalls in commercially available and discontinued devices to provide the reader with a comprehensive overview of the current landscape of mobile ECG devices as well as their clinical purposes, clinical outcomes, and benefits. In addition, we addressed the disadvantages per type of device to highlight the most promising devices or technology used and the areas where there is room for improvement.
Methods
Device Searches
First, we performed a PubMed database search for ECG devices. We searched for articles published between September 6, 2012, and September 6, 2022. Article titles, keywords, and abstracts were searched using the following search terms: “Wearable Electronic Devices” (medical subject headings [MeSH] term) AND (“Electrocardiography” [MeSH term] OR “electrocardiography, ambulatory” [MeSH term]). We only included articles published in English, and the identified devices needed to be capable of recording ECGs. We excluded devices that are only capable of recording photoplethysmography.
In addition, internet searches for mobile ECG devices using the Google search engine were performed. The search words used were “electrocardiography” combined with “mobile,” “wearable,” or “handheld.” Only ECG devices were included.
Device Characteristics
Once the mobile ECG devices were identified, we consulted their manufacturer websites to gather technical datasheets as well as user manuals and then summarized their characteristics and technical and user specifications. We also consulted the Food and Drug Administration (FDA) 510(k) Premarket Notification database and ClinicalTrials.gov to review data on devices when no data were available from the manufacturers.
After we identified the ECG devices, based on their characteristics, all devices found were classified into three types of mobile ECG devices: (1) handheld, (2) patch, and (3) wearable (as summarized in Figure 1 and Textbox 1).
Figure 1.
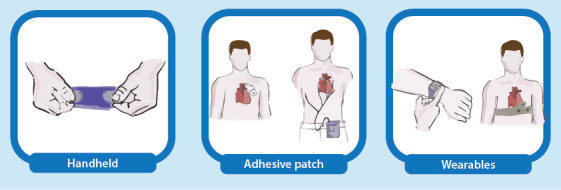
Presentation of mobile electrocardiogram device categories.
Types of mobile electrocardiogram (ECG) devices.
Handheld devices
These devices, which have embedded dry electrodes, are required to be carried separately by users. To perform ECG recordings, users place the device on their chest or hold the device in their hands. This type of device performs ECG recordings when it is activated by users. Users perform intermittent recordings lasting <1 minute.
Patch devices
These devices, which have disposable embedded electrodes or disposable surface electrodes, are usually attached to the left chest of patients. They can perform continuous recordings for up to 30 days.
Wearables
These devices, which use dry metal, textile, or single-use electrodes, are used for continuous wearing during normal daily activities. Wearables are worn on the chest as a garment (eg, T-shirt), as a harness, or on the wrist as a smartwatch. Depending on the area of measurement, these devices can perform for 24 hours or obtain recordings lasting <1 minute.
For each device, we identified the intended use, recording time, and number of electrodes (instead of leads because the number of leads was not specified for some devices; it should be noted that for 3-electrode devices, 6 leads could be obtained from the calculation of the limb leads). We also detailed whether the device is stand-alone.
We registered the user characteristics (user environment, multiple areas of measurement, and setup difficulty), as well as technical characteristics (sampling rate, sampling resolution, and signal bandwidth) and compliance characteristics for use, such as the level of protection of the device against the ingress of hazardous parts and water (the ingress protection [IP] rating).
Clinical Evidence
Finally, we searched for available clinical information by performing a search per device with the aim to identify the available clinical evidence regarding its capabilities. We identified the type of studies performed per device, whether the device had been validated for detection of certain heart conditions, and whether these studies had compared the device against 12-lead ECG devices or other mobile ECG devices.
We also analyzed the feasibility of these devices for home use while guaranteeing the safety of patients. We looked at home use compliance as well as analyzed the available clinical evidence for detection of heart disorders.
Results
Overview
With the PubMed search, we identified 434 articles (Figure 2), of which we excluded 317 (73%) owing to publication date as well as not being written in English and after an examination of titles and abstracts, leaving 117 (27%) for analysis. Of these 117 publications, 73 (62.4%) were excluded because they referred to prototype devices (n=35, 48%), non-ECG devices (n=17, 23%), and design and validation of artificial intelligence algorithms (n=17, 23%) or were opinion articles (n=4, 5%). From the remaining articles (44/117, 37.6%), we identified 48 ECG devices, of which 22 (46%) were patch-based devices, 8 (17%) were handheld devices, and 18 (38%) were wearables. Subsequently, from the internet search, another 33 devices were identified: 16 (49%) were patch-based devices, 11 (33%) were handheld devices, and 6 (18%) were wearables. In total, 82 devices were identified for this review (Figure 2). For 58 (70%) of these 82 devices, we were able to find characteristics from manufacturer websites. We summarized and grouped the devices into continuous recording devices and intermittent recording devices (Figure 3).
Figure 2.
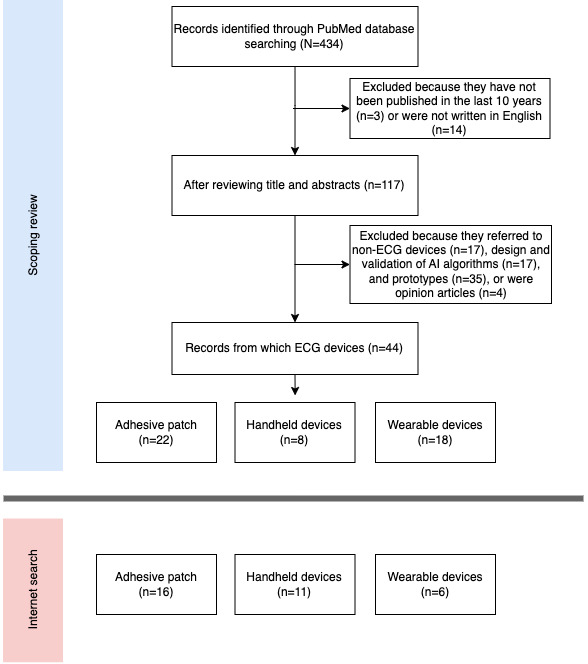
Schematic view of the methodology used for the scoping review and the internet search results. AI: artificial intelligence; ECG: electrocardiogram.
Figure 3.
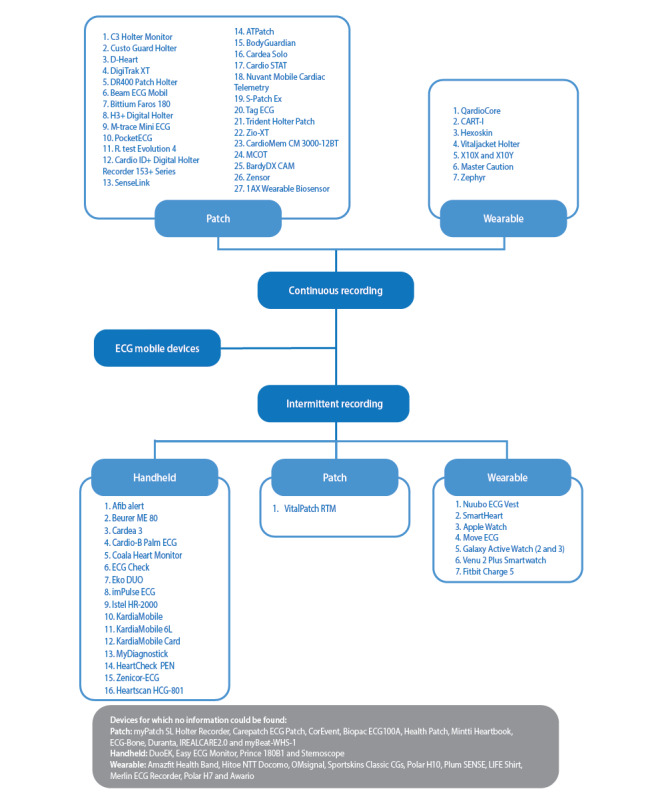
Electrocardiogram (ECG) device classification based on type of recording and type of device.
Clinical Purpose
For 3% (2/58) of the devices, the intended use information was not available from the manufacturer (Tables 1 and 2). Of the 58 devices, 21 (36%) do not state intended use regarding the detection of rhythm disorders; they are intended to be used for measuring and recording ECGs in general. However, more than half (31/58, 53%) of the devices are intended to be used when there is suspicion of arrhythmias (25/58, 43%), more specifically AF (6/58, 10%).
Table 1.
Functionality characteristics per device (continuous recording devices).
| Device | Manufacturer | Intended use | Recording time | Number of electrodes | Stand-alone | Source of clinical evaluation evidence | ||
| Patch | ||||||||
|
|
C3 Holter Monitora | Cortrium ApS | M+Rb | 1 week | 3 | Yes | Clinical trials websitec | |
|
|
Custo Guard Holtera | Custo Med GmbH | M+R+PMDd | >1 day | 4 | Yes | —e | |
|
|
D-Heart | D-Heart Srl | M+R | 1 day | 6 | No | — | |
|
|
DigiTrak XT | Koninklijke Philips NV | M+R+HRDf,g | 1 week | 5 | Yes | Clinical trials websitec and PNDh | |
|
|
DR400 Patch Holter | NorthEast Monitoring Inc | ERi+ADj | >1 week | 3 | Yes | PND | |
|
|
Beam ECG Mobila | IEM GmbH | M+R+ERg | >1 minute | 8 | Yes | — | |
|
|
Bittium Faros 180 | Bittium | M+R+ADg | >1 week | 2 | Yes | PND | |
|
|
H3+ Digital Holtera | Welch Allyn | M+R+AD | >1 day | 5 | Yes | PND | |
|
|
M-trace Mini ECGa | M4Medical | M+R+ADg | 1 minute | 4 | Yes | Clinical trials websitec | |
|
|
PocketECGa | Medicalgorithmics SA | M+R+AD | 1 day | 3 | Yes | Clinical trials websitec and PND | |
|
|
R.Test Evolution 4 | Novacor | AD | >1 month | 2 | Yes | Clinical trials website and PND | |
|
|
Cardio ID+ Digital Holter Recorder 153+ Series | Rozinn | M+R | >1 day | 3 | Yes | PND | |
|
|
SenseLinka | Temeco | AD+ER | >1 week | 5 | Yes | — | |
|
|
Zensor | Renew Health Ltd | AD | >1 week | 7 | Yes | Clinical trials websitec and PND | |
|
|
1AX Wearable Biosensora | LifeSignals Inc | M+R | >1 day | 6 | No | Clinical trials websitec and PND | |
|
|
ATPatch | Atsens | AD | >1 week | 3 | No | Clinical trials website and PND | |
|
|
BodyGuardian | Preventice Technologies Inc | AD | 1 day | 4 | No | Clinical trials website and PND | |
|
|
Cardea Soloa | Cardiac Insight Inc | AD | 1 week | 2 | Yes | Clinical trials websitec and PND | |
|
|
Cardio STAT | Icentia Inc | AD | >1 week | 2 | Yes | Clinical trials website | |
|
|
Nuvant Mobile Cardiac Telemetry Monitor | Corventis | AD+CDk | >1 week | — | — | Clinical trials websitec and PND | |
|
|
S-Patch Exa | Wellysis | M+R | >1 day | 2 | No | Clinical trials websitec | |
|
|
Tag ECGa | Welch Allyn | AD | 1 week | 2 | Yes | — | |
|
|
Trident Holter Patcha | TZ Medical Inc | M+Rg | 1 week | — | Yes | — | |
|
|
Zio XT | iRhythm Technologies Inc | M+R | >1 week | 2 | Yes | Clinical trials website and PND | |
|
|
CardioMem CM 3000-12BTa | GE Healthcare | AD | >1 day | 12 | Yes | Clinical trials website and PND | |
|
|
MCOTa | Koninklijke Philips NV | AD | >1 day | 4 | No | Clinical trials websitec | |
|
|
BardyDX CAMa | Bardy Diagnostics Inc | AD | >1 week | 2 | Yes | PND | |
| Wearable | ||||||||
|
|
QardioCorea | Qardio Inc | M+R | 1 day | 4 | No | PND | |
|
|
CART-Ia | Sky Labs Inc | AFDl | 1 day | — | No | Clinical trials websitec | |
|
|
Hexoskin | Carré Technologies Inc | Researchg | >1 day | — | No | Clinical trials websitec | |
|
|
VitalJacket Holter | BioDevices SA | AD+CD | >1 day | 6 | Yes | — | |
|
|
X10X and X10Y | L.I.F.E. Italia Srl | — | 1 day | 8 | Yes | Clinical trials websitec | |
|
|
Master Caution | HealthWatch Ltd | M+R | — | 12 | No | Clinical trials websitec and PND | |
|
|
Zephyr | Medtronic | M+R | >1 day | 2 | Yes | PND | |
aDevice found via internet search.
bM+R: measure and record electrocardiogram.
cNo results available.
dPMD: pacemaker detection.
eNot available.
fHRD: heart rate detection.
gData found via internet search.
hPND: Food and Drug Administration 510(k) Premarket Notification database.
iER: event recorder.
jAD: arrhythmia detection.
kCD: conduction disorder detection.
lAFD: atrial fibrillation detection.
Table 2.
Functionality characteristics per device (intermittent recording devices).
| Device | Manufacturer | Intended use | Recording time | Number of electrodes | Stand-alone | Source of clinical evaluation evidence | ||||||||||
| Handheld | ||||||||||||||||
|
|
Afib Alert | Lohman Technologies LLC | AFDa | <1 minute | 2 | Yes | PNDb | |||||||||
|
|
Beurer ME 80 | Beurer GmbH | ADc | <1 minute | 1 | Yes | —d | |||||||||
|
|
Cardea 3e | Human Medical Solutions Inc | AD | <1 minute | 4 | No | — | |||||||||
|
|
Cardio-B Palm ECGe | Shanghai International Holding Corp | M+Rf | <1 minute | 2 | Yes | — | |||||||||
|
|
Coala Heart Monitor | Coala Life AB | AFD | <1 minute | 3 | No | Clinical trials website and PND | |||||||||
|
|
ECG Checke | Cardiac Designs Inc | AD | <1 minute | 2 | No | PND | |||||||||
|
|
Eko DUOe | Eko Devices Inc | M+R | >1 minute | 2 | No | Clinical trials website and PND | |||||||||
|
|
Impulse ECG | — | — | <1 minute | 2 | No | — | |||||||||
|
|
Istel HR-2000e | Diagnosis SA | M+R | <1 minute | 4 | No | Clinical trials website | |||||||||
|
|
KardiaMobile | AliveCor, Inc | AD | <1 minute | 2 | No | Clinical trials website and PND | |||||||||
|
|
KardiaMobile 6L | AliveCor, Inc | AD | <1 minute | 3 | No | Clinical trials website and PND | |||||||||
|
|
KardiaMobile Card | AliveCor, Inc | AD | <1 minute | 2 | No | Clinical trials website and PND | |||||||||
|
|
MyDiagnostick | MyDiagnostick Medical BV | AFD | 1 minute | 2 | Yes | Clinical trials website | |||||||||
|
|
HeartCheck PEN | CardioComm Solutions, Inc | AD | <1 minute | 2 | No | PND | |||||||||
|
|
Zenicor-ECG | Zenicor Medical Systems | M+R | <1 minute | 2 | No | Clinical trials website | |||||||||
|
|
Heartscan HCG-801e | Omron | M+R | <1 minute | 3 | Yes | Clinical trials website | |||||||||
| Patch | ||||||||||||||||
|
|
VitalPatch RTMd | VitalConnect Inc | M+R | <1 week | 2 | Yes | PND | |||||||||
| Wearable | ||||||||||||||||
|
|
Nuubo ECG Vest | Nuubo Wearable Technologies | M+R | >1 week | 4 | Yes | Clinical trials website | |||||||||
|
|
SmartHearte | SHL Telemedicine International Ltd | M+R | <1 minute | 18 | No | PND | |||||||||
|
|
Apple Watch | Apple Inc | AFD | <1 minute | 2 | Yes | Clinical trials website and DNDg | |||||||||
|
|
Move ECGe | Withings | AFD | <1 minute | 2 | No | Clinical trials website and PND | |||||||||
|
|
Galaxy Active Watch (2 and 3)e | Samsung Electronics Co, Ltd | AFD | <1 minute | 2 | Yes | Clinical trials website and PND | |||||||||
|
|
Venu 2 Plus Smartwatche | Garmin Ltd | AFD | <1 minute | 2 | Yes | PND | |||||||||
|
|
Fitbit Charge 5 e | Alphabet Inc | AFD | <1 minute | 2 | Yes | Clinical trials website and PND | |||||||||
aAFD: atrial fibrillation detection.
bPND: Food and Drug Administration 510(k) Premarket Notification database.
cAD: arrhythmia detection.
dNot available.
eDevice found via internet search.
fM+R: measure and record electrocardiogram.
gDND: Food and Drug Administration 510(k) De Novo database.
Use Characteristics
Adhesive patch devices are intended to be placed either at the left side (11/28, 39%) or center of the chest (17/28, 60%). Table 2 shows that these devices require setup for positioning the devices on patients, with the steps including skin preparation (shaving and removal of nonconductive skin layer via skin abrasion) as well as templates for the correct device placement, and for performing successful patient recordings. These steps are performed once because these devices are used on a longer-term basis (from >1 day up to >30 days). There are 2 types of patch devices: those in which the whole system, including 2 or 3 electrodes, is embedded in the patch and those that use disposable single electrodes attached through a cable. In the latter case, the devices aim to provide recordings that resemble 12-lead clinical ECG recordings. Patch devices are usually managed by health care centers, and analyses are performed by the manufacturer, specialized companies, or at health care centers.
The wearables category has shown to be more versatile because some of the devices (7/14, 50%) in this category are intended for intermittent use, whereas others (7/14, 50%) are intended for continuous recording. Devices in the former category are often used as daily accessories, such as the Apple Watch (Apple Inc), Amazfit Band (Zepp Health Corporation), and CART-I smart ring (Sky Labs Inc). As for the wearable devices that offer continuous recording, they can be used as garments such as T-shirts. These devices have embedded textile electrodes and can perform recordings lasting 24 hours. Although these devices offer prolonged recordings compared with the accessory wearables, only 2 (29%) of the 7 devices allow simultaneous recording and analysis.
Handheld devices are designed for patients, both for clinical and home use. Of the 14 devices, 11 (79%) rely on limb (including lower limbs) recordings, whereas 3 (21%) perform chest recordings. To record ECGs using handheld devices, no extra steps are required for preparing the area of contact, and ECGs can be recorded in <1 minute.
Technical Characteristics
Patch and wearable devices can be used for at least 24 hours continuously, and these devices can include the feature to detect cardiac events automatically. By contrast, handheld devices have recording durations, initiated by patients, ranging from 15 to 120 seconds. Patients are typically instructed to perform recordings at the onset of symptoms or at specified times.
Handheld devices record ECGs via dry electrodes. These metal electrodes are manufactured from stainless steel, copper, silver or silver chloride (Ag or AgCl), or other unspecified materials. By contrast, patch devices use disposable electrodes, either commercially available or as part of the product.
Of the 58 devices with available manufacturer information, 43 (74%) are intended to be used at home. Of these 43 devices, only 21 (49%) disclosed their IP rating. Of these 21 devices, 5 (24%) have been tested for IP22 (protected from touch by fingers and objects >12 mm and protected from water spray <15° from the vertical) and 10 (48%) for higher IP, whereas 6 (29%) devices have been tested only for water IP (Tables 3 and 4).
Table 3.
Technical characteristics per device (continuous recording devices).
| Device | Use environment | Multiple measures | Requires setup | Ingress protection rating | Sampling rate (Hz) | Resolution (bits) | Signal bandwidth (Hz) | |
| Patch | ||||||||
|
|
C3 Holter Monitor | Home and clinical | N/Aa | ✓ | —b | 256 | 24 | — |
|
|
Custo Guard Holter | Clinical | ✓ | ✓ | 65 | 533 | 18 | 0-105 |
|
|
D-Heart | Home and clinical | N/A | ✓ | — | 640 | — | — |
|
|
DigiTrak XT | Home | N/A | ✓ | — | 175 | 10 | 0.05-60 |
|
|
DR400 Patch Holter | Home | ✓ | ✓ | 44 | 180 | 12 | 0.05-70 |
|
|
Beam ECG Mobil | Home | ✓ | ✓ | — | 200 | 12 | Event: 0.3-75; loop: 0.1-75 |
|
|
Bittium Faros 180 | Home | ✓ | ✓ | 67 | 100 | — | — |
|
|
H3+ Digital Holter | Clinical | N/A | ✓ | — | 180 | 12 | — |
|
|
M-trace Mini ECG | Home | N/A | ✓ | — | 1000 | 24 | 0.5-100 |
|
|
PocketECG | — | N/A | ✓ | 22 | 300 | — | 0.05-60 |
|
|
R.Test Evolution 4 | — | N/A | ✓ | X4 | 200 | 10 | — |
|
|
Cardio ID+ Digital Holter Recorder 153+ Series | — | N/A | N/A | — | 1024 | 12 | 0.05-60 |
|
|
SenseLink | Home and clinical | N/A | ✓ | 22 | 1000 | 16 | — |
|
|
Zensor | — | N/A | ✓ | 22 | 360 | 12 | 0.67-40 |
|
|
1AX Wearable Biosensor | Home | N/A | ✓ | 24 | 244.14 | 16 | 0.2-40 |
|
|
ATPatch | Home | N/A | ✓ | 57 | 250 | 10 | 0.05-40 |
|
|
BodyGuardian | Home | ✓ | ✓ | X4 | 256 | 12 | — |
|
|
Cardea Solo | Home | N/A | ✓ | — | — | — | — |
|
|
Cardio STAT | Home | N/A | N/A | — | — | — | — |
|
|
Nuvant Mobile Cardiac Telemetry Monitor | — | N/A | ✓ | — | 200 | 10 | — |
|
|
S-Patch Ex | — | N/A | ✓ | 55 | 256 | 12 | — |
|
|
Tag ECG | Home | ✓ | ✓ | X7 | 250 | — | 0.05-65 |
|
|
Trident Holter Patch | — | N/A | N/A | — | — | — | — |
|
|
Zio XT | — | ✓ | ✓ | X4 | 200 | 10 | 0.05-30 |
|
|
CardioMem CM 3000-12 BT | Home | N/A | ✓ | 20 | 1024 | 12 | 0.05-120 |
|
|
MCOT | Home | N/A | ✓ | X4 | 250 | 12 | — |
|
|
BardyDX CAM | Home | N/A | ✓ | 23 | 171 | — | 0.67-25 |
| Wearable | ||||||||
|
|
QardioCore | Home | N/A | N/A | 65 | 600 | 16 | 0.05-40 |
|
|
CART-I | Home | N/A | N/A | 58 | — | — | — |
|
|
Hexoskin | Home | N/A | N/A | — | 256 | 12 | — |
|
|
VitalJacket Holter | — | N/A | ✓ | — | 500 | 10 | 0.03-150 |
|
|
X10X and X10Y | — | N/A | N/A | — | — | — | — |
|
|
Master Caution | Home | N/A | N/A | — | 1000 | — | — |
|
|
Zephyr | Home | N/A | N/A | 55 | 250 | 12 | — |
aN/A: not applicable.
bNot available.
Table 4.
Technical characteristics per device (intermittent recording devices).
| Device | Use environment | Multiple measures | Requires setup | Ingress protection rating | Sampling rate (Hz) | Resolution (bits) | Signal bandwidth (Hz) | ||||||||
| Handheld | |||||||||||||||
|
|
Afib Alert | Home | ✓ | ✓ | —a | — | — | — | |||||||
|
|
Beurer ME 80 | Home | ✓ | N/Ab | — | 256 | — | — | |||||||
|
|
Cardea 3 | Home and clinical | N/A | N/A | — | 500 | — | 1-75 | |||||||
|
|
Cardio-B Palm ECG | Home | ✓ | N/A | — | — | — | 1-40 | |||||||
|
|
Coala Heart Monitor | Home and clinical | ✓ | N/A | 22 | 1000 | 24 | — | |||||||
|
|
ECG Check | Home | N/A | N/A | — | 200 | — | 0.5-25 | |||||||
|
|
Eko DUO | Clinical | N/A | N/A | 55 | — | — | — | |||||||
|
|
Impulse ECG | Home | N/A | N/A | — | — | — | — | |||||||
|
|
Istel HR-2000 | Home | N/A | N/A | 22 | 160, 320, and 640 | 24 | 0.05-32, 0.05-35, and 0.05-130 | |||||||
|
|
KardiaMobile | Home | N/A | N/A | 64 | 300 | 16 | 0.5-40 | |||||||
|
|
KardiaMobile 6L | Home | ✓ | N/A | 22 | 300 | 16 | 0.5-40 | |||||||
|
|
KardiaMobile Card | Home | N/A | N/A | X8 | 300 | 16 | 0.5-40 | |||||||
|
|
MyDiagnostick | Clinical | N/A | N/A | 24 | — | — | — | |||||||
|
|
HeartCheck PEN | Home | ✓ | N/A | — | 250 | — | 1-40 | |||||||
|
|
Zenicor-ECG | Home | N/A | N/A | 22 | — | — | — | |||||||
|
|
Heartscan HCG-801 | Home | ✓ | N/A | 20 | 125 | — | 0.05-40 | |||||||
| Patch | |||||||||||||||
|
|
VitalPatch RTM | Home and clinical | ✓ | ✓ | 24 | — | — | — | |||||||
| Wearable | |||||||||||||||
|
|
Nuubo ECG Vest | Home and clinical | N/A | N/A | 22 | 250 | — | 0-65 | |||||||
|
|
SmartHeart | Home | N/A | ✓ | — | — | — | 0.05-150 | |||||||
|
|
Apple Watch | Home | N/A | N/A | — | — | — | — | |||||||
|
|
Move ECG | Home | N/A | N/A | — | — | — | — | |||||||
|
|
Galaxy Active Watch (2 and 3) | Home | N/A | N/A | — | — | — | — | |||||||
|
|
Venu 2 Plus Smartwatch | Home | N/A | N/A | — | — | — | — | |||||||
|
|
Fitbit Charge 5 | Home | N/A | N/A | — | — | — | — | |||||||
aNot available.
bN/A: not applicable.
Clinical Evidence
Through the individual searches with regard to all 58 devices for which we were able to find characteristics from manufacturer websites, we found articles (n=36) that covered 22 (38%) of the devices, demonstrating their capabilities to record cardiac disorders. Of these 22 devices, 8 (36%) are handheld devices, 8 (36%) are patch devices, and 6 (28%) are wearables (Tables 5 and 6).
Table 5.
Summary of device study objectives (continuous recording devices).
| Device, study, heart disorder or ECGa abnormality | Comparators | Participants, n | Sensitivity, % | Specificity, % | Accuracy, % | ||||||||
| Patch | |||||||||||||
|
|
D-Heart | ||||||||||||
|
|
|
Maurizi et al [14] | |||||||||||
|
|
|
|
Quality recordings | 12-lead device | 117 | —b | — | — | |||||
|
|
Bittium Faros 180 | ||||||||||||
|
|
|
Müller et al [15] | |||||||||||
|
|
|
|
AFc | PPGd devices | 144 | 90 | 84.2 | — | |||||
|
|
R.Test Evolution 4 | ||||||||||||
|
|
|
Eysenck et al [16] | |||||||||||
|
|
|
|
AF | Zio XT, Nuubo ECG Vest, and BardyDX CAM | 21 | — | — | 30-second recording: 50.8; 6-minute recording: 77.3 | |||||
|
|
CardioSTAT | ||||||||||||
|
|
|
Nault et al [17] | |||||||||||
|
|
|
|
AF | 12-lead Holter | 212 | — | — | 99 | |||||
|
|
Zio XT | ||||||||||||
|
|
|
Eysenck et al [16] | |||||||||||
|
|
|
|
AF | Zio XT, Nuubo ECG Vest, BardyDX CAM, and R.Test Evolution 4 | 21 | — | — | 30-second recording: 86.7; 6-minute recording: 80.8 | |||||
|
|
|
Hannun et al [18] | |||||||||||
|
|
|
|
AF and flutter | — | 53,549 | 71 | 94.1 | — | |||||
|
|
|
|
AVBe | — | 53,549 | 73.1 | 98.1 | — | |||||
|
|
|
|
Bigeminy | — | 53,549 | 82.9 | 99.6 | — | |||||
|
|
|
|
EARf | — | 53,549 | 38 | 99.3 | — | |||||
|
|
|
|
IVRg | — | 53,549 | 61.1 | 99.1 | — | |||||
|
|
|
|
Junctional rhythm | — | 53,549 | 63.4 | 98.4 | — | |||||
|
|
|
|
Noise | — | 53,549 | 74.9 | 98.3 | — | |||||
|
|
|
|
Sinus rhythm | — | 53,549 | 90.1 | 85.9 | — | |||||
|
|
|
|
SVTh | — | 53,549 | 40.8 | 98.3 | — | |||||
|
|
|
|
Ventricular tachycardia | — | 53,549 | 65.2 | 99.6 | — | |||||
|
|
|
|
Wenckebach | — | 53,549 | 54.1 | 98.6 | — | |||||
|
|
BardyDX CAM | ||||||||||||
|
|
|
Eysenck et al [16] | |||||||||||
|
|
|
|
AF | Zio XT, Nuubo ECG Vest, BardyDX CAM, and R.Test Evolution 4 | 21 | — | — | 30-second recording: 99.9; 6-minute recording: 95.3 | |||||
|
|
ATPatch | ||||||||||||
|
|
|
Choi et al [19] | |||||||||||
|
|
|
|
Quality recordings | 12-lead device | 10 | — | — | 0.1 | |||||
|
|
BodyGuardian | ||||||||||||
|
|
|
Bruce et al [20] | |||||||||||
|
|
|
|
Quality recordings | — | 10 | 97 | 77 | — | |||||
| Wearable | |||||||||||||
|
|
Amazfit | ||||||||||||
|
|
|
Chen et al [21] | |||||||||||
|
|
|
AF | PPG devices | 451 | 87.3 | 99.2 | 94.76 | ||||||
|
|
|
Zhang et al [22] | |||||||||||
|
|
|
|
Rhythm disorders and AF | 12-lead device | 291 | 93.3 | 95.3 | — | |||||
|
|
|
|
PACi | 12-lead device | 291 | 84 | 96.6 | — | |||||
|
|
|
|
PVCj | 12-lead device | 291 | 89.3 | 93.9 | — | |||||
|
|
|
|
First-degree AVB | 12-lead device | 291 | 32.1 | 97.7 | — | |||||
aECG: electrocardiogram.
bNot available.
cAF: atrial fibrillation.
dPPG: photoplethysmography.
eAVB: atrioventricular block.
fEAR: ectopic atrial rhythm.
gIVR: idioventricular rhythm.
hSVT: supraventricular tachycardia.
iPAC: premature atrial contraction.
jPVC: premature ventricular contraction.
Table 6.
Summary of device study objectives (intermittent recording devices).
| Device, study, heart disorder or ECGa abnormality | Comparators | Participants, n | Sensitivity, % | Specificity, % | Accuracy, % | ||||||||
| Handheld | |||||||||||||
|
|
Beurer ME 80 | ||||||||||||
|
|
|
Nigolian et al [23] | |||||||||||
|
|
|
|
AFb | 12-lead device | 16 | 100 | 94 | 96 | |||||
|
|
|
|
AVBc | 12-lead device | 13 | 85 | 97 | 94 | |||||
|
|
|
|
LBBBd | 12-lead device | 7 | 71 | 100 | 96 | |||||
|
|
|
|
RBBBe | 12-lead device | 10 | 90 | 100 | 98 | |||||
|
|
|
|
LVHf | 12-lead device | 5 | 80 | 100 | 98 | |||||
|
|
|
|
ST-segment elevation | 12-lead device | 11 | 64 | 93 | 87 | |||||
|
|
|
|
ST-segment depression | 12-lead device | 13 | 54 | 95 | 85 | |||||
|
|
|
|
Prolonged QTc | 12-lead device | 4 | 50 | 91 | 88 | |||||
|
|
Coala Heart Monitor | ||||||||||||
|
|
|
Insulander et al [24] | |||||||||||
|
|
|
|
AF | —g | 1000 | 95.1 | 97.6 | 97.3 | |||||
|
|
ECG Check | ||||||||||||
|
|
|
Aljuaid et al [25] | |||||||||||
|
|
|
|
AF | Holter | 90 | 100 | 97 | — | |||||
|
|
Eko DUO | ||||||||||||
|
|
|
Bokma et al [26] | |||||||||||
|
|
|
|
CHDh | 12-lead device, Move ECG, KardiaMobile | 176 | 100 | 99 | — | |||||
|
|
|
Bachtiger et al [27] | |||||||||||
|
|
|
|
LVEFi of ≤40% | 12-lead device | 1050 | 91.9 | 80.2 | — | |||||
|
|
Istel HR-2000 | ||||||||||||
|
|
|
Krzowski et al [28] | |||||||||||
|
|
|
|
Sinus rhythm | 12-lead device and KardiaMobile | 98 | 91.5 | 84.6 | — | |||||
|
|
|
|
AF | 12-lead device and KardiaMobile | 98 | 77.3 | 98.7 | — | |||||
|
|
KardiaMobile | ||||||||||||
|
|
|
Krzowski et al [28] | |||||||||||
|
|
|
|
Sinus rhythm | 12-lead device and Istel HR-2000 | 98 | 88.1 | 89.7 | — | |||||
|
|
|
|
AF | 12-lead device and Istel HR-2000 | 98 | 86.4 | 97.4 | — | |||||
|
|
|
Palà et al [29] | |||||||||||
|
|
|
|
AF | WatchBP, MyDiagnostick, and FibriCheck | 359 | 80 | 95.5 | — | |||||
|
|
|
Lau et al [30] | |||||||||||
|
|
|
|
AF | 12-lead device | 109 | 97.5 | 92 | 94.5 | |||||
|
|
|
Desteghe et al [31] | |||||||||||
|
|
|
|
AF (cardiology ward) | 12-lead device and MyDiagnostick | 445 | 54.5 | 97.5 | — | |||||
|
|
|
|
AF (geriatric ward) | 12-lead device and MyDiagnostick | 445 | 78.9 | 97.9 | — | |||||
|
|
|
Bokma et al [26] | |||||||||||
|
|
|
|
CHD | 12-lead device, Move ECG, and Eko DUO | 176 | 100 | 99 | — | |||||
|
|
|
Scholten et al [32] | |||||||||||
|
|
|
|
AF | 12-lead device, Apple Watch, and Move ECG | 220 | 99 | 97 | — | |||||
|
|
|
Bumgarner et al [33] | |||||||||||
|
|
|
|
AF | 12-lead device | 100 | 99 | 83 | — | |||||
|
|
|
Ford et al [34] | |||||||||||
|
|
|
|
AF | Apple Watch | 125 | 94 | 90 | 91 | |||||
|
|
|
Wasserlauf et al [35] | |||||||||||
|
|
|
|
AF | Implantable Cardiac Monitor | 24 | 83.3 | 83.3 | — | |||||
|
|
|
Himmelreich et al [36] | |||||||||||
|
|
|
|
AF and AFLj | 12-lead device | 23 | 100 | 100 | — | |||||
|
|
|
|
AF | 12-lead device | 44 | 90.9 | 93.5 | — | |||||
|
|
|
|
AF | 12-lead device | 28 | 46.4 | 100 | — | |||||
|
|
MyDiagnostick | ||||||||||||
|
|
|
Tieleman et al [37] | |||||||||||
|
|
|
|
AF | 12-lead device | 192 | 100 | 95.9 | — | |||||
|
|
|
Palà et al [29] | |||||||||||
|
|
|
|
AF | WatchBP, KardiaMobile, and FibriCheck | 359 | 76.9 | 97.1 | — | |||||
|
|
|
Verbiest-van Gurp et al [38] | |||||||||||
|
|
|
|
AF | 12-lead device and WatchBP | 4339 | 90.1 | 97.9 | — | |||||
|
|
|
Vaes et al [39] | |||||||||||
|
|
|
|
AF | 12-lead device | 191 | 94 | 93 | — | |||||
|
|
|
Yeo et al [40] | |||||||||||
|
|
|
|
AF | 12-lead device | 671 | 100 | 96.2 | — | |||||
|
|
|
Karregat et al [41] | |||||||||||
|
|
|
|
Paroxysmal AF | 12-lead Holter | 270 | 66.7 | 68.8 | — | |||||
|
|
|
Desteghe et al [31] | |||||||||||
|
|
|
|
AF (cardiology ward) | 12-lead device and KardiaMobile | 445 | 81.8 | 94.2 | — | |||||
|
|
|
|
AF (geriatric ward) | 12-lead device and KardiaMobile | 445 | 89.5 | 95.7 | — | |||||
|
|
Zenicor-ECG | ||||||||||||
|
|
|
Doliwa et al [42] | |||||||||||
|
|
|
|
AF | 12-lead device | 49 | 96 | 92 | — | |||||
| Wearable | |||||||||||||
|
|
Apple Watch | ||||||||||||
|
|
|
Abu-Alrub et al [43] | |||||||||||
|
|
|
|
AF | Galaxy Watch Active 3, and Move ECG | 100 | 87 | 86 | — | |||||
|
|
|
Nasarre et al [44] | |||||||||||
|
|
|
|
Cardiac abnormalities and cardiac arrest | 12-lead device | 67 | 100 | 100 | — | |||||
|
|
|
|
Brugada Syndrome | 12-lead device | 67 | 92 | 100 | — | |||||
|
|
|
|
Long QT | 12-lead device | 67 | 80 | 100 | — | |||||
|
|
|
|
HCMk | 12-lead device | 67 | 92 | 85 | — | |||||
|
|
|
|
ARVC/Dl | 12-lead device | 67 | 100 | 99 | — | |||||
|
|
|
Caillol et al [45] | |||||||||||
|
|
|
|
Bradyarrhythmias | 12-lead device | 40 | 96 | 91 | — | |||||
|
|
|
|
Tachyarrhythmias | 12-lead device | 40 | 25 | 99 | — | |||||
|
|
|
|
Cardiac Ischemia | 12-lead device | 40 | 7 | 100 | — | |||||
|
|
|
Spaccarotella et al [46] | |||||||||||
|
|
|
|
Measurements of the QT interval | 12-lead device | — | 69 | 88 | — | |||||
|
|
|
Scholten et al [32] | |||||||||||
|
|
|
|
AF | 12-lead device, Move ECG, and KardiaMobile | 220 | 96 | 94 | — | |||||
|
|
|
Ford et al [34] | |||||||||||
|
|
|
|
AF | KardiaMobile | 125 | 68 | 93 | 87 | |||||
|
|
Move ECG | ||||||||||||
|
|
|
Bokma et al [26] | |||||||||||
|
|
|
|
CHD | 12-lead device, KardiaMobile, and Eko DUO | 176 | 100 | 98 | — | |||||
|
|
Abu-Alrub et al [43] | ||||||||||||
|
|
|
AF | Apple Watch, Galaxy Watch Active 3 | 100 | 88 | 81 | — | ||||||
|
|
Scholten et al [32] | ||||||||||||
|
|
|
AF | 12-lead device, Apple Watch, and KardiaMobile | 220 | 95 | 95 | — | ||||||
|
|
Nuubo ECG Vest | ||||||||||||
|
|
|
Eysenck et al [16] | |||||||||||
|
|
|
|
AF | Zio XT, Nuubo ECG Vest, BardyDX CAM, and R.Test Evolution 4 | 21 | — | — | 30-second recording: 97; 6-minute recording: 89.7 | |||||
|
|
Fitbit | ||||||||||||
|
|
|
Rajagopalan [47] | |||||||||||
|
|
|
|
AF | 12-lead device | 475 | 98.7 | 100 | — | |||||
|
|
Galaxy Active Watch | ||||||||||||
|
|
|
Yang [48] | |||||||||||
|
|
|
|
AF | 12-lead device | 544 | 98.1 | 100 | — | |||||
aECG: electrocardiogram.
bAF: atrial fibrillation.
cAVB: atrioventricular block.
dLBBB: left bundle branch block.
eRBBB: right bundle branch block.
fLVH: left ventricular hypertrophy.
gNot available.
hCHD: congenital heart defect.
iLVEF: low ventricular ejection fraction.
jAFL: atrial flutter.
kHCM: hypertrophic cardiomyopathy.
lARVC/D: arrhythmogenic right ventricular cardiomyopathy/dysplasia.
Of the 36 articles, 24 (66%) evaluated the devices’ capabilities to diagnose rhythm disorders [15-17,21,24,25,29-35,37-43,49-56], whereas 7 (20%) reported on capabilities to detect rhythm disorders and other heart conditions, such as cardiomyopathy, conduction disorders, and cardiac ischemia [18,22,23,28,36,45,57]. Finally, for 3 (14%) of the 22 devices, the evidence found was regarding the evaluation of the quality of the signals recorded by them [14,19,20].
In 23 (63%) of the 36 articles, the feasibility to detect AF was studied. Studies on the Istel HR-2000 (Diagnosis SA), AliveCor heart monitor (AliveCor Inc), MyDiagnostick (MyDiagnostick Medical BV), Apple Watch (Apple Inc), Amazfit (Zepp Health Corporation), and Move ECG (Withings France SA) have reported sensitivities of <94% (range 54.5%-94%) for the detection of AF [21,28,29,31,34,43]. The Apple Watch, KardiaMobile (AliveCor), Bittium Faros 180 (Bittium), and Move ECG studies reported specificities of <90% (range 81%-86%) for AF detection [15,33,43]. For other rhythm disorders, the studies reported specificities of >85% (range 85.9%-99,6%), whereas the sensitivities ranged from 25% to 96% [18,22,45]. For cardiac ischemia detection, the Apple Watch and Beurer ME 80 (Beurer GmbH) were evaluated, and the studies reported specificities of >90% (range 93%-100%) and sensitivities of <65% (range 7%-64%); these studies included 40 and 13 participants, respectively [23,45].
Discussion
Overview
The aim of this review was to provide an overview of the mobile ECG devices available in the market, including the technology used, their clinical application, and the published clinical evidence. In this review, we have identified 58 mobile ECG devices with available manufacturer information and observed that the main intended use of these devices is the detection of rhythm disorders, more specifically AF. We analyzed the relation of the technical characteristics and how these design decisions influence the capabilities of the devices to record cardiac disorders. In terms of clinical evidence, upon reviewing 2 FDA databases, we noted that most of the devices (33/58, 57%) did not require clinical validation because they have been found to be equivalent to other ECG devices in the market or to their previous versions. The published studies we found focused on the evaluation of the devices for the detection of rhythm disorders, more specifically AF.
Clinical Purpose and Technical Capabilities of ECG Devices
To detect rhythm disorders, especially AF, one may only need to be able to capture basic heart rhythms. However, according to the European Society of Cardiology guidelines for the diagnosis of AF, an irregular R-R interval, absence of distinct repeating P waves, and irregular atrial activations indicate AF [58]. For home monitoring devices, it is not completely clear whether the detection of AF is solely based on the detection of irregular R-R intervals or whether they include P-wave detection as well.
For the diagnosis of AF, a 12-lead ECG is recorded only if the physician suspects AF, and the diagnosis will be provided if the ECG records an AF episode [59]. With our analysis, it is also possible to note that continuous monitoring devices show better performance in terms of sensitivity and specificity than intermittent monitoring devices. By using continuous recording systems, the chances of recording AF events are high, but it is necessary to consider that the amounts of data generated while continuous recordings could make it cumbersome to see when the AF events have been detected, as recordings are performed over periods higher than 24 hours.
For wearable devices with continuous recording systems, 16% of the recordings have been considered inconclusive by cardiologists, according to third-party comparisons [60]. It has also been reported that owing to signal processing and algorithm settings, devices such as the Apple Watch are limited in terms of diagnosing and misdiagnosing AF in comparison with medical grade devices [61]. This brings into question the value of using wearable devices with continuous recording systems for early detection of AF because these devices add more challenges to already stressed health care systems and clinical workflows [12].
Considering the clinical importance of the detection of AF as well as cardiac ischemia, the difference in the number of devices that can detect either condition is striking. The overwhelming number of devices is aimed at detecting AF, whereas no devices are intended for the detection of ischemia, and only 2 (9%) of the 22 devices have published studies for the detection of ST-segment elevation. From a technical point of view, many of the devices may not be completely limited in their capability to detect ischemia.
To detect other cardiac disorders such as ischemia, one also needs to be able to capture morphological details of the ECG. For either case, there are some technical challenges that require further discussion and are discussed in the following paragraphs.
A low number of electrodes and limited measurement area impose restrictions on the detection of all heart diseases [45,62,63]. Caillol et al [45] have shown how single-lead devices such as the Apple Watch could miss ST-segment elevation caused by ischemia in specific parts of the heart. The authors were able to demonstrate that ST-segment elevations and depressions were visible for lateral and inferior infarction, but when they attempted to record an anterior infarction, no ST-segment elevations or depressions were visible on the recordings [45]. Samol et al [64] demonstrated that performing ECG recordings with the Apple Watch (placed on the chest) allowed 6 precordial channels to be recorded in a serial manner [64]. A study using the AliveCor heart monitor showed that with only 2 electrodes, the device is capable of recording ST-segment elevations, once again by performing serial recordings [65,66]. The other device studied for ischemia detection (Beurer ME 80) also requires serial measurements. Considering the acute nature of the condition, we do not see any feasible application of this method of measuring ECGs for ischemia detection.
There is a need for ECG devices intended to detect ischemic diseases, but, as our search has shown, there are no devices intended for this purpose available in the market. There is evidence showing that methods for measurements and technologies are moving toward the detection of ischemic diseases; for example, the RELF method (in which the RELF leads record the voltage differences from the right shoulder [R] to an exploratory electrode [E], to the left shoulder [L], and to the left iliac crest [F]) has been developed using a 3-lead detection system, and when this device was tested for the detection of acute coronary artery occlusion, it showed a specificity of 96% during daily life recordings, and when ST-segment elevation myocardial infarction (STEMI) criteria on a 12-lead ECG device were observed during the interventions, the RELF method had a sensitivity of 100% [67,68].
For heart diseases other than rhythm disorders, single-lead devices allow preliminary recordings to be made, but to obtain a deeper understanding and to allow physicians to provide a diagnosis, more information should be recorded. However, one can imagine that performing studies on conditions such as myocardial infarction in acute settings, is more complex. Furthermore, the approval of a device aiming to detect a high-risk cardiac disorder would require compliance with more stringent requirements; for example, upon the detection of heart disorders such as acute coronary syndrome, it is necessary to provide rapid attention and therapy for patients. If such disorders are undetected, the life and quality of life of patients will be highly affected.
Other characteristics such as signal processing and acquisition may affect the capabilities of the device to detect various cardiac disorders. Applying filters to the captured ECG affects the waveform, which could lead to misinterpretation and misdiagnosis. Signal processing is a design characteristic that has been specified in the International Electrotechnical Commission (IEC) standard [69]. For the detection and interpretation of ischemic diseases, devices need to be able to record changes in the ST segment. The suggested update on the current standard for ECG devices specifies that devices that contain a filter with a high-pass cutoff frequency of 0.67 Hz can detect ST-segment deviations as long as filters are not modifying the phase of the ECG signal [70]. Bailey et al [71] have performed measurements to demonstrate that zero-phase filters indeed do not modify the phase of the ECG recordings. For 45% (26/58) of the devices that specify signal bandwidth, the lower limit ranges from 0.03 to 1 Hz, whereas the upper limit ranges from 25 to 1000 Hz. According to the suggested update of the standard, 17 (65%) of the 26 devices would be suitable for ischemia detection. In addition, the applied filter (zero phase or not) will influence the ability to detect ischemia; however, this is not specified for any of the devices.
Regarding signal acquisition, one of the influencing factors is the sampling frequency, which refers to the time interval of the discrete digital points transformed from the cardiac biopotentials [72]. In general, mobile ECG devices have a sample frequency of at least 250 samples per second. The applicable standard for home use does not specify the required sample frequencies [73]. The general standard for ECG devices used in the clinical environment recommends sample frequencies of at least 500 samples per second (there is no specification for devices used at home) [69,74]. According to Kligfield et al [72], most of the diagnostic information in the ECG is contained below 100 Hz in adults. We noted that all devices analyzed are capable of recording cardiac biopotentials at adequate sample rates.
Besides sampling frequency, signal resolution influences the quality of the ECG. The signal resolution refers to how biopotential signals are expressed in digits into which the input signal can be converted, based on the number of discrete steps. When a device has a resolution of 16 bits, it means that the number of measured steps between the minimum and maximum values that can be recorded is 216=65,536. In other words, if a device can only capture signals between −2.5 V and +2.5 V (5 V at full-scale deflection), the detail that can be recorded at a resolution of 24 bits is 0.298 µV, also referred to as the least significant bit (LSB). Thus, the combination of full-scale deflection and resolution determine how little of the heart biopotentials can be captured. In fact, the question is this: what is the maximum value of the LSB that provides enough detail on the morphology of the ECG signals (the standard defines an LSB of ≤1 µV [69])?
The relationship of sampling frequency and signal resolution is relevant for diagnosis because of the added information that these features provide to physicians; for example, regarding the relationship of fragmented QRS (fQRS) and heart disorders, fQRS can only be observed when the sample rate and resolution are sufficient to capture the detailed signals. It has been demonstrated that the use of fQRS is a key feature for detecting myocardial scars in patients [75-77].
Device Features and Technical Characteristics
It is observed that the prioritization of a device’s characteristics depends upon its intended use. For handheld home-use devices, usability and easy-of-use characteristics are a priority in comparison with patches, where the recording is a priority and the comfort of the patient is secondary.
We believe that the prioritization regarding users starts from design decisions, such as the selection of electrodes. Adhesive patch devices use wet electrodes, whereas handheld devices and wearables use a mix of electrodes, ranging from embedded metal dry electrodes to textile electrodes that do not require skin preparation. According to electrode comparisons and reviews, wet gel electrodes provide good signal quality for short-term recordings because the gel improves the electrode-skin contact, allowing the formation of a conductive path between skin and electrode [78,79]. However, it has also been noted that long-term use of these types of electrodes can cause skin irritation, and the signal quality decreases as the conductive gels dry out [80]. Hickey et al [81] have reported that by using devices that include multiple adhesive electrodes or patch-type devices, user compliance is diminished owing to application and wearing complexity. Dry electrodes do not require a medium for conduction because the substrate is in direct contact with the skin. This metal-skin interface has been reported to influence the quality of recorded signals owing to movement artifact and charge sensitivity [79]. When biocompatible, the use of dry electrodes prevents undesirable chemical effects and skin irritation on patients [78,82].
For home-use medical electrical devices, their enclosures should provide the user protection against access of hazardous parts inside the enclosure and against harmful effects owing to ingress of water [83]. To designate a device’s degree of protection, the IP rating is disclosed. Devices must comply with the minimum IP rating of 22, which is applicable to medical home-use and health care devices [73]. Compliance with the features specified in the standard helps to guarantee the essential device performance as well as basic device safety to users and patients. Of the 38 devices meant for home use, only 14 (37%) have disclosed their IP rating in compliance with the applicable IEC standard. For the remaining devices (24/38, 63%), the IP rating has not been registered on the available device patient information; however, this requirement might be covered by the checklist of general safety and performance requirements. Of note, the devices carry the Conformité Européenne (CE) marking and meet the requirements specified by IEC standards [73,84].
Clinical Evidence
Of the 58 devices with available manufacturer information, only 18 (31%) have published feasibility and reliability studies on diagnosing heart conditions. Patch devices are used as the benchmark for comparison in clinical studies, specifically if these devices have a continuous recording function (Holter devices). As for other devices with published clinical evidence, these devices perform recordings in positions that are not similar to those of the 12-lead clinical ECG. The studies are part of the clinical evidence on the route to compliance with medical device regulations for clinical testing to show the capability of the device to achieve its intended purpose, clinical performance, and benefits [85]. Upon performing the search in the FDA 510(k) Premarket Notification database, we noted that most devices do not include clinical evidence in the submissions because they show evidence of their similarities to other devices in the market or previous versions of the device in question. However, for recently released devices, such as the Apple Watch, we were able to see detailed clinical evidence summaries. We believe that because compliance requirements for new devices have become more stringent, we can expect to see more clinical evidence for new devices. Our belief is also based on the changes made to the European Union regulations governing medical devices; as other researchers have pointed out, the new regulations focus on the need for more clinical data for all medical devices [86,87].
In 2017, the medical device directive was updated to a new version, which is more stringent and aims to improve the safety and effectiveness of medical devices. One of the main changes made to the regulations concerns the additional emphasis placed on the clinical evidence of medical devices [83] to ensure their safety and effectiveness [85]. For devices without available clinical evidence, we could argue that for them to be available in the market, an important step is the clinical evaluation. Before 2017, owing to their similarities to other products already available in the market, these devices’ clinical investigations could have been based on the clinical evidence presented by similar devices. This is specifically the case if these devices have been certified before May 2021, when the European Union Medical Device Regulation became fully applicable. Nowadays, another source of information regarding the performance and safety of medical devices as well as the risks involved in using them is the postmarket surveillance; however, these activities are normally confidential, which could be the reason for the lack of available public clinical data for these devices [88].
The studies have shown promising evidence of the capabilities of the ECG devices, but they have been tested on small populations, which is a limitation in terms of investigating their full functionality and use in broader scenarios. As has been specified in the guidance regarding sufficient clinical evidence, these types of publications could be sufficient if there are no concerns regarding the safety of the patient and performance of the device [88].
For handheld devices, another observation concerned the design of the studies owing to the use characteristics of these devices. In the study by Magnusson et al [89], the recordings were limited and scheduled at certain times of the day, limiting the comparison with patch devices, which were used on a continuous basis, whereas in another study, the approach was based on patient management, with patients instructed to perform recordings when symptoms were present, which, as Doliwa et al [90] have shown, is an improvement with regard to detecting paroxysmal AF in patients who have had a recent stroke. These data were confirmed by other studies with similar approaches and outcomes [41,91]. The design of clinical studies should take into account user case scenarios that approximate to the intended use of the device in daily life. By designing studies based on user scenarios, it would be possible to compare the capabilities of handheld devices with those of patch devices when their performance is evaluated for the detection of symptomatic cardiac diseases. There is a marked lack of studies for the vast majority of the devices (35/58, 60%) included in this review, with, as mentioned previously, only 38% (22/58) of the devices having been investigated in studies regarding their capabilities to detect cardiac disorders.
Limitations
To the best of our ability, we tried to perform an exhaustive search to identify all available devices; however, we cannot guarantee that all were indeed identified. In terms of the analysis performed, we were not able to summarize all technical and clinical information related to the devices owing to the lack of availability of data for such devices.
As this review shows, there is a wide range of mobile ECG devices available for home use, but as mentioned, technologies are moving toward the use of other sensors. One limitation of this review is that we have not analyzed other devices containing other types of sensors used for cardiac monitoring, such as photoplethysmography or consumer electronics not intended for detection of cardiac disorders such as the Fitbit (Google LLC); however, this was a choice because we decided to include only ECG devices.
Finally, in our analysis, we decided not to include the fact that for some devices (ie, KardiaMobile and Fitbit), users are required to sign up for subscription services to obtain further diagnosis of ECGs. We decided not to analyze the availability of these types of services because they depend on location, and prices may change over time.
Future Perspectives
We believe that the inclusion of other sensors will help to improve ECG devices’ capabilities to detect disorders. As noted by Sana et al [92], certain heart conditions are difficult to detect with ECG recordings [92]. Structural heart abnormalities can potentially be diagnosed with the help of other sensors (eg, by analyzing sound, accelerometer, and gyroscope recordings), which suggests that phonocardiograms or seismocardiograms could be added to the ECG recording [92]. We noted that, of the 58 devices included in this review, a few (n=2, 3%), such as the Eko DUO (Eko Devices Inc) and Coala Heart Monitor (Coala Life AB), already include these features. We also noted that there is a trend toward acquiring more information on the heart. During the systematic search, we came across prototypes, which included microphones, accelerometers, and gyroscope sensors, that are currently in development and in early stages of testing [93-97].
Conclusions
In this review, we have explored the current scope of mobile ECG devices available in the market for use at home. We have summarized the usability, technical, and clinical characteristics that could allow selection of an ECG device for patients and home use. Devices available in the market are mainly intended for the detection of arrhythmias, more specifically AF, but no devices are intended for the detection of cardiac ischemic disorders. We showed that this is due to the capabilities of the devices, such as the limited measurement areas, limited number of electrodes, and recording capabilities. Clinical research concerning the devices has been primarily focused on rhythm disorders, with few studies focusing on other heart disorders, involving small test populations. Trends in the development of mobile ECG devices are inclusion of other sensors on ECG devices to increase cardiac information collected by them and a movement toward the inclusion of embedded algorithms, allowing the diagnosing of rhythm disorders.
Acknowledgments
This research was funded by ZonMw Innovative Medical Devices Initiative-Dutch CardioVascular Alliance—Heart for Sustainable Care (104021004) and the Dutch Heart Foundation (2019B011).
Abbreviations
- AF
atrial fibrillation
- CE
Conformité Européenne
- ECG
electrocardiogram
- FDA
Food and Drug Administration
- fQRS
fragmented QRS
- IEC
International Electrotechnical Commission
- IP
ingress protection
- LSB
least significant bit
- MeSH
medical subject headings
- STEMI
ST-segment elevation myocardial infarction
Footnotes
Conflicts of Interest: PAD is cofounder of HeartEye BV. All other authors declare no other conflicts of interest.
References
- 1.Cardiovascular diseases (CVDs) World Health Organization. 2021. Jun, [2021-06-04]. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015 Apr 02;372(14):1333–41. doi: 10.1056/NEJMoa1406656. https://europepmc.org/abstract/MED/25830423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meek S, Morris F. ABC of clinical electrocardiography. introduction. I-leads, rate, rhythm, and cardiac axis. BMJ. 2002 Feb 16;324(7334):415–8. doi: 10.1136/bmj.324.7334.415. https://europepmc.org/abstract/MED/11850377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall ME, Hall JE, Guyton A. Fundamentals of electrocardiography. In: Hall JE, editor. Guyton and Hall Textbook of Medical Physiology. 14th edition. Philadelphia, PA, USA: Saunders; 1956. pp. 135–41. [Google Scholar]
- 5.Holst H, Ohlsson M, Peterson C, Edenbrandt L. A confident decision support system for interpreting electrocardiograms. Clin Physiol. 1999 Sep;19(5):410–8. doi: 10.1046/j.1365-2281.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 6.Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Recording of bipolar multichannel ECGs by a smartwatch: modern ECG diagnostic 100 years after Einthoven. Sensors (Basel) 2019 Jun 30;19(13):2894. doi: 10.3390/s19132894. https://www.mdpi.com/resolver?pii=s19132894 .s19132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung E, Järvelin MR, Doshi RN, Shinbane JS, Carlson SK, Grazette LP, Chang PM, Sangha RS, Huikuri HV, Peters NS. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol. 2015 May 27;6:149. doi: 10.3389/fphys.2015.00149. https://europepmc.org/abstract/MED/26074823 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019 Nov;7(11):922–32. doi: 10.1016/j.jchf.2019.08.008. https://linkinghub.elsevier.com/retrieve/pii/S2213-1779(19)30725-5 .S2213-1779(19)30725-5 [DOI] [PubMed] [Google Scholar]
- 9.Serhani MA, el Kassabi HT, Ismail H, Nujum Navaz A. ECG monitoring systems: review, architecture, processes, and key challenges. Sensors (Basel) 2020 Mar 24;20(6):1796. doi: 10.3390/s20061796. https://www.mdpi.com/resolver?pii=s20061796 .s20061796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal A, Joshi R. Portable out-of-hospital electrocardiography: a review of current technologies. J Arrhythm. 2018 Feb 23;34(2):129–38. doi: 10.1002/joa3.12035. https://europepmc.org/abstract/MED/29657588 .JOA312035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung CC, Davies B, Gibbs K, Laksman ZW, Krahn AD. Patch monitors for arrhythmia monitoring in patients for suspected inherited arrhythmia syndrome. J Cardiovasc Electrophysiol. 2021 Mar;32(3):856–9. doi: 10.1111/jce.14917. [DOI] [PubMed] [Google Scholar]
- 12.Ding EY, Marcus GM, McManus DD. Emerging technologies for identifying atrial fibrillation. Circ Res. 2020 Jun 19;127(1):128–42. doi: 10.1161/CIRCRESAHA.119.316342. https://europepmc.org/abstract/MED/32716695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Alusi MA, Ding E, McManus DD, Lubitz SA. Wearing your heart on your sleeve: the future of cardiac rhythm monitoring. Curr Cardiol Rep. 2019 Nov 25;21(12):158. doi: 10.1007/s11886-019-1223-8. https://europepmc.org/abstract/MED/31768764 .10.1007/s11886-019-1223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurizi N, Faragli A, Imberti J, Briante N, Targetti M, Baldini K, Sall A, Cisse A, Berzolari FG, Borrelli P, Avvantaggiato F, Perlini S, Marchionni N, Cecchi F, Parigi G, Olivotto I. Cardiovascular screening in low-income settings using a novel 4-lead smartphone-based electrocardiograph (D-Heart®) Int J Cardiol. 2017 Jun 01;236:249–52. doi: 10.1016/j.ijcard.2017.02.027.S0167-5273(16)33297-1 [DOI] [PubMed] [Google Scholar]
- 15.Müller C, Hengstmann U, Fuchs M, Kirchner M, Kleinjung F, Mathis H, Martin S, Bläse I, Perings S. Distinguishing atrial fibrillation from sinus rhythm using commercial pulse detection systems: the non-interventional BAYathlon study. Digit Health. 2021 May 22;7:20552076211019620. doi: 10.1177/20552076211019620. https://journals.sagepub.com/doi/10.1177/20552076211019620?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_20552076211019620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eysenck W, Freemantle N, Sulke N. A randomized trial evaluating the accuracy of AF detection by four external ambulatory ECG monitors compared to permanent pacemaker AF detection. J Interv Card Electrophysiol. 2020;57(3):361–9. doi: 10.1007/s10840-019-00515-0. https://link.springer.com/article/10.1007/s10840-019-00515-0 . [DOI] [PubMed] [Google Scholar]
- 17.Nault I, André P, Plourde B, Leclerc F, Sarrazin J, Philippon F, O'Hara G, Molin F, Steinberg C, Roy K, Blier L, Champagne J. Validation of a novel single lead ambulatory ECG monitor - Cardiostat™ - compared to a standard ECG Holter monitoring. J Electrocardiol. 2019 Mar;53:57–63. doi: 10.1016/j.jelectrocard.2018.12.011.S0022-0736(18)30567-3 [DOI] [PubMed] [Google Scholar]
- 18.Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019 Jan;25(1):65–9. doi: 10.1038/s41591-018-0268-3. https://europepmc.org/abstract/MED/30617320 .10.1038/s41591-018-0268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Kim SH, Lee W, Kang SH, Yoon CH, Youn TJ, Chae IH. Comparison of continuous ECG monitoring by wearable patch device and conventional telemonitoring device. J Korean Med Sci. 2020 Nov 16;35(44):e363. doi: 10.3346/jkms.2020.35.e363. https://jkms.org/DOIx.php?id=10.3346/jkms.2020.35.e363 .35.e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce CJ, Ladewig DJ, Somers VK, Bennet KE, Burrichter S, Scott CG, Olson LJ, Friedman PA. Remote electrocardiograph monitoring using a novel adhesive strip sensor: a pilot study. World J Cardiol. 2016 Oct 26;8(10):559–65. doi: 10.4330/wjc.v8.i10.559. https://www.wjgnet.com/1949-8462/full/v8/i10/559.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou J, Huo Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm. 2020 May;17(5 Pt B):847–53. doi: 10.1016/j.hrthm.2020.01.034. https://linkinghub.elsevier.com/retrieve/pii/S1547-5271(20)30089-8 .S1547-5271(20)30089-8 [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Xian H, Chen Y, Liao Y, Zhang N, Guo X, Yang M, Wu J. The auxiliary diagnostic value of a novel wearable electrocardiogram-recording system for arrhythmia detection: diagnostic trial. Front Med (Lausanne) 2021 Jun 24;8:685999. doi: 10.3389/fmed.2021.685999. https://europepmc.org/abstract/MED/34249976 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigolian A, Dayal N, Nigolian H, Stettler C, Burri H. Diagnostic accuracy of multi-lead ECGs obtained using a pocket-sized bipolar handheld event recorder. J Electrocardiol. 2018 Mar;51(2):278–81. doi: 10.1016/j.jelectrocard.2017.11.004.S0022-0736(17)30438-7 [DOI] [PubMed] [Google Scholar]
- 24.Insulander P, Carnlöf C, Schenck-Gustafsson K, Jensen-Urstad M. Device profile of the Coala Heart Monitor for remote monitoring of the heart rhythm: overview of its efficacy. Expert Rev Med Devices. 2020 Mar;17(3):159–65. doi: 10.1080/17434440.2020.1732814. [DOI] [PubMed] [Google Scholar]
- 25.Aljuaid M, Marashly Q, AlDanaf J, Tawhari I, Barakat M, Barakat R, Zobell B, Cho W, Chelu MG, Marrouche NF. Smartphone ECG monitoring system helps lower emergency room and clinic visits in post-atrial fibrillation ablation patients. Clin Med Insights Cardiol. 2020 Jan 20;14:1179546820901508. doi: 10.1177/1179546820901508. https://journals.sagepub.com/doi/10.1177/1179546820901508?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_1179546820901508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pengel LK, Robbers-Visser D, Groenink M, Winter MM, Schuuring MJ, Bouma BJ, Bokma JP. A comparison of ECG-based home monitoring devices in adults with CHD. Cardiol Young (Forthcoming) 2022 Jul 18;:1–7. doi: 10.1017/S1047951122002244.S1047951122002244 [DOI] [PubMed] [Google Scholar]
- 27.Bachtiger P, Petri CF, Scott FE, Ri Park S, Kelshiker MA, Sahemey HK, Dumea B, Alquero R, Padam PS, Hatrick IR, Ali A, Ribeiro M, Cheung WS, Bual N, Rana B, Shun-Shin M, Kramer DB, Fragoyannis A, Keene D, Plymen CM, Peters NS. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health. 2022 Feb;4(2):e117–25. doi: 10.1016/S2589-7500(21)00256-9. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(21)00256-9 .S2589-7500(21)00256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krzowski B, Skoczylas K, Osak G, Żurawska N, Peller M, Kołtowski Ł, Zych A, Główczyńska R, Lodziński P, Grabowski M, Opolski G, Balsam P. Kardia mobile and ISTEL HR applicability in clinical practice: a comparison of Kardia Mobile, ISTEL HR, and standard 12-lead electrocardiogram records in 98 consecutive patients of a tertiary cardiovascular care centre. Eur Heart J Digit Health. 2021 May 12;2(3):467–76. doi: 10.1093/ehjdh/ztab040. https://europepmc.org/abstract/MED/36713595 .ztab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palà E, Bustamante A, Clúa-Espuny JL, Acosta J, González-Loyola F, Santos SD, Ribas-Segui D, Ballesta-Ors J, Penalba A, Giralt M, Lechuga-Duran I, Gentille-Lorente D, Pedrote A, Muñoz MÁ, Montaner J. Blood-biomarkers and devices for atrial fibrillation screening: lessons learned from the AFRICAT (Atrial Fibrillation Research in CATalonia) study. PLoS One. 2022 Aug 23;17(8):e0273571. doi: 10.1371/journal.pone.0273571. https://dx.plos.org/10.1371/journal.pone.0273571 .PONE-D-21-36016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013 Apr 30;165(1):193–4. doi: 10.1016/j.ijcard.2013.01.220.S0167-5273(13)00280-5 [DOI] [PubMed] [Google Scholar]
- 31.Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, Schurmans J, Vanduynhoven P, Dendale P, Heidbuchel H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2017 Jan;19(1):29–39. doi: 10.1093/europace/euw025.euw025 [DOI] [PubMed] [Google Scholar]
- 32.Scholten J, Jansen WP, Horsthuis T, Mahes AD, Winter MM, Zwinderman AH, Keijer JT, Minneboo M, de Groot JR, Bokma JP. Six-lead device superior to single-lead smartwatch ECG in atrial fibrillation detection. Am Heart J. 2022 Nov;253:53–8. doi: 10.1016/j.ahj.2022.06.010.S0002-8703(22)00143-0 [DOI] [PubMed] [Google Scholar]
- 33.Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018 May 29;71(21):2381–8. doi: 10.1016/j.jacc.2018.03.003. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(18)33486-7 .S0735-1097(18)33486-7 [DOI] [PubMed] [Google Scholar]
- 34.Ford C, Xie CX, Low A, Rajakariar K, Koshy AN, Sajeev JK, Roberts L, Pathik B, Teh AW. Comparison of 2 smart watch algorithms for detection of atrial fibrillation and the benefit of clinician interpretation: SMART WARS study. JACC Clin Electrophysiol. 2022 Jun;8(6):782–91. doi: 10.1016/j.jacep.2022.02.013. https://linkinghub.elsevier.com/retrieve/pii/S2405-500X(22)00220-1 .S2405-500X(22)00220-1 [DOI] [PubMed] [Google Scholar]
- 35.Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ Arrhythm Electrophysiol. 2019 Jun;12(6):e006834. doi: 10.1161/CIRCEP.118.006834. https://www.ahajournals.org/doi/abs/10.1161/CIRCEP.118.006834?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 36.Himmelreich JC, Karregat EP, Lucassen WA, van Weert HC, de Groot JR, Handoko ML, Nijveldt R, Harskamp RE. Diagnostic accuracy of a smartphone-operated, single-lead electrocardiography device for detection of rhythm and conduction abnormalities in primary care. Ann Fam Med. 2019 Sep;17(5):403–11. doi: 10.1370/afm.2438. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=31501201 .17/5/403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tieleman RG, Plantinga Y, Rinkes D, Bartels GL, Posma JL, Cator R, Hofman C, Houben RP. Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace. 2014 Sep;16(9):1291–5. doi: 10.1093/europace/euu057. https://europepmc.org/abstract/MED/24825766 .europace/euu057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbiest-van Gurp N, Uittenbogaart SB, Lucassen WA, Erkens PM, Knottnerus JA, Winkens B, Stoffers HE, van Weert HC. Detection of atrial fibrillation in primary care with radial pulse palpation, electronic blood pressure measurement and handheld single-lead electrocardiography: a diagnostic accuracy study. BMJ Open. 2022 Jun 29;12(6):e059172. doi: 10.1136/bmjopen-2021-059172. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=35768092 .bmjopen-2021-059172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaes B, Stalpaert S, Tavernier K, Thaels B, Lapeire D, Mullens W, Degryse J. The diagnostic accuracy of the MyDiagnostick to detect atrial fibrillation in primary care. BMC Fam Pract. 2014 Jun 09;15:113. doi: 10.1186/1471-2296-15-113. https://bmcfampract.biomedcentral.com/articles/10.1186/1471-2296-15-113 .1471-2296-15-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeo C, Mon AA, Tan VH, Wong K. Validation of MyDiagnostick tool to identify atrial fibrillation in a multi-ethnic Asian population. Singapore Med J (Forthcoming) 2022 Feb 24; doi: 10.11622/smedj.2022028. doi: 10.11622/smedj.2022028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karregat EP, Gurp NV, Bouwman AC, Uittenbogaart SB, Himmelreich JC, Lucassen WA, Krul SP, van Kesteren HA, Luermans JG, van Weert HC, Stoffers HE. Screening for paroxysmal atrial fibrillation in primary care using Holter monitoring and intermittent, ambulatory single-lead electrocardiography. Int J Cardiol. 2021 Dec 15;345:41–6. doi: 10.1016/j.ijcard.2021.10.021. https://linkinghub.elsevier.com/retrieve/pii/S0167-5273(21)01575-8 .S0167-5273(21)01575-8 [DOI] [PubMed] [Google Scholar]
- 42.Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J. 2009 Jun;43(3):163–8. doi: 10.1080/14017430802593435.906991912 [DOI] [PubMed] [Google Scholar]
- 43.Abu-Alrub S, Strik M, Ramirez FD, Moussaoui N, Racine HP, Marchand H, Buliard S, Haïssaguerre M, Ploux S, Bordachar P. Smartwatch electrocardiograms for automated and manual diagnosis of atrial fibrillation: a comparative analysis of three models. Front Cardiovasc Med. 2022 Feb 04;9:836375. doi: 10.3389/fcvm.2022.836375. https://europepmc.org/abstract/MED/35187135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasarre M, Strik M, Daniel Ramirez F, Buliard S, Marchand H, Abu-Alrub S, Ploux S, Haïssaguerre M, Bordachar P. Using a smartwatch electrocardiogram to detect abnormalities associated with sudden cardiac arrest in young adults. Europace. 2022 Mar 02;24(3):406–12. doi: 10.1093/europace/euab192.6361020 [DOI] [PubMed] [Google Scholar]
- 45.Caillol T, Strik M, Ramirez FD, Abu-Alrub S, Marchand H, Buliard S, Welte N, Ploux S, Haïssaguerre M, Bordachar P. Accuracy of a smartwatch-derived ECG for diagnosing Bradyarrhythmias, Tachyarrhythmias, and Cardiac Ischemia. Circ Arrhythm Electrophysiol. 2021 Jan;14(1):e009260. doi: 10.1161/CIRCEP.120.009260. [DOI] [PubMed] [Google Scholar]
- 46.Spaccarotella CA, Migliarino S, Mongiardo A, Sabatino J, Santarpia G, de Rosa S, Curcio A, Indolfi C. Measurement of the QT interval using the Apple watch. Sci Rep. 2021 May 24;11(1):10817. doi: 10.1038/s41598-021-89199-z. doi: 10.1038/s41598-021-89199-z.10.1038/s41598-021-89199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.510(k) premarket notification - Fitbit ECG app. U.S. Food and Drug Administration. 2020. [2023-03-17]. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K200948 .
- 48.510(k) premarket notification - Samsung ECG monitor app. U.S. Food and Drug Administration. 2020. [2023-03-17]. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K201168 .
- 49.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, Saliba W, Wolski K, Hussein A, Lindsay BD, Wazni OM, Tarakji KG. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm. 2018 Oct;15(10):1561–5. doi: 10.1016/j.hrthm.2018.06.037.S1547-5271(18)30661-1 [DOI] [PubMed] [Google Scholar]
- 50.Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L, Teh AW. Accuracy of a smartwatch based single-lead electrocardiogram device in detection of atrial fibrillation. Heart. 2020 May;106(9):665–70. doi: 10.1136/heartjnl-2019-316004.heartjnl-2019-316004 [DOI] [PubMed] [Google Scholar]
- 51.Zaprutko T, Zaprutko J, Baszko A, Sawicka D, Szałek A, Dymecka M, Telec W, Kopciuch D, Ratajczak P, Michalak M, Rafał D, Szyszka A, Nowakowska E. Feasibility of atrial fibrillation screening with mobile health technologies at pharmacies. J Cardiovasc Pharmacol Ther. 2020 Mar;25(2):142–51. doi: 10.1177/1074248419879089. [DOI] [PubMed] [Google Scholar]
- 52.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014 Jun;111(6):1167–76. doi: 10.1160/TH14-03-0231.14-03-0231 [DOI] [PubMed] [Google Scholar]
- 53.Koltowski L, Balsam P, Glowczynska R, Rokicki JK, Peller M, Maksym J, Blicharz L, Maciejewski K, Niedziela M, Opolski G, Grabowski M. Kardia Mobile applicability in clinical practice: a comparison of Kardia Mobile and standard 12-lead electrocardiogram records in 100 consecutive patients of a tertiary cardiovascular care center. Cardiol J. 2021;28(4):543–8. doi: 10.5603/CJ.a2019.0001. https://europepmc.org/abstract/MED/30644079 .VM/OJS/J/58839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marinucci D, Sbrollini A, Marcantoni I, Morettini M, Swenne CA, Burattini L. Artificial neural network for atrial fibrillation identification in portable devices. Sensors (Basel) 2020 Jun 24;20(12):3570. doi: 10.3390/s20123570. https://www.mdpi.com/resolver?pii=s20123570 .s20123570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013 Mar;36(3):328–33. doi: 10.1111/pace.12053. https://europepmc.org/abstract/MED/23240827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campo D, Elie V, de Gallard T, Bartet P, Morichau-Beauchant T, Genain N, Fayol A, Fouassier D, Pasteur-Rousseau A, Puymirat E, Nahum J. Atrial fibrillation detection with an analog smartwatch: prospective clinical study and algorithm validation. JMIR Form Res. 2022 Nov 04;6(11):e37280. doi: 10.2196/37280. https://formative.jmir.org/2022/11/e37280/ v6i11e37280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN, Chang PM, Saxon LA. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015 May;26(5):520–6. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 58.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GY, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb 01;42(5):373–498. doi: 10.1093/eurheartj/ehaa612.5899003 [DOI] [PubMed] [Google Scholar]
- 59.Magnusson P, Samuelsson MV, Pergolizzi Jr JV, Annabi H, LeQuang JA. Atrial fibrillation and the role of thumb ECGs. In: Min M, editor. Cardiac Pacing and Monitoring - New Methods, Modern Devices. London, UK: IntechOpen; 2019. Apr, [Google Scholar]
- 60.Mannhart D, Lischer M, Knecht S, du Fay de Lavallaz J, Strebel I, Serban T, Vögeli D, Schaer B, Osswald S, Mueller C, Kühne M, Sticherling C, Badertscher P. Clinical validation of 5 direct-to-consumer wearable smart devices to detect atrial fibrillation: BASEL wearable study. JACC Clin Electrophysiol. 2023 Feb;9(2):232–42. doi: 10.1016/j.jacep.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Turakhia MP. Wearable devices in cardiovascular medicine. In: Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 12th edition. Amsterdam, Netherlands: Elsevier; 2018. pp. 117–22. [Google Scholar]
- 62.Ben-Dov N, Gladstone DJ, Micieli A, Crystal E, Newman D. Single‑lead patch recording systems: the expanding role for long-term ECG recording systems. J Electrocardiol. 2019 Jul;55:54–8. doi: 10.1016/j.jelectrocard.2019.03.004. https://www.sciencedirect.com/science/article/abs/pii/S002207361830846X . [DOI] [Google Scholar]
- 63.Behzadi A, Sepehri Shamloo A, Mouratis K, Hindricks G, Arya A, Bollmann A. Feasibility and reliability of SmartWatch to obtain 3-lead electrocardiogram recordings. Sensors (Basel) 2020 Sep 07;20(18):5074. doi: 10.3390/s20185074. https://www.mdpi.com/resolver?pii=s20185074 .s20185074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: a new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors (Basel) 2019 Oct 10;19(20):4377. doi: 10.3390/s19204377. https://www.mdpi.com/resolver?pii=s19204377 .s19204377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muhlestein JB, Anderson JL, Bethea CF, Severance HW, Mentz RJ, Barsness GW, Barbagelata A, Albert D, Le VT, Bunch TJ, Yanowitz F, May HT, Chisum B, Ronnow BS, Muhlestein JB, Duke University Cooperative Cardiovascular Society (DUCCS) investigators Feasibility of combining serial smartphone single-lead electrocardiograms for the diagnosis of ST-elevation myocardial infarction. Am Heart J. 2020 Mar;221:125–35. doi: 10.1016/j.ahj.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Muhlestein JB, Le V, Albert D, Moreno FL, Anderson JL, Yanowitz F, Vranian RB, Barsness GW, Bethea CF, Severance HW, Ramo B, Pierce J, Barbagelata A, Muhlestein JB. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol. 2015 Mar;48(2):249–59. doi: 10.1016/j.jelectrocard.2014.11.005.S0022-0736(14)00444-0 [DOI] [PubMed] [Google Scholar]
- 67.van Heuverswyn F, de Buyzere M, Coeman M, de Pooter J, Drieghe B, Duytschaever M, Gevaert S, Kayaert P, Vandekerckhove Y, Voet J, el Haddad M, Gheeraert P. Feasibility and performance of a device for automatic self-detection of symptomatic acute coronary artery occlusion in outpatients with coronary artery disease: a multicentre observational study. Lancet Digit Health. 2019 Jun;1(2):e90–9. doi: 10.1016/s2589-7500(19)30026-3. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(19)30026-3 .S2589-7500(19)30026-3 [DOI] [PubMed] [Google Scholar]
- 68.el Haddad M, Vervloet D, Taeymans Y, de Buyzere M, Bové T, Stroobandt R, Duytschaever M, Malmivuo J, Gheeraert P. Diagnostic accuracy of a novel method for detection of acute transmural myocardial ischemia based upon a self-applicable 3-lead configuration. J Electrocardiol. 2016 Mar;49(2):192–201. doi: 10.1016/j.jelectrocard.2015.11.007.S0022-0736(15)00375-1 [DOI] [PubMed] [Google Scholar]
- 69.IEC 60601-2-25:2011: medical electrical equipment - part 2-25: particular requirements for the basic safety and essential performance of electrocardiographs. International Electrotechnical Commission. 2011. [2022-09-30]. https://webstore.iec.ch/publication/2636 .
- 70.Young B. New standards for ECG equipment. J Electrocardiol. 2019 Nov;57S:S1–4. doi: 10.1016/j.jelectrocard.2019.07.013.S0022-0736(19)30394-2 [DOI] [PubMed] [Google Scholar]
- 71.Bailey JJ, Berson AS, Garson A, Horan LG, Macfarlane PW, Mortara DW, Zywietz C. Recommendations for standardization and specifications in automated electrocardiography: bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the committee on electrocardiography and cardiac electrophysiology of the council on clinical cardiology, American Heart Association. Circulation. 1990 Feb;81(2):730–9. doi: 10.1161/01.cir.81.2.730. [DOI] [PubMed] [Google Scholar]
- 72.Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, van Herpen G, Kors JA, Macfarlane P, Mirvis DM, Pahlm O, Rautaharju P, Wagner GS, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. American College of Cardiology Foundation. Heart Rhythm Society. Josephson M, Mason JW, Okin P, Surawicz B, Wellens H. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society For Computerized Electrocardiology. Circulation. 2007 Mar 13;115(10):1306–24. doi: 10.1161/CIRCULATIONAHA.106.180200.CIRCULATIONAHA.106.180200 [DOI] [PubMed] [Google Scholar]
- 73.IEC 60601-1-11:2015/AMD1:2020: amendment 1 - medical electrical equipment - part 1-11: general requirements for basic safety and essential performance - collateral standard: requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment. International Electrotechnical Commission. 2015. [2022-09-30]. https://webstore.iec.ch/publication/59650 .
- 74.IEC 60601-2-47:2012: medical electrical equipment - part 2-47: particular requirements for the basic safety and essential performance of ambulatory electrocardiographic systems. International Electrotechnical Commission. 2012. [2022-09-30]. https://webstore.iec.ch/publication/2666 .
- 75.Take Y, Morita H. Fragmented QRS: what is the meaning? Indian Pacing Electrophysiol J. 2012 Sep;12(5):213–25. doi: 10.1016/s0972-6292(16)30544-7. https://europepmc.org/abstract/MED/23071383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Redfors B, Kosmidou I, Crowley A, Maehara A, Ben-Yehuda O, Arif A, Dizon JM, Mintz GS, Stone GW. Prognostic significance of QRS fragmentation and correlation with infarct size in patients with anterior ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: insights from the INFUSE-AMI trial. Int J Cardiol. 2018 Feb 15;253:20–4. doi: 10.1016/j.ijcard.2017.10.051.S0167-5273(17)33370-3 [DOI] [PubMed] [Google Scholar]
- 77.Floré V. QRS fragmentation after acute myocardial infarction. Int J Cardiol. 2018 Feb 15;253:27–8. doi: 10.1016/j.ijcard.2017.11.051.S0167-5273(17)36357-X [DOI] [PubMed] [Google Scholar]
- 78.Searle A, Kirkup L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol Meas. 2000 May;21(2):271–83. doi: 10.1088/0967-3334/21/2/307. [DOI] [PubMed] [Google Scholar]
- 79.Meziane N, Webster JG, Attari M, Nimunkar AJ. Dry electrodes for electrocardiography. Physiol Meas. 2013 Sep;34(9):R47–69. doi: 10.1088/0967-3334/34/9/r47. [DOI] [PubMed] [Google Scholar]
- 80.Lee EK, Kim MK, Lee CH. Skin-mountable biosensors and therapeutics: a review. Annu Rev Biomed Eng. 2019 Jun 04;21(1):299–323. doi: 10.1146/annurev-bioeng-060418-052315. [DOI] [PubMed] [Google Scholar]
- 81.Hickey KT, Biviano AB, Garan H, Sciacca RR, Riga T, Warren K, Frulla AP, Hauser NR, Wang DY, Whang W. Evaluating the utility of mHealth ECG heart monitoring for the detection and management of atrial fibrillation in clinical practice. J Atr Fibrillation. 2017 Feb 28;9(5):1546. doi: 10.4022/jafib.1546. https://europepmc.org/abstract/MED/29250277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Button V, Costa E. Electrodes for biopotential recording and tissue stimulation. In: Button V, editor. Principles of Measurement and Transduction of Biomedical Variables. Cambridge, MA, USA: Academic Press; 2015. pp. 25–76. [Google Scholar]
- 83.IEC 60529:1989+AMD1:1999+AMD2:2013 CSV consolidated version: degrees of protection provided by enclosures (IP Code) International Electrotechnical Commission. 2013. [2022-09-30]. https://webstore.iec.ch/publication/2452 .
- 84.IEC 60601-1:2005+AMD1:2012+AMD2:2020 CSV consolidated version: medical electrical equipment - part 1: general requirements for basic safety and essential performance. International Electrotechnical Commission. 2015. [2022-09-30]. https://webstore.iec.ch/publication/67497 .
- 85.Regulation 2017/745 of the European Parliament and of the Conuncil of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EE. Official Journal of the European Union. 2017. [2022-09-30]. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 .
- 86.Letourneur D, Joyce K, Chauvierre C, Bayon Y, Pandit A. Enabling MedTech translation in academia: redefining value proposition with updated regulations. Adv Healthc Mater. 2021 Jan;10(1):e2001237. doi: 10.1002/adhm.202001237. [DOI] [PubMed] [Google Scholar]
- 87.Fink M, Akra B. Regulatory clearance: how are outcome measurements critical? Injury. 2020 May;51 Suppl 2:S67–70. doi: 10.1016/j.injury.2019.10.071.S0020-1383(19)30678-3 [DOI] [PubMed] [Google Scholar]
- 88.MDCG 2020-6 Regulation (EU) 2017/745: clinical evidence needed for medical devices previously CE marked under directives 93/42/EEC or 90/385/EEC a guide for manufacturers and notified bodies. Medical Device Coordination Group Document. 2020. Apr, [2022-09-30]. https://health.ec.europa.eu/system/files/2020-09/md_mdcg_2020_6_guidance_sufficient_clinical_evidence_en_0.pdf .
- 89.Magnusson P, Lyren A, Mattsson G. Diagnostic yield of chest and thumb ECG after cryptogenic stroke, Transient ECG Assessment in Stroke Evaluation (TEASE): an observational trial. BMJ Open. 2020 Sep 24;10(9):e037573. doi: 10.1136/bmjopen-2020-037573. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=32973062 .bmjopen-2020-037573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, von Arbin M, Wallen H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace. 2012 Aug;14(8):1112–6. doi: 10.1093/europace/eur431.eur431 [DOI] [PubMed] [Google Scholar]
- 91.Orrsjö G, Cederin B, Bertholds E, Nasic S, Welin L. Screening of paroxysmal atrial fibrillation after ischemic stroke: 48-hour Holter monitoring versus prolonged intermittent ECG recording. Int Sch Res Notices. 2014 Mar 04;2014:1–6. doi: 10.1155/2014/208195. https://www.hindawi.com/journals/isrn/2014/208195/ [DOI] [Google Scholar]
- 92.Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Apr 07;75(13):1582–92. doi: 10.1016/j.jacc.2020.01.046. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(20)30523-4 .S0735-1097(20)30523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klum M, Urban M, Tigges T, Pielmus AG, Feldheiser A, Schmitt T, Orglmeister R. Wearable cardiorespiratory monitoring employing a multimodal digital patch stethoscope: estimation of ECG, PEP, LVET and respiration using a 55 mm single-lead ECG and phonocardiogram. Sensors (Basel) 2020 Apr 04;20(7):2033. doi: 10.3390/s20072033. https://www.mdpi.com/resolver?pii=s20072033 .s20072033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazaro J, Reljin N, Hossain MB, Noh Y, Laguna P, Chon KH. Wearable armband device for daily life electrocardiogram monitoring. IEEE Trans Biomed Eng. 2020 Dec;67(12):3464–73. doi: 10.1109/tbme.2020.2987759. [DOI] [PubMed] [Google Scholar]
- 95.Di Rienzo M, Vaini E, Castiglioni P, Merati G, Meriggi P, Parati G, Faini A, Rizzo F. Wearable seismocardiography: towards a beat-by-beat assessment of cardiac mechanics in ambulant subjects. Auton Neurosci. 2013 Nov;178(1-2):50–9. doi: 10.1016/j.autneu.2013.04.005.S1566-0702(13)00080-5 [DOI] [PubMed] [Google Scholar]
- 96.Di Rienzo M, Rizzo G, Işılay ZM, Lombardi P. SeisMote: a multi-sensor wireless platform for cardiovascular monitoring in laboratory, daily life, and telemedicine. Sensors (Basel) 2020 Jan 26;20(3):680. doi: 10.3390/s20030680. https://www.mdpi.com/resolver?pii=s20030680 .s20030680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D'Mello Y, Skoric J, Xu S, Roche PJ, Lortie M, Gagnon S, Plant DV. Real-time cardiac beat detection and heart rate monitoring from combined seismocardiography and gyrocardiography. Sensors (Basel) 2019 Aug 08;19(16):3472. doi: 10.3390/s19163472. https://www.mdpi.com/resolver?pii=s19163472 .s19163472 [DOI] [PMC free article] [PubMed] [Google Scholar]


