Abstract
Background
Postpartum Hemorrhage (PPH) is one of the main causes of maternal mortality, mainly in the poorest regions of the world, drawing attention to the need for strategies for preventing it. This study aims to evaluate the efficacy of prophylactic administration of Tranexamic Acid (TXA) in decreasing blood loss in pregnant women in delivery, preventing PPH.
Methods
Systematic review of randomized clinical trials. We searched for publications in PubMed, EMBASE and Cochrane Library databases, with the uniterms “postpartum, puerperal hemorrhage” and “tranexamic acid”, published between January of 2004 and January of 2020. The eligibility criteria were trials published in English with pregnant women assessed during and after vaginal or cesarean delivery about the effect of prophylactic use of TXA on bleeding volume. The random-effects model was applied with the DerSimonian-Laird test and the Mean Difference (MD) was calculated for continuous variables together with each 95% CI. This systematic review was previously registered in the PROSPERO platform under the registration n° CRD42020187393.
Results
Of the 630 results, 16 trials were selected, including one with two different doses, performing a total of 6731 patients. The intervention group received a TXA dose that varied between 10 mg.kg−1 and 1g (no weight calculation). The TXA use was considered a protective factor for bleeding (MD: -131.07; 95% CI: -170.00 to -92.78; p = 0.000) and hemoglobin variation (MD: -0.417; 95% CI: -0.633 to -0.202; p = 0.000). In the subgroup analysis related to the cesarean pathway, the effect of TXA was even greater.
Conclusion
The prophylactic use of tranexamic acid is effective in reducing the post-partum bleeding volume.
PROSPERO registration ID
CRD42020187393.
KEYWORDS: Postpartum hemorrhage, Prophylaxis, Tranexamic acid
Introduction
The occurrence of post-partum hemorrhage is a leading cause of maternal morbidity and mortality. With an incidence that can reach 6%, Post-Partum Hemorrhage (PPH) is, according to the World Health Organization (WHO), responsible for almost one quarter of all maternal deaths in the world, and can reach one third in developing nations.1, 2, 3 The difference in mortality related to socioeconomic indices reflects how the quality of the health structure and treatment are factors that might impact this outcome.1 Thus, the development of actions that are not only effective, but also of reasonable cost, is essential to improving these numbers.
Classically, PPH is defined as a volume of postpartum blood loss higher than expected: ≥500 mL after vaginal birth or ≥1000 mL after cesarean delivery. However, this definition is criticized because bleeding may not be externally visible, or blood volume may be wrongly measured in a mixture with amniotic fluid. In addition, postpartum morbidity is relatively infrequent among women with blood loss from 500 to 999 mL.4 The definition of PPH was revised by the American College of Obstetricians and Gynecologists (ACOG) in 2017, and a new one was proposed: cumulative blood loss ≥1000 mL or bleeding associated with signs/symptoms of hypovolemia within 24 hours of the birth process regardless of delivery route.5 However, they kept the recommendation to consider abnormal a blood loss greater than 500 mL and proceed investigation in a vaginal delivery.
Several risk factors are associated with PPH, including a history of prior PPH, overdistended uterus (multiple gestations, polyhydramnios, and a macrosomic fetus), nulliparity, induction and augmentation of labor, placental abnormalities (placenta praevia, placenta accreta), coagulation disorders, and prolonged labor. Some studies even suggest epidural anesthesia as a risk factor.6 Nevertheless, only one third of PPH cases have identifiable risk factors, and there is no isolated risk factor that helps to identify which patient is likely to respond to initial treatment, which is mostly based on the use of traditional uterotonics, such as oxytocin and methylergometrine, assuming that the main cause of PPH is uterine atony.3
Tranexamic Acid (TXA) is a synthetic lysine analogue that inhibits fibrinolysis. In recent years, due to its relatively low cost and ease of use, this drug started to be used in the treatment of acute bleeding in several situations, with low incidence of collateral effects.7 Solid evidence recommends its use in some situations, and the best known examples are the CRASH-2 and WOMAN trials which, respectively, validated the use of TXA in situations like trauma and the treatment of PPH itself.8,9 However, it is not yet defined whether the prophylactic use of TXA brings benefits, leaving open the possibility of its administration even before the diagnosis of PPH is established. Previous meta-analyses failed to define this question, and new clinical trials have been published since then.10,11
The aim of this systematic review and meta-analysis was to evaluate the effectiveness of the prophylactic use of TXA in reducing postpartum bleeding.
Methods
Eligibility criteria
This study is a systematic review with meta-analysis of blinded and randomized clinical trials about the effect of prophylactic use of TXA on bleeding volume in patients undergoing vaginal or cesarean delivery. The search included studies in English published between January of 2004 and January of 2020.
Information sources
The search was performed in the PubMed/Medline, EMBASE and Cochrane Library databases. PRISMA statement guidelines were followed for planning and preparing the study and the research protocol was previously registered on the PROSPERO platform under registration CRD42020187393.12
Data items
The primary outcome assessed was bleeding volume, in milliliters, in the peripartum period. As secondary outcomes, hemoglobin variation, incidence of side effects, use of uterotonics, and need of transfusion of blood products were evaluated. Sensitivity assessments were performed for type of delivery, presence of risk factors for PPH and time of drug administration.
Search strategy
The search in the database was carried out between March 10th and 12th, 2020. The search strategy consisted of the keywords “postpartum, puerperal hemorrhage” and “tranexamic acid” or synonyms, adopting AND and OR as interlocutors. The search strategies at EMBASE and Cochrane Library were as follows: "postpartum hemorrhage" in Title Abstract Keyword AND tranexamic acid in Title Abstract Keyword - (Word variations have been searched)” with filter for clinical trials. In PubMed/Medline, the search comprised: ((("postpartum period"[MeSH Terms] OR ("postpartum"[All Fields] AND "period"[All Fields]) OR "postpartum period"[All Fields] OR "postpartum"[All Fields]) OR ("postpartum period"[MeSH Terms] OR ("postpartum"[All Fields] AND "period"[All Fields]) OR "postpartum period"[All Fields] OR "puerperium"[All Fields])) AND ("tranexamic acid"[MeSH Terms] OR ("tranexamic"[All Fields] AND "acid"[All Fields]) OR "tranexamic acid"[All Fields])) AND (("hemorrhage"[All Fields] OR "hemorrhage"[MeSH Terms] OR "hemorrhage"[All Fields]) OR ("hemorrhage"[MeSH Terms] OR "hemorrhage"[All Fields] OR "bleeding"[All Fields])) AND Clinical Trial[ptyp].
Selection process
Two independent researchers (IC and RF) made a preliminary assessment of the title and abstracts with the aid of the Rayyan© tool.13 In this phase, all inaccessible studies, duplicates, only research protocols, in non-English language, or which used active control protocols were initially excluded. In a second phase, the articles were read in full text for inclusion consideration and the eligibility of each study was determined. In the case of a disagreement, a third researcher (CG) made the final assessment.
Study risk of bias assessment
As a way of assessing the risk of bias and the quality of the studies included in this meta-analysis, the Risk of Bias 2.0© tool was used.14
Data collection process, effect measures, and synthesis methods
The data regarding bleeding volume and the other secondary outcomes were recorded in a spreadsheet. A random effects model was used with the DerSimonian-Laird test. Mean Difference (MD) and 95% Confidence Intervals (95% CI) were calculated for continuous variables and a p-value of 5% was considered for statistical significance. Statistical heterogeneity was calculated using the Chi-Square method (X²) and Higgins test (I²).15 The presence of heterogeneity was considered if p < 0.05 and I² ≥ 50%. Evaluation of potential publication bias was made through visual analysis of the funnel graph and by Begg16 and Egger tests.17
Certainty assessment
To assess the impact of the study, the GRADE protocol (Grading of Recommendations, Assessment, Development and Evaluations) was applied to the GRADEpro GDT©18 program, according to the GRADE System Methodological Guidelines.19
As for the secondary outcomes, in those in which there was not enough data to perform a meta-analysis, a qualitative analysis was performed.
Results
Results for study selection
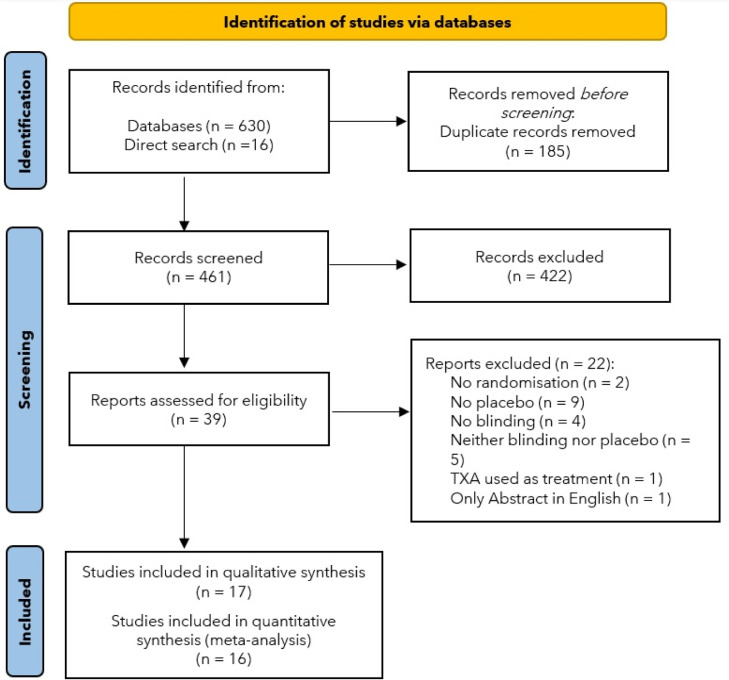
The search in the databases resulted in 630 trials. Initially, 185 results were identified as duplicates. In addition to these, another 16 studies found through direct search were also included. Upon reading the abstracts of the 461 remaining results, only 39 studies were qualified for full text reading. Of these, two were excluded for not having or not reporting randomization,20,21 nine for not using placebo in the control group,22, 23, 24, 25, 26, 27, 28, 29, 30 four for not having or not reporting blinding,31, 32, 33, 34 five for not reporting neither blinding nor placebo,35, 36, 37, 38, 39 one for using TXA as treatment and not as prophylaxis,40 and one because only the abstract was written in English.41 Thus, 17 trials were selected to comprise the systematic review, however, one of them could not be part of the meta-analysis, as the volume measurements did not report standard deviation42 (Fig. 1)
Figure 1.
PRISMA 2020 flow diagram.
Study characteristics
The 16 trials selected for the meta-analysis11,43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 included 6701 patients, with 3361 patients in the intervention group and 3340 in the control group. However, in the study by Goswami et al.,49 two different doses of TXA were used in comparison with the control group (10 mg.kg−1 and 15 mg.kg−1); so, for the purposes of statistical analysis, each dose was regarded as an independent trial, but the control group was the same. As such, the final analysis considered the inclusion of 6731 patients from 17 trials,11,43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 with 3361 patients in the intervention group and 3370 in the control arm. Characteristics of each study are described in Table 1.
Table 1.
Characteristics of the included studies.
| Autor | Year | Country | Type of delivery | TXA Dose | Control | Patients TXA (n) | Patients Control (n) | Type of anesthesia (cesarean) |
|---|---|---|---|---|---|---|---|---|
| Gungorduk et al. | 2011 | Turkey | Cesarean | 1g, 10 min before incision | 30 mL glucose 5% | 330 | 330 | Non described |
| Movafegh et al. | 2011 | Iran | Cesarean | 10 mg.kg−1, 20 min before anesthesia | Saline IV | 50 | 50 | Spinal |
| Sentürk et al. | 2012 | Turkey | Cesarean | 1 g, 5 min before incision | Dextrose 5% | 101 | 122 | Spinal |
| Gungorduk et al. | 2013 | Turkey | Vaginal | 1 g, for 5 min after extracting the anterior shoulder | 30 mL glucose 5% | 220 | 219 | ‒ |
| Xu et al. | 2013 | China | Cesarean | 10 mg.kg−1 + 200 mL saline | 200 mL saline | 88 | 86 | Spinal |
| Goswami et al. A | 2013 | India | Cesarean | 10 mg.kg−1 + 20 mL dextrose 5% | 5 mL DW + 20 mL dextrose 5% | 30 | 30 | Spinal |
| Goswami et al. B | 2013 | India | Cesarean | 15 mg.kg−1 + 20 mL dextrose 5% | 5 mL DW + 20 mL de dextrose 5% | 30 | 30 | Spinal |
| Shahid et al. | 2013 | Pakistan | Cesarean | 1g + 20 mL glucose 5% | 10 mL DW + 20 mL glucose 5% | 38 | 36 | Spinal |
| Ghosh et al. | 2014 | India | Cesarean | 1g | 10 mL DW | 70 | 70 | Spinal |
| Maged et al. | 2015 | Egypt | Cesarean | 1g | 30 mL glucose 5% | 100 | 100 | Non described |
| Mirghafourvand et al. | 2015 | Iran | Vaginal | 1g, after extracting the anterior shoulder | DW | 60 | 60 | ‒ |
| Sujata et al.a | 2016 | India | Cesarean | 10 mg.kg−1 | Saline IV | 30 | 30 | Non described |
| Ismail et al. | 2017 | Egypt | Vaginal | 1g, soon after delivery | 30 mL glucose 5% | 100 | 100 | ‒ |
| Abbas et al. | 2018 | Egypt | Cesarean | 1g before incision | Saline IV | 31 | 31 | General |
| Sentilhes et al. | 2018 | France | Vaginal | 1g, 2 min after delivery | Saline IV | 1931 | 1927 | ‒ |
| Sujita et al. | 2018 | Thailand | Vaginal | 1g + 20 mL saline (30 mL total) | 30 mL saline | 72 | 69 | ‒ |
| Millani et al. | 2019 | Iran | Cesarean | 1g + 20 mL of DW, 15 min before incision | Glucose 5% 10 mL + 20 mL DW | 30 | 30 | Spinal |
| Shah et al. | 2019 | Nepal | Cesarean | 1g | 10 mL saline | 80 | 80 | Non described |
This trial was not included in the meta-analysis because lack of data; IV, Intravenous; DW, Distilled Water; A and B, In Goswami et al. the analysis was divided into two groups because two different doses of TXA were used (10 mg.kg−1 and 15 mg.kg−1).
Regarding the trial countries, Turkey, Iran, India, and Egypt had three studies each. China, Pakistan, France, Thailand, and Nepal had one trial each.
Results of synthesis and qualitative assessment of individual studies
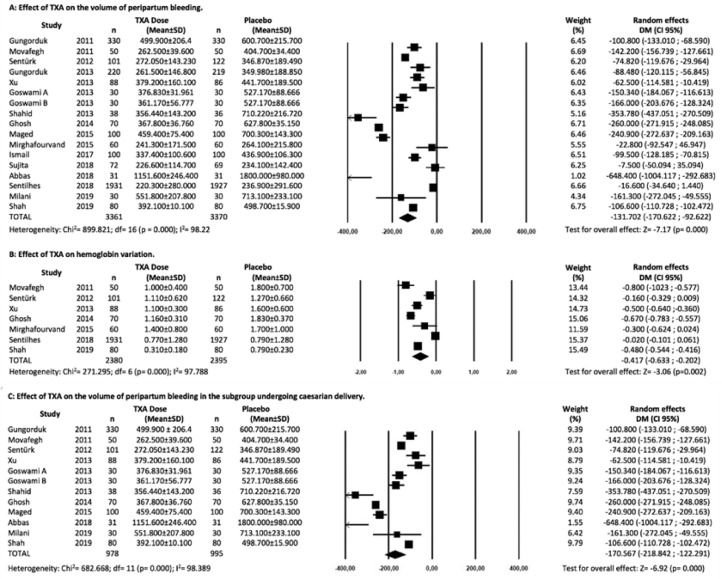
The assessment of peripartum bleeding in the 17 trials of the meta-analysis11,43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 showed a reduction in volume sufficient to consider TXA as a protection factor (MD: -131.70; 95% CI: -170.62 to -92.78; p = 0.000), as shown in Figure 2A.
Figure 2.
(A) Meta-analysis of the effect of TXA on the volume of peripartum bleeding in difference of means and 95% CI. (B) Meta-analysis of the effect of TXA on hemoglobin variation in difference of means and 95% CI. (C) Meta-analysis of the effect of TXA on the volume of peripartum bleeding in the subgroup undergoing cesarean delivery in difference of means and 95% CI.
Regarding secondary outcomes, seven of the total studies underwent hemoglobin variation assessment11,43,46,48,51,53,55 allowing comparison of results. The TXA group also showed a lower variation in hemoglobin, expressed as g.dL−1 (MD: -0.417; 95% CI: -0.633 to -0.202; p = 0.00) (Fig. 2B).
As for side effects, in the qualitative assessment, nine articles did not report or did not have side effects.42, 43, 44, 45, 46,50,52,54,55 Two trials reported a rare occurrence of serious side effects,11,48 such as Xu et al. who described two thromboembolic events in each arm of the study (RR = 0.98; 95% CI: 0.14 to 6.78; p = 0.38), and Sentilhes et al. who reported one event in TXA group and four in the control group (RR = 0.25; 95% CI: 0.03 to 2.24; p = 0.37). Only Sentilhes et al. described a case of seizure in the TXA group. The remaining studies only described mild side effects, with higher incidence of gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
In the qualitative analysis of the use of different uterotonics, nine trials found a reduction in their need.11,43, 44, 45,47,49,51,52,57 The highlights were Gungorduk et al. from 2013,47 with a 69% reduction in the need for additional uterotonics (p = 0.007), and Sentilhes et al. with a reduction of 25% (RR = 0.75; 95% CI: 0.71 to 0.92; p = 0.006). The need to use blood products was assessed by eleven studies. However, only a few found statistical difference, such as Abbas et al.,45 who observed a 76% reduction (p = 0.0001) in the need for blood transfusion in the TXA group, but this trial used a very small sample (n = 62) and evaluated patients with high risk of PPH (placental accretism).
Considering only the 11 trials in which the cesarean delivery was studied, the quantitative subgroup analysis related to this type of birth showed that the effect of TXA is even greater on the reduction of bleeding (MD: -170.56; 95% CI: -218.84 to -122.29; p = 0.000) (Fig. 2C). Regarding the five trials that assessed vaginal delivery, a quantitative analysis in this restricted group showed a much smaller effect of TXA (MD: -48.99; 95% CI: -91.42 to -6.57; p = 0.024), with all the caveats that must be considered when conducting a meta-analysis in such a small group of trials.
The presence of risk factors for bleeding was an inclusion criterion in three trials, as in the study by Abbas et al., performed only in women with placental accretism, in the study by Goswami at al. that selected only patients with anemia, and in the trial by Sujata et al. that considered most risk factors as inclusion criteria.42,45,47 The remaining articles considered these predisposing factors as exclusion criteria, with great variation in the quality and quantity of elements considered. The risk elements most used as exclusion criteria were coagulation disorders, being considered by 12 trials,11,42,43,46,48,49,51, 52, 53, 54, 55,57 followed by factors that cause uterine hyperdistention, with 11 studies.11,43,44,46,48,50, 51, 52, 53, 54,56 In third place, there were placental abnormalities11,43,47,50,52,56 and anemia,50, 51, 52,54,57 in six trials each. Even though some of the literature does not consider multiparity as risk factor, five studies used it as exclusion criteria.11,44,50,53,57
There was also heterogeneity of the time of drug administration; however, all trials were centered at some point between 20 minutes before incision and five minutes after fetus extraction.
Risk of bias in studies
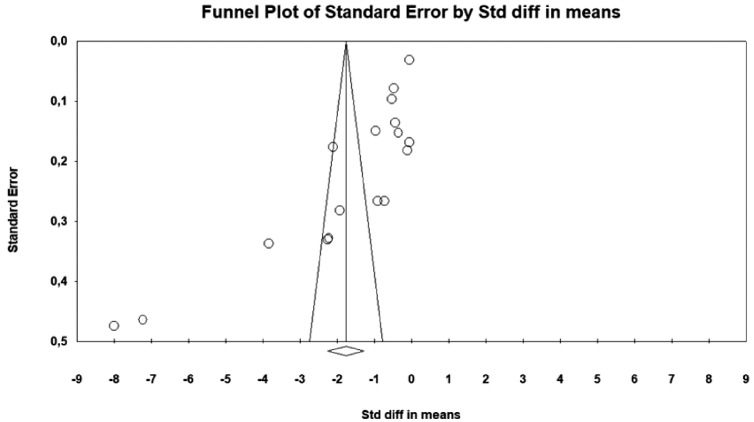
In Figure 3, the funnel plot for the sixteen trials shows a smaller distribution of studies to the left of the summary effect, with a distribution mostly beyond the limits of the funnel. The Egger (p < 0.001) and Begg (p < 0.001) tests reinforce the presence of publication bias.
Figure 3.
Funnel plot with publication bias analysis.
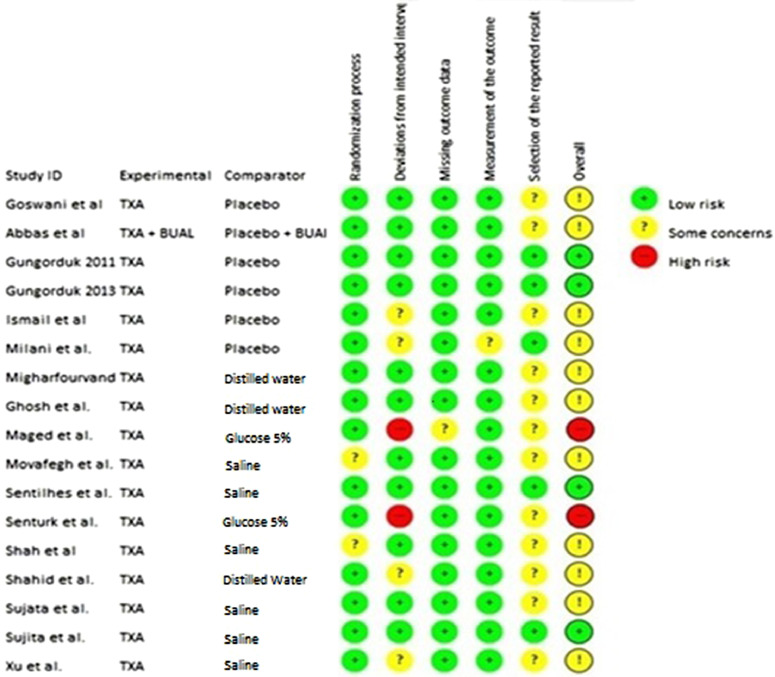
The evaluation with the Risk of Bias tool considered all trials in the systematic review. Two studies, the one from Maged et al. and the one from Sentürk et al.46,52 were classified as “high risk” for bias in at least one of the domains analyzed. Another eleven trials presented “some concerns” in at least one of the domains. The remainders were classified as “low risk” (Fig. 5).
Figure 5.
RoB 2.0 analysis framework.
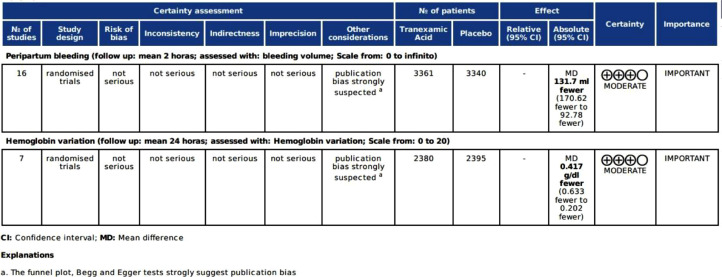
Certainty of evidence
The analysis of the quality of evidence by GRADE protocol17 indicates that the certainty of evidence is “moderate”, both for the primary outcome of bleeding and for the secondary outcome of hemoglobin variation (Fig. 4).
Figure 4.
Graduation of the certainty of evidence by the GRADE protocol.
Discussion
This meta-analysis carried out with 16 trials published between 2011 and 2019 showed a favorable result for the prophylactic use of TXA in the reduction of PPH.
There is plausibility for the use of TXA in this context, from a logic of the mechanism of action to its establishment as another option for the treatment of PPH after the WOMAN trial.8 This famous multicenter and international RCT, with more than twenty thousand patients, was a milestone from which this medication started to be adopted in protocols for the control of PPH worldwide.58
As with any disease, the idea of developing preventive strategies is more interesting than investing in the treatment itself, especially when it comes to a health problem like PPH. This condition demands a large amount of human and material resources for its treatment, and it is more prevalent precisely in regions where such resources are scarce. This systematic review shows that the TXA may indeed be one of the recommended strategies.
The results of this study are in line with previous systematic reviews,10,59 including a systematic Cochrane review conducted in 2015 with twelve trials and a total of 3285 patients, where the authors concluded that the TXA decreased postpartum bleeding prevented PPH, and reduced the need for blood components. Since then, other RCTs have been published, including the study by Sentilhes et al.,11 a large multicenter RCT performed in France, with almost four thousand patients who underwent vaginal delivery and is, by far, the RCT with the highest weight of this meta-analysis. However, this study failed to identify a statistically significant reduction in the diagnosis of PPH between the two groups (RR = 0.83; 95% CI 0.68 to 1.01; p = 0.07), despite the reduction in the use of uterotonics (RR = 0.75; 95% CI 0.61 to 0.92; p = 0.04). It was important that a new meta-analysis showed the effect that these new clinical trials would have on the results of the prophylactic use of TXA.
This meta-analysis showed high heterogeneity (I² = 98.22), which can be attributed to several factors. One of them is related to the different intervals during which the bleeding was recorded. While some trials measured bleeding after the removal of the placenta up to hours after the delivery,51 others measured it from the beginning of the cesarean section,53 and some only after the fetus extraction.11,48 The different TXA dosages administered, always within usual clinical range and with small differences in the administration technique, may also have influenced. There were also differences in populations, as already mentioned, with some trials including patients with recognized risk factors for PPH and others considering its presence as an exclusion criterion. In addition to the different routes of delivery, even differences in the methods of measuring volume can be embroiled as causes of heterogeneity (calculation based on hematocrit, weighting surgical dressings, aspirated contents, etc.).
The statistical analysis of the hemoglobin variation showed a significant effect in favor of the TXA administration, corroborating and being consistent with the result of the main outcome.
Overall, there was no increase in serious adverse events, with only two trials reporting a low incidence of serious complications.11,48 When it comes to TXA, thromboembolic events are the most worrying complication, but in this systematic review this concern does not represent an impediment to the adoption of the intervention. It is important to remember that many of the trials did not aim to identify these events and even establish risk factors for thromboembolic episodes as exclusion criteria. It is also important to consider that pregnancy and postpartum represent, by themselves, risk factors for thromboembolic events, due to the physiological prothrombotic state of these conditions.
Regarding the use of uterotonics and blood products, there was a reduction in the incidence of needs of both interventions in the patients receiving TXA in the qualitative analysis, which, as well as the variation of hemoglobin, is consistent with the main outcome.
The specific analysis of the cesarean delivery showed an enhanced effect of TXA in this subgroup, which is consistent with the nature of a procedure in which major bleeding is expected. In a context of prevention, such a result could justify the use of TXA in this particular group instead of the entire universe of patients. However, it should be noted that this result may have been influenced by the fact that the trial by Sentilhes et al., which had the largest population in the meta-analysis and studied only vaginal delivery, did not show significant effect and its simple withdrawal from any analysis would tend to favor the intervention group. Due to heterogeneity in the other sensitivity analyses, regarding the time of administration, it was not possible to perform specific statistical analysis. However, it is believed that the variation between trials is too small to affect the outcome in this matter in any way.
Several strategies have been adopted to minimize bias; however, even so, due to the very nature of the meta-analysis, there is no way to rule out its presence. The evaluation by the RoB 2.0 tool showed that almost all trials had at least one domain with some problem, mainly in the “selection of reported results” domain, which assesses the bias that may arise because the result is selected among the various measures of effect that were initially evaluated by the clinical trial. This is due, in large measure, to the fact that few trials have previously registered their research protocols on independent platforms, corroborating the finding. The evaluation with the GRADE protocol indicated that the certainty of the evidence is moderate. However, this analysis also shows that the study has no inconsistency bias, because even with such different trials, the result tended to the same direction, that is, the conclusion of the intervention was consistent.60
Finally, the present systematic review helps to make it clear that the use of this medication can indeed be beneficial for the prevention of PPH and that the evidence is robust in favor of its effectiveness. The inclusion of prophylactic TXA in the active management of the third stage of vaginal delivery (along with oxytocin administration and controlled traction of the umbilical cord) may be considered, as well as it may be even more beneficial in cesarean sections, considering the higher blood loss expected during this procedure. Even so, more studies are still necessary, mainly under a more adequate design for detecting adverse effects and considering what would be the effective dose with the lowest incidence of adverse events, in addition to what would be the most appropriate administration form.
A general recommendation for the prophylactic use of TXA in preventing postpartum bleeding still lacks more weight of evidence, not because of efficacy, which was present in this study, but because of its cost-effectiveness. The widespread adoption of medication in such a common event around the world, even if relatively inexpensive, would mean a significant increase in the cost and complexity of healthcare. Perhaps clinical outcomes that really represent an improvement in care, such as mortality and morbidity, should be the focus of the next clinical trials.
Conclusion
The prophylactic use of tranexamic acid is effective in reducing the bleeding post-partum volume.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Professor Gabriel Magalhães Nunes Guimarães for substantial help in conceptualization and suggestions in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bjane.2022.08.002.
Appendix. Supplementary materials
References
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Global Health. 2014;2:323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Sheldon WR, Blum J, Vogel JP, Souza JP, Gülmezoglu AM, Winikoff B, et al. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl 1):5–13. doi: 10.1111/1471-0528.12636. [DOI] [PubMed] [Google Scholar]
- 4.Anger H, Durocher J, Dabash R, Winikoff B. How well do postpartum blood loss and common definitions of postpartum hemorrhage correlate with postpartum anemia and fall in hemoglobin? PLoS ONE. 2019;14:1–13. doi: 10.1371/journal.pone.0221216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists ACOG Practice Bulletin: postpartum hemorrhage. Am Coll Obstet Gynecol. 2017;130:168–186. [Google Scholar]
- 6.Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after cesarean delivery: An analysis of risk factors. South Med J. 2005;98:681–685. doi: 10.1097/01.SMJ.0000163309.53317.B8. [DOI] [PubMed] [Google Scholar]
- 7.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: A randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technology Assessment. 2013;17:1–80. doi: 10.3310/hta17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015;(6) doi: 10.1002/14651858.CD007872.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731–742. doi: 10.1056/NEJMoa1800942. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan ‒ a web and mobile app for systematic reviews. Systematic Reviews. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMaster University 2020 (developed by Evidence Prime Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool [Software] [Internet]. [cited 2020 Dec 21]. Available from: www.gradepro.org.
- 19.Brasil. Ministério da Saúde. Secretaria de Ciência T e IEstratégicosD de C e Tecnologia. Methodological guideline: GRADE System – Manual graduation quality of evidence and strength of recommendation for decision making process in health.
- 20.Bhatia S, Deshpande H. Role of tranexamic acid in reducing blood loss during and after caesarean section. Med J Dr DY Patil University. 2015;8:21. [Google Scholar]
- 21.Sahu J, Mishra N. Role of intravenous tranexamic acid in reducing blood loss during caesarean section: Study at tribal-dominated area hospital in Chhattisgarh, India. J Obstet Gynaecol Res. 2019;45:841–848. doi: 10.1111/jog.13915. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: Randomized clinical trial. J Mat-Fetal Neonatal Med. 2013;26:1705–1709. doi: 10.3109/14767058.2013.794210. [DOI] [PubMed] [Google Scholar]
- 23.Salas MT. A single-blinded randomized controlled trial on the effect of prophylactic intravenous administration of tranexamic acid on the reduction of blood loss during and after primary cesarean section ‒ Perinatal Medicine and General Obstetrics. J Obstet Gynaecol Res. 2017;43:20–55. [Google Scholar]
- 24.Ramani B, Nayak L. Intravenous 1-gram tranexamic acid for prevention of blood loss and blood transfusion during caesarean section: a randomized case control study. Inter J Reproduction Contraception Obstet Gynecol. 2014;3:366–369. [Google Scholar]
- 25.Gobbur V, Shiragur S, Jhanwar U, Tehalia M. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. Inter J Reproduction Contraception Obstet Gynecol. 2014;3:414–417. [Google Scholar]
- 26.Sadek S, Mahesan A, Ramadan H, Dad N, Movva V, Kanaan C. Prophylactic tranexamic acid usage in prevention of post-partum hemorrhage a prospective cohort study. Res Square. 2019:1–13. [Google Scholar]
- 27.Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after cesarean section: A multi-center, randomized trial. Euro J Obstet Gynecol Reproductive Biol. 2004;112:154–157. doi: 10.1016/s0301-2115(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 28.Yehia AH, Koleib MH, Abdelazim IA, Atik A. Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial. Asian Pacific J Reproduction. 2014;3:53–56. [Google Scholar]
- 29.Singh T, Burute SB, Deshpande HG, Jethani S, Ratwani K. Efficacy of tranexamic acid in decreasing blood loss during and after caesarean section: a randomized case control prospective study. J Evolution Med Dental Sci. 2014;3:2780–2788. [Google Scholar]
- 30.Mayur G, Purvi Patel, Desai A. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randamized case controlled prospective study. J Obstet Gynecol India. 2007;57:12. [Google Scholar]
- 31.Taj N, Fiardus A, Akhtar N, Chaudhary MH, Sarah Bajwa Z, et al. Efficacy of tranexamic acid in reducing blood loss during and after cesarean section. Rawal Med J. 2014;39:311–313. [Google Scholar]
- 32.Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of Intravenous Tranexamic Acid on Caesarean Blood Loss: A Prospective Randomised Study. J Obstet Gynecol India. 2016;66:347–352. doi: 10.1007/s13224-016-0915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Maternal-Fetal Neonatal Med. 2009;22:72–75. doi: 10.1080/14767050802353580. [DOI] [PubMed] [Google Scholar]
- 34.G B, MV A, Mittal S. Efficacy of prophylactic tranexamic acid in reducing blood loss during and after caesarean section. Inter J Reproduction Contraception Obstet Gynecol. 2016;39:2011–2016. [Google Scholar]
- 35.Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. J Maternal-Fetal Neonatal Med. 2015;28:1014–1018. doi: 10.3109/14767058.2014.941283. [DOI] [PubMed] [Google Scholar]
- 36.Sharma R, Najam R, Misra MK. Efficacy of Tranexamic Acid in Decreasing Blood Loss During and After Cesarean Section. Biomed Pharmacol J. 2011;4:231–235. [Google Scholar]
- 37.Roy P, Sujatha MS, Bhandiwad A, Biswas B. Role of Tranexamic Acid in Reducing Blood Loss in Vaginal Delivery. J Obstet Gynecol India. 2016;66:246–250. doi: 10.1007/s13224-016-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhivya Lakshmi SJ, Abraham R. Role of prophylactic tranexamic acid in reducing blood loss during elective caesarean section: A randomized controlled study. J Clin Diagnost Res. 2016;10:17–21. doi: 10.7860/JCDR/2016/21702.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashmi PS, Sudha TR, Prema P, Patil Rajashri, Vijayanath V. Role of Tranexamic acid in reducing blood loss during and after cesarean section: A randomized case control prospective study. J Med Res Practice. 2012;1:40–43. [Google Scholar]
- 40.Ayedi M, Jarraya A, Smaoui M, Zouari J, Smaoui L, Kolsi K. Effect of tranexamic acid on post-partum hemorrhage by uterine atony: A preliminary result of a randomized, placebocontrolled trial: 11AP4-7. Euro J Anaesthesiol. EJA. 2011;28:293. [Google Scholar]
- 41.Moradan S. Prophylactic effect of misoprostol versus tranexamic acid in conjunction with oxytocin in reduction of post-partum hemorrhage after cesarean section in: A randomized clinical trial. J Semnan University Med Sci. 2018;20:603–807. [Google Scholar]
- 42.Sujata N, Tobin R, Kaur R, Aneja A, Khanna M, Hanjoora VM. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing cesarean delivery. Inter J Gynecol Obstet. 2016;133:312–315. doi: 10.1016/j.ijgo.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 43.Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Inter J Gynecol Obstet. 2011;115:224–226. doi: 10.1016/j.ijgo.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Ismail A, Abbas AM, Shahat. Mohamed A. Evaluation of subendometrial and intramyometrial blood flow after intravenous tranexamic acid for prevention of postpartum hemorrhage in vaginal delivery: a randomized controlled study. J Gynecol Res Obstet. 2017;3:046–050. [Google Scholar]
- 45.Abbas AM, Shady NW, Sallam HF. Bilateral uterine artery ligation plus intravenous tranexamic acid during cesarean delivery for placenta previa: a randomized double-blind controlled trial. J Gynecol Obstet Human Reproduction. 2019;48:115–119. doi: 10.1016/j.jogoh.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: A double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet. 2013;287:641–645. doi: 10.1007/s00404-012-2624-8. [DOI] [PubMed] [Google Scholar]
- 47.Gungorduk K, Ascoǧlu O, Yldrm G, Ark C, Tekirdaǧ AI, Besmoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. Obstet Gynecol Surv. 2013;68:673–675. doi: 10.1055/s-0032-1326986. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: A double-blind randomization trial. Arch Gynecol Obstet. 2013;287:463–468. doi: 10.1007/s00404-012-2593-y. [DOI] [PubMed] [Google Scholar]
- 49.Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: A double-blind randomized case control prospective trial. Saudi J Anaesth. 2013;7:427–431. doi: 10.4103/1658-354X.121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. Journal of the College of Physicians and Surgeons – Pakistan. JCPSP. 2013;23:459–462. [PubMed] [Google Scholar]
- 51.Ghosh A, Chaudhuri P, Muhuri B. Efficacy of intravenous tranexamic acid before cesarean section in preventing post partum hemorrhage- a prospective randomised double blind placebo controlled study. Inter J Biol Med Res. 2014;5:4461–4464. [Google Scholar]
- 52.Maged AM, Helal OM, Elsherbini MM, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Inter J Gynecol Obstet. 2015;131:265–268. doi: 10.1016/j.ijgo.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Mirghafourvand M, Mohammad-Alizadeh S, Abbasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: A double-blind randomised controlled trial. Australian New Zealand J Obstet Gynaecol. 2015;55:53–58. doi: 10.1111/ajo.12262. [DOI] [PubMed] [Google Scholar]
- 54.Milani F, Haryalchi K, Sharami SH, Atrkarroshan Z, Farzadi S. Prophylactic effect of tranexamic acid on hemorrhage during and after the cesarean section. Inter J Women's Health Reproduction Scienc. 2019;7:74–78. [Google Scholar]
- 55.Shah P, Agrawal A, Chhetri S, Rijal P, Bhatta NK. Tranexamic acid in prevention of postpartum hemorrhage in elective cesarean section. Inter J Reproduction Contraception Obstet Gynecol. 2019;8:372. [Google Scholar]
- 56.Sujita A, Songthamwat S, Songthamwat M. Effectiveness of tranexamic acid for reducing postpartum blood loss in the first two hours after vaginal delivery: A randomised controlled trial. J Clin Diagnost Res. 2018;12:QC01–QC04. [Google Scholar]
- 57.Gungorduk K, Yıldırım G, Asıcıoğlu O, Gungorduk O, Sudolmus S, Ark C. Efficacy of Intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28:233–240. doi: 10.1055/s-0030-1268238. [DOI] [PubMed] [Google Scholar]
- 58.No GG. Prevention and management of postpartum haemorrhage. BJOG. 2017;124:e106–e149. doi: 10.1111/1471-0528.14178. [DOI] [PubMed] [Google Scholar]
- 59.Franchini M, Mengoli C, Cruciani M, et al. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: An updated systematic review and meta-analysis. Blood Transfusion. 2018;16:329–337. doi: 10.2450/2018.0026-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence - Inconsistency. Journal of Clinical Epidemiology. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.