Abstract
Introduction
Polycystic ovary syndrome (PCOS) is an ovarian health condition as well as a long-term endocrine dysfunction that affects reproductive-aged women. Toll-like receptor 2 (TLR2) gene was linked to PCOS and chronic inflammation, and the prevalence of obesity was rising in Saudi women. Previous studies on rs5743708 polymorphism were documented in the obesity as well as in PCOS women.
Aim
In this study, we investigated the molecular role of rs5743708 polymorphism in TLR2 gene among Saudi women diagnosed with PCOS using the Rotterdam criteria.
Methods
Blood samples were collected from 220 Saudi women in this hospital-based case-control study; 110 were PCOS women and remaining 110 were non-PCOS (control women). Biochemical analysis was performed on serum samples, and molecular analysis was performed on EDTA blood. Genotyping for rs5743708 polymorphism was performed with polymerase chain reaction-restriction fragment length polymorphism analysis.
Results
In both groups, clinical data was calculated using t-test, which revealed both positive (p < 0.05) and negative (p > 0.05) associations. HWE analysis supported the rs5743708 polymorphism (p < 0.05). In the rs5743708 polymorphism, none of the genotypes, genetic models, or allele frequencies were found to be associated with PCOS and non-PCOS women. However, both ANOVA and regression analyses revealed a positive relationship in PCOS with weight and BMI (p < 0.0001).
Conclusion
The rs5743708 polymorphism was not associated to PCOS in Saudi women. One of the predictions could be that 42.7% of PCOS and 73.6% of non-PCOS women were obese, and the rs5743708 polymorphism has been linked to both obesity and PCOS in the previous studies. This study suggests screening for additional polymorphisms with a large sample size.
Keywords: PCOS, non-PCOS, TLR2 gene, Rs5743708 polymorphism, Saudi women
1. Introduction
Among reproductive aged women, polycystic ovary syndrome (PCOS) is considered as a prevalent endocrine, metabolic dysfunction and multifaceted disease (Jabarpour et al., 2023). The common cause of PCOS is through anovulatory infertility affecting 10% of adult women. The incident of PCOS in the global population has attained 1.55 Million cases among women (Liu et al., 2023). The initial definition of PCOS was started using two of the three Rotterdam criteria, which include oligo-anovulation, polycystic ovaries, and hyperandrogenism (ESHRE and Group 2004). Still the etiology of PCOS is not updated; however, the main cause was ovarian and endocrine disturbances, and young women primarily manifest with hyperandrogenism, morphology of polycystic ovary, irregular menstruation, and infertility as reproductive symptoms, and if woman becomes pregnant, additional pregnancy complications will enhance (Udesen et al., 2023). Around 10% of genetic factors accounted for heritability and PCOS etiology interacts with genetic, epigenetic, and developmental factors (Pei et al., 2023). The pathophysiological mechanism remains still unclear for PCOS but it is associated with an increased prevalence of hypertension, insulin resistance (IR), type 2 diabetes, metabolic syndrome (MetS), nonalcoholic fatty liver, and cardiovascular disease (Butler et al., 2023). Chronic low-grade inflammation, which is linked to PCOS, is challenging to correlate to long-term cardiovascular complications and their risks (Aboeldalyl et al., 2021). Obesity is a major contributor to the development of PCOS. Obesity worsens the metabolic phenotype of PCOS, and the prevalence of PCOS is higher in overweight and obese women (Xing et al., 2022).
Single nucleotide polymorphisms (SNPs) may alter the susceptibility to PCOS caused by lifestyle factors. Several genetic loci associated with PCOS traits have been identified through genome-wide association studies (GWAS), but the majority of these studies have been conducted in global populations other than Saudi Arabia (Hiam et al., 2019). PCOS is a polygenic and complex symptomatic condition; hence, population-based case-control studies and GWAS can reveal potential associations (Al-Mutawa 2023). An imminent factor in PCOS is inflammation. The link between PCOS, chronic inflammation, and SNPs was discovered via toll-like receptors, which are inflammatory receptors. Previous reports on the Toll-like receptor 2 (TLR2) gene was linked it to obesity, IR, and metabolic disorders. The TLR2 gene was related to a higher body mass index (BMI) and proinflammatory cytokine markers. Prior studies have connected the rs5743708 polymorphism in the TLR2 gene to obesity (Soydas et al., 2016) and PCOS (Kuliczkowska-Płaksej et al., 2021). The current study focused on rs5743708 polymorphism in the context of PCOS and obesity in Saudi women because the prevalence of obesity is higher in women than in men (Alkhorayef et al., 2023), and rs5743708 polymorphism has been linked to a potential role in women. We investigated the molecular role of the rs5743708 polymorphism in PCOS women in Saudi Arabia using a case-control study.
2. Materials and methods
2.1. Saudi subejcts
A total of 220 Saudi women were enrolled in the study, with 110 cases of PCOS and 110 healthy controls or non-PCOS women. Both PCOS and non-PCOS women were selected from Capital city of Saudi Arabia. This study was carried out in outpatient clinic (OPC), Department of Obstetrics and Gynecology at King Khalid University Hospital (KKUH). The study recruitment took place at OPC premises during the first 11 months of 2021. All women participants involved in this study has provided their written informed consent. This study was provided based on Helsinki Declaration and ethical grant was sanctioned from Institutional Review Board in Medical College at King Saud University. The inclusion criteria of confirmed PCOS women are on the basis of Saudi nationality, signed consent form, Rotterdam criteria, polycystic and oligo or amenorrhea/anovulation. The Saudi women (i) who did not meet the Rotterdam criteria, (ii) were on medication, (iii) had other hormonal diseases, or (iv) were of another nationalities were excluded from PCOS cases (Al-Mutawa 2023). Non-PCOS Saudi women aged 18–40 years old with normal ovulation and a regular menstrual cycle between the 4th and 5th weeks were chosen. In terms of cases and controls, women from a mixed population were excluded from this study (Shetty et al., 2022). Saudi women who were pregnant were not considered for either PCOS or non-PCOS groups. PCOS and non-PCOS women were selectted between 18-40 years of age criteria. Among 220 Saudi women, no one was diagnosed with any type of infections (Based on filled questionnaire details). We also ruled out premature ovarian insufficiency.
2.2. BMI analysis
The investigation of this study was carried out by collecting the participants' health histories and documenting the information based on anthropmetric, biochemical, and clinical data from both PCOS and non-PCOS women. BMI information was defined as weight in kilograms and height in centimeters, with the formula kg/m2, and both overweight and obesity were defined according to WHO guidelines.
2.3. Varying types of blood analysis
A fasted blood sample was taken from 220 women recruited for this study. Peripheral blood was collected in 2 ml EDTA tubes, 4 ml plain vacutainers was used for collecting fasting serum in a coagulant tube.
2.4. Biochemical analysis
Fasting blood glucose (FBG), fasting insulin (FI), serum creatinine/Creatinine, follicle stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), total testosterone (TT), aspartate aminotransferase (AST), alanine transaminase (ALT), and lipid profile parameters including total cholesterol (TC), triglycerides (TG), high and low-density lipoprotein cholesterol (HDLc/LDLc) levels were measured.
2.5. rs5743708 analysis
A total of 220 blood samples were extracted for Molecular analysis. The Qiagen kit and protocol were used to extract genomic DNA (Qiagen DNA isolation kit, Hilden, Germany). A NanoDrop spectrophotometer was used to measure the quality of extracted genomic DNA. Polymerase chain reaction (PCR) was used for genotyping, which was then followed by Acil restriction enzyme. Our previous publication went into detailed explanation about the PCR protocol and methodology between CATALASE gene in the vitiligo subjects. The annealing temperature used in this study was 64 °C, and the following primers were used: (F: GCCTACTGGGTGGAGAACCT R: GGCCACTCCAGGTAGGTCTT). On a 2.5% agarose gel stained with ethidium bromide, both the PCR (341 bp) and digested products (G-305/36 bp and A-341 bp) were run.
2.6. Statistical analysis
Percentages, mean and standard deviations were calculated for both numerical and categorical data. To calculate the t-tests, Table 1 was compared between PCOS and non-PCOS women. In Table 2, Hardy-Weinberg Equilibrium (HWE) analysis was studied on rs5743708 polymorphism. In both groups, genotype and allele frequencies, as well as various genetic models (Table 3), was investigated using odds ratios (OR), 95% confidence intervals (CI), and p values. Multiple logistic regression analysis and one-way ANOVA was used to investigate the relationship between the rs5743708 polymorphism and demographic features in PCOS women (Table 4, Table 5). Fig. 1 was generated with origin software and p < 0.05 was considered as positive association.
Table 1.
Demographic details for PCOS and non-PCOS involved in this study.
| Reference range | PCOS (n = 110) | Non-PCOS (n = 110) | P value | |
|---|---|---|---|---|
| Age (Years) | 18–40 Years | 30.26 ± 5.77 | 31.47 ± 5.02 | 0.09 |
| Weight (kgs) | 44–112 | 74.26 ± 12.07 | 76.45 ± 11.18 | 0.16 |
| Height (cms) | 148–171 | 158.91 ± 4.97 | 158.17 ± 4.32 | 0.67 |
| BMI (kg/m2) | 17.5–42.7 | 29.29 ± 5.03 | 30.91 ± 5.19 | 0.71 |
| FBG (mmol/L) | 4.07–5.83 | 5.04 ± 0.85 | 4.38 ± 0.67 | < 0.0001 |
| Fasting Insulin (µIU/ml) | < 25 | 11.04 ± 6.40 | 9.17 ± 5.38 | 0.01 |
| Creatinine (mcmol/L) | 53–115 | 53.76 ± 13.24 | 47.39 ± 11.39 | 0.0001 |
| FSH (IU/mL) | 1.5–12.4 | 6.87 ± 2.71 | 6.41 ± 2.67 | 0.21 |
| LH (IU/mL) | 7.41 ± 4.55 | 7.10 ± 3.87 | 0.58 | |
| TSH (mIU/L) | 0.250–5.000 | 2.17 ± 0.85 | 2.08 ± 0.78 | 0.41 |
| Total testosterone (nmol/L) | 0.50–2.40 | 1.93 ± 0.89 | 0.92 ± 0.75 | < 0.0001 |
| AST (U/L) | 15–37 | 20.52 ± 8.14 | 18.67 ± 7.14 | 0.07 |
| ALT (U/L) | 16.0–61.0 | 25.34 ± 12.62 | 23.47 ± 12.06 | 0.26 |
| TC (mmol/L) | 1.63–3.20 | 5.14 ± 1.10 | 3.17 ± 0.98 | < 0.0001 |
| TG (mmol/L) | 0.50–2.27 | 1.85 ± 1.12 | 1.57 ± 1.04 | 0.055 |
| HDLc (mmol/L) | 0.80–1.63 | 0.69 ± 0.31 | 0.49 ± 0.28 | 0.0001 |
| LDLc (mmol/L) | 1.81–4.27 | 3.69 ± 0.93 | 3.15 ± 0.89 | 0.0001 |
| Menarche (Age) | 9–15 | 12.34 ± 1.19 | NA | NA |
| Other family Histories | NA | 38 (34.5%) | 28 (25.5%) | 0.01 |
| Family History of PCOS | NA | 35 (31.9%) | 0(0) | < 0.0001 |
NA = Not applicable/Not analyzed.
Table 2.
HWE analysis studied in rs5743708 polymorphism.
| PCOS women | non-PCOS women | |
|---|---|---|
| HWE-analysis | 0.09 | 0.07 |
| χ 2 | 22.275 | 13.948 |
| P value | 0.00002 | 0.0001 |
Table 3.
Genotype and allele frequencies in rs5743708 polymorphism in PCOS and non-PCOS women.
| Genotype and allele | PCOS (n = 110) | non-PCOS (n = 110) | χ 2 | ORs | 95%CI | P value |
|---|---|---|---|---|---|---|
| GG Genotype | 95 (86.4%) | 98 (89.1%) | Reference | Reference | Reference | Reference |
| GA Genotype | 10 (9.1%) | 09 (8.2%) | 0.08 | 1.14 | 0.44–2.94 | 0.77 |
| AA Genotype | 05 (4.5%) | 03 (2.7%) | 0.54 | 1.71 | 0.39–7.35 | 0.46 |
| GG vs GA + AA | 95 (86.4%) | 98 (89.1%) | 0.38 | 0.77 | 0.34–1.74 | 0.53 |
| GG + AA vs GA | 100 (90.9%) | 101 (91.8%) | 0.05 | 0.89 | 0.34–2.28 | 0.81 |
| GG + GA vs AA | 105 (95.5%) | 107 (97.3%) | 0.51 | 0.58 | 0.13–2.52 | 0.47 |
| G allele | 200 (90.1%) | 205 (93.2%) | Reference | Reference | Reference | Reference |
| A allele | 20 (8.9%) | 15 (6.8%) | 0.77 | 1.36 | 0.68–2.74 | 0.37 |
Table 4.
Multiple logistic regression analysis performed between rs5743708 polymorphism and PCOS women.
| Dependent variables | R-value | Adjusted R square | F | p value |
|---|---|---|---|---|
| Age | 0.015 | −0.009 | 0.025 | 0.874 |
| Weight | 0.487 | 0.230 | 33.53 | < 0.001 |
| BMI | 0.481 | 0.224 | 32.45 | < 0.001 |
| FBG | 0.154 | 0.015 | 2.62 | 0.108 |
| FI | 0.183 | 0.025 | 3.71 | 0.057 |
| Creatinine | 0.110 | 0.003 | 1.32 | 0.252 |
| Total testosterone | 0.042 | −0.008 | 0.18 | 0.666 |
| TG | 0.005 | −0.009 | 0.003 | 0.957 |
| TC | 0.006 | −0.009 | 0.004 | 0.952 |
| HDL-C | 0.131 | 0.008 | 1.882 | 0.173 |
| LDL-C | 0.003 | −0.009 | 0.001 | 0.979 |
Table 5.
Anova analysis studied between GG, GA and AA genotypes and PCOS demographics.
| GG (n = 95) | GA (n = 10) | AA (n = 05) | P value | |
|---|---|---|---|---|
| Age (Years) | 30.32 ± 5.48 | 28.60 ± 7.57 | 32.40 ± 7.64 | 0.46 |
| Weight (kgs) | 71.91 ± 10.57 | 87.60 ± 9.75 | 92.42 ± 11.93 | 0.0002 |
| BMI (kg/m2) | 28.26 ± 4.44 | 35.90 ± 3.30 | 35.66 ± 4.16 | 0.0001 |
| FBG (mmol/L) | 5.01 ± 0.80 | 5.13 ± 0.74 | 5.65 ± 1.79 | 0.25 |
| FI (µIU/ml) | 10.68 ± 6.06 | 11.50 ± 7.46 | 16.84 ± 9.04 | 0.10 |
| Creatinine | 53.22 ± 13.36 | 56.20 ± 15.60 | 59.20 ± 7.26 | 0.52 |
| TC (mmol/L) | 5.13 ± 1.08 | 5.49 ± 1.46 | 4.83 ± 0.73 | 0.51 |
| TG (mmol/L) | 1.81 ± 1.03 | 2.58 ± 1.74 | 1.15 ± 0.26 | 0.03 |
| HDLc (mmol/L) | 0.68 ± 0.28 | 0.80 ± 0.50 | 0.80 ± 0.20 | 0.36 |
| LDLc (mmol/L) | 3.69 ± 0.95 | 3.80 ± 0.98 | 3.60 ± 0.66 | 0.91 |
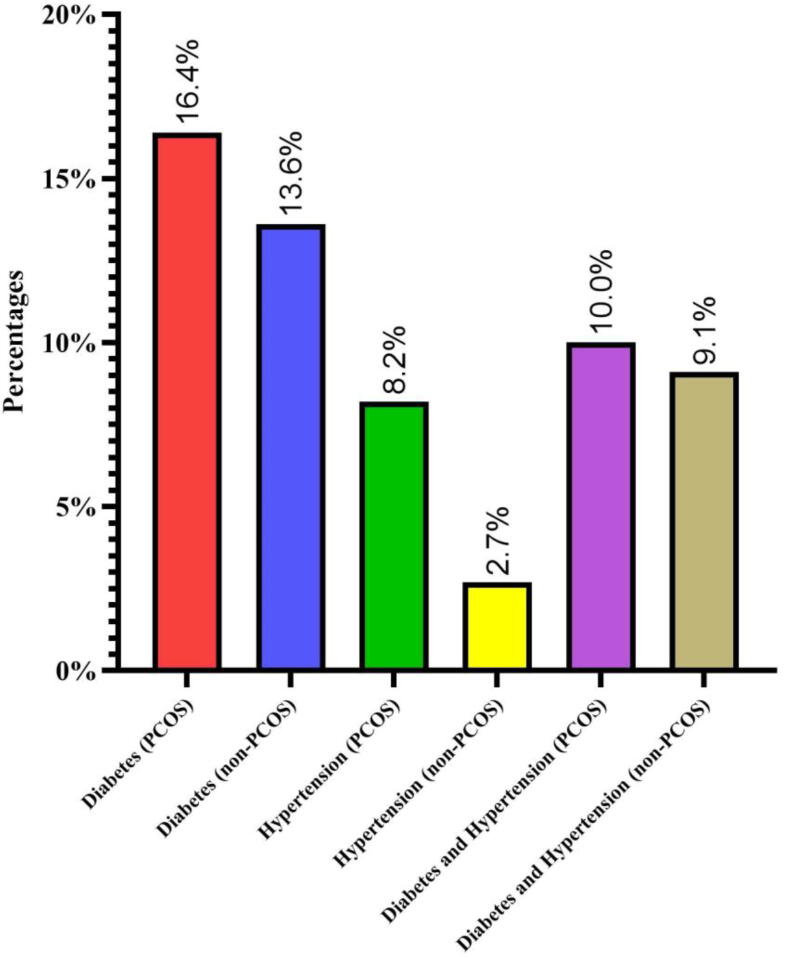
Fig. 1.
Presence of additional family histories in PCOS and non-PCOS women.
3. Results
3.1. PCOS and non-PCOS characteristics
In this case-control study, 110 PCOS and 110 non-PCOS women were selected. The main concept of this study was to enroll age and BMI matching subjects and the mean age of PCOS and non-PCOS was 30.26 ± 5.77 and 31.47 ± 5.02 (p = 0.09). Weight (p = 0.16) and BMI (p = 0.71) was found to have elevated levels in non-PCOS when compared with PCOS subjects. Height was found to be almost similar in both the groups (p = 0.67). PCOS has the elevated levels of FBG (p < 0.0001), FI (p = 0.01) and serum creatinine levels (p = 0.0001) in comparison with non-PCOS (p < 0.05). Thyroid parameters such as FSH, LH and TSH were non-associated in both the groups (p > 0.05). High levels of TT were found in PCOS subjects and associated (p < 0.0001). Both AST and ALT were not associated in PCOS vs non-PCOS women (p > 0.05). In lipid profile, TG was not associated (p = 0.055), whereas, HDLc, LDLc and TC was associated (p < 0.05). The details of base line features were shown in Table 1.
3.2. Analysis of HWE
Table 2 defines the HWE analysis for PCOS and non-PCOS subjects in rs5743708 polymorphism. The χ2 values for both PCOS and non-PCOS was found to be 22.275 and 13.948. Both PCOS (p = 0.00002) and non-PCOS (p = 0.0001) subjects showed statistical significance.
3.3. Genetic analysis for rs5743708 polymorphism
The GG, GA and AA genotypes of PCOS women were found to be 86.4%, 9.1%, 4.5% and 89.1%, 8.2%, 2.7% were found in non-PCOS women. Genotype analysis showed negative associated with heterozygous and AA homozygous genotypes i.e., (GA vs GG: OR-1.14[95%CI:0.44–2.94]; p = 0.77 and AA vs GG: OR-1.71 [95%CI: 0.39–7.35]; p = 0.46). Different genetic models (GG vs GA + AA: OR-0.77 [95%CI: 0.34–1.74]; p = 0.53, GG + AA vs GA: OR-0.89 [95%CI: 0.34–2.28]; p = 0.81 and GG + GA vs AA: OR-0.58 [95%CI: 0.13–2.52]; p = 0.47) failed to show the association between cases and controls. G and A alleles in PCOS and non-PCOS women had 90.9%/ 8.9% and 93.2%/6.8%. Allelic association was not present (A vs G: OR-1.36 [95%CI: 0.68–2.74]; p = 0.37). All the details were showed in Table 3.
3.4. Regression results
Linear regression analysis was performed with 10 dependent variables and rs5743708 polymorphism as independent variable. The 10 variables included in this study were Age, Weight, BMI, FBG, FI, Serum creatinine, TT, TG, TC, HDLc and LDLc. The current study results for regression model confirmed both weight and BMI was strongly associated (p < 0.0001). However, other parameters remain constant with non-significant association (p > 0.05). The complete details of regression analysis were showed in Table 4.
3.5. Anova analysis
Table 5 describes Anova analysis between GG, GA and AA genotypes in rs5743708 polymorphism and 10 dependent variables as defined in Table 4. In this analysis, 50% of each elevated levels were found in GA and AA genotypes. BMI (35.90 ± 3.30), TC (5.49 ± 1.46), TG (2.58 ± 1.74), HDLc (0.80 ± 0.50) and LDLc (3.80 ± 0.98) levels were found to be high in GA genotypes. The elevated levels in AA genotypes were found to be Age (32.40 ± 7.64), Weight (92.42 ± 11.93), FBG (5.65 ± 1.79), FI (16.84 ± 9.04) and serum creatinine (59.20 ± 7.26). Anova analysis showed weight (p = 0.0002), BMI (p = 0.0001) and TG (p = 0.03) was associated in comparison with 3 genotypes present in rs5743708 polymorphism.
4. Discussion
PCOS is a syndrome characterized by multiple cysts in the ovary. Acne and hirsutism, or excessive hair growth, were frequently observed in PCOS women. In general, PCOS is associated with obesity & IR, affecting 65–80% of women, and there is a link between hyperandrogenism, hyperinsulinemia, and obesity. PCOS is also associated with chronic inflammation, which leads to increased adipose tissue defined by a pro-inflammatory mechanism. The TLR gene family has been linked to chronic inflammation, and we chose rs5743708 polymorphism in the TLR2 gene for this study. This polymorphism (R753Q) has an amino acid substitution from cytosine to thymidine. PCOS increases the risk of human health diseases in women, lowering the patient's quality of life. In obstetrics and gynecology, treating PCOS women remains difficult. PCOS is primarily treated with antiandrogen drugs (Wu et al., 2023).
Among all the forms, there was no genetic correlation between PCOS and non-PCOS (p > 0.05). The results of HWE study supported the rs5743708 polymorphism (p < 0.05). Weight and BMI were frequently associated in both linear regression and Anova analysis (p < 0.05), while TG was discovered to be positively associated in the latter (p < 0.05). One of the common reasons could be female obesity has played a role in rs5743708 polymorphism in Saudi subjects (n = 220). Anthropometric measurements such as weight, height and BMI was not associated between PCOS and non-PCOS women (p > 0.05). On other side, the mean of BMI was found to be obese in the control women (30.91 ± 5.19) when compared with PCOS women (29.29 ± 5.03). This indicates rs5743708 polymorphism plays a role in the Saudi obese subejcts.
Previous studies conducted with PCOS women around the globe revealed similar findings to our current study, which showed no association (Ojeda-Ojeda et al., 2016, Kuliczkowska-Płaksej et al., 2021). However, other polymorphisms such as rs3804100, rs4986790 and rs4986791 was associated in the Poland women (Kuliczkowska-Płaksej et al., 2021). Unfortunately, no meta-analysis studies were documented between rs5743708 polymorphism and PCOS. In the human diseases, the rs5743708 polymorphism was studied using various meta-analysis studies (Wang et al., 2013, Gao et al., 2017, Shan et al., 2020, Zhou and Zhang, 2020).
Family history plays an important role in the Saudi Arabia. In general, family history of any specific diseases can be used to prevent, diagnose and treat human diseases running in the families. Additionally, consanguinity plays an additional role in replicating the inheritance. In this study, PCOS women had a family history of 34.5% with documented chronic diseases in which 16.4% was confirmed as diabetes, 8.2% with hypertension (HTN) and 10% with the combination of both diabetes and HTN. The non-PCOS women had 25.5% of family history of chronic diseases such as diabetes (13.6%), HTN (2.7%) and combination of both diabetes and HTN (9.1%). Figure-1 was designed to show various prevalence of family history of chronic diseases in both PCOS and non-PCOS. The family history of PCOS was found to be 31.9% in PCOS group and there was no family history of PCOS in the control group which was one of the designed criteria of this study.
Elevated TT levels was found in PCOS women along with free testosterone. The reference range of TT was in between 0.5 and 2.4 nmol/L and in PCOS women were documented with 1.93 ± 0.89 and in control women, it was 0.92 ± 0.75. In this study, a strong correlation was observed (p < 0.0001). Previous studies have documented the relation between FBG and FI which leads to MetS (Fu et al., 2023). In our study, FBG and FI was found to be significantly associated (p < 0.05). Serum creatinine is also connected with both MetS and chronic kidney disease (Al-Hail et al., 2019). In this study, PCOS women has confirmed the positive association in serum creatinine levels. The overall, analysis concludes as the individual combination of TT, FBG, FI and serum creatinine will lead to future complications in the PCOS women in the Saudi Arabia.
One of the limitations of this study was biochemical data was transferred from KKUH as these tests were routinely performed when patient visits to OPC and it can be considered as secondary data. Screening of a single polymorphism and missing of serum levels will be another limitations of this study. One of the strengths of this study can be confirmed as an enrollment of Saudi women (n=220) towards this study rather mixed women study.
5. Conclusion
This study concludes as rs5743708 polymorphism has no role in PCOS women in the Saudi Arabia. This may be due to obesity plays a role in both PCOS and non-PCOS subjects. The accurate prevalence of PCOS in Saudi Arabia was not documented till now and future studies should work on it.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSPD2023R375), King Saud University, Riyadh, Saudi Arabia, for funding this project.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Amal F. Alshammary, Email: aalshammary@ksu.edu.sa.
Abdulrahman M. Alshammari, Email: abdalshammari@ksu.edu.sa.
Raed Farzan, Email: rfarzan@ksu.edu.sa.
Sarah F. Alsobaie, Email: salsobaie@ksu.edu.sa.
Arwa A. Alageel, Email: aaalageel@ksu.edu.sa.
Imran Ali Khan, Email: imkhan@ksu.edu.sa.
References
- Aboeldalyl S., James C., Seyam E., et al. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int. J. Mol. Sci. 2021;22(5):2734. doi: 10.3390/ijms22052734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hail N., Butler A.E., Dargham S.R., et al. Creatine kinase is a marker of metabolic syndrome in Qatari women with and without polycystic ovarian syndrome. Front. Endocrinol. 2019;10:659. doi: 10.3389/fendo.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhorayef, N., Almutery, F.T., Rasheed, Z., et al., 2023. Regulatory effects of ketogenic diet on the inflammatory response in obese Saudi women. Journal of Taibah University Medical Sciences. [DOI] [PMC free article] [PubMed]
- Al-Mutawa J. Genetic contribution between APE1 variants in Polycystic Ovarian Syndrome. Saudi J. Biol. Sci. 2023:103563. doi: 10.1016/j.sjbs.2023.103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A.E., Moin A.S.M., Reiner Ž., et al. HDL-associated proteins in subjects with Polycystic Ovary Syndrome: a proteomic study. Cells. 2023;12(6):855. doi: 10.3390/cells12060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE, T.R., Group, A.-S.P.C.W., 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility 81 (1), 19–25. [DOI] [PubMed]
- Fu L., Xie N., Qu F., et al. The association between polycystic ovary syndrome and metabolic syndrome in adolescents: a systematic review and meta-analysis. Reprod. Sci. 2023;30(1):28–40. doi: 10.1007/s43032-022-00864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Xiao H., Wang Y., et al. Association of single-nucleotide polymorphisms in toll-like receptor 2 gene with asthma susceptibility: a meta-analysis. Medicine. 2017;96(20) doi: 10.1097/MD.0000000000006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiam D., Moreno-Asso A., Teede H.J., et al. The genetics of polycystic ovary syndrome: an overview of candidate gene systematic reviews and genome-wide association studies. J. Clin. Med. 2019;8(10):1606. doi: 10.3390/jcm8101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabarpour M., Aleyasin A., Nashtaei M.S., et al. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: a randomized clinical trial. Sci. Rep. 2023;13(1):3376. doi: 10.1038/s41598-023-28956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliczkowska-Płaksej J., Jończyk M., Jawiarczyk-Przybyłowska A., et al. The frequency of TLR2 (rs3804099, rs3804100, and rs5743708) and TLR4 (rs4986790 and rs4986791) polymorphisms in women with polycystic ovary syndrome–preliminary study. Gynecol. Endocrinol. 2021;37(11):1027–1034. doi: 10.1080/09513590.2021.1952975. [DOI] [PubMed] [Google Scholar]
- Liu D., Gao X., Pan X.-F., et al. The hepato-ovarian axis: genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023;21(1):62. doi: 10.1186/s12916-023-02775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda-Ojeda M., Martínez-García M.Á., Alpañés M., et al. Association of TLR2 S450S and ICAM1 K469E polymorphisms with polycystic ovary syndrome (PCOS) and obesity. J. Reprod. Immunol. 2016;113:9–15. doi: 10.1016/j.jri.2015.09.072. [DOI] [PubMed] [Google Scholar]
- Pei Y., Risal S., Jiang H., et al. Transcriptomic survey of key reproductive and metabolic tissues in mouse models of polycystic ovary syndrome. Commun. Biol. 2023;6(1):69. doi: 10.1038/s42003-022-04362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, C., Aisaiti, A., Wu, Z.P., et al., 2020. Association of TLR-2 gene polymorphisms with the risk of periodontitis: a meta-analysis. Disease Markers 2020. [DOI] [PMC free article] [PubMed]
- Shetty S.S., Kumari N.S., Hegde P., et al. Leptin gene polymorphism Rs7799039; G2548A, metabolic and oxidative stress markers in polycystic ovarian syndrome. J. King Saud Univ.-Sci. 2022;34(6) [Google Scholar]
- Soydas T., Karaman O., Arkan H., et al. The correlation of increased CRP levels with NFKB 1 and TLR 2 polymorphisms in the case of morbid obesity. Scand. J. Immunol. 2016;84(5):278–283. doi: 10.1111/sji.12471. [DOI] [PubMed] [Google Scholar]
- Udesen P.B., Sørensen A.E., Svendsen R., et al. Circulating miRNAs IN Women with Polycystic Ovary Syndrome: a longitudinal cohort study. Cells. 2023;12(7):983. doi: 10.3390/cells12070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-Q., Liu L., Liu Y., et al. TLR-2 gene polymorphisms and susceptibility to cancer: evidence from meta-analysis. Genet. Test. Mol. Biomarkers. 2013;17(12):864–872. doi: 10.1089/gtmb.2013.0246. [DOI] [PubMed] [Google Scholar]
- Wu M., Liu H., Zhang J., et al. The mechanism of Leonuri Herba in improving polycystic ovary syndrome was analyzed based on network pharmacology and molecular docking. J. Pharm. Pharm. Sci. 2023;6 doi: 10.3389/jpps.2023.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C., Zhang J., Zhao H., et al. Effect of sex hormone-binding globulin on polycystic ovary syndrome: mechanisms, manifestations, genetics, and treatment. Int. J. Womens Health. 2022:91–105. doi: 10.2147/IJWH.S344542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang M. Associations between genetic polymorphisms of TLRs and susceptibility to tuberculosis: a meta-analysis. Innate Immun. 2020;26(2):75–83. doi: 10.1177/1753425919862354. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Shah, M.Z.u.h., Shrivastava, V.K., 2023. Ameliorative effects of quercetin on endocrine and metabolic abnormalities associated with experimentally induced polycystic ovary syndrome in mice. Comparative Clinical Pathology. 1–9.