Abstract
Yellow cosmos (Cosmos sulphureus Cav.) is a specific flowering plant and considered a suitable genetic engineering model. Agrobacterium-mediated plant transformation is commonly used for plant genetic engineering. Floral dip transformation is one of the plant genetic transformation methods, and it involves dipping flower buds into an Agrobacterium suspension. Studies on floral dip transformation of yellow cosmos have never been reported. Therefore, an efficient method in plant genetic engineering must be established. This study developed an effective and efficient floral dip transformation method for yellow cosmos.
In this study, flower buds with sizes of 5–7 mm were used. Several parameters have been observed to optimize the floral dip method. These parameters included the optical density (OD600) of Agrobacterium culture, concentration of surfactant, and duration of flower bud dipping into the Agrobacterium suspension.
The results showed that the floral dip method was most efficient when the flower buds were dipped into Agrobacterium suspension with OD600 = 0.8 and containing 5% sucrose and 0.1% Silwet L-77 for 30 s. This method enhanced the transformation efficiency at a rate of 12.78 ± 1.53%. The neomycin phosphotransferase II and green fluorescent protein genes with sizes of 550 and 736 bp, respectively, were confirmed by polymerase chain reaction. In addition, the transgenic plants were kanamycin resistant and fluorescent under ultraviolet light observation. This finding suggests that the proposed floral dip transformation provides new insights into efficient plant genetic engineering methods for yellow cosmos.
Keywords: Floral dip, OD, Surfactant, Transgenic, Silwet L-77, Yellow cosmos
1. Introduction
Yellow cosmos (Cosmos sulphureus Cav.) is an annual tropical plant that belongs to the Asteraceae family and has a golden-yellow flower. In general, each flower has approximately 15–30 flower tubes with a stalk length of 15 cm (Hilmi et al., 2020, Win, 2016). Recently, yellow cosmos has been reported to contain high levels of secondary metabolites, such as flavonoids, phenolics, tannins, and gallic acid, which can be used as natural pesticides and herbicides (Aftab et al., 2021, Respatie et al., 2019, Saleem et al., 2017). Yellow cosmos produces daisy-like flowers with seeds atop long and slender stems. Moreover, the cosmos plant has many flowers, pollination can be controlled, and seeds can be produced quickly. This plant is a suitable model for genetic engineering using the floral dip method.
Several methods, including Agrobacterium-mediated plant transformation, are used for plant genetic engineering (Keshavareddy et al., 2018, Mayavan et al., 2015). Agrobacterium-mediated transformation is the most widely used plant genetic engineering method because this technique is low cost, simple, and efficient for plant genetic transformation methods. Agrobacterium tumefaciens from soil bacteria causes crown gall disease through open tissues to colonize plant cells. The advantage of Agrobacterium is that it contains a tumor-inducing plasmid (Ti-plasmid), virulence genes (vir), and chromosomal virulence (chv) (Silalahi et al., 2021, Subramanyam et al., 2015). Therefore, plant genetic transformation heavily relies on A. tumefaciens as a powerful tool for the transfer of genes into the plant genome.
To date, genetic transformation methods are often carried out through plant tissue culture. The disadvantages of plant genetic transformation using tissue culture-based methods are the high cost, laboriousness, and easy contamination by bacteria and fungi Morphogenesis and organogenesis in tissue culture processes require a considerable long time from culture until acclimatization (Handayani et al., 2022; Jakhar et al., 2019). Therefore, an efficient genetic transformation method must be developed. Tissue culture-independent–based transformation using the in planta method may be an option to overcome this problem without conducting tissue culture (Chumakov and Moiseeva, 2012, Yaroshko et al., 2020).
Floral dip method is one of the in planta genetic transformation procedures, and it is performed by dipping flowers into an Agrobacterium suspension in a time-dependent manner (Bastaki and Cullis, 2014, Rod-In et al., 2014). The floral dip method does not require a particular tissue culture skill, eliminates soma-clonal variations, and is affordable (Chumakov et al., 2006, Eck, 2018, Zhang et al., 2017). The floral dip method is influenced by several factors, such as the density of bacteria, the presence of sucrose, concentration of surfactants, and dipping duration. Bacterial density indicates the amount of bacterial growth in a medium suspension (Clough and Bent, 1998). Sucrose serves as an energy source for bacterial growth (Subramanyam et al., 2015). The duration of target organ dipping into the Agrobacterium suspension provides the bacteria with the opportunity to process gene insertion (Zhang et al., 2006). Meanwhile, surfactants can reduce the surface tension of target organs and act as attractants to increase the penetration of bacteria into plant tissues (Curtis and Nam, 2001). Studies of floral dip transformation has been reported for Arabidopsis thaliana (Bent, 2006, Clough and Bent, 1998, Narusaka et al., 2010, Zhang et al., 2006), Raphanus sativus (Curtis and Nam, 2001), Scherenkiella parvula (Wang et al., 2019), Amaranthus caudatus L. (Yaroshko et al., 2018) and Zea mays (Guiqin et al., 2012). However, the transformation efficiency was<5%. A higher transformation efficiency was obtained in Solanum lycopersicum L.(Yasmeen et al., 2009), Oryza sativa (Rod-In et al., 2014), A. thaliana (Ali et al., 2022a, Ali et al., 2022b) and Linum usitatissimum (Bastaki and Cullis, 2014).
The application of floral dip transformation method in yellow cosmos has not been reported. Therefore, this study is important for improving the efficiency of plant breeding through a biotechnological approach. This research aimed to develop an effective and efficient floral dip transformation method for yellow cosmos.
2. Materials and methods
2.1. Plant material
C. sulphureus Cav. Plants were planted in a mixture of soil medium and organic manure (1:1) in a polybag at the Green House, Faculty of Agriculture, Universitas Gadjah Mada, Yogyakarta, Indonesia. The plants were maintained until the generative phase after 10 weeks for floral dip transformation.
2.2. Agrobacterium tumefaciens preparation
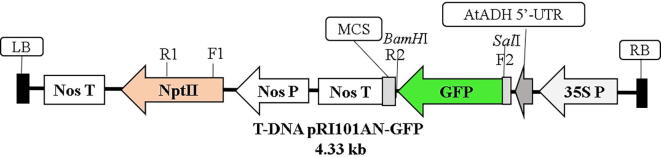
An Agrobacterium tumefaciens strain GV3101 containing the recombinant vector pRI101AN- green fluorescent protein (GFP) was provided by Prof. Bambang Sugiharto of The Center for Development of Advanced Science and Technology, University of Jember, Indonesia. Agrobacterium containing the GFP-encoding gene was confirmed by polymerase chain reaction (PCR) colony using two pairs of primers: neomycin phosphotransferase II (NPTII) and GFP. Detection vector using two genes was utilized to confirm the presence of T-DNA (Fig. 1). The composition of the PCR mix will be described Section 2.7. The NPTII gene had a band size of 550 bp, and the full-length GFP had band size of 736 bp.
Fig. 1.
T-DNA physical map of the recombinant vector pRI101AN-GFP.
2.3. Floral dip transformation
The Agrobacterium suspension was prepared by the addition of 5% sucrose and Silwet L-77. To establish the floral dip transformation method in yellow cosmos, we applied two different optical densities (OD600) of Agrobacterium culture, i.e., 1.5 and 0.8, in the first experiment. The different durations of bud dipping were 30, 60, and 90 s. In the second experiment, the suspension was added with varied concentrations of Silwet L-77, i.e., 0%, 0.05% and 0.1%. Ten buds were used in each treatment.
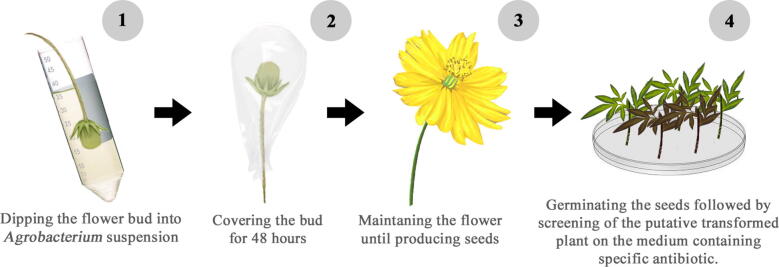
The floral dip transformation method was started by preparing an Agrobacterium suspension and then dipping the flower buds until they were covered by the suspension. Fig. 2 presents the procedure of the floral dip transformation method. A colony of A. tumefaciens strain GV3101 carrying the recombinant vector pRI101AN-GFP was cultured in a starter medium. It was cultured in 5 mL Luria Bertani (LB) medium containing 50 ppm kanamycin, 100 ppm rifampicin, and 12.5 ppm gentamicin in the dark for overnight and with shaking at 200 rpm at 27 °C. The starter was transferred to 50 mL LB medium containing the same antibiotics and then shaken at 250 rpm at 27 °C to obtain OD600 = 1.5, 0.8. Afterward, the suspension at OD600 = 0.8 was transferred to a new conical tube, and 5% sucrose and Silwet L-77 were added to each treatment. Flower buds with sizes of 0.5–0.7 mm were used (Fig. 3a). The infection was carried out by dipping the flower buds into the Agrobacterium suspension for each treatment period (Fig. 3b) and covering them with a paper for 48 h to maintain high humidity (Fig. 3c). Finally, the flowers were maintained for 3 weeks until the seeds were physiologically ripe. The seeds were harvested and dried at room temperature.
Fig. 2.
Floral dip transformation of yellow cosmos C. sulphureus Cav.
Fig. 3.
Materials used in floral dip transformation of yellow cosmos C. sulphureus Cav. a) Cosmos flower buds with sizes of 5–7 mm, b) floral dip transformation process, and c) covering of flower buds for 48 h.
2.4. Screening of seedling on the medium containing kanamycin
The harvested seeds were soaked in 2 gL−1 fungicide (DhitaneTM M−45) containing mancozeb 80% and bactericide (Agrept® 20WP) containing 20% streptomycin sulfate for 30 min. Then, the seeds were rinsed with distilled water and germinated on a medium using a filter paper with 50 ppm kanamycin for 7 days. Kanamycin-resistant seedlings were transplanted to the growth medium composed of mixtures of soil and manure in a screen house until the generative period.
2.5. Detection of GFP in the assay of T1 cosmos resistant to kanamycin
Both leaves and steam collected from one month after planting screened and wild-type plants were used for the detection of fluorescence GFP gene. The samples were visualized using an ultraviolet (UV) transilluminator (Scope WD, Japan). Then, they were documented in light and dark conditions using a digital camera.
2.6. Genomic DNA extraction
Yellow cosmos leaves were extracted using the cetyl trimethyl ammonium bromide (CTAB) method (Doyle and Doyle, 1990) with modifications. Then, 100 mg leaves were grinded using pastel stick added with liquid N2 in a 2 mL tube. The samples were added with 1.400 µL buffer (2% CTAB, 0.1 M Tris-HCl pH 8.0, 1.4 M NaCl, 0.02 M ethylenediaminetetraacetic acid, 2% polyvinylpyrrolidone, 2% β-mercapto-ethanol, and ddH2O) and then incubated in a water bath at 65 °C for 60 min. Afterward, the samples were centrifuged at 12,000 rpm for 10 min using Minispin® (Eppendorf, Germany). The supernatants were transferred to a new 1.5 mL tube and added with 500 µL chloroform isoamyl alcohol (CIA). The samples were vortexed and centrifuged at 12,000 rpm for 15 min. The supernatants were transferred to a new tube and added with 500 µL CIA followed by vortexing and centrifugation in a similar manner. The supernatants were transferred to a 1.5 mL new tube and added with 1/10 from a solution of sodium acetate and 2/3 from a total solution of cold isopropanol. The solutions were stored overnight at − 20 °C. Afterward, the solutions were centrifuged at 12,000 rpm for 15 min, and the flowthrough was discarded. The tubes were added with 500 µL 70% ethanol and centrifuged at 12,000 rpm for 5 min. The flowthrough was discarded, and 500 µL ethanol absolute was added. The solutions were centrifuged at 12,000 rpm for 5 min, the flowthrough was discarded, and the pellets were dried at room temperature. Finally, the pellets were added with 50 µL ddH2O, and the DNA samples were stored at − 20 °C.
2.7. PCR analysis
PCR was used to detect NPTII and GFP genes in transgenic plants. The PCR reaction mixture consisted of 5 µL Powerpol 2X PCR Mix with Dye® (ABclonal, USA), 2 µL nuclease-free water, 1 µL DNA sample, and 1 µL of each forward and reverse primer (10 mM). Amplification of the NPTII gene was performed using a forward primer and a reverse primer with a size product of 550 bp. The GFP gene was amplified using a forward primer and a reverse primer with a product size of 736 bp (Table 1). DNA amplification was conducted using a PCR T100TM Thermal Cycler (Bio-Rad, USA). Amplification was carried out with thermocycling settings consisting of predenaturation at 95 °C for 3 min, followed by 39 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for the NPTII gene and 58 °C for the GFP gene, elongation at 72 °C for 1 min, and final elongation 72 °C for 5 min. The PCR products were separated by electrophoresis Powerpac-BasicTM (Bio-Rad, USA) in 1% agarose gel added with 5 µL DNA stain for a 30 mL solution gel. The plate was added with 5 µL ladder AccuBandTM 100 bp + 3 K DNA Ladder II (Smobio, Taiwan) as the marker band, 10 µL PCR product of the plasmid as the positive marker, wild-type plant, and each putative transgenic plant. Lastly, the agarose gel was visualized in a UV transilluminator (Scope WD, Japan).
Table 1.
Primers used for PCR analysis of NPTII and GFP genes in putative transformed plants.
| Primer | Sequence | GC contents | Tm (°C) | Size product |
|---|---|---|---|---|
| NPTII-F1 | 5′- GTCATCTCACCTTGCTCCTGCC −3′ | 59% | 60 | 550 bp |
| NPTII-R1 | 5′- GTCCGTTGGTCGGTCATTTCG −3′ | 57.14% | ||
| GFP-F2 | 5′- GCTGTCGACATGCGTAAAGG −3′ | 52.38% | 58 | 736 bp |
| GFP-R2 | 5′- GCGCGGATCCTTATTTGTATAG −3′ | 45.45% |
2.8. Data analysis
Data from different levels of OD, durations, and Silwet L-77 were analyzed using the analysis of variance. The significant data were analyzed using DMRT α < 0.05. Then, the comparison of level bacterial density was analyzed by t test with α < 0.05 using R studio.
3. Results
3.1. Confirmation of the presence of binary vector in Agrobacterium tumefaciens
A. tumefaciens strain GV3101 containing the binary vector pRI101AN-GFP was cultured in LB medium with selective antibiotics. The results of the PCR colony A. tumefaciens showed that the single amplicon size was 550 bp for NPTII and 736 bp for GFP (Fig. 4). The target sequences were amplified using NPTII and GFP genes as selectable markers. This A. tumefaciens strain can be used for genetic transformation using the floral dip method.
Fig. 4.
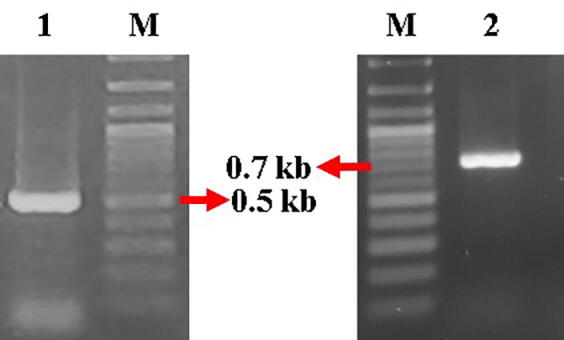
Confirmation of A. tumefaciens colonies containing the recombinant vector pRI101AN-GFP in PCR using the primers of NPTII and GFP. M: 100 bp ladder, 1: amplicon NPTII gene with a band size 550 bp; 2: amplicon GFP gene with a band size 736 bp.
3.2. Effect of floral dipping on the number of seeds produced
The results showed that the difference in OD and dipping duration had an effect on the average number of seeds produced per flower. A high OD resulted in a small number of seeds. Likewise, the increase in dipping duration produced a few seeds (Table 2). This result showed that the high density of Agrobacterium suspension and long dipping period caused a decrease in the amount of seeds produced.
Table 2.
Effect of OD, dipping duration, and Silwet L-77 on the average number of seeds and plants resistant to kanamycin and transformation efficiency of T1 C. sulphureus Cav.
|
Treatments |
Results |
||||||
|---|---|---|---|---|---|---|---|
| Experiment | Agrobacterium suspension | Silwet L-77 (%) | Duration (s) | Number of seeds | Number of seedlings | Resistant to Kanamycin (%) | Transformation efficiency (%) |
| Control | 0 | 0 | 14.5 ± 2.7a | 0 ± 0d | 0 ± 0c | 0 ± 0b | |
| I | 0 | 30 | 3.40 ± 1.5d | 0.8 ± 1.0c | 23.06 ± 23.6b | 0 ± 0b | |
| OD600 = 1.5 | 0 | 60 | 10.2 ± 3.2bc | 4.6 ± 1.6a | 45.32 ± 11.7a | 0 ± 0b | |
| 0 | 90 | 2.00 ± 1.7d | 0.9 ± 1.1c | 26.00 ± 28.6b | 0 ± 0b | ||
| 0 | 30 | 12.3 ± 2.0a | 1.8 ± 1.3bc | 14.63 ± 11.9b | 8.26 ± 6.5a | ||
| OD600 = 0.8 | 0 | 60 | 9.00 ± 2.7c | 2.2 ± 1.4b | 24.97 ± 15.8b | 2.26 ± 4.9b | |
| 0 | 90 | 11.1 ± 2.4ab | 1.3 ± 1.4bc | 11.71 ± 10.5b | 0 ± 0b | ||
| II | OD600 = 0.8 + 5% sucrose | 0 | 30 | 15.4 ± 4.0a | 4.2 ± 3.2a | 25.35 ± 19.2a | 9.74 ± 2.0b |
| 0.05 | 30 | 12.9 ± 2.5a | 4.2 ± 3.6a | 32.52 ± 29.2a | 9.38 ± 2.3b | ||
| 0.1 | 30 | 13.3 ± 4.8a | 4.5 ± 3.0a | 35.11 ± 19.4a | 12.78 ± 1.5a | ||
Note that the number followed by the same letters is not significantly different based on the DMRT test; α < 0.05.
3.3. Screening of seedlings resistant to kanamycin
Based on experiment 1 (Table 2), a lower percentage of kanamycin-resistant plants were obtained using Agrobacterium suspension at OD600 = 0.8 for 30 s obtained compared with those at OD600 = 1.5, whose percentage reached 45.32%. The plants survived on the selection media were suggested as putative transformants. These results indicate that the plants contained the NPTII gene as a kanamycin resistance antibiotic. In vivo plant screening using filter paper media with kanamycin was more efficient than in vitro screening (Fig. 5a). The grown plants were then transferred to the soil media in polybags maintained in the greenhouse until T2 seeds were obtained (Fig. 5b).
Fig. 5.
Screening of C. sulphureus Cav. putative transformants in the selection medium containing 50 ppm kanamycin. a) Wild-type seeds did not germinate on the selection medium after 7 days. b) Seedlings selected from the selection medium containing 50 ppm kanamycin after 7 days. c) Transformed cosmos plants at age of 6 weeks. WT: Wild type; T1a and T1b: first-generation transgenic cosmos plants.
3.4. GFP assay of T1 putative transformant plants
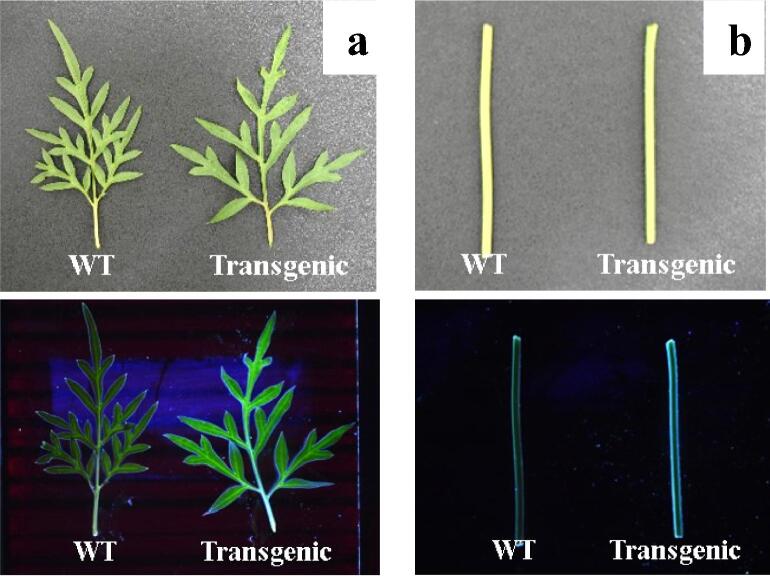
Based on the results and detection of GFP signals, some plants have been selected from the medium containing kanamycin. Their leaf and stem samples were fluorescent under UV light. In addition, other plant and wild-type samples showed no fluorescence (Fig. 6). This finding indicates that the GFP gene has been inserted into the genome of the cosmos plant. GFP is a reporter gene whose expression can be detected visually without molecular analysis. It can assist in the selection process of transgenic plants. To ensure that transgenic plants were obtained, we performed PCR to detect the NPTII and GFP genes in the plant DNA.
Fig. 6.
GFP assay of T1 yellow cosmos organs using UV light: a) leaves from wild-type and transgenic plants in light condition (upper) and UV transilluminator (lower); b) stems from wild-type and transgenic plants in light condition (upper) and UV transilluminator (lower).
3.5. PCR analysis of T1 transgenic yellow cosmos
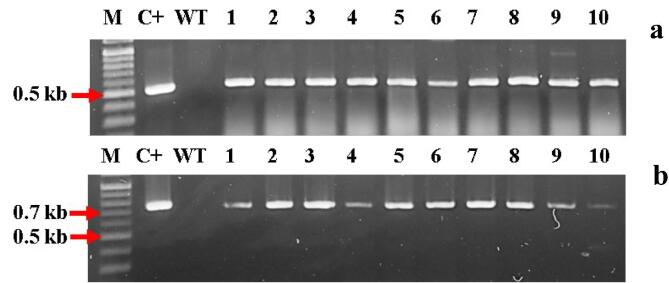
Molecular analysis of DNA genome from putative transformed plants in the selection medium and wild-type plants was carried out by amplification of specific primers for NPTII and GFP genes. Based on the first experiment, bacterial density OD600 = 0.8 for 30 s resulted in a high average transformation efficiency (8.26 ± 6.5%). The results showed that transformed plants were indicated by the presence of approximately 550 bp amplicon region of NPTII gene (Fig. 7a) and 736 bp amplicon GFP gene in ten samples (Fig. 7b) of 44 T1 transgenic plants in the second experiment. This result indicated that the genomic DNA-presenting gene amplicon was detected in the transgenic plants.
Fig. 7.
Amplicon genes from PCR product in 1% agarose gel, a)NPTII gene with a size of 550 bp, b) GFP gene with a size of 736 bp. M: 100 bp ladder, C+: control (recombinant plasmid), WT: wild-type; No. 1–10: DNA transgenic plant samples.
3.6. Factors affecting the transformation efficiency of floral dip on C. sulphureus Cav.
3.6.1. Effect of bacterial density
Different bacterial densities were used in floral dip transformation in Agrobacterium suspension (OD600 = 0.8, 1.5). The flower buds infected in Agrobacterium suspension at OD600 = 0.8 obtained the highest transformation efficiency of (3.74 ± 3.24%) than those at OD600 = 1.5, which caused no plant transformation according to the t test (Fig. 8a). In addition, the high bacterial density caused browning of the buds after dipping into the Agrobacterium suspension. This step disturbed flower blooming, which decreased the number of seeds produced.
Fig. 8.
Effect of different treatments on transformation efficiency of floral dipping of C. sulphureus Cav. in the presence of NPTII and GFP genes. a) Effect of Agrobacterium OD600, b) dipping duration (s) in the first experiment; c) effect of Silwet L-77 (%) in the second experiment. (*): significant based on the T-test and DMRT test α < 0.05.
3.6.2. Effect of dipping duration
The flower buds were infected by Agrobacterium suspensions for various intervals from 30 to 90 s. Dipping duration for 30 s obtained the highest transformation efficiency of (4.13 ± 6.16%) (Fig. 8b). The low duration effectively increased the transformation efficiency of floral dipping of yellow cosmos.
3.6.3. Effect of silwet L-77 concentration
Bacterial density of OD600 = 0.8 with 5% sucrose and duration dipping of 30 s were used in various concentration levels of Silwet L-77 (0%, 0.05%, and 0.1%). The different concentration levels of Silwet-77 affected the increase in the transformation efficiency. The best treatment was observed with 0.1% concentration of Silwet L-77, with a transformation efficiency of (12.78 ± 1.53%). The 0% and 0.05% concentrations of Silwet L-77 attained transformation efficiencies of (9.74 ± 2.01%) and (9.38 ± 2.38%), respectively (Fig. 8c). Silwet L-77 as surfactant assisted in the increase in insertion level.
4. Discussion
The Agrobacterium strain GV3101 containing the recombinant vector pRI101AN-GFP was successfully used for floral dip transformation of yellow cosmos (C. sulphureus Cav.). Floral dip transformation is a simple and efficient genetic transformation method. The floral dip transformation method does not require a particular skill and rapidly obtains transgenic plants. This method is faster than tissue culture in obtaining genetically modified organisms (Chumakov and Moiseeva, 2012, Eck, 2018). In addition, it can be performed outside the laboratory without using laminar air flow and unsterile conditions (Ali et al., 2022b, Yaroshko et al., 2020). Several factors, such as flower bud size, bacterial density, sucrose content, dipping duration, and concentration of surfactant agent (Silwet L-77), affect the increase in transformation efficiency.
A. tumefaciens strain GV3101 has a c5b chromosomal background with the Ti-plasmid pTiC5BD, opine of nopaline, and genes resistant to rifampicin and gentamicin (Yadav et al., 2014). A high bacterial density causes necrosis in the target organ. Necrosis can inhibit the development of flower tubes until they dry out. This condition provides a hypersensitive response that reduces the potential for postinfection cell regeneration and recovery (Jaberi et al., 2018, Yong et al., 2006). Moreover, the dipping duration causes flower bud damage and inhibits seed development (Smagur et al., 2009). A significant reduction in the number of seeds has been reported in the floral dipping of Triticum sp., which was caused by the long duration of infection (Zale et al., 2009).
The recombinant vector pRI101AN-GFP has two inserted genes, namely, NPTII and GFP, as selectable marker and reporter gene, respectively. NPTII is used as a selectable marker for resistance to kanamycin antibiotics. In this study, the plants were germinated as putative transformants in a selection medium. Thus, the seedlings germinated had kanamycin resistance. The NPTII gene cannot inhibit protein synthesis and ribosome binding in transformant plants (Irsyadi et al., 2022). However, the selected plants can be affected by their resistance properties. High concentrations caused seedling etiolation and plant death (Chen et al., 2020). A concentration of 50 ppm kanamycin was effectively used in the selection of transformants of Glycine max L. (Isda, 2012) and Saccharum officinarum (Fibriani et al., 2019).
Plants selected from the selection medium containing kanamycin lacked NPTII and GFP gene amplicons according to the PCR. Thus, the seedlings escaped at 50 ppm kanamycin concentration. Irsyadi et al. (2022) reported that in 50 ppm kanamycin concentration, selected putative transformants of C. sulphureus that escaped kanamycin reached 3.84%. Dalton et al., (1995) reported the extremely low concentration of selective agents, which allowed the undesirably high numbers of escape and loss of resistant transformants. Moreover, each plant species has a different tolerance level for various concentrations of kanamycin antibiotics. Seedlings of wild-type A. thaliana tolerated up to 1,400 ppm kanamycin (Chen et al., 2020), Gossypium hirsutum L. tolerated up to < 50 ppm kanamycin (Unbeck et al., 1989), Lycopersicon esculentum Mill was putatively transformed under 100 ppm kanamycin (Subaila and Saleh, 2010), and Artemisia annua L. tolerated up to < 20 ppm kanamycin (Chen et al., 2000).
GFP is a reporter gene that can be detected directly without cofactors and certain substrates to exhibit fluorescence in UV or blue light (Chen et al., 2015, Zhao et al., 2016). However, GFP was not detected in all transgenic plants. Such result was due to the autofluorescence of chloroplasts and plant cell walls; solids become a nuisance when detecting GFP fluorescence in plants. In addition, the physiological conditions of the transformant plant and the number of copies of the GFP gene affect the green glow intensity (Cheng et al., 2019). The eYGFPuv gene was successfully expressed in fluorescence of A. thaliana transformant plants resulting from a floral dip during growth. The fluorescence of the leaf, root, and stem can be observed under UV or blue lights (Yuan et al., 2021).
In floral dip transformation, flower size influences the success of transformation efficiency. As a result, flower buds transform into young organs that have meristem tissues, which can enhance bacterial insertion into plant tissues. Flower buds are still undergoing meiosis to form male and female flowers (Mayavan et al., 2015, Yasmeen et al., 2009). Unpollinated flower buds with sizes of 5–7 mm were used as target organs in this study. In addition, bacterial density affects the increase in the transformation efficiency. Agrobacterium growth in the log phase is often used in Agrobacterium-mediated transformation. In this phase, the bacteria remain actively growing. This finding usually indicates that the level of bacterial density OD600 = 0.4–0.8, which is effective for genetic transformation (Clough and Bent, 1998). Moreover, the use of extremely high bacterial density affects the transformation efficiency level. The bacterial density of OD = 1.5 did not produce transformed plants in yellow cosmos. In addition, the sucrose content can increase the transformation efficiency. Sucrose is a source of energy and additional nutrient for bacterial growth (Rizal et al., 2019, Subramanyam et al., 2015).
The duration of floral bud dipping into the Agrobacterium suspension can affect the transformation efficiency. A long dipping duration causes a decrease in the number of seeds and inhibits the development of flower buds (Bastaki and Cullis, 2014, Jaberi et al., 2018). This condition was reported for Triticum sp. (Zale et al., 2009). Furthermore, surfactants affected the transformation efficiency. Silwet L-77 is a surfactant with low phytotoxicity and can reduce surface tension and open the pores of plant tissues. Moreover, Silwet L-77 acts as an attractant that can attract bacteria into the plant genome (Clough and Bent, 1998).
The bud size, sucrose content, and Silwet L-77 content have been reported in the floral dipping of L. usitatissimum with bud sizes of 5–10 mm in Agrobacterium suspension containing 5% sucrose and 0.1% Silwet L-77, which resulted in the transformation efficiency of 50% (Bastaki and Cullis, 2014), the flower target before pollination with the same suspension content in S. lycopersicum was 23% (Yasmeen et al., 2009) and a value of 12.7% was obtained for O. sativa (Rod-In et al., 2014). A concentration of 0.1% Silwet L-77 was effective in the floral dipping of A. thaliana and obtained a transformation efficiency of 10% (Ali et al., 2022a, Ali et al., 2022b). In addition, flower buds with a diameter of 2 mm were effective in floral dipping of Brassica rapa (Hu et al., 2019). A high OD of 1.4 has been reported for the floral dipping of Capsella grandiflora, which obtained a transformation efficiency of 0.52% (Dew-Budda et al., 2019). At an OD of 0.8, the floral dipping of R. sativus L. (Curtis and Nam, 2001), Scherenkiella parvula (Wang et al., 2019), and A. thaliana was performed (Zhang et al., 2006). Moreover, 5% sucrose was effectively used in the floral dipping of S. officinarum (Mayavan et al., 2015) and Tricosanthes cucumerina L. (Subramanyam et al., 2015).
5. Conclusion
The flower bud target organ dipped in Agrobacterium suspension at OD600 = 0.8 + 5% sucrose + 0.1% Silwet L-77 for 30 s was effective as a floral dipping method for yellow cosmos (C. sulphureus Cav.), with a transformation efficiency of (12.78 ± 1.53%). Simple and effective transformation methods enhance the development of plant genetic engineering methods.
CRediT authorship contribution statement
Aziz Purwantoro: Conceptualization, Supervision. Muhammad Burhanuddin Irsyadi: Methodology. Widhi Dyah Sawitri: Conceptualization. Nor Chamidah Fatumi: Methodology. Shania Nur Fajrina: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Prof. Bambang Sugiharto from the Center for Development and Advanced Science and Technology, University of Jember, Indonesia for providing the binary vector pRI101AN-GFP and Agrobacterium strain. We thank “Badan Penerbit dan Publikasi” Universitas Gadjah Mada for their language editing services.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Aziz Purwantoro, Email: azizp@ugm.ac.id.
Muhammad Burhanuddin Irsyadi, Email: burhanuddin2020@mail.ugm.ac.id.
Widhi Dyah Sawitri, Email: widhi.d.s@ugm.ac.id.
Nor Chamidah Fatumi, Email: n.chamidah.fatumi@mail.ugm.ac.id.
Shania Nur Fajrina, Email: shanianur00@mail.ugm.ac.id.
References
- Aftab N., Saleem K., Khan A.H.A., Butt T.A., Mirza C.R., Hussain J., Farooq G., Tahir A., Yousaf S., Zafar M.I., Nawaz I., Iqbal M. Cosmos sulphureus Cav. is more tolerant to lead than copper and chromium in hydroponics system. International Journal of Environmental Science and Technology. 2021;18(8):2325–2334. doi: 10.1007/s13762-020-02981-w. [DOI] [Google Scholar]
- Ali I., Salah K.B.H., Sher H., Ali H., Ullah Z., Ali A., Alam N., Shah S.A., Iqbal J., Ilyas M., Al-Quwaie D.A.H., Khan A.A., Mahmood T. Drought stress enhances the efficiency of floral dip method of Agrobacterium-mediated transformation in Arabidopsis thaliana. Braz. J. Biol. 2022;84(e259326) doi: 10.1590/1519-6984.259326. [DOI] [PubMed] [Google Scholar]
- Ali I., Sher H., Ali A., Hussain S., Ullah Z. Simplified floral dip transformation method of Arabidopsis thaliana. J. Microbiol. Methods. 2022;197 doi: 10.1016/j.mimet.2022.106492. [DOI] [PubMed] [Google Scholar]
- Bastaki N.K., Cullis C.A. Floral-dip transformation of flax (Linum usitatissimum) to generate transgenic progenies with a high transformation rate. J. Vis. Exp. 2014;94:1–10. doi: 10.3791/52189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent, A. 2006. Arabidopsis thaliana Floral Dip Transformation Method. In: Methods in Molecular Biology. 343. Humana Press. https://doi.org/10.1385/1-59745-130-4:87. [DOI] [PubMed]
- Chen C., Fu X., Peng R., Tian Y., Yao Q. Detoxifying processes during kanamycin-induced stress to Arabidopsis thaliana seedling growth. Biotechnology and Biotechnological Equipment. 2020;34(1):673–679. doi: 10.1080/13102818.2020.1798811. [DOI] [Google Scholar]
- Chen L., Sun G., Wu S., Liu H., Wang H. Efficient transformation and expression of GFP gene in Valsa mali var. mali. World J Microbiol Biotechnol. 2015;31:227–235. doi: 10.1007/s11274-014-1780-3. [DOI] [PubMed] [Google Scholar]
- Chen D., Ye H., Li G. Expression of chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plant via Agrobacterium-mediated transformation. Plant Sci. 2000;155(2):179–185. doi: 10.1016/s0168-9452(00)00217-x. [DOI] [PubMed] [Google Scholar]
- Cheng X., Huang C., Zhang X., Lyu Y. Establishment of transgenic marigold using the floral dip method. Acta Physiol. Plant. 2019;41(8):1–8. doi: 10.1007/s11738-019-2937-3. [DOI] [Google Scholar]
- Chumakov M.I., Moiseeva E.M. Technologies of Agrobacterium plant transformation In planta. Appl. Biochem. Microbiol. 2012;48:657–666. [Google Scholar]
- Chumakov M.I., Rozhok N.A., Velikov V.A., Tyrnov V.S., Volokhina I.V. Agrobacterium-mediated in planta transformation of maize via pistil filaments. Russ. J. Genet. 2006;42(8):893–897. doi: 10.1134/S1022795406080072. [DOI] [PubMed] [Google Scholar]
- Clough J.S., Bent A.F. Floral dip a simplified method for Agrobacterium-mediated.pdf. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Curtis I.S., Nam H.G. Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method - Plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res. 2001;10:363–371. doi: 10.1023/A:1016600517293. [DOI] [PubMed] [Google Scholar]
- Dalton S.J., Bettany A.J.E., Timms E., Morris P. The effect of selection pressure on transformation frequency and copy number in transgenic plants of tall fescue (Festuca arundinacea Schreb.) Plant Sci. 1995;108(1):63–70. doi: 10.1016/0168-9452(95)04127-G. [DOI] [Google Scholar]
- Dew-Budda K., Davida B., Beilstein M.A. Agrobacterium-mediated floral-dip transformation of the obligate outcrosser Capsella grandiflora. BioRxiv. 2019 doi: 10.1101/757328. [DOI] [Google Scholar]
- Doyle J.J., Doyle J.L. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Eck J.V. The Status of Setaria viridis transformation : Agrobacterium - mediated to floral dip. Fronti. Plant Sci. 2018;9(652):1–5. doi: 10.3389/fpls.2018.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibriani S., Agustien I., Sawitri W.D., Sugiharto B. Genetic transformation and expression of sucrose phosphate synthase mutant in tomato plant. J. Bioteknol Biosains Indones. 2019;6(1):134–143. [Google Scholar]
- Guiqin M., Chang N., Xiang K., Sheng Y., Zhang Z., Pan G. Genetic transformation of maize female inflorescence following floral dip method mediated by Agrobacterium. Biotechnology. 2012;11(3):178–183. doi: 10.3923/biotech.2012.178.183. [DOI] [Google Scholar]
- Handayani E., Irsyadi M.B., Alawiyah R.L.M.N., Aris I. Effect of explants sterilization and plant growth regulators on embryo culture of kepel (Stelechocarpus burahol) IOP Conference Series: Earth and Environmental Science. 2022;985(012016) doi: 10.1088/1755-1315/985/1/012016. [DOI] [Google Scholar]
- Hilmi M.I., Taryono, Sayekti R.S. Characterization of cosmos (Cosmos spp.) accessions from the special region of yogyakarta and riau origin. Agrinova: Journal of Agriculture. Innovation. 2020;3(1):1–5. doi: 10.22146/a.58345. [DOI] [Google Scholar]
- Hu D., Bent A.F., Hou X., Li Y. Agrobacterium-mediated vacuum infiltration and floral dip transformation of rapid-cycling Brassica rapa. BMC Plant Biol. 2019;19(246):1–9. doi: 10.1186/s12870-019-1843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irsyadi M.B., Sawitri W.D., Purwantoro A. Molecular and phenotypic characteristics of T1 transgenic yellow cosmos (Cosmos sulphureus) carrying neomycin phosphotransferase II. Biodiversitas. 2022;23(12):6097–6105. doi: 10.13057/biodiv/d231202. [DOI] [Google Scholar]
- Isda, M.N. 2012. Optimization of concentration Kanamycin in the soybean explants (Glycine max L.) to transformation TcPIN gene. Semirata PTN Barat.
- Jaberi M., Azadi P., Gharehyazi B., Khosrowchahli M., Sharafi A., Aboofazeli N., Bagheri H. Silvere nitrate and adenine sulphate induced high regeneration frequency in the recalcitrant plant Cosmos bipinatus using cotyledone explants. J. Hortic Sci. Biotech. 2018;93(2):204–208. [Google Scholar]
- Keshavareddy, G., A.R.V., Kumar, and Ramu, V.S. 2018. Methods of plant transformation- A review. International Journal of Current Microbiology and Applied Sciences. 7(7), 2656–2669.
- Jakhar M.L., Verma R., Dixit D. Effect of antioxidant on in vitro degree of browning and culture establishment of Guggul (Commiphora wightii (Arnott)): A valuable dessert medicinal plant. J. Pharmacognosy and Phytochemistry. 2019;5:250–254. [Google Scholar]
- Mayavan S., Subramanyam K., Jaganath B., Sathish D., Manickavasagam M., Ganapathi A. Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep. 2015;34(10):1835–1848. doi: 10.1007/s00299-015-1831-8. [DOI] [PubMed] [Google Scholar]
- Narusaka M., Shiraishi T., Iwabuchi M., Narusaka Y. The floral inoculating protocol: A simplified Arabidopsis thaliana transformation method modified from floral dipping. Plant Biotechnology. 2010;27(4):349–351. doi: 10.5511/plantbiotechnology.27.349. [DOI] [Google Scholar]
- Respatie D.W., Yudono P., Purwantoro A., Trisyono Y.A. The potential of Cosmos sulphureus flower extract as a bioherbicide for Cyperus rotundus. Biodiversitas. 2019;20(12):3568–3574. doi: 10.13057/biodiv/d201215. [DOI] [Google Scholar]
- Rizal S., Suharyono A.S., Amelia J.R. The effect of addition of sucrose solution on the antibacterial activities of green grass jelly extract sinbiotic beverages during storage in cold temperature. Agric Jurnal Ilmu Pertanian. 2019;31(1):53–66. [Google Scholar]
- Rod-In, Sujipuli, W., and Ratanasut, K. 2014. The floral-dip method for rice (Oryza sativa) transformation. Journal of Agricultural Technology. 10(2), 467–474.
- Saleem M., Ali H.A., Furqan M.A., Uzma S., Ammara S., Irshad I. Chemical characterisation and hepatoprotective potential of Cosmos sulphureus Cav. and Cosmos bipinnatus Cav. Natural Product Research. 2017:1–4. doi: 10.1080/14786419.2017.1413557. [DOI] [PubMed] [Google Scholar]
- Silalahi D., Wirawan I.G.P., Sritamin M. Transformasi genetik tanaman kentang (Solanum tuberosum L.) dengan gen acvb menggunakan vektor Agrobacterium tumefaciens. Agrotrop : Journal on Agriculture Science. 2021;11(1):63. 10.24843/ajoas.2021.v11.i01.p07. [Google Scholar]
- Smagur A.W., Konka K.H., Kononowicz A.K. Flower bud dipping or vacuum infiltration two methods of Arabidopsis thaliana transformation. Russ. J. Plant Physiol. 2009;56(4):560–568. [Google Scholar]
- Subaila S., Saleh. Inhibitory effect of kanamycin on in vitro culture of Lycopersicon esculantum Mill cv. Mt11. J. Agrobiotech. 2010;1:79–86. [Google Scholar]
- Subramanyam K., Chinnathambi A., Thaneswari R.M., Ganapathi A.A.S., Manickavasagam M., Ganapathi A. Highly efficient Agrobacterium-mediated in planta genetic transformation of snake gourd (Tricosanthes cucumerina L.) Plant Cell Tiss Organ Cult. 2015;123:133–142. doi: 10.1007/s11240-01508214. [DOI] [Google Scholar]
- Unbeck P., Swain W., Yang N. Inheritance and expression of genes for kanamycin and chloromphenicol resistance in transgenic catton plants. Crop Sci. 1989;29(1):196–201. [Google Scholar]
- Wang G., Pantha P., Tran K., Oh D., Dassanayake M. Plant growth and Agrobacterium mediated floral-dip transformation of the extremophyte Schrenkiella parvula. J Vis. Exp. 2019;143:e58544. doi: 10.3791/58544. [DOI] [PubMed] [Google Scholar]
- Win N.W. Anatomical characteristics of Cosmos sulphureus Cav. from family Asteraceae. Meral Portal. 2016;1–19 [Google Scholar]
- Yadav S., Sharma P., Trivastava A., Desai P., Shrivastava N. Strain specific Agrobacterium-mediated genetic transformation of Bacopa monnieri. J. Genet. Eng. Biotechnol. 2014;12:89–94. doi: 10.1016/j.jgeb.2014.11.003. [DOI] [Google Scholar]
- Yaroshko O.M., Morgun B.V., Velykozhon L.G., Gajdošova A., Andrushenko O.L., Kuchuk M.V. PCR analyses of first-generation plants of amaranthus caudatus l. after «floral-dip» genetic transformation. Fiziol. Rast. Genet. 2020;52(2):128–139. doi: 10.15407/frg2020.02.128. [DOI] [Google Scholar]
- Yaroshko O., Vasylenko M., Gajdošová A., Morgun B., Khrystan O., Velykozhon L., Kuchuk M. “Floral-dip” transformation of Amaranthus caudatus L. and hybrids A. caudatus × A. paniculatus L. Biologija. 2018;64(4):321–330. doi: 10.6001/biologija.v64i4.3904. [DOI] [Google Scholar]
- Yasmeen A., Mirza B., Inayatullah S., Safdar N., Jamil M., Ali S., Choudhry M.F. In planta transformation of tomato. Plant Mol. Biol. Report. 2009;27(1):20–28. doi: 10.1007/s11105-008-0044-5. [DOI] [Google Scholar]
- Yong W.T.L., Abdullah J.O., Mahmood M. Optimization of Agrobacterium-mediated transformation parameters for Melastomataceae spp. using green fluorescent protein (GFP) as a reporter. Sci Hort. 2006;109:78–85. [Google Scholar]
- Yuan G., Lu H., Tang D., Hassan M.M., Li Y., Chen J.G., Tuskan G.A., Yang X. Expanding the application of a uv-visable reporter for transient gene expression and stable transformation in plant. Hortic. Res. 2021;8(234):1–11. doi: 10.1038/S41438-021-00663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale J.M., Agrawal S., Loar S., Steber C.M. Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep. 2009;28:903–913. doi: 10.1007/s00299-009-0696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S.S., Niu Q.W., Chua N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006;1(2):641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang D., Zhong Y., Chang X., Hu M., Cheng C. A simple and efficient in planta transformation method for pommelo (Citrus maxima) using Agrobacterium tumefaciens. Sci. Hortic. 2017;214:174–179. doi: 10.1016/j.scienta.2016.11.033. [DOI] [Google Scholar]
- Zhao C., Mao K., Youa C.X., Zhaoa X.Y., Wanga S.H., Li Y.Y., Hao Y.J. Molecular cloning and functional analysis of a UV-B photoreceptor gene, MdUVR8 (UV Resistance Locus 8), from apple. Plant Sci. 2016;247:115–126. doi: 10.1016/j.plantsci.2016.03.006. [DOI] [PubMed] [Google Scholar]