Abstract
Background
Up to 60% of pediatric surgical patients develop high levels of preoperative anxiety. This study compared the effects of oral combinations of midazolam and ketamine with oral midazolam alone for pediatric preanesthetic medication.
Methods
The study protocol was registered in PROSPERO as CRD42020172920. A systematic literature search was conducted using Medline, Cochrane, EMBASE, CENTRAL, and Web of Science for randomized controlled trials comparing oral combinations of midazolam and ketamine with midazolam alone as preanesthetic medication in elective surgical pediatric patients. Meta-analyses included the following outcomes: anxiety and sedation levels, child...s behavior during separation from parents, face mask acceptance, and venipuncture. The quality of evidence was assessed using GRADE criteria.
Results
Twenty studies were included. The following effects (RR (95% CI)) were observed for combinations of ketamine and midazolam relative midazolam alone: anxiolysis (1.2 (0.94...1.52); p.ß=.ß0.15; I2.ß=.ß80%; GRADE.ß=.ßvery low); satisfactory sedation (1.2 ( 1.10...1.31); p.ß<.ß0.001; I2.ß=.ß71%; GRADE.ß=.ßvery low); behavior during parental separation (1.2 (1.06...1.36); p.ß=.ß0.003; I2.ß=.ß88%; GRADE.ß=.ßvery low); facial mask acceptance (1.13 (1.04...1.24); p.ß=.ß0.007; I2.ß=.ß49%; GRADE.ß=.ßvery low); behavior during venipuncture (1.32 (1.11...1.57); p.ß=.ß0.002; I2.ß=.ß66%; GRADE.ß=.ßvery low).
Conclusions
While similar probabilities of obtaining anxiolysis were found, adequate sedation, calm behavior during child...s separation from parents, low levels of fear during face mask adaptation, and cooperative behavior during peripheral venous cannulation were more likely with midazolam-ketamine combinations.
Keywords: Medication, preanesthetic; Child; Pediatrics, surgery; Cyclohexanes, ketamine; Benzodiazepines, midazolam
Introduction
Up to 60% of children undergoing anesthesia and surgery develop high levels of preoperative anxiety.1 Preoperative high-anxiety levels are associated with emergence delirium, increased postoperative pain, analgesic consumption, general anxiety, sleeping problems and postoperative eating disorders, and maladaptive behavior.2, 3 For these reasons, managing preoperative anxiety and fear is of utmost clinical relevance.
The effectiveness of the preanesthetic medication has been measured as a composite outcome that includes the child...s levels of anxiety4 and sedation in the preanesthesia holding area and her behavior during critical events that precede the anesthetic induction: the separation from parents, the adaptation of the face mask during preoxygenation or induction of inhalational anesthesia, and the venipuncture for peripheral vein cannulation.5
An ideal preanesthetic medication agent should exhibit consistent, predictable results, good patient acceptance, and be free of significant side effects (e.g., hemodynamic instability, respiratory obstruction, or delayed recovery).6 Midazolam and ketamine fulfill many of such characteristics. Midazolam, a GABA-A agonist, is among the most commonly used drugs for preanesthetic medication. Good or excellent sedation, however, is observed only in 60 to 80% of cases.7 Ketamine, an antagonist of the N-methyl-D-aspartate (NMDA) receptor, has also been administered orally as preanesthetic medication to pediatric patients. Doses of 3 or 6.ßmg.kg-1 produce adequate sedation with no prolongation of recovery time, time to discharge, or increased incidence of postoperative nausea and vomiting (PONV), although nystagmus occurs frequently.8 The rationale for combining midazolam and ketamine rests on the assumption of maintaining the anxiolysis provided by midazolam while adding the sedative and analgesic properties of ketamine without increasing side-effects.5 To date, only small studies have investigated the efficacy of orally-administered combinations of midazolam and ketamine compared to midazolam alone as preanesthetic medication in children. This systematic review with meta-analysis aimed to estimate the pooled effect sizes of the currently available studies that compared oral combinations of midazolam and ketamine relative to oral midazolam alone used for preanesthetic medication of pediatric surgical patients according to the adequacy of the outcomes of anxiolysis and sedation, behavior during separation from parents, facial mask acceptance during preoxygenation or anesthesia induction, and behavior at venipuncture for intravenous cannulation.
Methods
The study complied with Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA).9 The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42020172920.10
Sources of information and search strategy
MEDLINE (from 1946), Web of Science (from 1945), EMBASE (from 1947), Scholar Google, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for articles, abstracts, theses, and conference reports of randomized controlled trials (RCTs). Filters were applied to identify studies performed on humans and children with no language restriction. Searches were conducted from November 2019 through January 2020. The following keywords and index terms were used: "midazolam," "ketamine," "preanesthetic medication," "premedication," "randomized clinical trial," "pediatric," and "children."
The search string used on the PubMed engine was ..ú((((ketamine[MeSH Terms]) AND midazolam[MeSH Terms]) AND preanesthetic medication[MeSH Terms])) Filters: Clinical Trial; Humans; Child: birth-18 years..Ñ.
The EMBASE search was done with the following string: "children:ab AND 'oral midazolam' AND 'ketamine'/exp AND 'midazolam'/mj AND 'sedation'/exp AND ('randomized clini' OR 'premedication'/exp).
The CENTRAL search string was: "midazolam" AND "ketamine" in Record Title AND ("preanesthetic" OR "preanesthesia" OR "premedication") in Title Abstract Keyword AND "oral" NOT "intranasal" NOT "transmucosal" in Title Abstract Keyword AND ("children" OR "pediatric") in Title Abstract Keyword AND "randomized control trial" in Publication Type.
Scholar Google was searched with the following terms: "allintitle: midazolam ketamine children oral preanesthetic OR premedication OR oral -intranasal -transmucosal -intramuscular -rectal."
...The Web Of Science search terms were: "TS.ß=.ß(midazolam AND ketamine) AND TS.ß=.ß(preanesthesia medication OR preanesthetic medication) AND TS.ß=.ßchild* Indexes.ß=.ßSCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan.ß=.ßAll years."
The reference lists of the selected articles were manually screened for additional relevant articles. Study protocols, unpublished studies, conference abstracts, or unpublished theses were also searched. Abstracts, conference proceedings, and theses were included if providing complete data. Clarifying information or study data were requested from authors when necessary.
Eligibility criteria
The studies eligible for inclusion in quantitative analyses were randomized clinical trials. The clinical question addressed the following PICOT elements: P, pediatric patients scheduled for elective surgical procedures; I, ketamine combined with midazolam administered orally; C, midazolam administered orally; O, scoring of anxiolysis, sedation, emotional status, behavior during separation from parents, facemask acceptance, and behavior at venipuncture; T, in the immediate preanesthetic period.
Study selection
Three authors conducted independent literature searches (GROF, CMC, JPK). All authors (GROF, CMC, JPK, and GNB) screened titles, abstracts, and full papers of the retrieved references. Studies were rejected at title screening, after reading abstracts, or after reading the full articles. Reasons for exclusion for individual studies included: not being a prospective randomized trial; both oral midazolam and oral ketamine groups were not included in the same study; other than oral administration routes were used; study samples did not include pediatric surgical patients; study outcomes did not include at least one of the primary outcomes of this systematic review (anxiety and sedation levels, child...s behavior during separation from parents, face mask acceptance, or behavior at venipuncture). Controversies about the inclusion of studies were resolved by consensus among the authors (GROF, CMC, JPK, GNB).
Data extraction process and data items
The authors independently extracted data from the included studies on dedicated forms consolidated for inclusion in the analysis software. The software Engauge Digitizer was used to extract data presented as graphs in the original articles.11
Assessment of the risk of bias within studies
Within-study risk of bias was assessed according to the revised Cochrane risk-of-bias tool for randomized trials (ROB 2).12 Studies were considered to have a high risk of bias if a high risk of bias was assigned to any domain or ..úsome concerns..Ñ were assigned to the ROB 2 tool...s multiple domains.12
Summary measures
Effect-sizes were summarized as relative risks (RR) because outcomes were reported as categorical, dichotomized variables in the included studies. Ninety-five percent confidence intervals (95% CI) were estimated for summary measures.
Synthesis of results
Random effects meta-analyses were used to estimate pooled effect sizes based on the following assumptions: the included studies comprised distinct treatment protocols (e.g., varying dose combinations of midazolam and ketamine in intervention groups), and different scales were used to measure outcomes. Consequently, variability among the different effect estimates could be attributed to both within-study sampling error and between-study heterogeneity in real effects. Cochrane Q tests and I2 statistics were used to assess statistical heterogeneity in effect sizes across the studies included in the meta-analyses.
Assessment of risk of bias across studies
Potential publication bias was evaluated through visual inspection of contour-enhanced funnel plots and quantified by Harbord...s zero-slope regression asymmetry test. Duval & Tweedie...s trim-and-fill method was used to identify missing studies and adjust the effect size13, 14 if publication bias was detected. Fixed-effects meta-analyses were performed on the log-transformed RR and standard errors to apply the trim-and-fill method.13, 15 Contour-enhanced funnel plots incorporating the trim-and-fill method results were constructed plotting the log-transformed RR against the log-transformed standard error. Additionally, filled studies were included in the funnel plots whenever indicated by the trim-and-fill procedures, and the predicted log-transformed effect-size was presented beside the observed log-transformed effect size.15
Sensitivity analyses
The pooled estimates... robustness was assessed by sequentially removing each study...s data and re-analyzing the remaining data (leave-one-out analysis) to confirm that the pooled effect-sizes did not result from single-study dominance.
The studies included in meta-analyses differed regarding the dosing regimens of midazolam and ketamine. Such methodological differences might affect the clinical effectiveness of the different dosing regimens. Therefore, subgroup analyses were conducted to explore the different dosing regimens... effect on the primary outcomes. Chi-square tests were used to assess subgroup differences. The quality of evidence provided by the meta-analyses was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.16
Software
Review Manager software 5.3 (Review Manager (RevMan), Copenhagen) was used for meta-analyses. STATA 14/MP (StataCorp, College Station, TX, USA) was used to conduct Harbord...s tests (metabias module) and Duval & Tweedie...s trim-and-fill analyses (metatrim module).13, 17 The GRADEpro GDT software was used to construct a summary of findings (SoF) table.18
Results
Study selection
Twenty studies were included in the meta-analyses (Fig. 1). Twelve of them were published in the English language, two articles in the Persian language, and one article in the Russian language. The search strings used to identify studies in each database and the complete list of retrieved articles and abstracts with reasons for rejection or acceptance are provided in e-component 1.
Figure 1.
Study flow diagram.
Characteristics of the studies
The 20 studies included in the meta-analyses provided data relative to 1540 patients, 834 received orally administered combinations of midazolam and ketamine, and 706 received midazolam alone. The main characteristics of the studies included in quantitative analyses are shown in Table 1 and detailed below.
Table 1.
Characteristics of the studies.
| Study | Source | Country | Language | n MIKE1 | n MDZ2 | Age range | Type of surgery | MIKE Doses | MDZ dose | Vehicle | Volume | Timing | Reported outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astuto 2002 | Article | Italy | English | MK1.ß=.ß40; MK2.ß=.ß42 | 38 | 2...6 years | Urologic | Group MK1: M 0.3.ß+.ßK 1.ßmg.kg-1 and Group MK2: M 0.3.ß+.ßK 2.ßmg.kg-1 | 0.5.ßmg.kg-1 | Glucose syrup | 1...2 teaspoons | 30.ßmin before induction | sedation, anxiolysis, parental separation, face mask acceptance, adverse events: PONV, headache, diplopia, hallucinations |
| Darlong 2004 | Article | India | English | 24 | 24 | 1...9 years | Eye surgery | M 0.25.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | 50% dextrose | Up to 0.3.ßmL.kg-1 | 30.ßmin before induction | sedation, parental separation, face mask acceptance, adverse events: PONV, salivation, irrelevant talking, breath holding |
| Darlong 2011 | Article | India | English | MKL.ß=.ß29; MKH.ß=.ß29 | 29 | 1...10 years | Eye surgery | Group MKL M 0.25.ß+.ßK 3.ßmg.kg-1 and Group MKH: M 0.5.ß+.ßK 6.ßmg.kg-1 | 0.5.ßmg.kg-1 | Honey | Up to 0.5.ßmL.kg-1 | 30.ßmin before induction | sedation, parental separation, face mask acceptance, adverse events: PONV, salivation, irrelevant talking breath holding |
| Foroutan 2007 | Article | Iran | English | 50 | 59 | 2...8 years | Cardiac | M 0.25.ß+.ßK 3.ßmg.kg-1.ß+.ßatropine 0.02.ßmg.kg-1 | 0.5.ßmg.kg-1 | Apple juice | Up to 5...8.ßmL | 45.ßmin before induction | parental separation, behavior at venepuncture, face mask acceptance |
| Funk 2000 | Article | Germany | English | 39 | 38 | 2...10 years | Elective surgery more than 30.ßminute-expected duration | M 0.5.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | Strawberry-flavored glucose syrup | Up to 12.5.ßmL | 30.ßmin before induction | Sedation, anxiolysis, parental separation, behavior at venepuncture; PONV, vertigo,psychodelic symptoms, salivation |
| Ghai 2005 | Article | India | English | 49 | 48 | 10 months...6 years | Elective surgery | M 0.25.ß+.ßK 2,5.ßmg.kg-1 | 0.5.ßmg.kg-1 | Honey | Not reported | 20.ßmin prior to induction | sedation, anxiolysis, parental separation, face mask acceptance, adverse events: PONV |
| Hasani 2000 | Article | Iran | Persian | 50 | 50 | Elective surgery | M 0.25.ß+.ßK 2,5.ßmg.kg-1 | 0.5.ßmg.kg-1 | Not reported | Not reported | 30.ßmin before induction | sedation, parental separation, face mask acceptance. | |
| Jain 2010 | Article | India | English | 31 | 29 | 1...5 years | CT imaging | M 0.25.ß+.ßK 1.ßmg.kg-1 | 0.5.ßmg.kg-1 | Honey | 5.ßmL | 20...30.ßmin before venepunction | behavior at venepuncture, sedation, parental satisfaction |
| Kulikov 2010 | Article | Russia | Russian | 80 | 20 | Elective neurosurgery | M 0.5.ß+.ßK 3.ßmg.kg-1 | 0.75.ßmg.kg-1 | Honey or thick syrup (e.g.hawthorn) | 10.ßm.ßL | 40 min before induction | sedation, behavior at venepuncture | |
| Kumar 2009 | Article | India | English | 20 | 20 | 3...10 years | Elective surgery | M 0.3.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | Apple juice | Up to 0.5.ßmL.kg-1 | 30.ßmin before induction | Sedation, anxiolysis, parental separation, behavior at venepuncture; facial mask acceptance PONV |
| Lin 1993 | ASA Meeting Abstract | USA | English | 15 | 15 | under 8 years | Ambulatory surgeries | M 0.5.ß+.ßK 3.ßmg.kg-1 | 0.75.ßmg.kg-1 | Apple juice | 3.ßmL | 20...30.ßmin before induction | Sedation, parental separation, face mask acceptance, behavior at venepuncture adverse events: salivation, nistagmus. |
| Magar 2016 | Article | India | English | 30 | 30 | 3...10 years | Surgeries under general anesthesia | M 0.5.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | Midazolam syrup (2.ßmg.mL-1) / parenteral preparation of ketamine dissolved in 5% dextrose | According to the total dose of midazolam for each group. | 30.ßmin before surgery | Sedation, anxiolysis, parental separation, behavior at venepuncture; Face mask acceptance hallucinations, salivation, excessive sedation |
| Majidinejad 2015 | Article | Iran | English | 33 | 33 | 6 months...6 years | Brain CT | M 0.2.ß+.ßK 5.ßmg.kg-1 | 0.5.ßmg.kg-1 | Sugar syrup | 5.ßmL | 40.ßmin before the scheduled procedure | sedation. PONV |
| Mithun 2018 | Article | India | English | 50 | 50 | 2...10 years | Elective surgeries between 20.ßminutes to 2 hours | M 0.5.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | Orange syrup | 0.5.ßmL.kg-1 up to a maximum of 10.ßm.ßL | 30.ßmin before induction | Sedation, anxiolysis, parental separation, PONV, nystagmus, salivation, tachycardia, bradycardia, exitement, involuntary movements, respiratory depression |
| Rabie 2005 | Article | Egypt | English | 30 | 30 | 3...8 years | Tonsillectomy with or without adenoidectomy | M 0.25.ß+.ßK 4.ßmg.kg-1 | 0.5.ßmg.kg-1 | Cherry or orange syrup | 5.ßmL | 20.ßmin prior to induction | sedation, parental separation, face mask acceptance, adverse events: PONV |
| Ramakrishna 2018 | Article | India | English | 50 | 50 | 2...10 years | Surgeries lasting more than 30.ßminutes | M 0.5.ß+.ßK 3.ßmg.kg-1 + atropine 0.02.ßmg.kg-1 | 0.5.ßmg.kg-1 + atropine 0.02.ßmg.kg-1 | Honey | Up to 0,5.ßmL.kg-1 | 30.ßmin before induction | Sedation, anxiolysis, parental separation, behavior at venepuncture. |
| Sajedi 2014 | Article | Iran | Persian | 68 | 68 | 6 months...6 years | Outpatient eye surgery | M 0.25.ß+.ßK 2.5.ßmg.kg-1 | 0.5.ßmg.kg-1 | Strawberry-flavored juice | Up to 0,5.ßmL.kg-1 | 30.ßmin before induction | Sedation, anxiolysis, parental separation, behavior at venepuncture. PONV |
| Sathyan 2006 | Doctoral thesis | India | English | 25 | 25 | 1...12 years | Surgeries lasting at least 30.ßminutes | Group A - M 0.3.ß+.ßK 1.ßmg.kg-1; Group B- M 0.3.ß+.ßK 2.ßmg.kg-1 | 0.5.ßmg.kg-1 | Solution mixed with sugar crystals.ß+.ß2-3.ßg of sugar crystal to chew | Not reported | 30.ßmin before induction | Sedation, parental separation, behavior at venepuncture, face mask acceptance. Hiccoughs, delayed recovery |
| Walia 2017 | Article | India | English | 30 | 30 | 1...8 years | Elective surgeries lasting less than 3 hours | M 0.25.ß+.ßK 3.ßmg.kg-1 | 0.5.ßmg.kg-1 | Acetaminophen syrup (5.ßmL.ß=.ß120.ßmg) | Up to 0,4.ßmL.kg-1 | 30.ßmin before induction | Sedation, parental separation, face mask acceptance, emergence score. Hiccoughs, delayed recovery |
| Warner 1995 | Article | USA | English | 20 | 20 | 1.5...7 years | Minor outpatient surgeries | M 0.4.ß+.ßK 4.ßmg.kg-1 + atropine 0.02.ßmg.kg-1 | 0.5.ßmg.kg-1 + atropine 0.02.ßmg.kg-1 | Cherry syrup | 1 - 2 teaspoons | 20...30.ßmin before surgery | Sedation, anxiolysis, parental separation, facial mask acceptance |
MIKE, Group that received the combination of midazolam and ketamine.
MDZ, Group that received the combination of midazolam and ketamine; M, Midazolam; K, ketamine.
Primary outcomes of the included studies
Anxiolysis was reported in 5 studies (436 patients), sedation was reported in 18 studies (1556 patients); behavior at separation from parents was reported in 16 studies (1302 patients); mask acceptance was reported in 11 studies (832 patients); behavior at venipuncture was reported in 9 studies (735 patients).
Midazolam and midazolam ketamine combination dosing regimens
Several dosing combinations of midazolam and ketamine (MIKE) were used in the intervention groups. The combination of midazolam 0.25.ßmg.kg-1 with ketamine 3.ßmg.kg-1 was used in 4 studies.6, 19, 20, 21 Midazolam 0.5.ßmg.kg-1 with ketamine 3.ßmg.kg-1 was used in 5 studies.5, 22, 23, 24, 25 Midazolam 0.25.ßmg.kg-1 with ketamine 2.5.ßmg.kg-1 was used in 3 studies.26, 27, 28 Midazolam 0.3.ßmg.kg-1 with ketamine 1.ßmg.kg-1 was used in 2 studies.29, 30 Midazolam 0.3.ßmg.kg-1 with ketamine 2.ßmg.kg-1 was used in 2 studies.29, 30 Midazolam 0.3.ßmg.kg-1 with ketamine 3.ßmg.kg-1, midazolam 0.5.ßmg.kg-1 with ketamine 6.ßmg.kg-1, midazolam 0.25.ßmg.kg-1 with ketamine 1.ßmg.kg-1 midazolam 0.25.ßmg.kg-1 with ketamine 4.ßmg.kg-1, and midazolam 0.2.ßmg.kg-1 with ketamine 5.ßmg.kg-1 were used in one study each.19, 31, 32, 33, 34 Except for two studies in which the dose of midazolam was 0.75.ßmg.kg-1 in the midazolam groups (MDZ),22, 35 0.5.ßmg.kg-1 was used in the remaining eighteen studies.
Types of surgery
The studies included patients undergoing urologic,29 ophthalmologic,19, 20, 28, 29 cardiac,21 CT imaging,31, 33 neurosurgery,22 tonsillectomy,34 or miscellaneous elective pediatric surgical procedures lasting 20 through 180.ßminutes.5, 23, 24, 25, 26, 27, 30, 32, 35,36
Measurements
Measurement of sedation
Three or four-point categorical scales were used in the included studies to measure anxiolysis level (e.g., 1.ß=.ßpanicky, 2.ß=.ßmoaning, 3.ß=.ßcomposed, 4.ß=.ßfriendly); sedation (e.g., 1.ß=.ßalert and active, 2.ß=.ßawake, 3.ß=.ßdrowsy, but responds to verbal command, 4.ß=.ßasleep); behavior at separation from parents (e.g., 1.ß=.ßcombative and clinging, 2.ß=.ßanxious, 3.ß=.ßcalm, 4.ß=.ßsleeping); facial mask acceptance (e. g., 1.ß=.ßterrified, crying with mask, 2.ß=.ßfear of mask, not reassured, 3.ß=.ßslight fear of mask, reassured; 4.ß=.ßunafraid, accepts face mask), and behavior at venipuncture (e.g., 1.ß=.ßcrying and uncooperative, not able to start IV line, 2.ß=.ßwithdraw for painful stimulus, but allows IV cannulation; 3.ß=.ßcalm, awake not crying, no withdrawal for IV cannulation, 4.ß=.ßasleep, no response to painful stimulus).6, 24, 25 Results were presented in the included studies as dichotomized categories ..úsatisfactory..Ñ or ..úunsatisfactory..Ñ anxiolysis, sedation, behavior at separation from parents, facial mask acceptance, and behavior at venipuncture, according to criteria established by the respective authors.
Side-effects attributable to preanesthetic medication
Six studies reported postoperative nausea and vomiting (PONV).6, 21, 23, 29,32, 34 Three studies reported excessive salivation.6, 23, 35 Two studies reported6, 23, 35 hallucination or diplopia or nystagmus.23, 29 Excessive sedation and peripheral oxygen desaturation,23 and headache29 were reported in one study each.
Synthesis of results
Primary outcomes
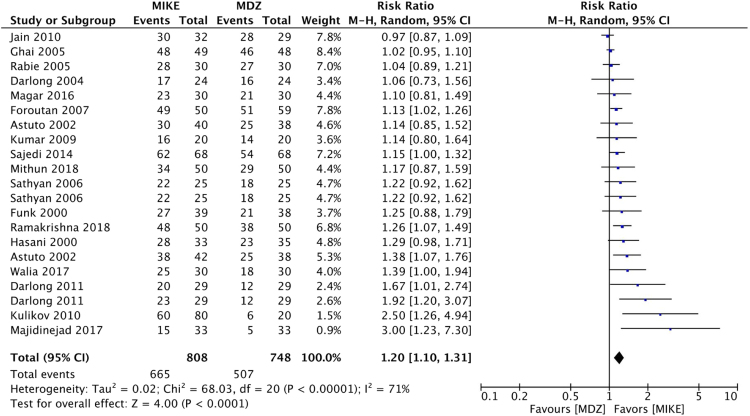
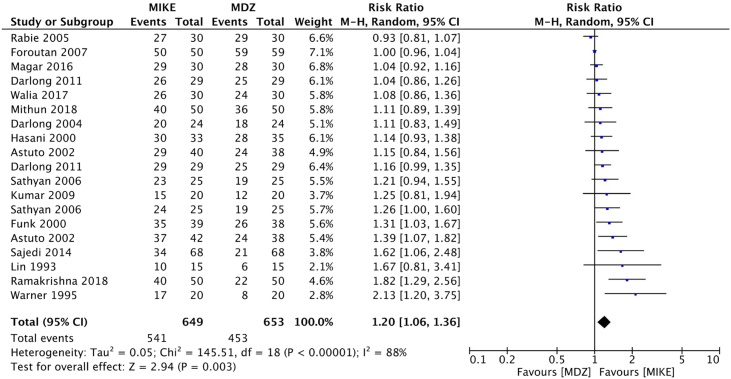
There was no difference between the treatments regarding RR of anxiolysis (RR.ß=.ß1.2 (0.94...1.52; p.ß=.ß0.15; I2.ß=.ß80%; GRADE.ß=.ßvery low) (Fig. 2). The probabilities of obtaining a ..úsatisfactory rating..Ñ were higher among patients who received combinations of ketamine and midazolam relative to patients who received midazolam alone as preanesthetic medication for the following outcomes: sedation (RR.ß=.ß1.20; 95% CI.ß=.ß1.10...1.31; p.ß<.ß0.001; I2.ß=.ß71%; GRADE.ß=.ßvery low) (Fig. 3), behavior during parental separation (RR.ß=.ß1.2; 95% CI %.ß=.ß1.06...1.36; p.ß=.ß0.003; I2.ß=.ß88%; GRADE.ß=.ßvery low) (Fig. 4), facial mask acceptance (RR.ß=.ß1.13; 95% CI.ß=.ß1.04...1.24; p.ß=.ß0.007; I2.ß=.ß49%; GRADE.ß=.ßvery low) (Fig. 5), and venipuncture (RR.ß=.ß1.32; 95% CI.ß=.ß1.11...1.57; p.ß=.ß0.002; I2.ß=.ß66%; GRADE.ß=.ßvery low) (Fig. 6).
Figure 2.
Forest plots of pooled comparisons of the frequency of satisfactory anxiolysis. Black boxes relative risks (RR). Black lines surrounding boxes represent the respective 95% CI. The black diamond represents the combined RR estimate, and its width corresponds to the 95% CI bounds. MDZ, midazolam; MIKE, Combinations of midazolam and ketamine; M-H, Mantel-Haenszel.
Figure 3.
Forest plots of pooled comparisons of the frequency of satisfactory sedation. Black boxes relative risks (RR). Black lines surrounding boxes represent the respective 95% CI. The black diamond represents the combined RR estimate, and its width corresponds to the 95% CI bounds. MDZ, midazolam; MIKE, Combinations of midazolam and ketamine; M-H, Mantel-Haenszel.
Figure 4.
Forest plots of pooled comparisons of the frequency of satisfactory behavior during separation from parents. Black boxes relative risks (RR). Black lines surrounding boxes represent the respective 95% CI. The black diamond represents the combined RR estimate, and its width corresponds to the 95% CI bounds. MDZ, midazolam; MIKE, Combinations of midazolam and ketamine; M-H, Mantel-Haenszel.
Figure 5.
Forest plots of pooled comparisons of the frequency of satisfactory facial mask acceptance. Black boxes relative risks (RR). Black lines surrounding boxes represent the respective 95% CI. The black diamond represents the combined RR estimate, and its width corresponds to the 95% CI bounds. MDZ, midazolam; MIKE, Combinations of midazolam and ketamine; M-H, Mantel-Haenszel.
Figure 6.
Forest plots of pooled comparisons of the frequency of satisfactory behavior during venipuncture. Black boxes relative risks (RR). Black lines surrounding boxes represent the respective 95% CI. The black diamond represents the combined RR estimate, and its width corresponds to the 95% CI bounds. MDZ, midazolam; MIKE, Combinations of midazolam and ketamine; M-H, Mantel-Haenszel.
Adverse effects
Treatments did not differ regarding the probabilities of PONV (RR.ß=.ß1.37; 95% CI.ß=.ß0.59...3.18; p.ß=.ß0.46; I2.ß=.ß0%), hallucinations (RR.ß=.ß4.54; 95% CI.ß=.ß0.53...38.89; p.ß=.ß0.17; I2.ß=.ß0%), excessive salivation (RR.ß=.ß1.90; 95% CI.ß=.ß0.71...5.08; p.ß=.ß0.20; I2.ß=.ß0%), diplopia/nystagmus (RR.ß=.ß1.77; 95% CI.ß=.ß0.58...5.41; p.ß=.ß0.31; I2.ß=.ß0%), or oxygen desaturation (RR.ß=.ß1.36; 95% CI.ß=.ß0.10...19.39; p.ß=.ß0.82; I2.ß=.ß33%). The following adverse effects were extracted from one study each, so that the I2 statistic was not applicable: excessive sedation (RR.ß=.ß5; 95% CI.ß=.ß0.25...99.95; p.ß=.ß0.29); headache (RR.ß=.ß2.72; 95% CI.ß=.ß0.11...64.85; p.ß=.ß0.54), tachycardia (RR.ß=.ß2; 95% CI.ß=.ß0.38...10.43; p.ß=.ß0.31), bradycardia (RR.ß=.ß0.33; 95% CI.ß=.ß0.001...7.99; p.ß=.ß0.50), involuntary movements (RR.ß=.ß5; 95% CI.ß=.ß0.25...101.58; p.ß=.ß0.29), hiccoughs (RR.ß=.ß0.20; 95% CI.ß=.ß0.01...3.97; p.ß=.ß0.29), and delayed recovery (RR.ß=.ß0.20; 95% CI.ß=.ß0.01...3.97; p.ß=.ß0.29).
Sensitivity analyses
Leave-one-out procedures
No changes in p-values of the z-tests for overall effects were observed during leave-one-out procedures conducted on the studies included in the primary outcomes... meta-analyses.
Subgroup analyses
Subgroup analyses disclosed significant differences in effect sizes attributable to the dose combinations of the midazolam and ketamine in the MIKE treatment regarding sedation (chi...ß=.ß22.38, df.ß=.ß9; p.ß=.ß0.008); I...ß=.ß59.8%) and behavior at separation from parents (chi...ß=.ß19.73; df.ß=.ß8; p.ß=.ß0.01; I...ß=.ß59.4%). No significant differences across dose combinations of the midazolam and ketamine subgroups were found for the outcomes anxiolysis (chi...ß=.ß2.81; df.ß=.ß2; p.ß=.ß0.24; I...ß=.ß28.9%), face mask acceptance (chi...ß=.ß10.76; df.ß=.ß7; p.ß=.ß0.15; I...ß=.ß34.9%), and behavior at venipuncture (chi...ß=.ß10.87; df.ß=.ß6; p.ß=.ß0.09; I...ß=.ß44.8%) (e-component 2).
Assessment of risk of bias within studies
Because ..úhigh risk..Ñ or ..úsome concerns..Ñ grade was assigned to one or more domains of the Cochrane Collaboration ROB 2 tool, 11 studies (55%) were classified as having a high overall risk of bias.6, 20, 22, 23,27, 29, 30, 32,33, 35, 36 The ..úsome concerns..Ñ class was assigned to the overall risk of bias of 9 studies (45%).5, 19, 21, 24, 25, 26, 28, 31, 34 (e-component 3).
Assessment of risk of publication bias across studies
Harbord...s test detected evidence of publication bias or small-study effects in meta-analyses of sedation (p.ß<.ß0.001), behavior at separation from parents (p.ß<.ß0.001), and behavior at venipuncture (p.ß=.ß0.04). No evidence of publication bias or small-study effect was detected for the face mask acceptance (p.ß=.ß0.07) and anxiolysis (p.ß=.ß0.30) meta-analyses.
As a result of the trim-and-fill method, the sedation meta-analysis produced ten filled studies, seven of which in the region of p.ß>.ß0.10, and three filled studies in the region of 0.05.ß<.ßp.ß<.ß0.1 of the contour-enhanced funnel plot. The adjusted intervention effect, that is, the risk ratio that would have been found in the absence of publication bias, was estimated as 1.07 (95% CI.ß=.ß0.99...1.55; p.ß=.ß0.057; I2.ß=.ß79.07%). Despite the lack of evidence of small-study or publication bias produced by the Harbord...s test, applying the trim-and-fill method, the mask acceptance meta-analysis produced five filled studies, two of which in the region of p.ß>.ß0.10, two filled studies in the region of 0.05.ß<.ßp.ß<.ß0.1, and one study in the p.ß<.ß0.01 region of the contour-enhanced funnel plot. The adjusted risk ratio was 1.043 (95% CI.ß=.ß0.945...1.151; p.ß=.ß0.40; I2.ß=.ß62.2%). The separation from parents meta-analysis produced eight filled studies, five of which in the region of p.ß>.ß0.10, and three studies in the region of 0.05.ß<.ßp.ß<.ß0.1 of the contour-enhanced funnel plot. The adjusted risk ratio was 1.075 (95% CI.ß=.ß0.987...1.171; p.ß=.ß0.096; I2.ß=.ß62.5%). The behavior at venipuncture meta-analysis produced four filled studies, two of which in the region of p.ß>.ß0.10, and two studies in the region of 0.05.ß<.ßp.ß<.ß0.1 of the contour-enhanced funnel plot. The adjusted risk ratio was 1.14 (95% CI.ß=.ß0.946...1.340; p.ß=.ß0.16; I2.ß=.ß73.8%). No filled studies resulted from applying the trim-and-fill method to the anxiolysis meta-analysis. Contour-enhanced funnel plots, including filled studies, are shown in e-component 4.
Quality of evidence
Very low confidence was assigned to the meta-analyses of all primary outcomes at GRADE assessment, driven by the high statistical heterogeneity, the severe risk of bias within the included studies, and the high risk of publication bias (e-component 5).
A completed PRISMA checklist is provided in e-component 6.
Discussion
This systematic review with meta-analyses pooled the results of 20 studies that addressed preanesthetic medication effects with combinations of midazolam and ketamine compared with midazolam alone orally administered to surgical pediatric patients 20 to 45.ßminutes before anesthetic induction. The primary outcomes were satisfactory anxiolysis and sedation, satisfactory behavior at separation from parents, satisfactory facial mask acceptance, and satisfactory behavior at venipuncture, as defined by each study...s authors.
The main results were that oral combinations of midazolam and ketamine were associated with a similar probability of achieving satisfactory anxiolysis and higher probabilities of satisfactory sedation, calm behavior during parental separation, no fear during facial mask adaptation, and cooperative behavior during venipuncture relative to oral midazolam alone.
Midazolam is a water-soluble benzodiazepine and the most commonly used sedative in pediatric surgical patients. After orally-administered doses of 0.5...0.75.ßmg.kg-1 (maximum of 20.ßmg), sedation and anxiolysis are achieved within 20.ßminutes, and an elimination half-time of 2.2 to 6.8.ßhours.37 Because of the injectable form...s bitter taste, several vehicles such as honey, glucose, apple juice, and paracetamol syrup have been used to increase palatability and acceptance. Currently, a cherry-flavored syrup formulation is available for oral use (2.ßmg.mL-1).38 However, oral midazolam may fail to produce sedation in 20...40% of patients.7, 37
Intramuscular ketamine has long been used for preanesthetic medication in children.39 Less painful routes have been studied, including the oral transmucosal,40, 41 the intranasal,42, 43 and the rectal routes.44, 45 Orally administered ketamine undergoes significant first-pass effects that result in the formation of norketamine and dehydronorketamine. Norketamine crosses the blood-brain barrier and has about one-fifth to one-third the potency of ketamine, contributing to prolonging its analgesic effect.46
The rationale for using combinations of oral midazolam and ketamine was not consistent across the studies included in this systematic review. While some studies used lower doses of midazolam in the group that received the drug combination compared to the control group,6, 19, 20, 21, 26, 27, 28, 29, 30, 31, 32, 33, 34, 36 others used the same doses of midazolam in the control group and in the group that received the drug combination,5, 19, 22, 23, 24, 25, 35 suggesting that while the former sought to study the effectiveness based on the synergism between midazolam and ketamine, the later based their hypotheses on additive effects. Although these different rationales could have been associated with differences in side effects related to the potentiation of midazolam effects, such as excessive sedation, respiratory depression, and prolonged awakening from anesthesia,47 the scarcity of data regarding those outcomes prevented comparisons.
The variety of dosing regimens was associated with variations in effect sizes larger than those expected by chance, as suggested by the highly significant heterogeneity measures associated with meta-analyses of all primary outcomes and confirmed in subgroup analyses.
Some concerns and/or high suspicion of methodological biases were found in critical aspects of randomized controlled trials methodology, mainly because little information was provided in most articles about randomization methods, allocation concealment, and participants and investigators... blinding. Because no study had a clear statistical plan or protocol registration, biases in selecting reported results could not be discarded. Moreover, except for the anxiety outcome, the high risk of publication bias and small-study effects pervaded the included studies.
The trim-and-fill method assumes that publication bias is the only cause of funnel plot asymmetry and performs poorly whenever high within-studies heterogeneity exists. By combining contour-enhanced funnel plots with the trim-and-fill method, it is possible to assign other causes to funnel plot asymmetry depending on the location of the imputed missing studies on the funnel plot regions plot. Missing studies in the region of p.ß>.ß0.10 indicate that publication bias is a plausible cause of the observed asymmetry, whereas missing studies in the region of 0.05 < p < 0.1 or p.ß<.ß0.1 suggest that other causes may have also contributed to the funnel-plot asymmetry, e.g., high heterogeneity or the effects of one-sided comparisons.15 Accordingly, both publication and high within-studies heterogeneity are plausible causes of funnel plot asymmetry in meta-analyses of all primary outcomes, except anxiolysis. Moreover, caution must be exercised in interpreting the adjusted risk rates produced by the trim-and-fill meta-analyses because they are based on imputed intervention effect estimates.48
According to GRADE, this systematic review provides a very low quality of evidence (certainty) for the preanesthetic use of oral combinations of the ketamine/midazolam relative to oral midazolam alone for providing anxiolysis, sedation, or calm and cooperative behavior during separation from parents, facial mask acceptance, or venipuncture, suggesting that the actual effect is probably markedly different from the estimated effect.
In conclusion, based on this study...s relative risks that pooled the effect sizes of studies included in the meta-analyses, similar probabilities of obtaining satisfactory anxiolysis were found for the midazolam-ketamine combinations relative to midazolam alone. However, the probabilities of obtaining satisfactory sedation, calm behavior during the child...s separation from parents, low levels of fear during face mask adaptation, and cooperative behavior during peripheral venous cannulation were higher for the midazolam-ketamine combinations administered orally 20 to 45.ßminutes before induction of anesthesia compared to oral midazolam alone. However, because of the small effect sizes, high within-studies risk of bias, high methodological and statistical heterogeneity, and high risk of publication bias found in meta-analyses, a weak level of recommendation is provided for replacing oral midazolam alone with oral combinations of midazolam and ketamine for the preanesthetic medication of pediatric surgical patients.49, 50
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.bjane.2021.07.026.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Kain Z.N., Mayes L.C., O...Connor T.Z., et al. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–1245. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 2.Kain Z.N., Mayes L.C., Caldwell-Andrews A.A., et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651–658. doi: 10.1542/peds.2005-2920. [DOI] [PubMed] [Google Scholar]
- 3.Kain Z.N., Caldwell-Andrews A.A., Maranets I., et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–1654. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 4.Manyande A., Cyna A.M., Yip P., et al. Non-pharmacological interventions for assisting the induction of anaesthesia in children. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD006447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk W., Jakob W., Riedl T., et al. Oral preanaesthetic medication for children: double-blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. Br J Anaesth. 2000;84:335–340. doi: 10.1093/oxfordjournals.bja.a013435. [DOI] [PubMed] [Google Scholar]
- 6.Walia C., Pankaj, Prabhu M, et al. Oral premedication in children: Comparison of combination of midazolam-ketamine and oral midazolam-A Randomised trial. Indian J Clin Anaesth. 2018;5:249–254. [Google Scholar]
- 7.Manso M.A., Guittet C., Vandenhende F., et al. Efficacy of oral midazolam for minimal and moderate sedation in pediatric patients: A systematic review. Pediatric Anesthesia. 2019;29:1094–1106. doi: 10.1111/pan.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutstein H.B., Johnson K.L., Heard M.B., et al. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76:28–33. doi: 10.1097/00000542-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. National Institute of Health Research. PROSPERO. International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed 09/23/2019.
- 11.Engauge Digitizer Software [computer program]. Version 62015.
- 12.Sterne J.A.C., Savovi.. J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Duval S., Tweedie R. Trim and Fill: A Simple Funnel-Plot...Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Palmer T.M., Sutton A.J., Peters J.L., et al. Contour-enhanced funnel plots for meta-analysis. Stata J. 2008;8:242–254. [Google Scholar]
- 15.Peters J.L., Sutton A.J., Jones D.R., et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa R.A., Wiercioch W., Santesso N., et al. Decision-Making about Healthcare Related Tests and Diagnostic Strategies: User Testing of GRADE Evidence Tables. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbord R.M., Harris R.J., Sterne J.A.C. Updated Tests for Small-study Effects in Meta-analyses. Stata J. 2009;9:197–210. [Google Scholar]
- 18.GRADEpro GDT [computer program] McMaster University; Hamilton (ON): 2015. [Google Scholar]
- 19.Darlong V., Shende D., Singh M., et al. Low-versus high-dose combination of midazolam-ketamine for oral premedication in children for ophthalmologic surgeries. Singapore Med J. 2011;52:512–516. [PubMed] [Google Scholar]
- 20.Darlong V., Shende D., Subramanyam M.S., et al. Oral ketamine or midazolam or low dose combination for premedication in children. Anaesth Intensive Care. 2004;32:246–249. doi: 10.1177/0310057X0403200214. [DOI] [PubMed] [Google Scholar]
- 21.Foroutan A., Yazdanian F., Panahipour A.A., et al. Oral premedication for pediatric cardiac surgery: a comparison of midazolam, ketamine and midazolam plus ketamine. Iran Heart J. 2008;8:17–23. [Google Scholar]
- 22.Kulikov A.S., Sorokin V.S., Lubnin A. Oral premedication with midasolam and ketamine in children with neurosurgical diseases. Anesteziol Reanimatol. 2010:6–10. [PubMed] [Google Scholar]
- 23.Magar J., Kotwani M.B.K., Kotak S. A double blinded comparative study of oral premedication in children with midazolam alone or in combination with ketamine. Int J Contemp Pediatr. 2016;3:8. [Google Scholar]
- 24.Mithun B., Anand B. A comparative study between midazolam, ketamine and combination of both as a premedication in pediatric surgeries. MedPulse Int J Anesthesiol. 2018;6:27–32. [Google Scholar]
- 25.Ramakrishna R., Hemanth K.J., Sunil B.V., et al. Oral premedication in children: A randomized study of a combination of oral midazolam, ketamine with atropine vs midazolam and atropine vs ketamine and atropine. Indian J Clin Anaesth. 2018;5:261–265. [Google Scholar]
- 26.Ghai B., Grandhe R.P., Kumar A., et al. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Paediatr Anaesth. 2005;15:554–559. doi: 10.1111/j.1460-9592.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 27.Hasani M. Comparison Of Oral Premedication With Combination Of Midazolam With Ketamine Vs Midazolam Ketamine Alone In Children Children Medical Center (year 2000) Tehran Univ Med J. 2002;60:423–428. [Google Scholar]
- 28.Sajedi P., Aghadavoudi O., Salimi-Jazi F. Oral midazolam alone or in combination with ketamine as oral premedication in pediatric ophthalmologic surgeries. J Isfahan Med Sch. 2014;31:1901–1909. [Google Scholar]
- 29.Astuto M., Disma N., Crimi E. Two doses of oral ketamine, given with midazolam, for premedication in children. Minerva Anestesiol. 2002;68:593–598. [PubMed] [Google Scholar]
- 30.Sathyan N. Madurai Medical College; Madurai: 2006. Comparative Evaluation of Two Doses of Ketamine with Midazolam, Ketamine alone and Midazolam alone as Oral Premedication in Children: A Study of 100 cases. [Google Scholar]
- 31.Jain K., Ghai B., Saxena A.K., et al. Efficacy of two oral premedicants: Midazolam or a low-dose combination of midazolam-ketamine for reducing stress during intravenous cannulation in children undergoing CT imaging. Paediatr Anaesth. 2010;20:330–337. doi: 10.1111/j.1460-9592.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A., Shah Z.A., Anuradha, et al. Comparative evaluation of ketamine, midazolam and combination of both as oral premedicants in children. J Anaesthesiol Clin Pharmacol. 2009;25:449–453. [Google Scholar]
- 33.Majidinejad S., Taherian K., Esmailian M., et al. Oral midazolam-ketamine versus midazolam alone for procedural sedation of children undergoing computed tomography; a randomized clinical trial. Emergency. 2015;3:64–69. [PMC free article] [PubMed] [Google Scholar]
- 34.Rabie ME. Combination of oral ketamine and midazolam versus midazolam alone as a premedication in children undergoing tonsillectomy. AJAIC. 2005;8:58–64. [Google Scholar]
- 35.Lin Y.C., Moynihan R.J., Hackel A. A comparison of oral midazolam, oral ketamine, and oral midazolam combined with ketamine as preanesthetic medication for pediatric outpatients. Anesthesiology. 1993;79:A1177. [Google Scholar]
- 36.Warner D.L., Cabaret J., Velling D. Ketamine plus midazolam, a most effective paediatric oral premedicant. Paediatr Anaesth. 1995;5:293–295. doi: 10.1111/j.1460-9592.1995.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 37.Dave NM. Premedication and Induction of Anaesthesia in paediatric patients. Indian J Anaesth. 2019;63:713–720. doi: 10.4103/ija.IJA_491_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall J., Rodarte A., Blumer J., et al. Pediatric pharmacodynamics of midazolam oral syrup. Pediatric Pharmacology Research Unit Network. J Clin Pharmacol. 2000;40:578–589. [PubMed] [Google Scholar]
- 39.Ryh.±nen P., Kangas T., Rantakyl.± S. Premedication for outpatient adenoidectomy: comparison between ketamine and pethidine. Laryngoscope. 1980;90:494–500. doi: 10.1002/lary.5540900317. [DOI] [PubMed] [Google Scholar]
- 40.Cioac.. R., Canavea I. Oral transmucosal ketamine: an effective premedication in children. Paediatr Anaesth. 1996;6:361–365. doi: 10.1046/j.1460-9592.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 41.Horiuchi T., Kawaguchi M., Kurehara K., et al. Evaluation of relatively low dose of oral transmucosal ketamine premedication in children: a comparison with oral midazolam. Paediatr Anaesth. 2005;15:643–647. doi: 10.1111/j.1460-9592.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 42.Poonai N., Canton K., Ali S., et al. Intranasal ketamine for anesthetic premedication in children: a systematic review. Pain Manag. 2018;8:495–503. doi: 10.2217/pmt-2018-0039. [DOI] [PubMed] [Google Scholar]
- 43.Weksler N., Ovadia L., Muati G., et al. Nasal ketamine for paediatric premedication. Can J Anaesth. 1993;40:119–121. doi: 10.1007/BF03011307. [DOI] [PubMed] [Google Scholar]
- 44.Marhofer P., Freitag H., H..chtl A., et al. S(+)-ketamine for rectal premedication in children. Anesth Analg. 2001;92:62–65. doi: 10.1097/00000539-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Zhou Z.J., Zhang X.F., et al. A comparison of two different doses of rectal ketamine added to 0.5 mg x kg(-1) midazolam and 0.02 mg x kg(-1) atropine in infants and young children. Anaesth Intensive Care. 2010;38:900–904. doi: 10.1177/0310057X1003800515. [DOI] [PubMed] [Google Scholar]
- 46.Dinis-Oliveira RJ. Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2017;2:2–10. doi: 10.1080/20961790.2017.1285219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway A., Rolley J., Sutherland J.R. Midazolam for sedation before procedures. Cochrane Database Syst Rev. 2016;2016 doi: 10.1002/14651858.CD009491.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi L., Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine. 2019;98 doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyatt G.H., Oxman A.D., Sultan S., et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Andrews J., Guyatt G., Oxman A.D., et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.