This systematic review and meta-analysis evaluates the type, timing, frequency, and duration of physical therapy for Parkinson disease and whether outcomes are durable over time.
Abstract
Importance
Parkinson disease (PD) is a neurodegenerative syndrome affecting approximately 1% of the population older than 60 years, and a major goal of treatment is preservation of physical function through physical therapy (PT). Although PT outcomes for PD are well documented, aggregate information on the parameters of PT are needed to guide implementation.
Objective
To evaluate current evidence on the types, timing, frequency, duration, and outcomes of PT regimens applied for PD.
Data Sources
PubMed, Embase, Medline, and the Web of Science Core Collection were searched for articles published from January 1, 2000, to August 10, 2022. Search terms included terms related to Parkinson disease, PT interventions, and PT-related outcomes.
Study Selection
Included studies were peer-reviewed randomized clinical trials available in English of any PT intervention for patients with PD that included PT-related outcomes. The Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline was followed.
Data Extraction and Synthesis
Two reviewers extracted data and assessed quality using the Cochrane Risk of Bias Tool. Data were analyzed using a random-effects model.
Main Outcomes and Measures
A meta-analysis compared outcomes of nonstandard PT vs standard PT and standard PT vs no intervention for Unified Parkinson’s Disease Rating Scale (UPDRS) score and measures of gait and balance.
Results
A total of 46 trials with 3905 patients were included (range of mean ages, 61-77 years). Ten trials (22%) compared 2 types of nonstandard PT interventions; 26 (57%), nonstandard PT vs standard PT; and 10 (22%), PT vs no intervention. The most common nonconventional PT intervention was aquatic physiotherapy (5 trials [11%]). Durations of PT regimen ranged from 2 to 12 weeks in 39 trials (85%), and PT was most commonly performed with frequencies of either twice or 3 times weekly (27 [59%]). In most trials (39 [85%]), PT session length ranged from 30 to 60 minutes. Across trials, PT outcomes were reported for gait (14 trials [30%]), balance (10 [22%]), quality of life (3 [9%]), and cognition (1 [2%]). Approximately half of the trials (22 [48%]) documented durability of some level of benefit after completion of the prescribed regimen. Meta-analysis showed no significant difference for PT vs no intervention in UPDRS scores (standardized mean difference [SMD], −1.09; 95% CI, −2.50 to 0.33) or for nonstandard PT vs standard PT in measures of gait (SMD, 0.03; 95% CI, −0.53 to 0.59), balance (SMD, 0.54; 95% CI, −0.03 to 1.12), and UPDRS score (SMD, −0.49; 95% CI, −1.04 to 0.06). Meta-analytic regression of moderators revealed no significant differences in outcomes by frequency of PT per week (SMD, 0.17; 95% CI, –0.03 to 0.36).
Conclusions and Relevance
The findings suggest that although a wide range of types and regimens of PT for PD have been tested, comparative effectiveness of different models of care and implementation strategies as well as long-term durability of their outcomes remain undetermined.
Key Points
Question
What is the optimal type, timing, frequency, and duration of physical therapy (PT) for Parkinson disease (PD)?
Findings
This systematic review and meta-analysis of 46 randomized clinical trials of 3905 patients with PD found no differences between standard and nonstandard PT in gait, balance, and motor outcomes. Although many PT regimens were tested for PD, data were sparse on optimal type, timing, frequency, and duration and on the durability of outcomes.
Meaning
Although PT for PD has been shown to be associated with mobility benefits and there are guidelines for implementation, research is needed to better define the ideal type, timing, frequency, and duration.
Introduction
Parkinson disease (PD) is a progressive neurodegenerative syndrome affecting approximately 1% of the population older than 60 years.1 Prevalence estimates for PD increased from 2.5 million in 1990 to approximately 6 million in 2016,2 and PD is now the fastest growing neurological disease worldwide.3 Additionally, a 2022 analysis revealed that PD prevalence is underestimated by approximately 50% and new cases are diagnosed every 6 minutes.4 The burden of a progressive PD diagnosis is usually accompanied by functional disability and impaired quality of life.5 Symptoms include mobility deficits, problems with transfers, progressive balance dysfunction, and significant challenges in walking.6 A multidisciplinary approach has been recommended by many experts,7 and physical therapy (PT) is considered a cornerstone of PD treatment plans. Despite published guidelines from the American Physical Therapy Association,8 implementation of long-term PT programs for PD has been hampered by availability of information.
One of the crucial treatment goals of PD is maintenance of mobility and physical function. This goal may possibly be achieved through the application of PT,6 a medical intervention that helps restore functional movement, such as standing, walking, or moving different body parts, that is delivered by physical therapists and/or movement experts. Multiple trials and systematic reviews have documented the benefits of PT for PD.9,10 However, previous reviews addressing PT for PD have focused on specific PT types and exercise modalities (eg, aquatic therapy11 or virtual reality12) rather than methods of administration and maintenance of outcomes. There is a paucity of comprehensive studies examining PT modalities and methods for application and maintenance of PT. Agreement on how to administer PT for PD has been elusive, and potentially important variables, such as PT timing, frequency, and duration, may prove to be important to maintenance of outcomes.13 To address these knowledge gaps, we sought to evaluate the current evidence on methods of PT delivery for PD inclusive of type, timing, and frequency as well as the durability of outcome.
Methods
Identification of Eligible Publications
To be included in this systematic review and meta-analysis, studies must have been peer-reviewed and available in English. Eligible studies were randomized clinical trials published between January 1, 2000, and August 10, 2022, that included people with PD and compared a PT intervention with another intervention or no intervention. Duplicate abstracts were excluded, as were abstracts describing reviews, editorials, commentaries, protocols, conference abstracts, and dissertations. Articles from non–peer-reviewed journals and articles with untranslated full texts written in a language other than English were excluded. The review protocol was registered in PROSPERO. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Search Strategy
An electronic search of the literature was conducted in multiple databases: PubMed, Embase, Medline, and the Web of Science Core Collection. Search strategies included terms about the study population (Parkinson disease, idiopathic Parkinson disease, and Parkinson), the intervention (eg, physical therapy, physical therapy modalities, resistance training, strength training, and weight training), and some PT-related outcomes (ie, quality of life, activities of daily living, and motor skills). The full search strategy is available in eAppendix 1 in Supplement 1. We combined text and, where appropriate, Medical Subject Headings terms from all 3 categories. All sources were last searched on August 10, 2022.
Screening and Data Extraction
Study screening and data extraction were performed in Covidence. All abstracts and full-text articles were screened by 3 independent reviewers (M.E.H., J.L.M.L.J.L., and A.-M.A.N.) according to the inclusion and exclusion criteria. Conflicts were resolved by discussion and consensus among the 3 reviewers. Data were extracted from each article by 1 of the 3 reviewers, with another of the reviewers independently auditing the article to ensure accuracy of data extraction. The following variables were extracted: title, publication year, country, hospital, setting, funding sources, start and end date, primary and secondary aims, total number of participants, baseline population characteristics (age, gender, race and ethnicity, and educational level), PD characteristics (disease duration, baseline disease severity, medications, and cognitive impairment), PT characteristics (type, frequency, time intervals, session length, and duration), and PT outcomes (motor performance, mobility and gait, balance, and recent falls). Quality assessment was performed by 4 independent reviewers (M.E.H., J.L.M.L.J.L., J.H.L., and A.-M.A.N.) using the Cochrane Risk of Bias Tool, a standardized tool used to evaluate research bias in systematic reviews.14
Statistical Analysis
After exclusion of all trials with high risk of bias, a meta-analysis was separately conducted for studies comparing nonstandard vs standard PT and studies comparing standard PT vs no intervention. Trials comparing different types of nonstandard PT were excluded due to the heterogeneity of the various types of nonstandard PT. Trials with a design of 3 or more arms were excluded. Included studies must have stated either the effect sizes or the means and SDs of the outcome in both the experimental and the control groups. The meta-analysis was conducted using Review Manager, version 5.4.15 We implemented the inverse variance statistical method, with an analysis model of random effects and an effect measure of standardized mean difference (SMD), and 95% CIs were considered. The most commonly reported outcomes were categorized as follows: the Unified Parkinson’s Disease Rating Scale (UPDRS), measures of gait (timed up and go test and 10-m walking test), and measures of balance (Berg Balance Scale [BBS] and Mini Balance Evaluation System Test [mini-BESTest]). Other outcome types were reported too infrequently to be included in the meta-analysis. Across all studies in the meta-analysis, we conducted follow-up meta-regressions to assess whether duration of PT, frequency per week, and total number of PT sessions (computed by multiplying the 2 prior values) were significant moderators. We also dichotomized duration into subgroups of less than 12 or 12 or more weeks and of 6 or fewer or more than 6 weeks. For the meta-regressions, the metafor package in R, version 4.2 (R Project for Statistical Computing) was used.
Results
Study Selection
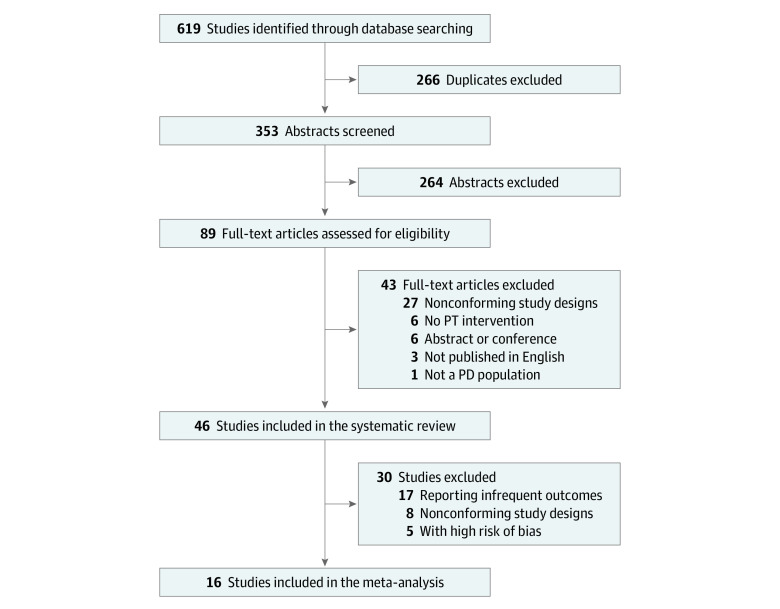
The search returned 353 abstracts after excluding all duplicates; 264 abstracts were excluded based on the eligibility criteria. Of the remaining 89 studies, 43 were excluded for the following reasons: nonconforming study design (n = 27), no PT intervention (n = 6), abstract only or conference presentation (n = 6), article not published in English (n = 3), and/or no PD patient population (n = 1) (Figure 1). The remaining 46 studies with a total of 3905 patients were included in the review.
Figure 1. PRISMA Flow Diagram.
PD indicates Parkinson disease; PT, physical therapy.
Included Studies
Most studies (27 [59%])16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 were published after 2016 (Table 1 and eAppendix 2 in Supplement 1). More than half of the trials were conducted in Italy (19 [41%]),17,19,20,21,23,24,26,29,30,31,34,40,41,46,49,51,52,59,60 Brazil (5 [11%]),18,22,27,44,45 or the US (5 [11%]).39,47,48,54,56 A fraction of the remaining trials was conducted in the UK (4 [9%]).16,35,53,61 The number of participants per trial ranged between 1547 and 76216 participants. Thirty-two studies (70%) involved 50 participants or less,17,18,19,20,21,22,23,24,25,26,27,28,29,32,33,37,39,40,41,42,44,45,46,47,49,51,54,55,58,59,60,61 and 8 studies (17%) enrolled more than 100 participants.16,35,36,38,50,53,57,62 Only 2 studies had sample sizes exceeding 500.16,57 The mean age of participants ranged from 6117 to 7718 years. With respect to baseline disease severity, more than half of the trials (25 [54%])17,21,24,25,27,29,30,31,38,39,40,41,42,43,44,45,46,48,50,51,53,56,58,59,60 included participants with a Hoehn and Yahr stage between 2 and 3. Overall, 44 trials (96%)16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60 used a parallel design and 2 (5%) used a crossover design.19,61 Of the 44 parallel design trials, 43 (98%)16,17,18,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60 were single-blinded and only 1 (2%) was double-blinded.20 Overall, 45 trials (98%)16,17,18,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 were conducted in an outpatient setting and only 1 (2%) in an inpatient setting.21
Table 1. Summary of the 46 Included Trials.
| Source (country) | Sample size (mean age, y) [H&Y stage] | Intervention | Control | Primary outcome | Main findings |
|---|---|---|---|---|---|
| Acarer et al,43 2015 (Turkey) | 60 (67) [2-3] | Vestibular rehabilitation | No intervention | Not specified | Vestibular rehabilitation was effective for improving balance (BBS, ABC, DGI) after 8 wk |
| Agosti et al,23 2016 (Italy) | 20 (62.9) [NA]a | GPR | No intervention | Kinematic parameters of gait, UPDRS III | GPR group had significant improvement in kinematic parameters of gait and UPDRS III persisting after 12 wk |
| Akhila et al,33 2022 (India) | 32 (70.8) [1.5-3.0]a | Task-related trunk training | Standard PT | Mini-BESTest | Task-related trunk training appeared more beneficial than standard physiotherapy in balance capacity after 6 wk |
| Alagumoorthi et al,36 2022 (India) | 192 (69.7) [1.5-3.0]a | Wii training | Balance training | Number of participants who fell | Wii group significantly improved over control group at 12th and 36th week in number of fallers, fall rate, and bodily discomfort component of PDQ-39 |
| Au et al,39 2022 (US) | 22 (69.7) [2.1]a | Spaced PT | Burst PT | TUG | Spaced PT group had stability of the TUG at 6 mo; burst group had significant worsening once PT was discontinued after 6 wk |
| Bonnì et al,20 2019 (Italy) | 16 (71.8) [NA]a | Blindfolded balance training | Standard PT | Gait, neurophysiological | Decrease of stance and double stance phase and increase of swing phase with respect to gait cycle in BBT group vs PT group after 2 wk |
| Capato et al,18 2020 (Brazil) | 35 (77) [4]a | PT, RAS | Standard PT | Mini-BESTest | RAS and regular balance training improved balance in patients with PD after 5 wk, but long-term effects, up to 6 mo, were sustained only in the RAS group |
| Capecci et al,44 2014 (Brazil) | 20 (73.4) [3] | Postural rehabilitation or postural rehabilitation plus kinesiology taping | No intervention | BBS, TUG, trunk bending | All treated patients showed significant improvement in trunk posture, gait, and balance at 1 mo; benefits persisted at 2 mo for all measures except lateral trunk bend |
| Carpinella et al,29 2017 (Italy) | 42 (73) [2.7]a | Balance and gait training with gamepad | Structured PT | BBS, 10MWT | Gamepad-based training was superior to PT in improving BBS performance and retaining it for 1 mo; 10MWT data were comparable between groups after training |
| Carvalho et al,45 2015 (Brazil) | 22 (64.1) [2.1] | Strength training or aerobic training | Regular PT | UPDRS III | Strength and aerobic training groups had UPDRS III improved by 27.5% and 35%, respectively; regular PT improved by 2.9% after 12 wk |
| Cholewa et al,28 2017 (Poland) | 42 (61.3) [3.2]a | Rehabilitation exercises | No intervention | Gait speed, mean step length, step frequency | Rehabilitation group showed significant improvement in gait speed (12.35%), mean step length (18.00%), and frequency step (2.40%) vs control group after 9 mo |
| Clarke et al,16 2016 (UK) | 762 (70) [NA]a | PT | No intervention | NEADL scale score | PT and OT were not associated with clinically meaningful improvements in ADLs or QOL at 3 mo |
| Clerici et al,30 2019 (Italy) | 60 (67) [2.7] | AT plus motor cognitive rehabilitation | Motor cognitive rehabilitation | FOG | MIRT improved FOG at 4 wk; AT added no further benefits |
| de Natale et al,40 2017 (Italy) | 16 (67) [2.5]a | DT | Traditional PT | 9-HPT | DT had motor and cognitive outcomes significantly improved at 2 wk of treatment and retained after follow-up of 8 wk vs TR |
| Dipasquale et al,24 2017 (Italy) | 40 (69.9) [2]a | Standard PT | General exercises | FIM, HRS, TUG, UPDRS | PT seemed more effective than a generic exercise program at 4 mo in patients with H&Y stage II PD |
| Feng et al,25 2019 (China) | 28 (67.5) [3.1]a | Virtual reality training | Conventional PT | BBS, TUG, UPDRS III, FGA | 12 wk of VR training improved gait and balance vs conventional PT (significant for BBS, TUG, and FGA but not UPDRS III) |
| Ferrazzoli et al,26 2018 (Italy) | 36 (64.5) [NA] | Multidisciplinary intensive rehabilitation plus rotiogotine | Rotigotine only | UPDRS III | During 18 mo, no differences in UPDRS III between the 2 groups, but MIRT plus rotigotine group improved in 6MWT and TUG |
| Ferreira et al,22 2018 (Brazil) | 35 (64.1) [1]a | Resistance training | No intervention | Anxiety symptoms and QOL | Significant reduction in anxiety symptoms and increase in QOL after 24 wk |
| Frazzitta et al,46 2012 (Italy) | 50 (72) [3]a | IRT | Walking instructions at home | UPDRS, UPDRS II, UPDRS III | IRT group preserved UPDRS, UPDRS II, and UPDRS III values over 1 y; control group had a significant increase in those variables in same period |
| Frisaldi et al,17 2021 (Italy) | 38 (60.7) [2]a | Conventional PT and dance therapy | Conventional PT | MDS-UPDRS III | DT method was more effective than intensive program of conventional PT in improving motor impairment in patients with mild PD after 5 wk |
| de Oliveira Gondim et al,27 2017 (Brazil) | 28 (65) [2]a | Individualized orientation and phone monitoring | Usual exercise instructions | ADLs, UPDRS-motor, PDQ-39 | Significant improvement in ADL and UPDRS-motor, PDQ-39 total and dimensions mobility, emotional well-being, and bodily discomfort in the experimental vs control group at 12 wk |
| Hirsch et al,47 2003 (US) | 15 (70.8) [1.8]a | Balance and resistance training | Balance training | SOT, muscle strength | Both types of training improved SOT performance for at least 4 wk; effect was larger in combined group |
| King et al,48 2015 (US) | 58 (63.9) [2.4] | Home exercise program, individual PT, or group class | No intervention | 7-PPT | Only the individual group significantly improved in the 7-PPT after 4 wk, mostly in functional and balance measures |
| Marchese et al,49 2000 (Italy) | 20 (65) [1.5-3]a | Cued PT | Noncued PT | Not specified | Both groups had significant reduction of ADLs and motor sections of UPDRS after 6 wk of rehabilitation; this improvement largely faded in the noncued group but was still significant in the cued group after 12 wk |
| McGinley et al,50 2012 (Australia) | 210 (67.9) [2.5] | Progressive strength training or movement strategy training (both with fall prevention) | Life skills | Feasibility of rehabilitation and PT programs | All 3 programs proved feasible, suggesting they may be safely implemented for people with PD in community-based clinical practice |
| Modugno et al,51 2010 (Italy) | 24 (62) [3]a | Active theater program | Standard PT | Clinical disability and QOL | Theater group showed progressive significant improvements at the end of the third year in all clinical scales |
| Monticone et al,52 2015 (Italy) | 70 (74.1) [2.5-4]a | Multidisciplinary rehabilitation care | General PT | MDS-UPDRS III | Multidisciplinary rehabilitation care positively changed the course of motor impairment, balance, and activities of daily living; effects lasted for at least 1 y after the intervention |
| Morris et al,38 2017 (Australia) | 133 (71) [2] | Progressive resistance strength training | Nonspecific life skills training | Rate of falls | Home program of strength and movement strategy training and falls education did not prevent falls in 12 mo when applied at the study dose |
| Nieuwboer et al,53 2007 (UK, Belgium, the Netherlands) | 153 (67.5) [2.5]a | Home cueing program followed by 3 wk with no training | 3 wk of no training followed by home cueing program | Posture and gait score | Significant improvements after intervention on the posture and gait scores; severity of freezing was reduced in freezers only; gait speed, step length, and timed balance tests improved in the full cohort; effects of intervention were reduced at 6-wk follow-up |
| Pazzaglia et al,34 2020 (Italy) | 51 (72) [NA]a | VR rehabilitation program | Conventional rehabilitation program | BBS | VR rehabilitation program led to increase in BBS and DGI scores vs conventional rehabilitation program at 6 wk |
| Pelosin et al,31 2018 (Italy) | 70 (70.4) [2.4]a | Action observation therapy | Standard PT | FOG | AOT was feasible, safe, and efficacious in improving FOG at 5 wk |
| Pérez-de la Cruz,37 2018 (Spain) | 29 (65.9) [NA]a | Aquatic Ai Chi program | Dry land conventional PT | TUG, PDQ-39-SS, LS, VAS, FTSTS, Yesavage test | Ai Chi program reduced limb bradykinesia and joint rigidity, decreased pain, and improved self-reported QOL at 11 wk in patients with H&Y stage 1-3 PD |
| Qutubuddin et al,54 2007 (US) | 22 (71.2) [NA]a | CDP therapy | Balance PT | BBS, CDP variables | No differences found between treatment groups, but both groups demonstrated improvement on selected outcome measures |
| Raciti et al,21 2022 (Italy) | 30 (65.7) [2] | Experimental robotic therapy | Conventional PT | 9-HPT | Exoskeleton-assisted therapy may be a safe and effective strategy for delivering highly intensive and repetitive training |
| San Martín Valenzuela et al,32 2020 (Spain) | 40 (66.4) [1-3]a | Dual task training | Single task training | Velocity and spatiotemporal parameters of gait | Dual-task group demonstrated improved velocity and stride length time in all assessment conditions after 5 wk of training and improved perceived QOL |
| Chivers Seymour,35 2019 (UK, Belgium) | 474 (71) [1-4]a | PDSAFE | DVD plus counseling | Risk of repeat falling | PDSAFE did not reduce falling at 6 mo |
| Tamir et al,55 2007 (Israel) | 23 (67.4) [NA]a | Combination of imagery and physical practice | Physical practice | Not specified | Significantly faster performance of movement sequences at 12 wk and higher gains in mental and motor subsets of the UPDRS and in cognitive tests vs the control group |
| Tickle-Degnen et al,56 2010 (US) | 117 (66.3) [2-3] | 18-h Rehabilitation or 27-h rehabilitation | No intervention | HRQOL | Both groups had significant beneficial effects in HRQOL at 6 wk, with benefits persisting at 2- and 6-mo follow-up |
| van Nimwegen et al,57 2013 (the Netherlands) | 586 (65.1) [1-3]a | ParkFit program | Standard PT | Activity diary and monitor | ParkFit did not change the overall volume of physical activities in older, sedentary patients with PD |
| Varalta et al,19 2021 (Italy) | 20 (70.8) [NA]a | Consecutive PT and cognitive training | Standard PT | MOCA, UPDRS III | Improvements in walking abilities were noted after both interventions, but only the patients treated with consecutive training showed better performance on functional mobility and memory tasks |
| Vivas et al,58 2011 (Spain) | 12 (65.7) [2.67]a | Water-based therapy | Land-based therapy | Not specified | Main effect of both therapies seen at 4 wk for the FRT; only the AT group improved in the BBS and the UPDRS |
| Volpe et al,59 2014 (Italy) | 34 (68) [2.82]a | Hydrotherapy | Land-based standard rehabilitation | Center of the pressure sway area | Better improvement in patients who underwent hydrotherapy than land-based therapy in the center of pressure sway area closed eyes, BBS, ABC, Falls Efficacy Scale, PDQ-39, and falls diary at 2 mo |
| Volpe et al,41 2017 (Italy) | 30 (70.6) [2-3]a | Water-based physiotherapy | Non–water-based physiotherapy | Cervical and dorsal flexion, lateral trunk inclination | After 8 wk of treatment, only the water-based group showed a significant improvement of trunk posture with a significant reduction of cervical flexion, dorsal flexion, and lateral inclination of the trunk |
| Volpe et al,60 2013 (Italy) | 24 (61.6) [2-3]a | Irish set dancing classes plus home program | Standard PT | UPDRS | Irish dancing and PT were safe and feasible, with good adherence over 6 mo; although improvements were made in both groups, the dance group showed results superior to standard physiotherapy for FOG, balance, and motor disability |
| Wade et al,61 2003 (UK) | 24 (71.3) [NA] | Active rehabilitation | No intervention | Not specified | Patients with PD declined significantly over 6 mo, but short-term multidisciplinary rehabilitation may improve mobility |
| Wróblewska et al,42 2019 (Poland) | 40 (72.1) [2-3]a | Nordic walking | No intervention | FOG | NW training had a beneficial effect on FOG in PD at 3 mo that persisted to 6 mo |
Abbreviations: 7-PPT, 7-item physical performance test; 9-HPT, hole peg test; 10MWT, 10-m walking test; ABC, activities-specific balance confidence scale; ADLs, activities of daily living; AOT, action observation therapy; AT, aquatic therapy; BBS, Berg Balance Scale; CDP, computerized dynamic posturography; DGI, dynamic gait index; DT, dance therapy; FGA, functional gait assessment; FIM, functional independence measure; FOG, freezing of gait; FRT, functional reach test; FTSTS, 5 times sit-to-stand test; GPR, global postural reeducation; H&Y, Hoehn and Yahr; HRQOL, health-related quality of life; HRS, Hamilton Rating Scale; IRT, intensive rehabilitation treatment; LS, leg standing; MDS-UPDRS III, Movement Disorder Society Sponsored Revision of the Unified Parkinson Disease Rating Scale; mini-BESTest, Mini Balance Evaluation System Test; MIRT, multidisciplinary intensive rehabilitation treatment; MOCA, Montreal Cognitive Assessment Test; NA, not applicable; NEADL, Nottingham Extended Activities of Daily Living; NW, Nordic walking; OT, occupational therapy; PD, Parkinson disease; PDQ-39, Parkinson Disease Questionnaire; PDQ-39-SS, Parkinson Disease Questionnaire–Social Support; PT, physical therapy; QOL, quality of life; RAS, rhythmical auditory stimulation; SOT, sensory orientation test; TR, traditional rehabilitation; TUG, timed up and go test; UPDRS, Unified Parkinson’s Disease Rating Scale; UPDRS II, Unified Parkinson Disease Rating Scale for activities of daily living; UPDRS III, Unified Parkinson Disease Rating Scale for motor skills; VAS, visual analog scale; VR, virtual reality.
Variables of the experimental group were included in the table if the mean age and/or baseline H&Y stage of the trial participants was not mentioned.
Outcome Measures
The primary outcomes varied across the included trials. Some trials measured patient-reported outcomes (eg, quality of life),16,22,51 and others targeted functional outcomes (eg, 10-m walking test).29 The most common primary outcome was the UPDRS, including its different components (ie, UPDRS II [activities of daily living], UPDRS III [motor skills], and Movement Disorder Society Sponsored Revision of the UPDRS). The UPDRS measures were used in 14 studies (30%).17,19,23,24,25,26,27,43,45,46,52,55,58,60 Other common primary outcomes included gait (14 studies [30%]),20,23,24,25,28,29,30,31,32,42,44,53,54,58 using measurements such as the 10-m walking test,58 the timed up and go test,44 or other gait parameters.20 One other category, in 10 studies (22%), addressed balance measures such as the BBS or the mini-BESTest.18,25,29,33,34,43,44,54,55,58 Other outcomes included falls or postural stability (4 studies [9%]),35,36,43,53 the Parkinson Disease Questionnaire-39 (4 [9%]),27,37,43,56 other quality of life measures (3 [7%]),22,43,51 and cognition (1 [2%]).40
The included trials used 1 of 3 types of comparison groups. Some trials (10 [22%]29,30,32,35,36,39,47,53,54,55) compared 2 different types of PT (eg, dance therapy vs conventional PT17), while 10 (22%)16,22,23,28,42,43,44,48,56,61 compared PT with a no-intervention control group.28,49 The remaining studies (26 [57%]17,18,19,20,21,24,25,26,27,31,33,34,37,38,40,41,45,46,49,50,51,52,57,58,59,60) compared PT with general exercise.24,46 One trial included PT delivered in a group setting.48
When comparing 2 different types of PT, it was not uncommon to measure UPDRS and/or balance as primary outcomes. In fact, multiple studies showed that 1 type of PT was superior to another in improving balance in patients with PD.18,25,29,33,34 For example, virtual reality training had significantly better balance outcomes compared with standard PT,34 and task-related trunk training with emphasis on spine exercises proved to be superior to standard PT based on the mini-BESTest.33 One trial showed that water-based PT improved balance (BBS) compared with land-based PT in patients with PD.58 Safety and feasibility of PT interventions in patients with PD were assessed in 2 different trials, and both had affirmative results.21,50 However, 5 of the 46 trials (11%) did not find a difference in their primary outcome(s).16,26,35,38,57 Two of those trials targeted falls in their outcomes.35,38
Physical Therapy Regimens
Physical therapy modalities varied considerably across studies (Table 2). Twenty-one trials (46%) described 1 of their comparison arms as standard or conventional PT. Generally, standard or conventional PT is delivered by a physical therapist in a regular outpatient center and consists of general body movements and exercises. Any variation in the setting, type, intensity, or mode of delivery was categorized as nonstandard or nonconventional. Those interventions included water-based PT (5 studies [11%]),30,37,41,59,60 followed by multidisciplinary therapy (4 [9%])18,19,26,52; balance, resistance, or strength training (4 [9%])22,38,45,47; and dance therapy (3 [7%]).17,40,60 Other interventions included virtual reality rehabilitation (2 [4%]),25,34 walking (2 [4%]),35,42 cueing PT (2 [4%]),49,53 and game-based rehabilitation (2 [4%]).29,36
Table 2. Summary of the Type, Duration, Frequency, Session Length, and Durability of Outcome of the Physical Therapy Regimen in the Intervention and Control Groups.
| Source | Intervention group | Control group | Durability of outcome | ||||
|---|---|---|---|---|---|---|---|
| Frequency | Session length | Regimen duration | Frequency | Session length | Regimen duration | ||
| Acarer et al,43 2015 | NA | 30-40 min | 8 wk | NA | NA | NA | NA |
| Agosti et al,23 2016 | 3 Times/wk | 40 min | 4 wk | NA | NA | NA | 4 and 8 wk |
| Akhila et al,33 2022 | 3 Times/wk | 60 min | 6 wk | 3 Times/wk | 60 min | 6 wk | NA |
| Alagumoorthi et al,36 2022 | 3 Times/wk | 30-40 min | 12 wk | 3 Times/wk | 30-40 min | 12 wk | 24 wk |
| Au et al,39 2022 | 2 Times/wk | NA | 6 wk | 1 Time every 2 wk | NA | 6 mo | 18 wk |
| Bonnì et al,20 2019 | 5 Times/wk | 40 min | 2 wk | 5 Times/wk | 45 min | 2 wk | NA |
| Capato et al,18 2020 | 2 Times/wk | 45 min | 5 wk | 2 Times/wk | 45 min | 5 wk | 1 and 6 mo |
| Capecci et al,44 2014 | 3 Times/wk | 40 min | 4 wk | NA | NA | NA | 4 wk |
| Carpinella et al,29 2017 | 3 Times/wk | 45 min | 7 wk | 3 Times/wk | 45 min | 7 wk | 4 wk |
| Carvalho et al,45 2015 | 2 Times/wk | 40 min | 12 wk | NA | NA | NA | NA |
| Cholewa et al,28 2017 | 2 Times/wk | 60 min | 36 mo | NA | NA | NA | NA |
| Clarke et al,16 2016 | Median sessions, 4 | 58 min | 8 wk | NA | NA | NA | NA |
| Clerici et al,30 2019 | 4 Times/d for 5 d, PT the 6th day | 60 min | 4 wk | 4 Times/d for 5 d, PT on the 6th day, 3 AT weekly | 60 min | 4 wk | NA |
| De Natale et al,40 2017 | 2 Times/wk | 60 min | 10 wk | 2 Times/wk | 60 min | 10 wk | 8 wk |
| Dipasquale et al,24 2017 | 2 Times/wk | 60 min | 4 mo | 2 Times/wk | 60 min | 4 mo | 135 d |
| Feng et al,25 2019 | 5 d/wk | 45 min | 12 wk | 5 Times/wk | 45 min | 12 wk | NA |
| Ferrazzoli et al,26 2018 | 4 Sessions for 5 d plus 1 h of exercise on the 6th day | 60 min | 4 wk | NA | NA | NA | 6, 12, and 18 mo |
| Ferreira et al,22 2018 | 2 Times/wk | 30-40 min | 24 wk | NA | NA | NA | NA |
| Frazzitta et al,46 2012 | 3 Times daily, 5 d/wk | 60 min | 4 wk | NA | NA | NA | NA |
| Frisaldi et al,17 2021 | 3 Times/wk | 60 min | 5 wk | 3 Times/wk | 60 min | 5 wk | NA |
| de Oliveira Gondim et al,27 2017 | 3 Times/wk | Up to 1 h | 12 wk | 3 Times/wk | Up to 1 h | 12 wk | NA |
| Hirsch et al,47 2003 | 3 Times/wk | 15 min Resistance, 30 min balance | 10 wk | 3 Times/wk | 30 min | 10 wk | 4 wk |
| King et al,48 2015 | 3 Times/wk | 60 min | 6 wk | 3 Times/wk | 60 min | 6 wk | NA |
| Marchese et al,49 2000 | 3 Times/wk | 60 min | 6 wk | 3 Times/wk | 60 min | 6 wk | 6 wk |
| McGinley et al,50 2012 | 1 Time/wk | 120 min | 8 wk | 1 Times/wk | 120 min | 8 wk | NA |
| Modugno et al,51 2010 | 2 Consecutive days either 1 or 2 times per mo | 6 h | 3 y | 3 Times/wk | 2 to 3 h | 3 y | NA |
| Monticone et al,52 2015 | Daily | 90 min | 8 wk | Daily | 90 min | 8 wk | 12 mo |
| Morris et al,38 2017 | 2 Times/wk | 60 min | 6 wk | 2 Times/week | 60 min | 6 wk | 12 mo |
| Nieuwboer et al,53 2007 | 9 Sessions over 3 wk | 30 min | 3 wk | 9 Sessions over 3 wk | 30 min | 3 wk | 6 wk |
| Pazzaglia et al,34 2020 | 3 Times/wk | 40 min | 6 wk | 3 Times/wk | 40 min | 6 wk | NA |
| Pelosin et al,31 2018 | 2 Times/wk | 45 min | 5 wk | 2 Times/wk | 45 min | 5 wk | 4 wk |
| Pérez-de la Cruz,37 2018 | 2 Times/wk | 45 min | 11 wk | 2 Times/wk | 45 min | 11 wk | 4 wk |
| Qutubuddin et al,54 2007 | 2 Times/wk | 30 min | 4 wk | 2 Times/wk | 30 min | 4 wk | NA |
| Raciti et al,21 2022 | 6 Times/wk | 45 min for each arm (90 min total) | 8 wk | 6 Times/wk | 45 min | 8 wk | NA |
| San Martín Valenzuela et al,32 2020 | 2 Times/wk | 60 min | 10 wk | 2 Times/wk | 60 min | 10 wk | 8 wk |
| Chivers Seymour et al,35 2019 | 12 Sessions in 6 mo plus daily exercise | 1-1.5 h Supervised session, 30 min exercise | 72 wk | 1 Session on fall avoidance | NA | NA | 6 mo |
| Tamir et al,55 2007 | 2 Times/wk | 1 h | 12 wk | 2 Times/wk | 1 h | 12 wk | NA |
| Tickle-Degnen et al,56 2010 | 3 Times/wk | 90 min | 6 wk | 2 Times/wk | 90 min | 6 wk | 2 and 18 wk |
| van Nimwegen et al,57 2013 | Maximum of 35 sessions/y | NA | 12 mo | Maximum 35 sessions/y | NA | 12 mo | NA |
| Varalta et al,19 2021 | PT once weekly; cognitive once weekly | 50 min | 12 wk | 2 Times/wk | 50 min | 12 wk | NA |
| Vivas et al,58 2011 | 2 Times/wk | 45 min | 4 wk | 2 Times/wk | 45 min | 4 wk | 17 d |
| Volpe et al,59 2014 | 5 Times/wk | 60 min | 8 wk | 5 Times/wk | 60 min | 8 wk | NA |
| Volpe et al,41 2017 | 5 Times/wk | 60 min | 8 wk | 5 Times/wk | 60 min | 8 wk | 8 wk |
| Volpe et al,60 2013 | 1 Time/wk | 90 min | 24 wk | 1 Time/wk | 90 min | 24 wk | NA |
| Wade et al,61 2003 | 1 Time/wk | NA | 6 wk | 1 Time/wk | NA | 6 wk | NA |
| Wróblewska et al,42 2019 | 2 Times/wk | 60 min | 12 wk | NA | NA | NA | 3 mo |
Abbreviations: AT, aquatic therapy; NA, not available.
Physical therapy regimens differed in duration, frequency, and session length. The total duration ranged from 2 weeks20 to 3 years,28,51 whereas most studies had regimen durations of 2 to 12 weeks (39 [85%]16,17,18,19,20,21,23,25,26,27,29,30,31,32,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,53,54,55,56,58,59,61). Frequency ranged from once weekly60,61 to daily52; however, most of the trials had frequencies of either 2 or 3 times weekly (27 studies [59%]).17,18,22,23,24,27,28,29,31,32,33,34,36,37,38,39,40,42,44,45,47,48,49,54,55,56,58 Only 9 studies (20%) had frequencies greater than or equal to 5 times weekly.20,21,25,30,35,41,46,52,59 The session lengths were somewhat similar, with 40 trials (87%) reporting sessions that lasted from 30 to 60 minutes.16,17,18,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,53,54,57,58,59,60,61 Of the other 6 trials (13%), 4 had session lengths of 90 minutes,35,52,56,60 1 had a session length of 120 minutes,50 and 1 had sessions lasting for 6 hours.51 Only 1 trial had 1 arm that administered PT in group sessions48; all other trials administered PT individually.
Durability of Outcome
We investigated durability of the outcome of the PT interventions (ie, whether the observed outcome was maintained after PT was discontinued). We identified all trials that followed up patients for a minimum of 1 visit after the end of the intervention period. About half of the included trials (22 [48%]) addressed this issue.18,23,24,26,29,31,32,35,36,37,38,39,40,41,42,44,47,49,52,53,56,58 Patients receiving multiple cycles of PT with follow-up after each cycle were not included in the subgroup evaluation. The follow-up intervals, measured from the end of the PT intervention, are included in Table 2. Follow-up periods ranged from 17 days58 to 18 months.26 Most of the 22 trials had 1 maintenance follow-up visit (18 [82%]).24,29,31,32,35,36,37,38,39,40,41,42,44,47,49,52,53,58 Some trials had 2 maintenance follow-up visits (3 [14%]),18,23,56 and only 1 trial (5%) had 3 visits.26 The findings at each follow-up interval are included in Table 1. Seventeen of the 22 trials (77%) reported that the observed improvement at the end of treatment was durable until the subsequent follow-up.18,23,24,29,31,32,36,37,40,41,42,44,47,49,52,56,58 The remaining 5 trials (23%) revealed worsening of outcomes after stopping PT39,53 or that the PT intervention group lost durability of outcome over time.26,35,38
Quality Assessment
Quality assessment was conducted using the Cochrane Risk of Bias Tool.14 Every section of the assessment tool was divided into high risk of bias, low risk of bias, or some concerns. Overall, 17 articles (37%) had a low risk of bias,16,19,24,26,30,31,32,35,37,38,39,41,48,49,59,60,61 24 (52%) had some concerns,17,18,20,21,22,23,25,27,29,33,34,40,44,45,46,50,51,52,53,54,55,56,57,58 and 5 (11%) had a high risk of bias28,36,42,43,47(eAppendix 3 in Supplement 1). Studies were rated with high risk due to bias in the measurement of the outcome,28,47 deviations from intended interventions,28,42 or selection of the reported result.30,43 Studies classified as having some concerns mainly had insufficient reporting on the randomization process or on deviations from the intended interventions.
Meta-Analysis
There were enough studies to meta-analyze all 3 categories of outcomes when comparing nonstandard vs standard PT (k = 6 for gait,17,19,25,29,37,40 k = 9 for balance,17,18,25,29,33,34,40,52,58 and k = 8 for UPDRS17,18,19,25,40,45,52,60). However, when comparing standard PT vs no intervention, only UPDRS measures could be analyzed (k = 322,23,26) as there were not enough studies for gait or balance outcomes. For gait, results indicated no difference between nonstandard and standard PT (SMD, 0.03; 95% CI, −0.53 to 0.59). For balance and UPDRS, the results also indicated no significant difference between nonstandard and standard PT (balance: SMD, 0.54; 95% CI, −0.03 to 1.12; UPDRS: SMD, −0.49; 95% CI, −1.04 to 0.06). When comparing standard PT vs no intervention for UPDRS, results also lacked statistical significance (SMD, –1.09; 95% CI, −2.50 to 0.33). Across the 4 meta-analyses, between-study variance (τ2) ranged from 0.32 to 1.32, with most of this variance explained by actual differences rather than random error (I2 = 68%-88%). Forest plots are presented in Figure 2. Results of the meta-analytic moderator analyses were not statistically significant (eAppendix 4 in Supplement 1), and differences in outcomes by frequency of PT per week were not significant (SMD, 0.17; 95% CI, –0.03 to 0.36).
Figure 2. Forest Plots of Measures of Gait, Balance, and Unified Parkinson Disease Rating Scale (UPDRS) Scores.
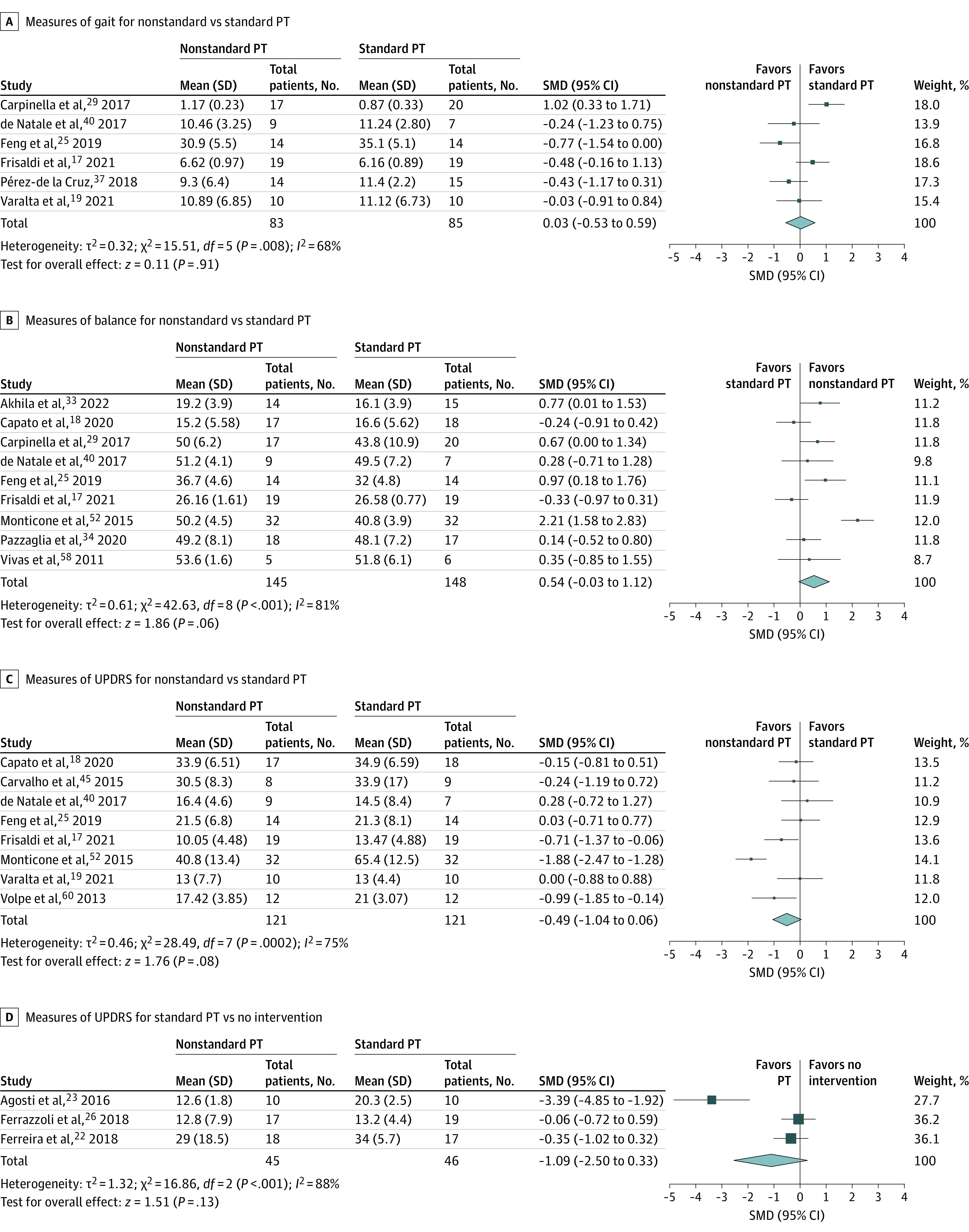
Gait was assessed by the 10-m walk test and timed up and go test and balance by the Berg Balance Scale and Mini Balance Evaluation System Test. A random-effects model was used with inverse variance. Squares represent standardized mean differences (SMDs), with horizontal lines representing 95% CIs. Diamonds represent pooled SMDs, with outer points of the diamonds representing 95% CIs. PT indicates physical therapy.
Discussion
This comprehensive systematic review and meta-analysis revealed that many types of PT have shown benefit in the care of persons with PD. The study also revealed that there are gaps in our understanding of the effects of the type, timing, and frequency of PT for PD care, although maintenance of effect remains largely unexplored. Although a wide range of PT techniques and regimens have been commonly applied, there is scant head-to-head evidence comparing different techniques. A recently published expert viewpoint revealed no consensus on optimal timing and maintenance of PT for PD.62 Although many studies compared different interventions using comparative arms such as exercise therapy,24,27,38,46,55 few focused on uncovering the optimal implementation strategies for standard PT regimens with respect to type, timing, frequency, and maintenance of effect.39
Physical therapy has been established as an effective nonpharmacologic approach for the treatment of mobility deficits in patients with PD; however, there is a wide range of options. Complicating the choice is uncertainty on how exercise should be woven into implementation of PT. Previously published systematic reviews11,12 have focused on specific types of PT rather than the nature of the PT regimens. The 2 most common published outcomes in the current study were for gait (11 trials [24%]23,25,26,28,30,31,32,42,44,53,61) and balance (10 trials [22%]18,25,29,33,43,44,48,52,53,58). The evidence supported that different types of PT can be beneficial, and these ranged from general physiotherapy16 to specific tailored PT regimens.42,43 A wide range of PT durations, frequencies, and session lengths were applied across studies. Many PT regimens showed improvement in at least 1 area of physical function. Our findings aligned with the systematic review by Tomlinson et al (2012),9 which revealed benefits in speed and in BBS and UPDRS scores. The field of neurology has continued to be challenged with how to evaluate and control for home-based exercise in PT. For example, a systematic review and meta-analysis of exercise for PD published in 2021 showed that moderate aerobic exercise was associated with improved balance and gait; however, the outcomes for other PD motor symptoms were less clear.10 We deliberately focused our review on PT and not on exercise therapy. Our review included 5 trials with evidence supporting improvement in a primary outcome that was motor based.17,27,40,49,52 There were fewer studies showing benefits of other modalities such as dance40 and vestibular rehabilitation.43 This review addressed whether PT outcomes are durable after PT discontinuation, a largely unexplored topic in the literature. Many of the trials addressing this issue reported positive results on longer-term follow-up18,23,24,29,31,32,36,37,40,41,42,44,47,49,52,56,58; however, none of the trials followed up participants for more than 18 months.
Most trials in this study used PT regimens of 2 to 12 weeks with frequencies of 2 to 3 times weekly and with session lengths of 30 to 60 minutes. Although clinicians may consider this a reasonable standard when prescribing PT, without trial comparators, clinicians can only craft a reasonable guess. In addition, clinicians may be challenged to choose a therapy that will have the best chance for durability of outcome. Further complicating this is the nonsignificant results obtained from our meta-analytic moderator tests of duration, frequency, and number of sessions. Due to the limited number of studies amenable to meta-analysis, it remains unclear whether these factors were truly not associated with outcomes or whether findings were prone to type II error. More studies in this area may help to provide clarity.
The findings from this study may inform clinical practice and help physical therapists and physicians to implement PT for PD. Across geographical regions such as in the US, persons with PD can seek PT without a prescription from a physician. However, there are limited data for implementing gamepad-based training for balance29 or action observation treatment for freezing of gait.31 More comparative effectiveness research is needed. Also, many clinicians likely will not be aware of whether a facility offers specific interventions that require training or specialized equipment. Home-based exercises46,48 might improve access and adherence; however, few outcomes data are available on these regimens and how implementation could be weaved together with direct access to physical therapists or through physician prescriptions.
Limitations
There were several limitations in this study. First, our search strategy might have missed publications addressing outcomes other than activities of daily living, quality of life, and/or motor outcomes. In addition, the diversity of tests used to measure outcomes rendered head-to-head comparisons challenging. One example of this limitation would be gait, which could be measured through speed,28 kinematic parameters,23 the timed up and go test,44 and the 10-m walk test.29 Although all these variables measure gait performance, each outcome should be interpreted as independent and specific to a clinical characteristic. Furthermore, pooling all trials and then reporting and analyzing all PT parameters for different PT types is a limitation. Ideally, one should aim for a better categorization of PT types and analysis of PT subcategories separately. However, this was not possible due to uncertain categorizations as well as numerous categories that each contained few studies. Another important limitation was the heterogeneity in PT types, timing, frequencies, and duration and the limited number of studies that compared these factors. As a result, our meta-analyses were based on a limited number of studies and thus were prone to type II error. More work needs to be done to confirm or refute our meta-analytic findings. Additionally, only a minority of trials included adequate long-term outcomes to assess durability of the intervention. Future studies should account for home exercise programs and their effects on outcomes.
Conclusions
This systematic review and meta-analysis found that although the literature supports the use of PT as an effective treatment for PD and despite guidelines for inclusion of PT from the American Physical Therapy Association,8 implementation factors such as type, timing, frequency, and durability of outcomes remain largely unexplored. Our meta-analysis revealed no significant difference between standard PT and nonstandard PT for balance, gait, and UPDRS scores. These findings should be interpreted with caution and confirmed with better-powered studies. More controlled trials and comparative effectiveness studies are needed to evaluate the risks, benefits, and durability of each type of PT intervention and to guide better implementation.
eAppendix 1. Search Strategy Used to Retrieve Eligible Articles for the Review
eAppendix 2. Summary of Systematic Review Findings
eAppendix 3. Summary of the Risk of Bias in Trials Implementing an Intention-to-Treat Analysis and Trials Implementing a Per-Protocol Analysis
eAppendix 4. Methods Used to Perform the Meta Moderators Analysis
Data Sharing Statement
References
- 1.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna). 2017;124(8):901-905. doi: 10.1007/s00702-017-1686-y [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Vos T, Alahdab F, et al. ; GBD 2017 US Neurological Disorders Collaborators . GBD 2017 US Neurological Disorders Collaborators. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3-S8. doi: 10.3233/JPD-181474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis AW, Roberts E, Beck JC, et al. ; Parkinson’s Foundation P4 Group . Incidence of Parkinson disease in North America. NPJ Parkinsons Dis. 2022;8(1):170. doi: 10.1038/s41531-022-00410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1-12. doi: 10.1016/j.cger.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radder DLM, Sturkenboom IH, van Nimwegen M, Keus SH, Bloem BR, de Vries NM. Physical therapy and occupational therapy in Parkinson’s disease. Int J Neurosci. 2017;127(10):930-943. doi: 10.1080/00207454.2016.1275617 [DOI] [PubMed] [Google Scholar]
- 7.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284-2303. doi: 10.1016/S0140-6736(21)00218-X [DOI] [PubMed] [Google Scholar]
- 8.Osborne JA, Botkin R, Colon-Semenza C, et al. Physical therapist management of Parkinson disease: a clinical practice guideline from the American Physical Therapy Association. Phys Ther. 2022;102(4):pzab302. doi: 10.1093/ptj/pzab302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. doi: 10.1136/bmj.e5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Song H, Shen L, Wang Y. The efficacy and safety of moderate aerobic exercise for patients with Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med. 2021;10(3):2638-2649. doi: 10.21037/apm-20-1661 [DOI] [PubMed] [Google Scholar]
- 11.Cugusi L, Manca A, Bergamin M, et al. Aquatic exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: a systematic review. J Physiother. 2019;65(2):65-74. doi: 10.1016/j.jphys.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Dockx K, Bekkers EM, Van den Bergh V, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev. 2016;12(12):CD010760. doi: 10.1002/14651858.CD010760.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord. 2013;28(11):1587-1596. doi: 10.1002/mds.25658 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 16.Clarke CE, Patel S, Ives N, et al. ; PD REHAB Collaborative Group . Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease: a randomized clinical trial. JAMA Neurol. 2016;73(3):291-299. doi: 10.1001/jamaneurol.2015.4452 [DOI] [PubMed] [Google Scholar]
- 17.Frisaldi E, Bottino P, Fabbri M, et al. Effectiveness of a dance-physiotherapy combined intervention in Parkinson’s disease: a randomized controlled pilot trial. Neurol Sci. 2021;42(12):5045-5053. doi: 10.1007/s10072-021-05171-9 [DOI] [PubMed] [Google Scholar]
- 18.Capato TTC, Nonnekes J, de Vries NM, IntHout J, Barbosa ER, Bloem BR. Effects of multimodal balance training supported by rhythmical auditory stimuli in people with advanced stages of Parkinson’s disease: a pilot randomized clinical trial. J Neurol Sci. 2020;418:117086. doi: 10.1016/j.jns.2020.117086 [DOI] [PubMed] [Google Scholar]
- 19.Varalta V, Poiese P, Recchia S, et al. Physiotherapy versus consecutive physiotherapy and cognitive treatment in people with Parkinson’s disease: a pilot randomized cross-over study. J Pers Med. 2021;11(8):687. doi: 10.3390/jpm11080687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnì S, Ponzo V, Tramontano M, et al. Neurophysiological and clinical effects of blindfolded balance training (BBT) in Parkinson’s disease patients: a preliminary study. Eur J Phys Rehabil Med. 2019;55(2):176-182. doi: 10.23736/S1973-9087.18.05126-2 [DOI] [PubMed] [Google Scholar]
- 21.Raciti L, Pignolo L, Perini V, et al. Improving upper extremity bradykinesia in Parkinson’s disease: a randomized clinical trial on the use of gravity-supporting exoskeletons. J Clin Med. 2022;11(9):2543. doi: 10.3390/jcm11092543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira RM, Alves WMGDC, de Lima TA, et al. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson’s disease: a randomized controlled trial. Arq Neuropsiquiatr. 2018;76(8):499-506. doi: 10.1590/0004-282x20180071 [DOI] [PubMed] [Google Scholar]
- 23.Agosti V, Vitale C, Avella D, et al. Effects of global postural reeducation on gait kinematics in parkinsonian patients: a pilot randomized three-dimensional motion analysis study. Neurol Sci. 2016;37(4):515-522. doi: 10.1007/s10072-015-2433-5 [DOI] [PubMed] [Google Scholar]
- 24.Dipasquale S, Meroni R, Sasanelli F, et al. Physical therapy versus a general exercise programme in patients with Hoehn Yahr stage II Parkinson’s disease: a randomized controlled trial. J Parkinsons Dis. 2017;7(1):203-210. doi: 10.3233/JPD-161015 [DOI] [PubMed] [Google Scholar]
- 25.Feng H, Li C, Liu J, et al. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: a randomized controlled trial. Med Sci Monit. 2019;25:4186-4192. doi: 10.12659/MSM.916455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrazzoli D, Ortelli P, Riboldazzi G, Maestri R, Frazzitta G. Effectiveness of rotigotine plus intensive and goal-based rehabilitation versus rotigotine alone in “de-novo” parkinsonian subjects: a randomized controlled trial with 18-month follow-up. J Neurol. 2018;265(4):906-916. doi: 10.1007/s00415-018-8792-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira Gondim ITG, Lins CCDSA, Asano NMJ, Asano AGC, Cabral ED, de Sales Coriolano MDGW. Individualized guidance and telephone monitoring in a self-supervised home-based physiotherapeutic program in Parkinson. Fisioter Mov. 2017;30(3):559-568. doi: 10.1590/1980-5918.030.003.ao14 [DOI] [Google Scholar]
- 28.Cholewa J, Cholewa J, Gorzkowska A, Malecki A, Stanula A. Can rehabilitation influence the efficiency of control signals in complex motion strategies? Biomed Res Int. 2017;2017:3631624. doi: 10.1155/2017/3631624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpinella I, Cattaneo D, Bonora G, et al. Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(4):622-630.e3. doi: 10.1016/j.apmr.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 30.Clerici I, Maestri R, Bonetti F, et al. Land plus aquatic therapy versus land-based rehabilitation alone for the treatment of freezing of gait in Parkinson disease: a randomized controlled trial. Phys Ther. 2019;99(5):591-600. doi: 10.1093/ptj/pzz003 [DOI] [PubMed] [Google Scholar]
- 31.Pelosin E, Barella R, Bet C, et al. Effect of group-based rehabilitation combining action observation with physiotherapy on freezing of gait in Parkinson’s disease. Neural Plast. 2018;2018:4897276. doi: 10.1155/2018/4897276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San Martín Valenzuela C, Moscardó LD, López-Pascual J, Serra-Añó P, Tomás JM. Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: results of randomized controlled DUALGAIT trial. Arch Phys Med Rehabil. 2020;101(11):1849-1856.e1. doi: 10.1016/j.apmr.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 33.Akhila R, Karthikbabu S, Mohan D, Prem V, Kumar Roy A. Task-related trunk training on balance, trunk control, pulmonary function and quality of life in patients with Parkinson’s disease: a randomised controlled trial. Int J Ther Rehabil. Published online April 6, 2022. doi: 10.12968/ijtr.2020.0146 [DOI] [Google Scholar]
- 34.Pazzaglia C, Imbimbo I, Tranchita E, et al. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: a randomised controlled trial. Physiotherapy. 2020;106:36-42. doi: 10.1016/j.physio.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 35.Chivers Seymour K, Pickering R, Rochester L, et al. Multicentre, randomised controlled trial of PDSAFE, a physiotherapist-delivered fall prevention programme for people with Parkinson’s. J Neurol Neurosurg Psychiatry. 2019;90(7):774-782. doi: 10.1136/jnnp-2018-319448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alagumoorthi G, Jebakani BD, Thirunavukarasu S, Ramachandaran V, Kumaresan A. Effectiveness of Wii sports-based strategy training in reducing risk of falling, falls and improving quality of life in adults with idiopathic Parkinson’s disease—a randomized comparative trial. Clin Rehabil. 2022;36(8):1097-1109. doi: 10.1177/02692155221089030 [DOI] [PubMed] [Google Scholar]
- 37.Pérez-de la Cruz S. A bicentric controlled study on the effects of aquatic Ai Chi in Parkinson disease. Complement Ther Med. 2018;36:147-153. doi: 10.1016/j.ctim.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 38.Morris ME, Taylor NF, Watts JJ, et al. A home program of strength training, movement strategy training and education did not prevent falls in people with Parkinson’s disease: a randomised trial. J Physiother. 2017;63(2):94-100. doi: 10.1016/j.jphys.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 39.Au KLK, Lopes JLMLJ, Kraus A, et al. A randomized clinical trial of burst vs spaced physical therapy for Parkinsons disease. Parkinsonism Relat Disord. 2022;97:57-62. doi: 10.1016/j.parkreldis.2022.02.021 [DOI] [PubMed] [Google Scholar]
- 40.de Natale ER, Paulus KS, Aiello E, et al. Dance therapy improves motor and cognitive functions in patients with Parkinson’s disease. NeuroRehabilitation. 2017;40(1):141-144. doi: 10.3233/NRE-161399 [DOI] [PubMed] [Google Scholar]
- 41.Volpe D, Giantin MG, Manuela P, et al. Water-based vs non–water-based physiotherapy for rehabilitation of postural deformities in Parkinson’s disease: a randomized controlled pilot study. Clin Rehabil. 2017;31(8):1107-1115. doi: 10.1177/0269215516664122 [DOI] [PubMed] [Google Scholar]
- 42.Wróblewska A, Gajos A, Smyczyńska U, Bogucki A. The therapeutic effect of Nordic walking on freezing of gait in Parkinson’s disease: a pilot study. Parkinsons Dis. 2019;2019:3846279. doi: 10.1155/2019/3846279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acarer A, Karapolat H, Celebisoy N, Ozgen G, Colakoglu Z. Is customized vestibular rehabilitation effective in patients with Parkinson’s? NeuroRehabilitation. 2015;37(2):255-262. doi: 10.3233/NRE-151258 [DOI] [PubMed] [Google Scholar]
- 44.Capecci M, Serpicelli C, Fiorentini L, et al. Postural rehabilitation and Kinesio taping for axial postural disorders in Parkinson’s disease. Arch Phys Med Rehabil. 2014;95(6):1067-1075. doi: 10.1016/j.apmr.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 45.Carvalho A, Barbirato D, Araujo N, et al. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson’s disease: pilot study. Clin Interv Aging. 2015;10:183-191. doi: 10.2147/CIA.S68779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazzitta G, Bertotti G, Riboldazzi G, et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: a randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair. 2012;26(2):144-150. doi: 10.1177/1545968311416990 [DOI] [PubMed] [Google Scholar]
- 47.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(8):1109-1117. doi: 10.1016/S0003-9993(03)00046-7 [DOI] [PubMed] [Google Scholar]
- 48.King LA, Wilhelm J, Chen Y, et al. Effects of group, individual, and home exercise in persons with Parkinson disease: a randomized clinical trial. J Neurol Phys Ther. 2015;39(4):204-212. doi: 10.1097/NPT.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchese R, Diverio M, Zucchi F, Lentino C, Abbruzzese G. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord. 2000;15(5):879-883. doi: [DOI] [PubMed] [Google Scholar]
- 50.McGinley JL, Martin C, Huxham FE, et al. Feasibility, safety, and compliance in a randomized controlled trial of physical therapy for Parkinson’s disease. Parkinsons Dis. 2012;2012:795294. doi: 10.1155/2012/795294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modugno N, Iaconelli S, Fiorlli M, Lena F, Kusch I, Mirabella G. Active theater as a complementary therapy for Parkinson’s disease rehabilitation: a pilot study. ScientificWorldJournal. 2010;10:2301-2313. doi: 10.1100/tsw.2010.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord. 2015;30(8):1050-1058. doi: 10.1002/mds.26256 [DOI] [PubMed] [Google Scholar]
- 53.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134-140. doi: 10.1136/jnnp.200X.097923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qutubuddin AA, Cifu DX, Armistead-Jehle P, Carne W, McGuirk TE, Baron MS. A comparison of computerized dynamic posturography therapy to standard balance physical therapy in individuals with Parkinson’s disease: a pilot study. NeuroRehabilitation. 2007;22(4):261-265. doi: 10.3233/NRE-2007-22402 [DOI] [PubMed] [Google Scholar]
- 55.Tamir R, Dickstein R, Huberman M. Integration of motor imagery and physical practice in group treatment applied to subjects with Parkinson’s disease. Neurorehabil Neural Repair. 2007;21(1):68-75. doi: 10.1177/1545968306292608 [DOI] [PubMed] [Google Scholar]
- 56.Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(2):194-204. doi: 10.1002/mds.22940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Nimwegen M, Speelman AD, Overeem S, et al. ; ParkFit Study Group . Promotion of physical activity and fitness in sedentary patients with Parkinson’s disease: randomised controlled trial. BMJ. 2013;346:f576. doi: 10.1136/bmj.f576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vivas J, Arias P, Cudeiro J. Aquatic therapy versus conventional land-based therapy for Parkinson’s disease: an open-label pilot study. Arch Phys Med Rehabil. 2011;92(8):1202-1210. doi: 10.1016/j.apmr.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 59.Volpe D, Giantin MG, Maestri R, Frazzitta G. Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson’s disease: a randomized controlled pilot study. Clin Rehabil. 2014;28(12):1210-1217. doi: 10.1177/0269215514536060 [DOI] [PubMed] [Google Scholar]
- 60.Volpe D, Signorini M, Marchetto A, Lynch T, Morris ME. A comparison of Irish set dancing and exercises for people with Parkinson’s disease: a phase II feasibility study. BMC Geriatr. 2013;13:54. doi: 10.1186/1471-2318-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2003;74(2):158-162. doi: 10.1136/jnnp.74.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salloum RG, Au KLK, Okun MS. Timing of physical therapy sessions for individuals with Parkinson disease may unlock benefits. JAMA Neurol. 2022;79(12):1219-1220. doi: 10.1001/jamaneurol.2022.2649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy Used to Retrieve Eligible Articles for the Review
eAppendix 2. Summary of Systematic Review Findings
eAppendix 3. Summary of the Risk of Bias in Trials Implementing an Intention-to-Treat Analysis and Trials Implementing a Per-Protocol Analysis
eAppendix 4. Methods Used to Perform the Meta Moderators Analysis
Data Sharing Statement