Highlights
-
•
Short-term (≤12 weeks) aerobic exercise significantly enhanced general health for digestive system cancer (DSC) survivors.
-
•
Short-term aerobic exercise, with or without resistance exercise tended to benefit to physical health, mental health, role function and relieved cancer-related symptoms for patients with DSC.
-
•
Long-term (>12 weeks) resistance exercise was negatively associated with general health, physical health, and role function.
Keywords: Digestive system cancer, Exercise, Network meta-analysis, Quality of life
Abstract
Background
There is scant evidence regarding the effects of exercise type and duration on quality of life (QoL) in digestive system cancer (DSC) survivors. We aim to investigate the optimal type and duration of exercise to improve QoL for DSC survivors through a systematic review and network meta-analysis.
Methods
A systematic literature search of PubMed, Embase, and Web of Science was performed. Eligibility for study inclusion was limited to studies that were randomized controlled trials involving all kinds of exercise in adult patients with DSCs, and the comparator was in standard care or other types of exercise. The primary outcome was QoL, including general health, physical health, mental health, and role function. Secondary outcomes included cancer-related symptoms such as fatigue, insomnia, depression, anxiety, and duration of hospital stay. The network meta-analyses were performed using a random-effect model.
Results
The analysis included 32 eligible articles and a total of 2558 participants. Our primary outcome indicated that short-term aerobic exercise significantly enhanced general health (standardized mean difference (SMD) = 0.66, 95% credible intervals (CrIs): 0.05 to 1.30), and also contributed to a better mental health (SMD = 0.38, 95%CrI: –0.05 to 0.81) and role function (SMD = 0.48, 95%CrI: –0.27 to 1.20). Although without significant changes, short-term resistance exercise tended to increase the physical health of patients with DSCs (SMD = 0.69, 95%CrI: –0.07 to 1.50) and effective in alleviating fatigue (SMD = –0.77, 95%CrI: –1.50 to 0.01). Short-term aerobic exercise was related to a lower score of insomnia (SMD = –1.20, 95%CrI: –2.40 to 0.06), depression (SMD = –0.51, 95%CrI: –1.50 to 0.45), and anxiety (SMD = –0.45, 95%CrI: –1.30 to 0.34). All types of exercise related to a trend of declined hospital stays (–0.87 to –5.00 day). Long-term resistance exercise, however, was negatively associated with general health (SMD = –0.33, 95%CrI: –1.70 to 1.00), physical health (SMD = –0.18, 95%CrI: –1.30 to 0.90), and role function (SMD = –1.20, 95%CrI: –2.50 to 0.11).
Conclusion
This study suggests that short-term aerobic exercise, with or without resistance exercise programs, enhances QoL (especially for general health) as well as relieves cancer-related symptoms for DSC survivors, while long-term resistance exercise may have negative effects, and thus should be adopted cautiously. These results provide important evidence for the management of DSCs.
Graphical Abstract
1. Introduction
Digestive system cancers (DSCs) are the most aggressive cancers with the highest mortality worldwide, which contribute to a massive burden globally.1, 2, 3 Although the treatments for DSCs, such as surgery and chemoradiotherapy, have made some breakthroughs in recent years, improving the overall survival rate significantly, symptoms related to the disease and treatments (e.g., insomnia, pain, depression, and anxiety) can severely affect patients’ quality of life (QoL) and potentially decrease their length and quality of survival.4,5 Therefore, there is a need to identify effective interventions for improving QoL in patients with DSCs.
Exercise, a common intervention that influences one's physiologic and psychological status, has been proven to enhance QoL in patients with various non-communicable chronic diseases, such as depression,6 cognitive impairment,7 myocardial infarction,8 and spinal cord injury.9 As for the digestive conditions, exercise may influence the composition of gut microbiota, promote lipid metabolism in liver, and reduce insulin resistance and systematic inflammation.10,11 Moreover, immune function can be regulated through exercise; by stimulating gastrointestinal motility, it can result in reduced exposure of the digestive tract to carcinogens, lowering the overall risk of DSCs significantly.12 In terms of QoL of patients with cancer, guidelines have indicated that exercise may benefit physical function and improve cancer-related symptoms, such as fatigue and psychological distress.13,14 Previous research has demonstrated that exercise, as an independent factor, is associated with improved liver frailty index in patients with hepatocarcinoma15 and is consistently associated with improvements in cancer-related fatigue, mental disorders, and physical function in patients with pancreatic cancer.16
An exercise program usually consists of type and duration components. At present, however, no comprehensive study has investigated the effects of exercise type and duration on QoL and cancer-related symptoms in patients with DSCs. Also, whether or not aerobic exercise can improve cardiovascular function in patients with abdominal cancer is still controversial.17 Given the generally low activity levels of cancer survivors, the duration of training should also be considered. Therefore, we performed a systematic review and network meta-analysis of multiple studies to determine the optimal type and duration of exercise to effectively improve QoL and cancer-related symptoms for DSC survivors.
2. Methods
2.1. Data source and search strategy
This systematic review and network meta-analysis was registered with International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022319731) and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis–Network Meta-Analyses (PRISMA–NMA).18 The database of PubMed, Embase, and Web of Science were searched online for English-language publications from inception to March 2022. The search strategy for each database was presented in Supplementary material 1. Additionally, the references of the latest reviews on exercise for DSCs were screened and hand searches were performed to supplement the included publications.
2.2. Study selection
Included studies met the following criteria: (a) the study design was randomized controlled trials, (b) the effects of all kinds of exercise in adult patients (≥18 years) with any types of DSCs were evaluated; trials that reported mixed cancers were also involved if the trial contained only DSCs or presented separate data on DSCs, (c) the comparator was standard care, or there was mutual comparison between involved forms of exercise, and (d) at least one of the following outcomes was reported: general health, physical health, mental health, role function, fatigue, insomnia, depression, anxiety, and duration of hospital stay.
The exclusion criteria were: (a) duplicated studies, (b) studies focused on children with DSCs, (c) participants consisted of patients with mixed types of cancer (such as prostate cancer, breast cancer, lung cancer, etc.), without separate data available for DSCs, (d) did not include the aforementioned outcomes, (e) written in a language other than English, and (f) abstracts, conferences, letter to the editor, case reports, and non-randomized controlled trials.
Two researchers (CQL and YCW) independently screened the titles and abstracts to determine inclusion. Full texts of potentially eligible publications were further identified and assessed by the same reviewers. Any disagreements between the reviewers were resolved by discussion, and a third researcher (RLG) was consulted to reach consensus.
2.3. Outcomes
The primary outcome was mean change from baseline to endpoint in QoL in patients with DSCs. We included general health, physical health, mental health, and role function, the 4 main health dimensions incorporated into QoL instruments.19 As shown in Supplementary material 2, higher primary outcome scores indicate a higher QoL. The secondary outcomes were defined as the mean change from baseline to endpoint in cancer-related conditions, including fatigue, insomnia, depression, anxiety, and duration of hospital stay, which are common conditions for patients with DSCs. Lower scores for cancer-related conditions or shorter hospital stay duration indicate a better condition.
2.4. Data extraction and definition
Data extraction was independently performed by 2 researchers (YCW and CQL). The extracted data, including study characteristics (first author, publication year, and area), participants characteristics (DSC type and sample size), intervention characteristics (exercise type and exercise duration), and target outcomes (the QoL scores and secondary outcomes), were organized using standardized tables.
Scored data for all outcomes of interest were extracted from the measurement instruments in Supplementary material 2. Change values were extracted from means and standard deviations (SDs) of the changes, or by calculating from the available data. When means and SDs were not available, calculations were performed based on reported data (e.g., using p values, confidence intervals, or extracting data from figures); for studies that only reported medians and interquartile ranges, means were estimated by the median, SDs were converted by dividing interquartile ranges by 1.35.20 Any discrepancies were resolved through discussion with 2 independent researchers (WBZ and RLG).
This study focused on the effects of different types of exercise and exercise duration on patients with DSCs. DSCs were defined as cancers of the digestive tract (esophagus, stomach, small intestine, colon, and rectum) and digestive accessory organs (pancreas, liver, and gallbladder). Exercise interventions were firstly classified into the following categories: AE (defined as aerobic exercise, which recruits large groups of muscles and improves cardiovascular ability; includes walking, cycling, swimming, high intensity interval training, and Qigong),21 RE (defined as resistance exercise, which aims to enhance muscular strength and power by using muscular strength to move a weight or to work against a resistive load), and AE&RE (defined as combining both aerobic exercise and resistance exercise). We further classified the exercise categories according to intervention duration: long-term exercise was defined as exercise duration longer than 12 weeks, and short-term exercise was defined as that less than 12 weeks.22 We finally classified 6 exercise groups (long-term AE, short-term AE, long-term AE&RE, short-term AE&RE, long-term RE, and short-term RE) and a no-exercise control group (participants under standard care).
2.5. Risk of bias assessment
The risk of bias was assessed according to the second version of the Cochrane risk-of-bias tool for randomized trials (ROB 2).23 The risk of bias was evaluated in duplicate (CQL and YCW). The assessment included bias in the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. The discrepancies were resolved by expert consensus.
2.6. Data synthesis and analysis
The network meta-analysis was conducted using R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) to compare the relative effectiveness of the different interventions under investigation. The Just Another Gibbs Sampler was designed to work with R for statistical computation, and we used the gemtc and rjags packages in R.24
Considering different assessment instruments were involved, standardized mean differences (SMDs) calculated by RevMan software (Version 5.3; The Cochrane Collaboration, Oxford, UK) were allowed for this network meta-analysis. For duration of hospital stay, mean differences (MDs) were calculated in days. To estimate effect sizes, we used both consistency and inconsistency models, running 4 Markov chains simultaneously with different initial values. If the included data did not meet the requirements of the inconsistency assessment, a consistency model was applied to estimate the effect sizes of included interventions and evaluate the ranking probabilities of each exercise. Based on the node-splitting analysis, if its p value was over 0.05, we selected a consistency model.25 To assess the heterogeneity among studies, we calculated I2. Effect sizes were estimated with SMDs and 95% credible intervals (95%CrIs) using post-intervention scores generated by the random effect model. Effect sizes were classified according to the Cochrane handbook as large (SMD > 0.70), moderate (SMD: 0.40–0.70), or small (SMD < 0.40).26 The comparisons were considered statistically significant when 95%CrI did not include the value of 0 effect. Sensitivity analyses were performed to explore outliers, and Egger's test was used to check for publication bias. Results of comparisons between the no-exercise control group and all interventions were presented in forest plots by using forestplot package. To rank the exercise intervention, rankograms were established, and the surface under the cumulative ranking curve (SUCRA) was calculated for each exercise category.27 A SUCRA value of 1 indicates that the exercise type ranks first, and a 0 means that intervention ranks last. The rank of the intervention mirrors the SUCRA value.
3. Results
3.1. Study selection and characteristics
A total of 6429 articles were retrieved from the databases and hand searched. After removing duplicates and unrelated publications, the full text of 461 potentially relevant studies were evaluated for eligibility. Of these, 425 studies were excluded based on the predefined inclusion and exclusion criteria, and 4 studies were excluded due to unavailable mean or standard deviation (neither reported directly nor able to be derived indirectly by calculation). Finally, 32 trials with 2558 participants were included in this network meta-analysis (Fig. 1).28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 Of the included articles, 1128,30,32,35,38,41,44,45,52,54,59 articles estimated the effects of short-term AE&RE, 7 studies31,37,43,46,48,50,58 determined the effects of short-term AE, 5 studies33,36,39,42,49 reported long-term AE, 2 studies47,51 reported both short-term AE and long-term AE, 2 studies29,55 reported both short-term AE&RE and long-term AE&RE, 1 study56 evaluated the effects of long-term AE&RE, 1 study40 determined the effects of short-term RE, 1 study53 reported both short-term RE and long-term RE, 1 study57 reported both long-term AE and long-term AE&RE, and 1 study34 directly compared the effects of short-term AE&RE and short-term AE. Most of the trials reported on patients with colorectal cancer (n = 13, 40.63%), followed by studies reporting on colon cancer (n = 4, 12.50%), esophageal cancer (n = 4, 12.50%), mixed DSCs (n = 4, 12.50%), rectal cancer (n = 3, 9.38%), hepatocarcinoma (n = 2, 6.25%), and pancreatic cancer (n = 2, 6.25%). The details of included studies and study characteristics are shown in Supplementary materials 3 and 4.
Fig. 1.
Flowchart of the study selection process. RCTs = randomized controlled trials.
3.2. Risk of bias
The randomization process was adequate in most trials (90.63%). Deviations from intended interventions were problematic in 62.50% of the trials, and 28.13% of the studies showed high risk. No involved study missed outcome data, and all the trials described the measurement of outcomes in detail. All included studies were evaluated as low risk in terms of the selection of the reported results. Overall, a high risk of bias was found in 9 studies (28.13%)32,35,41,42,49,50,53,55,59, for reasons mostly related to the deviation and randomization process of intended interventions. A summarized and individual risk of bias assessment is presented in Supplementary material 5.
3.3. Primary outcomes
The network plot of QoL was shown in Fig. 2. The thickness of the line connecting interventions indicates the number of enrolled studies with comparable results.60 Only a single study conducted direct comparisons of different exercise interventions (long-term AE vs. long-term AE&RE) and their effects on physical health.57 We used node-splitting analysis to assess consistency; all p values between direct and indirect effects were over 0.05 (Supplementary material 6). All the potential scale reduced factor values were equal to 1, indicating the model was convergent and the results stable; the consistency models were adopted for the following network meta-analyses. The results for the primary outcomes are shown in Fig. 3, and the observed statistical heterogeneity (I2) ranged from 70% to 99% in the pairwise comparisons, with high heterogeneity. Therefore, we used a random effects model to calculate SMD. The comparative effectiveness results of various exercise interventions are presented in Supplementary material 7. Ranks of various exercises in terms of primary outcomes are presented in Supplementary material 8.
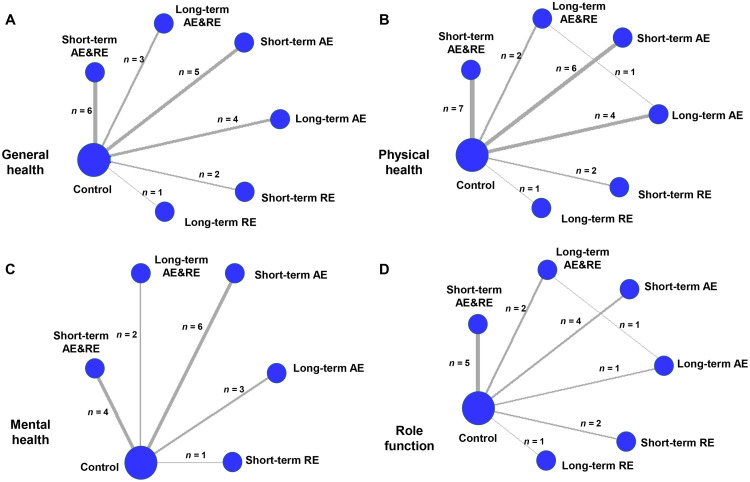
Fig. 2.
Network plot of life quality. (A) general health, (B) physical health, (C) mental health, and (D) role functional well-being. The thickness of the line connecting interventions indicates the number of enrolled studies with comparable results. Ref. 29, 51, 53, 55, and 57 included more than 1 arm. AE = aerobic exercise; AE&RE = aerobic exercise combined with resistance exercise; RE = resistance exercise.
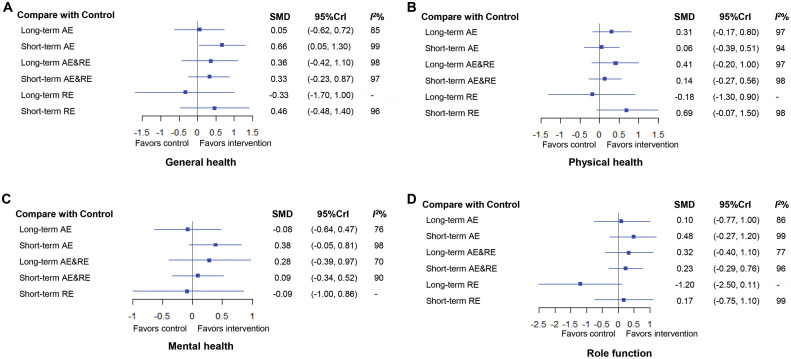
Fig. 3.
Forest plots for comparisons between controls and exercise intervention types for life quality. (A) general health, (B) physical health, (C) mental health, and (D) role function. I2% was calculated to measure the heterogeneity among studies. - means the values of I2% that are not provided/observed. 95%CrI = 95% credible interval; AE = aerobic exercise; AE&RE = aerobic exercise combined with resistance exercise; RE = resistance exercise; SMD = standardized mean difference.
First, for general health, a total of 17 studies were included. Outcomes for all exercise types involved in the trials were directly compared with those of control participants (Fig. 2A). Fig. 3A shows that, among all interventions, long-term AE&RE, short-term AE&RE, and short-term RE tended to increase the general health of participants as compared to the no-exercise group (SMD = 0.36, 95%CrI: –0.42 to 1.10; SMD = 0.33, 95%CrI: –0.23 to 0.87; SMD = 0.46, 95%CrI: –0.48 to 1.40; respectively), while only the short-term AE significantly enhanced general health (SMD = 0.66, 95%CrI: 0.05 to 1.30, with a SUCRA value of 83.67). In contrast, long-term RE tended to decrease the general health (SMD = –0.33, 95%CrI: –1.70 to 1.00).
Second, for physical health, 20 studies were involved (Fig. 2B). Fig. 3B shows that, although none of the exercise types showed significant advantages compared to the no-exercise control group, short-term RE, long-term AE, and long-term AE&RE tended to increase the physical health of patients with DSCs (SMD = 0.69, 95%CrI: –0.07 to 1.50 for short-term RE, SMD = 0.31, 95%CrI: –0.17 to 0.80 for long-term AE; SMD = 0.41, 95%CrI: –0.20 to 1.00 for long-term AE&RE). Fig. 3B shows that short-term RE had the highest SUCRA value (SUCRA = 87.13), followed by long-term AE&RE (SUCRA = 70.44), and long-term AE (SUCRA = 63.38). However, similar to what we saw with general health, the trend associated with long-term RE was a decrease in physical health (SMD = –0.18, 95%CrI: –1.30 to 0.90).
Third, for mental health, a total of 15 studies were involved (Fig. 2C). Short-term AE (SMD = 0.38, 95%CrI: –0.05 to 0.81) and long-term AE&RE (SMD = 0.28, 95%CrI: –0.39 to 0.97) potentially contributed to a higher measure of mental health as compared to the no-exercise control group. Short-term AE was the most promising exercise type for mental health (SUCRA = 83.25), while long-term AE and short-term RE were associated with decreased mental health (SMD = –0.08, 95%CrI: –0.64 to 0.47 for long-term AE; SMD = –0.09, 95%CrI: –1.00 to 0.86 for short-term RE) (Fig. 3C).
Fourth, 14 studies were involved in the analysis of role function (Fig. 2D). Compared to the non-exercise group, short-term AE, long-term AE&RE, and short-term AE&RE tended to promote role function, with SMD ranging from 0.23 (95%CrI: –0.29 to 0.76) for short-term AE&RE to 0.48 (95%CrI: –0.27 to 1.20) for short-term AE. Short-term AE was still the most promising exercise type for role function (SUCRA = 78.66). Long-term RE tended toward a decline in role function (SMD = –1.20, 95%CrI: –2.50 to 0.11) (Fig. 3D).
3.4. Secondary outcomes
All p values between the direct and indirect effects in node-splitting analyses were over 0.05 (Supplementary material 6), and potential scale reduced factor values of all interventions in secondary outcomes were equal to 1. Therefore, consistency models were used for the subsequent network meta-analyses of secondary outcomes. The specific network plots of secondary outcomes are presented in Supplementary material 9. Results of the network meta-analyses of secondary outcomes are shown in Table 1 and Supplementary materials 10–12. The observed statistical heterogeneity (I2) ranged from 43%–99% in the pairwise comparisons (Supplementary material 10), and we used random effects models to calculate SMD and MD.
Table 1.
Comparisons between exercise interventions and control group for fatigue, insomnia, depression, anxiety, and duration of hospital stay.
| Fatiguea | Insomniaa | Depressiona | Anxietya | Duration of hospital stay (day)b | |
|---|---|---|---|---|---|
| Long-term AE | –0.23 (–0.67 to 0.22) | –0.89 (–2.60 to 0.82) | –0.24 (–1.20 to 0.71) | –0.04 (–0.76 to 0.69) | –1.70 (–6.00 to 2.80) |
| Short-term AE | –0.26 (–0.75 to 0.24) | –1.20 (–2.40 to 0.07) | –0.51 (–1.50 to 0.45) | –0.45 (–1.30 to 0.34) | –0.87 (–3.40 to 0.99) |
| Long-term AE&RE | –0.15 (0.77 to 0.49) | –0.12 (–2.57 to 2.30) | –0.13 (–1.30 to 1.00) | –0.17 (–0.91 to 0.58) | –5.00 (–14.00 to 3.40) |
| Short-term AE&RE | 0.12 (–0.42 to 0.68) | –0.13 (–1.90 to 1.50) | –0.47 (–1.30 to 0.38) | –0.37 (–1.00 to 0.29) | –0.96 (–3.10 to 0.89) |
| Long-term RE | 0.05 (–1.10 to 1.10) | –0.51 (–2.90 to 2.00) | — | — | — |
| Short-term RE | –0.77 (–1.50 to 0.01) | –0.49 (–2.20 to 1.20) | — | — | — |
Abbreviations: 95%CrI = 95% credible interval; AE = aerobic exercise; AE&RE = aerobic exercise combined with resistance exercise; MD = mean difference; RE = resistance exercise; SMD = standardized mean difference.
Presented SMD with 95%CrI.
Presented MD with 95%CrI.
The network of eligible comparisons for fatigue consisted of 17 studies (Supplementary Fig. 6A of Supplementary material 9). Compared with the no-exercise control group, short-term RE was more effective at alleviating fatigue (SMD = –0.77, 95%CrI: –1.50 to 0.01), and long-term AE (SMD = –0.23, 95%CrI: –0.67 to 0.22), short-term AE (SMD = –0.26, 95%CrI: –0.75 to 0.24) also had better outcomes. In contrast, short-term AE&RE tended to slightly aggregate fatigue condition (SMD = 0.12, 95%CrI: –0.42 to 0.68) (Supplementary Fig. 7A of Supplementary material 10).
Nine studies estimated the effects of exercise types on the insomnia of patients with DSCs (Supplementary Fig. 6B of Supplementary material 9). Although none of the interventions impacted outcomes significantly, the short-term AE (SMD = –1.20, 95%CrI: –2.40 to 0.06) and long-term AE (SMD = –0.89, 95%CrI: –2.60 to 0.82) showed reductions in insomnia (Supplementary Fig. 7B of Supplementary material 10).
The network meta-analysis of depression consisted of 10 studies with 4 types of exercise (Supplementary Fig. 6C of Supplementary material 9). The network meta-analysis of anxiety consisted of 7 studies and 4 exercise types (Supplementary Fig. 6D of Supplementary material 9). When compared with the no-exercise control group, short-term AE (SMD = –0.51, 95%CrI: –1.50 to 0.45 for depression; SMD = –0.45, 95%CrI: –1.30 to 0.34 for anxiety) and short-term AE&RE (SMD = –0.47, 95%CrI: –1.30 to 0.38 for depression; SMD = –0.37, 95%CrI: –1.00 to 0.29 for anxiety) were associated with reductions in both depression and anxiety (Supplementary Fig. 7C–D of Supplementary material 10).
For duration of hospital stay, the network meta-analysis consisted of 14 studies with 4 types of exercise (Supplementary Fig. 6E of Supplementary material 9). Regardless of duration, aerobic exercise and the combination of both aerobic and resistance exercise, presented trends toward a reduction in the duration of hospital stay as compared with the no-exercise control group (MD = –0.87 day, 95%CrI: –3.40 to 0.99 of short-term AE to MD = –5.00 day, 95% CrI: –14.00 to 3.40 of long-term AE&RE) (Table 1, Supplementary Fig. 7E of Supplementary material 10).
4. Discussion
Exercise has shown promising effects on physical function, and various enhancements have been observed in cardiovascular endurance, muscle strength, and psychological health. It would no doubt be valuable to determine the attributes of exercise that are essential for optimizing effects on QoL depending on cancer type. However, there is a paucity of qualitative evidence on the QoL of patients with DSCs according to exercise type and duration in the previous literature. To our knowledge, this systematic review and network meta-analysis is the first study to explore the optimal type and duration of exercises to effectively improve QoL and cancer-related symptoms for patients with DSCs.
The results of our study demonstrated that aerobic exercise and the combination of aerobic exercise and resistance exercise had the highest probability to enhance patients’ QoL. Aerobic exercise significantly improved general health (SMD ranged from 0.33 to 0.66), and aerobic exercise with or without resistance exercise related to a trend of alleviated cancer-related symptoms. Regular aerobic exercise promotes gastrointestinal tract motility, providing positive effects on the gut and decreasing the contact time between pathogens and the gastrointestinal mucosa layer.61 By reducing prostaglandins production, exercise significantly protects the integrity of the intestine, and prevents the development of DSCs and promotes survivors’ general health.62 Moreover, similar to our results showing that aerobic exercise (with or without resistance exercise) trended toward improving patients’ mental health, previous studies have suggested that this type of exercise can improve self-efficacy beliefs by elevating body temperature and cerebral blood flow, which is associated with higher levels of endorphins and lower rates of mental disorders.63, 64, 65 Aerobic exercise, indicated by a randomized intervention, has shown an increase in the apoptotic potential of the crypt vs. no-exercise participants, which could be in line with the maintenance of the balance between apoptosis and cell proliferation, necessary to prevent the development of malignant tissue.66 Moreover, a previous trial demonstrated that exercise could significantly reduce fasting insulin levels and insulin resistance in colorectal cancer survivors67 and that aerobic exercise could improve cardiopulmonary fitness, immune function, total antioxidant capacity, anti-inflammatory plasma adiponectin, and gut microbiota composition.68 By way of the mechanisms mentioned above, aerobic exercise with or without resistance exercise could prevent carcinogenesis and prognosis, which is reflected by lower levels of fatigue, insomnia, depression, and anxiety, and a reduced number of days in the hospital.69,70
There is still scant scientific evidence with respect to how duration of exercise affects QoL in patients with DSCs. The results of our network meta-analysis showed that short-term aerobic exercise with or without resistance exercise was the most promising exercise type for the improvement of general health and tended to benefit measures of mental health, role function, and cancer-related symptoms. Longstanding management of insulin resistance and glucose metabolism to lower the production of lipid peroxide and reactive oxygen species are beneficial to the prevention of DSCs.71,72 Given that long-term aerobic exercise reduces visceral fat and insulin resistance continuously,42 it contributes to an enhancement of physical health. The results we saw in patients who participated in long-term exercise support this viewpoint. However, levels of self-efficacy in exercise play an essential role in exercise management, and physical ability determines the duration of exercise. During chemotherapy, patients who suffer from DSCs reported more fatigue and a negative attitude toward exercise, and fatigue itself may hinder patients from becoming active.73,74 Additionally, prolonged exercise might limit other recreational and social activities, which could negatively impact the role–emotional aspects of the participants’ QoL.70 The agreement to participate in long-term exercise intervention is easily encumbered for many participants suffering from DSCs, most of whom are elderly and already tend to present poor health, low self-efficacy, perceived lack of time, and to lack self-motivation and self-management skills.75 Therefore, a short-term exercise is much more likely to persevere and, indeed, was reported to have a higher adherence rate.76
The present study, however, found that although short-term resistance exercise was the most promising exercise intervention for alleviating fatigue (SMD = –0.77), long-term resistance exercise was associated with lowered QoL in patients with DSCs, including in terms of general health (SMD = –0.33), physical health (SMD = –0.19), and role function (SMD = –1.20). Fatigue is one of the most common symptoms experienced during the course of a malignancy. It is caused by a cachexia-related decrease in body mass index resulting from a reduction in skeletal muscle mass.77 Resistance exercise causes muscle contraction against external resistance, which contributes to muscle mass, strength, and bone density and offsets progressive muscle wasting and disruptions to muscle metabolism. Skeletal muscle, an essential factor in counteracting pro-inflammatory effects, secretes muscle-derived interleukin 6, interleukin 8, interleukin 15, and interleukin 1 receptor antagonist, all of which act as antagonists to the generally pro-inflammatory cytokines, thereby decreasing the level of tumor necrosis factor α and hindering the activation of nuclear factor kappa-B.78,79 Additionally, the effects of exercise, particularly resistance exercise, can reduce leptin concentration, further exerting an anti-inflammatory response.80 Given that chronic low-grade inflammation contributes to the development of cancer, the anti-inflammatory effects of exercised skeletal muscle may contribute to a decrease in cancer risk and help regulate cancer proliferation and survival. Previous studies have found that resistance exercise has high effect sizes when it comes to modulating cancer-related fatigue in patients with breast and prostate cancer.81,82 Moreover, surgery and chemotherapy could significantly change the body composition (i.e., loss of weight and muscle mass) of cancer survivors,13 which may decrease patients' exercise endurance thereby limiting the beneficial effects of exercise. In those postoperative or post-chemotherapy patients, resistance exercise could be provided to improve muscle strength and mitigate the side effects of treatment.83 In terms of why long-term resistance exercise is associated with a lower QoL in patients with DSCs while short-term resistance exercise is shown to be beneficial, the favorable effects appear to be canceled out over time by disease progression, with the adherence rate showing a continuous decline over the course of the intervention period.53
There are some limitations to our network meta-analysis. First, the assessment tools used to evaluate QoL and other cancer-related symptoms were very diverse, which may increase heterogeneity and hamper interpretation of the current evidence. Second, there are only 2 studies focusing solely on resistance exercise, and no publications reported the effects of exercise on patients with gastric cancer. Additional randomized controlled trials reporting resistance exercise in larger sample sizes of patients with DSCs, especially gastric cancer, are warranted. Also, we did not further classify the group according to a measurement of training volume (i.e., training frequency × intensity × time × duration) nor did we classify based on whether a metastasis or prescription antidepressant medications (e.g., diazepam, anxiolytic, analgesic) were present for DSC patients since few studies could be involved in each group. Moreover, we have not analyzed the effect of different orders when 2 types of exercises were combined. Finally, further classifications of exercise types according to intensity and frequency have not been considered but would be worthwhile to discuss in future studies.
5. Conclusion
Our study provides evidence that short-term aerobic exercise, with or without resistance exercise programs, may be the optimal intervention strategy to improve QoL in patients with DSCs, especially in terms of general health enhancement. Short-term aerobic exercise with or without resistance exercise also potentially alleviates cancer-related symptoms, such as fatigue, insomnia, depression, and anxiety, and substantially reduces hospital length of stay. Long-term resistance exercise, however, was associated with lower measures of general health, physical health, and role function. Thus this type of intervention should be adopted cautiously by patients with DSCs. These results are of clinical importance as they highlight an area for future research into DSCs management.
Acknowledgments
Acknowledgments
This study was supported by the Medical–Engineering Cross Project between University of Shanghai for Science & Technology and Naval Medical University (No. 2020-RZ05) and Wu Mengchao talent plan fund (to RLG).
Authors' contributions
RLG and WBZ made equal contributions to the conception and design; CQL and YCW contributed to the literature search, study selection, data extraction, risk of bias assessment, data analysis, and drafting of the manuscript; SQS, YLZ, and JQZ participated in the conception and design of the study and contributed to the revision of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.12.008.
Contributor Information
Wen-Bin Zou, Email: dr.wenbinzou@hotmail.com.
Rui-Liang Ge, Email: geruiliang@smmu.edu.cn.
Supplementary materials
References
- 1.Keum N, Bao Y, Smith-Warner SA, et al. Association of physical activity by type and intensity with digestive system cancer risk. JAMA Oncol. 2016;2:1146–1153. doi: 10.1001/jamaoncol.2016.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S, Liu T, Cheng Y, Bai Y, Liang G. Immune cell infiltration as a biomarker for the diagnosis and prognosis of digestive system cancer. Cancer Sci. 2019;110:3639–3649. doi: 10.1111/cas.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tantoy IY, Cooper BA, Dhruva A, et al. Quality of life of patients with gastrointestinal cancers undergoing chemotherapy. Qual Life Res. 2018;27:1865–1876. doi: 10.1007/s11136-018-1860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang MM, Chen DM, Zhang O, et al. Effect of family support on quality of postoperative life in patients with digestive cancer. Ann Palliat Med. 2020;9:2072–2078. doi: 10.21037/apm-20-1129. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y, Wu Z, Sun L, et al. The effects and mechanisms of exercise on the treatment of depression. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.705559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Zhao X, Li B, et al. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J Sport Health Sci. 2022;11:212–223. doi: 10.1016/j.jshs.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campo G, Tonet E, Chiaranda G, et al. Exercise intervention improves quality of life in older adults after myocardial infarction: Randomised clinical trial. Heart. 2020;106:1658–1664. doi: 10.1136/heartjnl-2019-316349. [DOI] [PubMed] [Google Scholar]
- 9.Nightingale TE, Rouse PC, Walhin JP, Thompson D, Bilzon JLJ. Home-based exercise enhances health-related quality of life in persons with spinal cord injury: A randomized controlled trial. Arch Phys Med Rehabil. 2018;99:1998–2006. doi: 10.1016/j.apmr.2018.05.008. e1. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z, Xu M. Exercise and organ cross talk. Adv Exp Med Biol. 2020;1228:63–76. doi: 10.1007/978-981-15-1792-1_4. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Zhang Y, Taaffe DR, et al. Protective effects of physical activity in colon cancer and underlying mechanisms: A review of epidemiological and biological evidence. Crit Rev Oncol Hematol. 2022;170 doi: 10.1016/j.critrevonc.2022.103578. [DOI] [PubMed] [Google Scholar]
- 12.Xie F, You Y, Huang J, et al. Association between physical activity and digestive-system cancer: An updated systematic review and meta-analysis. J Sport Health Sci. 2021;10:4–13. doi: 10.1016/j.jshs.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J Sport Health Sci. 2021;10:201–210. doi: 10.1016/j.jshs.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchihashi J, Koya S, Hirota K, et al. Effects of in-hospital exercise on frailty in patients with hepatocellular carcinoma. Cancers (Basel) 2021;13:194. doi: 10.3390/cancers13020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo H, Galvão DA, Newton RU, et al. Exercise medicine in the management of pancreatic cancer: A systematic review. Pancreas. 2021;50:280–292. doi: 10.1097/MPA.0000000000001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franssen RFW, Janssen-Heijnen MLG, Barberan-Garcia A, Vogelaar FJ, Van Meeteren NLU, Bongers BC. Moderate-intensity exercise training or high-intensity interval training to improve aerobic fitness during exercise prehabilitation in patients planned for elective abdominal cancer surgery? Eur J Surg Oncol. 2022;48:3–13. doi: 10.1016/j.ejso.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson NK. Quantitative issues in health-related quality of life assessment. Health Policy. 1988;10:217–230. doi: 10.1016/0168-8510(88)90058-9. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Li T, Deeks JJ. Cochrane; 2022. Chapter 6: Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions version 6.3.www.training.cochrane.org/handbook Available at: [accessed 02.12.2022] [Google Scholar]
- 21.Kuo CC, Wang CC, Chang WL, Liao TC, Chen PE, Tung TH. Clinical effects of baduanjin qigong exercise on cancer patients: A systematic review and meta-analysis on randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/6651238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. 2017;13:689–703. doi: 10.1038/nrneurol.2017.128. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.R Foundation for Statistical Computing . R Foundation for Statistical Computing; Vienna: 2015. R: A language and environment for statistical computing [program] [Google Scholar]
- 25.Dias S, Welton NJ, Caldwell DM, Ads AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thomas J, Chandler J, et al. Cochrane; 2022. Cochrane handbook for systematic reviews of interventions version 6.3.www.training.cochrane.org/handbook Available at: [accessed 02.12.2022] [Google Scholar]
- 27.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Ahn KY, Hur H, Kim DH, et al. The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: Randomized controlled trial. Int J Colorectal Dis. 2013;28:643–651. doi: 10.1007/s00384-013-1665-1. [DOI] [PubMed] [Google Scholar]
- 29.Allen SK, Brown V, White D, et al. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: Effect on cardiopulmonary exercise test performance, muscle mass and quality of life-a pilot randomized clinical trial. Ann Surg Oncol. 2022;29:1839–1850. doi: 10.1245/s10434-021-11002-0. [DOI] [PubMed] [Google Scholar]
- 30.Ausania F, Senra P, Meléndez R, Caballeiro R, Ouviña R, Casal-Núñez E. Prehabilitation in patients undergoing pancreaticoduodenectomy: A randomized controlled trial. Rev Esp Enferm Dig. 2019;111:603–608. doi: 10.17235/reed.2019.6182/2019. [DOI] [PubMed] [Google Scholar]
- 31.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: A randomized blinded controlled trial. Ann Surg. 2018;267:50–56. doi: 10.1097/SLA.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 32.Berkel AEM, Bongers BC, Kotte H, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: Results of a randomized clinical trial. Ann Surg. 2022;275:e299–e306. doi: 10.1097/SLA.0000000000004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JC, Damjanov N, Courneya KS, et al. A randomized dose–response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology. 2018;27:1221–1228. doi: 10.1002/pon.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–1197. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 35.Christensen JF, Simonsen C, Banck-Petersen A, et al. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open. 2019;3:74–84. doi: 10.1002/bjs5.50110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 37.Dunne DF, Jack S, Jones RP, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–512. doi: 10.1002/bjs.10096. [DOI] [PubMed] [Google Scholar]
- 38.Fagevik Olsén M, Kjellby Wendt G, Hammerlid E, Smedh U. Effects of a training intervention for enhancing recovery after Ivor-Lewis esophagus surgery: A randomized controlled trial. Scand J Surg. 2017;106:116–125. doi: 10.1177/1457496916655499. [DOI] [PubMed] [Google Scholar]
- 39.Ho M, Ho JWC, Fong DYT, et al. Effects of dietary and physical activity interventions on generic and cancer-specific health-related quality of life, anxiety, and depression in colorectal cancer survivors: A randomized controlled trial. J Cancer Surviv. 2020;14:424–433. doi: 10.1007/s11764-020-00864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong Y, Wu C, Wu B. Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420954912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houborg KB, Jensen MB, Rasmussen P, Gandrup P, Schroll M, Laurberg S. Postoperative physical training following colorectal surgery: A randomised, placebo-controlled study. Scand J Surg. 2006;95:17–22. doi: 10.1177/145749690609500104. [DOI] [PubMed] [Google Scholar]
- 42.Kaibori M, Matsui K, Yoshii K, et al. Perioperative exercise capacity in chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson E, Farahnak P, Franzén E, et al. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery—A randomized controlled design. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Lee MK, Lee DH, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: A randomized controlled trial. Support Care Cancer. 2019;27:2933–2940. doi: 10.1007/s00520-018-4588-0. [DOI] [PubMed] [Google Scholar]
- 45.López-Rodríguez-Arias F, Sánchez-Guillén L, Aranaz-Ostáriz V, et al. Effect of home-based prehabilitation in an enhanced recovery after surgery program for patients undergoing colorectal cancer surgery during the COVID-19 pandemic. Support Care Cancer. 2021;29:7785–7791. doi: 10.1007/s00520-021-06343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loughney L, West MA, Moyses H, et al. The effects of neoadjuvant chemoradiotherapy and an in-hospital exercise training programme on physical fitness and quality of life in locally advanced rectal cancer patients: A randomised controlled trial (The EMPOWER Trial) Perioper Med (Lond) 2021;10:23. doi: 10.1186/s13741-021-00190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Qu HQ, Chen FY, et al. Effect of baduanjin qigong exercise on cancer-related fatigue in patients with colorectal cancer undergoing chemotherapy: A randomized controlled trial. Oncol Res Treat. 2019;42:431–439. doi: 10.1159/000501127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morielli AR, Boulé NG, Usmani N, et al. Effects of exercise during and after neoadjuvant chemoradiation on symptom burden and quality of life in rectal cancer patients: A phase II randomized controlled trial. J Cancer Surviv. 2021 doi: 10.1007/s11764-021-01149-w. [DOI] [PubMed] [Google Scholar]
- 49.Moug SJ, Mutrie N, Barry SJE, et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: Results from the REx trial. Colorectal Dis. 2019;21:548–562. doi: 10.1111/codi.14560. [DOI] [PubMed] [Google Scholar]
- 50.Onerup A, Andersson J, Angenete E, et al. Effect of short-term homebased pre- and postoperative exercise on recovery after colorectal cancer surgery (PHYSSURG-C): A randomized clinical trial. Ann Surg. 2022;275:448–455. doi: 10.1097/SLA.0000000000004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 52.Steffens D, Young J, Beckenkamp PR, et al. Feasibility and acceptability of a preoperative exercise program for patients undergoing major cancer surgery: Results from a pilot randomized controlled trial. Pilot Feasibility Stud. 2021;7:27. doi: 10.1186/s40814-021-00765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steindorf K, Clauss D, Tjaden C, et al. Quality of life, fatigue, and sleep problems in pancreatic cancer patients—A randomized trial on the effects of exercise. Dtsch Arztebl Int. 2019;116:471–478. doi: 10.3238/arztebl.2019.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taha A, Taha-Mehlitz S, Staartjes VE, et al. Association of a prehabilitation program with anxiety and depression before colorectal surgery: A post hoc analysis of the pERACS randomized controlled trial. Langenbecks Arch Surg. 2021;406:1553–1561. doi: 10.1007/s00423-021-02158-0. [DOI] [PubMed] [Google Scholar]
- 55.van Vulpen JK, Hiensch AE, van Hillegersberg R, et al. Supervised exercise after oesophageal cancer surgery: The PERFECT multicentre randomized clinical trial. Br J Surg. 2021;108:786–796. doi: 10.1093/bjs/znab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc. 2016;48:767–775. doi: 10.1249/MSS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 57.van Waart H, Stuiver MM, van Harten WH, et al. Recruitment to and pilot results of the PACES randomized trial of physical exercise during adjuvant chemotherapy for colon cancer. Int J Colorectal Dis. 2018;33:29–40. doi: 10.1007/s00384-017-2921-6. [DOI] [PubMed] [Google Scholar]
- 58.Yang LH, Duan PB, Hou QM, Wang XQ. Qigong exercise for patients with gastrointestinal cancer undergoing chemotherapy and at high risk for depression: A randomized clinical trial. J Altern Complement Med. 2021;27:750–759. doi: 10.1089/acm.2020.0531. [DOI] [PubMed] [Google Scholar]
- 59.Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support Care Cancer. 2018;26:615–624. doi: 10.1007/s00520-017-3875-5. [DOI] [PubMed] [Google Scholar]
- 60.Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: Application and practice using R software. Epidemiol Health. 2019;41 doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Oliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2009;12:533–538. doi: 10.1097/MCO.0b013e32832e6776. [DOI] [PubMed] [Google Scholar]
- 63.Schönfeld P, Brailovskaia J, Bieda A, Zhang XC, Margraf J. The effects of daily stress on positive and negative mental health: Mediation through self-efficacy. Int J Clin Health Psychol. 2016;16:1–10. doi: 10.1016/j.ijchp.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chekroud SR, Gueorguieva R, Zheutlin AB, et al. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 65.Mikkelsen K, Stojanovska L, Polenakovic M, Bosevski M, Apostolopoulos V. Exercise and mental health. Maturitas. 2017;106:48–56. doi: 10.1016/j.maturitas.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Campbell KL, McTiernan A, Li SS, et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: A randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16:1767–1774. doi: 10.1158/1055-9965.EPI-07-0291. [DOI] [PubMed] [Google Scholar]
- 67.Lee MK, Kim JY, Kim DI, et al. Effect of home-based exercise intervention on fasting insulin and Adipocytokines in colorectal cancer survivors: A randomized controlled trial. Metabolism. 2017;76:23–31. doi: 10.1016/j.metabol.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Jurdana M. Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol Oncol. 2021;55:7–17. doi: 10.2478/raon-2020-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao R, Yu T, Liu L, et al. Exercise intervention for post-treatment colorectal cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2020;14:878–893. doi: 10.1007/s11764-020-00900-z. [DOI] [PubMed] [Google Scholar]
- 70.Imayama I, Alfano CM, Mason CE, et al. Exercise adherence, cardiopulmonary fitness and anthropometric changes improve exercise self-efficacy and health-related quality of life. J Phys Act Health. 2013;10:676–689. doi: 10.1123/jpah.10.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown JC, Rickels MR, Troxel AB, et al. Dose–response effects of exercise on insulin among colon cancer survivors. Endocr Relat Cancer. 2018;25:11–19. doi: 10.1530/ERC-17-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown JC, Zemel BS, Troxel AB, et al. Dose–response effects of aerobic exercise on body composition among colon cancer survivors: A randomised controlled trial. Br J Cancer. 2017;117:1614–1620. doi: 10.1038/bjc.2017.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Waart H, van Harten WH, Buffart LM, et al. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2016;25:964–970. doi: 10.1002/pon.3936. [DOI] [PubMed] [Google Scholar]
- 74.Strandberg E, Bean C, Vassbakk-Svindland K, et al. Who makes it all the way? Participants vs. decliners, and completers vs. drop-outs, in a 6-month exercise trial during cancer treatment. Results from the Phys-Can RCT. Support Care Cancer. 2022;30:1739–1748. doi: 10.1007/s00520-021-06576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vassbakk-Brovold K, Berntsen S, Fegran L, et al. Individualized comprehensive lifestyle intervention in patients undergoing chemotherapy with curative or palliative intent: Who participates? PloS One. 2015;10 doi: 10.1371/journal.pone.0131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Norton LH, Norton KI, Lewis NR. Adherence, compliance, and health risk factor changes following short-term physical activity interventions. Biomed Res Int. 2015;2015 doi: 10.1155/2015/929782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang S, Chu S, Gao Y, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019;8:738. doi: 10.3390/cells8070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia L, Tan S, Zhou Y, et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018;11:2063–2073. doi: 10.2147/OTT.S161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kistner TM, Pedersen BK, Lieberman DE. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. 2022;4:170–179. doi: 10.1038/s42255-022-00538-4. [DOI] [PubMed] [Google Scholar]
- 80.de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441–450. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- 81.Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 82.Soriano-Maldonado A, Carrera-Ruiz Á, Díez-Fernández DM, et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors: Study protocol for the EFICAN randomized controlled trial. Medicine (Baltimore) 2019;98:e17625. doi: 10.1097/MD.0000000000017625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J. The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: A systematic review and meta-analysis. J Sport Health Sci. 2022;11:194–201. doi: 10.1016/j.jshs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.