Highlights
-
•
Multiple cell linages contribute to the effects of exercise on the heart.
-
•
Cross-talk among non-cardiomyocytes and non-cardiomyocytes to cardiomyocytes are necessary in cardiac response to exercise.
Keywords: Angiogenesis, Cardiac fibrosis, Cardioprotection, Hypertrophy, Non-cardiomyocytes, Proliferation
Abstract
Cardiomyocytes comprise ∼70% to 85% of the total volume of the adult mammalian heart but only about 25% to 35% of its total number of cells. Advances in single cell and single nuclei RNA sequencing have greatly facilitated investigation into and increased appreciation of the potential functions of non-cardiomyocytes in the heart. While much of this work has focused on the relationship between non-cardiomyocytes, disease, and the heart's response to pathological stress, it will also be important to understand the roles that these cells play in the healthy heart, cardiac homeostasis, and the response to physiological stress such as exercise. The present review summarizes recent research highlighting dynamic changes in non-cardiomyocytes in response to the physiological stress of exercise. Of particular interest are changes in fibrotic pathways, the cardiac vasculature, and immune or inflammatory cells. In many instances, limited data are available about how specific lineages change in response to exercise or whether the changes observed are functionally important, underscoring the need for further research.
Graphical abstract
1. Overview of exercise: Key cardiovascular adaptations
Exercise, a subcategory of physical activity, is planned, structured, repetitive, and intentional movement intended to promote health. Most forms of exercise induce molecular, structural, and functional changes that protect the mammalian heart against pathological stress. While there are different forms of exercise (e.g., resistance, strength, and plyometric training), aerobic exercise is one effective way to help prevent cardiovascular disease.1, 2, 3 Despite the long-recognized cardiovascular benefits of exercise in both healthy and diseased humans, less is known about the optimal duration and intensity of exercise or the dose–response relationship.4 In part this reflects the challenges of long-term, large-scale adherence to randomized lifestyle interventions. As a consequence, most of our current recommendations are based on observational data, which are potentially confounded by many factors. The Physical Activity Guidelines for Americans set forth by the United States (U.S.) Department of Health and Human Services suggests children and adolescents complete at least 1 h of moderate-to-vigorous intensity physical activity on a daily basis; this includes aerobic, muscle, and bone-strengthening exercise.5 For adults to receive cardiovascular benefit, at least 150 min of moderate intensity or 75 min of vigorous intensity activity per week is recommended. Moreover, current guidelines suggest the addition of strength training will likely provide adjunctive benefits.5 The Centers for Disease Control and Prevention reported that, despite these recommendations in support of cardiovascular health, only 23% of adults in the U.S. have been shown to meet the aerobic and muscle-strengthening exercise guidelines.
Cardiac enlargement in endurance athletes was first described over a century ago.6,7 Marked by increases in wall thickness and dilation of all 4 cardiac chambers with preservation of function, these features are common to the “athlete's heart”. The structural and functional changes observed with exercise, also called exercise-induced cardiac remodeling or physiological hypertrophy, represent a form of reversible “physiological” heart growth. These changes are accompanied by normal or sometimes modestly improved contractile function and associated predominantly with beneficial cardiovascular outcomes. In contrast, disease states including hypertension, myocardial infarction, or stenotic valve lesions lead to “pathological hypertrophy”, which commonly precedes impaired systolic and/or diastolic function, interstitial fibrosis, and unfavorable cardiovascular outcomes. Analyses of animal models of physiological and pathological remodeling have revealed these growth states are associated with distinct and often opposing transcriptional programs, signaling, and cellular effects. Generally distinct from changes seen in pathological hypertrophy, functionally important changes seen in exercise protect against pathological cardiac stress with remarkable consistency. Examples of pathways important in the hearts response to exercise that also protect against pathological stress when the changes seen in exercise are mimicked pharmacologically or genetically include phosphoinositide 3-kinase (PI3K),8,9 serine/threonine kinase 1 (Akt1),10, 11, 12 endothelial nitric oxide synthase (eNOS),13 peroxisome-proliferator-activated receptor coactivator 1α (PGC1α),14,15 CCAAT/enhancer-binding protein β (C/EBPβ),16 CBP/p300-interacting transactivators with E (glutamic acid)/D (aspartic acid)-rich-carboxyl terminal domain (CITED4),17 microRNA-222 (miR-222),18 and long noncoding exercise associated cardiac transcript 1 (lncExACT1).19 Cardiomyocyte-specific overexpression of PI3K (which is activated in the heart during exercise20) protects the heart against transverse aortic constriction (TAC)-induced pathological growth and cardiac functional decline.9 In contrast, C/EBPβ, a member of the basic helix–loop–helix (bHLH) gene family of DNA-binding transcription factors, was downregulated in the hearts of mice with 14 days of swimming but upregulated in TAC-induced pathological hypertrophy.16 Mice heterozygous for C/EBPβ (50% reduction) developed a gene-expression pattern consistent with physiological hypertrophy, with increased cardiomyocyte size and protection against TAC-induced heart failure.16 Reduction of C/EBPβ conferred these effects partially through increasing CITED4, a transcriptional regulator upregulated in the heart after exercise.16 Cardiac-specific overexpression of CITED4 increased heart weight and cardiomyocyte size, with normal fractional shortening consistent with physiological hypertrophy. Moreover, cardiac-specific overexpression of CITED4 protected against myocardial ischemia-reperfusion-induced cardiac injury while cardiac-specific CITED4 knockout accelerated dilation and heart failure after TAC.16,21 Most recently, we identified a long noncoding RNA (lncRNA), lncExACT1, whose cardiac expression was downregulated by exercise but upregulated by TAC.19 Inhibition of lncExACT1, to mimic the change seen in exercise, protected against TAC-induced heart failure and cardiac dysfunction after ischemic injury.19 These effects were conferred, in part, through the binding of lncExACT1 to miR-222, an miR that increases in the heart after exercise and is required for exercise-induced physiological cardiac growth.18 Together these observations suggest exercise studies can yield a novel list of possible therapeutic targets.
The cardinal feature of physiological hypertrophy is an increase in cardiomyocyte size, which accounts for most of the growth of the heart in this setting. Intriguingly, we found that exercise also induces an increase in cardiomyocyte proliferation in both the young adult22 and aged23 mammalian heart. Given the centrality of changes in cardiomyocyte phenotype, it was understandable that initial studies focused on this cell. In mice with cardiomyocyte-specific expression of dominant negative PI3K, 4 weeks of swimming nullified its protective effect against TAC-induced pathological cardiac growth.9 This suggests that cardiomyocyte PI3K is necessary for exercise's beneficial cardiac effects. LncRNA cardiac physiological hypertrophy-associated regulator (CPhar) was increased in the hearts of mice after 8 weeks of swimming.24 Knockdown of CPhar by short hairpin RNA canceled out 3 weeks of swimming-induced cardiomyocyte hypertrophy and markers of proliferation.24 These results provide direct evidence of the effect of exercise on cardiomyocytes and suggest that CPhar is necessary in this process. However, in this study, short hairpin RNA was delivered by adeno-associated virus 9 under the control of U6, which is not cardiomyocyte specific. Thus, it is possible that CPhar from sources other than cardiomyocytes could also contribute to the attenuation of exercise's effect on the heart. For more on exercise-mediated changes in cardiomyocytes, the interested reader is referred to prior reviews on that topic.25, 26, 27, 28 There is less information available, however, on the roles of other cell types in the heart's response to exercise. This partially reflects technical considerations, such as the challenges of genetically manipulating specific cell types in the heart. It may also reflect a bias that is being addressed by an increasing number of studies highlighting the important roles played by non-cardiomyocytes in the heart in disease states, which we hope will increasingly be applied in studies of exercise. As illustrated below, even the limited data available on other cell types suggest that they are altered in exercised hearts and may also play important—and sometimes surprising—roles in the cardiac exercise response. In some instances, non-cardiomyocytes appear necessary for the cardiomyocyte responses to exercise that have been so well documented. In this review, we discuss the current state of knowledge with respect to non-cardiomyocytes in the exercise response. In many cases, existing data are ambiguous as to whether effects are cell-autonomous or indirect and related to cross-talk from other lineages, as well as to the functional importance of such effects. We also highlight lineages whose role in the cardiac exercise response is not well characterized but which represent fertile ground for future investigation. We have omitted some cell types (e.g., pericytes and smooth muscle cells) due to the paucity of data available and in the interest of brevity.
2. Non-cardiomyocytes and exercise
2.1. Fibroblasts
Fibroblasts originating from epicardial and endocardial resident populations in development and after injury28 comprise approximately 20% of all non-cardiomyocytes in the heart at baseline.29 They reside in the interstitial space of the myocardium, epicardium, and perivascular areas of the heart.30 Fibroblasts were previously thought to be the predominant cell of the adult mammalian heart, more numerous than endothelial cells.31, 32, 33, 34 This conclusion was likely influenced by a lack of fibroblast-specific genes, making it difficult to identify and study fibroblasts unambiguously.35, 36, 37 Litviňuková et al.38 used single-nucleus RNA sequencing (snRNA-seq) to identify 6 fibroblast subpopulations in the adult human heart, estimating that fibroblasts constitute ∼24% of the nuclei in atrial tissues and ∼15% in ventricular tissues. In adult mouse hearts, collagen Type 1 α 1-green fluorescent protein reporter labeling identified <5% of all cells as fibroblasts in healthy, unperturbed hearts. After myocardial infarction, this number increased to ∼8% and 15% at Day 7 and Day 30, respectively.39 At 7 days after myocardial infarction, the percentage of fibroblasts increased in both infarct zone (∼40%) and border zone (∼30%).39 In cardiac homeostasis, fibroblasts play a major role producing extracellular matrix, an important structural scaffold for all cardiac lineages that influences the distribution of mechanical stress.30,40, 41, 42, 43 The role of cardiac fibroblasts has been extensively studied under pathological conditions such as ischemic injury or infarction,44 heart failure,45 hypertrophic cardiomyopathy,46 and other disease states. In these contexts, fibroblasts aid in scar formation, often replacing necrotic or dead cardiomyocytes and marking resolution of the early inflammatory phases. This “reparative” fibrosis can be critical to maintaining overall myocardial structural integrity after ischemic injury and infarction.47 In contrast, exercise does not typically induce fibrotic remodeling as seen in disease states.48, 49, 50, 51 Moreover, exercise appears to mitigate fibrosis in some settings (e.g., TAC-induced pathological growth and left anterior descending coronary artery ligation-induced myocardial infarction) and induces transcriptional changes in expression of both protein-coding (e.g., CITED416,17,21) and noncoding RNA (both miRNAs (e.g., miR-29,52,53 miR-22218) and lncRNA (e.g., lncExACT119)) that suppress the fibrotic response.
Previous studies have reported minimal or no significant changes in collagen expression, total collagen concentration, collagen ratios (Type III to Type I), or cross-linking54, 55, 56, 57 after 10−13 weeks of treadmill or 4 months of voluntary wheel running exercise. These studies suggest exercise induces physiological responses in cardiac fibroblasts that are distinct from the fibrotic programs induced in disease (Table 1 and Fig. 1). A seminal study by Lighthouse et al.48 investigated fibroblasts in the exercised heart, highlighting this concept and implicating potential underlying mechanisms. The authors compared transcriptomes in cardiac non-cardiomyocytes (∼77% of which were fibroblasts) isolated from mice with 4 days or 10 days of swimming-induced cardiac growth, TAC-induced pathological growth, and left anterior descending coronary artery ligation-induced myocardial infarction.48 In fibroblasts from the pathological models, well-known myofibroblast gene programs such as Rho/Rho-kinase, serum response factor-dependent, and integrin-linked kinase signaling were upregulated, while nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent reactive oxygen species scavenging pathways were downregulated. Opposite changes in these signaling pathways (e.g., upregulation of pro-inflammatory transcription factors like signal transducer and activator of transcription 1, nuclear factor of activated T cells 2, and interferon regulatory factor 3 (IRF3), IRF5, and IRF749) were observed in fibroblasts from exercised hearts. No changes in typical fibroblast genes, including those encoding extracellular matrix proteins and myofibroblast activation markers (e.g., periostin, Col1α1, Col1α2, Col3α1), were observed. This suggests fibroblasts may contribute to the beneficial effects of exercise by mitigating oxidative damage through Nrf2 and potentially by interfering with myofibroblast activation. The authors further showed that 2 key genes in the Nrf2 signaling pathway, metallothionein 1 (Mt1) and Mt2, were upregulated in cardiac fibroblasts by exercise, yet they were downregulated in pathological animal models and samples from heart failure patients.48 In fibroblasts isolated from the cardiac tissue of heart failure patients, p38 inhibition increased Mt1/Mt2 expression, suggesting a role for MAPK signaling in Mt1/Mt2 suppression. In mice lacking Mt1/Mt2, 4 weeks of swimming did not induce physiological cardiac growth but did lead to increased fibrosis, vascular rarefaction, and diastolic and systolic dysfunction, which suggests that Mt1/Mt2 are required for physiological hypertrophy and at least some of the cardiac benefits of exercise. Of note, these mice were global Mt1/Mt2 knockouts, leaving open the possibility that Mt1/Mt2 from other lineages also contributes to these phenotypes and the normal exercise response. However, in mouse cardiac fibroblasts in vitro, Mt1 overexpression reduced myofibroblast activation supporting important cell autonomous effects. Interestingly, conditioned media from cardiac fibroblasts overexpressing Mt1 protected cardiomyocytes from H2O2-induced oxidative injury and cell death, thereby underscoring the potential importance of cellular cross-talk to the cardiac benefits of exercise.
Table 1.
Cardiac fibrosis in response to exercise.
| Exercise model | Outcome | Species | Age (week) | Sex |
|---|---|---|---|---|
| Swimming (50 min/day for 4 weeks)57 |
Exercise reduced isoproterenol-induced cardiac fibrosis in wild-type but not in AMPK knockout mice | Mice | 10 | Male |
| Treadmill (40 min, 4 days/week for 5 weeks)58 |
Exercise did not prevent doxorubicin-induced fibrosis or ejection fraction. It preserved cardiac strain, alleviated doxorubicin-induced atrophy | Mice | 8 | Male |
| Treadmill (velocity increased every 3 min to max after warmup at Weeks 0 and 4)60 |
Exercise reduced doxorubicin-induced fibrosis and preserved myofibril integrity and sarcomere organization | Rats | 12 | Male |
| Treadmill (1 week of acclimation and 4 weeks of moderate intensity for 60 min/day for 28 days)59 |
Exercise reduced doxorubicin-induced gene expressions of fibrosis factors, cardiac remodeling factors, and inflammatory mediators | Mice | Unclear | Male |
| Swimming (1 h/day, 5 days/week for 14 weeks, with 4% body weight load attached to tail)61 |
Exercise improved myocardial tolerance to doxorubicin-induced damaged | Mice | 6–8 | Male |
| Treadmill (1 week of training, 50 min, 5 days/week for 8 weeks)63 |
Exercise preserved cardiac function and reduced collagen volume fraction after myocardial infarction | Rats | 7 | Male |
| Treadmill (speeds increased every 2 weeks until reaching 27 m/min, 60 min, 5 days/week for 8 weeks)64 |
Exercise reduced cardiac fibrotic-related protein expressions in spontaneously hypertensive ovariectomized rats | Rats | 15 | Female |
| Swimming (speeds increased for 2 weeks, then 90 min/day, twice daily for 2 weeks)50 |
Exercise increased heart weight and succinate dehydrogenase but did not change myofibrillar ATPase | Mice | 8 | Female |
| Treadmill (training for 1 week then 45 min, 5 days/week for 10 weeks up to 70% of max oxygen capacity)65 |
Exercise reduced collagen deposition and cross-linking in left ventricular septum but not left ventricular or right ventricular free wall in older rats | Rats | 23 and 111 | Male |
| Treadmill (15, 13, and 10 m/min, respectively, for 50 min/day, progressively increased with 30, 24, and 15 m/min for 60 min/day, 5 days/week at termination)66 |
Exercise prevented age-related decline mRNA expressions of fibrillar collagens Type 1 and Type 3 in left ventricle | Rats | 20, 60, and 104 | Female |
| Treadmill (1 week of training, 60 min, 5 days/week for 8 weeks, then 4 days/week until 48 h before sacrifice)67 |
Exercise reduced fibrosis and advanced glycation end products associated with aging and diabetes | Rats | 28 and 116 | Male |
| Treadmill (1 week of training 15 min/day, then 8 weeks of moderate intensity exercise 60 min/day, 5 days/week)68 |
Exercise reversed diabetes-induced alterations in factors of myocardial fibrosis | Rats | 8 | Male |
| Treadmill (4 weeks starting at 30 min/day working up to 60 min/day by Week 5, 5 days/week)69 |
Exercise restored bioavailability of hydrogen sulfide in aged rats; likely protective against cardiac fibrosis. Exercise also reduced expression of Collagen I and α-smooth muscle actin in hearts of aged rats | Rats | 12 and 92 | Male |
| Treadmill (45 min, 5 days/week for 7 weeks)70 |
Exercise reduced aged-related fibrosis and dysregulation of matrix metalloproteinase | Rats | 12 and 124 | Unclear |
| Treadmill (60 min, 5 days/week for 12 weeks)71 |
Exercise reduced age-related cardiac remodeling and fibrosis in the left ventricle | Rats | 12 and 96 | Unclear |
| Treadmill (60 min, 5 days/week for 10 weeks)54 |
Exercise did not change the ratio of Collagen III/Collagen I in the heart after isoproterenol stimulation | Rats | Unclear | Male |
Abbreviation: AMPK = adenosine monophosphate-activated protein kinase.
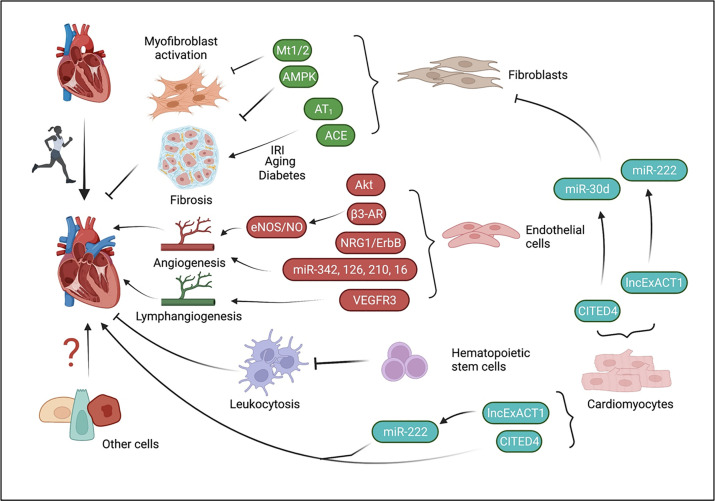
Fig. 1.
Potential roles of different types of cells in cardiac response to exercise. The schematic depicts ways that exercise may confer its cardiac effects working through distinct cell lineages and molecular pathways. In cardiomyocytes, exercise increases the expression of the transcriptional co-regulator CITED4 and downregulates the long noncoding RNA lncExACT1, increasing available miR-222 and decreasing its transcriptional target, DCHS2. These effects contribute to hypertrophic and proliferative cardiomyocyte responses to exercise. Exercise-induced upregulation of CITED4 in cardiomyocytes negatively regulates cardiac fibroblast activation via cross-talk mediated in part by the miR-30d family and miR-222. In fibroblasts themselves, exercise inhibits myofibroblast activation via Mt1/Mt2 and fibrosis via AMPK, while also inhibiting pathologic fibrosis in ischemic injury, aging, and diabetes through modulation of renin-angiotensin signaling. In hematopoietic stem cells, aerobic exercise exerts a cardioprotective effect by reducing hematopoietic stem cell recruitment of leukocytes to the heart, thereby attenuating cardiac inflammation. Cardiac endothelial cells are both key regulators and responders to exercise, directing beneficial angiogenesis and, in part, cardiac hypertrophy via β3-adrenergic/eNOS, neuregulin/ErbB, and Akt signaling. MicroRNAs, miR-16, -126, -210, and -342 also contribute to the cardiac angiogenic response to aerobic exercise. Lastly, it was found that endothelial VEGFR3 participates in cardiac lymphangiogenesis, leading to increased lymphatic density and growth of the exercised heart. ACE = angiotensin-converting enzyme; Akt = serine/threonine kinase; AMPK = AMP-activated protein kinase; AT1 = angiotensin II type 1 receptor; CITED4 = CBP/p300-interacting transactivators with E (glutamic acid)/D (aspartic acid)-rich-carboxylterminal domain4; DCHS2 = Dachsous cadherin-related 2; eNOS = endothelial nitric oxide synthase; ErbB = Erb-B2 receptor tyrosine kinase; IRI = ischemia reperfusion injury; lncExACT1 = long noncoding exercise-associated cardiac transcripts; miR = microRNA; Mt1 = metallothionein 1; NRG1 = neuregulin-1; VEGFR3 = vascular endothelial growth factor receptor 3.
Other work has examined the role of fibroblasts in exercise-mediated mitigation of cardiac disease and aging. For example, 4 weeks of swim training attenuated isoproterenol-induced (isoproterenol injected during the last 2 weeks of swim training) cardiac fibrosis and induction of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase-reactive oxygen species in wild-type but not adenosine monophosphate-activated protein kinase knockout mice, suggesting that exercise alleviates cardiac fibrosis and oxidative stress in an adenosine monophosphate-activated protein kinase (AMPK)-dependent manner.57 Similarly, swim training (doxorubicin injected 4 days after swim training;58 at the same time as swim training;59,60 after 14 weeks of swim training;61 4 days before swim training62) has been shown to have a multitude of protective effects against doxorubicin-induced cardiac injury. Exercised hearts treated with doxorubicin show a reduction in fibrosis, potentially via protein expression of transforming growth factor-β (TGF-β), α smooth muscle actin, Collagens I and III, NAPDH oxidase-4 and p53, as well as markers of oxidative stress.58, 59, 60, 61, 62, 63
Starting exercise 1 week after myocardial infarction has also been shown as effective in reducing fibrosis following myocardial infarction.63 Inflammatory and fibrotic genes (TGF-β, p-Smad2/3, connective tissue growth factor, tissue-type plasminogen activator, matrix metalloproteinase-9 (MMP-9), and Collagen I) expression were also reduced in ovariectomized hypertensive rats after 8 weeks of treadmill exercise training.64 The authors posited this occurs via downregulation of the angiotensin II type I receptor. In rats subjected to myocardial infarction, 8 weeks of treadmill running (starting 1 week after myocardial infarction) reduced cardiac angiotensin-converting enzyme expression in remote zones of the left ventricle, with lower collagen volumes, thus attenuating fibrosis in non-infarcted myocardial tissue.63 In aged (25.5- to 26.0-month-old) mice, 10 weeks of treadmill exercise reduced aging-related collagen deposition and cross-linking in the left ventricular septum of rats.65,66 Exercise treadmill training in late middle-aged (29-month-old) rats attenuated fibrosis and advanced glycation end products associated with aging and diabetes.67 There was a reduction in collagen cross-linking and an age-related rise in cardiac fibrosis, but no change in fibrotic gene expression programs. A similar study using a streptozotocin and high fat diet-induced type 2 diabetes mellitus cardiomyopathy in rats showed that 8 weeks of moderate-intensity exercise (starting 5 weeks after high fat diet feeding) reduced myocardial oxidative stress and fibrosis through TGF-β1/Smad signaling, which downregulated expression of MMP-2, connective tissue growth factor, TGF-β1, p-Smad2, p-Smad3 while increasing tissue inhibitor of MMP Type 1 and Smad7 expression.68 Other studies have shown that exercise reduces age-related cardiac fibrosis partly through restoring bioavailability of hydrogen sulfide in aged (23-month-old) mice.69 Twelve weeks of exercise training reduced collagen staining as well as extra-myocyte interstitial space in aging (24- and 31-month-old) rat hearts.70,71 The downregulated MMP activation in these aged hearts was reversed by exercise, likely through a reduction in the protein expression of tissue inhibitor of MMP Type 1 and TGF-β1.
Recently, the CITED4 signaling pathway, which plays an established role in exercise-induced growth and the proliferation of cardiomyocytes,16,17 was shown to regulate an expansive network of microRNAs governing fibrosis in mice with TAC-induced pressure overload.21 In particular, miR-30d, a mediator of fibroblast-cardiomyocyte cross-talk, was found to be downregulated in cardiomyocyte-specific CITED4 knockout mice after TAC, and its induction was sufficient to induce fibrotic response and cardiac dysfunction in this model.21
Other exercise-modulated miRNA pathways have been shown to play important roles in cardiac fibrosis. In Wistar rats subjected to 10 weeks of swimming, left ventricle miR-29c expression was positively correlated with training duration and early and late ventricular filling velocity ratio while negatively correlated with Col1α1 and Col3α1 expression, suggesting involvement of miR-29c in exercise-mediated reduction in cardiac fibrosis and diastolic dysfunction.52 Conversely, in adult mice subjected to TAC, global genetic deletion or pharmacological inhibition (antimir, daily for 3 days, starting 1 day after TAC) of miR-29 attenuated cardiac hypertrophy and fibrosis.53 These results suggest cardiomyocyte miR-29 plays a dominant role in regulating fibrosis in pathological hypertrophy. While there are studies examining the functional role of miR-29 in regulating fibrosis with gain- and loss-of-function of miR-29 across a variety of organs,72,73 further investigation is needed to decipher the causal role of miR-29 in regulating fibrosis in different organs, particularly during exercise.
It should be emphasized that in many of these reports it is unclear whether the anti-fibrotic effects of exercise are due to cell-autonomous effects on fibroblast biology, cross-talk with other cell lineages, or some combination of the two. For example, cardiomyocyte-specific deletion of the exercise-induced transcriptional co-activator CITED4 results in a dramatic increase in fibrosis after TAC. Logically, this suggests cardiomyocyte-fibroblast cross-talk, an inference supported by mechanistic in vitro studies.21 Similarly, overexpression of the exercise-induced miR-222 reduces fibrosis even when expression is confined to cardiomyocytes.18 In contrast, inhibition of lncExACT1 (which decreases in hearts after exercise training) reduces fibrosis after aortic constriction and ischemic injury most likely through a combination of direct effects on fibroblasts and indirect effects on cardiomyocytes.19
2.2. Endothelial cells
Vascular endothelial cells are the most abundant cell type in the adult heart, constituting approximately 40% of cardiac cells and 60% of total non-cardiomyocytes in the heart.29 The endothelium serves as an interface between circulating blood and cardiac tissues, controlling diffusion and transport of nutrients and other circulating molecules from the blood into the surrounding cardiomyocytes and other cell types to meet the demands of homeostasis and cardiac growth.74,75 It has been suggested that the coupling of angiogenesis to cardiac growth may occur early on in pathological hypertrophy, but may not persist, potentially contributing to the development of heart failure.76 Prior work demonstrated that this failure of angiogenesis may be related to p53-mediated inhibition of hypoxia-inducible factor 1-α.77 In contrast, macroscopic and microscopic vascular adaptations occur in athletes and animals with physiological growth. Enlarged coronary lumens78,79 and expansion of the vascular bed cross-sectional area are both observed with exercise-induced physiological cardiac hypertrophy in proportional response to the increased demand for oxygen and nutrients75 (Table 2 and Fig. 1), which suggests the involvement of endothelial cells in exercise-induced physiological cardiac hypertrophy. Indeed, exercise-induced physiological cardiac hypertrophy is associated with increases in cardiac eNOS expression and NO production. Inhibition of NOS80 or eNOS knockout81 abolishes exercise-induced cardiac hypertrophy. These findings indicate the necessary role endothelial cells play in cardiac growth during exercise, despite there being a possibility the changes in eNOS and NO seen in these studies may come from multiple sources (e.g., endothelial cells, cardiomyocytes). Taken together, this work underscores the essential role of endothelial cells and coupled angiogenesis in supporting cardiac growth and preventing the transition to heart failure.
Table 2.
The roles of endothelial cells in cardiac response to exercise.
| Exercise model | Outcome | Species | Age (week) | Sex |
|---|---|---|---|---|
| Treadmill (30 min at 70%–80% of maximal heart rate at Week 1. In weeks 2–8, exercise effort was increased by 5 min. After 8 weeks: 70 min, 5 days/week)158 |
Exercise increased endothelial cell proliferation up to 16 weeks and increased vessel density at Week 3 until a decrease at Week 8 | Yucatan minipigs | 3–24 | Male |
| Swimming (90 min, 5 days/week for 8 weeks)159 |
Exercise improved age-related reduction in VEGF angiogenic signals | Rats | 16 and 92 | Male |
| Wheel running (8 weeks, voluntary)160 |
Exercise improved left ventricular function after myocardial infarction in wild-type but not eNOS knockout mice | Mice | 12 | Male and female |
| Swimming (5 days/week, increased from 30 min/day to 180 min/day for 10 weeks)80 |
Exercise increased eNOS expression and insulin-induced myocardial NO expression | Rats | 8 | Male |
| Wheel running (4 weeks, voluntary)13 |
Exercise increased cardiac eNOS phosphorylation and NO production, which protected against myocardial reperfusion injury in wild-type but not β3 receptor knockout mice | Mice | 8–10 | Male |
| Treadmill (5 days/week for 4 weeks at 60% of max aerobic velocity)86 |
Exercise normalized iNOS expression and conveyed a cardioprotective effect against reperfusion injury | Mice | 8 | Male |
| Treadmill (3 days of training, then 5 days/week for 4 weeks)91 |
Exercise increased post-ischemic cardiac function and increased ErbB/PI3K/Akt, which were blocked by ErbB inhibitor | Rats | 12 | Male |
| Wheel running (6 weeks, voluntary)97 |
Exercise and garlic improved angiogenesis through miR-126 and miR-210 | Rats | Unclear | Male |
| Swimming (5 days/week for 10 weeks with 4% caudal body weight workload)98 |
Exercise restored cardiac miR-16 and VEGF expression as well as angiogenesis in obese rats | Rats | 20 | Male |
| Swimming (10 min twice/day, then increased by 10 min/day until reaching 90 min/day for a total of 3 weeks)112 |
Exercise induced physiological cardiac growth with increases in cardiac lymphangiogenesis, which were blocked by VEGFR3 inhibitor | Mice | 8–10 | Male |
Abbreviations: Akt = serine/threonine kinase; eNOS = endothelial nitric oxide synthase; ErbB = Erb-B2 receptor tyrosine kinase; iNOS = inducible nitric oxide synthase; miR = microRNA; NO = nitric oxide; PI3K = phosphoinositide 3-kinase; VEGF = vascular endothelial growth factor; VEGFR3 = vascular endothelial growth factor receptor 3.
Other work suggests vascular endothelium may play an even more direct role in regulating cardiac hypertrophy. In mice with inducible expression of the pro-angiogenetic, macrophage-derived, secrete peptide PR39 in cardiomyocytes, Tirziu et al.82 demonstrated that 3 weeks of forced PR39 secretion from cardiomyocytes increased vascular density. Both increases in vascular density and cardiac growth were observed after 6 weeks of PR39 induction, while these changes were reversed by NOS inhibition with NG-nitro-L-arginine methyl ester (L-NAME).82 In addition, in mice with cardiomyocyte-specific overexpression of placental growth factor (a member of the vascular endothelial growth factor (VEGF) family that induces angiogenesis), Jaba et al.83 observed increased angiogenesis and endothelial cell-derived NO production and subsequent cardiac growth, likely via G protein βγ/PI3Kγ/Akt/ mammalian target of rapamycin complex 1 signaling.83 These effects of placental growth factor were attenuated by L-NAME. Taken together, these studies provide evidence that angiogenesis can regulate cardiac hypertrophy.
In addition to its signaling effects, which as discussed above can regulate cardiomyocyte growth,83 NO is the dominant vasodilator released by the endothelium. The endothelium's production of L-arginine by way of NOS enzymes is enhanced by exercise—likely a response to increased blood flow and shear stress.84,85 In rats, 2 weeks of swim training increased heart weight to body weight ratios, promoted cardiac function, and increased cardiac eNOS activation/phosphorylation and NO production.80 These beneficial effects were abolished by the NOS inhibitor, L-NAME,80 indicating the essential role of eNOS-mediated NO production in the cardiac benefits of exercise. Similarly, Vettor et al.81 showed that 6 weeks of swim training enhanced cardiac eNOS expression, mitochondrial biogenesis, and insulin sensitivity in wild-type mice, while such beneficial effects of exercise were lost in eNOS knockout mice. Of note, Akt inhibition abrogated exercise-induced cardiac eNOS phosphorylation and NO production, while NOS inhibition by L-NAME had no impact on exercise-induced cardiac Akt phosphorylation.80 Taken together, these data suggest that exercise confers some of its effects on the heart through Akt-dependent eNOS activation and NO production. In addition to Akt-dependent NO generation, exercise can also enhance NO generation in a β3-adrenergic receptor-dependent manner. Calvert et al.13 showed that, in wild-type mice, 4 weeks of voluntary wheel running increased cardiac β3-adrenergic receptor protein expression and eNOS phosphorylation at Ser1177 as well as increased cardiac and plasma NO production and protected the heart against ischemia−reperfusion injury. However, these effects of voluntary wheel running were lost in β3-adrenergic receptor knockout mice, indicating the essential role of β3-adrenergic receptor in exercise-mediated activation of NO production and cardioprotection. Interestingly, in high fat/high sucrose diet-induced diabetic mice, cardiac β3-adrenergic receptor and eNOS phosphorylation was reduced and ischemia-reperfusion injury was increased. Four weeks of treadmill running attenuated post-ischemic injury without affecting cardiac β3-adrenergic receptor and eNOS phosphorylation,86,87 suggesting that in diabetic hearts, exercise confers its protective effects through pathways independent of β3-adrenergic receptor/eNOS. Indeed, in diabetic hearts, cardiac inducible NOS was accompanied by enhanced nitrosative stress and reactive oxygen species production. All these changes were attenuated by exercise, suggesting that exercise protected the diabetic heart through inducible NOS and nitro-oxidative stress normalization. These differences may be due to the type of exercise and/or disease studied (e.g., non-diabetic vs. diabetic), but they highlight the diverse mechanisms contributing to exercise-induced NO production. It is also worth noting that despite the name, “eNOS” is not specific to endothelial cells and is expressed, for example, in cardiomyocytes; therefore, many of the results above could reflect changes in non-endothelial lineages.
In the adult heart, endothelial cells also synthesize and secrete neuregulin-1 (NRG1), a member of the epidermal growth factor family,87, 88 which binds to erythroblastic leukemia viral oncogene homolog 3 (ErbB3) and ErbB4 to form heterodimers with ErbB2 and confer effects on cardiomyocytes through diverse signaling pathways.88 The NRG1–ErbB axis has been studied in pregnant rodents, a model and condition that produces physiologic cardiac growth, although often with differences in gene expression when compared to exercise-induced hypertrophy.89 From early (7–14 days) and late (18–20 days) pregnancy, protein expression of NRG1, ErbB2, and ErbB4 in left ventricles increased in association with left ventricular mass and preserved fractional shortening as well as enhanced phosphorylation of Akt and extracellular signal-regulated kinase 1/2.90 Moreover, inhibition of ErbB2 by Lapatinib inhibited ErbB2 and extracellular signal-regulated kinase 1/2 phosphorylation, reduced fractional shortening, and exacerbated left ventricular dilation but did not affect left ventricular mass. These results suggest a role of NRG1/ErbB signaling in the physiological cardiac growth seen in pregnancy. In rats subjected to ischemia−reperfusion, 4 weeks of treadmill exercise also increased cardiac NRG1 and phosphorylation of ErbB2, ErbB4, and Akt in conjunction with improved cardiac function, reduced cardiac fibrosis, and reduced cardiomyocyte apoptosis.91 These beneficial effects of exercise were abrogated by ErbB inhibition,91 demonstrating an important functional role for exercise-induced NRG1/ErbB signaling. These data are consistent with other studies documenting a role for NRG1/ErbB4 in protecting the heart against ischemia-reperfusion injury92 and heart failure,93, 94 as well as in enhancing cardiomyocyte proliferation94 and cardiac angiogenesis.95
Endothelial cells also express miRNAs, small noncoding RNAs that generally inhibit gene expression. While many miRNAs have been implicated in the beneficial effects of exercise, we focus here on miRNAs likely to originate in the endothelium. In exosomes isolated from the plasma of Sprague-Dawley rats subjected to 4 weeks of swimming exercise, 12 differentially expressed miRNAs were identified. Among them, miR-342-5p was the only one that exerted protective effects in cardiomyocytes subjected to hypoxic reoxygenation.96 Increased exosomal miR-342-5p was also observed in the plasma of student-athletes after 1 year of rowing training when compared to untrained controls.96 While both mature and pre-miR-342-5p were increased in many tissues after 4 weeks of exercise training, the largest increases were seen in the aorta, and removal of aortic endothelium reduced exercise-induced miR-342-5p,96 suggesting that aortic endothelial cells are a major source of miR-342-5p whose expression and release appear to be induced by laminar shear stress. Further, inhibition of miR-342-5p in exercised rats abolished the protective effects of exercise against ischemia-reperfusion injury. Mechanistically, exosomal miR-342-5p conferred its effects by binding Ppm1f and enhancing Akt phosphorylation, thus attenuating cell death.96 This study provides some of the most direct evidence for a causal role of an endothelial miRNA in exercise-mediated cardioprotection.
Cardiac miR-126 was downregulated and cardiac miR-210 was upregulated in diabetic rats as compared to non-diabetic rats, in association with reduced cardiac angiogenesis and elevated serum triglycerides. These changes were reversed by 6 weeks of voluntary wheel running.97,98 The results indicate potential roles of miR-126 and miR-210 in exercise-mediated enhancement of angiogenesis and lipid metabolism in diabetic hearts. Similarly, cardiac miR-16, which targets VEGF-A, was upregulated in obese Zucker rats, which manifest cardiac hypertrophy with capillary rarefaction.98 This was reversed by 10 weeks of swim training. Of note, the benefits of exercise were seen in obese but not lean Zucker rats,98 which likely reflects higher baseline cardiac miR-16 levels in lean rats. Importantly, while many of the studies discussed in this section document important endothelial phenotypes seen in the exercised heart, the miRNAs discussed were not shown to be endothelial-specific, making it impossible to infer that they solely reflect contributions from endothelial cells. In addition to providing evidence for the role of non-cardiomyocyte lineages, identification of endothelial-specific miRNAs or networks could give rise to targetable approaches for human translation.
Recent work suggests that the lymphatic endothelium may also play important and potentially distinct roles in the cardiac exercise response. The cardiac lymphatic vasculature is essential in interstitial fluid homeostasis, lipid transport, and immune surveillance, mediating antigen clearance and inflammatory resolution.99, 100, 101, 102, 103 The network of cardiac lymphatics is extensive,103,104 and therapeutic activation of lymphangiogenesis through overexpression of VEGF-C has shown benefits in models of myocardial infarction in limiting injury, accelerating repair, and improving cardiac function.105, 106, 107, 108 Exercise as an intervention to promote lymphatic flow and vessel integrity has been studied clinically, principally in patients with peripheral edema after oncologic surgery,108, 109, 110, 111 and in animal models of obesity.111 Exercise leads to a higher density of lymphatic vessels in the heart, as assessed by lymphatic-specific markers endothelial hyaluronan receptor 1 and podoplanin.112 A major driver of lymphangiogenesis is VEGF-C and -D activation of vascular endothelial growth factor receptor 3 (VEGFR3),112 and chemical inhibition of VEGFR3 not only attenuates exercise-induced increase in lymphatic vessel density in the heart but also blocks cardiomyocyte growth.112 This suggests that cardiac lymphangiogenesis may be necessary for physiological hypertrophy. Indeed, conditioned media from lymphatic endothelial cells promotes the proliferation and hypertrophy of neonatal rat cardiomyocytes in vitro.112 Interestingly, this cross-talk from lymphatic endothelial cells to cardiomyocytes leads to activation of Akt and CITED4-dependent pathways of hypertrophy.112 Further, in vivo studies of cardiac lymphatic vasculature size, function, and content will help elucidate the critical role these vessels play in exercise-mediated growth and repair after injury.
2.3. Immune cells
The important role of immune cells in mediating cardiac adaptions to pathological stresses is well documented.113, 114, 115, 116, 117, 118, 119, 120, 121 However, changes in immune cell signatures in the heart have not yet been thoroughly investigated, nor has the direct involvement of cardiac resident or recruited immune cells in the heart's response to physiological stress (e.g., exercise), though there are some reports pointing to the possible roles immune cells play to that effect Table 3.
Table 3.
Cardiac immune cell responses in exercise.
| Exercise model | Outcome | Animal model |
|---|---|---|
| Voluntary wheel running (6 weeks)120 |
Exercise increased hematopoietic stem cell quiescence; and reduced hematopoietic proliferation, myeloid and lymphoid colony production, and mobilization of circulating precursor immune cells, primarily via reduced leptin production in adipose tissue | 7- to 8-week-old C57BL/6J male mice, leptin receptor knockout (Leprfl/fl mice) |
| Wheel running (2 weeks, 1 h of daily exercise at a speed of 5.2 m/min)126 | Exercise upregulated PGC-1α and reduced cardiac (macrophage infiltration, iNOS and TNF-α expression) and systemic inflammation | 8-month-old male diabetic mice |
| Voluntary wheel running (12 weeks), ladder resistance training (3 times/week, 12 weeks), using a climbing ladder (90 cm, 1 cm grid, 80° incline, increased to 100% body weight)125 | Both forms of exercise prevented increase in circulating TNF-α, with decreases in tissue cytokine (TNF-α and IL-1β) mRNA expression in the heart, attenuating age-related chronic inflammation | 38-week-old male SAMP1 mice |
| Isoproterenol-induced cardiac stimulation, treadmill running (6 times/week, 4 weeks)127 | Isoproterenol increased IL-10 release via cardiac macrophage activation, producing heart failure phenotype; exercise reversed cardiac dysfunction and fibrosis. Beneficial effect was absent in macrophage/monocyte IL-10 knockout mice | 6- to 8-week-old C57BL/6 male mice |
| Wheel running (11 weeks),133 treadmill training (9 weeks),134 treadmill training (12 weeks, max of 21 m/min, 30 min twice a day, 5 days/week at 5.5% grade, 65%–75% max oxygen consumption)135 |
All 3 exercise models reduced cardiac mast cell activation and degranulation in ovariectomized rats Treadmill training for 9 weeks reduced angiotensin II-induced cardiac fibrosis, mast cell degranulation and activation Treadmill training for 12 weeks did not reduce doxorubicin-induced cardiac dysfunction, but reduced mast cell activation |
8- to 9-week-old Sprague-Dawley female rats, ovariectomized |
Abbreviations: IL = interleukin; iNOS = inducible nitric oxide synthase; Leprfl/fl = leptin receptor flox/flox mice; PGC-1α = peroxisome proliferator-activated receptor-gamma coactivator -1alpha; SAMP1 = senescence-accelerated prone mouse 1; TNF-α = tumor necrosis factor-α.
2.4. Hematopoietic stem cells
Recent work by Frodermann et al.120 examined the relationship between immune cells and exercise. In this study, they found that 6−10 weeks of voluntary exercise can influence immunologic signatures in the adult mouse heart through modulation of cell signaling in adipose tissue and bone marrow.120 They found that 6 weeks of voluntary wheel running in mice increased hematopoietic stem cell quiescence while reducing hematopoietic proliferation, myeloid and lymphoid colony production, and mobilization of precursor immune cells into the circulation when compared to sedentary mice.120 These effects of exercise protected mice from chronic leukocytosis, atherosclerosis, and maladaptive systemic leukocytosis in myocardial infarction.120 However, it should be noted that these changes in the heart were primarily due to a reduction in leptin production in adipose tissue and subsequently reduced leptin signaling in bone marrow immune cell niches. Leptin receptor knockout mice had reduced cardiac inflammation and improved recovery from myocardial infarction compared to wild-type controls. Interestingly, abrupt withdrawal of mice from 6 weeks of wheel running reversed these changes, but long-term epigenetic and transcriptional changes remained, including reduced chromatin accessibility in hematopoietic precursor cells.120 These results suggest that the regulation of circulating immune cells by exercise may occur through modulating leptin signaling in adipose tissues.
2.5. Cardiac macrophages
Macrophages make up 7%–8% of the total noncardiomyocyte cell population and are distributed throughout the entire heart.121 There are 2 distinct lineages of cells with unique origins, characterized by the cellular expression of a key cell surface marker known as chemokine (C–C motif) receptor-2 (CCR2) in the adult heart.122 It has been shown that CCR2-negative macrophages are present in the embryo and are maintained locally in the heart postnatally, while CCR2-positive macrophages derive from circulating monocytes and represent 5%–15% of total cardiac macrophages.113,119 Additional subsets within the CCR2-positive and negative populations are grouped according to high or low expression of lymphocyte antigen 6C and major histocompatibility complex II.38,119 Cardiac macrophages have important housekeeping functions within the adult heart, influencing extracellular matrix microenvironment, phagocytosis of dead cardiomyocytes, and cell turnover. In contrast, monocytes found within the adult heart likely represent mobilized or circulating cells.123
Disease states such as hypertension, heart failure, myocarditis, diastolic dysfunction, and myocardial infarction are associated with cardiac immune activation and disruptions in cardiac macrophage homeostasis.35,124 This often occurs via CCR2-positive macrophage secretion of inflammatory cytokines (tumor necrosis factor-α, interleukin 1β (IL-1β), IL-6, IL-8) and MMPs, recruitment of myeloid cells (primarily neutrophils) to the heart, and growth factors (e.g., TGF-α and TGF-β) that influence acute inflammation, thrombosis, and fibrosis in response to various cardiac injuries.122 Importantly, macrophages also participate in reparative phases after inflammation in both physiologic and pathologic states via local proliferation within the heart and secretion of anti-inflammatory proteins, such as IL-10 and VEGF, to promote beneficial cardiac remodeling through angiogenesis and cardiomyocyte proliferation.35
Prior studies have shown that exercise models like wheel running and ladder climbing reduced systemic inflammation and macrophage infiltration in the heart of aged mice.125,126 These studies demonstrate that resident cardiac macrophage populations are affected by exercise. However, the results do not conclusively establish a primary role for macrophages in mediating the benefits of exercise. A recent study by Feng et al.127 showed that isoproterenol-induced heart failure was accompanied by an increase in IL-10 release, mainly secreted by macrophages in the heart. They further found that 4 weeks of treadmill running attenuated isoproterenol-induced (isoproterenol injected 5 days after running for a total of 2 weeks) cardiac functional decline and fibrosis.127 These beneficial effects of exercise were lost in mice with macrophage/monocyte-specific IL-10 knockout (C-X3-C motif chemokine receptor 1 (CX3CR1)CreER crossed with IL-10flox/flox mice).127 These results highlight the importance of macrophage in the beneficial effects of exercise on the heart. However, it should be noted that the knockout mice were systemic macrophage and monocyte knockout, meaning that IL-10 from non-cardiac macrophage sources may contribute to the effects of exercise seen in this study.
2.6. Cardiac mast cells
Cardiac mast cells have pathological and physiological protective roles within cardiac remodeling and can be found in the myocardium, aortic valve, coronary arterial walls, atherosclerotic lesions, and near nerves.128 Exercise is a physiologic trigger for mast cell activation, also called degranulation, in skeletal muscle.129 Mast cells also expand in the heart after cardiac injury.130 Mast cell production and release of histamine induces a potent post-exercise vasodilatory response to increase local blood flow to tissues.131,132 In ovariectomized rats, where ovarian hormone deprivation led to an increased density of cardiac mast cells and degranulation, regular exercise (11 weeks of running) reduced cardiac mast cell activation and degranulation without affecting mast cell density.133 Similarly, in ovariectomized rats treated with angiotensin II, 9 weeks of treadmill training reduced angiotensin II-induced cardiac fibrosis and cardiac mast cell degranulation but had no effect on mast cell density.134 However, in ovariectomized rats treated with doxorubicin, 12 weeks of treadmill running had no effect on doxorubicin-induced cardiac dysfunction and cardiac mast cell density but did partially reduce mast cell hyperactivation.135 In mice subjected to 4 weeks of swim training, there was an upregulation of CD34+ cells, a marker expressed by mast cells, along with physiological cardiac growth, suggesting a possible association between mast cells and exercise.
2.7. Neutrophils
Neutrophils, the body's first responders to injury and inflammation and the predominant blood leukocyte, have reported roles in angiotensin II-induced cardiac hypertrophy and fibrosis.136, 137, 138, 139, 140, 141, 142, 143 However, there are few studies on the role of neutrophils in the heart in exercise. After high-intensity acute bouts of exercise, neutrophils demarginate from the vasculature in response to shear stress and circulating catecholamines, producing a neutrophilia that is maintained by cortisol's influence on bone marrow release of additional neutrophils.142,143 Changes to neutrophil demargination and activation after exercise appear to be dose-dependent, with some studies reporting maladaptive neutrophil effects with longer-duration and higher-intensity bouts of exercise.144, 145, 146, 147, 148 Aerobic exercise can activate neutrophil responses in the absence of pathogenic stimuli, and after exposure to inflammatory challenges, this pro-inflammatory response is dampened.143,147,148 It remain unknown whether these changes to circulating neutrophils influence recruitment into the heart during exercise, as has been shown in skeletal muscle.149
2.8. Lymphocytes
The predominant adaptive immune cells in the mammalian heart are B and T lymphocytes, which are recruited to sites of cardiac injury to promote acute and chronic inflammatory responses.150,151 It appears that Th1 CD8+ cells exert pro-inflammatory effects, while Th2 CD4+ cells promote fibrotic effects in the heart.152 Regulatory T cells (Tregs) are associated with suppression of pro-inflammatory signaling and expand locally in the heart after infarction and in heart failure states to attenuate hypertrophic remodeling.150 Patients with heart failure have lower levels of circulating Tregs, which positively correlate with overall cardiac function.151 Another important subpopulation of cardiac T cells includes Th17 cells. These are inactivated by Tregs and secrete IL-17, which is pathogenic in hypertensive cardiac remodeling.153 There is also a pathologic role for CD8+ T cells after myocardial infarction, which have been shown to influence maladaptive ischemic remodeling in murine models using left anterior descending artery ligation.154 Adamo et al.155,156 have shown that B lymphocytes—which are the most numerous of all leukocytes within the murine heart, with 95% lying within the vasculature and 5% extravasating into myocardial tissue—are important regulators of the heart's immunologic response to injury.
Despite these provocative studies on the roles played by immune cells in the effects of exercise on the heart, many crucial questions remain to be addressed. First, there are multiple sources of immune cells in the body, so further study is warranted to determine whether exercise has an impact on immune cells native to the heart (i.e., not recruited or influenced by external cross-talk pathways) and whether these changes contribute to the cardiac benefits of exercise. Second, diverse effects of immune cells (e.g., macrophages in angiogenesis, fibrosis, and sympathetic and parasympathetic nervous system influence on cardiomyocytes) have been reported to affect cardiomyocytes. An important area of future research may be to better characterize their role, if any, in physiologic stress.121 Third, because of a lack of specificity of markers for certain immune cells (e.g., CD34 is not specific to mast cells134), additional work utilizing multiple markers and/or advanced cell identity measurements is needed to understand whether exercise modulates the function of specific immune cells or whether their signaling directly contributes to the cardiac benefits of exercise. Finally, current studies reveal only a small aspect of the role immune cells play in exercise's effects on the hearts. More work is needed to fully elucidate the function of immune cells (e.g., T and B lymphocytes of the adaptive immune system) in the physiologic exercise response and cardioprotective benefits of exercise.
3. Cellular cross-talk during exercise
As noted above, there is increasing recognition that multiple cell lineages and subtypes contribute to cardiac biology, although detailed information about their roles in exercise is available for only a few of these cell types. It is perhaps not surprising that even less is known about the potentially complex interactions among these lineages in exercise in vivo. Lighthouse et al.48 showed that Mt1/Mt2 were highly expressed in cardiac fibroblasts and were upregulated by exercise. In vitro overexpression of Mt1 in NIH-3T3 fibroblasts partially blocked TGF-β1-induced myofibroblast markers. Further, they showed that conditioned media from fibroblast overexpressing Mt1 protected cardiomyocytes against H2O2-induced oxidative stress and cell death,48 suggesting a potential exercise-induced paracrine cardioprotective mechanism. We recently showed that conditioned media from ventricular cardiomyocytes with knockdown of CITED4, a mediator of exercise-induced cardiac benefits,16 promoted expression of extracellular matrix genes and myofibroblast gene markers in ventricular fibroblasts.21 This cardiomyocyte–fibroblast cross-talk was mediated by alterations in networks of microRNAs, including miR-30d.21 Of note, conditioned media from cardiomyocytes overexpressing miR-30d blocked fibroblast activation.21 This suggests CITED4-driven paracrine transfer of miR-30d as well as the possibility that other factors (e.g., cardiomyocytes, fibroblasts) could contribute to the antifibrotic effects of exercise (Fig. 1). An interesting avenue for future research is investigating changes that occur to non-cardiomyocyte cell populations in the cardiac interstitium in response to exercise. A 1980 study from Guski et al.157 used ultrastructural morphometric examination of rat myocardium in the left ventricle to show that it contains about 15% interstitial connective tissue, 6% of which is interstitial cells. This percentage was found to vary based on the number of hours of swim training the rats experienced. The volume ratio of extracellular space to interstitium in the left ventricle decreased after 45 h of training, suggesting increased cellular density with aerobic exercise. The specific cellular lineages involved in the expansion of the interstitium in exercise are not well characterized; however, as highlighted in this review, observed changes include increased capillary density (endothelial cell lineage) and reduction in fibrosis (fibroblast cell lineage). Ultimately, studies will need to be extended beyond in vitro models to document the in vivo importance of cellular cross-talk in exercise.
4. Conclusions and perspectives
The potent cardiac beneficial effects of exercise have been observed in different animal models and human subjects, and they involve the manipulations of different signaling pathways on different types of cells in the heart. We are just beginning to uncover the mechanisms underlying the benefits of exercise. Non-cardiomyocytes, including fibroblasts, endothelial cells, immune cells, and others, have been less extensively studied in the exercised heart. However, available information suggests they have roles distinct from cardiomyocytes. In some instances, notably with respect to fibroblasts and endothelial cells, it appears these non-cardiomyocytes actively contribute to the functional benefits of exercise. In other cases, the biology of non-cardiomyocytes is modified by signals for cardiomyocytes or other cell types in ways that contribute to the effects of exercise. At this point, however, the role of many of these non-cardiomyocyte populations in the physiological adaptation to exercise remains unknown. Nevertheless, given the demonstrated importance of other cell lineages in other contexts, it may be reasonable to assume these lineages have an additional role to play in the exercise response. Rapidly evolving technologies such as transcriptomics provide single-cell resolution for molecular analyses. These technologies have the potential to uncover previously unrecognized roles for non-cardiomyocyte lineages, thereby enhancing our understanding of the benefits of exercise and aiding in the identification of new therapeutic strategies for cardiovascular disease.
Acknowledgments
Supported by the NIH (R01AG061034 (AR), R35HL15531 (AR), R21AG077040 (HL), and K08HL140200 (JR)), the American Heart Association (20CDA35310184 (HL) and 19AMFDP34990046 (JSG)), Sarnoff Cardiovascular Research Foundation Fellowship award (LET and CS), and Massachusetts General Hospital Sanchez-Ferguson Faculty Scholar Program (JSG). Fig. 1 and the graphical abstract were created with BioRender (https://biorender.com/).
Authors’ contributions
LET and AR conceptualized the manuscript; HL conceptualized the manuscript and drew the figure. All authors performed the literature search, drafted the manuscript, and contributed to the writing of the manuscript. All the authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Connor EA, Evans CV, Rushkin MC, Redmond N, Lin JS. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: Updated evidence report and systematic review for the us preventive services task force. JAMA. 2020;324:2076–2094. doi: 10.1001/jama.2020.17108. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Chen C, Spanos M, et al. Exercise training maintains cardiovascular health: Signaling pathways involved and potential therapeutics. Signal Transduct Target Ther. 2022;7:306. doi: 10.1038/s41392-022-01153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, Liu X, Rosenzweig A. What do we know about the cardiac benefits of exercise? Trends Cardiovasc Med. 2015;25:529–536. doi: 10.1016/j.tcm.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piercy KL, Troiano RP. Physical activity guidelines for Americans from the U.S. Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.005263. [DOI] [PubMed] [Google Scholar]
- 6.Henschen Skidlauf und skidwettlauf. Eine medizinische sportstudie. Mitt Med Klin Upsala. 1899;2:15–88. [in Germany] [Google Scholar]
- 7.Darling EA. The effects of training. A study of the Harvard University crews. Boston Med Surg J. 1899;141:229–233. [Google Scholar]
- 8.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks KL, Gao X, Du XJ, et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Li L, del MonteF, et al. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T, Tao J, del Monte F, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 12.DeBosch B, Treskov I, Lupu TS, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 13.Calvert JW, Elston M, Aragón JP, et al. Exercise protects against myocardial ischemia−reperfusion injury via stimulation of β(3)-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 16.Boström P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezzerides VJ, Platt C, Lerchenmuller C, et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1:e85904. doi: 10.1172/jci.insight.85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Xiao J, Zhu H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Trager LE, Liu X, et al. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation. 2022;145:1218–1233. doi: 10.1161/CIRCULATIONAHA.121.056850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrino C, Naga Prasad SV, Mao L, et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerchenmüller C, Rabolli CP, Yeri A, et al. CITED4 protects against adverse remodeling in response to physiological and pathological stress. Circ Res. 2020;127:631–646. doi: 10.1161/CIRCRESAHA.119.315881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vujic A, Lerchenmüller C, Wu TD, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerchenmüller C, Vujic A, Mittag S, et al. Restoration of cardiomyogenesis in aged mouse hearts by voluntary exercise. Circulation. 2022;146:412–426. doi: 10.1161/CIRCULATIONAHA.121.057276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao R, Wang L, Bei Y, et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation. 2021;144:303–317. doi: 10.1161/CIRCULATIONAHA.120.050446. [DOI] [PubMed] [Google Scholar]
- 25.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerchenmüller C, Rosenzweig A. Mechanisms of exercise-induced cardiac growth. Drug Discov Today. 2014;19:1003–1009. doi: 10.1016/j.drudis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Platt C, Rosenzweig A. The role of microRNAs in the cardiac response to exercise. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore-Morris T, Guimarães-Camboa N, Banerjee I, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: The fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souders CA, Borg TK, Banerjee I, Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol. 2012;181:1226–1235. doi: 10.1016/j.ajpath.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 33.Nag AC. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 34.Bergmann O, Zdunek S, Felker A, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol. 2005;202:891–899. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 37.LaFramboise WA, Scalise D, Stoodley P, et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292:C1799–C1808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 38.Litviňuková M Talavera-López C, Maatz H, et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Villalba A, Romero JP, Hernandez SC, et al. Single-cell RNA sequencing analysis reveals a crucial role for CTHRC1 (collagen triple helix repeat containing 1) cardiac fibroblasts after myocardial infarction. Circulation. 2020;142:1831–1847. doi: 10.1161/CIRCULATIONAHA.119.044557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: The renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: At the center of it all. J Mol Cell Cardiol. 2010;48:474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janbandhu V, Tallapragada V, Patrick R, et al. HIF-1α suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction. Cell Stem Cell. 2022;29:281–297. doi: 10.1016/j.stem.2021.10.009. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humeres C, Shinde AV, Hanna A, et al. Smad7 effects on TGF-β and ErbB2 restrain myofibroblast activation and protect from postinfarction heart failure. J Clin Invest. 2022;132 doi: 10.1172/JCI146926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng Q, Bhandary B, Bhuiyan MS, et al. Myofibroblast-specific TGFβ receptor II signaling in the fibrotic response to cardiac myosin binding protein C-induced cardiomyopathy. Circ Res. 2018;123:1285–1297. doi: 10.1161/CIRCRESAHA.118.313089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 48.Lighthouse JK, Burke RM, Velasquez LS, et al. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4:e92098. doi: 10.1172/jci.insight.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan ML, Cheslow Y, Vikstrom K, et al. Cardiac adaptations to chronic exercise in mice. Am J Physiol. 1994;267:H1167–H1173. doi: 10.1152/ajpheart.1994.267.3.H1167. [DOI] [PubMed] [Google Scholar]
- 51.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: Novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 52.Soci UP, Fernandes T, Hashimoto NY, et al. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. 2011;43:665–673. doi: 10.1152/physiolgenomics.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sassi Y, Avramopoulos P, Ramanujam D, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating wnt signaling. Nat Commun. 2017;8:1614. doi: 10.1038/s41467-017-01737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess ML, Buggy J, Price RL, et al. Exercise- and hypertension-induced collagen changes are related to left ventricular function in rat hearts. Am J Physiol. 1996;270:H151–H159. doi: 10.1152/ajpheart.1996.270.1.H151. [DOI] [PubMed] [Google Scholar]
- 55.Woodiwiss AJ, Oosthuyse T, Norton GR. Reduced cardiac stiffness following exercise is associated with preserved myocardial collagen characteristics in the rat. Eur J Appl Physiol Occup Physiol. 1998;78:148–154. doi: 10.1007/s004210050400. [DOI] [PubMed] [Google Scholar]
- 56.Jin H, Yang R, Li W, et al. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol. 2000;279:H2994–H3002. doi: 10.1152/ajpheart.2000.279.6.H2994. [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Fu Y, Xiao H, et al. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PloS One. 2015;10 doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes-Santos IL, Jordao CP, Passos CS, et al. Exercise training preserves myocardial strain and improves exercise tolerance in doxorubicin-induced cardiotoxicity. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.605993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang HL, Hsieh PL, Hung CH, et al. Early moderate intensity aerobic exercise intervention prevents doxorubicin-caused cardiac dysfunction through inhibition of cardiac fibrosis and inflammation. Cancers (Basel) 2020;12:1102. doi: 10.3390/cancers12051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sequeira CM, Martins MA, Alves R, et al. Aerobic exercise training attenuates doxorubicin-induced ultrastructural changes in rat ventricular myocytes. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118698. [DOI] [PubMed] [Google Scholar]
- 61.Ascensão A, Magalhaes J, Soares J, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–460. doi: 10.1016/j.ijcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Calvé A Haddad R, Barama SN, Meilleur M, Sebag IA, Chalifour LE. Cardiac response to doxorubicin and dexrazoxane in intact and ovariectomized young female rats at rest and after swim training. Am J Physiol Heart Circ Physiol. 2012;302:H2048–H2057. doi: 10.1152/ajpheart.01069.2011. [DOI] [PubMed] [Google Scholar]
- 63.Xu X, Wan W, Powers AS, et al. Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. J Mol Cell Cardiol. 2008;44:114–122. doi: 10.1016/j.yjmcc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin YY, Hong Y, Zhou MC, et al. Exercise training attenuates cardiac inflammation and fibrosis in hypertensive ovariectomized rats. J Appl Physiol (1985) 2020;128:1033–1043. doi: 10.1152/japplphysiol.00844.2019. [DOI] [PubMed] [Google Scholar]
- 65.Thomas DP, Cotter TA, Li X, McCormick RJ, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–169. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- 66.Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: Interactive effect of age and exercise training. J Appl Physiol (1985) 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- 67.Wright KJ, Thomas MM, Betik AC, Belke D, Hepple RT. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp Gerontol. 2014;50:9–18. doi: 10.1016/j.exger.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Wang SQ, Li D, Yuan Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J Physiol Sci. 2019;69:861–873. doi: 10.1007/s12576-019-00696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma N, Liu HM, Xia T, Liu JD, Wang XZ. Chronic aerobic exercise training alleviates myocardial fibrosis in aged rats through restoring bioavailability of hydrogen sulfide. Can J Physiol Pharmacol. 2018;96:902–908. doi: 10.1139/cjpp-2018-0153. [DOI] [PubMed] [Google Scholar]
- 70.Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–1117. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–793. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- 72.He Y, Huang C, Lin X, Li J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie. 2013;95:1355–1359. doi: 10.1016/j.biochi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Deng Z, He Y, Yang X, et al. MicroRNA-29: A crucial player in fibrotic disease. Mol Diagn Ther. 2017;21:285–294. doi: 10.1007/s40291-016-0253-9. [DOI] [PubMed] [Google Scholar]
- 74.Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–3179. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of HIF-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 78.Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol. 1968;24:485–490. doi: 10.1152/jappl.1968.24.4.485. [DOI] [PubMed] [Google Scholar]
- 79.Wyatt HL, Mitchell J. Influences of physical conditioning and deconditioning on coronary vasculature of dogs. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:619–625. doi: 10.1152/jappl.1978.45.4.619. [DOI] [PubMed] [Google Scholar]
- 80.Zhang QJ, Li QX, Zhang HF, et al. Swim training sensitizes myocardial response to insulin: Role of Akt-dependent eNOS activation. Cardiovasc Res. 2007;75:369–380. doi: 10.1016/j.cardiores.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Vettor R, Valerio A, Ragni M, et al. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: Role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab. 2014;306:E519–E528. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- 82.Tirziu D, Chorianopoulos E, Moodie KL, et al. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaba IM, Zhuang ZW, Li N, et al. No triggers RGS4 degradation to coordinate angiogenesis and cardiomyocyte growth. J Clin Invest. 2013;123:1718–1731. doi: 10.1172/JCI65112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- 85.Miller VM, Vanhoutte PM. Enhanced release of endothelium-derived factor(s) by chronic increases in blood flow. Am J Physiol. 1988;255:H446–H451. doi: 10.1152/ajpheart.1988.255.3.H446. [DOI] [PubMed] [Google Scholar]
- 86.Kleindienst A, Battault S, Belaidi E, et al. Exercise does not activate the β3 adrenergic receptor-eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res Cardiol. 2016;111:40. doi: 10.1007/s00395-016-0559-0. [DOI] [PubMed] [Google Scholar]
- 87.Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 88.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung E, Heimiller J, Leinwand LA. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One. 2012;7:e42297. doi: 10.1371/journal.pone.0042297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–H942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 91.Cai MX, Shi XC, Chen T, et al. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016;149:1–9. doi: 10.1016/j.lfs.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 92.Hedhli N, Huang Q, Kalinowski A, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Gu X, Li Z, et al. Neuregulin-1/ErbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 94.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 95.Gui C, Zeng ZY, Chen Q, Luo YW, Li L, Chen LL. Neuregulin-1 promotes myocardial angiogenesis in the rat model of diabetic cardiomyopathy. Cell Physiol Biochem. 2018;46:2325–2334. doi: 10.1159/000489622. [DOI] [PubMed] [Google Scholar]
- 96.Hou Z, Qin X, Hu Y, et al. Longterm exercise-derived exosomal miR-342-5p: A novel exerkine for cardioprotection. Circ Res. 2019;124:1386–1400. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 97.Naderi R, Mohaddes G, Mohammadi M, et al. The effect of garlic and voluntary exercise on cardiac angiogenesis in diabetes: The role of miR-126 and miR-210. Arq Bras Cardiol. 2019;112:154–162. doi: 10.5935/abc.20190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandes T, Casaes L, Soci Ú, et al. Exercise training restores the cardiac microRNA-16 levels preventing microvascular rarefaction in obese Zucker rats. Obes Facts. 2018;11:15–24. doi: 10.1159/000454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis K, Laine G, Geissler H, Mehlhorn U, Brennan M, Allen S. Effects of myocardial edema on the development of myocardial interstitial fibrosis. Microcirculation. 2000;7:269–280. [PubMed] [Google Scholar]
- 100.Mehlhorn U, Geissler HJ, Laine GA, Allen SJ. Role of the cardiac lymph system in myocardial fluid balance. Eur J Cardiothorac Surg. 2001;20:424–425. doi: 10.1016/s1010-7940(01)00772-2. [DOI] [PubMed] [Google Scholar]
- 101.Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 102.Huggenberger R, Siddiqui SS, Brander D, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shore L. The lymphatic drainage of the human heart. J Anat. 1929;63:291–313. [PMC free article] [PubMed] [Google Scholar]
- 104.Tewalt E, Cohen J, Rouhani S, Engelhard V. Lymphatic endothelial cells-key players in regulation of tolerance and immunity. Front Immunol. 2012;3:305. doi: 10.3389/fimmu.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Houssari M, Dumesnil A, Tardif V, et al. Lymphatic and immune cell cross-talk regulates cardiac recovery after experimental myocardial infarction. Arterioscler Thromb Vasc Biol. 2020;40:1722–1737. doi: 10.1161/ATVBAHA.120.314370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klotz L, Norman S, Vieira JM, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bok SK, Jeon Y, Hwang PS. Ultrasonographic evaluation of the effects of progressive resistive exercise in breast cancer-related lymphedema. Lymphat Res Biol. 2016;14:18–24. doi: 10.1089/lrb.2015.0021. [DOI] [PubMed] [Google Scholar]
- 109.Baumann F, Reike A, Reimer V, et al. Effects of physical exercise on breast cancer-related secondary lymphedema: A systematic review. Breast Cancer Res Treat. 2018;170:1–13. doi: 10.1007/s10549-018-4725-y. [DOI] [PubMed] [Google Scholar]
- 110.Kim M, Park IH, Lee KS, et al. Breast cancer-related lymphedema after neoadjuvant chemotherapy. Cancer Res Treat. 2015;47:416–423. doi: 10.4143/crt.2014.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hespe GE, Kataru RP, Savetsky IL, et al. Exercise training improves obesity-related lymphatic dysfunction. J Physiol. 2016;594:4267–4282. doi: 10.1113/JP271757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bei Y, Huang Z, Feng X, et al. Lymphangiogenesis contributes to exercise-induced physiological cardiac growth. J Sport Health Sci. 2022;11:466–478. doi: 10.1016/j.jshs.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frangogiannis NG, Lindsey ML, Michael LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 115.Gwechenberger M, Mendoza LH, Youker KA, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 116.Wu L, Ong S, Talor MV, et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J Exp Med. 2014;211:1449–1464. doi: 10.1084/jem.20132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anzai A, Choi JL, He S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swirski FK, Nahrendorf M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 120.Frodermann V, Rohde D, Courties G, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25:1761–1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leuschner F, Nahrendorf M. Novel functions of macrophages in the heart: Insights into electrical conduction, stress, and diastolic dysfunction. Eur Heart J. 2020;41:989–994. doi: 10.1093/eurheartj/ehz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derivedresident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hulsmans M, Sager HB, Roh JD, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uchida M, Horii N, Hasegawa N, et al. Gene expression profiles for macrophage in tissues in response to different exercise training protocols in senescence mice. Front Sports Act Living. 2019;1:50. doi: 10.3389/fspor.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Botta A, Laher I, Beam J, et al. Short term exercise induces PGC-1α, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PloS One. 2013;8:e70248. doi: 10.1371/journal.pone.0070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feng L, Li G, An J, et al. Exercise training protects against heart failure via expansion of myeloid-derived suppressor cells through regulating IL-10/STAT3/S100A9 pathway. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.008550. [DOI] [PubMed] [Google Scholar]
- 128.Zhang W, Chancey AL, Tzeng HP, et al. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2011;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero SA, McCord JL, Ely MR, et al. Mast cell degranulation and de novo histamine formation contribute to sustained postexercise vasodilation in humans. J Appl Physiol (1985) 2017;122:603–610. doi: 10.1152/japplphysiol.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Varricchi G, Marone G, Kovanen PT. Cardiac mast cells: Underappreciated immune cells in cardiovascular homeostasis and disease. Trends Immunol. 2020;41:734–746. doi: 10.1016/j.it.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 131.Chen HI, Poduri A, Numi H, et al. VEGF-C and aortic cardiomyocytes guide coronary artery stem development. J Clin Invest. 2014;124:4899–4914. doi: 10.1172/JCI77483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henri O, Pouehe C, Houssari M, et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. 2016;133:1484–1497. doi: 10.1161/CIRCULATIONAHA.115.020143. [DOI] [PubMed] [Google Scholar]
- 133.Phungphong S, Kijtawornrat A, Wattanapermpool J, Bupha-Intr T. Regular exercise modulates cardiac mast cell activation in ovariectomized rats. J Physiol Sci. 2016;66:165–173. doi: 10.1007/s12576-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jitmana R, Raksapharm S, Kijtawornrat A, Saengsirisuwan V, Bupha-Intr T. Role of cardiac mast cells in exercise training-mediated cardiac remodeling in angiotensin II-infused ovariectomized rats. Life Sci. 2019;219:209–218. doi: 10.1016/j.lfs.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 135.Phungphong S, Kijtawornrat A, Kampaengsri T, Wattanapermpool J, Bupha-Intr T. Comparison of exercise training and estrogen supplementation on mast cell-mediated doxorubicin-induced cardiotoxicity. Am J Physiol Regul Integr Comp Physiol. 2020;318:R829–R842. doi: 10.1152/ajpregu.00224.2019. [DOI] [PubMed] [Google Scholar]
- 136.Okyere AD, Tilley DG. Leukocyte-dependent regulation of cardiac fibrosis. Front Physiol. 2020;11:301. doi: 10.3389/fphys.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J, Duan Y, Sluijter JP, Xiao J. Lymphocytic subsets play distinct roles in heart diseases. Theranostics. 2019;9:4030–4046. doi: 10.7150/thno.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Daseke MJ, 2nd, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML. Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res Cardiol. 2019;114:37. doi: 10.1007/s00395-019-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 140.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest. 2017;127:1600–1612. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang HM, Wang HX, Yang H, et al. Role for granulocyte colony stimulating factor in angiotensin II-induced neutrophil recruitment and cardiac fibrosis in mice. Am J Hypertens. 2013;26:1224–1233. doi: 10.1093/ajh/hpt095. [DOI] [PubMed] [Google Scholar]
- 142.Morozov VI, Pryatkin SA, Kalinski MI, Rogozkin VA. Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Eur J Appl Physiol. 2003;89:257–262. doi: 10.1007/s00421-002-0755-5. [DOI] [PubMed] [Google Scholar]
- 143.Peake JM. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: Possible mechanisms of action. Exerc Immunol Rev. 2002;8:49–100. [PubMed] [Google Scholar]
- 144.Giraldo E, Garcia JJ, Hinchado MD, Ortega E. Exercise intensity-dependent changes in the inflammatory response in sedentary women: Role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. Neuroimmunomodulation. 2009;16:237–244. doi: 10.1159/000212384. [DOI] [PubMed] [Google Scholar]
- 145.Smith JA, Pyne DB. Exercise, training, and neutrophil function. Exerc Immunol Rev. 1997;3:96–116. [PubMed] [Google Scholar]
- 146.Pyne DB. Regulation of neutrophil function during exercise. Sports Med. 1994;17:245–258. doi: 10.2165/00007256-199417040-00005. [DOI] [PubMed] [Google Scholar]
- 147.Chuong P, Wysoczynski M, Hellmann J. Do changes in innate immunity underlie the cardiovascular benefits of exercise? Front Cardiovasc Med. 2019;6:70. doi: 10.3389/fcvm.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Robson PJ, Blannin AK, Walsh NP, Castell LM, Gleeson M. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med. 1999;20:128–135. doi: 10.1055/s-2007-971106. [DOI] [PubMed] [Google Scholar]
- 149.Nunes-Silva A, Bernardes PT, Rezende BM, et al. Treadmill exercise induces neutrophil recruitment into muscle tissue in a reactive oxygen species-dependent manner. An intravital microscopy study. PloS One. 2014;9:e96464. doi: 10.1371/journal.pone.0096464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang TT, Yuan J, Zhu ZF, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 151.Tang TT, Ding YJ, Liao YH, et al. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–458. doi: 10.1159/000303050. [DOI] [PubMed] [Google Scholar]
- 152.Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: Friendly guests or unwelcome visitors? Am J Physiol Heart Circ Physiol. 2019;317:H124–H140. doi: 10.1152/ajpheart.00028.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Santos-Zas I, Lemarié J Zlatanova I, et al. Cytotoxic CD8(+) T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat Commun. 2021;12:1483. doi: 10.1038/s41467-021-21737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Adamo L, Rocha-Resende C, Mann DL. The emerging role of B lymphocytes in cardiovascular disease. Annu Rev Immunol. 2020;38:99–121. doi: 10.1146/annurev-immunol-042617-053104. [DOI] [PubMed] [Google Scholar]
- 156.Adamo L, Rocha-Resende C, Lin CY, et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guski H. The effect of exercise on myocardial interstitium. An ultrastructural morphometric study. Exp Pathol (Jena) 1980;18:141–150. doi: 10.1016/s0014-4908(80)80013-5. [DOI] [PubMed] [Google Scholar]
- 158.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol (1985) 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 159.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1290–H1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 160.de Waard MC, van Haperen R, SoulliéT Tempel D, de Crom R, Duncker DJ. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol. 2010;48:1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.