Despite regulatory advances over the past two decades, low levels of environmental heavy metal contamination remain widespread. Because lead is predominantly excreted by the kidney, patients with chronic kidney disease (CKD) may be particularly susceptible to lead toxicity. We have recently used regulatory data to demonstrate associations between higher levels of lead exposure in community drinking water and lower hemoglobin concentrations and higher erythropoietin stimulating agent (ESA) utilization among those with end-stage kidney disease (ESKD) [1]. To further investigate these findings, we used portable X-ray fluorescence (XRF) to measure levels of tibial lead in 69 patients seen for routine nephrology care. Tibial lead is widely regarded to reflect total lead accumulation [2]. While standard K-shell fluorescence remains the standard for measuring bone lead, recent advances in portable XRF techniques offer a more clinically feasible methodology which is under clinical evaluation [3–6]. We compared tibial lead levels to hemoglobin concentrations taken at the time of the clinic visit. Gender-specific thresholds [<11.2 g/dL (women) and 13.7 g/dL (men)] were used to define anemia. We hypothesized that tibial lead levels would be indirectly associated with hemoglobin concentrations, particularly for those with more severe CKD, among whom the myelosuppressive effects of lead would be synergized by the decreased erythropoietin functionality that accompanies anemia of CKD.
We used multivariate linear and logistic regression to examine the association of tibial lead with hemoglobin concentrations and odds of anemia, respectively, adjusted for age, gender and ethnicity, and explored for effect modification by cohort median estimated glomerular filtration (eGFR) (43 mL/min/1.73 m²). The 2021 CKD Epidemiology Collaboration equation was used to generate race-free estimates of glomerular filtration. In addition, we described the associations of tibial lead with iron concentrations, % transferrin saturation and ferritin among individuals with available laboratory data. As a model control, we examined whether tibial measures of copper, a heavy metal predominantly metabolized by the liver, was associated with hemoglobin concentrations.
The average patient age was 68.2 (8.4) years; 36% (n = 25) were female and 17% (n = 12) were African American. Large proportions of patients had hypertension (83%, n = 56) and diabetes (44%, n = 29). The mean values for eGFR and hemoglobin were 42 (4.3) mL/min/1.73 m² and 12.6 (2.2) g/dL, respectively. Tibial lead levels did not relate to renal function (correlation –0.06; P = .60). When stratified by median cohort tibial lead level (5.1 µg/g), those with higher levels of tibial lead had lower hemoglobin concentrations [12.3 (2.6) versus 12.9 (1.7) g/dL; P = .31] and higher proportions of anemia [50% (n = 17) vs 34% (n = 12); P = .20], respectively.
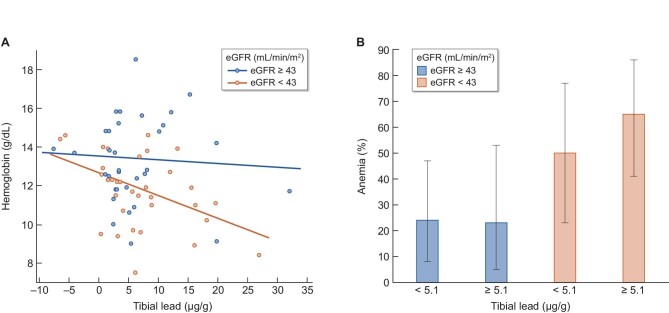
Among those with eGFR below the cohort median of 43 mL/min/1.73 m², among whom hemoglobin concentrations were lower [11.1 (1.9) vs 13.3 (2.1) g/dL; (P < .001)] and anemia proportions were higher [58% (n = 20) vs 25% (n = 9)], tibial lead was associated with lower hemoglobin concentrations and higher proportions of anemia (Fig. 1). In adjusted analyses, the associations between tibial lead and hemoglobin concentrations and anemia proportions were both significantly more robust in those with lower eGFR (multiplicative interaction between continuous measures of tibial lead and eGFR P-values .04 for hemoglobin and .01 for anemia, respectively). In those with eGFR below 43 mL/min/1.73 m², a 1 µg/g higher tibial lead concentration was associated with a 0.12 g/dL (–0.21, –0.02) lower hemoglobin concentration and a 23% (1.03, 1.47) higher odds of anemia. Tibial lead did not meaningfully associate with hemoglobin or anemia among those with eGFR ≥43 mL/min/1.73 m². Tibial lead was not meaningfully associated with measures of iron deficiency. Log transformation of data and correction for ESA use resulted in similar findings. Tibial levels of copper did not associate with hemoglobin concentrations or odds of anemia, and were not modified by eGFR.
Figure 1:
Association of tibial lead levels with hematologic outcomes according to CKD severity. Univariate change in hemoglobin concentration per 1 µg/g higher tibial lead concentration, and proportions with anemia according to cohort median tibial lead concentration, stratified by CKD severity. Multiplicative interaction P-values between tibial lead and eGFR were .04 and .01 for hemoglobin and anemia, respectively.
Our findings suggest that among patients with advanced CKD, environmental lead exposure is associated with a higher risk of anemia and lower hemoglobin concentrations. The effect modification observed in our analysis is consistent with a synergistic effect of lead toxicity in those already susceptible to myelosuppression due to CKD. Accumulating in the proximal tubule, the site of erythropoietin production, lead inhibits erythropoietin production [7–9], which is already compromised in CKD. Furthermore, lead inhibits heme synthesis and decreases erythrocyte survival [10, 11], which are similarly affected by uremia [12]. Accordingly, that the associations between tibial lead and myelosuppression are most robust in those with advanced CKD adds biologic plausibility and specificity to our findings, and suggests that even low levels of lead exposure, as found commonly in our environment, may have important clinical consequence for those with CKD.
Whether tibial lead will associate with other lead-related adverse outcomes warrants study. In particular, given that lead toxicity has been associated with cognitive decline [13, 14], which is a highly prevalent condition in CKD [15], a putative role of lead in CKD-related cognitive dysfunction needs to be examined. Limitations of our study included its relatively small size, which precluded further stratification by renal function and limited the analytic power. In addition, we did not have measures of circulating lead, nor could we fully account for lead exposure, limiting conclusions about whether renal function modifies the risk to any given exposure.
In summary, for individuals with CKD, environmental lead exposure may be a meaningful and preventable determinant of anemia. Whether tissue lead levels associate with other lead-related diseases will require further study.
Contributor Information
Subhash Paudel, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Parvathy Geetha, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Periklis Kyriazis, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Aaron Specht, Harvard T.H. Chan School of Public Health, Boston, MA, USA; School of Health Sciences, Purdue University, West Lafayette, IN, USA.
Howard Hu, Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, USA.
John Danziger, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
FUNDING
This research was supported, in part, by the University of Southern California Environmental Health Sciences Center, funded by the National Institute for Environmental Health Sciences (P30-ESES007048).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Danziger J, Mukamal KJ, Weinhandl E.. Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD. J Am Soc Nephrol 2021;32:2425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu H, Shih R, Rothenberg Set al. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect 2007;115:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Specht AJ, Dickerson AS, Weisskopf MG.. Comparison of bone lead measured via portable x-ray fluorescence across and within bones. Environ Res 2019;172:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Specht AJ, Parish CN, Wallens EKet al. Feasibility of a portable X-ray fluorescence device for bone lead measurements of condor bones. Sci Total Environ 2018;615:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang X, Specht AJ, Wells Eet al. Evaluation of a portable XRF device for in vivo quantification of lead in bone among a US population. Sci Total Environ 2021;753:142351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson KM, Specht AJ, Hart JMet al. Lead exposure and association with angiogenic factors and hypertensive disorders of pregnancy. Pregnancy Hypertens 2020;22:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn LG, Liu X, Rajovic Bet al. Blood lead concentration and thyroid function during pregnancy: results from the Yugoslavia prospective study of environmental lead exposure. Environ Health Perspect 2014;122:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camaj PR, Graziano JH, Preteni Eet al. Long-term effects of environmental lead on erythropoietin production in young adults: a follow-up study of a prospective cohort in Kosovo. J Environ Public Health 2020;2020:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graziano JH, Slavkovic V, Factor-Litvak Pet al. Depressed serum erythropoietin in pregnant women with elevated blood lead. Arch Environ Health 1991;46:347–50. [DOI] [PubMed] [Google Scholar]
- 10. Hernberg S, Nurminen M, Hasan J.. Nonrandom shortening of red cell survival times in men exposed to lead. Environ Res 1967;1:247–61. [DOI] [PubMed] [Google Scholar]
- 11. Goldman RH, Weissmann L.. A diagnosis to chew on. N Engl J Med 2019;381:466–73. [DOI] [PubMed] [Google Scholar]
- 12. Hamza E, Metzinger L, Metzinger-Le Meuth V. Uremic toxins affect erythropoiesis during the course of chronic kidney disease: a review. Cells 2020;9:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farooqui Z, Bakulski KM, Power MCet al. Associations of cumulative Pb exposure and longitudinal changes in Mini-Mental Status Exam scores, global cognition and domains of cognition: the VA Normative Aging Study. Environ Res 2017;152:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Power MC, Korrick S, Tchetgen Tchetgen EJet al. Lead exposure and rate of change in cognitive function in older women. Environ Res 2014;129:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Zwieten A, Wong G, Ruospo Met al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: the COGNITIVE-HD study. Nephrol Dial Transplant 2018;33:1197–203. [DOI] [PubMed] [Google Scholar]