Abstract
DNA polymerase activities in fractionated cell extract of Aeropyrum pernix, a hyperthermophilic crenarchaeote, were investigated. Aphidicolin-sensitive (fraction I) and aphidicolin-resistant (fraction II) activities were detected. The activity in fraction I was more heat stable than that in fraction II. Two different genes (polA and polB) encoding family B DNA polymerases were cloned from the organism by PCR using degenerated primers based on the two conserved motifs (motif A and B). The deduced amino acid sequences from their entire coding regions contained all of the motifs identified in family B DNA polymerases for 3′→5′ exonuclease and polymerase activities. The product of polA gene (Pol I) was aphidicolin resistant and heat stable up to 80°C. In contrast, the product of polB gene (Pol II) was aphidicolin sensitive and stable at 95°C. These properties of Pol I and Pol II are similar to those of fractions II and I, respectively, and moreover, those of Pol I and Pol II of Pyrodictium occultum. The deduced amino acid sequence of A. pernix Pol I exhibited the highest identities to archaeal family B DNA polymerase homologs found only in the crenarchaeotes (group I), while Pol II exhibited identities to homologs found in both euryarchaeotes and crenarchaeotes (group II). These results provide further evidence that the subdomain Crenarchaeota has two family B DNA polymerases. Furthermore, at least two DNA polymerases work in the crenarchaeal cells, as found in euryarchaeotes, which contain one family B DNA polymerase and one heterodimeric DNA polymerase of a novel family.
DNA polymerases are indispensable components of the molecular machinery responsible for replicating and repairing the genome of every organism. Studies of members of the domains Bacteria and Eucarya show that for these fundamental processes of life, multiple DNA polymerases are required (18). However, in the third domain of life, Archaea, the mechanisms involved in DNA replication and repair remain cryptic.
Two major subdomains, Euryarchaeota and Crenarchaeota, are now known to exist in Archaea (1). In the complete genome sequences of Methanococcus jannaschii and Pyrococcus horikoshii in Euryarchaeota, one DNA polymerase gene each was predicted from the similarity of the deduced amino acid sequences to family B DNA polymerases, and no more pol-like genes were found (4, 16). The fact that these euryarchaeotes may survive with just a single DNA polymerase was very perplexing (8, 11, 19). Recently, however, a novel two-subunit DNA polymerase whose sequence has no similarity to that of any DNA polymerase family was identified in Pyrococcus furiosus (28), in addition to a family B DNA polymerase isolated earlier from this organism (30). Through a protein homology search in the complete genome sequences of M. jannaschii, Archaeoglobus fulgidus, Methanobacterium thermoautotrophicum, and P. horikoshii, homologs of this novel DNA polymerase were found (7, 15). Based on the conservation of this DNA polymerase in these euryarchaeotes, members of Euryarchaeota seem to possess at least two DNA polymerases, one of family B and a second, heterodimeric DNA polymerase.
On the other hand, the demonstration of more than one DNA polymerase in cell extracts of the organisms in Crenarchaeota has often been attributed to proteolytic degradation or contamination (20). The evidence, at the gene level, suggesting that multiple DNA polymerases occur in this subdomain originates from research with Sulfolobus solfataricus P2 and Pyrodictium occultum PL-19. Two genes which seemed to encode family B DNA polymerases were cloned from S. solfataricus (23); then two family B DNA polymerase genes were cloned from P. occultum, and the gene products were proved to be functional DNA polymerases (29). Edgell et al. predicted from the deduced amino acid sequence that the sulfolobales have three family B DNA polymerase genes, and they proposed that the DNA polymerases encoded by these genes be designated B1, B2, and B3 (9). From these reports, it is possible that the organisms in Crenarchaeota have multiple family B DNA polymerases. However, there is no report on the expression of any of the three DNA polymerase genes of S. solfataricus P2, even though a B1 ortholog of S. solfataricus P2 has been cloned from S. solfataricus MT4, expressed, and characterized in detail (21, 22).
For an understanding of archaeal DNA replication, it is necessary to know how many DNA polymerases function in the cells. To obtain further experimental evidence that Crenarchaeota may contain multiple DNA polymerases in general, we designed an experiment to investigate the existence of multiple DNA polymerases in Aeropyrum pernix, a hyperthermophilic member of this subdomain, both in vivo and at the gene level. In this report, we demonstrate the presence of two family B DNA polymerases in this organism. In addition, we discuss the biochemical properties of the two groups of family B DNA polymerases found in Archaea, one of which exists only in Crenarchaeota whereas the other exists in Euryarchaeota as well.
MATERIALS AND METHODS
Preparation of cell extracts for enzyme assay.
A. pernix K1 was cultivated as described earlier (26). Ten grams of cells was suspended in buffer A (50 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 10% glycerol, 0.5 mM dithiothreitol) to a volume of 40 ml and disrupted by three passages through a French pressure cell (SLM Aminco, Rochester, N.Y.). Cell debris was removed by centrifugation at 48,000 × g for 30 min at 4°C. The supernatant was dialyzed against buffer A overnight, and 2.5 ml of the dialysate was applied to an anion-exchange column (HiTrap Q; Pharmacia Biotech, Uppsala, Sweden) fitted to a high-pressure liquid chromatograph (AKTA Explorer 10S; Pharmacia Biotech). After being loaded with the lysate, the column was washed with 4 column volumes of equilibration buffer (buffer A), followed by elution with a linear NaCl gradient (0 to 100%) developed with 50 ml of buffer B (buffer A containing 1 M NaCl) at a flow rate of 2 ml/min. The effluents were monitored by absorbance at 280 nm, and fractions of volume 1.5 ml were collected. The contents of each fraction was analyzed for DNA polymerase activity, and the positive fractions were pooled for rechromatography.
DNA polymerase assay.
The DNA polymerase assay was carried out as described previously (15). In brief, the assay mixture contained, in a volume of 30 μl, 20 mM Tris-HCl (pH 8.8), 2 mM MgCl2, 2 mM β-mercaptoethanol, 0.2 μg of activated calf thymus DNA per ml, 40 μM deoxynucleoside triphosphates containing 60 nM [methyl-3H]TTP (Amersham, Buckinghamshire, United Kingdom), and 3 μl of each fraction. Activated DNA was prepared as outlined by Richardson (24), and the amount of radioactivity incorporated into DNA strands was counted by a scintillation counter.
Activity gel analysis.
In situ analysis of DNA polymerase activity to identify the active polypeptides in the peak fractions of the A. pernix cell extracts or recombinant DNA polymerases I and II [Pol I and Pol II] after purification were done as described earlier (12, 31). (The family B DNA polymerase in euryarchaeotes is designated Pol I [the first DNA polymerase discovered in this subdomain] to distinguish it from the euryarchaeal heterodimeric DNA polymerase referred to as Pol II.) The conditions were the same as those described in previous reports (14, 28).
PCR and DNA sequencing.
Genomic DNA from A. pernix K1 was prepared as described by Sako and others (26). Degenerate primers were designed based on two conserved motifs A (SLYPSII) and C (VIYGDTD), which are found in polymerase regions I and II of archaeal family B DNA polymerases (29). The primers were used to amplify, via PCR, an approximately 400-bp fragment from genomic DNA of A. pernix. PCR was performed in a thermal cycler; the thermal profiles involved 30 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 50 s, and extension at 72°C for 50 s. Approximately 20 ng of genomic DNA served as the template in the reaction. All PCR reagents were purchased from a commercial source (TaKaRa Shuzo, Kyoto, Japan), and each was used as described by the manufacturer. The PCR fragment obtained was ligated into a TA cloning vector (pT7 Blue; Novagen, Milwaukee, Wis.), and the product was used in transforming E. coli JM109 cells. The nucleotide sequences of cloned DNA fragments were determined by a capillary sequencer (ABI Prism 310 genetic analyzer; Applied Biosystems, Foster City, Calif.), and the BLAST search program (19a) was used to identify DNA fragments coding for polypeptides with similarities to known archaeal family B DNA polymerases.
Genomic Southern hybridization.
Aliquots of A. pernix genomic DNA (1.2 μg) were independently digested with BamHI, HindIII, PstI, XbaI, and ScaI. The products of digestion were resolved by electrophoresis on 0.7% agarose gel and transferred onto a nylon membrane (Hybond-N+; Amersham). Two different DNA fragments obtained by PCR (ApeI and ApeII; approximately 400 bp each, which translated into amino acid sequences showing high similarity to archaeal family B DNA polymerases) were used as probes for Southern hybridization as described elsewhere (29). A chemiluminescent labeling and detection system (Gene Images; Amersham) was applied in the procedure to visualize signals.
Genomic walking library construction.
The genomic walking library of A. pernix was constructed by using the Universal Genome Walker kit (Clontech Laboratories, Palo Alto, Calif.) as described by the manufacturer. Briefly, the genomic DNA of A. pernix was digested to completion with EcoRV, PvuII, DraI, ScaI, and StuI. Thus, the DNA fragments generated were all blunt ended. The product of each restriction digest was ligated at 16°C to a blunt-ended adapter, which is a double-stranded deoxyoligonucleotide with a known sequence, in a volume of 8 μl. Ligation mixtures were heated at 70°C for 5 min to end the reaction, and the volume was increased to 80 μl. One microliter of each of the five libraries served as a template in the genome walking PCR.
Genomic walking to sequence the entire pol genes.
To obtain the complete nucleotide sequence of the gene which ApeI constituted part of, two primers, ApeI-F1 (5′ CTGAAGTCTCTCAGCATGC-3′) and ApeI-R1 (5′-TATGAAAGTGTATATTAACGCGAG-3′) were designed for PCR to obtain nucleotide sequence upstream and downstream of ApeI, respectively. The primers were combined individually with the adapter primer (AP1; 5′ CCTGTAGTCTATTCCAACCCTC-3′), supplied by the manufacturer, for the PCR amplifications. Five different reactions, each containing an aliquot of a different library as the template, were carried out for ApeI-F1 and likewise ApeI-R1. In the case of ApeII, three primers were used together with the adapter primer for PCR to obtain the entire gene from which it originated. Two of the three primers (ApeII-F1 [5′-ACCTCTCAAGTATTTTCTTGA-3′] and ApeII-F2 [5′-ACAGCCTCCCTATACTCCC-3′]) were used to obtain nucleotide sequence upstream of ApeII. To obtain nucleotide sequence at the downstream region, ApeII-R1 (5′-TTCAGGAAGAGCCCTCCCGGCTTCTTCAA-3′) was used. The complete structural gene (polA) for Pol I was amplified by using ApeI-N (5′-GTTACCATGGCTGGTCCTGCTAAGCCTAAG-3′, NcoI tagged) and ApeI-C (5′-GAATCCATATGTGGTTATCACGAGTCGAAA-3′, NdeI tagged) as forward and reverse primers, respectively (restriction enzyme recognition sequences are underlined). The entire gene (polB) coding for Pol II was amplified with ApeII-N (5′-ATACATATGAGGGGGTCAACCCCCGTTATC-3′, NdeI tagged) as the forward primer and ApeII-C (5′ GATGCGGCCGCATAAGGTACTTCATCCTCCTACACACCC-3′, NotI tagged) as the reverse primer. In all of these PCR amplifications, the thermal profiles involved 30 cycles of denaturation at 95°C for 30 s, annealing temperature of 58°C for 1 min, and extension at 72°C for 2 min 30 s. The PCR fragments were ligated into a TA cloning vector (pT7 Blue; Novagen), and DNA inserts in isolated recombinant plasmids were sequenced in both orientations as described above. To ensure accuracy of PCR, TaKaRa LA Taq (TaKaRa Shuzo) was used for amplifications, and several clones were sequenced independently to correct possible errors.
Cloning and expression of A. pernix polA and polB.
The plasmid containing polA in pT7 Blue was digested with NcoI and NdeI to release polA, while the one harboring polB in pT7 Blue was digested with NdeI and NotI to obtain polB. The polA was inserted into an NcoI/NdeI-digested pET15b (Novagen), and the recombinant plasmid, pAPP1, was used for transformation of Escherichia coli BL21(DE3) cells. The gene was expressed by incubating transformed cells in Luriani-Bertani broth supplemented with ampicillin (100 μg/ml) at 37°C for 18 h. The polB was expressed in a similar fashion except that the expression plasmid, pAPP2, was made by insertion of the gene into the NdeI-NotI digested pET21a (Novagen).
Purification of A. pernix Pol I and Pol II.
Pol I and Pol II were prepared from E. coli BL21(DE3)/pAPP1 and BL21(DE3)/pAPP2, respectively. One-liter cultures of cells harboring the pol genes were harvested by centrifugation at 7,000 × g for 10 min. The cell pellets were suspended in 30 ml of buffer A and lysed by two passages through a French pressure cell. The lysates were each centrifuged at 48,000 × g for 30 min, and the supernatant from each preparation was heated at 80°C for 15 min followed by recentrifugation. The supernatant containing Pol I was applied to a gel filtration column (Superdex 200; Pharmacia) equilibrated with buffer A, followed by elution with the same buffer. Fractions were examined for DNA polymerase activity, and the proteins eliciting activity were identified by the in situ method as described above. In the case of Pol II, after gel filtration, the fractions containing DNA polymerase activity were pooled, dialyzed against buffer A, and applied to an anion-exchange column (HiTrap Q; Pharmacia), and the chromatography was developed with a 0 to 1 M NaCl gradient.
Primer extension analysis.
The primer extension abilities of the purified Pol I and Pol II were demonstrated by using a M13 single-stranded DNA primed with a 5′-labeled oligonucleotide as the substrate (28). To anneal the primer to the template, 1.0 μg of M13 single-stranded DNA and 1.0 pmol of 32P-labeled primer (45 bases in length) in a buffer containing 20 mM Tris-HCl (pH 6.3) and 1.5 mM MgCl2 were heated at 95°C for 3 min to ensure denaturation. The mixture was gently cooled to room temperature. Primer extension was carried out at 68°C. Each reaction mixture at this temperature received 0.05 U of either A. pernix Pol I or Pol II, and the reaction was initiated by adding deoxynucleoside triphosphates (dNTPs) to a concentration of 250 μM. The total volume of the mixture was 20 μl, and aliquots (5 μl) were taken at 1, 2, and 3 min after initiation of the reaction. Each aliquot was dispensed into an Eppendorf tube containing 3 μl of stop solution (98% deionized formamide, 1 mM EDTA, 0.1% xylene cyanol, 0.1% bromphenol blue), and 2.5 μl of each was analyzed by polyacrylamide gel electrophoresis (PAGE) in the presence of 8 M urea. The same amount of Thermus aquaticus (Taq) DNA polymerase (TaKaRa Taq) was used to compare the primer extension abilities. One unit was defined as the amount of enzyme catalyzing the incorporation of 10 nmol of dTMP per 30 min at 70°C into DNA, using the conditions described earlier (15). The amount of Taq DNA polymerase used in this experiment corresponds to 2.5 U according to the manufacturer’s specification.
Computer analysis.
With the exception of A. pernix Pol I and Pol II, all DNA polymerases were retrieved from GenBank. The proteins and their accession numbers are as follows: P. occultum Pol I (B1; D38573) and Pol II (B3; D38574), S. solfataricus B1 (U92875) and B3 (X71597), Sulfurisphaera ohwakuensis B1 (AB008894), Sulfolobus acidocaldarius B1 (U33846), Cenarchaeum symbiosum B1 (AF028831), Thermococcus litoralis Pol I (M47198), P. furiosus Pol I (D12983), and A. fulgidus Pol I (AE001070). Amino acid sequence comparisons were carried out at a web site (7a).
Nucleotide sequence accession numbers.
The nucleotide sequences of A. pernix Pol I and Pol II have been submitted to the DDBJ and assigned accession no. AB017500 and AB017501, respectively.
RESULTS
Detection of DNA polymerase activities in cell extracts of A. pernix.
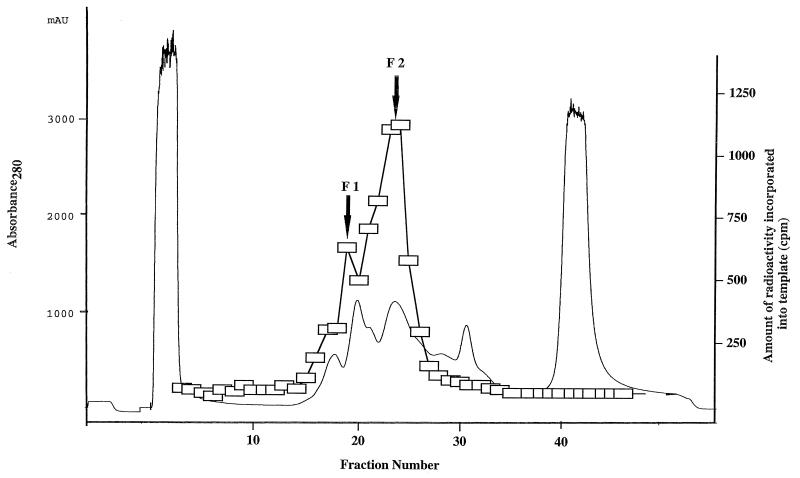
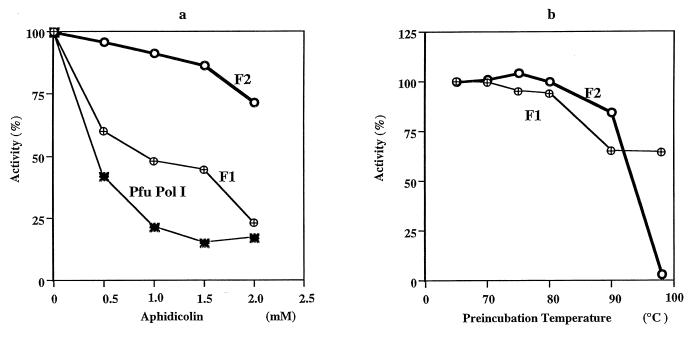
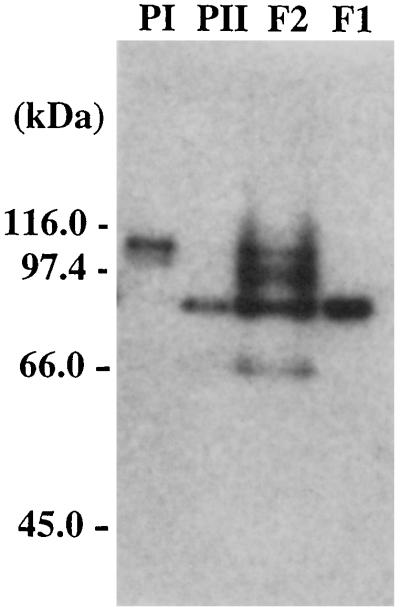
Cell extracts of A. pernix were fractionated by an anion-exchange chromatography, and aliquots from the fractions were analyzed for DNA polymerase activity. Two peaks of activity were identified in the NaCl gradient elution profiles (Fig. 1). The peaks coincided with NaCl concentrations of 12 and 20 mM and will be referred to as fractions I and II, respectively. These two fractions were subjected to rechromatography on the anion-exchange column. To determine if the two DNA polymerase activities originated from different proteins, we investigated their responses to aphidicolin, a tetracyclic diterpenoid. This compound is a specific inhibitor of eucaryal DNA polymerase α and most of the α-like (family B) DNA polymerases (13). Previous results suggest that crenarchaeotes contain aphidicolin-sensitive and aphidicolin-resistant DNA polymerases (10, 17, 25, 29). Therefore, despite being only partially purified, the DNA polymerase activities in fractions I and II were subjected to the aphidicolin sensitivity test. As shown in Fig. 2a, the polymerase activity in fraction II was resistant, whereas the activity in fraction I was sensitive, to the compound to a concentration of 2 mM. The thermostabilities of the proteins responsible for the DNA polymerase activities in fractions I and II were investigated. As shown in Fig. 2b, both activities were stable at a preincubation temperature of 80°C for 30 min. However, while about 65% of the activity in fraction I remained after preincubation at 98°C for 30 min, fraction II lost all activity. Because of using partially purified proteins, we can envisage the possibility of a single DNA polymerase eluting in different fractions due to modifications to the protein or interactions with other proteins. An in situ method for detecting DNA polymerase activity (activity gel analysis) was therefore used to investigate the source of activity in each fraction. After sodium dodecyl sulfate (SDS)-PAGE, a single band with an approximate size of 90 kDa was detected in fraction I. In contrast, four different bands of approximately 105, 98, 90, and 66 kDa were detected in fraction II. The largest size in fraction II and the size of the single band in fraction I corresponded to those of recombinant Pol I and Pol II, respectively, produced after gene cloning as described below (Fig. 3).
FIG. 1.
Chromatographic profile of A. pernix cell extract determined on an anion-exchange column (HiTrap Q) and corresponding DNA polymerase activity. Procedures are described in Materials and Methods —, absorbance at 280 nm monitored throughout the chromatography; □, incorporation of [3H]dTTP into activated DNA.
FIG. 2.
Effects of aphidicolin and preincubation temperature on DNA polymerizing activity. The standard assay mixture including fraction I (F1) or II (F2) was incubated at 70°C for 5 min in the presence of the indicated levels of aphidicolin (a), or the enzyme fractions were incubated at the indicated temperatures for 30 min before the activities were measured under the conditions described above (b). P. furiosus (Pfu) Pol I served as a control.
FIG. 3.
Activity gel analysis of DNA polymerizing activities in fractionated A. pernix cell extracts, fraction I and fraction II, and recombinant Pol I and Pol II. DNA polymerizing activity was detected by in situ incorporation of [α-32P]dCTP as described in Materials and Methods. Proteins in fraction I (F1), fraction II (F2), purified Pol I (PI), and Pol II (PII) were separated by SDS-PAGE, and 32P incorporated into DNA strands in the gel was detected by autoradiography. The molecular masses indicated on the left were derived from a size standard marker (Bio-Rad, Hercules, Calif.).
Cloning of two family B DNA polymerase genes from A. pernix.
Two different gene fragments were amplified by PCR with a set of degenerated primers based on two conserved sequences from family B DNA polymerases. Genomic Southern hybridization analysis using these two fragments as probes suggested that each gene was present as a single copy in the genome (data not shown). We then cloned the entire structural genes as described in Materials and Methods. In the P. occultum study, the two genes and their functional products were named polA and polB (genes) and Pol I and Pol II (products), as the first and second discovered pol genes and DNA polymerases, respectively, in accordance with the custom of bacterial genetics. It was then proposed that crenarchaeal family B DNA polymerases be referred to as B1, B2, and B3 because of the finding of three family B DNA polymerase genes (11). In accordance to this proposed nomenclature, P. occultum Pol I and Pol II correspond to B1 and B3, respectively. The polA gene from A. pernix was composed of 2,769 nucleotides and coded for a protein of 923 amino acid residues with an estimated molecular mass of 105,406 Da (Fig. 4a); polB comprised 2,316 bases and coded for a protein of 772 amino acid residues with an estimated molecular mass of 87,905 Da (Fig. 4b). The amino acid sequences of both proteins contained the conserved motifs for 3′→5′ exonuclease and polymerase activities. As shown in Table 1, at the amino acid sequence level, the product of the polA gene showed the highest identity (47%) to P. occultum Pol I (B1). In addition, its identities to the Pol I (B1) homologs found in other thermophilic crenarchaeotes were above 40%. Despite originating from a psychrophile, the Pol I homolog of C. symbiosum (27) showed 37% identity with the product of A. pernix polA. The product of polB and P. occultum Pol II (B3) exhibited a higher amino acid sequence identity (56%), and both polymerases shared identities ranging from 29 to 40% with family B DNA polymerases found in both Euryarchaeota and Crenarchaeota. Therefore, we named these gene products of A. pernix Pol I and Pol II, as the first and second discovered DNA polymerases in this organism, after confirmation of their DNA polymerase activities (see below). The results of our sequence comparison of the archaeal family B DNA polymerases is consistent with the recent phylogenetic analysis involving archaeal and eucaryal family B DNA polymerases that shows that known crenarchaeal DNA polymerases fall into two groups (9). Group I consists of only crenarchaeal members, while group II comprises members from both crenarchaeotes and euryarchaeotes. A. pernix Pol I (B1) and Pol II (B3) belong to group I and group II, respectively. Among the crenarchaeotes used in this study, only P. occultum and A. pernix are capable of growth at 100°C. Therefore, the high amino acid sequence identities observed between their proteins were not surprising. The G+C contents of A. pernix polA and polB were 49.8 and 57.4%, respectively. These values were distinctly lower than the originally published value of 67% for A. pernix K1 genomic DNA (26). However, G+C content from the total genome sequencing project now in progress in Japan seems to be lower (ca. 56%) (unpublished results). Given this information, the values of polA and polB are reasonable.
FIG. 4.
Nucleotide and deduced amino acid sequences of A. pernix polA (a) and polB (b) genes. The regions used in designing primers for genome walking PCR are indicated by black arrows; grey arrows indicated the primers used in amplifying the structural gene for each polymerase. The regions including the exonuclease motifs (3) and the polymerase motifs (32) are underlined by solid and broken lines, respectively; boldface letters represent putative box A sequences.
TABLE 1.
Distribution of family B DNA polymerases in crenarchaeotes and euryarchaeotes
| Archaeal subdomain | Organism | % identity
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ApeI | PocI | Sso B1 | Soh B1 | Sac B1 | Csy B1 | ApeII | PocII | Sso B3 | Afu | Tli | Pfu | ||
| Crenarchaeota | A. pernix I (B1) | ||||||||||||
| P. occultum I (B1) | 47 | ||||||||||||
| S. solfataricus B1 | 43 | 49 | |||||||||||
| S. ohwakuensis B1 | 43 | 49 | 71 | ||||||||||
| S. acidocaldarius B1 | 41 | 48 | 67 | 75 | |||||||||
| C. symbiosum B1 | 37 | 37 | 36 | 35 | 35 | ||||||||
| A. pernix II (B3) | |||||||||||||
| P. occultum II (B3) | 56 | ||||||||||||
| S. solfataricus B3 | 29 | 31 | |||||||||||
| Euryarchaeota | A. fulgidus Pol I | 38 | 40 | 28 | |||||||||
| T. litoralis Pol I | 33 | 32 | 23 | 35 | |||||||||
| P. furiosus Pol I | 32 | 33 | 23 | 34 | 74 | ||||||||
Expression of A. pernix polA and polB.
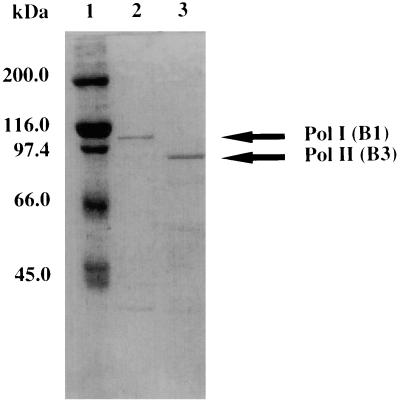
The polA and polB genes were cloned into pET15b and pET21a vectors, respectively. Induction of the expression of these genes with isopropyl-β-d-thiogalactopyranoside (IPTG) in E. coli BL21(DE3) cells yielded very low levels of thermostable DNA polymerase activities. In contrast, constitutive expression (without IPTG) of the genes resulted in a higher protein yield. A two-step purification procedure for Pol I and the three-step purification procedure for Pol II resulted in fairly purified proteins (Fig. 5). The detected protein bands of approximate sizes of 106 and 87 kDa, respectively, in the purified fraction of Pol I and Pol II were confirmed to have DNA polymerase activity by the in situ assay (activity gel analysis) as shown in Fig. 3.
FIG. 5.
Production of A. pernix Pol I and Pol II. Proteins were loaded on an SDS–10% polyacrylamide gel and stained with Coomassie brilliant blue. Lanes: 1, molecular mass markers (sizes are indicated on the left); 2, Pol I; 3, Pol II.
Biochemical properties of A. pernix Pol I and Pol II.
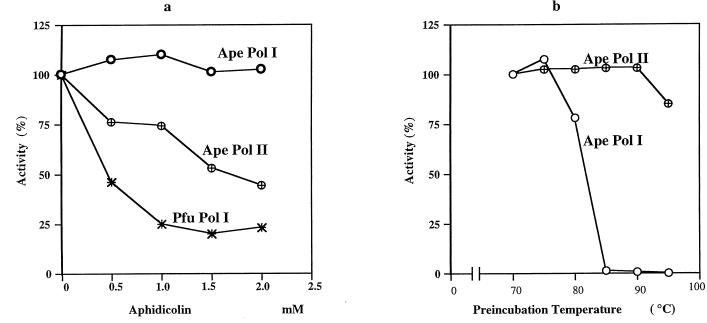
To relate the purified Pol I and Pol II to the DNA polymerase activities observed in the A. pernix cell extract, both proteins from Fig. 5 were subjected to the aphidicolin sensitivity test as described for fractions I and II. As shown in Fig. 6a, Pol II was sensitive to aphidicolin, while Pol I was completely resistant to the compound at the concentrations tested. In the thermostability test, Pol II was found to be more thermotolerant than Pol I (Fig. 6b). The sizes and properties of A. pernix Pol I and Pol II corresponded well with those of the proteins responsible for the DNA polymerase activity in fractions II and I, respectively, of the cell extract. Thus, we may conclude that in cloning polA and polB, we isolated the genes responsible for the activities in the two fractions of the A. pernix cell extract. This hypothesis may need confirmation by N-terminal sequencing of the native proteins.
FIG. 6.
Effects of aphidicolin and preincubation temperature on DNA polymerizing activity of A. pernix Pol I and Pol II. Aliquots of purified A. pernix (Ape) Pol I or Pol II were incubated at 70°C for 5 min in the presence of the indicated levels of aphidicolin (a), or the enzyme fractions were incubated at indicated temperatures for 30 min before the activities were measured under the conditions described above (b). P. furiosus (Pfu) Pol I served as a control.
We compared the primer extension abilities of A. pernix Pol I and Pol II with that of Taq DNA polymerase. Similar to other family B DNA polymerases, both Pol I and Pol II exhibited far less in vitro primer elongation ability compared with Taq polymerase, a member of family A. Pol I seemed to have a stronger ability than Pol II, in agreement with the case of P. occultum (29).
DISCUSSION
A complete understanding of DNA replication in Archaea requires identification of the major proteins involved in the process, and of cardinal importance are the DNA polymerases. While previous experiments used either gene products expressed in E. coli or cell extract to provide the evidence for the existence of different kinds of DNA polymerases in Crenarchaeota, in the present study both methods were used to demonstrate that a crenarchaeote, A. pernix, contains multiple DNA polymerases. A. pernix Pol I (B1) is aphidicolin resistant and heat labile above 75°C, and Pol II (B3) is aphidicolin sensitive and more thermostable. The results corroborated the findings from P. occultum (29). Hence, the crenarchaeal Pol I (B1) and Pol II (B3) homologs may represent the aphidicolin-resistant and aphidicolin-sensitive DNA polymerases detected previously in other crenarchaeal cells.
In the in situ DNA polymerase activity analysis with the fractionated cell extract (fraction II), three smaller bands corresponding to 98-, 90-, and 65-kDa proteins were detected in the autoradiogram in addition to the band corresponding to Pol I (105 kDa). These proteins may represent the degradation products of Pol I, or they may be entirely different DNA polymerases. The band of 90 kDa seems to be from unseparated Pol II. Some contamination of Pol II in fraction II can be predicted from the observations that to compare with purified Pol I (Fig. 6), the activity from fraction II was less aphidicolin resistant and more heat stable (Fig. 2). The properties of purified Pol I and fraction II may be comparable when some of aphidicolin-sensitive and heat-stable activity is subtracted from the total activity of fraction II. It should be noted here that Pol II is very stable, and it always gave a band signal much stronger than that of the same amount of Pol I in the activity gel analysis. Edgell and coworkers (9) have suggested the existence of three family B DNA polymerase in S. solfataricus P2 from their work and previous literature (25). Therefore, it would be noteworthy to detect a third DNA polymerase from A. pernix. The size of the expected third DNA polymerase in S. solfataricus is around 74 kDa from the nucleotide sequence of the third gene (23). The sizes of the bands observed in our experiment are somewhat different from this size. From the assay profile in the anion-exchange chromatography shown in Fig. 1, another activity may exist in fraction 21. However, in situ activity gel analysis using fractions around fraction II (e.g., fraction 23) gave basically the same band signals as in Fig. 3 (data not shown). The existence of a third DNA polymerase could not be confirmed due to insufficient evidence. Further experiments are necessary to answer this interesting question.
We have attempted to isolate from A. pernix and other crenarchaeotes the genes that code for the heterodimeric DNA polymerase found thus far in the euryarchaeotes (7), with consistently negative results. It is our hypothesis that the heterodimeric DNA polymerase occurs only in Euryarchaeota. We propose here that in the archaeal domain, the crenarchaeotes at least have two family B DNA polymerases, while the euryarchaeotes have one (Table 1). Instead, the euryarchaeotes have, in addition to their family B DNA polymerase, a heterodimeric DNA polymerase. It will be interesting to know the biological roles of each DNA polymerase in Archaea, especially why the forms of DNA polymerases in the cells are so different between Euryarchaeota and Crenarchaeota.
Even though their amino acid sequences are typically that of family B DNA polymerases, comparison of the structure-function relationship of DNA polymerases Pol I and Pol II in Crenarchaeota will be of further interest, and the results are likely to contribute to our understanding of the basic mechanism of DNA synthesis in these organisms. In vitro primer extension abilities of Pol I and Pol II by themselves were obviously lower than that of Taq DNA polymerase (Fig. 7). The low processivity of the archaeal family B DNA polymerases is known (20). In Eucarya, proliferating cell nuclear antigen (PCNA) functions as an accessory factor to enhance the processivity of DNA polymerase δ (and ɛ), the replicative DNA polymerase, severalfold (2). The PCNA homologs in Archaea may stimulate the processivity of these DNA polymerases in vivo as observed in eucaryal cells. We confirmed the functional interaction between P. furiosus Pol I (B3) and its PCNA homologs in vitro (5). Furthermore, we have found two putative homologs of PCNA from A. pernix and cloned them (6). There is only one PCNA homolog each in the euryarchaeal genomes. It is possible that each DNA polymerase in A. pernix has a specific PCNA as the partner for elongation reaction, and the next step is to investigate the effect of each homolog on Pol I and Pol II. The results will help to clarify the role of each DNA polymerase in the process of DNA replication in Crenarchaeota.
FIG. 7.
Chain elongation ability of A. pernix Pol I and Pol II. A 32P-labeled primer annealed to M13 DNA served as the template. Aliquots (5 μl) of reaction mixture were sampled at 1, 2, and 3 min after initiation of reaction, and their products were resolved on an 8% polyacrylamide gel containing 8 M urea. Taq DNA polymerase was used as a control.
ADDENDUM IN PROOF
The complete genome sequence of Aeropyrum pernix has been published (Y. Kawarabayasi et al., DNA Res. 6:83–101, 1999).
REFERENCES
- 1.Barns S M, Delwiche J D, Palmer J D, Pace N R. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer G A, Burgers P M J. The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase delta. Proc Natl Acad Sci USA. 1988;85:7506–7510. doi: 10.1073/pnas.85.20.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernad A, Blanco L, Lazaro J M, Martin G, Salas M. A conserved 3′→5′ exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989;59:219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- 4.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J-F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Presley E A, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Hurst M A, Roberts K M, Kaine B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 5.Cann, I. K. O., S. Ishino, I. Hayashi, K. Komori, H. Toh, K. Morikawa, and Y. Ishino. Unpublished data.
- 6.Cann, I. K. O., S. Ishino, Y. Kawarabayashi, H. Kikuchi, Y. Sako, and Y. Ishino. Unpublished data.
- 7.Cann I K O, Komori K, Toh H, Kanai S, Ishino Y. A heterodimeric DNA polymerase: evidence that members of euryarchaeota possess a distinct DNA polymerase. Proc Natl Acad Sci USA. 1998;95:14250–14255. doi: 10.1073/pnas.95.24.14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.CLUSTALW. revision date. [Online.] 5 February 1998. http://www.genome.ad.jp/SIT/CLUSTALW.html http://www.genome.ad.jp/SIT/CLUSTALW.html. [15 March 1999, last date accessed.] . [15 March 1999, last date accessed.] [Google Scholar]
- 8.Edgell D R, Doolittle W F. Archaebacterial genomics: the complete genome sequence of Methanococcus jannaschii. Bioessays. 1996;19:1–4. [Google Scholar]
- 9.Edgell D R, Klenk H-P, Doolittle W F. Gene duplications in evolution of archaeal family B DNA polymerases. J Bacteriol. 1997;179:2632–2640. doi: 10.1128/jb.179.8.2632-2640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elie C, De Rocondo A M, Forterre P. Thermostable DNA polymerase from the archaebacterium Sulfolobus acidocaldarius. Eur J Biochem. 1989;178:619–626. doi: 10.1111/j.1432-1033.1989.tb14490.x. [DOI] [PubMed] [Google Scholar]
- 11.Gray M W. The third form of life. Nature. 1996;383:299. doi: 10.1038/383299a0. [DOI] [PubMed] [Google Scholar]
- 12.Hamatake R K, Hasegawa H, Clark A B, Bebenek K, Kunkel T A, Sugino A. Purification and characterization of DNA polymerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;265:4072–4083. [PubMed] [Google Scholar]
- 13.Huberman J A. New view of the biochemistry of eukaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase α. Cell. 1981;23:647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- 14.Imamura M, Uemori T, Kato I, Ishino Y. A non-α-like DNA polymerase from the hyperthermophilic archaeon Pyrococcus furiosus. Biol Pharm Bull. 1995;18:1647–1652. doi: 10.1248/bpb.18.1647. [DOI] [PubMed] [Google Scholar]
- 15.Ishino Y, Komori K, Cann I K O, Koga Y. A novel DNA polymerase family found in Archaea. J Bacteriol. 1998;180:2232–2236. doi: 10.1128/jb.180.8.2232-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Yoshizawa T, Nakamura Y, Robb F T, Horikoshi K, Masuchi Y, Shizuya H, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 17.Klimczak L J, Grummt F, Burger K J. Purification and characterization of DNA polymerase from the archaeabacterium Sulfolobus acidocaldarius. Nucleic Acids Res. 1985;13:5269–5281. doi: 10.1093/nar/13.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg A, Baker T A. DNA replication. 2nd ed. New York, N.Y: W. H. Freeman and Co.; 1992. [Google Scholar]
- 19.Morell V. Life’s last domain. Science. 1996;273:1043–1045. doi: 10.1126/science.273.5278.1043. [DOI] [PubMed] [Google Scholar]
- 19a.National Center for Biotechnology Information. revision date. BLAST. [Online.] 13 April 1999. http://www.ncbi.nlm.nih.gov/ http://www.ncbi.nlm.nih.gov/. National Center for Biotechnology Information, National Institutes of Health, Bethesda, Md. [1 March 1999, last date accessed.] . National Center for Biotechnology Information, National Institutes of Health, Bethesda, Md. [1 March 1999, last date accessed.] [Google Scholar]
- 20.Perler F B, Kumar S, Kong H. Thermostable DNA polymerases. Adv Protein Chem. 1996;48:377–435. doi: 10.1016/s0065-3233(08)60367-8. [DOI] [PubMed] [Google Scholar]
- 21.Pisani F, De Martino C, Rossi M. A DNA polymerase from the archaeon Sulfolobus solfataricus shows sequence similarity to family B DNA polymerases. Nucleic Acids Res. 1992;20:2711–2716. doi: 10.1093/nar/20.11.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisani F, Rossi M. Evidence that an archaeal α-like DNA polymerase has a molecular organization of its associated catalytic activities. J Biol Chem. 1994;269:7887–7892. [PubMed] [Google Scholar]
- 23.Prangishvili D, Klenk H-P. The gene for a 74 kDa DNA polymerase from the archaeon Sulfolobus solfataricus. Syst Appl Microbiol. 1994;16:665–671. doi: 10.1093/nar/21.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson C C. DNA polymerase from Escherichia coli. In: Cantoni G L, Davies D R, editors. Procedures in nucleic acid research. New York, N.Y: Harper & Row; 1966. pp. 263–276. [Google Scholar]
- 25.Rossi M, Rella R, Pensa M, Bartolucci S, De Rosa M, Gambacorta A, Raia C A, Dell’Aversano Orabona N. Structure and properties of a thermophilic and thermostable DNA polymerase isolated from Sulfolobus solfataricus. Syst Appl Microbiol. 1986;7:337–341. [Google Scholar]
- 26.Sako Y, Nomura N, Uchida A, Ishida Y, Morii H, Koga Y, Hoaki T, Maruyama T. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int J Syst Bacteriol. 1996;46:1070–1077. doi: 10.1099/00207713-46-4-1070. [DOI] [PubMed] [Google Scholar]
- 27.Schleper C, Swanson R V, Mathur E J, Delong E F. Characterization of a DNA polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1997;179:7803–7811. doi: 10.1128/jb.179.24.7803-7811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uemori T, Sato Y, Kato I, Doi H, Ishino Y. A novel DNA polymerase in the hyperthermophilic archaeon, Pyrococcus furiosus: gene cloning, expression, and characterization. Genes Cells. 1997;2:499–512. doi: 10.1046/j.1365-2443.1997.1380336.x. [DOI] [PubMed] [Google Scholar]
- 29.Uemori T, Ishino Y, Doi H, Kato I. The hyperthermophilic archaeon Pyrodictium occultum has two α-like DNA polymerases. J Bacteriol. 1995;177:2164–2177. doi: 10.1128/jb.177.8.2164-2177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemori T, Ishino Y, Toh H, Asada K, Kato I. Organization and nucleotide sequence of the DNA polymerase gene from the archaeon Pyrococcus furiosus. Nucleic Acids Res. 1993;21:259–265. doi: 10.1093/nar/21.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wernette C M, Kaguni L S. A mitochondrial DNA polymerase from embryos of Drosophila melanogaster. J Biol Chem. 1986;261:14764–14770. [PubMed] [Google Scholar]
- 32.Wong S W, Wahl A F, Yuan P-M, Arai N, Pearson B E, Arai K-L, Korn D, Hunkapiller M W, Wang T S-F. Human DNA polymerase a gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988;7:37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]