Summary
Early weaning usually causes small intestine epithelial development abnormality, increasing the risk of gastrointestinal diseases. Glutamine (Gln), enriching in plasma and milk, is widely reported to benefit intestinal health. However, whether Gln affects intestinal stem cell (ISC) activity in response to early weaning is unclear. Here, both the early weaning mice and intestinal organoids were used to study the role of Gln in regulating ISC activities. Results showed that Gln ameliorated early weaning-induced epithelial atrophy and augmented the ISC-mediated epithelial regeneration. Gln deprivation disabled ISC-mediated epithelial regeneration and crypt fission in vitro. Mechanistically, Gln augmented WNT signaling in a dose-dependent manner to regulate ISC activity, while WNT signaling blockage abolished the effects of Gln on ISCs. Together, Gln accelerates stem cell-mediated intestinal epithelial development associated with the augmentation of WNT signaling, which provides novel insights into the mechanism by which Gln promotes intestinal health.
Keywords: glutamine, intestinal stem cell, early weaning, intestinal epithelial development, WNT signaling
Graphical abstract
Highlights
-
•
A novel early weaning mice model that undergoes growth retardation was established
-
•
Gln ameliorates early weaning-induced gut atrophy and growth retardation
-
•
Gln is essential for ISC self-renewal and epithelial differentiation in vitro
-
•
Gln augments Wnt to facilitate ISC-mediated intestinal epithelial development
Early weaning usually disrupts small intestinal epithelial development in humans and livestock. In this article, using early weaning mice and organoid models, Yao, Jiang et al. demonstrate that glutamine is essential for stem cell-mediated intestinal epithelial regeneration and boosts intestinal stem cell activity associated with the augmentation of WNT signaling, thereby improving small intestinal epithelial development during early weaning.
Introduction
Early weaning is a significant physiologic event that influences the development of the small intestine in humans and livestock. Although the World Health Organization recommends that infants be exclusively breastfed before the age of 6 months after birth and continue breastfeeding until 24 months, a proportion of young mothers have to apply early weaning to their babies because of insufficient milk supply, social stress, and work burdens (Maviso et al., 2022; Victora et al., 2016). Studies have shown that early weaning practice increases the risk of gastrointestinal diseases, such as diarrhea, gastroenteritis, necrotizing enterocolitis, and infectious diseases (Hall et al., 2020; Rollins et al., 2016). In parallel, to improve the sow’s productivity in current swine husbandry, piglets are usually early weaned at age 3 or 4 weeks (Verdile et al., 2019), which usually causes small intestinal epithelial dysplasia and further leads to dysfunction in barrier and absorption (Lalles and Montoya, 2021). As a result, early weaning piglets commonly experience poor growth performance and an increase in diarrhea and mortality (Wei et al., 2021). Accumulated evidence suggests nutritional intervention is an effective and safe strategy to alleviate early weaning-induced small intestinal epithelial dysplasia (Wei et al., 2021).
Glutamine (Gln), a functional amino acid that is normally enriched in mammalian serum and milk, is known as a preferred energy substrate for intestinal epithelial cells, which in turn participate in many key metabolic processes including nucleotide and protein biosynthesis (Deters and Saleem, 2021; Wu, 2010). Small intestinal epithelium not only catabolizes most of the Gln in the enteral diet but also takes up a large amount of Gln from the arterial blood (Jang et al., 2019; Reeds and Burrin, 2000). Interestingly, Gln is the most abundant free amino acid in milk, suggesting that Gln may contribute to intestinal health and development during lactation (Wu et al., 1996). Consistently, it has been reported that Gln supplementation can alleviate the small intestinal epithelial morphology damages, and improve growth performance in early weaning piglets (Ma et al., 2021; Wu et al., 1996). However, whether Gln supplementation contributes to small intestinal epithelial development in response to early weaning is still unclear.
Small intestinal epithelium undergoes rapid renewal, with one regeneration every 2–5 days, and acts as a dynamic barrier against the external environment (Smith et al., 1984). Small intestinal epithelial development is driven by intestinal stem cells (ISCs) (Sato et al., 2009). ISCs are located in crypt regions and asymmetrically differentiate into one ISC and one transit-amplifying cell. The latter further differentiates into mature functional cells such as enterocytes, Paneth cells, enteroendocrine cells, and goblet cells. These mature intestinal epithelial cells, except for Paneth cells that stay in the crypt, further migrate up to villi and eventually die programmatically (van der Flier and Clevers, 2009). Specifically, previous studies suggest that nutritional level, early life stress, gut microbe, and aging factors can affect intestinal epithelial homeostasis by regulating ISC function (Alonso and Yilmaz, 2018; Pentinmikko et al., 2019; Wong et al., 2019). Recent studies have shown that Gln modulates macrophage polarization and regulates the pluripotency in human embryonic stem cells (Liu et al., 2017; Vardhana et al., 2019; Vigeland et al., 2021). These findings raise the question of whether Gln regulates ISC function to facilitate intestinal epithelial homeostasis during early weaning.
WNT/β-catenin signaling plays an indispensable role in ISC activities (Clevers and Nusse, 2012; Guillermin et al., 2021; Zou et al., 2018). The activity of WNT signaling is not only regulated by its ligands, inhibitors, and amplifiers secreted by intestinal epithelium or mesenchyme but also affected by other factors such as amino acid metabolism, microbial metabolites, nerve growth factor, and inflammatory cytokines (Frick et al., 2021; Lee et al., 2018; Wong et al., 2019; Zha et al., 2019). Recently, WNT/β-catenin signaling has been reported to regulate aging, early life stress, and pathogen infection-interfered ISC function (Nalapareddy et al., 2022; Pentinmikko et al., 2019; Wong et al., 2019).
Here, we establish an excellent early weaning mice model and sought to determine how Gln regulates ISC activities to mediate intestinal development in vivo, ex vivo, and in vitro. We discovered that Gln supplementation not only acts on mature enterocytes but also augments the activity of ISCs to accelerate intestinal epithelial regeneration, which is associated with the enhancement of WNT signaling. Our findings disclose a mechanism by which Gln promotes intestinal epithelial development during early weaning, which may provide a basal theory for the application of Gln in infant and young domestic animal nutrients.
Results
Ala-Gln supplementation ameliorates intestinal epithelial development and growth retardation of early weaning mice
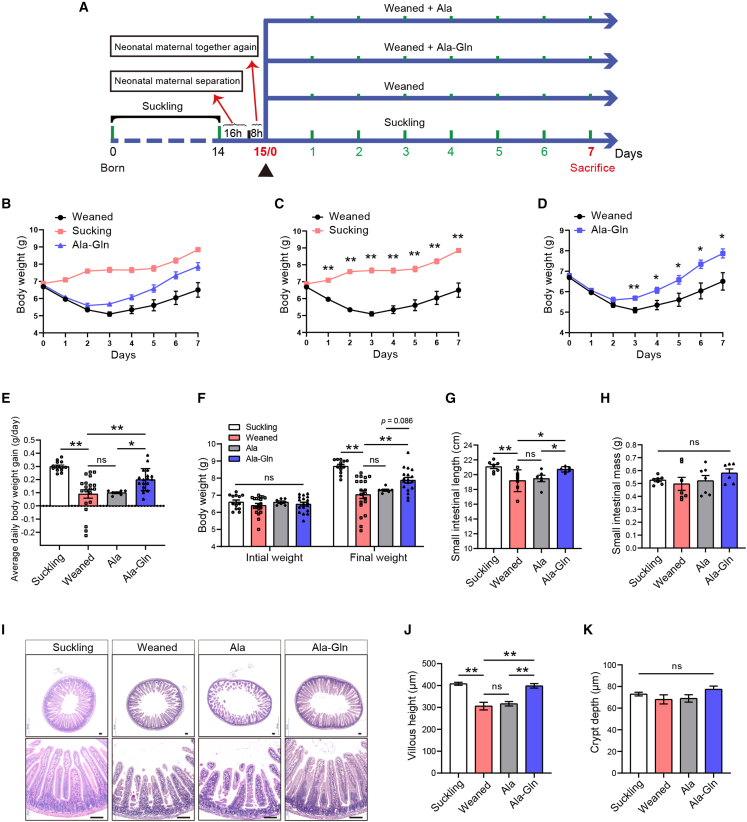
Firstly, we established the early weaning stressed mice model as shown in Figure 1A. Our results showed that early weaning leads to growth retardation and intestinal epithelial atrophy in the first week (Figures 1B–1D and 1I). Therefore, in our experiment, all mice were early weaned at age 15 days to establish an early weaning model. Compared with the weaned group, the average daily body weight gain, final weight, and small intestinal length of mice in the Ala-Gln group were significantly increased (Figures 1E–1G), while small intestinal mass and average daily food and water intake of mice in the Ala-Gln group were numerically increased (Figures 1H, S1A, and S1B). However, there was no difference in the average daily body weight gain, final weight, small intestinal length/mass, or average daily food/water intake between the weaned group and the Ala group (Figures 1E–1H, S1A, and S1B). The results of jejunum H&E staining showed that early weaning decreased the villus height of the jejunum. Ala-Gln supplementation effectively ameliorated this decrease in villus height induced by early weaning, whereas there was no difference in villus height between the weaned group and the Ala group (Figures 1I and 1J). There was no significant difference in the crypt depth among the groups (Figure 1K). Collectively, we successfully established an early weaning model in mice, while Ala-Gln supplementation effectively ameliorated early weaning-induced intestinal atrophy and growth retardation.
Figure 1.
Ala-Gln supplementation alleviates early weaning-induced growth retardation and intestinal morphology damage
(A) Experimental procedure.
(B–D) The average weight of suckling and post-weaning mice was monitored during the experiment (n = 5–8 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; t test, results of 3–4 independent experiments).
(E and F) The average daily body weight gain (E), initial weight, and final weight (F) were measured during the experiment (n = 7 to 20 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(G and H) The small intestinal length (G) and small intestinal mass (H) were determined (n = 6–7 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(I) Representative images of H&E staining of jejunum in mice on day 7 post-weaning are shown. Scale bars, 100 μm.
(J and K) The villous height (J) and crypt depth (K) in mice were measured on day 7 post-weaning (n = 8 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
Ala-Gln supplementation ameliorates early weaning-induced plasma Gln deficiency
To explore whether plasma Gln is relatively deficient in early weaning mice, we compared the plasma amino acid profile of weaned and unweaned mice from the same litter on day 4 post-weaning. The results showed that early weaning significantly decreased the concentration of plasma Gln in early weaned mice (Figure S2). Next, to evaluate whether Ala-Gln supplementation can alleviate early weaning-induced Gln deficiency, the profile of plasma amino acids was detected on day 7 post-weaning without fasting or water prohibition. Concentrations of plasma Gln in the Ala-Gln group were 19% greater than those in the weaned group (Figure S3). Meanwhile, the Ala-Gln group had 72% higher concentrations of plasma Ala than that in the weaned group, indicating that Ala-Gln in drinking water can be effectively absorbed by weaned mice (Figure S3). These results demonstrate that Ala-Gln supplementation alleviates the Gln deficiency in weaned mice caused by early weaning.
Ala-Gln supplementation ameliorates the proliferation and self-renewal of ISCs during early weaning
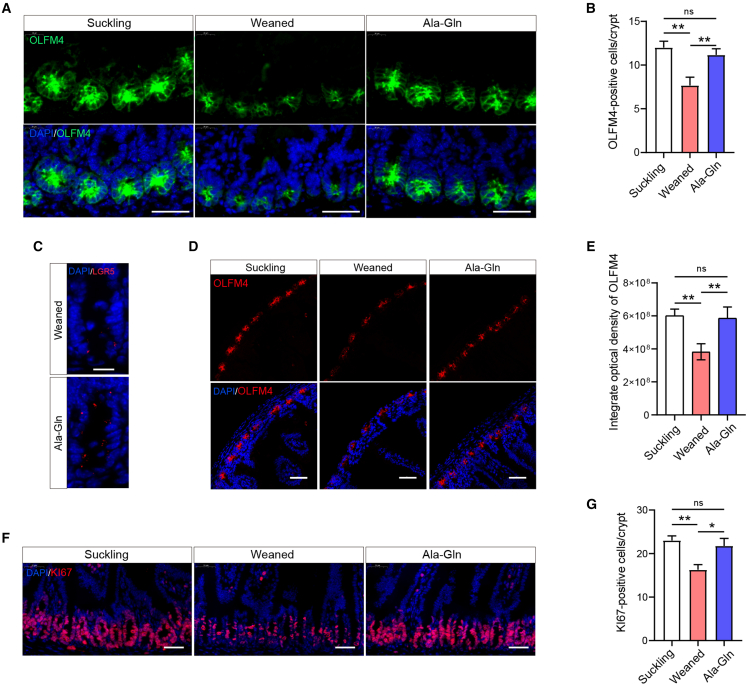
To address whether Ala-Gln supplementation affects the frequency of ISCs during early weaning, we performed immunofluorescence using ISC-specific markers LGR5 and OLFACTOMEDIN-4 (OLFM4, an LGR5 co-expressed protein) in the mice jejunum. Ala-Gln supplementation reversed early weaning-induced decrease of OLFM4-positive ISCs (Figures 2A–2C). We next analyzed the integrated density of OLFM4-positive fluorescence in low-magnification images, which showed that Ala-Gln supplementation alleviated the decrease in the protein level of OLFM4 induced by early weaning (Figures 2D and 2E). To investigate the effect of Ala-Gln supplementation on the ISC proliferation in response to early weaning, we performed immunofluorescence analysis for KI67, a marker to label proliferating cells. Ala-Gln supplementation effectively alleviated the decrease in KI67-positive crypt base columnar cells caused by early weaning (Figures 2F and 2G). These data indicate that Ala-Gln supplementation ameliorates the inhibition of ISC self-renewal caused by early weaning.
Figure 2.
Ala-Gln supplementation increases the number and proliferation rate of ISCs in vivo
(A) Intestinal stem cells labeled with OLFM4 (green) in the section of the proximal jejunum. Scale bars, 40 μm.
(B) The number of ISC were quantified (n = 6 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(C) Intestinal stem cells were labeled with LGR5 (red) in the section of the proximal jejunum. Scale bar, 50 μm.
(D) Representative immunofluorescence images of ISC stained with OLFM4 (red) and DAPI (blue) are shown in the section of the proximal jejunum. Scale bars, 50 μm.
(E) Quantification of integrate optical density (n = 6 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(F) Representative images of crypt base columnar cells of immunofluorescence stained with KI67 (red) and DAPI (blue). Scale bars, 50 μm.
(G) Quantification of KI67-positive crypt base columnar cells (n = 6 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
Enteral Ala-Gln supplementation confers ex vivo ISCs better capability to form enteroids
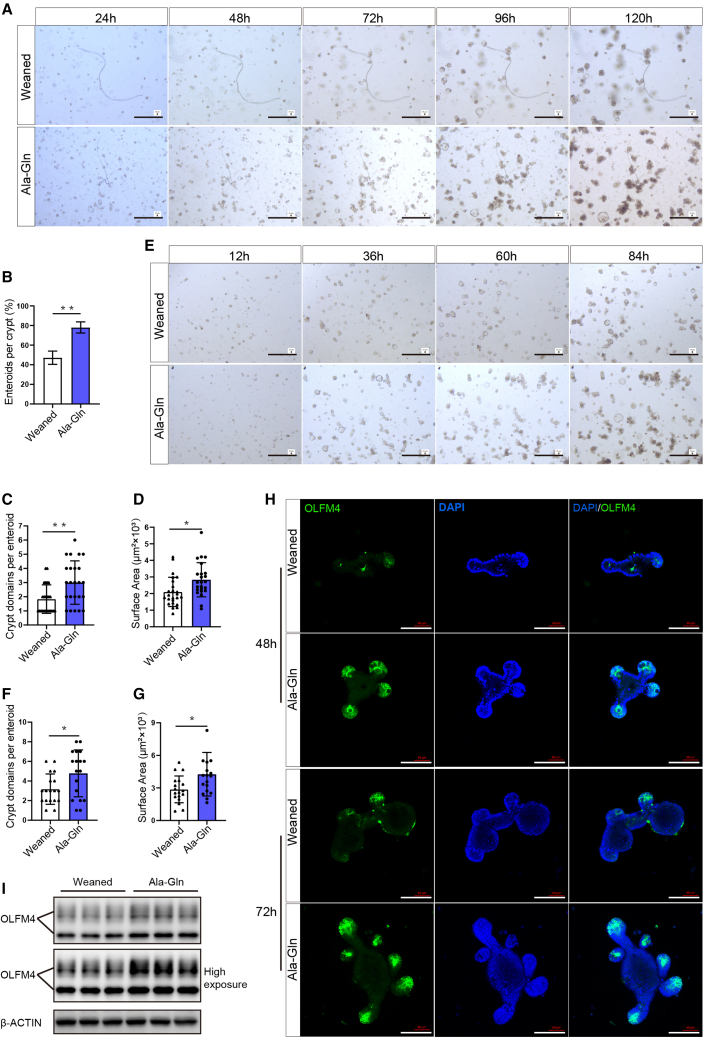
Given that Ala-Gln supplementation increases ISC self-renewal activity in vivo, we ask whether it also boosts the ISC-mediated regeneration of small intestinal epithelium. The small intestinal organoid (enteroid)-forming ability of the isolated crypts from jejunum, which is a proxy for ISC function, was examined ex vivo. We found that the ex vivo crypts isolated from the Ala-Gln group exhibit better enteroid-forming capability (Figures 3A and 3B). Furthermore, enteroids differentiated from the crypts of the Ala-Gln group had more structure domains and bigger sizes than these of weaned group (Figures 3A, 3C, and 3D), indicating a better epithelial regeneration ability and a higher crypt fission rate in the Ala-Gln group. Next, we performed OLFM4 immunostaining to compare ISC quantity in these groups. As shown in Figure 3H, enteroids differentiated from the crypts of the Ala-Gln group had more OLFM4-positive ISCs when compared with the weaned group. Western blot data further showed that Ala-Gln supplementation increased the protein levels of OLFM4 in primary enteroids (Figure 3I). Interestingly, when sub-cloned, secondary enteroids from the Ala-Gln group also exhibited a better regeneration capacity than those from the weaned group (Figures 3E–3G). These data indicate that enteral Ala-Gln supplementation confers ex vivo ISC better capability to form enteroids and support intestinal development.
Figure 3.
Enteral Ala-Gln supplementation augments the activity of ex vivo ISCs to form enteroids
(A) Representative images of the morphology of primary enteroids expanded from crypt cells at 24, 48, 72, 96, and 120 h are shown. Scale bars, 200 μm.
(B) Enteroid-forming capacity of mice jejunum crypts (n = 16, 16 wells of separated crypts from 4 independent mice per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; t test, results of 3 independent experiments).
(C and D) Quantification of crypt domains (C) and surface area (D) per primary enteroids at 96 h (n = 24, 24 enteroids from 4 independent mice per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; t test, results of 3 independent experiments).
(E) Representative images of the morphology of secondary enteroids expanded from crypt cells at 12, 36, 60, and 84 h are shown. Scale bars, 200 μm.
(F and G) Quantification of crypt domains (F) and surface area (G) per secondary enteroid (n = 18, 18 enteroids from 3 independent mice per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; t test, results of 3 independent experiments).
(H) Representative images of primary enteroids of immunofluorescence stained with OLFM4 (green) and DAPI (blue) are shown. Scale bars, 100 μm.
(I) Western blot analysis of intestinal stem cells marker OLFM4 protein expression in primary enteroids (n = 3, primary enteroids from 3 independent mice per condition, results of 3 independent experiments).
Gln deprivation disables the function of ISCs ex vivo and in vitro
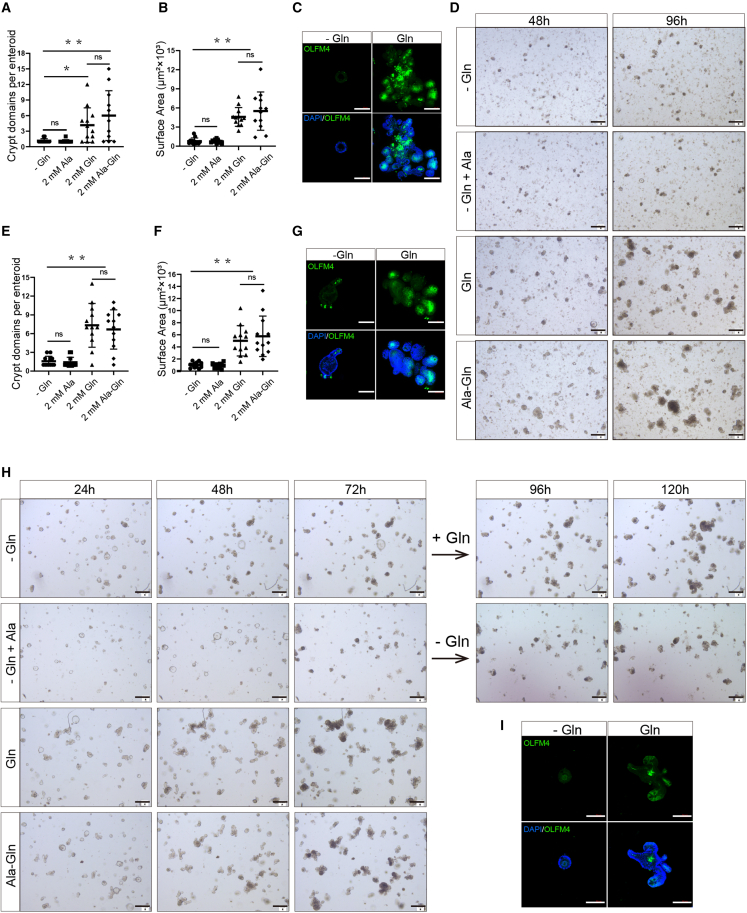
To verify the function of Gln in ISCs, we cultured primary enteroids in media containing 0 mM Gln, 2 mM Ala, 2 mM Ala-Gln, or 2 mM Gln. We found that Gln deprivation significantly reduced the size and crypt domains of enteroids (Figures 4A, 4B, and 4D). However, there was no difference between 2 mM Ala-Gln and 2 mM Gln groups, or between 0 mM Gln and 2 mM Ala groups (Figures 4A, 4B, and 4D). These data suggested that Ala does not affect the functions of ISCs, while the Ala-Gln acts as an efficient substitute for Gln in the enteroid medium. To assess the effects of Gln deprivation on ISC proliferation, we performed immunofluorescence for OLFM4 and found that Gln deprivation inhibited the expression of ISC marker protein (Figure 4C). To investigate the effects of Gln deprivation on the function of ISCs in vitro, we sub-cultured enteroids (more than 50 time passages from the primary crypts) in the medium containing 0 mM Gln, 2 mM Ala, 2 mM Ala-Gln, or 2 mM Gln. Consistent with the results ex vivo, the in vitro results show that Gln deprivation significantly decreased enteroid size and crypt domains (Figures 4E, 4F, and 4H), indicating an essential role of Gln in crypt fission. We also found that Gln deprivation inhibited ISC marker protein expression in the sub-cultured (in vitro) enteroids (Figure 4G). Interestingly, the re-supplementation of Gln was able to rescue the enteroid growth defects in the Gln deprivation condition (Figure 4H). Instead, prolonged Gln deprivation for more than 96 h led to the atrophy of enteroids (Figure 4I). Taken together, these results suggest that Gln is essential for ISC function both ex vivo and in vitro.
Figure 4.
Gln deprivation disables the functions of ISCs ex vivo and in vitro
(A and B) Quantification of crypt domains (A) and surface area (B) per primary enteroids cultured in Gln-free medium supplemented with 2 mM Ala, 2 mM Gln, or 2 mM Ala-Gln were measured at 96 h (n = 12, 12 primary enteroids from 3 independent mice per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(C) Representative images of primary enteroids of immunofluorescence stained with OLFM4 (green) and DAPI (blue) at 96 h. Scale bars, 100 μm.
(D) Representative of images of primary enteroids at 48 and 96 h. Scale bars, 100 μm.
(E and F) Quantification of crypt domains (E) and surface area (F) of the stable-passaged enteroids in Gln-free medium supplemented with 2 mM Ala, 2 mM Gln, or 2 mM Ala-Gln were measured at 96 h (n = 12, 12 enteroids per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 4 independent experiments).
(G) Representative images of enteroids immunofluorescence stained with OLFM4 (green) and DAPI (blue) at 96 h. Scale bars, 100 μm.
(H) Representative bright-field images of the 50 more time-passaged enteroids in medium containing 0 mM Gln, 2 mM Gln, 2 mM Ala-Gln, or 2 mM Ala for 72 h, and the enteroids that were growing in 0 mM Gln medium were switched to 2 mM Gln supplemented medium for an additional 48 h culture. Scale bars, 100 μm.
(I) Representative images of the in vitro enteroids immunofluorescence stained with OLFM4 (green) and DAPI (blue) at 120 h. Scale bars, 100 μm.
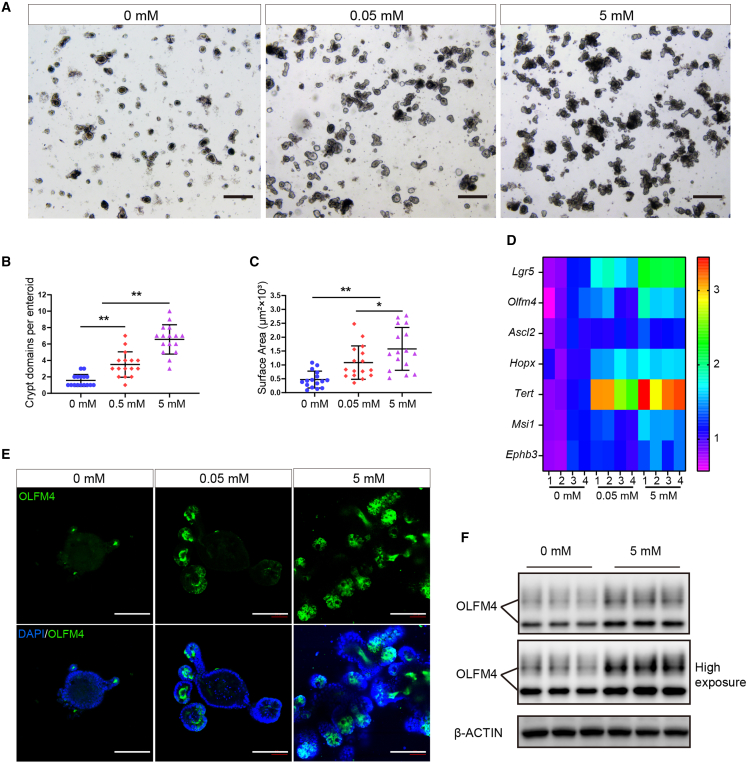
Gln supplementation augments ISC stemness in vitro
To investigate the effects of Gln on the stemness of ISCs in vitro, we cultured in vitro enteroids (more than 50 passages from the primary crypts) in medium containing 0 mM Gln, 0.05 mM Gln, or 5 mM Gln, respectively. We found that Gln supplementation increased crypt domains and surface area of enteroids in a dose-dependent manner (Figures 5A–5C). In addition, qRT-PCR results showed that Gln promotes a subset of ISC marker gene expression in enteroids in a dose-dependent manner (Figure 5D). Moreover, OLFM4 immunostaining showed that Gln supplementation elevates the number of OLFM4-positive ISCs in enteroids in a similar pattern (Figure 5E). Western blot further confirmed that 5 mM Gln supplementation increased OLFM4 expression in the enteroids when compared with the control (Figure 5F). These results suggest that Gln supplementation promotes ISC stemness in vitro.
Figure 5.
Gln supplementation augments the activity of ISCs in vitro
(A). Representative bright-field images of the stable-passaged enteroids grown for 72 h in medium containing 0, 2, or 5 mM Gln. Scale bars, 100 μm.
(B and C) Crypts domains and surface area of stable-passaged enteroids were measured (n = 12, 12 enteroids per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 4 independent experiments).
(D) Heat maps were generated based on levels of the stemness marker genes of ISC cells in vitro. The data represent fold-change differences relative to the 0 mM Gln group. Genes with a corresponding adjusted p value less than 0.05 were considered statistically significant (n = 4, 4 independent wells of enteroids, ∗p < 0.05, one-way ANOVA, results of 3 independent experiments).
(E) Representative images of enteroid immunofluorescence stained with OLFM4 (green) and DAPI (blue) at 72 h. Scale bars, 100 μm.
(F) Western blot analysis of intestinal stem cell marker OLFM4 protein expression (n = 4, 4 independent wells of enteroids per group, results of 3 independent experiments).
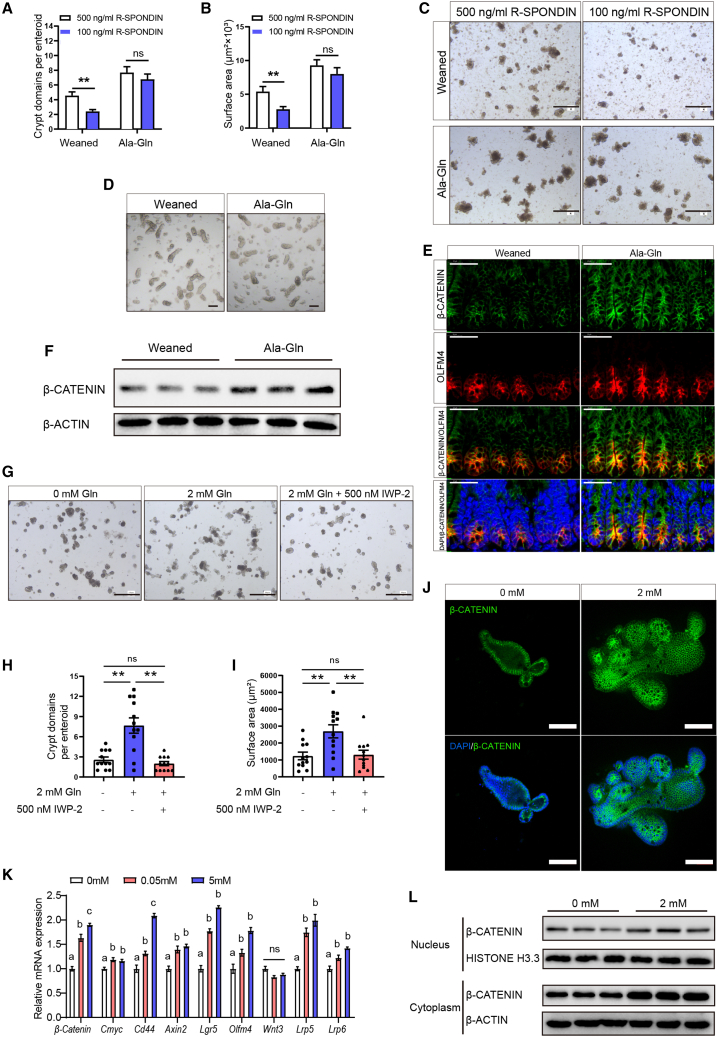
Gln supplementation augments the WNT/β-catenin signaling pathway in ISCs
Next, we ask whether the WNT/β-catenin pathway is involved in regulating Gln supplementation-mediated ISC function. R-Spondin is a WNT amplifier, which is added to the enteroid culture medium to support the growth of enteroids. We found that a relatively low dosage of R-spondin is sufficient to drive the growth of primary enteroids from the Ala-Gln supplementation group (Figures 6A–6D). Then, we measured the level of β-catenin (a surrogate for canonical WNT signaling) in the primary crypts of early weaning mice with or without Ala-Gln supplementation. Immunostaining data also found that both OLFM4 and β-catenin are much more accumulated in the Ala-Gln supplementation group than that in the weaned group (Figure 6E). In addition, western blot results showed that Ala-Gln supplementation increased the β-catenin expression in jejunum crypts (Figure 6F). To examine whether Gln supplementation-mediated ISC function is dependent on the WNT signaling pathway, a specific WNT antagonist IWP-2 was applied for in vitro enteroids. As expected, the budding efficiency and expansion of 2 mM Gln-supplemented enteroids were dramatically increased, while co-treatment with Gln and IWP-2 significantly reduced enteroid budding efficiency and expansion (Figures 6G–6I). Besides, qRT-PCR results showed that the mRNA expression levels of a group of WNT-responsive genes, including β-Catenin, Cmyc, Cd44, Axin2, Lgr5, and Olfm4, increased in a dose-dependent manner (Figure 6K). Gln supplementation increased the transcription of WNT receptors Lrp5 and Lrp6, but it did not affect the transcription of WNT ligand Wnt3 (Figure 6K). Western blot results showed that the Gln supplementation increases both cytoplasmic β-catenin and nuclear β-catenin levels in the enteroid crypt domains (Figure 6L). Immunofluorescence data also showed that Gln supplementation increased the β-catenin expression in the enteroid crypt domains (Figure 6J).
Figure 6.
Gln enhances ISC WNT signaling in vivo and in vitro
(A and B) Crypt domains and surface areas quantification of primary enteroids from the mice in the weaned group and the Ala-Gln group cultured with indicated concentrations of R-spondin (n = 18, 18 primary enteroids from 3 independent mice per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(C) Representative images of primary enteroids at 96 h in vitro culture are shown. Scale bars, 100 μm.
(D) Representative image of crypts isolated from the jejunum of the early weaning mice with or without Ala-Gln supplementation. Scale bars, 10 μm.
(E) Representative image of jejunum crypts immunofluorescence stained with β-catenin (green), OLFM4 (red), and DAPI (blue). Scale bars, 40 μm.
(F) Western blot analysis of β-catenin protein expression in jejunum crypts (n = 3, separated crypts from 3 independent mice per group, results of 3 independent experiments).
(G) Representative bright-field images of enteroids treated with/without Gln and IWP-2. Scale bars, 200 μm.
(H and I) Crypt domains and surface area of enteroids were measured at 72 h (n = 12, 12 enteroids per condition were analyzed, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(J) Representative images of primary enteroids immunofluorescence stained with β-catenin (green) and DAPI (blue) at 96 h. Scale bars, 100 μm.
(K) qRT-PCR analyses on the expression of multiple WNT-target genes in the enteroid crypt domains at 72 h (n = 8, 8 wells of enteroids per group, means ± SD; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(L) Western blot analysis of cytoplasm and nucleus β-catenin levels of the enteroid crypt domains at 96 h (n = 3, 3 wells of enteroids per group, results of 3 independent experiments).
To further demonstrate whether the WNT/β-catenin pathway is involved in Gln’s action on ISCs, we conducted the co-treatment of Gln and IWP-2 experiment in vivo. Both early weaning and IWP-2 treatment attenuated the capability of ISCs to drive the growth of primary enteroids, including the size and crypt domains of enteroids (Figures S4A–S4C). Gln supplementation effectively ameliorated early weaning-induced inhibition of the capability of ISCs to drive the growth of primary enteroids, but co-treatment with IWP-2 abolished this action of Gln on ISCs (Figures S4A–S4C). The results of immunohistochemistry and immunofluorescence of β-catenin showed that IWP-2 co-treatment antagonized the increase of β-catenin levels in crypts induced by Gln supplementation (Figures S4D–S4F). IWP-2 co-treatment also nullified the relief effect of Gln in preventing the reduction of ISC number (Figures S4G and S4H) and proliferation (Figures S4I and S4J) caused by early weaning. Accordingly, the beneficial effects of Gln supplementation on ISC-derived epithelial regeneration (Figures S4K and S4N), villous height (Figure S4L), and body weight (Figure S4M) were removed by co-treatment with IWP-2. In summary, Gln dose dependently enhances the WNT/β-catenin signaling pathway in ISCs, while WNT signaling blockage abolishes the effects of Gln on ISCs.
Gln supplementation promotes ISC-mediated epithelial regeneration in vivo and in vitro
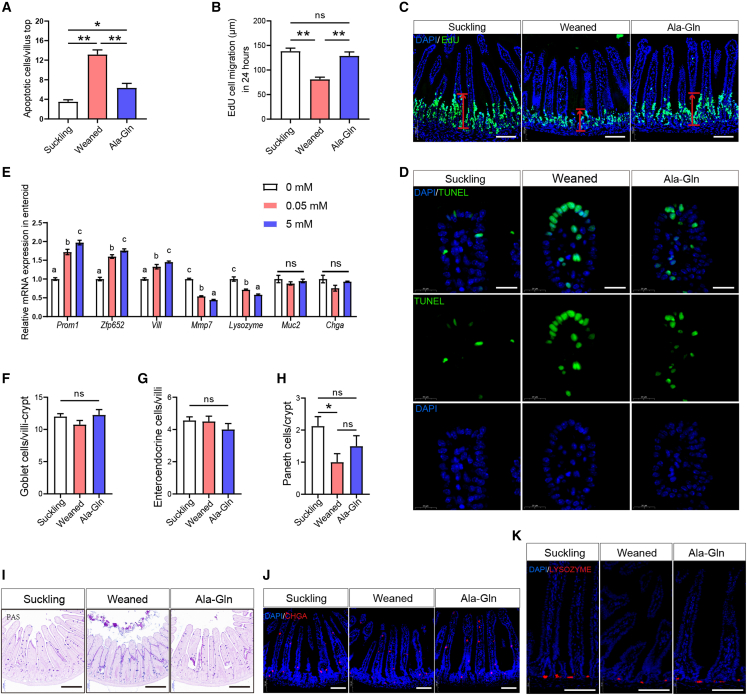
To understand the mechanism of how enteral Ala-Gln supplementation rescues the small intestinal epithelial atrophy induced by early weaning, we first carried out a TUNEL experiment. The results showed that early weaning leads to apoptosis in the small intestinal epithelium, with a high-density accumulation of TUNEL-positive cells on the top of the villus, whereas Gln supplementation partially alleviated this phenotype (Figures 7A and 7D). Next, to assess the rate of intestinal epithelial renewal, we measured the migration height of proliferating and differentiating cells from the crypt to the villus compartment by EdU labeling. We found that Gln supplementation reversed the decrease in the migration height of basal cells caused by early weaning (Figures 7B and 7C). Therefore, Gln supplementation not only decreased the amount of apoptosis of villous top epithelial cells but also boosted the regeneration of the small intestinal epithelium, which jointly ameliorates small intestinal villus atrophy caused by early weaning.
Figure 7.
Effects of Gln supplementation on epithelial regeneration and ISC differentiation in vivo and in vitro
(A) Quantification of apoptotic cells per villus top (n = 6 mice, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(B) Quantification of cell migration after 24 h EdU labeling. EdU cell migration distance was used as an absolute measure of distance from the crypt bottom to the cell that had migrated the furthest (n = 4 mice, means ± SEM, ∗∗p < 0.01, one-way ANOVA, results of 3 independent experiments).
(C) Representative confocal images of cell migration in suckling and weaned mice with 24 h EdU labeling. Scale bars, 100 μm.
(D) Representative images of immunofluorescence represent the overlap of positive signal (green) and nuclear signal (blue). Scale bars, 20 μm.
(E) Relative mRNA levels of transit-amplifying cells Prom1 and Zfp652, enterocyte marker Vill, Paneth cells markers Lysozyme and Mmp7, enteroendocrine Chga and Globet cells marker Muc2 in enteroids were analyzed by qPCR (n = 8, 8 wells of enteroids per group, means ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, results of 3 independent experiments).
(F–H) The number of goblet cells (F), enteroendocrine cells (G), and Paneth cells (H) per crypt-villus were quantified (n = 6 mice, means ± SEM, ∗p < 0.05, one-way ANOVA, results of 3 independent experiments).
(I) Representative images of goblet cells. Scale bars, 200 μm. (J and K) Representative immunofluorescence images of lysozyme (red), CHGA (red), and DAPI (blue) in the jejunum. Scale bars, 100 μm.
To further study the effects of Gln supplementation on differentiation directions of ISC in vitro enteroids, we cultured enteroids in medium containing 0 mM Gln, 0.05 mM Gln, or 5 mM Gln, respectively, for 3 days, and examined the marker gene expression of transit-amplifying cells, enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. qRT-PCR results showed that Gln supplementation increased the expression of transit-amplifying cell markers Prom1 and Zfp652, and enterocyte cell marker Vill in a dose-dependent manner, confirming that Gln supplementation promotes intestinal epithelial regeneration in vitro, but the Gln supplement did not change the expression of goblet cell marker (MUC2) and enteroendocrine cell marker (CHGA) in enteroids (Figure 7E). PAS and immunofluorescence results also showed that neither early weaning nor Ala-Gln supplementation changed the number of goblet cells and enteroendocrine cells in small intestinal epithelium in vivo (Figures 7F, 7G, 7I, and 7J). Gln decreased the expression of Paneth cell markers Lysozyme and Mmp7 in vitro (Figure 7E), while Ala-Gln supplementation alleviated the decrease of Paneth cells in vivo caused by early weaning (Figures 7H and 7K). To summarize, Gln supplementation promotes the differentiation of ISCs into transit-amplifying cells and further into enterocyte cells, which may contribute to the development of small intestinal epithelium in early weaning mice.
Discussion
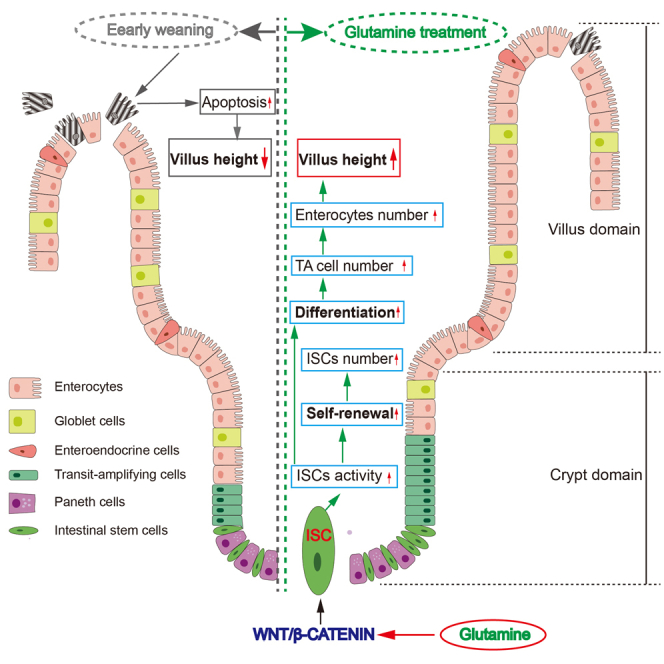
The beneficial role of Gln in intestinal health has been widely demonstrated in animal models and patients. The mechanism by which Gln regulates mature enterocytes is well understood. However, whether Gln affects ISC activity to mediate intestinal epithelial homeostasis in response to early weaning-induced small epithelial development retardation has yet to be elucidated. In this study, both the early weaning mice model and the three-dimensional enteroids model were used to explore how Gln affects ISC function to mediate epithelial development in vivo, ex vivo, and in vitro. We discovered that Gln is essential for ISCs to mediate intestinal epithelial development. Gln supplementation augments ISC activity to accelerate intestinal epithelial regeneration, which is associated with the augmentation of WNT signaling. For the first time, our findings provide insights into the mechanism by which Gln promotes intestinal development in response to early weaning from the perspective of ISCs.
Early weaning usually causes abnormal intestinal morphology, dysfunction, and growth arrest in early weaning piglets (Lalles and Montoya, 2021). To facilitate a mechanism study, we established a novel early weaning mice model with significant intestinal growth retardation. In the present model, early weaning mice developed similar symptoms to early weaning piglets (Wei et al., 2021), including decreased villus height, increased enterocyte apoptosis, and growth retardation. During weaning, Gln is the most abundant free amino acid in sows’ milk (Wu et al., 1996). In addition, up to 67%–70% of Gln in the enteral diet is catabolized by the small intestinal epithelium of weaning piglets (Wu, 2010). These findings suggest that Gln may play a crucial role in small intestinal development and homeostasis at this growth stage. In this study, Ala-Gln supplementation alleviated early weaning-caused intestinal epithelial atrophy and growth retardation in weaned mice, which is consistent with previous findings in malnourished children and mice and weaning piglets (Lima et al., 2007; Ma et al., 2021). These findings prompt us to explore the potential mechanism of Gln in ISC-mediated small intestinal development using early weaning mice.
The concentration of plasma Gln in early weaning piglets gradually increases over the 0- to 14-day period post-weaning, which implies that the requirement for Gln in piglets rises subsequently to weaning (Wu et al., 1996). In this study, we directly confirmed that plasma Gln is relatively deficient in early weaning mice by comparing the plasma Gln levels between suckling and weaned mice on day 4 post-weaning. The previous finding indicated that free Gln supplementation can increase the concentration of plasma Gln in human volunteers (Boza et al., 2000). In our study, we found that Ala-Gln supplementation only increased the concentration of Gln in plasma by 19% (from 634.47 to 765.98 μmol/L), while alanine was increased by 73% (from 205.70 to 356.13 μmol/L), which may be due to extensive catabolism of Gln by the small intestinal epithelium during the first pass. Crypt structure is considered to be a barrier to maintaining the homeostasis of ISCs, including a metabolic barrier (Gehart and Clevers, 2019; Lee et al., 2018). For example, studies have shown that oral feeding of lactate can reach the bottom of intestinal crypts, but butyrate and hydroxyl-butyrate cannot (Kaiko et al., 2016; Lee et al., 2018). Thus, it is reasonable to question whether the enteral Gln reaches the crypt. Although we cannot determine whether oral feeding of Gln directly reaches intestinal crypts, it can be assumed that oral feeding of Gln can provide more Gln (arterial or enteral Gln) for ISCs. First, enteral Gln supplementation would provide sufficient amounts of Gln to the enterocytes and, hence, would spare Gln in mucosal arterial blood for ISC needs. Second, we found that Ala-Gln supplementation indeed increased the concentration of Gln in the arteriovenous mixed blood of early weaning mice. Third, it has previously been reported that enteral administration of proteins rich in Gln does not elevate Gln concentration in plasma but improves its concentration in the intestinal mucosa (Preiser et al., 2003). Future studies are needed to determine whether enteral Gln can directly reach intestinal crypts for ISC-derived small intestinal epithelial regeneration.
Gln is a nonessential amino acid that can be synthesized by transamination in cells, and it is considered a conditionally essential/functional amino acid (Wu, 2010). In this study, we found that Gln deprivation disabled ISCs from mediating intestinal epithelial regeneration, which may partly explain the phenomenon that depleting blood Gln by glutaminase eventually leads to intestinal necrosis and ulcerations (Baskerville et al., 1980). Interestingly, we observed that reintroduction of Gln after 72 h of deprivation reactivated ISCs to support enteroid size expansion and new crypt domain formation, while Gln deprivation beyond 96 h led to enteroid atrophy. Therefore, it can be concluded that Gln is essential for the function of ISCs. Gln is the substrate for mature intestinal epithelial cells to support intestinal functions and the synthesis of pyrimidines and purines in rapidly dividing cells (Hosios et al., 2016). Until now, little is known about the effect of Gln on the ISC self-renewal homeostasis. Recently accumulated findings suggest that ISC self-renewal homeostasis, including their number and proliferation, is easily disrupted in response to changes in the luminal environment (Gehart and Clevers, 2019). In the present study, we found that Gln supplementation reversed the decrease in the proliferation rate and number of ISCs caused by early weaning. In parallel, the ISC marker levels and crypt domains of the in vitro enteroids were also increased by the Gln supplement in a dose-dependent manner. Therefore, we conclude that Gln supplementation enhances the self-renewal of ISC activity, thus providing sufficient ISCs to differentiate and replenish the intestinal epithelial cells.
Intestinal epithelial injury caused by different factors, such as chemoradiotherapy, stress, inflammation, and malnutrition, is usually accompanied by epithelial atrophy (Shaw et al., 2012). Studies have shown that Gln supplementation can effectively alleviate intestinal epithelium atrophy (Ma et al., 2021; Wu et al., 1996). Given that mature enterocytes no longer proliferate and the regeneration of intestinal epithelium is commonly driven by ISCs, we hypothesize that Gln supplementation improves the ability of ISCs to mediate epithelial regeneration. Testing the ability of ISCs to form enteroids is a reliable ex vivo method for evaluating the function of ISCs in driving epithelial regeneration. Small intestinal crypts have a high rate of fission to support the growth of the small intestine in young animals and contribute to intestinal length (Baker et al., 2019; Bruens et al., 2017; Brunsgaard, 1997). In this study, we found that early weaning weakened the capability of ISCs to drive the growth of primary enteroids, whereas Gln supplementation increased the efficiency of enteroid formation, enteroid size, and crypt domains ex vivo, and this phenomenon even exists in the secondary enteroids. In vitro, enteroid size and crypt domains were also increased by the Gln supplement in a dose-dependent manner. These results may partly explain why Gln supplementation increased the length of the small intestine in early weaning mice. In vivo, we found that Gln supplementation increased the heights of EdU-positive cells migrating from crypts to villi during 24 h. This is consistent with previous reports that Gln supplementation increased the number of EdU-positive cells in crypts (Moore et al., 2015; Ueno et al., 2011). Therefore, these results verify that Gln supplementation improves the ability of ISCs to mediate epithelial regeneration.
Accumulating findings underline the importance of WNT signaling in ISCs for epithelial regeneration in response to intestinal damage (Guillermin et al., 2021; Zou et al., 2018). We further hypothesized that the WNT signaling is involved in the augments of ISC activity by Gln. In this study, we first found that a relatively low dosage of WNT amplifier is sufficient to support the growth of primary enteroids from the Ala-Gln supplementation group. In in vitro enteroids, Gln increased the expression of WNT-responsive genes and the level of nuclear β-catenin, a surrogate for canonical WNT signaling (MacDonald et al., 2009). Furthermore, WNT signaling blockage inhibited Gln-stimulated enhancement of ISC function in enteroids. Interestingly, co-treatment with a WNT inhibitor in vivo abolished the Gln supplementation-boosted capability of ISCs to drive the growth of primary enteroids and the enhancement of β-catenin levels in crypts during early weaning. Furthermore, the beneficial action of Gln supplementation on ISC self-renewal activity, ISC-derived epithelial regeneration, villous height, and body weight was negated by co-treatment with a WNT inhibitor in vivo. Thus, we proposed that Gln promotes the function of ISCs, which is associated with the enhancement of WNT/β-catenin signaling. Future studies are needed to elucidate the mechanism by which Gln augments WNT signaling in ISCs.
Although we have clarified that Gln promotes the function of ISCs, the process of Gln promoting intestinal epithelial homeostasis is still incompletely understood. Furthermore, we found that Ala-Gln supplementation alleviated the excessive apoptosis of enterocytes at the top of villi. This is consistent with the previous studies that Gln supplementation decreased epithelial apoptosis in malnourished weanling mice and early weaning piglets (Ma et al., 2021; Ueno et al., 2011). In vitro, Gln promoted the differentiation of ISCs into transit-amplifying cells and then into enterocytes, which is consistent with our in vivo results that Ala-Gln supplementation accelerated the migration of crypt cells to the top of villi. Therefore, these findings suggest that Gln supplementation not only reduces excessive apoptosis of mature enterocytes but also accelerates the differentiation of ISCs into enterocyte cells migrating to villi, which jointly ameliorates early weaning-induced epithelium abnormality. Overall, Gln supplementation confers intestinal epithelium a stronger regenerative capacity to counteract the disruption of homeostasis caused by early weaning.
In summary, our findings reveal that Gln accelerates stem cell-mediated small intestinal epithelial development, which is associated with the augmentation of WNT signaling. Our findings provide novel insights into the mechanism by which Gln supplementation promotes intestinal health in response to early weaning practice, which may guide Gln’s application in infant and young domestic animal nutrition.
Experimental procedures
Resource availability
Corresponding author
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Qian Jiang (jiangqian@hunau.edu.cn).
Materials availability
This study did not generate unique reagents.
Data and code availability
All data are contained within the article.
Early weaning model of mice
All pups were weaned on postnatal day 15. On postnatal day 14, the mother was taken away from their pup’s cage for 18 h consecutively (3:00 p.m. to 9:00 a.m.). After separation, the mother was returned to their pup’s cage for 6 h (9:00 a.m. to 3:00 p.m.), which is to make the pup adapt better to life after weaning, and then the mother was taken away from her pups forever. All animal protocols were approved by the Laboratory Animal Ethical Commission of the Institute of Subtropical Agriculture (Permit No. ISA-267/2019 for the mice experiment), the Chinese Academy of Sciences.
Animal experiments design
To establish the early weaning mice model, pups from the same litter were randomly divided into two groups on the basis of similar body weights (n = 3–4 per group). The suckling group lived with their mothers, whereas the weaned group lived alone. To investigate the effects of Gln supplementation on early weaning mice, early weaning pups from the same litter were randomly divided into two groups on the basis of similar body weights (n = 3–4 per group). Mice in the weaned group were free to drink water, while the Ala-Gln group was free to drink 2% L-alanyl-L-glutamine (Solarbio, Beijing, China) supplemented water, equaling a solution of 111 mM Ala-Gln. To assess the effects of alanine (Solarbio) supplementation on early weaning mice, early weaning pups from the same litter were randomly divided into two groups on the basis of similar body weights (n = 3–4 per group). The weaned group was free to drink water, while the Ala group was free to drink a solution of 111 mM of L-alanine (Solarbio) in water. Three or more weaning mice were caged together in each group. We measured the body weight every day and recorded food consumption weekly in the groups. The water was changed every 3 days. On day 7 post-weaning, the mice were sacrificed to collect jejunual tissues and blood for further analysis.
Statistical analyses
Data, expressed as means ± SEM or means ± SD, were analyzed statistically using SPSS 22.0 software. An independent t test was used to compare the two groups. Data from three or more groups were analyzed by one-way ANOVA followed by LSD multiple comparisons. The “n,” noted in the figure legends, represents the replicated number of mice, enteroids, or enteroid cultures in the experiments depicted. ∗p < 0.05 and ∗∗p < 0.01 were considered statistically significant. All data are representative of at least three independent experiments.
Author contributions
Conceptualization, J.T., Q.J., and K.Y.; investigation, J.T. and Y.L.; data curation, J.T.; project administration, Y.L., Q.J., Y.Y., and K.Y.; methodology and resources, X.B.; validation and visualization, F.Y.; writing – original draft, J.T.; writing – review & editing, X.T., Q.J., C.Y., and K.Y.; supervision, Y.Y.; funding acquisition, Q.J., Y.Y., and K.Y.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32130099), the Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), and TaiShan Industrial Experts Program (tscy20190121).
Declaration of interests
The authors declare no competing interests.
Published: June 15, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.05.012.
Contributor Information
Qian Jiang, Email: jiangqian@hunau.edu.cn.
Kang Yao, Email: yaokang@isa.ac.cn.
Supplemental information
References
- Alonso S., Yilmaz O.H. In: Stover P.J., Balling R., editors. Vol 38. 2018. Nutritional regulation of intestinal stem cells; pp. 273–301. (Annual Review of Nutrition). [DOI] [PubMed] [Google Scholar]
- Baker A.-M., Gabbutt C., Williams M.J., Cereser B., Jawad N., Rodriguez-Justo M., Jansen M., Barnes C.P., Simons B.D., McDonald S.A.C., et al. Crypt fusion as a homeostatic mechanism in the human colon. Gut. 2019;68:1986–1993. doi: 10.1136/gutjnl-2018-317540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville A., Hambleton P., Benbough J.E. Pathological features of glutaminase toxicity. Br. J. Exp. Pathol. 1980;61:132–138. [PMC free article] [PubMed] [Google Scholar]
- Boza J.J., Maire J., Bovetto L., Ballèvre O. Plasma glutamine response to enteral administration of glutamine in human volunteers (free glutamine versus protein-bound glutamine) Nutrition. 2000;16:1037–1042. doi: 10.1016/s0899-9007(00)00433-0. [DOI] [PubMed] [Google Scholar]
- Bruens L., Ellenbroek S.I.J., van Rheenen J., Snippert H.J. In vivo imaging reveals existence of crypt fission and fusion in adult mouse intestine. Gastroenterology. 2017;153:674–677.e3. doi: 10.1053/j.gastro.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsgaard G. Morphological characteristics, epithelial cell proliferation, and crypt fission in cecum and colon of growing pigs. Dig. Dis. Sci. 1997;42:2384–2393. doi: 10.1023/a:1018899625022. [DOI] [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/beta-Catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Deters B.J., Saleem M. The role of glutamine in supporting gut health and neuropsychiatric factors. Food Sci. Hum. Wellness. 2021;10:149–154. doi: 10.1016/j.fshw.2021.02.003. [DOI] [Google Scholar]
- Frick A., Khare V., Jimenez K., Dammann K., Lang M., Krnjic A., Gmainer C., Baumgartner M., Mesteri I., Gasche C. A novel PAK1-Notch1 Axis regulates crypt homeostasis in intestinal inflammation. Cell. Mol. Gastroenterol. Hepatol. 2021;11:892–907.e1. doi: 10.1016/j.jcmgh.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehart H., Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- Guillermin O., Angelis N., Sidor C.M., Ridgway R., Baulies A., Kucharska A., Antas P., Rose M.R., Cordero J., Sansom O., et al. Wnt and Src signals converge on YAP-TEAD to drive intestinal regeneration. EMBO J. 2021;40 doi: 10.15252/embj.2020105770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Walton M., Van Ogtrop F., Guest D., Black K., Beardsley J. Factors influencing undernutrition among children under 5 years from cocoa-growing communities in Bougainville. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosios A.M., Hecht V.C., Danai L.V., Johnson M.O., Rathmell J.C., Steinhauser M.L., Manalis S.R., Vander Heiden M.G. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell. 2016;36:540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C., Hui S., Zeng X., Cowan A.J., Wang L., Chen L., Morscher R.J., Reyes J., Frezza C., Hwang H.Y., et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 2019;30:594–606.e3. doi: 10.1016/j.cmet.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko G.E., Ryu S.H., Koues O.I., Collins P.L., Solnica-Krezel L., Pearce E.J., Pearce E.L., Oltz E.M., Stappenbeck T.S. The colonic crypt protects stem cells from microbiota-derived metabolites (vol 165, pg 1708, 2016) Cell. 2016;167:1137. doi: 10.1016/j.cell.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallès J.P., Montoya C.A. Dietary alternatives to in-feed antibiotics, gut barrier function and inflammation in piglets post-weaning: where are we now? Anim. Feed Sci. Technol. 2021;274 doi: 10.1016/j.anifeedsci.2021.114836. [DOI] [Google Scholar]
- Lee Y.S., Kim T.Y., Kim Y., Lee S.H., Kim S., Kang S.W., Yang J.Y., Baek I.J., Sung Y.H., Park Y.Y., et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe. 2018;24:833–846.e6. doi: 10.1016/j.chom.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Lima N.L., Soares A.M., Mota R.M.S., Monteiro H.S.A., Guerrant R.L., Lima A.A.M. Wasting and intestinal barrier function in children taking alanyl-glutamine-supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr. 2007;44:365–374. doi: 10.1097/MPG.0b013e31802eecdd. [DOI] [PubMed] [Google Scholar]
- Liu P.S., Wang H., Li X., Chao T., Teav T., Christen S., Di Conza G., Cheng W.C., Chou C.H., Vavakova M., et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- Ma D., Guedes J.M., Duttlinger A.W., Johnson J.S., Zuelly S.M., Lay D.C., Richert B.T., Kim Y.H.B. Impact of L-glutamine as replacement of dietary antibiotics during post weaning and transport recovery on carcass and meat quality attributes in pigs. Livest. Sci. 2021;244 doi: 10.1016/j.livsci.2020.104350. [DOI] [Google Scholar]
- MacDonald B.T., Tamai K., He X. Wnt/beta-Catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviso M.K., Ferguson B., Kaforau L.M., Capper T. A qualitative descriptive inquiry into factors influencing early weaning and breastfeeding duration among first-time mothers in Papua New Guinea's rural eastern highlands. Women Birth. 2022;35:E68–E74. doi: 10.1016/j.wombi.2021.01.006. [DOI] [PubMed] [Google Scholar]
- Moore S.R., Guedes M.M., Costa T.B., Vallance J., Maier E.A., Betz K.J., Aihara E., Mahe M.M., Lima A.A.M., Oriá R.B., Shroyer N.F. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G831–G839. doi: 10.1152/ajpgi.00422.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K., Zheng Y., Geiger H. Aging of intestinal stem cells. Stem Cell Rep. 2022;17:734–740. doi: 10.1016/j.stemcr.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N., Iqbal S., Mana M., Andersson S., Cognetta A.B., Suciu R.M., Roper J., Luopajärvi K., Markelin E., Gopalakrishnan S., et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571:398–402. doi: 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser J.C., Peres-Bota D., Eisendrath P., Vincent J.L., Van Gossum A. Gut mucosal and plasma concentrations of glutamine: a comparison between two enriched enteral feeding solutions in critically ill patients. Nutr. J. 2003;2:13. doi: 10.1186/1475-2891-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P.J., Burrin D.G. The gut and amino acid homeostasis. Nutrition. 2000;16:666–668. doi: 10.1016/s0899-9007(00)00354-3. [DOI] [PubMed] [Google Scholar]
- Rollins N.C., Bhandari N., Hajeebhoy N., Horton S., Lutter C.K., Martines J.C., Piwoz E.G., Richter L.M., Victora C.G., Lancet Breastfeeding Series Group Why invest, and what it will take to improve breastfeeding practices? Lancet. 2016;387:491–504. doi: 10.1016/s0140-6736(15)01044-2. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shaw D., Gohil K., Basson M.D. Intestinal mucosal atrophy and adaptation. World J. Gastroenterol. 2012;18:6357–6375. doi: 10.3748/wjg.v18.i44.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.W., Patterson J.Y., Peacock M.A. A comprehensive description of brush-border membrane-development applying to enterocytes taken from a wide variety of mammalian-species. Comp. Biochem. Physiol. A Comp. Physiol. 1984;77:655–662. doi: 10.1016/0300-9629(84)90180-4. [DOI] [PubMed] [Google Scholar]
- Ueno P.M., Oriá R.B., Maier E.A., Guedes M., de Azevedo O.G., Wu D., Willson T., Hogan S.P., Lima A.A.M., Guerrant R.L., et al. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G612–G622. doi: 10.1152/ajpgi.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. Annual Review of Physiology. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium; pp. 241–260. [DOI] [PubMed] [Google Scholar]
- Vardhana S.A., Arnold P.K., Rosen B.P., Chen Y., Carey B.W., Huangfu D., Carmona-Fontaine C., Thompson C.B., Finley L.W.S. Glutamine independence is a selectable feature of pluripotent stem cells. Nat. Metab. 2019;1:676–687. doi: 10.1038/s42255-019-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile N., Mirmahmoudi R., Brevini T.A.L., Gandolfi F. Evolution of pig intestinal stem cells from birth to weaning. Animal. 2019;13:2830–2839. doi: 10.1017/s1751731119001319. [DOI] [PubMed] [Google Scholar]
- Victora C.G., Bahl R., Barros A.J.D., França G.V.A., Horton S., Krasevec J., Murch S., Sankar M.J., Walker N., Rollins N.C., Lancet Breastfeeding Series Group Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/s0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- Vigeland C.L., Beggs H.S., Dang H., Doerschuk C.M. Differences in metabolic activity between macrophage populations in response to LPS-stimulation and glutamine inhibition. Am. J. Respir. Crit. Care Med. 2021;203 [Google Scholar]
- Wei X., Tsai T., Howe S., Zhao J. Weaning induced gut dysfunction and nutritional interventions in Nursery pigs: a partial review. Animals. 2021;11:1279. doi: 10.3390/ani11051279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.L.X., Qin H.Y., Tsang S.W., Zuo X., Che S., Chow C.F.W., Li X., Xiao H.T., Zhao L., Huang T., et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun. 2019;10:1745. doi: 10.1038/s41467-019-09744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010;1:31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Meier S.A., Knabe D.A. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J. Nutr. 1996;126:2578–2584. doi: 10.1093/jn/126.10.2578. [DOI] [PubMed] [Google Scholar]
- Zha J.M., Li H.S., Lin Q., Kuo W.T., Jiang Z.H., Tsai P.Y., Ding N., Wu J., Xu S.F., Wang Y.T., et al. Interleukin 22 expands transit-amplifying cells while depleting Lgr5(+) stem cells via inhibition of wnt and Notch signaling. Cell. Mol. Gastroenterol. Hepatol. 2019;7:255–274. doi: 10.1016/j.jcmgh.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W.Y., Blutt S.E., Zeng X.L., Chen M.S., Lo Y.H., Castillo-Azofeifa D., Kein O.D., Shroyer N.F., Donowitz M., Estes M. Epithelial-secreted wnt ligands are essential drivers of intestinal stem cell response to virus-induced villus damage. Gastroenterology. 2018;154:S96. doi: 10.1016/j.celrep.2017.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.