Summary
The adult subventricular zone (SVZ) is a neurogenic niche that continuously produces newborn neurons. Here we show that serine racemase (SR), an enzyme that catalyzes the racemization of L-serine to D-serine and vice versa, affects neurogenesis in the adult SVZ by controlling de novo fatty acid synthesis. Germline and conditional deletion of SR (nestin precursor cells) leads to diminished neurogenesis in the SVZ. Nestin-cre+ mice showed reduced expression of fatty acid synthase and its substrate malonyl-CoA, which are involved in de novo fatty acid synthesis. Global lipidomic analyses revealed significant alterations in different lipid subclasses in nestin-cre+ mice. Decrease in fatty acid synthesis was mediated by phospho Acetyl-CoA Carboxylase that was AMP-activated protein kinase independent. Both L- and D-serine supplementation rescued defects in SVZ neurogenesis, proliferation, and levels of malonyl-CoA in vitro. Our work shows that SR affects adult neurogenesis in the SVZ via lipid metabolism.
Keywords: serine racemase,; adult neurogenesis,; lipid metabolism,; subventricular zone,; D-serine,; L-serine,; malonyl-CoA
Graphical abstract
Highlights
-
•
Serine racemase mediates adult subventricular zone neurogenesis via lipid metabolism
In this article, Roychaudhuri et al. show that serine racemase controls adult SVZ neurogenesis by mediating a non-AMPK-dependent control of lipid metabolism.
Introduction
Adult neurogenesis is the production of new neurons in the subventricular zone (SVZ) of the lateral ventricles and the sub granular zone of the hippocampus in the brain (Kempermann, 2006; Ming and Song, 2011; Tong and Alvarez-Buylla, 2014). Newborn neurons in the SVZ migrate along the rostral migratory stream (RMS) and eventually differentiate into olfactory bulb (OB) interneurons feeding the precursor cell population (Lim and Alvarez-Buylla, 2016). Recent work has shown that adult neurogenesis depends on metabolic control of fatty acid synthesis (Knobloch, 2017; Knobloch et al., 2013). Defects in fatty acid metabolism play a crucial role in the quiescent and proliferative behavior of neural progenitor cells (Clemot et al., 2020; Knobloch et al., 2017). Serine racemase (SR) is a pyridoxal phosphate (PLP)-dependent enzyme that synthesizes D-serine from L-serine and vice versa (Wolosker et al., 1999a, 1999b). D-serine functions as a neurotransmitter during brain development (Schell et al., 1997). SR-deficient mice show NMDA receptor hypofunction and is considered a model for schizophrenia (Balu et al., 2013; Basu et al., 2009); however, little is known of D-serine’s functions outside the NMDA receptor. D-serine regulates proliferation and differentiation of neural stem cells (NSCs) from postnatal mouse forebrain (Huang et al., 2012). D-serine has been implicated in adult hippocampal neurogenesis, with exogenous treatment of D-serine increasing the density of NSCs, transit amplifying progenitors and increased survival of newborn neurons (Sultan et al., 2013). We investigated the metabolic role of SR in NSCs from the adult SVZ. We hypothesized that SR may influence metabolism in the SVZ due to L- and D-serine synthesis and transport via the astrocyte-neuron shuttle (Wolosker et al., 2017; Ehmsen et al., 2013).
Using lipidomic analysis and biochemical approaches, we show that the SVZ of nestin-cre+ mice have significant alterations in the different classes of lipids, decreased expression of fatty acid synthase (FASN) and malonyl-CoA, a precursor for de novo fatty acid synthesis. Our work also shows that SR mediates its effects in the SVZ via activation of acetyl-CoA carboxylase (ACC). SR may influence lipid metabolism by regulating astrocytic L-serine and consequently neuronal D-serine synthesis in NSCs. These findings may have therapeutic implications in the treatment of disorders of adult neurogenesis like schizophrenia and Alzheimer’s disease (Le Douce et al., 2020; Moreno-Jimenez et al., 2019; Schoenfeld and Cameron, 2015; Yun et al., 2016).
Results
Lack of SR leads to defects in adult SVZ neurogenesis and proliferation
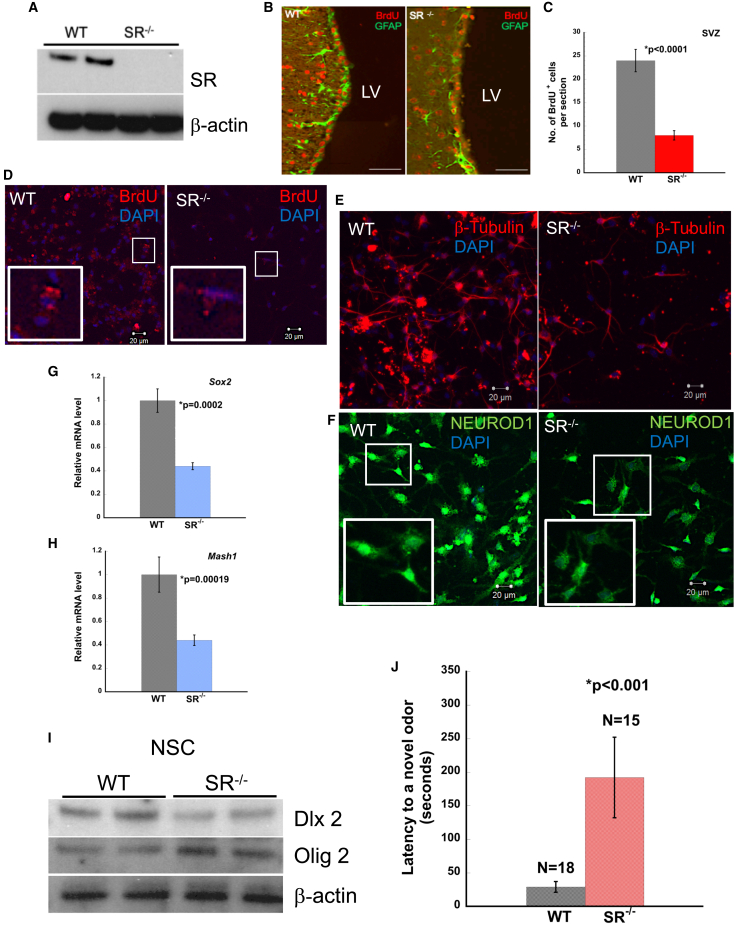
SR is a PLP-dependent enzyme, which racemizes D-serine in neurons from L-serine in astrocytes (Wolosker et al., 1999b, 2017). We determined neurogenesis in the SVZ of adult wild-type (WT) and SR−/− mice (Figures 1A, 1B, S1A and S4D). We monitored BrdU incorporation in age-matched WT and SR−/− mice for 14 days (Doetsch et al., 1999). Our data showed significantly reduced incorporation of BrdU in SR−/− mice (Figures 1B and 1C). Quantitative analysis showed 4-fold reduction in BrdU-positive cells in SR−/− mice (Figure 1C). Glial fibrillary acidic protein (GFAP)-positive staining (mature astrocytes) was decreased in the SVZ of SR−/− mice (Figure 1B).
Figure 1.
Lack of Serine Racemase leads to defects in adult SVZ neurogenesis and proliferation in vivo
(A) Expression of SR in WT and SR−/− mice SVZ lysates.
(B) WT and SR−/− SVZ lateral ventricle showing BrdU incorporation (red) and GFAP expression (green). Scale bar, 100 μm.
(C) Quantitative estimation of BrdU+ cells.
(D–H) BrdU incorporation in NSC from WT and SR−/− SVZ. Inset shows incorporation of BrdU in NSCs (red). Expression of (E) β-III Tubulin and (F) NEUROD1 in NSCs from WT and SR−/− mice after differentiation. Relative mRNA levels of (G) Sox2 and (H) Mash1 in olfactory bulb of WT and SR−/− mice. Error bars represent SD. Data are representative of three independent experiments and tissues pooled from n = 5–6 mice/experiment.
(I) Expression of DLX2 and OLIG 2 in NSC lysates.
(J) Mouse olfactory behavior test in age-matched WT and SR−/− mice. N = number of mice.
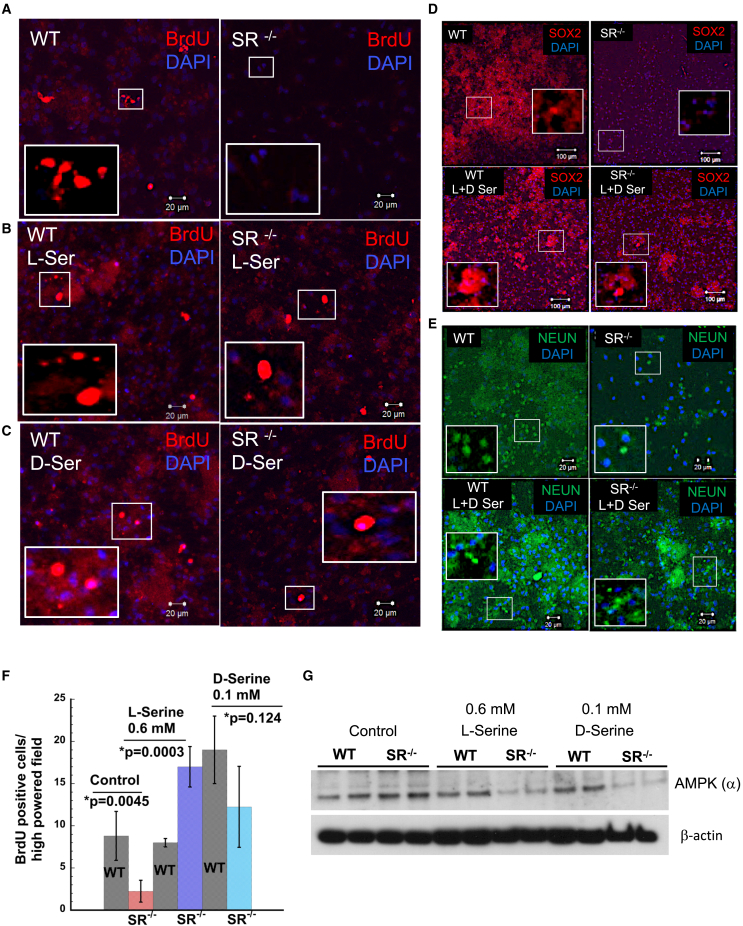
The neuroblast assay serves as an in vitro model of neurogenesis (Azari and Reynolds, 2016; Azari et al., 2012). To determine neurogenesis in vitro, we performed the neuroblast assay. NSCs were isolated from the SVZ of age-matched adult WT and SR−/− mice and grown as adherent monolayer cultures for 10–12 days (Walker and Kempermann, 2014). After 4 days in media with reduced and eventually no growth factors, neuroblasts were observed above a layer of astrocytes (Figure S2A) (Azari and Reynolds, 2016). We examined BrdU incorporation in NSCs as an indicator of cell division. BrdU incorporation in NSCs from WT and SR−/− SVZ showed 4-fold lower incorporation in SR−/− (∗p = 0.0084) (Figures 1D and S2D).
Neuroblasts (type A cells) migrate rostrally and differentiate to OB interneurons. We determined the expression of β-III Tubulin (marker for immature neurons) and NEUROD1 (marker for neuroblasts). β-III Tubulin staining in NSCs was reduced in SR−/− mice (Figure 1E). Quantitative analysis showed 5-fold reduction of β-III tubulin positive staining in SR−/− mice (∗p < 0.001) (Figure S2B). A similar trend was observed with NEUROD1 (∗p < 0.001) (Figures 1F and S2C). We examined the differentiation of NSCs to astrocytes and neurons. GFAP expression was reduced in SR−/− mice (Figure S1C). NEUN expression (marker for newborn post mitotic neurons) was also reduced in SR−/− NSCs (Figure S1D). Gene expression for markers of proliferation in the OB, Sox2, and Mash1 showed 2-fold reduction in SR−/− mice (Figures 1G and 1H). To substantiate our findings, we determined and observed reduced expression of DLX2 in SR−/− NSCs (Figures 1I and S6A). We also observed increased expression of OLIG2 in SR−/− NSCs (Figures 1I and S6B), suggesting a propensity for oligodendrocyte differentiation (Wang et al., 2020). We determined the expression of Ki67 in primary neurons derived from NSCs. SR−/− neurons expressed 2-fold lower levels of Ki67 (Figure S1B). To determine the effects of SVZ neurogenesis on olfactory behavior, we performed olfactory behavior test in age-matched WT and SR−/− mice (Witt et al., 2009). In the novel odor test, we determined the amount of time the mice spent locating and sniffing the novel odor. WT mice spent a shorter time exploring and sniffing the novel odor compared with SR−/− mice that took approximately eight times longer (∗p < 0.001) (Figure 1J). Collectively, these results show that SR−/− mice have defects in adult SVZ neurogenesis and proliferation in vitro and in vivo.
Conditional deletion of SR leads to defects in adult SVZ neurogenesis and proliferation
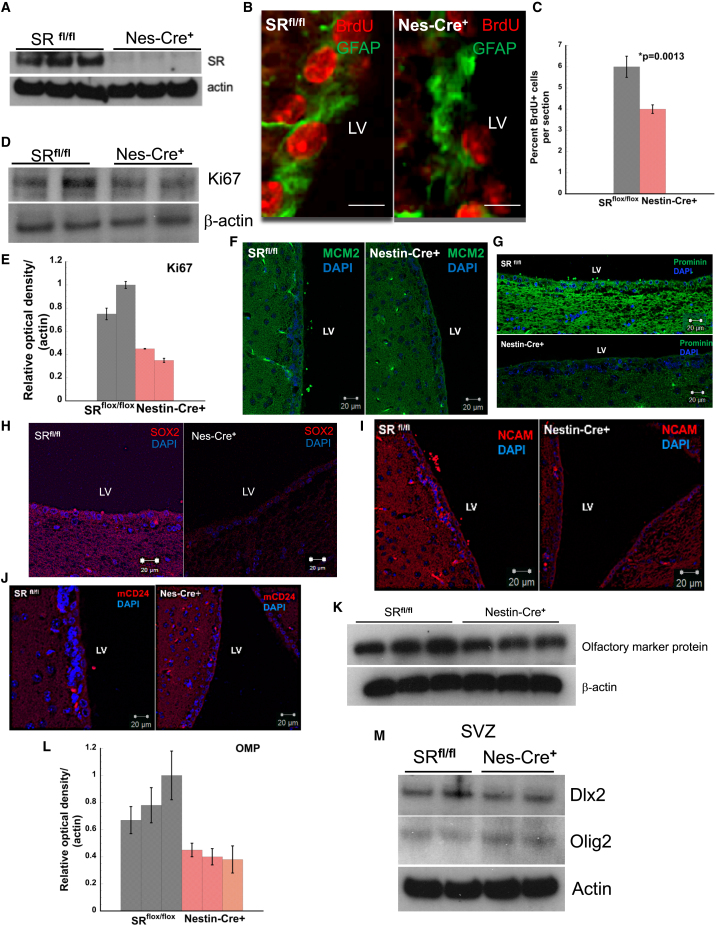
We conditionally deleted Srr in nestin precursor cells (nestin-cre+) to elucidate its function in neural progenitor cells (Bernal and Arranz, 2018; Wiese et al., 2004). Our rationale was 2-fold first, D-serine the product of Sr may control neuronal development and generation of newborn neurons (Schell et al., 1997). Second, neuronal D-serine synthesis dependent on astrocytic L-serine may influence proliferation and metabolic homeostasis. SR expression was reduced in the brains of nestin-cre+ mice compared with age-matched SRflox/flox (Figure 2A). We ascertained levels of neurogenesis in the SVZ of adult mice by monitoring BrdU incorporation (Doetsch et al., 1999). BrdU imaging showed 1.5-fold reduced incorporation in nestin-cre+ mice (Figures 2B and 2C). Expression of Ki67, a proliferative marker for early adult neurogenesis, showed approximately 2-fold decreased expression in the SVZ of nestin-cre+ mice (Figures 2D and 2E) (Kee et al., 2002).
Figure 2.
Conditional deletion of Srr leads to defects in adult SVZ neurogenesis and proliferation
(A) Expression of SR in SRflox/flox and nestin-cre+ mice brain.
(B) BrdU incorporation (red) and GFAP expression (green) in the SVZ of SRflox/flox and nestin-cre+ mice. Scale bar, 100 μm.
(C) Quantitative estimation of BrdU+ cells counted in different high-powered fields per section. n = 4–5 mice.
(D) Expression of Ki67 in SVZ.
(E–J) Densitometric analysis of blot in (D). Expression of NSC and proliferation markers (F) MCM2, (G) Prominin, (H) SOX2, (I) NCAM, and (J) mCD24 in SRflox/flox and nestin-cre+ mice. Scale bar, 20 μm.
(K) Expression of OMP in SVZ.
(L) Densitometry of OMP blot.
(M) Expression of DLX2 and OLIG 2 in SVZ.
We monitored expression of MCM2 (DNA Replication Licensing Factor; marker for proliferation of mitotic progenitor cells) in the SVZ. We observed approximately 3-fold reduction of MCM2 expression in nestin-cre+ mice (Figures 2F and S7F). Prominin 1 is a marker for NSCs in the adult SVZ (Faigle and Song, 2013). Prominin 1 expression was reduced 4-fold in nestin-cre+ mice (Figures 2G and S7G). We determined expression of mCD24 (glycosyl-phosphatidyl inositol membrane anchored glycoprotein) that regulates the proliferation of committed neuronal precursors in the SVZ and is a negative regulator of cell proliferation (Belvindrah et al., 2002; Doetsch et al., 1999). Nestin-cre+ mice showed 3-fold higher expression of mCD24 (Figures 2J and S4E). SRY-box 2 (SOX-2) is a transcription factor essential for self-renewal and differentiation of neural progenitor cells (Pevny and Nicolis, 2010). SOX-2 expression in the SVZ was 3-fold lower in nestin-cre+ mice (Figures 2H and S7I). Expression of polysialylated specific antigen-neuronal cell adhesion molecule (PSA-NCAM) (Figures 2I and S7H) and GFAP (Figure S5C) was 6- and 5-fold lower in nestin-cre+ mice. We also observed reduced PSA-NCAM expression along the RMS in nestin-cre+ mice (Figure S5B).
We determined expression of olfactory marker protein (OMP) present on olfactory receptor neurons and observed 2-fold decrease in nestin-cre+ mice suggesting defects in adult SVZ neurogenesis (Figures 2K and 2L). We observed reduced expression of proliferation marker DLX2 in the SVZ of nestin-cre+ mice (Figures 2M and S6C). Expression of OLIG2 (cell fate determination of motor neurons and oligodendrocytes) was increased in nestin-cre+ mice (Figures 2M and S6D), suggesting that conditional deletion of Srr may increase propensity for oligodendrocyte differentiation (Wang et al., 2020). Collectively our data show that nestin-cre+ mice have defects in SVZ neurogenesis and proliferation and support the data with SR−/− mice (Figures 1B and 1C).
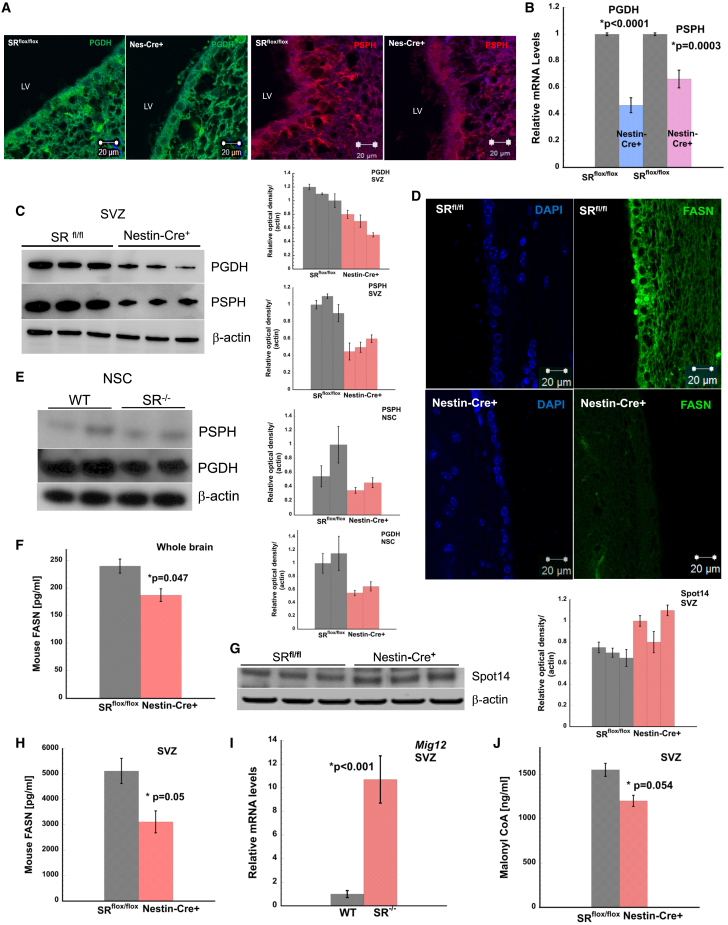
SR alters L-serine and de novo fatty acid synthesis in the SVZ of nestin-Cre+ mice
L-serine synthesized by astrocytes regulates neuronal development (Furuya et al., 2000; Kalhan and Hanson, 2012; Mitoma et al., 1998; Reid et al., 2018). SR racemizes L-serine and D-serine (Wolosker et al., 1999b, 2017). We hypothesized that deletion of Srr may have metabolic implications for neuronal development. L-serine synthesis is regulated by phosphoglycerate dehydrogenase (PGDH) and phosphoserine phosphatase (PSPH) via the phosphorylated pathway (Fell and Snell, 1988; Snell and Fell, 1990; Yamasaki et al., 2001). STRING analysis followed by k means clustering shows that SR interacts with PSPH and PHGDH in both mice and humans (Figures S6E and S6F). We determined expression of Pgdh and Psph in the SVZ and observed decreased expression of both enzymes in nestin-cre+ mice (Figures 3A, S7J, and S7K). Relative mRNA levels showed more than 50% reduction in enzymes in nestin-cre+ mice (Figure 3B). Protein expression was also decreased in the SVZ of nestin-cre+ mice (Figure 3C), supporting a consistent trend (Ehmsen et al., 2013). To determine if 0.4 mM L-serine in the culture media alters expression of PGDH and PSPH, we determined their expression in WT and SR−/− NSCs. We found decreased expression of both enzymes in SR−/− NSCs (Figure 3E). FASN controls the synthesis of fatty acids by synthesizing palmitate from acetyl- and malonyl-CoA. We observed 3-fold reduction in FASN expression in nestin-cre+ SVZ (Figures 3D and S7E). In whole brain we observed approximately 50% lower FASN expression in nestin-cre+ mice (Figures S2E and S2F). To confirm, we estimated FASN levels using ELISA. We found lower expression in the brain and significantly lower levels in the SVZ of nestin-cre+ mice (Figures 3F and 3H). These data strongly suggest that nestin-cre+ mice have defects in de novo fatty acid synthesis. Spot 14 (thyroid hormone responsive spot14) is a regulator of de novo lipogenesis and is involved in proliferation of adult NSCs (Knobloch et al., 2014). It negatively regulates malonyl-CoA, an essential substrate for FASN in de novo lipogenesis. We observed higher expression of SPOT 14 in nestin-cre+ mice (Figures 3G and S5A). MIG12 (Midline-1-Interacting G12 like protein) complexes with SPOT14 to regulate ACC (Knobloch et al., 2013; Park et al., 2013). We determined mRNA levels of Mig12 in the SVZ and observed 10-fold higher expression in nestin-cre+ mice (Figure 3I). We estimated levels of malonyl-CoA in the SVZ and observed that SRflox/flox levels were 1,500 ng/mL, while nestin-cre+ levels were 1,200 ng/mL (∗p = 0.054 relative to SRflox/flox control) (Figure 3J). Levels of malonyl-CoA suggest that de novo lipogenesis fueled by FASN and its substrate is impaired in nestin-cre+ mice.
Figure 3.
Serine Racemase alters L-serine and de novo fatty acid synthesis in the SVZ of nestin-cre+ mice
(A) Expression of PGDH (green) and PSPH (red) in the SVZ of SR flox/flox and nestin-cre+ mice. Scale bar, 20 μm.
(B) Relative mRNA levels of Pgdh and Psph. Data are representative of three independent experiments. Tissues were pooled from n = 5–8 mice.
(C) Expression of PSPH and PGDH in SVZ.
(D) Expression of mouse FASN in SVZ. Scale bar, 20 μm.
(E) Expression of PSPH and PGDH in NSC lysates.
(F) Mouse FASN expression in whole brain homogenates. ∗p = 0.047 relative to SRflox/flox.
(G) Expression of SPOT14 in SVZ.
(H) Expression of mouse FASN in SVZ. ∗p = 0.05 relative to SR flox/flox.
(I) mRNA levels of SPOT14 regulator Mig12 in SVZ of WT and SR−/− mice.
(J) Estimation of malonyl-CoA in SVZ by ELISA. ∗p = 0.054 relative to SR flox/flox. Error bars are SD. Data are representative of three independent experiments. Tissues were pooled from n = 5–8 mice per experiment.
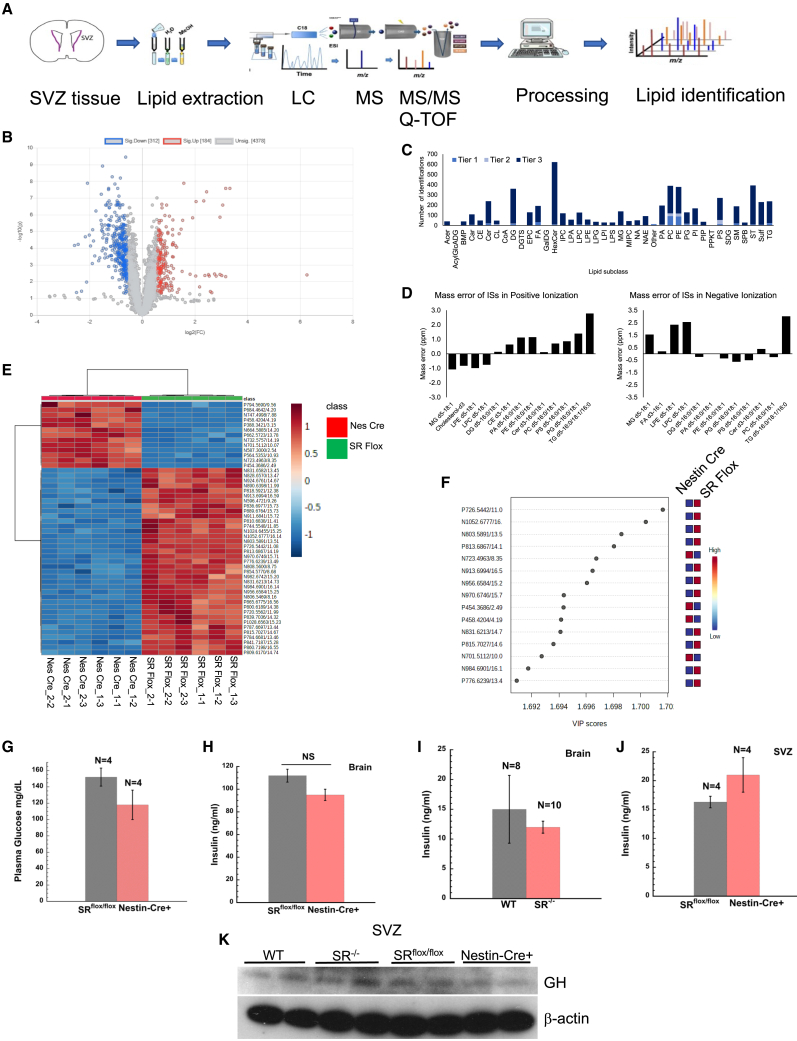
Global lipidomic profile altered in the SVZ of adult SRflox/flox and Nestin-Cre+ mice
Recent evidence implicates lipids in stem cell development and proliferation (Knobloch, 2017; Knobloch et al., 2013; Knobloch and Jessberger, 2017; Lodhi et al., 2011). Our rationale stems from serine metabolism regulating ceramide and sphingolipid levels including cell proliferation (Furuya et al., 2000; Gao et al., 2018; Kalhan and Hanson, 2012). We performed global lipidomic analysis in SVZ of age-matched SRflox/flox and nestin-cre+ mice by lipid extraction followed by UPLC-MS/MS identification (Figure 4A). Volcano plot with fold change (mean nestin-cre+/mean SRflox/flox) ≥1.5 or ≤0.667 and p value adjusted for false discovery rate <0.05 (criteria for significance), resulted in 184 lipids with fold change ≥1.5 (red) and 312 lipids with fold change ≤0.667 (blue) (Figure 4B). The different lipid classes were identified in three tiers of MS/MS analysis (Figure 4C). In tier one (404 features with MS/MS match score threshold of 500), two (146 features with MS/MS match score threshold of 100), and three (remaining features in the lipidome database for putative mass match) with a mass error threshold of 5.0 mDa were identified. Out of 8,648 unique features detected, 5,262 features (60.6%) were identified. The summed intensities showed good stability in data acquisition (Figure S4C). Thirteen internal standards were detected in positive ionization and 12 internal standards in negative ionization (Figure 4D). The mass error was less than 2.8–3.0 ppm. Heatmap of the lipidomic analysis with the top 50 lipids (ranked by p value) is shown in Figure 4E. The heatmap data show substantial downregulation of highly ranked lipids (by p value) in the SVZ of nestin-cre+ mice. Out of the top 50 lipids, 37 were downregulated and 13 upregulated in nestin-cre+ mice compared with SRflox/flox. The identified lipids are listed in Tables S4 and S5. Partial least squares-discriminant analysis with variable importance in prediction scores of 15 of the most important lipids (Figure 4F, Table S1) identified a diverse group of lipids that were significantly altered. A caveat of nestin-cre mouse strain is the presence of metabolic phenotypes (Declercq et al., 2015; Harno et al., 2013). Nestin-cre strain show lower levels of growth hormone, impaired insulin sensitivity, and impaired glucose levels on a high-fat diet. In order to rule out confounding effects, we estimated plasma glucose and insulin levels in age-matched SR flox/flox and nestin-cre+ mice fed a normal diet. Our data show no difference in plasma glucose levels in SRflox/flox and nestin-cre+ mice (Figure 4G). Total brain and SVZ insulin showed no differences (Figures 4H and 4J). We determined brain insulin levels in age-matched WT and SR−/− mice (background on which SRflox/flox mice were generated) to rule out mice background effects. Our data showed no difference in brain insulin in WT and SR−/− mice (Figure 4I). We determined expression of growth hormone in the SVZ and found no change in WT and SR−/− mice except a modest decrease in nestin-cre+ mice (Figure 4K). Control experiments suggest that the metabolic differences in our work are due to conditional deletion of Srr. The lipidomics data clearly highlight major differences among the different lipid classes in the SVZ of nestin-cre+ mice.
Figure 4.
Global lipidomic profile in the SVZ of nestin-cre+ mice
(A) Schematic of lipidomics workflow.
(B) Parametric volcano plot showing fold change against p value adjusted for false discovery rate. Blue circles indicate downregulation (312 lipids), red indicates upregulation (184 lipids) of lipids. Unassigned lipids are shown in gray.
(C) Lipid identification tier. In tier 1 (404 features), 2 (146 features), and 3 (remaining features).
(D) Mass error of internal standards.
(E) Heatmap of top 50 lipids ranked by p value.
(F) Plot shows partial least squares-discriminant analysis with variable importance in prediction scores of 15 most important lipids.
(G) Plasma glucose levels in SRflox/flox and nestin-cre+ mice.
(H and I) Insulin concentration (ng/mL) in mice whole brain homogenates.
(J) Insulin concentration (ng/mL) in mice SVZ homogenates. Error bars represent SD. NS = nonsignificant.
(K) Expression of growth hormone in mice SVZ. Error bars represent SD. Data are representative of three independent experiments. Tissues were pooled from n = 5–8 mice per experiment. For SVZ lipidomics, each genotype comprised tissues from 5 to 8 mice and injections in triplicate (see method for details).
Major lipid classes altered in the SVZ of nestin-cre+ mice
Global lipidomic analysis in the SVZ of nestin-cre+ and SRflox/flox mice by UPLC-MS/MS showed significant alterations in the different classes of lipids. We plotted lipidomics data as fold change relative to SRflox/flox (significant). The different lipid classes were fatty acid conjugates, sphingomyelins, phosphatidylcholine, phosphatidic acid, ceramides, sterols, carnitine, tri, di, and monoacylglycerols, hexosylceramides, and lysophosphatidic acid. Fatty acid conjugates are polyunsaturated fatty acids with conjugate double bonds. Out of the three fatty acid conjugates, two showed modest decrease in nestin-cre+ while FA 12:0; O4 showed 2-fold higher levels in nestin-cre+ mice (Figure S3A, Table S2). L-serine availability affects the synthesis of ceramides, sphingomyelin, and phosphatidylserine (Esaki et al., 2015; Gao et al., 2018). Our data show reduction by >50% in all the sphingomyelin species identified (Figure S3B, Table S2). Most notable decrease was seen in sphingomyelin 38:1; O2 in nestin-cre+ mice. Phosphatidylcholine synthesis was significantly reduced (>40%) in the SVZ of nestin-cre+ mice (Figures S3C, S4A and Table S2). Phosphatidic acid (low abundance phospholipid) showed increases (≥2-fold) in PA 8:0_25:0, PA 36:1 and PA 19:2_19:2 and decrease (≥2-fold) in the remaining molecules in nestin-cre+ mice (Figure S3D, Table S2). All identified ceramides in our screen showed approximately 40% or higher reduction in nestin-cre+ mice (Figure S3E, Table S2). Sterol expression was consistently increased above 3-fold in all the identified species with the exception of ST 22:1; O4 whose expression was down 10-fold in nestin-cre+ mice (Figure S3F, Table S2). Carnitine (a branched non-essential amino acid) expression was reduced by 50% for three of the identified four species with the exception of CAR 24:1; O2, which was increased 2-fold in nestin-cre+ mice (Figure S3G, Table S2). Both tri and diacylglycerols (synthesized from fatty acyl-CoA and glycerol 3 phosphate) were reduced by 50% or more in nestin-cre+ mice with the exception of TG 70:3, TG 75:4 (Figure S3H, Table S2) and DG 34:1 and DG O-24:1 (Figure S3I, Table S2) that were increased (≥2.5-fold). Monoacylglycerols were increased 2-fold in nestin-cre+ mice (Figure S3J, Table S2). Hexosylceramides are sphingolipids with hexose ring attached to a ceramide. Nestin-cre+ mice showed consistent downregulation (≥40%) in all the hexosylceramide molecules identified (Figure S3K, Table S2). Lysophosphatidic acid (a bioactive phospholipid produced during cell membrane synthesis) showed bidirectional trends with LPA 30:2 and LPA 32:3 being reduced 2-fold and LPA O-18:3, LPA O-22:3 and LPA O-26:3 increased more than 5-fold in nestin-cre+ mice (Figure S3L, Table S2). Our lipidomic screen showed substantial alterations in most lipid classes in the SVZ of nestin-cre+ mice.
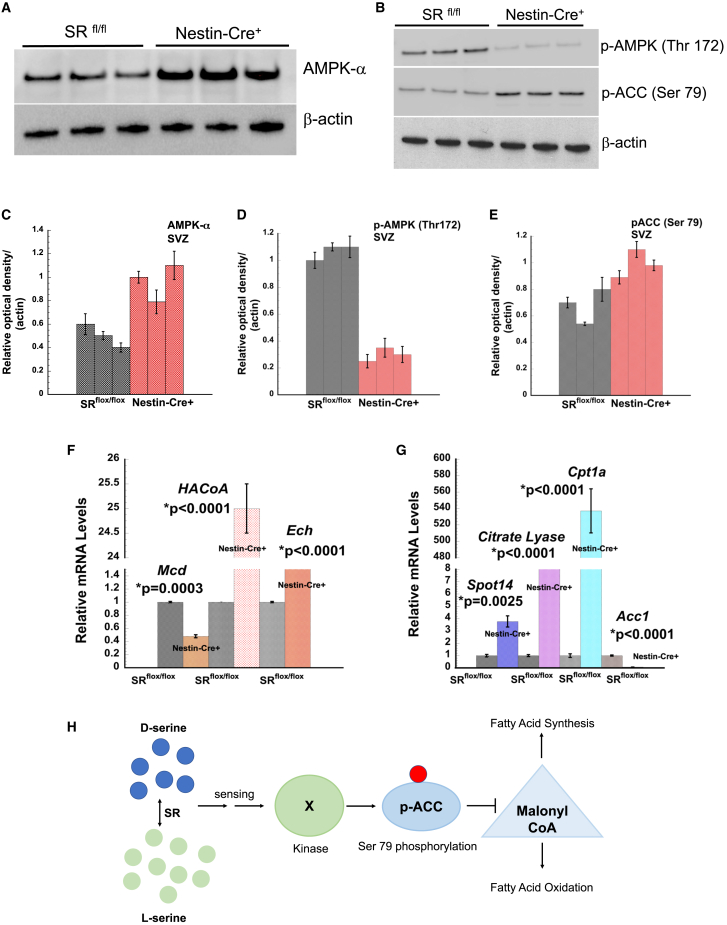
Non-AMPK-dependent ACC control of fatty acid metabolism in nestin-cre+ SVZ
ACC is a regulator of de novo fatty acid synthesis (Garcia and Shaw, 2017; Steinberg et al., 2006). We observed increased activation of phosho-ACC (Ser79) in nestin-cre+ mice (Figures 5B and 5E). AMP-activated protein kinase (AMPK) is a cellular sensor of nutrient availability and ATP. It controls overall cellular lipid metabolism through direct phosphorylation of ACC1 and ACC2 at Ser 79, suppressing fatty acid synthesis and simultaneously promoting fatty acid oxidation by relieving suppression of carnitine palmitoyl transferase 1 (CPT1) (Gonzalez et al., 2020; Garcia and Shaw, 2017). We observed more than 2-fold increased expression of AMPK (α) in SVZ of nestin-cre+ mice (Figures 5A and 5C). Expression of p-AMPK (Thr172) levels were, surprisingly, 5-fold lower in nestin-cre+ SVZ, suggesting a non-AMPK-dependent phosphorylation of ACC (Figures 5B and 5D). Based on the observed trends in de novo fatty acid synthesis, we determined expression of key genes in the synthesis and β-oxidation pathways. Citrate lyase (Cl) converts citrate from carbohydrate metabolism to acetyl-CoA. We observed an 8-fold increase in Cl mRNA expression in nestin-cre+ mice (Figure 5G). CPT1a is a key rate-limiting enzyme in long chain fatty acid oxidation and its activity is negatively regulated by malonyl-CoA. We observed approximately 500-fold increase in Cpt1a mRNA expression in nestin-cre+ SVZ (Figure 5G). ACC1 catalyzes the biotin dependent irreversible carboxylation of acetyl-CoA to malonyl-CoA, which serves as a key precursor in fatty acid synthesis. Our data showed 8-fold downregulation of Acc1 and 4-fold upregulation of Spot14 in the SVZ of nestin-cre+ mice (Figure 5G). We determined mRNA levels of key genes involved in the β-oxidation pathway. Malonyl-CoA decarboxylase (Mcd), which converts malonyl-CoA to acetyl-CoA, was downregulated by 50% in nestin-cre+ mice (Figure 5F). Hydroxyacyl-CoA (HaCoA) dehydrogenase, which is involved in β-oxidation of hydroxyacyl-CoA to oxoacyl-CoA, was elevated 30-fold in nestin-cre+ mice (Figure 5F). Enoyl-CoA Hydratase (Ech) catalyzes the hydration of a double bond between the second and third carbon atoms on 2-trans/cis enoyl-CoA thioester to produce β-hydroxyacyl-CoA thioester. We observed >8-fold expression of Ech in the SVZ of nestin-cre+ mice (Figure 5F). Collectively, these data suggest that SR may alter serine synthesis and availability, compromising the de novo fatty acid synthesis pathway. A concomitant increase in β-oxidation pathway may compensate for energy production in the SVZ of nestin-cre+ mice (Figure 5H).
Figure 5.
Expression of AMPK-α, phospho-AMPK, phospho-ACC, and fatty acid metabolism enzymes
(A) Expression of AMPK-α in the mice SVZ.
(B) Expression of p-ACC and phospho-AMPK in mice SVZ.
(C) Densitometry of AMPK-α blot shown in (A).
(D) Densitometry of p-AMPK blot in (B).
(E) Densitometry plot of p-ACC blot in (B).
(F) Relative mRNA levels of Mcd, HaCoA, and Ech in the SVZ of mice.
(G) Relative mRNA levels of Cpt1a, Acc1, Spot 14, and Cl in SVZ of mice. Error bars represent SD. Data are representative of three independent experiments. RNA was isolated from n = 5–8 mice per genotype in each experiment.
(H) Schematic of SR-mediated phosphorylation of ACC.
L- and D-serine rescue defects in adult SVZ neurogenesis and proliferation in vitro
In order to rescue defects in adult SVZ neurogenesis and proliferation in nestin-cre+ and SR−/− mice, we performed experiments in adherent NSCs treated with exogenous L-serine (0.6 mM) and D-serine (0.1 mM) individually and in an equimolar racemic mixture (0.1 mM each). Since neurobasal media contains basal L-serine concentration of 0.4 mM, and no D-serine, we used L-serine at a concentration of 0.6 mM. Control was media containing 0.4 mM basal L-serine with no exogenous L- and/or D-serine. We determined incorporation of BrdU in WT and SR−/− NSCs. SR−/− NSCs show significantly reduced incorporation of BrdU (Figure 6A). However, exogenous L- and D-serine increased the number of proliferating cells incorporating BrdU in SR−/− NSCs (Figures 6B, 6C, 6F). L- and D-serine treatment showed comparable BrdU incorporation in SR−/− NSCs. We also performed rescue of SOX2 in NSCs and observed lower expression of SOX2 in SR−/− NSCs (Figure 6D). Treatment with equimolar L- and D-serine, showed increased expression of SOX2 in both WT and SR−/− NSC (Figures 6D and S7B). We observed decreased NEUN expression in SR−/− control NSCs, but upon treatment with equimolar mixture of L- and D-serine, NEUN expression was higher than WT. We also observed that treated WT NSCs showed increased NEUN expression compared with control (Figures 6E and S7A). Nestin-cre+ mice showed increased expression of AMPK-α (Figure 5A). To determine if L- and/or D-serine alters levels of AMPK-α, we determined its expression in a rescue experiment. SR−/− NSCs grown in presence of L- and D-serine (0.6 mM and 0.1 mM, respectively) showed decreased expression of AMPK-α compared with untreated controls, indicating that AMPK-α expression is regulated by L- and D-serine. Interestingly, D-serine appeared to have a more potent effect on AMPK-α expression compared with L-serine (Figure 6G).
Figure 6.
L- and D-serine rescue defects in SVZ neurogenesis and proliferation in vitro
(A) BrdU incorporation in WT and SR−/− NSCs treated with 10 μM BrdU for 18 h at 37°C.
(B) BrdU incorporation in cells treated with 0.6 mM L-serine.
(C) BrdU incorporation in cells treated with 0.1 mM D-serine.
(D) Expression of SOX2 in control (top) and NSCs treated with equimolar mixture of L- and D-serine (100 μM) (lower panel).
(E) Expression of NEUN in differentiating neurons in control (top) and mixture of L- and D-serine (bottom). Scale bar, 100 μm.
(F) Number of BrdU-positive cells per high-powered field in NSCs from WT and SR−/− mice SVZ. An average of 4–5 fields were counted. Error bars represent SD.
(G) Expression of AMPK-α in WT and SR−/− NSCs.
L- and D-serine rescue defects in de novo fatty acid synthesis and differentiation
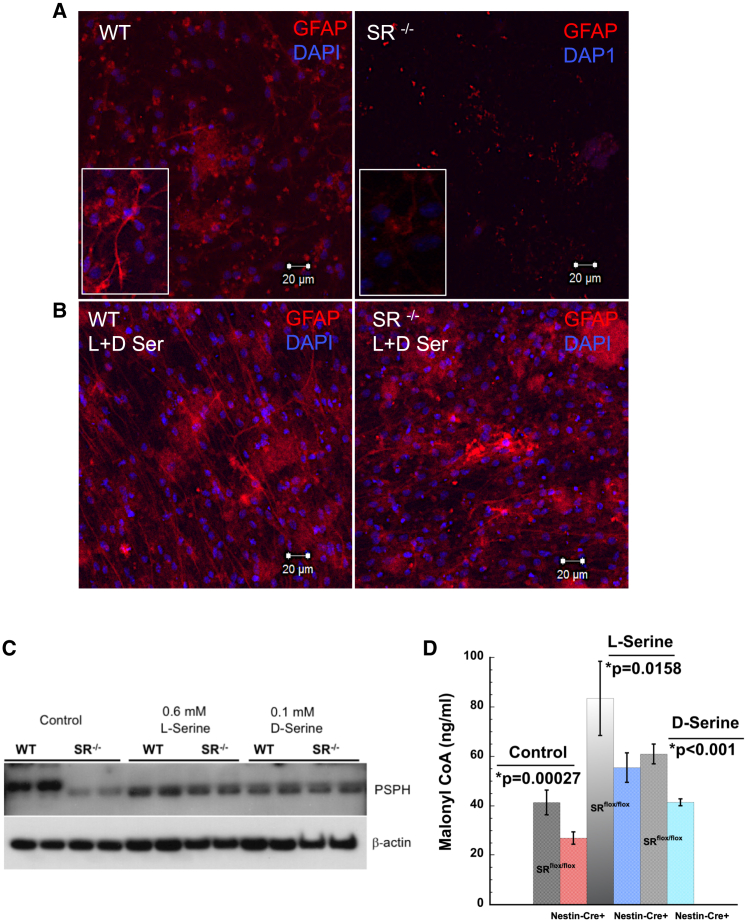
To determine if L- and D-serine regulate PSPH, we treated NSCs with L- and D-serine (0.6 mM and 0.1 mM, respectively). Expression of PSPH was decreased in SR−/− NSCs (Figure 7C). Treatment with D-serine showed modest increase in PSPH compared with L-serine in SR−/− NSCs and a decrease in WT. The magnitude of decrease was greater in WT NSCs, while the increased expression in SR−/− NSCs was smaller compared with untreated controls (Figure 7C). These data suggest that L- and D-serine regulate expression of PSPH via a feedback mechanism (Fell and Snell, 1988; Snell and Fell, 1990). Our data on NSCs from SR−/− showed differences in GFAP expression during differentiation (Figure S1C), suggesting that defects in adult neurogenesis in SR−/− mice may result from defects in astrocytic differentiation (Doetsch, 2003). To determine mature astrocytic differentiation, expression of GFAP was examined. Results show decreased expression of GFAP in differentiating SR−/− NSCs under control conditions (Figure 7A). Treatment with equimolar racemic mixture of L- and D-serine (100 μM) showed increased GFAP expression in SR−/− and modest increase in WT NSCs (Figures 7B and S7C). Similar rescue of NCAM expression (Figure S4B) was observed in WT and SR−/− NSCs treated with L- and D-serine (Figure S7D). Rescue of NCAM expression was greater in WT and SR−/− NSCs treated with L-serine than D-serine (Figure S7D). To determine if levels of malonyl-CoA can be rescued with similar treatment, NSCs grown under control conditions showed approximately 2-fold lower levels in nestin-cre+ compared with SRflox/flox, while treatment with exogenous L- and D-serine showed 2-fold and higher levels of malonyl-CoA in both SRflox/flox and nestin-cre+ NSCs. The magnitude of increase was greater with L-serine than D-serine (Figure 7D). These data show both L- and D-serine treatment rescue defects in astrocytic, neuronal differentiation and malonyl-CoA levels.
Figure 7.
L- and D-serine rescue defects in de novo fatty acid synthesis and differentiation
(A) Expression of GFAP (red) in differentiating NSCs from WT and SR−/− mice.
(B) Expression of GFAP in differentiating NSCs from WT and SR−/− mice cultured in the presence of equimolar mixture of L-serine and D-serine (100 μM). Scale bar, 20 μm.
(C) Expression of PSPH in NSCs treated with L-serine and D-serine.
(D) Levels of malonyl-CoA (ng/mL) in NSCs cultured from SRflox/flox and nestin-cre+ mice in the presence of 0.6 mM L-serine and 0.1 mM D-serine. Error bars represent SD. Data are representative of three independent experiments. Tissues for each experiment were pooled from n = 5–8 mice per genotype.
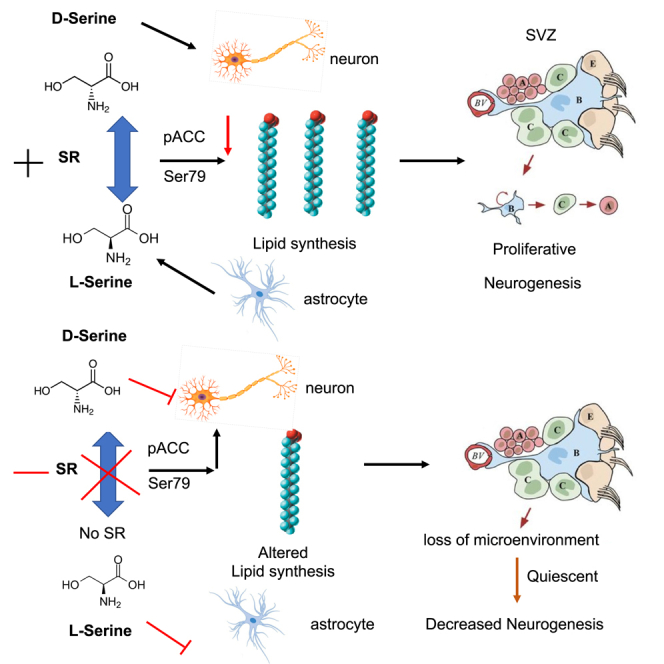
Model of SR mediated metabolic control of adult SVZ neurogenesis
We present a simplified summary elucidating the role of SR in mediating adult SVZ neurogenesis via lipid metabolism (Figure S6G). SR controls the availability of L-serine and D-serine via the neuron-strocyte shuttle (Wolosker et al., 2017; Ehmsen et al., 2013). Availability of L- and D-serine affects the synthesis of lipids by activation of ACC by an unidentified kinase. Overexpression of Spot14 and Mig12 in nestin-cre+ and SR−/− SVZ regulates the catalytic activity of ACC (Knobloch et al., 2013; Park et al., 2013). Activation of ACC by phosphorylation at Ser 79 leads to inhibition of de novo fatty acid synthesis by decreasing levels of malonyl-CoA and FASN. Reduction in de novo fatty acid synthesis affects the formation of newborn neurons in the SVZ (Type A and B cells) and their proliferation, due to loss of their membrane microenvironment (Clemot et al., 2020; Knobloch et al., 2017).
Discussion
Homeostatic energy changes in the neurogenic niches appear to influence the proliferative properties of adult NSCs. Lipid metabolism plays a major role in adult neurogenesis (Clemot et al., 2020; Knobloch et al., 2013; Knobloch and Jessberger, 2017). D-serine functions as a co-agonist at the NMDA receptor (Balu et al., 2013; Snyder and Kim, 2000). D-serine also mediates adult hippocampal neurogenesis (Sultan et al., 2013) and is involved in proliferation and differentiation of NSCs (Huang et al., 2012). D-cysteine, synthesized by SR plays a role in neurodevelopment (Semenza et al., 2021).
Conditionally deleting Srr elicits global changes in lipid profile in the adult SVZ. Our results indicate that SR activates ACC at Ser 79 by an unknown kinase (non-AMPK dependent) and at the tertiary level by affecting polymerization and catalytic activity of ACC due to its interaction with SPOT14 and MIG12 heterocomplex (Knobloch et al., 2013). While L-serine influences cellular proliferation and 1C metabolism, the role of D-serine in mammalian metabolism is unknown.
Lipid rafts are enriched in cholesterol and sphingolipids and involved in stem cell maintenance (Lingwood and Simons, 2010). Our lipidomics data show significant alterations in important lipids in nestin-cre+ SVZ. SR interacts with PSD95 and stargazin in activation of AMPA receptor (Ma et al., 2014). Deletion of Srr may impact neuronal metabolism due to serine availability and alteration in membrane signaling pathways. Downregulation of sphingomyelins, ceramides, hexosylceramides, and phosphatidylcholines, a constituent of cell membranes, was seen in nestin-cre+ SVZ. This affects proliferative properties of adult stem cells due to loss of membrane microenvironment.
Deletion of Srr downregulated PGDH and PSPH indicating alterations in serine levels. L-serine is a precursor for the synthesis of phosphatidylserine, sphingosine, and ceramide (Esaki et al., 2015). Our lipidomics data reveal 2-fold lower levels of a C22 ceramide species Cer22:0_21:0; O3 in nestin-cre+ SVZ. Reduction in C16, C18, C20, C22, and C24 acyl chain ceramide species were reported in mouse embryonic fibroblasts under L-serine deprivation (Esaki et al., 2015). Marked reduction in sphingomyelins, ceramides, and hexosylceramides in addition to other lipids was observed under L-serine deprivation in nestin-cre+ SVZ. Deletion of Srr results in significant reduction of D-serine synthesis (Balu et al., 2013; Basu et al., 2009). D-serine levels in both neurons and glia are dependent on PGDH, with lower expression leading to decrease in not only L-serine but also D-serine (Ehmsen et al., 2013; Esaki et al., 2015). Altered levels of L- and D-serine may locally affect cellular metabolism and synthesis of lipid precursors like sphingosine affecting the microenvironment of NSCs. Deficits in NSC malonyl-CoA was rescued by L- and D-serine. Rescue experiments showed metabolic compensation, resulting in restored expression of NCAM, SOX2, GFAP, and NEUN respectively.
BrdU incorporation identifies new neurons. SR−/− and nestin-cre+ mice show reduced incorporation of BrdU. Endogenous proliferation marker Ki-67 was also decreased in nestin-cre+ SVZ and in SR−/− primary neurons, highlighting their quiescent nature.
While we observed defects in astrocyte and neuronal differentiation, oligodendrocyte differentiation (OLIG2 expression) was enhanced both in SVZ and in NSCs. Deletion of Srr protected against cerebral ischemia and excitotoxicity in mice (Mustafa et al., 2010). Our observations highlight a role for SR in myelination repair with implications for multiple sclerosis and in stroke post recovery (Jia et al., 2019).
Hematopoietic stem cells rely on fatty acid oxidation for maintenance of the stem cell pool (Ito et al., 2012). While D-amino acids comprise peptidoglycans in prokaryotes, their function in mammals is unknown. SR phosphorylates ACC at Ser79, inhibiting synthesis of malonyl-CoA leading to decreased fatty acid synthesis. Interestingly, our data also showed that activation of ACC is independent of AMPK. While nestin-cre+ mice showed increased expression of AMPK dependent on L- and D-serine, it did not undergo activation by phosphorylation at Thr 172, suggesting that SR may activate additional hubs independent of AMPK. We have yet to identify the kinase responsible for ACC activation. Our data show that increased expression of SPOT14 and Mig12 in nestin-cre+ SVZ may regulate the catalytic activity of ACC due to its effect on ACC polymerization (Knobloch et al., 2013; Park et al., 2013).
Alterations in SVZ fatty acid synthesis affect the NSC microenvironment and their stemness, resulting in reduced newborn neurons and quiescence. We speculate on possible connections between serine and lipid metabolism. Glutamate is a known activator of ACC (Brownsey et al., 2006). Due to structural similarities with glutamate, serine may serve as an activator of ACC. PLP is a structural analog of citrate, which also regulates ACC. PLP inhibits both isoforms of ACC (Brownsey et al., 2006). In the absence of SR, excess unbound PLP may inhibit the activity of ACC, thereby regulating fatty acid synthesis.
In summary, SR plays a role in lipid-mediated adult SVZ neurogenesis via regulation of ACC. Deletion of Srr alters levels of L- and D-serine impacting proliferative properties in NSCs. Metabolic control of neurogenesis by supplementation of L- and D-serine may prove beneficial in Alzheimer’s disease and schizophrenia that have an underlying component of adult neurogenesis (Le Douce et al., 2020; Schoenfeld and Cameron, 2015; Yun et al., 2016).
Experimental procedures
See supplemental information for detailed protocols.
Resource availability
Corresponding author
Robin Roychaudhuri (robinroychaudhuri@gmail.com) Current address: University of Maryland School of Medicine, Department. of Obstetrics, Gynecology and Reproductive Sciences, Center for Birth Defects, Baltimore, MD 21201.
Materials availability
All materials will be available upon request to the authors.
Data and code availability
The lipidomics data are available at the National Metabolomics Data Repository (NMDR), the Metabolomics Workbench, https://www.metabolomicsworkbench.org where it has been assigned Study ID ST002705. The data can be accessed directly via its Project DOI: https://doi.org/10.21228/M82H8Q. This work is supported by NIH grant U2C-DK119886 and OT2-OD030544 grant.
Generation of nestin-cre+ SR mice
Nestin-cre+ mice were generated by breeding SRflox/flox mice (Basu et al., 2009) with nestin-cre mice obtained from Jackson labs (number 003771). All animal experiments were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine.
BrdU (bromodeoxyuridine) labeling
Eight-week-old WT and age-matched SR−/− mice were administered 1 mg/mL BrdU (BromodeoxyUridine) in water by means of a water bottle within the cage for 14 days (ad libitum).
Micro dissection of adult mouse brain SVZ
Mice brains were dissected, removed, and kept in sterile PBS. The Petri dish was placed under a dissecting microscope with light source at low magnification on the ventral surface and the olfactory bulbs were removed by holding the cerebellum. The brain was then rotated onto the dorsal aspect and using a sterile blade a coronal cut was made at the level of the optic chiasm (Walker and Kempermann, 2014).
Adherent monolayer stem cell culture
The NSC adherent monolayer culture system is a valuable tool for determining the proliferative and differentiative potential of adult neural stem cells in vitro. Cell culture and differentiation of precursor cells in adherent monolayer cultures was followed as per the protocol of Walker and Kempermann (2014).
Neuroblast assay
Neuroblasts (Type A cells) were visualized after plating the cells obtained from dissociation of adherent monolayer cultures following accutase treatment. The dissociated monolayer cells were plated in a 15-μm 24-well plate at 2–3 × 105 cells/mL in complete NSC medium supplemented with 20 ng/mL EGF and 10 ng/mL b-FGF.
Mouse FASN ELISA
Mouse FASN (fatty acid synthase) expression in mice brain and SVZ was measured quantitatively using a mouse FASN ELISA kit.
Mouse malonyl-CoA ELISA
Estimation of mouse malonyl-CoA in SVZ lysates was measured quantitatively using a mouse malonyl-CoA ELISA kit.
Quantitative lipidomic analysis
Quantitative lipidomic analysis on SVZ tissue from SRflox/flox and nestin-cre+ mice was performed at The Metabolomics Innovation Center University of Alberta, Canada.
Analysis of gene expression
RNA was isolated from SVZ of WT and SR−/−, SRflox/flox, and nestin-cre+ mice using an RNA isolation kit. Gene expression studies were performed using the SYBR green method.
Rescue in monolayer adherent cultures
Rescue experiments in adherent monolayer cultures were performed by isolating NSCs from the SVZ of age-matched adult mice. The cells were grown in culture for 7–10 days, following which differentiation was induced by gradual removal of growth factors as mentioned in Walker and Kempermann (2014).
Time spent sniffing novel odor
Mouse olfactory behavior was tested in age-matched WT and SR−/− mice. Mice (n = 20–25 per group) were collected and brought to the behavior suite and the mice were placed in clean cages with new bedding.
Blood glucose estimation
Blood glucose level was monitored by tail bleed using Contour glucometer and Contour blood glucose test strips. Blood glucose measurements were obtained from tail veins at indicated time points post injection.
Insulin ELISA
Quantitative estimation of insulin from brain homogenates and SVZ of age-matched WT, SR−/−, SRflox/flox, and nestin-cre+ mice was performed using the Ultra Sensitive Mouse Insulin ELISA Kit.
Statistics
Statistical tests were computed using KaleidaGraph (Synergy Software, Reading, PA). Data are represented as mean ± SD. Unpaired Student’s t test was used for pairwise comparison with a fixed control condition. For multiple pairwise comparisons with different control and treatment conditions, one-way ANOVA analysis followed by Tukey’s post hoc test was used. Values with p < 0.05 were considered significant.
Author contributions
R.R. conceived, designed, and performed all experiments and analyzed all the data. R.R. wrote, edited, and revised the manuscript. H.A. performed stem cell differentiation experiments. All authors reviewed the manuscript.
Acknowledgments
The authors acknowledge Barbara Smith at The Johns Hopkins Microscopy Core Facility for confocal imaging, Lauren Albacarys for performing olfactory behavior tests, Dr. Paul Kim for initial technical assistance, and Evan R. Semenza for helpful discussions. The authors acknowledge Prof. Peixin Yang at the University of Maryland School of Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences for contributing reagents during revision. The work was funded by grant P50 DA044123 from NIDA to S.H.S.
Conflict of interests
The authors declare no competing interests.
Published: June 22, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.05.015.
Supplemental information
Shown are the unique identification number, lipid molecule, and the class, related to Figure 4F
Number in each table indicates the lipid molecule identified in the respective class (plotted in Supplemental Figure 3 on the x axis), followed by the lipid ID and fold change in nestin-cre+ mice relative to SRflox/flox (on the y axis), related to Figure 4E
References
- Azari H., Reynolds B.A. In vitro models for neurogenesis. Cold Spring Harbor Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari H., Sharififar S., Fortin J.M., Reynolds B.A. The neuroblast assay: an assay for the generation and enrichment of neuronal progenitor cells from differentiating neural stem cell progeny using flow cytometry. J. Vis. Exp. 2012 doi: 10.3791/3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu D.T., Li Y., Puhl M.D., Benneyworth M.A., Basu A.C., Takagi S., Bolshakov V.Y., Coyle J.T. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl. Acad. Sci. USA. 2013;110:E2400–E2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A.C., Tsai G.E., Ma C.L., Ehmsen J.T., Mustafa A.K., Han L., Jiang Z.I., Benneyworth M.A., Froimowitz M.P., Lange N., et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatr. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R., Rougon G., Chazal G. Increased neurogenesis in adult mCD24-deficient mice. J. Neurosci. 2002;22:3594–3607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal A., Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018;75:2177–2195. doi: 10.1007/s00018-018-2794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R.W., Boone A.N., Elliott J.E., Kulpa J.E., Lee W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Clémot M., Sênos Demarco R., Jones D.L. Lipid mediated regulation of adult stem cell behavior. Front. Cell Dev. Biol. 2020;8:115. doi: 10.3389/fcell.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq J., Brouwers B., Pruniau V.P.E., Stijnen P., de Faudeur G., Tuand K., Meulemans S., Serneels L., Schraenen A., Schuit F., Creemers J.W.M. Metabolic and behavioural phenotypes in nestin-cre mice are caused by hypothalamic expression of human growth hormone. PLoS One. 2015;10:e0135502. doi: 10.1371/journal.pone.0135502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat. Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F., Caillé I., Lim D.A., García-Verdugo J.M., Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ehmsen J.T., Ma T.M., Sason H., Rosenberg D., Ogo T., Furuya S., Snyder S.H., Wolosker H. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J. Neurosci. 2013;33:12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki K., Sayano T., Sonoda C., Akagi T., Suzuki T., Ogawa T., Okamoto M., Yoshikawa T., Hirabayashi Y., Furuya S. L-serine deficiency elicits intracellular accumulation of cytotoxic deoxysphingolipids and lipid body formation. J. Biol. Chem. 2015;290:14595–14609. doi: 10.1074/jbc.M114.603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R., Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D.A., Snell K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 1988;256:97–101. doi: 10.1042/bj2560097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S., Tabata T., Mitoma J., Yamada K., Yamasaki M., Makino A., Yamamoto T., Watanabe M., Kano M., Hirabayashi Y. L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc. Natl. Acad. Sci. USA. 2000;97:11528–11533. doi: 10.1073/pnas.200364497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Lee K., Reid M.A., Sanderson S.M., Qiu C., Li S., Liu J., Locasale J.W. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 2018;22:3507–3520. doi: 10.1016/j.celrep.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., Shaw R.J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Hall M.N., Lin S.C., Hardie D.G. AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metabol. 2020;31:472–492. doi: 10.1016/j.cmet.2020.01.015. [DOI] [PubMed] [Google Scholar]
- Harno E., Cottrell E.C., White A. Metabolic pitfalls of CNS Cre-based technology. Cell Metabol. 2013;18:21–28. doi: 10.1016/j.cmet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Huang X., Kong H., Tang M., Lu M., Ding J.H., Hu G. D-Serine regulates proliferation and neuronal differentiation of neural stem cells from postnatal mouse forebrain. CNS Neurosci. Ther. 2012;18:4–13. doi: 10.1111/j.1755-5949.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Carracedo A., Weiss D., Arai F., Ala U., Avigan D.E., Schafer Z.T., Evans R.M., Suda T., Lee C.H., Pandolfi P.P. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Kamen Y., Pivonkova H., Káradóttir R.T. Neuronal activity-dependent myelin repair after stroke. Neurosci. Lett. 2019;703:139–144. doi: 10.1016/j.neulet.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Kalhan S.C., Hanson R.W. Resurgence of serine: an often neglected but indispensable amino Acid. J. Biol. Chem. 2012;287:19786–19791. doi: 10.1074/jbc.R112.357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N., Sivalingam S., Boonstra R., Wojtowicz J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Oxford University Press; 2006. Adult Neurogenesis Stem Cells and Neuronal Development in the Adult Brain. [Google Scholar]
- Knobloch M. The role of lipid metabolism for neural stem cell regulation. Brain Plast. 2017;3:61–71. doi: 10.3233/BPL-160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M., Braun S.M.G., Zurkirchen L., von Schoultz C., Zamboni N., Araúzo-Bravo M.J., Kovacs W.J., Karalay O., Suter U., Machado R.A.C., et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M., Jessberger S. Metabolism and neurogenesis. Curr. Opin. Neurobiol. 2017;42:45–52. doi: 10.1016/j.conb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Knobloch M., Pilz G.A., Ghesquière B., Kovacs W.J., Wegleiter T., Moore D.L., Hruzova M., Zamboni N., Carmeliet P., Jessberger S. A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity. Cell Rep. 2017;20:2144–2155. doi: 10.1016/j.celrep.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M., von Schoultz C., Zurkirchen L., Braun S.M.G., Vidmar M., Jessberger S. SPOT14-positive neural stem/progenitor cells in the hippocampus respond dynamically to neurogenic regulators. Stem Cell Rep. 2014;3:735–742. doi: 10.1016/j.stemcr.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douce J., Maugard M., Veran J., Matos M., Jégo P., Vigneron P.A., Faivre E., Toussay X., Vandenberghe M., Balbastre Y., et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in alzheimer's disease. Cell Metabol. 2020;31:503–517.e8. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Lim D.A., Alvarez-Buylla A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harbor Perspect. Biol. 2016;8:a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lodhi I.J., Wei X., Semenkovich C.F. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol. Metabol. 2011;22:1–8. doi: 10.1016/j.tem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T.M., Paul B.D., Fu C., Hu S., Zhu H., Blackshaw S., Wolosker H., Snyder S.H. Serine racemase regulated by binding to stargazin and PSD-95: potential N-methyl-D-aspartate-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (NMDA-AMPA) glutamate neurotransmission cross-talk. J. Biol. Chem. 2014;289:29631–29641. doi: 10.1074/jbc.M114.571604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma J., Furuya S., Hirabayashi Y. A novel metabolic communication between neurons and astrocytes: non-essential amino acid L-serine released from astrocytes is essential for developing hippocampal neurons. Neurosci. Res. 1998;30:195–199. doi: 10.1016/s0168-0102(97)00113-2. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Mustafa A.K., Ahmad A.S., Zeynalov E., Gazi S.K., Sikka G., Ehmsen J.T., Barrow R.K., Coyle J.T., Snyder S.H., Doré S. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J. Neurosci. 2010;30:1413–1416. doi: 10.1523/JNEUROSCI.4297-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Hwang I.W., Makishima Y., Perales-Clemente E., Kato T., Niederländer N.J., Park E.Y., Terzic A. Spot14/Mig12 heterocomplex sequesters polymerization and restrains catalytic function of human acetyl-CoA carboxylase 2. J. Mol. Recogn. 2013;26:679–688. doi: 10.1002/jmr.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L.H., Nicolis S.K. Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Reid M.A., Allen A.E., Liu S., Liberti M.V., Liu P., Liu X., Dai Z., Gao X., Wang Q., Liu Y., et al. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 2018;9:5442. doi: 10.1038/s41467-018-07868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M.J., Brady R.O., Jr., Molliver M.E., Snyder S.H. D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld T.J., Cameron H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza E.R., Harraz M.M., Abramson E., Malla A.P., Vasavda C., Gadalla M.M., Kornberg M.D., Snyder S.H., Roychaudhuri R. D-cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2110610118. e2110610118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Fell D.A. Metabolic control analysis of mammalian serine metabolism. Adv. Enzym. Regul. 1990;30:13–32. doi: 10.1016/0065-2571(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Snyder S.H., Kim P.M. D-amino acids as putative neurotransmitters: focus on D-serine. Neurochem. Res. 2000;25:553–560. doi: 10.1023/a:1007586314648. [DOI] [PubMed] [Google Scholar]
- Steinberg G.R., Macaulay S.L., Febbraio M.A., Kemp B.E. AMP-activated protein kinase--the fat controller of the energy railroad. Can. J. Physiol. Pharmacol. 2006;84:655–665. doi: 10.1139/y06-005. [DOI] [PubMed] [Google Scholar]
- Sultan S., Gebara E.G., Moullec K., Toni N. D-serine increases adult hippocampal neurogenesis. Front. Neurosci. 2013;7:155. doi: 10.3389/fnins.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C.K., Alvarez-Buylla A. SnapShot: adult neurogenesis in the V-SVZ. Neuron. 2014;81:220–220.e1. doi: 10.1016/j.neuron.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.L., Kempermann G. One mouse, two cultures: isolation and culture of adult neural stem cells from the two neurogenic zones of individual mice. J. Vis. Exp. 2014:e51225. doi: 10.3791/51225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Z., Fan H., Ji Y., Reynolds K., Gu R., Gan Q., Yamagami T., Zhao T., Hamad S., Bizen N., et al. Olig2 regulates terminal differentiation and maturation of peripheral olfactory sensory neurons. Cell. Mol. Life Sci. 2020;77:3597–3609. doi: 10.1007/s00018-019-03385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C., Rolletschek A., Kania G., Blyszczuk P., Tarasov K.V., Tarasova Y., Wersto R.P., Boheler K.R., Wobus A.M. Nestin expression--a property of multi-lineage progenitor cells? Cell. Mol. Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt R.M., Galligan M.M., Despinoy J.R., Segal R. Olfactory behavioral testing in the adult mouse. J. Vis. Exp. 2009:949. doi: 10.3791/949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., Balu D.T., Coyle J.T. Astroglial versus neuronal D-serine: check your controls. Trends Neurosci. 2017;40:520–522. doi: 10.1016/j.tins.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., Blackshaw S., Snyder S.H. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., Sheth K.N., Takahashi M., Mothet J.P., Brady R.O., Jr., Ferris C.D., Snyder S.H. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc. Natl. Acad. Sci. USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M., Yamada K., Furuya S., Mitoma J., Hirabayashi Y., Watanabe M. 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J. Neurosci. 2001;21:7691–7704. doi: 10.1523/JNEUROSCI.21-19-07691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Reynolds R.P., Masiulis I., Eisch A.J. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 2016;22:1239–1247. doi: 10.1038/nm.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown are the unique identification number, lipid molecule, and the class, related to Figure 4F
Number in each table indicates the lipid molecule identified in the respective class (plotted in Supplemental Figure 3 on the x axis), followed by the lipid ID and fold change in nestin-cre+ mice relative to SRflox/flox (on the y axis), related to Figure 4E
Data Availability Statement
The lipidomics data are available at the National Metabolomics Data Repository (NMDR), the Metabolomics Workbench, https://www.metabolomicsworkbench.org where it has been assigned Study ID ST002705. The data can be accessed directly via its Project DOI: https://doi.org/10.21228/M82H8Q. This work is supported by NIH grant U2C-DK119886 and OT2-OD030544 grant.