Abstract
An immunosuppressive state is a typical feature of the tumor microenvironment. Despite the dramatic success of immune checkpoint inhibitor (ICI) therapy in preventing tumor cell escape from immune surveillance, primary and acquired resistance have limited its clinical use. Notably, recent clinical trials have shown that epigenetic drugs can significantly improve the outcome of ICI therapy in various cancers, indicating the importance of epigenetic modifications in immune regulation of tumors. Recently, RNA modifications (N6-methyladenosine [m6A], N1-methyladenosine [m1A], 5-methylcytosine [m5C], etc.), novel hotspot areas of epigenetic research, have been shown to play crucial roles in protumor and antitumor immunity. In this review, we provide a comprehensive understanding of how m6A, m1A, and m5C function in tumor immunity by directly regulating different immune cells as well as indirectly regulating tumor cells through different mechanisms, including modulating the expression of immune checkpoints, inducing metabolic reprogramming, and affecting the secretion of immune-related factors. Finally, we discuss the current status of strategies targeting RNA modifications to prevent tumor immune escape, highlighting their potential.
Graphical abstract

Public summary
-
•
RNA modification is a novel hotspot of epigenetic research, affecting a wide range of physiological and pathological processes.
-
•
RNA modification plays an important role in tumor immunity.
-
•
RNA modification may be a potential clinical therapeutic target to prevent tumor immune escape.
Introduction
The immune system plays a crucial role in host defense against tumors. Antitumor responses mainly rely on activated CD8+ T cells recognizing tumor antigens. In addition, innate immune cells are pivotal in eliminating tumors. Despite the immune system aiming to eliminate tumor cells, tumor cells with reduced immunogenicity in an immunosuppressive tumor microenvironment (that is, one that includes immunosuppressive cells and immunosuppression-related molecules) can escape T cell attack. Immune checkpoint inhibitor (ICI) therapy can overcome immune escape and provide significant benefits to patients, and its application has revolutionized the field of tumor therapy. However, despite ICI effectiveness, most patients have a poor response to ICIs (primary resistance), and some patients who initially respond develop resistance after a period of treatment (secondary resistance), highlighting the necessity to explore novel treatment strategies to enhance the anticancer potency of immunotherapies.
RNA modification, a crucial epigenetic change, has become a research hotspot since its discovery in the 1950s.1 To date, more than 100 chemical modifications of RNA have been identified;2 these modifications include methylation to generate N1-methyladenosine (m1A),3 N6-methyladenosine (m6A),4 5-methylcytosine (m5C),5 and N7-methylguanosine (m7G);6 RNA cap methylation;7 pseudouridine formation;8 and uridylation.9 The enzymes that catalyze the addition and removal of these modifications from RNA are called “writers” and “erasers,” respectively, and the RNA-binding proteins that identify modified RNA modifications are called “readers.” All types of RNA modifications are regulated by “readers,” “writers,” and “erasers.” These modifications play multifunctional roles in the RNA life cycle, affecting factors such as translation efficiency, transcript stabilization, pre-mRNA splicing, nuclear export, and mRNA storage under stress.4,10,11,12,13,14,15 Hence, RNA modifications affect a wide range of physiological and pathological processes.13,16,17
Notably, the combination of epigenetic drugs with immunotherapy may be a useful strategy to circumvent ICI resistance. For example, the combination of pembrolizumab (a PD-L1 inhibitor) plus entinostat (a histone deacetylase inhibitor) provides a clinically meaningful benefit to non-small cell lung cancer (NSCLC) patients who have shown resistance to PD-L1 inhibitors.18 In addition, administration of guadecitabine (a DNA methylation inhibitor) combined with pembrolizumab to patients with an advanced solid tumor can reverse previous resistance to ICIs.19 The positive results of these clinical trials indicate the importance of epigenetics in tumor immune regulation. Notably, RNA modifications have also been found to play a critical role in tumor immunity. For example, methyltransferase-like protein 3 (METTL3), a typical m6A reader, inhibits tumor growth and metastasis by enhancing the YTHDF1-mediated translation of SPRED2.20 Moreover, YTHDF1, a typical m6A reader, impairs tumor antigen cross-presentation of dendritic cells (DCs) by enhancing the translation of lysosomal cathepsin transcripts.21 Although numerous studies have been performed, details regarding the roles of RNA modifications in regulating tumor immunity remain unclear. Some reviews have summarized the critical roles of m6A in tumorigenesis, progression,22 and cancer metabolism.23 However, the roles of m6A and other RNA modifications (including m1A and m5C) in tumor immunity and the underlying mechanisms have not been systematically described, and we attempt to provide an up-to-date and comprehensive overview of these topics in this review.
In this review, we outline the evidence showing that m6A, m1A, and m5C modifications participate in tumor immunity by directly regulating different immune cells as well as by indirectly regulating tumor cells. Our review emphasizes the essential roles played by RNA modifications in shaping tumor immunity and highlights potential therapeutic strategies to prevent immune escape of tumor cells by targeting RNA modifications.
The m6A modification in tumor immunity
Overview of the m6A RNA modification
The m6A modification is the most prevalent RNA modification on mRNA and regulates the fate of mRNA by affecting its stability, splicing, translation, and export. It plays an important role in tumour immunity (Table 1). M6A was first discovered in 197424 and is now considered the most representative type of RNA modification, accounting for 0.1%–0.4% of all adenosine molecules in total cellular RNA, with an average of 3–5 m6A modifications per mRNA.25 RRACH (R = G or A; H = A, C, or U) is the consensus sequence in mRNA on which the m6A modification is found, and a combination of immunoprecipitation and high-throughput sequencing results has revealed that the m6A modification is enriched at stop codons and 3′ UTRs.26 However, the consensus sequence for the m6A modification is different on noncoding RNAs (ncRNAs), including long ncRNAs (lncRNAs), microRNAs (miRNAs), circular RNAs (circRNAs), small nuclear RNAs (snRNAs), and ribosomal RNAs (rRNAs).27,28 Based on the known m6A modification sites, several RNA modification databases have been established (Table 2), and various m6A modification site prediction algorithms have been proposed (Table 3), such as WHISTLE,29 SRAMP,30 BERMP,31 iRNA-PseColl,32 and Gene2vec.33 Despite these advances, the specificities of m6A modification sites are still poorly understood.
Table 1.
Functions of m6A regulators in tumor immunity
| Regulators | Cancer type | Function | References | |
|---|---|---|---|---|
| Writers | METTL3 | breast cancer | enhance PD-L1 expression | Wan et al.104 |
| bladder cancer | enhance PD-L1 expression | Ni et al.105 | ||
| NSCLC | reduce degradation of PD-L1 | Liu et al.106 | ||
| colorectal carcinoma | reduce recruitment of CD8+ T cells and the levels of IFN-γ, CXCL9, and CXCL10 | Wang et al.121 | ||
| colorectal cancer | facilitate MDSC accumulation by promoting CXCL1 expression | Chen et al.122 | ||
| RBM-15 | ccRCC | increase macrophage infiltration and M2 polarization by promoting CXCL11 expression | Zeng et al.123 | |
| METTL14 | CCA | reduce degradation of PD-L1 | Zheng et al.107 | |
| HCC | enhance PD-L1 expression | Peng et al.108 | ||
| colorectal carcinoma | reduce recruitment of CD8+ T cells and the levels of IFN-γ, CXCL9, and CXCL10 | Wang et al.121 | ||
| Readers | IGF2BP1 | HCC | enhance PD-L1 expression | Liu et al.109 |
| YTHDF1 | colon cancer | enhance PD-L1 expression | Li et al.110 | |
| GC | reduce DC recruitment and T cell infiltration by reducing expression of IFNGR1 | Bai et al.115 | ||
| YTHDF3 | downregulate PD-L1 expression | Zhao et al.113 | ||
| Erasers | ALKBH5 | ICC | enhance PD-L1 expression | Qiu et al.111 |
| melanoma/colon cancer | reduce sensitivity to immunotherapy | Li et al.118 | ||
| GBM | promote secretion of CXCL8/IL8 and recruitment of TAMs | Dong et al.124 | ||
| HCC | increase PD-L1+ macrophage recruitment | You et al.125 | ||
| Promote tumor progression by reducing RIG-I expression | Jin et al.126 | |||
| FTO | melanoma | enhance PD-1 (PDCD1), CXCR4, and SOX10 expression | Yang et al.112 | |
| leukemia | maintain immune evasion by enhancing LILRB4 expression | Su et al.114 | ||
| melanoma | suppress infiltration and function of CD8+ T cells | Liu et al.119 |
Table 2.
RNA modification databases
| Database | Resources | Website | References |
|---|---|---|---|
| m6A2Target | m6A modifies the associated writers/erasers/readers | http://m6a2target.canceromics.org/#/ | Bao et al.220 |
| m6A-Atlas | m6A sites and quantitative epitranscriptome profiles | http://180.208.58.66/m6A-Atlas/index.html | Tang et al.221 |
| REPIC | m6A-seq and MeRIP-seq data | https://repicmod.uchicago.edu/repic | Liu et al.222 |
| DirectRMDB | Oxford Nanopore Technologies (ONT)-based database of quantitative RNA modification profiles | http://www.rnamd.org/directRMDB/ | Zhang et al.223 |
| MODOMICS | Modified residues, enzymes, guide RNAs, RNA modification pathways, sequences of modified RNAs, diseases | https://iimcb.genesilico.pl/modomics/ | Boccaletto et al.202 |
| RMVar | Multiple modification types, lncRNA/circRNA/miRNA methylation-associated SNPs/SNVs, RNA bing proteins (RBPs), diseases | http://rmvar.renlab.org/ | Luo et al.224 |
| RMDisease V2.0 | 1,366,252 RM-associated variants that may affect 16 different types of RNA modifications, diseases | www.rnamd.org/rmdisease2 | Song et al.225 |
| RMBase | Multiple methylation types, SNP/SNVs, RBPs, miRNA modifications | http://rna.sysu.edu.cn/rmbase/ | Xuan et al.226 |
| m5C-Atlas | m5C sites, associated SNPs, relevance to RNA secondary structure, RBPs | https://www.xjtlu.edu.cn/biologicalsciences/m5c-atlas | Ma et al.227 |
Table 3.
RNA modification site prediction methods
To understand the roles of RNA modifications, appropriate analytical methods or techniques are essential. The lack of specific antibodies limits functional exploration to some extent. However, considerable improvements in methods for deciphering RNA modifications have been achieved in the last decade.34,35,36 The methods for detection of RNA modifications have evolved from liquid chromatography,37 reverse-transcriptase polymerase chain reaction (RT-PCR),38 and thin-layer chromatography (TLC)39 to mass spectrometry (MS)40 and sequencing analysis.41 Liquid chromatography (LC) coupled to MS (LC-MS) or tandem MS (LC-MS/MS) are the most commonly used MS methods to quantify RNA modifications on single nucleosides.36 Recently, an LC-electrospray ionization (ESI)-MS/MS method was developed. It was used to profile and characterize the RNA modifications in plant 18S rRNA and 25S rRNA.42 In addition, this method was also used to detect modifications of RNA in rat peripheral blood during alcohol exposure43 and in thyroid carcinoma tissue.44
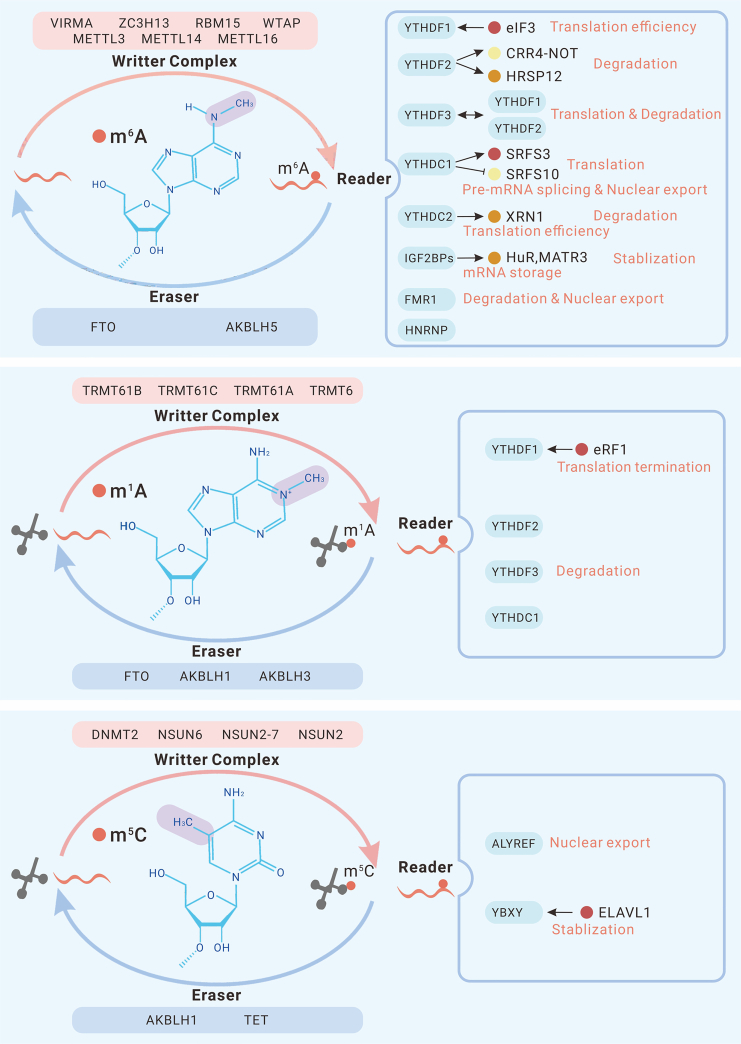
m6A modifications are reversible and regulated by three types of enzymes: m6A methyltransferases (“writers”), m6A demethylases (“erasers”), and m6A-binding proteins (“readers”). The deposition of a methyl group to form m6A relies on the m6A methyltransferase complex (MTC), comprising the METTL3-methyltransferase-like protein 14 (METTL14) heterodimer and other adaptor proteins, including WT1-associated protein (WTAP), RNA-binding motif protein 15 (RBM15), vir-like m6A methyltransferase associated (VIRMA) subunits, and zinc-finger CCCH domain-containing protein 13 (ZC3H13).45,46 METTL3, discovered in 1999, was the first identified m6A methyltransferase.47 An S-adenosyl methionine (SAM)-dependent methyltransferase catalyzes RNA methylation using SAM, a prevalent methyl donor in vivo, and is critical for almost all m6A modifications on mRNA. In 2014, METTLE14 and WTAP were identified as other important MTC subunits.48,49 In the complex, METTL3 is the catalytic core, METTL14 is the RNA-binding platform, and WTAP is crucial for recruitment of the MTC.45,49 In addition to the aforementioned complexes and factors, the functions of methyltransferase-like protein 16 (METTL16) and the METTL5-TRMT112 complex have also been validated.50 m6A demethylases can remove a methyl group from m6A modifications on mRNA and cooperate with m6A methyltransferases to maintain the dynamic balance of m6A modifications. Fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5) are two well-known m6A demethylases.51,52 In general, m6A modifications are involved in functions via their recognition by m6A-binding proteins, including five members of the YTH domain-containing family (YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2),53 insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1, IGF2BP2, and IGF2BP3),15 heterogeneous nuclear ribonucleoprotein proteins (HNRNPA2B1 and HNRNPC),54,55 leucine-rich pentatricopeptide repeat-containing (LRPPRC),56 fragile X mental retardation 1 (FMR1),57 and proline-rich coiled-coil 2 C (Prrc2c).58 Their roles are described in detail below.
m6A modifications affect the fate of mRNAs by regulating and controlling gene expression in various ways in different subcellular locations, as facilitated by reader proteins. (Figure 1) YTH domain-containing family members are the principal readers. The YTH structural domain recognizes and binds m6A modification sites. YTHDF1 interacts with translation initiation factor complex 3 (eIF3) and enhances mRNA translation efficiency,4 whereas YTHDF2 accelerates target transcript degradation in two ways: deadenylation mediated by carbon catabolite repression 4 (CCR4)-negative on TATA-less (NOT)10 complex action and endoribonucleolytic cleavage mediated by heat-responsive protein 12 (HRSP12).11 YTHDF3 cooperates with YTHDF1 to enhance protein synthesis by interacting with ribosomal proteins and increases YTHDF2-mediated mRNA decay,59 suggesting that YTHDF proteins interact with each other to synthetically influence fundamental biological processes associated with m6A RNA modification.60 Moreover, multiple m6A-modified mRNAs form phase-separated YTHDF-m6A-mRNA complexes by binding to YTHDF1–YTHDF3, which, in turn, form phase-separated structures in cells and may function during cellular stress to induce storage of certain mRNAs encoding cellular repair proteins.61 YTHDC1 facilitates exon inclusion in targeted mRNAs by recruiting arginine/serine-splicing factor 3 (SRFS3) and blocking serine/arginine-splicing factor 10 (SRSF10) binding, thus promoting mRNA translation.62 In addition, YTHDC1 affects mRNA density by splicing pre-mRNA transcripts13 and mediating the export of mRNA.14 YTHDC2 also enhances the translation efficiency of target mRNAs, shows 3'→5′ RNA helicase activity, and recruits XRN1, which is a 5'→3′ exoribonuclease, by inserting ankyrin repeats.63 In contrast to the decay-promoting function of YTHDF2, IGF2BPs (IGF2BP1–IGF2BP3) have been shown to stabilize target mRNAs in a manner facilitated by their cofactors (HuR and MATR3) and to promote the storage of target mRNAs under stress.15 In a previous study, FMR1 was found to bind to m6A to enhance mRNA stability.12 However, a recent study revealed that Drosophila FMR1 bound preferentially to mRNAs containing the m6A-tagged “AGACU” motif, forming FMR1-ribonucleoprotein (RNP) granules that then underwent a dynamic phase switch to promote mRNA decay.64 FMR1 has also been proven to promote nuclear export of m6A-modified mRNAs.65 As mentioned above, m6A-binding proteins play different or even contradictory roles in mRNA modification. YTHDF2 promotes mRNA degradation, but IGF2BPs have the opposite effect. In addition, in older and more recent studies, FMR1 has been reported to exert the opposite effect on mRNA stability. In addition, interactions between different RNA-binding proteins can be either cooperative or competitive.57,59 These complex regulatory roles may explain the diversity of m6A mechanisms of action and biological effects.
Figure 1.
Regulators of RNA modifications and their molecular functions
All RNA modifications are catalyzed by writers and removed by erasers. They can be recognized by their respective reader proteins and affect the fate of target RNAs by regulating their nuclear export, splicing, translation, and degradation.
In addition to influencing the fate of mature mRNAs, m6A modifications regulate the generation and function of ncRNAs, which are also of physiological importance. Among miRNAs, HNRNPA2B1 is involved in regulating variable shearing of exons and subsequent processing of certain primary miRNAs (pri-miRNAs) by binding to the microprocessor complex DGCR8.54 Some lncRNAs are closely related to cancers and are regulated by RNA-modification-related enzymes, such as DMDRMR and MALAT1.66 Moreover, recent studies on m6A have focused on its interaction with the nucleus and chromatin as well as its role in nascent RNA processes, by which it can regulate gene transcription. m6A modifications on the lncRNA X-inactive-specific transcript (XIST) placed by RBM15 participate in transcriptional repression of X chromosome gene expression.67 m6A modifications on chromosome-associated regulatory RNAs (carRNAs) deposited by METTL3 and YTHDC1 accelerate the decay of these RNAs.68 This process is involved in regulating the state of nearby chromatin and downstream transcription.68 Regarding enhancer RNAs (eRNAs) and nascent transcripts, m6A-modified eRNAs can recruit the nuclear m6A reader YTHDC1 and promote formation of BRD4, which is related to enhancer and gene activation,69 and the m6A modification of nascent transcripts protects them from termination mediated by the integrator complex, thus enhancing gene expression.70 Moreover, m6A can bind to YTHDC1, and YTHDC1 can recruit KDM3B via physical interaction, which promotes histone dimethylated histone H3 Lys9 (H3K9me2) demethylation and gene expression.71
Overall, m6A modification of mRNAs and ncRNAs is involved in regulation of almost all important life processes. Recently, m6A modifications have been shown to regulate tumor immunity, and details regarding the regulatory network housing tumor immune factors and m6A modifications are described below. Collectively, m6A modifications regulate the immune response through two main processes: by directly affecting the function of immune cells and by indirectly regulating tumor cells. In the following sections, both of these mechanisms are outlined.
m6A regulates tumor immunity by directly affecting immune cells
Macrophages
Macrophages, vital components of innate immunity, can be polarized into different phenotypes depending on their genetic background and stimuli in the environment. A general classification of macrophages places these cells into the classically activated macrophage (M1) and alternatively activated macrophage (M2) categories.72 M1 macrophages show high antigen-presenting and T cell activation abilities, and M2 macrophages are related to immunosuppression.73 Recent studies have revealed that m6A is involved in regulating polarization of macrophages under physiological and inflammatory conditions. METTL3 drives M1 polarization by enhancing the stability of signal transducer and activator of transcription 1 (STAT1) mRNA.74 However, IGF2BP2 facilitates the switch of M1 macrophages into M2 macrophages by stabilizing tuberous sclerosis complex 1 (TSC1) and peroxisome proliferator-activated receptor γ (PPARγ).75 In addition to affecting polarization, m6A regulates the activation of macrophages. METTL3 has been identified as a positive regulator. For example, deletion of METTL3 in macrophages impairs their ability to eliminate pathogens because of reduced degradation of Irakm transcripts and subsequent inhibition of the Toll-like receptor 4 (TLR4) signaling pathway.76 However, FTO, a demethylase, activates macrophages by enhancing the stability of STAT1 and PPARγ in a YHTDF2-dependent manner.77 Moreover, m6A inhibits activation of certain cellular programs, with METTL14 and FTO preventing their hyperactivation. METTL14 mediates m6A methylation of suppressor of cytokine signaling 1 (SOCS1) mRNA and promotes translation of SOCS1 by binding to YTHDF1, an interaction that is essential for sustaining the negative feedback loop established via macrophage activation.78 In addition, knocking out FTO in macrophages mediates similar inflammation inhibition and leads to increased m6A abundance on SOCS1 mRNA, thereby facilitating transcript stability and subsequent translation in a YHTDF1-dependent manner79 (Figure 2).
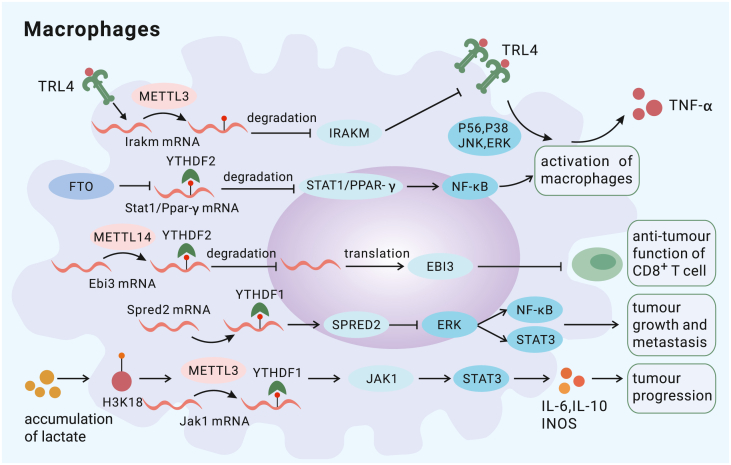
Figure 2.
The mechanisms by which m6A modification regulates macrophages
METTL3 (a writer) promotes production of TNF-α by enhancing degradation of Irakm (an inhibitor of TRL4) mRNA, and it also inhibits tumor growth by targeting SPRED2 (an inhibitor of the ERK pathway). However, METTL3 enhances immunosuppression through the Jak1/STAT3 pathway. METTL14 (a writer) increases degradation of Ebi3 mRNA, promoting the antitumor function of CD8+ T cells. FTO (an eraser) reduces degradation of STAT1/PPAR-γ mRNA, promoting macrophage activation.
Tumor-associated macrophages (TAMs) acquire an M2 phenotype and either infiltrate tumors or reside near tumor tissues. TAMs promote tumor progression and subvert antitumor immunity.80 Recently, the mechanisms by which m6A regulates the function of TAMs have been further elucidated. METTL3 and METTL14 are crucial for maintaining the antitumor function of macrophages. Deletion of METTL3 in myeloid cells creates an environment conducive to tumor growth and metastasis by increasing infiltration of immunosuppressive cells.20 Further research revealed that METTL3 deficiency in macrophages leads to a reduction in YTHDF1-mediated translation of SPRED2 and inhibition of the nuclear factor κB (NF-κB) pathway.20 Another study highlighted that C1q+ TAMs with high expression of METTL14 and YTHDF2 express diverse immunomodulatory ligands, which enhances the recruitment and function of CD8+ effector T cells (TEFFs).81 Moreover, METTL14-deficient C1q+ TAMs show elevated Ebi3, a cytokine subunit that negatively regulates T cell function, which results in CD8+ T cell dysfunction.81 On the other hand, m6A readers, including IGF2BP2 and YTHDF1, have been shown to play a significant inhibitory role in macrophage antitumor ability. By binding to IGF2BP2, the lncRNA PACERR induces pro-oncogenic effects and promotes M2 polarization in pancreatic cancer by stabilizing KLF12 and c-myc in the cytoplasm.82 In addition, METTL3 enhances the immunosuppressive function of tumor-infiltrating myeloid cells (TIMs) through the Jak1/STAT3 pathway by promoting Jak1 translation through the m6A-YTHDF1 axis.83 This process may be driven by lactate accumulation in the form of H3K18 lactylation marks83 (Figure 2).
DCs
DCs are antigen-presenting cells (APCs) that process antigenic information, thereby connecting innate and adaptive immune responses.84 Recent studies have provided some clues about how m6A regulates stimulation, migration, and presentation of DCs under physiological conditions. METTL3-mediated mRNA m6A methylation enhances DC-mediated stimulation of T cell activation and cytokine production by enhancing the translation efficiency of CD40, CD80, and TLR4 signaling adaptor (Tirap) mRNA.85 In addition to affecting stimulation, m6A regulates DC migration. CC-chemokine receptor 7 (CCR7) is critical for DC migration to draining lymph nodes,86 and a feedback mechanism that inhibits CCR7-mediated DC migration in a manner mediated via m6A modification has been identified.87 Demethylation of the lncRNA Dpf3 induced by CCR reduces Dpf3 decay. Dpf3 binds to hypoxia-inducible factor 1 alpha (HIF-1α), impairing the glycolytic metabolism and migratory capacity of DCs by attenuating expression of the glycolytic gene Ldha87 (Figure 3).
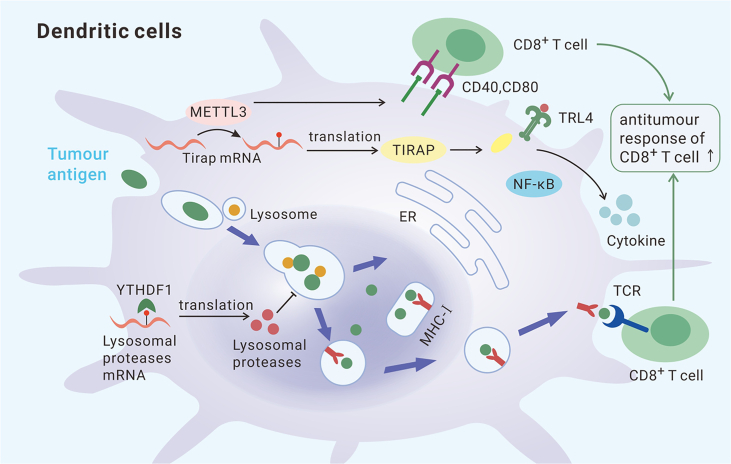
Figure 3.
The mechanisms by which m6A modification regulates DCs
METTL3 positively regulates the function of dendritic cells (DCs) by increasing CD40, CD80, and Tirap (TLR4 signaling adaptor) translation. YTHDF1 inhibits the cross-priming ability of DCs targeting lysosomal proteases, suppressing the CD8+ T cell antitumor response.
Other studies have focused on the tumor background. For example, on the basis of positive regulation of DC function by m6A, researchers have designed nanomedicines containing an FTO inhibitor, and such agents promote DC maturation and antigen presentation after thermal ablation of hepatocellular carcinoma (HCC) cells.88 However, contrary to previous findings, YTHDF1 has been found to be a negative regulator of DC antitumor effects. YTHDF1-silenced mice show improved tumor antigen cross-presentation by DCs and antitumor responses by CD8+ T cells.21 Mechanistically, YTHDF1 enhances translation of lysosomal cathepsin transcripts, which contributes to antigen degradation, thereby reducing DC cross-priming capacity21 (Figure 3).
Natural killer cells
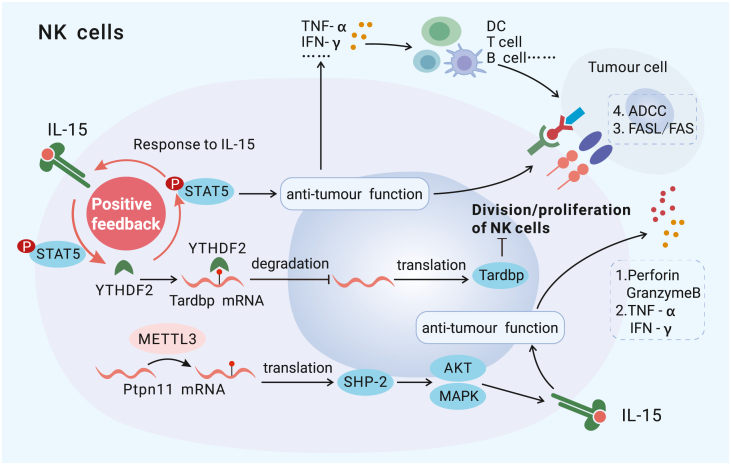
Natural killer (NK) cells constitute an important part of the innate immune system and are essential effector cells in antitumor immunity because they exert cytotoxic effects on tumor cells through surface receptors.89,90 They also regulate the antitumor immune response by expressing cytokines and chemokines that influence the recruitment and action of other immune cells, such as T cells and DCs.91,92 Recent studies have shown that regulators of m6A consistently activate NK cells. YTHDF2 deficiency profoundly impairs the antitumor and antiviral capacities of NK cells.93 YTHDF2 plays multifaceted roles in NK cells, including maintaining homeostasis, regulating terminal maturation, and favoring response to interleukin-15 (IL-15) by enhancing the stability of Tardbp mRNA.93 In addition, METTL3 is an important positive regulator of NK cell antitumor effects because it maintains homeostasis and infiltration of NK cells.94 Conditional ablation of METTL3 in NK cells weakened the immune response to IL-15 exposure, and this mitigated response was related to reduced expression of Src-homology phosphotyrosine phosphatase-2 (SHP-2) mediated by a reduction in m6A abundance on Ptpn11 mRNA94 (Figure 4).
Figure 4.
The mechanisms by which m6A modification regulates NK cells
METTL3 enhances the antitumor function of NK cells by promoting SHP-2 (a critical mediator of IL-15-induced activation) translation. YTHDF2 (a writer) positively regulates NK cell division/proliferation by increasing Tardbp mRNA degradation. The STAT5–YTHDF2 positive feedback loop, which is downstream of IL-15, controls NK cell effector functions.
T cells
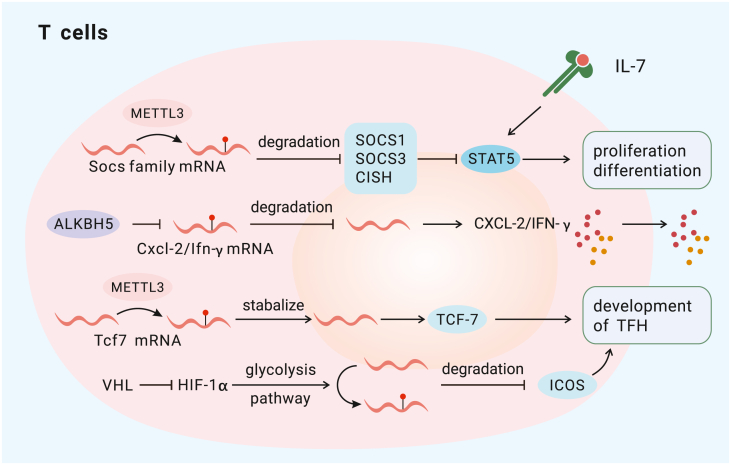
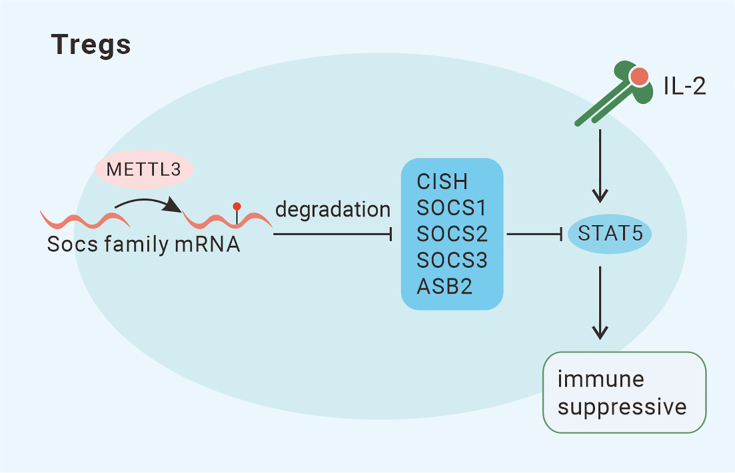
T cells, including CD4+ T cells and CD8+ T cells, play essential roles in adaptive immunity. Regulatory T cells (Tregs) constitute a special T cell subpopulation that controls self-tolerance and is associated with immunosuppression.95 Recent studies have provided some clues to explain the association between m6A regulators and the differentiation, homeostasis, survival, and pathogenicity of T cells. First, deleting METTL3 in T cells disrupts CD4+ T cell homeostasis and differentiation by upregulating the expression of SOCS family members (Socs1, Socs3, and Cish), which encode proteins that inhibit STAT signaling and thus downregulate IL-7-mediated STAT5 activation96 (Figure 5). Similarly, in Tregs, METTL3 has been shown to contribute to immune suppression through the IL-2/STAT5 signaling pathway by regulating the expression of SOCS family members (Cish, Socs1, Socs2, Socs3, and Asb2)97 (Figure 6). Second, after targeted knockdown of WTAP, cell death is induced by activation of the T cell receptor (TCR) signaling pathway.98 WTAP knockdown stabilizes Orai1 and Ripk1 mRNAs, and subsequent cell death is mediated by more intense and sustained Ca2+ signaling.98 Third, ALKBH5 controls the pathogenicity of CD4+ T cells.99 Ablation of ALKBH5 in naive CD4+ T cells suppresses development of colitis and autoimmune diseases by reducing interferon-γ (IFN-γ) and CXCL2 mRNA stability99 (Figure 5).
Figure 5.
The mechanisms by which m6A modification regulates T cells
METTL3 promotes proliferation and differentiation of T cells via the JAK/STAT5 pathway by decreasing the expression of SOCS family proteins (SOCS1, SOCS3, and CISH). ALKBH5 (an eraser) enhances production of CXCL-2 and IFN-γ by targeting CXCL-2 and IFN-γ mRNA degradation. METTL3 also promotes T follicular helper (Tfh) development by stabilizing Tcf-7 mRNA to increase TCF-1 (a regulator of Tfh differentiation) expression. The VHL-HIF-1α axis regulates Tfh cell development in an m6A-dependent manner.
Figure 6.
The mechanisms by which m6A modification regulates T cells
METTL3 mediates immune suppression via the JAK/STAT5 pathway by decreasing the expression of SOCS family proteins (SOCS1, SOCS2, SOCS3, CISH, and Asb2).
T follicular helper (Tfh) cells are another specific subpopulation of CD4+ T cells; they initiate formation of germinal centers and promote secretion of high-affinity antibodies by B cells.100 Knocking out METTLE3 in CD4+ T cells leads to poor differentiation of Tfh cells and, thus, impaired germinal center responses.101 Further experiments elucidated that m6A modification of the 3′ UTR of Tcf7 mRNA is mediated by METTL3 and that this modification stabilizes Tcf7 transcripts and subsequently ensures activation of Tfh cells.101 However, in another study, m6A modifications have been shown to exert the opposite effects on Tfh cell development. Specifically, m6A modification induced by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) accelerates degeneration of inducible costimulator (ICOS) mRNA and, thus, impairs Tfh cell initiation when von Hippel-Lindau (VHL) activity is impaired102 (Figure 5).
In addition to its effect on CD4αβ T cells, m6A regulates the development of γδ T cells. γδ T cells are involved in intrinsic immunity and are abundant in the mucosae, where they function in immune surveillance. m6A has been identified as a developmental checkpoint for immature γδ T cell development, and the number of γδ T cells has been shown to increase significantly when ALKBH5 is absent.103 Moreover, ALKBH5-mediated m6A demethylation decreases the stability and expression of Jagged1 and Notch2 mRNAs, inactivating the Jagged1/Notch2 signaling pathway.103
Above all, different m6A modification regulators play a variety of roles in different cells. For example, METTL3, the most classic m6A writer, has the most extensively studied function in immune cells, and its knockdown in macrophages, DCs, NK cells, and T cells impairs their functions. However, other m6A regulators, such as YTHDF2 in DCs, negatively regulate antitumor functions; therefore, inhibitors of these m6A regulators may be effective antitumor agents. Despite the diversity of roles of m6A modifications, there are common mechanisms regulating functions across different immune cells. First, m6A, as the most abundant modification on mRNA, regulates gene expression by regulating the stability, splicing, translation, and export of mRNAs. In immune cells, m6A mainly influences the translation efficiency and stability of mRNAs of related genes. Second, although the regulatory pathways are different, m6A modifications generally influence similar cellular processes in different immune cells, such as cell proliferation, differentiation, and secretion of cytokines, which ultimately affects their antitumor capacity.
In addition, interactions and synergies between different immune cells are worthy of attention. For example, CD8+ T cells, as the main antitumor effector cells, are downstream of many immune cell functions. Therefore, regulation of macrophage and DC function by m6A ultimately affects their antitumor function by influencing the recruitment, function, and activation of CD8+ T cells.21,81,85
m6A regulates the immune response by affecting tumor cells
In addition to its direct regulatory role in a variety of immune cells, m6A regulates antitumor immunity by indirectly regulating tumor cell function. As recent studies have shown, three main mechanisms, surface molecule expression, cytokine secretion, and metabolism reprogramming, are involved in m6A regulation of tumor cells, and these mechanisms are described below.
Expression of surface molecules
Tumor cells express surface molecules associated with tumor immunity, especially immune checkpoints such as PD-L1, CTLA-4, and CD276, which play important roles in regulating antitumor immunity by costimulating or coinhibiting the killing effect of T cells and are thus key targets for immunotherapy.
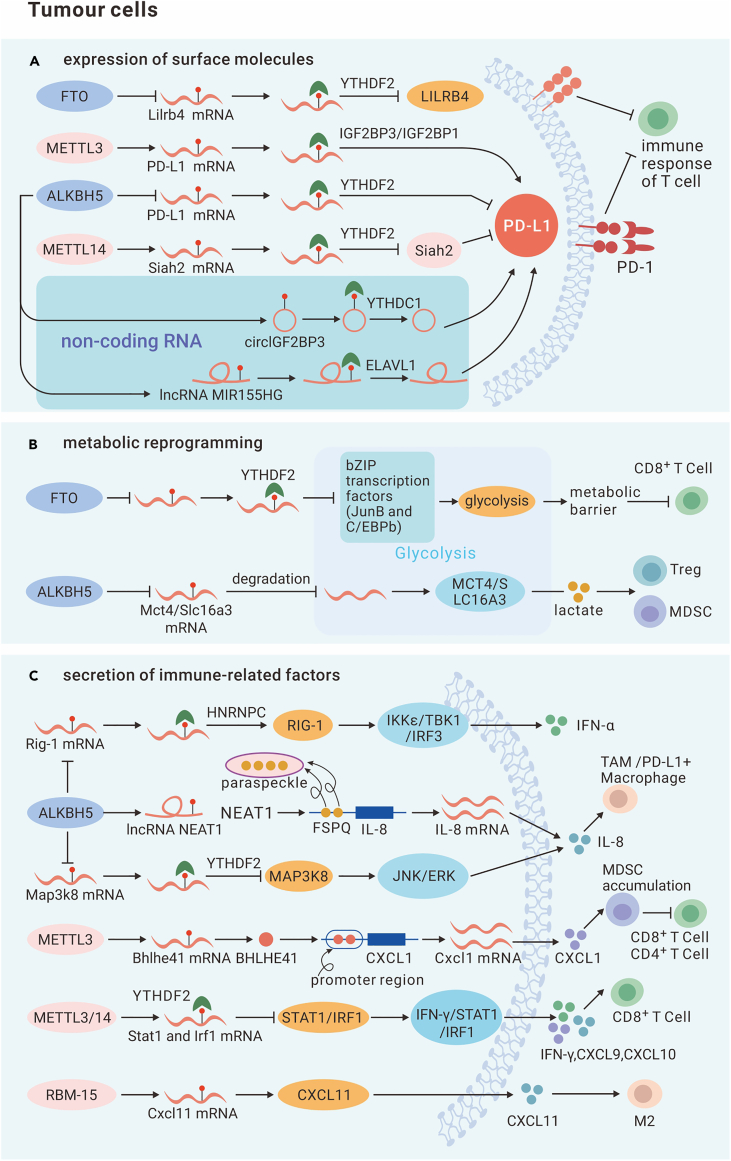
Several studies have demonstrated that PD-L1 expression in tumor cells is closely correlated with m6A modification of RNA. METTL3, a typical m6A methyltransferase, suppresses tumor immune surveillance in breast cancer.104 Knocking out METTL3 results in decreased m6A modification abundance on PD-L1 mRNA and, thus, reduced PD-L1 mRNA stability, leading to less IGF2BP3 binding to PD-L1 mRNA and downregulating PD-L1 expression.104 Similarly, in bladder cancer, METTL3 induced c-Jun N-terminal kinase (JNK) signaling activation to stabilize PD-L1 mRNA by promoting the interaction between the m6A site and IGF2BP1.105 In addition, METTL3 also mediates immune escape by affecting ncRNA modifications. METTL3 mediates the m6A modification of circIGF2BP3 in NSCLC and promotes cyclization of circIGF2BP3 by binding to YTHDC1, thereby increasing the content of circIGF2BP3.106 This increase in the circIGF2BP3 level in NSCLC contributes to tumor cell immune evasion by increasing PD-L1 levels through reduced PD-L1 ubiquitination and subsequent proteasomal degradation.106 METTL14 is another important m6A writer. METTL14 reduces the degradation of PD-L1 via the ubiquitination-dependent pathway by destabilizing Siah2 mRNA in a YTHDF2-dependent manner in cholangiocarcinoma (CCA).107 In addition, in HCC, LPS-induced METLL14 expression increased m6A modification abundance on the lncRNA MIR155HG, thereby stabilizing MIR155HG by binding to ELAVL1 (HuR) and subsequently increasing PD-L1 expression via the miR-223/STAT1 axis.108 Regarding reader proteins, knockdown of IGF2BP1 reduced PD-L1 expression and promoted immune infiltration in HCC.109 In addition to the abovementioned regulators, methionine and YTHDF1 have been shown to promote PD-L1 expression.110 FTO and ALKBH5 are the two main m6A demethylases. Conditional deletion of the ALKBH5 gene in intrahepatic CCA (ICC) cells increases m6A abundance on PD-L1 mRNA and increases PD-L1 mRNA decay by binding to YTHDF2.111 Knocking down FTO in melanoma enhances sensitivity to anti-PD-1 and IFN therapies by suppressing PD-1 (PDCD1), CXCR4, and SOX10 expression through YTHDF2-mediated mRNA decay, but it does not affect PD-L1 (CD274).112 In general, METTL3, METTL14, IGF2BP1, FTO, and ALKBH increase the level of PD-L1 on the membrane and thereby promote tumor cell immune escape. In summary, these mechanisms of PD-L1 regulation can either directly upregulate PD-L1 expression or reduce PD-L1 protein degradation. Therefore, speculating that inhibitors of METTL3, IGF2BP, FTO, and ALKBH may be promising targets for cancer therapy is reasonable, and moreover, combination of these inhibitors with PD-1 inhibitors may enhance the efficacy of anti-PD-1 therapy to overcome drug resistance. This possibility has been confirmed by numerous studies.109110 However, m6A on CBX1 mRNA decreases its stability via YTHDF3 recognition, and CBX1 upregulates PD-L1 expression via the IFN-γ-STAT1 pathway,113 suggesting diverse functions of m6A in tumor immunity (Figure 7A; Table 1).
Figure 7.
The mechanisms by which m6A modification induces immune evasion in tumor cells
(A) FTO promotes ILIRB4 (an immune checkpoint) expression by targeting its mRNA. METTL3 and ALKBH5 upregulate the expression of PD-L1 by targeting PD-L1 mRNA, thus enhancing immunosuppression. METTL3 also regulates ncRNAs (circIGF2BP3 and lncMIR155HG). METTL14 negatively regulates Siah2 (a RING E3 ubiquitin ligase that enhances PD-L1 degradation) to increase PD-L1.
(B) FTO increases the metabolic barrier for T cell activation and inhibits the function of CD8+ T cells targeting JunB and C/EBPb (glycolysis regulators). ALKBH5 enhances lactate content in tumor-infiltrating Tregs and MDSCs by targeting Mct4/Slc16a3.
(C) ALKBH5 inhibits production of IFN-γ and IL-8 via the ALKBH5/RIG-I/IFNα axis, the ALKBH5/paraspeckle/CXCL8 axis, and the ALKBH5/MAP3K8 axis. METTL3 promotes CXCL1 production by targeting BHLHE4 (which binds to the promoter region of CXCL1) and induces immunosuppression. METTL3/14 also inhibits IFN-γ, CXCL9, and CXCL10 by inhibiting IFN-γ-STAT1-IRF1 signaling. RBM-15 promotes CXCL11 production, thus inducing M2 polarization.
In addition to that of PD-1/PD-L1, the expression of other immune checkpoint ligands is regulated by m6A modifications. FTO has been shown to play a role in maintaining immune evasion in leukemia.114 LILRB4 is an important immune checkpoint, and reduced LILRB4 expression enhances T cell toxicity and antitumor immune responses. Inhibition or depletion of FTO increases degradation of m6A-modified LILRB4 mRNA, primarily by promoting m6A-modified LILRB4 mRNA binding to YTHDF2114 (Table 1).
In addition to the checkpoint ligands discussed above, expression of many other surface molecules regulates the immune response. YTHDF1 has been found to be overexpressed in gastric cancer (GC) and has been identified as an oncogene because of its induction of cell proliferation, and it has also been found to be associated with the antitumor immune response.115 Deletion of YTHDF1 in GC cells led to increased DC recruitment and T cell infiltration because of increased levels of IFNγ receptor 1 (IFNGR1) on the surface of tumor cells.115 However, it is not clear whether this effect is mediated by m6A.
Metabolic reprogramming
Metabolic reprogramming of tumor cells can reshape the tumor microenvironment and thus affect the immune response; moreover, the vital role played by m6A in cancer metabolism has been fully proven.116,117 Notably, m6A controls tumor immunity via metabolic reprogramming, which is discussed below. Deletion of the m6A demethylase ALKBH5 has been found to increase the sensitivity of melanoma and colon cancer to immunotherapy.118 ALKBH5 deletion alters the metabolic profile of tumor cells and reduces lactate levels in the tumor immune microenvironment by altering the splicing and expression of target genes (Mct4/Slc16a3) via an m6A-dependent mechanism.118 This altered metabolism, in turn, leads to attenuation of recruitment of immunosuppressive cells such as Tregs and myeloid-derived suppressor cells (MDSCs).118 Another m6A demethylase, FTO, has also been found to be associated with tumor cell escape from immune surveillance through its effect on glycolytic metabolism.119 Notably, the FTO gene reduces the levels of basic leucine zipper (bZIP) transcription factors (JunB and CCAAT/enhancer binding protein-b(C/EBPb) ) in the m6A/YTHDF2 pathway in B16-OVA cells, which reduces gluconeogenic activity and facilitates the infiltration and function of CD8+ T cells by removing the metabolic barrier to their activation.119 YTHDF1 also potentiates glycolysis. circRHBDD1 is a novel circRNA that enhances the glycolysis rate and glycolysis capacity via the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and its inhibition enhances the anti-PD-L1 therapeutic effect.120 Mechanistically, circRHBDD1 promotes YTHDF1 binding to PIK3R1 mRNA, thereby facilitating PIK3R1 translation and activating downstream signaling pathways.120 At present, studies of how m6A modification affects metabolic reprogramming to alter immune responses are focused mainly on glucose metabolism, and the regulation of other tumor metabolism pathways needs to be investigated further (Figure 7B; Table 1).
Secretion of immune-related factors and other mechanisms
The immune response can be regulated by immune-related factors, including chemokines, cytokines, and IFNs, in the tumor microenvironment. An increasing number of studies have proven the connection between m6A and these immune-related factors. For example, METTL3 or METTL14 deficiency in colorectal carcinoma cells increases recruitment of CD8+ T cells and the levels of IFN-γ, CXCL9, and CXCL10 in the tumor microenvironment.121 Mechanistically, STAT1 and INF1 mRNA stability is negatively regulated in an m6A-Ythdf2-dependent manner, and IFN-γ-STAT1-IRF1 signaling affect the immune response.121 In another study, METTLE3 facilitated MDSC accumulation in colorectal cancer by promoting CXCL1 expression and thereby compromised the function of CD4+ and CD8+ T cells.122 Notably, m6A regulates CXCL1 expression not by directly regulating CXCL1 mRNA but by promoting the translation efficiency of BHLHE41, a transcription factor that binds the promoter region of CXCL1.122 RBM-15 has been identified as an essential component of writer complexes. Similarly, RBM-15 has been shown to promote CXCL11 expression, although it accomplishes this through its direct regulation of CXCL11 mRNA stability, thereby increasing macrophage infiltration and M2 polarization.123 ALKBH5 has been identified as another regulator that fosters immunosuppression. It promotes secretion of CXCL8/IL-8 and recruitment of TAMs under anoxic conditions in a glioblastoma multiforme (GBM) model.124 ALKBH5 regulates CXCL8/IL-8 not by directly removing m6A modifications on CXCL8 mRNA but by stabilizing the lncRNA NEAT1; hence, the transcriptional repressor splicing factor proline and glutamine rich (SFPQ) is removed from the CXCL8 promoter and transferred to paraspeckles.124 Similarly, another study showed that ALKBH5 upregulates IL-8 expression in HCC, thereby increasing PD-L1+ macrophage recruitment and facilitating immunosuppressive microenvironment formation.125 Mechanistically, ALKBH5 destabilizes MAP3K8 mRNA in a YTHDF2-mediated manner and activates the JNK/extracellular signal-regulated kinase (ERK) pathway.125 In head and neck squamous cell carcinoma (HNSCC), knocking down ALKBH5 inhibits tumor progression. Depletion of ALKBH5 increases m6A abundance on DDX58 mRNA and increases retinoic acid-inducible gene I (RIG-I) expression by increasing DDX58 mRNA maturation in a manner dependent on the function of the m6A reader HNRNPC.126 RIG-I positively regulates secretion of IFNα through the inhibitor of kappa B kinase ε (IKKε)/TBK1/IRF3 pathway (Figure 7C; Table 1).
In addition, tumor cells regulate antigen expression and the IFN response through modulation of m6A modifications. The YY1-CDK9 transcription elongation complex affects the expression levels of METTL3 and YTHDF2, thus inhibiting the IFN response and antigen presentation in glioblastoma stem cells (GSCs).127 Mechanistically, IFN-related genes (IFNB1, STAT1, and IRF1) are stabilized in an m6A-dependent manner, and IFN signaling is enhanced when the complex is inhibited.127
In summary, the roles played by m6A regulators in tumor cells are more consistent than the complex roles they play in immune cells. As an immune evasion regulator in tumors, m6A upregulates the expression of immune checkpoints and promotes recruitment of immunosuppressive cells such as Tregs, MDSCs, and M2 macrophages, thus contributing to an immunosuppressive tumor microenvironment. Therefore, targeting the m6A pathway may be a new strategy for tumor immunotherapy.
The m1A RNA modification in tumor immunity
Overview of the m1A RNA modification
The m1A modification, which was first discovered decades ago, was initially recognized as a reliable way to regulate the function and stability of transfer RNA (tRNA) and rRNA.128,129,130 More recently, m1A modifications have been shown to mark eukaryotic mRNAs.131 However, in contrast to the m6A modification, which preferentially decorates stop codons and 3′ UTRs, the m1A modification is enriched mainly on start codons upstream of the first splice site and 5′ UTRs of mRNA transcripts.131,132 M1ARegpred133 and RAMPred134 are powerful tools for predicting m1A modification sites (Table 3).
Similar to the m6A modification, the m1A modification is also regulated by specific regulators. The methyl group in the m1A modification is mainly added to mitochondrial and cytoplasmic RNAs by specific writers. tRNA methyltransferase 6 noncatalytic subunit (TRMT6) and RNA methyltransferase 61A (TRMT61A) form the α2β2 heterotetramer TRMT6/61A,135 which is a methyltransferase targeting cytoplasmic tRNAs, while TRMT61B136 and TRMT10C137 are tRNA methyltransferases that act mainly in mitochondria.138 Moreover, TRMT6/TRMT61A and TRMT10C catalyze the m1A modification of mRNAs in the cytoplasm and mitochondria.138 In addition, nucleomethylin (NML) has been discovered to be a writer for 28S rRNA m1A modification.139 Some readers have also been identified. YTHDF proteins, first identified as m6A readers, have been shown to recognize and bind to the m1A modification.140 Their roles as m1A-binding proteins remain to be further elucidated. In terms of erasers, ALKBH1,141 ALKBH3,142 ALKBH7,143 and FTO144 can remove m1A on tRNA, while ALKBH3 can also target modifications on mRNA.132
Generally, m1A is crucial for the structure and function of tRNAs and mRNAs. m1A modifications were first identified on tRNAs, and the positive electrostatic charge carried by m1A has been shown to be essential for maintaining the structure and function of tRNAs.145 In a subsequent study, ALKBH1 was found to act as a demethylase that removes methyl groups from m1A modifications on tRNAs and tRNAiMet, thereby reducing translation initiation and translation elongation.141 Recently, m1A modifications were found on mRNAs and disrupted Watson-Crick base pairing.131 Their function on mRNAs is still being explored. Li et al.132 found that m1A on 5′ UTRs is associated with higher translational efficiency, whereas m1A on mitochondrial mRNAs, located mainly in the coding sequence (CDS), inhibits translation. Consistent with these findings, ALKBH3 knockdown results in the downregulation of ErbB2 and AKT1S1 expression.146 However, Safra et al.138 found that only low m1A abundance on mRNA in the cytosol results in translational repression. In addition to affecting the translation efficiency of the target mRNA, ALKBH3, a targeted mRNA m1A demethylase, reduces the stabilization of macrophage colony-stimulating factor (CSF-1) mRNA.147 RNA-binding proteins may play essential roles in the function of m1A. YTHDF1 binds to m1A on ATP5D mRNA and subsequently forms a complex with eRF1 to terminate translation.148 In addition, YTHDF2 and YTHDF3 have been demonstrated to reduce the stability of target transcripts149,150 (Figure 1).
Aberrant m1A RNA modification in tumor immunity
Previous studies have revealed a relationship between m1A modification and tumorigenesis; for example, m1A modification of tRNAs drives liver tumorigenesis through activation of cholesterol synthesis.151 However, the relationship between m1A methylation and tumor immunity remains largely unknown. A recent study reported that tRNA-m1A modification was essential for rapid T cell proliferation.152 TRMT61A, which has been recognized as a typical m1A writer, is crucial for CD4+ T cell immune function, as indicated by an experiment in which its absence led to arrest of activated CD4+ T cells.152 Further studies revealed that this effect is mediated by tRNA-m1A enhancement of the translation efficiency of Myc mRNA, mainly mediated by an improved elongation process, which enhances expression of Myc and ultimately regulates cell cycling and metabolism.152 In addition, the analysis showed that m1A regulatory genes are positively correlated with immune cell infiltration and that YTHDF3, which had been identified previously as an m1A reader, may regulate macrophage activation.153 Bioinformatics studies have largely aimed to study m1A regulators, including three writers (TRMT6, TRMT61A, and TRMT10C), two erasers (ALKBH1 and ALKBH3), and four readers (YTHDF1, YTHDF2, YTHDF3, and YTHDC1). In oral squamous cell carcinoma,154 colon cancer,155 and HCC,156 the m1A score was found to be negatively correlated with patient prognosis and immune cell infiltration. In contrast, in ovarian cancer157 and lung adenocarcinoma,158 the m1A score was found to be positively correlated with prognosis and immunotherapy outcome. In ovarian cancer, innate immune cells were increased in the high m1A score subpopulation, whereas adaptive immune cells were increased in the low m1A score subpopulation.157 The m1A score showed a positive correlation with almost all immune cells in lung adenocarcinoma.158 The differences in these outcomes are rarely studied, and further research regarding recent theories is urgently needed.
The m5C RNA modification in tumor immunity
Introduction to the m5C RNA modification
The m5C RNA modification involves methylation of cytidine residues at position 5. It is widespread on rRNAs, tRNAs, other ncRNAs, and mRNAs. The m5C modification is conserved in tRNAs and rRNAs, but in mRNAs, the m5C modification is located primarily in 5′ UTRs and 3′ UTRs.159 Various m5C prediction models based on different machine learning algorithms have emerged; for example, Chen et al.160 predicted other modification sites with various machine learning models based on known m5C modification sites on mRNAs in humans and mice. Further predictive models are described in Table 3.
Similar to m6A and m1A modifications, m5C modifications are regulated by writers, readers, and erasers. m5C modification relies mainly on the NOL1/NOP2/sun (NSUN) family, which includes NSUN1,161 NSUN2,162 NSUN3,163 NSUN4,164 NSUN5, NSUN6,165 NSUN7, and the DNA methyltransferase homologue DNMT2.166 These writers mainly catalyze methylation on tRNAs (NSUN2, NSUN3, NSUN6, and DNMT2), rRNAs (NSUN1, NSUN4, and NSUN5), and other ncRNAs (NSUN2 and NSUN7) in the nucleus and mitochondria, while NSUN2167 and NSUN6168 mediate m5C modification on mRNAs. m5C function partially depends on RNA-binding proteins, and research in this area is still in an early stage. RNA and export factor-binding protein 2 (ALYREF), Y-box-binding protein 1 (YBX1), ypsilon schachtel (YPS), and YTHDF2 are the currently known m5C-binding proteins,167,169,170,171 and their functions are described below. Recently, ten-eleven translocation (TET) family members and ALKBH1 were identified as m5C demethylases that dynamically regulate m5C content.172
m5C controls the fate of modified RNA. Research on m5C initially focused on rRNAs and tRNAs. Regarding its effect on rRNAs, m5A regulates rRNA maturation,171 rRNA stability,161 and protein translation.161,173 In tRNAs, m5A is involved in stabilizing tRNA structure174 and regulating selective translation during oxidative stress.175 NSUN2 mediates methylation of tRNAs, reducing tRNA cleavage by nucleic acid endonucleases and thereby increasing tRNA stability, ultimately affecting translation efficiency.176 In addition, TET mediates oxidation of m5C in tRNAs and promotes translation.177 In recent years, with advances in detection methods, studies on m5C modification of mRNAs have gradually increased. NSUN2 has been found to also mediate m5C modification of mRNAs; ALYREF is an mRNA export adaptor that specifically binds to the m5C site after mRNA m5C modification to regulate targeted mRNA export.167 In addition, ALYREF enhances the stability of pyruvate kinase M2 (PKM2) mRNA.178 Similarly, YBX1 has been shown to stabilize mRNA by recruiting ELAVL1, which is another important m5C reader protein.179 In addition to affecting stability and export, YBX1 regulates translation of mRNAs. m5C methylation of IL-17A mRNA and intercellular adhesion molecule 1 (ICAM-1) mRNA promotes their translation.180,181 Moreover, NSUN2-mediated m5C methylation has also been shown to synergize with METTL3/METTL14-mediated m6A methylation to promote translation of p21 mRNA.182 However, m5C methylation catalyzed by NSUN2 had the opposite effect on p27 mRNA translation, suppressing its gene expression.183 Furthermore, m5C modifications are involved in regulation of mitochondrial function, stem cell development and differentiation, neurological development, cellular senescence, and other processes184,185,186 (Figure 1).
Aberrant m5C RNA modifications in tumor immunity
m5C modification is associated with development of GC,187 bladder cancer,179 HCC,188 esophageal squamous cell carcinoma,189 and other tumors. In addition, in previous studies, the m5C demethylase TET has been proven to affect the function of immune cells.190,191,192 However, research on m5C in tumor immunity is still in its infancy. Previous studies have shown that NSUN2 methylates IL-17A mRNA and thereby increases IL-17 expression in T lymphocytes,180 and it can also increase leukocyte adhesion by methylating ICAM-1 mRNA.181 Regarding tumor immunity, the m5C reader YBX1 has been identified as a positive regulator of PD-L1 that induces immune evasion.193 Recent studies have shown that m5C RNA methylation regulators are prognostic predictors in certain cancers and can regulate immune cells in the tumor microenvironment. NSUN2 and NSUN6 have been identified as risk and protective factors, respectively, in triple-negative breast cancer (TNBC) and have been associated with six major immune cells, with the highest correlation between NSUN6 and CD4+ T cells.194 In another study, NSUN2 was found to negatively regulate immune cell infiltration, while high NSUN2 expression was associated with downregulation of multiple immune checkpoints.195 NSUN3 and NSUN4 have also been found to be related to immune cells in lung squamous cell carcinoma (LUSC); notably, NSUN3 was closely associated with CD8+ T cell infiltration, and NSUN4 was closely associated with neutrophils.196 m5C modifications may enhance humoral immunity, and in HNSCC, DNMT1 expression levels promote peptide cross-linking.197 In addition, the m5C eraser TET2 has also been shown to be significantly associated with infiltration of many immune cells. In papillary thyroid carcinoma,198 oral squamous cell carcinoma,199 and renal cell carcinoma,200 m5C scores were found to correlate with different immune phenotypes. A high m5C score (associated with an immunosuppressive phenotype) predicted a poor prognosis, while a low m5C score (associated with an immune-activating phenotype) predicted a good prognosis. Furthermore, in a study in prostate cancer, naive B cells, CD8+ T cells, M1 macrophages, and M2 macrophages were identified as key immune cells involved in distinguishing m5C-related immune phenotypes, and CTLA4 was differentially expressed between the two phenotypes.201
Discussion
In this review, we focused on the relationship between m6A, m1A, and m5C modifications and tumor immunity and outlined different mechanisms by which RNA modifications affect antitumor immunity. Although RNA has over 100 modifications, the majority of studies have focused on m6A. In this review, we not only discussed m6A extensively but also focused on m1A and m5C, which have differences and similarities with m6A. On the one hand, these RNA modifications are all dependent on regulation of writers, erasers, and readers. In the presence of these regulators, RNA modifications are reversible and are precisely regulated to perform biological functions. All modifications are able to regulate the stability and translational efficiency of target RNAs. On the other hand, most of the more than 170 RNA modifications identified so far have been identified to occur on ncRNAs.202 m6A modifications are mainly found on mRNAs, whereas m1A and m5C modifications are mainly found on tRNAs and rRNAs203 and are present at much lower levels on mRNAs.204 While numerous studies have revealed the role played by m6A in tumor immunity, studies of m1A and m5C are still in the initial stages, and more investigation is needed. Recent studies have demonstrated the crucial roles played by m1A and m5C in tumorigenesis and immunity. For instance, the m5C modification at position 34 on mitochondrial tRNAMet promotes metastasis by driving mitochondrial mRNA translation, increasing mitochondrial function, and affecting metabolic plasticity in tumors,205 indicating a pivotal function of m5C in tumorigenesis. TRMT61A mediates tRNA-m1A58 modification and is crucial for rapid T cell activation because it enhances the translation efficiency of Myc mRNA,152 indicating an essential role of m1A in regulating immunity. Therefore, it has been speculated that m1A modification is a vital regulator of tumor immunity. More studies should be carried out to determine the exact function of m1A and m5C in tumor immunity.
In addition, other types of RNA modifications have also been found to play a key role in regulation of tumor immunity. For example, the immunomodulatory function of A-to-I editing, which is catalyzed by adenosine deaminases acting on RNA (ADARs), has been described in previous studies.206 Recently, a new mechanism underlying the cytotoxicity of 6-thioguanine (6TG), a widely used chemotherapeutic agent, was found.207 6TG increased A-to-I editing in bladder cancer-associated protein (BLCAP) mRNA, eventually decreasing cell viability.207 Moreover, knockdown of ADAR1 in tumor cells was found to enhance the response to ICIs by overcoming inactivation of antigen presentation.208 In addition to A-to-I editing, m7G modification of tRNA has recently been found to promote formation of an immunosuppressive microenvironment in HCC tumors mediated via the METTL1-transforming growth factor β2 (TGF-β2)-MDSC axis.209 Further exploration focusing on other types of RNA modifications is also needed.
Notably, increasing evidence suggests the potential for cross-talk between different RNA modifications. m6A, m1A, m5C, and other RNA modifications, including A-to-I editing and m7G210 and pseudouridine (Ψ)211 modification, have been found to modify mRNA. The cross-talk of mRNA modifications includes interactions of content and function. First, the abundance of RNA modifications on mRNA is modulated by other modifications. For instance, there is a negative correlation between m6A and A-to-I editing, two of the most abundant RNA modifications affecting mRNA, and both occur at adenosine.212 Second, different modifications on mRNA cooperate in regulating the fate of mRNA. For example, NSUN2-mediated m5C methylation promotes METTL3/METTL14-mediated m6A methylation in p21 mRNA and enhances p21 expression and vice versa.182 HRSP12 binds to m1A and promotes the interaction between m6A modifications and YTHDF2, thus cooperatively promoting rapid mRNA degradation.213 Additionally, modifications of ncRNAs can also cross-talk with modifications of mRNAs. For example, the tRNA methyltransferase TRMT10A not only installs N1-methylguanosine (m1G) on tRNA but also facilitates the m6A demethylase activity of FTO and potentially accelerates degradation of mRNA in a YTHDF2-dependent manner.214 However, the precise coregulation networks involving different RNA modifications and the impact of these interactions on tumor immunity remain largely unclear; these topics are promising areas for further research.
Furthermore, studies have revealed that different epigenetic modifications, including DNA methylation, chromatin remodeling, histone modification, and RNA modification, do not function independent of each other and share interaction networks.215 For example, m6A modification of CBX1 (a histone methylation regulator) mRNA destabilized the transcript via the m6A reader YTHDF3.113 In addition, METTL3/METTL14 downregulates H3K9me2 modification by YTHDC1-mediated recruitment of the H3K9me2 demethylase KDM3B to m6A-associated chromatin regions, which indicates that there is direct information flow from RNA to chromatin.71 On the other hand, m6A modification of mRNA can be regulated by histone modifications.216 For example, histone H3 trimethylation at Lys36 (H3K36me3) can be directly recognized by METTL14 and promote m6A modification of nascent RNAs to coregulate transcription, with MTC interacting with adjacent RNA polymerase II molecules.217 Recent studies have shown a similar regulatory mechanism between DNA methylation and RNA modifications. For instance, RNA m6A guides DNA 5-methylcytosine (5mC) demethylation via the m6A reader FXR1, recruiting TET1 to genomic loci and reprogramming chromatin accessibility.218 However, the precise cross-talk between different RNA modifications that regulate the antitumor immune response needs to be further explored and verified.
In summary, RNA modifications are closely associated with tumor immunity and may be novel targets for preventing immune escape. The regulation of oncogenic signaling pathways by RNA modifications revealed in previous studies has identified potential drug targets, such as the Wnt/β-catenin pathway, PI3K-Akt-mTOR pathway, and JAK-STAT pathway.219 In this review, we examined RNA modifications and tumor immunity, offering ideas for therapy for many patients who have shown poor responses to existing immunotherapies. Inhibitors of ALKBH5/FTO and targeted deletion of METTL3/14 have been shown to enhance the effects of PD-L1 inhibitor immunotherapy by enhancing the expression of immune checkpoints and secretion of cytokines.112118121 In addition, RNA-modified regulators hold promise for development as biological indicators of cancer prognosis and response to immune checkpoint blockade. Further clinical studies are urgently needed. However, current applications generally remain limited by the lack of relevant small-molecule inhibitors and unknown mechanisms of action. A deeper understanding of the biological mechanisms is conducive to selection of more efficient targets and drugs. These outcomes might further improve the survival time of cancer patients and their quality of life.
Acknowledgments
We appreciate helpful discussions with Dr. Huabing Li. This work was supported by the Natural Science Foundation of China (NSFC 82000939) and the Science and Technology Commission of Shanghai (20DZ2270800 and 22Y31900700).
Author contributions
X.F. and S.G. provided direction and guidance throughout the preparation of this manuscript. Y.K. collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. J.Y. reviewed and made significant revisions to the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: May 29, 2023
Contributor Information
Shengfang Ge, Email: geshengfang@hotmail.com.
Xianqun Fan, Email: fanxq@sjtu.edu.cn.
Lead contact website
References
- 1.Davis F.F., Allen F.W. Ribonucleic acids from yeast which contain a fifth nucleotide. J. Biol. Chem. 1957;227:907–915. [PubMed] [Google Scholar]
- 2.Roundtree I.A., Evans M.E., Pan T., et al. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shima H., Igarashi K. N 1-methyladenosine (m1A) RNA modification: the key to ribosome control. J. Biochem. 2020;167:535–539. doi: 10.1093/jb/mvaa026. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhao B.S., Roundtree I.A., et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnsack K.E., Höbartner C., Bohnsack M.T. Eukaryotic 5-methylcytosine (m5C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes. 2019;10:102. doi: 10.3390/genes10020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enroth C., Poulsen L.D., Iversen S., et al. Detection of internal N7-methylguanosine (m7G) RNA modifications by mutational profiling sequencing. Nucleic Acids Res. 2019;47:e126. doi: 10.1093/nar/gkz736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowling V. Regulation of mRNA cap methylation. Biochem. J. 2010;425:295–302. doi: 10.1042/BJ20091352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charette M., Gray M.W. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 9.De Almeida C., Scheer H., Zuber H., et al. RNA uridylation: a key posttranscriptional modification shaping the coding and noncoding transcriptome. Wiley Interdiscip. Rev. RNA. 2018;9 doi: 10.1002/wrna.1440. [DOI] [PubMed] [Google Scholar]
- 10.Du H., Zhao Y., He J., et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun. 2016;7:12626–12711. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park O.H., Ha H., Lee Y., et al. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol. Cell. 2019;74:494–507.e8. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F., Kang Y., Wang M., et al. Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum. Mol. Genet. 2018;27:3936–3950. doi: 10.1093/hmg/ddy292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasowitz S.D., Ma J., Anderson S.J., et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roundtree I.A., Luo G.Z., Zhang Z., et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6 doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H., Weng H., Sun W., et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C.X., Cui G.S., Liu X., et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu P.J., Zhu Y., Ma H., et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmann M.D., Jänne P.A., Opyrchal M., et al. Entinostat plus pembrolizumab in patients with metastatic NSCLC previously treated with anti–PD-(L) 1 TherapyEntinostat plus pembrolizumab in previously treated mNSCLC. Clin. Cancer Res. 2021;27:1019–1028. doi: 10.1158/1078-0432.CCR-20-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadatos-Pastos D., Yuan W., Pal A., et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. cancer. 2022;10 doi: 10.1136/jitc-2022-004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H., Zhang X., Yang P., et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 2021;12:1394–1415. doi: 10.1038/s41467-021-21514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han D., Liu J., Chen C., et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–274. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu C., Shi X., Dai C., et al. RNA m6A modification in cancers: molecular mechanisms and potential clinical applications. Innov. 2020;1 doi: 10.1016/j.xinn.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Huang H., Wu S., et al. The role of RNA m6A modification in cancer glycolytic reprogramming. Curr. Gene Ther. 2023;23:51–59. doi: 10.2174/1566523222666220830150446. [DOI] [PubMed] [Google Scholar]
- 24.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rottman F., Shatkin A.J., Perry R.P. Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: possible implications for processing. Cell. 1974;3:197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- 26.Meyer K.D., Saletore Y., Zumbo P., et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D., Qiao J., Wang G., et al. N 6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–3920. doi: 10.1093/nar/gky130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Hou C., Chen C., et al. The role of N6-methyladenosine (m6A) modification in the regulation of circRNAs. Mol. Cancer. 2020;19:105–111. doi: 10.1186/s12943-020-01224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen K., Wei Z., Zhang Q., et al. WHISTLE: a high-accuracy map of the human N 6-methyladenosine (m6A) epitranscriptome predicted using a machine learning approach. Nucleic Acids Res. 2019;47:e41. doi: 10.1093/nar/gkz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y., Zeng P., Li Y.-H., et al. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91. doi: 10.1093/nar/gkw104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y., He N., Chen Y., et al. BERMP: a cross-species classifier for predicting m6A sites by integrating a deep learning algorithm and a random forest approach. Int. J. Biol. Sci. 2018;14:1669–1677. doi: 10.7150/ijbs.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng P., Ding H., Yang H., et al. iRNA-PseColl: identifying the occurrence sites of different RNA modifications by incorporating collective effects of nucleotides into PseKNC. Mol. Ther. Acids. 2017;7:155–163. doi: 10.1016/j.omtn.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Q., Xing P., Wei L., et al. Gene2vec: gene subsequence embedding for prediction of mammalian N6-methyladenosine sites from mRNA. Rna. 2019;25:205–218. doi: 10.1261/rna.069112.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen B., Yuan B.-F., Feng Y.-Q. Analytical methods for deciphering RNA modifications. Anal. Chem. 2018;91:743–756. doi: 10.1021/acs.analchem.8b04078. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L.-Y., Song J., Liu Y., et al. Mapping the epigenetic modifications of DNA and RNA. Protein Cell. 2020;11:792–808. doi: 10.1007/s13238-020-00733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moshitch-Moshkovitz S., Dominissini D., Rechavi G. The epitranscriptome toolbox. Cell. 2022;185:764–776. doi: 10.1016/j.cell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Buck M., Connick M., Ames B.N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal. Biochem. 1983;129:1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- 38.Motorin Y., Muller S., Behm-Ansmant, et al. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol. 2007;425:21–53. doi: 10.1016/S0076-6879(07)25002-5. [DOI] [PubMed] [Google Scholar]
- 39.Grosjean H., Keith G., Droogmans L. Detection and quantification of modified nucleotides in RNA using thin-layer chromatography. Methods Mol. Biol. 2004;265:357–391. doi: 10.1385/1-59259-775-0:357. [DOI] [PubMed] [Google Scholar]
- 40.Wetzel C., Limbach P.A. Mass spectrometry of modified RNAs: recent developments. Analyst. 2016;141:16–23. doi: 10.1039/c5an01797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz S., Motorin Y. Next-generation sequencing technologies for detection of modified nucleotides in RNAs. RNA Biol. 2017;14:1124–1137. doi: 10.1080/15476286.2016.1251543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang X.M., Ye T.T., You X.J., et al. Mass spectrometry profiling analysis enables the identification of new modifications in ribosomal RNA. Chinese Chem. Lett. 2023;34 [Google Scholar]
- 43.Chen M.Y., Gui Z., Chen K.K., et al. Adolescent alcohol exposure alters DNA and RNA modifications in peripheral blood by liquid chromatography-tandem mass spectrometry analysis. Chinese Chem. Lett. 2022;33:2086–2090. [Google Scholar]
- 44.Chen M.Y., Qi C.B., Tang X.M., et al. Comprehensive profiling and evaluation of the alteration of RNA modifications in thyroid carcinoma by liquid chromatography-tandem mass spectrometry. Chinese Chem. Lett. 2022;33:3772–3776. [Google Scholar]
- 45.Wang X., Feng J., Xue Y., et al. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 46.Wen J., Lv R., Ma H., et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell. 2018;69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bokar J.A., Shambaugh M.E., Polayes D., et al. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Yue Y., Han D., et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N 6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ping X.L., Sun B.F., Wang L., et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warda A.S., Kretschmer J., Hackert P., et al. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia G., Fu Y.E., Zhao X.U., et al. N 6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng G., Dahl J.A., Niu Y., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao S., Sun H., Xu C. YTH domain: a family of N6-methyladenosine (m6A) readers. Dev. Reprod. Biol. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alarcón C.R., Goodarzi H., Lee H., et al. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu N., Dai Q., Zheng G., et al. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arguello A.E., DeLiberto A.N., Kleiner R.E. RNA chemical proteomics reveals the N6-methyladenosine (m6A)-regulated protein–RNA interactome. J. Am. Chem. Soc. 2017;139:17249–17252. doi: 10.1021/jacs.7b09213. [DOI] [PubMed] [Google Scholar]
- 57.Edupuganti R.R., Geiger S., Lindeboom R.G.H., et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 2017;24:870–878. doi: 10.1038/nsmb.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu R., Li A., Sun B., et al. A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29:23–41. doi: 10.1038/s41422-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li A., Chen Y.S., Ping X.L., et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi H., Wang X., Lu Z., et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ries R.J., Zaccara S., Klein P., et al. m6A enhances the phase separation potential of mRNA. Nature. 2019;571:424–428. doi: 10.1038/s41586-019-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao W., Adhikari S., Dahal U., et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Wojtas M.N., Pandey R.R., Mendel M., et al. Regulation of m6A transcripts by the 3ʹ→ 5ʹ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol. Cell. 2017;68:374–387.e12. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Zhang G., Xu Y., Wang X., et al. Dynamic FMR1 granule phase switch instructed by m6A modification contributes to maternal RNA decay. Nat. Commun. 2022;13:859–916. doi: 10.1038/s41467-022-28547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu P.J., Shi H., Zhu A.C., et al. The RNA-binding protein FMRP facilitates the nuclear export of N6-methyladenosine–containing mRNAs. J. Biol. Chem. 2019;294:19889–19895. doi: 10.1074/jbc.AC119.010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu Y., Niu S., Wang Y., et al. DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression. Cancer Res. 2021;81:923–934. doi: 10.1158/0008-5472.CAN-20-1619. [DOI] [PubMed] [Google Scholar]
- 67.Patil D.P., Chen C.K., Pickering B.F., et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J., Dou X., Chen C., et al. N 6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367:580–586. doi: 10.1126/science.aay6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J.H., Wang R., Xiong F., et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell. 2021;81:3368–3385.e9. doi: 10.1016/j.molcel.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu W., He C., Kaye E.G., et al. Dynamic control of chromatin-associated m6A methylation regulates nascent RNA synthesis. Mol. Cell. 2022;82:1156–1168.e7. doi: 10.1016/j.molcel.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y., Xia L., Tan K., et al. N6-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat. Genet. 2020;52:870–877. doi: 10.1038/s41588-020-0677-3. [DOI] [PubMed] [Google Scholar]
- 72.Cassetta L., Cassol E., Poli G. Macrophage polarization in health and disease. Sci. World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Liu Z., Tang H., et al. The N 6-methyladenosine (m6A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am. J. Physiol. Physiol. 2019;317:C762–C775. doi: 10.1152/ajpcell.00212.2019. [DOI] [PubMed] [Google Scholar]
- 75.Wang X., Ji Y., Feng P., et al. The m6A reader IGF2BP2 regulates macrophage phenotypic activation and inflammatory diseases by stabilizing TSC1 and PPARγ. Adv. Sci. 2021;8 doi: 10.1002/advs.202100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tong J., Wang X., Liu Y., et al. Pooled CRISPR screening identifies m6A as a positive regulator of macrophage activation. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gu X., Zhang Y., Li D., et al. N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell. Signal. 2020;69 doi: 10.1016/j.cellsig.2020.109553. [DOI] [PubMed] [Google Scholar]
- 78.Du J., Liao W., Liu W., et al. N6-Adenosine methylation of Socs1 mRNA is required to sustain the negative feedback control of macrophage activation. Dev. Cell. 2020;55:737–753.e7. doi: 10.1016/j.devcel.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu Z., Li Y., Yuan W., et al. N6-methyladenosine of Socs1 modulates macrophage inflammatory response in different stiffness environments. Int. J. Biol. Sci. 2022;18:5753–5769. doi: 10.7150/ijbs.74196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pathria P., Louis T.L., Varner J.A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Dong L., Chen C., Zhang Y., et al. The loss of RNA N6-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8+ T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957.e10. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y., Shi M., He X., et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2022;15:52. doi: 10.1186/s13045-022-01272-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiong J., He J., Zhu J., et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell. 2022;82:1660–1677.e10. doi: 10.1016/j.molcel.2022.02.033. [DOI] [PubMed] [Google Scholar]
- 84.Qian C., Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin. Immunol. 2018;35:3–11. doi: 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Wang H., Hu X., Huang M., et al. Mettl3-mediated mRNA m 6 A methylation promotes dendritic cell activation. Nat. Commun. 2019;10:1898. doi: 10.1038/s41467-019-09903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ulvmar M.H., Werth K., Braun A., et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 87.Li F., Cao Y., Luo Y., et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;43:600–605. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Xiao Z., Li T., Zheng X., et al. Nanodrug enhances post-ablation immunotherapy of hepatocellular carcinoma via promoting dendritic cell maturation and antigen presentation. Bioact. Mater. 2023;21:57–68. doi: 10.1016/j.bioactmat.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodgins J.J., Khan S.T., et al. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Invest. 2019;129:3499–3510. doi: 10.1172/JCI129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smyth M.J., Thia K.Y.T., Street S.E.A., et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morandi B., Mortara L., Chiossone L., et al. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Böttcher J.P., Bonavita E., Chakravarty P., et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma S., Yan J., Barr T., et al. The RNA m6A reader YTHDF2 controls NK cell antitumor and antiviral immunity. J. Exp. Med. 2021;218 doi: 10.1084/jem.20210279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song H., Song J., Cheng M., et al. METTL3-mediated m6A RNA methylation promotes the anti-tumour immunity of natural killer cells. Nat. Commun. 2021;12:5522–5615. doi: 10.1038/s41467-021-25803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M.O., Rudensky A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H.B., Tong J., Zhu S., et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tong J., Cao G., Zhang T., et al. m6A mRNA methylation sustains Treg suppressive functions. Cell Res. 2018;28:253–256. doi: 10.1038/cr.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ito-Kureha T., Leoni C., Borland K., et al. The function of Wtap in N6-adenosine methylation of mRNAs controls T cell receptor signaling and survival of T cells. Nat. Immunol. 2022;23:1208–1221. doi: 10.1038/s41590-022-01268-1. [DOI] [PubMed] [Google Scholar]
- 99.Zhou J., Zhang X., Hu J., et al. m6A demethylase ALKBH5 controls CD4+ T cell pathogenicity and promotes autoimmunity. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vinuesa C.G., Linterman M.A., et al. Follicular helper T cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 101.Yao Y., Yang Y., Guo W., et al. METTL3-dependent m6A modification programs T follicular helper cell differentiation. Nat. Commun. 2021;12:1333–1416. doi: 10.1038/s41467-021-21594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu Y., Zhao Y., Zou L., et al. The E3 ligase VHL promotes follicular helper T cell differentiation via glycolytic-epigenetic control. J. Exp. Med. 2019;216:1664–1681. doi: 10.1084/jem.20190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding C., Xu H., Yu Z., et al. RNA m6A demethylase ALKBH5 regulates the development of γδ T cells. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2203318119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wan W., Ao X., Chen Q., et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol. Cancer. 2022;21:1–16. doi: 10.1186/s12943-021-01447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ni Z., Sun P., Zheng J., et al. JNK signaling promotes bladder cancer immune escape by regulating METTL3-mediated m6A modification of PD-L1 mRNA. Cancer Res. 2022;82:1789–1802. doi: 10.1158/0008-5472.CAN-21-1323. [DOI] [PubMed] [Google Scholar]
- 106.Liu Z., Wang T., She Y., et al. N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer. 2021;20:105–125. doi: 10.1186/s12943-021-01398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng H., Zheng W.J., Liang B. Decreased expression of programmed death ligand-L1 by seven in absentia homolog 2 in cholangiocarcinoma enhances T-cell–mediated antitumor activity. Front. Immunol. 2022;13:845193. doi: 10.3389/fimmu.2022.845193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peng L., Pan B., Zhang X., et al. Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via m6A modification of lncRNA MIR155HG to upregulate PD-L1 expression. Cell Biol. Toxicol. 2022;38:1159–1173. doi: 10.1007/s10565-022-09718-0. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y., Guo Q., Yang H., et al. Allosteric regulation of IGF2BP1 as a novel strategy for the activation of tumor immune microenvironment. ACS Cent. Sci. 2022;8:1102–1115. doi: 10.1021/acscentsci.2c00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li T., Tan Y.T., Chen Y.X., et al. Methionine deficiency facilitates antitumour immunity by altering m6A methylation of immune checkpoint transcripts. Gut. 2022;72:501–511. doi: 10.1136/gutjnl-2022-326928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiu X., Yang S., Wang S., et al. M6A demethylase ALKBH5 regulates PD-L1 expression and tumor immunoenvironment in intrahepatic cholangiocarcinoma. Cancer Res. 2021;81:4778–4793. doi: 10.1158/0008-5472.CAN-21-0468. [DOI] [PubMed] [Google Scholar]
- 112.Yang S., Wei J., Cui Y.H., et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 2019;10:2782–2814. doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao Y., Huang S., Tan X., et al. N6-Methyladenosine-Modified CBX1 regulates nasopharyngeal carcinoma progression through heterochromatin formation and STAT1 activation. Adv. Sci. 2022;9 doi: 10.1002/advs.202205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su R., Dong L., Li Y., et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bai X., Wong C.C., Pan Y., et al. Loss of YTHDF1 in gastric tumors restores sensitivity to antitumor immunity by recruiting mature dendritic cells. J. Immunother. cancer. 2022;10 doi: 10.1136/jitc-2021-003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dey P., Kimmelman A.C., DePinho R.A. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021;11:1067–1081. doi: 10.1158/2159-8290.CD-20-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.An Y., Duan H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer. 2022;21:14–24. doi: 10.1186/s12943-022-01500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li N., Kang Y., Wang L., et al. ALKBH5 regulates anti–PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc. Natl. Acad. Sci. USA. 2020;117:20159–20170. doi: 10.1073/pnas.1918986117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Liang G., Xu H., et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metabol. 2021;33:1221–1233.e11. doi: 10.1016/j.cmet.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Cai J., Chen Z., Zhang Y., et al. CircRHBDD1 augments metabolic rewiring and restricts immunotherapy efficacy via m6A modification in hepatocellular carcinoma. Mol. Ther. 2022;24:755–771. doi: 10.1016/j.omto.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L., Hui H., Agrawal K., et al. m6A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO J. 2020;39 doi: 10.15252/embj.2020104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H., Pan Y., Zhou Q., et al. METTL3 inhibits antitumor immunity by targeting m6A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. 2022;163:891–907. doi: 10.1053/j.gastro.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 123.Zeng X., Chen K., Li L., et al. Epigenetic activation of RBM15 promotes clear cell renal cell carcinoma growth, metastasis and macrophage infiltration by regulating the m6A modification of CXCL11. Free Radic. Biol. Med. 2022;184:135–147. doi: 10.1016/j.freeradbiomed.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 124.Dong F., Qin X., Wang B., et al. ALKBH5 facilitates hypoxia-induced paraspeckle assembly and IL8 secretion to generate an immunosuppressive tumor microenvironment. Cancer Res. 2021;81:5876–5888. doi: 10.1158/0008-5472.CAN-21-1456. [DOI] [PubMed] [Google Scholar]
- 125.You Y., Wen D., Zeng L., et al. ALKBH5/MAP3K8 axis regulates PD-L1+ macrophage infiltration and promotes hepatocellular carcinoma progression. Int. J. Biol. Sci. 2022;18:5001. doi: 10.7150/ijbs.70149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin S., Li M., Chang H., et al. The m6A demethylase ALKBH5 promotes tumor progression by inhibiting RIG-I expression and interferon alpha production through the IKKε/TBK1/IRF3 pathway in head and neck squamous cell carcinoma. Mol. Cancer. 2022;21:97. doi: 10.1186/s12943-022-01572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu Z., Zhao L., Shen J.Z., et al. Transcription elongation machinery is a druggable dependency and potentiates immunotherapy in glioblastoma stem CellsTargeting transcription machinery potentiates immunotherapy. Cancer Discov. 2022;12:502–521. doi: 10.1158/2159-8290.CD-20-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dunn D.B. The occurence of 1-methyladenine in ribonucleic acid. Biochim. Biophys. Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 129.Helm M., Giegé R., Florentz C.A. Watson− Crick Base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 130.Sharma S., Watzinger P., Kötter P., et al. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dominissini D., Nachtergaele S., Moshitch-Moshkovitz S., et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li X., Xiong X., Wang K., et al. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat. Chem. Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 133.Yao J.H., Lin M.X., Liao W.J., et al. M1ARegpred: epitranscriptome target prediction of N1-methyladenosine (m1A) regulators based on sequencing features and genomic features. Front. Biosci. 2022;27:269. doi: 10.31083/j.fbl2709269. [DOI] [PubMed] [Google Scholar]
- 134.Chen W., Feng P., Tang H., et al. RAMPred: identifying the N1-methyladenosine sites in eukaryotic transcriptomes. Sci. Rep. 2016;6:31080–31088. doi: 10.1038/srep31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ozanick S., Krecic A., Andersland J., et al. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. Rna. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chujo T., Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. Rna. 2012;18:2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vilardo E., Nachbagauer C., Buzet A., et al. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase—extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–11593. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safra M., Sas-Chen A., Nir R., et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 139.Waku T., Nakajima Y., Yokoyama W., et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J. Cell Sci. 2016;129:2382–2393. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 140.Dai X., Wang T., Gonzalez G., et al. Identification of YTH Domain-containing proteins as the readers for N 1-Methyladenosine in RNA. Anal. Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu F., Clark W., Luo G., et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:1897–2828. doi: 10.1016/j.cell.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z., Qi M., Shen B., et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L.S., Xiong Q.P., Peña Perez S., et al. ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat. Cell Biol. 2021;23:684–691. doi: 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei J., Liu F., Lu Z., et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell. 2018;71:973–985.e5. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- 146.Zhao Y., Zhao Q., Kaboli P.J., et al. m1A regulated genes modulate PI3K/AKT/mTOR and ErbB pathways in gastrointestinal cancer. Transl. Oncol. 2019;12:1323–1333. doi: 10.1016/j.tranon.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Woo H.-H., Chambers S.K. Human ALKBH3-induced m1A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim. Biophys. Acta. Gene Regul. Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 148.Wu Y., Chen Z., Xie G., et al. RNA m 1 A methylation regulates glycolysis of cancer cells through modulating ATP5D. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2119038119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seo K.W., Kleiner R.E. YTHDF2 recognition of N1-methyladenosine (m1A)-modified RNA is associated with transcript destabilization. ACS Chem. Biol. 2019;15:132–139. doi: 10.1021/acschembio.9b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng Q., Gan H., Yang F., et al. Cytoplasmic m1A reader YTHDF3 inhibits trophoblast invasion by downregulation of m1A-methylated IGF1R. Cell Discov. 2020;6:12. doi: 10.1038/s41421-020-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y., Wang J., Li X., et al. N1-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat. Commun. 2021;12:6314–6319. doi: 10.1038/s41467-021-26718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y., Zhou J., Li X., et al. tRNA-m1A modification promotes T cell expansion via efficient MYC protein synthesis. Nat. Immunol. 2022;23:1433–1444. doi: 10.1038/s41590-022-01301-3. [DOI] [PubMed] [Google Scholar]
- 153.Wu Y., Jiang D., Zhang H., et al. N1-Methyladenosine (m1A) regulation associated with the pathogenesis of abdominal aortic aneurysm through YTHDF3 modulating macrophage polarization. Front. Cardiovasc. Med. 2022;9:883155. doi: 10.3389/fcvm.2022.883155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao L., Chen R., Sugimoto M., et al. The Impact of m1A methylation modification patterns on tumor immune microenvironment and prognosis in oral squamous cell carcinoma. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao Y., Wang H., Li H., et al. Integrated analyses of m1A regulator-mediated modification patterns in tumor microenvironment-infiltrating immune cells in colon cancer. OncoImmunology. 2021;10 doi: 10.1080/2162402X.2021.1936758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao M., Shen S., Xue C. A novel m1A-score model correlated with the immune microenvironment predicts prognosis in hepatocellular carcinoma. Front. Immunol. 2022;13:805967. doi: 10.3389/fimmu.2022.805967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu J., Chen C., Wang Y., et al. Comprehensive of N1-methyladenosine modifications patterns and immunological characteristics in ovarian cancer. Front. Immunol. 2021;12:746647. doi: 10.3389/fimmu.2021.746647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bao G., Li T., Guan X., et al. Comprehensive analysis of immune profiles and clinical significance of m1A regulators in lung adenocarcinoma. Front. Oncol. 2022;2269 doi: 10.3389/fonc.2022.882292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen Y.S., Yang W.L., Zhao Y.L., et al. Dynamic transcriptomic m5C and its regulatory role in RNA processing. Wiley Interdiscip. Rev. RNA. 2021;12:e1639. doi: 10.1002/wrna.1639. [DOI] [PubMed] [Google Scholar]
- 160.Chen L., Li Z., Zhang S., et al. Predicting RNA 5-methylcytosine sites by using essential sequence features and distributions. BioMed Res. Int. 2022;2022:4035462. doi: 10.1155/2022/4035462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sharma S., Yang J., Watzinger P., et al. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–9076. doi: 10.1093/nar/gkt679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hussain S., Sajini A.A., Blanco S., et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakano S., Suzuki T., Kawarada L., et al. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNAMet. Nat. Chem. Biol. 2016;12:546–551. doi: 10.1038/nchembio.2099. [DOI] [PubMed] [Google Scholar]
- 164.Metodiev M.D., Spåhr H., Loguercio Polosa P., et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haag S., Warda A.S., Kretschmer J., et al. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. Rna. 2015;21:1532–1543. doi: 10.1261/rna.051524.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goll M.G., Kirpekar F., Maggert K.A., et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 167.Yang X., Yang Y., Sun B.F., et al. 5-methylcytosine promotes mRNA export—NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Selmi T., Hussain S., Dietmann S., et al. Sequence-and structure-specific cytosine-5 mRNA methylation by NSUN6. Nucleic Acids Res. 2021;49:1006–1022. doi: 10.1093/nar/gkaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang Y., Wang L., Han X., et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol. Cell. 2019;75:1188–1202.e11. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 170.Zou F., Tu R., Duan B., et al. Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc. Natl. Acad. Sci. USA. 2020;117:3603–3609. doi: 10.1073/pnas.1910862117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dai X., Gonzalez G., Li L., et al. YTHDF2 binds to 5-methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal. Chem. 2019;92:1346–1354. doi: 10.1021/acs.analchem.9b04505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fu L., Guerrero C.R., Zhong N., et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J. Am. Chem. Soc. 2014;136:11582–11585. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Heissenberger C., Liendl L., Nagelreiter F., et al. Loss of the ribosomal RNA methyltransferase NSUN5 impairs global protein synthesis and normal growth. Nucleic Acids Res. 2019;47:11807–11825. doi: 10.1093/nar/gkz1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Y., Sierzputowska-Gracz H., Guenther R., et al. 5-Methylcytidine is required for cooperative binding of magnesium (2+) and a conformational transition at the anticodon stem-loop of yeast phenylalanine tRNA. Biochemistry. 1993;32:10249–10253. doi: 10.1021/bi00089a047. [DOI] [PubMed] [Google Scholar]
- 175.Chan C.T.Y., Pang Y.L.J., Deng W., et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937–939. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blanco S., Dietmann S., Flores J.V., et al. Aberrant methylation of t RNA s links cellular stress to neuro-developmental disorders. EMBO J. 2014;33:2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shen H., Ontiveros R.J., Owens M.C., et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J. Biol. Chem. 2021;296:100087. doi: 10.1074/jbc.RA120.014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang J.Z., Zhu W., Han J., et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021;41:560–575. doi: 10.1002/cac2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen X., Li A., Sun B.F., et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 180.Wang N., Tang H., Wang X., et al. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem. Biophys. Res. Commun. 2017;493:94–99. doi: 10.1016/j.bbrc.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 181.Luo Y., Feng J., Xu Q., et al. NSun2 deficiency protects endothelium from inflammation via mRNA methylation of ICAM-1. Circ. Res. 2016;118:944–956. doi: 10.1161/CIRCRESAHA.115.307674. [DOI] [PubMed] [Google Scholar]
- 182.Li Q., Li X., Tang H., et al. NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. J. Cell. Biochem. 2017;118:2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang H., Fan X., Xing J., et al. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging (Albany NY) 2015;7:1143. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Blanco S., Kurowski A., Nichols J., et al. The RNA–methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Flores J.V., Cordero-Espinoza L., Oeztuerk-Winder F., et al. Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Rep. 2017;8:112–124. doi: 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai X., Hu Y., Tang H., et al. RNA methyltransferase NSUN2 promotes stress-induced HUVEC senescence. Oncotarget. 2016;7:19099–19110. doi: 10.18632/oncotarget.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu Y., Chen C., Tong X., et al. NSUN2 modified by SUMO-2/3 promotes gastric cancer progression and regulates mRNA m5C methylation. Cell Death Dis. 2021;12:842–913. doi: 10.1038/s41419-021-04127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sun Z., Xue S., Zhang M., et al. Aberrant NSUN2-mediated m5C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39:6906–6919. doi: 10.1038/s41388-020-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Su J., Wu G., Ye Y., et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene. 2021;40:5814–5828. doi: 10.1038/s41388-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li H., Lu T., Sun W., et al. Ten-eleven translocation (TET) enzymes modulate the activation of dendritic cells in allergic rhinitis. Front. Immunol. 2019;10:2271. doi: 10.3389/fimmu.2019.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schoeler K., Aufschnaiter A., Messner S., et al. TET enzymes control antibody production and shape the mutational landscape in germinal centre B cells. FEBS J. 2019;286:3566–3581. doi: 10.1111/febs.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yue X., Lio C.-W.J., Samaniego-Castruita Y., et al. Loss of TET2 and TET3 in regulatory T cells unleashes effector function. Nat. Commun. 2019;10:2011–2014. doi: 10.1038/s41467-019-09541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tao Z., Ruan H., Sun L., et al. Targeting the YB-1/PD-L1 Axis to enhance chemotherapy and antitumor immunityYB-1 promotes tumor immune evasion. Cancer Immunol. Res. 2019;7:1135–1147. doi: 10.1158/2326-6066.CIR-18-0648. [DOI] [PubMed] [Google Scholar]
- 194.Huang Z., Pan J., Wang H., et al. Prognostic significance and tumor immune microenvironment heterogenicity of m5C RNA methylation regulators in triple-negative breast cancer. Front. Cell Dev. Biol. 2021;9:657547. doi: 10.3389/fcell.2021.657547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tong X., Xiang Y., Hu Y., et al. NSUN2 promotes tumor progression and regulates immune infiltration in nasopharyngeal carcinoma. Front. Oncol. 2022;12:788801. doi: 10.3389/fonc.2022.788801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pan J., Huang Z., Xu Y. m5C RNA methylation regulators predict prognosis and regulate the immune microenvironment in lung squamous cell carcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.657466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xue M., Shi Q., Zheng L., et al. Gene signatures of m5C regulators may predict prognoses of patients with head and neck squamous cell carcinoma. Am. J. Transl. Res. 2020;12:6841–6852. [PMC free article] [PubMed] [Google Scholar]
- 198.Li F., Deng Q., Pang X., et al. m5C regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in papillary thyroid carcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.729887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gao L., Chen R., Sugimoto M., et al. The RNA methylation modification 5-Methylcytosine impacts immunity characteristics, prognosis and progression of Oral squamous cell carcinoma by bioinformatics analysis. Front. Bioeng. Biotechnol. 2021;9:760724. doi: 10.3389/fbioe.2021.760724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu W., Zhu W., Tian X., et al. Integrative 5-methylcytosine modification immunologically reprograms tumor microenvironment characterizations and phenotypes of clear cell renal cell carcinoma. Front. Cell Dev. Biol. 2021;9:772436. doi: 10.3389/fcell.2021.772436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yu G., Bao J., Zhan M., et al. Comprehensive analysis of m5C methylation regulatory genes and tumor microenvironment in prostate cancer. Front. Immunol. 2022;13:914577. doi: 10.3389/fimmu.2022.914577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Boccaletto P., Stefaniak F., Ray A., et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–D235. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cui L., Ma R., Cai J., et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct. Target. Ther. 2022;7:334. doi: 10.1038/s41392-022-01175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wiener D., Schwartz S. The epitranscriptome beyond m6A. Nat. Rev. Genet. 2021;22:119–131. doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 205.Delaunay S., Pascual G., Feng B., et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature. 2022;607:593–603. doi: 10.1038/s41586-022-04898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nakahama T., Kawahara Y. Adenosine-to-inosine RNA editing in the immune system: friend or foe? Cell. Mol. Life Sci. 2020;77:2931–2948. doi: 10.1007/s00018-020-03466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.You X.J., Li L., Ji T.T., et al. 6-Thioguanine incorporates into RNA and induces adenosine-to-inosine editing in acute lymphoblastic leukemia cells. Chinese Chem. Lett. 2023;34 [Google Scholar]
- 208.Ishizuka J.J., Manguso R.T., Haining W.N. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature. 2019;565:43–48. doi: 10.1038/s41586-018-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zeng X., Liao G., Li S., et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents HCC recurrence after radiofrequency ablation. Hepatology. 2023;77:1122–1138. doi: 10.1002/hep.32585. [DOI] [PubMed] [Google Scholar]
- 210.Zhang L.S., Liu C., Ma H., et al. Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA. Mol. Cell. 2019;74:1304–1316.e8. doi: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Carlile T.M., Rojas-Duran M.F., Zinshteyn B., et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiang J.F., Yang Q., Liu C.X., et al. N6-Methyladenosines modulate A-to-I RNA editing. Mol. Cell. 2018;69:126–135.e6. doi: 10.1016/j.molcel.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 213.Boo S.H., Ha H., Kim Y.K. m1A and m6A modifications function cooperatively to facilitate rapid mRNA degradation. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111317. [DOI] [PubMed] [Google Scholar]
- 214.Ontiveros R.J., Shen H., Stoute J., et al. Coordination of mRNA and tRNA methylations by TRMT10A. Proc. Natl. Acad. Sci. USA. 2020;117:7782–7791. doi: 10.1073/pnas.1913448117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhao Y., Chen Y., Jin M., et al. The crosstalk between m6A RNA methylation and other epigenetic regulators: a novel perspective in epigenetic remodeling. Theranostics. 2021;11:4549–4566. doi: 10.7150/thno.54967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Q., Chen C., Ding Q., et al. METTL3-mediated m6A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–1205. doi: 10.1136/gutjnl-2019-319639. [DOI] [PubMed] [Google Scholar]
- 217.Huang H., Weng H.,, Zhou K., et al. Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature. 2019;567:414–419. doi: 10.1038/s41586-019-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deng S., Zhang J., Su J., et al. RNA m6A regulates transcription via DNA demethylation and chromatin accessibility. Nat. Genet. 2022;54:1427–1437. doi: 10.1038/s41588-022-01173-1. [DOI] [PubMed] [Google Scholar]
- 219.Uddin M.B., Wang Z., Yang C. The m6A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol. Cancer. 2021;20:61. doi: 10.1186/s12943-021-01356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bao X., Zhang Y., Li H.,, et al. RM2Target: a comprehensive database for targets of writers, erasers and readers of RNA modifications. Nucleic Acids Res. 2023;51:D269–D279. doi: 10.1093/nar/gkac945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tang Y., Chen K., Song B., et al. m6A-Atlas: a comprehensive knowledgebase for unraveling the N 6-methyladenosine (m6A) epitranscriptome. Nucleic Acids Res. 2021;49:D134–D143. doi: 10.1093/nar/gkaa692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu S., Zhu A., He C., et al. REPIC: a database for exploring the N 6-methyladenosine methylome. Genome Biol. 2020;21:100–113. doi: 10.1186/s13059-020-02012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang Y., Jiang J., Ma J., et al. DirectRMDB: a database of post-transcriptional RNA modifications unveiled from direct RNA sequencing technology. Nucleic Acids Res. 2023;51:D106–D116. doi: 10.1093/nar/gkac1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Luo X., Li H., Liang J., et al. RMVar: an updated database of functional variants involved in RNA modifications. Nucleic Acids Res. 2021;49:D1405–D1412. doi: 10.1093/nar/gkaa811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Song B., Wang X., Liang Z., et al. RMDisease V2. 0: an updated database of genetic variants that affect RNA modifications with disease and trait implication. Nucleic Acids Res. 2023;51:D1388–D1396. doi: 10.1093/nar/gkac750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xuan J.J., Sun W.J., Lin P.H., et al. RMBase v2. 0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018;46:D327–D334. doi: 10.1093/nar/gkx934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ma J., Song B., Wei Z., et al. m5C-Atlas: a comprehensive database for decoding and annotating the 5-methylcytosine (m5C) epitranscriptome. Nucleic Acids Res. 2022;50:D196–D203. doi: 10.1093/nar/gkab1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen W., Feng P., Yang H., et al. iRNA-3typeA: identifying three types of modification at RNA’s adenosine sites. Mol. Ther. Acids. 2018;11:468–474. doi: 10.1016/j.omtn.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lv H., Zhang Z.M., Li S.H., et al. Tan J.X., Chen W., Lin H. Evaluation of different computational methods on 5-methylcytosine sites identification. Brief. Bioinform. 2020;21:982–995. doi: 10.1093/bib/bbz048. [DOI] [PubMed] [Google Scholar]
- 230.Li J., Huang Y., Yang X., et al. Zhou Y., Zhou Y. RNAm5Cfinder: a web-server for predicting RNA 5-methylcytosine (m5C) sites based on random forest. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-35502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang M., Xu Y., Li L., et al. Accurate RNA 5-methylcytosine site prediction based on heuristic physical-chemical properties reduction and classifier ensemble. Anal. Biochem. 2018;550:41–48. doi: 10.1016/j.ab.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 232.Jiang J., Song B., Chen K., et al. m6AmPred: identifying RNA N6, 2′-O-dimethyladenosine (m6Am) sites based on sequence-derived information. Methods. 2022;203:328–334. doi: 10.1016/j.ymeth.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 233.Song Z., Huang D., Song B., et al. Attention-based multi-label neural networks for integrated prediction and interpretation of twelve widely occurring RNA modifications. Nat. Commun. 2021;12:4011. doi: 10.1038/s41467-021-24313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]