Abstract
Background
Dyslipidemia is one of the important contributors to cardiovascular disease and type 2 diabetes. There is little or no information on dyslipidemia among academic staff and students in Bangladesh. Therefore, this study aimed to investigate the prevalence and factors related to dyslipidemia among university academic staff and students in Bangladesh.
Methods
A total of 533 participants (302 academic staff and 231 students) were enrolled in this cross-sectional study. A simple random sampling technique was used to enrol the participants. Fasting blood samples were obtained from the participants, and serum levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured using the standard methods. Dyslipidemia was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP-III) model guideline. Multivariable logistic regression was conducted to identify the factors related to lipid marker abnormalities.
Results
Overall, the prevalence of dyslipidemia was 81.5%, of which 85% was in staff and 76.5% in students. A significant difference was found in the prevalence of dyslipidemia between males and females only in the student group (p < 0.01). Among staff, hypertriglyceridemia prevalence was 49.7%, hypercholesterolemia 23%, high LDL-C 24.7% and low HDL-C 77.3%. On the other hand, hypertriglyceridemia prevalence was 39%, hypercholesterolemia 25.6%, high LDL-C 26.5% and low HDL-C 69.3% among students. The most common lipid abnormality was low HDL-C in both groups. The prevalence of mixed dyslipidemia was 14.2% and 14.1% in staff and students, respectively. According to the regression analysis, increased age, obesity, diabetes, and inadequate physical activity were significantly associated with dyslipidemia.
Conclusions
Dyslipidemia was prevalent among the majority of the study participants. Increased age, obesity, diabetes, and inadequate physical activity were significantly associated with dyslipidemia. The study’s results highlight the importance of implementing interventions to address the associated risk factors of dyslipidemia among academic staff and students in Bangladesh.
Keywords: Dyslipidemia, University staff and students, Prevalence, Risk factors, Bangladesh
Background
Dyslipidemia is a state that occurs due to the abnormalities of lipids in the blood, such as elevated total cholesterol (TC), elevated triglycerides (TG), low level of high-density lipoprotein cholesterol (HDL-C) and elevated low-density lipoprotein cholesterol (LDL-C). These abnormalities can occur either single or combinedly [1]. Dyslipidemia, especially high levels of LDL-C, is a significant risk factor for cardiovascular disease (CVD), but other forms, such as hypertriglyceridemia, are related to acute pancreatitis and non-alcoholic fatty liver disease [2]. Hypercholesterolemia is the most prevalent form of dyslipidemia and is associated with an increased risk of CVD, with higher levels of LDL-C being the 8th leading risk factor for global death in 2019 [2].
The prevalence of dyslipidemia has increased over the last 3 decades and is considered a health burden globally [2]. CVD is a major cause of global death with a significant number of people dying every year from it than from any other reason [3]. A recent literature review indicated a high prevalence of CVD in the Bangladeshi adult population [4].
The nature of work and working environment may be linked with dyslipidemia. There are many aspects of work that involve less physical activity, unhealthy diets, and physical and mental stress. Generally, employed workers spend a significant portion of their lives at work, and the demands and pressure of the work may affect their food habits, lifestyle and daily activity patterns, which may affect their overall health. A study by Catalina-Romero et al. [5] showed a relationship of job stress with dyslipidemia, even after adjusting for multiple covariates. In another study, job-related mental stress was found to be related to increased levels of blood cholesterol and triglycerides among company workers [6]. Similarly, work at academic institutions is not out of mental and physical stress besides being a significant time of sedentary work; hence academic staff may be at risk of dyslipidemia and related CVD. Sedentary behaviours affect the metabolic profiles that are frequently seen in dyslipidemia [7].
A higher prevalence of dyslipidemia has also been reported among young adults in different countries [8–10]. An increased prevalence of dyslipidemia in young adulthood is a concern as it increases the risk of coronary heart disease in later life [11]. It has been suggested that about 50% of young adults with elevated total cholesterol have five times the risk of coronary heart disease and nine times risk of myocardial infarction than those having low total cholesterol levels over 30 to 40 years of age [12, 13]. As both academic staff and young students are very important groups of the national population. Therefore, determining dyslipidemia prevalence and its related risk factors in these special population groups will be an important step in increasing awareness and prevention of dyslipidemia and related health effects. Therefore, this study was conducted to measure dyslipidemia prevalence and associated factors among university students and academic staff in Bangladesh.
Methods
Participant recruitment and study design
This study was a cross-sectional design conducted between February 2019 and January 2020. A total of 533 participants (302 academic staff and 231 students) were recruited from the two universities located in Sylhet and Dhaka districts. All the analyses were conducted at the Biochemistry and Molecular Biology Department of SUST, Sylhet, Bangladesh. Inclusion criteria: (i) willingness to participate; (ii) both sexes and (iii) ≥ 18 years of age. Exclusion criteria: (i) participants with physical dysfunction (ii) participants with infectious disease and liver and kidney diseases (iii) women with pregnancy and nursing mothers and (iv) subjects with incomplete questionnaires or missing blood samples. PASS version 15.0 was used for sample size calculation. A sample size of 480 (270 males and 210 females) was needed to achieve 90% statistical power. A simple random sampling technique was used to enrol the participants. The Ethics Review Committee at the BMB Department, School of Life Sciences, SUST approved this study protocol (ID 02/BMB/2019). Written informed consent was obtained from all study subjects before study commencement. All methods of the study were carried out in accordance with institutional guidelines and regulations.
Data collection
We used a structured questionnaire for collecting the anthropometric, demographic and lifestyle information described elsewhere [14–22]. Individuals’ body height, weight, and waist and hip circumference (WC and HC, respectively) were measured by trained personnel who were experienced in health-related research. Body mass index (BMI) was determined as the person’s weight in kilograms divided by the square of height in meters. Before blood pressure measurements, the participants were asked to rest for 10 min, and then three consecutive blood pressure measurements were taken 5 min apart. The average of 2nd and 3rd measurements were taken for systolic and diastolic blood pressures (SBP and DBP, respectively). An automated blood pressure measuring device was used for blood pressure measurement (Omron M10, Omron Corporation, Tokyo, Japan).
Specimen collection and lipid markers measurements
After overnight fasting, venous blood samples were collected from the study subjects in the morning. After centrifugation, serum was isolated and stored at -20oC until biochemical analysis. A semi-automatic analyzer (Humalyzer 3000, USA) was used to measure biochemical parameters. Serum levels of TG, TC, HDL-C, LDL-C, and fasting blood glucose were measured using enzymatic colourimetric techniques [23–26].
Diagnostic criteria
Dyslipidemia was defined according to the NCEP-ATP-III model guideline [27]. Dyslipidemia was defined as having one or more of the following: TC ≥ 200 mg/dL; TG: ≥ 150 mg/dL; LDL-C ≥ 130 mg/dL and HDL-C < 40 mg/dL. Mixed hyperlipidemia was defined as TC ≥ 150 mg/dL plus TG ≥ 200 mg/dL. Isolated dyslipidemia was defined as isolated hypercholesterolemia - a combination of high TC (≥ 200 mg/dL) and normal/low TG (< 150 mg/dL); isolated hypertriglyceridemia - a combination of high TG (> 150 mg/dL) and normal/low TC (< 200 mg/dL); isolated low HDL-C was defined as a combination of low HDL-C (≤ 40 mg/dL) with normal TG and TC. Hypertension was defined as SBP above or equal to 140 mmHg and DBP above or equal to 90 mmHg or self-reported use of antihypertensive medications [28, 29]. Participants with diabetes were identified by checking prescriptions provided by physicians and/or self-reported use of anti-diabetic medications. BMI was divided into normal (18.5–23.0 kg/m2), overweight (23.1–27.5 kg/m2), and obesity (≥ 27.5 kg/m2) [30, 31]. Healthy individuals were defined as both non-hypertensive and non-diabetic. Physical activity was grouped as inadequate (comfortable office work and housework), medium (walking, swimming) and adequate (carrying, lifting, jogging, and/or sports). Smoking was classified as never smokers and current smokers.
Statistical analyses
Data analyses were performed using SPSS Version 25.0 (IBM, Chicago, IL, USA). Data were presented as mean, frequencies and percentages. Independent sample t-test was used to compare the mean of two given samples and the chi-square test was used to compare categorical variables. Multivariable logistic regression was performed to determine the factors independently associated with lipid marker abnormalities. In regression models, elevated lipid profiles were dependent variables and anthro-demographics and behavioural factors were considered the independent variables. All p-values were two-sided and a p-value < 0.05 was considered statistically significant.
Results
Characteristics of the study participants
Table 1 shows the general characteristics of the participants. Among 533 participants, 354 were males and 179 were females. The mean age of the staff and students was 40.5 ± 10.0 years and 21.8 ± 2.0 years, respectively, and there was a significant difference between genders (p < 0.001). Among staff, the mean of BMI, WC, SBP and DBP were higher in males than in females (p < 0.01 at least for all cases). Among students, only WC and DBP showed a significant difference between the gender groups (p < 0.05 at least for both cases). Based on blood pressure and blood glucose concentrations, 34.7% and 14.2% of the academic staff were hypertensive and diabetic, respectively; whereas, 7.9% and 2.6% of the students were hypertensive and diabetic, respectively. Regarding biochemical parameters, the mean level of TC and LDL were higher in male staff; whereas mean TG and HDL were slightly higher in female staff but the differences were not statistically significant between the genders. On the other hand, the mean TG level was significantly higher in male students; whereas the mean HDL level was higher in female students (p < 0.001). About 79% of the academic staff and 81.2% of the students were used to either medium or adequate physical activity. About 11% of the academic staff and 16% of the students were used to smoking.
Table 1.
Baseline characteristics of the study participants
| Variables | Academic staff | Students | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | P-value | Total | Male | Female | P-value | |
| N | 302 | 216 | 86 | 231 | 138 | 93 | ||
| Age (years) | 40.5 ± 10.0 | 41.8 ± 10.0 | 37.3 ± 8.0 | 0.000 | 21.8 ± 2.0 | 22.2 ± 2.0 | 21.2 ± 2.0 | 0.000 |
| Weight (kg) | 68.4 ± 9.4 | 70.2 ± 8.5 | 63.7 ± 10.0 | 0.000 | 59.5 ± 11.9 | 64.4 ± 11.0 | 52.2 ± 9.2 | 0.000 |
| Height (cm) | 162.9 ± 8.2 | 166.6 ± 5.5 | 153.8 ± 6.3 | 0.000 | 162.3 ± 10.9 | 167.4 ± 6.4 | 154.7 ± 11.8 | 0.000 |
| BMI (kg/m2) | 25.7 ± 3.12 | 25.26 ± 2.6 | 26.8 ± 3.8 | 0.000 | 22.6 ± 4.4 | 22.9 ± 3.5 | 22.1 ± 5.6 | 0.214 |
| WC (cm) | 86.6 ± 8.1 | 88.3 ± 6.7 | 84.4 ± 9.4 | 0.005 | 79.9 ± 8.6 | 80.8 ± 8.7 | 77.7 ± 8.1 | 0.047 |
| HC (cm) | 94.9 ± 7.8 | 94.0 ± 5.6 | 96.1 ± 9.9 | 0.117 | 92.3 ± 7.9 | 92.1 ± 7.8 | 92.6 ± 8.1 | 0.766 |
| SBP (mmHg) | 120.9 ± 13.4 | 123.3 ± 13.1 | 115.2 ± 12.4 | 0.000 | 120.4 ± 66.8 | 121.1 ± 11.9 | 119.2 ± 104.9 | 0.855 |
| DBP (mmHg) | 82.1 ± 10.2 | 83.9 ± 9.6 | 77.7 ± 10.4 | 0.000 | 74.0 ± 9.2 | 76.3 ± 8.5 | 70.5 ± 9.1 | 0.000 |
| Glucose (mg/dL) | 100.7 ± 41.4 | 99.2 ± 34.2 | 104.4 ± 54.0 | 0.292 | 78.1 ± 19.8 | 79.2 ± 21.6 | 75.6 ± 19.8 | 0.105 |
| TG (mg/dL) | 164.4 ± 81.2 | 159.4 ± 83.6 | 176.5 ± 74.0 | 0.119 | 146.6 ± 94.9 | 166.2 ± 102.3 | 114.1 ± 70.7 | 0.000 |
| TC (mg/dL) | 167.4 ± 50.2 | 169.6 ± 51.1 | 161.9 ± 47.7 | 0.204 | 161.3 ± 52.5 | 166.3 ± 54.2 | 152.8 ± 48.9 | 0.069 |
| LDL (mg/dL) | 101.3 ± 48.4 | 105.2 ± 48.4 | 91.6 ± 47.3 | 0.027 | 100.2 ± 46.3 | 104.7 ± 47.4 | 92.8 ± 43.7 | 0.072 |
| HDL (mg/dL) | 34.2 ± 10.8 | 34.0 ± 10.4 | 34.6 ± 12.0 | 0.685 | 35.9 ± 15.0 | 33.4 ± 13.42 | 40.1 ± 16.6 | 0.003 |
| Hypertensive (%) | 104 (34.7) | 84 (39.3) | 20 (23.3) | 0.008 | 18 (7.9) | 14 (10.3) | 4 (4.4) | 0.107 |
| Diabetic (%) | 43 (14.2) | 31 (14.4) | 12 (14.0) | 0.929 | 6 (2.6) | 3 (2.2) | 3 (3.2) | 0.662 |
| Physical activity (%) | ||||||||
| Inadequate | 62 (20.6) | 42 (19.3) | 20 (23.2) | 0.482 | 44 (18.8) | 18 (13.0) | 26 (27.6) | 0.126 |
| Medium/ Adequate | 240 (79.4) | 174 (80.7) | 66 (76.8) | 187 (81.2) | 120 (87.0) | 67 (72.4) | ||
| Smoking status (%) | ||||||||
| No | 268 (89.0) | 182 (84.7) | 86 (100) | 0.000 | 194 (83.8) | 106 (77.4) | 93 (100.0) | 0.000 |
| Yes | 34 (11.0) | 34 (15.3) | 0 (0) | 37 (16.2) | 37 (22.6) | 0 (0.0) | ||
Data are presented as mean ± SD or %. P-values are obtained from independent sample t-test for continuous variables and Chi-square test for categorical variables. TG: Triglyceride; TC: Total cholesterol; LDL: Low density lipoprotein; HDL: High density lipoprotein
Dyslipidemia among study participants
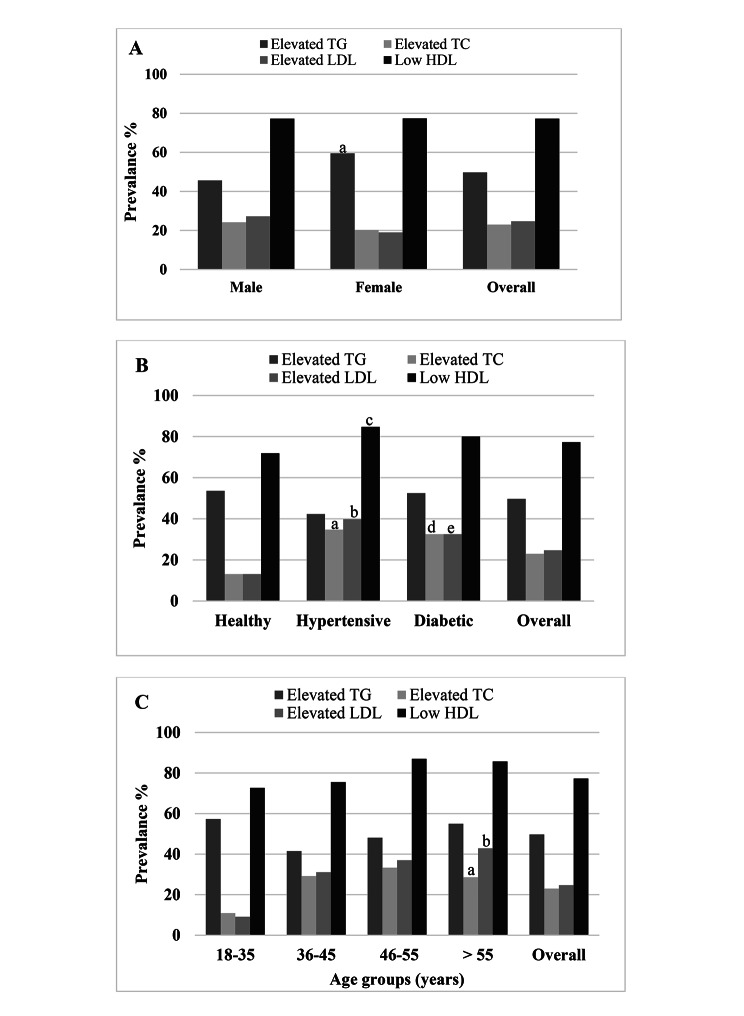
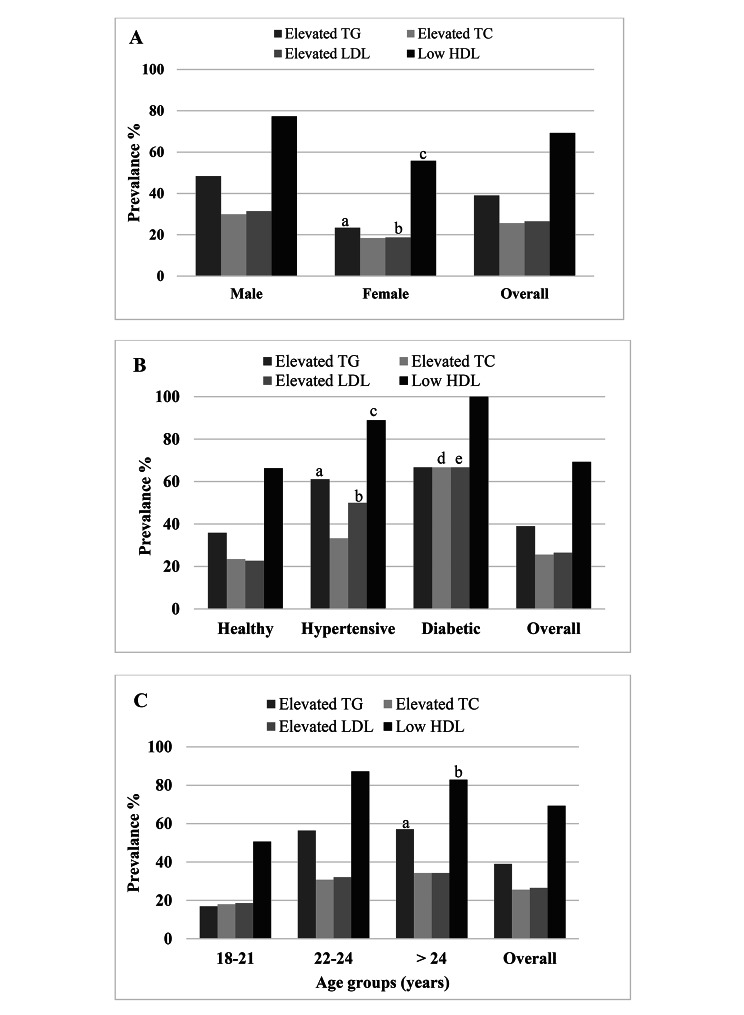
Overall, the prevalence of dyslipidemia was 81.5% of which 85% was in academic staff and 76.5% in students (Table 2). No significant difference was found for dyslipidemia prevalence between genders in academic staff (83.5% vs. 88.7%). Whereas, males had a higher prevalence of dyslipidemia than females among the students (82.9% vs. 65.8%, p < 0.01). The lipid levels and prevalence of lipids abnormalities were higher in diabetic and hypertensive individuals than in the control healthy individuals. Among staff, the prevalence of hypertriglyceridemia, hypercholesterolemia, high LDL and low HDL was 49.7%, 23%, 24.7% and 77.3%, respectively (Table 3). On the other hand, this prevalence was 39%, 25.6%, 26.5% and 69.3%, respectively among the students. Among staff, isolated hypertriglyceridemia was 35.5%, isolated hypercholesterolemia was 8.9%, and isolated low HDL-C was 29.6% (Table 4). Mixed hyperlipidemia was prevalent among 14.2% of the staff. In the student’s group, isolated hypertriglyceridemia was 24.9%, isolated hypercholesterolemia 11.3%, and isolated low HDL-C 29.9% (Table 4). Mixed hyperlipidemia was prevalent among 14.1% of the students. Low HDL levels were the main prevalent dyslipidemia in both staff members and students. Among staff, an increasing trend of dyslipidemia was observed in the > 35 years age group and the highest trend was found in the 46–55 years and > 55 years age groups (Fig. 1). In contrast, an increasing trend of dyslipidemia was observed in the > 21 years age group and the highest trend was found in the 22–24 years and > 24 years age groups among the students (Fig. 2).
Table 2.
Dyslipidemia in different groups
| N | Gender | Dyslipidemia, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | P-value | ||
| Academic staff | 302 | 216 | 86 | 85.0 | 83.5 | 88.7 | 0.217 |
| Students | 231 | 138 | 93 | 76.5a | 82.9 | 65.8b | 0.003 |
| Total | 533 | 354 | 179 | 81.5 | 83.3 | 77.7 | 0.112 |
P-values are derived from the chi-square test. aP < 0.01 and bP < 0.001 when the prevalence of dyslipidemia in the staff group is compared to the student group
Table 3.
Prevalence and levels of lipid markers in different groups
| Variables | Overall | Healthy | Hypertensive | Diabetic | aP-value | bP-value |
|---|---|---|---|---|---|---|
| Academic staff, n | 302 | 155 | 104 | 43 | - | - |
| TG (mg/dL) | 164.4 ± 81.2 | 169.4 ± 81.5 | 153.5 ± 78.2 | 171.7 ± 86.1 | 0.125 | 0.879 |
| Elevated TG, (%) | 49.7 | 53.6 | 42.3 | 52.5 | 0.081 | 0.902 |
| TC (mg/dL) | 167.4 ± 50.2 | 152.7 ± 47.1 | 185.1 ± 47.5 | 180.0 ± 51.5 | 0.000 | 0.004 |
| Elevated TC, n (%) | 23.0 | 13.1 | 34.7 | 32.5 | 0.000 | 0.004 |
| LDL (mg/dL) | 101.3 ± 48.4 | 84.7 ± 43.7 | 123.6 ± 44.3 | 110.1 ± 51.6 | 0.000 | 0.006 |
| Elevated LDL, n (%) | 24.7 | 13.1 | 39.8 | 32.5 | 0.000 | 0.004 |
| HDL (mg/dL) | 34.2 ± 10.8 | 35.4 ± 11.6 | 32.4 ± 8.2 | 33.8 ± 12.9 | 0.029 | 0.493 |
| Low HDL, n (%) | 77.3 | 71.9 | 84.7 | 80.0 | 0.019 | 0.301 |
| Students, n | 231 | 207 | 18 | 6 | - | - |
| TG (mg/dL) | 146.6 ± 94.9 | 141.9 ± 96.3 | 183.2 ± 81.8 | 178.5 ± 64.9 | 0.057 | 0.233 |
| Elevated TG, n (%) | 39.0 | 35.9 | 61.1 | 66.7 | 0.036 | 0.125 |
| TC (mg/dL) | 161.3 ± 52.5 | 158.0 ± 53.7 | 179.9 ± 34.6 | 203.0 ± 32.9 | 0.023 | 0.019 |
| Elevated TC, n (%) | 25.6 | 23.5 | 33.3 | 66.7 | 0.325 | 0.016 |
| LDL (mg/dL) | 100.2 ± 46.3 | 96.5 ± 46.9 | 121.8 ± 31.2 | 143.4 ± 25.5 | 0.005 | 0.005 |
| Elevated LDL, n (%) | 26.5 | 22.7 | 50.0 | 66.7 | 0.011 | 0.013 |
| HDL (mg/dL) | 35.9 ± 15.0 | 36.9 ± 15.6 | 27.4 ± 6.3 | 30.6 ± 2.2 | 0.11 | 0.000 |
| Low HDL, n (%) | 69.3 | 66.3 | 88.9 | 100.0 | 0.049 | 0.083 |
Healthy: both non-hypertensive and non-diabetic. Data are presented as mean ± SD or n (%). aP-value is the difference between the healthy and hypertensive group and bP-value is the difference between the healthy and diabetic group. P-values for mean concentrations are derived from independent sample t-test and P-values for prevalence (%) are obtained from the chi-square test. Elevated TG: TG ≥ 150 mg/dL; Elevated TC: TC ≥ 200 mg/dL; Elevated LDL: LDL ≥ 130 mg/dL and Low HDL: HDL < 40 mg/dL in men and < 50 in women; (National Cholesterol Education Program, ATP III, 2001)
Table 4.
Dyslipidemia prevalence based on isolated and mixed phenotypes
| Phenotypes | Total (%) |
Male (%) |
Female (%) |
P- value | |
|---|---|---|---|---|---|
| Academic staff | Isolated hypertriglyceridemia | 35.5 | 31.6 | 45.2 | 0.027 |
| Isolated hypercholesterolemia | 8.9 | 10.1 | 6.0 | 0.256 | |
| Isolated low HDL | 29.6 | 30.9 | 26.2 | 0.423 | |
| Mixed dyslipidemia | 14.2 | 14.0 | 14.3 | 0.951 | |
| Students | Isolated hypertriglyceridemia | 24.9 | 28.9 | 18.2 | 0.085 |
| Isolated hypercholesterolemia | 11.3 | 10.2 | 13.2 | 0.525 | |
| Isolated low HDL | 29.3 | 27.3 | 32.5 | 0.435 | |
| Mixed dyslipidemia | 14.1 | 19.5 | 5.2 | 0.004 |
P-values are obtained from the chi-square test. Isolated hypertriglyceridemia (Isolated hyperTG): TG ≥ 150 mg/dL and TC < 200 mg/dL; isolated hypercholesterolemia (Isolated hyperTC): TC ≥ 200 mg/dL and TG < 150 mg/dL; isolated low HDL-C: HDL-C ≤ 40 mg/dL in men and ≤ 50 mg/dL in women without hypertriglyceridemia or hypercholesterolemia and Mixed hyperlipidemia: TG ≥ 150 mg/dL and TC ≥ 200 mg/dL (National Cholesterol Education Program, ATP III, 2001)
Fig. 1.
Dyslipidemia prevalence in the sex (A), health status (B) and age (C) groups among academic staff. In Fig. 1(A), ap<0.05 when the male group is compared to the female group. In Fig. 1(B), a,bp<0.001; cp<0.05 and d,ep<0.01 when the healthy group is compared to hypertensive and diabetic groups respectively. In Fig. 1(C), ap<0.01; bp<0.001 when the lower age groups are compared to the highest age group. P-values are obtained from the chi-square test
Fig. 2.
Dyslipidemia prevalence in the sex (A), health status (B) and age (C) groups among students. In Fig. 2(A), a,cp<0.001; bp<0.05 when the male group is compared to the female group. In Fig. 2(B), a,b,cp<0.05 and d,ep<0.05 when the healthy group is compared to the hypertensive and diabetic groups respectively. In Fig. 2(C), a,bp<0.001 when the lower age groups are compared to the highest age group. P-values are obtained from the chi-square test
Factors associated with dyslipidemia
The results of the multivariable logistic regression analysis for staff and students are presented in Table 5 and Table 6, respectively. Among staff members, hypertriglyceridemia was positively and independently associated with the 36–45 years age group. Hypercholesterolemia showed a positive association with 46–55 years and > 55 years age groups and inadequate physical activity. Elevated LDL showed a positive association with 36–45 years and 46–55 years and > 55 years age groups, and inadequate physical activity. Low HDL showed a positive association with general obesity and inadequate physical activity. In contrast, among students, hypertriglyceridemia showed a positive association with > 21 years of age groups and diabetes. Hypercholesterolemia was positively associated with abdominal obesity. Elevated LDL was positively associated with abdominal obesity and inadequate physical activity. Low HDL showed a positive association with > 21 years of age groups, diabetes, and inadequate physical activity.
Table 5.
Assessing factors associated with dyslipidemia among academic staff
| Variables | Elevated TG | P-value | Elevated TC | P-value | Elevated LDL | P-value | Low HDL | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||
| Age (years) | |||||||||
| 18–35 | Reference | Reference | Reference | Reference | |||||
| 36–45 | 0.38 (0.16–1.46) | 0.046 | 3.38 (0.64–7.67) | 0.149 | 3.49 (0.90-13.48) | 0.070 | 0.93 (0.48–1.82) | 0.832 | |
| 46–55 | 1.68 (0.45–6.24) | 0.433 | 6.63 (1.21–8.34) | 0.029 | 4.18 (0.98–17.74) | 0.045 | 1.91 (0.73–4.96) | 0.184 | |
| > 55 | 1.86 (0.27–6.70) | 0.798 | 7.91 (0.45–9.70) | 0.042 | 5.77 (1.23–19.65) | 0.048 | 1.55 (0.40–6.08) | 0.528 | |
| Gender | |||||||||
| Male | Reference | Reference | Reference | Reference | |||||
| Female | 0.52 (0.23–1.19) | 0.120 | 1.11 (0.05–13.43) | 0.945 | 0.55 (0.04-8.00) | 0.661 | 0.76 (0.30–1.93) | 0.569 | |
| BMI (kg/m2) | |||||||||
| Normal | Reference | Reference | Reference | Reference | |||||
| Overweight | 1.77 (0.58–5.42) | 0.316 | 1.78 (0.17–20.33) | 0.998 | 6.89 (0.07–17.23) | 0.998 | 1.58 (0.53–4.70) | 0.409 | |
| Obese | 1.60 (0.40–6.32) | 0.503 | 1.53 (0.07–17.16) | 0.998 | 7.5 (0.05–21.87) | 0.998 | 8.88 (1.83–23.10) | 0.007 | |
| Diabetes | |||||||||
| No | Reference | Reference | Reference | Reference | |||||
| Yes | 0.73 (0.31–1.72) | 0.471 | 0.93 (0.17–4.95) | 0.930 | 1.31 (0.31–5.52) | 0.706 | 1.51 (0.63–3.65) | 0.353 | |
| Smoking | |||||||||
| No | Reference | Reference | Reference | Reference | |||||
| Yes | 0.77 (0.12–4.95) | 0.779 | 1.44 (0.03–13.56) | 0.996 | 1.48 (0.01–8.78) | 0.867 | 0.56 (0.03–9.58) | 0.689 | |
| Physical activity | |||||||||
| Medium/Adequate | Reference | Reference | Reference | Reference | |||||
| Inadequate | 0.73 (0.32–3.62) | 0.435 | 0.76 (0.16–2.56) | 0.032 | 0.75 (0.21–2.70) | 0.036 | 2.53 (1.01–6.38) | 0.049 | |
Values are presented as OR (95% CI). OR = Odds ratio, CI = Confidence Interval. Multivariable logistic regression was applied to evaluate the relationship between elevated lipid profile markers and associated factors
Table 6.
Assessing factors associated with dyslipidemia among students
| Variables | Elevated TG | P-value | Elevated TC | P-value | Elevated LDL | P-value | Low HDL | P-value |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Age (years) | ||||||||
| 18–21 | Reference | Reference | Reference | Reference | ||||
| 22–24 | 4.38 (1.92–9.91) | 0.000 | 7.46 (0.77–5.45) | 0.089 | 1.59 (0.47–5.32) | 0.451 | 5.75 (2.39–13.84) | 0.000 |
| > 24 | 3.59 (1.34–9.54) | 0.010 | 7.75 (0.67–9.17) | 0.104 | 0.91 (0.19–4.38) | 0.913 | 4.44 (1.56–12.68) | 0.005 |
| Gender | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.34 (0.07–1.73) | 0.195 | 0.57 (0.02–17.94) | 0.748 | 0.60 (0.02–16.58) | 0.762 | 0.45 (0.09–2.34) | 0.341 |
| BMI (kg/m2) | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| Overweight | 2.97 (0.80–11.00) | 0.103 | 0.67 (0.02–12.82) | 0.821 | 3.39 (0.01–11.62) | 0.998 | 1.48 (0.27–8.22) | 0.656 |
| Obese | 1.46 (0.86–15.10) | 0.065 | 1.79 (0.05–9.14) | 0.998 | 1.02 (0.01–1.32) | 0.998 | 0.56 (0.03–9.08) | 0.680 |
| Diabetes | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.86 (1.31–2.62) | 0.000 | 1.46 (0.78–2.72) | 0.234 | 1.88 (1.14–3.11) | 0.013 | 1.57 (1.19–2.04) | 0.001 |
| Smoking | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 8.29 (0.56–12.67) | 0.122 | 1.46 (0.03–14.80) | 0.999 | 1.18 (0.03–10.38) | 0.998 | 0.79 (0.05–12.26) | 0.867 |
| Physical activity | ||||||||
| Medium/Adequate | Reference | Reference | Reference | Reference | ||||
| Inadequate | 0.44 (0.07–2.75) | 0.380 | 5.18 (0.26–10.56) | 0.278 | 3.39 (1.22–5.71) | 0.032 | 3.30 (0.31–4.84) | 0.032 |
Values are presented as OR (95% CI). OR = Odds ratio, CI = Confidence Interval. Multivariable logistic regression was applied to evaluate the relationship between elevated lipid profile markers and associated factors
Discussion
The prevalence of dyslipidemia is steadily increasing over the past few decades and has become a global public health problem. Its prevalence varies widely according to ethnicity, socioeconomic status, culture, lifestyle, and dietary habits. This study determined the prevalence and associated risk factors among university academic staff members and students in Bangladesh. In this study, the prevalence of dyslipidemia was 85% in academic staff and 76.5% in students. In both academic staff and students, the most prevalent form of dyslipidemia was low HDL-C.
Some previous studies also determined the prevalence of dyslipidemia among staff and students in Asian countries. For example, a recent study conducted by Zhou et al. reported the prevalence of dyslipidemia among university staff members in China [32]. The authors reported comparatively an increased prevalence of dyslipidemia in male (51.49%) than in female (41.77%) staff members, with no significant difference between the genders [32]. In the present study, the prevalence of dyslipidemia was higher than those reported by Zhou et al. [32]. Similar to that study findings, we also did not find a significant difference in the prevalence of dyslipidemia between male (83.5%) and female staff (88.7%) members. This might be a reason the study participant’s education levels were high. It has been suggested that both lifestyle and biological factors are associated with dyslipidemia, which can be changed by education level; and a significant impact of higher education level was observed on TC and LDL-C components [33]. However, the exact mechanism by which the education level has an impact on dyslipidemia is not well understood, they likely involve psychological stress, unhealthy dietary habits and an unbalanced lifestyle [32]. A recent study showed that about 89% of university staff had moderate/high stress and only 25% of staff slept at least 8 h nightly [34]. There is also evidence that stress can increase the risk of obesity, diabetes, hypertension, and CVD [35]. Similar findings were found in other studies, showed that workers in pressurized environments or previous work stress history were possibly to have CVD [36, 37]. There are some possible mechanisms by which work-related stress can influence the pathways of cardiovascular pathology at molecular level, including high secretion of inflammatory cytokines and cortisol [35, 38, 39]. On the other hand, some studies have demonstrated a higher prevalence of dyslipidemia and a lower rate of awareness and treatment among males than among females [40, 41]. There are many disciplines in a university; however, health-related education is covered in a few disciplines, therefore, health education and intervention programs for university staff members are needed to reduce and prevent dyslipidemia.
The prevalence of dyslipidemia in our student cohort is close to the prevalence rate found among students (86.7%) of a Yemeni University [10]. Comparatively, a lower prevalence of dyslipidemia was found among university students (60%) in Saudi Arabia [9]. Another study also reported a low prevalence of dyslipidemia among students (63.8%) in a university in Egypt [42]. In our study, the prevalence of lipid profile abnormalities is comparatively higher (except high LDL) than those reported in a recent study conducted on undergraduate Medical College students (n = 100) in Dhaka, where the prevalence of hypertriglyceridemia, hypercholesterolemia, high level of LDL and low level of HDL were: 28.0%, 22.0%, 30.0%, 31.0%, and respectively [43]. This lower prevalence in that study [43] might be related to the small number of participants enrolled from a single institution or some differences in food habits. In the present study, male students had a comparatively higher prevalence of dyslipidemia than females, although the prevalence difference was not statistically significant between the genders. A study in Pakistan showed an increased tendency of dyslipidemia in younger males compared to younger females [44]. In other studies, an insignificant difference was also found in dyslipidemia prevalence between the genders [45, 46]. This variation between the gender groups in young adults might be related to food consumption habits and/or some physiological differences. In the present study, we also observed a high prevalence of dyslipidemia in female staff than the female students. This higher level of dyslipidemia among female staff might be related to increased age and postmenopausal effects on lipid levels. Epidemiological studies suggested that menopause may have a potential role in altering TC, LDL-C and HDL-C levels [47–49].
In this study, the prevalence of high TC, high LDL, and low HDL in the 36–55 years age group in the staff member is a concern. However, the prevalence of hypertriglyceridemia was higher in the 18–35 years age group, although it differs within the age groups. In our study subjects, the factor that contributed to hypertriglyceridemia might be a carbohydrate-rich diet [50]. In our study, hypercholesterolemia and elevated LDL were significantly associated with increased age. The possible reason might be the excess deposition of visceral fat which leads to releasing of high levels of free fatty acids and pro-inflammatory cytokines from the adipocytes and related macrophages, which further influence insulin resistance with increased age [51–53]. A retrospective study that included a large number of participants showed a peak prevalence of dyslipidemia in 40–59 years of age in males and 60–69 years of age in females [54]. However, the exact mechanisms behind the differences in dyslipidemia risk in males and females are not clear yet.
It is well established that obesity is one of the vital contributors to developing dyslipidemia. In our study, both general and abdominal obesity were significantly associated with dyslipidemia. Similar results were found in previous studies in other countries [32, 42, 55, 56]. Increased weight gain among our participants may be related to a significant portion of sedentary behaviours and more deskbound activities. In epidemiological studies, it has been suggested that the association of abdominal obesity with dyslipidemia is mediated via an etiopathological mechanism [57]. So, increased BMI and WC are considered primary screening tools for detecting dyslipidemia individuals. Considering these aspects, controlling body weight and reducing body fat can be effective strategies to control dyslipidemia and hypertension.
Inadequate physical activities were related to dyslipidemia in our analysis. Similar findings were observed in several early studies [42, 46, 58]. Intervention studies indicated that less exercise as physical activity may elevate lipid profile marker levels, resulting in a decrease in TG concentrations and an increase in HDL-C concentrations [59, 60]. Thus, encouraging regular physical activities may be effective in controlling and reducing dyslipidemia. The most prevalent form of dyslipidemia was low HDL in our study, which was in line with other studies conducted in neighbouring countries. Whether low HDL-C contributed to the increased cardiovascular risk in the South Asian population remains unknown, more studies are required to evaluate this further.
The association of dyslipidemia with hypertension and diabetes has been reported in numerous studies [61–65]. Similar to these previous studies, we also found an increased prevalence of dyslipidemia among hypertensive and diabetic participants than among the healthy control participants. Dyslipidemia may affect arteries’ structure and function, impairs endothelial function, interrupt nitric oxide production and blood pressure regulation, and promotes atherosclerosis [63, 66]. It is well known that dyslipidemia is a significant risk factor for CVD. Increased blood glucose levels combined with dyslipidemia increase atherosclerosis-related inflammation and make it more complicated [67]. On the other hand, the accumulation of visceral fat leads to insulin resistance which may play a key role in inducing diabetic dyslipidemia [68].
Our study had also some limitations which need to consider. Firstly, due to the nature of the cross-sectional design, causality could not be established in our study. Secondly, our findings may not apply to other populations, as the study subjects were mainly university staff and students. Thirdly, we did not have detailed data on participants’ food habits. Finally, the sample size was relatively small; therefore, further large-scale studies are needed to determine the actual scenario of the prevalence and risk factors of dyslipidemia in these special population groups in Bangladesh. The major strength of the present study is that it provided important information on the increased prevalence of dyslipidemia and potential associated factors in university academic staff and students in Bangladesh. Furthermore, this study’s findings might be a foundation for further investigations to reduce the burden of dyslipidemia and related complications among these special groups of the national population.
Conclusion
This study indicated a high prevalence of dyslipidemia among academic staff and students in Bangladesh. Low HDL-C was the most prevalent form of dyslipidemia among the study participants, followed by higher TG. The risk of dyslipidemia was significantly related to increased age, obesity, diabetes and inadequate physical activity. These results suggest the need for a screening program for the study of blood lipids and proper intervention programs to reduce and prevent dyslipidemia among academic staff and students in Bangladesh.
Acknowledgements
The authors would like to thank all participants for their cooperation and participation in the study.
Authors’ contributions
N.A. contributed in the conception, study design, execution, acquisition of data, analysis and interpretation, drafting and revision of the article. R.R.K., K.A.F., and A.T., contributed to experiments and data analysis. F.I., contributed to the manuscript revision. All authors read and approved the submitted version.
Funding
This study did not receive any external funding. It was supported by an internal grant from the SUST research centre (LS/2018/2/07).
Data Availability
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors have no conflict of interest to declare.
Ethics approval and consent to participate
The BMB Department, School of Life Sciences, SUST Ethics Review Committee approved this study protocol (ID 02/BMB/2019). Written informed consent was obtained from all study subjects before study commencement.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–90. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 2.Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18:689–700. doi: 10.1038/s41569-021-00541-4. [DOI] [PubMed] [Google Scholar]
- 3.McAloon CJ, Osman F, Glennon P, Lim PB, Hayat SA. Global epidemiology and incidence of Cardiovascular Disease. Cardiovascular Diseases. Elsevier; 2016. 57–96.
- 4.Chowdhury MZI, Haque MA, Farhana Z, Anik AM, Chowdhury AH, Haque SM, et al. Prevalence of cardiovascular disease among bangladeshi adult population: a systematic review and meta-analysis of the studies. Vasc Health Risk Manag. 2018;14:165. doi: 10.2147/VHRM.S166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalina-Romero C, Calvo E, Sánchez-Chaparro MA, Valdivielso P, Sainz JC, Cabrera M, et al. The relationship between job stress and dyslipidemia. Scand J Public Health. 2013;41:142–9. doi: 10.1177/1403494812470400. [DOI] [PubMed] [Google Scholar]
- 6.Mg K, Sb K, Bs C, Jk P, Sk B, Sj C. Job stress and cardiovascular risk factors in male workers. Prev Med. 2005;40. [DOI] [PubMed]
- 7.Ezeukwu AO, Agwubike EO. Anthropometric measures of adiposity as correlates of atherogenic index of plasma in non-obese sedentary nigerian males. Libyan J Med. 2014;9:23798. doi: 10.3402/ljm.v9.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawant AM, Shetty D, Mankeshwar R, Ashavaid TF. Prevalence of dyslipidemia in young adult indian population. Japi. 2008;56:99–102. [PubMed] [Google Scholar]
- 9.Hamam F. Dyslipidemia and related risk factors in a Saudi University Community. FNS. 2017;08:56–69. doi: 10.4236/fns.2017.81004. [DOI] [Google Scholar]
- 10.Al-Duais MA, Al-Awthan YS. Prevalence of dyslipidemia among students of a yemeni University. J Taibah Univ Med Sci. 2019;14:163–71. doi: 10.1016/j.jtumed.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK, et al. Prevalence of dyslipidemia in urban and rural India: the ICMR–INDIAB study. PLoS ONE. 2014;9:e96808. doi: 10.1371/journal.pone.0096808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleeman JI, Grundy SM. National cholesterol Education Program recommendations for cholesterol testing in young adults. A science-based approach. Circulation. 1997;95:1646–50. doi: 10.1161/01.CIR.95.6.1646. [DOI] [PubMed] [Google Scholar]
- 13.Washington RL. Interventions to reduce cardiovascular risk factors in children and adolescents. Am Fam Physician. 1999;59:2211–8. [PubMed] [Google Scholar]
- 14.Ali N, Mahmood S, Manirujjaman M, Perveen R, Al Nahid A, Ahmed S, et al. Hypertension prevalence and influence of basal metabolic rate on blood pressure among adult students in Bangladesh. BMC Public Health. 2018;18:1–9. doi: 10.1186/s12889-017-4617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman S, Islam S, Haque T, Kathak RR, Ali N. Association between serum liver enzymes and hypertension: a cross-sectional study in bangladeshi adults. BMC Cardiovasc Disord. 2020;20:1–7. doi: 10.1186/s12872-020-01411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saadi MM, Roy MN, Haque R, Tania FA, Mahmood S, Ali N. Association of microalbuminuria with metabolic syndrome: a cross-sectional study in Bangladesh. BMC Endocr Disord. 2020;20:153. doi: 10.1186/s12902-020-00634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molla NH, Kathak RR, Sumon AH, Barman Z, Mou AD, Hasan A, et al. Assessment of the relationship between serum uric acid levels and liver enzymes activity in bangladeshi adults. Sci Rep. 2021;11:1–9. doi: 10.1038/s41598-021-99623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mou AD, Barman Z, Hasan M, Miah R, Hafsa JM, Das Trisha A, et al. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. 2021;11:1–9. doi: 10.1038/s41598-021-00839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali N, Ahmed S, Mahmood S, Trisha AD, Mahmud F. The prevalence and factors associated with obesity and hypertension in university academic staff: a cross-sectional study in Bangladesh. Sci Rep. 2023;13:7309. doi: 10.1038/s41598-023-34574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barman Z, Hasan M, Miah R, Mou AD, Hafsa JM, Trisha AD, et al. Association between hyperuricemia and chronic kidney disease: a cross-sectional study in bangladeshi adults. BMC Endocr Disord. 2023;23:45. doi: 10.1186/s12902-023-01304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan M, Fariha KA, Barman Z, Mou AD, Miah R, Habib A, et al. Assessment of the relationship between serum xanthine oxidase levels and type 2 diabetes: a cross-sectional study. Sci Rep. 2022;12:20816. doi: 10.1038/s41598-022-25413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miah R, Fariha KA, Sony SA, Ahmed S, Hasan M, Mou AD, et al. Association of serum xanthine oxidase levels with hypertension: a study on bangladeshi adults. Sci Rep. 2022;12:21727. doi: 10.1038/s41598-022-26341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali N, Rahman S, Islam S, Haque T, Molla NH, Sumon AH, et al. The relationship between serum uric acid and lipid profile in bangladeshi adults. BMC Cardiovasc Disord. 2019;19:1–7. doi: 10.1186/s12872-019-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali N, Miah R, Hasan M, Barman Z, Mou AD, Hafsa JM, et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in bangladeshi adults. Sci Rep. 2020;10:1–7. doi: 10.1038/s41598-020-64884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque T, Rahman S, Islam S, Molla NH, Ali N. Assessment of the relationship between serum uric acid and glucose levels in healthy, prediabetic and diabetic individuals. Diabetol Metab Syndr. 2019;11:1–8. doi: 10.1186/s13098-019-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathak RR, Sumon AH, Molla NH, Hasan M, Miah R, Tuba HR, et al. The association between elevated lipid profile and liver enzymes: a study on bangladeshi adults. Sci Rep. 2022;12:1–8. doi: 10.1038/s41598-022-05766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleeman JI, Grundy SM, Becker D, Clark L. Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol Education Program (NCEP) Adult Treatment Panel (ATP III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 29.Ali N, Mahmud F, Akter SA, Islam S, Sumon AH, Barman DN et al. The prevalence of general obesity, abdominal obesity, and hypertension and its related risk factors among young adult students in Bangladesh. J Clin Hypertens. 2022. [DOI] [PMC free article] [PubMed]
- 30.WHO Appropriate body-mass index for asian populations and its implications for policy and intervention strategies. Lancet (London England) 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali N, Mohanto NC, Nurunnabi SM, Haque T, Islam F. Prevalence and risk factors of general and abdominal obesity and hypertension in rural and urban residents in Bangladesh: a cross-sectional study. BMC Public Health. 2022;22:1–14. doi: 10.1186/s12889-022-14087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhang J, Liu R-H, Xie Q, Li X-L, Chen J-G, et al. Association between Health-Related physical fitness and risk of Dyslipidemia in University Staff: a cross-sectional study and a ROC curve analysis. Nutrients. 2021;14:50. doi: 10.3390/nu14010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho SMJ, Lee HJ, Shim JS, Song BM, Kim HC. Associations between age and dyslipidemia are differed by education level: the Cardiovascular and metabolic Diseases Etiology Research Center (CMERC) cohort. Lipids Health Dis. 2020;19:1–12. doi: 10.1186/s12944-020-1189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Sayegh N, Al-Enezi K, Nadar M, Dean E. Health status, behaviors, and beliefs of health sciences students and staff at Kuwait University: toward maximizing the health of future health professionals and their patients. Int J Environ Res Public Health. 2020;17:8776. doi: 10.3390/ijerph17238776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabi H, Kivimäki M, Batty GD, Shipley MJ, Britton A, Brunner EJ, et al. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J. 2013;34:2697–705. doi: 10.1093/eurheartj/eht216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steptoe A, Kivimäki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–54. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- 37.Satyjeet FNU, Naz S, Kumar V, Aung NH, Bansari K, Irfan S et al. Psychological stress as a risk factor for cardiovascular disease: a case-control study. Cureus. 2020;12. [DOI] [PMC free article] [PubMed]
- 38.von Känel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med. 2001;63:531–44. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–65. doi: 10.1161/01.CIR.0000038364.26310.BD. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Xiao J, Yang Z, Ji L, Jia W, Weng J, et al. Serum lipids and lipoproteins in chinese men and women. Circulation. 2012;125:2212–21. doi: 10.1161/CIRCULATIONAHA.111.065904. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Yu S, Mao Z, Li Y, Zhang H, Yang K, et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in chinese rural population: the Henan rural cohort study. Lipids Health Dis. 2018;17:1–12. doi: 10.1186/s12944-018-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahed WYA, El-Khashab K, Hassan SK. Prevalence of Dyslipidemia among Healthy University Students: Fayoum Governorate, Egypt. Epidemiology, Biostatistics, and Public Health. 2016;13.
- 43.Hossain MS, Islam MD, Galib A, Malek R, Akter K, Khanam M. Lipid Profile in Relation to Body Mass Index among Medical College students in Dhaka, Bangladesh. Med Today. 2021;33:114–9. doi: 10.3329/medtoday.v33i2.56055. [DOI] [Google Scholar]
- 44.Basit A, Sabir S, Riaz M, Fawwad A, members Abro NDSP. NDSP 05: prevalence and pattern of dyslipidemia in urban and rural areas of Pakistan; a sub analysis from second National Diabetes Survey of Pakistan (NDSP) 2016–2017. J Diabetes Metab Disord. 2020;19:1215–25. doi: 10.1007/s40200-020-00631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabauskas V, Miseviciene I, Klumbiene J, Petkeviciene J, Milasauskiene Z, Plieskiene A, et al. Prevalence of dyslipidemias among lithuanian rural population (CINDI program) Med (Kaunas) 2003;39:1215–22. [PubMed] [Google Scholar]
- 46.Al-Kaabba AF, Al-Hamdan NA, El Tahir A, Abdalla AM, Saeed AA, Hamza MA. Prevalence and correlates of dyslipidemia among adults in Saudi Arabia: results from a national survey. 2012.
- 47.Carr MC, Kim KH, Zambon A, Mitchell ES, Woods NF, Casazza CP, et al. Changes in LDL density across the menopausal transition. J Investig Med. 2000;48:245–50. [PubMed] [Google Scholar]
- 48.Hjortland MC, McNamara PM, Kannel WB. Some atherogenic concomitants of menopause: the Framingham Study. Am J Epidemiol. 1976;103:304–11. doi: 10.1093/oxfordjournals.aje.a112228. [DOI] [PubMed] [Google Scholar]
- 49.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 50.Krishnaswami V, Radhakrishnan T, John BM, Mathew A. Pattern of ischaemic heart disease: a clinical study. J Indian Med Assoc. 1970;55:153–7. [PubMed] [Google Scholar]
- 51.Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, et al. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58:1452–8. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 52.Shanmugasundaram M, Rough SJ, Alpert JS. Dyslipidemia in the elderly: should it be treated? Clin Cardiol. 2010;33:4–9. doi: 10.1002/clc.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab. 2014;99:E2269–2276. doi: 10.1210/jc.2013-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu T, Zhou W, Sun J, Wang J, Zhu D, Bi Y. Gender and age differences in lipid Profile among chinese adults in Nanjing: a retrospective study of over 230,000 individuals from 2009 to 2015. Exp Clin Endocrinol Diabetes. 2018;126:429–36. doi: 10.1055/s-0043-117417. [DOI] [PubMed] [Google Scholar]
- 55.Bays HE, Chapman RH, Grandy S, SHIELD Investigators’ Group The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61:737–47. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma U, Kishore J, Garg A, Anand T, Chakraborty M, Lali P. Dyslipidemia and associated risk factors in a resettlement colony of Delhi. J Clin Lipidol. 2013;7:653–60. doi: 10.1016/j.jacl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Paccaud F, Schlüter-Fasmeyer V, Wietlisbach V, Bovet P. Dyslipidemia and abdominal obesity: an assessment in three general populations. J Clin Epidemiol. 2000;53:393–400. doi: 10.1016/S0895-4356(99)00184-5. [DOI] [PubMed] [Google Scholar]
- 58.Shawar SM, Al-Bati NA, Al-Mahameed A, Nagalla DS, Obeidat M. Hypercholesterolemia among apparently healthy university students. Oman Med J. 2012;27:274–80. doi: 10.5001/omj.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erem C, Hacihasanoglu A, Deger O, Kocak M, Topbas M. Prevalence of dyslipidemia and associated risk factors among turkish adults: Trabzon lipid study. Endocrine. 2008;34:36–51. doi: 10.1007/s12020-008-9100-z. [DOI] [PubMed] [Google Scholar]
- 60.Kang W-M, Zhang J-S, Liu X-X, Wang M-S, Zhao M-L, Yu J-C. Prevalence of abnormity of blood lipid and associated factors in health examination population in Beijing. Chin Med Sci J. 2009;24:142–6. doi: 10.1016/S1001-9294(09)60078-X. [DOI] [PubMed] [Google Scholar]
- 61.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, et al. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition examination surveys II, III, and 1999–2006. Am J Cardiol. 2010;106:969–75. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 62.Choudhury KN, Mainuddin AKM, Wahiduzzaman M, Islam SMS. Serum lipid profile and its association with hypertension in Bangladesh. Vasc Health Risk Manag. 2014;10:327–32. doi: 10.2147/VHRM.S61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otsuka T, Takada H, Nishiyama Y, Kodani E, Saiki Y, Kato K, et al. Dyslipidemia and the risk of developing hypertension in a Working-Age Male Population. J Am Heart Assoc. 2016;5:e003053. doi: 10.1161/JAHA.115.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhowmik B, Siddiquee T, Mujumder A, Afsana F, Ahmed T, Mdala IA, et al. Serum lipid Profile and its Association with Diabetes and Prediabetes in a Rural Bangladeshi Population. Int J Environ Res Public Health. 2018;15:E1944. doi: 10.3390/ijerph15091944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao C-Y, Ye X-F, Zhang W, Ji L-N, Wang J-G, ATTEND investigators Association between dyslipidemia and antihypertensive and antidiabetic treatments in a China multicenter study. J Clin Hypertens (Greenwich) 2021;23:1399–404. doi: 10.1111/jch.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–11. doi: 10.1016/S0735-1097(02)01723-0. [DOI] [PubMed] [Google Scholar]
- 67.Taskinen M-R, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–95. doi: 10.1016/j.atherosclerosis.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 68.Yanai H, Hirowatari Y, Yoshida H. Diabetic dyslipidemia: evaluation and mechanism. GHM. 2019;1:30–5. doi: 10.35772/ghm.2019.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.