Abstract
Triplet DCF (docetaxel, cisplatin and 5-flurouracil) and doublet CP/CF (carboplatin and paclitaxel/cisplatin and 5-fluorouracil) regimens were prospectively evaluated in advanced squamous anal cell carcinoma (SCCA), and validated as standard treatments. Even though the high efficacy and good tolerance of DCF regimen were confirmed in 3 independent prospective trials, doublet CP regimen is still recommended in several guidelines based in its better safety profile with similar efficacy compared to CF regimen. We performed a propensity score-adjusted method with inverse probability of treatment weighted (IPTW) and matched case control (MCC) comparison among patients with metastatic or non-resectable locally advanced recurrent SCCA, treated with chemotherapy as first line regimen. The primary endpoint was the overall survival (OS), and the secondary endpoint was the progression-free survival (PFS). 247 patients were included for analysis. 154 patients received DCF and 93 patients received a doublet regimen. The median OS was 32.3 months with DCF and 18.3 months with doublet regimens (HR 0.53, 95%CI 0.38–0.74; p = 0.0001), and the median PFS was 11.2 months with DCF versus 7.6 months with doublet regimens (HR 0.53, 95%CI 0.39–0.73; p < 0.0001). The hazard ratios by IPTW and MCC analyses were 0.411 (95% CI, 0.324–0.521; p < 0.0001) and 0.406 (95% CI, 0.261–0.632; p < 0.0001) for OS, and 0.466 (95% CI, 0.376–0.576; p < 0.0001) and 0.438 (95% CI, 0.298–0.644; P < 0.0001) for PFS. The triplet DCF regimen provides a high and significant benefit in OS and PFS over doublet regimens, and should be considered as upfront treatment for eligible patients with advanced SCCA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-023-00413-2.
Keywords: Anal carcinoma, Advanced, Metastatic, Chemotherapy, Docetaxel
To the editor. The advanced squamous cell carcinoma of the anus (SCCA) is a rare entity but its incidence is steadily increasing [1, 2]. For metastatic or non-resectable locally advanced recurrence, two chemotherapy regimens were prospectively validated [3, 4]. First, the triplet DCF regimen has consistently demonstrated a high objective response rate (ORR, ~85%) and complete response rate (CRR, ~45%), as well as a long-term PFS (24.5% at 5 years) and OS (44.4% at 5 years) rates in three independent prospective trials [3, 5–7], and became standard [8]. The modified biweekly DCF (mDCF) regimen is preferred to the standard DCF (sDCF) regimen due to its good tolerance (grade 3/4 toxicity rate of 36 to 53% with mDCF vs. 83% with sDCF) [3, 7]. Second, carboplatin and paclitaxel (CP) regimen, despite its similar predefined efficacy (ORR 59% vs. 57%) and toxicity (grade 3/4 toxicity rate 71% vs. 76%) endpoints compared to cisplatin and 5-fluorouracil (CF) regimen, was considered as the preferred regimen in a randomized phase 2 study due to its significantly lower serious adverse events [4]. Thus, while there is no safety argumentation to prefer doublet over DCF regimen, and the efficacy data of DCF is encouraging, no direct comparison is currently available.
We used 3 independent large French SCCA databases. All SCCA patients with metastatic or non-resectable locally advanced recurrence, and treated in first-line with at least one cycle of DCF, or a doublet chemotherapy regimen were included in the analysis. The primary outcome was OS, and the secondary outcome was PFS. In order to limit bias due to potential confounding factors unbalanced between treatment groups we applied a propensity score method, considered as the best available tool to minimize the difference of the characteristics among non-randomized groups [9] (Additional File 1).
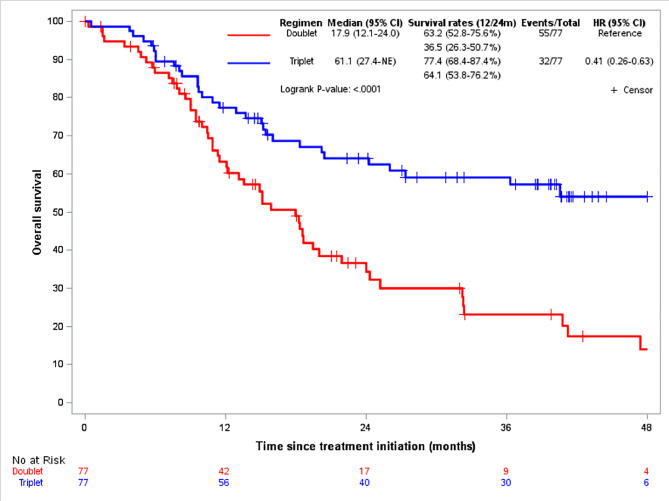
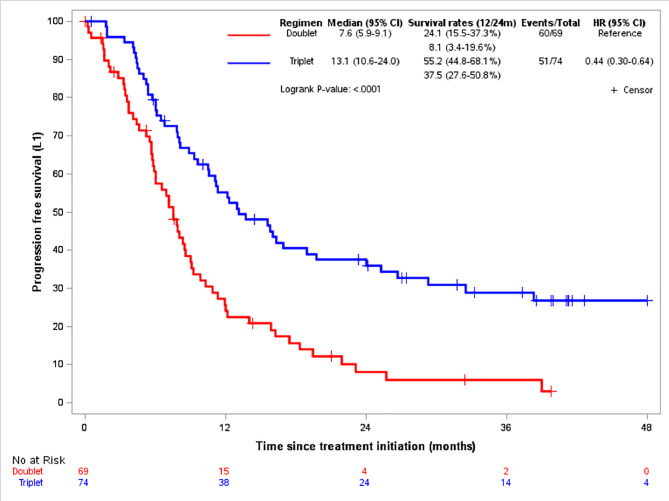
247 patients fulfilled the eligibility criteria and were included for analysis. 93 patients received a doublet chemotherapy, and 154 patients received DCF (table S1). The median OS was 32.3months (95%CI, 24.8–61.1) in the DCF arm, and 18.3months (95%CI, 13.6–24.0) in the doublet arm (HR 0.53, 95%CI 0.38–0.74; p = 0.0001) (Figure S1). The median PFS was 11.2months (95%CI, 10.1–13.7) in the DCF arm, and 7.6months (95%CI, 6.0-9.1) in the doublet arm (HR 0.53, 95%CI 0.39–0.73; p < 0.0001) (Figure S2). In the matched population (77 patients in each arm) with well-balanced characteristics at baseline (Table S2), the median OS was 61.1months (95%CI, 27.4-NE) in the DCF arm compared to 17.9months (95%CI, 12.1–24.0) in the doublet arm (Fig. 1). The median PFS was 13.1months in the DCF arm (95% CI, 10.6–24.0) versus 7.6months (95%CI, 5.9–9.1) in the doublet arm (Fig. 2). HR for OS and PFS were 0.406 (95%CI, 0.261–0.632; p < 0.0001) and 0.438 (95% CI, 0.298–0.644; p < 0.0001), respectively. In the IPTW analysis, the HR for OS and PFS were 0.411 (95%CI, 0.324–0.521; P < 0.0001) and 0.466 (95%CI, 0.376–0.576; p < 0.0001), respectively. The benefit of DCF regimen was observed irrespectively of doublet chemotherapy regimen used. The HR for OS was 2.34 (95%CI, 1.46–3.73) with CF, 3.07 (95%CI, 1.06–8.84) with CP, and 2.88 (95%CI, 1.41–5.90) with mitomycin and fluoropyrimidine (MF) compared to DCF regimen (Figure S3).
Fig. 1.
overall survival according to regimens in matched population
Fig. 2.
progression-free survival according to regimens in matched population
In this study, the patients’ characteristics and outcomes observed with doublet chemotherapy is comparable to those of published data (Table S3) [4]. Then, DCF regimen provided a high and significant benefit over doublet chemotherapy regimens in the upfront treatment of advanced SCCA patients, irrespective of different doublet regimens. The long-term outcomes also favored DCF: at 4 years, ~55% of patients were alive in the DCF arm, compared to ~15% in the doublet arm. PFS rates were 55.2% vs. 24.1% at 1 year, and 37.5% vs. 8.1% at 2 year, and ~30% vs. <5% at 4 years. These efficacy data are in line with published biological results. In Epitopes-HPV02 and InterAACT trials, the clearance of HPV ctDNA, which was significantly correlated to a better survival, was observed in 61.1% of patients after DCF [10], and 17.9% after doublet CP/CF regimens [4]. Even though there are obvious limitations in our study mainly related to the absence of the randomization and the retrospective nature of the analysis, the magnitude of the adjusted OS benefit was around 60% in favor of DCF. Thus, in the absence of a randomized trial, DCF should be considered as an upfront treatment for eligible patients with advanced SCCA.
In second-line, anti-PD1 immunotherapy is effective in 10–20% of patients. However, new immunotherapy combination regimens currently being evaluated seem more promising. New line of chemotherapy is also an option in patients with good performance status. Besides, ablative treatments should always be considered as part of first and second-line strategies in selective patients, especially in good responders with oligometastatic disease [11].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary data: methods, tables and figures
Acknowledgements
The authors would like to thank the investigators and their team.
The authors would like to thank Guadalupe Tizón for the English writing assistance.
Abbreviations
- CF
cisplatin and 5-fluorouracil
- CP
carboplatin and paclitaxel
- ctDNA
circulating tumor DNA
- DCF
docetaxel, cisplatin and 5-flurouracil
- HPV
human papillomavirus
- HR
hazard ratio
- IPTW
inverse probability of treatment weighted
- MCC
matched case control
- mDCF
modified DCF
- MF
mitomycin and fluoropyrimidine
- NE
not evaluable
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- SCCA
squamous anal cell carcinoma
- sDCF
standard DCF
Authors’ contributions
Conceptualization, SK, DV, CB; methodology, KLM, DV, AM; validation, SK, AV, MJ, CB; investigation and resources, SK, VV, AS, TA, PV, ES, SP, OB, MZ, JD, CdlF, DS, FG, AV, EK, TN, EF, JT, CB; data curation, SK, AM; writing-original draft preparation, SK, AM; writing-review and editing, SK, DV, CB; supervision project administration, SK, MJ, CB.All authors have read and agreed to the published version of the manuscript.
Funding
None.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Islami F, Ferlay J, Lortet-Tieulent J, et al. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46:924–38. doi: 10.1093/ije/dyw276. [DOI] [PubMed] [Google Scholar]
- 2.Deshmukh AA, Suk R, Shiels MS, et al. Recent Trends in squamous cell carcinoma of the Anus incidence and mortality in the United States, 2001–2015. J Natl Cancer Inst. 2020;112:829–38. doi: 10.1093/jnci/djz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, François E, André T, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–106. doi: 10.1016/S1470-2045(18)30321-8. [DOI] [PubMed] [Google Scholar]
- 4.Rao S, Sclafani F, Eng C, et al. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J Clin Oncol. 2020;38:2510–8. doi: 10.1200/JCO.19.03266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Jary M, Mansi L, et al. DCF (docetaxel, cisplatin and 5-fluorouracil) chemotherapy is a promising treatment for recurrent advanced squamous cell anal carcinoma. Ann Oncol. 2013;24:3045–50. doi: 10.1093/annonc/mdt396. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Meurisse A, Spehner L, et al. Pooled analysis of 115 patients from updated data of Epitopes-HPV01 and Epitopes-HPV02 studies in first-line advanced anal squamous cell carcinoma. Ther Adv Med Oncol. 2020;12:1758835920975356. doi: 10.1177/1758835920975356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Ghiringhelli F, De La Fouchardiere C, et al. Atezolizumab plus modified DCF (docetaxel, cisplatin, and 5-fluorouracil) as first-line treatment for metastatic or locally advanced squamous cell anal carcinoma: a SCARCE-PRODIGE 60 randomized phase II study. J Clin Oncol. 2022;40:3508. doi: 10.1200/JCO.2022.40.16_suppl.3508. [DOI] [Google Scholar]
- 8.Moureau-Zabotto L, Vendrely V, Abramowitz L, et al. Anal cancer: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SNFCP) Dig Liver Dis. 2017;49:831–40. doi: 10.1016/j.dld.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Haukoos JS, Lewis RJ. The Propensity score. JAMA. 2015;314:1637. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard-Tessier A, Jeannot E, Guenat D, et al. Clinical validity of HPV circulating tumor DNA in Advanced Anal Carcinoma: an ancillary study to the Epitopes-HPV02 trial. Clin Cancer Res. 2019;25:2109–15. doi: 10.1158/1078-0432.CCR-18-2984. [DOI] [PubMed] [Google Scholar]
- 11.Stouvenot M, Meurisse A, Saint A, Buecher B, et al. Second-line treatment after docetaxel, cisplatin and 5-fluorouracil in metastatic squamous cell carcinomas of the anus. Pooled analysis of prospective Epitopes-HPV01 and Epitopes-HPV02 studies. Eur J Cancer. 2022;162:138–47. doi: 10.1016/j.ejca.2021.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data: methods, tables and figures