Abstract
Background
Although sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated cardiovascular benefits in patients with type 2 diabetes mellitus, real-world evidence regarding their benefits to diabetic patients with acute myocardial infarction (AMI) is insufficient. This study evaluated cardiovascular outcomes by comparing SGLT2i with dipeptidyl peptidase-4 inhibitors (DPP-4i) in combination with metformin in diabetic patients with AMI.
Methods
This study involved 779 diabetic participants with AMI from a Korean nationwide multicenter observational cohort, who were divided into two groups: (1) metformin plus SGLT2i group (SGLT2i group, n = 186) and (2) metformin plus DPP-4i (DPP-4i group, n = 593). The primary endpoint was one year of major adverse composite events (MACEs), a composite outcome of all-cause mortality, non-fatal myocardial infarction, any revascularization, cerebrovascular accident, and stent thrombosis. To balance the baseline differences, inverse probability of treatment weighting (IPTW) was performed.
Results
After IPTW, the rate of MACEs in the SGLT2i group was not significantly lower than that in the DPP-4i group (hazard ratio [HR], 0.99; 95% confidence interval [Cl], 0.46 to 2.14, p = 0.983). In the unadjusted and adjusted analyses, all items for clinical outcomes were comparable between the two groups. In our exploratory analysis, the left ventricular ejection fraction showed a significant improvement in the SGLT2i group than in the DPP-4i group before achieving statistical balancing (6.10 ± 8.30 versus 2.95 ± 10.34, p = 0.007) and after IPTW adjustment (6.91 ± 8.91 versus 3.13 ± 10.41, p = 0.027).
Conclusions
Our findings demonstrated that SGLT2i did not influence the rate of MACEs compared with DPP-4i in combination with metformin in diabetic patients with AMI but did improve left ventricular ejection fraction.
Trial registration
Not applicable (retrospectively registered).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01914-4.
Keywords: Antidiabetic agents, Diabetes mellitus, Hypoglycemic agents, Myocardial infarction
Background
Diabetes mellitus (DM) is the main risk factor for the onset of atherosclerotic cardiovascular disease (ASCVD). Conversely, cardiovascular disease is the leading cause of mortality in patients with DM [1]. Moreover, patients with DM are known to have poor short- and long-term prognoses following acute myocardial infarction (AMI) compared with those without DM [2]. Therefore, preventing myocardial infarction (MI) in diabetic patients is crucial, and if an AMI does occur, administering aggressive treatment is necessary to ensure optimal outcomes.
Combination therapy with oral hypoglycemic agents (OHAs) is frequently prescribed to optimize glucose control. Many clinicians use sodium-glucose cotransporter 2 inhibitors (SGLT2i) or dipeptidyl peptidase-4 inhibitors (DPP-4i) in patients with DM as a second-line therapy in combination with metformin (MET) monotherapy. However, these strategies have different mechanisms of action and clinical benefits. SGLT2i, a novel class of OHAs, reduce serum glucose levels via an insulin-independent mechanism by inhibiting the reabsorption of glucose from the proximal convoluted tubule in the kidney, which expedites renal glucosuria [3]. In recently published cardiovascular outcome trials (CVOTs), SGLT2i have proven cardiovascular benefits in patients with DM in both primary and secondary prevention [4–7], which is driven by a low incidence of cardiovascular death and hospitalization for heart failure (HF). Based on these results, the 2022 American Diabetes Association guidelines have recommended the use of SGLT2i in patients diagnosed with type 2 DM and who have a history of, or are at high risk for, ASCVDs and HF [8]. SGLT2i have been successful in improving glycemic control without causing hypoglycemia and effectively lowering body weight. DPP-4i have a different glucose-lowering effect, which works by deactivating the two main incretin hormones (i.e., glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1). This leads to an increase in insulin and a decrease in glucagon [9]. DPP-4i have demonstrated neutral cardiovascular effects in CVOTs [10–13]. Nonetheless, in Korea, they are commonly used as second-line OHAs in combination with MET owing to their favorable tolerability, neutral effect on weight gain, and low risk of hypoglycemia [14–17].
In patients with AMI, the combination of OHAs appears to be an important strategy for achieving glycemic goals and long-term cardiovascular benefits. Determining the optimal combination of OHAs is important for the desired clinical outcomes of AMI. In a literature review, a few articles have compared the clinical outcomes of SGLT2i and DPP-4i [18–21]. However, most of these studies have focused on the investigation of surrogate markers of cardiovascular disease, such as metabolic risk factors, glycated hemoglobin (HbA1c), and lipid profiles, and have included the general population rather than specifically patients with AMI. Therefore, real-world evidence of a combination of OHAs in AMI patients is limited. This study aimed to evaluate cardiovascular outcomes by comparing the use of SGLT2i versus DPP-4i in combination with MET in patients with AMI and concomitant DM.
Methods
Data collection of study population and study scheme
All relevant information was gathered from the Korea Acute Myocardial Infarction Registry-V (KAMIR-V). The KAMIR-V is a nationwide, multicenter, observational cohort supported by the Korean Working Group of Acute Myocardial Infarction, which included Korean AMI patients from January 2016 to June 2020. In total, 43 tertiary medical facilities equipped with percutaneous coronary intervention (PCI) capabilities and on-site coronary artery bypass graft services participated in this registry [22, 23]. It gathers demographic and biological information including the characteristics and clinical outcomes of the Korean population with AMI. The study protocol was approved by the institutional review boards of the participating institutions.
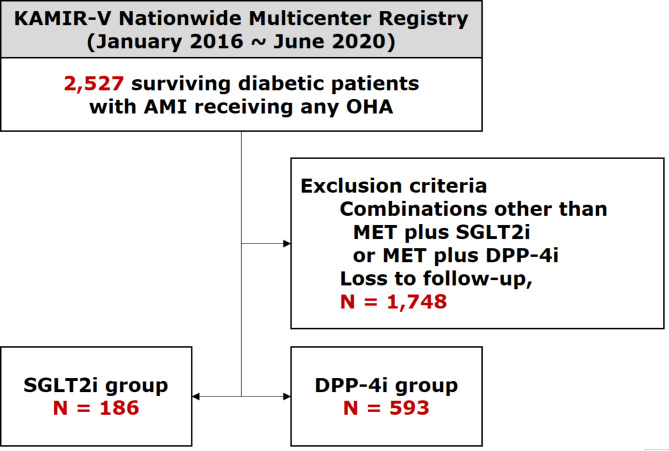
Among a total of 15,628 cohort participants with suspected AMI in the KAMIR-V, 2,527 surviving participants with AMI and concomitant DM receiving any OHA were screened. After excluding (1) those who received combinations of OHA other than MET plus SGLT2i or MET plus DPP-4i and (2) those who were lost to follow-up, 779 participants were finally included in the analysis. These participants were subdivided into two groups based on their OHA types at the point of discharge: (1) MET plus SGLT2i group (SGLT2i group, n = 186) and (2) MET plus DPP-4i group (DPP-4i group, n = 593). The flowchart of the enrollment of participants is illustrated in Fig. 1.
Fig. 1.
Study flowchart
AMI = acute myocardial infarction; DPP-4i = dipeptidyl peptidase-4 inhibitors; KAMIR-V = Korea Acute Myocardial Infarction Registry-V; MET = metformin; OHA = oral hypoglycemic agent; SGLT2i = sodium-glucose cotransporter 2 inhibitors
Definitions
According to the international guidelines [24], AMI is defined as myocardial injury characterized by a rise or fall in cardiac markers and related clinical manifestations as follows: (1) myocardial ischemia-related symptoms and/or signs; (2) new-onset changes in the 12-lead electrocardiogram (ECG), including deviated ST-segments (either elevation or depression), inverted T-waves, or the development of pathological Q-waves; (3) imaging evidence for loss of viable myocardium or regional wall motion abnormalities; or (4) existence of intracoronary thrombi during coronary angiography. ST-segment elevation MI (STEMI) is defined as AMI with new-onset ST-segment elevation in at least two continuous leads on the 12-lead surface ECG (> 0.2 mV in leads V1-3 or > 0.1 mV in all other leads on the 12-lead surface ECG) [25, 26]. As mentioned in previous studies [22, 27], the definitions of left main coronary artery (LMCA) disease and multivessel coronary artery disease (CAD) were defined as a disease with a ≥ 50% reduction in the diameter of LMCA and a disease with ≥ 70% reduction in the diameter of ≥ 2 native coronary vessels or ≥ 70% reduction in the diameter of one native coronary vessel with ≥ 50% reduction in the diameter of LMCA, respectively. Body mass index (BMI) was calculated using the mass (weight) and height values of the participants. Left ventricular ejection fraction (LVEF) was measured using 2-dimensional transthoracic echocardiography during hospitalization.
In addition, we recorded the angiographic and procedural characteristics in both groups. Intracoronary imaging guidance in PCI refers to the use of two currently available devices (intravascular ultrasound or optical coherence tomography) in the PCI procedure. The infarct-related artery, typically referred to as the culprit artery, is defined as a native coronary artery that is responsible for the development of AMI with occlusion or stenosis due to atherothrombotic changes. Thrombolysis In Myocardial Infarction (TIMI) flow grade was used as a quantitative and stratified indicator of antegrade intracoronary flow [28]. The characteristics of intracoronary lesions were defined according to the American College of Cardiology/American Heart Association classification (ACC/AHA) [29, 30]. Treatment strategies for PCI were subdivided as follows: (1) drug-eluting stent (DES) implantation, (2) bioresorbable vascular scaffold implantation, (3) balloon angioplasty only, and (4) other treatments. Infarct-related artery (IRA) was categorized into two types: (1) LMCA or left anterior descending coronary artery and (2) left circumflex coronary artery and right coronary artery.
Clinical outcomes
Clinical follow-up of each participant was conducted for 12 months after enrollment. As previously described, it was performed at 6 months and 1 year through outpatient visits or whenever any cardiovascular event occurred [23]. The primary endpoint was a major adverse composite event (MACE), a composite outcome of all-cause mortality, non-fatal MI (NFMI), revascularization, cerebrovascular accident, and rehospitalization. The secondary endpoints included each individual component of MACE including all-cause mortality, cardiac/non-cardiac death, NFMI, any revascularization, cerebrovascular accident, and rehospitalization. Any revascularization refers to repeated PCI for any segment of the entire native coronary artery or coronary artery bypass graft. Rehospitalization was defined as the initial occurrence of an unplanned hospital admission due to different etiologies such as angina, HF, uncontrolled elevated blood glucose levels, hypoglycemia, or other factors.
Exploratory outcomes
In addition to 1-year clinical outcomes, we performed a comparative analysis of the degree of improvement in left ventricular systolic function and glycemic control in patients following treatment in both groups. These investigations were based on the 1-year follow-up levels of LVEF and HbA1c and their changes from baseline during the 1-year follow-up period.
Statistical analysis
All statistical analyses were conducted using SPSS (version 25.0; SPSS Inc., Armonk, New York, United States of America) and STATA (version 15.0; StataCorp, College Station, TX, USA). All continuous parameters were reported as means with standard deviations and examined by the Student’s t-test or Mann–Whitney test. All categorical (discrete) parameters were reported as frequencies with proportions (percentages) and examined by Pearson’s chi-square test or Fisher’s exact test.
The primary intention of this analysis was to determine whether there was a clear difference in clinical outcomes between the two combinations of OHAs (i.e., MET plus SGLT2i versus MET plus DPP-4i). To balance the baseline differences in this observational study, we employed a statistical matching technique, called inverse probability of treatment weighting (IPTW). In IPTW, the propensity score was constructed using multiple logistic regression analysis with the following baseline covariates: age, sex, use of emergency medical services, BMI, Killip classification, medical history, smoking history, family history of CAD, use of thrombolysis, LMCA disease, multivessel CAD, PCI, final diagnosis (STEMI or non-STEMI), initial HbA1c level, post-discharge medications, vascular approach (femoral or non-femoral), use of glycoprotein IIb/IIIa inhibitors, thrombus aspiration, intracoronary imaging guidance, preprocedural TIMI flow grade, ACC/AHA lesion characteristics, treatment strategies, and IRA.
We applied the Cox proportional hazard models to estimate hazard ratios and their corresponding 95% confidence intervals for each component of clinical outcomes, comparing DPP-4i group with SGLT2i group. In Cox models, we adjusted for all baseline covariates mentioned earlier.
In the present study, we plotted survival curves for MACEs using the Kaplan–Meier method. We also used the log-rank test to compare outcomes between the two groups. Participants with missing data for these baseline covariates or those who were lost to follow-up were precluded from these analyses.
Results
Baseline demographic and clinical characteristics
In this study, 779 participants with AMI and concomitant DM were included in the statistical analysis. In total, 186 (23.9%) and 593 (76.1%) subjects were allocated to SGLT2i group and DPP-4i group, respectively (Fig. 1).
All the relevant baseline characteristics are outlined in Tables 1 and 2. Patients in the SGLT2i group were more likely to be male, younger, and smokers than those in the DPP-4i group. Prior CAD was more frequent in the DPP-4i group than in the SGLT2i group. The SGLT2i group had more instances of STEMI as a final diagnosis than the DPP-4i group. Initial HbA1c levels were higher in the SGLT2i group than in the DPP-4i group. Among discharge medications, beta-blockers were more frequently prescribed to individuals in the SGLT2i group compared to those in the DPP-4i group. A comparison of the procedural characteristics showed that the SGLT2i group received more PCI with more DES implantation and a lower preprocedural TIMI flow grade than the DPP-4i group. All these differences were statistically balanced after the adjustment of baseline covariates with IPTW (Supplemental Table 1).
Table 1.
Baseline clinical characteristics of the patients
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Characteristics | SGLT2i group | DPP-4i group | p -value | SGLT2i group | DPP-4i group | p -value |
| (n = 186) | (n = 593) | (n = 537) | (n = 532) | |||
| Male patients | 150 (80.7) | 422 (71.2) | 0.011 | 358 (66.7) | 388 (72.9) | 0.364 |
| Age, years | 59.11 ± 11.52 | 66.12 ± 10.86 | < 0.001 | 63.21 ± 11.17 | 63.74 ± 11.33 | 0.694 |
| Age ≥ 75 years | 20 (10.7) | 153 (25.8) | < 0.001 | 76 (14.2) | 105 (19.8) | 0.269 |
| EMS utilization | 45 (24.2) | 122 (20.6) | 0.294 | 109 (20.2) | 105 (19.7) | 0.914 |
| BMI ≥ 25 kg/m2 | 76 (43.4) | 221 (40.0) | 0.426 | 212 (39.4) | 215 (40.4) | 0.880 |
| Killip class III-IV | 17 (9.1) | 51 (8.6) | 0.830 | 52 (9.7) | 55 (10.3) | 0.877 |
| Previous history | ||||||
| Hypertension | 113 (60.8) | 395 (66.7) | 0.136 | 350 (65.1) | 344 (64.6) | 0.935 |
| DM | 186 (100.0) | 593 (100.0) | NA | 537 (100.0) | 532 (100.0) | NA |
| Dyslipidemia | 48 (25.8) | 134 (22.6) | 0.367 | 125 (23.3) | 133 (25.0) | 0.741 |
| Prior CAD | 19 (10.2) | 116 (19.6) | 0.003 | 46 (8.6) | 72 (13.5) | 0.176 |
| Prior heart failure | 6 (3.2) | 9 (1.5) | 0.140 | 10 (1.8) | 9 (1.8) | 0.970 |
| Prior CVA | 12 (6.5) | 56 (9.5) | 0.214 | 33 (6.1) | 49 (9.2) | 0.310 |
| DM duration | 0.078 | |||||
| 0 to 10 years | 98 (73.7) | 259 (65.4) | NA | NA | NA | |
| > 10 years | 35 (26.3) | 137 (34.6) | NA | NA | NA | |
| Smoking | 110 (61.1) | 278 (48.7) | 0.004 | 254 (47.2) | 283 (53.2) | 0.350 |
| Family history of CAD | 16 (8.9) | 44 (7.6) | 0.580 | 40 (7.5) | 42 (7.9) | 0.885 |
| Use of thrombolysis | 2 (1.1) | 2 (0.3) | 0.243 | 3 (0.5) | 2 (0.4) | 0.811 |
| LVEF, % | 51.07 ± 12.20 | 52.58 ± 11.40 | 0.126 | 53.04 ± 12.55 | 52.00 ± 11.57 | 0.502 |
| LVEF < 40% | 29 (15.8) | 70 (12.2) | 0.205 | 79 (14.7) | 78 (14.7) | 0.984 |
| STEMI diagnosis | 100 (53.8) | 227 (38.3) | < 0.001 | 244 (45.5) | 241 (45.3) | 0.976 |
| HbA1c, % | 8.33 ± 1.87 | 7.70 ± 1.53 | < 0.001 | 7.88 ± 1.65 | 7.85 ± 1.62 | 0.886 |
| Discharge medications | ||||||
| Aspirin | 185 (99.5) | 593 (100.0) | 0.239 | 156 (99.7) | 532 (100.0) | 0.327 |
| P2Y12 inhibitor | 186 (100.0) | 586 (98.8) | 0.207 | 537 (100.0) | 532 (100.0) | NA |
| Beta-blocker | 163 (87.6) | 455 (76.7) | 0.001 | 431 (80.2) | 416 (78.3) | 0.734 |
|
ACE inhibitor or ARB |
142 (76.3) | 461 (77.7) | 0.691 | 444 (82.5) | 427 (80.3) | 0.603 |
| Statin | 182 (97.9) | 564 (95.1) | 0.143 | 520 (96.7) | 516 (97.0) | 0.901 |
| Ezetimibe | 23 (12.4) | 67 (11.3) | 0.691 | 56 (10.5) | 62 (11.6) | 0.786 |
Values are presented as number (percentage) for categorical values and means ± standard deviation for continuous variables
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body-mass index; CAD = coronary artery disease; CVA = cerebrovascular accidents; DM = diabetes mellitus; DPP-4i = dipeptidyl peptidase-4 inhibitors; EMS = emergency medical service; HbA1c = hemoglobin A1c; IPTW = inverse probability of treatment weighting; LMCA = left main coronary artery; LVEF = left ventricular ejection fraction; NA = not applicable; PCI = percutaneous coronary intervention; SGLT2i = sodium-glucose cotransporter 2 inhibitors; STEMI = ST-segment elevation myocardial infarction
Table 2.
Baseline procedural characteristics of the patients
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Characteristics | SGLT2i group | DPP-4i group | p -value | SGLT2i group | DPP-4i group | p -value |
| (n = 186) | (n = 593) | (n = 537) | (n = 532) | |||
| Use of PCI | 184 (98.9) | 551 (92.9) | 0.001 | 537 (100.0) | 532 (100.0) | NA |
| LMCA disease | 11 (5.9) | 38 (6.4) | 0.797 | 33 (6.1) | 33 (6.2) | 0.978 |
| Multivessel CAD | 111 (59.7) | 353 (59.8) | 0.970 | 344 (64.1) | 320 (60.1) | 0.521 |
| Use of transfemoral approach | 88 (47.8) | 248 (45.0) | 0.507 | 241 (44.9) | 246 (46.2) | 0.851 |
| Use of GPIIb/IIIa inhibitor | 11 (6.0) | 46 (8.3) | 0.298 | 41 (7.7) | 43 (8.0) | 0.926 |
| Use of thrombus aspiration | 12 (6.5) | 63 (11.4) | 0.057 | 79 (14.7) | 54 (10.2) | 0.460 |
|
Intracoronary imaging guidance (OCT or IVUS) |
63 (34.2) | 154 (27.9) | 0.105 | 151 (28.0) | 163 (30.7) | 0.627 |
| Preprocedural TIMI flow grade 0-I | 81 (44.0) | 206 (35.6) | 0.041 | 175 (32.5) | 209 (39.2) | 0.240 |
|
ACC/AHA lesion characteristics B2/C |
151 (84.4) | 444 (84.4) | 0.987 | 471 (87.6) | 448 (84.2) | 0.344 |
| Treatment strategies | 0.039 | 0.160 | ||||
| DES implantation | 178 (97.3) | 523 (94.9) | 528 (98.2) | 512 (96.1) | ||
| BVS implantation | 1 (0.5) | 1 (0.2) | 1 (0.2) | 0 (0.0) | ||
| Balloon angioplasty only | 3 (1.6) | 27 (4.9) | 7 (1.3) | 20 (3.9) | ||
| Others | 1 (0.5) | 0 (0.0) | 1 (0.3) | 0 (0.0) | ||
| Infarct-related artery | 0.306 | 0.614 | ||||
| LMCA or LAD | 98 (53.6) | 271 (49.2) | 253 (47.1) | 268 (50.4) | ||
| LCX or RCA | 85 (46.4) | 280 (50.8) | 284 (52.9) | 264 (49.6) | ||
Values are presented as number (percentage) for categorical values and means ± standard deviation for continuous variables
Categorical values are presented as number (percentage)
ACC = American College of Cardiology; AHA = American Heart Association; BVS = bioresorbable vascular scaffold; DES = drug-eluting stent; DPP-4i = dipeptidyl peptidase-4 inhibitors; GPIIb/IIIa = glycoprotein IIb/IIIa; IVUS = intravascular ultrasound; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LMCA = left main coronary artery; NA = not applicable; OCT = optical coherence tomography; PCI = percutaneous coronary intervention; RCA = right coronary artery; SGLT2i = sodium-glucose cotransporter 2 inhibitors; TIMI = Thrombolysis in Myocardial Infarction
Clinical and exploratory outcomes
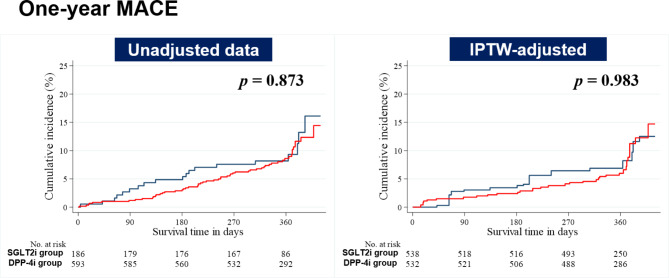
The 1-year clinical outcomes of all participants are outlined in Table 3. The median follow-up interval was 361 days. These outcomes included MACE and its individual components including all-cause mortality (cardiac death and non-cardiac death), NFMI, revascularization, and rehospitalization. Kaplan–Meier survival curves of 1-year MACE were also illustrated in the unadjusted and IPTW-adjusted datasets (Fig. 2). In the unadjusted and IPTW-adjusted analyses, all items for clinical outcomes were comparable between the two groups.
Table 3.
1-year clinical outcomes in propensity score matched patients
| Outcomes | SGLT2i group | DPP-4i group | Unadjusted analysis | IPTW-adjusted analysis | ||
|---|---|---|---|---|---|---|
| (n = 186) | (n = 593) | HR (95% CI) | p -value | HR (95% CI) | p -value | |
| MACE | 19 (10.2) | 58 (9.8) | 0.96 (0.57–1.61) | 0.873 | 0.99 (0.46–2.14) | 0.983 |
| All-cause mortality | 0 (0.0) | 11 (1.8) | NA | NA | NA | NA |
| Cardiac death | 0 (0.0) | 5 (0.8) | NA | NA | NA | NA |
| Non-cardiac death | 0 (0.0) | 6 (1.0) | NA | NA | NA | NA |
| Myocardial infarction | 2 (1.1) | 9 (1.5) | 1.47 (0.32–6.82) | 0.621 | 2.78 (0.31–24.90) | 0.362 |
| Any revascularization | 12 (6.4) | 22 (3.7) | 0.56 (0.28–1.13) | 0.108 | 0.68 (0.26–1.74) | 0.418 |
| CVA | 4 (2.1) | 8 (1.3) | 0.67 (0.20–2.23) | 0.513 | 1.43 (0.24–8.71) | 0.697 |
| Rehospitalization | 3 (1.6) | 19 (3.2) | 2.05 (0.61–6.94) | 0.247 | 1.58 (0.28–8.99) | 0.603 |
Categorical values are presented as percentage (number)
CI = confidence interval; CVA = cerebrovascular accident; DPP-4i = dipeptidyl peptidase-4 inhibitors; HR = hazard ratio; NA = not applicable; IPTW = inverse probability of treatment weighting; MACE = major adverse composite event; SGLT2i = sodium-glucose cotransporter 2 inhibitors
Fig. 2.
1-year event rates of MACE in the study population
DPP-4i = dipeptidyl peptidase-4 inhibitors; IPTW = inverse probability of treatment weighting; MACE = major adverse composite event; SGLT2i = sodium-glucose cotransporter 2 inhibitors
We further investigated the exploratory outcomes according to LVEF and HbA1c levels, which are summarized in Table 4. Both HbA1c and LVEF levels at the 1-year follow-up were comparable between the groups. However, group A had lower Δ HbA1c but higher Δ LVEF than group B. These differences were statistically attenuated in the covariate-adjusted data, except for Δ LVEF after IPTW adjustment.
Table 4.
1-year exploratory outcomes in propensity score matched patients
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Characteristics | SGLT2i group | DPP-4i group | p -value | SGLT2i group | DPP-4i group | p -value |
| (n = 186) | (n = 593) | (n = 537) | (n = 532) | |||
| 1-year follow-up | ||||||
| HbA1c, % | 7.03 ± 1.39 | 7.08 ± 1.45 | 0.804 | 7.06 ± 1.18 | 6.95 ± 1.36 | 0.483 |
| LVEF, % | 53.97 ± 10.71 | 55.41 ± 10.12 | 0.284 | 56.41 ± 11.06 | 55.08 ± 10.29 | 0.448 |
| LVEF < 40% | 7 (9.2) | 14 (5.4) | 0.232 | 15 (8.0) | 16 (5.9) | 0.559 |
| Change from baseline during 1-year follow-up | ||||||
| Δ HbA1c | -1.15 ± 2.00 | -0.65 ± 1.90 | 0.041 | -0.76 ± 1.73 | -0.80 ± 2.00 | 0.910 |
| Δ LVEF | 6.10 ± 8.30 | 2.95 ± 10.34 | 0.007 | 6.91 ± 8.91 | 3.13 ± 10.41 | 0.027 |
Values are presented as number (percentage) for categorical values and means ± standard deviation for continuous variables
DPP-4i = dipeptidyl peptidase-4 inhibitors; HbA1c = hemoglobin A1c; IPTW = inverse probability of treatment weighting; LVEF = left ventricular ejection fraction; SGLT2i = sodium-glucose cotransporter 2 inhibitors
Discussion
This study aimed to compare the cardiovascular outcomes of SGLT2i and DPP-4i as add-on therapies in patients with AMI and concomitant DM receiving MET. Our results showed that SGLT2i use was not associated with a reduced risk of MACEs compared with DPP-4i use in patients with AMI. However, our study demonstrated the potential benefit of SGLT2i over DPP-4i in improving LVEF, implying that the use of SGLT2i may exert beneficial effects in the clinical setting of AMI, although it did not reduce MACE.
Considering the cardiovascular benefits of SGLT2i in diabetic patients as established in recently published CVOTs [4–7], guidelines recommend prioritizing the use of SGLT2i in patients with type 2 DM and concurrent cardiovascular disease. However, these CVOTs of SGLT2i enrolled subjects with chronic ASCVDs and not AMI; therefore, evidence of SGLT2i use in AMI and type 2 DM is still limited. Only one retrospective observational study evaluated patients with AMI and concomitant type 2 DM, revealing that the use of SGLT2i was related with a reduced risk of adverse cardiovascular events [31]. One limitation of this study was that the control group consisted of patients not taking SGLT2i rather than those taking an active competitor. Moreover, it was conducted at a single center in Taiwan and had a small sample size, which means that it is somewhat difficult to generalize their results. However, our study expanded on previous retrospective observational studies using active competitors such as DPP-4i prevalently described for use in type 2 DM patients and providing evidence in Korean patients using the KAMIR-V, a nationwide multicenter observational cohort of patients with AMI undergoing PCI.
Our study showed no significant difference in the incidence of cardiovascular events between SGLT2i and DPP-4i. However, the results of our study were significantly different from those of previous observational [32, 33] and network meta-analyses comparing SGLT2i and DPP-4i [34], demonstrating that SGLT2i have a beneficial effect on cardiovascular disease compared with DPP-4i. This disparity seems to be interesting and may be expounded by the different features of enrolled participants, such as the acute stage of MI in our study versus the chronic stage of MI in previous studies. In addition, it might not be sufficient to demonstrate statistical significance in our study because of the insufficient number of subjects and the relatively short follow-up period. Meanwhile, DPP-4i may have some beneficial effects on AMI, the acute stage of MI. This hypothesis has been supported in several studies. Wang et al. reported that DPP-4i therapy improved the three-year survival rates of patients with first-time AMI and concomitant DM [35]. Vildagliptin, a DPP-4i, reduced acute mortality in post-AMI and concomitant type 2 DM in a rat study by restoring the autophagic response through the attenuation of Bcl-2-Beclin-1 interaction [36]. However, explaining this clearly even with this assumption is difficult; therefore, further study is needed with a large number of subjects and long-term follow-up.
HF, a chronic complication of DM, is important for the prognosis of DM but has been underdiagnosed [37, 38]. The benefits of SGLT2i were first established in patients with HF with reduced ejection fraction [39, 40], and a recently published large-scale study revealed that these effects were also significant in patients with HF with preserved ejection fraction [41, 42]. Based on these studies, recent guidelines specify that SGLT2i should be considered as a first-line treatment for HF, regardless of ejection fraction [43, 44]. Similarly, in our study, LVEF was significantly improved in patients treated with SGLT2i than in patients treated with DPP-4i before achieving statistical balancing and after IPTW adjustment. This implies that SGLT2i use may achieve more improvement of left ventricular systolic function. These findings appear to be consistent with the beneficial effects of SGLT2 inhibitors on HF. This improvement in systolic function is indeed beneficial, particularly in patients with AMI who are at a high risk of HF.
Although not fully understood, the effect of SGLT2i on HF can be explained by several mechanisms in addition to hypoglycemic and diuretic effects. These theories include the following potential benefits of sodium-glucose cotransporter 2 inhibition: inhibiting the cardiac Na+/H + exchanger, decreasing oxidative stress, and improving cardiac energy metabolism. SGLT2i have been found to have an inhibitory effect on the Na+/H + exchanger in cardiac cells. The Na+/H + exchanger is a protein that regulates the exchange of sodium and hydrogen ions across cell membranes, including those of cardiac cells [45]. This exchange is essential for maintaining the proper intracellular pH and ion balance in the heart. In HF, the Na+/H + exchanger is frequently upregulated or overactivated leading to increased sodium and calcium levels within cardiac cells [46]. This elevated intracellular sodium and calcium can contribute to cellular dysfunction, impaired relaxation, and increased workload on the heart, further exacerbating HF. SGLT2i helps to restore the balance of sodium and calcium within cardiac cells by inhibiting the Na+/H + exchanger. This inhibition leads to a reduction in intracellular sodium and calcium levels, thereby improving cellular function and alleviating the workload on the heart [45, 47]. By targeting this specific mechanism, SGLT2i have the potential to ameliorate the adverse effects of Na+/H + exchanger overactivation in HF.
Chronic inflammation and increased oxidative stress also play a significant role in the development and progression of HF [48, 49]. SGLT2i have been shown to possess anti-inflammatory and antioxidant properties. These drugs can reduce the production of pro-inflammatory molecules [50, 51] and inhibit oxidative stress [52]. By dampening inflammation and oxidative stress, SGLT2i protect cardiac cells and mitigate the detrimental effects of HF [50, 52, 53]. Finally, HF is associated with alterations in cardiac energy metabolism. SGLT2i have been found to modulate cardiac metabolism by increasing reliance on fatty acids as an energy source and promoting ketone body utilization [54, 55]. This metabolic shift can improve energy efficiency, preserve myocardial function, and enhance overall cardiac performance in individuals with HF [56, 57].
Besides, despite SGLT2i, with their aforesaid cardiovascular benefits, are superior in the improvement in LVEF compared with DPP-4i, clinical outcomes became comparable in both groups before and after IPTW. As previously mentioned, this discrepancy may be explained by the relatively small sample size with the relatively short follow-up interval. Moreover, LVEF at baseline seemed to be relatively good with the inclusion of less than 20% of patients with LVEF < 40% in each group, which may significantly offset the superior cardiovascular safety of SGLT2i. Since this issue is still not fully explainable, further investigations should be performed in the future.
Our results demonstrated that DPP4-i had similar but numerically low risk of any revascularization to SGLT2i. It is still contentious whether DPP4-i have beneficial effects in terms of coronary revascularization or not. A meta-analysis of CVOTs for DPP-4i showed that DPP-4i resulted in a non-significant decrease in coronary revascularization [58], whereas some CVOTs on SGLT2i showed robust evidence for the prevention of HF but not for atherosclerotic changes such as coronary revascularization or peripheral revascularization [6, 59]. In contrast to these publications, recent evidence from administrative data in Taiwan revealed that the use of SGLT2i was associated with a lower risk of revascularization compared with the use of DPP-4i [60]. These disparities between the studies may be owing to the variations in study designs and patient enrollment criteria. Therefore, further investigations are needed to confirm the effect of SGLT2i versus DPP-4i on atherosclerotic changes, including coronary revascularization.
We should also note that less than a quarter of the study population received SGLT2i. Given that SGLT2i are one of promising classes of OHAs with well-established cardiovascular benefits [61, 62], their prescription was still insufficient compared with other cardio-protective medications. In the real-world practice, SGLT2i have been more under-prescribed than DPP-4i worldwide [16, 63]. In a Korean population-based cohort study, the prescription of SGLT2i has been suboptimal among patients with established ASCVDs or HF. Similarly, in data claims by the United States of America, patients were less likely to initiate treatment with SGLT2i than with DPP-4i [64]. These trends may be explained by the “occupation effect,” considering that DPP4-i were introduced in 2006 but SGLT2i in 2012 [63]. According to the Diabetes Fact Sheets in Korea, DPP-4i, one of previously established OHAs, still occupy a large proportion of total OHA market shares [17]. They are the third most common OHA monotherapy after MET and sulfonylurea, and the combination of both MET and DPP-4i has been the most common dual combination therapy since 2014. Furthermore, the use of DPP-4i may be one of independent factors affecting the non-initiation of SGLT2i [16]. This can be partly explained given the context that the combination of both SGLT2i and DPP-4i was not covered by the Korean national health insurance [16], as well as the “occupation effect” of DPP-4i over SGLT2i. Hence, the revision of healthcare insurance policy will be required to enable extensive insurance coverage of a combination of OHAs to bridge this evidence-practice gap in real-world practice.
Another notable finding is that the incidence of all-cause mortality was generally low in the present study. There are several possible explanations for this finding. First, as summarized in Tables 1 and 2, all participants received high rates of cardio-protective medications and high rates of PCI, which may account for their low mortality rate to some extent. Second, potential selection bias may influence these findings. As illustrated in the study scheme (Fig. 1), “survivor-cohort effect” may partially account for these findings, given that we included participants who survived during the index hospitalization. Furthermore, our study excluded patients who were lost to follow-up, which also contributed to the selection bias. That is, there may have been more deaths than were included.
To the best of our knowledge, there have been no head-to-head comparison studies of novel OHAs in the acute phase of MI. Therefore, our study offers important information in real-world settings and provides useful information when selecting a second-line OHA as add-on therapies with MET in patients who need additional glucose-lowering effects. An additional strength of our study is that we used the statistical matching technique, IPTW, to balance the different baseline characteristics.
Study limitations
Our study had some key limitations. First, the KAMIR-V was conducted only in tertiary medical centers that treat high-volume AMI patients. Therefore, generalizing the clinical outcomes, including cardiovascular outcomes, to all medical institutions with patients experiencing AMI is difficult. Second, although this study was based only on a prospective, observational registry, it was a nonrandomized study. Despite employing two different propensity score weighting methods to reduce selection bias, the problem of selection bias may still persists owing to several reasons, such as data selection by inclusion and exclusion criteria, the presence of data with missing values, and the possibility of unmeasured confounders. Third, the patient follow-up period in our study was one year, which is a relatively short period for statistical significance. Fourth, several important factors that may affect clinical outcomes were unavailable, which could have been clinically crucial. That is, we could not collect detailed information about OHAs such as adherence to or duration of these medications, their initiation timing, their dosages, or transitions to another class of OHA. Furthermore, glucose-lowering medications used before enrollment are unknown; therefore, it is not possible to confirm the effect of starting a new diabetes drug. Fifth, as our study has a relatively low statistical power with a small sample size and it excludes the untreated group (patients treated with MET monotherapy) from the statistical analysis, large-scale randomized controlled trials should be conducted to perform a head-to-head comparison between drugs in the future.
Conclusions
The use of SGLT2i compared with DPP-4i over one year did not demonstrate a statistically significant difference in composite cardiovascular outcomes in patients with AMI and concomitant DM. However, the use of SGLT2i appeared to improve left ventricular systolic function compared with the use of DPP-4i.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All authors would like to thank all members of each participating institution of the Korea Myocardial Infarction Registry-V (KAMIR-V) registry for their invaluable contributions. We politely disclose that this research is based on one-year analysis of clinical data from the KAMIR-V registry.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association classification
- AMI
Acute myocardial infarction
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- CAD
Coronary artery disease
- CVOT
Cardiovascular outcome trials
- DES
Drug-eluting stent
- DM
Diabetes mellitus
- DPP-4i
Dipeptidyl peptidase-4 inhibitors
- ECG
Electrocardiogram
- HbA1c
Glycated hemoglobin
- HF
Heart failure
- IPTW
Inverse probability of treatment weighting
- KAMIR-V
Korea Acute Myocardial Infarction Registry-V
- LMCA
Left main coronary artery
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse composite event
- MET
Metformin
- MI
Myocardial infarction
- NFMI
Non-fatal myocardial infarction
- OHA
Oral hypoglycemic agent
- PCI
Percutaneous coronary intervention
- SGLT2i
Sodium-glucose cotransporter 2 inhibitors
- STEMI
ST-segment elevation myocardial infarction
- TIMI
Thrombolysis In Myocardial Infarction
Authors’ contributions
Lyu YS and Oh S: conceptualization
Lyu YS and Oh S: data curation
Lyu YS and Oh S: formal analysis
Jeong MH: Funding acquisition
Lyu YS and Oh S: Investigation
Lyu YS and Oh S: Methodology
Jeong MH: Supervision
Lyu YS and Oh S: Writing ? original draft
Kim JH, Kim SY, and Jeong MH: Writing ? review & editing
Funding
This work was supported by research funds from Chosun University Hospital, 2022.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
We affirm that the present study was conducted in accordance with the ethical standards of the World Medical Association’s Declaration of Helsinki. This study was approved by the Institutional Review Boards of Chonnam National University Hospital (IRB No. CNUH-2023-016) and Chosun University Hospital (IRB No. 2023-01-010).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Young Sang Lyu and Seok Oh contributed equally to this work.
Change history
9/5/2023
A Correction to this paper has been published: 10.1186/s12933-023-01960-y
References
- 1.International Diabetes Federation . Diabetes and cardiovascular disease report. Brussels: IDF; 2016. [Google Scholar]
- 2.Avogaro A, Bonora E, Consoli A, Del Prato S, Genovese S, Giorgino F. Glucose-lowering therapy and cardiovascular outcomes in patients with type 2 diabetes mellitus and acute coronary syndrome. Diabetes and Vascular Disease Research. 2019;16(5):399–414. doi: 10.1177/1479164119845612. [DOI] [PubMed] [Google Scholar]
- 3.Rosenwasser RF, Sultan S, Sutton D, Choksi R, Epstein BJ. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes metabolic syndrome and obesity: targets and therapy. 2013;6:453. doi: 10.2147/DMSO.S34416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 8.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S. 9. Pharmacologic approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Supplement1):125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 9.Ahrén B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016;59(5):907–17. doi: 10.1007/s00125-016-3899-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 12.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 13.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl j Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 14.Lee KA, Jin HY, Kim YJ, Im YJ, Kim EY, Park TS. Treatment patterns of type 2 diabetes assessed using a Common Data Model based on Electronic Health Records of 2000–2019. J Korean Med Sci. 2021;36(36):e230. doi: 10.3346/jkms.2021.36.e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 2019;10:389. doi: 10.3389/fendo.2019.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek JH, Yang YS, Ko SH, Han KD, Kim JH, Moon MK, Park JS, Lee BW, Oh TJ, Chon S, et al. Real-world prescription patterns and barriers related to the use of sodium-glucose cotransporter 2 inhibitors among korean patients with type 2 diabetes Mellitus and Cardiovascular Disease. Diabetes Metab J. 2022;46(5):701–12. doi: 10.4093/dmj.2022.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, Won KC, Kim DJ, Park KS. Diabetes fact sheets in Korea, 2018: an Appraisal of current status. Diabetes Metab J. 2019;43(4):487–94. doi: 10.4093/dmj.2019.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheen AJ. SGLT2 versus DPP4 inhibitors for type 2 diabetes. Lancet Diabetes Endocrinol. 2013;1(3):168–70. doi: 10.1016/S2213-8587(13)70095-0. [DOI] [PubMed] [Google Scholar]
- 19.Kawalec P, Mikrut A, Lopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30(4):269–83. doi: 10.1002/dmrr.2494. [DOI] [PubMed] [Google Scholar]
- 20.Mishriky BM, Tanenberg RJ, Sewell KA, Cummings DM. Comparing SGLT-2 inhibitors to DPP-4 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2018;44(2):112–20. doi: 10.1016/j.diabet.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Whitelaw J, Kishore K, Neto AS, Holmes NE, Marhoon N, Bellomo R, Ekinci EI. The comparative epidemiology and outcomes of hospitalized patients treated with SGLT2 or DPP4 inhibitors. J Diabetes Complications. 2021;35(12):108052. doi: 10.1016/j.jdiacomp.2021.108052. [DOI] [PubMed] [Google Scholar]
- 22.Oh S, Jeong MH, Cho KH, Kim MC, Sim DS, Hong YJ, Kim JH, Ahn Y. Outcomes of Nonagenarians with Acute myocardial infarction with or without coronary intervention. J Clin Med 2022, 11(6). [DOI] [PMC free article] [PubMed]
- 23.Kim MH, Yuan SL, Lee KM, Jin X, Song ZY, Cho Y-R, Lee MS, Kim JH, Jeong MH. Clinical outcomes of Calcium-Channel blocker vs Beta-blocker: from the korean Acute Myocardial Infarction Registry. JACC: Asia; 2023. [DOI] [PMC free article] [PubMed]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force, Members C, Thygesen K, Alpert JS et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012, 60(16):1581–1598. [DOI] [PubMed]
- 25.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. : 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 26.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of Acute Coronary Syndromes in patients presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 27.Oh S, Kim JH, Cho KH, Kim MC, Sim DS, Hong YJ, Ahn Y, Jeong MH. Association between baseline smoking status and clinical outcomes following myocardial infarction. Front Cardiovasc Med. 2022;9:918033. doi: 10.3389/fcvm.2022.918033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone GW, Brodie BR, Griffin JJ, Morice MC, Costantini C, St Goar FG, Overlie PA, Popma JJ, McDonnell J, Jones D, et al. Prospective, multicenter study of the safety and feasibility of primary stenting in acute myocardial infarction: in-hospital and 30-day results of the PAMI stent pilot trial. Primary angioplasty in myocardial infarction stent pilot trial investigators. J Am Coll Cardiol. 1998;31(1):23–30. doi: 10.1016/s0735-1097(97)00439-7. [DOI] [PubMed] [Google Scholar]
- 29.Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82(4):1193–202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- 30.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL, editors. Jr. : Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988, 78(2):486–502. [DOI] [PubMed]
- 31.Chang T-Y, Lu C-T, Huang H-L, Chou R-H, Chang C-C, Liu C-T, Huang P-H, Lin S-J. Association of Sodium-Glucose cotransporter 2 (SGLT2) inhibitor Use with Cardiovascular and renal outcomes in type 2 diabetes Mellitus patients with stabilized Acute myocardial infarction: a propensity score matching study. Front Cardiovasc Med 2022, 9. [DOI] [PMC free article] [PubMed]
- 32.Kohsaka S, Lam CS, Kim DJ, Cavender MA, Norhammar A, Jørgensen ME, Birkeland KI, Holl RW, Franch-Nadal J, Tangri N. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. The Lancet Diabetes & Endocrinology. 2020;8(7):606–15. doi: 10.1016/S2213-8587(20)30130-3. [DOI] [PubMed] [Google Scholar]
- 33.Persson F, Nyström T, Jørgensen ME, Carstensen B, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Eriksson JW, Norhammar A. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metabolism. 2018;20(2):344–51. doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319(15):1580–91. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M-T, Lin S-C, Tang P-L, Hung W-T, Cheng C-C, Yang J-S, Chang H-T, Liu C-P, Mar G-Y, Huang W-C. The impact of DPP-4 inhibitors on long-term survival among diabetic patients after first acute myocardial infarction. Cardiovasc Diabetol. 2017;16(1):1–11. doi: 10.1186/s12933-017-0572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, Ishikawa S, Tobisawa T, Ogasawara M, Nishizawa K. Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol. 2015;14(1):1–16. doi: 10.1186/s12933-015-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 38.Boonman-de Winter L, Rutten F, Cramer M, Landman M, Liem A, Rutten G, Hoes A. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55(8):2154–62. doi: 10.1007/s00125-012-2579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 40.McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SD, McMurray JJ, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CS, Martinez F. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 42.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 43.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 44.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–726. [Google Scholar]
- 45.Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic na(+) through inhibition of the cardiac na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–73. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakabayashi S, Hisamitsu T, Nakamura TY. Regulation of the cardiac na(+)/H(+) exchanger in health and disease. J Mol Cell Cardiol. 2013;61:68–76. doi: 10.1016/j.yjmcc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Direct cardiac actions of Sodium glucose cotransporter 2 inhibitors Target pathogenic mechanisms underlying heart failure in Diabetic Patients. Front Physiol. 2018;9:1575. doi: 10.3389/fphys.2018.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–76. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 49.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128(9):3716–26. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, Mayer G. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–66. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grubic Rotkvic P, Cigrovski Berkovic M, Bulj N, Rotkvic L. Minireview: are SGLT2 inhibitors heart savers in diabetes? Heart Fail Rev. 2020;25(6):899–905. doi: 10.1007/s10741-019-09849-3. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iannantuoni F, Diaz-Morales AMdM, Falcon N, Banuls R, Abad-Jimenez C, Victor Z, Hernandez-Mijares VM, Rovira-Llopis A. S: The SGLT2 inhibitor Empagliflozin ameliorates the Inflammatory Profile in type 2 Diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med 2019, 8(11). [DOI] [PMC free article] [PubMed]
- 54.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–5. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 56.Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: a “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39(7):1108–14. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 57.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 58.Patoulias DI, Boulmpou A, Teperikidis E, Katsimardou A, Siskos F, Doumas M, Papadopoulos CE, Vassilikos V. Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of cardiovascular outcome trials. World J Cardiol. 2021;13(10):585. doi: 10.4330/wjc.v13.i10.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonaca MP, Wiviott SD, Zelniker TA, Mosenzon O, Bhatt DL, Leiter LA, McGuire DK, Goodrich EL, De Mendonca Furtado RH, Wilding JP. Dapagliflozin and cardiac, kidney, and limb outcomes in patients with and without peripheral artery disease in DECLARE-TIMI 58. Circulation. 2020;142(8):734–47. doi: 10.1161/CIRCULATIONAHA.119.044775. [DOI] [PubMed] [Google Scholar]
- 60.Lee H-F, Chen S-W, Liu J-R, Li P-R, Wu L-S, Chang S-H, Yeh Y-H, Kuo C-T, Chan Y-H, See L-C. Major adverse cardiovascular and limb events in patients with diabetes and concomitant peripheral artery disease treated with sodium glucose cotransporter 2 inhibitor versus dipeptidyl peptidase-4 inhibitor. Cardiovasc Diabetol. 2020;19(1):1–12. doi: 10.1186/s12933-020-01118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015, 373(22):2117–2128. [DOI] [PubMed]
- 62.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 63.Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in antidiabetic drug utilization and expenditure in Denmark: a 22-year nationwide study. Diabetes Obes Metab. 2020;22(2):167–72. doi: 10.1111/dom.13877. [DOI] [PubMed] [Google Scholar]
- 64.McCoy RG, Van Houten HK, Karaca-Mandic P, Ross JS, Montori VM, Shah ND. Second-line therapy for type 2 diabetes management: the Treatment/Benefit Paradox of Cardiovascular and kidney comorbidities. Diabetes Care. 2021;44(10):2302–11. doi: 10.2337/dc20-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.