Introduction
Spontaneous regression (SR) of melanocytic neoplasms is a rare but fascinating subject in dermatology. This event occurs frequently in both benign and malignant melanocytic tumors in the skin.1 Regression is defined as partial or complete fading of a tumor that can be verified by histopathological examination.2,3
We present a patient with prior history of melanoma and cyclin-dependent kinase inhibitor 2A (CDKN2A) mutation who developed rapid regression of 5 melanocytic lesions in a short time period after COVID vaccination. We discuss the potential significance of our observations.
Case report
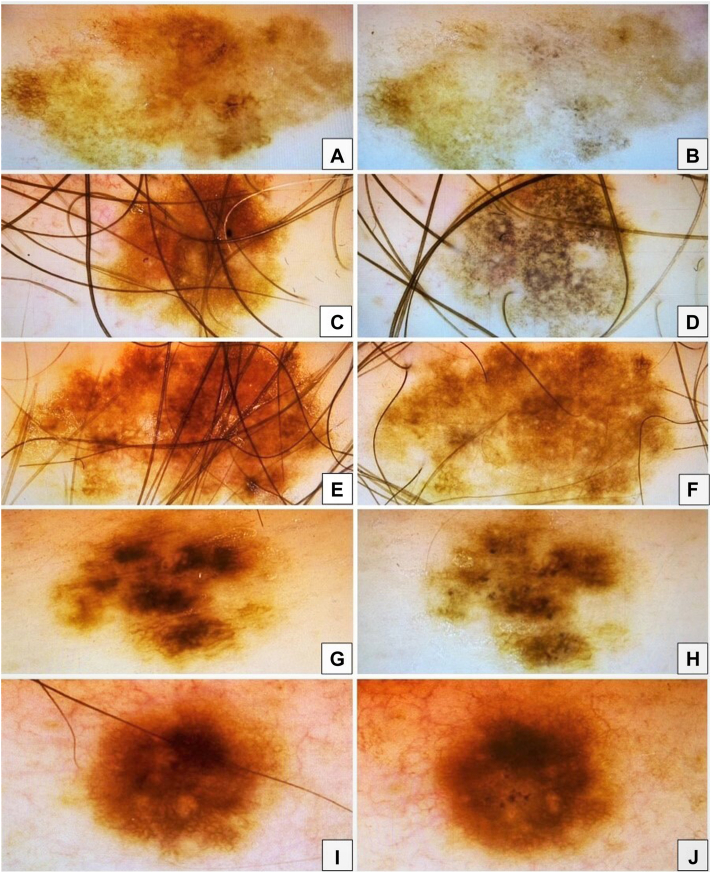
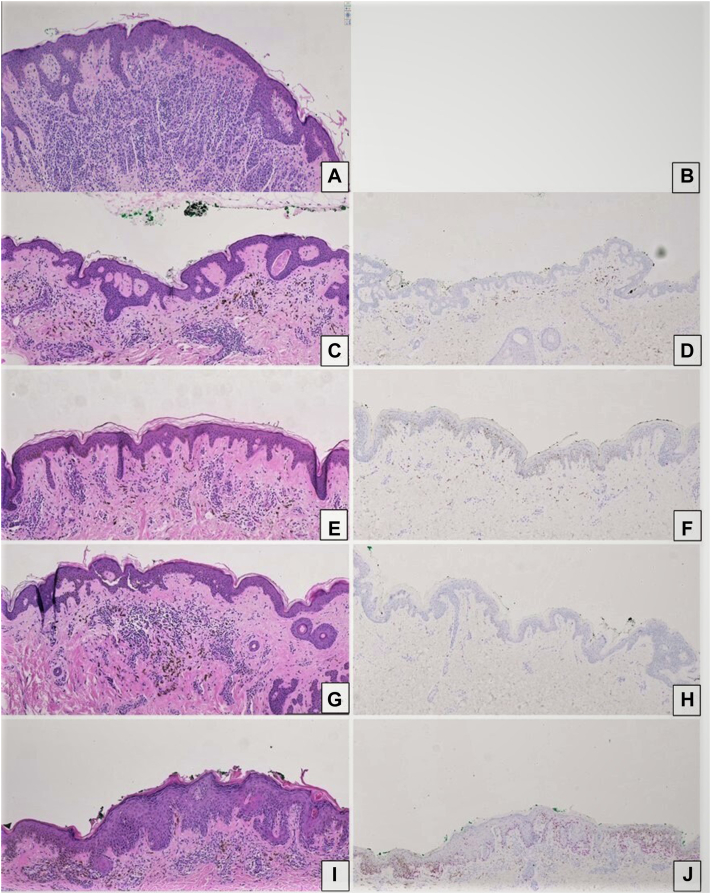
A healthy 56-year-old male on no medications was diagnosed with melanoma on the left shoulder in 2003, where histopathology confirmed superficial spreading type, with 0.8 mm thickness with pathologic stage T1b according to the eighth edition AJCC (American Joint Committee on Cancer) staging system. The melanoma was surgically excised with 1 cm margins. However, no sentinel lymph node biopsy was performed. The patient underwent targeted analysis for CDKN2A mutation as a result of a familial history of melanoma. The test results confirmed a positive mutation status. In early 2022, a high-grade dysplastic nevus was excised from the left breast. In March 2022 we started to follow the patient according to the national care program for malignant melanoma (family melanoma), due to detection of CDKN2A mutation. Several atypical melanocytic lesions were photographed using a digital dermatoscope (FotoFinder Systems GmbH) and documented in Fig 1, A, C, E, G, and I. At the follow-up visit 4 months later, the 5 lesions were photo-documented again (Fig 1, B, D, F, H, and J) and excised due to atypical dermoscopic changes with a similar character of regression—progressive disappearance of the reticular pigment network with changes in color and grayish peppering in the majority of the lesions, with signs of gradual involution of the nevi clinically. No clinical sign of a halo was observed. The histopathology showed only postinflammatory hyperpigmentation without signs of nevus or melanoma in 2 lesions (Figs 1 and 2, C and G), high-grade dysplastic nevus with regression in 1 lesion (Figs 1 and 2, I), and melanocytic nevi in regression without signs of atypia in 2 lesions (Figs 1 and 2, A and E). Per the patient's report, he received the third dose of the Spikevax vaccine (Moderna COVID-19 mRNA vaccine) in the same week as his March 2022 visit. Prior to this, he had received 2 doses of the same vaccine, 6 months earlier. He had no history of previous SARS-CoV-2 infection. We performed a total skin exam including the oral mucosa. A magnetic resonance scan of the abdomen including magnetic resonance cholangiopancreatography and ophthalmic evaluation were performed. Investigations did not confirm any underlying malignancy; therefore, we hypothesized a possible association between SR and the recent COVID-19 vaccination. The patient declined to undergo positron emission tomography and computed tomography scan due to concerns about radiation risk but agreed to regular follow-up appointments.
Fig 1.
A, C, E, G, and I, Close-up of dermoscopic features of atypical pigmented nevus during the first visit. B, D, F, H, and J, Dermoscopic features of the same nevi during the 3-month follow-up visit. These nevi display progressive disappearance of the reticular pigment network with grayish peppering in most of the lesions, which might be explained by a gradual involution of the nevi.
Fig 2.
A, C, E, G, and I, Hematoxylin and eosin stain of the previously shown pigmented nevus. A, C, E, and G, Histopathology showing orthokeratosis, perivascular round cell infiltrated with scattered pigment macrophages. I, High-grade dysplastic nevus. D, F, H, and J, Immunohistochemical staining with SOX10 of the same lesions. B, Immunohistochemical staining for lesion A was not performed. A and C, No melanocytes visible. J, A limited presence of small groups of melanocytes in the superficial layers of the epidermis (pagetoid spread) but without significant cytologic atypia. (A, C, E, G, and I, Hematoxylin-eosin stain; original magnifications: A, C, E, G, and I, ×10; D, F, H, and J, SOX10; original magnifications: D, F, H, and J, ×14).
Discussion
It is well known and common that acquired nevi regress over the years. Furthermore, regression always involves an immunological response.2 Finally, regression of nevi can occur either quickly or slowly. Most commonly, it regresses slowly and then remains “invisible” to both the patient and dermatoscopists. When it occurs rapidly, as in our case, peppering can be observed dermatoscopically, as well as several histopathological features that are characteristic of regression, including the absence of epidermal atrophy and the presence of macrophages, dermal fibrosis, and lymphocytic inflammation.2,3
Prodromal nevi regression is not fully understood. Possible causes include immune destruction, involution, and genetics. Increased immune cell activity can destroy nevus cells, and involution is caused by fewer pigment-producing cells. Mutations in the BRAF (v-raf murine sarcoma viral oncogene homolog B1) gene may also increase regression. Benign nevi regression may be harmless, but also may signal the presence of an underlying melanoma, and thus close clinical surveillance is warranted. COVID-19 vaccine may trigger autoimmune diseases in some people, leading to antibody production against melanocytes.4 Furthermore, an interesting case report shows an eruption of multiple halo nevi in correlation with the SARS-CoV-2 vaccine, suggesting a potential role in triggering this sudden eruption.5 While the presence of an underlying melanoma triggering SR in this patient cannot be entirely excluded, it is less favored after seeking a second dermatopathology opinion for the nevi illustrated in Figs 1 and 2, which agreed that features of melanoma were not present. The absence of pre-2022 nevus photos presents a challenge in assessing any regression reaction. Therefore, awareness of this phenomenon is critical for optimizing clinical practice and avoiding unnecessary diagnostic procedures.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: Futurum, The Academy for Healthcare, Region Jönköping County.
IRB approval status: Not applicable.
Patient consent: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Martín J.M., Rubio M., Bella R., Jordá E., Monteagudo C. Complete regression of melanocytic nevi: correlation between clinical, dermoscopic, and histopathologic findings in 13 patients. Actas Dermosifiliogr. 2012;103:401–410. doi: 10.1016/j.ad.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Thomas J.A., Badini M. The role of innate immunity in spontaneous regression of cancer. Indian J Cancer. 2011;48:246–251. doi: 10.4103/0019-509X.82887. [DOI] [PubMed] [Google Scholar]
- 3.Jessy T. Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med. 2011;2:43–49. doi: 10.4103/0976-9668.82318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccarese G., Drago F., Boldrin S., Pattaro M., Parodi A. Sudden onset of vitiligo after COVID-19 vaccine. Dermatol Ther. 2022;35 doi: 10.1111/dth.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Giorgi V., Colombo J., Salvati L., et al. Eruptive halo nevi: a new COVID-19 vaccine-related cutaneous adverse event or a paraneoplastic phenomenon? Dermatol Ther. 2022;35 doi: 10.1111/dth.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]