Abstract
Purpose
To determine whether the rare NLRP3-Associated Autoinflammatory Disease (NLRP3-AID) is associated with retinal changes and to assess the ocular involvement.
Methods
A retrospective cohort study of 20 patients(40 eyes) diagnosed with rare NLRP3-AID at Peking Union Medical College Hospital, from April 2015 to August 2022. Patients underwent a comprehensive ophthalmological examination, including visual acuity, intraocular pressure examination, slit-lamp examination, fundus photography, optical coherence tomography(OCT), and fluorescence angiography (FA). Some patients also underwent optical coherence tomography angiography (OCTA).
Results
This study analyzed 40 eyes of 20 patients (11 [55.0%] male; median age, 25.0 years [range, 12–52 years]) and 13 patients (26 eyes, 65%) demonstrated ocular involvement. The most common ophthalmologic manifestation was conjunctivitis (22 eyes, 84.6%), followed by papilledema (14 eyes, 53.8%), retinopathy (10 eyes, 38.5%), optic atrophy (6 eyes, 23.1%), uveitis (4 eyes, 15.4%), reduced pupil light reflex (3 eyes, 11.5%) and cataracts (2 eyes, 7.7%). Ocular involvement was bilateral in 11 patients (55.0%). Five kinds of retinal lesions were seen in 5 patients (10 eyes, 25%) with NLRP3-AID, including peripheral retinal vascular leakage, microaneurysms, macular ischemia, macular epiretinal membrane formation and drusen.
Conclusions
Peripheral retinal vascular leakage, macular ischemia, microaneurysms and drusen are newly identified retinal findings in patients with NLRP3-AID, which suggests the importance of detailed retinal examination in these patients.
Keywords: NLRP3-associated autoinflammatory disease (NLRP3-AID), Cryopyrin-associated periodic syndrome (CAPS), Retina, Retinal vascular leakage, Macular ischemia, Microaneurysms
Introduction
NLRP3-associated autoinflammatory disease (NLRP3-AID), often referred to as cryopyrin-associated periodic syndrome (CAPS), is a rare, heterogeneous disease entity caused by variants in the NLR family pyrin domain containing-3 (NLRP3) gene on chromosome 1q44 [1]. NLRP3-AID, which affects one to three in a million children and adults worldwide,[1, 2] is characterized by repeated episodes of fever and a variety of inflammatory signs [3, 4]. The longstanding uncontrolled inflammation results in irreversible organ damage, including sensorineural hearing loss, amyloidosis, vision loss, skeletal deformities and cognitive disability [1].
Previous publications have noted that 71% of patients with NLRP3-AID demonstrate ophthalmologic manifestations [5, 6]. Patients with NLRP3-AID have been reported to present with conjunctivitis, uveitis, papilledema, optic atrophy, cataract and glaucoma [5, 7]. To the best of our knowledge, retinopathy in NLRP3-AID patients has rarely been documented. In our previous study, we identified a middle-aged woman with NLRP3-AID and early onset drusen [8]. Retinopathy in NLRP3-AID is a surprising finding that deserves attention, as it could help deepen the understanding of this disorder. To date, we have found five kinds of retinal changes in NLRP3-AID patients.
The objectives of the present study were to report four newly recognized kinds of retinopathy in NLRP3-AID patients, as well as to provide all the ophthalmologic manifestations of NLRP3-AID in a single-center cohort of NLRP3-AID patients diagnosed after 2015.
Methods
Study participants
All participants provided written informed consent. The study was approved by the Peking Union Medical College Hospital ethics committee (JS-3421) and performed according to the Declaration of Helsinki. A total of 20 patients were diagnosed with NLRP3-AID at the Department of Rheumatology and Immunology, Peking Union Medical College Hospital, from April 2015 to August 2022. The diagnostic criteria for NLRP3-AID included (1) elevated inflammatory markers (CRP/SAA) and (2) at least two of six typical symptoms (urticaria-like rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis and skeletal abnormalities) [1]. All the participants met the above diagnostic criteria and underwent genetic tests.
Ophthalmic evaluations
All participants underwent comprehensive ophthalmic evaluations, including best-corrected visual acuity testing (BCVA), intraocular pressure (IOP), slit-lamp examination, ophthalmoscope examination, fundus photography, fluorescein angiography (FA) and optic coherence tomography (OCT). Color fundus images were obtained using the Visucam 224 (Carl Zeiss Meditec, Dublin, CA), FA was performed using the Spectralis HRA + OCT device (Heidelberg Engineering, Heidelberg, Germany), and OCT examinations were performed using a VG200 SS-OCT (SVision Imaging, Ltd., Luoyang, China). Some patients also underwent optical coherence tomography angiography (OCTA) examinations (VG200; SVision Imaging, Ltd., Luoyang, China) and visual field testing (Humphrey Field Analyzer, Zeiss, Dublin, CA).
Statistical analysis
The incidence of ocular manifestations was calculated form the total number eyes of participants (n1 = 40). The frequency of each manifestation was calculated from the total number of eyes with manifestations (n = 26).
Results
Ocular manifestations in patients with NLRP3-AID
This study analyzed 20 patients (11 [55.0%] male; median age, 25.0 years [range, 12–52 years]). Ocular involvement was observed in a total of 13 patients (26 eyes, 65%). Twelve of them had confirmed variants in the NLRP3 gene. One patient was NLRP3-variant negative but had characteristic clinical features of NLRP3-AID. The most common ophthalmologic manifestation was conjunctivitis (22 eyes, 84.6%), followed by papilledema (14 eyes, 53.8% ), retinopathy (10 eyes,38.5%), optic atrophy (6 eyes, 23.1%), uveitis (4 eyes, 15.4%), reduced pupil light reflex (3 eyes,11.5%) and cataracts (2 eyes, 7.7%). Eleven patients manifested bilateral ocular involvement (55.0%).
Retinal changes in patients with NLRP3-AID
Five patients showed distinct retinal changes. Among them, two patients manifested peripheral retinal vascular leakage, while scattered microaneurysms, macular ischemia, focal epiretinal membrane, and macular drusen were found in one patient each (Table 1).
Table 1.
Demographics and clinical characteristics of NLRP3-AID patients with retinal findings
| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Gender | F | M | F | M | F |
| Age at onset, years old | 2 | 6 | 3 | 2 | 7 |
| Age at diagnosis, years old | 39 | 32 | 12 | 20 | 18 |
| BCVA (OD) | 20/25 | 20/20 | 20/200 | 20/20 | 20/20 |
| BCVA (OS) | 20/25 | 20/20 | 20/32 | 20/20 | 20/20 |
| Family history | + | + | + | - | - |
| Conjunctivitis | + | + | + | - | + |
| Uveitis | |||||
| Papilledema | + | + | - | + | - |
| Optic atrophy | - | - | + | - | - |
| Retinopathy | + | + | + | + | + |
| NLRP3 variants | p.T348M | p.G326E | p.A439V | p.K829T | - |
NLRP3-AID, NLRP3-Associated Autoinflammatory Disease; No., number; BCVA, best corrected visual acuity
Patient 1 was a 39-year-old Chinese woman who had NLRP3-AID with widespread drusen at the posterior poles of both eyes; she was described in our previous case report [8].
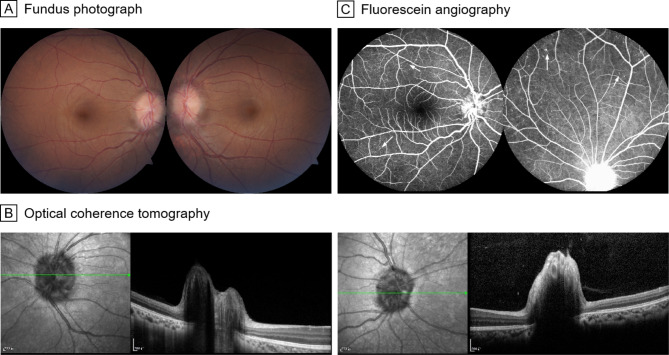
Patient 2 was a 32-year-old Chinese man who presented with recurrent rash and fever since the age of six. He was first admitted to the Department of Rheumatology and then referred to the Department of Ophthalmology due to red eyes. Best corrected visual acuity (BCVA) was 20/20 bilaterally. Ophthalmoscopy and OCT revealed papilledema in both eyes (Fig. 1AB). FA examination demonstrated early-stage hyperfluorescence of the optic disc in both eyes and scattered microaneurysms in the mid-peripheral retina of both eyes (Fig. 1C).
Fig. 1.
Fundus photograph, FA and OCT of patient 2. A and B, Fundus photographs and OCT reveal papilledema in both eyes. C, FA images demonstrate early-stage hyperfluorescence of the optic disc in both eyes and scattered microaneurysms (white arrows) in the mid-peripheral retina of both eyes
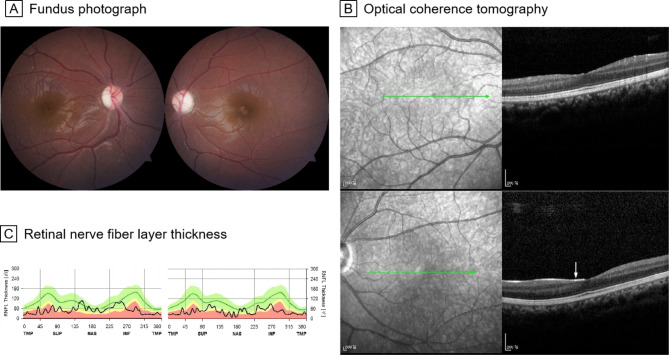
Patient 3 was a 12-year-old Chinese girl who presented with recurrent rash and fever since the age of three. BCVA was 20/200 in the right eye and 20/32 in the left eye. Ophthalmoscopy examination showed pale discs in both eyes (Fig. 2A). A focal epiretinal membrane in the left eye was also found on OCT (Fig. 2B). OCT demonstrated thinning of the retinal nerve fiber layer in both eyes, which was consistent with optic disc atrophy (Fig. 2C).
Fig. 2.
Fundus photograph, OCT and retinal nerve fiber layer thickness of patient 3. A, Fundus photographs show pale discs in both eyes. B, OCT images shows a focal epiretinal membrane (white arrow) in the left eye. C, Retinal nerve fiber layer thinning was found in both eyes
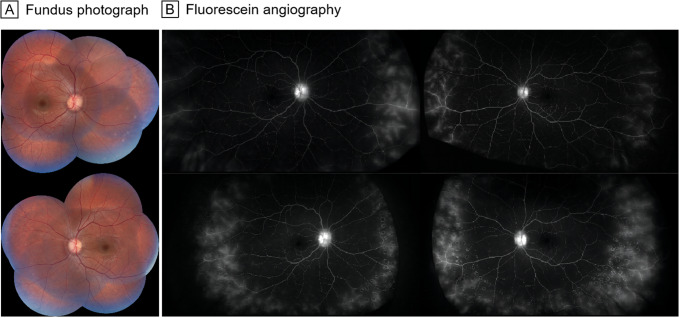
Patient 4 was a 20-year-old Chinese man who presented with recurrent rash and fever since the age of two. BCVA was 20/20 bilaterally. Ophthalmoscopy showed mild papilledema without obvious retinal changes (Fig. 3A). FA revealed vascular leakage at the peripheral retina of both eyes (Fig. 3B). Scattered laser treatment at the peripheral retina vascular leakage area and sub-Tenon’s capsule injection of 20 mg triamcinolone acetonide were performed bilaterally to attempt to alleviate the leakage. However, two months after retinal laser photocoagulation, when we repeated the FA, there was no improvement in the vascular leakage. He then received 150 mg of canakinumab subcutaneously every 8 weeks. Although his clinical symptoms improved remarkably after the treatment, unfortunately, the vascular leakage had not resolved according to FA.
Fig. 3.
Fundus photograph and FA of patient 4. A, Fundus photographs show mild papilledema without obvious retinal changes. B, FA (top row) reveal vascular leakage at the peripheral retina of both eyes. Two months after retinal laser photocoagulation (bottom row), there is no improvement in vascular leakage
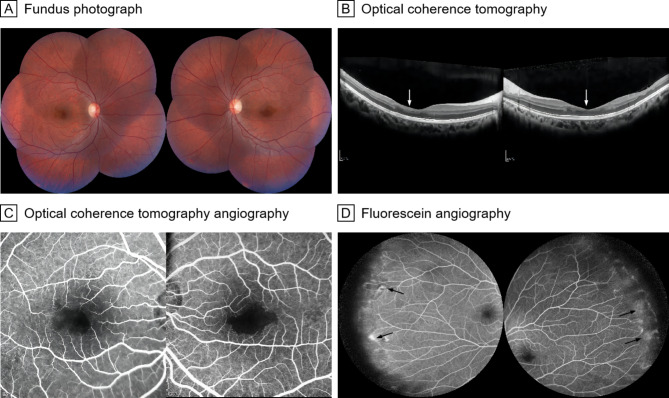
Patient 5 was an 18-year-old Chinese woman with a history of blurred vision for two years. The BCVA was 20/20 bilaterally. Ophthalmoscopy showed no obvious retina changes (Fig. 4A). OCT demonstrated disruption of the outer plexiform layer (Fig. 4B). OCTA demonstrated an enlarged and irregular macula foveal avascular zone and nonperfusion area (Fig. 4C). FA showed vascular leakage in the periphery of the retina (Fig. 4D).
Fig. 4.
Fundus photograph, OCT, OCTA and FA of patient 5. A, Fundus photographs show no obvious retina changes. B, OCT images demonstrate disruption of the outer plexiform layer (white arrows). C, OCTA images demonstrate an enlarged and irregular macula foveal avascular zone and nonperfusion area. D, FA showed vascular leakage (black arrows) in the periphery of the retina
The retinal findings of NLRP3-AID in our study were variable, both clinically and genetically. Patient 1, who presented with drusen, carried pathogenic NLRP3 (NM_001243133.1) variant T348M. Patient 2, who presented with microaneurysms, carried pathogenic variant G326E. Patient 3, who had an epiretinal membrane, carried pathogenic variant A439V. Patient 4 showed peripheral retinal vascular leakage on fluorescein fundus angiography (FA) and carried pathogenic variant K829T. Patient 5 demonstrated peripheral retinal vascular leakage, nonperfusion and macular ischemia; despite her clinical diagnosis, however, she carried no known NLRP3 variant.
Discussion
To the best of our knowledge, this is the first study reporting four new retinal findings in NLRP3-AID, including peripheral retinal vascular leakage, drusen, microaneurysms and macular ischemia. Early onset drusen was reported in our previous study [8].
Previous studies have reported ocular involvement in NLRP3-AID, including conjunctivitis, papilledema, optic atrophy, keratitis, uveitis, cataract and glaucoma [5–7, 9]. The most common ocular involvements in NLRP3-AID are conjunctivitis and papilledema, which are consistent with our study findings. However, although many kinds of ocular manifestations have been reported in NLRP3-AID patients, retinal changes have rarely been mentioned. Previous studies have reported retinal vasculitis, retinal atrophy, chorioretinitis, cystoid macular edema and epiretinal membrane in NLRP3-AID patients [7, 10]. An epiretinal membrane was also found in our study. Additionally, retinal vascular sheathing was detected in previous studies,[10, 11] where it was associated with papilledema but not real retinal change.
Although the retinal findings and NRLP3 variants differed among in these patients, there may be some commonalities. For patients with retinal findings, retinopathies were not their only signs; indeed, they all demonstrated other ocular abnormalities, such as conjunctivitis, papilledema and uveitis. This inspired us to perform fundus examinations for NLRP3-AID patients who already showed any ocular abnormalities. Additionally, the patients in this study were all Chinese. However, it is not clear whether retinopathy is related to race.
Notably, 3 out of 5 patients with retinal changes showed a normal retina appearance on ophthalmoscope examination, except for one patient with drusen and one patient with a focal epiretinal membrane. The visual acuity of most patients with ocular manifestations was generally good. Our study suggested that retinal changes could be detected on FA or OCT/OCTA examination in patients with good visual acuity and a normal retinal appearance on fundus photography. This indicates the importance of multimodal ophthalmic imaging, including FA,[12] OCT/OCTA imaging,[13] and ultra-widefield imaging in NLRP3-AID patients.
The NLRP3 inflammasome is a major component of innate immunity, playing a critical role in the inflammatory response. It provides a molecular platform that can be activated by multiple endogenous and exogenous stimuli, including ATP, microbial agonists, particulate matter and pore-forming toxins [14–18]. Researchers have suggested that the NLRP3 inflammasome contributes to endothelial dysfunction and causes vascular injury [19–22]. Our study found retinal vascular impairment in NLPR3-AID patients, such as vascular leakage and macular ischemia. Chronic inflammation could explain the vascular damage, and the presence of a focal epiretinal membrane may also be secondary to chronic inflammation. We proposed conjectures on the relationship between drusen and NLPR3-AID in our previous study [8].
With respect to treatment, therapeutic intervention might not be required for epiretinal membranes, drusen and microaneurysms. Patients with peripheral retinal vascular leakage and good visual acuity could be seen at regular intervals to monitor the progression of the condition. Patient 4 agreed to local and systemic treatments because his peripheral retinal vascular leakage reached 360 degrees, even though his vision was good. Scattered laser treatment at the peripheral retina vascular leakage area and sub-Tenon’s capsule injection of 20 mg triamcinolone acetonide were performed bilaterally, but there was no improvement in the vascular leakage. His clinical symptoms improved remarkably after subcutaneous administration of 150 mg of canakinumab every 8 weeks,[23] but the vascular leakage remained unaffected. Therefore, further research is needed to elucidate whether and how peripheral retina vascular leakage can be treated.
Our study is limited by the small sample size due to the rarity of NLRP3-AID. Additionally, the follow-up periods were not long enough to observe the progression of the retinal changes. Despite the above limitations, this is the largest cohort study of NLRP3-AID in China, and the first study to report four new retinal changes in NLRP3-AID.
Conclusions
Peripheral retinal vascular leakage, macular ischemia, microaneurysms and drusen are newly identified retinal findings in patients with NLRP3-AID, which increased the understanding of this rare disease. It also suggests the importance of detailed retinal examination in NLRP3-AID patients.
Acknowledgements
We would like to acknowledge all the patients for their consents to participate in the study.
Authors’ contributions
ZW analyzed the data and wrote the first draft. DL and WYY took the images. XFZ, BW and BL collected the data. ZY, NW, and XW prepared the materials. XZ, WHY and MS performed the validation. All authors read and approved the final manuscript.
Funding
This work was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-C-61, 2022-PUMCH-D-002, 2022-PUMCH-B-013), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences(2021-RW320-015), and Xinjiang Production and Construction Corps Science and Technology Project, Science and Technology Development Program in Major Fields (2022AB021).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants provided written informed consent. The study was approved by the Peking Union Medical College Hospital ethics committee (JS-3421) and performed according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interest
The authors declared there was no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhangwanyu Wei and Zhikun Yang contributed equally as co-first authors.
Contributor Information
Zhangwanyu Wei, Email: margaret9999@163.com.
Zhikun Yang, Email: yangzhikun@pumch.cn.
Weihong Yu, Email: yuweihongpumch@163.com.
References
- 1.Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS) Ann Rheum Dis. 2017;76(6):942–7. doi: 10.1136/annrheumdis-2016-209686. [DOI] [PubMed] [Google Scholar]
- 2.Hua Y, Wu D, Shen M, Yu K, Zhang W, Zeng X. Phenotypes and genotypes of chinese adult patients with systemic autoinflammatory diseases. Semin Arthritis Rheum. 2019;49(3):446–52. doi: 10.1016/j.semarthrit.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubartelli A. Autoinflammatory diseases. Immunol Lett. 2014;161(2):226–30. doi: 10.1016/j.imlet.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Levy R, Gérard L, Kuemmerle-Deschner J, Lachmann HJ, Koné-Paut I, Cantarini L, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2015;74(11):2043–9. doi: 10.1136/annrheumdis-2013-204991. [DOI] [PubMed] [Google Scholar]
- 6.Meng T, Wu D, Luo Y, Wu N, Zhao M, Shen M, et al. Ocular manifestations in chinese adult patients with NLRP3-associated autoinflammatory disease. Sci Rep. 2021;11(1):11904. doi: 10.1038/s41598-021-91315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dollfus H, Häfner R, Hofmann HM, Russo RA, Denda L, Gonzales LD, et al. Chronic infantile neurological cutaneous and articular/neonatal onset multisystem inflammatory disease syndrome: ocular manifestations in a recently recognized chronic inflammatory disease of childhood. Arch Ophthalmol. 2000;118(10):1386–92. doi: 10.1001/archopht.118.10.1386. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Yang Z, Zhao X, Chen Y, Li D, Zhang L et al. Early onset drusen and RPE dysfunction in a patient with NLRP3-AID. Ocul Immunol Inflamm. 2022:1–4. [DOI] [PubMed]
- 9.Zhou Y, Wang W, Zhong L, Wang L, Ma M, Tang X, et al. Clinical and genetic spectrum of 14 cases of NLRP3-associated autoinflammatory disease (NLRP3-AID) in China and a review of the literature. Orphanet J Rare Dis. 2022;17(1):214. doi: 10.1186/s13023-022-02364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigante D, Stabile A, Minnella A, Avallone L, Ziccardi L, Bersani G, et al. Post-inflammatory retinal dystrophy in CINCA syndrome. Rheumatol Int. 2010;30(3):389–93. doi: 10.1007/s00296-009-0939-y. [DOI] [PubMed] [Google Scholar]
- 11.Hirano M, Seguchi J, Yamamura M, Narita A, Okanobu H, Nishikomori R, et al. Successful resolution of stromal keratitis and uveitis using canakinumab in a patient with chronic infantile neurologic, cutaneous, and articular syndrome: a case study. J Ophthalmic Inflamm Infect. 2015;5(1):34. doi: 10.1186/s12348-015-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G, Aaberg MT, Patel TP, Iyengar RS, Powell C, Tran A, et al. Quantification of Retinal Nonperfusion and Neovascularization with Ultrawidefield Fluorescein Angiography in patients with diabetes and Associated characteristics of Advanced Disease. JAMA Ophthalmol. 2020;138(6):680–8. doi: 10.1001/jamaophthalmol.2020.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, et al. Automated quantification of Capillary Nonperfusion using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016;134(4):367–73. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, Warner N, Viani K, Nuñez G. Function of nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227(1):106–28. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol. 2019;10:276. doi: 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawan S, Backlund MP, Immonen AT, Kivelä TT, Turunen JA. Functional consequences of pathogenic variant c.61G > C in the inflammasome gene NLRP3 underlying keratitis fugax hereditaria. Br J Ophthalmol. 2022. [DOI] [PubMed]
- 19.Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, et al. NLRP3 Inflammasome mediates Aldosterone-Induced vascular damage. Circulation. 2016;134(23):1866–80. doi: 10.1161/CIRCULATIONAHA.116.024369. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res. 2022;118(2):372–85. doi: 10.1093/cvr/cvab010. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira NS, Bruder-Nascimento T, Pereira CA, Zanotto CZ, Prado DS, Silva JF et al. NLRP3 inflammasome and mineralocorticoid receptors are Associated with Vascular Dysfunction in type 2 diabetes Mellitus. Cells. 2019;8(12). [DOI] [PMC free article] [PubMed]
- 22.Zhang Y, Li X, Pitzer AL, Chen Y, Wang L, Li PL. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal. 2015;22(13):1084–96. doi: 10.1089/ars.2014.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu N, Wu D, Zhao M, Miao J, Yu W, Wang Y, et al. Clinical benefits of TNF-α inhibitors in chinese adult patients with NLRP3-associated autoinflammatory disease. J Intern Med. 2021;290(4):878–85. doi: 10.1111/joim.13334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.