Abstract
The rapidly increasing use of high-throughput screening had produced a plethora of expanding knowledge on the molecular basis of natural killer/T-cell lymphoma (NKTCL), which in turn has revolutionized the treatment. Specifically, the use of asparaginase-containing regimens has led to substantial improvement in survival outcomes in NKTCL patients. Novel treatment strategies that are currently under development include cell-surface-targeted antibodies, immune checkpoint inhibitors, Epstein-Barr virus targeted cytotoxic T lymphocyte, immunomodulatory agents, chimeric antigen receptor T cells, signaling pathway inhibitors and epigenetic targeted agents. In almost all cases, initial clinical studies of newly developed treatment are conducted in patients relapsed, and refractory NKTCL due to very limited treatment options. This review summarizes the results of these novel treatments for NKTCL and discusses their potential for likely use in NKTCL in a wider setting in the future.
Keywords: Natural killer/T-cell lymphoma, Targeted therapy, Immunotherapy, Novel agents
Background
Natural killer/T-cell lymphoma (NKTCL) is a rare and highly aggressive subtype of non-Hodgkin lymphoma (NHL) strongly associated with Epstein-Barr virus (EBV) infection and characterized by extranodal involvement [1, 2]. NKTCL cells express high level of P-glycoprotein and are thus resistant to anthracycline-containing therapies, such as the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen [3]. Based on improved survival outcomes in NKTCL patients, therapeutic regimens based on L-asparaginase are now recommended by the NCCN guidelines [4]. These regimens include SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) regimen [5], modified SMILE (use of pegaspargase instead of L-asparaginase) [6], P-GemOx (pegaspargase, gemcitabine, and oxaliplatin) [7] and DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) [8, 9]. Despite these advances, survival outcome in patients with relapsed or refractory (r/r) disease remains poor. In patients who relapsed after initial non-anthracycline-based treatment, the median overall survival is only 6 months [10].
Genetic testing, particularly high-throughput sequencing, has radically changed the landscape of treatment of malignant tumors, including NKTCL [11, 12]. The current review summarizes the key molecular hallmarks of NKTCL and the corresponding targeted therapies (e.g., immune checkpoint inhibitors, cell surface-targeted agents, epigenetic targeted agents, signaling pathway inhibitors), mostly tested in patients with r/r NKTCL. This review also provides a perspective on future development.
Cell-surface-targeted antibodies
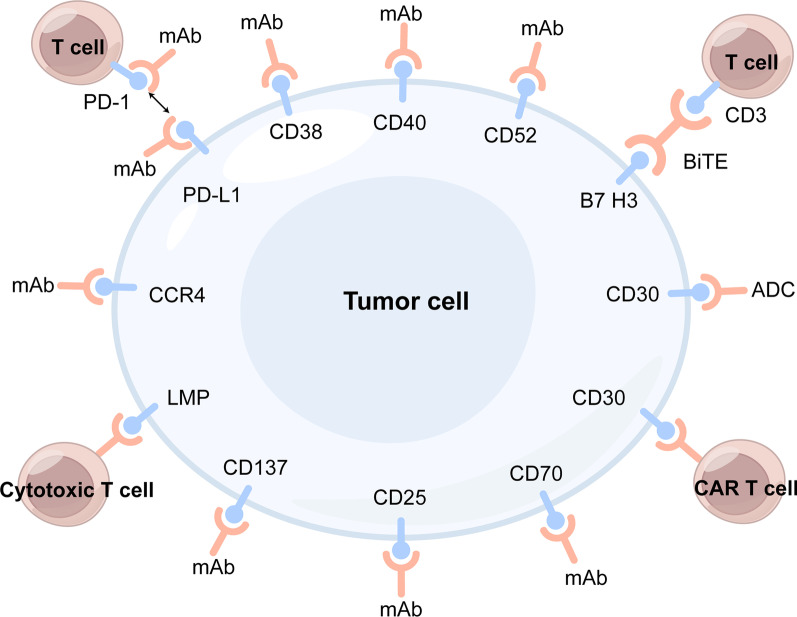
A variety of cell surface antigens have been used to develop targeted therapy for NKTCL (Fig. 1). Monoclonal antibodies (mAbs) against cell surface antigens and their conjugated forms, such as antibody–drug conjugates (ADCs) and bispecific T-cell engagers (BiTEs), under development for NKTCL treatment are listed in Table 1.
Fig. 1.
Cell-surface antigens as potential therapeutic targets for NKTCL. mAb: monoclonal antibody, ADC: antibody–drug conjugate, BiTE: bispecific T cell antigen, CAR: chimeric antigen receptor, LMP: latent membrane protein, PD-1: programmed cell death protein 1, PD-L1: programmed cell death ligand 1
Table 1.
Summary of cell-surface molecules for targeted therapy and ongoing clinical trials in NKTCL patients
| Agent | Target | Trial ID | Patient number (evaluable /estimate) | Study phase | Combined agents | Indication | Results for NKTCL | References | |
|---|---|---|---|---|---|---|---|---|---|
| Cell-surface-targeted antibodies | Daratumumab | CD38 | NCT02927925 | 32 | 2 | / | r/r NKTCL |
ORR:25%, CR:0% 4-m PFS:13% 6-m OS:42.9% |
[16] |
| Isatuximab | CD38 | NCT04763616 | 37 | 2 | Cemiplimab | r/r NKTCL | / | / | |
| Basiliximab | CD25 | NCT04337593 | 30 | 2 | Pegaspargase | r/r NKTCL | / | / | |
| Alemtuzumab | CD52 | NCT00069238 | 31 | 2 | EPOCH | Untreated T and NK-cell lymphoma | / | / | |
| Brentuximab vedotin | CD30 | NCT02280785 | 33 (7 NKTCL) | 2 | / | r/r CD30-expressing NHL | ORR:29% | [22] | |
| Brentuximab vedotin | CD30 | NCT03246750 | 36 | 1/2 | MAD | Newly diagnosed ENKTL | / | / | |
| CAR-T Therapy | CD30.CAR-T | CD30 | NCT04526834 | 21 | 1 | r/r CD30 positive NHL | / | / | |
| CD30.CAR-T | CD30 | NCT03049449 | 26 | 1 | Cyclophosphamide Fludarabine | CD30 expressing lymphomas | / | / | |
| CD30.CAR-EBVSTs | CD30 | NCT04288726 | 18 | 1 | r/r CD30 positive NHL | / | / | ||
| EBV targeted CTL | Baltaleucel-T | EBV antigens | NCT01948180 | 15 | 2 | Advanced ENKTL |
Salvage cohort: ORR:50% CR:30% |
[73] | |
| VT-EBV-N | LMP | NCT03671850 | 48 | 2 | EBV positive NKTCL | ||||
NKTCL: natural killer/T-cell lymphoma, ORR: objective response rate, CR: complete remission, OS: overall survival, PFS: progression-free survival, EPOCH: etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin, NHL: non-Hodgkin lymphoma, MAD: methotrexate, L-asparaginase, and dexamethasone, ENKTL: extranodal natural killer/T- cell lymphoma, CAR: chimeric antigen receptor, CTL: cytotoxic T lymphocyte, EBV: Epstein-Barr virus, LMP: latent membrane protein
CD38-targeted mAbs
CD38 is a glycoprotein primarily expressed on the surface of T cells, NK cells, B cells, and other immune cells. CD38 functions as a lymphocyte receptor and transducer of signals to regulate the proliferation and differentiation of these cells [13]. A study of 94 patients with NKTCL found CD38 expression on NKTCL cells in majority of the cases and very high CD38 expression in half of the patients [14]. Daratumumab is an anti-CD38 mAb approved by the US FDA for use in patients with multiple myeloma (MM). The action of daratumumab is mediated by various Fc-dependent immune mechanisms, including antibody dependent cytotoxicity (ADCC), antibody dependent phagocytosis (ADCP), and complement dependent cytotoxicity (CDC) [15]. However, a phase 2 single-arm trial of 32 patients with r/r NKTCL reported limited efficacy: at a dose of 16 mg/kg, daratumumab monotherapy demonstrated 25% objective response rate (ORR), no complete remission (CR), 13% 4-month progression-free survival (PFS) rate and 43% 6-month overall survival (OS) rate [16]. Efforts are ongoing in developing anti-CD38 mAb (e.g., isatuximab) in combination with immunotherapy (e.g., cemiplimab, a programmed cell death protein 1 (PD-1) inhibitor) for NKTCL (NCT04763616) since CD38 has been shown to attenuate immune response to immune checkpoint therapy [17].
CD30-targeted ADCs
CD30 is expressed on activated lymphocytes and can mediate multiple signaling pathways to modulate cell growth, proliferation and apoptosis [18]. CD30 is expressed in 40–75% of NKTCL patients [19–21]. Brentuximab vedotin (BV) is an ADC that combines an anti-CD30 mAb with monomethyl auristatin E, a microtubule-targeting cytotoxic agent, and has been studied in a variety of NHLs and demonstrated satisfactory efficacy and safety [22]. The ECHELON-2 compared a BV-CHP regimen (BV, cyclophosphamide, doxorubicin, and prednisone) versus CHOP regimen in patients with peripheral T-cell lymphoma (PTCL), and reported improved long-term patient survival with BV-CHP [23], facilitating the exploration of BV in NKTCL treatment. Complete remission after BV monotherapy or in combination with bendamustine in patients with refractory NKTCL has been reported in scattered case series [24, 25]. A phase 2 single-arm trial evaluated BV monotherapy in r/r NHL patients with high CD30 expression; in the 7 NKTCL patients, CR and partial remission (PR) was reported in 1 patient each [22]. One trial (NCT03246750) is ongoing to examine the efficacy of BV in combination with methotrexate, L-asparaginase, and dexamethasone (MAD) in patients with newly diagnosed NKTCL.
CD52-targeted mAbs
CD52, a cell surface marker in mature lymphocytes, is expressed in 25% to 47% of NKTCL patients [26, 27]. The anti-CD52 mAb alemtuzumab has demonstrated encouraging efficacy and acceptable safety as monotherapy in patients with T cell lymphomas and other malignant hematopoietic diseases [28]. A trial of 116 patients with PTCL compared alemtuzumab in combination with CHOP versus CHOP regimen alone, and found higher CR rate with the combination regimen (60%) versus CHOP alone (43%) [29]. A trial of alemtuzumab plus EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) regimen (NCT00069238) is currently ongoing in patients with untreated T and NK-cell lymphomas that include a subpopulation of NKTCL patients.
CD25-targeted mAbs
CD25 is the alpha chain of interleukin-2 receptor (IL-2Rα) that increases the affinity of IL-2R complex to IL-2 by combining to the β and γ chains of IL-2R (IL-2R β and IL-2Rγ). The activation of IL-2R promotes cell proliferation and immune response [30]. In comparison to healthy volunteers, NKTCL patients had significantly higher serum CD-25 [31]. Elevated serum CD25 has been associated with poor response to chemotherapy and survival in NKTCL patients [31]. On the basis of PR after treatment with an anti-CD25 mAb basiliximab, plus pegaspargase in a patient with relapsed NKTCL [32], a phase 2 clinical trial of basiliximab plus pegaspargase is currently ongoing in NKTCL patients (NCT04337593).
C-C chemokine receptor (CCR4)-targeted mAbs
Chemokines are implicated in hematologic malignancies, including progression, metastasis, and angiogenesis [33]. NKTCL patients have elevated serum chemokine (CC motif) ligand (CCL) 17 and CCL22 as well as expression of their receptor CCR4 in tumor tissues [34]. The anti-CCR4 mAb mogamulizumab has been shown to enhance the ADCC activity of NK cells against NKTCL cell lines [35]. In a phase 3 randomized trial of 372 patients with cutaneous T-cell lymphoma, the ORR was 28% in the mogamulizumab versus 5% in the vorinostat group [36]. Currently, there is no clinical trials of anti-CCR4 mAbs in NKTCL patients.
CD40-targeted mAbs
CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is broadly expressed on the surface of both immune and non-immune cells [37]. CD40 is expressed in EBV-infected NKTCL cell lines, and CD40-CD40 ligand (CD40L) signaling has been shown to protect EBV-infected T/NK cells from apoptosis [38]. Dacetuzumab is a humanized immunoglobulin G1 mAb against CD40 developed initially for diffuse large B-cell lymphoma (DLBCL). A phase 2b trial in patients with relapsed DLBCL, however, failed to show improved survival with dacetuzumab [39]. Currently, there is no trial of CD40-targeted agent in NKTCL patients.
B7-H3-targeted BiTEs
B7-H3 (also known as CD276) is a member of the B7 ligand family, and functions as a protumorigenic factor to inhibit immune response in malignant tissues [40]. Zheng et al. [41] discovered that B7-H3 is highly expressed in NKTCL cell lines, and constructed a BiTE antibody that connects B7-H3 to the CD3 chain of T cell receptor (TCR) complex to achieve specific T cell cytotoxicity against NKTCL cells [42]. Preclinical studies demonstrated encouraging results in cultured NKTCL cells as well as a mouse model of NKTCL [41]. Currently, there is no trial of B7-H3 targeted therapy in NKTCL patients.
Other potential targets
A previous study confirmed the high expression of CD70 in SNK6 and SNK8 cell lines as well as tumor cells from NKTCL patients [43]. In that study, anti-CD70 mAb induced complement-dependent killing of SNK-6 cells. CD137 is also highly expressed in NKTCL cell lines, likely due to the induction by latent membrane protein 1 (LMP1) encoded by EBV [44]. CD137 deficiency hampers T cell proliferation [45, 46]. The studies of CD70 and CD137 are in the very early stage of preclinical development.
Immune checkpoint inhibitors (ICIs)
Immune checkpoints, e.g., PD-1 and programmed cell death ligand 1 (PD-L1), are critical in the development and maintenance of immune tolerance in tumor microenvironment [47]. PD-L1 is expressed in 39% to 100% of NKTCL patients [48–51]. Previous studies demonstrated a close association between EBV infection and PD-L1 expression in various malignancies [52, 53]. PD-L1 expression in NKTCL is increased by EBV-driven LMP1 through the nuclear factor κB (NF-κB) signaling pathway [54]. Many ICIs, including the PD-1 mAbs pembrolizumab, sintilimab, tislelizumab, and toripalimab, the PD-L1 mAbs sugemalimab and avelumab, and the dual-targeting anti-PD1/PD-L1 antibody IBI318, have been investigated for NKTCL (Table 2).
Table 2.
Summary of immune checkpoint inhibitors and ongoing clinical trials in NKTCL patients
| Agent | Target | Trial ID | Patient number (evaluable /estimate) | Study phase | Combined agents | Indication | Results for NKTCL | References |
|---|---|---|---|---|---|---|---|---|
| Avelumab | PD-L1 | NCT03439501 | 21 | 2 | / | r/r NKTCL |
ORR: 38% CR:24% |
[60] |
| Sugemalimab (CS1001) | PD-L1 | NCT05700448 | 150 | 3 | P-GemOx | r/r NKTCL | / | / |
| Sugemalimab (CS1001) | PD-L1 | NCT03595657 | 80 | 2 | / | r/r ENKTL |
ORR:46.2%, CR:30.4% 1y OS:68.6% 2y OS:54.6% |
[61, 62] |
| IMC-001 | PD-L1 | NCT04414163 | 20 | 2 | / | r/r NKTCL | / | / |
| Camrelizumab (SHR-1210) | PD-1 | NCT03363555 | 97 | 2 | / | r/r NKTCL | / | / |
| Toripalimab | PD-1 | NCT04365036 | 207 | 3 | P-GemOx | Newly diagnosed early stage NKTCL | / | / |
| Sintilimab | PD-1 | NCT03228836 | 28 | 2 | / | r/r NKTCL |
ORR: 75.0% 1y OS:82.1% 2y OS: 78.6% |
[58] |
| Sintilimab | PD-1 | NCT04279379 | 20 | 2 | Decitabine | r/r or advanced NKTCL | / | / |
| Sintilimab | PD-1 | NCT04127227 | 63 | 2 | P-GemOx | Newly diagnosed advanced ENKTL |
ORR:100% CR:87.5% 1y OS:100% 1y PFS:95% |
[126] |
| Sintilimab | PD-1 | NCT05008666 | 37 | 2 |
Chidamide Azacitidine L-DEP |
ENKTL-HLH | / | / |
| Sintilimab | PD-1 | NCT04676789 | 30 | 2 | Pegaspargase | Limited stage NKTCL | / | / |
| Sintilimab | PD-1 | NCT03936452 | 55 | 2 |
Pegaspargase Anlotinib |
Untreated, limited stage NKTCL |
ORR:87.8% CR:87.8% 2y PFS:87.6% 2y OS:97.9% |
[127] |
| Tislelizumab | PD-1 | NCT05477264 | 38 | 2 | / | Newly diagnosed NKTCL | / | / |
| Immune Tislelizumab | PD-1 | NCT05254899 | 54 | 2 | P-GemOx | High-risk early stage ENKTL | / | / |
| Tislelizumab | PD-1 | NCT05464433 | 46 | 1/2 | Mitoxantrone hydrochloride liposome | r/r NKTCL | / | / |
| Tislelizumab | PD-1 | NCT05058755 | 62 | NA | / | r/r NKTCL | / | / |
| Tislelizumab | PD-1 | NCT04038411 | 50 | 2 | Chidamide, Lenalidomide, Etoposide | r/r NKTCL |
CR:50.0% 1y PFS:86.8% |
[76] |
| Tislelizumab | PD-1 | NCT03493451 | 77 (22 NKTCL) | 2 | / | r/r mature T- and NK-cell neoplasms |
ORR 31.8% CR 18.2% |
[128] |
| Pembrolizumab | PD-1 | NCT04417166 | 30 | 2 | / | Untreated, limited stage NKTCL | / | / |
| Pembrolizumab | PD-1 | NCT03728972 | 19 | 2 | / | Untreated, early-stage ENKTL | / | / |
| Pembrolizumab | PD-1 | NCT03107962 | 20 | 2 | / | r/r NKTCL | / | / |
| IBI318 | PD-1 and PD-L1 bispecific | NCT04602065 | 129 | 1/2 | / | r/r NKTCL | / | / |
PD-1: programmed cell death protein 1, PD-L1: programmed cell death ligand 1, NKTCL: natural killer/T-cell lymphoma, ORR: objective response rate, CR: complete remission, PR: partial remission, OS: overall survival, PFS: progression-free survival, P-GemOx: pegaspargase, gemcitabine, and oxaliplatin, ENKTL: extranodal natural killer/T-cell lymphoma, L-DEP: L-asparaginase, doxorubicin liposome, etoposide and methylprednisolone
In a retrospective study of 7 r/r NKTCL patients, pembrolizumab monotherapy at a dose of 2 mg/kg every 3 weeks showed 100% ORR without significant toxicities; notably, 5 patients (71%) achieved CR, and all remained in CR after a median follow-up of 6 months [55]. In another retrospective study in 7 r/r NKTCL patients, pembrolizumab monotherapy at a dose of 100 mg every 3 weeks showed 57% ORR [56]. Two trials (NCT04417166, NCT03728972) are ongoing to examine pembrolizumab monotherapy in patients with untreated early-stage NKTCL. Nivolumab was evaluated in a study that included 3 patients who had failed previous L-asparaginase-based regimens [57]. In this study, all 3 patients showed initial response, but only 1 patient remained in CR and the remaining 2 patients died from infections. In the phase 2 single-arm ORIENT-4 trial [58], monotherapy with the anti-PD-1 mAb sintilimab demonstrated 75.0% ORR and 78.6% 2-year OS in 28 patients with r/r NKTCL. Several other ICIs, including toripalimab, camrelizumab, tislelizumab and IBI318, are being investigated in ongoing clinical trials (Table 2). In a study of 9 patients with advanced NKTCL [59], PD-1 inhibitors in combination with P-GemOx chemotherapy demonstrated 88.9% ORR, 77.8% CR rate, 66.7% 1-year PFS rate and 100.0% 1-year OS rate. A phase 2 trial (NCT04127227) is ongoing to examine PD-1 inhibitors in combination P-GemOx chemotherapy in patients with advanced NKTCL (Table 2).
In a phase 2 trial of 21 patients with r/r NKTCL, monotherapy with the anti-PD-L1 mAb avelumab demonstrated 38% ORR and 24% CR rate [60]. However, the responders in this trial had relatively long remission, with the maximum that exceeded 25 months [60]. This study also demonstrated a positive correlation between PD-L1 expression and treatment response, suggesting that evaluating the level of PD-L1 expression could potentially help identify patients who are more likely to benefit from PD-L1 inhibitors. In a single-arm phase 2 trial in 80 patients with r/r NKTCL, the anti-PD-L1 mAb sugemalimab demonstrated 46.2% ORR, 30.4% CR rate, 68.6% 1-year OS rate and 54.6% 2-year OS rate [61, 62]. In addition to EBV-driven LMP1, PD-L1 can also be increased via the signal transducer and activator of transcription 3 (STAT3) [63]. Given these results, it is possible to combine PD-L1 inhibitors with other therapies such as STAT3 inhibitors and EBV-targeted cytotoxic T lymphocytes (CTL). Other PD-L1 inhibitors (e.g., IMC-001) are summarized in Table 2.
Many other immune checkpoint molecules, including transforming growth factor-β1 (TGF-β1), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), T-cell immunoglobulin-3 (TIM-3), T-cell immunoglobulin and ITIM domain (TIGIT), B/T lymphocyte attenuator (BTLA), and lymphocyte-activation gene 3 (LAG-3), are upregulated in NKTCL patients [64, 65]. However, there are currently no targeted therapies based on these molecules [64, 65].
EBV targeted cellular therapy
EBV is a one of the most ubiquitous viruses that infect human beings, with an estimated prevalence of 90% in the world population [66]. The latency II pattern EBV antigens, including LMP1, LMP2 and EBV nuclear antigen 1 (EBNA1), are widely expressed in EBV-positive tumor cells [67, 68], and have been implicated in the survival and proliferation of NKTCL cells [69]. LMP1 promotes the survival, proliferation, invasion and migration of NKTCL cells through the NF-κB pathway [70]. In a phase 1 single-arm trial of 52 patients with EBV-associated lymphomas, treatment with CTLs that target LMP2 or LMP1/2 resulted in long-lasting responses without significant toxicity [71]. This study included 11 patients with NKTCL, among whom 6 had active disease and 5 were in remission but at high risk for relapse. Four out of the 6 patients with active disease achieved CR and 3 of them remained in CR for at least four years. All 5 patients in remission remained in remission for at least 2 years after CTLs infusion. In a study of 10 NKTCL patients (8 with localized disease and 2 with advanced disease) who received autologous LMP-specific CTLs after achieving CR with induction therapy, the 4-year OS and PFS rate were 100% and 90%, respectively [72]. While the results of this study are impressive, further research is necessary since most patients had localized-stage NKTCL. Kim et al. [73] conducted a phase 2 trial to examine autologous EBV-specific T cells (baltaleucel-T) in 54 patients with advanced, relapsed NKTCL. The attempt to expand baltaleucel-T cells failed in 39 out of the 54 patients. The remaining 15 patients received a median of 4 doses of baltaleucel-T. In the 10 patients with active disease, the therapy achieved 50% ORR and 30% CR rate. In the remaining 5 patients with no measurable disease at the baseline, 2 remained in remission during the follow-up of 5 months.
Overall, these studies showed that EBV targeted cellular therapies could induce sustained treatment response in NKTCL patients. However, there is a major need to optimize the CTL expansion protocol.
Immunomodulatory agents
A randomized trial in NKTCL patients reported moderate efficacy of immunomodulatory agent thalidomide in combination with conventional chemotherapy: 8 out the 11 patients achieved CR, and one achieved PR [74]. Successful treatment of relapsed NKTCL with lenalidomide was described in a case report. This patient experienced relapse after hematopoietic stem cell transplantation (HSCT), and achieved CR with lenalidomide monotherapy [75]. Further studies with larger sample sizes are needed to verify these preliminary findings. In a phase 2 single-arm trial of in 20 r/r NKTCL patients, lenalidomide plus tislelizumab, chidamide and etoposide demonstrated 50.0% CR and 86.8% 1-year PFS rate [76]. A phase 3 trial (NCT02085655) is ongoing to compare P-GemOx plus thalidomide with AspaMetDex (pegaspargase, methotrexate, dexamethasone) in previously untreated or r/r NKTCL (Table 3).
Table 3.
Summary of epigenetic targeted therapy, and immunomodulatory agents and ongoing clinical trials in NKTCL patients
| Agent | Target | Trial ID | Number of estimated enrollment | Study phase | Combined agents | Indication | Results for NKTCL | References | |
|---|---|---|---|---|---|---|---|---|---|
| Immuno-modulatory agent | Thalidomide | / | NCT02085655 | 264 | 3 | P-Gemox or methotrexate, dexamethasone | Previously untreated or r/r NKTCL | / | / |
| Epigenetic targeted agents | Chidamide | HDACi | NCT03820596 | 37 | 1/2 | Sintilimab | r/r ENKTL |
CR:59.5% PR:48.6% 1.5yPFS:52.5% 1.5y OS:76.2% |
[120] |
| Chidamide | HDACi | NCT03630731 | 32 | 2 | / | Chemotherapy responded stage IV or r/r NKTCL | / | / | |
| Chidamide | HDACi | NCT04414969 | 35 | 2 | Anti PD-1 antibody, Pegaspargase | Stage IE and IIE ENKTL | / | / | |
| Chidamide | HDACi | NCT04994210 | 30 | 2 | Sintilimab | Newly diagnosed ENKTL | / | / | |
| Chidamide | HDACi | NCT02878278 | 24 | 2 | / | r/r NKTCL | CR: 33% | [118] | |
| Vorinostat | HDACi | NCT00336063 | 18 | 1 | Azacitidine | Nasal NKTCL | / | / | |
| Romidepsin | HDACi | NCT01913119 | 16 | 1 | / | r/r NKTCL | / | / | |
NKTCL: natural killer/T-cell lymphoma, HDACi: histone deacetylase inhibitor, CR: complete remission, ENKTL: extranodal natural killer/T-cell lymphoma, HLH: hemophagocytic lymphohistiocytosis, L-DEP: L-asparaginase, doxorubicin liposome, etoposide and methylprednisolone, P-GemOx: pegaspargase, gemcitabine, and oxaliplatin
Chimeric antigen receptor T-cell (CART) therapy
CART therapy has been successfully used in the treatment of aggressive B cell lymphomas [77, 78]. However, the use of CART therapy for NKTCL is limited. In a mouse model for NKTCL, CAR-T cells that target B7-H3 demonstrated robust cytotoxicity against NKTCL cells [79]. Several trials of CD30 CART therapy are currently ongoing in patients with CD30-positive NHL (NCT04526834, NCT03049449, NCT04288726). Since NKTCL is closely associated with EBV infection [1], LMP1 may represent another target for CART therapy for NKTCL.
Signaling pathway inhibitors
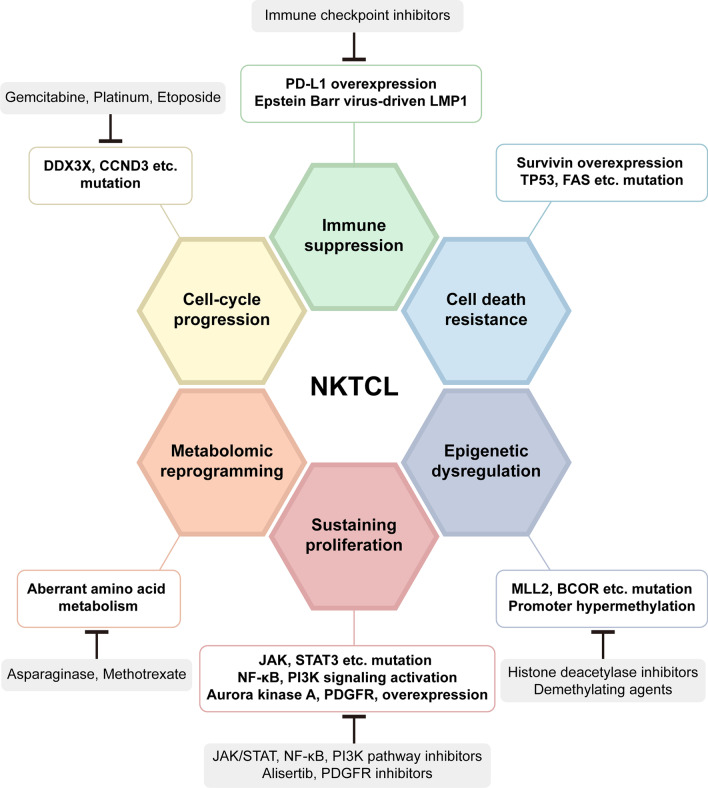
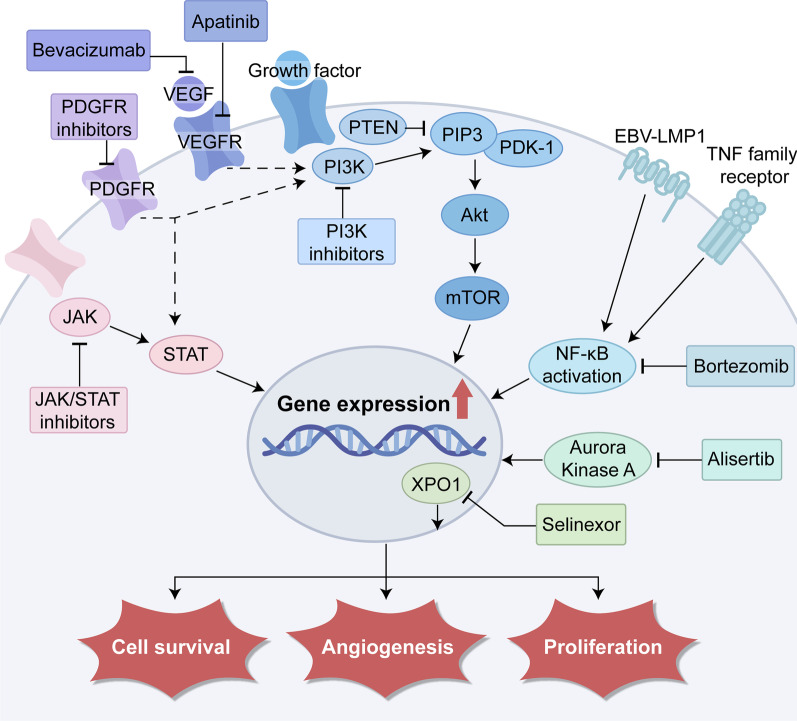
Genomic expression profiling (GEP) has unveiled a variety of mechanisms that underlie the pathogenesis of NKTCL [80], and holds the potential for developing individualized treatment strategies. Figure 2 is a schematic overview of the six main hallmark characteristics in the pathogenesis of NKTCL and the corresponding targeted therapies. Relevant signaling pathways include the Janus-associated kinase/signal transducer and activator of transcription (JAK/STAT), vascular endothelial growth factor (VEGF), platelet-derived growth factor receptor (PDGFR), phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt)/ mammalian target of rapamycin (mTOR) pathway and NF-κB pathways [11] (Fig. 3). In 2020, Xiong et al. [81] identified three molecular subtypes (TSIM, MB, and HEA) in NKTCL using an integrated approach combining whole-genome/exome sequencing, array-based copy number variation analysis, and RNA sequencing. In this study, activation of JAK/STAT and NF-κB pathway was involved in TSIM and HEA subtype, respectively, and these molecular subtypes were sensitive to different targeted treatments. In the following sections, we summarize the potential signaling pathway inhibitors against NKTCL (also in Table 4).
Fig. 2.
An overview of the six hallmark characteristics in the pathogenesis of NKTCL and targeted therapies. NKTCL: natural killer/T-cell lymphoma, PD-L1: programmed cell death ligand 1, MLL2: mixed lineage leukemia 2, BCOR: BCL-6 corepressor, JAK/STAT: Janus-associated kinase/signal transducer and activator of transcription, PDGFR: platelet-derived growth factor receptor, PI3K: phosphatidylinositol 3-kinase, NF-κB: nuclear factor κB, LMP1: latent membrane protein 1
Fig. 3.
Novel agents targeting signaling pathways, including JAK/STAT, NF-κB, PDGFR, VEGF/VEGFR, PI3K/Akt/ mTOR, XPO1 and AURKA. PDGFR: platelet-derived growth factor receptor, VEGF: vascular endothelial growth factor, PI3K/Akt: phosphatidylinositol 3-kinase/protein kinase B, mTOR: mammalian target of rapamycin, XPO1: exportin-1, AURKA: Aurora kinase A
Table 4.
Summary of signaling pathway inhibitors and ongoing clinical trials in NKTCL patients
| Signaling pathway inhibitors | Target | Trial ID | Number of estimated enrollment | Study phase | Combined agents | Indication | Results for NKTCL | References |
|---|---|---|---|---|---|---|---|---|
| Bortezomib | NF-κB | NCT02808091 | 7 | 2 | GIFOX | Newly diagnosis NKTCL | ORR:43% | [95] |
| Anlotinib | VEGFR | NCT04004572 | 37 | 2 | Sintilimab Pegaspargase | Stage IV NKTCL | / | / |
| Apatinib | VEGFR2 | NCT04366128 | 60 | NA | Camrelizumab Pegaspargase | Stage IE/IIE ENKTL | / | / |
| Avastin (Bevacizumab) | VEGF | NCT01921790 | 30 | 2 | GemAOD | Untreated NKTCL | / | / |
| Tofacitinib | JAK1/3 | NCT03598959 | 20 | 2 | Chidamide | r/r NKTCL | / | / |
| Ruxolitinib | JAK1/2 | NCT02974647 | 82 | 2 | r/r NKTCL | / | / | |
| Selinexor (ATG-010) | XPO1 | NCT04425070 | 97 (10 NKTCL) | 1/2 | GemOx or ICE or Tislelizumab | Peripheral T- and NK/T-cell lymphoma | ORR: 60% CR: 20% | [109] |
NKTCL: natural killer/T-cell lymphoma, GIFOX: gemcitabine, ifosfamide and oxaliplatin, ORR: objective response rate, CR: complete remission, GemAOD: gemcitabine, oxaliplatin, pegaspargase and dexamethasone, VEGFR: vascular endothelial growth factor receptor, VEGF: vascular endothelial growth factor, NA: not available, JAK: Janus-associated kinase, NF-κB: nuclear factor κB, ENKTL: extranodal natural killer/T-cell lymphoma, XOP1: exportin-1, GemOx: gemcitabine and oxaliplatin, ICE: ifosfamide, carboplatin and etoposide
JAK/STAT pathway inhibitors
Aberrant activation of JAK/STAT pathway is responsible for sustained proliferation of tumor cells under the stimulation by various cytokines [82, 83], and is a crucial factor in the pathogenesis of NKTCL [84]. In a study of 65 patients with NKTCL by Koo et al. [85], JAK3 mutation was detected in 35.4% of the cases. In contrast, STAT3 mutation is much less common (about 20%), but phosphorylated STAT3 (pSTAT3) is constitutively expressed in about 75% of NKTCL patients [86, 87]. These findings suggest that JAK/STAT pathway may be a potential therapeutic target in NKTCL. The pan-JAK inhibitor CP-690550 [85], the JAK1/3 inhibitor tofacitinib [87] and the selective STAT3 inhibitor WP1066 [86] could inhibit the proliferation and induce apoptosis in several NKTCL cell lines. STAT3 mutations sensitize NKTCL cells to the STAT3 inhibitor static [87]. NKTCL cells with mutations in JAK3 and STAT3 are more susceptible than wild-type controls to treatment with tofacitinib and stattic, respectively [88]. Patients with STAT3 mutation tend to have high expression of CD30 [21], suggesting that combining JAK/STATs inhibitors with an anti-CD30 antibody may be more effective. Two trials are currently ongoing to evaluate JAK inhibitors, tofacitinib in one trial (NCT03598959) and ruxolitinib in another (NCT02974647) in patients with r/r NKTCL. A phase 2 multinational global study evaluated the JAK1 selective inhibitor golidocitinib in r/r PTCL patients; in the 3 NKTCL patients, ORR was reported in 2 patients [89].
NF-κB pathway inhibitors
NF-κB plays an important role in the proliferation and survival of immune cells [90]. Aberrant activation of the NF-κB pathway (e.g., elevated expression of TNFRSF21 and c-Rel) has been recognized as a hallmark of NKTCL [91]. Both non-canonical pathway [92] and canonical pathway [93] have been implicated. Bortezomib, a proteasome inhibitor that indirectly inhibits the NF-κB pathway, has been shown to impair the viability and induce apoptosis of NKTCL cell lines [94]. In a phase 2 trial that enrolled only 7 NKTCL patients before termination due to slow recruitment [95], a combination of bortezomib and GIFOX (gemcitabine, ifosfamide, oxaliplatin) regimen demonstrated 43% ORR (CR in one patient). Bortezomib has been shown to trigger EBV into the lytic cycle from latency [96], suggesting the possibility of combination treatment with bortezomib and EBV targeted CTLs. Despite these findings, evidence for NF-κB targeted therapy for NKTCL is rather limited.
VEGF/VEGFR inhibitors
GEP analysis revealed overexpression of angiogenesis-related genes in NKTCL cell lines, including VEGF-A and KDR (encoding VEGF-A and VEGF receptor 2 (VEGFR2), respectively) [91]. In a phase 2 trial of 39 patients with T cell lymphomas, the anti-VEGF mAb bevacizumab in combination with CHOP chemotherapy achieved 90% ORR [97]. Unfortunately, this trial did not include NKTCL patients. A phase 2 trial (NCT01921790) is ongoing to evaluate bevacizumab in combination with chemotherapy in NKTCL patients. VEGFR inhibitors apatinib and anlotinib are also being tested in several ongoing trials (NCT04004572, NCT04366128).
PDGFR inhibitors
PDGFRα is a receptor tyrosine kinase that interacts with key proteins in both the JAK/STAT and PI3K/Akt signaling pathways [98]. GEP analysis revealed overexpression of PDGFRα and enhanced PDGFRα phosphorylation in NKTCL cell lines [91]. High PDGFRα expression has been associated with poor prognosis in NKTCL patients [99]. The PDGFR tyrosine kinase inhibitor imatinib has been shown to inhibit the viability of NKTCL cells and induce an arrest of cell cycle at G0/G1 stage [100]. However, few PDGFR pathway-related gene mutations have been reported in NKTCL cells so far, limiting the enthusiasm on PDGFR as a target for the treatment of NKTCL.
PI3K/Akt/mTOR pathway inhibitors
The PI3K/Akt/mTOR pathway plays an important role in the regulation of cell proliferation and survival, and dysregulated PI3K/Akt/mTOR pathway is a hallmark of a variety of cancers [101]. A study showed high expression of several PI3K isoforms (PIK3α, PIK3β, PIK3γ, PIK3δ) in majority of NKTCL samples as well as an association between high PI3Kα expression with poor patient prognosis. This study also showed that copanlisib (a pan-class I inhibitor against PI3Kα and PIK3δ) could reduce the phosphorylation of Akt and inhibit the tumor growth both in vivo and in vitro [102]. In a study by Kawada et al., mTOR inhibitors (rapamycin and CCI-779) arrested NKTCL cells in the G1 phase and reduced cell viability [103], suggesting that mTOR inhibitor may also be a potential therapeutic option in NKTCL treatment. Overall, the PI3K/Akt/mTOR pathway represents promising target in developing new treatment of NKTCL, but no trials have been or are currently being conducted.
Exportin-1 inhibitors
Exportin-1 (XPO1) facilitates the transport of various proteins and RNAs from the nucleus to the cytoplasm [104]. XPO1 has been reported to be overexpressed in NHLs and high expression of XPO1 has been associated with poor prognosis [105]. Selinexo, a selective XPO1 inhibitor, has been widely explored and demonstrated satisfactory efficacy in the treatment of DLBCL [106] and MM [107], but studies on NKTCL are limited. In a phase 1 trial of 10 patients with PTCL and 1 patient with NKTCL, selinexor in combination with DICE (dexamethasone, ifosfamide, carboplatin, and etoposide) regimen achieved 91% ORR and 82% CR rate, but 45% of patients discontinued the treatment due to the significant toxicities [108]. A phase 1b trial (NCT04425070) is currently ongoing to examine selinexor in combination with GemOx, ICE or tislelizumab in patients with PTCL and NKTCL. The latest results about the regimen of selinexor with GemOx are encouraging: 60% ORR and 20% CR in 10 NKTCL patients [109].
Aurora kinase A inhibitors
Aurora kinase A (AURKA) plays a crucial role in the regulation of cell cycle, primarily during mitosis [110]. Overexpression of AURKA has been found in various hematological malignancies, including acute myeloid leukemia, MM and NHL [111]. A previous study observed high expression of AURKA in NKTCL cell lines as well as in NKTCL patients [112]. In this study, MK-8745 (a small-molecule AURKA inhibitor) significantly increased the apoptosis of NKTCL cells and induced the cell cycle arrest. However, there is no trial of AURKA targeted agents in the treatment of NKTCL patients.
Epigenetic targeted agents
Epigenetic dysregulation has been described in a wide variety of solid and hematological malignancies [113, 114]. Mutations and aberrant expression patterns of BCL-6 corepressor (BCOR) and mixed lineage leukemia 2 (MLL2) have been implicated in NKTCL [115, 116]. Treatment approaches based on epigenetics for NKTCL are summarized in Table 3.
Histone deacetylase inhibitors (HDACi) produce multiple cytotoxic effects on cancer cells through histone acetylation of tumor suppressors [117]. Chidamide is a selective inhibitor of HDAC1, 2, 3 and 10 [118], and has been tested as monotherapy in a phase 2 trial in patients with r/r NKTCL (NCT02878278). In the most recent report of this trial [118], chidamide achieved 33% CR with a median follow-up of 3.7 months; all patients who achieved CR remained disease-free for > 6 months. In a non-randomized study of 37 patients with advanced NKTCL, the ORR was 40% in the 19 patients who received chidamide plus chemotherapy versus 15% in the remaining 18 who received chidamide monotherapy [119]. In a phase 1b/2 single-arm trial in r/r NKTCL patients, combination treatment with chidamide and sintilimab achieved 59.5% ORR, 48.6% CR, 52.5% 18-month PFS and 76.2% 18-month OS [120]. Several trials are currently being conducted to test chidamide in combination with other agents for NKTCL (NCT04994210, NCT05008666). In a trial of NKTCL patients, panobinostat, (an oral non-selective HDACi) in combination with bortezomib resulted in PR in one out of the two patients with r/r NKTCL [121]. Inhibition of histone deacetylase may trigger EBV reactivation and the use of romidepsin (another HDACi) has been reported to cause EBV reactivation [122, 123]. The risk of EBV reactivation when using HDACi therapy must be carefully considered and future strategy using anti-EBV drugs in combination with HDACi could be further explored. The ongoing trials evaluating HDACi in patients with NKTCL are summarized in Table 3.
Global promoter methylation analysis revealed hypermethylation of the promoters for a number of tumor suppressor genes, including BCL2L11 (BIM), DAPK1, and TET2, in NKTCL cell lines and patient samples; treatment with decitabine induced the re-expression of methylated genes [124], suggesting possible therapeutic action of demethylating agents.
Conclusions
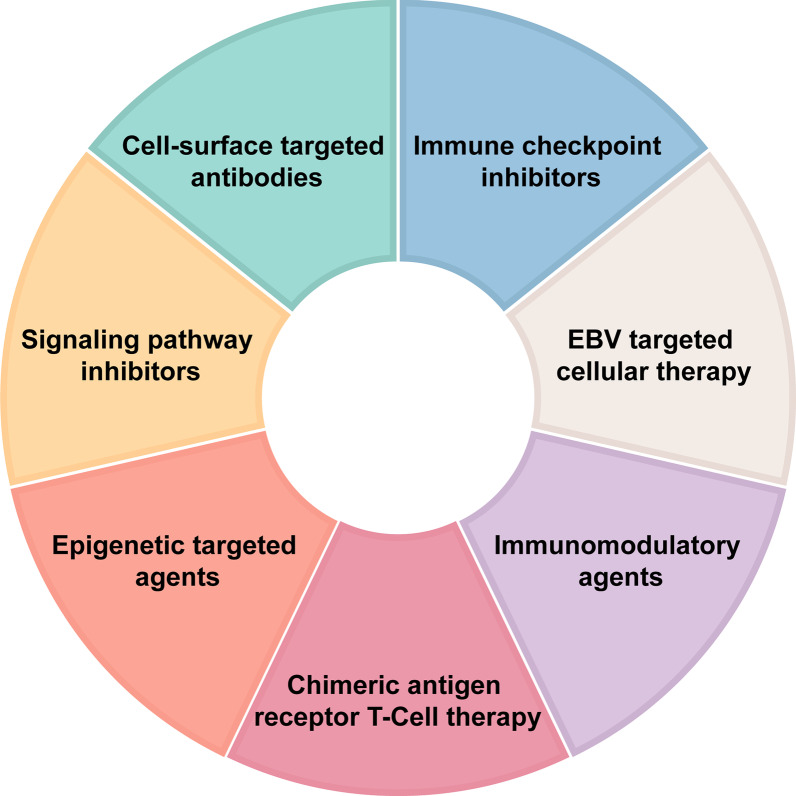
Asparaginase-based chemotherapy has improved survival outcomes in patients with localized NKTCL. Advanced NKTCL, however, remains a major challenge, with disease progression within 5 years of diagnosis in over 70% of the patients [125]. A variety of novel agents have been developed for r/r NKTCL (summarize in Fig. 4). Among these novel treatments, immunotherapies (ICIs, cell-surface-targeted antibodies and EBV-specific CTL) have demonstrated promising results. Evidence of signaling pathway inhibitors and epigenetic targeted agents are currently limited. In our opinion, it is unlikely that signaling pathway inhibitors and epigenetic targeted agents could achieved satisfactory efficacy as monotherapy in r/r NKTCL. Combination strategies with ICIs, cell-surface-targeted antibodies and anthracycline-containing chemotherapy may help to enhance therapeutic efficacy in the management of r/r NKTCL and hold the potential for future development. Other important obstacles include limited number of patients available for clinical trials and distinct gene mutations in different patients. An individualized approach is thus required.
Fig. 4.
An overview of the seven main categories of novel agents for the treatment of NKTCL
Acknowledgements
Dr. Kehong Zhang from the Ivy Medical Editing edited this review article.
Abbreviations
- NKTCL
Natural killer/T-cell lymphoma
- EBV
Epstein-Barr virus
- r/r
Relapsed and refractory
- NHL
Non-Hodgkin lymphoma
- CHOP
Cyclophosphamide, doxorubicin, vincristine, and prednisone
- SMILE
Dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide
- P-GemOx
Pegaspargase, gemcitabine, and oxaliplatin
- DDGP
Dexamethasone, cisplatin, gemcitabine, and pegaspargase
- mAb
Monoclonal antibody
- ADC
Antibody-drug conjugate
- BiTE
Bispecific T-cell engager
- MM
Multiple myeloma
- ADCC
Antibody dependent cytotoxicity
- ADCP
Antibody dependent phagocytosis
- CDC
Complement dependent cytotoxicity
- ORR
Objective response rate
- CR
Complete remission
- PR
Partial remission
- PFS
Progression-free survival
- OS
Overall survival
- PD-1
Programmed cell death protein 1
- BV
Brentuximab vedotin
- CHP
Cyclophosphamide, doxorubicin, and prednisone
- PTCL
Peripheral T-cell lymphoma
- MAD
Methotrexate, l-asparaginase, and dexamethasone
- EPOCH
Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin
- IL-2R
Interleukin-2 receptor
- CCR4
C-C chemokine receptor
- CCL
CC motif ligand
- TNFR
Tumor necrosis factor receptor
- DLBCL
Diffuse large B-cell lymphoma
- TCR
T cell receptor
- ICI
Immune checkpoint inhibitors
- PD-L1
Programmed cell death ligand 1
- LMP1
Latent membrane protein 1
- NF-κB
Nuclear factor κB
- STAT3
Signal transducer and activator of transcription 3
- CTL
Cytotoxic T lymphocyte
- EBNA1
EBV nuclear antigen 1
- HSCT
Hematopoietic stem cell transplantation
- AspaMetDex
Pegaspargase, methotrexate, and dexamethasone
- CTLA-4
Cytotoxic T lymphocyte-associated antigen 4
- TIM-3
T-cell immunoglobulin-3
- TIGIT
T-cell immunoglobulin and ITIM domain
- BTLA
B/T lymphocyte attenuator
- LAG-3
Lymphocyte-activation gene 3
- CART
Chimeric antigen receptor T-cell
- GEP
Genomic expression profiling
- JAK/STAT
Janus-associated kinase/signal transducer and activator of transcription
- VEGF
Vascular endothelial growth factor
- PDGFR
Platelet-derived growth factor receptor
- PI3K
Phosphatidylinositol 3-kinase
- Akt
Protein kinase B
- mTOR
Mammalian target of rapamycin
- GIFOX
Gemcitabine, ifosfamide, oxaliplatin
- XPO1
Exportin-1
- DICE
Dexamethasone, ifosfamide, carboplatin, and etoposide
- AURKA
Aurora kinase A
- BCOR
BCL-6 corepressor
- MLL2
Mixed lineage leukemia 2
- HDACi
Histone deacetylase inhibitors
Author contributions
Q-QC conceived and designed the study. X-PT, YC, JC and Y-C-Z contributed to research performance, provision of study thought, data analysis, manuscript writing and final approval of manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program (2022YFC2502602), the Key Program of National Natural Science Foundation of China (82230001), the National Natural Science Foundation of China (82270199), the Sun Yat-Sen University Clinical Research 5010 Program (2020009), the Special Support Program of Sun Yat-sen University Cancer Center (PT19020401), and the Clinical Oncology Foundation of Chinese Society of Clinical Oncology (Y-XD2019-124 and Y-SY2021ZD-0110).
Availability of data and materials
The datasets supporting the conclusions of this study are included in the figures and tables.
Declarations
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
All authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao-Peng Tian, Yi Cao, Jun Cai and Yu-Chen Zhang contributed equally to this work.
References
- 1.Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi: 10.1016/S0140-6736(16)32407-2. [DOI] [PubMed] [Google Scholar]
- 2.Cai Q, Cai J, Fang Y, Young KH. Epstein-Barr virus-positive natural killer/T-cell lymphoma. Front Oncol. 2019;9:386. doi: 10.3389/fonc.2019.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76(11):2351–2356. doi: 10.1002/1097-0142(19951201)76:11<2351::AID-CNCR2820761125>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Guidelines T-Cell Lymphomas Version 1. 2023.
- 5.Yamaguchi M, Suzuki R, Kwong YL, Kim WS, Hasegawa Y, Izutsu K, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008;99(5):1016–1020. doi: 10.1111/j.1349-7006.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi S, Yahalom J, Hsu M, Chelius M, Lunning M, Moskowitz A, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016;57(11):2575–2583. doi: 10.1080/10428194.2016.1180689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Ma S, Cai J, Yang Y, Jing H, Shuang Y, et al. Sequential P-GEMOX and radiotherapy for early-stage extranodal natural killer/T-cell lymphoma: a multicenter study. Am J Hematol. 2021;96(11):1481–1490. doi: 10.1002/ajh.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Zhang L, Liu X, Li X, Li L, Fu X, et al. Efficacy and safety of a pegasparaginase-based chemotherapy regimen versus an l-asparaginase-based chemotherapy regimen for newly diagnosed advanced extranodal natural killer/T-cell lymphoma: a randomized clinical trial. JAMA Oncol. 2022;8(7):1035–1041. doi: 10.1001/jamaoncol.2022.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse E, Zhao WL, Xiong J, Kwong YL. How we treat NK/T-cell lymphomas. J Hematol Oncol. 2022;15(1):74. doi: 10.1186/s13045-022-01293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim SH, Hong JY, Lim ST, Hong H, Arnoud J, Zhao W, et al. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28(9):2199–2205. doi: 10.1093/annonc/mdx316. [DOI] [PubMed] [Google Scholar]
- 11.de Mel S, Hue SS, Jeyasekharan AD, Chng WJ, Ng SB. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol. 2019;12(1):33. doi: 10.1186/s13045-019-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somasundaram N, Lim JQ, Ong CK, Lim ST. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL) J Hematol Oncol. 2019;12(1):28. doi: 10.1186/s13045-019-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwivedi S, Rendón-Huerta EP, Ortiz-Navarrete V, Montaño LF. CD38 and regulation of the immune response cells in cancer. J Oncol. 2021;2021:6630295. doi: 10.1155/2021/6630295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Wang H, Li PF, Lu Y, Xia ZJ, Huang HQ, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann Hematol. 2015;94(8):1381–1388. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Liang L, Liao Q, Li Y, Zhou Y. CD38: an important regulator of T cell function. Biomed Pharmacother. 2022;153:113395. doi: 10.1016/j.biopha.2022.113395. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Zhu J, Yao M, Kim TM, Yoon DH, Cho SG, et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: an open-label, single-arm, multicenter, phase 2 study. J Hematol Oncol. 2021;14(1):25. doi: 10.1186/s13045-020-01020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. CD38-mediated Immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 2018;8(9):1156–1175. doi: 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muta H, Podack ER. CD30: from basic research to cancer therapy. Immunol Res. 2013;57(1–3):151–158. doi: 10.1007/s12026-013-8464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GN, Zhao WG, Zhang XD, Jian XY, Zhang CL, Zhang MZ, et al. A retrospective study on the clinicopathological and molecular features of 22 cases of natural killer/T-cell lymphoma in children and adolescents. Sci Rep. 2022;12(1):7118. doi: 10.1038/s41598-022-11247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamoto K, Miyoshi H, Suzuki T, Sasaki Y, Yamada K, Yanagida E, et al. Frequent expression of CD30 in extranodal NK/T-cell lymphoma: potential therapeutic target for anti-CD30 antibody-based therapy. Hematol Oncol. 2018;36(1):166–173. doi: 10.1002/hon.2482. [DOI] [PubMed] [Google Scholar]
- 21.Oishi N, Satou A, Miyaoka M, Kawashima I, Segawa T, Miyake K, et al. Genetic and immunohistochemical profiling of NK/T-cell lymphomas reveals prognostically relevant BCOR-MYC association. Blood Adv. 2023;7(1):178–189. doi: 10.1182/bloodadvances.2022007541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Yoon DH, Kim JS, Kang HJ, Lee HW, Eom HS, et al. Efficacy of brentuximab vedotin in relapsed or refractory high-CD30-expressing non-Hodgkin lymphomas: results of a multicenter, open-labeled phase II trial. Cancer Res Treat. 2020;52(2):374–387. doi: 10.4143/crt.2019.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz S, O'Connor OA, Pro B, Trümper L, Iyer S, Advani R, et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol. 2022;33(3):288–298. doi: 10.1016/j.annonc.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HK, Moon SM, Moon JH, Park JE, Byeon S, Kim WS. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015;50(4):254–256. doi: 10.5045/br.2015.50.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol. 2016;95(5):847–849. doi: 10.1007/s00277-016-2627-9. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Yuan CM, Hubacheck J, Janik JE, Wilson W, Morris JC, et al. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol. 2009;145(2):173–179. doi: 10.1111/j.1365-2141.2009.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang ST, Lu CL, Chuang SS. CD52 expression in non-mycotic T- and NK/T-cell lymphomas. Leuk Lymphoma. 2007;48(1):117–121. doi: 10.1080/10428190601016167. [DOI] [PubMed] [Google Scholar]
- 28.Poggio T, Duyster J, Illert AL. Current immunotherapeutic approaches in T cell non-Hodgkin lymphomas. Cancers (Basel). 2018;10:9. doi: 10.3390/cancers10090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wulf GG, Altmann B, Ziepert M, D'Amore F, Held G, Greil R, et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: the DSHNHL2006-1B/ACT-2 trial. Leukemia. 2021;35(1):143–155. doi: 10.1038/s41375-020-0838-5. [DOI] [PubMed] [Google Scholar]
- 30.Damoiseaux J. The IL-2 - IL-2 receptor pathway in health and disease: the role of the soluble IL-2 receptor. Clin Immunol. 2020;218:108515. doi: 10.1016/j.clim.2020.108515. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Liao DZ, Zhang J, Xia ZJ, Peng XW, Lu Y. Clinical significance of serum soluble interleukin-2 receptor-α in extranodal natural killer/T-cell lymphoma (ENKTL): a predictive biomarker for treatment efficacy and valuable prognostic factor. Med Oncol. 2013;30(4):723. doi: 10.1007/s12032-013-0723-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Bi XW, Zhu YJ, He YZ, Lai QY, Xia ZJ, et al. IL-2Rα up-regulation is mediated by latent membrane protein 1 and promotes lymphomagenesis and chemotherapy resistance in natural killer/T-cell lymphoma. Cancer Commun (Lond) 2018;38(1):62. doi: 10.1186/s40880-018-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurence AD. Location, movement and survival: the role of chemokines in haematopoiesis and malignancy. Br J Haematol. 2006;132(3):255–267. doi: 10.1111/j.1365-2141.2005.05841.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumai T, Nagato T, Kobayashi H, Komabayashi Y, Ueda S, Kishibe K, et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. 2015;64(6):697–705. doi: 10.1007/s00262-015-1675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanazawa T, Hiramatsu Y, Iwata S, Siddiquey M, Sato Y, Suzuki M, et al. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin Cancer Res. 2014;20(19):5075–5084. doi: 10.1158/1078-0432.CCR-14-0580. [DOI] [PubMed] [Google Scholar]
- 36.Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 37.Karnell JL, Rieder SA, Ettinger R, Kolbeck R. Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv Drug Deliv Rev. 2019;141:92–103. doi: 10.1016/j.addr.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Imadome K, Shimizu N, Arai A, Miura O, Watanabe K, Nakamura H, et al. Coexpression of CD40 and CD40 ligand in Epstein-Barr virus-infected T and NK cells and their role in cell survival. J Infect Dis. 2005;192(8):1340–1348. doi: 10.1086/466530. [DOI] [PubMed] [Google Scholar]
- 39.Fayad L, Ansell SM, Advani R, Coiffier B, Stuart R, Bartlett NL, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. 2015;56(9):2569–2578. doi: 10.3109/10428194.2015.1007504. [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, et al. Immune checkpoint of B7–H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15(1):153. doi: 10.1186/s13045-022-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng M, Yu L, Hu J, Zhang Z, Wang H, Lu D, et al. Efficacy of B7-H3-redirected BiTE and CAR-T immunotherapies against extranodal nasal natural killer/T cell lymphoma. Transl Oncol. 2020;13(5):100770. doi: 10.1016/j.tranon.2020.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou S, Liu M, Ren F, Meng X, Yu J. The landscape of bispecific T cell engager in cancer treatment. Biomark Res. 2021;9(1):38. doi: 10.1186/s40364-021-00294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshino K, Kishibe K, Nagato T, Ueda S, Komabayashi Y, Takahara M, et al. Expression of CD70 in nasal natural killer/T cell lymphoma cell lines and patients; its role for cell proliferation through binding to soluble CD27. Br J Haematol. 2013;160(3):331–342. doi: 10.1111/bjh.12136. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimori M, Imadome K, Komatsu H, Wang L, Saitoh Y, Yamaoka S, et al. CD137 expression is induced by Epstein-Barr virus infection through LMP1 in T or NK cells and mediates survival promoting signals. PLoS ONE. 2014;9(11):e112564. doi: 10.1371/journal.pone.0112564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somekh I, Thian M, Medgyesi D, Gülez N, Magg T, Gallón Duque A, et al. CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis. Blood. 2019;134(18):1510–1516. doi: 10.1182/blood.2019000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez R, Fournier B, Cordeiro DJ, Winter S, Izawa K, Martin E, et al. Concomitant PIK3CD and TNFRSF9 deficiencies cause chronic active Epstein-Barr virus infection of T cells. J Exp Med. 2019;216(12):2800–2818. doi: 10.1084/jem.20190678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–742. [PMC free article] [PubMed] [Google Scholar]
- 48.Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469(5):581–590. doi: 10.1007/s00428-016-2011-0. [DOI] [PubMed] [Google Scholar]
- 49.Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, et al. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96(1):25–31. doi: 10.1007/s00277-016-2818-4. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, Feng X, Jing C, Yu X, Zheng Y, Xu C. The expression and clinical significance of programmed cell death receptor 1 and its ligand in tumor tissues of patients with extranodal nasal NK/T cell lymphoma. Sci Rep. 2022;12(1):36. doi: 10.1038/s41598-021-02515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurent C, Fabiani B, Do C, Tchernonog E, Cartron G, Gravelle P, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients. Haematologica. 2016;101(8):976–984. doi: 10.3324/haematol.2016.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang W, Hong S, Chen N, He X, Zhan J, Qin T, et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget. 2015;6(32):33019–33032. doi: 10.18632/oncotarget.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9(1):109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1):15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing l-asparaginase: efficacy and safety. Ann Hematol. 2018;97(1):193–196. doi: 10.1007/s00277-017-3127-2. [DOI] [PubMed] [Google Scholar]
- 58.Tao R, Fan L, Song Y, Hu Y, Zhang W, Wang Y, et al. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: a multicenter, single-arm, phase 2 trial (ORIENT-4) Signal Transduct Target Ther. 2021;6(1):365. doi: 10.1038/s41392-021-00768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai J, Liu P, Huang H, Li Y, Ma S, Zhou H, et al. Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Signal Transduct Target Ther. 2020;5(1):289. doi: 10.1038/s41392-020-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136(24):2754–2763. doi: 10.1182/blood.2020007247. [DOI] [PubMed] [Google Scholar]
- 61.Huang H, Tao R, Yang Y, Cen H, Zhou H, Guo Y, et al. GEMSTONE-201: preplanned primary analysis of a multicenter, single-arm, phase 2 study of sugemalimab (suge) in patients (pts) with relapsed or refractory extranodal natural killer/T cell lymphoma (R/R ENKTL) J Clin Oncol. 2022;40(16):7501–7501. doi: 10.1200/JCO.2022.40.16_suppl.7501. [DOI] [Google Scholar]
- 62.Huang H, Tao R, Hao S, Yang Y, Cen H, Zhou H, et al. Sugemalimab monotherapy for patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (GEMSTONE-201): results from a single-arm, multicenter, phase II study. J Clin Oncol. 2023;41:3032–3041. doi: 10.1200/JCO.22.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132(11):1146–1158. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao Z, Lv X, Liu S, He Z, Chen S, Wang L, et al. Different aberrant expression pattern of immune checkpoint receptors in patients with PTCL and NK/T-CL. Asia Pac J Clin Oncol. 2018;14(5):e252–e258. doi: 10.1111/ajco.12850. [DOI] [PubMed] [Google Scholar]
- 65.Lin R, Li X, Wu S, Qian S, Hou H, Dong M, et al. Suppression of latent transforming growth factor-β (TGF-β)-binding protein 1 (LTBP1) inhibits natural killer/ T cell lymphoma progression by inactivating the TGF-β/Smad and p38(MAPK) pathways. Exp Cell Res. 2021;407(1):112790. doi: 10.1016/j.yexcr.2021.112790. [DOI] [PubMed] [Google Scholar]
- 66.Dunmire SK, Verghese PS, Balfour HH., Jr Primary Epstein-Barr virus infection. J Clin Virol. 2018;102:84–92. doi: 10.1016/j.jcv.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Fox CP, Haigh TA, Taylor GS, Long HM, Lee SP, Shannon-Lowe C, et al. A novel latent membrane 2 transcript expressed in Epstein-Barr virus-positive NK- and T-cell lymphoproliferative disease encodes a target for cellular immunotherapy. Blood. 2010;116(19):3695–3704. doi: 10.1182/blood-2010-06-292268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, et al. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996;77(10):2137–2149. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 69.Haverkos BM, Coleman C, Gru AA, Pan Z, Brammer J, Rochford R, et al. Emerging insights on the pathogenesis and treatment of extranodal NK/T cell lymphomas (ENKTL) Discov Med. 2017;23(126):189–199. [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L, Zhao Y, Shi H, Ma C, Wei L. LMP1 promotes nasal NK/T-cell lymphoma cell function by eIF4E via NF-κB pathway. Oncol Rep. 2015;34(6):3264–3271. doi: 10.3892/or.2015.4305. [DOI] [PubMed] [Google Scholar]
- 71.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho SG, Kim N, Sohn HJ, Lee SK, Oh ST, Lee HJ, et al. Long-term outcome of extranodal NK/T cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mol Ther. 2015;23(8):1401–1409. doi: 10.1038/mt.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim WS, Oki Y, Kim SJ, Yoon SE, Ardeshna KM, Lin Y, et al. Autologous EBV-specific T cell treatment results in sustained responses in patients with advanced extranodal NK/T lymphoma: results of a multicenter study. Ann Hematol. 2021;100(10):2529–2539. doi: 10.1007/s00277-021-04558-0. [DOI] [PubMed] [Google Scholar]
- 74.Wu H, Zhao C, Gu K, Jiao Y, Hao J, Sun G. Thalidomide plus chemotherapy exhibit enhanced efficacy in the clinical treatment of T-cell non-Hodgkin's lymphoma: a prospective study of 46 cases. Mol Clin Oncol. 2014;2(5):695–700. doi: 10.3892/mco.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Wang ZH, Chen XQ, Wang KF, Huang HQ, Xia ZJ. First-line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: an updated analysis with long-term follow-up. Oncol Lett. 2015;10(2):1036–1040. doi: 10.3892/ol.2015.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L, Liu X, Wang X, Chang Y, Fu X, Li X, et al. Anti-PD-1-antibody (Tislelizumab) combined with deacetylase inhibitor (Chidamide), lenalidomide and etoposide for the treatment of refractory/relapsed extranodal natural killer/T cell lymphoma, Nasal. European Hematology Association 2022, Poster Abstract P1240.
- 77.Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–1312. doi: 10.1002/ajh.26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86. doi: 10.1186/s13045-020-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, et al. B7–H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. 2021;27(5):1227–1235. doi: 10.1158/1078-0432.CCR-20-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong J, Zhao WL. Advances in multiple omics of natural-killer/T cell lymphoma. J Hematol Oncol. 2018;11(1):134. doi: 10.1186/s13045-018-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong J, Cui BW, Wang N, Dai YT, Zhang H, Wang CF, et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020;37(3):403–419.e406. doi: 10.1016/j.ccell.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Awasthi N, Liongue C, Ward AC. STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. J Hematol Oncol. 2021;14(1):198. doi: 10.1186/s13045-021-01214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bouchekioua A, Scourzic L, de Wever O, Zhang Y, Cervera P, Aline-Fardin A, et al. JAK3 deregulation by activating mutations confers invasive growth advantage in extranodal nasal-type natural killer cell lymphoma. Leukemia. 2014;28(2):338–348. doi: 10.1038/leu.2013.157. [DOI] [PubMed] [Google Scholar]
- 85.Koo GC, Tan SY, Tang T, Poon SL, Allen GE, Tan L, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2(7):591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 86.Geng L, Li X, Zhou X, Fang X, Yuan D, Wang X. WP1066 exhibits antitumor efficacy in nasal-type natural killer/T-cell lymphoma cells through downregulation of the STAT3 signaling pathway. Oncol Rep. 2016;36(5):2868–2874. doi: 10.3892/or.2016.5091. [DOI] [PubMed] [Google Scholar]
- 87.Liu J, Liang L, Li D, Nong L, Zheng Y, Huang S, et al. JAK3/STAT3 oncogenic pathway and PRDM1 expression stratify clinicopathologic features of extranodal NK/T-cell lymphoma, nasal type. Oncol Rep. 2019;41(6):3219–3232. doi: 10.3892/or.2019.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sim SH, Kim S, Kim TM, Jeon YK, Nam SJ, Ahn YO, et al. Novel JAK3-activating mutations in extranodal NK/T-cell lymphoma, Nasal type. Am J Pathol. 2017;187(5):980–986. doi: 10.1016/j.ajpath.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Zhu J, Song Y, Zhao W, Zhou K, Wu J, Zhang H, et al. Golidocitinib in treating refractory or relapsed peripheral T-cell lymphoma: primary analysis of the multinational pivotal study results (JACKPOT8) J Clin Oncol. 2023;41(16):7503–7503. [Google Scholar]
- 90.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109(7):2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 91.Huang Y, de Reyniès A, de Leval L, Ghazi B, Martin-Garcia N, Travert M, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115(6):1226–1237. doi: 10.1182/blood-2009-05-221275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X, Wang B, Ma X, Guo Y. NF-kappaB activation through the alternative pathway correlates with chemoresistance and poor survival in extranodal NK/T-cell lymphoma, nasal type. Jpn J Clin Oncol. 2009;39(7):418–424. doi: 10.1093/jjco/hyp037. [DOI] [PubMed] [Google Scholar]
- 93.Ng SB, Selvarajan V, Huang G, Zhou J, Feldman AL, Law M, et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011;223(4):496–510. doi: 10.1002/path.2823. [DOI] [PubMed] [Google Scholar]
- 94.Iwata S, Yano S, Ito Y, Ushijima Y, Gotoh K, Kawada J, et al. Bortezomib induces apoptosis in T lymphoma cells and natural killer lymphoma cells independent of Epstein-Barr virus infection. Int J Cancer. 2011;129(9):2263–2273. doi: 10.1002/ijc.25873. [DOI] [PubMed] [Google Scholar]
- 95.Tang T, Tay K, Tao M, Quek RHH, Farid M, Lim ST. A Phase II study of bortezomib-GIFOX (Gemcitabine, Ifosfamide, Oxaliplatin) in patients with newly diagnosed natural-killer/T-cell lymphoma. Blood. 2016;128(22):5353–5353. doi: 10.1182/blood.V128.22.5353.5353. [DOI] [Google Scholar]
- 96.Granato M, Romeo MA, Tiano MS, Santarelli R, Gonnella R, Gilardini Montani MS, et al. Bortezomib promotes KHSV and EBV lytic cycle by activating JNK and autophagy. Sci Rep. 2017;7(1):13052. doi: 10.1038/s41598-017-13533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404) Leuk Lymphoma. 2014;55(4):768–772. doi: 10.3109/10428194.2013.816700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J, Chen P, Liu W, Xia Z, Shi F, Zhong M. Expression and significance of Ku80 and PDGFR-α in nasal NK/T-cell lymphoma. Pathol Res Pract. 2016;212(3):204–209. doi: 10.1016/j.prp.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Lu L, Fu X, Li Z, Qiu Y, Li W, Zhou Z, et al. Platelet-derived growth factor receptor alpha (PDGFRα) is overexpressed in NK/T-cell lymphoma and mediates cell survival. Biochem Biophys Res Commun. 2018;504(2):525–531. doi: 10.1016/j.bbrc.2018.08.181. [DOI] [PubMed] [Google Scholar]
- 101.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang D, Song TL, Nairismägi ML, Laurensia Y, Pang WL, Zhe DCM, et al. Evaluation of the PIK3 pathway in peripheral T-cell lymphoma and NK/T-cell lymphoma. Br J Haematol. 2020;189(4):731–744. doi: 10.1111/bjh.16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawada J, Ito Y, Iwata S, Suzuki M, Kawano Y, Kanazawa T, et al. mTOR inhibitors induce cell-cycle arrest and inhibit tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Clin Cancer Res. 2014;20(21):5412–5422. doi: 10.1158/1078-0432.CCR-13-3172. [DOI] [PubMed] [Google Scholar]
- 104.Azizian NG, Li Y. XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol. 2020;13(1):61. doi: 10.1186/s13045-020-00903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trkulja KL, Manji F, Kuruvilla J, Laister RC. Nuclear export in non-Hodgkin lymphoma and implications for targeted XPO1 inhibitors. Biomolecules. 2023;13:1. doi: 10.3390/biom13010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–e522. doi: 10.1016/S2352-3026(20)30120-4. [DOI] [PubMed] [Google Scholar]
- 107.Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738. doi: 10.1056/NEJMoa1903455. [DOI] [PubMed] [Google Scholar]
- 108.Tang T, Martin P, Somasundaram N, Lim C, Tao M, Poon E, et al. Phase I study of selinexor in combination with dexamethasone, ifosfamide, carboplatin, etoposide chemotherapy in patients with relapsed or refractory peripheral T-cell or natural-killer/T-cell lymphoma. Haematologica. 2021;106(12):3170–3175. doi: 10.3324/haematol.2020.251454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang H, Gao Y, Zhang H, Zhou K, Wu J, Cai Z, et al. XPO1 inhibitor (ATG-010) plus GemOx regimen for heavily pretreated patients with relapsed or refractory (R/R) T and NK-cell lymphoma: updates of the phase Ib touch study. Blood. 2022;140(Supplement 1):6554–6555. doi: 10.1182/blood-2022-165797. [DOI] [Google Scholar]
- 110.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5(1):42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 111.Farag SS. The potential role of Aurora kinase inhibitors in haematological malignancies. Br J Haematol. 2011;155(5):561–579. doi: 10.1111/j.1365-2141.2011.08898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iqbal J, Weisenburger DD, Chowdhury A, Tsai MY, Srivastava G, Greiner TC, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic γδ T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25(2):348–358. doi: 10.1038/leu.2010.255. [DOI] [PubMed] [Google Scholar]
- 113.Berglund A, Putney RM, Hamaidi I, Kim S. Epigenetic dysregulation of immune-related pathways in cancer: bioinformatics tools and visualization. Exp Mol Med. 2021;53(5):761–771. doi: 10.1038/s12276-021-00612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang P, Zhang M. Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma. Clin Epigen. 2020;12(1):169. doi: 10.1186/s13148-020-00962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dobashi A, Tsuyama N, Asaka R, Togashi Y, Ueda K, Sakata S, et al. Frequent BCOR aberrations in extranodal NK/T-Cell lymphoma, nasal type. Genes Chromosomes Cancer. 2016;55(5):460–471. doi: 10.1002/gcc.22348. [DOI] [PubMed] [Google Scholar]
- 116.Lee S, Park HY, Kang SY, Kim SJ, Hwang J, Lee S, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget. 2015;6(19):17764–17776. doi: 10.18632/oncotarget.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bose P, Dai Y, Grant S. Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther. 2014;143(3):323–336. doi: 10.1016/j.pharmthera.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan G, Huang H-Q, Li P, Bing B, Xiaoxiao W, Qixiang R, et al. Chidamide, oral subtype-selective histone deacetylase inhibitor (HDACI) monotherapy was effective on the patients with relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma. Blood. 2017;130(Supplement 1):2797–2797. [Google Scholar]
- 119.Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69. doi: 10.1186/s13045-017-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang H, Gao Y, Wang X, Bai B, Zhang L, Xiao Y, et al. Sintilimab plus chidamide for relapsed/refractory (R/R) extranodal NK/T cell lymphoma (ENKTL): a prospective, multicenter, single-arm, phase Ib/II trial (SCENT) Hematol Oncol. 2021;39:S2. doi: 10.1002/hon.127_2880. [DOI] [Google Scholar]
- 121.Tan D, Diong CP, Loh Y, Goh YT. Histone deacetylase (HDAC) inhibitors when combined with a proteasome inhibitor are safe and effective in patients with extranodal natural killer/T-cell lymphoma (ENKTL) Ann Oncol. 2016;27(9):1811–1812. doi: 10.1093/annonc/mdw231. [DOI] [PubMed] [Google Scholar]
- 122.Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016;27(3):508–513. doi: 10.1093/annonc/mdv596. [DOI] [PubMed] [Google Scholar]
- 123.Tsai PF, Lin SJ, Weng PL, Tsai SC, Lin JH, Chou YC, et al. Interplay between PKCδ and Sp1 on histone deacetylase inhibitor-mediated Epstein-Barr virus reactivation. J Virol. 2011;85(5):2373–2385. doi: 10.1128/JVI.01602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Küçük C, Hu X, Jiang B, Klinkebiel D, Geng H, Gong Q, et al. Global promoter methylation analysis reveals novel candidate tumor suppressor genes in natural killer cell lymphoma. Clin Cancer Res. 2015;21(7):1699–1711. doi: 10.1158/1078-0432.CCR-14-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu W, Yang Y, Qi S, Wang Y, He X, Zhang L, et al. Treatment, survival, and prognosis of advanced-stage natural killer/T-cell lymphoma: an analysis from the China Lymphoma Collaborative Group. Front Oncol. 2020;10:583050. doi: 10.3389/fonc.2020.583050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai Q, Liu P, Xia Y, Cai J, Huang H, Li Z, et al. Sintilimab plus P-gemox (Pegaspargase, Gemcitabine and Oxaliplatin) regimen for newly-diagnosed advanced, extranodal natural killer/T cell lymphoma, Nasal type: a multicenter, phase 2 cohort of the open-label spirit study. Blood. 2022;140(Supplement 1):2299–2301. doi: 10.1182/blood-2022-168457. [DOI] [Google Scholar]
- 127.Sun P, Li Y, Li C, Ren K, Wang Y, Yang H, et al. A phase II study of sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy as first-line therapy in patients with newly diagnosed, stage I–II extranodal natural-killer/T-cell lymphoma. Am J Hematol. 2023;98(7):1043–1051. doi: 10.1002/ajh.26922. [DOI] [PubMed] [Google Scholar]
- 128.Bachy E, Savage KJ, Huang H, Kwong Y-L, Gritti G, Zhang Q, et al. Tislelizumab, a PD-1 inhibitor for relapsed/refractory mature T/NK-cell neoplasms: Results from a phase 2 study. J Clin Oncol. 2022;40(16):7552–7552. doi: 10.1200/JCO.2022.40.16_suppl.7552. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this study are included in the figures and tables.