Abstract
Harnessing the immunogenic potential of senescent cells may be a viable but context-dependent opportunity to boost anti-tumor immunity.
One-sentence summary:
Harnessing the immunogenic potential of senescent cells may be a viable but context-dependent opportunity to boost anti-tumor immunity.
Tumorigenesis is a multi-step process driven by numerous critical biological activities, including the evasion of immune system-mediated destruction. Tumor cells acquire several mechanisms to escape elimination, foremost the expression of inhibitory immune checkpoint proteins and the modulation of antigen presentation machinery (1). Targeting these tumor-induced immunosuppressive pathways to harness anti-tumor immunity has emerged as a prominent therapeutic strategy against cancer. Such immunotherapies are exceptionally powerful for a subset of tumor types, but they are not effective for most malignancies (1). To design effective new therapeutic interventions, a deeper understanding of the molecular underpinnings underlying anti-tumor immune responses and resistance to those responses by tumors is imperative (1).
Although typically rare in healthy tissue, senescent cells are common in the tumor microenvironment at many, if not all, stages of tumorigenesis. While most of our understanding on the pathophysiological roles of senescent cells in tissues is based on experimental murine models, evidence of senescent cells in human health and diseases is emerging. Several subsets of senescent cells are likely relevant during human and murine tumorigenesis, such as oncogene-induced senescence (caused by activating mutations in oncogenes or loss of tumor suppressors) or therapy-induced senescence (generated during chemotherapeutic interventions or radiation therapy). Based on their tumor-promoting property, senescent cells were recently recognized as a ‘Hallmark of Cancer’ (2). Recent studies have explored in greater detail how senescent cells modulate anti-tumor immune responses. Chen et al. (3), Marin et al. (4), and Shabandi et al. (5) uncovered that senescent tumor cells are proficient in inducing antigen presentation machinery. By increasing sensitivity to interferon signaling, senescent tumor cells enlist innate and adaptive immune cells to support tumor regression (3, 4). Conversely, senescent cells also engage powerful immunosuppressive mechanisms to inhibit anti-cancer immune responses (5), highlighting the complex and dual role senescent cells play.
Senescent tumor cells heat up the tumor microenvironment
Although the phenotype and function of senescent cells can vary substantially, their core features encompass a durable cell cycle arrest and a bioactive secretome that typically includes immune-modulatory components. This senescence-associated secretory phenotype plays disease-relevant but context-dependent roles (2) and is thought to promote immunosurveillance of senescent cells. For example, murine hepatocytes induced to senesce through oncogenic KRASG12V undergo cell cycle arrest, produce a secretome that prompts macrophage and T cell engagement, and are eventually eliminated by cytotoxic CD8+ T cells (6). Similarly, p53 restoration in established murine hepatocellular carcinoma causes senescence and tumor regression that is dependent on various immune cells (7). Considering the immunogenic potential of senescent cells demonstrated by these and other studies, a deeper understanding of how the immune system clears or ignores senescent tumor cells may facilitate the development of additional therapeutic strategies for cancer.
Two recent complementary studies described important features by which senescent cancer cells become visible to immune cells (3, 4). In a liver tumor mouse model that allows for p53 suppression or restoration, induction of p53-mediated senescence modestly decreased the proportions of myeloid-derived suppressor cells and neutrophils while simultaneously increasing macrophage and lymphocyte numbers, indicating a switch from an immune ‘cold’ to an immune ‘hot’ microenvironment (3). Senescent cells underwent major remodeling in their cell surface proteome, which had at least two important consequences. First, senescent cells became hyper-sensitive to type II interferon (IFN-γ) signaling due to induction of the IFN-γ receptor, IFNGR1. Secondly, senescent cells augmented the expression of major histocompatibility complex (MHC) class I molecules, which was further boosted by IFN-γ signaling, to heighten their antigen presentation capacity (3, 4). Interestingly, antigen processing of senescent tumor cells may also differ from non-senescent counterparts. Accordingly, IFNGR1 and CD8+ T cells were required for tumor regression after p53-mediated senescence induction (3). Building on the immunogenic potential of senescent cells, Marin et al. (4) explored whether pre-treatment of senescent tumor cells could protect mice against tumor development. Provided evidence suggests that senescent tumor cells prime the tissue or immune system towards tumor control, providing a moderate effect in slowing tumor growth.
Collectively, these studies illuminate how senescent tumor cells control their clearance versus persistence, which appears to be, at least in part, dictated by the integrity of IFN-γ signaling. Additional studies are needed to understand the full spectrum of senescent cell functions in immunity and anti-cancer therapy. Such emphasis is expected to unravel additional senescent cell-mediated pathways, both beneficial and detrimental, to modulate various parts of the immune system during tumorigenesis.
Fighting over the thermostat
If senescent cells express potent features facilitating T cell engagement and anti-tumor immunity, why then are some tumors with a high fraction of senescent cells not being eradicated? A recent study set out to answer this question using p53WT breast tumors (5). The wildtype status of p53 is thought to be a culprit underlying the overall poor survival of affected individuals. Chemotherapy in such tumors activates a p53-dependent senescence response that prevents mitotic catastrophe. Cells in this scenario harbor typical senescence traits, including desirable immunogenic features to potentially facilitate immune-mediated tumor elimination. Additionally, they induce a secretome containing immune-modulatory factors, upregulate antigen presentation machinery, and express CD137L, OX40L, and other co-stimulatory receptors that support T cell activation and effector function. However, these tumors are not eliminated by T cells. One important reason is that tumor cells after chemotherapy concomitantly express potent immuno-suppressive signals such as checkpoint proteins programmed cell death ligand 1 (PD-L1) and CD80. Upon engagement with their respective receptors on T cells, programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4), these proteins prohibit T cell activity, expansion, and cytotoxic activities. The unfortunate outcome that results is no anti-tumor T cell activity and, eventually, tumor relapse.
Evasion of senescent cells from immune-mediated clearance has been widely described beyond the cancer context. In fact, immune evasion has been postulated to be the major cause underlying senescent cell accumulation in chronic conditions including aging or disease (8, 9). Recently, induction of checkpoint proteins, PD-L1 or PD-L2, on senescent cells have been causally implicated in senescent cell immunosurveillance (8, 9), suggesting that T cell inhibition is a conserved feature of chronically present senescent cells. Considering that checkpoint inhibition has made tremendous progress in cancer immunotherapy, these findings may present an opportunity to target detrimental senescent cells in a variety of settings.
Interestingly, PD-L1 and CD80 were predominantly found to be mutually exclusively expressed in murine p53WT mammary tumor cells after chemotherapy (5), suggesting that senescent tumor cell subsets with divergent properties can be generated. One subset induced PD-L1 due to IFN-γ sensitivity, whereas the CD80-expressing subset depended on p53 signaling. Chemotherapy followed by immune checkpoint blockade targeting PD-L1 or CD80 with antibodies provided a substantial benefit over chemotherapy alone in a murine mammary tumor transplant model. T cells, especially CD8+ effector T cells, were able to infiltrate tumors, resulting in a consistent delay in tumor relapse, with a subset of tumors never relapsing. However, most tumors eventually did relapse, even under conditions of combinatorial immune checkpoint blockade of both PD-L1–PD-1 and CD80–CTLA-4 pathways. These observations suggest additional signals, either senescent cell-dependent or -independent, that enable tumor cell evasion and relapse of p53WT tumors.
From cold to colder
The above and other studies highlight a potent crosstalk between senescent cells and T cells that contribute to shaping tumorigenesis and the tumor microenvironment. Whereas immediate-early senescent cell responses facilitate T cell recruitment and clearance of neoplastic cells (3, 4, 6), senescent tumor cells may acquire T cell-inhibitory properties at later stages after senescence induction (5, 8, 9). Beyond time-dependent kinetics as a variable, additional complexity can be expected based on the cell or tumor type, mode of chemotherapy and senescence induction, and potentially sex- and species-related dependencies.
Consequently, a holistic view of cellular senescence is required to allow careful consideration when senescent cells may be beneficial during anti-tumor therapies. In this regard, it is important to add that several lines of evidence indicate additional immunosuppressive, pro-tumorigenic properties of pre-neoplastic senescent cells that are also proficient in attracting various myeloid cell types. Although senescent cell-recruited monocytes and macrophages appear initially beneficial to encourage tumor cell clearance (6, 7, 10), senescent cells can also skew immature myeloid cells towards suppressive phenotypes (3, 8, 10). This property appears dependent on the senescent cell burden in the tumor microenvironment and context. Still, such suppressive myeloid subsets are proficient in inhibiting not only T cell infiltration and expansion but also natural killer cell-mediated cytotoxicity. Collectively, this favors a tumor-permissive environment that allows tumor growth and progression (8, 10).
Concluding considerations
Cellular senescence is a complex cell fate that can substantially vary in its phenotypic and functional properties. Causes and consequences of senescent cell heterogeneity are becoming increasingly apparent, as are their pathophysiological effects in aging and diseases. Similarly, the fields of anti-tumor immunity and tumor evolution harbor considerable complexities as well, causing the intersection of cellular senescence, immunity and, tumor initiation and progression to be exceptionally convoluted. Although much additional effort is required to translate discoveries into human cancer therapy, it has become increasingly apparent that senescent cells have an immunogenic potential that may be leveraged to elicit anti-tumor immunity and potentially complement anti-cancer therapies. However, this potential will need to be carefully wielded to avoid the immune-suppressive, pro-tumorigenic capacities of senescent cells.
Figure 1. Senescent tumor cells modulate anti-tumor immunity.
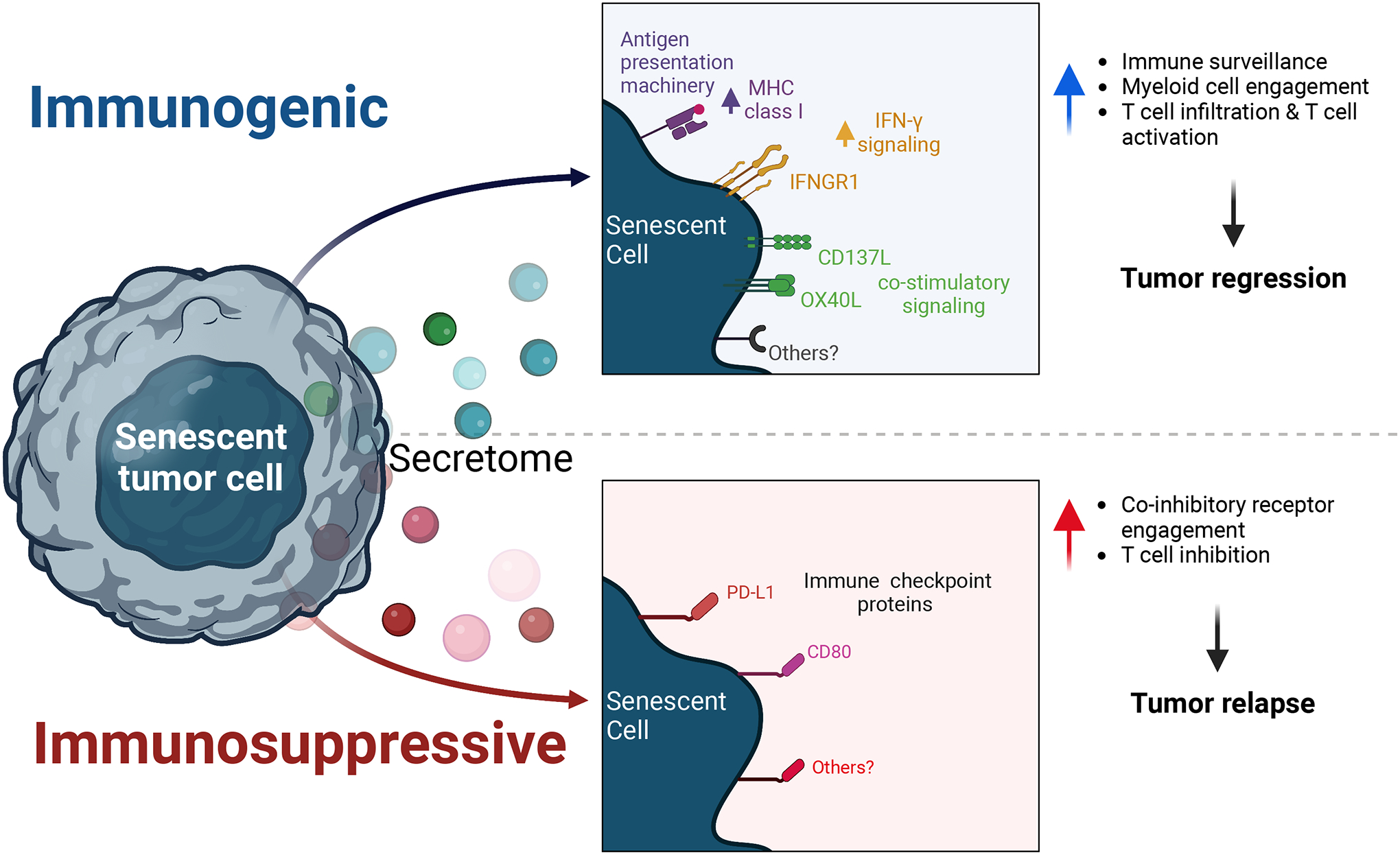
Senescent tumor cells play dual and opposing roles in cancer. The acute induction of senescence remodels their cell surface proteome to express antigen presentation machinery and costimulatory molecules. This increases immune cell engagement and can facilitate immune-mediated tumor cell killing. However, in some cancers, subsets of senescent tumor cells express immune checkpoint proteins such as PD-L1 and CD80; these engage co-inhibitory receptors on immune cells and may inhibit anti-tumor immunity.
Funding:
This work was supported by National Institutes of Health grant R01AG053229 (to DJB), National Institutes of Health grant R01AG068076 (to DJB), National Institutes of Health grant R01AG045779 (to JJG), National Institutes of Health grant T32AG049672 (to IS), and the Glenn Foundation for Medical Research (to DJB and IS). This research was conducted while IS was a Glenn Foundation for Medical Research Postdoctoral Fellow.
Footnotes
Competing interests:
D.J.B. and I.S. have a potential financial interest related to this research. D.J.B. and I.S are co-inventors on patents held by Mayo Clinic. D.J.B is a co-inventor on patent applications licensed to or filed by Unity Biotechnology and a Unity Biotechnology shareholder. Research in the Baker laboratory has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies. J.J.G. is a scientific advisor and consultant for Retro Biosciences. L.I.P. reports no competing interests.
References and Notes:
- 1.Sharma P, Allison JP, Immune checkpoint therapy: Forging ahead. Sci Transl Med 14, eadf2947 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Hallmarks of Cancer: New Dimensions. Cancer Discov 12, 31–46 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Chen HA, Ho YJ, Mezzadra R, Adrover JM, Smolkin R, Zhu C, Woess K, Bernstein N, Schmitt G, Fong L, Luan W, Wuest A, Tian S, Li X, Broderick C, Hendrickson RC, Egeblad M, Chen Z, Alonso-Curbelo D, Lowe SW, Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov 13, 432–453 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini CS, Prats N, Lopez-Dominguez JA, Kovatcheva M, Garralda E, Munoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M, Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov 13, 410–431 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahbandi A, Chiu FY, Ungerleider NA, Kvadas R, Mheidly Z, Sun MJS, Tian D, Waizman DA, Anderson AY, Machado HL, Pursell ZF, Rao SG, Jackson JG, Breast cancer cells survive chemotherapy by activating targetable immune-modulatory programs characterized by PD-L1 or CD80. Nat Cancer 3, 1513–1533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturmlechner I, Zhang C, Sine CC, van Deursen EJ, Jeganathan KB, Hamada N, Grasic J, Friedman D, Stutchman JT, Can I, Hamada M, Lim DY, Lee JH, Ordog T, Laberge RM, Shapiro V, Baker DJ, Li H, van Deursen JM, p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 374, eabb3420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW, Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaib S, López-Domínguez JA, Lalinde M, Prats N, Marin I, Meyer K, Muñoz MI, Aguilera M, Mateo L, Attolini CS, Llanos S, Pérez-Ramos S, Escorihuela M, Al-Shahrour F, Cash TP, Tchkonia T, Kirkland JL, Arribas J, Serrano M, The efficacy of chemotherapy is limited by intratumoural senescent cells that persist through the upregulation of PD-L2. bioRxiv, (2022). [Google Scholar]
- 9.Wang TW, Johmura Y, Suzuki N, Omori S, Migita T, Yamaguchi K, Hatakeyama S, Yamazaki S, Shimizu E, Imoto S, Furukawa Y, Yoshimura A, Nakanishi M, Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, Heikenwalder M, Wang XW, Zender L, Greten TF, Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 30, 533–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


