Abstract
Long-beaked common dolphin (Delphinus delphis bairdii) distribution is limited to the Eastern North Pacific Ocean. Its whistle repertoire is poorly investigated, with no studies in the Gulf of California. The aim of the present study is to characterize the whistles of this species and compare their parameters with different populations. Acoustic monitoring was conducted in La Paz Bay, Gulf of California. Recordings were inspected in spectrogram view in Raven Pro, selecting good quality whistles (n = 270). In the software Luscinia, contours were manually traced to obtain whistle frequencies and duration. Number of steps, inflection points and contour type were visually determined. We calculated the descriptive statistics of the selected whistle parameters and we compared the results with a dolphins population from the Eastern Pacific Ocean. Permutational multivariate analysis of variance (PERMANOVA) was performed to test the intraspecific variation of the whistle parameters among groups. In the present study the mean values (±SD) of the whistle parameters were: maximum frequency = 14.13 ± 3.71 kHz, minimum frequency = 8.44 ± 2.58 kHz and duration = 0.44 ± 0.31 s. Whistles with the upsweep contour were the most common ones (34.44%). The coefficient of variation (CV) values for modulation parameters were high (>100%), in accordance with other studies on dolphins. Whistle parameters showed significant differences among groups. Finally, ending and maximum frequencies, duration and inflection points of the whistles recorded in the present study were lower compared with the parameters of the long-beaked common dolphins from the Eastern Pacific Ocean. This study provides the first whistle characterization of long-beaked common dolphin from the Gulf of California and it will help future passive acoustic monitoring applications in the study area.
Keywords: Bioacoustics, Dolphins, Mexico, Gulf of California, Vocal repertoire
Introduction
In the Mexican Pacific, two subspecies of common dolphins are recognized, the short-beaked common dolphin (Delphinus delphis delphis) with a cosmopolitan distribution; and the long-beaked common dolphin (Delphinus delphis bairdii) with a distribution limited to the Eastern North Pacific (ENP) Ocean (Braulik, Jefferson & Bearzi, 2021; Carretta, Chivers & Perryman, 2011), including the Gulf of California (Urbán et al., 2005). The taxonomy of the common dolphin (Delphinus sp.) has been historically troubled. Cunha et al. (2015) noted that because the sympatric/parapatric long-beaked and short-beaked common dolphins off California may not interbreed, the ENP long-beaked common dolphins might be recognized as separate species D. d. bairdii (Dall, 1873). However, the molecular analysis of the common dolphins from the ENP (Rosel, Dizon & Heyning, 1994) did not include populations from the contiguous southern regions such as the eastern tropical Pacific and the eastern South Pacific. A complete global review and revision of the common dolphins is still pending and nowadays the Committee of Taxonomy of the Society for Marine Mammalogy (CTSMM, 2023) considers provisionally the long-beaked common dolphins distributed in the ENP as a subspecies D. delphis bairdii following the suggestion of Hershkovitz (1966).
Dolphins live in fission–fusion societies and use whistles to communicate during social interactions (Au & Hastings, 2008; Madsen et al., 2012). Whistles can vary in frequency, typically between 1 and 35 kHz (May-Collado & Wartzok, 2008; Richardson et al., 2013). Whistle parameters of dolphins showed intra and interspecific differences and they can be used to classify and distinguish species and populations (Ansmann et al., 2007; Azevedo & Van Sluys, 2005; Morisaka et al., 2005; Rendell et al., 1999). While studies investigating the whistle repertoire of short-beaked common dolphins are relatively abundant (Ansmann et al., 2007; Azzolin et al., 2021; Fearey et al., 2019; Gannier et al., 2020; Gannier et al., 2010; Pagliani et al., 2022; Papale et al., 2014; Petrella et al., 2012), only few focused on the acoustics of long-beaked common dolphins (Caldwell & Caldwell, 1968; Oswald, Barlow & Norris, 2003; Oswald et al., 2007; Oswald et al., 2021a; Oswald et al., 2021b), of which none in the Gulf of California. Indeed, in the study area the investigations on this species have mainly been isotopic analyses and population studies (Aurioles-Gamboa et al., 2013; Niño Torres et al., 2006; Vidal & Gallo-Reynoso, 2012). A study focused on the acoustics of the long-beaked common dolphin will allow for comparisons with other species inhabiting the same area, and will help their acoustic identification using passive acoustic monitoring in the future.
Here we present the first whistle characterization of long-beaked common dolphins from the Gulf of California. The aim of this study is to describe frequencies, duration and modulation parameters of the whistles of long-beaked common dolphins encountered in La Paz Bay and to compare our results with a different population inhabiting the Eastern Pacific Ocean.
Materials & Methods
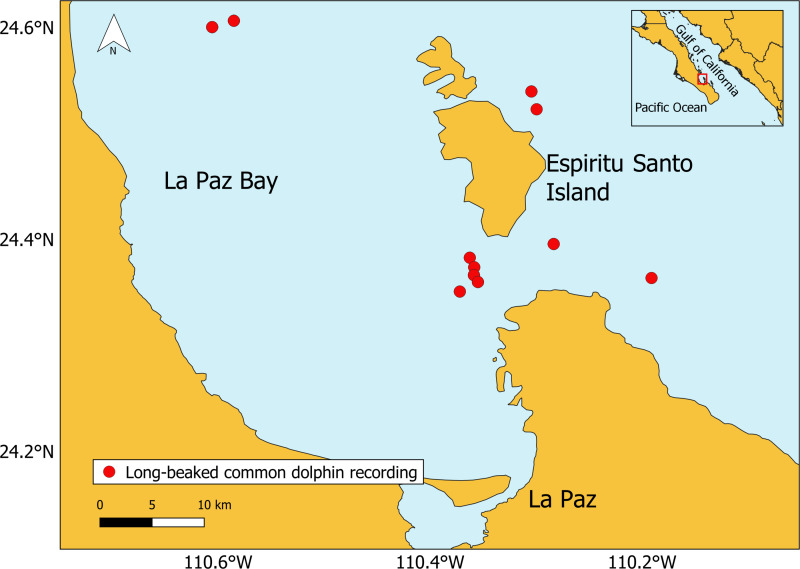
Data were collected in La Paz Bay (Fig. 1), Gulf of California, Mexico, between November 2020 and September 2021. Surveys were conducted in daylight hours during calm sea conditions (Beaufort scale ≤ 2), with a motorized research vessel (7.3 m long, 75 HP). Groups of long-beaked common dolphins were recorded with the engine off, using Reson TC4013.1 omnidirectional hydrophone (sensitivity −211 dB Rms ± 3dB re 1 V/µPa, 1 Hz to 170 kHz) connected to a preamplifier Reson VP2000 Voltage EC6081 (50 dB gain, 500 Hz high-pass filter, 50 kHz low-pass filter). A Marantz PMD661 (data format 24-bits WAV, sampling rate 96 kHz) was used for recording. For each recording session the predominant behavior of the group (displayed by more than 50% of the dolphins) was recorded using continuous scan sampling method (Altmann, 1974; Mann, 1999). The behavior was categorized into five behavioral state categories based on dolphin ethograms (Baker et al., 2017; Bearzi, 1994; Heiler et al., 2016) (Data S1). All behaviors were mutually exclusive. The study was fully observational following the “Guidelines for the treatment of marine mammals in field research” supported by the Society for Marine Mammalogy (Gales et al., 2009). No ethical permit was required by the competent bodies. To ensure consistency of the data collection, the same trained researcher collected the observational data throughout all the study.
Figure 1. Location of the long-beaked common dolphin recordings (generated with QGIS, version 3.6.3).
The base map shapefile of Mexico is provided by Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO).
The recording effort was 1 h and 34 min from a total of 5 groups of long-beaked common dolphins recorded (Table 1). No other dolphin species were present during the recordings. A total of 270 good quality whistles (non-overlapped and clearly visible in the spectrogram) were firstly selected in Raven pro (version 1.5 Cornell University, Laboratory of Ornithology, New York) and then whistle frequencies and duration were extracted using Luscinia software (version 2.16.10.29.01) (Lachlan, 2007) as previously described in Antichi et al. (2022b). In addition, number of steps, inflection points, and contour, were visually determined. A step was considered as a period of constant frequency between two periods of the same frequency modulation (i.e., two periods of rising or two periods of falling frequency) (Ansmann et al., 2007; Petrella et al., 2012). Inflection points were defined as shift from falling to rising or rising to falling contour slope (Ansmann et al., 2007; Petrella et al., 2012; Pires et al., 2021). Moreover, each whistle contour was classified into six categories following Ansmann et al. (2007) (Fig. 2).
Table 1. Recording effort of the study.
Only good quality whistles (non-overlapped with the contour clearly visible in the spectrogram) were considered. To avoid pseudo-replication, whistles with identical time-frequency contours were considered only once.
| Dolphin group | Date (dd/mm/yyyy) | No recordings | Recording effort (min) | Group size | No whistles |
|---|---|---|---|---|---|
| I | 18/11/2020 | 2 | 20 | 150 | 30 |
| II | 09/12/2020 | 2 | 16 | 250 | 14 |
| III | 25/04/2021 | 1 | 10 | 300 | 10 |
| IV | 25/04/2021 | 1 | 10 | 80 | 3 |
| V | 07/09/2021 | 5 | 38 | 140 | 213 |
Figure 2. Spectrogram examples of the six whistle contours considered during the study.
(A) upsweep, (B) downsweep, (C) convex, (D) concave, (E) constant frequency, (F) sine (1,024 points FFT, Hann window, 50% overlap).
A chi-squared test was used to investigate the relative frequency of occurrence of the six contour forms. Due to the non-normal distribution of the whistle parameters (Shapiro–Wilks, p < 0.05) non-parametric tests were used for any further analyses. Permutational multivariate analysis of variance (PERMANOVA) was conducted to test if the whistle parameters varied among dolphin groups. Variables were transformed to y = ln(y+1) to reduce differences in scale among the variables. P values for all PERMANOVA tests were calculated based on Euclidean distances using 999 permutations to estimate the probability of group differences. Multilevel pairwise post hoc tests (Martinez Arbizu, 2020) with Bonferroni adjustment were performed to calculate differences between pairs of groups. One-sample Wilcoxon test was used to compare the whistle parameters of long-beaked common dolphins collected in our study with the ones recorded in Eastern Pacific Ocean by Oswald et al. (2007). Statistical tests were performed in R software (version 4.2.1; R Core Team, 2022) with RStudio interface (Version 2022.12.0; RStudio Team, 2022).
Results
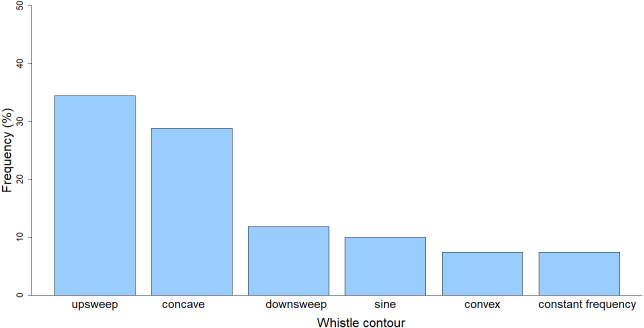
Descriptive statistics (maximum, minimum, mean, standard deviation, coefficient of variation) of the whistle parameters were calculated (Table 2). The majority of the whistles (n = 260; 96%) were recorded while the dolphins were “traveling” (the group was moving in a consistent direction with regular surfacing intervals) while only during one encounter (n = 10; 4%) the predominant behavior was “feeding” (the group was pursuing prey, sometimes with deep dives). Out of the six whistle contour categories, the most common type of contour was upsweep (n = 93; 34.44%), followed by concave (n = 78; 28.89%), downsweep (n = 32; 11.85%), sine (n = 27; 10%), convex (n = 20; 7.41%), and constant frequency (n = 20; 7.41%) (χ2 = 114.13, df. = 5, p < 0.001) (Fig. 3).
Table 2. Whistle parameters comparison between long-beaked common dolphin from the Gulf of California and from the Eastern Pacific Ocean.
| Whistle parameter | Present study (n = 270) | Oswald et al. (2007) (n = 174) | |||
|---|---|---|---|---|---|
| Min | Max | Mean ± SD | CV | Mean ± SD | |
| Duration (s)* | 0.05 | 2.17 | 0.44 ± 0.31 | 70.45% | 0.62 ± 0.34 |
| Maximum frequency (kHz)* | 3.52 | 26.12 | 14.13 ± 3.71 | 26.26% | 16.21 ± 4.94 |
| Minimum frequency (kHz) | 2.62 | 18.47 | 8.44 ± 2.58 | 30.57% | 8.48 ± 2.70 |
| Frequency range (kHz) | 0.43 | 19.93 | 5.69 ± 3.40 | 59.75% | – |
| Starting frequency (kHz) | 2.62 | 26.12 | 10.81 ± 4.25 | 39.32% | 10.87 ± 4.89 |
| Ending frequency (kHz)* | 3.28 | 21.58 | 12.50 ± 3.78 | 30.24% | 14.46 ± 5.12 |
| Peak frequency (kHz) | 3.00 | 20.55 | 10.46 ± 2.62 | 25.05% | – |
| No. of inflection points* | 0.00 | 8.00 | 0.92 ± 0.99 | 107.61% | 1.59 ± 3.29 |
| No. of steps | 0.00 | 4.00 | 0.40 ± 0.68 | 170.00% | – |
Notes.
Significantly different parameters between the two long-beaked common dolphin populations (One-sample Wilcoxon test, p <0.001).
Figure 3. Frequency of whistle contours of the long-beaked common dolphin from the Gulf of California.
Whistle parameters showed significant differences among groups (F = 4.762, df = 4, p = 0.001). Five pairs of groups showed significant differences in whistle parameters (Table 3). The group IV showed no differences with the other groups. The whistles recorded in the present study showed lower ending frequency (V = 8, 582, p < 0.001), maximum frequency (V = 7, 786, p <0.001), duration (V = 7, 142, p < 0.001) and inflection points (V = 3, 378, p < 0.001) than the whistles from Oswald et al. (2007). No differences were found for starting frequency (V = 16, 607, p = 0.09476) and minimum frequency (V = 16, 921, p = 0.1429) (Table 2).
Table 3. Multilevel post hoc pairwise tests with Bonferroni adjustment of the whistle parameters between group pairs.
| Group pairs | R 2 | p | padjusted |
|---|---|---|---|
| I vs II | 0.023576317 | 0.402 | 1.00 |
| I vs IV | 0.057779319 | 0.112 | 1.00 |
| I vs III | 0.143076262 | 0.001 | 0.01* |
| I vs V | 0.037041288 | 0.001 | 0.01* |
| II vs IV | 0.125803616 | 0.067 | 0.67 |
| II vs III | 0.220604483 | 0.001 | 0.01* |
| II vs V | 0.022609014 | 0.003 | 0.03* |
| IV vs III | 0.132934612 | 0.153 | 1.00 |
| IV vs V | 0.003419664 | 0.524 | 1.00 |
| III vs V | 0.020883659 | 0.004 | 0.04* |
Notes.
Significant differences between group pairs (p adjusted <0.05).
Discussion
This study represents the first whistle characterization of long-beaked common dolphin from the Gulf of California. Indeed, in the study area only the acoustic behavior of the common bottlenose dolphin (Tursiops truncatus) was studied (Antichi et al., 2023; Antichi et al., 2022a; Antichi et al., 2022b; Gauger, Caraveo-Patiño & Romero-Vivas, 2021; Gauger et al., 2022). Group size of the groups encountered ranged between 80 and 300 individuals with a mean of 184 dolphins. This is in accordance with the study of Oswald et al. (2021b) that also encountered large groups of long-beaked common dolphins, superior to 100 individuals. The whistles of the recorded long-beaked common dolphin ranged from 2.62 kHz to 26.12 kHz, with durations from 0.05 s to 2.17 s. In the present study, the coefficients of variation (CV) of the modulation parameters (inflection points and steps) were greater than 100%. This result indicates high variability in the modulation patterns of the whistles, and might be the consequence of either social or ecological factors or even a combination of both (Azevedo & Van Sluys, 2005; Azzolin et al., 2013; Bazúa-Durán & Au, 2004; May-Collado & Wartzok, 2008; Rendell et al., 1999). The high CV values for modulation parameters and low CV values for frequencies and duration has been previously reported, not only in Delphinus sp. (Azzolin et al., 2021; Panova, Agafonov & Logominova, 2021; Papale et al., 2014) but also in other dolphin species (Azevedo & Van Sluys, 2005; La Manna et al., 2020; Oswald, Barlow & Norris, 2003; Pires et al., 2021; Wang et al., 2013).
In regard to the contours, the upsweep category was the most commonly recorded. This result seems to agree with other studies that found upsweep to be the most common contour in common dolphins, always accounting for around 30% of the whistle composition (Ansmann et al., 2007; Pagliani et al., 2022). However, Petrella et al. (2012) reported downsweep whistles as the most common ones, followed closely by the upsweep contour. The biological meaning of the different contour categories still needs to be fully understood (Bazúa-Durán & Au, 2002) but it seems to be associated with different behaviors (Díaz López, 2011; Hawkins & Gartside, 2010; Kuczaj et al., 2015). Upsweep whistle type was found to be highly associated with social behavior in common bottlenose dolphins (Díaz López, 2011). In Australia, Indo-Pacific bottlenose dolphins (Tursiops aduncus) showed high correlation between upsweep whistles and socializing, while sine whistle type appeared to be used for group contact call and was more associated with traveling behaviors (Hawkins & Gartside, 2009; Hawkins & Gartside, 2010). In the present study the majority of the whistles were recorded while the dolphins were traveling. This result might help to disclose the relationship between the traveling behavior and the upsweep contour for this species in the study area. Additional behavioral studies are needed to better associate the different whistle contours to behaviors.
The intraspecific variation of whistle parameters among the recorded groups could be due to the fluid society structure in which this species lives (Tyack, 1986; Bazúa-Durán & Au, 2002; May-Collado & Wartzok, 2008). This variability could represent the ability of dolphins to adapt their whistles to constant changes in their biotic and abiotic environment. It has been previously found that greater variation in whistle repertoire can be expected in dolphins that live in fluid societies (Tyack, 1986; Tyack, 2000). The result could be due to the difference in number of whistles analyzed for each group. A more homogeneous number of whistles per group is needed to better assess the possible intraspecific variation of the whistle parameters.
The whistles recorded in the present study seem to differ from the ones collected by Oswald et al. (2007). Specifically, the whistles of the long-beaked common dolphins from La Paz Bay showed lower ending frequency, maximum frequency, duration and inflection points compared to the ones recorded by Oswald et al. (2007). Whistle variation has been previously reported at interspecific (Oswald et al., 2007; Papale et al., 2021; Steiner, 1981) and intraspecific level, for common dolphins (Ansmann et al., 2007; Azzolin et al., 2021; Gannier et al., 2010; Papale et al., 2014; Petrella et al., 2012) and other dolphin species (Akkaya et al., 2023; Azzolin et al., 2013; Luís et al., 2021; May-Collado & Wartzok, 2008; Yuan et al., 2021). The high plasticity of dolphin whistles can be attributed to many factors, including geographical variability, behavioral state, general environment, group size, social context and individual variability (Bazúa-Durán & Au, 2002; Bazúa-Durán & Au, 2004; Camargo Jr et al., 2006; La Manna et al., 2020; May-Collado & Wartzok, 2008; May-Collado, 2010).
Characterizing the whistle parameters of the long-beaked common dolphin, together with the whistles of the oceanic ecotype of common bottlenose dolphin in the study area (Antichi et al., 2023), will eventually assist the acoustic identification of these two species that share the same oceanic habitat, using passive acoustic monitoring. The whistles of the long-beaked common dolphins from this study and the oceanic common bottlenose dolphins (Antichi et al., 2023) appear to be, after a preliminary analysis, distinguishable from each other based on the lower duration and frequency range of the former. Additional studies would help to effectively differentiate the two species, especially when combining the differences between whistle frequency parameters and whistle contours.
Conclusions
This study presents the first whistle characterization of long-beaked common dolphins from the Gulf of California, bringing us closer to filling the knowledge gap of this poorly studied species, while also providing important information for future studies about its taxonomic status. Whistles produced by long-beaked common dolphins from the Gulf of California differ from the ones recorded in the Eastern Pacific Ocean. Future comparison of the whistle parameters (duration, frequencies and modulation) and contours between the long-beaked common dolphins and the oceanic ecotype of common bottlenose dolphins in the study area may enable their acoustic identification through passive acoustic monitoring. The identification of this species would be crucial to design more focused conservation actions.
Supplemental Information
Acknowledgments
We would like to thank Citlali Cuevas, Mario Márquez Segovia and Minerva Valerio Conchas for their support in the data collection. A special thanks to Maia Austin and Laura May-Collado for their support with the Luscinia software.
Funding Statement
This research was part of the PhD program of Simone Antichi conducted at the UABCS (Universidad Autónoma de Baja California Sur). The PhD scholarship to Simone Antichi is provided by the Consejo Nacional de Ciencia y Tecnología (CONACyT) of Mexico (Grant number 759498). The fieldwork activities were financed by internal funds of the UABCS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Óscar Carlón-Beltrán performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Lorena Viloria-Gómora conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Jorge Urbán R. conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Sergio Martínez-Aguilar conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Simone Antichi conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The research was conducted with the permit issued by the Secretariat of Environment and Natural Resources of Mexico (SEMARNAT) (Permit SGPA/DGVS/00657/21.).
Data Availability
The following information was supplied regarding data availability:
The dolphin ethogram, the raw data and the script used for the analysis are available in the Supplementary Files.
References
- Akkaya et al. (2023).Akkaya A, Awbery T, Medcalf K, Lyne P, Cipriano G, Alvarenga M, Israpilova L, Atalan Y, Eikelenboom O, Ricci P, Crugliano R, Papale E, Fanizza C, Carlucci R. Initial results on the variation of whistle characteristics of bottlenose dolphins from two neighbouring regions of the Mediterranean Sea: Northern Ionian and Southern Adriatic Sea. Frontiers in Marine Science. 2023;10:1–15. doi: 10.3389/fmars.2023.1099576. [DOI] [Google Scholar]
- Altmann (1974).Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–266. doi: 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- Ansmann et al. (2007).Ansmann IC, Goold JC, Evans PG, Simmonds M, Keith SG. Variation in the whistle characteristics of short-beaked common dolphins, Delphinus delphis, at two locations around the British Isles. Journal of the Marine Biological Association of the United Kingdom. 2007;87:19–26. doi: 10.1017/S0025315407054963. [DOI] [Google Scholar]
- Antichi et al. (2023).Antichi S, Austin M, May-Collado LJ, Urbán RJ, Martínez-Aguilar S, Viloria-Gómora L. Differences in the whistles of two ecotypes of bottlenose dolphins from the Gulf of California. JASA Express Letters. 2023;3:051201. doi: 10.1121/10.0019502. [DOI] [PubMed] [Google Scholar]
- Antichi et al. (2022a).Antichi S, Jaramillo-Legorreta AM, Urbán RJ, Martínez-Aguilar S, Viloria-Gómora L. Small vessel impact on the whistle parameters of two ecotypes of common bottlenose dolphin (Tursiops truncatus) in La Paz Bay, Mexico. Diversity. 2022a;14:712–724. doi: 10.3390/d14090712. [DOI] [Google Scholar]
- Antichi et al. (2022b).Antichi S, Urbán J, Martínez-Aguilar S, Viloria-Gómora L. Changes in whistle parameters of two common bottlenose dolphin ecotypes as a result of the physical presence of the research vessel. PeerJ. 2022b;10:e14074. doi: 10.7717/peerj.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au & Hastings (2008).Au WW, Hastings MC. Principles of marine bioacoustics. Springer; New York: 2008. [Google Scholar]
- Aurioles-Gamboa et al. (2013).Aurioles-Gamboa D, Rodríguez-Pérez MY, Sánchez-Velasco L, Lavín MF. Habitat, trophic level, and residence of marine mammals in the Gulf of California assessed by stable isotope analysis. Marine Ecology Progress Series. 2013;488:275–290. doi: 10.3354/meps10369. [DOI] [Google Scholar]
- Azevedo & Van Sluys (2005).Azevedo AF, Van Sluys M. Whistles of tucuxi dolphins (Sotalia fluviatilis) in Brazil: comparisons among populations. The Journal of the Acoustical Society of America. 2005;117:1456–1464. doi: 10.1121/1.1859232. [DOI] [PubMed] [Google Scholar]
- Azzolin et al. (2021).Azzolin M, Gannier A, Papale E, Buscaino G, Mussi B, Ardizzone G, Giacoma C, Pace DS. Whistle variability of the Mediterranean short beak common dolphin. Aquatic Conservation: Marine and Freshwater Ecosystems. 2021;31:36–50. doi: 10.1002/aqc.3168. [DOI] [Google Scholar]
- Azzolin et al. (2013).Azzolin M, Papale E, Lammers MO, Gannier A, Giacoma C. Geographic variation of whistles of the striped dolphin (Stenella coeruleoalba) within the Mediterranean Sea. The Journal of the Acoustical Society of America. 2013;134:694–705. doi: 10.1121/1.4808329. [DOI] [PubMed] [Google Scholar]
- Baker et al. (2017).Baker I, O’Brien J, McHugh K, Berrow S. An ethogram for bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Aquatic Mammals. 2017;43:594–613. doi: 10.1578/AM.43.6.2017.594. [DOI] [Google Scholar]
- Bazúa-Durán & Au (2002).Bazúa-Durán C, Au WW. The whistles of Hawaiian spinner dolphins. The Journal of the Acoustical Society of America. 2002;112:3064–3072. doi: 10.1121/1.1508785. [DOI] [PubMed] [Google Scholar]
- Bazúa-Durán & Au (2004).Bazúa-Durán C, Au WW. Geographic variations in the whistles of spinner dolphins (Stenella longirostris) of the Main Hawaiıan Islands. The Journal of the Acoustical Society of America. 2004;116:3757–3769. doi: 10.1121/1.1785672. [DOI] [PubMed] [Google Scholar]
- Bearzi (1994).Bearzi G. Behavioural states: terminology and definitions. In: Notarbartolo di Sciara G, Evanse E Politi PGH, editors. Methods for the study of bottlenose dolphins in the wild. European Cetacean Society Newsletter. Vol. 23. 1994. pp. 9–12. [Google Scholar]
- Braulik, Jefferson & Bearzi (2021).Braulik G, Jefferson T, Bearzi G. Delphinus delphis. The IUCN Red List of Threatened Species 2021: e. T134817215A50352620 2021.
- Caldwell & Caldwell (1968).Caldwell MC, Caldwell DK. Vocalization of naive captive dolphins in small groups. Science. 1968;159:1121–1123. doi: 10.1126/science.159.3819.1121. [DOI] [PubMed] [Google Scholar]
- Camargo Jr et al. (2006).Camargo Jr FS, Rollo MM, Giampaoli V, Bellini C. Whistle variability in South Atlantic spinner dolphins from the Fernando de Noronha Archipelago off Brazil. The Journal of the Acoustical Society of America. 2006;120:4071–4079. doi: 10.1121/1.2359704. [DOI] [PubMed] [Google Scholar]
- Carretta, Chivers & Perryman (2011).Carretta JV, Chivers SJ, Perryman WL. Abundance of the long-beaked common dolphin (Delphinus capensis) in California and western Baja California waters estimated from a 2009 ship-based line-transect survey. Bulletin, Southern California Academy of Sciences. 2011;110:152–165. doi: 10.3160/0038-3872-110.3.152. [DOI] [Google Scholar]
- CTSMM (2023).CTSMM . List of marine mammal species and subspecies. Society for Marine Mammalogy; 2023. . http://www.marinemammalscience.org. [Google Scholar]
- Cunha et al. (2015).Cunha HA, de Castro RL, Secchi ER, Crespo EA, Lailson-Brito J, Azevedo AF, Lazoski C, Solé-Cava AM. Molecular and morphological differentiation of common dolphins (Delphinus sp.) in the Southwestern Atlantic: testing the two species hypothesis in sympatry. PLOS ONE. 2015;10:e0140251. doi: 10.1371/journal.pone.0140251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz López (2011).Díaz López B. Whistle characteristics in free-ranging bottlenose dolphins (Tursiops truncatus) in the Mediterranean Sea: Influence of behaviour. Mammalian Biology. 2011;76:180–189. doi: 10.1016/j.mambio.2010.06.006. [DOI] [Google Scholar]
- Fearey et al. (2019).Fearey J, Elwen SH, James B, Gridley T. Identification of potential signature whistles from free-ranging common dolphins (Delphinus delphis) in South Africa. Animal cognition. 2019;22:777–789. doi: 10.1007/s10071-019-01274-1. [DOI] [PubMed] [Google Scholar]
- Gales et al. (2009).Gales N, Bowen W, Johnston D, Kovacs K, Littnan C, Perrin W, Reynolds III J, Thompson P. Guidelines for the treatment of marine mammals in field research. Marine Mammal Science. 2009;25:725–736. doi: 10.1111/j.1748-7692.2008.00279.x. [DOI] [Google Scholar]
- Gannier et al. (2020).Gannier A, Fuchs S, Gannier A, Fernandez M, Azevedo JM. Dolphin whistle repertoires around São Miguel (Azores): are you common or spotted? Applied Acoustics. 2020;161:107169. doi: 10.1016/j.apacoust.2019.107169. [DOI] [Google Scholar]
- Gannier et al. (2010).Gannier A, Fuchs S, Quèbre P, Oswald JN. Performance of a contour-based classification method for whistles of Mediterranean delphinids. Applied Acoustics. 2010;71:1063–1069. doi: 10.1016/j.apacoust.2010.05.019. [DOI] [Google Scholar]
- Gauger, Caraveo-Patiño & Romero-Vivas (2021).Gauger MF, Caraveo-Patiño J, Romero-Vivas E. Passive acoustic monitoring of the bottlenose dolphin Tursiops truncatus to determine continuous presence in Ensenada de La Paz, Mexico. Revista de Biología Marina y Oceanografía. 2021;55:238–249. doi: 10.22370/rbmo.2020.55.3.2588. [DOI] [Google Scholar]
- Gauger et al. (2022).Gauger MF, Romero-Vivas E, Peck MA, Balart EF, Caraveo-Patiño J. Seasonal and diel influences on bottlenose dolphin acoustic detection determined by whistles in a coastal lagoon in the southwestern Gulf of California. PeerJ. 2022;10:e13246. doi: 10.7717/peerj.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins & Gartside (2009).Hawkins ER, Gartside DF. Patterns of whistles emitted by wild indo-pacific bottlenose dolphins (Tursiops aduncus) during a provisioning program. Aquatic Mammals. 2009;35:171–186. [Google Scholar]
- Hawkins & Gartside (2010).Hawkins ER, Gartside DF. Whistle emissions of Indo-Pacific bottlenose dolphins (Tursiops aduncus) differ with group composition and surface behaviors. The Journal of the Acoustical Society of America. 2010;127:2652–2663. doi: 10.1121/1.3308465. [DOI] [PubMed] [Google Scholar]
- Heiler et al. (2016).Heiler J, Elwen SH, Kriesell H, Gridley T. Changes in bottlenose dolphin whistle parameters related to vessel presence, surface behaviour and group composition. Animal Behaviour. 2016;117:167–177. doi: 10.1016/j.anbehav.2016.04.014. [DOI] [Google Scholar]
- Hershkovitz (1966).Hershkovitz P. Catalog of living whales. Bulletin of the United States National Museum. 1966;246:1–259. [Google Scholar]
- Kuczaj et al. (2015).Kuczaj SA, Frick EE, Jones BL, Lea JS, Beecham D, Schnöller F. Underwater observations of dolphin reactions to a distressed conspecific. Learning & Behavior. 2015;43:289–300. doi: 10.3758/s13420-015-0179-9. [DOI] [PubMed] [Google Scholar]
- Lachlan (2007).Lachlan R. Luscinia: a bioacoustics analysis computer program. 2007. https://luscinia.sourceforge.net/ https://luscinia.sourceforge.net/
- La Manna et al. (2020).La Manna G, Rako-Gospić N, Sarà G, Gatti F, Bonizzoni S, Ceccherelli G. Whistle variation in Mediterranean common bottlenose dolphin: the role of geographical, anthropogenic, social, and behavioral factors. Ecology and Evolution. 2020;10:1971–1987. doi: 10.1002/ece3.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luís et al. (2021).Luís A, May-Collado L, Rako-Gospić N, Gridley T, Papale E, Azevedo A, Silva M, Buscaino G, Herzing D, Dos Santos M. Vocal universals and geographic variations in the acoustic repertoire of the common bottlenose dolphin. Scientific Reports. 2021;11:1–9. doi: 10.1038/s41598-021-90710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen et al. (2012).Madsen PT, Jensen FH, Carder D, Ridgway S. Dolphin whistles: a functional misnomer revealed by heliox breathing. Biology Letters. 2012;8:211–213. doi: 10.1098/rsbl.2011.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann (1999).Mann J. Behavioral sampling methods for cetaceans: a review and critique. Marine Mammal Science. 1999;15:102–122. doi: 10.1111/j.1748-7692.1999.tb00784.x. [DOI] [Google Scholar]
- Martinez Arbizu (2020).Martinez Arbizu P. 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. https://rdrr.io/github/gauravsk/ranacapa/
- May-Collado (2010).May-Collado LJ. Changes in whistle structure of two dolphin species during interspecific associations. Ethology. 2010;116:1065–1074. doi: 10.1111/j.1439-0310.2010.01828.x. [DOI] [Google Scholar]
- May-Collado & Wartzok (2008).May-Collado LJ, Wartzok D. A comparison of bottlenose dolphin whistles in the Atlantic Ocean: factors promoting whistle variation. Journal of Mammalogy. 2008;89:1229–1240. doi: 10.1644/07-MAMM-A-310.1. [DOI] [Google Scholar]
- Morisaka et al. (2005).Morisaka T, Shinohara M, Nakahara F, Akamatsu T. Geographic variations in the whistles among three Indo-Pacific bottlenose dolphin Tursiops aduncus populations in Japan. Fisheries Science. 2005;71:568–576. doi: 10.1111/j.1444-2906.2005.01001.x. [DOI] [Google Scholar]
- Niño Torres et al. (2006).Niño Torres CA, Gallo-Reynoso JP, Galván-Magaña F, Escobar-Briones E, Macko SA. Isotopic analysis of δ13C, δ15N, and δ34S a feeding tale in teeth of the longbeaked common dolphin, Delphinus capensis. Marine Mammal Science. 2006;22:831–846. doi: 10.1111/j.1748-7692.2006.00065.x. [DOI] [Google Scholar]
- Oswald, Barlow & Norris (2003).Oswald JN, Barlow J, Norris TF. Acoustic identification of nine delphinid species in the eastern tropical Pacific Ocean. Marine Mammal Science. 2003;19:20–037. doi: 10.1111/j.1748-7692.2003.tb01090.x. [DOI] [Google Scholar]
- Oswald et al. (2007).Oswald JN, Rankin S, Barlow J, Lammers MO. A tool for real-time acoustic species identification of delphinid whistles. The Journal of the Acoustical Society of America. 2007;122:587–595. doi: 10.1121/1.2743157. [DOI] [PubMed] [Google Scholar]
- Oswald et al. (2021a).Oswald JN, Walmsley SF, Casey C, Fregosi S, Southall B, Janik VM. Oscillatory whistles—the ups and downs of identifying species in passive acoustic recordings. The Journal of the Acoustical Society of America. 2021a;149:A40–A40. [Google Scholar]
- Oswald et al. (2021b).Oswald JN, Walmsley SF, Casey C, Fregosi S, Southall B, Janik VM. Species information in whistle frequency modulation patterns of common dolphins. Philosophical Transactions of the Royal Society B: Biological Sciences. 2021b;376:20210046. doi: 10.1098/rstb.2021.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliani et al. (2022).Pagliani B, Amorim TO, De Castro FR, Andriolo A. Intraspecific variation in short-beaked common dolphin’s whistle repertoire. Bioacoustics. 2022;31:1–16. doi: 10.1080/09524622.2020.1858449. [DOI] [Google Scholar]
- Panova, Agafonov & Logominova (2021).Panova E, Agafonov A, Logominova I. First description of whistles of Black Sea short-beaked common dolphins, Delphinus delphis ponticus. Bioacoustics. 2021;30:662–679. doi: 10.1080/09524622.2020.1842245. [DOI] [Google Scholar]
- Papale et al. (2014).Papale E, Azzolin M, Cascão I, Gannier A, Lammers M, Martin V, Oswald J, Perez-Gil M, Prieto R, Silva M. Macro-and micro-geographic variation of short-beaked common dolphin’s whistles in the Mediterranean Sea and Atlantic Ocean. Ethology Ecology & Evolution. 2014;26:392–404. doi: 10.1080/03949370.2013.851122. [DOI] [Google Scholar]
- Papale et al. (2021).Papale EB, Azzolin MA, Cascão I, Gannier A, Lammers MO, Martin VM, Oswald JN, Perez-Gil M, Prieto R, Silva MA. Dolphin whistles can be useful tools in identifying units of conservation. BMC Zoology. 2021;6:1–13. doi: 10.1186/s40850-021-00065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella et al. (2012).Petrella V, Martinez E, Anderson MG, Stockin KA. Whistle characteristics of common dolphins (Delphinus sp.) in the Hauraki Gulf, New Zealand. Marine Mammal Science. 2012;28:479–496. doi: 10.1111/j.1748-7692.2011.00499.x. [DOI] [Google Scholar]
- Pires et al. (2021).Pires CR, Rossi-Santos MR, Paro AD, Wedekin LL. Whistles of the pantropical spotted dolphin (Stenella attenuata) in Santos Basin, western South Atlantic Ocean. The Journal of the Acoustical Society of America. 2021;149:3241–3249. doi: 10.1121/10.0004950. [DOI] [PubMed] [Google Scholar]
- R Core Team (2022).R Core Team . Version 4.2.1. Vienna: R Foundation for Statistical Computing; 2022. R: A language and environment for statistical computing. [Google Scholar]
- Rendell et al. (1999).Rendell L, Matthews J, Gill A, Gordon J, Macdonald D. Quantitative analysis of tonal calls from five odontocete species, examining interspecific and intraspecific variation. Journal of Zoology. 1999;249:403–410. doi: 10.1111/j.1469-7998.1999.tb01209.x. [DOI] [Google Scholar]
- Richardson et al. (2013).Richardson WJ, Greene Jr CR, Malme CI, Thomson DH. Marine mammals and noise. Academic Press; San Diego, CA: 2013. [Google Scholar]
- Rosel, Dizon & Heyning (1994).Rosel P, Dizon A, Heyning J. Genetic analysis of sympatric morphotypes of common dolphins (genus Delphinus) Marine Biology. 1994;119:159–167. doi: 10.1007/BF00349552. [DOI] [Google Scholar]
- RStudio Team (2022).RStudio Team . Version 2022.12.0. Boston: RStudio, Inc; 2022. RStudio: integrated development for R. [Google Scholar]
- Steiner (1981).Steiner WW. Species-specific differences in pure tonal whistle vocalizations of five western North Atlantic dolphin species. Behavioral Ecology and Sociobiology. 1981;9:241–246. doi: 10.1007/BF00299878. [DOI] [Google Scholar]
- Tyack (1986).Tyack P. Population biology, social behavior and communication in whales and dolphins. Trends in Ecology & Evolution. 1986;1:144–150. doi: 10.1016/0169-5347(86)90042-X. [DOI] [PubMed] [Google Scholar]
- Tyack (2000).Tyack PL. Cetacean societies: field studies of dolphins and whales. The University of Chicago Press; Chicago, IL: 2000. Functional aspects of cetacean communication; pp. 270–307. [Google Scholar]
- Urbán et al. (2005).Urbán R, Rojas-Bracho L, Guerrero-Ruiz M, Jaramillo-Legorreta A, Findley LT. Biodiversity, ecosystems, and conservation in Northern Mexico. Oxford University Press; New York, USA: 2005. Cetacean diversity and conservation in the Gulf of California; pp. 276–297. [Google Scholar]
- Vidal & Gallo-Reynoso (2012).Vidal O, Gallo-Reynoso JP. Composition by sex and size of long-beaked common dolphin (Delphinus capensis) from a die-off in the Gulf of California, México. Marine Biodiversity Records. 2012;5:e82. doi: 10.1017/S1755267212000395. [DOI] [Google Scholar]
- Wang et al. (2013).Wang Z, Fang L, Shi W, Wang K, Wang D. Whistle characteristics of free-ranging Indo-Pacific humpback dolphins (Sousa chinensis) in Sanniang Bay, China. The Journal of the Acoustical Society of America. 2013;133:2479–2489. doi: 10.1121/1.4794390. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2021).Yuan J, Wang Z, Duan P, Xiao Y, Zhang H, Huang Z, Zhou R, Wen H, Wang K, Wang D. Whistle signal variations among three Indo-Pacific humpback dolphin populations in the South China Sea: a combined effect of the Qiongzhou Strait’s geographical barrier function and local ambient noise? Integrative Zoology. 2021;16:499–511. doi: 10.1111/1749-4877.12531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Martinez Arbizu P. 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. https://rdrr.io/github/gauravsk/ranacapa/
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The dolphin ethogram, the raw data and the script used for the analysis are available in the Supplementary Files.