Abstract
Background
The COVID-19 pandemic continues, and this condition has caused many cases in various countries around the world, resulting in more than 6 million deaths worldwide. Herbal medicines can act as immunomodulators, anti-inflammatories, antioxidants, antimicrobials, and others depending on the type and content of the herbs used. Previous studies have shown that several types of herbs, such as Echinacea purpurea, Curcumin or Turmeric, Nigella sativa, and Zingiber officinale, have proven their effectiveness as herbal plants for COVID-19.
Methods
We conducted a comprehensive literature search through five databases, namely, PubMed, Scopus, Embase, Wiley, and ProQuest to assess the efficacy of phytopharmaceuticals until July 12, 2022. We used the Cochrane RoB 2.0 for the quality assessment of the study.
Results
Phytopharmaceuticals significantly improved patients’ recovery rate (OR = 3.54; p < 0.00001) and reduced deaths (OR = 0.24; p < 0.0001) compared to the control group. Phytopharmaceuticals also performed as a protective factor for COVID-19 clinical symptoms, such as dyspnea (OR = 0.42; p < 0.05) and myalgia (OR = 0.31; p = 0.02) compared to the control group. However, there is no statistically significant effect on cough (OR = 0.76; p = 0.61) and fever (OR = 0.60; p < 0.20). The results were not affected by patients’ covariates [hypertension, diabetes mellitus, and cardiovascular diseases (meta-regression p > 0.05)].
Conclusion
Herbal medicine has the potential as an adjuvant therapy in the management of COVID-19.
Keywords: adjuvant therapy, COVID-19, herbal medicine, systematic review
Introduction
Coronavirus disease 2019 (COVID-19), a highly infectious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a catastrophic impact on the world’s population, resulting in over 6.9 million deaths as recorded on April 26, 2023.1 The paradox of SARS-CoV-2 transmission lies in the uncertainty about its mode of transmission with droplet versus airborne transmission continuing to be a topic of debate.2 Although the contact and droplet routes are still being advocated as the main modes by leading public health agencies, the emerging evidence of viable SARS-CoV-2 in the absence of aerosol-generating procedures suggests airborne transmission as an important mode of transmission.2,3 This had only been recognized for aerosol-generating procedures within healthcare settings. However, as aerosols are generated from activities such as exhalation, speaking, coughing, and sneezing, it is vital to recognize the indoor and outdoor airborne transmission.2,4
SARS-CoV-2 attacks lower respiratory tract, so the symptoms can be mild symptoms such as fever, cough, or fatigue, or moderate to severe such as difficulty breathing, chest pain, or severe hypoxia that can develop into acute respiratory distress syndrome (ARDS), total organ failure, even death.5 The infectious process can result in the activation of immune system and secretion of pro-inflammatory mediators, known as cytokine storms.5,6 Therefore, changes in the immune response in a person with COVID-19 have a vital role in modulating and protecting the individual’s self.5,7 Antiviral drugs, such as remdesivir, tocilizumab, dexamethasone, and several nutritional supplements or natural products with immunomodulatory properties as potential adjuvant therapies, are included in efforts to stop the spread of the virus.8
Herbal medicines as complementary therapy can potentially act as immunomodulator, anti-inflammatory, antioxidant, antimicrobial, and others, depending on the type and content.9 Herbs can be processed and consumed in many different ways such as whole herbs in the original form, teas, syrups, essential oils, ointments, salves, rubs, and even in capsules or tablets containing powdered or dry herbal extracts.10 These might be effective solutions to deal COVID-19. Besides, complementary therapies are parts of health-related practices with usage history outside of standard biomedical practice.11 In Indonesia, the availability of herbal medicines is vast, varied, and has received positive responses from various circles of society. The healthcare professional, particularly nurse, faces a challenge to understand on the efficacy of herbal medicine as adjunct therapy for COVID-19 treatment.
Nurses together with pharmacist and physician play a vital role as part and parcel of responsible healthcare provider team in ensuring that complementary therapies should be included as part of holistic care. The healthcare providers should recognize in holistic care that it requires not only physical but also included psychosocial and spiritual aspect. Hence, the nurse as well as pharmacist should collaborate together in promoting complementary therapy as part of their patient-centered care. Indirectly they will be as an advocator for the patient rather than patient taking it without guidance which may produce harm to theirself.12
Researchers and scientific organizations started to consider the use of herbal medicine as the treatment of COVID-19 and has been widely used in relieving symptoms, enhancing laboratory markers, and raising the clinical cure rate.13–17 Unfortunately, to date, no systematic review has been accompanied by a meta-regression to assess the effectiveness of herbal medicines as adjuvants in managing COVID-19 and the covariate factors. Therefore, this systematic review, meta-analysis, and meta-regression aimed to assess the effectiveness of herbal medicines as potentially adjuvant solid therapies to be included in the management of COVID-19.
Methods
Study Design
This systematic review and the meta-analysis were conducted according to the Cochrane Handbook for Systematic Reviews of Intervention, following the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) framework.18
Literature Search
A comprehensive literature search was conducted through PubMed, Scopus, Embase, Wiley, and ProQuest to assess the efficacy studies of herbal medicines in the management of COVID-19 therapy in Indonesia, until July 12, 2022, using the search keywords: (COVID-19 OR SARS-CoV-2) AND (Herbal medicine OR Medicinal plant OR phytopharmaca OR Phytomedicine). Search details for each database can be seen in Supplementary file 1.
Eligibility Criteria
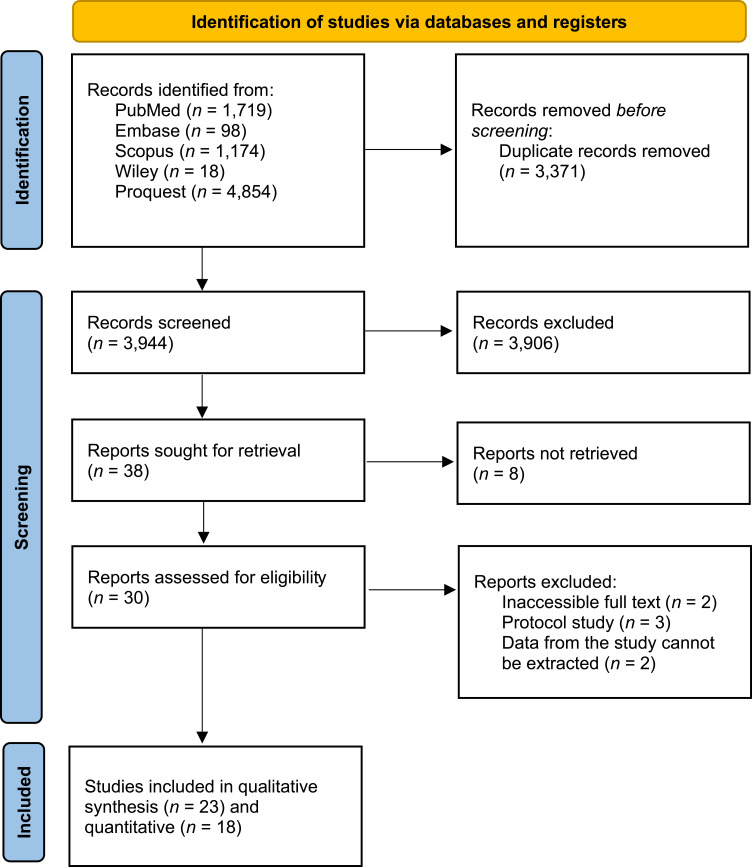
In terms of study feasibility, study inclusion criteria include (1) Type of study, randomized control trials; (2) Study population, patients with COVID-19; (3) Intervention, herbal medicine including Echinacea purpurea, Curcumin, Turmeric, Nigella sativa, and Zingiber officinale; (4) Study outcomes, recovery, death, and improvement in clinical symptoms of COVID-19 patients; (5) Accompanied by control. Meanwhile, the exclusion criteria included (1) Studies that were not completed at the time of the search; (2) Studies that cannot be accessed by full paper; (3) Studies using other languages, other than English and Indonesian. In addition, duplicate removal is performed using Microsoft Office Excel. Screening of titles and abstracts from studies was performed according to accessibility criteria by two independent authors (SA and MFA). Any differences are discussed into a consensus. The planned procedure is illustrated in Figure 1.
Figure 1.
PRISMA flow diagram.
Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. Creative Commons.18
Data Extraction
We extracted the included studies in tabular form to include data on (1) Author and year of publication; (2) Study characteristics, including study locations; (3) Study population, including number of intervention and control samples, number of males, and the average age; (4) Intervention, including herbal medicine name, content, dose, and frequency of intervention; (5) Study outcomes, recovery, death, and improvement of clinical symptoms in COVID-19 patients.
Quality Assessment
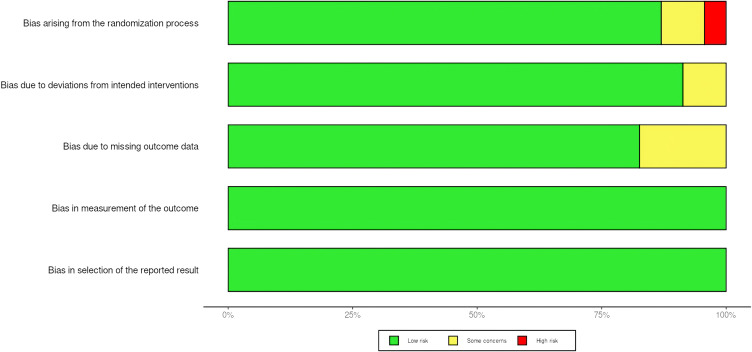
We also assessed the quality of each study using the Cochrane Revised Risk of Bias (ROB 2.0) for randomized controlled trials. This evaluated 5 domains including randomization bias, bias due to deviation from the intended intervention, missing outcome data, outcome measures, and bias in reporting outcomes. A study is classified as good quality if the research meets the requirements as good research converted through the AHRQ standard. Assessment was carried out by two independent authors, and disagreement will be discussed between the two authors. It is important to note that we did exclude studies with low quality in our review. The study quality assessment can be seen in Figure 2.
Figure 2.
Summary risk of bias of included studies.
Statistical Analysis
Data analysis was performed using Review Manager 5.4 (Cochrane Collaboration, Oxford, UK). The clinical outcome of dichotomous data is reported as an odd ratio (OR). An appropriate 95% confidence interval (95% CI) was calculated, and it was assumed that the significant p-value was less than 5% (p < 0.05). Statistical heterogeneity was calculated using the I2 method (<25% considered low heterogeneity, 25–50% moderate heterogeneity, and >50% high heterogeneity). If high heterogeneity is found in the meta-analysis, additional analysis will be carried out using the random effects model described by DerSimonian and Laird, which provides a more conservative view of the analysis. For all meta-analyses, data will be presented in the forest plots. Moreover, we also conducted a restricted-maximum likelihood random-effects meta-regression to examine the impact of the following covariates: diabetes mellitus (DM), hypertension, coronary artery disease/cardiovascular disease (CAD/CVD).
Results
Characteristic of Included Studies
Twenty-three randomized controlled trials with a total of 3516 COVID-19 patients in the intervention and control groups were included in both qualitative and quantitative analyses.19–41 This study was carried out in various countries, of which 10 studies came from Iran, 7 from China, 4 from India, 1 from Saudi Arabia, and 1 from Vietnam. All studies were published in the 2020–2022 timeframe, precisely during the COVID-19 pandemic. The characteristics of the included studies can be seen in Table 1.
Table 1.
Characteristics of Included Studies
| Author | Location | Samples (Male/ Woman) | Age Mean (SD) | Covariate | Control | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current Smoking (%) | Diabetes (%) | Ht (%) | CVD (%) | Obes (%) | COPD (%) | |||||||
| Name of Intervention and Frequency | Contents | |||||||||||
| Hu K, et al (2020)26 | China | IG: 142 (79/63) CG: 142 (71/71) |
IG: 50.4 (15.2) CG: 51.8 (14.8) |
N/A | N/A | N/A | N/A | N/A | N/A | Treatment according to The Protocol for Diagnosis and Treatment of Novel Coronavirus Pneumonia (4th edition) | LH capsules (Lianhuaqingwen), 4 capsules, 3 times a day, for 14 days | Forsythia suspensa; Lonicera japonica; Ephedra sinica;Isatis indigotica; Pogostemon cablin; Rheum palmatum; Glycyrrhiza uralensis; Dryopteris; crassirhizoma; Rhodiola crenulata; Houttuynia cordata; Prunus sibirica; gypsum and 1-menthol |
| Valizadeh, et al (2020)27 | Iran | IG: 20 (15/5) CG: 20 (16/4) |
IG: 53.3 (8.4) CG: 51.4 (7.9) |
17.5 | 7.5 | 5 | 12.5 | N/A | N/A | Placebo Capsules Betaferon 300 g subcutaneously for 5 days Bromhexine 8 mg tablet every 8 hours Atorvastatin 40 mg daily |
160 mg Nano-curcumin capsules as much as 40 mg/capsule daily for 14 days; Betaferon 300 g subcutaneously for 5 days; Bromhexine 8 mg tablet every 8 hours; Atorvastatin 40 mg daily | Nano-curcumin |
| Koshak AE, et al (2021)28 | Saudi Arabia | IG: 91 (48/43) CG: 92 (49/43) |
IG: 35 (10) CG: 36 (12) |
11 | 8 | 9 | 0.5 | N/A | 4 | Treatment according to The Protocol for Diagnosis and Treatment of Novel Coronavirus Pneumonia (4th edition) | Applicable standard treatment + Nigella sativa orally 500 mg twice daily for 10 days | Nigella sativa |
| Karimi, et al (2021)25 | Iran | IG: 184 (106/76) CG: 174 (91/85) |
IG: 48.72 (14.86) CG: 50.79 (15.87) |
N/A | N/A | N/A | N/A | N/A | N/A | Receive routine interventions according to the instructions of the Iranian Ministry of Health (2020) such as azithromycin, hydroxychloroquine, KALETRA®(lopinavir/ritonavir) | Receive herbal medicine and routine intervention for 7 days | Rheum palmatum L.; Glycyrrhiza glabra L.; Punica granatum L.;Nigella sativa; Matricaria chamomilla; Zataria multiflora Boiss.; G. glabra; Ziziphus jujubamill.; Ficus carica; Urtica dioica; Althaea officinalis; Nepeta bracteata |
| Xiao M, et al (2020)29 | China | IG: 95 (47/48) CG: 94 (50/44) |
IG: 54.31 (11.63) CG: 54.06 (13.90) |
14.8 | 6.6 | 18.1 | 3.3 | N/A | 1.6 | Antiviral with oral oseltamivir (75 mg per tablet) one tablet per day; Arbidol (100 mg per tablet) orally, 2 tablets, 3 times daily; Ribavirin (100 mg per tablet) orally, one and a half tablets 3 times daily; Antimicrobial therapy | Huoxiang Zhengqi twice a day and Lianhua Qingwen three times a day + control group medication for 14 days | Pericarpium Arecae; Radix Angelicae; Dahuricae; Perillae; Poria; Rhizoma Pinelliae; Rhizoma Atractylodis; Macrocephalae; Pericarpium Citri; Reticulatae; Cortex Magnoliae Officinalis; Radix Platycodonis; Herba Pogostemonis; Radix Glycyrrhizae |
| Thakar, et al (2022)24 | India | IG: 41 (26/15) CG: 39 (27/12) |
IG: 40 (12.9) CG: 35.31 (11.68) |
N/A | N/A | N/A | N/A | N/A | N/A | Applicable standard treatment | 3 g AYUSH 64 orally per day divided into 3 appropriate doses after each meal for 14 days | Picrorhiza kurroa Royle ex Benth.; Caesalpinia bonduc (L.) Roxb.; Swertia chirata Buch. Ham. ex Wall.; Alstonia scholaris (L.) R. Br. |
| Zhou S, et al (2021)23 | China | I: 54 (38/16) C: 57 (33/24) |
66.0 (IQR 56.0–72.0) | N/A | N/A | N/A | N/A | N/A | N/A | Applicable standard treatment | Shenhuang Granule 2 times a day for 14 days | Panax ginseng C. A. Mey; Rheum palmatum L.; Sargentodoxa cuneata; Taraxacum mongolicum; Aconiti Lateralis Radix Praeparata; Whitmania pigra Whitman |
| Zhang X-Y, et al (2021)22 | China | I: 65 (32/33) C: 65 (28/37) |
I: 44.31 (13.45) C: 48.25 (14.22) |
N/A | 7.7 | 16.2 | N/A | N/A | N/A | Applicable standard treatment | Xiyanping injeksi 10mg/kg once a day for 14 days | A. paniculate |
| Chen, et al (2021)21 | China | I: 64 (31/33) C: 65 (29/36) |
I: 54.16 (12.11) C: 52.51 (12.31) |
N/A | N/A | N/A | 19.3 | N/A | 2.3 | Placebo capsule with oral dose of 1.4 g concurrently with rehabilitation therapy | Bufei Huoxue Capsule | Psoralen; Astragalus; Red peony; paeoniflori; iso-psoralen; verbasil glucoside; pentagalloyl glucose; verbasil isoflavones; tonic osteostatin; isopsorale |
| Devpura G, et al (2021)30 | India | I: 45 (35/10) C: 50 (42/8) |
I: 33.4 (9.4) C: 35.4 (10.4) |
N/A | 1 | 1 | N/A | N/A | N/A | Placebo | Ayurvedic twice a day for 7 days | Tinospora cordifolia; Withania somnifera; Ocimum sanctum |
| Xu, et al (2021)41 | China | I: 77 (43/34) C: 80 (44/36) |
I: 49.1 (15.7) C: 50.4 (16.0) |
N/A | N/A | N/A | N/A | N/A | N/A | Applicable standard treatment | Reduning injection | Artemisia annua; Lonicera japonica Thunb; Gardenia jasminoides Ellis. |
| Loc, et al (2022)40 | Vietnam | I: 34 (18/ 16) C: 35 (14/ 21) |
I: 34 C: 35 |
N/A | N/A | N/A | N/A | N/A | N/A | Placebo 3 tablets 3 times a day for 14 days | Oral Kovir capsules | Bupleurum chinense DC.; Poria cocos (Schw.) Wolf.; Codonopsis pilosula (Franch.) Nannf.; Peucedanum decursivum Maxim; (or)Peucedanum praeruptorum Dunn.; Glycyrrhiza uralensis Fisch. or; Glycyrrhiza glabra L.; Platycodon; grandiflorum (Jacq.) A. DC.; Ligusticum wallichii Franch.; Citrus aurantium L. |
| Mesri, et al (2021)39 | Iran | I: 50 (31/19) C: 50 (21/29) |
I: 47.1 (15.53) C: 45.46 (13.46) |
N/A | N/A | N/A | N/A | N/A | N/A | Standard medication (Hydroxychloroquine) | Herbal medicine + standard medicine | Zingiber officinale; Echinacea herbal medicines |
| Liu J (2021)32 | China | I: 99 (36/63) C: 96 (37/59) |
I: 56.00 (48.50–62.00) (IQR) C: 56.50 (48.75–62.25) (IQR) |
N/A | 13.8 | 60 | 5.1 | N/A | 2 | Standard treatment for 14 days | Q-14 10 g twice a day for 14 days | Ephedra Herba, Amygdalus Communis Vas, Gypsum Fibrosum, licorice, Pogostemon Cablin (Blanco) Benth., Magnolia Officinalis Rehd Et Wils, Atractylodes Lancea (Thunb.)Dc., Amomum Tsao-Ko Crevostet, Pinelliae rhizoma preparata, Poria Cocos(Schw.) Wolf., Radix Rhei Et Rhizome, Hedysarum Multijugum Maxim. |
| Fazlju, et al (2022)38 | Iran | I: Polium: 267 (140/ 127) - Hyssop: 231 (112/ 119) C: 252 (137/115) |
I: - Polium: 36.4 (17,4) - Hyssop: 36.2 (19,6) C: 37.2 (18,1) |
Polium: 18% Hyssop: 11.3% |
N/A | N/A | N/A | N/A | N/A | Placebo | Hyssop and Polyum as much as 5 (for under 12 years) or 10 cc (for over 12 years) per day after breakfast for 20 days | POLIUM: Teucrium polium L.; Cuscuta epithymum Murr;Cichorium intybus L Hyssop: Hyssopus officinalis L.; Echium amoenum Fisch and C.A. Mey; Glycyrrhiza glabra L. |
| Hasheminasab, et al (2022)37 | Iran | I: 35 (14/ 18) C: 35 (17/ 15) |
I: 51.49 (11,61) C: 53.28 (13,22) |
N/A | N/A | N/A | N/A | N/A | N/A | Conventional therapy | Barley-based remedy for 5 days | H. vulgare; Z. Jujuba; C. Myxa |
| Varnasseri M, et al (2022)33 | Iran | I: 30 (12/ 18) C: 30 (17/ 13) |
I: 47.87 (14.31) C: 44.27 (11.20) |
N/A | N/A | N/A | N/A | N/A | 40 | 200 mg hydroxychloroquine sulfate; Lopinavir/ritonavir (Kaletra) | 2g Amla sachets every 12 hours for 10 days | Phyllanthus Emblica |
| Asadirad A, et al (2021)36 | Iran | I: 30 (24/6) C: 30 (24/6) |
I: 56 (14.02) C: 50.2 (12.01) |
N/A | N/A | N/A | N/A | N/A | N/A | (lopinavir/ritonavir (Kaletra®) 400/100 mg subcutaneously 44μgIFN-β-1a | 40 mg/day nano-curcumin ie, 40 mg nano-mcellar curcumin oral capsules SinaCurcumin® every 6 hours for 7 days | Curcuma longa |
| Askari G, et al (2022)34 | Iran | I: 23 (14/9) C: 23 (13/10) |
I: 43.74 (12.9) C: 51.52 (13.8) |
N/A | 26 | 24 | 15.2 | N/A | N/A | Two placebo capsules each containing 505 mg maltodextrin | Two curcumin-piperine capsules; each capsule contains 500 mg of curcumin plus 5 mg of piperine; At 9 and 18 for 14 days | Curcuma longa |
| Shafie EH, et al (2022)35 | Iran | I: 26 (15/11) C: 24 (14/10) |
I: 57.46 (11.61) C: 57.79 (11.45) |
3.80% | N/A | N/A | N/A | N/A | N/A | Standard treatments | 160 mg nano-curcumin; 4 capsules of 40 mg daily for 6 days | Curcuma longa |
| Tahmasebi, et al (2020)31 | Iran | I: 80 (48/32) C: 40 (24/16) |
I: 54.2 (9.1) C: 52.4 (8.5) |
N/A | N/A | N/A | N/A | N/A | N/A | Standard treatments | Sinacurcumin® 80 mg; 2 capsules daily (every 12 hours) for 21 days | Curcuma longa |
| Pawar KS, et al (2020)20 | India | I: 70 (45/25) C: 70 (54/16) |
18–85 (IQR) | N/A | N/A | N/A | N/A | N/A | N/A | Standard treatments | Curcumin with piperine, curcumin 252 mg; for 14 days | Curcuma longa |
| Sankhe AP, et al (2022)19 | India | I: 70 (44/26) C: 70 (45/15) |
I:51.29 (15.27) C: 50.92 (14.99) |
N/A | 25.8 | 30 | 10 | N/A | N/A | Standard treatments | Ayurcov containing 500 mg of curcumin; The first dose of this combination is started one hour after lunch, the second dose is given two hours after the first dose, and the third dose is given two hours after the second dose. | Curcuma longa |
Abbreviations: Ht, hypertension; CVD, cardiovascular diseases; Obes, obesity; COPD, chronic obstructive pulmonary diseases; I, intervention; C, control.
Study Outcome
Analysis of Phytopharmaceuticals Effectivity in COVID-19 Patients Recovery
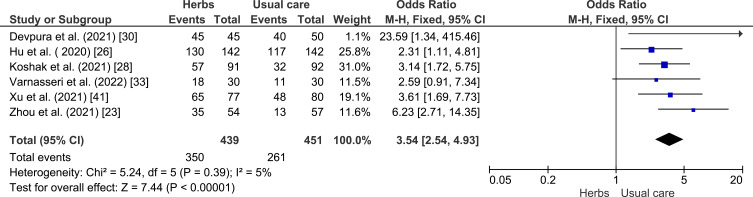
A meta-analysis was conducted to assess the potency of herbal plants compared to the control group in the recovery of COVID-19 patients (see Figure 3). Herbal plants showed a better significant healing rate (p < 0.00001) with OR = 3.54 (95% CI: 2.54–4.93), implied its protective effect against COVID-19 patients, so it can be concluded that herbal plants can increase the healing effect in the intervention group compared to the control group. Low heterogeneity was found in this analysis with I2 = 5%.
Figure 3.
Analysis of control vs intervention effectiveness of herbal plants on COVID-19 recovery.23,26,28,30,33,41
Analysis of Phytopharmaceuticals Effectivity in Improving COVID-19 Clinical Symptoms
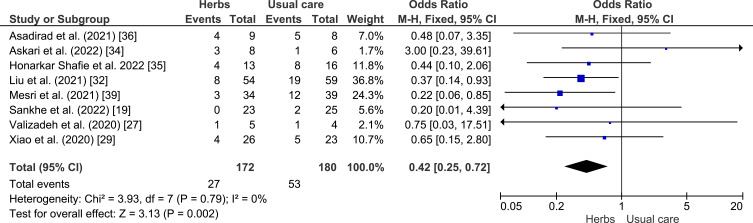
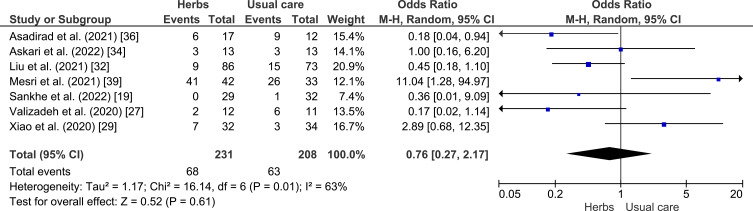
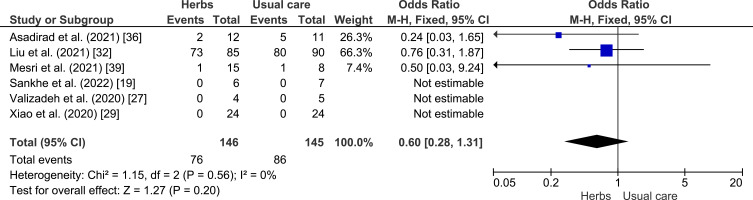
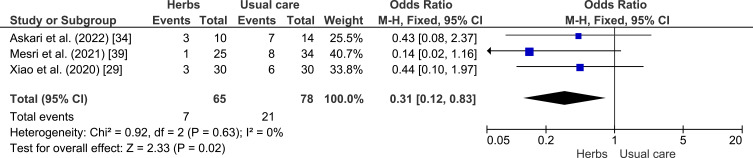
A meta-analysis was conducted to assess the potential of herbal plants in improving of clinical outcome, namely shortness of breath, cough, fever, and myalgia as shown in Figures 4–7. Herbal intervention showed as a strong protective factor in shortness of breath (p = 0.002) with OR of 0.42 (95% CI: 0.25–0.72) and muscle pain (p = 0.02) with OR of 0.31 (95% CI: 0.12–0.83). However, there is no statistically significant effect on cough (p = 0.61) with OR of 0.76 (95% CI: 0.27–2.17) and fever (p = 0.20) with OR of 0.60 (95% CI: 0.28–1.33), Heterogeneity was only found in the incidence of fever with I2 of 63%.
Figure 4.
Forest plot of the relationship between the incidence of shortness of breath in the intervention and control groups.19,26,29,32,34–36,39
Figure 5.
Forest plot of the relationship between the incidence of cough in the intervention and control groups.19,27,29,32,34,36,39
Figure 6.
Forest plot of the relationship between the incidence of heat (fever) in the intervention and control groups.19,26,27,29,36,39
Figure 7.
Forest plot of the relationship between the incidence of muscle pain (myalgia) in the intervention and control groups.29,34,39
Analysis of the Effectiveness of Phytopharmaceuticals in the Prevention of Death of COVID-19 Patients
A meta-analysis was conducted to assess the potency of herbs in reducing mortality as shown in Figure 8. The herbal intervention showed a strong correlation with lower mortality rate (p < 0.00001) with OR 0.24 (95% CI: 0.14–0.41). Heterogeneity was not found.
Figure 8.
Forest plot of the relationship between the incidence of death in the intervention and control groups.20,23,27,31,36,41
Meta-Regression
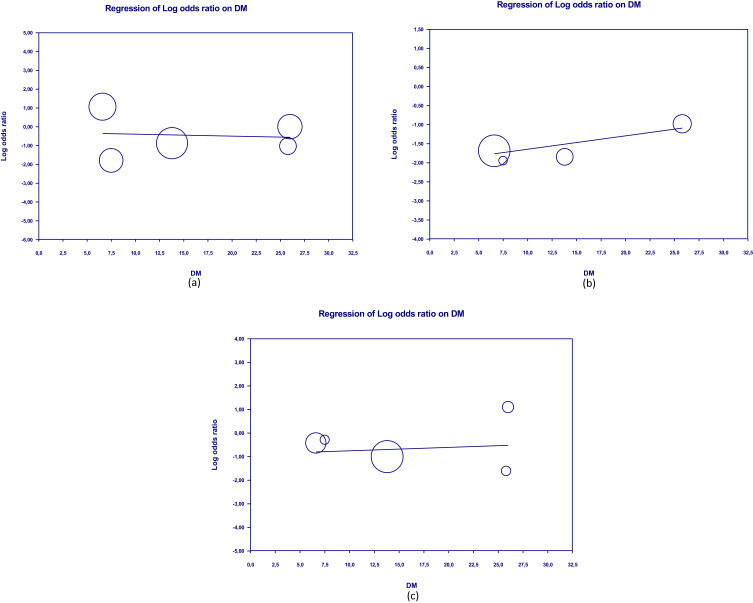
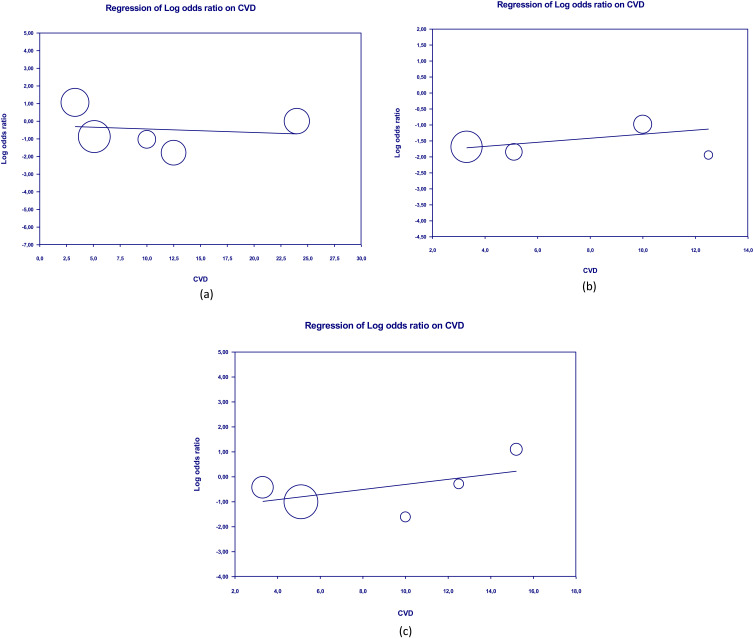
Meta-regression showed that the clinical outcomes of patients were not significantly influenced by covariate factors such as diabetes mellitus, hypertension, and CVD on shortness of breath (p = 0.321, p = 0.818, p = 0.413, respectively) and cough (p = 0.888; p = 0.859, p = 0.799). Furthermore, there was no significant influence of diabetes mellitus, hypertension, and CVD on death (p = 0.413, p = 0.971, p = 0.551). Table 2 presents the detailed results of the meta-regression, while Figures 9–11 show the scatterplots of the log regression on the covariate variables. The results of the meta-regression indicate that the effectiveness of herbal interventions is not significantly influenced by other factors in improving the clinical outcomes of COVID-19 patients.
Table 2.
Association Between Clinical Outcomes and Covariate Variable in Population Given Herbs
| Variable | SE | 95% CI | z value | 2-Sided p value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Hypertension | SoB | 0.0175 | −0.0518 | 0.0170 | −0.99 | 0.321 |
| Cough | 0.0302 | −0.0645 | 0.0538 | −0.18 | 0.859 | |
| Death | 0.0203 | −0.0405 | 0.0391 | −0.04 | 0.971 | |
| DM | SoB | 0.0631 | −0.1091 | 0.1381 | 0.23 | 0.818 |
| Cough | 0.0735 | −0.1544 | 0.1336 | −0.14 | 0.888 | |
| Death | 0.0429 | −0.0489 | 0.1192 | 0.82 | 0.413 | |
| CVD | SoB | 0.1063 | −0.1068 | 0.3101 | 0.96 | 0.339 |
| Cough | 0.0786 | −0.1740 | 0.1340 | −0,25 | 0.799 | |
| Death | 0.1069 | −0.1458 | 0.2732 | 0.60 | 0.551 | |
Abbreviations: DM, diabetes mellitus; CVD, cardiovascular-diseases; SE, standard error; SoB, shortness of breath.
Figure 9.
Scatterplot of regression of clinical outcome and death’s log odd ratio on DM.
Notes: (a) Shortness of breath; (b) Cough; (c) Death.
Figure 10.
Scatterplot of regression of clinical outcome and death’s log odd ratio on hypertension.
Notes: (a) Shortness of breath; (b) Cough; (c) Death.
Figure 11.
Scatterplot of regression of clinical outcome and death’s log odd ratio on CVDs.
Notes: (a) Shortness of breath; (b) Cough; (c) Death.
Discussion
Principal Finding
Herbal medicine has become a part of primary medicine, supported by many studies for several diseases. Likewise, many studies have also been conducted to assess the potency of herbal medicine in COVID-19 pandemic. Herbal medicine consists of plant parts or extracts that work synergistically.42 The plant parts or plant extracts allow herbal medicines to be used as therapy for various diseases.9,42
The results of meta-regression analysis conducted showed that there is no significant influence of covariate factors on the clinical outcomes of the patients. This could be interpreted as herbal therapy producing a significant improvement on the outcomes of the patients with COVID-19. The effectiveness of the herbal therapy on reducing symptoms of COVID-19 has been demonstrated in several studies. In a study conducted by Demeke et al on the use of herbal medicine as a management strategy for COVID-19, the effectiveness of herbal medicine in reducing fever and cough was found to be a result of its anti-inflammatory effects. Other studies also found the significant use of herbal plants in the prevention and treatment of COVID-19 in the general public with a total of 1747 respondents (aged 20–70), where 80.2% used herbal plants for prevention and 71% used herbal medicine to treat respiratory symptoms. Nugraha et al have reviewed at least 4 herbal medicines that served as a therapy for COVID-19. Echinacea purpurea, a top-rated herbal medicine in Europe and North America, shows promising effects due to its antiviral, immunomodulatory, and anti-inflammatory role.9 Next is curcumin or turmeric, which is often used in Indonesia. Turmeric consists of a group of curcuminoids, most of which are curcumin. In a study conducted by Pulido-Moran et al, it can act as an immunomodulator as an anti-inflammatory, antioxidant, antidiabetic, and cholesterol-lowering.9,43 Curcumin is also very abundant in Indonesia, so it can be a herbal medicine with great potential as a therapy for COVID-19.9,44 The following herbal medicine is Cinchona sp., which can produce quinine. Quinine formerly acts as an antimalarial drug, nowadays it is also potentially known as an inhibitor for viral infections. Next is Xanthorrhizol, which can act as antimicrobial, anti-inflammatory, antioxidant, antihyperglycemic, antihypertensive, antiplatelet, nephroprotective, anticancer, and supplementary agents for systemic lupus erythematosus (SLE).9
In a study conducted by Karimi et al regarding the effectiveness of Persian herbal medicine against 184 COVID-19 patients who underwent intervention, herbal medicine significantly (p < 0.01) decreased cough, shortness of breath, and myalgia.25 Besides, in another study conducted by Hu et al regarding the effectiveness of Lianhuaqingwen capsules in 142 COVID-19 patients who were given the intervention compared to 142 patients as controls, it was found that recovery on day 14 in the intervention group reached 130 (91.5%) with recovery time of 7.0 (6.0–8.0) days. Meanwhile, in the control group, only 117 (82.4%) patients successfully recovered with symptom recovery time of 10.0 (9.0–11.0) days.26 From the research conducted, it can be concluded that herbal medicine is a promising therapy for COVID-19.
Herbal medicines can be derived from natural ingredients, either plants, animals or minerals, which the utilization varies depending on the current needs. The use of herbal medicines in Indonesia itself is not a new thing. Among them are turmeric, kencur rice, ginger, and other herbal drinks commonly referred to as jamu.42,45 This is also supported by wide availability of herbal medicines in Indonesia. For example, according to the Indonesian Central Statistics Agency, curcumin or tumeric availability reaches more than 190,000 tons per year.44
Various studies have carried out the safety profile of herbal medicine. In a study by Hu et al using Lianhuaqingwen capsules, no severe side effects were found. The most common side effect was an increase in alanine aminotransferase levels or aspartate aminotransferase levels, where no significant difference in side effects was found between the intervention and control groups (p > 0.05).26 Another study by Koshak et al with Nigella sativa showed minimum side effects, where only 3 out of 183 people with gastrointestinal symptoms were found.28 Another study by Askari et al also reported similar results where no severe side effects were found in herbal medicines.34 From here, the safety and application of herbal medicines can be implied as good and can be implemented as an adjuvant in the management of COVID-19. According to sub-group analysis in this present study, we found no association between covariate variables and clinical outcomes in people with COVID-19 who received herbal medicine.
Medicinal plant extracts contain hundreds or even thousands of bioactive compounds and identifying bioactive compounds responsible for certain biological activities is a significant challenge.46 In fact, all the extra activities of medicinal plants are the result of the combined action of several compounds with synergistic, additive or antagonistic activity.47 It is unacceptable to use bioactive compounds as drugs without understanding the intra-extract interactions on a wider scale. In some cases, the activity of medicinal plant extracts exhibits a better effect than equivalent doses of the isolated compounds and cannot be predicted on what is known about the individual compounds. Whether it is synergies, increased bioavailability, cumulative effects, or simply additive properties requires further research.48
The scientific search for extracts of medicinal plants is challenging because of their complexity and enormous variability. Because plant extracts are complex and no single bioactive molecule is frequently used for medicinal purposes, understanding the interactions between active compounds can be very important in this regard.49–51
In order to maximize the effects of drug action, synergistic interactions between individual compounds or mixtures or medicinal plant extracts are an important part of their therapeutic efficacy. Therefore, the synergy of medicinal plant extracts needs to be evaluated using rigorous analytical methods and validated in clinical trials. In addition, the specific bioactive compounds responsible for these effects and the basic mechanisms of their interactions are still not fully understood and require further research.52
Herbal agents may be useful as a treatment against COVID-19. Advice for patients is still not recommended to use supplements that contain one of these compounds to prevent COVID-19 or cure disease without special advice or under the direct supervision of a doctor. Advice for clinicians is that the administration of this herbal medicine must be given carefully to patients, even if they are in good health.9 Pharmacists, doctors, and nurses often have little training and understanding of how herbal medicines affect the health of their patients. Many of them also lack information about this product and how to use it. Adequate training is now very important because most patients frequently use other types of prescription or non-prescription drugs.42
Implication to Clinical Practice and Further Study
There is evidence that the addition of herbal medicine to standard treatment for people with COVID-19 resulted in significant benefits, but the risks are highly unknown and inconclusive. Due to poor reporting of safety results, the evidence pertaining to the harmful effects of herbal medicine therapies is likewise highly ambiguous. After their efficacy and safety issues are thoroughly addressed, herbal medicine interventions may be recommended.53 Despite the promising results, there is a need for more randomized controlled trials to be conducted globally to validate these findings. Further research is also recommended to analyze the long-term effects of herbal treatments on COVID-19 recovery and its impacts on the immune system of patients. With these recommendations, future studies have the potential to provide a more comprehensive understanding of the efficacy of herbs as complementary and alternative therapy in COVID-19 recovery, and contribute to the development of effective treatment strategies for the current COVID-19 pandemic and potential future pandemics.
Currently, vaccines occupy a prominent position and primary method in disease prevention methods due to their ability to prime the immune system for potential threats from harmful pathogens such as viruses. We consider the potential of mRNA vaccines in managing COVID-19 as a complementary approach to herbal therapy.54 The combination of herbal therapy and mRNA vaccines might work synergistically to improve clinical outcomes and recovery among COVID-19 patients.
Strengths and Limitation
This study has several advantages, such as the various countries of origin and large number of studies. In addition, most study results are also homogeneous. However, this study also has limitations, namely only extracting data from English-language papers and the herbal plants in the included studies varied their types and doses.
Conclusion
The cultural and historical value of herbal medicine has made it more preferable for a number of patients. The natural properties of herbs as a natural substance add to the positive value of alternative medicine. The effects of herbal plants on healing, clinical improvement, and reducing mortality in COVID-19 patients proved to be more beneficial and statistically significant than treatment with standard therapy. The same goes for safety so that herbal medicines can be applied as adjuvants in handling COVID-19, especially since their availability is large in Indonesia. To improve the quality of data, effective trial designs with a sufficient sample size, randomization or blinding methods, and outcome measurements with accurate reporting are required despite the numerous obstacles presented by COVID-19 clinical research. Consequently, future well-designed RCTs on herbal therapy for the treatment of COVID-19 are eagerly expected.
In the future, to make herbal plant management of COVID-19 therapy, clinical trials need to be carried out with similar results, doses, and types of plants. In addition, it is necessary to determine the dosage and frequency that is safe for consumption according to the type of herbal medicine. If there are sufficient data, this research, in collaboration with the government and various health education institutions in Indonesia, can be continued by developing guidelines on herbal plants in managing COVID-19.
Funding Statement
This APC was paid by Directorate for Research and Community Engagement, Universitas Padjadjaran, Indonesia.
Disclosure
The author(s) declare that they have no conflict of interests in this study.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data; 2023. Available from: https://covid19.who.int/. Accessed July 10, 2023.
- 2.Priyanka COP, Singh I, Patra G. Aerosol transmission of SARS-CoV-2: the unresolved paradox. Travel Med Infect Dis. 2020;37:101869. doi: 10.1016/j.tmaid.2020.101869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lednicky JA, Lauzard M, Fan ZH, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson N, Corbett S, Tovey E. Airborne transmission of covid-19. BMJ. 2020;370:m3206. [DOI] [PubMed] [Google Scholar]
- 5.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotfi M, Hamblin MR, Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Tracking SARS-CoV-2 variants; 2022. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants. Accessed July 10, 2023. [PubMed]
- 8.World Health Organization. WHO therapeutics and COVID-19: living guideline. World Health Organization; 2022:128. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3. Accessed July 10, 2023. [Google Scholar]
- 9.Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Alternat Med. 2020;2020:2560645. doi: 10.1155/2020/2560645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton (FL): CRC press; 2011. [PubMed] [Google Scholar]
- 11.National Centre for Complementary and Integrative Health (NCCIH). Complementary, alternative, or integrative health: what’s in a name? 2021. Available from: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed July 10, 2023.
- 12.Hall H, Leach M, Brosnan C, Collins M. Nurses’ attitudes towards complementary therapies: a systematic review and meta-synthesis. Int J Nurs Stud. 2017;69:47–56. doi: 10.1016/j.ijnurstu.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 13.Dai T, Zhang L, Dai X, et al. Multimode participation of traditional Chinese medicine in the treatment of COVID-19. Integr Med Res. 2021;10:100781. doi: 10.1016/j.imr.2021.100781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang L, Song E, Zhang J, Lee HW, Lee MS. Herbal medicine for COVID-19: an overview of systematic reviews and meta-analysis. Phytomedicine. 2022;102:154136. doi: 10.1016/j.phymed.2022.154136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B-J, Lee JA, Kim K-I, Choi J-Y, Jung H-J. A consensus guideline of herbal medicine for coronavirus disease 2019. Integr Med Res. 2020;9(3):100470. doi: 10.1016/j.imr.2020.100470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar A, Dubey A, Saini D, Prasad CP. Role of complementary and alternative medicine in prevention and treatment of COVID-19: an overhyped hope. Chin J Integr Med. 2020;26(8):565. doi: 10.1007/s11655-020-2851-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo CS, Tan PM, Shu CSI, Choo ZX, Te KK. Challenges and strategies for implementing Chinese medicine during COVID-19 in Malaysia. Integr Med Res. 2021;10:100783. doi: 10.1016/j.imr.2021.100783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankhe AP, Memane NS, Gawali VP, et al. A randomized, controlled, blinded, parallel group, clinical trial to study the role of Ayurcov (AyurCoro3), one day regimen as an adjuvant therapy for COVID-19 disease management, at dedicated Covid Hospital (DCH) in India. Complement Ther Med. 2022;67:102824. doi: 10.1016/j.ctim.2022.102824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawar KS, Mastud RN, Pawar SK, et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. 2021;12:669362. doi: 10.3389/fphar.2021.669362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Liu C, Wang T, et al. Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: a multicentre, double-blind, and randomised controlled trial. J Ethnopharmacol. 2022;284:114830. doi: 10.1016/j.jep.2021.114830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X-Y, Lv L, Zhou Y-L, et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: a multicenter, prospective, open-label and randomized controlled trial. Phytother Res. 2021;35(8):4401–4410. doi: 10.1002/ptr.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Feng J, Xie Q, et al. Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: a randomized controlled multicenter trial. Phytomedicine. 2021;89:153612. doi: 10.1016/j.phymed.2021.153612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakar A, Goyal M, Bhinde S, Chhotala Y, Panara K, Chaudhari S. Impact of AYUSH 64 as an adjunctive to standard of care in mild COVID 19 - an open-label randomized controlled pilot study. J Ayurveda Integr Med. 2022;13(3):100587. doi: 10.1016/j.jaim.2022.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi M, Zarei A, Soleymani S, et al. Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother Res. 2021;35(11):6295–6309. doi: 10.1002/ptr.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu K, Guan W-J, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi: 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol. 2020;89(Pt B):107088. doi: 10.1016/j.intimp.2020.107088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshak AE, Koshak EA, Mobeireek AF, et al. Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial. Complement Ther Med. 2021;61:102769. doi: 10.1016/j.ctim.2021.102769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao M, Tian J, Zhou Y, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devpura G, Tomar BS, Nathiya D, et al. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine. 2021;84:153494. doi: 10.1016/j.phymed.2021.153494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahmasebi S, El-Esawi MA, Mahmoud ZH, et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J Cell Physiol. 2021;236(7):5325–5338. doi: 10.1002/jcp.30233 [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Yang W, Liu Y, et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91:153671. doi: 10.1016/j.phymed.2021.153671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varnasseri M, Siahpoosh A, Hoseinynejad K, et al. The effects of add-on therapy of Phyllanthus Emblica (Amla) on laboratory confirmed COVID-19 cases: a randomized, double-blind, controlled trial. Complement Ther Med. 2022;65:102808. doi: 10.1016/j.ctim.2022.102808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Askari G, Sahebkar A, Soleimani D, et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials. 2022;23(1):472. doi: 10.1186/s13063-022-06375-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honarkar Shafie E, Taheri F, Alijani N, et al. Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: a randomized double-blind placebo-controlled trial. Phyther Res. 2022;36(2):1013–1022. doi: 10.1002/ptr.7374 [DOI] [PubMed] [Google Scholar]
- 36.Asadirad A, Nashibi R, Khodadadi A, et al. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phyther Res. 2022;36(2):1023–1031. doi: 10.1002/ptr.7375 [DOI] [PubMed] [Google Scholar]
- 37.Hasheminasab FS, Azimi M, Khodadoost M, et al. Efficacy of the barley-based remedy, a Persian medicine formula, in coronavirus disease 2019 (COVID-19) hospitalized patients: an open-labeled randomized controlled trial. Adv Integr Med. 2022;9(3):185–190. doi: 10.1016/j.aimed.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fazlju SMB, Heris JA, Farshbaf-Khalili A, et al. HYSSOP and POLIUM could help to prevent COVID-19 in high-risk population: the results of a parallel randomized placebo-controlled field trial. Indian J Tradit Knowl. 2022;21(2):243–253. [Google Scholar]
- 39.Mesri M, Esmaeili Saber SS, Godazi M, et al. The effects of combination of Zingiber officinale and Echinacea on alleviation of clinical symptoms and hospitalization rate of suspected COVID-19 outpatients: a randomized controlled trial. J Complement Integr Med. 2021;18(4):775–781. doi: 10.1515/jcim-2020-0283 [DOI] [PubMed] [Google Scholar]
- 40.Loc HN, Lan TTN, Huong DTL, et al. Traditional Vietnamese medicine Kovir capsule in patients with mild COVID-19: a double-blind randomized controlled trial. Phytother Res. 2022;36(7):2878–2888. doi: 10.1002/ptr.7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Zhang J, Zheng W, et al. Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study. Ann Palliat Med. 2021;10(5):5146–5155. [DOI] [PubMed] [Google Scholar]
- 42.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Neurol. 2014;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21(3):264. doi: 10.3390/molecules21030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badan Pusat Statistika. Produksi tanaman biofarmaka (obat) 2019–2021; 2022. Available from: bps.go.id/indicator/55/63/1/produksitanaman-biofarmaka-obat-.html. Accessed July 10, 2023.
- 45.Rokhmah D, Ali K, Putri SMD, Khoiron K. Increase in public interest concerning alternative medicine during the COVID-19 pandemic in Indonesia: a google trends study. F1000Research. 2020;9:1201. doi: 10.12688/f1000research.25525.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enke CG, Nagels LJ. Undetected components in natural mixtures: how many? What concentrations? Do they account for chemical noise? What is needed to detect them? Anal Chem. 2011;83(7):2539–2546. doi: 10.1021/ac102818a [DOI] [PubMed] [Google Scholar]
- 47.Ulrich-Merzenich G, Panek D, Zeitler H, Vetter H, Wagner H. Drug development from natural products: exploiting synergistic effects. Indian J Exp Biol. 2010;48(3):208–219. [PubMed] [Google Scholar]
- 48.Abreu AC, McBain AJ, Simões M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep. 2012;29(9):1007–1021. doi: 10.1039/c2np20035j [DOI] [PubMed] [Google Scholar]
- 49.van Vuuren S, Viljoen A. Plant-based antimicrobial studies--methods and approaches to study the interaction between natural products. Planta Med. 2011;77(11):1168–1182. doi: 10.1055/s-0030-1250736 [DOI] [PubMed] [Google Scholar]
- 50.Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12(1):122–132. doi: 10.2174/138945011793591626 [DOI] [PubMed] [Google Scholar]
- 51.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2–3):97–110. doi: 10.1016/j.phymed.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, Seto SW, Chang D, et al. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol. 2016;7:201. doi: 10.3389/fphar.2016.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ang L, Song E, Hu X-Y, Lee HW, Chen Y, Lee MS. Herbal medicine intervention for the treatment of COVID-19: a living systematic review and cumulative meta-analysis. Front Pharmacol. 2022;13:906764. doi: 10.3389/fphar.2022.906764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priyanka CH, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis. 2023;52:102550. doi: 10.1016/j.tmaid.2023.102550 [DOI] [PubMed] [Google Scholar]