Abstract
Chronic stability of functional performance is a significant challenge to the success of implantable devices for neural stimulation and recording. Integrating wireless technology with typical microelectrode array designs is one approach that may reduce instances of mechanical failure and improve the long-term performance of neural devices. We have investigated the long-term stability of Wireless Floating Microelectrode Arrays (WMFAs) implanted in rat sciatic nerve, and their ability to selectively recruit muscles in the hind limb via neural stimulation. Thresholds as low as 4.1 μA were able to generate visible motion of the rear paw. Each implanted device (n=6) was able to selectively recruit plantar flexion and dorsiflexion of the rear paw, and selective stimulation of both movements was achieved throughout the study period. The evoked limb motion was electrode specific and was dependent on location within the fascicular structure of the nerve. Motor thresholds and movement patterns remained stable for more than 8 weeks after device implantation. No major changes in limb function were observed between the implanted and contralateral limb, or between implanted animals and control group animals. The results of this study show that WFMAs with intrafascicular electrodes implanted in a healthy peripheral nerve can provide stable and selective motor recruitment, without altering overall limb function.
I. Introduction
Many different approaches to neural prostheses have been developed with the goal of improving stimulation selectivity to achieve superior control of motor or sensory function. Methods for improving stimulation selectivity generally focus on current steering strategies (particularly with extraneural cuff electrodes), [1] the use of electrode arrays that flatten or reshape the nerve, [2–3] or the use of intrafascicular microelectrode arrays (MEAs). [4–7] Intrafascicular MEAs can allow customization of several design parameters to make devices more application specific. In particular, it can be advantageous to vary the penetration depth of electrodes in order to target specific areas within a nerve, to accommodate different sizes of peripheral nerves, or to facilitate scaling between animal and human subjects. Reducing the geometric surface area of implanted electrodes can also significantly improve stimulation selectivity, provided the resulting increase in current and charge densities to generate a neural response does not result in damage to the tissue or electrode.
In addition to improving selectivity of neural stimulation, many different strategies have been employed to improve device longevity and chronic performance. One strategy is to reduce the overall dimensions of tissue-penetrating electrodes to reduce tissue damage caused by implantation. The ultimate goal being to reduce or eliminate scar tissue formation, which is expected to improve the long-term performance of neural devices. This strategy has been studied more extensively for cortically implanted devices than those in peripheral nerve. Also, few studies have directly investigated the relationship between degree of tissue damage due to device implantation in healthy peripheral nerves and any changes in limb function. Another strategy for improving chronic performance of neural devices is to use wireless technology to reduce the number of implanted components and wired connections, thus reducing the chance for mechanical failures. Several wireless device designs have been investigated for peripheral nerve stimulation, [8–10] but few have been tested for chronic performance in vivo.
In this study, we investigated the chronic performance of Wireless Floating Microelectrode Arrays (WFMAs), which consist of an intrafascicular MEA with electrodes of two different insertion lengths combined with a compact wireless stimulation module bonded directly to the MEA. WFMAs were evaluated for selective stimulation of motor axons in peripheral nerve. During chronic implantation of WFMAs, we assessed sensory and motor function of the implanted limb, and we evaluated each electrode for the type of motion elicited and the required stimulation current threshold.
II. Methods
All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of The University of Texas at Dallas.
A. Device Design and Assembly
The overall design and manufacture of wireless floating microelectrode arrays was the same as previously described. [11–14] Briefly, WFMAs were fabricated by MicroProbes for Life Sciences (MLS; Gaithersburg, MD) and Illinois Institute of Technology (IIT; Chicago, IL) with 3 components: a ceramic substrate and ASIC, iridium microwire electrodes, and a telemetry coil. The iridium electrodes consisted of 2 uninsulated reference and counter electrodes plus 16 Parylene-insulated stimulating electrodes fabricated with an exposed surface area of 2000 μm2. The stimulating electrodes’ exposed iridium surfaces were activated as described previously [11] to form a high charge-injection capacity layer of activated iridium oxide film (AIROF). The stimulating electrodes were 100 μm in diameter (main shaft, tapered at the tip) with alternating lengths of 1150 μm and 1350 μm and center-to-center spacing of 400 μm. Fully-assembled WFMAs were encapsulated with a silane-enhanced PDMS to create a structure with an overall diameter of 5 mm. WFMAs were then placed inside a silicone channel nerve guide to prevent devices from migrating during chronic implantation. After the WFMA was placed in the silicone channel, the electrode lengths available for insertion were 650 μm and 850 μm. The electrode layout and completed WFMA with silicone nerve guide are shown in Fig. 1. The WFMAs were wirelessly powered at 4.5 MHz with forward commands at 1.1 Mbits/sec, and reverse telemetry transmitted at 145 kHz using FSK modulation of a Class-E converter and the NeuroTalk interface. [12–13]
Figure 1.
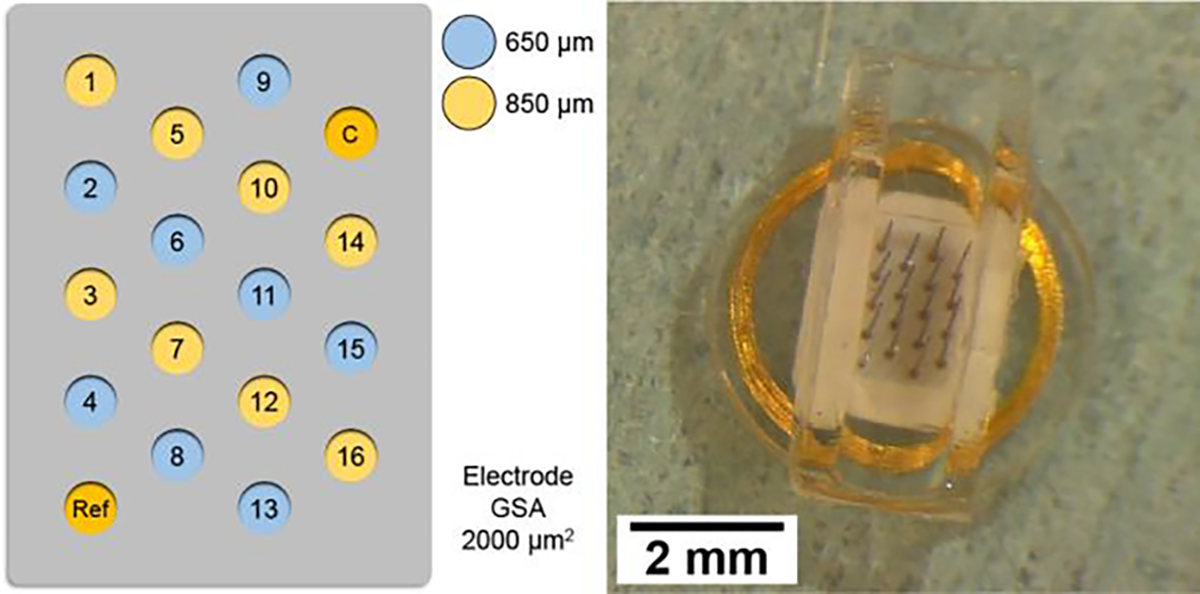
(Left) Microelectrode array layout with electrode lengths indicated by blue (650 μm) or yellow (850 μm) shading. (Right) Wireless floating microelectrode array after embedding within a silicone nerve guide (500 μm wall and base thickness).
B. Device Implantation
WFMA devices were implanted into the left sciatic nerve of n=6 female Sprague Dawley rats 10 to 16 weeks old. Animals were anesthetized with inhaled Isoflurane (2.0–3.0%) and the left hind limb was shaved and cleaned with 10% povidone-iodine and 70% alcohol. An incision was made in the skin parallel to the femur. The biceps femoris muscle was separated from the gluteus superficialis and vastus lateralis muscles by spreading the connective tissue between the muscles, and the biceps femoris was then retracted to reveal the sciatic nerve. To prepare for device implantation, the nerve was detached from the surrounding tissue by separating the connective tissue around the entire circumference of the nerve, ensuring any connecting neural branches were not damaged, until an exposed length of approximately 1.2 cm was achieved.
The WFMA was then placed under the nerve so that the flat coil surface rested on the underlying muscle tissue, and the nerve was placed inside the silicone channel. The nerve and WFMA device were then carefully pressed together until electrodes penetrated the sciatic nerve. The device was secured in place using a silicone sealant (Kwik-Cast™) that cures at body temperature and adheres to the silicone channel without attaching to the surrounding tissue. Care was taken to ensure the sealant did not enter the channel and contact (occlude) the electrodes. Devices were implanted with alternating orientations, such that the row of 5 electrodes was positioned to the left (towards the cranial direction) as in Fig. 1 for n=3 animals (A01, A03, and A05) and to the right (towards the caudal direction) for n=3 animals (A02, A04, and A06). Fig. 2 shows an implanted WFMA device after it was secured with silicone sealant (green). The success rate for device implantation was 100% and is generally successful if there are no postoperative infections and no complications recovering from anesthesia.
Figure 2.
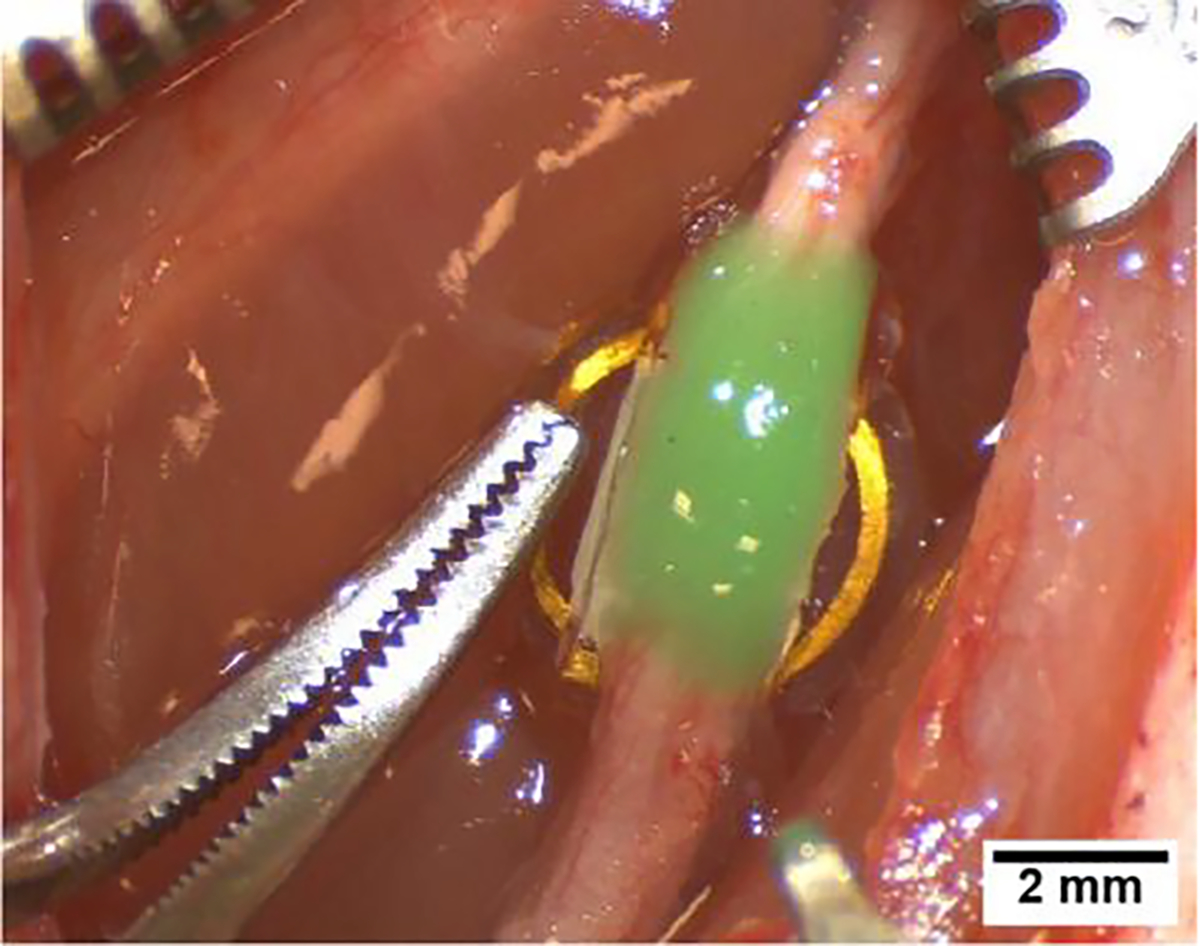
Wireless floating microelectrode array secured with silicone sealant (green) after implantation in the left sciatic nerve.
Voltage transients were recorded via the reverse telemetry feature of the WFMA in response to 4.7 μA, 200.2 μs current pulses (~1 nC/ph, 50 μC/cm2) at a 50 Hz frequency to confirm device function at the time of implantation. The muscle was then closed with 4–0 silk suture and the skin closed with 11 mm suture clips. Animals were given slow release buprenorphine immediately after surgery and every 72 hours as needed thereafter to manage post-op pain. Animals were also given cefazolin antibiotic immediately after surgery and water with sulfamethoxazole ad libitum for one week post-op as a prophylactic.
C. Neural Stimulation and Tracking Limb Motion
Stimulation trials were performed under isoflurane anesthesia (1.0–2.0%) and each testing session was limited to a maximum of 2 hours to avoid any potential complications related to repeated and prolonged anesthesia. Trials were completed once per week starting on post-op day 9. Trials were performed for 8 weeks after device implantation and we are continuing trials once every two weeks through 38 weeks post-implantation. The study will end at 38 weeks in an attempt to avoid age-related complications, as animals will reach 1 year of age near week 35 on average.
Animals were positioned on an elevated surface such that the left hind limb was hanging and free to move without restriction. Two Stingray F033C IRF CSM video cameras (Allied Vision Technologies GmbH, Germany) were positioned to view the lateral and dorsal surfaces of the foot for tracking limb motion (CinePlex, Plexon). The wireless telemetry coil was positioned over the implanted device to achieve consistent communication with the WFMA. For each trial, each electrode was tested individually with 200.2 μs pulses at 1 Hz. The current was gradually increased in increments of 2 to 10 μA until either reaching the maximum output capabilities of the device or observing a 1 Hz twitch in the left paw that was clearly distinguishable from motion of the animal due to breathing. Video of limb motion was then recorded at the threshold current (Ith) for generating a visible twitch and at one or two current values above threshold. Primary limb motion in each recording was classified as either plantar flexion or dorsiflexion, and any secondary limb motion evoked by stimulation was recorded as toe flexion, toe extension, lateral rotation, or medial rotation.
III. Results
The inter-electrode spacing resulted in the microelectrode array being slightly larger than the diameter of the sciatic nerve at the time of implantation. As a result, all six of the implanted arrays had one row of electrodes located either outside of the nerve or in the surrounding epineurium. Of the 96 total electrodes implanted across six animals, 21 appeared to be outside the nerve or within the epineurium upon visual inspection at the time of implantation. Of those 21 electrodes, 15 did not evoke a visible motor response at any current level at any point during the initial 8-week study period.
A. Motor Activation Thresholds
Thresholds for motor activation ranged from 4.1 μA to 84.6 μA, where electrodes located in rows at the outer edges of the nerve typically had higher motor activation thresholds than those at the center of the nerve.
At week 1 post-implantation, 72 electrodes (75% of all implanted electrodes) evoked a visible motor response: 55 electrodes (76.4 %; 57.3 % of total) evoked a motor response with Ith less than 20 μA (4nC/ph), 16 electrodes (22.2%; 16.7 % of total) evoked a motor response with Ith values between 20 and 40 μA, and only 1 electrode (1.4%; 1.0% of total) required Ith greater than 40 μA.
At week 8 post-implantation, 78 electrodes (81% of all implanted electrodes) evoked a visible motor response: 59 electrodes (75.6 %; 61.5 % of total) evoked a motor response with Ith less than 20 μA, 14 electrodes (17.9%; 14.6 % of total) evoked a motor response with Ith values between 20 and 40 μA, and only 5 electrodes (6.4%; 5.2% of total) required Ith greater than 40 μA.
B. Motor Recruitment Patterns
Plantar flexion and dorsiflexion of the left hind limb was evoked by stimulation of at least one electrode for each of the six implanted devices. The overall type of motion evoked by any one electrode did not change through 8 weeks postimplantation. Three electrodes evoked motion intermittently at only one or two time points during the study period. Five electrodes did not evoke motion within the first 1–2 weeks after implantation, but then consistently evoked a motor response through week 8. Electrodes that were inconsistent in whether or not they evoked a motor response were part of the group of 21 electrodes located either within the epineurium or completely outside the nerve.
Fig. 3 shows Ith values for all six animals for 8 weeks after implantation, and the type of movement evoked is indicated by either grey or blue shading for plantar flexion and dorsiflexion respectively. White shading indicates no motor response was observed when the electrode was tested up to the maximum output of the device. White shading with an “X” indicates data was not recorded at that time point or the electrode was nonfunctional when voltage transients were recorded at the time of device implantation, and so no testing was performed for that electrode.
Figure 3.
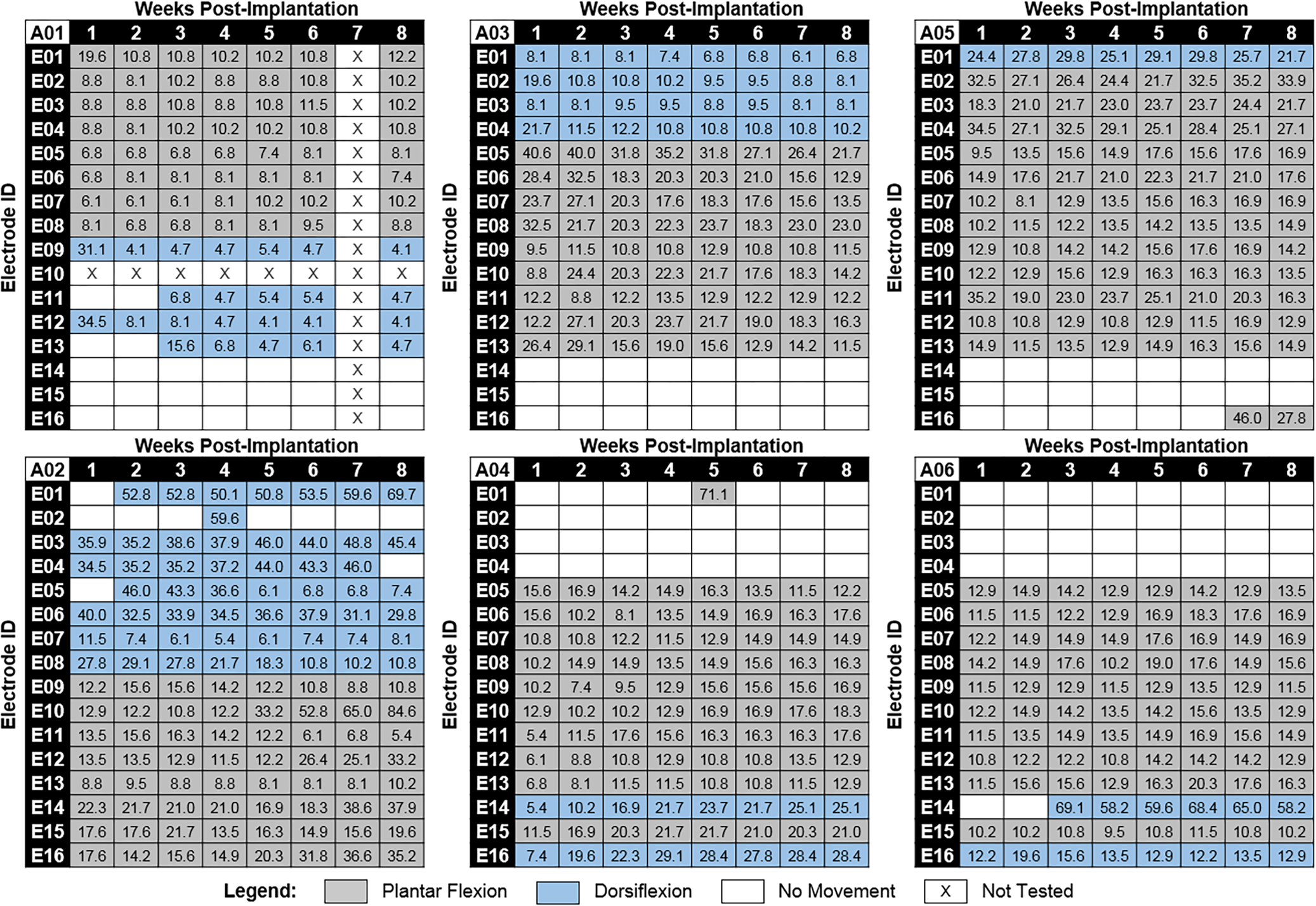
Motor activation current thresholds and elicited movement directions over time for all six implanted animals. Motor activation current thresholds are listed in μA for each electrode at each study time point. Elicited movement direction at each current threshold is indicated by either grey (plantar flexion) or blue (dorsiflexion) shading. Animal ID is indicated in the top left corner for each map. Animals A01, A03, and A05 (top row) had WFMAs implanted in the same orientation as shown in Fig. 1. Orientation for WFMAs implanted in animals A02, A04, and A06 (bottom row) was rotated 180 degrees such that E01-E04 were located on the right side of the array instead of the left.
IV. Discussion
We have conducted a chronic study of selective hind limb motor recruitment using a fully wireless implanted stimulator connected to an array of 16 penetrating microelectrodes placed in rat sciatic nerve. The magnitude and stability of motor recruitment thresholds for penetrating electrodes is highly dependent on ensuring implanted electrodes are inserted into the fascicles of the nerve. From our study results, we believe that a majority of the implanted electrodes are located very near motor fibers within the sciatic nerve due to the high percentage of electrodes generating a motor response. Reducing the electrode-electrode spacing when manufacturing WFMAs intended for rat sciatic nerve applications will greatly improve chances of successfully implanting all electrodes inside a nerve fascicle. Additionally, future development of imaging techniques or other methods might allow targeted device implantation that would improve subject to subject uniformity in recruitment of plantar flexion versus dorsiflexion.
The arrangement of electrodes recruiting plantar flexion versus dorsiflexion was generally consistent with the orientation of the device on the nerve but may have been influenced by slight rotation of the nerve during the implantation procedure. The mapping of patterns of evoked movement directions showed that electrodes that were located in the same row of the microelectrode array and were the same length (650 or 850 μm) evoked the same direction of paw movement for all but one electrode (animal A05, E01). This result is consistent with expected performance as electrodes within the same row should be located within the same fascicle along the length of the nerve, barring any major differences in depth of penetration.
No evidence of pain or discomfort was observed following device implantation for any animal throughout the study. Future work will continue to record motor activation thresholds and movement types as well as assessments of limb function through 38 weeks of device implantation. We also plan to attempt to confirm the location of each electrode within the cross section of the nerve using micro-computed tomography and standard histological analysis of sciatic nerve tissue samples removed after the final study day.
V. Conclusion
This study shows that Wireless Floating Microelectrode Arrays implanted in the sciatic nerve can selectively recruit muscles in the hind limb with current thresholds for most electrodes below 20 μA. Predictable recruitment of dorsiflexion and plantar flexion can be achieved with knowledge of the fascicular structure of the sciatic nerve and careful implantation of the device. Further, no obvious motor or sensory deficits were caused by implanting intrafascicular electrodes within a healthy sciatic nerve, or by weekly testing sessions with short-duration stimulation of each electrode.
Clinical Relevance—
This work establishes a compact wireless stimulation device as a versatile platform that can provide selective motor recruitment via peripheral nerve stimulation.
Acknowledgments
Research supported by a gift from Vanguard Charitable.
Contributor Information
Rebecca A. Frederick, Bioengineering Department at The University of Texas at Dallas, Richardson, TX 75080 USA..
Philip R. Troyk, Biomedical Engineering Department at the Illinois Institute of Technology, Chicago, IL 60616 USA. He is also with Sigenics, Inc., Chicago, IL 60616 USA..
Stuart F. Cogan, Bioengineering Department at The University of Texas at Dallas, Richardson, TX 75080 USA..
References
- [1].Grill WM and Mortimer JT, “Quantification of recruitment properties of multiple contact cuff electrodes,” IEEE Transactions on Rehabilitation Engineering, vol. 4, no. 2, June 1996, pp. 49–62. [DOI] [PubMed] [Google Scholar]
- [2].Tyler DJ and Durand DM, “Alteration of neural geometry for selective nerve stimulation,” Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, vol.5, 1997, pp. 2002–2003. [Google Scholar]
- [3].Tan DW, Schiefer MA, Keith MW, Anderson JR, and Tyler DJ, “Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees,” Journal of Neural Engineering, vol. 12, no. 2, 2015, 026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Badia J, Boretius T, Andreu D, Azevedo-Coste C, Stieglitz T, and Navarro X, “Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles,” Journal of Neural Engineering, vol. 8, no. 3, 2011, 036023. [DOI] [PubMed] [Google Scholar]
- [5].Mathews KS, Wark HA, and Normann RA, “Assessment of rat sciatic nerve function following acute implantation of high density Utah Slanted Electrode Array (25 electrodes/mm2) based on neural recordings and evoked muscle activity,” Muscle & Nerve, vol. 50, no. 3, 2014, pp. 417–424. [DOI] [PubMed] [Google Scholar]
- [6].Vasudevan S, Patel K, and Welle C, “Rodent model for assessing the long term safety of performance of peripheral nerve recording electrodes,” Journal of Neural Engineering, vol. 14, no. 1, 2017, 016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Overstreet CK, Cheng J, and Keefer EW, “Fascicle specific targeting for selective peripheral nerve stimulation,” Journal of Neural Engineering, vol. 16, no. 6, 2019, 066040. [DOI] [PubMed] [Google Scholar]
- [8].Sharma A, Rieth L, Tathireddy P, Harrison R, Oppermann H, Klein M, Töpper M, Jung E, Normann R, Clark G, and Solzbacher F, “Evaluation of the packaging and encapsulation reliability in fully integrated, fully wireless 100 channel Utah Slant Electrode Array (USEA): Implications for long term functionality,” Sensors and Actuators A: Physical, vol. 188, Dec. 2012, pp.167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Piech DK, Johnson BC, Shen K, Ghanbari MM, Li KY, Neely RM, Kay JE, Carmena JM, Maharbiz MM, and Muller R, “StimDust: A mm-scale implantable wireless precision neural stimulator with ultrasonic power and communication,” arXiv:1807.07590, 2018. [DOI] [PubMed] [Google Scholar]
- [10].Lee B, Koripalli MK, Jia Y, Acosta J, Sendi MSE, Choi Y, and Ghovanloo M, “An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects,” Scientific Reports, vol. 8, 2018, article no. 6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu Z, Troyk P, DeMichele G, Kayvani K, and Suh S, “Intrinsic activation of iridium electrodes over a wireless link,” 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, 2012, pp. 2788–2791. [DOI] [PubMed] [Google Scholar]
- [12].Troyk PR and DeMichele GA, “Inductively-coupled power and data link for neural prostheses using a class-E oscillator and FSK modulation,” Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, vol.4, 2003, pp. 3376–3379. [Google Scholar]
- [13].Troyk PR, Detlefsen DEA, and DeMichele GAD, “A multifunctional neural electrode stimulation ASIC using NeuroTalk™ interface,” 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, 2006, pp. 2994–2997. [DOI] [PubMed] [Google Scholar]
- [14].Bredeson S, Kanneganti A, Deku F, Cogan S, Romero-Ortega M, and Troyk P, “Chronic in-vivo testing of a 16-channel implantable wireless neural stimulator,” 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015, pp. 1017–1020. [DOI] [PubMed] [Google Scholar]


