Abstract
Aims:
Dual glucose-dependent insulinotropic polypeptide receptor (GIPR)/glucagon-like peptide-1 receptor (GLP-1R) agonists are an emerging class of therapeutics for the treatment of obesity and diabetes that reportedly offer superior weight loss and glycemic control over their GLP-1R agonist (GLP-1RA) monotherapy predecessors. GLP-1RAs enhance satiation and promote consumption of low-fat/low-sugar foods over palatable foods, yet the role of GIPR agonists (GIPRAs) alone or combined with GLP-1RAs to regulate palatable food intake and the role of specific macronutrients in these preferences has not been explored.
Methods:
To understand this regulation, we treated mice and rats on several choice diet paradigms of chow and a palatable food option with individual or dual GIPR and GLP-1R agonists.
Results:
In mice, the dual agonist tirzepatide suppressed total caloric intake, while promoting intake of chow over high fat/sucrose diet. Surprisingly, GIPR agonism alone did not alter food choice. The food intake shift seen with tirzepatide in wildtype mice was completely absent in GLP-1R knockout mice, suggesting that GIPR signaling does not regulate food preference. Tirzepatide also selectively suppressed intake of palatable food but not chow in a rat two-diet choice model. This suppression was specific to lipids, as GLP-1RA and dual agonist treatment in rats on a choice paradigm assessing individual palatable macronutrients robustly inhibited intake of Crisco (lipid) without decreasing intake of a sucrose (carbohydrate) solution.
Conclusions:
Decreasing preference for high-caloric, high-fat foods is a powerful action of GLP-1RA and dual GIPR/GLP-1R agonist therapeutics, which may contribute to the weight loss success of these drugs.
Keywords: Dual GIP/GLP-1 receptor agonists, tirzepatide, food intake, obesity, diet preference
Introduction
Glucagon-like peptide 1 (GLP-1) is a satiation signal synthesized predominantly in two locations in the body: L cells in the intestine and preproglucagon (PPG) neurons in the caudal brainstem 1. GLP-1, released in the gut during a meal, acts as an incretin hormone whose dominant physiologic effects are to increase glucose clearance and enhance satiation 2. These actions make the GLP-1 system an attractive target to treat people with obesity and type 2 diabetes (T2D). Accordingly, long-acting GLP-1 receptor (GLP-1R) agonists were the fastest growing class of anti-diabetic therapeutics in the past decade and offer patients improvements in glycemic control and weight loss 3,4. More recently, development of next generation co-agonist strategies targeting multiple hormone systems have been the focus of some academic labs and pharmaceutical companies, with GLP-1R agonists serving as a favorable foundation to pair with a co-agonist 5. To that end, glucose-dependent insulinotropic polypeptide (GIP), another GI-derived incretin hormone, has emerged as a natural and promising partner for GLP-1. Indeed, dual GIP/GLP-1 receptor agonism in pre-clinical models and clinical trials shows superior glucose-lowering and greater weight loss when compared to that of GLP-1R agonism 6–9. Therefore, there is major interest in understanding how GIPR agonism contributes to the enhanced therapeutic profile of dual GIP/GLP-1 receptor agonists.
In pre-clinical models maintained on a diet of exclusively either chow or high-fat diet (HFD), GLP-1R agonism decreases intake of either diet 10–12. More impressively however, GLP1-R agonist treatment in rats with ad libitum access to both chow and a highly palatable diet causes a shift in food preference to reduce intake of the highly palatable diet in favor of the chow diet 13–15. Given that over consumption of highly palatable food is linked with the development and perpetuation of obesity and T2D 16, these aforementioned findings are extremely provocative when thinking about therapeutic strategies to treat these metabolic diseases by limiting the intake of energy dense palatable foods.
The GIP receptor (GIPR) is expressed centrally in nuclei of relevance to energy balance and peripherally administered GIP analogues activate central GIPRs to reduce caloric intake 17–20. In fact, GIPR activation is required for the synergistic effects of dual GIP/GLP-1 receptor agonism on weight loss and appetite suppression 20. Despite these findings, the role of GIPR agonism alone or in combination with GLP-1 agonism on diet macronutrient preference has not been explored. Indeed, the mechanisms underlying the enhanced effects of GIPR/GLP-1R co-agonism are far from fully elucidated 5. The GIP component of this co-agonist pair has recently been found to provide unique benefits including, but not limited to, blockade of GLP-1-induced nausea and vomiting, weight-independent insulin sensitization, augmented insulin secretion, and lowering of triglycerides through a mechanism distinct from selective GLP-1RAs 21–25. Thus, GIPR agonism may modify or enhance the effects of GLP-1R agonism on food choice and macronutrient intake. As the unimolecular dual GIP and GLP-1 receptor agonist tirzepatide is currently in phase 3 clinical trials and is under regulatory review for indication of T2D in the US, Japan, and EU 8,26, how this investigational agent interacts with both receptor systems on food choice is critical to understanding its regulation of food intake, metabolism, and energy homeostasis. This work examines how individual and combined GLP-1R and GIPR agonism modulates preference for low and high palatable foods and the contribution of specific macronutrients in these preference choices.
Methods and Methods
Animal Models
Adult male wildtype (WT) and GLP-1R KO mice (C57BL/6 background, Taconic) and Sprague-Dawley and Long Evans male rats (Charles River Laboratories) were housed under 12h-light:12h-dark cycle in a temperature and humidity-controlled vivarium. All procedures were approved by the Institutional Care and Use Committee of the University of Pennsylvania and Eli Lilly and Company.
Peptide Synthesis
Long-acting GLP-1 (GLP-140) and GIP (GIP-085) receptor agonists and the dual GIP/GLP-1R agonist tirzepatide were synthesized at Eli Lilly and Company. Doses were chosen from previous preclinical food intake studies 21,22. Sibutramine (molecular weight=334.40 g/mol) was purchased from AH Diagnostics (Aarhus, Denmark).
Pharmacokinetic Studies
The pharmacokinetics of tirzepatide was determined following a single subcutaneous administration of 625 nmol/kg to male and female Crl:CD1(ICR) mice (2 animals/group/time point, 25 to 40 g), or 31 nmol/kg or 104 nmol/kg to female sprague dawley rats. Sparse sampling was conducted, and blood was collected at 4, 12, 24, 48, 72 and 96 hours post dose via cardiac puncture. Blood was collected into tubes containing K3EDTA anti-coagulant. Plasma was harvested then stored at approximately −70°C until bioanalysis. Plasma concentrations of intact tirzepatide were determined by a LC/MS method. The lower limit of quantitation was 3 nM for tirzepatide in mouse plasma. Pharmacokinetic parameters were calculated using the WinNonlin Professional (Version 3.2) software package (Pharsight Corporation, Mountain View, CA).
Tirzepatide Dose Response Studies
Diet-induced obese (DIO) male Long Evans rats were individually housed in a temperature-controlled (24°C) facility with a 12-hour light/dark photoperiod (lights off at 10:00 AM and lights on at 10:00 PM), and had free access to food (TD95217, Teklad, Indianapolis, IN) and water. After acclimatization to the facility, rats were randomized to treatment groups (n=5/group) based on body weight. Animals received daily subcutaneous administration of vehicle or tirzepatide (0.3, 1, 3, 10 and 30 nmol/kg) for 14-days, body weight and food intake were recorded daily.
Mouse Choice Diet Studies
For all studies, mice had ad libitum access to chow (diet #) and a HFD [either a 40% HFD (TD95217; Envigo) or a 60% HFD (D12492; Research diets)]. To avoid hyper-consumption of the HFD upon initial exposure, mice were exposed to their respective HFD for 48 hours followed by 5 days of only chow access before beginning the experiment. On study day one mice were restored ad libitum access to their choice diet and received the first drug treatment. Throughout the duration of the study, mice received daily subcutaneous injections of either vehicle (40mM Tris HCl buffer 0.01% Tween 20 pH 8.0; 0.1mL/10g BW), a long-acting GIPR agonist (GIP-085; 3 nmol/kg), or tirzepatide (3 nmol/kg). Body weight and food intake were recorded daily.
Gubra Diet Induced Obese Preference Study
The gubra preference study paradigm was conducted as previously described 13. Briefly, rats were fed a two-choice diet consisting of a standard rodent chow (Altromin #1324, Brogaarden, Denmark) and the gubra diet, a high palatable high-fat/high-sugar diet made up of a paste (1:1:1) of chocolate spread (Nutella, Ferrero, Italy), peanut butter (Skippy, Unilever, USA) and powdered regular rodent chow (Altromin #1324, Brogaarden, Denmark) for 19 weeks. Rats were stratified by body weight into 4 treatment groups and received daily subcutaneous injections of vehicle (40 mM Tris-HCl pH8 w/0.02% PS-80) or tirzepatide (10 nmol/kg), or oral gavage of sibutramine (5 mg/kg) for 21 days. Body weight and food intake were recorded daily. The week before drug treatments began and during week 3 of dosing, whole body composition was analyzed by non-invasive EchoMRI-900 (EchoMRI, USA). The scanner (QMR systems) measured whole body fat and lean tissue mass. During the scanning procedure, the rat was placed in a restrainer for approximately one minute.
Rat 4-Choice Intake Study
Rats were randomized into treatment group based on initial body weight. Drug treatments and access to a 4-choice high-caloric diet paradigm adapted from 27 began on study day one. Rats received daily intraperitoneal injections of either vehicle (40mM Tris HCl buffer 0.01% Tween 20 pH 8.0; 0.1mL/100g BW), long-acting GIPR agonist (GIP-085; 300 nmol/kg), long-acting GLP-1R agonist (GLP-140; 100 nmol/kg), or a combination of GIP-085 and GLP-140 (combo) one hour prior to the onset of the dark cycle. Throughout the duration of the study, rats had ad libitum access to chow (5001, LabDiet), 10% sucrose solution (w/v), Crisco® (B&G Foods), and water. All components were weighed every 24h prior to the onset of the dark cycle to determine daily intake.
Statistical Analysis
All food intake and body weight data were analyzed using ordinary or repeated measures one-way or two-way ANOVA, followed by Tukey post-hoc tests. All food intake data are expressed as Kcals consumed. All data are expressed as mean ± SEM. For all statistical tests, P < 0.05 was considered significant. All data were analyzed using GraphPad Prism 9.3.1 software (GraphPad Software, San Diego, CA).
Results
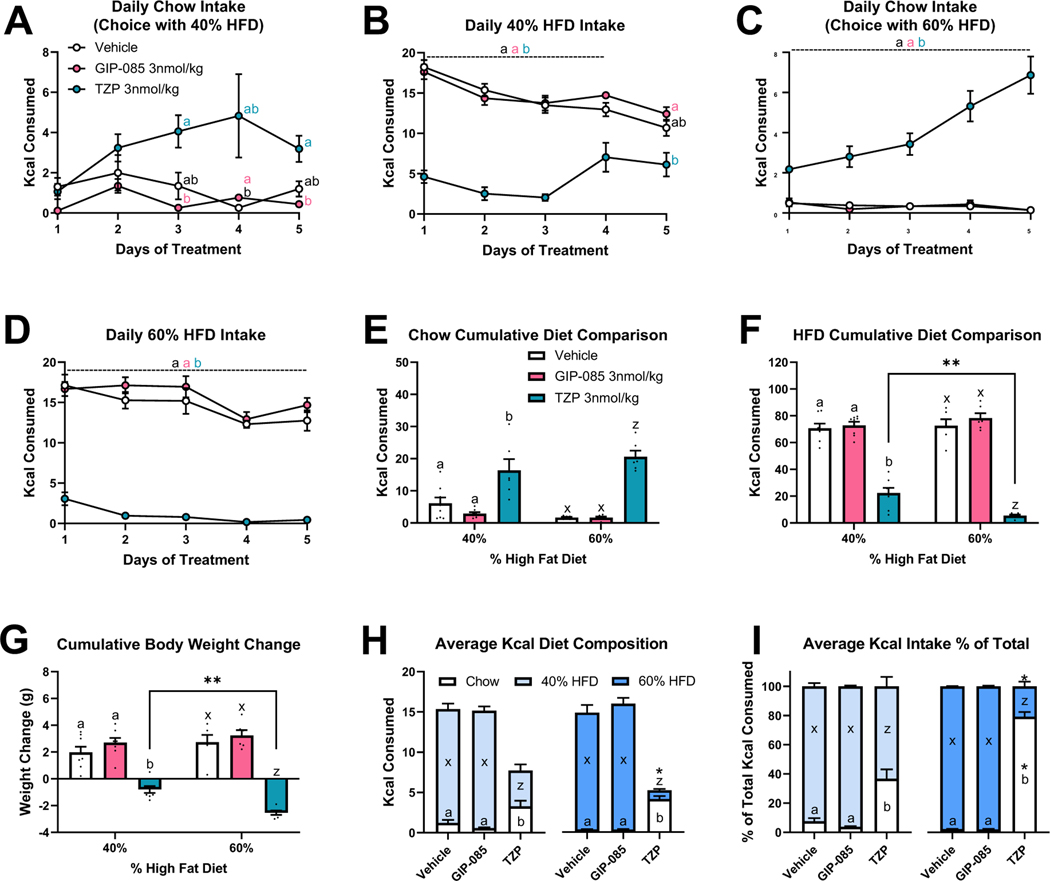
We first investigated the effect of selective GIPR agonism or dual GIPR/GLP-1R agonism on ad libitum choice intake between chow and high-fat diet (HFD). Mice received daily administration of vehicle, a long-acting GIPR agonist GIP-085, or the dual GIP/GLP-1 receptor agonist tirzepatide for 5 days. To determine whether the treatment effect differed based on the content of fat in the diet, one group of mice had access to chow and 40% HFD and a second group of mice had access to chow and 60% HFD. In mice given a choice of chow or 40% HFD, there was an overall effect of chow intake to differ by treatment group (Fig. 1A). Specifically, tirzepatide increased chow intake on days 3 and 5 compared to GIP-085 (Fig. 1A). Additionally, tirzepatide decreased daily 40% HFD intake compared to GIP-085 and vehicle for the first 3 days and compared to GIP-085 on day 5 (Fig. 1B). For mice given a choice of chow or 60% HFD, tirzepatide increased daily chow and decreased daily 60% HFD intake compared to GIP-085 and vehicle treated mice across all 5 days (Figs. 1C–1D). For both diet choice experiments, tirzepatide increased 5-day cumulative chow intake and decreased 5-day cumulative HFD intake compared to vehicle and GIP-085, and tirzepatide suppressed cumulative intake of 60% HFD more than 40% HFD (Figs. 1E–1F). In both the 40% and 60% HFD choice paradigms, tirzepatide treated mice lost weight over the 5 treatment days and tirzepatide-induced weight loss was more robust in mice on the 60% HFD vs the 40% HFD choice diet (Fig. 1G).
Figure 1. Individual GIP and Dual GIP/GLP-1 Receptor Agonism on Choice Diet Preference between Chow and 40% High-Fat Diet (HFD) or Chow and 60% HFD for 5 Days in Mice.
Effect of once daily injections [vehicle, GIP-085, or tirzepatide (TZP)] on daily intake of choice between chow (A) and 40% HFD (B) and in a separate group of mice choice between chow (C) and 60% HFD (D). Five-day cumulative intake of chow (E), intake of HFD (F), and body weight change (G) for both choice experiments. Daily average intake of chow and HFD as Kcals (H) and as a percentage of total Kcal intake (I) for both choice experiments. For panels E-I, comparisons made within a choice experiment are indicated with letter sets (a,b or x,z) and comparisons of the same treatment between choice experiment are indicated by asterisks. N=6–8 per treatment group.
To examine how GIPR and GLP-1R agonism altered total diet composition, we assessed daily average Kcal intake of both chow and HFD as absolute intake and as a % of total intake. Tirzepatide more effectively suppressed average HFD intake in mice with a choice of 60% HFD compared to 40% HFD, both as absolute intake (Fig. 1H) and when expressed as a % of total intake (Fig. 1I). Moreover, while tirzepatide increased average chow intake in both choice diets, the percent of chow intake was significantly higher for mice paired with 60% HFD (79 ± 3.1 %) versus 40% HFD (36.7 ± 8.8 %).
To help interpret these data, we determined the pharmacokinetic profile of tirzepatide in mice. Following a single subcutaneous administration of 625 nmol/kg tirzepatide, the maximum concentration was achieved by 12 hours post dose. The elimination half-life of tirzepatide was approximately 12 hours and the mean clearance of tirzepatide was 8.72 mL/hr/kg (Table 1).
Table 1.
Pharmacokinetic Parameters of Tirzepatide in Male and Female CD-1 mice Following a Single Subcutaneous Administration.
| Dose | Tmax | Cmax | AUC0-inf | T1/2 | CL/F |
|---|---|---|---|---|---|
| nmol/kg | (hr) | (nmol/L) | (hr*nmol/L) | (hr) | (mL/hr/kg) |
| 625 | 12 | 2538 | 71698 | 11.9 | 8.72 |
Abbreviations: AUC0–inf = area under the plasma concentration versus time curve from 0 to infinity, Cmax = maximum plasma concentration, Tmax = time of Cmax, T1/2 = half-life, CL/F = clearance.
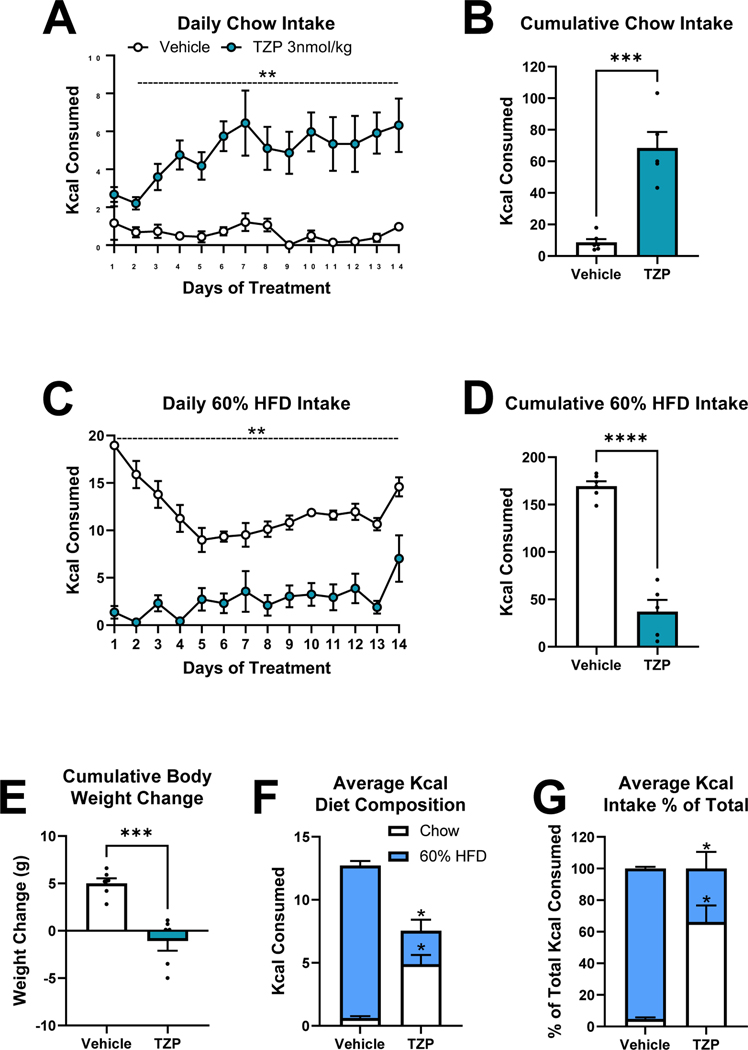
To determine whether the tirzepatide treatment-induced preference shift was an acute response or persisted during chronic treatment, we next treated mice on a choice diet of chow or 60% HFD with vehicle or tirzepatide for 14 days. Tirzepatide treatment elevated daily and 14-day cumulative chow intake, suppressed daily and 14-day cumulative 60% HFD intake, and caused weight loss over the study period (Figs. 2A–2E). The composition of total average daily intake was dramatically shifted in tirzepatide treated mice. Dual GIPR/GLP-1R agonist treatment increased the consumption of chow and decreased the intake of 60% HFD when expressed as absolute Kcal and as a % of total intake (Fig. 2F–2G). In fact, while vehicle treated mice chose to eat only 4.7 ± 1.0 % of Kcal from chow, tirzepatide treated animals increased chow intake to make up 66.1 ± 15.3 % of total Kcal consumed.
Figure 2. Chronic 14-day Dual GIP/GLP-1 Receptor Agonism on Choice Diet Preference between Chow and 60% High-Fat Diet (HFD) in Mice.
Effect of once daily injections [vehicle or tirzepatide (TZP)] on daily (A) and 14-day cumulative (B) chow intake, and daily (C) and 14-day cumulative (D) 60% HFD intake. 14-day cumulative weight change (E). Daily average intake of chow and 60% HFD as Kcals (F) and as a percentage of total Kcal intake (H). Direct comparisons are indicated with asterisks. N=5–6 per treatment group.
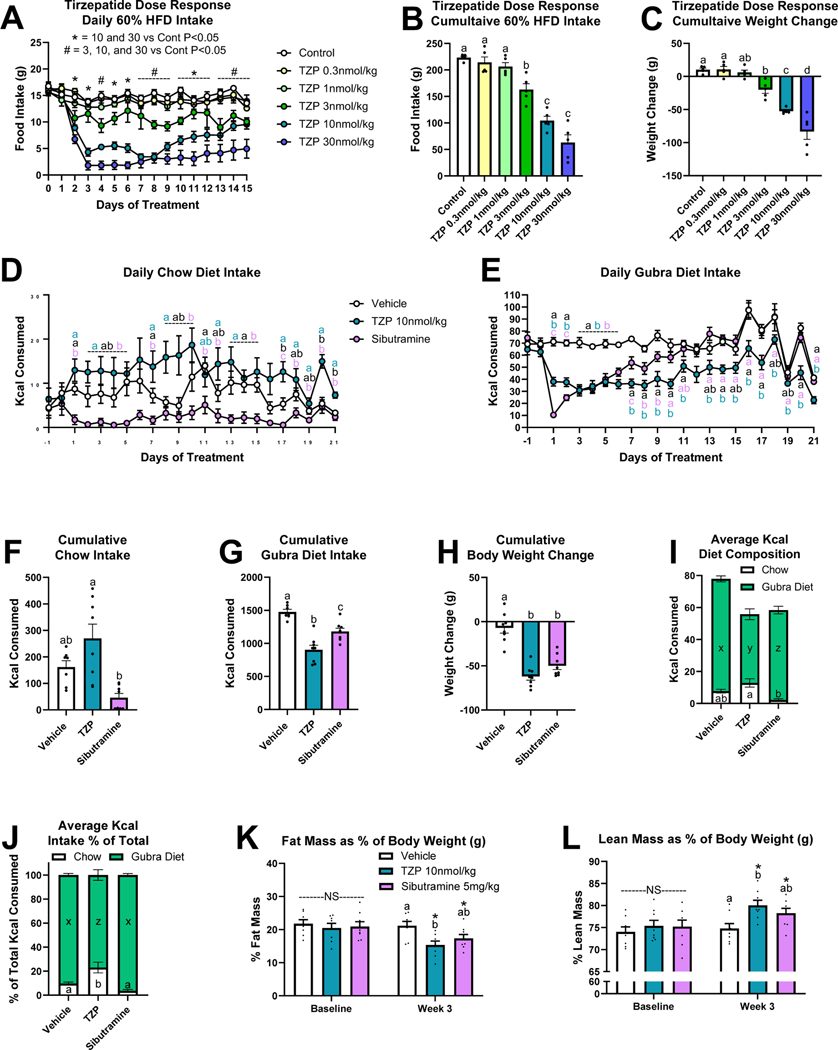
Because dual GLP-1R/GIPR agonist therapeutics are being evaluated as anti-obesity agents in phase 3 clinical trials, we next wanted to compare the actions of tirzepatide on diet preference in obese rats. To select an effective dose of tirzepatide, we first performed a dose response study in diet-induced obese (DIO) rats maintained on only 60% HFD diet. Of the doses tested (0.3, 1, 3, 10, 30 nmol/kg), 10 nmol/kg consistently reduced daily and cumulative food intake and induced weight loss (Figs. 3A–3C). Following a single subcutaneous dose of tirzepatide at 31 nmol/kg and 104 nmol/kg, the mean apparent clearance and mean half-life in rats were 8.31 mL/h/kg and 9.3 h and 10.3 mL/h/kg and 10.3 h, respectively (Table 2). Using the gubra DIO model, rats were fed a choice diet of chow and a highly palatable fat- and sugar-rich diet (gubra diet) composed of equal amounts of the chocolate spread Nutella, peanut butter and powdered chow 13. Following 19 weeks on this choice diet, rats received daily administration of vehicle, tirzepatide, or the well-known anti-obesity agent and sympathomimetic sibutramine for 21 days.
Figure 3. Tirzepatide Dose Response and Two-Choice Preference Intake in Diet-Induced Obese Rats.
Once daily tirzepatide injection dose response [vehicle or 0.3, 1, 3, 10, 30 nmol/kg tirzepatide (TZP)] on daily food intake (A), 15-day cumulative food intake (B), and body weight change (C). N=5 per treatment group. Effect of once daily injections [vehicle, tirzepatide (TZP), or sibutramine] on daily chow (D) and gubra diet (E) intake. 21-day cumulative chow intake (F), gubra diet intake (G), and weight change (H). Daily average intake of chow and gubra diet as Kcals (I) and as a percentage of total Kcal intake (J). Fat mass (K) and lean mass (L) as a percentage of body weight at baseline and week 3 of treatment. Direct comparisons are indicated with letter sets (a,b,c or x,y,z). For panels K-L, comparisons of the same treatment between timepoints are indicated by asterisks. N=8 per treatment group.
Table 2.
Mean Pharmacokinetic Parameters of Tirzepatide in Female Sprague Dawley Rats Following a Single Subcutaneous Administration.
| Dose | Tmax | Cmax | AUC0–96 | T1/2 | CL/F |
|---|---|---|---|---|---|
| nmol/kg | (hr) | (nmol/L) | (hr*nmol/L) | (hr) | (mL/hr/kg) |
| 31 | 12 | 127 | 3742 | 9.3 | 8.31 |
| 104 | 12 | 310 | 10040 | 10.3 | 10.3 |
Abbreviations: AUC0–96hr = area under the plasma concentration versus time curve from 0 to 96 hours, Cmax = maximum plasma concentration, Tmax = time of Cmax, T1/2 = half-life, CL/F = clearance.
While daily chow and gubra diet intake varied among treatment groups over the 3-week period, all drug treatments suppressed intake of the gubra diet for the first 9-days compared to vehicle (Figs. 3D–3E). Dual GIPR/GLP-1R agonism most consistently decreased gubra diet intake, as tirzepatide treated rats ate less gubra diet than vehicle treated rats on 16 of the 21 study days (Fig. 3E). Tirzepatide treatment increased 21-day cumulative chow intake compared to sibutramine treatment, but neither group was different from vehicle (Fig. 3F). Both drug treatments suppressed 21-day cumulative gubra diet intake and body weight change relative to vehicle treated rats (Figs. 3G–3H). Average daily gubra diet intake was decreased by sibutramine treatment compared to vehicle and further suppressed by tirzepatide (Fig. 3I). However, when looking at each diet choice as a percentage of total average daily intake, only tirzepatide treated rats showed a shift in diet preference to eat more chow and less gubra diet than the vehicle group (Fig. 3J). Although not significant, sibutratmine treatment decreased chow intake (3.6 ± 1.2 % of total Kcal versus 9.7 ± 1.3 % of total Kcal for vehicle) while the tirzepatide treatment did not (23.0 ± 4.5 % of total Kcal). Thus, while sibutramine suppressed intake of both food choices, tirzepatide selectively suppressed gubra diet intake. Whole body composition measured at baseline and during week 3 of treatment determined that while both drug treatment groups lost fat mass and gained lean mass as a percentage of body weight relative to their baseline levels, only tirzepatide treated rats had lower fat mass and elevated lean mass relative to vehicle treated rats at week 3 (Figs. 3K–3L).
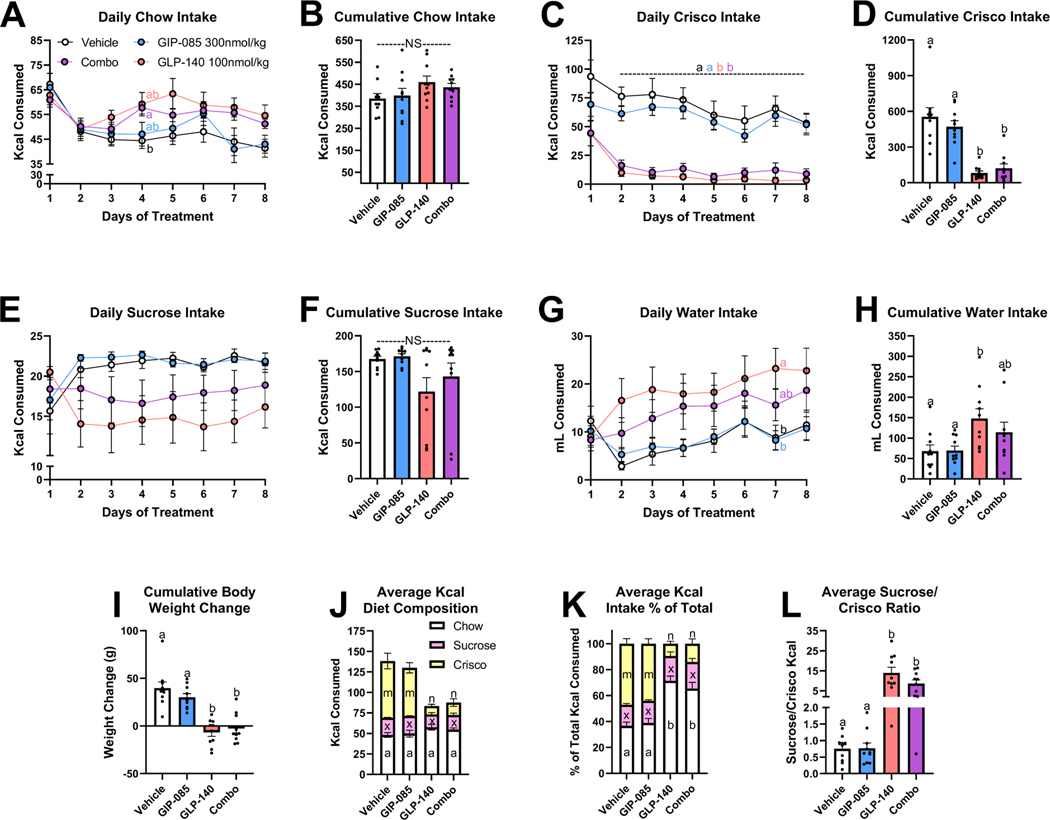
We’ve established that dual GIP/GLP-1 agonism selectively suppresses intake of the high-fat high-sugar palatable diet in a choice paradigm. However, as HFD and the gubra diet both have elevated sucrose and lipid content we wanted to determine whether this suppression was selective towards a certain palatable macronutrient. To test the individual and combined roles of GIPR and GLP-1R activation on altered preference for lipid and carbohydrate intake, rats were placed on an ad libitum diet with access to chow, Crisco, a 10% sucrose solution, and water for 8 days. Rats were treated daily with vehicle, the long-acting GIPR agonist GIP-085, a long-acting GLP-1R agonist (GLP-140) alone, or a combination of GIP-085 and GLP-140. In this case, having two separate molecules instead of a single dual GIP/GLP-1 analog allowed us to evaluate the effects of each individual component separately. Daily and 8-day cumulative chow intake was not different between treatment groups (Figs. 4A–4B). Starting on day 2 of treatment, daily intake of Crisco as well as 8-day cumulative Crisco intake was substantially reduced in GLP-140 and combo treated rats relative to vehicle and GIP-085 treated rats (Figs. 4C–4D). Interestingly, sucrose intake was not significantly affected by any drug treatment (Figs. 4E–4F). GLP-140 treatment increased 8-day cumulative water intake compared to vehicle and GIP-085 treatment (Figs. 4G–4F). GLP-140 and the combo treatment suppressed cumulative weight gain relative to vehicle and GIP-085 treatment (Fig. 4I).
Figure 4. Individual or Combined GIP and GLP-1 Receptor Agonism on Choice Diet Preference between Chow, Crisco, Sucrose, and Water in Rats.
Effect of once daily IP injections [vehicle, GIP-085 (300nmol/kg), GLP-140 (100nmol/kg), or combined GIP-085/GLP-140 (Combo)] of daily (A) and 8-day cumulative (B) chow intake, daily (C) and 8-day cumulative (D) Crisco intake, daily (E) and 8-day cumulative (F) sucrose intake (10% solution), and daily (G) and 8-day cumulative (H) water intake. 8-day cumulative body weight change (I). Daily average intake of chow, sucrose, and Crisco as total Kcals (J), as a % of total Kcal intake (K), and the ratio of sucrose to Crisco Kcal intake (L). Direct comparisons are indicated with letter sets (a,b or x,z or m,n).
Comparing absolute average daily Kcal intake of chow, Crisco, and sucrose, GLP-140 and combo treatment decreased the consumption of Crisco compared against vehicle or GIP-085, but there was no difference between treatment groups in the Kcal intake of chow or sucrose (Fig. 4J). However, when comparing the Kcal intake from chow, Crisco, and sucrose as a % of total Kcal intake, GLP-140 and combo treatment decreased intake of Crisco and increased intake of chow relative to vehicle and GIP-085 treatment (Fig. 4K). The percentage of total Kcal intake made up by sucrose was similar between all treatments. While vehicle and GIP-085 treated rats consumed roughly 25% less Kcals from sucrose than Crisco (Sucrose:Crisco ratio 0.75 ± 0.12 vehicle, 0.77 ± 0.16 GIP-085), GLP-140 and combo treated rats consumed over 800% more Kcals from sucrose than Crisco (Sucrose:Crisco ratio 13.93 ± 2.81 GLP-140, 8.59 ± 1.85 combo). Thus, rats treated with GLP-140 and the combination of GLP-140/GIP-085 robustly favored the intake of carbohydrates over lipids.
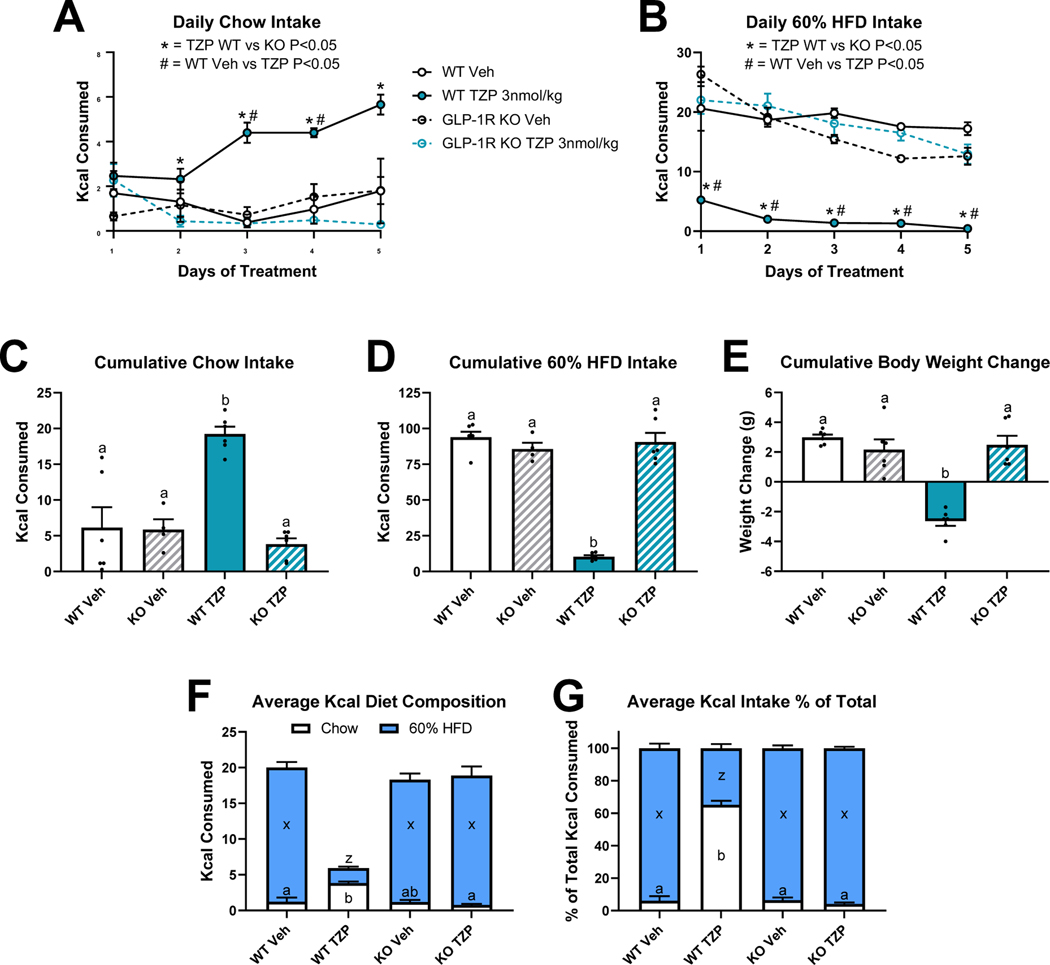
In rats and mice, we’ve observed that treatment with a GIPR agonist had no impact on food preference (Figs. 1 and 4). Furthermore, combined GIP/GLP-1 receptor agonism did not differentially suppress intake of Crisco beyond that seen with GLP-1R agonism. To determine whether the effects of dual GIPR and GLP-1 receptor agonism treatment on choice intake are mediated by engagement of the GLP-1R, we treated WT and GLP-1R knockout mice (GLP-1RKO) with ad libitum access to chow and 60% HFD with vehicle or tirzepatide for 5 days. Tirzepatide treated WT mice ate more chow than tirzepatide treated GLP-1RKO mice on treatment days 2–5, and more than vehicle treated WT mice on days 3 and 4 (Fig. 5A). Daily intake of 60% HFD was decreased by tirzepatide treatment in WT mice relative to tirzepatide treated GLP-1RKO mice and vehicle treated WT mice on all 5 days (Fig. 5B). On no day was chow or 60% HFD intake for tirzepatide treated GLP-1RKO mice different from vehicle treated mice. Tirzepatide treatment in WT mice increased 5-day cumulative chow intake, decreased cumulative intake of 60% HFD, and promoted cumulative body weight loss compared to all other treatment groups (Figs. 5C-5E). In WT mice, tirzepatide treatment increased the contribution of chow and respectively decreased the contribution of 60% HFD when expressed as absolute Kcal and as a % of total intake (Figs. 5F-5G).
Figure 5. GLP-1 Receptor Knockout Eliminates Dual GIP/GLP-1 Receptor Agonism-Induced Preference Shift between 5-Day Choice Chow and 60% High-Fat Diet (HFD) in Mice.
Effect of once daily injections [vehicle or tirzepatide (TZP)] in wildtype (WT) and GLP-1 receptor knockout (KO) mice on daily intake of chow (A) and 60% HFD (B). Five-day cumulative intake of chow (C), intake of 60% HFD (D), and body weight change (E). Daily average intake of chow and 60% HFD as Kcals (F) and as a percentage of total Kcal intake (G). Direct comparisons are indicated with letter sets (a,b or x,z). N=4–6 per treatment group.
Discussion
The food intake suppressive effects of dual GIP and GLP-1 receptor agonism have been investigated only in rodents maintained on one type of diet 6,9,19. However, this does not model the complexity of food choices in human eating behavior. Understanding how GIPR/GLP-1R co-agonism influences the preference for low and high palatability foods and the role of different macronutrients in these choices will help inform the effects of this treatment on energy homeostasis in clinical applications. Herein, we show for the first time that in mice and rats, treatment with the dual GIPR and GLP-1R agonist tirzepatide simultaneously reduced the intake of a palatable high-fat/high-sugar diet and increased the consumption a low-fat chow diet (Figs. 1–3). However, utilizing two different experimental approaches we found that GIPR agonism does not contribute the effect of tirzepatide on food choice in mice or rats. Specifically, we found that dosing a GIPR agonist alone had no impact on food or macronutrient choice (Figs. 1 and 4), and the effect of tirzepatide on food preference was absent in GLP-1RKO mice (Fig. 5). Together, these data suggests that despite GIPRAs established synergistic effect with GLP-1RAs to decrease overall caloric intake 7,28, in this choice paradigm GIPR agonism does not modify the action of GLP-1R agonism to strongly favor consumption of a low-palatability chow diet despite access to a highly palatable HFD.
GLP-1R agonists have been shown to promote intake of chow over high-fat/high-sugar diets or even candy 13–15. One study using the gubra DIO model reported that the GLP-1R agonist liraglutide decreased intake of the gubra diet and simultaneously increased intake of chow 13. In our study following the gubra DIO choice paradigm, we found that tirzepatide also increased chow intake and suppressed gubra diet intake, supporting that both GLP-1R agonism and dual GIPR/GLP-1R agonism selectively suppresses intake of palatable food under choice conditions. In fact, the magnitude of preference shift induced by liraglutide and tirzepatide in the gubra choice DIO model are very similar. Compared to controls, both liraglutide and tirzepatide decreased cumulative gubra diet intake by approximately 60% and approximately doubled cumulative chow intake 13. This supports the hypothesis that the effect of tirzepatide on food preference may be driven by GLP-1R agonism. Interestingly, we observed that tirzepatide more robustly shifted food preference away from HFD in mice when given a choice of chow and 60% HFD, compared to mice given a choice of chow and 40% HFD (Fig. 1). In agreement with these findings, treatment with the GLP-1R agonists in rats maintained on low-fat (17%), medium-fat (40%) or high-fat (81%) diets elicits a stronger and longer-lasting anorectic response and body weight loss as the percentage of fat in the diet increases 12. Collectively, these data suggest that GLP-1R activation may strongly regulate intake of lipids.
To distinguish the effects of individual or combined GIPR/GLP-1R agonism on preference of the high-fat versus high-sugar component of these diets, we tested rats with choice access to lipid (Crisco), carbohydrate (10% sucrose solution), and chow (Fig. 4). GLP-1R agonism alone or in combination with GIPR agonism selectively and effectively inhibited intake of Crisco while having no effect on sucrose intake and increased the contribution of chow to total Kcals consumed. Again, GIPR agonism alone did not alter food preference and had no effect to modify the actions of GLP-1R agonism. Vehicle and GIPR agonist treated rats consumed on average 30% more daily Kcals from Crisco than the sucrose solution. In contrast, GLP-1R agonist and combo treated rats vastly preferred sugar, consuming a daily average of 8–14 times more Kcals from sucrose solution than Crisco.
One study testing the role of liraglutide on macronutrient preference in rats presented with a cafeteria diet that included a low-fat low-sugar, low-fat high-sugar, high-fat low-sugar, and high-fat high-sugar option found that while GLP-1R agonism decreased total Kcals consumed, the intake of any one food type or macronutrient was not significantly affected 29. Differences between the results of Hyde et al. and our findings that GLP-1R agonism and GIPR/GLP-1R co-agonism do selectively suppress fat intake could be explained by several experimental differences in food choice, exposure paradigm, route of administration, drug, and dose. In addition to macronutrient composition, the taste, smell, and consistency of a diet likely influence the animal’s preference for consumption of that food. However, evidence in humans also support that GLP-1R agonists most strongly decreases intake of fat in a diet. Indeed, treatment with GLP-1R agonists in people with obesity, T2D, or type 1 diabetes not only show decreased overall food intake and body weight, but show specific reductions in the % of Kcals consumed from fat and a lower preference for high-fat foods 30–32. Fat is more energy dense (9 kcal/g) than carbohydrates or protein (4 kcal/g), and reducing the energy density of meals has been shown to induce weight loss even when people are not told to restrict calories 33. Thus, diminished caloric intake through specific reduction of fats in a diet may contribute to GLP-1R agonist-induced weight loss.
Food choices following GLP-1R agonist treatment could be a consequence of reduced preference for lipids or an increased preference for carbohydrates. Similarly, these effects could be due in part to an altered perceived taste and reward encoded valuation of these macronutrients that may influence these altered food choices. Liraglutide treatment in T2D patients not only decreased the pleasure response for fatty foods but increased their sensitivity for sweets 34. Interestingly, in addition to the brainstem preproglucagon neurons and L cells in the intestine, GLP-1 is also synthesized locally in taste bud cells of the tongue and modulates taste perception through GLP-1Rs expressed on neighboring taste nerve fibers 35. GLP-1 increases taste sensitivity to solutions of sucrose but not lipids, and surprisingly the addition of lipids to a sucrose solution further enhances the GLP-1R dependent attraction for sucrose 36. When rats are presented with only a sucrose solution or water, GLP-1R agonists do decrease intake of sucrose 15,37. Thus, while GLP-1R agonists may increase the sensitivity for sweet tastes, they don’t overtly increase the consumption of sugar. One explanation could be that given a choice between high-fat or high-sugar foods, GLP-1R agonists induce a preference for carbohydrates over lipids. However, the contribution of lingual GLP-1R signaling in food choice induced by systemic delivery of a GLP-1R agonist has not been directly investigated.
Central GIPRs and both peripheral and central GLP-1Rs mediate the anorectic actions of these hormones 10,20. Two feeding relevant nuclei that express both receptors and have circumventricular access to circulating ligands are the dorsal vagal complex in the brainstem and the hypothalamus 21,38,39. Interestingly, GIPR agonism attenuates GLP-1 induced neuronal activation in the hindbrain dorsal vagal complex, while subthreshold doses of GIP and GLP-1 synergistically increase hypothalamic neuronal activation 19,21. While these nuclei are involved in the hypophagic effects of these hormones, the hindbrain and hypothalamus primarily regulate homeostatic feeding behavior and are unlikely to directly regulate palatable food preferences. Third ventricle exendin-4 administration, which bathes both the hypothalamus and hindbrain, similarly suppress intake of diets with a low or high fat content 12. Higher-order feeding relevant nuclei like the ventral tegmental area (VTA) and nucleus accumbens (NAc) both express GLP-1Rs, receive PPG neuron projections from the nucleus of the solitary tract in the hindbrain, and strongly regulate palatable food intake 15,40. In fact, direct injections of a GLP-1R agonist into the VTA or NAc decreases intake of HFD and increase intake of chow in rats on a choice diet 15. Furthermore, GLP-1R antagonist treatment in the VTA partially blocks peripheral exendin-4 induced hypophagia in rats on a HFD, suggesting that direct VTA GLP-1R signaling is potentially clinically relevant for food intake control 41. Systemically administered exendin-4 was recently demonstrated to penetrate the brain to the mesolimbic reward system and bind VTA GLP-1Rs 41,42. The mechanism by which peripherally administered GLP-1R/GIPR co-agonists regulate palatable food intake, and specifically suppress lipid intake, may very well involve direct and/or polysynaptic routes to modulate the mesolimbic reward system 15,42,43. Future work utilizing GLP-1 and GIP receptor knockout/knockdown models in combination with targeted antagonism of GLP-1Rs will be necessary to elucidate the site of actions underlying incretin regulation of macronutrient preference.
This work is the first to investigate the role of GIPR agonism and dual GIPR/GLP-1R agonism on changes in food preference. Although we report that GIPR agonism does not affect food choice and does not contribute to the tirzepatide mediated preference shift in food intake, possible GIPR-specific effects may be evident under more chronic experimental conditions. As this work focused on the regulation of palatable macronutrients, we specifically investigated the effects of GIPR/GLP-1R agonism on lipid and sugar intake but did not investigate any possible regulation of protein intake. Still, these findings demonstrate that GIP/GLP-1 co-agonism promotes the consumption of healthier food choices over more palatable high-fat high-sugar foods. Given some of the emerging metabolic and antiemetic benefits of GIPR/GLP-1R co-agonism 21,22, understanding their regulation of food preference is pertinent to improving the control of food intake, body weight, and metabolic health of people with obesity and T2D.
Highlights:
Dual GIP/GLP-1 receptor agonists selectively suppress intake of palatable foods
GIPR signaling does not contribute to the suppression of palatable food intake
Dual GIP/GLP-1 receptor agonists suppress intake of lipids over carbohydrates
Funding
This work was supported by an investigator-initiated sponsored agreement from Eli Lilly & Co. (M.R.H.) and NIH-R01-DK021397 (M.R.H.) and -F32-DK127591 (C.E.G.).
This work was supported by an investigator-initiated sponsored agreement from Eli Lilly & Co. (M.R.H.) and NIH-R01-DK021397 (M.R.H.) and -F32-DK127591 (C.E.G.). M.R.H. receives research funding from Boehringer Ingelheim and Novo Nordisk that was not used in support of these studies. M.R.H. is a chief executive officer of Cantius Therapeutics, LLC, that pursues biological work unrelated to the current study. M.P.A., T.C., J.A.M., and R.J.S. are employees of Eli Lilly & Co.
Footnotes
Declaration of Interests
No other potential conflicts of interest relevant to this article were reported.
References
- 1.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(10):R885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell metabolism. 2018;27(4):740–756. [DOI] [PubMed] [Google Scholar]
- 3.Global diabetes therapeutic market has grown sixfold in last 20 years. GlobalData Pharma Intelligence Center 2021.
- 4.Muller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. 2021;46:101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mroz PA, Finan B, Gelfanov V, et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab. 2019;20:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. The New England journal of medicine. 2021;385(6):503–515. [DOI] [PubMed] [Google Scholar]
- 9.Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science translational medicine. 2013;5(209):209ra151. [DOI] [PubMed] [Google Scholar]
- 10.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen MSA, Holm JB, Palleja A, et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Scientific reports. 2019;9(1):15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mul JD, Begg DP, Barrera JG, et al. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(1):R68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen G, Jelsing J, Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO-rats. Acta Pharmacol Sin. 2012;33(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56(1):8–15. [DOI] [PubMed] [Google Scholar]
- 15.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(6):R1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan AM, Vigna SR. Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides. 1994;15(2):297–302. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PloS one. 2012;7(7):e40156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NamKoong C, Kim MS, Jang BT, Lee YH, Cho YM, Choi HJ. Central administration of GLP-1 and GIP decreases feeding in mice. Biochemical and biophysical research communications. 2017;490(2):247–252. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Delessa CT, Augustin R, et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell metabolism. 2021;33(4):833–844 e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borner T, Geisler CE, Fortin SM, et al. GIP Receptor Agonism Attenuates GLP-1 Receptor Agonist-Induced Nausea and Emesis in Preclinical Models. Diabetes. 2021;70(11):2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samms RJ, Christe ME, Collins KA, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. The Journal of clinical investigation. 2021;131(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samms RJ, Coghlan MP, Sloop KW. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends in endocrinology and metabolism: TEM. 2020;31(6):410–421. [DOI] [PubMed] [Google Scholar]
- 24.Pirro V, Roth KD, Lin Y, et al. Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes. J Clin Endocrinol Metab. 2022;107(2):363–378. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. [DOI] [PubMed] [Google Scholar]
- 26.Frias JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2020;15(6):379–394. [DOI] [PubMed] [Google Scholar]
- 27.Slomp M, Belegri E, Blancas-Velazquez AS, et al. Stressing the importance of choice: Validity of a preclinical free-choice high-caloric diet paradigm to model behavioural, physiological and molecular adaptations during human diet-induced obesity and metabolic dysfunction. Journal of neuroendocrinology. 2019;31(5):e12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norregaard PK, Deryabina MA, Tofteng Shelton P, et al. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes, obesity & metabolism. 2018;20(1):60–68. [DOI] [PubMed] [Google Scholar]
- 29.Hyde KM, Blonde GD, le Roux CW, Spector AC. Liraglutide suppression of caloric intake competes with the intake-promoting effects of a palatable cafeteria diet, but does not impact food or macronutrient selection. Physiology & behavior. 2017;177:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, obesity & metabolism. 2017;19(9):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dube MC, D’Amours M, Weisnagel SJ. Effect of liraglutide on food consumption, appetite sensations and eating behaviours in overweight people with type 1 diabetes. Diabetes, obesity & metabolism. 2020;22(8):1417–1424. [DOI] [PubMed] [Google Scholar]
- 32.Quast DR, Nauck MA, Schenker N, Menge BA, Kapitza C, Meier JJ. Macronutrient intake, appetite, food preferences and exocrine pancreas function after treatment with short- and long-acting glucagon-like peptide-1 receptor agonists in type 2 diabetes. Diabetes, obesity & metabolism. 2021;23(10):2344–2353. [DOI] [PubMed] [Google Scholar]
- 33.Rolls BJ. The relationship between dietary energy density and energy intake. Physiology & behavior. 2009;97(5):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brindisi MC, Brondel L, Meillon S, et al. Proof of concept: Effect of GLP-1 agonist on food hedonic responses and taste sensitivity in poor controlled type 2 diabetic patients. Diabetes Metab Syndr. 2019;13(4):2489–2494. [DOI] [PubMed] [Google Scholar]
- 35.Jensterle M, Rizzo M, Janez A. Glucagon-Like Peptide 1 and Taste Perception: From Molecular Mechanisms to Potential Clinical Implications. Int J Mol Sci. 2021;22(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin C, Passilly-Degrace P, Chevrot M, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. Journal of lipid research. 2012;53(11):2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang XJ, Wang YQ, Long Y, et al. Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide. Peptides. 2013;47:115–123. [DOI] [PubMed] [Google Scholar]
- 38.Adriaenssens AE, Biggs EK, Darwish T, et al. Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake. Cell metabolism. 2019;30(5):987–996 e986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burmeister MA, Ayala JE, Smouse H, et al. The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice. Diabetes. 2017;66(2):372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(14):4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American journal of physiology Endocrinology and metabolism. 2013;305(11):E1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez NS, Ige KY, Mietlicki-Baase EG, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018;43(10):2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]