Abstract
Primary tumors secrete a variety of factors to turn distant microenvironments into favorable and fertile ‘soil’ for subsequent metastases. Among these ‘seeding’ factors that initiate pre-metastatic niche (PMN) formation, tumor-derived extracellular vesicles (EVs) are of particular interest as tumor EVs can direct organotropism depending on their surface integrin profiles. In addition, EVs also contain versatile, bioactive cargo, which include proteins, metabolites, lipids, RNA, and DNA fragments. The cargo incorporated into EVs is collectively shed from cancer cells and cancer-associated stromal cells. Increased understanding of how tumor EVs promote PMN establishment and detection of EVs in bodily fluids highlight how tumor EVs could serve as potential diagnostic and prognostic biomarkers, as well as provide a therapeutic target for metastasis prevention. This review focuses on tumor-derived EVs and how they direct organotropism and subsequently modulate stromal and immune microenvironments at distal sites to facilitate PMN formation. We also outline the progress made thus far towards clinical applications of tumor EVs.
Keywords: Tumor-derived EVs, Organotropism, Pre-metastatic niche reprogramming, Biomarker, Therapy
Introduction
More than a century ago, Stephen Paget proposed the “seed and soil” hypothesis to explain the nonrandom pattern of cancer metastasis [1]. Paget’ s theory was strengthened when Isaiah Fidler provided the first experimental evidence for organotropic metastasis in the 1970’s. Fidler demonstrated that metastatic dissemination was not determined by nonspecific arrest of circulating tumor cells in the capillary bed of the first organ encountered, but rather it was dictated by both the properties of tumor cells (“the seed”) and distant organ environments (“the soil”) [2, 3]. Since then, much emphasis has centered on tumor cell biology while the impact of distant host tissues in metastasis remained elusive. However, in 2005, Lyden and colleagues discovered that tumors induced the formation of a pre-metastatic niche (PMN) prior to the arrival of metastatic cells. They showed that primary tumor-secreted factors promote vascular leakiness and upregulate the synthesis of extracellular matrix (ECM) components, including fibronection, in specific distal organ sites which in turn recruited VEGFR1 and VLA-4 (a fibronectin receptor) positive bone marrow-derived haematopoietic progenitor cells[4] to these sites. The changes in local stromal cells and the recruited bone marrow cells lead to the upregulation of pro-inflammatory mediators, including S100 proteins, creating an immune suppressive microenvironment and immune priveledged niche for incoming tumor cells.
In contrast to Paget’s theory where distant organs were thought to be inherently receptive to metastatic cells, Lyden’s further studies demonstrated that in addition to tumor-secreted soluble factors, including growth factors and chemokines[4, 5], tumor-derived extracellular vesicles (EVs)[6-8] also actively remodel these distal organs to support metastatic seeding.
Tumor-derived nano-sized EV subpopulations termed exosomes, contain a variety of proteins, lipids, RNA, and DNA, that are derived from the tumor cells and tumor-associated stromal cells. The heterogenous small EV subpopulations and their specific “cargoes” are not only responsible for creating the PMN but also for directing organotropic seeding of disseminated metastatic cells. Recent technical advances have allowed for high resolution separation of exosomes into large exosome vesicles (90-120 nm), small exosome vesicles (60-80 nm), and non-membranous exomeres (~35 nm) and has facilitated our understanding of molecular composition, biodistribution, and functions of the EV subtypes [9, 10]. The increased mechanistic understanding of tumor EV-mediated PMN formation and EV biogenesis have revealed potential opportunities to predict and prevent metastasis. For example, EVs may serve as surrogate biomarkers for the early detection of metastasis, and blocking EV biogenesis, secretion, and uptake may inhibit PMN formation to prevent metastasis.
This review will focus on how tumor-derived EVs facilitate PMN formation and direct organotropism and provide a brief summary of selective EV cargo molecules that support this process. In addition, therapeutic and diagnostic applications of tumor-derived EVs for the prevention and treatment of metastasis are also discussed.
EV cargos
EVs cargo consists of bioactive molecules, including proteins, glycans, lipids, and nucleic acids. While EV cargos generally reflect the biosynthetic status of the cell of origin, there are cases when EVs show an enrichment for specific bioactive molecules. For instance, ~35% of the entire spectrum of proteins produced in pancreatic ductal adenocarcinoma (PDAC) cells are present in EVs, which are associated with critical cancer pathways [11]. However, some proteins, lipids, and miRNA can be enriched in EVs as compared to the cell of origin. For example, EGFRvIII oncoprotein[12] and 11 miRNAs[13] are more highly concentrated in EVs than in the glioma cells of origin. While mechanisms detailing the trafficking of cellular molecules into EVs have been proposed[14], the underlying mechanism of cargo selection remains largely unknown.
Proteins are well-studied EV cargo and proteomic technologies have provided clues to EV biogenesis, targeting, and function. Notable EV protein classes include transmembrane proteins, such as tetraspanins, signaling receptors, and integrins, as well as intraluminal proteins, including heat shock proteins (HSPs), cytoskeletal proteins, endosomal sorting complex required for transport (ESCRT) proteins, RNA-binding proteins, and ribonucleoproteins [15]. Twenty-two consistently abundant proteins in EVs were recently identified from 14 cell lines irrespective of the isolation method, with most being transmembrane proteins and GTPases[16]. Secretory proteins such as growth factors and cytokines are normally exported through the classical endoplasmic reticulum (ER)/Golgi-dependent pathway[17, 18]. Whether these these secretory proteins can be packaged into EVs as an unconventional secretion pathway [19] remains controversial since proteomic detection of cytokines, chemokines, or growth factors is rarely reported. Meanwhile HSPs (e.g., HSPA8, HSP90AB1), tetraspanins (e.g., CD9), ESCRT proteins (e.g., TSG101), cytoskeletal proteins (e.g., ACTB, moesin), and GTPases (e.g., RAP1B) are common among EVs and, therefore, can serve as pan-EV markers. Other proteins, such as versican, tenascin C, and thrombospondin 2, are specific tumor tissue EV markers. Among various EV protein families, integrins are of particular interest due to their crucial role in guiding cellular tropism of EVs and their use as biomarkers of metastatic organotropism[8]. In contrast to integrins, some proteins can block EV uptake. For example, CD47, an anti-phagocytic signal, is ubiquitously expressed on tumor cell-derived EVs and prevents EV uptake by immune cells to prolong systemic EV circulation[20]. The enrichment of cancer-specific proteins in tumor-derived EVs provides a wealth of novel biomarkers for diagnosis and prognosis of various cancers. A number of EV protein biomarkers have proven clinical utility, including elevated levels of melanoma-specific protein TYRP2, VLA-4, HSP70, and the oncoprotein MET found in circulating EVs from melanoma patients [6]. Exploration of EV biomarkers specific to different cancer types can be a future direction to pursue due to the potential application to increase the yield of tumor-specific material and decrease unwanted background in downstream analyses.
Most types of EVs, including exosomes, microvesicles, and apoptotic bodies, are surrounded by a lipid bilayer originating from the endosome or cell membrane. As another example of cargo selection, EVs have been demonstrated to exhibit 2–3-fold enrichment of cholesterol, sphingomyelin, glycosphingolipids, and phosphatidylserine, but package lower concentrations of phosphatidylcholine and phosphatidylinositol compared to lipids in cellular membranes. The lipid composition of EVs also varies based on cell of origin[21]. EVs isolated from melanoma and osteosarcoma were enriched in multiple saturated and unsaturated fatty acid as compared to melanocytes and osteoblasts. The tumor EV fatty acid cargo, particularly palmitic acid, induces tumor necrosis factor alpha (TNFα) secretion by Kupffer cells, which generates a proinflammatory microenvironment and promotes fatty liver formation [22]. In another lipidomic comparison of EVs derived from high- and low-metastatic triple-negative breast cancer cells, increased accumulation of unsaturated diacylglycerols was found within EVs from high-metastatic cells, which is associated with enhanced angiogenesis[23]. In addition to membrane-bound vesicle structures, Lyden and colleagues discovered a minuscule (~35nm) non-membranous EV subpopulation termed “exomere”, which contains less lipid content than exosomes [10]. Exomeres, as compared to exosomes, selectively package metabolic-associated proteins and enzymes. Significant variations in the relative levels of ceramide, triglyceride, lysophosphatidylglycerol, glycosphingolipid, and mitochondrion-specific lipids between exomeres versus exosomes were detected across different cancer cell-types.
In EVs, lipid and protein cargo are heavily glycosylated and reflect the glycosylation patterns of host cells [24]. Glycosylation profiles differ markedly between normal and tumor or metastatic cells, and characterization of glycan structure and glycosylation sites in EV cargo, might also identify new biomarkers for cancer diagnosis and prognosis. For example, EVs derived from a metastatic colorectal cancer (CRC) cell line showed increased O-GlcNAcylation of cadherin and ATPase superfamilies of proteins compared to EVs derived from non-metastatic cells [25]. In contrast, increased amounts of bisecting GlcNAc branches on integrin β1 in breast cancer or cancer cell-derived EVs interferes with interactions with galectin-3 and FAK/AKT signaling activation to block carcinogenesis and metastasis[26].
Distinct glycolipids on tumor cell-derived EVs also interfere with biological processes in recipient cells. For example, the disialoganglioside GD3, a specific sialic acid-containing glycosphingolipid, is enriched in EVs secreted from melanoma and ovarian cancer cells and inhibits T cell activation and stimulates migration of normal cells [27, 28]. As glycosylated components are recognized by the ESCRT [29], manipulation of protein glycosylation via chemical or genetic engineering has been exploited to facilitate selective cargo sorting into EVs[30, 31]. A recent study overexpressing α (1,6) fucosyltranferase, an oncogenic-associated glycotransferase, in prostate cancer cells resulted in an increased abundance of proteins associated with metastasis in EVs derived from engineered cancer cells[32]. Glycosylation also impacts recognition and uptake of EVs by recipient cells and biodistribution. Surface glycosylation has been widely reported to suppress EV uptake[33-35]. Specifically, removal of N- and/or O-glycosylation in breast cancer cell-derived EVs enhances their uptake by endothelial cells[35]. O-deglycosylation of breast cancer cell-derived EVs enhanced EV accumulation in lungs, whereas N-deglycosylation did not alter biodistribution. Since N-glycosylation represents the majority of the cellular glycome, researchers developed nanosomes coated with N-glycans derived from various cancer cells as a model to study functional roles of surface N-glycosylation of tumor derived EVs and the impact on systemic dissemination and organotropic biodistribution[36]. High levels of mannose-type N-glycans on nanosomes leads to rapid clearance by C-type lectin-expressing tissue-resident dendritic cells (DCs) and macrophages. In contrast, reduced levels of mannose-type N-glycans and increased fraction of Neu5 Ac-terminated N-glycans prolong in vivo circulation and extrahepatic distribution.
Nucleic acid EV cargos can transfer genetic information to cells and alter the recipient cell’s gene expression and phenotype. The most widely studied nucleic acid in cargos of EVs is RNA. In EVs, RNA subtypes include mRNAs and various non-coding RNAs (ncRNAs), such as small non-coding RNAs, miRNAs, transfer RNA fragments, small nuclear RNA (snRNA), small nucleolar RNA, PIWI-interacting RNA, long non-coding RNA (lncRNA), ribosomal RNA, mitochondrial RNA, and circular RNA (circRNA) . The small ncRNAs have a peak size of 200bp but EV RNAs can extend to 5kb or more [37].
miRNAs are the most abundant small ncRNAs in EVs and inhibit translation of target mRNA in recipient cells. Various miRNAs within tumor-derived EVs have been identified which promote PMN formation. For example, breast cancer cell-derived EV miR-200b-3p[38] and miR-21[39] are taken up by alveolar epithelial type II cells and osteoclasts and facilitate PMN establishment in lung and bone, respectively. MiR-105 is another breast cancer-derived EV miRNA that disrupts endothelial tight junctions, thus promoting metastases in the lung and brain[40]. Interestingly, a liver-specific miRNA, miR-122-5p, is highly enriched in EVs derived from lung cancer cells, which promotes migration and epithelial-mesenchymal transition (EMT) in liver epithelial cells to create a liver PMN [41]. Other tumor-derived EV miRNAs involved in PMN generation include hepatocellular carcinoma (HCC)-derived EV miR-1247-3p for lung metastasis [42] and colorectal cancer-derived EV miR-934 for liver metastasis[43]. snRNA is another small ncRNA subtype enriched in lung tumor-derived EVs and induces chemokine release from lung alveolar epithelium via Toll-like receptor 3 (TLR3) to recruit neutrophils to support lung PMN formation [44].
LncRNAs are ncRNAs longer than 200bp that possess diverse regulatory functions, including negative regulation of miRNAs by serving as “miRNA sponges”, marking of mRNAs for degradation and transcriptional regulation of genes. Intriguingly, some lncRNAs, with relatively low cellular expression levels, such as HOX transcript antisense intergenic RNA (HOTAIR), are highly enriched in EVs[45], indicating an indispensable role of EV-transferred lncRNAs in biological processes. Elevated levels of HOTAIR in tumor EVs can facilitate metastases by blocking expression of the HoxD10 tumor suppressor gene which inhibits breast cancer cell migration and metastasis [46]. In addition, HOTAIR mediated loss of HoxD10 could lead to increased angiogenesis, although this has not been directly investigated [47]. A number of studies have demonstrated increased cancer cell proliferation, migration, and invasion upon transfer of EV lncRNA [48-52]. A large number of changes in lncRNA expression were observed in lung fibroblasts treated with breast cancer EVs, which resulted in increased proliferation and migration of fibroblasts[53]. While the impact of cancer-derived EV lncRNA on phenotypic changes in fibroblasts remains to be determined and awaits testing in vivo, this suggests cancer -derived EV lncRNAs could promote PMN formation.
In addition, some protein-coding RNAs, such as mRNA and circRNA, have been detected in tumor-derived EVs and expressed in EV recipient cells. Ovarian cancer cells secrete EVs containing mRNA encoding matrix metalloproteinase (MMP)1 and EV uptake in mesothelial cells leads to expression of MMP1 resulting in destruction of the peritoneal mesothelial barrier to allow tumor spread [54]. Similarly, circRNA encoding ubiquitin-like with PHD and ring finger domain 1 (UHRF1), a critical protein for regulating DNA methylation, is overexpressed in various cancer types, including HCC and was detected in HCC-derived EVs[55]. Upon EV uptake by NK cells, circUHRF1 is expressed and induces NK cell exhaustion by suppressing interferon (IFN)γ and TNFα secretion.
RNA packaging into EVs involves multiple mechanisms, including specific RNA sequence motifs and secondary configurations, differential affinity for membrane lipids, association with RNA-binding proteins (RBP), and other sorting signals such as ubiquitylation, sumoylation, phosphorylation, and uridylation RNA and RBP modifications [37].
EV-associated DNA was recently discovered within the past decade[56] and has not been as extensively investigated. Single-stranded DNA[57], double-stranded DNA (dsDNA)[58, 59], and mitochondrial DNA (mtDNA)[60, 61] have repeatedly been detected in EVs with the DNA located both within and on the surface of EVs. The predominant form of EV DNA is external dsDNA greater than 2.5kb in size, as indicated by >50% reduction in EV DNA post dsDNase digestion[58]. While the mechanism of DNA packaging into EVs remains largely unexplored, a recent study showed that dsDNA inclusion into tumor-derived microvesicles is regulated by activation of a small GTPase, ADP-ribosylation factor 6 [62]. EV-associated DNAs are derived from both nucleus and mitochondria and can reflect the genome mutational status of parental cells[58, 59]. This also raises the potential for using EV DNAs as biomarkers for cancer diagnosis and prognosis. As most cell-free DNA (cfDNAs) have been reported to associate with EVs [63, 64] and EV DNAs are relatively intact compared with non-vesicular cfDNA (~130bp in size), it is not surprising that EV DNAs show superior sensitivity and specificity compared to non-vesicular cfDNA in identifying mutations in patients with early-stage non-small-cell lung cancer [65]. In limited studies with EV DNAs derived from cancer cells, immune modulation was triggered by pathways downstream of EV DNAs in the recipient cell [56].Using chemotherapy-treated or irradiated tumor cells secreted EVs enriched with dsDNA, the cytosolic DNA damage receptor cyclic GMP-AMP synthase (cGAS) in recipient DCs was activated followed by subsequent recruitment and activation of stimulator of interferon genes (STING) resulting in cytokine release and anti-tumor immune responses[66, 67]. In addition, horizontal gene transfer and transcription of exogenous EV DNAs into recipient cells has also sparked much interest[56]. Donor cell-derived EV-genomic DNA localized to the nucleus of recipient HEK293 cells. Leukemia K562 cells secrete EVs containing the unique K562 genes, BCR/ABL hybrid gene involved in the pathogenesis of chronic myeloid leukemia. Uptake of K562-derived EVs by HEK293 cells induced the expression of BCR/ABL gene at both the mRNA and protein level[68]. The horizontal gene transfer of EV-associated DNAs is not limited to genomic DNA. Cancer-associated fibroblasts package mtDNA into EVs, and the subsequent uptake by hormone therapy-resistant breast cancer cells contributed to upregulation of mitochondrial genes necessary for oxidative phosphorylation[61]. Whether or how tumor-derived EV DNAs also contribute to PMN formation remains to be explored.
Metastatic organotropism
Metastatic organotropism refers to the ability of certain tumor types to disseminate to and colonize specific distant organs, such as lungs, liver, brain, and bone[69]. Although organotropism was first described by Stephen Paget well over a century ago, regulation of this crucial aspect of metastasis has largely remained a mystery. The poor understanding of the drivers of organotropism underlies the lack of effective predictive measures and therapies for metastasis. However, recent work has implicated tumor-derived EVs as critical determinants of metastatic organotropism. EV-dependent organotropism relies on the enrichment of specific molecules in organotropic cancer cell-derived EVs, as well as on reprogramming cell types unique to the various organ niches[7, 8, 44, 70]. Notably, EV-mediated organotropism depends on integrin adhesion molecules on the surface of tumor cell EVs, which bind specific extracellular matrices in metastatic organs. This adhesion enables uptake of EVs by cells at metastatic sites for PMN reprogramming.
Studies of lung and liver metastasis have been particularly insightful in revealing the important role of EV integrins in directing organotropic metastasis, illuminating how distinct EV integrin repertoires select for the specific extracellular matrices of these organs[8]. EVs derived from lung-tropic breast cancer cells have increased levels of integrins α6β1 and α6β4 compared to EVs from bone-tropic and brain-tropic breast cancer cells or liver-tropic pancreatic cancer cells. Importantly, these two integrins bind to the laminin-rich extracellular matrix of the lung microenvironment to promote targeting of breast cancer-derived EVs to the lung. Remarkably, education of mice with EVs derived from lung-tropic breast cancer cells was sufficient to confer lung metastatic outgrowth of bone-tropic breast cancer cells. By contrast, EVs from liver-tropic metastatic cancer cells carry αvβ5, which binds fibronectin in the liver, favoring hepatic uptake of EVs secreted by liver-tropic cancer cells.
Other EV integrins may also be essential for metastasis to additional organ sites. For instance, integrin α5 packaged into breast cancer derived EVs enabled breast cancer metastasis to bone by creating an osteogenic PMN following osteoblast uptake of integrin α5 EVs[71]. EVs secreted by brain-tropic metastatic breast cancer cells package integrins α2, α3, β1 and β3, suggesting a potential role in organtropic brain metastasis[8]. Many other integrins have been implicated in organotropic metastasis, indicating their loading into EVs may similarly promote EV-dependent PMN formation and organotropic metastasis[72]. Notably, EVs secreted by various cancers have been shown to participate in the preparation of a favorable lymphatic niche for tumor cell metastasis[73]. Tumor cell integrins that are critical for lymph node metastasis, namely integrin α4β1, may also support EV-mediated education of lymph nodes during cancer progression[72].
An additional key aspect of organotropism involves the uptake of EVs by particular cell types within each organ that contribute to generation of favorable niches for metastatic outgrowth. In lungs, EVs from lung-tropic cancer cells are uptaken by fibroblasts and epithelial cells[8, 44]. As a result, fibroblasts in the lung stromal niche become activated to express PMN factors, including fibronectin and S100 molecules. Fibroblast activation is mediated by integrin β4 which is enriched in lung-tropic EVs compared to other EVs[8]. Additionally, EV-educated type II alveolar epithelial cells in lungs increase secretion of chemokines, namely CXCL1, CXCL2, CXCL5, and CXCL12, that attract pro-metastatic neutrophils to the PMN[44]. In the liver, pancreatic cancer cell EVs are taken up by liver-resident macrophages, known as Kupffer cells, which then secrete TGFβ to initiate a cascade of intercellular crosstalk resulting in activation of hepatic stellate cells, increased fibronectin deposition, and recruitment of pro-metastatic bone marrow-derived cells[7]. Organotropic brain metastasis is also regulated by the uptake of EVs by cells unique to the brain microenvironment, including microglia and brain endothelial cells to influence the brain vascular niche[70]. Cell migration-inducing and hyaluronan-binding protein (CEMIP), is distinctly enriched in EVs from brain-tropic cells and mediates the EV-dependent formation of a metastasis supporting vascular niche in the brain.
Reprogramming PMNs
Reprogramming PMN-associated stromal cells
A key step in PMN formation is reprogramming endothelial cells to increase adhesion, vascular permeability, angiogenesis, and lymphangiogenesis, which all facilitate adhesion, extravasation, and colonization of tumor cells at secondary organs. Vascular endothelial growth factor (VEGF) was the first reported molecule promoting PMN formation via induction of angiogenesis as well as mobilization and recruitment of VEGFR1+ hematopoietic progenitors to the distant site[4]. In addition to being secreted as soluble factors, VEGF isoforms such as VEGF-A, VEGF-C and VEGF90k have been detected in EVs isolated from glioblastoma (GBM), pancreatic ductal adenocarcinoma (PDAC) and breast cancer cells, and increase vascular and/or lymphatic endothelial cell proliferation and permeability in vitro[74-76]. As EVs in circulating blood of GBM patients posess elevated levels of VEGF-A compared to healthy donors[74], the VEGF+EVs could enhance vascular permeability and angiogenesis both locally and at a distance. Compared to normal melanocytes or poorly metastatic melanoma cell-derived EVs, nerve growth factor receptor (NGFR) is markedly upregulated in small EVs secreted by highly metastatic melanoma cells [77]. The EV-associated NGFR induces lymphangiogenic gene expression and VEGFR-3 phosphorylation, ERK and nuclear factor-κB (NFκB) activation, as well as intracellular adhesion molecule (ICAM)-1 expression in lymphatic endothelial cells (LECs), promoting endothelial cell proliferation and tumor cell adhesion. Annexin II, a Ca2+-dependent phospholipid-binding protein associated with the plasma membrane and endosomal system, is one of the most highly expressed proteins in EVs that promotes breast cancer metastasis to the lung and brain via tissue plasminogen activator-dependent angiogenesis and activation of macrophages and proinflammatory signaling at the secondary organ[78]. Amphoterin-induced gene and open reading frame 2 (AMIGO2) is a novel cell adhesion molecule that can be transferred from tumor cells to the surface of endothelial cells via EVs. Hepatic sinusoidal endothelial uptake of AMGIO2-containing EVs derived from cancer cells, resulted in greater adhesion by gastric cancer cells which may facilitate liver metastasis [79]. Unsaturated diacylglycerols in EVs from highly metastatic breast cancer cells also promote angiogenesis and metastasis by activation of protein kinase D signaling in endothelial cells[23]. Several tumor cell-derived EV miRNAs and lncRNAs, such as miR-221-3p[80] and ELNAT1[81] correlate with lymphangiogenesis and lymphatic metastasis. EV-mediated ELNAT1, in particular, correlated with lymph node metastasis and poor prognosis in bladder cancer patients[81]. Other EV-associated ncRNAs (e.g., miR-23a[82], CCAT2[83]) suppress the tight junction protein ZO-1, upregulate VEGF-A expression, and inhibit apoptosis of endothelial cells, thereby enhancing vascular permeability and angiogenesis in the tumor microenvironment. Whether these EV-associated ncRNAs similarly promote PMN formation at distant sites remains to be determined.
As mentioned above, activated fibroblasts are another subset of heterogenous stromal cells that are largely reprogrammed from resident tissue fibroblasts to facilitate PMN formation. Activated fibroblasts secrete ECM molecules (e.g., fibronectin (FN)) and MMPs, as well as transforming growth factor β (TGFβ), S100 calcium binding protein A4 (S100A4), interleukin (IL)-6, C-C motif chemokine ligand (CCL)2, and stromal cell-derived factor 1 (SDF1), which attract bone marrow-derived haematopoietic progenitor cells and forge a proangiogenic and antiapoptotic microenvironment permissive to incoming tumor cells[76]. Various tumor-derived EV factors have been linked to fibroblast activation and heterogeneity in distal tissues [84]. For instance, the primary CRC-derived integrin beta-like 1 (ITGBL1)-enriched EVs can activate lung fibroblasts and hepatic stellate cells (HSCs ), the predominant resident fibroblasts in the liver, via TNFα-induced protein 3 (TNFAIP3)-mediated NFκB signaling[85]. The activated fibroblasts, in turn, produce high levels of pro-inflammatory cytokines, such as IL-6 and IL-8, and promote stemness, migration, and EMT of CRC cells. A similar integrin beta 1 (ITGB1)-NFκB signaling-mediated activation of lung fibroblasts was observed with miR-1247-3p-enriched EVs isolated from highly-metastatic HCC cells[42]. By directly targeting fibroblast β-1,4-galactosyltransferases III (B4GALT3), a glycosylation protein that inhibits ITGB1 activation and stability, exosomal miR-1247-3p promotes the formation of both intrahepatic metastasis niches and lung PMNs by primary HCC[42]. The correlation between nidogen 1 (NID1) in tumor cell-derived EVs or circulating EVs from HCC with metastatic potential, was recently identified. EV-NID1 not only destabilizes the vascular architecture and promotes angiogenesis in the lung, but also activates pulmonary fibroblasts to secrete soluble TNFR1, which facilitates HCC cell growth, mobility, and colonization into the lung[86]. A robust increase of tissue transglutaminase-2 (TG2), an ECM-crosslinking enzyme upregulated in metastatic cells, was detected in EVs derived from metastatic breast cancer cells. TG2 promotes FN dimerization to a fibrillar form on the EV surface, which in turn educates pulmonary fibroblasts to form a niche suitable for metastatic colonization[87]. In addition to tumor cells, cancer-associated fibroblasts (CAFs) at the primary tumor site also secrete EVs that may be more potent in inducing ECM remodeling of the PMN than tumor cell-derived EVs. For example, as compared to salivary adenoid cystic carcinoma (SACC) cell-derived EVs, EVs produced by SACC CAFs promote faster and more pronounced upregulation of known ECM-related PMN markers in the lung, including FN, MMP9, and lysyl oxidase (LOX), via activation of TGFβ signaling in lung fibroblasts[88].
Tumor-derived EVs can also directly orchestrate ECM architectural changes to facilitate PMN formation. Tumor EVs contain ECM molecules (ie., FN, tenascin-C [TnC]) and ECM-remodeling regulators (e.g., MMPs, LOXs, transglutaminases). EV secretion is required for appropriate extracellular deposition of TnC, an ECM glycoprotein that regulates cell adhesion to other ECM components, by several tumor cells and associated fibroblasts in breast cancer and PDAC[89]. EV TnC not only fosters distant ECM fiber nucleation, but it also promotes invasiveness of incoming tumor cells by activating WNT/β-catenin and NFκB signaling[90, 91]. Several studies also demonstrated enzymatically functional EV-associated MMPs including MMP2, MMP9, and MMP14, as well as other proteinases in EVs shed from ovarian cancer, fibrosarcoma, and melanoma. These proteases degrade and remodel existing ECM at distant sites to create favorable paths for metastatic cells to enter the PMN[92, 93].
Reprogramming PMN-associated immune profiles
Initiation of proinflammatory and immunosuppressive signaling at distant sites by tumor-derived EVs has been widely reported across multiple cancer types[6, 94-98]. The majority of EVs show a similar size and structure to liposomes, which are known to undergo rapid accumulation and clearance by the reticuloendothelial system. The innate immune system, particularly macrophages, engulf a large fraction of circulating EVs secreted from the primary tumor and undergo immunological reprogramming. Alterations in macrophage polarization by tumor-derived EVs are well documented[99-104]. For example, EVs secreted by metastatic osteosarcoma cells direct alveolar macrophages to adopt a pro-tumor phenotype by inducing the release of immunosuppressive factors IL-10, TGFβ2, and CCL2, which in turn decrease phagocytosis and efferocytosis of tumor cells[101]. Uptake of PDAC-derived EVs enriched for macrophage migration inhibitory factor (MIF) by liver-resident Kupffer cells induced TGFβ secretion, which subsequently stimulated hepatic stellate cell (HSC) activation, FN deposition, and recruitment of immunosuppressive myeloid cells to generate the liver PMN [7]. Moreover, tumor cell-derived EVs can induce metabolic reprogramming of macrophages. Lung cancer cell-derived EVs altered glycolytic metabolism in tissue resident interstitial macrophages and upregulated their expression of the immune checkpoint programmed death ligand-1 (PD-L1), which curtails effector T cell-mediated anti-tumor immune responses in the pre-metastatic lung and draining lymph node[105].
In addition to tissue resident macrophages, tumor EVs can directly educate and mobilize various bone marrow-derived cells to promote metastatic progression. Metastatic melanoma, for example, horizontally transfers MET, an oncogenic receptor tyrosine kinase, to bone marrow progenitor cells via EVs, which reprograms recipient bone marrow cells to a pro-vasculogenic phenotype and increases lung and bone metastases[6]. EVs derived from several cancer types, including acute myeloid leukemia (AML)[106, 107], esophageal squamous cell carcinoma[108], breast carcinoma[109], and glioma[110] promote conversion of immature bone marrow progenitor cells or bone marrow-derived monocytes to myeloid-derived suppressor cells (MDSCs), leading to T and natural killer cell (NK) dysfunction. Moreover, the immunosuppressive potential of MDSCs can be further augmented by tumor-derived EVs that are enriched with heat-shock proteins, including HSP86[96], HSP70[111] and HSP72[112], and miRNAs, including miR-21[96, 113], miR-10a[114], miR-29a, miR-92a[115], miR-107[116], miR-155[117], miR-1246[110]. Not only do tumor EVs guide the differentiation of bone marrow cells towards immuno-evasive phenotypes, but also lung and breast cancer cell EVs block the differentiation of bone marrow progenitor cells into DCs[118, 119] and can inhibit maturation and migration of DCs[119], thereby obstructing tumor antigen presentation.
Neutrophils, the most abundant innate immune cells, are also stimulated by tumor-derived EVs and are instrumental in driving PMN formation. Neutrophil extracellular traps (NETs) are web-like structures composed of chromatin DNA filaments coated with granule proteins and released by activated neutrophils. NETs promote vascular permeability, ECM remodeling, EMT, proliferation, migration, invasion, dormant cell awakening and can prevent NK cell- and effector T cell-mediated tumor cell killing, and serve as an emerging PMN hallmark [120-126]. EVs released by breast cancer cells induce NETs in neutrophils pretreated with granulocyte colony-stimulating factor[127]. In vitro treatment of neutrophils with metastatic melanoma cell-derived EVs also induces NET formation and prolongs neutrophil life span to promote a pro-tumorigenic phenotype marked by diminished phagocytic and cytotoxic activity and increased chemotaxis [128]. Gastric and colorectal cancer-derived EVs also enhanced neutrophil survival and polarization towards an N2 pro-tumorigenic phenotype [129, 130]. In addition to these in vitro studies, breast cancer cell-derived EVs have shown systemic effects on NET induction[127] and further support the contribution of tumor EVs in NET formation at the PMN.
NK and T cell-mediated cytotoxic immunity are pivotal sentinels of anti-tumor immune surveillance. Compared to T cells, NK cells respond early to malignant cells to counteract tumor progression and metastasis independent of additional activation. Nevertheless, cancer cells exploit various strategies, including tumor-derived EVs, to impair NK cell recruitment, proliferation, and survival, as well as cytotoxic function[131]. For instance, exposure of NK cells to AML EVs resulted in ligand-mediated downregulation of C-X-C motif chemokine receptor 3 (CXCR3), a main chemokine receptor responsible for NK cell recruitment[132] and decreased NK cell migration[133]. Similarly, EVs derived from mesothelioma, breast cancer, and melanoma block IL-2-induced NK cell proliferation by downregulating IL-2 receptor (IL-2R) or blocking IL-2R downstream pathways[134, 135]. Chronic exposure of NK cells to ligands of NKG2D, the predominant activating receptor on NK cells, induces NK cell tolerance by downregulating NKG2D expression[136, 137]. Interestingly, NKG2D ligands (NKG2DLs), such as MHC class I-related chain (MIC) A and MICB molecules are shed into EVs from NKG2DL+ tumor cells[138]. NK cells treated with these NKG2DL-enriched EVs display significantly reduced NKG2D and marked reduction in cytotoxic activity[138-140]. In addition to NKG2DLs, the high level of membrane-associated TGFβ carried by EVs isolated from tumor cells, including AML, renal cell carcinoma, mesothelioma, oral cancer, and PDAC, induce NK cell dysfunction by diminishing NKG2D levels[98, 133, 139, 141] and upregulating the expression of NKG2A (the main inhibitory receptor on NK cells) [142], which results in cancer progression. NK cells in PMNs generally show reduced maturation and cytotoxic effector functions[143]. However, the range of dynamic interactions between NK cells with tumor-derived EVs and the subsequent phenotype changes of NK cells within PMNs remain to be explored.
In addition, tumor-derived EVs also impact adaptive immune responses to favor metastatic progression. CD8+T cells are the dominant adaptive immune cell that conduct tumor-specific cytotoxicity. Interactions of tumor-derived EVs and CD8+ T cells have been extensively studied, and the EV-bearing immune checkpoint molecules, such as TNF and TNF receptor (TNFR) superfamily and PD-L1, contribute to escape from T cell-mediated immune surveillance. Tumor EVs enriched with Fas ligand (FasL, a member of the TNFR superfamily) and TNF-related apoptosis-inducing ligand (TRAIL, a member the TNF family of death factors) suppressed proliferation and promoted apoptosis in CD8+ T cells in melanoma[144-146], PDAC[147], CRC[148], oral cancer[149, 150], head and neck cancer[151-153], and ovarian cancer[154]. Moreover, FasL-bearing EVs can polarize other Fas-expressing macrophages to maintain an immunosuppressive environment[155]. PD-L1, a B7 family ligand, is a coinhibitory signal regulating T cell responses via binding to PD-1. PD-L1 is frequently enriched in EVs secreted from various cancer types[156-163]. Apart from inducing local and systemic immunosuppression by suppressing T cell proliferation, mobility, cytotoxic function, and cytokine release, tumor-derived PD-L1+ EVs can also compromise anti-PD-1/PD-F1 monoclonal antibody therapies[156, 164]. Other checkpoint molecules that have been detected on tumor-derived EVs or circulating EVs from cancer patient plasma include cytotoxic T-lymphocyte associated protein 4 (CTLA4)[155], as well as transmembrane, immunoglobulin, and mucin (TIM)3 and galectin-9 (the ligand for TIM3)[165], Interestingly, high levels of plasma EV TIM3 from non-small cell lung cancer (NSCLC) patients are associated with lymph node metastasis[165].
Adenosine is an ATP derivative that potently diminishes anti-tumor activities of CD4+ and CD8+ T cells and NK cells. Adenosine is generated by stepwise dephosphorylation of extracellular ATP via ectonucleotidases, including CD39 (ectonucleoside triphosphate diphosphohydrolase-1) and CD73 (5′-nucleotidase)[166]. CD39 and CD73 are present in EVs from breast, prostate, and colorectal cancers and glioblastoma, and contribute to ATP hydrolysis to adenosine and subsequent T cell dysfunction and inhibition of clonal expansion [167, 168]. Horizontal transfer of tumor cell-derived EV CD39/CD73 to NK and T cells leads to in situ deposition of adenosine and autocrine adenosine signaling-mediated loss of function[168, 169]. Other tumor-derived EV proteins that suppress T cell proliferation and activity include arginase I (ARGI) and TnC. Ovarian cancer-derived ARGI+ EVs enzymatically deplete L-arginine, which is essential for T cell function, and inhibit CD4+ and CD8+ T cells and accelerate tumor progression[170]. TnC+ EVs from glioblastoma inhibit T cell proliferation via interaction with α5β1 and αvβ6 integrins on T lymphocytes and suppress mammalian target of rapamycin (mTOR) signaling[171].
Tumor-derived EVs also expand immunosuppressive T regulatory cells (Tregs) to further restrict cytotoxic T cell function. Several studies have demonstrated that TGFβ-enriched tumor EVs skew expansion or induce phenotypic alteration of T cells towards Tregs[134, 172, 173]. Interestingly, following exposure to tumor cell EVs, transcriptional analysis in different T cell subsets showed that Tregs were more sensitive to EV treatment than other T cells, such as CD8+ T cells[174]. Together, these findings imply a central role for Tregs in mediating tumor EV-induced immunosuppression.
Numerous EV-associated ncRNAs are also implicated in the suppression of effector T cells and NK cells and induction of Treg cells. For example, CRC-derived EVs contain miR-424 that induced resistance to immune checkpoint blockade (ICB) therapy by disrupting the CD28-CD80/86 costimulatory pathway in T cells and DCs[175]. Metastatic CRC cells release EVs enriched for lncRNA-SNHG10 that activates TGFβ signaling by targeting inhibin subunit beta C (INHBC) and impairs proliferation and cytotoxicity in NK cells [176]. SNHG16 is another lncRNA transmitted via breast cancer EVs to γδT cells to induce a CD73+ Treg phenotype via sponging miR-16-5p and activating the TGFβ/SMAD5 pathway[177].
Antibody producing B cells are another major component of adaptive immunity but the role of B cells in cancer progression is controversial. There are several studies linking B cell infiltration to a favorable clinical outcome in patients with NSCLC, ovarian cancer, and breast cancer[178-180] and increased response to ICB therapy in those with melanoma[181], mediated by anti-tumoral antibody secretion, antigen presentation, and T cell activation. On the other hand, tumor-promoting humoral immunity facilitates tumor development and metastasis via engagement of Fc receptors on myeloid cells [182], antagonizing anti-tumor antibodies [183] and activating tumor antigens, such as HSPA4[184]. B cell subsets, such as B regulatory cells[185-187], can also inhibit T cell or NK cell-mediated anti-tumor response and induce resistance to cancer therapies. Interestingly, tumor EVs can interact with B cells within the lymph node cortex when the subcapsular sinus macrophage barrier is compromised during tumor progression or by therapeutic agents. This fosters plasma cell amplification and tumor-promoting autoantibody production and generates a PMN in the lymph node [188].
Clinical application
Diagnosis and prognosis
The most immediate clinical application of EVs in cancer are as diagnostic and prognostic biomarkers. Several ongoing clinical trials have focused on circulating EVs to identify new EV cancer biomarkers to predict early metastatic progression (Table 1). A number of pre-clinical studies have verified the essential role of tumor-derived EVs in establishing a distant PMN, and a recent comprehensive proteomic analysis of human tissue explant-derived EVs has confirmed the existence of unique damage-associated molecular patterns, including S100A4, S100A13, basigin, and galectin-9, packaged in EVs from tumor tissues but not non-tumor tissues. These damage-associated proteins potentiate induction of pro-inflammatory PMNs that support future metastasis[189]. Notably, the study demonstrated that proteomes of plasma-derived EVs from cancer patients reflect alterations in distant organs and the immune system and thus have the potential to serve as PMN biomarkers. For example, liver-derived selenoprotein P is frequently found in plasma-derived EVs from lung cancer patients, but not in plasma EVs in healthy donors nor is it present in EVs shed by lung cancer tissues. Thus, the presence of selenoprotein reflects specific alterations in liver function induced by the primary lung tumor and represents a liver PMN marker[189].
Table 1.
Summary of tumor EV-mediated reprogramming of stromal microenvironment in PMN
| Stromal cell being targeted |
PMN site | EV cargo | Reprogramming mechanism | Source tumor |
|---|---|---|---|---|
| Endothelial cells | Not defined | VEGF | Angiogenesis and endothelial permeability | GBM, breast cancer |
| Lymph nodes, liver | PDAC | |||
| Lymph node | NGFR | Angiogenesis and tumor cell adhesion | Melanoma | |
| Lung, brain | Annexin II | Tissue plasminogen activator-dependent angiogenesis | Breast cancer | |
| Liver | AMIGO2 | Tumor cell adhesion | Gastric cancer | |
| Not defined | Unsaturated diacylglycerols | Angiogenesis via protein kinase D signaling | Breast cancer | |
| lymph node | miR-221-3p, ELNAT1 | Lymphangiogenesis | Bladder cancer | |
| Lung fibroblasts | Lung | TG2 | Fibroblast activation via FN dimerization | Breast cancer |
| Endothelial cells, lung fibroblasts | NID1 | Angiogenesis, fibroblast activation | HCC | |
| Lung fibroblasts | miR-1247-3p | Fibroblast activation via inhibition of ITGB1 activation and stability | ||
| Lung fibroblasts, hepatic stellate cells | Lung, liver | ITGBL1 | fibroblast activation via TNFAIP3-mediated NFκB signaling | CRC |
| ECM | Not defined | TnC | Foster ECM fiber nucleation, promote invasiveness of tumor cells via WNT/β-catenin and NFκB signaling | PDAC, breast cancer |
| MMPs (MMP2, MMP9, MMP14) | Degrade and remodel existing ECM | Ovarian cancer, fibrosarcoma, melanoma |
Clinical observations also support the critical role of EV integrins in directing organotropism. Increased integrin β4 on plasma EVs was detected in breast cancer patients with lung metastases compared to patients with only primary breast tumors or those with liver metastases. High integrin α5 expression in primary breast tumors correlates with the presence of disseminated tumor cells in bone marrow in breast cancer patients[190]. In contrast to patients with lung or bone metastases, plasma EVs from breast cancer patients with liver metastases contained upregulated integrin αv[8]. Similarly, plasma EV-integrin α6A is a diagnostic marker that predicts PDAC recurrence and metastasis much earlier (e.g., one month after surgery) compared to conventional cancer antigen markers CEA and CA19-9[191]. Establishing which and how EV integrins direct metastatic targeting in specific organs in various cancer types could yield a panel of biomarkers for early prediction of metastasis occurrence at different sites.
ncRNAs, especially miRNAs, are the most widely studied EV cargo from cancer patient liquid biopsies and exhibit great potential for predicting metastasis. For example, circulating exosomal miR-25-3p is differentially expressed in healthy donors, CRC patients without metastases, and CRC patients with metastases[192]. Exosomal miR-25-3p targets VEGFR2 and tight junction proteins in endothelial cells to promote vascular permeability and angiogenesis at the distant pre-metastatic site. Therefore, quantitative evaluation of miR-25-3p levels in circulating EVs may serve as an early indicater of CRC patients at risk for metastasis and inform the subsequent treatment course. While most clinical studies focused on individual EV-associated miRNAs, using a panel of different EV miRNAS could increase prognostic accuracy in patients likely to develop metastasis. This approach has been supported by applying a panel of plasma EV miRNAs to distinguish lung cancer patients with or without metastasis[193].
Treatment
Therapeutic targeting of EVs and EV cargo could also provide an approach to impair metastasis, for which there are no specific treatments. Specifically, pathways of EV biogenesis and uptake may be prime targets for such intervention. Exosome biogenesis occurs via the multivesicular body (MVB) endosomal pathway, where inward budding of the endosome membrane leads to accumulation of intralumenal vesicles (ILVs) contained within the endosome[194, 195]. The MVB then traffics to the plasma membrane where it fuses and ILVs are released from the cell as exosomes. Alternatively, vesicles known as ectosomes and of similar size to exosomes can bud directly off the plasma membrane. The MVB pathway of biogenesis has been extensively studied and several essential regulatory factors have been identified including ESCRTs, Alix, syntenin, and lipids, such as ceramide, that participate in ILV formation, and proteins that control trafficking and plasma membrane fusion of MVBs, such as Rab27, Rab35, Ral GTPases, and SNAREs[196]. Ectosome biogenesis pathways are less studied, but involve key proteins, such as ARF6, ARRDC1, and ESCRTs[197]. Uptake of EVs may occur via direct fusion with recipient cell plasma membranes or through endocytosis[194, 196].
Functional studies on the impact of EV biogenesis and uptake on metastasis indicate that inhibiting these pathways can reduce metastatic burden. Depletion of Rab27[6] and Ral GTPases[198] diminished lung metastasis in mouse models of melanoma and breast cancer, respectively. Furthermore, treatment of melanoma-bearing mice with the drug reserpine blocked EV uptake and attenuated lung metastasis[199]. Interestingly, screening efforts to identify existing compounds that inhibit biogenesis have found several small molecule candidates that could be repurposed for therapeutic targeting of EV-dependent metastasis[200-202]. These drugs, which include manumycin A, tipifarnib, neticonazole, climbazole, ketoconazole, triadimenol, and simvastatin, could decrease the levels of biogenesis and reduce EV production, but their effect on in vivo cancer metastasis requires further study. Importantly, as EVs have developmental and physiological functions in non tumor cells, targeting biogenesis will require identifying potential cancer-specific pathways of EV formation.
Inhibiting EV uptake by recipient cells may be an additional therapeutic approach. In support, the anti-hypertensive drug, reserpine, was shown to block uptake of melanoma EVs, consequently diminishing PMN formation and impairing metastasis to lungs[199]. Organ-specific blockade of uptake could prevent unwanted side effects associated with systemic inhibition of EV uptake and perhaps be achieved by targeting EV integrins driving organotropic metastasis, such as α6β4 in lung-tropic EVs[8].
Conclusions and Perspective
The work discussed here emphasizes the critical role of circulating tumor-derived EVs in mediating communication between the primary tumor and distant organs (Figure 1). EVs direct the organotropic delivery of bioactive materials shed from primary tumors to recipient stromal and immune cells within the distant pre-metastatic site. Upon arriving at the distant organ, tumor-derived EVs modulate the function and phenotype of recipient cells via a broad array of pathways and mediators. Tumor EVs can initiate proinflammatory and immunosuppressive signaling as well as remodel the ECM to increase adhesiveness of incoming tumor cells. EVs can also promote angiogenesis and vascular permeability, thus allowing tumor cells to enter a nutrient-rich PMNs. Collectively tumor-derived EVs can reprogram hostile or neutral microenvironments into favorable “soil” for metastatic tumor cells to colonize and grow. Our knowledge of these EV-mediated events suggests several possibilities to improve diagnosis or treatment of metastatic disease. Blocking tumor-derived EV release and/or uptake by recipient cells could prevent the creation of the PMN and ultimately metastasis. More specifically, further investigation into the mechanisms of cargo loading into EVs, intracellular pathways supporting EV biogenesis as well as signaling events upon uptake of EVs in recipient cells, could identify unique targets. In addition, profiling of bioactive EV cargos in biological fluids is a non-invasive manner to evaluate their prognostic significance in cancer patients. Several practical issues remain, including the shed and elimination rates and whether the concentration of tumor-derived EVs in the circulation is sufficient to detect tumor EVs using current technologies. Recently, EVs from as little as 10 μL of patient plasma were successfully characterized and reflected EV phenotypic changes in patients with melanoma in response to treatment[203]. An asymmetric flow field-flow fractionation technique was developed to understand the heterogeneity of EVs with high resolution [10]. These technical advances will allow us to establish criteria for use of optimal EV subpopulations for therapeutics and prognostics and accelerate the clinical translation of the role of tumor EV-mediated PMN formation and metastatic progression.
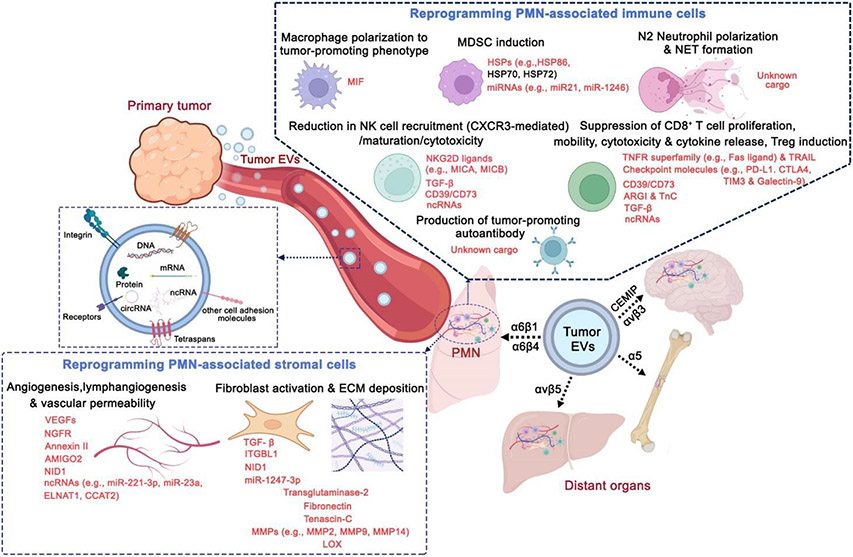
Figure 1.
Tumor EVs prime pre-metastatic niches (PMNs) by modulating stromal and immune cells at distal sites. Tumor EVs contain distinct integrins and cell adhesion molecules that determine their organotropism. Specific EV cargos attributed to stromal and immune microenvironment modulation at PMNs are highlighted in red.
Table 2.
Summary of tumor EV-mediated reprogramming of immune microenvironment in PMN
| Immune cell being targeted |
PMN site | EV cargo | Reprogramming mechanism | Source tumor |
|---|---|---|---|---|
| Macrophages | Lung, brain | Annexin II | Activate proinflammatory signaling | Breast cancer |
| Lung | Not defined | Secrete immunosuppressive factor release, decrease phagocytosis and efferocytosis | osteosarcoma cells | |
| Lymph node | Alter glycolytic metabolism, upregulate PD-L1 expression | Lung cancer | ||
| Kupffer cells | Liver | MIF | TGFβ secretion | PDAC |
| Hematopoietic progenitors | Lung, liver | VEGF | Mobilization and recruitment of hematopoietic progenitors to distant sites | Lung cancer, melanoma |
| Bone marrow derived cells | Lung, bone | MET | Pro-vasculogenic phenotype differentiation | Melanoma |
| Not defined | MUC1, palmitoylated proteins | MDSC differentiation | AML | |
| Lymph node | miR-21 | Monocytic MDSC differentiation | Esophageal squamous cell carcinoma, lung cancer, glioma | |
| Not defined | miR-10, miR-29a, miR-92a | MDSC differentiation | Glioma | |
| miR-107 | Gastric cancer | |||
| miR-155 | Chronic lymphocytic leukemia | |||
| Lung | miR-9, miR-181a | Breast cancer | ||
| Not defined | miR-1246 | GBM | ||
| HSP86 | Melanoma | |||
| HSP70 | Breast cancer, lung cancer, ovarian cancer | |||
| Lung | HSP72 | CRC, Lung cancer | ||
| Not defined | Not defined | Induce immuno-evasive phenotype differentiation, block DC differentiation, maturation and migration | Lung cancer, breast cancer | |
| Neutrophils | NET formation | Breast cancer | ||
| NET formation, promote life span | Melanoma | |||
| HMGB1 | Promote life span and N2 polarization | Gastric cancer | ||
| RNAs (long interspersed nuclear elements, short interspersed nuclear elements, and long terminal repeats) | Sustain survival and polarization towards a pro-tumorigenic phenotype | CRC | ||
| NK | Not defined | Reduce NK recruitment and migration by CXCR3 downregulation | AML | |
| TGFβ1 | Impair NK proliferation by downregulation of IL-2R | Mesothelioma, prostate cancer | ||
| NK dysfunction by downregulation of NKG2D | PDAC, AML, mesothelioma, prostate cancer, renal cell carcinoma | |||
| NK dysfunction by downregulation of NKG2D and upregulation of NKG2A | Oral cancer | |||
| Treg | Skew expansion or induce phenotypic alteration of T cells towards Tregs | CRC | ||
| NK | Lung | Not defined | Block IL-2-mediated NK proliferation | Breast cancer |
| Not defined | NKG2DLs (MICA/B) | NK tolerance by downregulation of NKG2D | Liver cancer, CRC, melanoma, mesothelioma, prostate cancer | |
| Liver | lncRNA-SNHG10 | Impaire NK proliferation and toxicity by activation of TGFβ signaling | CRC | |
| CD8+ T | Not defined | FasL, APO2L/TRAIL | Suppress CD8+ T cell proliferation and promote apoptosis | Melanoma, PDAC, Ovarian cancer |
| Liver | FasL, TRAIL | CRC | ||
| Lymph node | FasL | Oral cancer, head and neck cancer | ||
| Not defined | PD-L1 | Suppress T cell proliferation, mobility, cytotoxicity and cytokine release | Melanoma, lung cancer, gastric cancer, prostate cancer, CRC, GBM, head and neck cancer, breast cancer | |
| CD4+ T, CD8+ T, NK | FasL, TRAIL, CTLA4 | Suppresse CD8+ T cell proliferation and cytokine release and CD4+ T /NK cell activation and promote macrophage differentiation into M2 phenotype | GBM | |
| CD4+ T, CD8+ T | Lymph node | TIM3, galectin-9 | Promote T cell exhaustion | Lung cancer |
| Not defined | ARGI | T cell dysfunction by L-arginine depletion | Ovarian cancer | |
| T cell, NK | CD39, CD73 | T/NK cell dysfunction and inhibit clonoal expansion through adenosine production | Bladder cancer, CRC, prostate cancer, breast cancer, mesothelioma, GBM | |
| T cell | TnC | Inhibit T cell proliferation | GBM | |
| γδT cell | Lymph node | SNHG16 | Induce a CD73+ Treg phenotype | Breast cancer |
| B cell | Not defined | Promote plasma cell amplification and tumor-promoting autoantibody production | Melanoma |
Table 3.
Ongoing clinical trials involving EVs for the prediction of metastatic progression in cancer patients (data collected from http://clinicaltrials.gov, accessed in Dec 2022).
| Clinical Trial | Cancer Type | Identifier |
|---|---|---|
| Contents of circulating extracellular vesicles: biomarkers in colorectal cancer patients | CRC | NCT04523389 |
| Development of novel imaging and laboratory biomarkers to monitor the liver pre-metastatic niche and guide treatment of colon cancer: a pilot study | NCT03432806 | |
| Diagnostic and prognostic values of EUS-FNA specimens and circulating exosomal small RNA in patients with pancreatic cancer | PDAC | NCT04636788 |
| Circulating exosomes as potential prognostic and predictive biomarkers in advanced gastric cancer patients: a prospective observational study (“EXO-PPP Study”) | Gastric cancer | NCT01779583 |
| Exosomes-derived ncRNAs as biomarkers in cholangiocarcinoma patients | Cholangiocarcinoma | NCT03102268 |
| Non-coding RNA in the exosome of the epithelia ovarian cancer | Epithelia ovarian cancer | NCT03738319 |
| A prospective study of predicting prognosis and recurrence of thyroid cancer via new biomarkers, urinary exosomal thyroglobulin and galectin-3 | Thyroid cancer | NCT03488134 |
| Study of exosomes in monitoring patients with sarcoma (EXOSARC) | Sarcoma | NCT03800121 |
| A pilot study of circulating exosome RNA as diagnostic and prognostic markers in lung metastases of primary high-grade osteosarcoma | Osteosarcoma | NCT03108677 |
| Feasibility of exosome analysis in cerebrospinal fluid during the diagnostic workup of metastatic meningitis from breast cancer | Breast cancer | NCT05286684 |
| Interest of circulating tumor DNA in digestive and gynecologic/breast Cancer | Breast cancer/ Digestive cancer/ Gynecologic cancer | NCT04530890 |
| Validating the miR Scientific Sentinel™ platform (Sentinel PCC4 assay) in men undergoing core needle biopsy due to suspicion of prostate cancer for distinguishing between no cancer, low-, intermediate- and high-risk prostate cancer | Prostate cancer | NCT04100811 |
| Quantification and purification of circulating prostasomes as diagnostic tool for prostate cancer detection | NCT03694483 | |
| A prospective, randomized blinded, shared decision impact trial of the ExoDx Prostate (IntelliScore), EPI test, in men presenting for initial biopsy. | NCT03235687 | |
| To investigate the diagnostic accuracy of exosomal microRNA in predicting the aggressiveness of prostate cancer in Chinese patients | NCT03911999 | |
| Identification and characterization of predictive factors of onset of bone metastases in cancer patients | Not specified | NCT03895216 |
Acknowledgements
CTSC-TL1 training award (2TL1-TR-2386), National Cancer Institute (CA232093, CA163117 and CA207983), the Hartwell Foundation, the Thompson Family Foundation, the STARR Consortium, Alex’s Lemonade Stand Foundation, the Breast Cancer Research Foundation, the Feldstein Medical Foundation,the Tortolani Foundation, the Mary Kay Ash Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
No data was used for the research described in the article.
References
- [1].Paget S, The distribution of secondary growths in cancer of the breast, The Lancet 133(3421) (1889) 571–573. [PubMed] [Google Scholar]
- [2].Fidler IJ, Nicolson GL, Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines, Journal of the National Cancer Institute 57(5) (1976) 1199–1202. [DOI] [PubMed] [Google Scholar]
- [3].Hart IR, Fidler IJ, Role of organ selectivity in the determination of metastatic patterns of B16 melanoma, Cancer research 40(7) (1980) 2281–2287. [PubMed] [Google Scholar]
- [4].Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. , VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche, Nature 438(7069) (2005) 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peinado H, Lavotshkin S, Lyden D, The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts, Seminars in cancer biology, Elsevier, 2011, pp. 139–146. [DOI] [PubMed] [Google Scholar]
- [6].Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. , Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nature medicine 18(6) (2012) 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. , Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver, Nature cell biology 17(6) (2015) 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. , Tumour exosome integrins determine organotropic metastasis, Nature 527(7578) (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang H, Lyden D, Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization, Nature protocols 14(4) (2019) 1027–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. , Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nature cell biology 20(3) (2018) 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Han S, Huo Z, Nguyen K, Zhu F, Underwood PW, Basso KBG, et al. , The proteome of pancreatic cancer-derived exosomes reveals signatures rich in key signaling pathways, Proteomics 19(13) (2019) 1800394. [DOI] [PubMed] [Google Scholar]
- [12].Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. , Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells, Nature cell biology 10(5) (2008) 619–624. [DOI] [PubMed] [Google Scholar]
- [13].Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Curry WT, et al. , Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers, Nature cell biology 10(12) (2008) 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lucotti S, Kenific CM, Zhang H, Lyden D, Extracellular vesicles and particles impact the systemic landscape of cancer, The EMBO Journal 41(18) (2022) e109288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gurung S, Perocheau D, Touramanidou L, Baruteau J, The exosome journey: From biogenesis to uptake and intracellular signalling, Cell Communication and Signaling 19(1) (2021) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kugeratski FG, Flodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, et al. , Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker, Nature cell biology 23(6) (2021) 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aiello A, Giannessi F, Percario ZA, Affabris E, An emerging interplay between extracellular vesicles and cytokines, Cytokine & Growth Factor Reviews 51 (2020) 49–60. [DOI] [PubMed] [Google Scholar]
- [18].Park S, Arrell DK, Reyes S, Park EY, Terzic A, Conventional and unconventional secretory proteins expressed with silkworm bombyxin signal peptide display functional fidelity, Scientific reports 7(1) (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L, A system of cytokines encapsulated in extracellular vesicles, Scientific reports 8(1) (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. , Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature 546(7659) (2017) 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Skotland T, Sagini K, Sandvig K, Llorente A, An emerging focus on lipids in extracellular vesicles, Advanced drug delivery reviews 159 (2020) 308–321. [DOI] [PubMed] [Google Scholar]
- [22].Gang W, Jianlong L, Linda B, Haiyan C, Zhong L, Gabriel CT, et al. , Tumor extracellular vesicles and particles induce liver metabolic dysfunction, Nature (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nishida-Aoki N, Izumi Y, Takeda H, Takahashi M, Ochiya T, Bamba T, Lipidomic analysis of cells and extracellular vesicles from high-and low-metastatic triple-negative breast cancer, Metabolites 10(2) (2020) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsuda A, Kuno A, Yoshida M, Wagatsuma T, Sato T, Miyagishi M, et al. , Comparative glycomic analysis of exosome subpopulations derived from pancreatic cancer cell lines, Journal of Proteome Research 19(6) (2020) 2516–2524. [DOI] [PubMed] [Google Scholar]
- [25].Chaiyawat P, Weeraphan C, Netsirisawan P, Chokchaichamnankit D, Srisomsap C, Svasti J, et al. , Elevated O-GlcNAcylation of extracellular vesicle proteins derived from metastatic colorectal cancer cells, Cancer Genomics & Proteomics 13(5) (2016) 387–398. [PMC free article] [PubMed] [Google Scholar]
- [26].Tan Z, Cao L, Wu Y, Wang B, Song Z, Yang J, et al. , Bisecting GlcNAc modification diminishes the pro-metastatic functions of small extracellular vesicles from breast cancer cells, Journal of extracellular vesicles 10(1) (2020) e12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Otake AH, de Freitas Saito R, Duarte APM, Ramos AF, Chammas R, GD3 ganglioside-enriched extracellular vesicles stimulate melanocyte migration, Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1864(3) (2019) 422–432. [DOI] [PubMed] [Google Scholar]
- [28].Shenoy GN, Loyall J, Berenson CS, Kelleher RJ, Iyer V, Balu-Iyer SV, et al. , Sialic acid–dependent inhibition of t cells by exosomal ganglioside GD3 in ovarian tumor microenvironments, The Journal of Immunology 201(12) (2018) 3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin S, Zhou S, Yuan T, The “sugar-coated bullets” of cancer: Tumor-derived exosome surface glycosylation from basic knowledge to applications, Clinical and Translational Medicine 10(6) (2020) e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK, Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment, Journal of Biological Chemistry 289(47) (2014) 32526–32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hung ME, Leonard JN, Stabilization of exosome-targeting peptides via engineered glycosylation, Journal of Biological Chemistry 290(13) (2015) 8166–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Clark DJ, Schnaubelt M, Hoti N, Hu Y, Zhou Y, Gooya M, et al. , Impact of increased FUT8 expression on the extracellular vesicle proteome in prostate cancer cells, Journal of proteome research 19(6) (2020) 2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Royo F, Cossío U, de Angulo AR, Llop J, Falcon-Perez JM, Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice, Nanoscale 11(4) (2019) 1531–1537. [DOI] [PubMed] [Google Scholar]
- [34].Williams C, Pazos R, Royo F, González E, Roura-Ferrer M, Martinez A, et al. , Assessing the role of surface glycans of extracellular vesicles on cellular uptake, Scientific reports 9(1) (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nishida-Aoki N, Tominaga N, Kosaka N, Ochiya T, Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake, Journal of extracellular vesicles 9(1) (2020) 1713527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koide R, Hirane N, Kambe D, Yokoi Y, Otaki M, Nishimura S-I, Antiadhesive nanosome elicits role of glycocalyx of tumor cell-derived exosomes in the organotropic cancer metastasis, Biomaterials 280 (2022) 121314. [DOI] [PubMed] [Google Scholar]
- [37].O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO, RNA delivery by extracellular vesicles in mammalian cells and its applications, Nature reviews Molecular cell biology 21(10) (2020) 585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gu P, Sun M, Li L, Yang Y, Jiang Z, Ge Y, et al. , Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis Through Regulating CCL2 Expression in Lung Epithelial Cells, Frontiers in Cell and Developmental Biology 9 (2021) 1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, et al. , Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells, Theranostics 11(3) (2021) 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. , Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis, Cancer cell 25(4) (2014) 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li C, Qin F, Wang W, Ni Y, Gao M, Guo M, et al. , hnRNPA2B1-Mediated Extracellular Vesicles Sorting of miR-122-5p Potentially Promotes Lung Cancer Progression, International Journal of Molecular Sciences 22(23) (2021) 12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. , Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer, Nature communications 9(1) (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. , Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer, Journal of hematology & oncology 13(1) (2020) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. , Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils, Cancer cell 30(2) (2016) 243–256. [DOI] [PubMed] [Google Scholar]
- [45].Gezer U, Özgür E, Cetinkaya M, Isin M, Dalay N, Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes, Cell biology international 38(9) (2014) 1076–1079. [DOI] [PubMed] [Google Scholar]
- [46].Chen A, Cuevas I, Kenny PA, Miyake H, Mace K, Ghajar C, et al. , Endothelial cell migration and vascular endothelial growth factor expression are the result of loss of breast tissue polarity, Cancer research 69(16) (2009) 6721–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N, Sustained expression of homeobox D10 inhibits angiogenesis, The American journal of pathology 161(6) (2002) 2099–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hu X, Xia W, Liu Y, Cheng T, Xu T, Dong M, Extracellular vesicles carry lncRNA SNHG16 to promote metastasis of breast cancer cells via the miR-892b/PPAPDC1A axis, Frontiers in cell and developmental biology 9 (2021) 1319. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [49].Yang S.-j., Wang D.-d., Zhong S.-l., Chen W.-q., Wang F.-l., Zhang J, et al. , Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (cyclin D1) axis, Cell death & disease 12(5) (2021) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang P, Zhou H, Lu K, Lu Y, Wang Y, Feng T, Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer, OncoTargets and therapy 11 (2018) 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang Y-L, Liu L-C, Hung Y, Chen C-J, Lin Y-Z, Wu W-R, et al. , Long non-coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer, The Breast 46 (2019) 64–69. [DOI] [PubMed] [Google Scholar]
- [52].Ma X, Li Z, Li T, Zhu L, Li Z, Tian N, Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles, American journal of translational research 9(11) (2017) 5012. [PMC free article] [PubMed] [Google Scholar]
- [53].Feng T, Zhang P, Sun Y, Wang Y, Tong J, Dai H, et al. , High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation, Gene 710 (2019) 258–264. [DOI] [PubMed] [Google Scholar]
- [54].Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda S.-i., Kato T, et al. , Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer, Nature communications 8(1) (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang P-F, Gao C, Huang X-Y, Lu J-C, Guo X-J, Shi G-M, et al. , Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma, Molecular cancer 19(1) (2020) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Malkin EZ, Bratman SV, Bioactive DNA from extracellular vesicles and particles, Cell Death & Disease 11(7) (2020) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakefield XO, et al. , Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences, Nature communications 2(1) (2011) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. , Double-stranded DNA in exosomes: a novel biomarker in cancer detection, Cell research 24(6) (2014) 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, et al. , Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer, Journal of Biological Chemistry 289(7) (2014) 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guescini M, Genedani S, Stocchi V, Agnati LF, Astrocytes and Glioblastoma cells release exosomes carrying mtDNA, Journal of neural transmission 117(1) (2010) 1–4. [DOI] [PubMed] [Google Scholar]
- [61].Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. , Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer, Proceedings of the National Academy of Sciences 114(43) (2017) E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Clancy JW, Sheehan CS, Boomgarden AC, D’Souza-Schorey C, Recruitment of DNA to tumor-derived microvesicles, Cell Reports 38(9) (2022) 110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fernando MR, Jiang C, Krzyzanowski GD, Ryan WL, New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes, PloS one 12(8) (2017) e0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vagner T, Spinelli C, Minciacchi VR, Balaj L, Zandian M, Conley A, et al. , Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma, Journal of extracellular vesicles 7(1) (2018) 1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wan Y, Liu B, Lei H, Zhang B, Wang Y, Huang H, et al. , Nanoscale extracellular vesicle-derived DNA is superior to circulating cell-free DNA for mutation detection in early-stage non-small-cell lung cancer, Annals of Oncology 29(12) (2018)2379–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, et al. , DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity, The Journal of Immunology 198(4) (2017) 1649–1659. [DOI] [PubMed] [Google Scholar]
- [67].Diamond JM, Vanpouille-Box C, Spada S, Rudqvist N-P, Chapman JR, Ueberheide BM, et al. , Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs, Cancer immunology research 6(8) (2018) 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cai J, Han Y, Ren H, Chen C, He D, Zhou L, et al. , Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells, Journal of molecular cell biology 5(4) (2013) 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH, Metastasis Organotropism: Redefining the Congenial Soil, Dev Cell 49(3) (2019) 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. , Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis, Nat Cell Biol 21(11) (2019) 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li X-Q, Zhang R, Lu H, Yue X-M, Huang Y-F, Extracellular Vesicle-Packaged CDH11 and ITGA5 Induce the Premetastatic Niche for Bone Colonization of Breast Cancer Cells, Cancer Research 82(8) (2022) 1560–1574. [DOI] [PubMed] [Google Scholar]
- [72].Zhao L, Ma X, Yu J, Exosomes and organ-specific metastasis, Molecular Therapy-Methods & Clinical Development 22 (2021) 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hood JL, The association of exosomes with lymph nodes, Seminars in cell & developmental biology, Elsevier, 2017, pp. 29–38. [DOI] [PubMed] [Google Scholar]
- [74].Treps L, Perret R, Edmond S, Ricard D, Gavard J, Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles, Journal of extracellular vesicles 6(1) (2017) 1359479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, et al. , A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis, Nature communications 8(1) (2017) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang C-A, Li C-F, Huang R-C, Li Y-H, Liou J-P, Tsai S-J, Suppression of Extracellular Vesicle VEGF-C-mediated Lymphangiogenesis and Pancreatic Cancer Early Dissemination By a Selective HDAC1/2 Inhibitor, Molecular cancer therapeutics 20(9) (2021) 1550–1560. [DOI] [PubMed] [Google Scholar]
- [77].García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V, et al. , Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism, Nature Cancer 2(12) (2021) 1387–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al. , Exosomal annexin II promotes angiogenesis and breast cancer metastasis, Molecular Cancer Research 15(1) (2017) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Izutsu R, Osaki M, Nemoto H, Jingu M, Sasaki R, Yoshioka Y, et al. , AMIGO2 contained in cancer cell-derived extracellular vesicles enhances the adhesion of liver endothelial cells to cancer cells, Scientific reports 12(1) (2022) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhou C-F, Ma J, Huang L, Yi H-Y, Zhang Y-M, Wu X-G, et al. , Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1, Oncogene 38(8) (2019) 1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y, et al. , SUMOylation promotes extracellular vesicle–mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer, The Journal of Clinical Investigation 131(8) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hsu Y, Hung J, Chang W, Lin Y, Pan Y, Tsai P, et al. , Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1, Oncogene 36(34) (2017)4929–4942. [DOI] [PubMed] [Google Scholar]
- [83].Lang H-L, Hu G-W, Zhang B, Kuang W, Chen Y, Wu L, et al. , Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2, Oncology reports 38(2) (2017) 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Naito Y, Yamamoto Y, Sakamoto N, Shimomura I, Kogure A, Kumazaki M, et al. , Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts, Oncogene 38(28) (2019) 5566–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q, et al. , Primary tumors release ITGBLl-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation, Nature communications 11(1) (2020) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mao X, Tey SK, Yeung CLS, Kwong EML, Fung YME, Chung CYS, et al. , Nidogen 1-enriched extracellular vesicles facilitate extrahepatic metastasis of liver cancer by activating pulmonary fibroblasts to secrete tumor necrosis factor receptor 1, Advanced Science 7(21) (2020) 2002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shinde A, Paez JS, Libring S, Hopkins K, Solorio L, Wendt MK, Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche, Oncogenesis 9(2) (2020) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kong J, Tian H, Zhang F, Zhang Z, Li J, Liu X, et al. , Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts, Molecular cancer 18(1) (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Albacete-Albacete L, Sánchez-Álvarez M, Del Pozo MA, Extracellular vesicles: an emerging mechanism governing the secretion and biological roles of Tenascin-C, Frontiers in Immunology 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Albacete-Albacete L, Navarro-Lérida I, Lopez JA, Martín-Padura I, Astudillo AM, Ferrarini A, et al. , ECM deposition is driven by caveolin-1–dependent regulation of exosomal biogenesis and cargo sorting, Journal of Cell Biology 219(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Qian S, Tan X, Liu X, Liu P, Wu Y, Exosomal Tenascin-c induces proliferation and invasion of pancreatic cancer cells by WNT signaling, OncoTargets and therapy 12 (2019) 3197. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [92].Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA, Proinvasive properties of ovarian cancer ascites-derived membrane vesicles, Cancer research 64(19) (2004) 7045–7049. [DOI] [PubMed] [Google Scholar]
- [93].Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J, Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes, Journal of cellular biochemistry 105(5) (2008) 1211–1218. [DOI] [PubMed] [Google Scholar]
- [94].Gener Lahav T, Adler O, Zait Y, Shani O, Amer M, Doron H, et al. , Melanoma-derived extracellular vesicles instigate proinflammatory signaling in the metastatic microenvironment, International Journal of Cancer 145(9) (2019) 2521–2534. [DOI] [PubMed] [Google Scholar]
- [95].Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, et al. , Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis, Carcinogenesis 39(11) (2018) 1368–1379. [DOI] [PubMed] [Google Scholar]
- [96].Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, et al. , Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling, Cancer Research 79(18) (2019) 4715–4728. [DOI] [PubMed] [Google Scholar]
- [97].Hsu Y-L, Hung J-Y, Chang W-A, Jian S-F, Lin Y-S, Pan Y-C, et al. , Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN, Molecular Therapy 26(2) (2018) 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhao J, Schlößer HA, Wang Z, Qin J, Li J, Popp F, et al. , Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer, Cancers 11(6) (2019) 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M, Moon WK, Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis, Oncotarget 9(7) (2018) 7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liang Z.-x., Liu H.-s., Wang F.-w., Xiong L, Zhou C, Hu T, et al. , LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization, Cell death & disease 10(11) (2019) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wolf-Dennen K, Gordon N, Kleinerman ES, Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions, Oncoimmunology 9(1) (2020) 1747677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xun J, Du L, Gao R, Shen L, Wang D, Kang L, et al. , Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B, Theranostics 11(14) (2021) 6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang D, Wang X, Si M, Yang J, Sun S, Wu H, et al. , Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages, Cancer letters 474 (2020) 36–52. [DOI] [PubMed] [Google Scholar]
- [104].Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, et al. , Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages, Cell Communication and Signaling 16(1) (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. , Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming, Cell metabolism 33(10) (2021) 2040–2058. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pyzer AR, Stroopinsky D, Rajabi H, Washington A, Tagde A, Coll M, et al. , MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia, Blood, The Journal of the American Society of Hematology 129(13) (2017) 1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tohumeken S, Baur R, Böttcher M, Stoll A, Loschinski R, Panagiotidis K, et al. , Palmitoylated proteins on AML-derived extracellular vesicles promote myeloid-derived suppressor cell differentiation via TLR2/Akt/mTOR signaling, Cancer Research 80(17) (2020) 3663–3676. [DOI] [PubMed] [Google Scholar]
- [108].Zhao Q, Huang L, Qin G, Qiao Y, Ren F, Shen C, et al. , Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma, Cancer Letters 518 (2021) 35–48. [DOI] [PubMed] [Google Scholar]
- [109].Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, et al. , Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer, Oncogene 39(24) (2020) 4681–4694. [DOI] [PubMed] [Google Scholar]
- [110].Qiu W, Guo X, Li B, Wang J, Qi Y, Chen Z, et al. , Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells, Molecular Therapy 29(12) (2021) 3449–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gobbo J, Marcion G, Cordonnier M, Dias AM, Pernet N, Hammann A, et al. , Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer, Journal of the National Cancer Institute 108(3) (2016) djv330. [DOI] [PubMed] [Google Scholar]
- [112].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin J-P, et al. , Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells, The Journal of clinical investigation 120(2) (2010) 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang X, Li F, Tang Y, Ren Q, Xiao B, Wan Y, et al. , miR-21a in exosomes from Lewis lung carcinoma cells accelerates tumor growth through targeting PDCD4 to enhance expansion of myeloid-derived suppressor cells, Oncogene 39(40) (2020) 6354–6369. [DOI] [PubMed] [Google Scholar]
- [114].Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, et al. , Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways, Oncogene 37(31) (2018) 4239–4259. [DOI] [PubMed] [Google Scholar]
- [115].Guo X, Qiu W, Wang J, Liu Q, Qian M, Wang S, et al. , Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways, International journal of cancer 144(12) (2019) 3111–3126. [DOI] [PubMed] [Google Scholar]
- [116].Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, et al. , Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer, Cancer management and research 11 (2019) 4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bruns H, Böttcher M, Qorraj M, Fabri M, Jitschin S, Dindorf J, et al. , CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D, Leukemia 31(4) (2017) 985–988. [DOI] [PubMed] [Google Scholar]
- [118].Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. , Tumor exosomes inhibit differentiation of bone marrow dendritic cells, The Journal of Immunology 178(11) (2007) 6867–6875. [DOI] [PubMed] [Google Scholar]
- [119].Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, et al. , Tumor exosomes block dendritic cells maturation to decrease the T cell immune response, Immunology Letters 199 (2018) 36–43. [DOI] [PubMed] [Google Scholar]
- [120].Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. , Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps, Science translational medicine 8(361) (2016) 361ra138–361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. , DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25, Nature 583(7814) (2020) 133–138. [DOI] [PubMed] [Google Scholar]
- [122].Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. , Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice, Science 361(6409) (2018) eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kajioka H, Kagawa S, Ito A, Yoshimoto M, Sakamoto S, Kikuchi S, et al. , Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis, Cancer Letters 497 (2021) 1–13. [DOI] [PubMed] [Google Scholar]
- [124].Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee W-Y, Sanz M-J, et al. , Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature, Nature communications 6(1) (2015) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. , CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity, Immunity 52(5) (2020) 856–871. e8. [DOI] [PubMed] [Google Scholar]
- [126].De Meo ML, Spicer JD, The role of neutrophil extracellular traps in cancer progression and metastasis, Seminars in Immunology, Elsevier, 2022, p. 101595. [DOI] [PubMed] [Google Scholar]
- [127].Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. , Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis, Scientific reports 7(1) (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Guimarães-Bastos D, Frony AC, Barja-Fidalgo C, Moraes JA, Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype, Journal of Leukocyte Biology 111(3) (2022) 585–596. [DOI] [PubMed] [Google Scholar]
- [129].Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W, Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration, Molecular cancer 17(1) (2018) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hwang W-L, Lan H-Y, Cheng W-C, Huang S-C, Yang M-H, Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer, Journal of hematology & oncology 12(1) (2019) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, et al. , Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy, Molecular Cancer 21(1) (2022) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. , The trafficking of natural killer cells, Immunological reviews 220(1) (2007) 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, et al. , Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia, Scientific reports 7(1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Clayton A, Mitchell JP, Mason MD, Tabi Z, Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2, Cancer research 67(15) (2007) 7458–7466. [DOI] [PubMed] [Google Scholar]
- [135].Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. , Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function, The Journal of Immunology 176(3) (2006) 1375–1385. [DOI] [PubMed] [Google Scholar]
- [136].Coudert JD, Scarpellino L, Gros F, Vivier E, Held W, Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways, Blood, The Journal of the American Society of Hematology 111(7) (2008) 3571–3578. [DOI] [PubMed] [Google Scholar]
- [137].Groh V, Wu J, Yee C, Spies T, Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation, Nature 419(6908) (2002) 734–738. [DOI] [PubMed] [Google Scholar]
- [138].Ashiru O, Boutet P, Fernández-Messina L, Agüera-González S, Skepper JN, Valés-Gómez M, et al. , Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA* 008 that is shed by tumor cells in exosomes, Cancer research 70(2) (2010) 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Clayton A, Mitchell JP, Linnane S, Mason MD, Tabi Z, Human tumor-derived exosomes down-modulate NKG2D expression, The Journal of Immunology 180(11) (2008) 7249–7258. [DOI] [PubMed] [Google Scholar]
- [140].López-Cobo S, Campos-Silva C, Moyano A, Oliveira-Rodríguez M, Paschen A, Yáñez-Mó M, et al. , Immunoassays for scarce tumour-antigens in exosomes: detection of the human NKG2D-Ligand, MICA, in tetraspanin-containing nanovesicles from melanoma, Journal of nanobiotechnology 16(1) (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M, Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1, Haematologica 96(9) (2011) 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhu X, Qin X, Wang X, Wang Y, Cao W, Zhang J, et al. , Oral cancer cell-derived exosomes modulate natural killer cell activity by regulating the receptors on these cells, International journal of molecular medicine 46(6) (2020) 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, et al. , Primary Tumor Hypoxia Recruits CD11b+/Ly6Cmed/Ly6G+ Immune Suppressor Cells and Compromises NK Cell Cytotoxicity in the Premetastatic NicheThe Premetastatic Niche Is Induced by Tumor Hypoxia, Cancer research 72(16) (2012) 3906–3911. [DOI] [PubMed] [Google Scholar]
- [144].Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. , Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles, The Journal of experimental medicine 195(10) (2002) 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Martinez-Lorenzo MJ, Anel A, Alava MA, Piñeiro A, Naval J, Lasierra P, et al. , The human melanoma cell line MelJuSo secretes bioactive FasL and APO2L/TRAIL on the surface of microvesicles. Possible contribution to tumor counterattack, Experimental cell research 295(2) (2004) 315–329. [DOI] [PubMed] [Google Scholar]
- [146].Sharma P, Diergaarde B, Ferrone S, Kirkwood JM, Whiteside TL, Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells, Scientific reports 10(1) (2020) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. , Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis, Blood Cells, Molecules, and Diseases 35(2) (2005) 169–173. [DOI] [PubMed] [Google Scholar]
- [148].Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. , Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape, Gastroenterology 128(7) (2005) 1796–1804. [DOI] [PubMed] [Google Scholar]
- [149].Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL, Fas ligand–positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes, Clinical Cancer Research 11(3) (2005) 1010–1020. [PubMed] [Google Scholar]
- [150].Razzo BM, Ludwig N, Hong C-S, Sharma P, Fabian KP, Fecek RJ, et al. , Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma, Carcinogenesis 41(5) (2020) 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, et al. , Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression, Head & neck 31(3) (2009) 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL, Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes, The Journal of Immunology 183(6) (2009) 3720–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Ludwig S, Floros T, Theodoraki M-N, Hong C-S, Jackson EK, Lang S, et al. , Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer, Clinical Cancer Research 23(16) (2017)4843–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Taylor DD, Gerçel-Taylor ×, Lyons KS, Stanson J, Whiteside TL, T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by Fas ligand-containing membrane vesicles shed from ovarian tumors, Clinical cancer research 9(14) (2003)5113–5119. [PubMed] [Google Scholar]
- [155].Azambuja JH, Ludwig N, Yerneni S, Rao A, Braganhol E, Whiteside TL, Molecular profiles and immunomodulatory activities of glioblastoma-derived exosomes, Neuro-oncology advances 2(1) (2020) vdaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. , Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response, Nature 560(7718) (2018) 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim DH, Kim H, Choi YJ, Kim SY, Lee J-E, Sung KJ, et al. , Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer, Experimental & molecular medicine 51(8) (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. , Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis, Annals of surgical oncology 26(11) (2019) 3745–3755. [DOI] [PubMed] [Google Scholar]
- [159].Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, et al. , Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory, Cell 177(2) (2019) 414–427. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. , Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles, Science advances 4(3) (2018) eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL, Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients, Clinical cancer research 24(4) (2018) 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yang Y, Li C-W, Chan L-C, Wei Y, Hsu J-M, Xia W, et al. , Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth, Cell research 28(8) (2018) 862–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Daassi D, Mahoney KM, Freeman GJ, The importance of exosomal PDL1 in tumour immune evasion, Nature Reviews Immunology 20(4) (2020) 209–215. [DOI] [PubMed] [Google Scholar]
- [164].Shimada Y, Matsubayashi J, Kudo Y, Maehara S, Takeuchi S, Hagiwara M, et al. , Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer, Scientific reports 11(1) (2021) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Gao J, Qiu X, Li X, Fan H, Zhang F, Lv T, et al. , Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer, Biochemical and Biophysical Research Communications 498(3) (2018) 409–415. [DOI] [PubMed] [Google Scholar]
- [166].Hu M, Huang L, Strategies targeting tumor immune and stromal microenvironment and their clinical relevance, Advanced Drug Delivery Reviews (2022) 114137. [DOI] [PubMed] [Google Scholar]
- [167].Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z, Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production, The Journal of Immunology 187(2) (2011) 676–683. [DOI] [PubMed] [Google Scholar]
- [168].Wang M, Jia J, Cui Y, Peng Y, Jiang Y, CD73-positive extracellular vesicles promote glioblastoma immunosuppression by inhibiting T-cell clonal expansion, Cell death & disease 12(11) (2021) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Young A, Ngiow SF, Gao Y, Patch A-M, Barkauskas DS, Messaoudene M, et al. , A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment, Cancer research 78(4) (2018) 1003–1016. [DOI] [PubMed] [Google Scholar]
- [170].Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, et al. , Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma, Nature communications 10(1) (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A, et al. , Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity, Taylor & Francis, 2018, p. e1478647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y, Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression, Oncotarget 7(19) (2016) 27033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jiang L, Fei H, Yang A, Zhu J, Sun J, Liu X, et al. , Estrogen inhibits the growth of colon cancer in mice through reversing extracellular vesicle-mediated immunosuppressive tumor microenvironment, Cancer Letters 520 (2021) 332–343. [DOI] [PubMed] [Google Scholar]
- [174].Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL, Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets, Scientific reports 6(1) (2016) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zhao X, Yuan C, Wangmo D, Subramanian S, Tumor-Secreted extracellular vesicles regulate T-cell costimulation and can be manipulated to induce tumor-specific T-cell responses, Gastroenterology 161(2) (2021) 560–574. e11. [DOI] [PubMed] [Google Scholar]
- [176].Huang Y, Luo Y, Ou W, Wang Y, Dong D, Peng X, et al. , Exosomal lncRNA SNHG10 derived from colorectal cancer cells suppresses natural killer cell cytotoxicity by upregulating INHBC, Cancer cell international 21(1) (2021) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ni C, Fang Q-Q, Chen W-Z, Jiang J-X, Jiang Z, Ye J, et al. , Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+ γδ1 Treg cells, Signal transduction and targeted therapy 5(1) (2020) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. , CD20+ tumor-infiltrating lymphocytes have an atypical CD27-memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer, Clinical cancer research 18(12) (2012) 3281–3292. [DOI] [PubMed] [Google Scholar]
- [179].Schmidt M, Böhm D, Von Torne C, Steiner E, Puhl A, Pilch H, et al. , The humoral immune system has a key prognostic impact in node-negative breast cancer, Cancer research 68(13) (2008) 5405–5413. [DOI] [PubMed] [Google Scholar]
- [180].Lohr M, Edlund K, Botling J, Hammad S, Hellwig B, Othman A, et al. , The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer, Cancer letters 333(2) (2013) 222–228. [DOI] [PubMed] [Google Scholar]
- [181].Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. , B cells and tertiary lymphoid structures promote immunotherapy response, Nature 577(7791) (2020) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Andreu P, Johansson M, Affara N.l., Pucci F, Tan T, Junankar S, et al. , FcRγ activation regulates inflammation-associated squamous carcinogenesis, Cancer cell 17(2) (2010) 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, et al. , IgG4 subclass antibodies impair antitumor immunity in melanoma, The Journal of clinical investigation 123(4) (2013) 1457–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, et al. , Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG, Nature medicine 25(2) (2019) 312–322. [DOI] [PubMed] [Google Scholar]
- [185].Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. , Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells, Cancer research 71(10) (2011) 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF, A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses, Immunity 28(5) (2008) 639–650. [DOI] [PubMed] [Google Scholar]
- [187].Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. , B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis, Proceedings of the National Academy of Sciences 108(26) (2011)10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. , SCS macrophages suppress melanoma by restricting tumor-derived vesicle–B cell interactions, Science 352(6282) (2016) 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. , Extracellular vesicle and particle biomarkers define multiple human cancers, Cell 182(4) (2020) 1044–1061. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Pantano F, Croset M, Driouch K, Bednarz-Knoll N, Iuliani M, Ribelli G, et al. , Integrin alpha5 in human breast cancer is a mediator of bone metastasis and a therapeutic target for the treatment of osteolytic lesions, Oncogene 40(7) (2021)1284–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Asada T, Nakahata S, Fauzi YR, Ichikawa T, Inoue K, Shibata N, et al. , Integrin α6A (ITGA6A)-type Splice Variant in Extracellular Vesicles Has a Potential as a Novel Marker of the Early Recurrence of Pancreatic Cancer, Anticancer Research 42(4) (2022) 1763–1775. [DOI] [PubMed] [Google Scholar]
- [192].Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. , Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis, Nature communications 9(1) (2018) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, et al. , Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT, Molecular cancer 18(1) (2019) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kalluri R, LeBleu VS, The biology, function, and biomedical applications of exosomes, Science 367(6478) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Pegtel DM, Gould SJ, Exosomes, Annu Rev Biochem 88 (2019) 487–514. [DOI] [PubMed] [Google Scholar]
- [196].Mathieu M, Martin-Jaular L, Lavieu G, Thery C, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication, Nat Cell Biol 21(1) (2019) 9–17. [DOI] [PubMed] [Google Scholar]
- [197].Meldolesi J, Unconventional Protein Secretion Dependent on Two Extracellular Vesicles: Exosomes and Ectosomes, Front Cell Dev Biol 10 (2022) 877344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Ghoroghi S, Mary B, Larnicol A, Asokan N, Klein A, Osmani N, et al. , Ral GTPases promote breast cancer metastasis by controlling biogenesis and organ targeting of exosomes, Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, et al. , An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles, Cancer Cell 35(1) (2019) 33–45 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Datta A, Kim H, Lai M, McGee L, Johnson A, Moustafa AA, et al. , Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells, Cancer Lett 408 (2017) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, et al. , High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer, Sci Rep 8(1) (2018)8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Kulshreshtha A, Singh S, Ahmad M, Khanna K, Ahmad T, Agrawal A, et al. , Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions, Sci Rep 9(1) (2019) 16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wang J, Wuethrich A, Sina AAI, Lane RE, Lin LL, Wang Y, et al. , Tracking extracellular vesicle phenotypic changes enables treatment monitoring in melanoma, Science advances 6(9) (2020) eaax3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.