Abstract
Objectives
Acetaminophen has gained interest in the neonatal community for its use in the management of hemodynamically significant patent ductus arteriosus (HsPDA) in preterm infants. We conducted a systematic review of randomized controlled trials (RCTs) comparing the efficacy and safety of acetaminophen with indomethacin for the management of HsPDA in preterm infants.
Methods
We searched PROSPERO, OVID Medline, OVID EMBASE, Wiley Cochrane Library (CDSR and Central), EBSCO CINAHL, and SCOPUS from inception to June 15, 2021. Bibliographies of identified studies were searched for additional references. Data were analyzed with Review Manager (RevMan) Version 5.3.
Results
Four RCTs were identified, enrolling a total of 380 subjects. There was no difference between the interventions for the outcome of PDA closure after one course (RR 1.04 [95% CIs: 0.84, 1.29], P-value 0.70) or after two courses of treatment (RR 1.01 [95% CIs: 0.92, 1.12], P-value 0.77); and for the outcome of PDA ligation (RR 1.56 [95% CIs: 0.48, 5.12], P-value 0.46). However, patients who received acetaminophen had lower rates of necrotizing enterocolitis (RR 0.37 [95% CIs: 0.14, 0.95], P-value 0.04). There were no significant differences noted in the other clinical outcomes, that is, intraventricular hemorrhage, bronchopulmonary dysplasia, retinopathy of prematurity requiring treatment, and death. Two studies noted significant post-treatment elevation of serum creatinine and blood urea with indomethacin, as compared to none with acetaminophen use.
Conclusions
Acetaminophen has comparable efficacy to indomethacin for the outcome of HsPDA closure, with a better safety profile, that is, lesser rates of necrotizing enterocolitis and post-treatment azotemia noted with its use.
Keywords: Acetaminophen, Indomethacin, Meta-analysis, Patent ductus arteriosus, Prematurity, Systematic review
Premature infants with patent ductus arteriosus (PDA) have a high rate of morbidity and mortality (1). Approximately three-quarters of extreme premature infants demonstrate symptoms of hemodynamically significant PDA (HsPDA) (2). The ideal management for HsPDA in this vulnerable group of patients remains uncertain (3,4). Available pharmacological agents which are used to promote ductal closure act on the prostaglandin pathway, with indomethacin probably the most studied agent. Evidence shows that indomethacin is effective in closing the duct (5); however, its use is associated with numerous side effects related to its vasoconstrictive effects on renal, cerebral, and mesenteric circulation (6–8). Recently, acetaminophen (also known as paracetamol) has gained interest in the neonatal community for the indication of ductal closure due to its fewer side effects. Similar to indomethacin, it blocks the arachidonic acid pathway; however, it acts on a different enzyme (peroxidase) in the pathway. Acetaminophen could be an alternative to indomethacin if it has a similar efficacy for ductal closure.
We conducted a systematic review of randomized control trials (RCTs) enrolling preterm infants with HsPDA that compared acetaminophen with indomethacin, for the primary outcome of PDA closure.
MATERIALS AND METHODS
This systematic review of RCTs was designed as per the methodology provided in the Cochrane Handbook for Systematic Reviews (9) and is being reported as per the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) statement (10).
Search strategy
A search was executed by an expert librarian (SC) on the following databases: PROSPERO, OVID Medline, OVID EMBASE, Wiley Cochrane Library (CDSR and Central), EBSCO, CINAHL, and SCOPUS using controlled vocabulary (e.g., MeSH, Emtree, etc) and key words representing the concepts “preterm neonates” and “acetaminophen” and “indomethacin”. Variations of the randomized controlled trial filter by Lefebvre et al. (9) were used to limit each search. Animal studies were excluded. No language restrictions were applied. Databases were searched from inception to June 15, 2021. Results were exported to COVIDENCE review management software, where duplicates were removed. Detailed search strategies are available in Appendix 1. In addition, bibliography of the identified trials was searched for other potentially relevant studies.
Study selection
Two members (EB, AH) independently assessed the study eligibility for inclusion according to the pre-established criteria. Disagreements between the two reviewers were resolved through discussion with the third reviewer (MK). The studies were identified for inclusion if they satisfied the following criteria: randomized control trial, enrolling preterm infants with HsPDA, for treatment with acetaminophen (enteral or intravenous) or indomethacin (enteral or intravenous) for the outcome of PDA closure (defined as evidence of ductal closure or change to non-hemodynamically significant duct on an echocardiogram conducted within one week of the treatment completion). We recorded the following secondary outcomes: need for surgical closure of PDA, death, pulmonary hemorrhage, intraventricular hemorrhage (IVH), gastrointestinal bleeding, necrotizing enterocolitis (NEC), sepsis, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and laboratory markers of liver, or renal toxicity and platelet counts.
Risk of bias assessment
Cochrane risk-of-bias (RoB) tool for randomized trials were used to assess risk of bias in the included studies (11). Two reviewers (EB, MK) evaluated each study for RoB and the disagreements were resolved through discussion among the review team. The included studies were assessed for RoB for the following domains: Selection bias, performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias, reporting bias, and other biases.
What is known?
Indomethacin is commonly used for the closure of PDA in premature infants.
Data extraction
Two authors independently extracted the data from the included articles (EB, AH). Data were extracted for demographic characteristics (e.g., gestational age, birth weight, sex), clinical characteristics (e.g., diagnostic criteria, therapy courses, route of delivery), study characteristics (e.g., year of publication, setting, study design, sample size, comparison group, and blinding), reported efficacy and safety outcomes, and authors’ conclusions, using a standardized form.
Strategy for data synthesis
Data were analyzed with the help of Review Manager (RevMan) Version 5.3. [Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014]. We conducted meta-analyses using a random-effects model (9). We selected risk ratios as the effect measure for all our binary outcomes and mean difference for the continuous outcomes. Results are presented as summary estimate along with 95% confidence intervals. Sensitivity analyses were planned by including studies assessed as low risk of bias for the main outcome of treatment efficacy. Statistical heterogeneity was measured using I2 statistic and if substantial heterogeneity was noted (I2 > 50%), additional sensitivity analyses were planned.
RESULTS
Study selection and characteristics
We identified a total of 270 references that were exported to Covidence, a web-based software platform (Figure 1). Two researchers (EB, AH) independently screened the studies for eligibility based on pre-established inclusion and exclusion criteria. Four studies met criteria and were included in the analysis (12–15). Table 1 represents the characteristics of included studies. Mean GA ranged from 25 to 32 weeks across studies. PDA was diagnosed using echocardiography in all studies. Protocol for acetaminophen administration ranged from 15 mg/kg/dose four times daily for 3 days (3 studies) to 7 days (1 study). Indomethacin protocol involved the administration of 3 doses at 0.2 mg/kg/dose (2 studies), 0.1–0.2 mg/kg/dose (1 study) and 0.2–0.25 mg/kg/dose (1 study).
Figure 1.
Study flow diagram.
Table 1.
Characteristics of the included studies
| Dash 2015 | El Mashad 2017 | Meena 2020 | Davidson 2021 | |
|---|---|---|---|---|
| Country | India | Egypt | India | USA |
| Funding source | None | None | None | None |
| Inclusion criteria | BW ≤ 1500 g and Echo in ≤ 48 hrs of birth showing HsPDA | GA< 28 wks or BW< 1500 g and HsPDA diagnosed on basis of Echo and Clinical exam within < 2 wks of birth | GA < 37 wks and HsPDA diagnosed clinically and confirmed by Echo in first 28 postnatal days of life | GA between 22–32 wks and BW < 1500 g at ≤ 21 days of age with HsPDA diagnosed clinically and confirmed by Echo |
| HsPDA Echo criteria | PDA ≥ 1.5 mm with Left-to-right shunt, and LA:AO ratio > 1.5:1 | LA dilatation (LA:AO > 1.6), diastolic turbulence (backflow) on Doppler in the pulmonary artery, internal diameter of duct > 1.5 mm, and reverse end-diastolic flow in the descending aorta/mesenteric artery | Internal diameter of the duct > 1.5 mm, left atrial dilatation (LA/Ao > 1.4), diastolic turbulence (backflow) on Doppler in the pulmonary artery, and reversed end-diastolic flow in the descending aorta/mesenteric artery | Left-to-right ductal flow and 2 of the 3 following: ductal size ≥ 1.5 mm at smallest diameter, reversal of flow in descending aorta or LA:AO ratio ≥ 1.5 |
| INTERVENTION 1 | PO acetaminophen at 15 mg/kg/dose four times daily for 7 days | IV acetaminophen at 15 mg/kg/dose four times daily for 3 days | IV acetaminophen at 15 mg/kg/dose four times daily for 3 days | IV acetaminophen at 15 mg/kg/dose four times daily for 3 days |
| INTERVENTION 2 | IV indomethacin at 0.2 mg/kg/dose once daily for 3 days | IV indomethacin at 0.2 mg/kg/dose twice daily for 3 doses | PO indomethacin twice daily for 3 doses at: Starting dose: 0.2 mg/kg following doses: * Infants < 2 days: 0.1 mg/kg * Infants 2–7 days: 0.2 mg/kg * infants > 7 days: 0.25 mg/kg |
IV indomethacin twice daily for 3 doses at: * Infants 2–7 days: 0.2 mg/kg for all doses * Infants > 7 days: 0.2 mg/kg for 1st dose and 0.25 mg/kg for subsequent doses |
| Sample size PCM vs. Indo |
38 vs. 39 | 100 vs. 100 | 35 vs. 35 | 17 vs. 21 |
| Loss to follow-up for primary outcome | 4/77 (5.2%) | None | None | 1/38 (2.6%) |
| GA mean (SD) PCM vs. Indo |
28.5 (2.7) vs. 28.9 (2.6) | 26 (1.9) vs. 26 (2.1) | 32.14 (2.01) vs. 31.77(2.26) | 25.7 (1.4) vs. 25.3 (1.8) |
| BW mean (SD) PCM vs. Indo |
989 (299) vs. 1027 (262) | 1100 (130) vs. 1100 (140) | 1440 (340) vs. 1410 (320) | 785 (203) vs. 756 (241) |
| Male PCM vs. Indo |
36.9% vs. 33.3% | 60% vs. 60% | 51.4% vs. 42.9 | 53% vs. 40% |
| PDA size (mm) mean (SD) PCM vs. Indo |
2.02 (0.42) vs. 2.11 (0.53) | 2.7 (0.6) vs. 2.7 (0.7) | 1.85 (0.43) vs. 1.82 (0.28) | 2.7 (0.7) vs. 2.9 (0.7) |
| Postnatal age for diagnosis or Rx PCM vs. Indo |
Mean (SD) (hours) 14.7 (8.4) vs. 15.9 (11.8) |
Mean (SD) (days) 2.7 (4.4) vs. 3.1 (5.1) |
Mean (SD) (days) 9.02 (3.43) vs. 10.85 (4.25) |
Median (IQR) (days) 8 (7,11) vs. 6.5 (4,9.3) |
| Primary outcome | PDA closure | PDA closure | PDA closure | Successful PDA treatment (No longer HsPDA) |
AO, aortic root; BW, birth weight; g, gram; GA, gestational age; hr, hour; HsPDA, hemodynamically significant patent ductus arteriosus; Indo, indomethacin; IV, intravenous; LA, left atrial; PCM, paracetamol (acetaminophen); PDA, patent ductus arteriosus; PO, per oral; Rx, treatment.
What is New?
Our systematic review of the available RCTs shows that acetaminophen is as efficacious as indomethacin for the management of HsPDA in preterm infants with a better safety profile, that is, lesser rates of necrotizing enterocolitis and post-treatment azotemia.
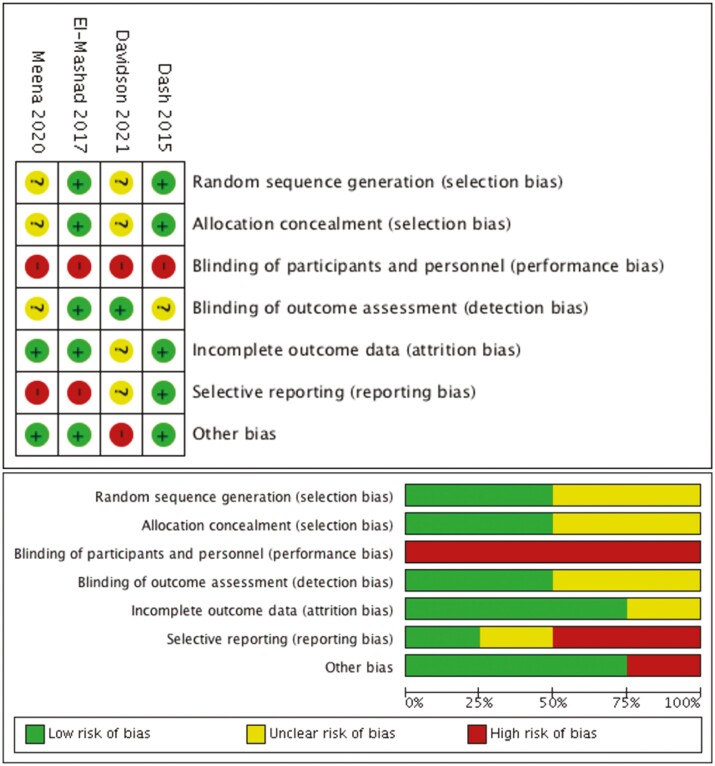
Risk of bias
Risk of bias for the four studies is reported in Figure 2. Cochrane Risk of bias assessment shows a range of low to high degrees of bias. The risk of performance bias was judged high for all studies, as none of the studies had undertaken blinding of participants and healthcare providers. Reporting bias was considered high for two studies (13,14) as they did not provide data for important clinical outcomes such as mortality and BPD. One trial (15) was assessed as high RoB in the domain of other biases as it was stopped early with < 50% of the targeted sample size enrolled.
Figure 2.
Risk of bias assessments of the included studies.
Primary outcome
The pooled estimates for the outcome of PDA closure shows that the closure rates were similar for acetaminophen and indomethacin groups following a single course of treatment [4 studies; 380 subjects; RR 1.04 (95% CIs: 0.84, 1.29); I2 = 69%] (Figure 3a) or after two courses of treatment [2 studies; 270 subjects; RR 1.01 (95% CIs: 0.92, 1.12); I2 = 0%] (Figure 3b). We observed significant heterogeneity with the pooled estimate of treatment effect following single course of intervention (I2 = 69%) which resulted from the extreme results noted in a small study (15). This study was assessed as at a high-risk of bias. In sensitivity analysis, we excluded the results of this study and noted the resolution of significant heterogeneity, with no significant change in the pooled effect estimate [3 studies; 343 patients; RR 1.05 (95% CIs: 0.92, 1.19); I2 = 44%].
Figure 3.
(a) Acetaminophen vs. Indomethacin: PDA closure rates after a single course of treatment. (b) Acetaminophen vs. Indomethacin: PDA closure rates after two courses of treatment. (c) Acetaminophen vs. Indomethacin: PDA ligation rates with each intervention.
There was no difference in PDA ligation rates noted between the two groups [3 studies; 310 subjects; RR 1.56 (95% CIs: 0.48, 5.12)); P-value 0.46] (Figure 3c).
Secondary outcomes
Two studies reported on the outcome of neonatal mortality (12,15) showing no difference among the intervention groups for the risk of death [RR 0.90 (95% CIs: 0.40, 2.02); P-value 0.79]. Incidence of NEC was reported in 3 studies (347 infants) (12–14), and was noted to be significantly lower in acetaminophen group as compared to indomethacin [RR 0.37 (95% CIs: 0.14, 0.95); P-value 0.04] [Absolute risk difference −0.06 (95% CIs: −0.11, −0.01)].
There was no difference noted between the two groups for the outcomes of pulmonary hemorrhage [3 studies; RR 0.92 (95% CIs: 0.14, 6.00); P-value 0.93], IVH [2 studies; RR 0.80 (95% CIs: 0.34, 1.84); P-value 0.59], gastrointestinal bleeding [3 studies; 0.43 (95% CIs: 0.06, 3.40); P-value 0.43], sepsis [3 studies; RR 1.02 (95% CIs: 0.58, 1.79); P-value 0.95], BPD [2 studies; RR 1.22 (95% CIs: 0.85, 1.76); P-value 0.28], ROP [2 studies; RR 0.71 (95% CIs: 0.27, 1.86); P-value 0.49] or ROP requiring treatment [2 studies; RR 1.35 (95% CIs: 0.60, 3.06); P-value 0.47] (Table 2).
Table 2.
Summary of results of the secondary outcomes: Acetaminophen vs. Indomethacin
| Outcome | Studies | Participants | Statistical method | Effect estimate | P-value |
|---|---|---|---|---|---|
| RR [95% CIs] | |||||
| GI Bleed | 3 | 347 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.06, 3.40] | 0.43 |
| Pulmonary hemorrhage | 3 | 347 | Risk Ratio (M-H, Random, 95% CI) | 0.92 [0.14, 6.00] | 0.93 |
| NEC | 3 | 347 | Risk Ratio (M-H, Random, 95% CI) | 0.37 [0.14, 0.95] | 0.04 |
| ROP | 2 | 259 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.27, 1.86] | 0.49 |
| ROP needing treatment | 2 | 91 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.60, 3.06] | 0.47 |
| Sepsis | 3 | 314 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.58, 1.79] | 0.95 |
| IVH | 2 | 275 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.34, 1.84] | 0.59 |
| BPD | 2 | 94 | Risk Ratio (M-H, Random, 95% CI) | 1.22 [0.85, 1.76] | 0.28 |
| Death | 2 | 114 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.40, 2.02] | 0.79 |
BPD, bronchopulmonary dysplasia; GI, gastrointestinal; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity.
Two studies reported on the results of the laboratory investigations that were conducted as part of the trial to assess for renal, hepatic, and hematological toxicity associated with the treatments (13,14). Indomethacin treatment was associated with significant elevation of blood urea and serum creatinine levels as compared to the acetaminophen treatment. However, the studies did not provide data for number of participants with acute kidney injury. There was no evidence of increased liver toxicity or thrombocytopenia noted with either of the treatments.
DISCUSSION
We have presented here an updated systematic review and meta-analyses of the available RCTs that compared acetaminophen with indomethacin for the management of HsPDA in preterm infants. The results reveal that treatment of HsPDA with acetaminophen is as effective as indomethacin for the outcome of ductus closure, with a better safety profile in terms of a lesser risk of NEC and post-treatment azotemia. There was no difference between the two interventions for the outcomes of death and other major neonatal morbidities. The duration of the acetaminophen treatment varied between 3 to 7 days in the included studies. A summary of findings table developed as per GRADE methodology (GRADEpro Guideline Development Tool [Software], available from gradepro.org) revealed low certainty of evidence for the majority of salient clinical outcomes (Supplementary Table).
Our results compare with the results of an existing Cochrane review that showed acetaminophen was as effective as indomethacin and ibuprofen for the outcome of PDA closure in premature infants (16). However, we are able to provide more precise estimates of the pooled effect size of all the clinical outcomes as we included two more RCTs that were published following the publication of the Cochrane review (14,15). As such, we are able to show that treatment of HsPDA with acetaminophen is associated with lesser risk of NEC [RR 0.37 (0.14, 0.95); P-value 0.04], an important clinical side-effect that was not identified in the Cochrane review. A few previous studies have suggested a possible link between indomethacin use and NEC (17,18). Indomethacin use has been shown to diminish splanchnic circulation with resultant mucosal hypoxia and increased risk for gastrointestinal perforations (17). On the other hand, acetaminophen acts at a more distal level in the prostaglandin synthesis pathway (peroxidase inhibition) and apparently, unlike cyclooxygenase inhibitors, its use in amounts needed for PDA closure doesn’t result in significant vasoconstriction and local hypoxia in other organs (19). Also, post-treatment azotemia is a well-known side-effect of indomethacin resulting from the reduction of prostaglandin synthesis (due to suppression of cyclooxygenase pathway in the kidneys) leading to a reduction in renal perfusion (20). However, the long-term effect of indomethacin on renal function remains uncertain.
Another systematic review (21) that compared the use of oral acetaminophen with oral ibuprofen for the management of PDA showed similar efficacy of the two agents for the outcome of PDA closure. However, the authors showed that acetaminophen use was associated with lesser incidence of renal dysfunction (OR 0.27 [0.10, 0.77]) and gastrointestinal bleeding (OR 0.31 [0.11, 0.88]), as compared to ibuprofen. The incidence of NEC was not different between the two agents.
Until now, the predominant use of acetaminophen in the neonatal practice has been for management of HsPDA following treatment with one to two courses of indomethacin or ibuprofen. Based on the results of this systematic review, the clinicians could consider using acetaminophen as the first line drug for the management of HsPDA in preterm infants for its better safety profile as compared to indomethacin. Although, the included trials enrolled ELBW infants and extremely low GA infants (mean GA < 28 weeks in 3 out of 4 included trials), but they did not separately provide data for this population. This subgroup is at higher risk of developing NEC and acute renal failure with the treatment of HsPDA with non-steroidal anti-inflammatory drugs (NSAIDs), and thus likely to benefit more from the use of acetaminophen as the first line drug for the treatment of HsPDA.
There is urgent need for methodologically rigorous trials that test the efficacy and safety profile of acetaminophen against other NSAIDs for the management of HsPDA in extremely preterm infants. Such a trial should avoid the pitfalls observed in the existing RCTs, especially the lack of masking of the trial interventions. Future research should also focus on the optimal duration of the acetaminophen treatment. In a systematic review that included several observational studies, it was noted that a 6-day course of acetaminophen was more efficacious, as compared to a 3-day course, for closure of PDA (22).
Our systematic review has a few limitations. First, we were able to include only four small to moderate size RCTs in this review, as such the majority of pooled effect estimates of our secondary outcomes have low certainty of evidence with wide 95% confidence intervals. On the other hand, all the included studies were conducted within the last 5–10 years and reflect the current understanding of PDA approach and management. Second, all the included trials did not employ masking of interventions to reduce the risk of bias. In addition, we assessed high-risk of bias for selective reporting in two of the included RCTs and extreme results noted in another RCT that was terminated early. As such, we are unable to make strong recommendations in favor of acetaminophen, despite the observed results of this systematic review. Lastly, we were unable to perform a test for publication bias in view of a small number of included studies in this review. However, it is unlikely that we missed any existing trial for inclusion, as our search strategy, undertaken with the help of an experienced research librarian, was exhaustive and included several electronic databases.
CONCLUSION
This systematic review of the small to moderate sized RCTs show that acetaminophen has comparable efficacy to indomethacin for the clincal outcome of HsPDA closure, with lesser rates of necrotizing enterocolitis and post-treatment azotemia. Based on the data presented here, the clinicians could consider using acetaminophen as the first line drug for the management of HsPDA in preterm infants. However, a few of the included trials were assessed at high-risk of bias and the treatment estimates of effect size for several secondary outcomes were imprecise. There is a need for a larger methodologically rigorous trial to confirm better risk-benefit profile of acetaminophen as compared to the NSAID agents in the sub-population of extremely preterm infants.
Supplementary Material
Abbreviations:
- BPD
Bronchopulmonary dysplasia
- GA
gestational age
- HsPDA
hemodynamically significant patent ductus arteriosus
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PDA
Patent ductus arteriosus
- OR
odds ratio
- RCT
randomized controlled trial
- ROP
retinopathy of prematurity
- RR
risk ratio
Contributor Information
Eyad Bitar, Department of Pediatrics, Division of Neonatal-Perinatal Care, University of Alberta, Edmonton.
Abbas Hyderi, Department of Pediatrics, Division of Neonatal-Perinatal Care, University of Alberta, Edmonton.
Sandra M Campbell, John W Scott Health Sciences Library, University of Alberta, Edmonton.
Manoj Kumar, Department of Pediatrics, Division of Neonatal-Perinatal Care, University of Alberta, Edmonton.
AUTHORS’ CONTRIBUTIONS
(EB) contributed to all stages of the review; wrote the initial draft of the manuscript; and approved the final manuscript as submitted. (AH) contributed to all stages of the review; reviewed all drafts of the manuscript; and approved the final manuscript as submitted. (SC) was associated with the planning of the study; developed and conducted study’s search strategy; and approved the final manuscript as submitted. (MK) contributed to all stages of the review, reviewed all drafts of the manuscript; and approved the final manuscript as submitted.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by the authors.
CONSENT FOR PARTICIPATION
No human subjects were enrolled as part of the study. A meta-analysis was done
CONSENT FOR PUBLICATION
All authors provide their consent for publication of this manuscript.
FUNDING
There are no funders to report.
POTENTIAL CONFLICTS OF INTEREST
The authors have no conflicts to disclose.
References
- 1. Noori S, McCoy M, Friedlich P.. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 2009;123(1):e1382138–e144. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 2. Hermes-DeSantis ER, Clyman RI.. Patent ductus arteriosus: Pathophysiology and management. J Perinatol 2006;26(Suppl 1):S14–8; discussion S22. doi: 10.1038/sj.jp.7211465. [DOI] [PubMed] [Google Scholar]
- 3. Mitra S, McNamara PJ.. Patent ductus arteriosus-time for a definitive Trial. Clin Perinatol 2020;47(3):617–39. doi: 10.1016/j.clp.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 4. Smith A, El-Khuffash A.. Patent Ductus Arteriosus clinical trials: Lessons learned and future directions. Children (Basel) 2021;8(1):47. doi: 10.3390/children8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans P, O’Reilly D, Flyer JN, Soll R, Mitra S.. Indomethacin for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev 2021;1(1):CD013133. doi: 10.1002/14651858.CD013133.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Austin NC, Pairaudeau PW, Haames TK.. Regional blood flow velocity changes after indomethacin infusion in preterm infants. Arch Dis Child 1992;67:851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coombs RC, Morgan ME, Durbin GM, Booth I W, McNeish A S.. Gut flow blood velocities in the newborn: Effect of patent ductus arteriosus and parenteral indomethacin. Arch Dis Child 1990;65:1067–71 doi: 10.1136/adc.65.10_spec_no.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Bel F, Guit GL, Schipper J, van de Bor M, Baan J.. Indomethacin-induced changes in renal blood flow velocity waveform in premature infants investigated with color Doppler imaging. J Pediatr 1991;118:621–6. doi: 10.1016/s0022-3476(05)83391-8. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook. Accessed June 16, 2022. [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA GroupPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Altman DG.. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley, 2008:187–241. [Google Scholar]
- 12. Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J.. Enteral paracetamol, or intravenous Indomethacin for closure of patent ductus arteriosus in preterm neonates: A randomized controlled trial. Indian Pediatr 2015;52(7):573–8. doi: 10.1007/s13312-015-0677-z. [DOI] [PubMed] [Google Scholar]
- 13. El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M.. Comparative study of the efficacy and safety of Paracetamol, ibuprofen, and Indomethacin in closure of patent Ductus Arteriosus in preterm neonates. Eur J Pediatr 2017;176(2):233–40. doi: 10.1007/s00431-016-2830-7. [DOI] [PubMed] [Google Scholar]
- 14. Meena V, Meena DS, Rathore PS, Chaudhary S, Soni JP.. Comparison of the efficacy and safety of indomethacin, ibuprofen, and paracetamol in the closure of patent ductus arteriosus in preterm neonates—a randomized controlled trial. Ann Pediatr Cardiol 2020;13(2):130–5. doi: 10.4103/apc.APC_115_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidson JM, Ferguson J, Ivey E, Philip R, Weems MF, Talati AJ.. A randomized trial of intravenous acetaminophen versus indomethacin for treatment of hemodynamically significant PDAs in VLBW infants. J Perinatol 2021;41:93–9. doi: 10.1038/s41372-020-0694-1. [DOI] [PubMed] [Google Scholar]
- 16. Ohlsson A, Shah PS.. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. no.: CD010061. doi: 10.1002/14651858.CD010061.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grosfeld JL, Chaet M, Molinari F, et al. Increased risk of necrotizing enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann Surg 1996;224(3):350–5; discussion 355. doi: 10.1097/00000658-199609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujii AM, Brown E, Mirochnick M, O’Brien S, Kaufman G.. Neonatal necrotizing enterocolitis with intestinal perforation in extremely premature infants receiving early Indomethacin treatment for patent Ductus Arteriosus. J Perinatol 2002;22(7):535–40. doi: 10.1038/sj.jp.7210795. [DOI] [PubMed] [Google Scholar]
- 19. Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D.. Ductal closure with Paracetamol: A surprising new approach to patent Ductus Arteriosus treatment. Pediatrics 2011;128(6):e1618–21. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 20. Akima S, Kent A, Reynolds GJ, Gallagher M, Falk MC.. Indomethacin and renal impairment in neonates. Pediatr Nephrol 2004;19(5):490–3. doi: 10.1007/s00467-003-1402-z. [DOI] [PubMed] [Google Scholar]
- 21. Pranata R, Yonas E, Vania R, Prakoso R.. The efficacy and safety of oral Paracetamol versus oral ibuprofen for patent Ductus Arteriosus closure in preterm neonates—a systematic review and meta-analysis. Indian Heart J 2020;72(3):151–9. doi: 10.1016/j.ihj.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terrin G, Conte F, Oncel MY, et al. Paracetamol for the treatment of patent Ductus Arteriosus in preterm neonates: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2016;101(2):F127–36. doi: 10.1136/archdischild-2014-307312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.