Abstract
Background:
Allergen specific immunotherapy (SIT) has been used for more than a century. Researchers have been working to improve efficacy and reduce the side effects.
Objective:
We have reviewed the literature about peptides immunotherapy for inhaled allergens. The mechanism of SIT is to induce regulatory T (Treg) cells and to reduce T helper (Th)2 cells to induce class switching from IgE to IgG and induce blocking antibodies to inhibit allergen binding of IgE.
Methods:
The relevant published literatures on the peptide SIT for aeroallergens have been searched on the medline.
Results:
Modification of allergens and routes of treatment has been performed. Among them, many researchers were interested in peptide immunotherapy. T-cell epitope peptide has no IgE epitope, that is able to bind IgE, but rather induces Treg and reduces Th2 cells, which was considered an ideal therapy. Results from cellular and animal model studies have been successful. However, in clinical studies, T-cell peptide immunotherapy has failed to show efficacy and caused side effects, because of the high effective rate of placebo and the development of IgE against T-cell epitope peptides. Currently, the modifications of IgE-allergen binding by blocking antibodies are considered for successful allergen immunotherapy.
Conclusion:
Newly developed hypoallergenic B cell epitope peptides and computational identification methods hold great potential to develop new peptide immunotherapies.
Keywords: aero-allergens, birch, blocking antibody, cedar, epitope, peptides, pollen, ragweed
Immunotherapy for allergic diseases, especially allergic diseases caused by inhaled allergens, was first reported by Noon1 and Freeman2 in 1911 on grass pollen allergies (Timothy grass [Phleum pretense], Poa trivialis, Holcus lanatus, Agropyrum caninum, Poaceae).3 Immunotherapy for hay fever has been widely used since then and has also been used to treat perennial allergic rhinitis and asthma caused by mites and animal allergens. In the 1920s, in the United States, weekly subcutaneous injections of crude allergen extracts over a period of years were used.3,4 However, the anaphylactic response develops even after years of apparent treatment success. Recently, technologic advances have led to the development of defined reagents for immunotherapy that carry less potential for reactivity and require fewer injections.
Mechanism of Action of Immunotherapy
Immunotherapy for allergic diseases has been considered effective by inducing blocking antibodies and regulatory T (Treg) cells. Two to 4 weeks after the start of low-dose allergen immunotherapy, the number of Treg cells producing interleukin (IL) 10 and transforming growth factor (TGF) β production increased, with accompanying suppression of allergen-specific T-helper (Th) 2 cells and production of immunoglobulin E (IgE). Induction of B-cell class switching induces production of allergen-specific IgG2, IgG4, and IgA.5–12 High-dose allergens can be administered within 1 year of immunotherapy, and the production of IL-12 and IL-27 predominates Th1 and increases the production of interferon γ, which induces IgE-producing B cells to be converted to IgG-producing cells.13–16 Results of recent studies suggest that these changes in cytokine production are due, at least in part, to epigenetic mechanisms.
In addition, regulatory B cells, a subtype of B cells that produces IL-10, suppress T cells and dendritic cells mediated inflammation and induce immune tolerance. Allergen-specific IgG4 production was confirmed in regulatory B cells induced after immunotherapy for bee venom, and it is presumed that similar reactions occur after immunotherapy with house-dust mite and grass pollen allergens.17
Treg cells are a subtype of CD4+ T cells. Treg cells include natural Treg cells derived from the thymus and induced Treg derived from the periphery. The induced Treg cells control the immune response against non–self-antigens by producing TGB-β. The pathogenic mechanism of allergic rhinitis is associated with Treg cells and Th1/Th2 imbalance. In patients with allergic nasal inflammation, the production of small molecules, especially prostaglandin E2 (PGE2), is high, and PGE2 activates the E prostanoid receptor (EP4) of Treg cells via the cyclic AMP (cAMP) dependent protein kinase A pathway. The PGE2-EP4-cAMP signaling pathway is involved in patients with allergic nasal inflammation. In these patients, the induction of Treg cells is suppressed via this PGE2-EP4-cAMP signaling pathway.18
Methylation of key genes may also indicate successful allergen specific immunotherapy (SIT). It has been reported that methylation of cytosine in the promoter region of forkhead box protein P3 (FOXP3) of Treg cells is closely associated with allergic diseases.18 Investigation of methylation of Th1-related genes interferon gamma (IFNG), RUNX family transcription factor 3 (RUNX3), and Th2 related genes interleukin 4 (IL4), interleukin 13 (IL13), CCL chemokine 17 (CCL17), and IL-2 in peripheral blood cells after house-dust SIT shows that DNA methylation increased overall, with particularly increased methylation in the promoter region of IL4.19
Problems with Immunotherapy
In the standard treatment of allergen immunotherapy, intradermal injection of a crude allergen solution is started from a low dose, and inoculation is performed twice a week to every other week. The amount of allergen is gradually increased, and the effect is seen several years later. The burden on the patient is heavy because frequent consultations are required and there is a rare but severe threat of anaphylaxis. In 2017, a European study reported systemic reactions in 90 of 4316 patients (2.1%), of which 65% were determined to be anaphylaxis.20 A North American study of 54.4 million injections showed a 0.1% adverse reaction with 0.8 fatal reactions per year (0.005% life-threatening reaction).21 A Canadian study with 380 patients showed 0.095% systemic reaction cases of patients who required epinephrine administration.22 A crude allergen extract contains IgE epitopes and hence the possibility of inducing an anaphylactic reaction. New immunotherapies have been developed to eliminate the risk of these adverse reactions, which largely depend on the ability to identify the amino acid sequences of major allergens and to produce recombinant allergens. The development of recombinant allergens has made it possible to mass produce allergens, fragment allergens, create peptides, and mutate and delete amino acids. In addition, even the synthesis of a short peptide that can inhibit FOXP3/ Nuclear factor of activated T cells (NFAT) interaction suppressor activity of conventional Tregs in vitro is possible.23
Development of Peptide Immunotherapy
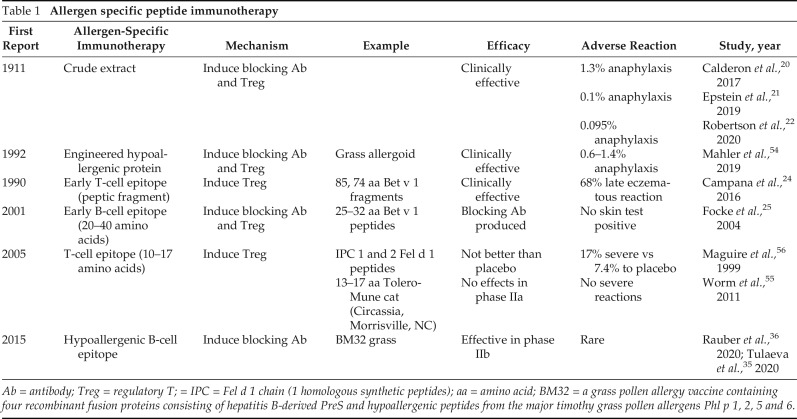
Recombinant proteins, less allergenic homologues of allergens, have been developed to shorten the duration of allergen immunotherapy and reduce adverse effects. These include early peptide immunotherapy, recently developed B-cell epitope peptide, and T-cell epitope peptide.24,25 (Table 1).
Table 1.
Allergen specific peptide immunotherapy
Ab = antibody; Treg = regulatory T; = IPC = Fel d 1 chain (1 homologous synthetic peptides); aa = amino acid; BM32 = a grass pollen allergy vaccine containing four recombinant fusion proteins consisting of hepatitis B-derived PreS and hypoallergenic peptides from the major timothy grass pollen allergens Phl p 1, 2, 5 and 6.
Early Peptide Immunotherapy
Allergen immunotherapy was mainly aimed at suppressing the reaction of Th2 cells, and peptide immunotherapy developed in the 1990s used allergen proteins cleaved into fragments. After that, the amino acid sequence of the allergen was identified, and a T-cell epitope peptide with low or non-reactivity to IgE was determined by using an overlapping peptide scanning. This method was used to treat birch (Betula platyphylla, Betulaceae, Bet v 1) and cat (Felis domesticus, Felidae, Fel d 1) allergies and was shown to be effective to some extent in clinical trials. However, patients treated with high concentrations of peptide showed a delayed respiratory response after inoculation, which confirmed the production of IgE antibodies against the inoculated peptide.26 The effectiveness of peptide immunotherapy was questioned because equivalent responses were frequently confirmed in the placebo-inoculated group.27 To solve these problems, immunotherapy that uses shorter peptides was developed.
B-Cell Epitope Peptide Immunotherapy
The B-cell epitope generally consists of 20–40 amino acids and depends on the tertiary structure of the protein. Some amino acid sequence changes or carrier proteins have been added. In many cases, noninflammatory allergen-specific immunoglobulin IgG4 is induced to act as a blocking antibody and inhibit IgE binding to the antigen.25
T-Cell Epitope Peptide Immunotherapy
T-cell epitope peptides consist of linear 10–17 amino acids and may not contain IgE epitopes. These T-cell epitope peptides bind to Major histocompatibility complex (MHC) class II on antigen-presenting cells and induce Th1 and Treg reactions. Because the T-cell epitope peptide is short, it cannot bind and cross-link IgEs bound to high affinity human IgE receptor (FcεRI). The development of T-cell epitope peptide therapy was advanced because the peptides can induce Th1 and Treg without causing adverse effects. T-cell immunotherapies was considered to have a huge benefit. However, their clinical results have been mixed with limitations. T-cell epitope peptide therapy for the birch allergen Bet v 1 also showed delayed respiratory symptoms after inoculation, similar to early peptide immunotherapy.28 One speculation was that the T-cell epitope stimulated the production of IgE.
In a recent study, peptides from the cat allergen Fel d 1 were used for immunotherapy with the 10 major peptides that bind to Human Leukocyte Antigen - DR isotype (HLA-DR), and the effect was observed even after 2 years without booster vaccination. However, a phase III cat allergy clinical trial revealed no significant difference from the placebo-inoculated group.29–31 Similarly, Phase IIb mite allergy clinical trials also showed no significant difference from placebo groups.32 The reason for these results is speculated to be that successful immunotherapy needs both T-cell alterations and induction of blocking antibodies.
Hypoallergenic B-Cell Epitope Peptide Immunotherapy
A hypoallergenic B-cell epitope peptide conjugated with a nonallergenic but immunogenic carrier was shown to be hypoallergenic. N-terminal region of surface antigen domain (PreS) from hepatitis B virus was developed for grass pollen allergens, Phl p 1, Phl p 2, Phl p 5, and Phl p 6 (BM32).33,34 PreS has the ability to provide T-cell help. Subsequent studies with BM32 showed its potential for clinical application.35,36 In this study,33 four injections, which included the three initial injections 3 months before the grass pollen season and a booster in the fall after the pollen season, were given during the first year. The next three injections were given before the next pollination season in the second year. A phase IIb study33 showed an increase in IgG, IgG1, and IgG4 in the patients who were treated. Even though IgG decreased after 5 months, the application of the booster significantly restored the titers of IgG1 and IgG4. T-cell activation was reduced after the BM32 immunotherapy, and mast cell/basophil degranulation may be prevented by these IgG1 and IgG4. BM32 did not substantially modify the IgE levels compared with the baseline. The quality of life of patients with asthma during the pollination season in the second year was improved because they observed only mild reactions.37,38
Innate Immunity and Toll-Like Receptor Stimulation
Toll-like receptors (TLR) are located on the cell surface or intracellularly and recognize pathogen-associated molecular patterns. A TLR9 stimulant by using an unmethylated cytosine and guanine separated by a phosphate (CpG) DNA motif, an agonist of TLR, coupled with the lipopolysaccharide, monophosphoryl lipid A, induced the innate immune system to lead a Th1 response. The combined use of CpG DNA and the ragweed (Ambrosia aetemisiifolia, Asteraceae) allergen Amb a 1 peptide enhances the effect of immunotherapy. Initial efficacy was observed in clinical trials, but the effectiveness was not confirmed in follow-up studies.39
Potential New Peptide Immunotherapy
We have investigated allergens of mountain cedar (Juniperus ashei, Cupressaseae) pollen, which contains Jun a 1, which is closely related to Japanese cedar (Cryptomeria japonica, Taxodiaceae) and is highly homologous to its major antigen Cry j 1.40–44 One of the monoclonal antibodies (mAb) against Jun a 1 is E58, which has unique characteristics.45 Initial binding of E58 to Jun a 1 inhibits the binding of other anti–Jun a 1 mAbs that bind to four different epitopes.46 When these four independent mAbs initially bind to Jun a 1, E58 can still bind to Jun a 1. From this characteristic, we hypothesized that the binding of E58 causes a change in the tertiary structure of Jun a 1 and a modification in the epitopes of the four mAbs, so that these four mAbs cannot bind. The effect of this E58 mAb was also observed in the IgE binding to Cry j 1 and the European cypress pollen allergen, Cup s 1 (Cupressus sempervirens, Cupressaseae). In an experiment that used human high-affinity receptor for IgE transgenic mice,47 a significant reduction in symptoms was observed after nasal instillation of Jun a 1 by reacting E58 Fab with Jun a 1 (Fig. 1). Because Jun a 1 belongs to the pectate lyase family of proteins, and this family of allergens includes allergens from grass, weed, and fungi, in addition to conifer pollen, E58 has the potential to be used widely for the treatment of these allergens. We are currently elucidating the tertiary structure of the E58 epitope that induces antibodies with better suppression of the binding of patient serum IgE to allergens and future clinical applications.
Figure 1.

A significant reduction in symptoms was observed after the preincubation of Jun a 1 with E58 Fab.
Future Development of New Peptide Immunotherapies
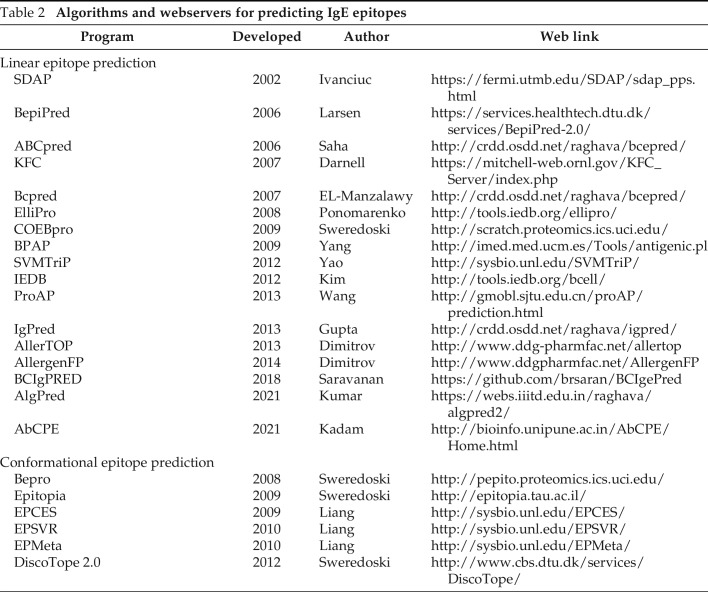
Instead of biologic experiments, computational identification methods have been proposed to analyze the characteristics of peptides that may help in the design of novel peptides for immunotherapy.48 The SDAP webserver49 (https://fermi.utmb.edu/ University of Texas Medical Branch, Galveston, TX) provides rapid, validated ways to define regions in known allergens that are similar (low property distance) to a linear IgE binding peptide. Mapping these on the parent or similar allergens reveals conformational epitopes that may be the basis for IgE binding. Other epitope algorithms and webservers for predicting linear and conformational IgE epitopes are listed in Table 2, whereby a full comparison of their results is beyond the scope of this review.
Table 2.
Algorithms and webservers for predicting IgE epitopes
Physical-chemical property motifs can be used to discriminate allergens from nonallergens and may help in limiting the size of allergen peptides for immunotherapy.50 Motif scores for Bet v 1 from birch pollen family, which cross-react with homologous fruit and nut allergens, correlated better than global sequence similarities with clinically observed cross-reactivities among those allergens.50 The physical-chemical property motifs also discriminated allergenic pectate lyases, including Jun a 1 from mountain cedar pollen, from pectate lyases in the human microbiome, most likely nonallergens.50 The latter lacked key motifs characteristic of the known allergens, some of which correlate with known IgE-binding sites.
Another approach is to use computational methods to make sense of large amounts of experimental data to identify motifs and sensitizing features of allergenic proteins. For example, an iterative motif-finding algorithm was applied to data obtained from 3142 serum samples, each tested against 103 highly purified natural or recombinant allergens, which revealed areas for potential cross-reactivity and sensitization.51 Suprun et al.52,53 recently reported that they successfully identified IgE epitopes and experimentally validated them for egg and peanut allergens. Identifying regions of allergens that are specific IgE epitopes or particular areas for sensitization can aid in designing hypoallergenic peptide immunotherapies that can be applied with a larger amount of peptides in shorter time periods without risking severe adverse effects.
CONCLUSION
Extensive efforts have been made to improve immunotherapy for aeroallergens for >100 years. T-cell epitope peptide immunotherapy was once considered highly effective without causing severe adverse effects. However, clinical studies failed to show the efficacy and safety of T-cell epitope peptide therapy. Currently, inducing both blocking IgG antibodies and Treg cells is considered essential for successful immunotherapy. Clinical studies of a hypoallergenic B-cell epitope peptide vaccines are ongoing. A better understanding of antibodies that can block allergen binding to IgE and computational methods to analyze large experimental data sets should lead to developing effective and safer peptide immunotherapies against aeroallergens.
Footnotes
This work was supported by the National Institutes of Health (R21 ES025406 to T.M.-H. and R01AI165866 to C.H.S.), and an Allergic Respiratory Disease Award from the American Lung Association/American Academy of Allergy, Asthma and Immunology to T. Midoro-Horiuti
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911; 177:1572–1573. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(00)78276-6/fulltext. [Google Scholar]
- 2. Freeman J. Further observation on the treatment of hay fever by hypodermic inoculations of pollen vaccine. Lancet. 1911; 178:814–817. https://www.sciencedirect.com/science/article/pii/S014067360140417X. [PubMed] [Google Scholar]
- 3. Finegold I. A brief history of allergen immunotherapy. Allergy Asthma Proc. 2022; 43:248–253. [DOI] [PubMed] [Google Scholar]
- 4. Veer AV, Cooke RA, Spain WC. Diagnosis and treatment of seasonal hayfever. Am J Medical Sci. 1927; 174:101–113. [Google Scholar]
- 5. Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003; 33:1205–1214. [DOI] [PubMed] [Google Scholar]
- 6. Akdis CA, Blesken T, Akdis M, et al. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008; 121:1120–1125.e2. [DOI] [PubMed] [Google Scholar]
- 8. O'Hehir RE, Gardner LM, de Leon MP, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009; 180:936–947. [DOI] [PubMed] [Google Scholar]
- 9. Heeringa JJ, McKenzie CI, Varese N, et al. Induction of IgG2 and IgG4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020; 75:1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004; 172:3252–3259. [DOI] [PubMed] [Google Scholar]
- 11. Pilette C, Nouri-Aria KT, Jacobson MR, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007; 178:4658–4666. [DOI] [PubMed] [Google Scholar]
- 12. Shamji MH, Kappen J, Abubakar-Waziri H, et al. Nasal allergen-neutralizing IgG4 antibodies block IgE-mediated responses: novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019; 143:1067–1076. [DOI] [PubMed] [Google Scholar]
- 13. Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997; 27:1007–1015. [DOI] [PubMed] [Google Scholar]
- 14. Bohle B, Kinaciyan T, Gerstmayr M, et al. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007; 120:707–713. [DOI] [PubMed] [Google Scholar]
- 15. Hamid QA, Schotman E, Jacobson MR, et al. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997; 99:254–260. [DOI] [PubMed] [Google Scholar]
- 16. Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int. 2013; 62:403–413. [DOI] [PubMed] [Google Scholar]
- 17. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015; 42:607–612. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Sha J, Sun L, et al. Contribution of regulatory T cell methylation modifications to the pathogenesis of allergic airway diseases. J Immunol Res. 2021; 2021:5590217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang C-M, Chang C-B, Chan MW, et al. Dust mite allergen-specific immunotherapy increases IL4 DNA methylation and induces Der p-specific T cell tolerance in children with allergic asthma. Cell Mol Immunol. 2018; 15:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calderon MA, Vidal C, Rodriguez Del Rio P, et al. European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real-life clinical assessment. Allergy. 2017; 72:462–472. [DOI] [PubMed] [Google Scholar]
- 21. Epstein TG, Liss GM, Berendts KM, et al. AAAAI/ACAAI Subcutaneous Immunotherapy Surveillance Study (2013–2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract. 2019; 7:1996–2003.e1. [DOI] [PubMed] [Google Scholar]
- 22. Robertson K, Montazeri N, Shelke U, et al. A single centre retrospective study of systemic reactions to subcutaneous immunotherapy. Allergy Asthma Clin Immunol. 2020; 16:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lozano T, Villanueva L, Durantez M, et al. Inhibition of FOXP3/NFAT interaction enhances T cell function after TCR stimulation. J Immunol. 2015; 195:3180–3189. [DOI] [PubMed] [Google Scholar]
- 24. Campana R, Moritz K, Marth K, et al. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016; 137:601–609.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Focke M, Linhart B, Hartl A, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004; 34:1525–1533. [DOI] [PubMed] [Google Scholar]
- 26. Michael JG, Litwin A, Hassert V, et al. Modulation of the immune response to ragweed allergens by peptic fragments. Clin Exp Allergy. 1990; 20:669–674. [DOI] [PubMed] [Google Scholar]
- 27. Wallner BP, Gefter ML. Peptide therapy for treatment of allergic diseases. Clin Immunol Immunopathol. 1996; 80:105–109. [DOI] [PubMed] [Google Scholar]
- 28. Spertini F, Perrin Y, Audran R, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014; 134:239–240.e13. [DOI] [PubMed] [Google Scholar]
- 29. Circassia announces top-line results from cat allergy phase III study. In Circassia. 2016. https://investors.niox.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study/.
- 30. Rudulier CD, Tonti E, James E, et al. Modulation of CRTh2 expression on allergen-specific T cells following peptide immunotherapy. Allergy. 2019; 74:2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018; 9:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Circassia announces top-line results from house dust mite allergy field study. In Circassia. 2017. https://investors.niox.com/media/press-releases/circassia-announces-top-line-results-from-house-dust-mite-allergy-field-study/.
- 33. Focke-Tejkl M, Weber M, Niespodziana K, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015; 135:1207–1207.e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niederberger V, Marth K, Eckl-Dorna J, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015; 136:1101–1103.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tulaeva I, Cornelius C, Zieglmayer P, et al. Quantification, epitope mapping and genotype cross-reactivity of hepatitis B preS-specific antibodies in subjects vaccinated with different dosage regimens of BM32. EBioMedicine. 2020; 59:102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rauber MM, Möbs C, Campana R, et al. Allergen immunotherapy with the hypoallergenic B-cell epitope-based vaccine BM32 modifies IL-10- and IL-5-secreting T cells. Allergy. 2020; 75:450–453. [DOI] [PubMed] [Google Scholar]
- 37. Niederberger V, Neubauer A, Gevaert P, et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J Allergy Clin Immunol. 2018; 142:497–509.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zieglmayer P, Focke-Tejkl M, Schmutz R, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016; 11:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaar O, Creticos PS, Kleine-Tebbe J, et al. One hundred ten years of allergen immunotherapy: a broad look into the future. J Allergy Clin Immunol Pract. 2021; 9:1791–1803. [DOI] [PubMed] [Google Scholar]
- 40. Czerwinski EW, Midoro-Horiuti T, White MA, et al. Crystal structure of Jun a 1, the major cedar pollen allergen from Juniperus ashei, reveals parallel beta-helical core. J Biol Chem. 2005; 280:3740–3746. PMCID: PMC2653420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Midoro-Horiuti T, Mathura V, Schein CH, et al. Major linear IgE epitopes of mountain cedar pollen allergen Jun a 1 map to the pectate lyase catalytic site. Mol Immunol. 2003; 40:555–562. [DOI] [PubMed] [Google Scholar]
- 42. Midoro-Horiuti T, Goldblum RM, Kurosky A, et al. Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. J Immunol. 2000; 164:2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Midoro-Horiuti T, Goldblum RM, Kurosky A, et al. Molecular cloning of the mountain cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin Immunol. 1999; 104(Pt 1):613–617. [DOI] [PubMed] [Google Scholar]
- 44. Midoro-Horiuti T, Goldblum RM, Kurosky A, et al. Isolation and characterization of the mountain cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin Immunol. 1999; 104(Pt 1):608–612. [DOI] [PubMed] [Google Scholar]
- 45. Goldblum RM, Ning B, Judy BM, et al. A single mouse monoclonal antibody, E58 modulates multiple IgE epitopes on group 1 cedar pollen allergens. Mol Immunol. 2016; 74:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goldblum RM, Ning B, Endsley MA, et al. IgE antibodies to mountain cedar pollen predominantly recognize multiple conformation epitopes on Jun a 1. J Allergy Clin Immunol. 2014; 134:967–969.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mueller GA, Glesner J, Daniel JL, et al. Mapping human monoclonal IgE epitopes on the major dust mite allergen Der p 2. J Immunol. 2020; 205:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ivanciuc O, Midoro-Horiuti T, Schein CH, et al. The property distance index PD predicts peptides that cross-react with IgE antibodies. Mol Immunol. 2009; 46:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schein CH, Negi SS, Braun W. Still SDAPing along: 20 years of the structural database of allergenic proteins. Front Allergy. 2022; 3:863172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu W, Negi SS, Schein CH, et al. Distinguishing allergens from non-allergenic homologues using physical-chemical property (PCP) motifs. Mol Immunol. 2018; 99:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfiffner P, Stadler BM, Rasi C, et al. Cross-reactions vs co-sensitization evaluated by in silico motifs and in vitro IgE microarray testing. Allergy. 2012; 67:210–216. [DOI] [PubMed] [Google Scholar]
- 52. Suprun M, Sicherer SH, Wood RA, et al. Mapping sequential IgE-binding epitopes on major and minor egg allergens. Int Arch Allergy Immunol. 2022; 183:249–261. [DOI] [PubMed] [Google Scholar]
- 53. Suprun M, Kearney P, Hayward C, et al. Predicting probability of tolerating discrete amounts of peanut protein in allergic children using epitope-specific IgE antibody profiling. Allergy. 2022; 77:3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mahler V, Zielen S, Rosewich M. Year-round treatment initiation for a 6-grasses pollen allergoid in specific immunotherapy of allergic rhinoconjunctivitis and asthma. Immunotherapy. 2019; 11:1569–1582. [DOI] [PubMed] [Google Scholar]
- 55. Worm M, Lee H-H, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011; 127:89–97, 97.e1-14. [DOI] [PubMed] [Google Scholar]
- 56. Maguire P, Nicodemus C, Robinson D, et al. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999; 93:222–231. [DOI] [PubMed] [Google Scholar]