Abstract
Neuroimaging studies have documented morphometric brain abnormalities in schizophrenia, but less is known about them in individuals at clinical high-risk for psychosis (CHR-P), including how they compare with those observed in early schizophrenia (ESZ). Accordingly, we implemented multivariate profile analysis of regional morphometric profiles in CHR-P (n=89), ESZ (n=93) and healthy controls (HC; n=122). ESZ profiles differed from HC and CHR-P profiles, including 1) cortical thickness: significant level reduction and regional non-parallelism reflecting widespread thinning, except for entorhinal and pericalcarine cortex, 2) basal ganglia volume: significant level increase and regional non-parallelism reflecting larger caudate and pallidum, and 3) ventricular volume: significant level increase with parallel regional profiles. CHR-P and ESZ cerebellar profiles showed significant non-parallelism with HC profiles. Regional profiles did not significantly differ between groups for cortical surface area or subcortical volume. Compared to CHR-P followed for ≥18 months without psychosis conversion (n=31), CHR-P converters (n=17) showed significant non-parallel ventricular volume expansion reflecting specific enlargement of lateral and inferolateral regions. Antipsychotic dosage in ESZ was significantly correlated with frontal cortical thinning. Results suggest that morphometric abnormalities in ESZ are not present in CHR-P, except for ventricular enlargement, which was evident in CHR-P who developed psychosis.
Keywords: profile analysis, cortical morphometry, subcortical morphometry, ventricles, thickness, schizophrenia
1. Introduction
Many prior magnetic resonance imaging (MRI) studies have documented morphometric brain abnormalities in schizophrenia (e.g., Haijma et al., 2013; Harvey et al., 1993; Kempton et al., 2010; Koshiyama et al., 2022; McCarley et al., 1999; Shenton et al., 2001; van Erp et al., 2016; van Erp et al., 2018; Wright et al., 2000), with abnormalities becoming more widespread over the illness course (Jung et al., 2011; van Haren et al., 2011). However, there is much research documenting brain morphometric alterations in schizophrenia across the illness course, from first-episode to chronic patients, less is known about these morphometric measures in individuals at clinical high-risk for psychosis (CHR-P) and how they compare with measures from schizophrenia patients early in their illness course (ESZ). Although still actively debated (Borgwardt et al., 2009; DeLisi, 2022; Fatemi & Folsom, 2009; Fusar-Poli et al., 2013; Mathalon et al., 2003; van Haren et al., 2011; Zipursky et al., 2013), many longitudinal studies have shown progressive worsening of morphometric brain abnormalities over time in schizophrenia over and above the effects of normal aging, consistent with progressive pathophysiological processes (Andreasen et al., 2011; Cahn et al., 2002; DeLisi, 2008; Dietsche et al., 2017; Fusar-Poli et al., 2013; Ho et al., 2003; Kempton et al., 2010; Mathalon et al., 2001; Vita et al., 2012). Moreover, while the presence of morphometric brain abnormalities in first-episode schizophrenia supports the prevailing theory they largely arise from faulty neurodevelopment (Andreasen et al., 2011; Cahn et al., 2002; Ho et al., 2003; Smucny et al., 2022; Weinberger, 1995), their neurodevelopmental origins are not incompatible with a progressive pathophysiology worsening these abnormalities over the illness course (Mathalon et al., 2003; Rapoport et al., 2005).
Previous neuroimaging studies have specifically documented cortical abnormalities early in the course of schizophrenia. Many studies have documented cortical thinning in ESZ, especially in frontal and temporal regions (e.g., Akudjedu et al., 2020; Crespo-Facorro et al., 2011; Gallardo-Ruiz et al., 2019; Hua & Mathalon, 2022; Lin et al., 2019; Rapoport et al., 2005; Wen et al., 2021; Zhao et al., 2022). In contrast, only three studies have examined regional cortical surface area, with two finding significant differences (Haring et al., 2016; Hua & Mathalon, 2022) and one finding no differences (Crespo-Facorro et al., 2011). Multiple studies have also implicated subcortical/ventricular abnormalities in ESZ, including reports of decreased caudate, hippocampus, amygdala, putamen, and thalamus volumes (Crespo-Facorro et al., 2009; Ebdrup et al., 2010; Fan et al., 2019; Hua & Mathalon, 2022) and enlarged 3rd and lateral ventricular volumes (HC, Cahn et al., 2002; Crespo-Facorro et al., 2009; Hua & Mathalon, 2022; Rosa et al., 2010). With regard to the cerebellum, there is mixed evidence of decreased cerebellar volume in ESZ with some finding significant differences (Bottmer et al., 2005; Chan et al., 2011; Kim et al., 2018) and others not (Morimoto et al., 2021; Steen et al., 2006). However, many studies focused on selective a priori regions, rather than examining deficits across a more comprehensive regional profile, making it difficult to evaluate inconsistent volumetric findings across studies.
A potential confound in most morphometric brain imaging studies of schizophrenia, including ESZ, is that most patients are currently taking, and have a history of treatment with, antipsychotic medications. Both animal and human studies provide evidence that dopamine D2 antagonists can induce brain morphometric changes (e.g., Dorph-Petersen et al., 2005; Konopaske et al., 2008; Lieberman et al., 2005; Roiz-Santiañez et al., 2015), including increased caudate volume (Albacete et al., 2019; Andersen et al., 2020; Chakos et al., 1994; Lang et al., 2004; Roiz-Santiañez et al., 2015) and, less consistently, reduced frontal cortex thickness (Cannon et al., 2016; Haijma et al., 2013; Lesh et al., 2015; Pies, 2018; Roiz-Santiañez et al., 2015). Nonetheless, cortical thickness reduction has been found in both antipsychotic-naïve and medicated ESZ patients, suggesting this reduction results, at least partly, from primary disease pathophysiology rather than exclusively resulting from antipsychotic exposure (Andreasen et al., 2011).
MRI studies examining whether morphometric brain abnormalities are present prior to psychosis onset in CHR-P individuals, as predicted by prevailing neurodevelopmental models of schizophrenia, have yielded mixed findings (e.g., Bois, Ronan, et al., 2015; Brent et al., 2013; Cannon et al., 2015; Jalbrzikowski et al., 2021). The strongest evidence comes from studies of cortical thickness (Bois, Whalley, et al., 2015; Brent et al., 2013; Collins et al., 2022; Jalbrzikowski et al., 2021; Zhao et al., 2022). Regional cortical thinning has been found in CHR-P individuals at baseline compared to HC, particularly with regard to bilateral medial frontal cortex (meta-analysis, Zhao et al., 2022). However, there is much variability across studies with some studies finding differences (Chung et al., 2019; Collins et al., 2022; del Re et al., 2021; Gisselgård et al., 2018; Jalbrzikowski et al., 2021; Jung et al., 2011; Kwak et al., 2019; Tomyshev et al., 2019), but not others (Bakker et al., 2016; Bois, Ronan, et al., 2015; Buechler et al., 2020; Cannon et al., 2015; Klauser et al., 2015; Ziermans et al., 2012). Relatively few CHR-P studies have examined cortical surface area and/or subcortical/ventricular/cerebellar volumes, which have great relevance to brain organization and functioning and may show important differential patterns of abnormalities. Recent evidence has been accumulating showing none to few cortical surface area abnormalities in CHR-P ((del Re et al., 2021; Jalbrzikowski et al., 2021; Tomyshev et al., 2019); but see (Bois, Ronan, et al., 2015)). There is mixed evidence of significant subcortical and/or ventricular volume abnormalities in CHR-P (Berger et al., 2017; Cannon et al., 2015; Chung et al., 2019; Jalbrzikowski et al., 2021; Konishi et al., 2018; Sasabayashi et al., 2020). Further, there is also mixed evidence of cerebellar volume abnormalities in CHR-P (Chan et al., 2011; Morimoto et al., 2021; Roman-Urrestarazu et al., 2014).
In addition, the potential for morphometric brain measures to serve as predictive biomarkers of future psychosis risk in CHR-P individuals remains unsettled, although this possibility may explain some of the inconsistent findings associated with CHR-P comparisons with HC. For example, morphometric abnormalities present during the CHR-P period may be subtle initially and only worsen over time. Alternatively, weak or inconsistent morphometric abnormalities in CHR-P individuals as a group may, in fact, reflect more prominent abnormalities present only in the small subset of CHR-P individuals who subsequently convert to a psychotic disorder (e.g., Andreou & Borgwardt, 2020; Collins et al., 2022; Ziermans et al., 2012). Consistent with the latter, decreased baseline cortical thickness (Buechler et al., 2020; del Re et al., 2021; Jalbrzikowski et al., 2021; Merritt et al., 2021; Walterfang et al., 2008) and steeper rates of cortical thinning (Cannon et al., 2015; Collins et al., 2022) have been found in CHR-P individuals who subsequently converted to psychosis relative to CHR-P non-converters. Additionally, baseline enlargement of 3rd and lateral ventricle volumes has been found in CHR-P converters relative to non-converters. (Cannon et al., 2015; Chung et al., 2017; Sasabayashi et al., 2020) Thus, structural brain abnormalities tend to be evident in those who subsequently convert to a psychotic disorder; however, this impression comes from a relatively small set of studies, many of which were underpowered or examined only a few select brain regions.
Accordingly, in the current structural MRI study, we examined multiple cortical and subcortical regional morphometric measures in CHR-P, ESZ, and HC individuals. Using multivariate profile analyses, we examined whether regional profiles of morphometric measures showed significant “non-parallelism” (i.e., pattern differences) or overall elevation or “level” differences between groups, with separate analyses conducted for cortical thickness, cortical surface area, subcortical volumes, and ventricular volumes. Moreover, longitudinal clinical follow-up data on CHR-P individuals allowed us to compare morphometric regional profiles in those who subsequently converted to psychosis relative to those who did not but were followed clinically for at least 18 months.
2. Methods
2.1. Participants
CHR-P individuals (n=89) were recruited from early psychosis research clinics at the University of California, San Francisco (UCSF; n=62) and the University of California, Davis (UCD; n=27). CHR-P individuals met Criteria of Psychosis-Risk Syndromes (COPS), based on the Structured Interview for Psychosis-Risk Syndromes (SIPS, McGlashan et al., 2010; Miller et al., 2003; Miller et al., 2002), for one or more of three non-mutually exclusive sub-syndromes: attenuated psychotic symptoms, brief intermittent psychotic states, and genetic risk with deterioration in social/occupational functioning. Within the CHR-P group, 17 individuals converted to a psychotic disorder and 31 did not convert to a psychotic disorder but had been followed clinically for at least 18 months from their scan date. The clinical outcomes of the 41 CHR-P who dropped out of the study prior to their 18-month follow-up assessment could not be determined with confidence, so they were excluded from the CHR-P clinical outcome analyses (but were retained in baseline group comparisons of CHR-P with ESZ and HC). Current symptom severity (positive, negative, disorganized, general) was assessed using the Scale of Psychosis-Risk Symptoms (SOPS, McGlashan et al., 2010; Miller et al., 2003; Miller et al., 2002). A subset of CHR-P individuals was taking antipsychotic medication at study entry (n=13/89; 14.61%) but were included because their past symptoms had never reached the threshold for full psychosis.
ESZ patients (n=93) were recruited from early psychosis clinics at UCSF (n=78) and UCD (n=15) and met criteria for a current diagnosis of schizophrenia or schizoaffective disorder based on the Structured Clinical Interview for DSM-IV (SCID, First et al., 2002), with illness onset within the last five years. The ESZ group had a mean illness duration of 2.35 years (SD = 1.45 years), with 48% being within the first 2 years of illness onset and 73% within the first 3 years of illness onset. Current symptom severity (positive, negative, general) was assessed with the Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987). Most ESZ patients were prescribed antipsychotic medication (n=75/93; 80.65%). HC participants (n=122) were recruited from the local UCSF (n=90) and UCD (n=32) communities. HC did not meet criteria for any Axis I diagnosis based on the SCID (for HC >16 years old) or the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (for HC ≤16 years old) (Kaufman et al., 1997).
Across both sites, participants had to be in good physical health, fluent in English, and between 12–35 years old. Exclusion criteria included a past year DSM-IV substance dependence diagnosis (except nicotine), head injury with loss of consciousness, neurological illness, or a first-degree relative with a psychotic illness (for HC). Study protocols were in accordance with UCSF’s and UCD’s Institutional Review Boards and the Declaration of Helsinki. Participants provided written informed consent, or in the case of minors, parental written consent and participant assent.
2.2. Structural MRI acquisition and processing
Participants were scanned at neuroimaging centers at UCSF and UCD using identical Siemens 3T TIM TRIO scanners and high-resolution structural T1-weighted MPRAGE image acquisition sequences using the following parameters: repetition time (TR)=2,300 ms, echo time (TE)=2.95 ms, flip angle=9°, field of view (FOV)=256×256, slice thickness=1.20 mm.
MRI images were processed using FreeSurfer (version 5.1, Han et al., 2006; Jovicich et al., 2006, see Supplemental Methods). Consistent with multi-site ENIGMA studies arguing for analytic standardization (van Erp et al., 2016; van Erp et al., 2018), the same FreeSurfer-provided parcellations were used to define regions of interest (ROIs). Cortical ROIs were based on the Desikan-Killiany atlas (Desikan et al., 2006), and subcortical/ventricular ROIs were from FreeSurfer’s atlas (Fischl et al., 2002). Thickness and surface area of cortical regions (68 unilateral ROIs–34 in each hemisphere) and volume of subcortical (14 unilateral ROIs–7 in each hemisphere), ventricular (5 unilateral ROIs–2 in each hemisphere, plus 3rd ventricle), and cerebellar (4 unilateral ROIs–2 in each hemisphere) regions were calculated.
2.3. Statistical analyses
2.3.1. Z-scoring adjustment procedure
To account for normal brain maturation (i.e., age, Mills et al., 2016), sex (Barnes et al., 2010; Hogstrom et al., 2013; Luders et al., 2004; Ritchie et al., 2018), scanning site, and intracranial volume (ICV, Barnes et al., 2010; Im et al., 2008; Toro et al., 2008), the effects of these variables were statistically removed from the morphometric brain measures. As cortical thickness is not as strongly correlated with ICV as surface area and volume, thickness measures were not statistically adjusted for ICV (Barnes et al., 2010) (FreeSurfer guidelines, https://surfer.nmr.mgh.harvard.edu/fswiki/eTIV). For the remaining regional morphometric measures, the effects of age, sex, ICV, and site were modeled in the HC group, and resulting parameter estimates were used to derive predicted values for each participant, reflecting the value expected for a healthy individual of a specific biological sex, age, and ICV assessed at a specific study site. By subtracting observed values from these predicted values and then dividing by the standard error of regression from the HC regression model (i.e., standard deviation of HC data points from the HC regression line), morphometric scores were transformed to adjusted z-scores (see Supplemental Methods, Hua et al., 2022; Pfefferbaum et al., 1992; Roach et al., 2020). Adjusted z-scores represent the participant’s deviation, in standard units, from the value for a HC of the same age, sex, ICV (except for cortical thickness measures), and study site as the participant.
2.3.2. Structural morphometric analyses
Using multi-factorial repeated measures multivariate analyses of variance (rmMANOVA), group differences for each type of morphometric ROI profile were assessed, including 1) cortical thicknesses, 2) cortical surface areas, 3) basal ganglia volumes (caudate, putamen, pallidum), 4) subcortical volumes (accumbens, hippocampus, amygdala, thalamus) 5) ventricular volumes, and 6) cerebellar volumes. Groups were compared on two aspects of each ROI profile: 1) non-parallelism of the profiles (i.e., Group x Region interaction effect) and 2) level differences between the profiles (i.e., Group main effect). Basal ganglia ROIs, which have been shown to be enlarged in schizophrenia due to the effect of antipsychotic medications (Albacete et al., 2019; Andersen et al., 2020; Chakos et al., 1994; Lang et al., 2004), were analyzed separately from other subcortical ROIs that typically show reductions in schizophrenia (van Erp et al., 2016). Given that adjusted z-scores re-express structural morphometric values as deviations from the expected normative value, profiles of z-scores for the HC group are flat and the mean for each ROI is approximately zero, whereas the profiles of CHR-P and ESZ groups reflect the extent and regional pattern of variation from the age norms derived from the HC group.
Initially, for each type of morphometric measure (cortical thickness, cortical surface area, basal ganglia volume, subcortical volume, ventricular volume, cerebellar volume), 3-way Group (HC, CHR-P, ESZ) x Hemisphere (Left, Right) x ROI rmMANOVA models were run. Due to non-significant 3-way interactions for all six models (ps>.119), ROIs were averaged between hemispheres, resulting in 34 cortical, 3 basal ganglia, 4 subcortical, 3 ventricular, and 2 cerebellar bilateral ROIs. Subsequent 2-way Group x ROI models focused on tests of regional profile non-parallelism between groups (i.e., Group x ROI interaction) and tests of profile level or elevation differences between groups (i.e., Group main effect). Significant non-parallelism of profiles was followed-up with 2-group Group x ROI rmMANOVAs comparing the profiles of HC vs. CHR-P, HC vs. ESZ, and CHR-P vs. ESZ. Lastly, following-up on significant non-parallelism of the 2-group rmMANOVAs, we examined Bonferroni-corrected simple effects comparing groups on each bilateral ROI.
2.3.3. Prediction of clinical outcomes in clinical high risk for psychosis group
We divided the CHR-P group into those who converted to a psychotic disorder and those who did not convert to a psychotic disorder but had been assessed clinically for at least 18 of the 24 months comprising the study follow-up period. Similar to the 3-group rmMANOVA models, CHR-P converter vs. non-converter morphometric regional profiles were compared using Group x ROI multivariate profile analyses
2.3.4. Antipsychotic medication and symptom severity analyses
To examine relationships between morphometric measures and clinical variables such as symptom severity ratings or antipsychotic medication without having to adjust for an excessive number of correlation tests, we first reduced the ROI measures to a smaller set of regional components by performing principal component analyses with promax rotation on unilateral ROIs separately for cortical thickness, cortical surface area, basal ganglia volume, subcortical volume, ventricular volume, and cerebellar volume in the combined sample of CHR-P, ESZ, and HC (for full details, see Supplemental Methods, Supplemental Tables 1–5, Supplemental Figures 1 and 2. Associations of these extracted ROI components with symptom severity and antipsychotic medication in CHR-P and ESZ groups using Pearson correlations. In CHR-P, clinical correlates included SOPS summary scales (positive, negative, disorganized, general). In ESZ, clinical correlates included antipsychotic dosage in chlorpromazine equivalents (CPZ) (Woods, 2003) and PANSS summary scales (positive, negative, general). Significance of these correlations within each morphometric measure type were adjusted for multiple tests with false discovery rate (FDR) correction, pFDR<.05.
3. Results
3.1. Participant demographics and clinical characteristics
See Table 1 for group demographics and clinical characteristics. Groups did not statistically differ on sex ((2, N=304) = 4.64, p=.098). However, as expected, groups differed in age (F(2,303)=9.05, p<.001), with the CHR-P group being younger than both HC (p=.011) and ESZ (p<.001) groups.
Table 1.
Demographic and Clinical Characteristics of Participant Groups
| HC Mean ± SD | CHR-P Mean ± SD | ESZ Mean ± SD | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Gender (% male) | 56.56 | 52.81 | 67.74 |
| Age (Range: 12–35)a | 20.86 ± 6.36 | 18.68 ± 4.59 | 22.01 ± 4.56 |
| Ethnicity (%) | |||
| White / Caucasian | 58.19 | 56.18 | 51.61 |
| Asian American | 20.49 | 13.48 | 22.58 |
| Black / African American | 6.56 | 10.11 | 6.45 |
| American Indian / Alaska Native | 2.46 | 2.25 | 0.00 |
| Native Hawaiian / Pacific Islander | 1.64 | 3.37 | 2.15 |
| More Than One Race | 10.66 | 10.11 | 11.83 |
| Not Reported | 0.00 | 4.49 | 5.38 |
| Medication (%) | |||
| Chlorpromazine equivalents (mg) | --- | b c | 285.34 ± 322.97b |
| Antipsychotic medication | --- | 14.61 | 80.65d |
| (typical, atypical, unknown) | --- | (0, 100, 0) | (1.33, 94.67, 4.00) |
| Symptom Severity Ratings e | |||
| SOPS Positive | --- | 10.00 ± 4.88 | --- |
| SOPS Negative | --- | 11.76 ± 5.78 | --- |
| SOPS Disorganized | --- | 5.78 ± 3.89 | --- |
| SOPS General | --- | 8.07 ± 4.82 | --- |
| PANSS Positive | --- | --- | 13.78 ± 4.70 |
| PANSS Negative | --- | --- | 16.55 ± 6.76 |
| PANSS General | --- | --- | 32.94 ± 8.49 |
Note. HC=healthy control; CHR-P=clinical high-risk for psychosis; ESZ=early illness schizophrenia; SD=standard deviation; SES=socioeconomic status; SOPS=Scale of Psychosis-Risk Symptoms; PANSS=Positive and Negative Syndrome Scale.
Groups were significantly different on age, F(2,303)=9.05, p<.001, with the CHR-P group significantly younger than HC (p=.011) and ESZ (p<.001) groups.
Chlorpromazine equivalents for 11 CHR-P participants and 12 ESZ participants was missing.
Morphometric results showed the same pattern when medicated CHR-P patients were dropped.
Antipsychotic medication status for 7 participants missing.
SOPS Negative for 4 participants missing; SOPS Disorganized and General for 3 participants missing; PANSS scores only for UCSF participants.
3.2. Cortical thickness profiles
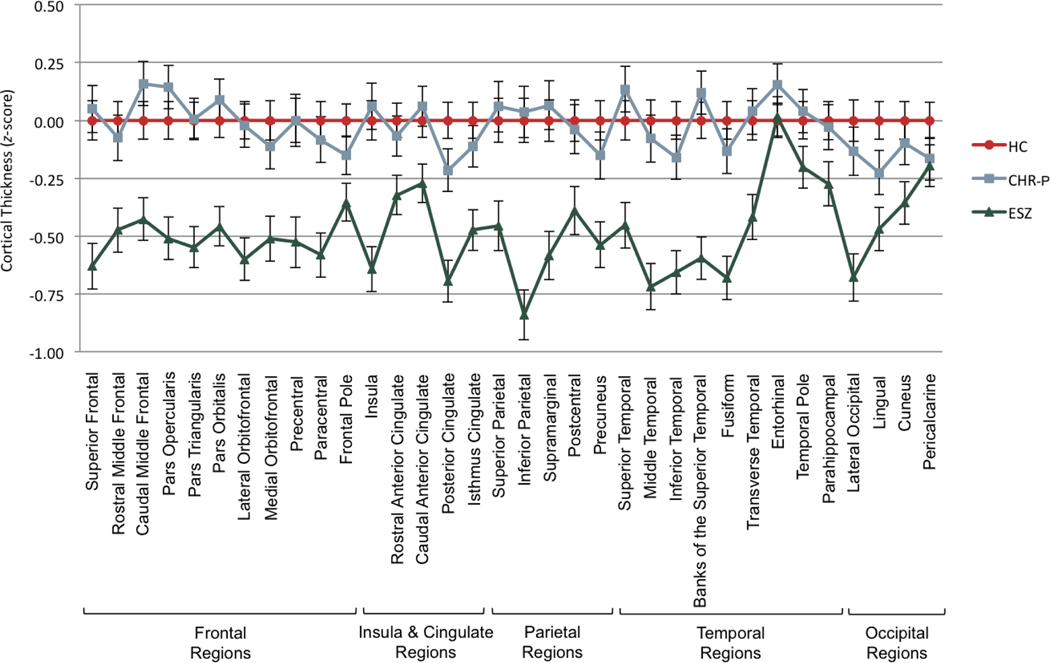
Cortical thickness ROI z-score profiles among the three groups (Figure 1 and Supplemental Table 6) showed significant non-parallelism (F[66,538]=1.86, Wilks’ =.66, p<.001, =.19) and level differences (F[2,301]=21.83, p<.001, =.13). In 2-group follow-up comparisons, the ESZ profile significantly deviated from the flat HC z-score profile (F[33,181]=2.31, Wilks’ =.42, p<.001, =.30) and from the CHR-P profile (F[33,148]=2.03, Wilks’ =.69, p=.002, =.31). Additionally, the ESZ group showed an overall reduction in profile level relative to HC (F[1,213]=40.68, p<.001, =.16) and CHR-P (F[1,180]=25.88, p<.001, =.13) groups, with the ESZ group having widespread cortical thinning across all regions except for entorhinal and pericalcarine ROIs (Supplemental Table 6). There were no significant differences between the HC and CHR-P groups (ps>.280, =.00 to .18).
Figure 1.
Cortical thickness profiles of healthy control (HC), clinical high-risk (CHR), and early illness schizophrenia (ESZ) groups. All groups were nonparallel and had significant level differences. In 2-group follow-up comparisons, HC vs. ESZ and CHR-P vs. ESZ were nonparallel and had significant level differences.
3.3. Cortical surface area profiles
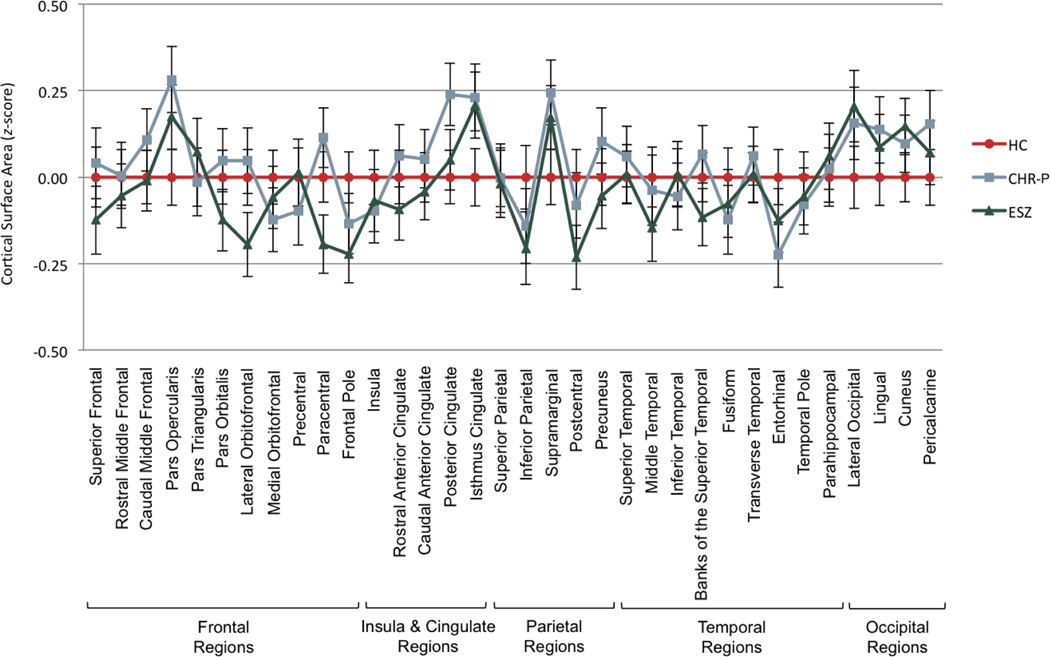
There was no significant non-parallelism (F[66,538]=1.24, Wilks’ =.75, p=.110, =.13) or level difference (F[2,301]=0.40, p=.671, =.00) of cortical surface area ROI z-score profiles between the HC, CHR-P, and ESZ groups (Figure 2 and Supplemental Table 7).
Figure 2.
Cortical surface area profiles of healthy control (HC), clinical high-risk (CHR), and early illness schizophrenia (ESZ) groups. Groups were parallel and had no significant level difference.
3.4. Basal ganglia volume profiles (caudate, putamen, pallidum)
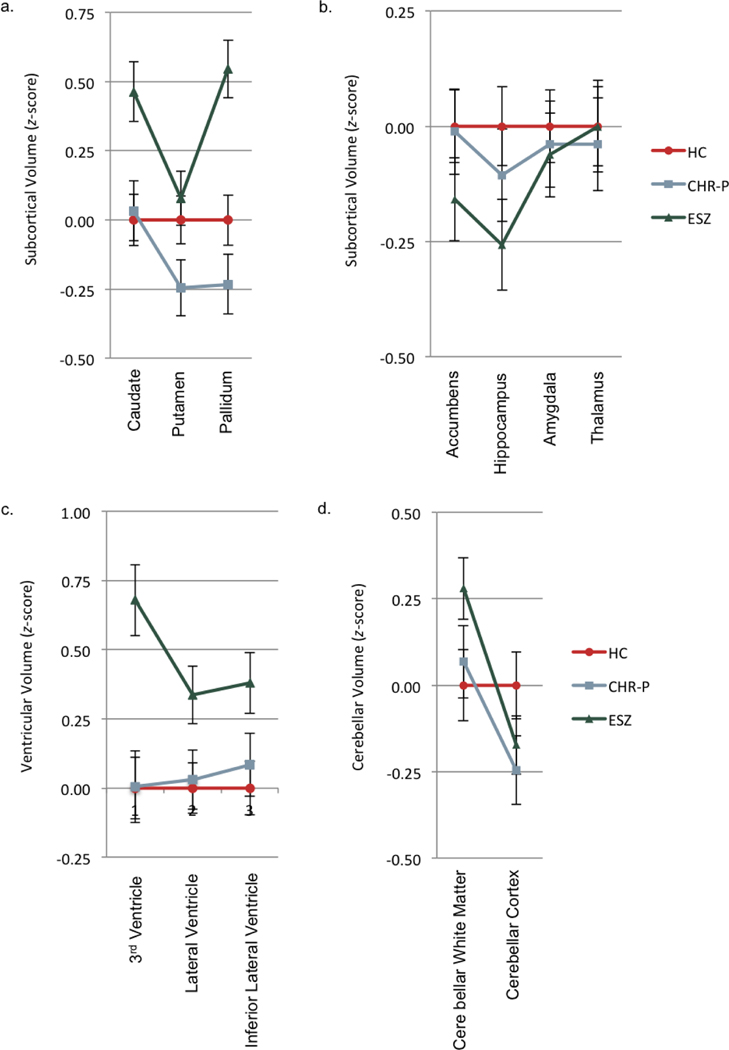
Basal ganglia volume ROI z-score profiles among the three groups (Figure 3a and Supplemental Table 8) showed significant non-parallelism (F[4,600]=4.21, Wilks’ =.95, p=.002, =.03) and level differences (F[2,301]=2.64, p<.001, =.07). In 2-group follow-up comparisons, the ESZ profile significantly deviated from the flat HC z-score profile (F[2,212]=5.77, Wilks’ =.95, p=.004, =.06) and the CHR-P profile (F[2,179]=4.32, Wilks’ =.95, p=.001,5 =.05). Additionally, the ESZ group showed overall greater volume relative to HC (F[1,213]=11.82, p<.001, =.01) and CHR-P (F[1,180]=18.861, p<.001, =.10) groups, with level differences driven by enlarged caudate and pallidum volumes in ESZ (Supplemental Table 8). There were no significant differences between HC and CHR-P groups (ps>.141, =.01 to .02).
Figure 3.
Subcortical/ventricular/cerebellar volume profiles of healthy control (HC), clinical high-risk (CHR), and early illness schizophrenia (ESZ) groups. a) All groups were nonparallel and had significant level differences. In 2-group follow-up comparisons, HC vs. ESZ and CHR-P vs. ESZ were nonparallel and had significant level differences. b) All groups were parallel and had no significant level differences. c) All groups were parallel and had significant level differences for ventricular volume. ESZ group had larger ventricular volumes relative to HC and CHR-P groups. d) All groups were nonparallel and had no significant level differences. In 2-group follow-up comparisons, HC vs. ESZ and HC vs. CHR were nonparallel.
3.5. Subcortical volume profiles (accumbens, hippocampus, amygdala, thalamus)
There were no significant differences in parallelism (F[6,598]=0.76, Wilks’ =.99, p=.600, =.01) or level (F[2,301]=0.97, p=.379, =.01) of subcortical volume ROI z-score profiles among the HC, CHR-P, and ESZ groups (Figure 3b and Supplemental Table 8).
3.6. Ventricular volume profiles
Ventricular volume ROI z-score profiles among the three groups (Figure 3c and Supplemental Table 8), were parallel (F[4,600]=1.96, Wilks’ =.97, p=.099, =.01), but showed a significant level difference (F[2,301]=7.99, p<.001, =.05). In post-hoc level comparisons between groups, ESZ had overall enlarged ventricles relative to HC (p<.001) and CHR-P (p=.005) groups. There was no significant group difference between HC and CHR-P groups (p=1.000).
3.7. Cerebellar volume profiles (white matter and cortex)
There was significant non-parallelism (F[2,301]=7.75, Wilks’ =.95, p<.001, =.05) but no level difference (F[2,301]=0.64, p=.531, =.00) of cerebellar volume ROI z-score profiles between the HC, CHR-P, and ESZ groups (Figure 3d and Supplemental Table 8). In 2-group follow-up comparisons, the ESZ profile significantly deviated from the flat HC z-score profile (F[1,213]=12.77, Wilks’ =.94, p<.001, =.06) but not the CHR-P profile (F[1,180]=1.23, Wilks’ =.99, p=.258, =.01). The ESZ did not show an overall difference in profile level relative to HC (F[1,213]=0.22, p-.642, =.00) or CHR-P (F[1,180]=1.26, p=.264, =.01) groups. Additionally, the CHR-P profile significantly deviated from the flat HC z-score profile (F[1,209]=7.60, Wilks’ =.97, p=.006, =.04) but showed no level difference (F[1,209]=0.52, p=.472, =.00).
3.8. Clinical high-risk for psychosis group: predicting clinical outcomes
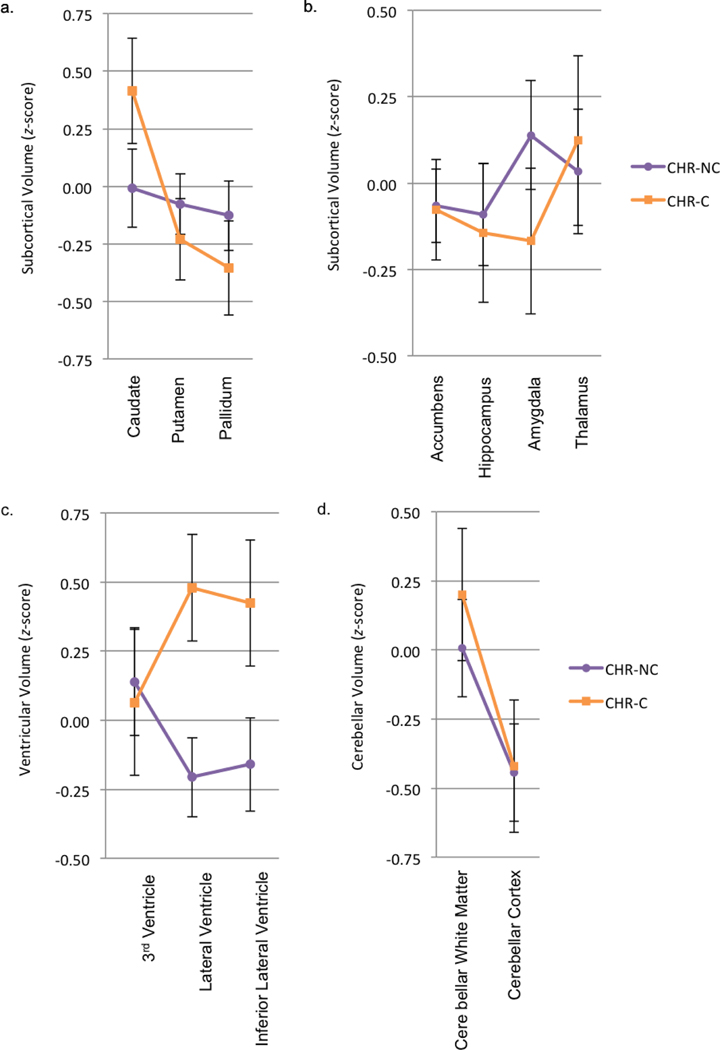
Analyses of morphometric ROI profile differences between CHR-P non-converters and CHR-P converters (Figure 4, Supplemental Tables 9 and 10, Supplemental Figures 3 and 4) showed no significant differences in parallelism or level for cortical thickness (ps>.828, =.00 to .61), cortical surface area (ps>.249, =.01 to .77), basal ganglia volume (ps>.068, =.00 to .11), subcortical volume (ps>.697, =.00 to .32), or cerebellar volume (ps>.172 =.01 to .05). For ventricular volume, there was significant non-parallelism (F[2,45]=3.49, Wilks’ = .87, p=.039, =.13), but no significant level difference (F[1,46]=3.17, p=.082, =.06), with the CHR-P converters having larger lateral ventricles and lateral inferior ventricles (Figure 4 and Supplemental Table 10) than the CHR-P non-converters.
Figure 4.
Subcortical/ventricular/cerebellar volume profiles of clinical high-risk non-converter (CHR-NC) and clinical high-risk converter (CHR-C) groups. a and b) Groups were parallel and had no significant level difference for subcortical volume. c) Groups were nonparallel and had no significant level difference for ventricular volume. d) Groups were parallel and had no significant level difference for cerebellar volume.
3.9. Antipsychotic medication dosage associations with structural morphometry in early illness schizophrenia
To examine whether significant structural morphometric abnormalities were accounted for by antipsychotic medication, we examined associations between CPZ dosage and morphometric component z-scores in the ESZ group only (Table 2). Higher CPZ dosage was significantly associated with cortical thinning of frontal regions (r=−.36, p<.001). These associations were still significant when controlling for symptom severity (r=−.34, p=.005). There was modest evidence that higher CPZ dosage equivalents was associated with decreased cortical surface area of occipital regions (r=−.27, p=.017) and decreased subcortical volume of the amygdala/hippocampus/accumbens (r=−.22, p=.046); however, these findings did not survive FDR correction.
Table 2.
Chlorpromazine (CPZ) Equivalents and Clinical Associations with Structural Brain Morphometry in Early Illness Schizophrenia
| Components | CPZ | PANSS Positive | PANSS Negative | PANSS General | Duration of Illness |
|---|---|---|---|---|---|
|
| |||||
| Cortical Thickness | |||||
| Frontal-Parietal | −.28† | .11 | −.09 | .10 | −.08 |
| Frontal | −.36*** | .05 | −.01 | .16 | −.15 |
| Temporal | −.20 | .07 | −.05 | .08 | −.10 |
| Occipital | −.14 | −.12 | .07 | −.05 | −.07 |
| Cingulate | −.17 | .15 | −.21 | .02 | −.17 |
| Cortical Surface Area | |||||
| Frontal-Parietal | −.09 | −.08 | .12 | −.02 | −.04 |
| Temporal | −.04 | −.04 | −.16 | −.18 | −.08 |
| Frontal | .01 | −.13 | −.06 | −.16 | .00 |
| Occipital | −.27† | −.03 | .01 | −.03 | −.10 |
| Subcortical Volume | |||||
| Hippocampus/Amygdala/Accumbens | −.22† | .10 | −.03 | .10 | −.13 |
| Thalamus/Pallidum | .07 | .00 | .08 | .00 | .02 |
| Caudate/Putamen | −.11 | .02 | .08 | .10 | −.18 |
| Ventricular Volume | |||||
| All Ventricles | .13 | −.02 | −.08 | .00 | .01 |
| Cerebellar Volume | |||||
| Whole Cerebellum | −.07 | .03 | −.02 | .00 | .08 |
Note. Bolded text denotes regions that are significant after false discovery rate correction (pFDR<.05). PANSS=Positive and Negative Syndrome Scale. Correlations for cortical thickness adjusted for age, scanning site, and sex. Correlations for cortical surface area and subcortical/ventricular/cerebellar volume adjusted for age, scanning site, sex, and intracranial volume.
p<.05 but did not survive FDR correction.
p<.001.
3.10. Clinical associations with structural morphometry in clinical groups
In the ESZ group, PANSS scores and duration of illness were not significantly associated with cortical thickness, cortical surface area, or basal ganglia/ventricular/cerebellar volume (Table 2). In the CHR-P group, higher SOPS negative symptom scores was significantly associated with decreased subcortical volume of the hippocampus/amygdala/accumbens (r=−.32, p=.003; Table 3), but this association did not survive multiple comparison correction.
Table 3.
Chlorpromazine (CPZ) Equivalents and Clinical Symptom Severity Associations with Structural Brain Morphometry in Clinical High-Risk
| SOPS | ||||
|---|---|---|---|---|
| Components | Positive | Negative | Disorganized | General |
|
|
|
|||
| Cortical Thickness | ||||
| Frontal-Parietal | .06 | −.11 | −.01 | −.06 |
| Frontal | .13 | .02 | .03 | .06 |
| Temporal | .03 | −.01 | −.05 | .00 |
| Occipital | −.01 | −.10 | .12 | −.07 |
| Cingulate | .17 | .05 | .08 | −.04 |
| Cortical Surface Area | ||||
| Frontal-Parietal | −.11 | .02 | −.12 | .05 |
| Temporal | −.11 | −.09 | −.12 | .02 |
| Frontal | −.10 | .10 | .07 | .10 |
| Occipital | .01 | .08 | −.03 | .08 |
| Subcortical Volume | ||||
| Hippocampus/Amygdala/Accumbens | −.10 | −.32† | −.20 | −.12 |
| Thalamus/Pallidum | .08 | −.04 | .16 | .07 |
| Caudate/Putamen | −.05 | .02 | .12 | .17 |
| Ventricular Volume | ||||
| All Ventricles | −.07 | .08 | −.09 | .00 |
| Cerebellar Volume | ||||
| Whole Cerebellum | .02 | .09 | .03 | .12 |
Note. SOPS=Scale of Psychosis-Risk Symptoms. Correlations for cortical thickness adjusted for age, scanning site, and sex. Correlations for cortical surface area and subcortical/ventricular/cerebellar volume adjusted for age, scanning site, sex, and intracranial volume.
p<.05 but did not survive FDR correction.
Overall, morphometric results showed the same pattern when medicated CHR-P individuals were dropped from analyses.
4. Discussion
In the current study, we examined the extent to which cortical thickness, cortical surface area, subcortical volumeventricular volume, and cerebellar volume are abnormal in CHR-P and ESZ patients relative to HC individuals after accounting for normal brain maturation. We found that ESZ individuals had widespread cortical thinning as well as increased basal ganglia and lateral ventricular volume compared to HC and CHR-P individuals, but there were no significant group differences in cortical surface area or cerebellar volume. Higher antipsychotic dosage was significantly associated with cortical frontal thinning in ESZ. In contrast to ESZ, only regional morphometric brain measure profiles significantly differed between HC and CHR-P groups for cerebellar volumes. When CHR-P converters were compared to non-converters followed for at least 18-months, CHR-P converters showed significantly larger lateral ventricles. Thus, results support the theory that schizophrenia is a progressive brain disorder, with little evidence of brain morphometric abnormalities predating psychosis onset, except for a finding larger ventricles in CHR-P individuals who subsequently converted to psychosis.
The profile of structural brain morphometric abnormalities in ESZ was consistent with literature finding widespread cortical thinning and subcortical/ventricular abnormalities in ESZ relative to HC (e.g., Akudjedu et al., 2020; Cahn et al., 2002; Crespo-Facorro et al., 2011; Fan et al., 2019; Gallardo-Ruiz et al., 2019; Lin et al., 2019; Merritt et al., 2021; Rosa et al., 2010; Zhao et al., 2022). Although some studies have found smaller cerebellar volumes in ESZ (Bottmer et al., 2005; Chan et al., 2011; Kim et al., 2018)), we did not replicate tis finding. Discrepancies in this phenomenon across studies (Bottmer et al., 2005; Chan et al., 2011; Kim et al., 2018; Morimoto et al., 2021; Steen et al., 2006) could be a result of heterogeneity in parcellating the cerebellum. Due to the relationship between structural morphometry and antipsychotic medications (Fusar-Poli et al., 2013; Ho et al., 2011; van Erp et al., 2018; Vita et al., 2015), we examined associations with antipsychotic dosage in the ESZ group. Higher CPZ dosage was associated with cortical thinning in the frontal cortex, with associations remaining when controlling for symptom severity. These findings are consistent with previous research (Cannon et al., 2016; Haijma et al., 2013). In contrast to this literature, we did not find higher CPZ dosage to be associated with basal ganglia enlargement (Ho et al., 2011; Taylor et al., 2007). It is possible that we did not find the expected association because we were only examining the effect of current antipsychotic mediation and not lifetime exposure. Although antipsychotic medication effects on structural morphometry cannot be ruled out, critically, ESZ differences were widespread and were not isolated to the frontal cortex. Previous research has also found both cortical structural deficits in ESZ individuals taking antipsychotic medications (e.g., Fan et al., 2019) and in antipsychotic-naïve ESZ individuals (Crespo-Facorro et al., 2011; Hazlett et al., 2008; Wei et al., 2022) but see (Lesh et al., 2015). Taken together, this suggests that structural morphometric differences are not merely a byproduct of antipsychotic medication, but are involved in the pathophysiology of the early course of schizophrenia.
With regard to the CHR-P group, there were no significant differences in structural morphometric profiles relative to the HC group. Although a meta-analysis found cortical thinning of the bilateral medial prefrontal cortex of CHR-P individuals relative to HC (Zhao et al., 2022), there is much variability in previous studies, with many studies finding no significant difference between groups (Bakker et al., 2016; Bois, Ronan, et al., 2015; Buechler et al., 2020; Cannon et al., 2015; Klauser et al., 2015; Ziermans et al., 2012). The largest multi-site CHR-P study to date found widespread cortical thinning in CHR-P compared to HC (Jalbrzikowski et al., 2021). However, between-group effect sizes were small (d=−0.09 to −0.17), which might explain why we did not detect abnormalities in our smaller sample CHR-P group. Interestingly, in contrast to our hypothesis of a level difference between the two clinical groups, morphometric CHR-P profiles were not just indicative of reduced levels of abnormalities present in ESZ, but profiles were also nonparallel. Given that CHR-P individuals are a heterogeneous group, with the majority not converting to a psychotic disorder (Cannon et al., 2008; Fusar-Poli et al., 2012; Simon et al., 2014), it is possible that this heterogeneity is resulting in both level differences and nonparallelism between ESZ and CHR-P. Subtle morphometric changes in the high-risk period may be more apparent and show a more similar pattern to the early illness phase in CHR-P individuals who subsequently develop a psychotic disorder.
Consistent with this idea, studies have found baseline cortical thinning (Buechler et al., 2020; del Re et al., 2021; Jalbrzikowski et al., 2021) and steeper rates of cortical thinning (Cannon et al., 2015) as well as ventricular expansion (Cannon et al., 2015; Chung et al., 2017; Sasabayashi et al., 2020) in CHR-P individuals who convert to a psychotic disorder versus those who do not. In the current study, our CHR-P converter group showed enlargement of lateral and inferolateral ventricles. Null cortical thickness findings should be interpreted with caution because of the low statistical power due to small samples of CHR-P converters and CHR-P non-converters followed for at least 18-months.
Lastly, structural morphometry was not significantly associated with clinical symptoms in the two clinical groups or duration of illness in ESZ. Research on structural morphometric associations with clinical symptoms in the high-risk period and early illness phase is limited. Of the few CHR-P studies that have examined clinical associations, most have not found them at all (Bakker et al., 2016; Buechler et al., 2020; del Re et al., 2021; Jung et al., 2011) or reported only weak associations (Kwak et al., 2019). For the early illness phase, Crespo-Faccoro et al. (2011) found no significant clinical symptom associations with cortical regions, but Fan et al. (2019) found PANSS positive symptoms to be inversely related to volumes of the amygdala and nucleus accumbens. Further, previous research in chronic schizophrenia patients has found evidence that greater illness duration is associated with cortical thinning of the insula (van Erp et al., 2018) and increased putamen and pallidum volume (van Erp et al., 2016), but not with cortical surface area (van Erp et al., 2018). Correlations in the current study could be attenuated by the restricted range of illness duration inherent in an ESZ sample.
Some current study limitations should be noted. Evidence of cortical thinning at baseline is mixed in CHR-P, with some longitudinal studies finding steeper rates of cortical thinning in CHR-P who later convert to psychosis compared to HC (Cannon et al., 2015). As such, longitudinal designs might be more sensitive to the subtle progressive structural changes developing in this group. Additionally, the current study was limited by insufficient follow-up CHR-P data and a relatively small CHR-P conversion group. Due to previous evidence of cortical thinning in CHR-P converters relative to CHR-P non-converters (Buechler et al., 2020; del Re et al., 2021; Jalbrzikowski et al., 2021), future studies should include larger samples in order to conduct sufficiently powered conversion analyses (Jalbrzikowski et al., 2021). Future studies should also examine how structural morphometry is related to other functional brain measures assessed with methods like functional MRI and electroencephalography measures (e.g., Hua et al., 2022).
In conclusion, the current study found widespread cortical thinning and subcortical/ventricular volumetric enlargement in the early illness phase of schizophrenia, but not in individuals at clinical high-risk for psychosis. Ventricle enlargement in the CHR-P group was only apparent in CHR-P individuals who converted to psychosis. By concurrently comparing structural morphometric profiles of HC, CHR-P, and ESZ groups, the current study extends previous morphometric research and found novel evidence that the CHR-P structural morphometric profile is not just a reduced level of the ESZ profile, but is also nonparallel to the ESZ profile. Although more longitudinal studies are needed to examine morphometric progression, results are consistent with the view that schizophrenia is a progressive brain disorder, with abnormalities being significantly worse during the early years of schizophrenia relative to those exhibiting symptoms of a psychosis-risk syndrome.
Supplementary Material
Highlights.
Compared clinical high-risk (CHR-P) and early illness schizophrenia (ESZ) profiles
ESZ morphometric profiles differed from healthy controls (HC) and CHR-P
Only cerebellar morphometric profile differences emerged between CHR-P and HC
CHR-P converters vs. non-converters showed regional ventricular volume expansion
Antipsychotic dosage in ESZ was correlated with frontal cortical thinning
Acknowledgements
Funding Details
This work was supported by the National Institute of Mental Health (MH076989 [DHM]) and University of Missouri dissertation funds (JPYH). Manuscript writing by JPYH was supported by the Department of Veterans Affairs Sierra Pacific Mental Illness Research, Education, and Clinical Center (MIRECC). The aforementioned funding sources had no role in the study design, data collection, analysis and interpretation of data, writing of the report, and in the decision to submit the article for publication.
Footnotes
Disclosure of Interest
DHM received compensation as a consultant for Boehringer-Ingelheim, Cadent Therapeutics, Neurocrine Biosciences, Gilgamesh Pharma, Recognify Life Sciences, and Syndesi Therapeutics. The other authors report no conflict of interest. JPYH, SLF, and DHM are United States Government employees. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akudjedu TN, Tronchin G, McInerney S, Scanlon C, Kenney JPM, McFarland J, Barker GJ, McCarthy P, Cannon DM, McDonald C, Hallahan B, 2020. Progression of neuroanatomical abnormalities after first-episode of psychosis: A 3-year longitudinal sMRI study. J. Psychiatr. Res 130, 137–151. 10.1016/j.jpsychires.2020.07.034 [DOI] [PubMed] [Google Scholar]
- Albacete A, Makowski C, Mallar Chakravarty M, Joober R, Malla AK, Contreras F, Menchón JM, Lepage M, 2019. The effect of second-generation antipsychotics on basal ganglia and thalamus in first-episode psychosis patients. Eur.Neuropsychopharmacol 29(12), 1408–1418. 10.1016/j.euroneuro.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Andersen HG, Raghava JM, Svarer C, Wulff S, Johansen LB, Antonsen PK, Nielsen M, Rostrup E, Vernon AC, Jensen LT, Pinborg LH, Glenthøj BY, Ebdrup BH, 2020. Striatal volume increase after six weeks of selective dopamine D(2/3) receptor blockade in first-episode, antipsychotic-naïve schizophrenia patients. Front. Neurosci 14, 484. 10.3389/fnins.2020.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC, 2011. Progressive brain change in schizophrenia: A prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry 70(7), 672–679. 10.1016/j.biopsych.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Borgwardt S, 2020. Structural and functional imaging markers for susceptibility to psychosis. Mol. Psychiatry 25(11), 2773–2785. 10.1038/s41380-020-0679-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker G, Caan MW, Vingerhoets WA, da Silva-Alves F, de Koning M, Boot E, Nieman DH, de Haan L, Bloemen OJ, Booij J, van Amelsvoort TA, 2016. Cortical morphology differences in subjects at increased vulnerability for developing a psychotic disorder: A comparison between subjects with ultra-high risk and 22q11.2 deletion syndrome. PLoS One 11(11), e0159928. 10.1371/journal.pone.0159928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC, 2010. Head size, age and gender adjustment in MRI studies: A necessary nuisance? NeuroImage 53(4), 1244–1255. 10.1016/j.neuroimage.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Berger GE, Bartholomeusz CF, Wood SJ, Ang A, Phillips LJ, Proffitt T, Brewer WJ, Smith DJ, Nelson B, Lin A, Borgwardt S, Velakoulis D, Yung AR, McGorry PD, Pantelis C, 2017. Ventricular volumes across stages of schizophrenia and other psychoses. Aust. N. Z. J. Psychiatry 51(10), 1041–1051. 10.1177/0004867417715914 [DOI] [PubMed] [Google Scholar]
- Bois C, Ronan L, Levita L, Whalley HC, Giles S, McIntosh AM, Fletcher PC, Owens DC, Johnstone EC, Lawrie SM, 2015. Cortical surface area differentiates familial high risk individuals who go on to develop schizophrenia. Biol Psychiatry, 78(6), 413–420. 10.1016/j.biopsych.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Bois C, Whalley HC, McIntosh AM, Lawrie SM, 2015. Structural magnetic resonance imaging markers of susceptibility and transition to schizophrenia: A review of familial and clinical high risk population studies. J. Psychopharmacol 29(2), 144–154. 10.1177/0269881114541015 [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Dickey C, Pol HH, Whitford TJ, DeLisi LE, 2009. Workshop on defining the significance of progressive brain change in schizophrenia: December 12, 2008 American College of Neuropsychopharmacology (ACNP) all-day satellite, Scottsdale, Arizona: The rapporteurs’ report. Schizophr. Res 112(1), 32–45. 10.1016/j.schres.2009.04.025 [DOI] [PubMed] [Google Scholar]
- Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schröder J, 2005. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res.: Neuroimag. 140(3), 239–250. 10.1016/j.pscychresns.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Brent BK, Thermenos HW, Keshavan MS, & Seidman LJ, 2013. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: A review of structural MRI findings. Child Adolesc. Psychiatr. Clin. N. Am 22(4), 689–714. 10.1016/j.chc.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler R, Wotruba D, Michels L, Theodoridou A, Metzler S, Walitza S, Hänggi J, Kollias S, Rössler W, & Heekeren K, 2020. Cortical volume differences in subjects at risk for psychosis are driven by surface area. Schizophr. Bull 46(6), 1511–1519. 10.1093/schbul/sbaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS, 2002. Brain volume changes in first-episode schizophrenia: A 1-year follow-up study. Arch. Gen. Psychiatry, 59(11), 1002–1010. 10.1001/archpsyc.59.11.1002 [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R, 2008. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Arch. Gen. Psychiatry 65(1), 28–37. 10.1001/archgenpsychiatry.2007.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, 2015. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry, 77(2), 147–157. 10.1016/j.biopsych.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry, 173(10), 980–988. 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M, 1994. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry 151(10), 1430–1436. 10.1176/ajp.151.10.1430 [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY, 2011. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: An activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull 37(1), 177–188. 10.1093/schbul/sbp073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Seidman LJ, Tsuang M, Walker E, Woods SW, McEwen S, van Erp TGM, Cannon TD, 2019. Cortical abnormalities in youth at clinical high-risk for psychosis: Findings from the NAPLS2 cohort. NeuroImage Clin., 23, 101862. 10.1016/j.nicl.2019.101862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Haut KM, He G, van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Seidman LJ, Tsuang M, Walker E, Woods SW, Cannon TD, 2017. Ventricular enlargement and progressive reduction of cortical gray matter are linked in prodromal youth who develop psychosis. Schizophr. Res 189, 169–174. 10.1016/j.schres.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Ji JL, Chung Y, Lympus CA, Afriyie-Agyemang Y, Addington JM, Goodyear BG, Bearden CE, Cadenhead KS, Mirzakhanian H, Tsuang MT, Cornblatt BA, Carrión RE, Keshavan M, Stone WS, Mathalon DH, Perkins DO, Walker EF, Woods SW, Powers AR, Anticevic A, Cannon TD, 2022. Accelerated cortical thinning precedes and predicts conversion to psychosis: The NAPLS3 longitudinal study of youth at clinical high-risk. Mol. Psychiatry 10.1038/s41380-022-01870-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santiáñez R, Pérez-Iglesias R, Rodriguez-Sanchez JM, Mata I, Tordesillas-Gutierrez D, Sanchez E, Tabarés-Seisdedos R, Andreasen N, Magnotta V, Vázquez-Barquero JL, 2011. Global and regional cortical thinning in first-episode psychosis patients: Relationships with clinical and cognitive features. Psychol. Med 41(7), 1449–1460. 10.1017/s003329171000200x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santiáñez R, Pérez-Iglesias R, Tordesillas-Gutiérrez D, Mata I, Rodríguez-Sánchez JM, de Lucas EM, & Vázquez-Barquero JL, 2009. Specific brain structural abnormalities in first-episode schizophrenia: A comparative study with patients with schizophreniform disorder, non-schizophrenic non-affective psychoses and healthy volunteers. Schizophr. Res 115(2–3), 191–201. 10.1016/j.schres.2009.09.007 [DOI] [PubMed] [Google Scholar]
- del Re EC, Stone WS, Bouix S, Seitz J, Zeng V, Guliano A, Somes N, Zhang T, Reid B, Lyall A, Lyons M, Li H, Whitfield-Gabrieli S, Keshavan M, Seidman LJ, McCarley RW, Wang J, Tang Y, Shenton ME, Niznikiewicz MA, 2021. Baseline cortical thickness reductions in clinical high risk for psychosis: Brain regions associated with conversion to psychosis versus non-conversion as assessed at one-year follow-up in the Shanghai-At-Risk-for-Psychosis (SHARP) study. Schizophr. Bull 47(2), 562–574. 10.1093/schbul/sbaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, 2008. The concept of progressive brain change in schizophrenia: Implications for understanding schizophrenia. Schizophr. Bull 34(2), 312–321. 10.1093/schbul/sbm164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, 2022. Commentary on whether progressive brain change underlies the pathology of schizophrenia: Should this even be debated? Schizophr. Res 244, 18–20. 10.1016/j.schres.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Dietsche B, Kircher T, Falkenberg I, 2017. Structural brain changes in schizophrenia at different stages of the illness: A selective review of longitudinal magnetic resonance imaging studies. Aust. N. Z. J. Psychiatry, 51(5), 500–508. 10.1177/0004867417699473 [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen K-A, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA, 2005. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology, 30(9), 1649–1661. 10.1038/sj.npp.1300710 [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, Lublin H, Skimminge A, Baaré W, 2010. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J. Psychiatry Neurosci 35(2), 95–104. 10.1503/jpn.090049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Xiang H, Tan S, Yang F, Fan H, Guo H, Kochunov P, Wang Z, Hong LE, Tan Y, 2019. Subcortical structures and cognitive dysfunction in first episode schizophrenia. Psychiatry Res. Neuroimaging, 286, 69–75. 10.1016/j.pscychresns.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, 2009. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull 35(3), 528–548. 10.1093/schbul/sbn187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2002. Structured Clinical Interview for DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P). Biometrics Research. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P, 2012. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry 69(3), 220–229. 10.1001/archgenpsychiatry.2011.1472 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S, 2013. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev, 37(8), 1680–1691. 10.1016/j.neubiorev.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Ruiz R, Crespo-Facorro B, Setién-Suero E, Tordesillas-Gutierrez D, 2019. Long-term grey matter changes in first episode psychosis: A systematic review. Psychiatry Investig. 16(5), 336–345. 10.30773/pi.2019.02.10.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselgård J, Lebedev AV, Dæhli Kurz K, Joa I, Johannessen JO, Brønnick K, 2018. Structural and functional alterations in the brain during working memory in medication-naïve patients at clinical high-risk for psychosis. PLoS One 13(5), e0196289. 10.1371/journal.pone.0196289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS, 2013. Brain volumes in schizophrenia: A meta-analysis in over 18000 subjects. Schizophr. Bull 39(5), 1129–1138. 10.1093/schbul/sbs118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, & Fischl B, 2006. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Haring L, Müürsepp A, Mõttus R, Ilves P, Koch K, Uppin K, Tarnovskaja J, Maron E, Zharkovsky A, Vasar E, Vasar V, 2016. Cortical thickness and surface area correlates with cognitive dysfunction among first-episode psychosis patients. Psychol Med, 46(10), 2145–2155. 10.1017/s0033291716000684 [DOI] [PubMed] [Google Scholar]
- Harvey I, Ron MA, Du Boulay G, Wicks D, Lewis SW, & Murray RM, 1993. Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol. Med 23(3), 591–604. 10.1017/s003329170002537x [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Newmark R, Goldstein KE, Zelmanova Y, Glanton CF, Torosjan Y, New AS, Lo JN, Mitropoulou V, Siever LJ, 2008. Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr Res, 101(1–3), 111–123. 10.1016/j.schres.2007.12.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M, 2003. Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry, 60(6), 585–594. 10.1001/archpsyc.60.6.585 [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V, 2011. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry, 68(2), 128–137. 10.1001/archgenpsychiatry.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM, 2013. The structure of the cerebral cortex across adult life: Age-related patterns of surface area, thickness, and gyrification. Cereb Cortex, 23(11), 2521–2530. 10.1093/cercor/bhs231 [DOI] [PubMed] [Google Scholar]
- Hua JPY, Karcher NR, Straub KT, Kerns JG, 2022. Associations between long-term psychosis risk, probabilistic category learning, and attenuated psychotic symptoms with cortical surface morphometry. Brain Imaging Behav. 16(1), 91–106. 10.1007/s11682-021-00479-8 [DOI] [PubMed] [Google Scholar]
- Hua JPY, Mathalon DH, 2022. Cortical and subcortical structural morphometric profiles in individuals with nonaffective and affective early illness psychosis. Schizophr. Bull. Open, 3(1), sgac028. 10.1093/schizbullopen/sgac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI, 2008. Brain size and cortical structure in the adult human brain. Cereb Cortex, 18(9), 2181–2191. 10.1093/cercor/bhm244 [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, Fusar-Poli P, Uhlhaas PJ, Takahashi T, Sugranyes G, Kwak YB, Mathalon DH, Katagiri N, Hooker CI, Smigielski L, Colibazzi T, Via E, Tang J, Koike S, Rasser PE, Michel C, Lebedeva I, Hegelstad WTV, de la Fuente-Sandoval C, Waltz JA, Mizrahi, … Hernaus D, 2021. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: An ENIGMA working group mega-analysis. JAMA Psychiatry 78(7), 753–766. 10.1001/jamapsychiatry.2021.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A, 2006. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage, 30(2), 436–443. 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, Han JY, Choi CH, Kang DH, Chung CK, Kwon JS, 2011. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr. Bull 37(4), 839–849. 10.1093/schbul/sbp151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13(2), 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Stahl D, Williams SC, DeLisi LE, 2010. Progressive lateral ventricular enlargement in schizophrenia: A meta-analysis of longitudinal MRI studies. Schizophr. Res 120(1–3), 54–62. 10.1016/j.schres.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Kim T, Lee KH, Oh H, Lee TY, Cho KIK, Lee J, Kwon JS, 2018. Cerebellar structural abnormalities associated with cognitive function in patients with first-episode psychosis. Front Psychiatry, 9, 286. 10.3389/fpsyt.2018.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser P, Zhou J, Lim JK, Poh JS, Zheng H, Tng HY, Krishnan R, Lee J, Keefe RS, Adcock RA, Wood SJ, Fornito A, Chee MW, 2015. Lack of evidence for regional brain volume or cortical thickness abnormalities in youths at clinical high risk for psychosis: Findings from the Longitudinal Youth at Risk Study. Schizophr. Bull 41(6), 1285–1293. 10.1093/schbul/sbv012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi J, Del Re EC, Bouix S, Blokland GAM, Mesholam-Gately R, Woodberry K, Niznikiewicz M, Goldstein J, Hirayasu Y, Petryshen TL, Seidman LJ, Shenton ME, McCarley RW, 2018. Abnormal relationships between local and global brain measures in subjects at clinical high risk for psychosis: A pilot study. Brain Imaging Behav. 12(4), 974–988. 10.1007/s11682-017-9758-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen K-A, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA, 2008. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol. Psychiatry 63(8), 759–765. 10.1016/j.biopsych.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D, Miura K, Nemoto K, Okada N, Matsumoto J, Fukunaga M, Hashimoto R, 2022. Neuroimaging studies within Cognitive Genetics Collaborative Research Organization aiming to replicate and extend works of ENIGMA. Hum. Brain. Mapp 43(1), 182–193. 10.1002/hbm.25040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YB, Kim M, Cho KIK, Lee J, Lee TY, Kwon JS, 2019. Reduced cortical thickness in subjects at clinical high risk for psychosis and clinical attributes. Aust. N. Z. J. Psychiatry, 53(3), 219–227. 10.1177/0004867418807299 [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Lapointe JS, Honer WG, 2004. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. Am. J. Psychiatry, 161(10), 1829–1836. 10.1176/ajp.161.10.1829 [DOI] [PubMed] [Google Scholar]
- Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Solomon M, Carter CS, 2015. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry 72(3), 226–234. 10.1001/jamapsychiatry.2014.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M, 2005. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry, 62(4), 361–370. 10.1001/archpsyc.62.4.361 [DOI] [PubMed] [Google Scholar]
- Lin Y, Li M, Zhou Y, Deng W, Ma X, Wang Q, Guo W, Li Y, Jiang L, Hu X, Zhang N, Li T, 2019. Age-related reduction in cortical thickness in first-episode treatment-naïve patients with schizophrenia. Neurosci. Bull 35(4), 688–696. 10.1007/s12264-019-00348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, Toga AW, 2004. Gender differences in cortical complexity. Nat Neurosci, 7(8), 799–800. 10.1038/nn1277 [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Rapoport JL, Davis KL, Krystal JH, 2003. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry. Arch. Gen. Psychiatry 60(8), 846–848. 10.1001/archpsyc.60.8.846 [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A, 2001. Progressive brain volume changes and the clinical course of schizophrenia in men: A longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry 58(2), 148–157. 10.1001/archpsyc.58.2.148 [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME, 1999. MRI anatomy of schizophrenia. Biol. Psychiatry, 45(9), 1099–1119. 10.1016/s0006-3223(99)00018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Walsh B, Woods S, 2010. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. Oxford University Press. [Google Scholar]
- Merritt K, Luque Laguna P, Irfan A, David AS, 2021. Longitudinal structural MRI findings in individuals at genetic and clinical high risk for psychosis: A systematic review. Front. Psychiatry, 12, 620401. 10.3389/fpsyt.2021.620401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull 29(4), 703–715. 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW, 2002. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry 159(5), 863–865. 10.1176/appi.ajp.159.5.863 [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Tamnes CK, 2016. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage 141, 273–281. 10.1016/j.neuroimage.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C, Uematsu A, Nakatani H, Takano Y, Iwashiro N, Abe O, Yamasue H, Kasai K, Koike S, 2021. Volumetric differences in gray and white matter of cerebellar Crus I/II across the different clinical stages of schizophrenia. Psychiatry Clin. Neurosci 75(8), 256–264. 10.1111/pcn.13277 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV, 1992. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin. Exp. Res 16(6), 1078–1089. 10.1111/j.1530-0277.1992.tb00702.x [DOI] [PubMed] [Google Scholar]
- Pies RW, 2018. Do antipsychotics “thin” the brain?: It is a rather gray matter. J. Clin. Psychopharmacol 38(3), 167–169. 10.1097/jcp.0000000000000879 [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR, 2005. The neurodevelopmental model of schizophrenia: Update 2005. Mol. Psychiatry, 10(5), 434–449. 10.1038/sj.mp.4001642 [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ, 2018. Sex differences in the adult human brain: Evidence from 5216 UK Biobank participants. Cereb Cortex, 28(8), 2959–2975. 10.1093/cercor/bhy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Hamilton HK, Bachman P, Belger A, Carrión RE, Duncan E, Johannesen J, Kenney JG, Light G, Niznikiewicz M, Addington J, Bearden CE, Owens EM, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman L, Tsuang M, Walker EF, Woods SW, Mathalon DH, 2020. Stability of mismatch negativity event-related potentials in a multisite study. Int. J. Methods Psychiatr. Res 29(2), e1819. 10.1002/mpr.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz-Santiañez R, Suarez-Pinilla P, Crespo-Facorro B. (2015). Brain structural effects of antipsychotic treatment in schizophrenia: A systematic review. Curr. Neuropharmacol 13(4), 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Urrestarazu A, Murray GK, Barnes A, Miettunen J, Jääskeläinen E, Mäki P, Nikkinen J, Remes J, Mukkala S, Koivukangas J, Heinimaa M, Moilanen I, Suckling J, Kiviniemi V, Jones PB, Veijola J, 2014. Brain structure in different psychosis risk groups in the Northern Finland 1986 birth cohort. Schizophr Res, 153(1–3), 143–149. 10.1016/j.schres.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Rosa PG, Schaufelberger MS, Uchida RR, Duran FL, Lappin JM, Menezes PR, Scazufca M, McGuire PK, Murray RM, Busatto GF, 2010. Lateral ventricle differences between first-episode schizophrenia and first-episode psychotic bipolar disorder: A population-based morphometric MRI study. World J. Biol. Psychiatry, 11(7), 873–887. 10.3109/15622975.2010.486042 [DOI] [PubMed] [Google Scholar]
- Sasabayashi D, Takayanagi Y, Takahashi T, Katagiri N, Sakuma A, Obara C, Katsura M, Okada N, Koike S, Yamasue H, Nakamura M, Furuichi A, Kido M, Nishikawa Y, Noguchi K, Matsumoto K, Mizuno M, Kasai K, Suzuki M. 2020. Subcortical brain volume abnormalities in individuals with an at-risk mental state. Schizophr. Bull 46(4), 834–845. 10.1093/schbul/sbaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW, 2001. A review of MRI findings in schizophrenia. Schizophr. Res 49(1–2), 1–52. 10.1016/s0920-9964(01)00163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Umbricht D, Lang UE, Borgwardt S. (2014, Nov). Declining transition rates to psychosis: The role of diagnostic spectra and symptom overlaps in individuals with attenuated psychosis syndrome. Schizophr Res, 159(2–3), 292–298. 10.1016/j.schres.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Smucny J, Dienel SJ, Lewis DA, Carter CS, 2022. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 47(1), 292–308. 10.1038/s41386-021-01089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA, 2006. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry 188, 510–518. 10.1192/bjp.188.6.510 [DOI] [PubMed] [Google Scholar]
- Taylor H, Ricciardi A, Dazzan P. (2007). A review of caudate nucleus volume in first episode psychosis. Clin. Neuropsychiatry, 4(5), 191–198. [Google Scholar]
- Tomyshev AS, Lebedeva IS, Akhadov TA, Omelchenko MA, Rumyantsev AO, Kaleda VG 2019. Alterations in white matter microstructure and cortical thickness in individuals at ultra-high risk of psychosis: A multimodal tractography and surface-based morphometry study. Psychiatry Res. Neuroimaging 289, 26–36. 10.1016/j.pscychresns.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T, 2008. Brain size and folding of the human cerebral cortex. Cereb. Cortex, 18(10), 2352–2357. 10.1093/cercor/bhm261 [DOI] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MW, Koenders L, de Haan, … Turner JA, 2016. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry, 21(4), 585. 10.1038/mp.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, Pearlson GD, Yao N, Fukunaga M, Hashimoto R, Okada N, Yamamori H, Bustillo JR, Clark VP, Agartz I, Mueller BA, Cahn W, de Zwarte SMC, Hulshoff Pol HE, Kahn RS, Ophoff RA, van Haren NEM, Andreassen OA, Dale AM, … Turner JA, 2018. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium. Biol. Psychiatry, 84(9), 644–654. 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS, 2011. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry, 68(9), 871–880. 10.1001/archgenpsychiatry.2011.88 [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Barlati S, Sacchetti E, 2015. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: Does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry, 78(6), 403–412. 10.1016/j.biopsych.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, & Sacchetti E, 2012. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry, 2(11), e190. 10.1038/tp.2012.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C, 2008. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophr. Res 103(1–3), 1–10. 10.1016/j.schres.2008.04.042 [DOI] [PubMed] [Google Scholar]
- Wei Q, Yan W, Zhang R, Yang X, Xie S, 2022. Aberrant cortical surface and cognition function in drug-naive first-episode schizophrenia. Annals Gen. Psychiatry 21(1), 4. 10.1186/s12991-022-00381-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, 1995. From neuropathology to neurodevelopment. The Lancet, 346(8974), 552–557. 10.1016/S0140-6736(95)91386-6 [DOI] [PubMed] [Google Scholar]
- Wen K, Zhao Y, Gong Q, Zhu Z, Li Q, Pan N, Fu S, Radua J, Vieta E, Kumar P, Kemp GJ, Biswal BB, 2021. Cortical thickness abnormalities in patients with first episode psychosis: a meta-analysis of psychoradiologic studies and replication in an independent sample. Psychoradiology, 1(4), 185–198. 10.1093/psyrad/kkab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry, 64(6), 663–667. 10.4088/jcp.v64n0607 [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET, 2000. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry 157(1), 16–25. 10.1176/ajp.157.1.16 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang Q, Shah C, Li Q, Sweeney JA, Li F, Gong Q, 2022. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry, 79(6), 560–570. 10.1001/jamapsychiatry.2022.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, Durston S, 2012. Progressive structural brain changes during development of psychosis. Schizophr. Bull 38(3), 519–530. 10.1093/schbul/sbq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky RB, Reilly TJ, Murray RM, 2013. The myth of schizophrenia as a progressive brain disease. Schizophr. Bull 39(6), 1363–1372. 10.1093/schbul/sbs135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.