Abstract
We have cloned the nap locus encoding the periplasmic nitrate reductase in Rhodobacter sphaeroides f. sp. denitrificans IL106. A mutant with this enzyme deleted is unable to grow under denitrifying conditions. Biochemical analysis of this mutant shows that in contrast to the wild-type strain, the level of synthesis of the nitrite and N2O reductases is not increased by the addition of nitrate. Growth under denitrifying conditions and induction of N oxide reductase synthesis are both restored by the presence of a plasmid containing the genes encoding the nitrate reductase. This demonstrates that R. sphaeroides f. sp. denitrificans IL106 does not possess an efficient membrane-bound nitrate reductase and that nitrate is not the direct inducer for the nitrite and N2O reductases in this species. In contrast, we show that nitrite induces the synthesis of the nitrate reductase.
Complete denitrification, i.e., reduction of nitrate into nitrous oxide or dinitrogen, is a bioenergetic process used by several species of bacteria. Four nitrogen oxide (N oxide) reductases (nitrate, nitrite, NO, and N2O reductases) are necessary to complete this reaction. The systems that regulate the synthesis of these enzymes are complex and vary from one denitrifier to another. In general, nitrate and N2O reductases are regulated independently with special regulators. In contrast, the regulation of nitrite and NO reductases is often linked at both the transcription and enzyme activity levels (45). Anaerobiosis and the presence of N oxides are the two essential factors that control the synthesis of the N oxide reductases (reviewed in reference 45).
Fumarate nitrate reductase factors and homologues are important elements of the denitrification regulation (45) and have been extensively studied in Escherichia coli (38). These trans-acting proteins activate, under anaerobic conditions, expression of operons such as nar. This operon encodes the membrane-bound nitrate reductase (5), an enzyme generally synthesized under anaerobic conditions. On the other hand, the expression of the periplasmic nitrate reductase, an enzyme first discovered in photosynthetic bacteria (21, 33, 35), is repressed during anaerobic growth for most of the denitrifiers (1, 42). This suggests a putative role for this enzyme in adaptation during a shift from aerobiosis to anaerobiosis (2, 36).
Anaerobic shift is sometimes not sufficient for the induction of denitrification enzymes (12, 17, 41). For example, the presence of N oxide is required, in addition to anaerobiosis, to induce the synthesis of the denitrification reductases in Pseudomonas stutzeri (17). More generally, nitrate acts as a good inducer for all N oxide reductases (17, 22), while nitrite, nitric oxide, and nitrous oxide at least induce their corresponding reductases (17, 18).
Regulations by nitrate and nitrite have been extensively studied in E. coli. Two two-component regulatory systems, NarXL and NarQP, have been shown to regulate the membrane-bound and periplasmic nitrate reductases (9, 39). NarX and NarQ are two sensors that can phosphorylate the two regulators NarL and NarP (25). In P. stutzeri, Paracoccus denitrificans, and Rhodobacter sphaeroides 2.4.3, coregulation of the nitrite and nitric oxide reductases has been demonstrated by the observation that mutation in the nitrite reductase gene affects norCB transcription (41). Fumarate nitrate reductase-like factors DNR, NNR, NnrR, and FnrD, belonging to the FixK group (45) and generally flanking the nor region, modulate both nirS and norCB genes (1, 44, 45). For R. sphaeroides 2.4.3, Kwiatkowski et al. (19) showed that NnrR, which is also present in R. sphaeroides 2.4.1 (19), regulates nirK and norCB in response to the presence of NO (18, 19, 40).
The presence of N2O can also induce the synthesis of some reductases: nitrate and N2O reductases in P. stutzeri (17) and N2O reductase in Rhodobacter capsulatus MT1131 (28). The membrane-bound component, NosR, necessary for the expression of the nitrous oxide reductase in this strain, may be implicated in this regulation (8).
R. sphaeroides f. sp. denitrificans IL106 is one of the few purple, nonsulfur denitrifying photosynthetic bacteria able to perform a complete denitrification process (16, 23, 29, 32, 35). A property of this bacterium is that the periplasmic nitrate reductase is synthesized under both aerobic and anaerobic conditions (20, 30). In contrast to the consensus reached for the other denitrifying species, the presence of a membrane-bound nitrate reductase is controversial for R. sphaeroides f. sp. denitrificans IL106.
In this work, we created a mutant deficient in the periplasmic nitrate reductase for R. sphaeroides f. sp. denitrificans IL106. The aim was to determine the role of this enzyme and to study the effect of nitrate on N oxide reductase synthesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
R. sphaeroides strains were grown at 30°C in Sistrom minimal medium supplemented with succinate as the carbon source (7) under anaerobic conditions, in the absence or presence (phototrophic conditions) of light (75 mol of photons m−2 s−1), or under aerobic conditions (100 ml of culture in 250-ml conical flasks, 275 rpm). Where indicated, the medium was supplemented with 20 mM KNO3 or NaNO2 or sparged with N2O (to saturation). E. coli strains were grown at 37°C in Luria-Bertani medium. When appropriate, tetracycline, spectinomycin, streptomycin, and kanamycin were added at concentrations of 1, 50, 50, and 25 μg/ml, respectively, for R. sphaeroides and at concentrations of 20, 50, 50, and 25 μg/ml for E. coli.
Preparation of cell extracts for electrophoresis.
Preparation of cell extracts, nondenaturing gel electrophoresis, and activity staining were performed as previously described (31).
Assays for nitrate reductase activity.
Cells were grown 48 h photosynthetically or under aerobic conditions, in the presence of N oxide, washed with 50 mM Tris-HCl, pH 8.2, and resuspended in the same buffer. They were broken up with a French press and centrifuged for 1 h 30 min at 200,000 × g. The nitrate reductase activity of the supernatant was measured as follows: the reaction mixture contained 0.85 ml of 50 mM Tris-HCl (pH 8.2), 2 mM methyl viologen, 20 mM KNO3, 100 μl of soluble fraction, and 50 μl of 10 mg of Na dithionite per ml of 200 mM NaHCO3. After 5 or 10 min at 30°C, the reaction was stopped by vigorous agitation until complete oxidation of methyl viologen. The nitrite formed was assayed by the diazo-coupling method (24).
Mass spectrometry measurements.
Mass spectrometry measurements were performed as previously described (30).
DNA manipulation and sequence analyses.
Isolation of plasmid DNA and restriction endonucleases and other enzymatic treatments of DNA were carried out according to standard protocols or manufacturers’ instructions. Sequence determination was performed by Genome Express S.A. (Grenoble). Sequence analyses were performed with the BISANCE computer program (Infobiogen).
Cosmid bank construction.
The cos vector SuperCos 1 and packaging extracts were obtained from Stratagene (La Jolla, Calif.). R. sphaeroides f. sp. denitrificans genomic DNA was partially digested with BamHI. The digested DNA was sized to yield 40-kb fragments, dephosphorylated, and then ligated into the BamHI site of SuperCos 1, previously linearized with XbaI. Packaging of the cosmids into phage heads and their subsequent infection in the E. coli strain XL1-Blue MR were performed as described by the manufacturer (SuperCos 1 and Gigapack II XL kits; Stratagene).
Probes.
From the sequence of two nitrate reductase peptides (31) and taking into account the codon bias of known R. sphaeroides genes, two degenerate primers, GA(C/T)TGGGA(T/C)GA(G/A)GC(G/C)TT(C/T)GA(C/T)GT and TC(G/A)AACCA(C/G)GG(C/G)AC(G/A)AA(C/G)AC(C/G)AC, were designed and used for PCR with Taq polymerase. The 1.9-kb product obtained was sequenced. From this sequence, a 104-bp oligonucleotide was synthesized and used as a probe for napA (Nitra104; nucleotides 2062 to 2165 from napA). Another oligonucleotide containing the first 52 nucleotides from napA was also synthesized and used as a probe (Nitra52; OligoExpress, Paris, France).
Southern DNA analysis.
DNA was transferred to nylon Hybond N+ membranes (Amersham) with a TE 80 Transvac vacuum blotter (Hoefer Scientific Instruments). The probes were labelled with digoxigenin (DIG)-dUTP (DIG high-prime or DIG oligonucleotide 3′-end tailing kits from Boehringer). Hybridizations and detection of hybridizing sequences by chemiluminescence with CDP-Star were performed according to the manufacturer’s protocols (Boehringer).
Nucleotide sequence accession numbers.
The nucleotide sequences of the nap, nos, and nor regions have been submitted to the GenBank and EMBL databases and were given accession no. AF069545, AF125260, and AF126490, respectively.
RESULTS
Cloning and sequencing of the periplasmic nitrate reductase gene of R. sphaeroides f. sp. denitrificans IL106.
In R. sphaeroides f. sp. denitrificans IL106, both a membrane-bound and a periplasmic nitrate reductase have been described and purified (6, 33). The evidence for two nitrate reductase activities is, however, controversial. Sawada and Satoh found 93% of nitrate reductase activity in the soluble fraction (33), whereas Byrne and Nicholas, using the same strain, detected 97% of the activity in the membrane fraction for cultures grown in the same conditions (6). Like Sawada and Satoh, we always observed a nitrate reductase activity in the periplasmic fraction of R. sphaeroides f. sp. denitrificans (31). On the other hand, we never detected any nitrate reductase activity in the membrane fraction that was not imputable to a contamination by the soluble fraction trapped in the chromatophores, even when we used the same procedure as Byrne and Nicholas. To determine the importance of the periplasmic enzyme in the denitrification pathway, we cloned, sequenced, and disrupted the nap locus.
NapA peptide sequences (31) provided the basis for the construction of one pair of degenerated oligonucleotides. From the sequence of the PCR product obtained, a 104-bp oligonucleotide was synthesized and used as a probe for napA. A cosmid library of genomic DNA (SuperCos 1 and Gigapack II XL kits; Stratagene) was screened. A positive signal was obtained with the cosmid pCOSIXE11, which was subcloned. A 4.6-kb EcoRI fragment was cloned into pUC18 (plasmid pCS1). Comparison of the sequence obtained with the sequence of R. sphaeroides 2.4.1 NapA (26) showed that the 5′-terminal part of napA was missing on the 4.6-kb EcoRI fragment. A 2.8-kb KpnI-EcoRI fragment was cloned into pBluescript (plasmid pMS578). The sequence of the nap locus of R. sphaeroides f. sp. denitrificans was obtained by sequencing the inserts of plasmids pCS1 and pMS578 (accession no. AF069545). This locus contains seven open reading frames, napKEFDABC, transcribed in the same direction. The entire nap operon has been cloned and sequenced in several bacteria (3, 11, 27). As expected, the closest similarities were found with the R. sphaeroides 2.4.1 locus (27), with 93, 98, 91, 89, 98, 95, and 98% of the amino acids identical for NapK, -E, -F, -D, -A, -B, and -C, respectively (data not shown).
Disruption of napA: effects on growth.
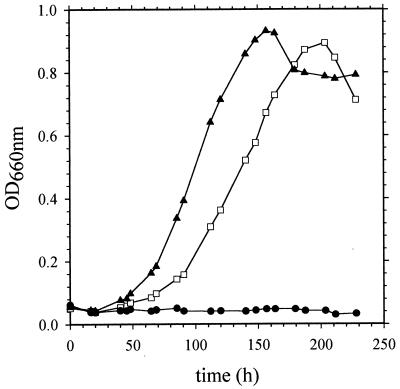
A 4.6-kb EcoRI fragment from cosmid pCOSIXE11, containing the last 798 nucleotides of napA and the entire napB and napC genes, was cloned into pSUP202Km (37) to yield pMS503 (NapC is a tetraheme cytochrome acting as an electron donor to the nitrate reductase) (19). An omega cartridge encoding resistance to spectinomycin and streptomycin was then cloned into the SacI site of napA. The resulting plasmid, pMS507, unable to replicate into R. sphaeroides, was moved from E. coli to R. sphaeroides f. sp. denitrificans by conjugation (10). The double crossover event was confirmed by Southern hybridization analysis. The resulting mutant, MS523, grew under both aerobic and phototrophic conditions but not under dark anaerobic conditions with nitrate as the electron acceptor (Fig. 1). When the plasmid pMS538 containing napABC (4.6-kb SmaI fragment cloned into pRK415 [14]) was introduced into the MS523 mutant, growth with nitrate was restored and the observed growth rate was even faster than it was for the wild type (Fig. 1). As expected, the mutant displays no nitrate reductase activity (Fig. 2), but the synthesis of the nitrate reductase was restored when the plasmid pMS538 was present in the MS523 strain (Fig. 3). The level of synthesis was high even in the absence of nitrate (compare Fig. 2, lane 1, and Fig. 3, lane 3). This may be either because multicopies (four to six) of the napAB genes are present with pMS538, compared with the wild-type strain without plasmid, or because some regulatory sequences upstream of napA are missing in the plasmid construct (the insert contains only 173 bp upstream of the napA start codon). We deduced from this series of experiments that the periplasmic nitrate reductase of R. sphaeroides f. sp. denitrificans is necessary for growth under denitrifying conditions.
FIG. 1.
Growth curves of R. sphaeroides f. sp. denitrificans wild type (□), MS523 mutant complemented with plasmid pRK415 (●), and MS523 mutant complemented with plasmid pMS538 (▴) under denitrifying conditions in the presence of 50 mM nitrate. OD660nm, optical density at 660 nm.
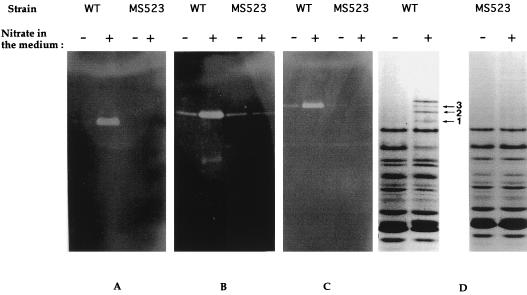
FIG. 2.
Nondenaturing electrophoresis of periplasmic extracts (50 μg of protein) of R. sphaeroides f. sp. denitrificans wild type (WT) and MS523 mutant grown under phototrophic conditions in the presence (+) or absence (−) of 20 mM nitrate. Gels were stained for nitrate (A), nitrite (B), and N2O (C) reductase activities with dithionite-reduced methyl viologen as the electron donor or with Coomassie R250 for protein detection (D).
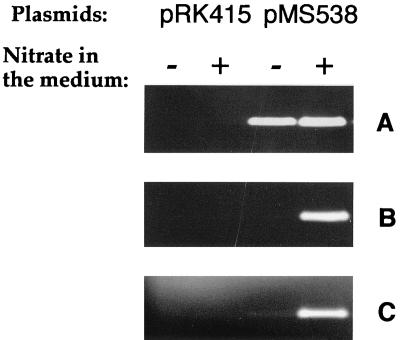
FIG. 3.
Nondenaturing electrophoresis of periplasmic extracts (50 μg of protein) of the MS523 mutant containing pRK415 or pMS538 in trans. Cells were grown under phototrophic conditions in the presence (+) or absence (−) of 20 mM nitrate. Gels were stained for nitrate (A), nitrite (B), and N2O (C) reductase activities with dithionite-reduced methyl viologen as the electron donor.
Effects of napA disruption on enzyme synthesis.
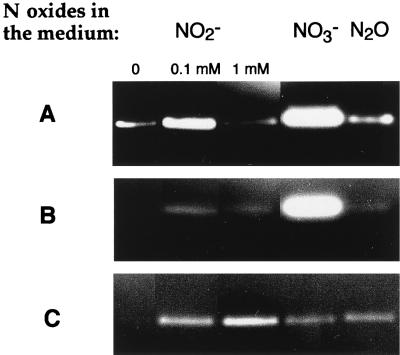
To further characterize this MS523 strain, nitrite reductase and N2O reductase activities of periplasmic extracts were analyzed, in addition to nitrate reductase activity, by nondenaturing polyacrylamide gel electrophoresis. In the wild type, the presence of nitrate in the medium strongly induced the synthesis of nitrate, nitrite, and N2O reductases (Fig. 2). An unexpected result of the disruption of the napA gene was that the synthesis of the nitrite and N2O reductases was no longer induced by the presence of nitrate in the medium (Fig. 2, lanes 8 and 12). These inductions were restored when the plasmid pMS538 was present in the MS523 strain (Fig. 3). These results show that nitrate itself is unable to induce the synthesis of nitrite and N2O reductases under anaerobic conditions, contrary to previous claims (22, 31). The induction of the synthesis of the nitrite and N2O reductases is observed only when both nitrate and nitrate reductase are present. This suggests that the real inducer is one of the products of nitrate reduction, i.e., nitrite, NO, or N2O. We investigated the effects of the presence of nitrite and N2O on reductase synthesis to test this hypothesis. Cells were grown under photosynthetic conditions for 18 h, and different concentrations of nitrite or N2O were then added. The cells were harvested 5 h after the addition of nitrite or N2O, and cell extracts were prepared. The addition of nitrite or N2O to the medium induced the synthesis of nitrate, nitrite, and N2O reductases. The dependence on nitrite concentration of the nitrate and nitrite reductase activities (maximal at 0.1 mM) was different from that observed for the N2O reductase synthesis, which peaked at 1 mM (Fig. 4). This experiment, however, does not enable us to differentiate between a direct effect of nitrite and an effect due to the NO or N2O produced during nitrite reduction. Analysis of the induction of the synthesis of the different reductases in the presence of N oxides for a mutant of R. sphaeroides f. sp. denitrificans with nitrite reductase deleted will be necessary to clarify this point.
FIG. 4.
Nondenaturing electrophoresis of periplasmic extracts (50 μg of protein) of R. sphaeroides f. sp. denitrificans grown under phototrophic conditions. Added to 18-h grown cultures were 0, 0.1, or 1 mM NaNO2 or 20 mM KNO3 or N2O. Cell extracts were prepared 5 h after addition of the N oxides. Gels were stained for nitrate (A), nitrite (B), and N2O (C) reductase activities with dithionite-reduced methyl viologen as the electron donor.
A partial answer can, however, be obtained by studying the induction of the nitrate reductase for R. sphaeroides 2.4.1 cells grown in the presence of nitrite. This strain possesses no nitrite reductase activity (15), and the induction effect cannot be attributed to the NO produced during the enzymatic reduction of nitrite. To avoid a chemical reduction of nitrite into NO, as suggested by Tosques et al. (41), we performed the experiment with aerobic cultures because NO reacts immediately with oxygen and thus cannot accumulate. As expected, in these cultures the 15NO concentration, measured with a mass spectrometer, was undetectable (lower than 0.2 μM) for cells grown for 48 h in the presence of 15NO2− or 15NO3−. Under such conditions, the presence of 1 mM nitrite doubled the level of nitrate reductase, from 21 to 41.5 nmol of nitrite formed per min per mg of protein. It is deduced that nitrite is a good inducer of the nitrate reductase synthesis.
DISCUSSION
The inability of R. sphaeroides f. sp. denitrificans to grow on nitrate in the absence of the periplasmic enzyme, plus the fact that we could detect no nitrate reductase activity in the membrane fraction, leads us to conclude that the membrane-bound nitrate reductase is absent in this strain. This conclusion is further substantiated by the absence of hybridization between genomic DNA of R. sphaeroides f. sp. denitrificans and the narG probe from E. coli (data not shown).
This shows that the periplasmic nitrate reductase is essential for this strain to grow on nitrate. What is this essential role? Generation of a proton motive force (PMF) or simply reduction of nitrate into nitrite, with the following reductions of nitrite into NO, NO into N2O, and N2O into N2 being the major components of the PMF? Experiments with the related strain R. sphaeroides 2.4.1, which possesses the periplasmic reductase but not the nitrite reductase, have shown that the reduction of nitrate by the periplasmic enzyme generates a PMF most probably formed by the coupling of the NADH-dehydrogenase to the periplasmic reduction via the quinone pool (4). This PMF is, however, insufficient to allow growth of this bacterium with nitrate as the sole electron acceptor (15). We obtained the same result even with an R. sphaeroides 2.4.1 strain containing multicopies of the reductase gene of R. sphaeroides f. sp. denitrificans (on plasmid pMS538) (data not shown). Similar behavior has been observed by McEwan et al. (21) for R. capsulatus N22DNAR+. We conclude that the main role of the periplasmic nitrate reductase is only to reduce nitrate into nitrite. The reduction of nitrite into N2 will then produce a PMF allowing bacterial growth.
Although the regulation of the synthesis of enzymes involved in denitrification appears to be quite different from one denitrifier to another, this synthesis is always increased by the addition of nitrate. In general, nitrate is assumed to be the direct inducer. In this study, however, we showed that in R. sphaeroides f. sp. denitrificans, nitrate is not the effector molecule for the nitrite and N2O reductase induction. The presence of nitrate no longer increases the level of synthesis of these enzymes in a mutant deficient in nitrate reductase activity (Fig. 2). This suggests that the real inducer for these enzymes is a product of nitrate reduction, i.e., N2O, NO, or nitrite.
In support of this hypothesis, we obtained experimental evidence that these compounds act as inducers of denitrifying enzymes. N2O is able to induce the synthesis of nitrite and N2O reductases under photosynthetic growth conditions to a small extent (Fig. 4). How N2O is sensed in the cell remains unknown. It has been suggested for other denitrifiers that NosR might be involved (8). nosR is present upstream of nosZ (encoding the N2O reductase) in P. stutzeri and Rhizobium meliloti (13, 43). We cloned and sequenced the nos locus (nosZDFYL) in R. sphaeroides f. sp. denitrificans (34), but the sequence upstream of nosZ is missing (GenBank accession no. AF125260). The identical organization of this locus and the close similarity between the deduced protein sequences for P. stutzeri or R. meliloti and R. sphaeroides suggest that nosR may be present in this latter species also. This still has to be verified.
The presence of nitrite strongly increases the synthesis of nitrate, nitrite, and N2O reductases (Fig. 4). However, we never obtained the same level of induction with nitrite as with nitrate, possibly owing to the accumulation of toxic concentrations of NO. We observed that adding nitrite in the millimolar range to growing cultures induced the production of NO. Such NO production was not induced by the addition of nitrate (data not shown). This observation can be readily explained given that the reduction of nitrite into NO is fast, compared with the reduction of NO into N2O and the reduction of nitrate into nitrite. In other words, to have a good induction of the synthesis of the reductases, it is necessary to have an adequate nitrite concentration but also a concentration of NO reductase relative to the concentration of nitrite reductase such that there is no toxic accumulation of NO. These conditions are reached when nitrate is added to the inoculum.
In R. sphaeroides 2.4.3, the transcription of nirK (encoding nitrite reductase) was reported to be increased in the presence of nitrite (41). However, Tosques et al. propose that the effector molecule is not nitrite but NO produced enzymatically or chemically from nitrite (41). They showed that NO was able to activate the transcription of the genes encoding nitrite and NO reductases (18). The NO-sensitive regulator is NnrR (18, 19, 40). We sequenced part of the nor cluster in R. sphaeroides f. sp. denitrificans (GenBank accession no. AF126490). It presents high homology with R. sphaeroides 2.4.3 (data not shown). An nnrR homolog was found upstream of norC. This suggests that NO also may be an inducer of nitrite and NO reductases in R. sphaeroides f. sp. denitrificans. However, experiments with R. sphaeroides 2.4.1 show that in Rhodobacter species, nitrite is probably an effector molecule for nitrate reductase induction.
Is the inability of nitrate to induce the synthesis of nitrite and N2O reductases a general feature of denitrifiers? In most of the studies concerning other denitrifiers, the enhancement of nitrite and N2O reductase synthesis by the addition of nitrate has been observed with wild-type strains possessing a nitrate reductase activity. It is therefore possible that in these strains, like in R. sphaeroides f. sp. denitrificans, the real effector molecule is not nitrate but a product of nitrate reduction, i.e., nitrite, nitric oxide, or nitrous oxide. To obtain a definite answer, the experiments we have conducted with the MS523 mutant of R. sphaeroides f. sp. denitrificans will have to be performed with similar mutants of other denitrifying species.
REFERENCES
- 1.Arai H, Kodama T, Igarashi Y. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol Microbiol. 1997;25:1141–1148. doi: 10.1046/j.1365-2958.1997.5431906.x. [DOI] [PubMed] [Google Scholar]
- 2.Bell L C, Richardson D J, Ferguson S J. Periplasmic and membrane-bound nitrate reductases in Thiosphaera pantotropha: the periplasmic enzyme catalyses the first step in aerobic denitrification. FEBS Lett. 1990;265:85–87. doi: 10.1016/0014-5793(90)80889-q. [DOI] [PubMed] [Google Scholar]
- 3.Berks B C, Richardson D J, Reilly A, Willis A C, Ferguson S J. The napEADBC gene cluster encoding the periplasmic nitrate reductase system of Thiosphaera pantotropha. Biochem J. 1995;309:983–992. doi: 10.1042/bj3090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berks B C, Ferguson S J, Moir J W B, Richardson D J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232:97–173. doi: 10.1016/0005-2728(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 5.Blasco F, Giordano G, Chippaux M, Bonnefoy V. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the α and β subunits in iron binding and electron transfer. Mol Gen Genet. 1989;218:249–256. doi: 10.1007/BF00331275. [DOI] [PubMed] [Google Scholar]
- 6.Byrne M D, Nicholas D J D. A membrane dissimilatory nitrate reductase from Rhodobacter sphaeroides f. sp. denitrificans. Biochim Biophys Acta. 1987;915:120–124. [Google Scholar]
- 7.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1956;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 8.Cuypers H, Viebrock-Sambale A, Zumft W G. NosR, a membrane-bound regulatory component necessary for expression of nitrous oxide reductase in denitrifying Pseudomonas stutzeri. J Bacteriol. 1992;174:5332–5339. doi: 10.1128/jb.174.16.5332-5339.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darwin A J, Stewart V. Nitrate and nitrite regulation of the Fnr-dependent aeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site. J Mol Biol. 1995;251:15–29. doi: 10.1006/jmbi.1995.0412. [DOI] [PubMed] [Google Scholar]
- 10.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a puf mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Härtig E, Zumft W G. Kinetics of nirS expression (cytochrome cd1 nitrite reductase) in Pseudomonas stutzeri during the transition from aerobic respiration to denitrification: evidence for a denitrification-specific nitrate- and nitrite-responsive regulatory system. J Bacteriol. 1999;181:161–166. doi: 10.1128/jb.181.1.161-166.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway P, McCormick W, Watson R J, Chan Y K. Identification and analysis of the dissimilatory nitrous oxide reduction genes nosRZDFY of Rhizobium meliloti. J Bacteriol. 1996;178:1505–1514. doi: 10.1128/jb.178.6.1505-1514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen N T, Tamaki D, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 15.Kerber N L, Cardenas J. Nitrate reductase from Rhodopseudomonas sphaeroides. J Bacteriol. 1982;150:1091–1097. doi: 10.1128/jb.150.3.1091-1097.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemme J H, Chyla I, Preuss M. Dissimilatory nitrate reduction by strains of the facultative phototrophic bacterium Rhodopseudomonas palustris. FEMS Microbiol Lett. 1980;9:137–140. [Google Scholar]
- 17.Körner J M, Zumft W G. Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol. 1989;55:1670–1676. doi: 10.1128/aem.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwiatkowski A V, Shapleigh J P. Requirement of nitric oxide for induction of genes whose products are involved in nitric oxide metabolism in Rhodobacter sphaeroides 2.4.3. J Biol Chem. 1996;271:24382–24388. doi: 10.1074/jbc.271.40.24382. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski A V, Laratta W P, Toffanin A, Shapleigh J P. Analysis of the role of the nnrR gene product in the response of Rhodobacter sphaeroides 2.4.1 to exogenous nitric oxide. J Bacteriol. 1997;179:5618–5620. doi: 10.1128/jb.179.17.5618-5620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Luque M, Dobao M, Castillo F. Characterization of the assimilatory and dissimilatory nitrate-reducing systems in Rhodobacter: a comparative study. FEMS Microbiol Lett. 1991;83:329–334. [Google Scholar]
- 21.McEwan A G, George C L, Fergusson S J, Jackson J B. A nitrate reductase activity in Rhodopseudomonas capsulata linked to electron transfer and generation of a membrane potential. FEBS Lett. 1982;150:277–280. [Google Scholar]
- 22.Michalski W P, Nicholas D J D. The adaptation of Rhodopseudomonas sphaeroides forma sp. denitrificans for growth under denitrifying conditions. J Gen Microbiol. 1984;130:155–165. [Google Scholar]
- 23.Michalski W P, Nicholas D J D. Identification of two denitrifying strains of Rhodobacter sphaeroides. FEMS Microbiol Lett. 1988;52:239–244. [Google Scholar]
- 24.Nicholas D J D, Nason A. Determination of nitrate and nitrite. Methods Enzymol. 1957;3:981–984. [Google Scholar]
- 25.Rabin R S, Stewart V. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate and nitrite-regulated gene expression in Escherichia coli K12. J Bacteriol. 1993;175:3259–3268. doi: 10.1128/jb.175.11.3259-3268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes F, Roldàn M D, Klipp W, Castillo F, Moreno-Viviàn C. Isolation of periplasmic nitrate reductase genes from Rhodobacter sphaeroides DSM 158: functional and structural differences among prokaryotic nitrate reductases. Mol Microbiol. 1996;19:1307–1318. doi: 10.1111/j.1365-2958.1996.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 27.Reyes F, Gavira M, Castillo F, Moreno-Viviàn C. Periplasmic nitrate-reducing system of the phototrophic bacterium Rhodobacter sphaeroides DSM 158: transcriptional and mutational analysis of the napKEFDABC gene cluster. Biochem J. 1998;331:897–904. doi: 10.1042/bj3310897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson D J, Bell L C, McEwan A G, Jackson J B, Ferguson S J. Cytochrome c2 is essential for electron transfer to nitrous oxide reductase from physiological substrates in Rhodobacter capsulatus and can act as electron donor to the reductase in vitro. Eur J Biochem. 1991;199:677–683. doi: 10.1111/j.1432-1033.1991.tb16170.x. [DOI] [PubMed] [Google Scholar]
- 29.Richardson D J, Bell L C, Moir J W B, Ferguson S J. A denitrifying strain of Rhodobacter capsulatus. FEMS Microbiol Lett. 1994;120:323–328. [Google Scholar]
- 30.Sabaty M, Gans P, Verméglio A. Inhibition of nitrate reduction by light and oxygen in Rhodobacter sphaeroides f. sp. denitrificans. Arch Microbiol. 1993;159:153–159. [Google Scholar]
- 31.Sabaty M, Gagnon J, Verméglio A. Induction by nitrate of cytoplasmic and periplasmic proteins in the photodenitrifier Rhodobacter sphaeroides forma sp. denitrificans under anaerobic or aerobic condition. Arch Microbiol. 1994;162:335–343. doi: 10.1007/BF00263781. [DOI] [PubMed] [Google Scholar]
- 32.Satoh T, Hoshino Y, Kitamura H. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch Microbiol. 1976;108:265–269. doi: 10.1007/BF00454851. [DOI] [PubMed] [Google Scholar]
- 33.Sawada E, Satoh T. Periplasmic location of dissimilatory nitrate and nitrite reductase in a denitrifying phototrophic bacterium. Plant Cell Physiol. 1980;21:205–210. [Google Scholar]
- 34.Schwintner C. Ph.D. thesis. Etude moléculaire de la dénitrification chez Rhodobacter sphaeroides f. sp. denitrificans, rôle de deux protéines inconnues. Marseille, France: Université d’Aix-Marseille II; 1997. [Google Scholar]
- 35.Shioi Y, Doi M, Arata H, Takamiya K. A denitrifying activity in an aerobic photosynthetic bacterium, Erythrobacter sp. strain OCh 114. Plant Cell Physiol. 1988;29:861–865. [Google Scholar]
- 36.Siddiqui R A, Warnecke-Eberz U, Hengsberger A, Schneider B, Freidrich B. Structure and function of a periplasmic nitrate reductase in Alcaligenes eutrophus H16. J Bacteriol. 1993;175:5867–5876. doi: 10.1128/jb.175.18.5867-5876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon R, Priefer U, Puhler A. A broad-host-range mobilization system for in vivo genetic engineering; transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 38.Spiro S, Guest J R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;75:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 39.Stewart V. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol Microbiol. 1983;9:425–434. doi: 10.1111/j.1365-2958.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 40.Tosques I E, Shi J, Shapleigh J P. Cloning and characterization of nnrR, whose product is required for the expression of proteins involved in nitric oxide metabolism in Rhodobacter sphaeroides 2.4.3. J Bacteriol. 1996;178:4958–4964. doi: 10.1128/jb.178.16.4958-4964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tosques I E, Kwiatkowski A V, Shi J, Shapleigh J P. Characterization and regulation of the gene encoding nitrite reductase in Rhodobacter sphaeroides 2.4.3. J Bacteriol. 1997;179:1090–1095. doi: 10.1128/jb.179.4.1090-1095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnecke-Eberz U, Friedrich B. Three nitrate reductase activities in Alcaligenes eutrophus. Arch Microbiol. 1993;159:405–409. [Google Scholar]
- 43.Zumft W G, Viebrock-Sambale A, Braun C. Nitrous oxide reductase from denitrifying Pseudomonas stutzeri: genes for copper-processing and properties of the deduced products, including a new member of the family of ATP/GTP-binding proteins. Eur J Biochem. 1990;192:591–599. doi: 10.1111/j.1432-1033.1990.tb19265.x. [DOI] [PubMed] [Google Scholar]
- 44.Zumft W G, Braun C, Cuypers H. Nitric oxide reductase from Pseudomonas stutzeri: primary structure and gene organization of a novel bacterial cytochrome bc complex. Eur J Biochem. 1994;219:481–490. doi: 10.1111/j.1432-1033.1994.tb19962.x. [DOI] [PubMed] [Google Scholar]
- 45.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]