Abstract
STUDY QUESTION
Is endometrial thickness (EMT) on the hCG trigger day related to the neonatal outcome of a single birth after fresh embryo transfer (ET)?
SUMMARY ANSWER
An EMT ≤7.8 mm was an independent predictor for greater odds of preterm delivery (PTD) of singletons born after fresh ET.
WHAT IS KNOWN ALREADY
There may be a positive association between live birth rates and EMT after fresh ET. It is still unknown whether a similar association is seen for the neonatal outcomes of singletons in fresh cycles.
STUDY DESIGN, SIZE, DURATION
This retrospective study involved singleton live births in women undergoing autologous IVF cycles during the period from 1 October 2016 to 31 July 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 2010 women who fulfilled the inclusion criteria were included. A multivariable regression analysis was performed to detect the relationship between EMT and neonatal outcomes after controlling for potential confounders. Smooth curve fitting and threshold effect analysis were used to evaluate the accurate cutoff value of EMT.
MAIN RESULTS AND THE ROLE OF CHANCE
The results of the multivariate regression analyses showed that the odds of PTD were reduced by 45% with an EMT of 9.00–9.90 mm (adjusted odds ratio (OR): 0.55, 95% CI: 0.13 to 0.98; P = 0.0451), reduced by 58% with an EMT of 10.00–10.90 mm (adjusted OR: 0.42, 95% CI: 0.06 to 0.87; P = 0.0211) and reduced by 75% with an EMT >11 mm (adjusted OR: 0.25, 95% CI: 0.04 to 0.66; P = 0.0034), compared to the group with an EMT of 6.00–8.90 mm. It could also be seen from the adjusted smooth curves that the odds of PTD decreased and gestational age (GA) increased with increasing EMT. Combined with the analysis of threshold effects, the results indicated that when the EMT was ≤7.6 mm, the incidence of PTD decreased as the EMT gradually increased (adjusted OR: 0.47, 95% CI: 0.03 to 0.99; P = 0.0107), and when the EMT was ≤7.8 mm, the GA increased (adjusted β: 1.94, 95% CI: 1.26 to 2.63; P < 0.0001) as the EMT gradually increased.
LIMITATIONS, REASONS FOR CAUTION
The main limitation of our study is its retrospective design. Although we found a significant decrease in PTD as the EMT increased, in terms of GA, the magnitude of the differences was modest, which may limit the clinical relevance of the findings.
WIDER IMPLICATIONS OF THE FINDINGS
Our data provide new insight into the relationship between EMT and neonatal outcomes by indicating that a thin endometrium of ≤7.8 mm is associated with an increased odds of PTD of singletons after fresh ET.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the National Natural Science Foundation of China (grant no. 82071717). There are no conflicts of interest.
Keywords: endometrial thickness (EMT), hCG trigger day, preterm delivery (PTD), gestational age (GA), fresh embryo transfer
WHAT DOES THIS MEAN FOR PATIENTS?
Endometrial thickness, monitored by a technique called transvaginal ultrasound, is a convenient and commonly used indicator closely related to pregnancy rate during IVF cycles. Previous studies showed a positive association between live birth rates and endometrial thickness after fresh embryo transfers. Is there a similar association for neonatal outcomes, such as birthweight and gestational age?
This analysis of 2010 women under 42 years old who became pregnant following fresh embryo transfers and gave birth to a single live baby between 1 October 2016 and 31 July 2021 at the Center of Assisted Reproduction at Tangdu Hospital of Air Force Military Medical University in China demonstrated that a thin endometrium of ≤7.8 mm was associated with an increased odds of preterm delivery of singletons. This information may be useful for patients to evaluate the risk of preterm birth based on endometrial thickness during fresh IVF treatments.
Introduction
In vitro fertilization has developed rapidly in more than 40 years since the first birth in 1978. Currently, the assessment of the success of IVF is not limited to a satisfactory live birth rate but also relates to fetal safety during pregnancy and the perinatal period. It is well known that singleton pregnancies after fresh IVF cycles have a higher risk of obstetric and perinatal complications, such as low birth weight (LBW), small for gestational age (SGA), placenta previa, and preeclampsia, compared with naturally born infants (Pinborg et al., 2013; Dunietz et al., 2015; Maheshwari et al., 2018). Some studies have shown that these risks are the result of intrinsic factors in subfertile couples or ART itself, including ovarian stimulation, in vitro embryo culture, and cryopreservation techniques (Pal et al., 2008; Vergouw et al., 2012; Sunkara et al., 2015). However, it is difficult to know which step mainly leads to adverse effects.
Endometrial thickness (EMT), which is closely related to pregnancy outcomes, is a convenient and commonly used indicator from transvaginal ultrasound monitoring during ovarian stimulation. Many studies have demonstrated that patients with a thin endometrium (<8 mm) have lower chances of becoming pregnant, whether in fresh embryo transfer (ET) cycles or in frozen-thawed ET cycles (Liu et al., 2018; Eftekhar et al., 2019; Shalom-Paz et al., 2021; Zhao et al., 2022), whereas researchers in other studies have claimed that women with a thick endometrium (>14 mm) have lower implantation and pregnancy rates and a higher rate of miscarriage (Weissman et al., 1999).
However, by searching the literature, we found that only a few studies reported the impact of EMT on neonatal outcomes. In singletons who underwent fresh ETs, Chung et al. (2006) reported, for the first time, that the risk of LBW in pregnant women with an EMT ≤10 mm was twice as high as that in women with an EMT >12 mm. Another study showed that an EMT <7.5 mm was related to increased risks of SGA babies resulting from fresh ET, while the incidence of LBW did not change (Oron et al., 2018). For maternal complications during the pregnancy period, Liu et al. (2021) observed that a thin endometrium (≤8 mm) was associated with an increased risk of hypertensive disorders of pregnancy (HDP). A more recent meta-analysis showed that an EMT <7.5 mm increased the incidence of both HDP and SGA infants and decreased the birthweight of babies (Liao et al., 2021). However, Huang et al. (2020) showed that EMT was not independently associated with adverse neonatal outcomes. The influence of EMT on neonatal outcomes is currently not fully understood. All previous studies were designed with simply three or four groups. Therefore, if a thin EMT is harmful to neonatal health, the exact cutoff value of the EMT is still unclear.
The aim of our study was to investigate the relationship between EMT on the hCG trigger day and neonatal outcomes of singletons after fresh ET. In addition, we tried to explore the exact cutoff value of the EMT for affected outcomes. To our knowledge, this is the first study in which smooth curve fitting and threshold effect analysis have been used to evaluate the accurate cutoff value of EMT.
Materials and methods
Study population
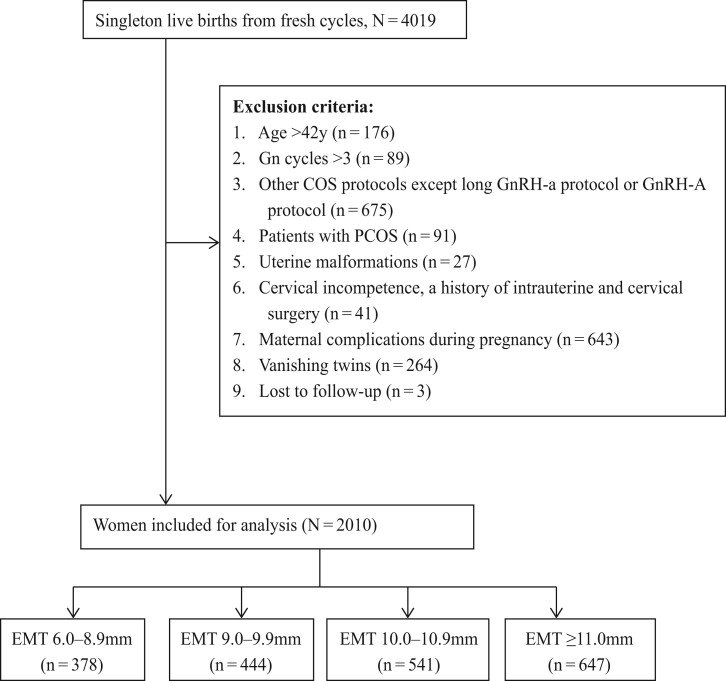
We conducted a retrospective cohort study covering the interval between 1 October 2016 and 31 July 2021, at the Center of Assisted Reproduction at Tangdu Hospital of Air Force Military Medical University in China. Our study population included all women who met the following criteria: autologous IVF/ICSI cycles, long luteal GnRH agonist (GnRH-a) protocol or antagonist protocol during the ovarian stimulation process, age at oocyte retrieval ≤42 years, and live singleton birth after fresh ET. All patients were in their first three gonadotrophin cycles. In this study, patients with known polycystic ovary syndrome (PCOS) diagnosed by the Rotterdam criteria or suffering from maternal complications during pregnancy were excluded from the analysis. This decision was made to prevent any basis for neonatal outcomes. In addition, patients with multiple births, vanishing twins, uterine malformations, cervical incompetence, or a history of intrauterine and cervical surgery were also excluded. A flow diagram of the patient-selection process is shown in Fig. 1. Finally, a total of 2010 cycles were included in this study.
Figure 1.
Flowchart of patient inclusion. Gn: gonadotrophin; COS: (controlled) ovarian stimulation; GnRH-a: GNRH agonist; GnRH-A: GnRH antagonist; PCOS: polycystic ovary syndrome; EMT: endometrial thickness.
Ethics approval
This study was approved by the Institutional Review Board of the hospital (assigned number: TDLL-202203-02).
Study procedures
Ovarian stimulation was achieved by using the GnRH agonist long protocol or the GnRH antagonist protocol. The starting dose and the type of ovarian stimulation protocol were determined by the patients’ characteristics and clinician preferences. All patients received both recombinant and urinary exogenous gonadotrophins. The daily gonadotrophins dose was decided on follicular growth in successive transvaginal sonograms and a blood test that included evaluation of the plasma levels of estrogen (E2), progesterone, and LH until the day of the hCG trigger. Ovulation was triggered in all patients with 250 μg of recombinant hCG when there were at least three follicles ≥17-mm diameter on transvaginal ultrasound. Ultrasound-guided oocyte retrieval was performed 36 h after the trigger injection. Approximately 12–17 h after insemination or sperm injection, the oocytes were examined for fertilization. Cleavage-stage embryos were transferred on the third day and blastocysts were transferred on the fifth day after oocyte retrieval (Pereira et al., 2015). There were no major changes in the clinical and laboratory conditions, culture media, or fresh ET techniques during the study period.
Outcome measures and definitions
Data on demographic and cycle characteristics were collected, including the ages of the couples, maternal BMI, anti-Müllerian hormone (AMH) levels, basal FSH levels, type of infertility, prior gonadotrophin cycles, infertility duration, infertility cause, total gonadotrophin dose, stimulation duration, estrogen (E2) levels on the trigger day, number of oocytes retrieved, stages and numbers of transferred embryos, and the thickness and type of endometrium.
The newborn height, weight, gender, gestational age (GA), and mode of delivery were recorded for all live infants. GA was counted from the day of ET, which was identified as Day 17 of the cycle for cleavage-stage ET and Day 19 for blastocyst transfer (Nelissen et al., 2012). Preterm delivery (PTD) and very PTD were identified as GA 32–36 weeks and <32 weeks, respectively. LBW, very LBW, and fetal macrosomia were identified as birthweight <2500, <1500, and ≥4000 g, respectively. SGA and very SGA were identified as birthweight <10th and <3rd percentiles. Large for gestational age (LGA) and very LGA were identified as birthweights >90th and >97th percentiles, respectively. In addition, the Z-score was introduced to calculate birthweight accounting for GA and neonatal sex according to the following equation: Z-score = (x − μ)/σ, in which x is the weight of newborns, and μ and σ are the average birthweight and the SD of infants of the same sex and the same GA, respectively. Birthweight percentiles and the calculation of Z-score were based on Chinese reference singleton newborns stratified by GA and neonatal sex (Dai et al., 2014).
Statistical analysis
Categorical variables are expressed as the number of cases (n) with the percentage of occurrence (%), and continuous variables are expressed as the median (interquartile range) or mean ± SD as appropriate. The patient demographics, cycle characteristics, and neonatal outcomes were compared between the four groups (according to EMT) via t tests (for continuous variables) or χ2 tests (for categorical variables).
Multiple linear regression analysis was performed to explore the relationship between EMT (mm) and GA (weeks). Logistic regression analysis was used to assess the adverse categorical outcomes, such as LBW, very LBW, fetal macrosomia, preterm, very preterm, SGA, very SGA, LGA, and very LGA, with adjustments for potential confounding factors.
We selected the confounders on the basis of their associations with the outcomes of interest or a change in the effect estimate of more than 10%, including the ages of the couples, maternal BMI, basal FSH levels, AMH levels, type of infertility, prior gonadotropin cycle, ovarian stimulation protocols, dosage of gonadotropins, E2 level on the hCG trigger day, number of oocytes retrieved, fertilization method, number and stage of embryos transferred, and gender of newborn.
All statistical analyses were performed by using EmpowerStats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and R software version 3.6.1 (http://www.r-project.org). A P-value of <0.05 was considered to be statistically significant.
Results
A total of 2010 fresh IVF cycles that met the inclusion criteria during the study period were included in this analysis. Of the births, 378, 444, 541, and 647 live-born singletons were categorized as Group 1 (6.00–8.90 mm), Group 2 (9.00–9.90 mm), Group 3 (10.00–10.90 mm), and Group 4 (>11.00 mm), respectively.
The patient baseline characteristics, cycle parameters, and neonatal outcomes are presented in Table 1. Compared to Group 1, women in the thicker endometrium groups were younger and had lower basal FSH levels, higher AMH values, and more primary infertility. Furthermore, in terms of the cycle parameters, the thicker endometrium groups had more GnRH agonist long protocols, higher E2 levels on the hCG trigger day, more total retrieved oocytes, lower dosages of gonadotropins, more ICSI cycles, and fewer cleavage embryos transferred.
Table 1.
Patient baseline characteristics, cycle parameters, and neonatal outcomes by different groups of endometrial thickness on the hCG trigger day.
| Characteristics | 6.00–8.90 mm | 9.00–9.90 mm | 10.00–10.90 mm | >11.00 mm | P-value |
|---|---|---|---|---|---|
| (n = 378) | (n = 444) | (n = 541) | (n = 647) | ||
| Baseline characteristics | |||||
| Maternal age (year), mean ± SD | 31.50 ± 4.32 | 30.78 ± 3.90 | 30.64 ± 3.88 | 30.58 ± 3.91 | 0.015* |
| Paternal age (year), mean ± SD | 33.00 ± 5.01 | 32.52 ± 4.85 | 32.43 ± 4.73 | 32.09 ± 4.59 | 0.105 |
| Maternal BMI (kg/m2), mean ± SD | 22.22 ± 2.94 | 22.22 ± 3.10 | 22.07 ± 2.96 | 22.60 ± 3.42 | 0.103 |
| Basal FSH (IU/l), mean ± SD | 7.83 ± 2.46 | 7.91 ± 2.33 | 7.66 ± 2.51 | 7.35 ± 2.44 | <0.001* |
| AMH (ng/ml), mean ± SD | 2.85 ± 2.34 | 2.64 ± 2.06 | 2.81 ± 2.20 | 3.03 ± 2.34 | 0.035* |
| Type of infertility, n (%) | <0.001* | ||||
| Primary | 146 (38.62%) | 220 (49.55%) | 282 (52.13%) | 371 (57.34%) | |
| Secondary | 232 (61.38%) | 224 (50.45%) | 259 (47.87%) | 276 (42.66%) | |
| Infertility duration (year), mean ± SD | 3.61 ± 2.96 | 3.59 ± 2.71 | 3.77 ± 2.70 | 3.74 ± 2.57 | 0.660 |
| Infertility cause, n (%) | 0.234 | ||||
| Female | 259 (68.52%) | 292 (65.77%) | 360 (66.54%) | 393 (60.74%) | |
| Male | 68 (17.99%) | 88 (19.82%) | 93 (17.19%) | 148 (22.87%) | |
| Mixed | 33 (8.73%) | 38 (8.56%) | 57 (10.54%) | 61 (9.43%) | |
| Unexplained | 18 (4.76%) | 26 (5.86%) | 31 (5.73%) | 45 (6.96%) | |
| Prior gonadotropin cycle, mean ± SD | 1.24 ± 0.65 | 1.24 ± 0.57 | 1.23 ± 0.53 | 1.20 ± 0.50 | 0.536 |
| Cycle parameters | |||||
| Ovarian stimulation protocols, n (%) | 0.025* | ||||
| GnRH-a long protocol | 230 (60.85%) | 268 (60.36%) | 343 (63.40%) | 434 (67.08%) | |
| Antagonist protocol | 148 (39.15%) | 176 (39.64%) | 198 (36.60%) | 213 (32.92%) | |
| Dosage of gonadotropins (IU), mean ± SD | 2309.84 ± 1111.45 | 2375.34 ± 1086.39 | 2307.30 ± 1150.48 | 2202.80 ± 1109.18 | 0.017* |
| Stimulation duration (days), mean ± SD | 11.40 ± 2.35 | 11.54 ± 2.11 | 11.62 ± 2.18 | 11.66 ± 2.18 | 0.324 |
| E2 level on HCG day (pg/ml), mean ± SD | 2809.38 ± 1299.41 | 2969.08 ± 1255.00 | 3089.74 ± 1316.63 | 3051.18 ± 1215.43 | 0.006* |
| Number of oocytes retrieved, mean ± SD | 9.17 ± 3.87 | 9.72 ± 3.90 | 9.62 ± 3.95 | 9.95 ± 3.64 | 0.018* |
| Retrieved MII oocytes, mean ± SD | 7.97 ± 3.40 | 8.27 ± 3.57 | 8.28 ± 3.60 | 8.54 ± 3.32 | 0.089 |
| Fertilization method, n (%) | 0.023* | ||||
| IVF | 273 (72.22%) | 292 (65.77%) | 363 (67.10%) | 403 (62.29%) | |
| ICSI | 93 (24.60%) | 129 (29.05%) | 146 (26.99%) | 212 (32.77%) | |
| IVF + ICSI | 12 (3.17%) | 23 (5.18%) | 32 (5.91%) | 32 (4.95%) | |
| Fertilization rate (%), mean ± SD | 83.58 ± 15.56 | 81.84 ± 16.92 | 83.07 ± 16.55 | 83.58 ± 16.07 | 0.320 |
| Number of available embryos, mean ± SD | 4.27 ± 2.30 | 4.55 ± 2.47 | 4.42 ± 2.33 | 4.70 ± 2.33 | 0.071 |
| Stage embryo transferred, n (%) | 0.001* | ||||
| D3 | 332 (87.83%) | 376 (84.68%) | 455 (84.10%) | 510 (78.83%) | |
| D5 | 46 (12.17%) | 68 (15.32%) | 86 (15.90%) | 137 (21.17%) | |
| Number of embryos transferred, n (%) | 0.188 | ||||
| 1 | 95 (25.13%) | 116 (26.13%) | 139 (25.69%) | 200 (30.91%) | |
| 2 | 282 (74.60%) | 328 (73.87%) | 401 (74.12%) | 444 (68.62%) | |
| 3 | 1 (0.26%) | 0 (0.00%) | 1 (0.18%) | 3 (0.46%) | |
| Endometrial thickness (mm), mean ± SD | 7.84 ± 0.68 | 9.11 ± 0.23 | 10.08 ± 0.20 | 11.70 ± 0.90 | <0.001* |
| Endometrial type, n (%) | 0.296 | ||||
| A | 7 (1.85%) | 11 (2.48%) | 7 (1.29%) | 5 (0.77%) | |
| A-B | 20 (5.29%) | 24 (5.41%) | 34 (6.28%) | 29 (4.48%) | |
| B | 120 (31.75%) | 142 (31.98%) | 154 (28.47%) | 204 (31.53%) | |
| B-C | 205 (54.23%) | 234 (52.70%) | 316 (58.41%) | 354 (54.71%) | |
| C | 26 (6.88%) | 33 (7.43%) | 30 (5.55%) | 55 (8.50%) | |
| Neonatal outcomes indicators | |||||
| Gender of newborn, n (%) | 0.507 | ||||
| Male | 203 (53.70%) | 215 (48.42%) | 277 (51.20%) | 327 (50.54%) | |
| Female | 175 (46.30%) | 229 (51.58%) | 264 (48.80%) | 320 (49.46%) | |
| Newborn height (cm), mean ± SD | 50.22 ± 2.23 | 50.22 ± 1.99 | 50.14 ± 1.98 | 50.19 ± 2.29 | 0.681 |
| GA (week), mean ± SD | 38.74 ± 2.00 | 38.96 ± 1.68 | 39.06 ± 1.52 | 39.08 ± 1.73 | 0.013* |
| Birthweight (g), mean ± SD | 3123.48 ± 491.04 | 3201.79 ± 556.65 | 3191.90 ± 446.38 | 3276.62 ± 495.47 | 0.025* |
| Z-score, mean ± SD | 0.05 ± 1.15 | 0.07 ± 1.13 | −0.05 ± 1.00 | 0.19 ± 1.02 | 0.220 |
| Birthweight, n (%) | 0.031* | ||||
| Normal birthweight | 342 (90.48%) | 373 (84.01%) | 494 (91.31%) | 574 (88.72%) | |
| Very low birth weight (<1500 g) | 6 (1.59%) | 5 (1.13%) | 0 (0.00%) | 6 (0.93%) | |
| Low birthweight (<2500 g) | 26 (6.88%) | 44 (9.91%) | 16 (2.96%) | 23 (3.55%) | |
| Fetal macrosomia (≥4000 g) | 4 (1.05%) | 22 (4.95%) | 31 (5.73%) | 44 (6.80%) | |
| Gestational age, n (%) | 0.023* | ||||
| Full-term (≥37 weeks) | 319 (84.39%) | 400 (90.09%) | 500 (92.42%) | 614 (94.90%) | |
| Preterm (32–36 weeks) | 48 (12.70%) | 39 (8.78%) | 41 (7.58%) | 24 (3.71%) | |
| Very preterm (<32 weeks) | 11 (2.91%) | 5 (1.13%) | 0 (0.00%) | 9 (1.39%) | |
| Small for gestational age, n (%) | 26 (6.88%) | 37 (8.33%) | 43 (7.95%) | 43 (6.65%) | 0.686 |
| Very small for gestational age, n (%) | 5 (1.32%) | 11 (2.48%) | 14 (2.59%) | 10 (1.55%) | 0.384 |
| Large for gestational age, n (%) | 40 (10.58%) | 39 (8.78%) | 53 (9.80%) | 51 (7.88%) | 0.469 |
| Very large for gestational age, n (%) | 16 (4.23%) | 13 (2.93%) | 19 (3.51%) | 24 (3.71%) | 0.789 |
| Mode of delivery, n (%) | 0.155 | ||||
| Vaginal | 121 (32.01%) | 154 (34.68%) | 183 (33.83%) | 249 (38.49%) | |
| Caesarean section | 257 (67.99%) | 290 (65.32%) | 358 (66.17%) | 398 (61.51%) | |
AMH: anti-Müllerian hormone; E2: estrogen; GA: gestational age; GnRH-a: GNRH agonist.
Statistically significant, with P < 0.05.
In terms of neonatal outcomes, with increasing EMT on the hCG trigger day, both the GA (weeks) and birthweight (g) gradually increased, and the proportion of LBW infants and the incidence of PTD babies decreased. The differences in all of the above-mentioned indices were significant (P < 0.05).
The neonatal outcomes of the multivariate analyses are shown in Table 2. GA (weeks) was positively associated with increasing EMT on the hCG trigger day (adjusted β: 0.13, 95% CI: 0.03 to 0.29; P < 0.001), even after accounting for confounding variables. The effective value β implied that for every 1 mm increase in EMT, GA increased by 0.13 weeks. Moreover, when EMT was taken as the categorical indicator, GA (weeks) still exhibited an increasing trend with the increase in EMT in the four groups (38.60 ± 1.99 vs 38.91 ± 1.68 vs 39.06 ± 1.52 vs 39.17 ± 1.73, P trend <0.0001). When the EMT was 9.0–9.9 mm (adjusted β: 0.29, 95% CI: 0.03 to 0.48; P = 0.0226), 10.0–10.9 mm (adjusted β: 0.47, 95% CI: 0.15 to 0.69; P = 0.0019) and >11 mm (adjusted β: 0.55, 95% CI: 0.20 to 0.83; P = 0.0005), the GA increase was significant compared to the 6.00–8.90 mm group.
Table 2.
Multivariable regression analysis for neonatal outcomes by endometrial thickness.
| Characteristics | Gestational age (weeks) | Non-adjusted β/OR | P-value | Adjusted β/OR | P-value |
|---|---|---|---|---|---|
| Adjust mean (95% CI) | (95% CI) | (95% CI) | |||
| Gestational age (week) | 0.07 (0.02, 0.12) | 0.0067 | 0.13 (0.03, 0.29) | <0.0001* | |
| Gestational age (EMT categorized into four groups) | |||||
| 6.00–8.90 mm (n = 378) | 38.60 ± 1.99 | Ref | Ref | ||
| 9.00–9.90 mm (n = 444) | 38.91 ± 1.68 | 0.22 (−0.01, 0.46) | 0.0659 | 0.29 (0.03, 0.48) | 0.0226* |
| 10.00–10.90 mm (n = 541) | 39.06 ± 1.52 | 0.32 (0.09, 0.55) | 0.0055 | 0.47 (0.15, 0.69) | 0.0019* |
| >11.00 mm (n = 647) | 39.17 ± 1.73 | 0.34 (0.12, 0.56) | 0.0021 | 0.55 (0.20, 0.83) | 0.0005* |
| P trend | <0.0001* | ||||
| Preterm (32–36 weeks) | 0.77 (0.63, 0.95) | 0.0129 | 0.55 (0.20, 0.75) | 0.0062* | |
| Preterm (EMT categorized into four groups) | |||||
| 6.00–8.90 mm (n = 378) | Ref | Ref | |||
| 9.00–9.90 mm (n = 444) | 0.66 (0.31, 1.41) | 0.2865 | 0.55 (0.13, 0.98) | 0.0451* | |
| 10.00–10.90 mm (n = 541) | 0.57 (0.26, 1.23) | 0.1512 | 0.42 (0.06, 0.87) | 0.0211* | |
| >11.00 mm (n = 647) | 0.26 (0.10, 0.65) | 0.0041 | 0.25 (0.04, 0.66) | 0.0034* | |
| Very preterm (<32 weeks) | 0.10 (0.01, 0.78) | 0.0287 | 0.37 (0.02, 1.44) | 0.6722 | |
| Birthweight (g) | 52.91 (27.77, 78.04) | <0.0001 | 19.11 (−2.48, 40.70) | 0.0832 | |
| Z-score | 0.03 (−0.03, 0.08) | 0.3333 | 0.03 (−0.03, 0.08) | 0.3750 | |
| LBW (<2500 g) | 0.60 (0.47, 0.77) | <0.0001 | 0.85 (0.55, 1.29) | 0.4411 | |
| Very LBW (<1500 g) | 0.19 (0.08, 0.47) | 0.0003 | 0.57 (0.01, 1.11) | 0.5997 | |
| Fetal macrosomia (≥4000 g) | 1.20 (0.97, 1.48) | 0.0982 | 1.22 (0.93, 1.61) | 0.1474 | |
| SGA (<10th percentile) | 0.86 (0.72, 1.03) | 0.1090 | 0.83 (0.67, 1.03) | 0.0922 | |
| Very SGA (<3rd percentile) | 0.76 (0.54, 1.09) | 0.1334 | 0.71 (0.44, 1.13) | 0.1434 | |
| LGA (>90th percentile) | 1.04 (0.86, 1.25) | 0.7138 | 1.14 (0.91, 1.42) | 0.2504 | |
| Very LGA (>97th percentile) | 0.93 (0.65, 1.33) | 0.6996 | 0.97 (0.63, 1.51) | 0.9101 | |
Analyses were adjusted for maternal age, paternal age, maternal BMI, basal FSH, anti-Müllerian hormone (AMH), type of infertility, prior gonadotropin cycle, ovarian stimulation protocols, dosage of gonadotropins, estrogen (E2) level on the hCG trigger day, number of oocytes retrieved, fertilization method, number and stage of embryos transferred, and gender of newborn; birthweight outcomes additionally adjusted for GA, preterm delivery (PTD), and very PTD.
LBW, low birthweight; LGA, large for gestational age; SGA, small for gestational age; OR, odds ratio; CI, confidence interval; Ref, reference group.
Statistically significant, with P < 0.05.
Furthermore, the incidence of PTD was significantly decreased with increasing EMT (adjusted odds ratio (OR): 0.55, 95% CI: 0.20 to 0.75; P = 0.0062). When the EMT was categorized into four groups, the odds of PTD were reduced by 45% with an EMT of 9.00–9.90 mm (adjusted OR: 0.55, 95% CI: 0.13 to 0.98; P = 0.0451), 58% with an EMT of 10.00–10.90 mm (adjusted OR: 0.42, 95% CI: 0.06 to 0.87; P = 0.0211) and 75% with an EMT >11.00 mm (adjusted OR: 0.25, 95% CI: 0.04 to 0.66; P = 0.0034) compared to the reference group (6.00–8.90 mm).
However, there was no significant difference in the rates of birthweight, LBW, very LBW, fetal macrosomia, very PTD, SGA, very SGA, LGA, or very LGA when the analyses were adjusted for confounding variables and birthweight outcomes were additionally adjusted for GA, PTD, and very PTD.
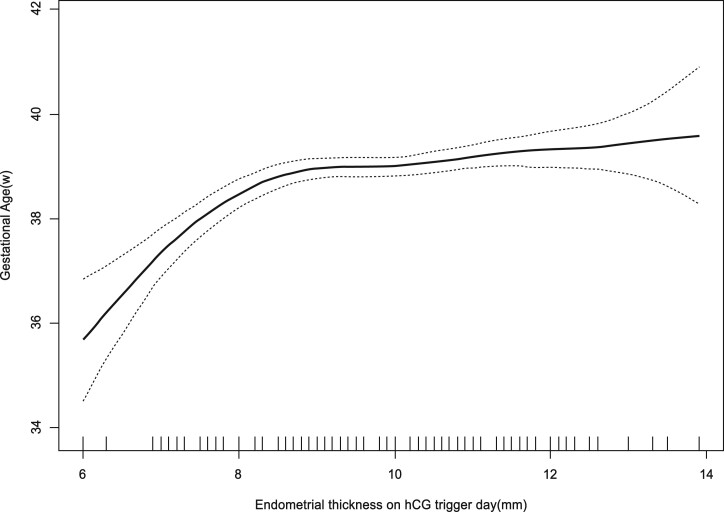
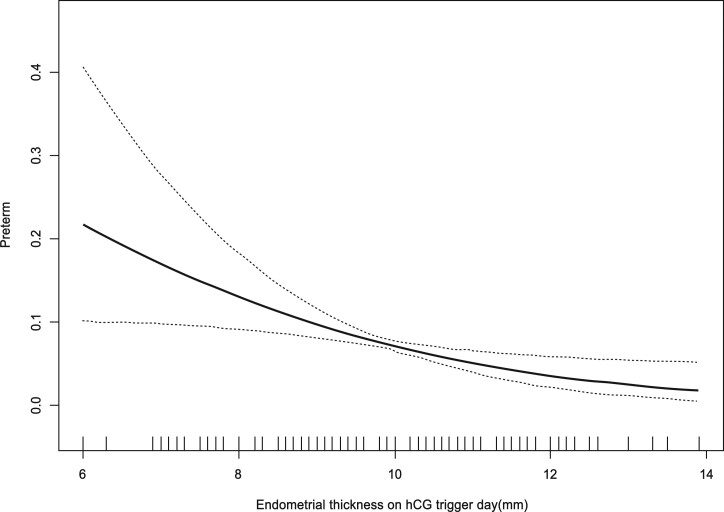
The curves in Figs 2 and 3 show the relationship between the EMT and the adjusted mean GA (weeks) and the adjusted PTD incidence, respectively. Combined with the threshold effect of the EMT (mm) on GA outcomes by using piecewise linear regression displayed in Table 3, the analysis indicated that when EMT was ≤7.8 mm, GA increased significantly with increasing EMT (adjusted β: 1.94, 95% CI: 1.26 to 2.63; P < 0.0001), and when EMT was ≤7.6 mm, the incidence of PTD decreased obviously with increasing EMT (adjusted OR: 0.47, 95% CI: 0.03 to 0.99; P = 0.0107). This may indicate that 7.8 mm is the inflection point where the EMT affects the outcome of GA.
Figure 2.
The relationship between endometrium thickness on the hCG trigger day and adjusted mean gestational age. Analyses were adjusted for maternal age, paternal age, maternal BMI, basal FSH, anti-Müllerian hormone (AMH), type of infertility, prior gonadotropin cycle, ovarian stimulation protocols, dosage of gonadotropins, estrogen (E2) level on the hCG trigger day, number of oocytes retrieved, fertilization method, number and stage of embryos transferred, and gender of newborn. GA: gestational age.
Figure 3.
The relationship between endometrium thickness on the hCG trigger day and adjusted preterm delivery incidence. Analyses were adjusted for maternal age, paternal age, maternal BMI, basal FSH, anti-Müllerian hormone (AMH), type of infertility, prior gonadotropin cycle, ovarian stimulation protocols, dosage of gonadotropins, estrogen (E2) level on the hCG trigger day, number of oocytes retrieved, fertilization method, number and stage of embryos transferred, and gender of newborn.
Table 3.
Threshold effects of endometrial thickness on gestational age and the incidence of preterm delivery using piece-wise linear regression.
| Characteristics | Crude β/OR | P-value | Adjust β/OR | P-value |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Gestational age (weeks) | ||||
| ≤7.8 mm | 1.56 (1.04, 1.97) | 0.0027 | 1.94 (1.26, 2.63) | <0.0001* |
| >7.8 mm | 0.11 (0.01, 0.21) | 0.0332 | 0.10 (−0.01, 0.20) | 0.0640 |
| Incidence of preterm delivery | ||||
| ≤7.6 mm | 0.63 (0.01, 0.99) | 0.0302 | 0.47 (0.03, 0.99) | 0.0107* |
| >7.6 mm | 0.85 (0.15, 3.37) | 0.2871 | 0.68 (0.32, 1.29) | 0.1589 |
Analyses were adjusted for maternal age, paternal age, maternal BMI, basal FSH, anti-Müllerian hormone (AMH), type of infertility, prior gonadotropin cycle, ovarian stimulation protocols, dosage of gonadotropins, estrogen (E2) level on the hCG trigger day, number of oocytes retrieved, fertilization method, number and stage of embryos transferred, and gender of newborn.
OR, odds ratio; CI, confidence interval.
Statistically significant, with P < 0.05.
The univariate linear analysis shown in Table 4 revealed that six factors significantly influenced GA (weeks), including maternal age (unadjusted β: −0.03, 95% CI: −0.04 to −0.01; P = 0.0085), paternal age (unadjusted β: −0.02, 95% CI: −0.03 to −0.00; P = 0.0323), maternal BMI (unadjusted β: −0.03, 95% CI: −0.05 to −0.00; P = 0.0240), prior gonadotropin cycles (unadjusted β: −0.24, 95% CI: −0.47 to −0.01; P = 0.0397), EMT (mm) (unadjusted β: 0.07, 95% CI: 0.02 to 0.12; P = 0.0067), and gender of the newborn (unadjusted β: 0.29, 95% CI: 0.01 to 0.56; P = 0.0391).
Table 4.
Univariate analysis of impact factors on gestational age (n = 2010).
| Exposure | Values |
Change in gestational age (weeks) |
|
|---|---|---|---|
| mean ± SD/n (%) | β (95% CI) | P-value | |
| Maternal age (year) | 30.92 ± 3.99 | −0.03 (−0.04, −0.01) | 0.0085* |
| Paternal age (year) | 32.45 ± 4.77 | −0.02 (−0.03, −0.00) | 0.0323* |
| Maternal BMI (kg/m2) | 22.30 ± 3.15 | −0.03 (−0.05, −0.00) | 0.0240* |
| Basal FSH (IU/l) | 7.64 ± 2.45 | 0.01 (−0.02, 0.04) | 0.6667 |
| AMH (ng/ml) | 2.85 ± 2.24 | 0.03 (−0.00, 0.06) | 0.0768 |
| Type of infertility | |||
| Primary | 1019 (50.70%) | Ref | |
| Secondary | 991 (49.30%) | 0.14 (−0.13, 0.41) | 0.3113 |
| Infertility duration (year) | 3.69 ± 2.71 | −0.04 (−0.09, 0.01) | 0.1149 |
| Infertility cause | |||
| Female | 1304 (64.88%) | Ref | |
| Male | 397 (19.75%) | −0.15 (−0.51, 0.21) | 0.4146 |
| Mixed | 189 (9.40%) | 0.23 (−0.30, 0.76) | 0.3891 |
| Unexplained | 120 (5.97%) | −0.25 (−0.78, 0.29) | 0.3663 |
| Prior gonadotropin cycle(s) | 1.22 ± 0.55 | −0.24 (−0.47, −0.01) | 0.0397* |
| Ovarian stimulation protocols | |||
| GnRH-a long protocol | 1275 (63.43%) | Ref | |
| Antagonist protocol | 735 (36.57%) | −0.14 (−0.30, 0.03) | 0.0982 |
| Dosage of gonadotropins (100 IU) | 22.89 ± 11.17 | −0.00 (−0.01, 0.00) | 0.4788 |
| Stimulation duration (days) | 11.57 ± 2.20 | 0.00 (−0.03, 0.04) | 0.7997 |
| E2 level on hCG day (100 pg/ml) | 29.98 ± 12.71 | 0.00 (−0.00, 0.01) | 0.3504 |
| Number of oocytes retrieved | 9.66 ± 3.83 | 0.01 (−0.03, 0.04) | 0.6245 |
| Fertilization method | |||
| IVF | 1331 (66.22%) | Ref | |
| ICSI | 580 (28.86%) | 0.12 (−0.19, 0.43) | 0.4520 |
| IVF + ICSI | 99 (4.93%) | −0.10 (−0.76, 0.56) | 0.7659 |
| Stage embryo transferred | |||
| D3 | 1673 (83.23%) | Ref | |
| D5 | 337 (16.77%) | 0.09 (−0.30, 0.49) | 0.6469 |
| Number of embryos transferred | |||
| 1 | 550 (27.36%) | Ref | |
| 2 | 1455 (72.39%) | −0.11 (−0.43, 0.20) | 0.4786 |
| 3 | 5 (0.25%) | 1.60 (−1.92, 5.12) | 0.3740 |
| Endometrial thickness (mm) | 9.97 ± 1.54 | 0.07 (0.02, 0.12) | 0.0067* |
| 6.00–8.90 mm (n = 378) | 378 (18.81%) | Ref | |
| 9.00–9.90 mm (n = 444) | 444 (22.09%) | 0.22 (−0.01, 0.46) | 0.0659 |
| 10.00–10.90 mm (n = 541) | 541 (26.92%) | 0.32 (0.09, 0.55) | 0.0055* |
| >11.00 mm (n = 647) | 647 (32.19%) | 0.34 (0.12, 0.56) | 0.0021* |
| Endometrial type | |||
| A | 30 (1.49%) | Ref | |
| A-B | 107 (5.32%) | 0.10 (−1.14, 1.34) | 0.8739 |
| B | 620 (30.85%) | −0.09 (−1.17, 0.98) | 0.8686 |
| B-C | 1109 (55.17%) | −0.28 (−1.34, 0.79) | 0.6075 |
| C | 144 (7.16%) | −1.15 (−2.33, 0.02) | 0.0555 |
| Gender of newborn | |||
| Male | 1022 (50.85%) | Ref | |
| Female | 988 (49.15%) | 0.29 (0.01, 0.56) | 0.0391* |
AMH: anti-Müllerian hormone; E2: estrogen; GNRH-a: GNRH agonist; Ref, reference group; SD, standard deviation; CI, confidence interval.
Statistically significant, with P < 0.05.
In order to adjust confounding factors as much as possible, we have performed propensity score analysis. Since the exposure indicator, EMT (mm) is a continuous variable, we used the outcome indicator PTD for propensity score matching (PSM). According to the size of samples, matching is carried out at a ratio of 1:10, allowing a difference range of 0.05 in PSM. We constructed a regression model after matching propensity scores. The results, presented in Supplementary Table S1, indicated that the incidence of PTD decreased significantly with increasing EMT (adjusted OR: 0.59, 95% CI: 0.35 to 0.87; P = 0.0052), which was consistent with the results in Table 2.
Discussion
The main objective of our current study, in which 2010 singleton births of non-PCOS infertile women under 42 years old were enrolled, was to evaluate the relationship between EMT on the hCG trigger day and neonatal outcomes after fresh ET. After adjusting for potential confounders, we found a strong negative correlation between EMT and the incidence of PTD, regardless of whether EMT was taken as a continuous or a categorical variable. In addition, the results of curve fitting and the threshold effect showed that the exact cutoff value was 7.8 mm. This may indicate that an EMT ≤7.8 mm is an independent factor for greater odds of PTD singletons born after fresh ET. To our knowledge, this is the first study in which the exact cutoff value of the impact of EMT on neonatal outcomes has been calculated through smooth curve fitting and threshold effect analysis using piecewise linear regression.
In recent years, increasing attention has been paid to the relationship between EMT and maternal and fetal safety, not just to pregnancy outcomes. Chung et al. (2006) first reported that suboptimal endometrial development with an EMT ≤10 mm was associated with a 2-fold increased risk of LBW compared with an EMT >12 mm in fresh IVF cycles. However, it is not clear whether this increased risk of LBW was adjusted for GA and PTD. The results of a recent study with a sample size of 5220 suggested that individuals with an EMT <8 mm had higher risks for both PTD (adjusted OR: 1.75, 95% CI: 1.30–2.34) and LBW (adjusted OR: 1.57, 95% CI: 1.09–2.26) than those with an EMT >8 mm (Hu et al., 2021). Nevertheless, the birthweight outcome was flawed without ruling out GA and pregnancy complications. In our study, we also found that the birthweight increased (3123.48 ± 491.04 vs 3201.79 ± 556.65 vs 3191.90 ± 446.38 vs 3276.62 ± 495.47, P = 0.025) with endometrial thickening. However, after adjusting for confounding factors, especially including GA and PTD, the birthweight gain was not significant (P > 0.05). This result indicated that the change in birthweight was closely related to gestational weeks, and there was no independent relationship between birthweight and EMT. Huang et al. (2020) demonstrated in IUI cycles, that EMT was also not independently associated with LBW. Previous studies have shown that a strong negative correlation between the incidence of PTD and EMT can also be seen in PCOS patients (Huang et al., 2021). However, to prevent any basis for adverse neonatal outcomes associated with abnormal glucose and lipid metabolism, we excluded patients with PCOS diagnosed via the Rotterdam criteria.
Different studies have generated different conclusions in terms of the cutoff value of EMT for the effect on neonatal outcomes. Chung et al. (2006) considered an EMT of 8 mm as the cutoff value (Hu et al., 2021; Huang et al., 2021), and some regarded the inflection point as 7.5 mm (He et al., 2022) or 10 mm (Chung et al., 2006). However, the cutoff values of EMT in these studies were almost based on empirical grouping. Our study is the first to use smooth curve fitting and threshold effect analysis to explore the exact inflection point. We found that when EMT was ≤7.8 mm, GA started to decline markedly with EMT thinning. This result indicated that the accurate cutoff value of the thin EMT that leads to an increase in the odds of PTD is 7.8 mm.
At present, the mechanism by which thin EMT affects neonatal outcomes is still being explored. Researchers in most previous studies have mentioned that this may be related to differences in oxygen tension. Early studies showed that after ovulation, the spiral artery contracts, resulting in a decrease in blood flow on the surface of the endometrium, thereby reducing the oxygen tension of the functional epithelium (Rossman and Bartelmez, 1957; Casper, 2011). This hypoxic stress in the villus space in the early pregnancy is the main prerequisite for normal embryogenesis and fetal development (Schoots et al., 2018). However, when the functional layer is thin or absent, the oxygen concentration in the blood vessels of the basal endometrium will be considerably increased. This high concentration of reactive oxygen species may create a harmful uterine environment for the implants and placenta, ultimately leading to adverse growth of the placenta and fetus (Ribeiro et al., 2018; Gupta et al., 2009).
Another mechanism may be related to defects in spiral artery remodeling. Deep placental formation involves trophoblasts invading the medial third of the myometrium of the uterus and completely transforming the spiral arteries in this area (Brosens et al., 2011). Low resistance of uteroplacental circulation is essential for the optimal development of the fetus (Brosens et al., 2002). A thin endometrium is characterized by high uterine artery blood flow impedance due to vascular dysplasia (Miwa et al., 2009). These abnormalities may change the remodeling of vascular spiral arteries and may affect the causes of abnormal placentation and adverse neonatal outcomes (Oron et al., 2018). Maternal complications are closely related to abnormal neonatal outcomes. Abnormal formation of the placenta and its blood vessels may cause placental diseases in the mother, such as placenta previa, cesarean section, and HDP. Jing et al. (2019) reported that after adjusting for confounders, they found that a thicker EMT was associated with a decreased risk of placenta previa (adjusted OR: 0.798, 95% CI: 0.651–0.979; P = 0.031) and a decreased risk of cesarean section (adjusted OR: 0.926, 95% CI: 0.889–0.965; P < 0.001). The results of a large sample study that involved 9266 women who had singleton livebirths after fresh IVF/ICSI-ET cycles confirmed that a thin endometrium (≤8 mm) was associated with an increased risk of HDP, indicating that the thin endometrium itself is a risk factor for HDP (Liu et al., 2021). The above-mentioned maternal complications may result in a decrease in the GA of newborns and increase in the incidence of PTD. In our study, women with maternal complications during pregnancy were excluded.
The main strength of this work is that this is the first study in which smooth curve fitting and threshold effect analysis, rather than just empirical grouping, have been used to explore the exact cutoff value of EMT. A second strength is that we made our best efforts to remove all confounding factors that could interfere with the results. We excluded patients with PCOS and patients with maternal complications during pregnancy, such as HDP, gestational diabetes, placenta previa, and placental abruption, which were likely to affect neonatal outcomes. In addition, to explore the independent effect of the EMT on neonatal outcomes, we also performed a regression analysis after PSM. The results of the PSM are consistent with the results of multivariable regression analysis in Table 2. This indicates that there is indeed a strong negative correlation between EMT and the incidence of PTD in fresh cycles.
However, the power of this study is limited owing to its retrospective design. A second limitation is that although we found a significant decrease in PTD as the EMT increased, in terms of GA, the magnitude of the differences was modest, possibly limiting the clinical relevance of the findings. In addition, it has still not been ruled out that the internal factors, such as previous medication, nutrition intake, and living habits, may cause bias in the neonatal outcomes.
Conclusion
In conclusion, in singletons born of non-PCOS patients after fresh ET, EMT on the hCG trigger day is negatively correlated with PTD. Our data also demonstrated that an EMT ≤7.8 mm was an independent predictor for greater odds of PTD after fresh ET. Therefore, we suggest that, to reduce the incidence of PTD, women with thin EMT after conception via fresh IVF-ET cycles should receive more attention from obstetricians and pediatricians.
Supplementary Material
Acknowledgements
The authors thank the nurses and laboratory staff of the Department of Assisted Reproduction for their contributions to this work. In addition, the authors thank the infertile couples who participated in this study.
Contributor Information
Jing Wu, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Jianlei Huang, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Jie Dong, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Xifeng Xiao, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Mao Li, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Xiaohong Wang, Department of Obstetrics and Gynecology, Reproductive Medicine Center, Tang Du Hospital, The Air Force Military Medical University, Xi’an, China.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Authors’ roles
X.W. and J.W. conceived and designed this study. J.W. and J.D. contributed to data acquisition and analysis and drafted the article. J.H., M.L., and X.X. were responsible for the collection of data. All authors interpreted the data.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 82071717).
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Brosens I, Pijnenborg R, Vercruysse L, Romero R.. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens JJ, Pijnenborg R, Brosens IA.. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies. a review of the literature. Am J Obstet Gynecol 2002;187:1416–1423. [DOI] [PubMed] [Google Scholar]
- Casper RF. It’s time to pay attention to the endometrium. Fertil Steril 2011;96:519–521. [DOI] [PubMed] [Google Scholar]
- Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, Freedman MF, Barnhart KT.. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril 2006;86:1634–1641. [DOI] [PubMed] [Google Scholar]
- Dai L, Deng C, Li Y, Zhu J, Mu Y, Deng Y, Mao M, Wang Y, Li Q, Ma S et al Birthweight reference percentiles for Chinese. PLoS One 2014;9:e104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunietz GL, Holzman C, McKane P, Li C, Boulet SL, Todem D, Kissin DM, Copeland G, Bernson D, Sappenfield WM. et al. Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil Steril 2015;103:974–979.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar M, Mehrjardi SZ, Molaei B, Taheri F, Mangoli E.. The correlation between endometrial thickness and pregnancy outcomes in fresh ART cycles with different age groups: a retrospective study. Middle East Fertil Soc J 2019;24:16–20. [Google Scholar]
- Gupta S, Malhotra N, Sharma D, Chandra A, Ashok A.. Oxidative stress and its role in female infertility and assisted reproduction: clinical implications. Int J Fertil Steril 2009;2:147–164. [Google Scholar]
- He T, Li M, Li W, Meng P, Xue X, Shi J.. Endometrial thickness is associated with low birthweight in frozen embryo transfer cycles: a retrospective cohort study of 8,235 singleton newborns. Front Endocrinol (Lausanne) 2022;13:929617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KL, Kawai A, Hunt S, Li W, Li X, Zhang R, Hu Y, Gao H, Zhu Y, Xing L. et al. Endometrial thickness in the prediction of neonatal adverse outcomes in frozen cycles for singleton pregnancies. Reprod Biomed Online 2021;43:553–560. [DOI] [PubMed] [Google Scholar]
- Huang J, Lin J, Lu X, Gao H, Song N, Cai R, Kuang Y.. Association between endometrial thickness and neonatal outcomes in intrauterine insemination cycles: a retrospective analysis of 1,016 live-born singletons. Reprod Biol Endocrinol 2020;18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lin J, Xia L, Tian L, Xu D, Liu P, Zhu J, Wu Q.. Decreased endometrial thickness is associated with higher risk of neonatal complications in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2021;12:766601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Li X, Zhang S, Gong F, Lu G, Lin G.. The risk of placenta previa and cesarean section associated with a thin endometrial thickness: a retrospective study of 5251 singleton births during frozen embryo transfer in China. Arch Gynecol Obstet 2019;300:1227–1237. [DOI] [PubMed] [Google Scholar]
- Liao Z, Liu C, Cai L, Shen L, Sui C, Zhang H, Qian K.. The effect of endometrial thickness on pregnancy, maternal, and perinatal outcomes of women in fresh cycles after IVF/ICSI: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:814648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N.. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod 2018;33:1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang J, Fu X, Li J, Zhang M, Yan J, Gao S, Ma J.. Thin endometrium is associated with the risk of hypertensive disorders of pregnancy in fresh IVF/ICSI embryo transfer cycles: a retrospective cohort study of 9,266 singleton births. Reprod Biol Endocrinol 2021;19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;1:35–58. [DOI] [PubMed] [Google Scholar]
- Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N.. Pathophysiologic features of “thin” endometrium. Fertil Steril 2009;91:998–1004. [DOI] [PubMed] [Google Scholar]
- Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, Land JA, Evers JL, Dumoulin JC.. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod 2012;27:1966–1976. [DOI] [PubMed] [Google Scholar]
- Oron G, Hiersch L, Rona S, Prag-Rosenberg R, Sapir O, Tuttnauer-Hamburger M, Shufaro Y, Fisch B, Ben-Haroush A.. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: a retrospective cohort study. Reprod Biomed Online 2018;37:341–348. [DOI] [PubMed] [Google Scholar]
- Pal L, Jindal S, Witt BR, Santoro N.. Less is more: increased gonadotropin use for ovarian stimulation adversely influences clinical pregnancy and live birth after in vitro fertilization. Fertil Steril 2008;89:1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z.. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J. Assist Reprod Genet 2015;32:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C.. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Ribeiro VC, Santos-Ribeiro S, De Munck N, Drakopoulos P, Polyzos NP, Schutyser V, Verheyen G, Tournaye H, Blockeel C.. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online 2018;36:416–426. [DOI] [PubMed] [Google Scholar]
- Rossman I, Bartelmez GW.. The injection of the blood vascular system of the uterus. Anat Rec 1957;128:223–231. [DOI] [PubMed] [Google Scholar]
- Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL.. Oxidative stress in placental pathology. Placenta 2018;69:153–161. [DOI] [PubMed] [Google Scholar]
- Shalom-Paz E, Atia N, Atzmon Y, Hallak M, Shrim A.. The effect of endometrial thickness and pattern on the success of frozen embryo transfer cycles and gestational age accuracy. Gynecol Endocrinol 2021;37:428–432. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, La Marca A, Seed PT, Khalaf Y.. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod 2015;6:1473–1480. [DOI] [PubMed] [Google Scholar]
- Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R.. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod 2012;27:2619–2626. [DOI] [PubMed] [Google Scholar]
- Weissman A, Gotlieb L, Casper RF.. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril 1999;71:147–149. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu D, Liu N, Li Y, Yao Z, Tian F, Xu A, Li Y.. An endometrial thickness < 8 mm was associated with a significantly increased risk of EP after freeze-thaw transfer: an analysis of 5,960 pregnancy cycles. Front Endocrinol 2022;13:884553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.