Abstract
Purpose
Hyperventilation is considered a major risk factor for hypoxic blackout during breath-hold diving, as it delays the apnea breaking point. However, little is known about how it affects oxygenation, the diving response, and spleen contraction during serial breath-holding.
Methods
18 volunteers with little or no experience in freediving performed two series of 5 apneas with cold facial immersion to maximal duration at 2-min intervals. In one series, apnea was preceded by normal breathing and in the other by 15 s of hyperventilation. End-tidal oxygen and end-tidal carbon dioxide were measured before and after every apnea, and peripheral oxygen saturation, heart rate, breathing movements, and skin blood flow were measured continuously. Spleen dimensions were measured every 15 s.
Results
Apnea duration was longer after hyperventilation (133 vs 111 s). Hyperventilation reduced pre-apnea end-tidal CO2 (17.4 vs 29.0 mmHg) and post-apnea end-tidal CO2 (38.5 vs 40.3 mmHg), and delayed onset of involuntary breathing movements (112 vs 89 s). End-tidal O2 after apnea was lower in the hyperventilation trial (83.4 vs 89.4 mmHg) and so was the peripheral oxygen saturation nadir after apnea (90.6 vs 93.6%). During hyperventilation, the nadir peripheral oxygen saturation was lower in the last apnea than in the first (94.0% vs 86.7%). There were no differences in diving response or spleen volume reduction between conditions or across series.
Conclusions
Serial apneas revealed a previously undescribed aspect of hyperventilation; a progressively increased desaturation across the series, not observed after normal breathing and could heighten the risk of a blackout.
Keywords: Hypoxia, Breath-hold diving, Blackout, Hypocapnia
Introduction
Hypoxic syncope or “blackout” (BO) is a potentially fatal condition associated with breath-hold diving. When fainting underwater, a diver must be immediately rescued by a fellow diver, or they will likely drown. In freediving competitions, this is resolved by the involvement of safety divers, but among recreational divers, such safety systems are often lacking. For example, between 2001 and 2013, 175 breath-hold diving-related deaths were reported in Australia of which 22 cases were considered to be associated with hypoxic BO (Lippmann 2019). The Divers Alert Network (DAN) collected information worldwide from 2004 to 2017, reporting 726 breath-hold diving-associated fatalities mainly in recreational snorkelers, despite not stating which deaths were due to BO. These cases surely are an underrepresentation of the real number of BO-related fatalities (DeWitt et al. 2019).
Single studies have reported a range of risk factors for BO, including extreme bradycardia (Joulia et al. 2013), arrhythmias (Wolf 1964), excess post-exercise oxygen consumption (Lindholm and Gennser 2005), and vascular collapse (Bouten et al. 2020). However, a more widely accepted risk factor is hyperventilation (Craig 1961, 1976; Edmonds and Walker 1999; Lippmann and Pearn 2012) as this breathing pattern reduces the arterial pressure of carbon dioxide (PaCO2). Lowered PaCO2 delays the physiological apnea breaking point when an urge to breathe arises, thus increasing apnea duration (Hill 1973; Lin et al. 1974; Bain et al. 2017). If breath-hold duration increases to the point that extreme hypoxia develops, BO may result.
As hypercapnia develops during apnea, and to some degree influenced by hypoxemia (Otis et al. 1984; Breskovic et al. 2012), the ventilatory drive is stimulated, giving rise to involuntary breathing movements (IBM). The start of IBM is considered a hallmark of the physiological breaking point of apnea (Fowler 1954; Agostoni 1963; Hill 1973; Lin et al. 1974). Divided by this point, a maximal effort apnea is characterized by two phases: the “easy phase” and the subsequent “struggle phase” (Dejours 1965). Hyperventilation thus increases the duration of the easy phase by overriding the physiological warning system which may potentially lead to severe hypoxia (Craig 1961; Lindholm and Gennser 2005; Kumar and Ng 2010; Lippmann and Pearn 2012). So far, it is unknown if the risk of BO associated with hyperventilation relies upon the delay of the apnea breaking point per se, or if it affects the depletion of oxygen stores (Sadler et al. 2020), e.g., by affecting the oxygen conserving cardiovascular diving response (Andersson et al. 2002) or the spleen contraction which enhances blood oxygen stores (Schagatay et al. 2001).
Recreational divers have a higher risk of death due to BO (Dunne et al. 2021). These divers do not perform single maximal dives—but typically make serial dives with short pauses. On expert freedivers, it was reported that hyperventilation before static apnea was not associated with an increased risk of BO even under extreme hypoxia (Lindholm and Lundgren 2006). Importantly, most of the studies of hyperventilation effects involve a single dive, and not serial freediving—the most common diving pattern in recreational divers. Professional freedivers like the Ama, who engage in breath-hold diving sessions of 4–5 h with serial dives, rarely report BO (Rahn et al. 1965). The Ama do not use hyperventilation before diving (Hong et al. 1963), while this is common in recreational divers (Craig 1976; Edmonds and Walker 1999; Lippmann and Pearn 2012).
There seems to be no information in the literature on the effects of hyperventilation during serial diving on the risk of BO. We, therefore, aimed to assess the development of hypoxia during a series of static apneas and determine whether hyperventilation before apnea affected oxygenation and apnea breaking points. We also aimed to reveal whether hyperventilation affects the diving response and the splenic contraction, mechanisms potentially protective against hypoxia.
Methods
Participants
The study included 18 adult participants (6 females and 12 males) with a mean ± SD age 30 ± 7 years, height 177 ± 8 cm, weight 76 ± 11 kg, and lung vital capacity of 5.4 ± 1.1 L. All participants were healthy and not training breath-hold diving; 15 were classified in class 2 indicating involvement in breath-hold diving during some period in life, but not during the last year, and 3 were in class 1, with no prior experience in breath-hold diving (Schagatay 1996). The participants received written and verbal information about the protocol after which they signed an informed consent. The protocol was approved by the regional human ethics board in Umeå and complied with the Helsinki Declaration of 2004.
Study design
The study evaluated a series of five simulated dives in two conditions: (1) normal breathing before apnea (NB) and (2) hyperventilation for 15 s before apnea (HV). All the participants were tested in the two conditions during a single visit to the laboratory (Fig. 1). The order of the conditions was weighted between the participants.
Fig. 1.
Apnea protocol used for normal breathing (NB) and hyperventilation (HV) series, which were done in weighted order. The green circle indicates 15 s of hyperventilation
Procedures
Participants arrived at the laboratory after 12 h without any alcohol consumption or strenuous exercise and 2 h without eating or drinking caffeine-containing beverages. Height and weight were measured. Slow vital capacity was measured in triplicate in standing conditions and the largest volume was used (Vitalograph Compact Expert® spirometer, Vitalograph, Buckingham, UK). The participants then rested for 15 min in the prone position while instrumented before the apnea series started. Between apneas, subjects rested prone with the head on a removable pillow covering the water container used for apneic face immersions, with arms on the sides of the container. The participants performed a series of five apneas, with face immersion in cold water (Schagatay and Andersson 1998) to voluntary maximal duration, with 2 min of recovery between apneas (Fig. 1). In the normal breathing condition (NB), the participants breathed normally before every apnea, while in the hyperventilation (HV) condition, they hyperventilated for 15 s before apnea. The participant was instructed in advance on how to perform the hyperventilation based on a previous protocol (de Bruijn et al. 2008). A researcher made a 2-min countdown before starting. At 30 s before apnea, a nose clip was applied, and 20 s before apnea, a mouthpiece was offered to breathe through. Ten seconds before the apnea, the countdown continued second by second.
Participants were instructed to exhale completely and make a large but not maximal inhalation before starting the apnea on volition, a technique that results in a volume of around 85% of the vital capacity (Schagatay and Holm 1996; Fig. 2). An experimenter closely monitored SpO2 and was ready to interrupt the apnea if it fell below 65%. When recordings of the first condition had ended the participants had a 10-min rest, before baseline recordings for the second condition started, thus the total time between the last apnea in one condition and the first of the other condition was 20 min (Fig. 1). The water temperature for face immersion was 15 ± 1 °C and the room temperature was 23 ± 1 °C. The barometric pressure (PB) was 762 ± 6 mmHg (102 ± 0.8 kPa).
Fig. 2.
Recordings from the chest bellows show breathing movements in the same participant before the fifth apnea during hyperventilation (top panel) and normal ventilation (bottom panel). The grey areas indicate apneas. AU, arbitrary units
Measurements
Continuous variables were recorded from 5 min before apneas until 5 min after the series. Peripheral arterial hemoglobin oxygen saturation (SpO2) and heart rate (HR) were measured on the second finger or third finger of the right hand via a pulse oximeter (3900, Datex-Ohmeda, Louisville, USA). Respiratory movements were measured via a lab-constructed pneumatic chest bellows connected to an amplifier and an analog recorder (Fig. 2). Skin blood flow (SkBF) was measured via laser-Doppler flowmeter (Periflux 5000, Perimed, Järfälla, Sweden) at the distal phalanx of the right thumb, where the probe was taped after confirming consistent readings. These measurements were recorded simultaneously with a time marker with a data acquisition system (MP160, Biopac Systems, Inc., Goleta, USA).
The fraction of exhaled gases was measured before and after every apnea. Exhaled end-tidal carbon dioxide (ETCO2) was measured via an infrared-based gas measurement module (CO2100C, Biopac Systems, Inc., Goleta, USA), and exhaled end-tidal oxygen (ETO2) was measured via a paramagnetic-based gas measurement module (O2100C, Biopac Systems, Inc., Goleta, USA) through a mouthpiece connected to a T-valve with 2 one-way valves (AFT21, Biopac Systems, Goleta, USA) that have a dead space of 80 mL, and a disposable bacterial filter. The partial pressures of the exhaled gases (PETCO2 and PETO2) were obtained by multiplying the fraction of the respective gases by the dry PB (PB—saturated water vapor pressure).
Spleen length and thickness were measured on frozen images taken every 15 s from the dorsal side via ultrasonic imaging (MyLab 25 gold equipped with a CA621 probe, Esaote, Genoa, Italy).
Data analysis
Baseline values used for SpO2, HR, and SkBF were averages for the 60–30 s before each apnea to minimize effects from the previous apnea and by the anticipation of the next apnea. SpO2 nadir was defined as the lowest SpO2 recorded after every apnea.
To assess the initial tachycardia during apnea, we calculated the average heart rate during the first 30 s of apnea (HRpeak). The “mean HR” during apnea, used to assess the bradycardia, excluded those first 30 s.
SkBF during apnea was defined as the mean value during the whole apnea duration. As the measurements are in perfusion units, percent change during apnea from baseline was used.
The onset of IBM was obtained by visual inspection of the respiratory movements curve and was defined as the first negative deflection after a flat line, which was followed by rhythmical negative deflections.
Spleen sectional area was calculated using length and thickness (Koga and Morlkawa 1975): S = 0.8 × a × b, where S is the sectional area, a is the length, and b is the thickness. Spleen volume was calculated as a function of the sectional area (Koga 1979): V = (7.53×S) − 77.56 Splenic volume baseline was defined as the average of the 5 min before the first apnea. Apnea values were defined as initial (first recorded value during apnea), middle (average of the measurements during apnea and final (last recorded value before resuming breathing).
To compare the SpO2 between conditions with different duration breath holds, the value at the end of apnea in NB was compared with the HV apnea at the same duration.
Statistical analysis
One outlier was detected in apnea duration, SpO2, and PETO2 during normal breathing, which had a studentized residual value of 3.36. It was considered a genuine value and the results of the analysis of variance (ANOVA) did not change without the outlier, so the participant was kept in the analysis.
The data are reported as mean ± standard deviation (SD). The data were tested for normality using Shapiro–Wilk’s test. A two-factor [time (apnea 1 to apnea 5) vs. group (normal breathing and hyperventilation)] repeated-measures ANOVA was used to compare apnea duration, PETO2, PETCO2, SpO2, HR, IBM-onset, and splenic contraction. When a two-way interaction was found, a one-way ANOVA was run to evaluate the simple main effects of conditions and apnea. A Bonferroni correction was applied for multiple comparisons. Effect sizes were estimated by the partial eta squared () and the generalized eta squared () and are presented with a 90% confidence interval (CI). An effect size of 0.01–0.05 was considered small, from 0.06 to 0.13 was considered medium and from and above 0.14 was considered large (Cohen 1988; Bakeman 2005; Lakens 2013). Significance was accepted at p < 0.05.
End-tidal gas variables (PETO2 and PETCO2) were lost for five participants due to dysfunction of the gas measurement modules; therefore, that analysis is made on 13 participants. Spleen measurements were collected in all participants, but due to technical failure of the ultrasonic machine, part of the recorded data was lost, and the complete data include 11 participants. The number of participants included is indicated in the results.
Results
All participants completed the apnea protocol successfully. The studied variables changed equally between sexes in response to apnea and hyperventilation, and therefore, men and women were analyzed as one single group.
Apnea duration
Mean (SD) apnea duration was longer in HV, at 133 ± 6 s, compared with 111 ± 12 s in NB (p < 0.001, = 0.61, 90% CI [0.30–0.73], = 0.05; Table 1, Fig. 3). There was an increase in duration across apnea A1–A5 in both conditions (p < 0.001, = 0.70, 90% CI [0.50–0.78], = 0.06; Table 1, Fig. 3).
Table 1.
Apnea duration between conditions and within apnea series
| Normal breathing (s) | Hyperventilation (s) | Time difference (s) | Time increase (%) | |
|---|---|---|---|---|
| A1 | 93 ± 48 | 111 ± 42 | 18 | 19 |
| A2 | 107 ± 51† | 127 ± 49 †* | 20 | 19 |
| A3 | 115 ± 52† | 134 ± 52†* | 19 | 16 |
| A4 | 118 ± 53† | 142 ± 58†* | 24 | 20 |
| A5 | 123 ± 55† | 152 ± 60†* | 29 | 24 |
| Mean | 111 ± 12 | 133 ± 6* | 22 | 20 |
Mean (SD) apnea duration values in seconds for A1–A5. Mean, an average of the five apneas in the same condition
*Significantly different from NB; †significantly different from A1 in the same condition. (n = 18)
Fig. 3.
Mean apnea duration for apnea 1–5 (A1–A5), with SD bars, for the two conditions: normal breathing (NB) and hyperventilation (HV). *p < 0.05, **p < 0.01 and ***p < 0.001 compared with A1 in the same condition (n = 18)
End-tidal carbon dioxide
At the start of each apnea in HV, the mean PETCO2 was 17.4 ± 3.4 mmHg (2.3 ± 0.5 kPa) which was lower than the 29.0 ± 4.0 mmHg (3.9 ± 0.5 kPa) in NB (p < 0.001, = 0.93, 90% CI [0.86–0.96], = 0.76; Fig. 4a, Fig. 6). In both conditions, PETCO2 at the start was lower in the last apnea compared with the first (p = 0.001, = 0.53, 90% CI [0.32–0.61], = 0.11; Fig. 4a).
Fig. 4.
End-tidal carbon dioxide pressures (PETCO2) at a the start of each apnea 1–5 and b at the end of apnea 1–5 in the two breathing conditions. End-tidal oxygen pressures (PETO2) at c the start of each apnea 1–5 and d at the end of apnea 1–5 in the two conditions. *p < 0.05, **p < 0.01, and ***p < 0.001 between conditions; †p < 0.05, ††p < 0.01, and †††p < 0.001 compared with A1 in the same condition (n = 13)
Fig. 6.
O2–CO2 diagram showing individual alveolar values during NB (a) and HV (b). Circles represent values at the start of apnea. Triangles represent values at the end of apnea. Filled symbols indicate NB (a) and empty symbols indicate HV (b). Colors show apnea series. Horizontal dashed lines show the range of normal PACO2. Black continuous lines represent the conventional apnea breaking point (Agostoni 1963) (n = 13)
At the end of apneas in HV, the mean PETCO2 was lower in HV with 38.5 ± 5.2 mmHg (5.1 ± 0.7 kPa), compared to after NB with 40.3 ± 5.5 mmHg (5.4 ± 0.7 kPa, p = 0.005, = 0.49, 90% CI [0.12–0.67], = 0.03; Fig. 4b, Fig. 6). PETCO2 at the end of apnea was similar across both series (p = 0.272, = 0.10, 90% CI [0.00–0.25], = 0.006; Fig. 4b).
Involuntary breathing movements
Of the 18 participants, 7 (39%) had IBM in A1–A5 in both conditions, allowing comparison of easy phase duration. The start of the struggle phase was delayed in HV, at a mean of 112 ± 49 s, compared with 89 ± 42 s in NB (p = 0.003, = 0.79, 90% CI [0.32–0.88], = 0.08; Fig. 5). There was no difference across the series in any condition (p = 0.446, = 0.34, 90% CI [0.02–0.46], = 0.04; Fig. 5).
Fig. 5.
Duration of the easy (a) and struggle (b) phases for apneas 1–5 (A1–A5) after normal breathing (NB) and hyperventilation (HV) for the seven subjects with involuntary breathing movements (IBM) in all apneas. ***p < 0.001, **p < 0.01, and *p < 0.05 between conditions
End-tidal oxygen
At the start of each apnea, PETO2 was higher in HV with 137.2 ± 7.4 mmHg (18.0 ± 1.0 kPa) compared with 121.6 ± 11.1 mmHg (16.2 ± 1.5 kPa) in NB (p < 0.001, = 0.917, 90% CI [0.80–0.94], = 0.43; Fig. 4c, Fig. 6). There was no difference across A1–A5 in any condition (p = 0.094, = 0.25, 90% CI [0.05–0.35], = 0.009; Fig. 4c).
At the end of each apnea, PETO2 was lower in HV with a mean of 83.4 ± 17.3 mmHg (11.1 ± 2.3 kPa), compared with 89.4 ± 15.6 mmHg (11.9 ± 2.1 kPa) in NB (p = 0.005, = 0.498, 90% CI [0.12–0.68], = 0.04; Fig. 4d, Fig. 6). The PETO2 at the end of apnea was also decreasing across the series in HV, being higher in A1 than in A5 (p < 0.001, = 0.426, 90% CI [0.16–0.55], = 0.03; Fig. 4d).
Peripheral oxygen saturation
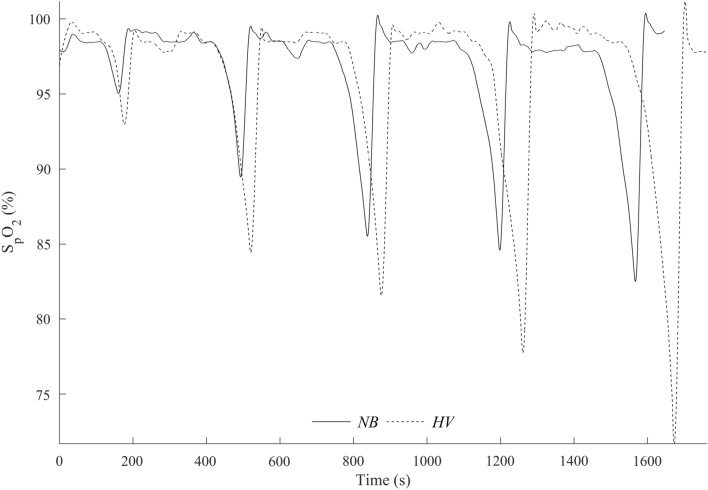
Figure 7 shows a representative recording of SpO2 across the NB and HV series from one subject, where the HV series results in progressive desaturation.
Fig. 7.
SpO2 recording in one participant during series of five dives after normal breathing (NB) and hyperventilation (HV)
Mean baseline SpO2 was similar between conditions and apnea series (p = 0.421, = 0.04, 90% CI [0.00–0.24], = 0.01; Fig. 8a). SpO2 nadir did not change across the apnea series in NB, (p = 0.576, = 0.03, 90% CI [0.00–0.14], = 0.10, Fig. 8b, Fig. 9a), but it decreased during the apnea series in HV (Fig. 8b, Fig. 9b) with the lowest value in A5 (p < 0.001, = 0.48, 90% CI [0.25–0.61], = 0.17, Fig. 9b). Mean SpO2 nadir was lower in HV with 90.6 ± 5.6%, compared with NB with 93.6 ± 4.9% (p < 0.001, = 0.644, 90% CI [0.35–0.76], = 0.10; Fig. 8b). In 4 participants, the SpO2 nadir resulting from the fifth apnea during HV was ≤ 75%. There was no difference between NB and HV when comparing the SpO2 values at the same time point as the average in NB at the end of apnea was 96.4 ± 3.5% and in HV was 96.0 ± 3.5% (p = 0.25, = 0.08, 90% CI [0.00–0.23], = 0.003).
Fig. 8.
Mean baseline pre-apnea SpO2 from A1–A5 (a) and at post-apnea nadir (b). *p < 0.05, **p < 0.01, ***p < 0.001 compared with A1 in the same condition; †p < 0.05, ††p < 0.01, †††p < 0.001 compared with HV (n = 18)
Fig. 9.
Mean (SE) SpO2 in NB (a) and HV (b) series during A1–A5. The grey boxes indicate apnea time. Negative time values before apnea time indicate breathing just before apneas. Positive values after apnea time indicate recovery time directly after apneas and partly overlap with pre-apnea values of the subsequent apnea. The gap in the middle of apnea time has variable duration, as apnea duration was different between participants. *p < 0.05, **p < 0.01, ***p < 0.001 indicates significance compared with A1 in the same condition; †††p < 0.001 indicates significance compared with HV (n = 18)
Diving response
The HR at baseline was similar in both conditions, with a mean of 68 ± 12 bpm in NB and 68 ± 9 bpm in HV (p = 0.923, = 0.001, 90% CI [0.00–0.04], = 0.0001; Fig. 10a). Recovery HR between apneas was also similar within series (p = 0.795, = 0.02, 90% CI [0.00–0.15], = 0.004; Fig. 10a).
Fig. 10.
Heart rate average for baseline (a), HRpeak (b), and during apnea (c). ***p < 0.001 between normal (NB) and hyperventilation (HV) conditions; †††p < 0.001, ††p < 0.01, and †p < 0.05 compared with A1 in the same condition
Mean HRpeak after apnea onset was higher in HV with 80 ± 15 bpm compared with 66 ± 13 bpm in NB (p < 0.001, = 0.76, 90% CI [0.54–0.84], = 0.23; Figs. 10b, 11b). During the NB series, HRpeak fell from 71 ± 14 bpm in A1 to 62 ± 11 bpm in A5 (p < 0.001, = 0.39, 90% CI [0.21–0.48], = 0.07; Figs. 10b, 11a). Across the HV series, HRpeak fell from A1, at 87 ± 17 bpm, to A5 at 75 ± 13 bpm, (p < 0.001, = 0.36, 90% CI [0.18–0.45], = 0.07; Figs. 10b, 11b).
Fig. 11.
Mean (SE) heart rate during apnea 1–5 at Normal (a) and HV (b). The grey boxes indicate apnea time. Negative time values before apnea time indicate breathing. Positive values after apnea time indicate recovery time and overlap with the previous values of the subsequent apnea. The gap in the middle of apnea time has variable duration, as apnea time was different between participants (n = 18)
The mean HR during apnea in NB was 54 ± 7 bpm and in HV was 55 ± 8 bpm, hence no differences (p = 0.205, = 0.09, 90% CI [0.00–0.32], = 0.01; Fig. 10c). Neither were there any differences in mean HR during apnea across the series (p = 0.232, = 0.08, 90% CI [0.00–0.19], = 0.01; Fig. 10c). The HR reduction in percent from baseline was also the same at 19% in NB and 18% in HV (p = 0.694, = 0.09, 90% CI [0.00–0.17], = 0.003).
There was no difference in the SkBF reduction between NB with − 10.0 ± 17.3% and HV with − 8.1 ± 18.3% (p = 0.10, = 0.10, 90% CI [0.00–0.33], = 0.01; Fig. 12a). Neither were any differences observed between series (p = 0.716, = 0.02, 90% CI [0.00–0.10], = 0.01; Fig. 12a).
Fig. 12.
SkBF reduction in percentage from baseline in both conditions from apnea 1 to apnea 5 (a) and recordings from one participant in the fifth apnea comparing both conditions (b). PU, perfusion units. (n = 18)
Spleen contraction
Mean (SD) baseline spleen volume before the apnea series was similar at 197 ± 54 mL in NB and 194 ± 56 mL in HV (p = 0.276; Fig. 13). The spleen contracted during all apneas in both NB and HV series and increased in size during breathing intervals, although not reaching baseline values. The average volume during recovery after every apnea was still 11% lower than baseline in NB, and 8% lower in HV, from A2 to A5 (p = 0.012; Fig. 13).
Fig. 13.
Mean spleen volume during the NB and HV apnea series. The grey boxes indicate apneas. Negative values before A1 indicate baseline measurements 5 min before starting. Positive values after every apnea indicate recovery time in minutes. The brackets indicate that an average period was compared. ***p < 0.001 compared with baseline; ###p < 0.001 compared with apnea values
There were no differences in mean splenic volume reductions during apnea between conditions or within series. During apneas, mean spleen volume was 131 ± 40 mL in NB and 126 ± 43 mL in HV series (p = 0.348), reflecting reductions from baseline by 33% and 35%, respectively (p < 0.001; Fig. 13). The mean spleen volume during the 2 min of recovery was similar at 176 ± 46 mL in NB and 179 ± 57 mL in HV series, and after 5 min recovery spleen volume was restored to normal in both series (Fig. 13).
Discussion
Hyperventilation compared to normal ventilation delayed the apnea breaking point and augmented the decrease in PETO2 and SpO2, which confirms conclusions from earlier studies that hyperventilation increases the risk of severe hypoxia (Craig 1961, 1976; Lanphier and Rahn 1963; Hill 1973; Lippmann 2019). A novel finding was that the serial apneas with hyperventilation lead to a progressively increased desaturation across the series, which resulted in more pronounced hypoxia in the fifth apnea, involving the development of severe hypoxia in several subjects. This finding could help explain why recreational divers are especially prone to BO, as they typically make a series of dives with short pauses, and slight hyperventilation is common in this group (Craig 1976; Edmonds and Walker 1999; Lippmann and Pearn 2012). They risk to, without notice, passing into the anaerobic phase of the dive, leading to the progressive development of oxygen debt.
This finding could be of great importance during serial diving as an increased oxygen debt would avert a sustainable diving session. This could reflect an inability of recreational divers to pace their diving efficiently. As a comparison, professional Ama divers can do more than 100 dives per dive session with pauses of similar duration as dives, apparently avoiding in a natural way reaching the aerobic dive limit (ADL), where anaerobic metabolism is required, and hence an oxygen debt develops (Hong et al. 1963, 1991; Radermacher et al. 1992; Mohri et al. 1995; Schagatay et al. 2011).
The increased desaturation in the HV did not happen during NB and apnea duration could explain this difference. NB apneas were shorter than HV apneas, but the apnea duration increased across the series in both conditions. The oxygen–hemoglobin dissociation curve does not change dramatically at the beginning of apnea, and therefore, shorter apneas do not lead to severe hypoxemia, while longer apneas are related to much greater hypoxemia per unit of time as the changes in SpO2 occur in the steep part of the curve. In HV, the extra work induced by spontaneous hyperventilation could contribute to an increased oxygen cost as the work of breathing (Wbreathing) is higher than increased ventilation due to hypercapnia (Otis 1954).
In HV, we also found an increase in HR in the first 30 s of apnea. This finding had been described before after spontaneous hyperventilation, especially when tidal volume is increased, via reduced parasympathetic activity, and possibly, by the pumping effect of the large swings on pleural pressure that increase cardiac preload (Cummin et al. 1986). Additionally, we speculate on another mechanism that links the increased HR at the beginning of apnea with hypoxemia. As spontaneous hyperventilation increases Wbreathing (Cummin et al. 1986; Coast and Krause 1993), it could increase oxygen consumption (). As minute ventilation was not measured, it is difficult to calculate the increased Wbreathing. If the apnea is started with a high , the oxygen stores would decrease faster. In a series of apneas, this could induce mixed venous desaturation that persists even after resuming breathing (Sands et al. 2010) and the following apnea could start with an oxygen debt. Despite starting with higher PETO2 values in HV, values were lower at the end of apnea compared to NB, but with no changes during the series of apneas. This finding could be explained by the measurement place, as it reflects changes in the alveolar pressure of oxygen (PAO2), while SpO2 is affected by local metabolism (Ellsworth et al. 2009), and as the PAO2 pathway during apnea is curvilinear, shorter apneas relate to higher values (Taboni et al. 2020). Regardless of the increase in PAO2 during hyperventilation and the effect it has on PaO2, the SpO2 was lower during HV and was of the same value when comparing same duration apneas. This emphasizes the lack of benefit of hyperventilation and could reflect the cost of using accessory expiratory muscles.
Apnea breaking points
Our model with 15 s of hyperventilation reached an average PETCO2 value of 17 mmHg, which is similar to other models that used 30 s (Landsberg 1974), 2 min (Craig 1961), and spontaneous duration of hyperventilation (Otis et al. 1984; Lindholm and Lundgren 2006). This finding shows that even short-period hyperventilation can induce significant hypocapnia and subsequent prolongation of apnea with the risk of developing severe hypoxia. As PETCO2 was lower at the end of hyperventilation apneas, it is evident that hypoxia contributes to triggering the respiratory drive.
The fact that our participants had limited experience in breath-hold diving could explain why IBM occurred only in seven participants, as many may have stopped at the first urge to breathe (Schagatay et al. 2000). Indeed, the longer apnea duration in HV was associated with an increase in the easy phase duration, clearly showing the effect of hyperventilation on the physiological breaking point. While many participants had a dive duration within the easy phase, the participants that surpassed this point are apparently at greater risk for developing dangerous hypoxia. In competition divers, increased sensitivity to hypoxia per se could be protective (Andersson and Schagatay 2009). The relative importance of hypercapnia and hypoxia, respectively, to the development of an urge to breathe should be further studied in the novice compared to trained divers for a better understanding of risk.
We did not find any difference in the diving response (bradycardia and vasoconstriction) between the breathing conditions or across the series of apneas. The HRpeak was lower in the last apneas in both conditions, which is likely related to more relaxation toward the end of the series. The reduction in SkBF displayed a great variation among participants, showing that the sympathetic response to apnea is highly individual (Busch et al. 2019), but there were no systematic differences between breathing conditions or across the series. As none of these main factors comprising the diving response were affected by hyperventilation, it can be concluded that the protective mechanism of the diving response is not enhanced to counteract the lower oxygen delivery during longer apneas.
Another studied protective response during apnea was spleen contraction, which elevates the circulating amount of erythrocytes resulting in enhanced oxygen stores, and has previously been associated with longer apneas (Schagatay et al. 2001, 2005, 2012; Baković et al. 2003). The spleen volume reduction during apnea in the present study was significant in both conditions, and the spleen did not return to baseline volume between the series of apneas. However, we did not find any differences in spleen volumes between normal breathing and hyperventilation or across the series of apneas, showing that neither did this protective mechanism counteract the enhanced desaturation with prolonged apneic duration. In most previous studies, spleen volumes were only measured between apneas (Hurford et al. 1990; Espersen et al. 2002; Baković et al. 2003; Schagatay et al. 2005, 2012; Prommer et al. 2007; Richardson et al. 2012; Elia et al. 2021) or during short-duration apneas (Palada et al. 2007), while we measured it during longer apneas. It is further interesting to note that this response, known to be initiated by hypoxia but enhanced by hypercapnia (Richardson et al. 2012), was similar in both conditions, and we suggest that this could be due to a balancing out between series by enhanced desaturation and reduced hypercapnia in HV.
Our study shows that even a short period of hyperventilation delays the physiological breaking point, prolongs apnea duration, and increases desaturation versus normal breathing. This effect increases progressively across the hyperventilation series, and a long series of dives could potentially lead to SpO2 levels associated with hypoxic BO. There is no change in the hypoxia-protective cardiovascular diving response or spleen contraction to compensate for this effect. The risk with hyperventilation appears multifactorial and includes a greater drop in SpO2 due to prolonged apnea duration, delayed apnea breaking point, and no changes in protective mechanisms. If the serial effect with progressively increased desaturation seen during hyperventilation could be due to incomplete central venous oxygenation between long dives despite normalized SpO2, requires further investigation.
As the experiment was conducted during static apnea, we avoided the increased metabolic demands from exercise associated with swimming dives, and thus, our conclusions may be limited to static apnea. Further studies are needed during repeated swimming dives to reveal if this imposes the same risk of BO, although we speculate that the enhanced metabolic rate with exercise would aggravate the situation, as oxygen depletion would be faster.
Conclusion
Serial dives revealed a previously undescribed aspect of hyperventilation; a progressively increased desaturation across the series, which was not observed after normal breathing. The fifth dive with hyperventilation resulted in desaturation below 75% in four subjects. This observation could help explain why hyperventilation increases the risk of BO, particularly in recreational divers, who typically perform serial dives.
The risk with hyperventilation appears multifactorial and includes a greater drop in SpO2 across a series of apneas. A monitoring system of SpO2 in freedivers could be developed to alert divers when serial dives lead to an increased desaturation and give a signal that a longer resting pause should be taken.
Acknowledgements
We are grateful to all the participants for their time and commitment to conducting the study.
Abbreviations
Generalized eta squared
Partial eta squared
- ADL
Aerobic dive limit
- ANOVA
Analysis of variance
- BO
Blackout
- CI
Confidence interval
- DAN
Divers alert network
- PETCO2
End-tidal carbon dioxide pressure
- PETO2
End-tidal oxygen pressure
- HR
Heart rate
- HV
Hyperventilation
- IBM
Involuntary breathing movements
- kPa
Kilopascal
- mL
Milliliters
- NB
Normal breathing
- PaCO2
Arterial pressure of carbon dioxide
- PACO2
Alveolar pressure of carbon dioxide
- PAO2
Alveolar pressure of oxygen
- PB
Barometric pressure
- SD
Standard deviation
- SE
Standard error
- SkBF
Skin blood flow
- SpO2
Peripheral oxygen saturation
- VO2
Oxygen consumption
- Wbreathing
Work of breathing
Author contributions
FP and ES: study design and conceptualization. FP, PH, EM, and ES: data collection. FP, PB, PH, and ES: data analysis. FP: writing. FP, PB, PH, EM, and ES: revision.
Funding
Funding was obtained through a donation from the Francis family in memory of their son/brother, who drowned from hypoxic blackout while snorkeling and holding his breath to dive underwater, and a grant from the Swedish Research Council for Sport Science (CIF). Open access funding provided by Mid Sweden University.
Data Availability
Data will be made available upon reasonable request.
Declarations
Conflict of interest
The authors of this manuscript report no conflict of interest. This manuscript has been approved by all the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agostoni E. Diaphragm activity during breath holding: factors related to its onset. J Appl Physiol. 1963;18:30–36. doi: 10.1152/jappl.1963.18.1.30. [DOI] [PubMed] [Google Scholar]
- Andersson J, Schagatay E. Repeated apneas do not affect the hypercapnic ventilatory response in the short term. Eur J Appl Physiol. 2009;105:569–574. doi: 10.1007/s00421-008-0936-y. [DOI] [PubMed] [Google Scholar]
- Andersson JPA, Linér MH, Rünow E, Schagatay E. Diving response and arterial oxygen saturation during apnea and exercise in breath-hold divers. J Appl Physiol. 2002;93:882–886. doi: 10.1152/japplphysiol.00863.2001. [DOI] [PubMed] [Google Scholar]
- Bain AR, Ainslie PN, Barak OF, et al. Hypercapnia is essential to reduce the cerebral oxidative metabolism during extreme apnea in humans. J Cereb Blood Flow Metab. 2017;37:3231–3242. doi: 10.1177/0271678X16686093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37:379–384. doi: 10.3758/BF03192707. [DOI] [PubMed] [Google Scholar]
- Baković D, Valic Z, Eterović D, et al. Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol. 2003;95:1460–1466. doi: 10.1152/japplphysiol.00221.2003. [DOI] [PubMed] [Google Scholar]
- Bouten J, Bourgois JG, Boone J. Hold your breath: peripheral and cerebral oxygenation during dry static apnea. Eur J Appl Physiol. 2020;120:2213–2222. doi: 10.1007/s00421-020-04445-y. [DOI] [PubMed] [Google Scholar]
- Breskovic T, Lojpur M, Maslov PZ, et al. The influence of varying inspired fractions of O2 and CO2 on the development of involuntary breathing movements during maximal apnoea. Respir Physiol Neurobiol. 2012;181:228–233. doi: 10.1016/j.resp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Busch SA, Bruce CD, Skow RJ, et al. Mechanisms of sympathetic regulation during Apnea. Physiol Rep. 2019;7:e13991. doi: 10.14814/phy2.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast JR, Krause KM. Relationship of oxygen consumption and cardiac output to work of breathing. Med Sci Sports Exerc. 1993;25:335–340. doi: 10.1249/00005768-199303000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- Craig ABJ. Causes of loss of consciousness during underwater swimming. J Appl Physiol. 1961;16:583–586. doi: 10.1152/jappl.1961.16.4.583. [DOI] [PubMed] [Google Scholar]
- Craig ABJ. Summary of 58 cases of loss of consciousness during underwater swimming and diving. Med Sci Sports. 1976;8:171–175. doi: 10.1249/00005768-197600830-00007. [DOI] [PubMed] [Google Scholar]
- Cummin AR, Iyawe VI, Mehta N, Saunders KB. Ventilation and cardiac output during the onset of exercise, and during voluntary hyperventilation, in humans. J Physiol. 1986;370:567–583. doi: 10.1113/jphysiol.1986.sp015951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn R, Richardson M, Schagatay E. Increased erythropoietin concentration after repeated apneas in humans. Eur J Appl Physiol. 2008;102:609–613. doi: 10.1007/s00421-007-0639-9. [DOI] [PubMed] [Google Scholar]
- Dejours P. Hazards of hypoxia during diving. In: Rahn H, Yokoyama T, editors. Physiology of breath-hold diving and the Ama of Japan. Tokyo: National Academy of Sciences; 1965. pp. 183–193. [Google Scholar]
- DeWitt H, Moore A, Tillmans F (2019) Breath-hold diving. In: Denoble PJ (ed) DAN 2019 annual diving report. Divers Alert Network, Durham, NC, pp 68–75
- Dunne CL, Madill J, Peden AE, et al. An underappreciated cause of ocean-related fatalities: a systematic review on the epidemiology, risk factors, and treatment of snorkelling-related drowning. Resusc plus. 2021;6:100103. doi: 10.1016/j.resplu.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds CW, Walker DG. Snorkelling deaths in Australia, 1987–1996. Med J Aust. 1999;171:591–594. doi: 10.5694/j.1326-5377.1999.tb123809.x. [DOI] [PubMed] [Google Scholar]
- Elia A, Barlow MJ, Wilson OJ, O’Hara JP. Splenic responses to a series of repeated maximal static and dynamic apnoeas with whole-body immersion in water. Exp Physiol. 2021;106:338–349. doi: 10.1113/EP088404. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, et al. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology. 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen K, Frandsen H, Lorentzen T, et al. The human spleen as an erythrocyte reservoir in diving-related interventions. J Appl Physiol. 2002;92:2071–2079. doi: 10.1152/japplphysiol.00055.2001. [DOI] [PubMed] [Google Scholar]
- Fowler WS. Breaking point of breath-holding. J Appl Physiol. 1954;6:539–545. doi: 10.1152/jappl.1954.6.9.539. [DOI] [PubMed] [Google Scholar]
- Hill PM. Hyperventilation, breath holding and alveolar oxygen tensions at the breaking point. Respir Physiol. 1973;19:201–209. doi: 10.1016/0034-5687(73)90078-9. [DOI] [PubMed] [Google Scholar]
- Hong SK, Rahn H, Kang DH, et al. Diving pattern, lung volumes, and alveolar gas of the Korean diving woman (ama) J Appl Physiol. 1963;18:457–465. doi: 10.1152/jappl.1963.18.3.457. [DOI] [PubMed] [Google Scholar]
- Hong SK, Henderson J, Olszowka A, et al. Daily diving pattern of Korean and Japanese breath-hold divers (ama) Undersea Biomed Res. 1991;18:433–443. [PubMed] [Google Scholar]
- Hurford WE, Hong SK, Park YS, et al. Splenic contraction during breath-hold diving in the Korean ama. J Appl Physiol. 1990;69:932–936. doi: 10.1152/jappl.1990.69.3.932. [DOI] [PubMed] [Google Scholar]
- Joulia F, Coulange M, Lemaitre F, et al. Plasma adenosine release is associated with bradycardia and transient loss of consciousness during experimental breath-hold diving. Int J Cardiol. 2013;168:e138–e141. doi: 10.1016/j.ijcard.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Koga T. Correlation between sectional area of the spleen by ultrasonic tomography and actual volume of the removed spleen. J Clin Ultrasound. 1979;7:119–120. doi: 10.1002/jcu.1870070208. [DOI] [PubMed] [Google Scholar]
- Koga T, Morlkawa Y. Ultrasonographic determination of the splenic size and its clinical usefulness in various liver diseases. Radiology. 1975;115:157–161. doi: 10.1148/115.1.157. [DOI] [PubMed] [Google Scholar]
- Kumar KR, Ng K. Don’t hold your breath: anoxic convulsions from coupled hyperventilation-underwater breath-holding. Med J Aust. 2010;192:663–664. doi: 10.5694/j.1326-5377.2010.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg PG. Carbon dioxide changes in hyperventilation and breath hold diving. South African Med J. 1974;48:18–22. [PubMed] [Google Scholar]
- Lanphier EH, Rahn H. Alveolar gas exchange during breath-hold diving. J Appl Physiol. 1963;18:471–477. doi: 10.1152/jappl.1963.18.3.471. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lally A, Moore TO, Hong SK. Physiological and conventional breath hold breaking points. J Appl Physiol. 1974;37:291–296. doi: 10.1152/jappl.1974.37.3.291. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Gennser M. Aggravated hypoxia during breath-holds after prolonged exercise. Eur J Appl Physiol. 2005;93:701–707. doi: 10.1007/s00421-004-1242-y. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Lundgren CEG. Alveolar gas composition before and after maximal breath-holds in competitive divers. Undersea Hyperb Med. 2006;33:463–467. [PubMed] [Google Scholar]
- Lippmann J. Snorkelling and breath-hold diving fatalities in Australia, 2001 to 2013. Demographics, characteristics and chain of events. Diving Hyperb Med. 2019;49:192–203. doi: 10.28920/dhm49.3.192-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann JM, Pearn JH. Snorkelling-related deaths in Australia, 1994–2006. Med J Aust. 2012;197:230–232. doi: 10.5694/mja11.10988. [DOI] [PubMed] [Google Scholar]
- Mohri M, Torii R, Nagaya K, et al. Diving patterns of ama divers of Hegura Island, Japan. Undersea Hyperb Med. 1995;22:137–143. [PubMed] [Google Scholar]
- Otis AB. The work of breathing. Physiol Rev. 1954;34:449–458. doi: 10.1152/physrev.1954.34.3.449. [DOI] [PubMed] [Google Scholar]
- Otis AB, Rahn H, Fenn WO. Alveolar gas changes during breath holding. Am J Physiol. 1984;152:674–686. doi: 10.1152/ajplegacy.1948.152.3.674. [DOI] [PubMed] [Google Scholar]
- Palada I, Eterović D, Obad A, et al. Spleen and cardiovascular function during short apneas in divers. J Appl Physiol. 2007;103:1958–1963. doi: 10.1152/japplphysiol.00182.2007. [DOI] [PubMed] [Google Scholar]
- Prommer N, Ehrmann U, Schmidt W, et al. Total haemoglobin mass and spleen contraction: a study on competitive apnea divers, non-diving athletes and untrained control subjects. Eur J Appl Physiol. 2007;101:753–759. doi: 10.1007/s00421-007-0556-y. [DOI] [PubMed] [Google Scholar]
- Radermacher P, Falke KJ, Park YS, et al. Nitrogen tensions in brachial vein blood of Korean ama divers. J Appl Physiol. 1992;73:2592–2595. doi: 10.1152/jappl.1992.73.6.2592. [DOI] [PubMed] [Google Scholar]
- Rahn H, Yokoyama T, Council NR. Physiology of breath-hold diving and the ama of Japan: papers. In: Rahn H, Yokoyama T, editors. Physiology of breath-hold diving and the ama of Japan. Tokyo: The National Academies Press; 1965. p. 387. [Google Scholar]
- Richardson MX, Engan HK, Lodin-Sundström A, Schagatay E. Effect of hypercapnia on spleen-related haemoglobin increase during apnea. Diving Hyperb Med. 2012;42:4–9. [PubMed] [Google Scholar]
- Sadler C, Brett K, Heerboth A, et al. Safety proposals for freediving time limits should consider the metabolic-rate dependence of oxygen stores depletion. Diving Hyperb Med. 2020;50:356–362. doi: 10.28920/dhm50.4.356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, Edwards BA, Kelly VJ, et al. Mechanism underlying accelerated arterial oxygen desaturation during recurrent apnea. Am J Respir Crit Care Med. 2010;182:961–969. doi: 10.1164/rccm.201003-0477OC. [DOI] [PubMed] [Google Scholar]
- Schagatay E (1996) The human diving response—effects of temperature and training(Doctoral thesis). Lund University
- Schagatay E, Andersson J. Diving response and apneic time in humans. Undersea Hyperb Med. 1998;25:13–19. [PubMed] [Google Scholar]
- Schagatay E, Holm B. Effects of water and ambient air temperatures on human diving bradycardia. Eur J Appl Physiol Occup Physiol. 1996;73:1–6. doi: 10.1007/BF00262802. [DOI] [PubMed] [Google Scholar]
- Schagatay E, Van Kampen M, Emanuelsson S, Holm B. Effects of physical and apnea training on apneic time and the diving response in humans. Eur J Appl Physiol. 2000;82:161–169. doi: 10.1007/s004210050668. [DOI] [PubMed] [Google Scholar]
- Schagatay E, Andersson JPA, Hallén M, Pålsson B. Selected contribution: role of spleen emptying in prolonging apneas in humans. J Appl Physiol. 2001;90:1623–1629. doi: 10.1152/jappl.2001.90.4.1623. [DOI] [PubMed] [Google Scholar]
- Schagatay E, Haughey H, Reimers J. Speed of spleen volume changes evoked by serial apneas. Eur J Appl Physiol. 2005;93:447–452. doi: 10.1007/s00421-004-1224-0. [DOI] [PubMed] [Google Scholar]
- Schagatay E, Lodin-Sundstrom A, Abrahamsson E. Underwater working times in two groups of traditional apnea divers in Asia: the Ama and the Bajau. Diving Hyperb Med. 2011;41:27–30. [PubMed] [Google Scholar]
- Schagatay E, Richardson MX, Lodin-Sundström A. Size matters: Spleen and lung volumes predict performance in human apneic divers. Front Physiol. 2012;3:1–8. doi: 10.3389/fphys.2012.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboni A, Fagoni N, Fontolliet T, et al. Breath holding as an example of extreme hypoventilation: experimental testing of a new model describing alveolar gas pathways. Exp Physiol. 2020;105:2216–2225. doi: 10.1113/EP088977. [DOI] [PubMed] [Google Scholar]
- Wolf S. The bradycardia of the dive reflex-a possible mechanism of sudden death. Trans Am Clin Climatol Assoc. 1964;76:192–200. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.