Abstract
The HemA enzyme (glutamyl-tRNA reductase) catalyzes the first committed step in heme biosynthesis in the enteric bacteria. HemA is mainly regulated by conditional protein stability; it is stable and, consequently, more abundant in heme-limited cells but unstable and less abundant in normally growing cells. Both the Lon and ClpAP energy-dependent proteases contribute to HemA turnover in vivo. Here we report that the addition of two positively charged lysine residues to the third and fourth positions at the HemA N terminus resulted in complete stabilization of the protein. By contrast, the addition of an N-terminal myc epitope tag did not affect turnover. This result confirms the importance of the N-terminal sequence for proteolysis of HemA. This region of the protein also contains a proline flanked by hydrophobic residues, a motif that has been suggested to be important for Lon-mediated proteolysis of UmuD. However, mutation of this motif did not affect the turnover of HemA protein. Cells expressing the stabilized HemA[KK] mutant protein display substantial defects in heme regulation.
Heme and related tetrapyrroles have several important functions in the enteric bacteria Salmonella typhimurium and Escherichia coli. Heme b (Fe protoporphyrin IX, or protoheme) and certain modified hemes are cofactors for cytochromes and are therefore required for respiration and growth on nonfermentable compounds as the source of carbon and energy (1a, 24). At the same time, respiration and some other intracellular processes produce the toxic substance H2O2, which can also be released by activated macrophages in the mammalian host. To combat these threats, enteric bacteria produce two catalases, which both contain heme. Two additional tetrapyrroles are produced from uroporphyrinogen III by a branch off the main heme biosynthetic pathway. Siroheme is the cofactor for an enzyme that reduces sulfite for use in cysteine biosynthesis (23). Additionally, the same branch of the heme pathway is used by S. typhimurium to synthesize vitamin B12, a cofactor utilized by several different enzymes (reviewed in reference 30).
The enzyme glutamyl-tRNA reductase (HemA) catalyzes the first committed step of the heme biosynthetic pathway, the reduction of charged glutamyl-tRNAGlu to form glutamate-1-semialdehyde, an unstable intermediate which is then converted to 5-aminolevulinic acid (ALA) by the product of the hemL gene (reviewed in references 3 and 21). The latter reaction can proceed slowly in vitro in the absence of an enzyme catalyst (19). HemA is also the target of heme-specific regulation. Recently we described the regulation of the HemA enzyme, in response to limitation for heme, by a mechanism that involves stabilization of the protein (36, 37). Experimentally, heme limitation is imposed by adaptation of a bradytrophic hemL null mutant to growth in the absence of ALA. The growth rate of adapted hemL cells is approximately 80% of that of ALA-supplemented hemL or wild-type cells. It is not yet clear how bacteria experience heme limitation in nature, but some possibilities include the secretion of heme pathway inhibitors by competitors, limitation for iron, or recovery from nongrowing states such as stationary phase.
Conditional stability of proteins is very rare in the enteric bacteria (excluding their accessory elements, such as plasmids and bacteriophages), although it is common in eukaryotes and is not unusual in some other bacteria. Examples of E. coli proteins exhibiting conditional stability include the sigma factors RpoH and RpoS (reviewed in reference 15), the addiction system component MazE (1), and UmuD (13). Recently a second instance in which a biosynthetic enzyme (LpxC) is regulated in this fashion has been discovered (27). It is of interest to discover what determines the conditional nature of this process for HemA as well as the other proteins. The energy-dependent proteases Lon and ClpAP are jointly responsible for HemA degradation in vivo. We have proposed that the N-terminal part of HemA, including the residues within the first 18 amino acids, functions as a degradation tag (37). This tag may constitute a sequence directly recognized by the proteases to initiate processive stepwise proteolysis. This model is based on the finding that a hybrid protein containing only these added amino acids, HemA1–18-LacZ, is degraded by the same two proteases in vivo. However, correct regulation by heme, which can be observed with the longer derivative HemA1–416-LacZ, is not seen with the short HemA1–18-LacZ protein (37).
There is considerable precedent for the idea that the N and C termini of individual proteins can determine stability against or sensitivity to proteolysis. For example, variants of the normally unstable phage P22 Arc repressor have altered C termini that confer stability (6). Conversely, nonpolar C-terminal tails destabilize variants of the DNA-binding domain of the phage lambda repressor (28), and unstable Mu repressor variants selected as vir mutants have acquired hydrophobic tails (38). The Tsp protease specifically targets the C-terminal determinant of the lambda repressor (33). A dramatic instance of C-terminal targeting was provided by the discovery of the ssrA-dependent tagging system, which allows the release of ribosomes from broken mRNA fragments and concomitantly adds a nonpolar C-terminal degradation tag to the incomplete polypeptide (22). The importance of the N terminus is shown by the N-end rule (35) and also by the instability conferred by an N-terminal fragment of the UmuD protein in transplant experiments (13). The finding that the function of the hydrophobic ssrA tag is blocked by charged C-terminal amino acids (16a) parallels the approach taken here.
If the N-terminal sequence of HemA constitutes a tag for protease recognition, it should be possible to find mutations which alter important residues and thereby interfere with degradation of native HemA by Lon and/or ClpAP. Here we report the isolation of a mutation that completely stabilizes HemA and HemA1–416-LacZ against Lon- and ClpAP-dependent turnover in vivo. The mutant was obtained by the addition of a pair of positively charged amino acids at the third and fourth positions. The simplest explanation for the mutant phenotype is that the charged amino acids block protease access to the initial cleavage site in HemA. The discovery of this mutant protein has allowed us to explore the importance of HemA turnover for correct heme regulation in vivo. In turn, this may lead to a simple screen that can be applied to recover a larger sample of mutations with effects on turnover.
MATERIALS AND METHODS
Growth of cultures.
All cultures were grown at 37°C in either Luria-Bertani (LB) medium (34) or minimal MOPS (morpholinepropanesulfonic acid) medium (26), as modified elsewhere (5), containing 0.2% glycerol as the carbon source. Plates were prepared by using nutrient agar (Difco) with 5 g of NaCl per liter or by using NCE medium (4) with 0.2% glycerol as the carbon source. ALA was used at 2 μM in minimal medium (11), and tryptophan was used at 0.002% to supplement Trp− mutants. Antibiotics were added to rich medium to final concentrations as follows: sodium ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml; kanamycin sulfate, 50 μg/ml; tetracycline hydrochloride, 20 μg/ml; and streptomycin sulfate, 200 μg/ml. For strains with F′ plasmids grown in minimal medium, the final antibiotic concentration was 100 μg of kanamycin sulfate/ml.
Construction of site-directed hemA mutations.
The plasmid pTE644 carries the promoter region upstream of hemA, extending from bp 1 to bp 734 of the sequence of GenBank accession no. J04243. This segment is bounded by the naturally occurring BamHI site on the upstream side and extends to an engineered NdeI site overlapping the hemA ATG initiation codon, followed by an EcoRI site. It is inserted into pUC120 between the BamHI and EcoRI sites. (The pUC120 plasmid used here has been modified to remove its NdeI site by a fill-in step.) The NdeI site in hemA was positioned by PCR (Pfu polymerase; Stratagene), and pTE644 has been sequenced to confirm the absence of mutations in the 258-bp hemA promoter region, between the StuI site and the NdeI site overlapping the hemA ATG initiation codon. The plasmid pTE647 is pTE644 carrying an additional NdeI-EcoRI fragment including the N-terminal 184 codons of hemA. This additional fragment was produced by PCR (Pfu polymerase) and includes the sequence from bp 729 to bp 1285 (GenBank accession no. J04243). Thus, pTE647 carries 731 bp upstream and 551 bp downstream of the hemA start site, modified to include an NdeI site overlapping the ATG initiation codon. The various mutations of hemA were constructed by PCR (Pfu polymerase) and used to replace the segment of hemA lying between the NdeI and MluI sites of pTE647. This region was then sequenced for each derivative plasmid. In some cases the substitution was made directly, but in other cases it proceeded through intermediate plasmids. All DNA fragments generated by PCR have been sequenced to confirm the absence of undesired mutations. Details of steps in plasmid construction and primer sequences are available from the authors on request.
To test the function of these mutated hemA segments, each was substituted into a plasmid bearing the wild-type hemA gene under the control of the PBAD (arabinose-inducible) promoter (17). Modification of the original plasmid, pBAD18, to give pTE570, which contains a ribosome binding site (RBS) and a unique NdeI site overlapping the ATG initiation codon, has been described previously (7). The plasmid pTE694 is pTE570 carrying hemA and the first 6 codons of prfA (bp 732 to bp 2048 of the sequence of GenBank accession no. J04243). This segment is bounded by the NdeI site overlapping the hemA ATG initiation codon and an EcoRI site on the downstream side. As before, the construction involved several steps, and all DNA fragments generated by PCR were subsequently sequenced to confirm the absence of mutations. Mutated hemA segments were substituted into pTE694 as NdeI-NheI fragments, and their identities were confirmed by sequencing.
Transfer of hemA mutations to the E. coli chromosome.
Three mutant alleles of hemA that retain the ability to complement a hemA mutant of E. coli when expressed from the PBAD promoter were transferred to the bacterial chromosome by linear transformation (31, 32). A wild-type control containing the NdeI site overlapping the ATG initiation codon was also transferred for comparison. The recipient strain for this transformation was constructed from E. coli TE3057, which has been described in detail previously (12). TE3057 carries a 7.5-kb fragment of S. typhimurium DNA including the wild-type hemA gene inserted into the trp operon of E. coli. It also carries a mutation in the E. coli hemA gene and so is dependent on the S. typhimurium copy of hemA for hemA function. A hemA::Kan disruption (at the MluI site at codon 19 of the S. typhimurium gene) was introduced into TE3057 by linear transformation to give TE6730. Subsequently, hemA alleles were introduced from the pTE647-based plasmids described above that contain upstream flanking DNA from the hemA promoter and the N-terminal half of hemA. Plasmids were digested with PstI, or in some cases BamHI, and then mixed with CaCl2-treated TE6730 cells and plated on nutrient (NB) agar selecting for Hem+ transformants. These Hem+ transformants were screened for a Kans Amps phenotype, and then DNA from candidate clones was analyzed by PCR. To prepare template DNA, 0.5 ml of an overnight culture grown in LB medium was centrifuged and the pelleted cells were resuspended in 1/10 volume of 10 mM Tris (pH 8.0)–0.1 mM EDTA. The resuspended cells were frozen at −70°C for 10 min, boiled for 10 min, and microcentrifuged for 10 min, and one-half the supernatant was retained. One microliter of this preparation was subjected to PCR (Taq polymerase) using S. typhimurium-specific primers. After PCR, the products were diluted threefold directly into the appropriate restriction enzyme digestion reaction mixture. All clones were tested for the presence of the NdeI and MluI sites and for a restriction site associated with the substitution when present.
Transfer to F′ plasmids.
Strains TE7590 and TE7591 contain F′ plasmids derived from strain TE4351, which has been described in detail previously (10). The original plasmid was constructed by insertion of a modified Tn10 transposon into F+. The transposon is Tn10d-put modified to carry a standard lac operon fusion construct and a Kanr marker (10). Strains TE7590 and TE7591 carry a hemA-lac protein fusion at codon 416 of the hemA gene and a hemA-prfA-lac protein fusion, respectively. The hemA genes of both plasmids carry a frameshift (fill-in) of the MluI site at codon 19 and are unable to confer a Hem+ phenotype. Each E. coli strain carrying a hemA mutation to be transferred onto the F′ plasmid was subjected to two preliminary steps: (i) pCDK30 (Ampr recD+) was introduced by electroporation and (ii) the F′ plasmids described above were introduced by conjugation, with selection for Kanr Tetr Ampr. Next, recombinant F′ plasmids that had repaired the hemA MluI site frameshift by transfer of material from the copy of the S. typhimurium hemA gene on the E. coli chromosome were isolated. These recombinants were obtained by mating the above-described strains with recipient strain TE2640 (E. coli hemA8) and selecting for Hem+ Trp+ exconjugants on minimal glycerol plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Repair of the frameshift mutation is accompanied by relief from polarity. Consequently, these recombinants exhibit increased expression of lacZ. Candidate clones were screened by PCR with S. typhimurium-specific primers followed by restriction digestion to confirm the presence of diagnostic restriction sites. The resulting F′ plasmids were then transferred to the final strain background in S. typhimurium by sequential conjugation with TE2279 and then with TE518. The final strains have a chromosomal hemA60 recA background and carry F′ plasmids that are marked with Kanr cassettes and express either native hemA or a hemA-lacZ protein fusion, each bearing the indicated change to the coding sequence for the HemA N terminus.
Western immunoblotting and pulse-labeling analysis.
Techniques for Western blotting were as described in reference 37, except that the primary antibodies were monoclonal antibodies (MAb) of the γ1 isotype, designated H17 and H23. The rates of synthesis and turnover of HemA protein were also examined by pulse-labeling and immunoprecipitation as described previously (37). Cells were grown to an optical density at 600 nm (OD600) of 0.4 in minimal MOPS medium containing 0.2% glycerol, 2 μM ALA, and kanamycin as necessary. Labeling, chase, sample preparation, immunoprecipitation, and gel electrophoresis were all performed exactly as described previously (37).
RESULTS
Site-directed mutations that alter the N terminus of HemA.
Previous transplant experiments have shown that the N-terminal 18 amino acids of HemA confer instability on LacZ in the context of the HemA1–18-LacZ hybrid protein (2, 37). This suggests a model in which the HemA N terminus contains a site recognized during the initial binding of the responsible proteases, after which proteolytic digestion would continue by a processive and relatively nonspecific mechanism. That the N terminus is also important in turnover of the native HemA protein is made more likely by the finding that both HemA1–18-LacZ and native HemA, as well as the full-length fusion protein HemA1–416-LacZ, are degraded in vivo by the same two proteases, Lon and ClpAP. These proteins are not appreciably degraded by other proteases in vivo under our standard conditions. Based on these considerations, several derivatives of the S. typhimurium hemA gene bearing alterations to the N-terminal region were constructed in the hope of finding variants whose products are stabilized against proteolysis by Lon or ClpAP. As discussed below, only mutants that encode functional enzymes as judged by in vivo complementation behavior were studied further. Retention of enzymatic activity in a particular mutant provides evidence that the defect in protease sensitivity is a specific effect.
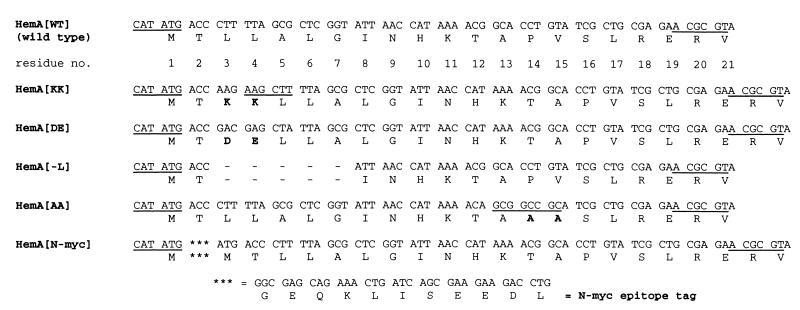
When scanning from the HemA N terminus, the first charged residue that is encountered is His-10. Since hydrophobicity is suspected to be an important aspect of Lon protease substrates (16, 18), we targeted three mutations to the hydrophobic N terminus of HemA. In the first set of experiments, two variants with inserts of a charged doublet of amino acids placed between Thr-2 and Leu-3 of HemA were constructed. One of these contains two lysine residues at this position (HemA[KK]), and the other contains two negatively charged amino acids (HemA[DE]). The name and sequence of each mutant hemA derivative constructed in this study are given in Fig. 1. In a third construct, five hydrophobic residues extending from Leu-3 to Gly-7 were deleted (HemA[−L]). The wild-type construct is designated HemA[WT]. Based on results from the HemA[KK] derivative and other considerations (see below), we subsequently constructed two additional mutants, which are also shown in Fig. 1. One of these has a substitution of two alanine residues for Pro-14 and Val-15 (HemA[AA]), and the other carries an additional 10-amino-acid epitope tag at the extreme N terminus (HemA[N-myc]).
FIG. 1.
Mutations constructed in this study. The DNA sequences of five mutant versions of the S. typhimurium hemA gene and their predicted protein products are shown together with those of the wild type (top two lines) for comparison. Each sequence begins with the NdeI site overlapping the ATG initiation codon and ends at an MluI site which lies at codons 19 to 21 of the wild-type gene. The five mutants are referred to in the text by the short designations listed in the left-hand column. The MluI and NdeI sites are underlined, as are two additional sites: a HindIII site in the HemA[KK] mutant and a NotI site in the HemA[AA] mutant. HemA[KK] and HemA[DE] bear insertions of two codons encoding the indicated amino acids between Thr-2 and Leu-3, and HemA[−L] has a deletion of five codons encoding Leu-3 through Gly-7 in wild-type hemA. In HemA[AA] the codons for Pro-14 and Val-15 are changed to encode two alanine residues, and HemA[N-myc] encodes HemA modified by addition of a c-myc epitope tag at the extreme N terminus of the protein. Details of construction and names of plasmids bearing these constructs are given in Materials and Methods. A mutation was inadvertently introduced to the fifth codon of HemA[DE] (CTT→CTA); this change is silent with respect to the amino acid sequence.
Plasmid expression system in E. coli.
The various hemA genes were placed under the control of the PBAD (arabinose-inducible) promoter in the plasmid pTE570 (7), derived from pBAD18 (17). An E. coli K-12 strain mutant for both hemA and ara (TE7054 [Table 1]) was transformed with each plasmid, and the ability to provide HemA function was tested. Cultures were plated in duplicate, and on one plate a disc containing arabinose was placed (Table 2). The HemA[WT], HemA[AA], and HemA[N-myc] constructs displayed very similar behavior, with weak complementation in the absence of the inducer and strong complementation in its presence. In contrast, the HemA[DE] plasmid complemented only poorly and HemA[−L] did not complement at all. These two constructs have not been studied further. The behavior of the HemA[KK] construct was unusual in that effective complementation was observed even in the absence of the inducer.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| S. typhimurium | ||
| LT-2 | Wild type | Lab collection |
| TE277 | zde-1858::Tn10d-Tet (75% linked to hemA) | 11 |
| TE299-1 | ΔhemL376 | 11 |
| TE315 | TR5877 = hsdL6 hsdSA29 (rLT− mLT+ rS− mS+) metA22 metE551 ilv-452 trpB2 xyl-404 rpsL120 (Strr) H1-b H2-e,n,x (Fels2−)nml | B. A. D. Stocker |
| TE518 | hemA60 recA1 | Lab collection |
| TE768 | araC1 DUP[(cob-4)*Tn10*(zdd-1852)] | 11 |
| TE1277 | hsdL (r− m+) galE542 (Fels?) | Lab collection |
| TE1303 | hemE1 env-53 | Lab collection |
| TE2279 | TE315 zde-1858::Tn10d-Tet hemA427 (Am) srl-203::Tn10d-Cam recA1 | Lab collection |
| TE3345 | TE315 putPA1303::Kanr-hemA (MluI fill-in)-lac [pr] (codon 416) | This study |
| TE3347 | TE315 putPA1303::Kanr-hemA (MluI fill-in)-prfA-lac [pr] (codon 6) | This study |
| TE3726 | LT2/pTE367 (AmprprfA+) | 12 |
| TE4351 | pyrD121 Δ(putPA)521/F+zzf-6807::[Tn10d-putPA1302::Cam] | 10 |
| TE7590 | pyrD121 Δ(putPA)521/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA (MluI fill-in)-lac [pr] (codon 416)] | P22.TE3345 × TE4351 |
| TE7591 | pyrD121 Δ(putPA)521/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA (MluI fill-in)-prfA-lac [pr] (codon 6)] | P22.TE3347 × TE4351 |
| TE7619 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[WT]lac [pr] (codon 416)] | This study |
| TE7620 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[WT]prfA-lac [pr] (codon 6)] | This study |
| TE7621 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[KK]lac [pr] (codon 416)] | This study |
| TE7622 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[KK]prfA-lac [pr] (codon 6)] | This study |
| TE7676 | hsdL (r− m+) galE542 (Fels?) DUP[(cob-4)*Tn10*(zdd-1852)] | P22.TE768 × TE1277 |
| TE7677 | hsdL (r− m+) galE542 (Fels?) DUP[(cob-4)*Tn10*(zdd-1852 hemA743::Kan(MluI)] | P1.TE6730 × TE7676 |
| TE7678 | hemA743::Kan (MluI)/pTE367 | P22.TE7677 × TE3726 |
| TE7679 | hemA744[KK]/pTE367 | P22.TE7622 × TE7678 |
| TE7691 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[AA]lac [pr] (codon 416)] | This study |
| TE7693 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[AA]prfA-lac [pr] (codon 6)] | This study |
| TE7695 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[N-myc]-lac [pr] (codon 416)] | This study |
| TE7696 | hemA60 recA1/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[N-myc]-prfA-lac [pr] (codon 6)] | This study |
| TE7697 | zde-1858::Tn10d-Tet hemA744[KK]/pTE367 | P22.TE277 × TE7679 |
| TE7700 | zde-1858::Tn10d-Tet hemA744[KK] | P22.TE7697 × LT2 |
| TE7701 | zde-1858::Tn10d-Tet hemA+ | P22.TE7697 × LT2 |
| TE7725 | hemEl env-53 zde-1858::Tn10d-Tet hemA744[KK] | P22.TE7700 × TE1303 |
| TE7726 | hemE1 env-53 zde-1858::Tn10d-Tet hemA[WT] | P22.TE7700 × TE1303 |
| TE7727 | ΔhemL376 zde-1858::Tn10d-Tet hemA744[KK] | P22.TE7700 × TE299-1 |
| TE7728 | ΔhemL376 zde-1858::Tn10d-Tet hemA[WT] | P22.TE7700 × TE299-1 |
| E. coli | ||
| LMG194 | K-12 F− λ− ΔlacX74 galE galK thi rpsL ΔphoA (PvuII) | L. Guzman |
| TE2331 | K-12 F− λ− IN(rrnD-rrnE) ΔlacX74 rpsL galK2 | R. Menzel |
| TE3057 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA-prfA-dorf-kdsA′] | 12 |
| TE2640 | K-12 F− λ− IN(rrnD-rrnE) ΔlacX74 rpsL galK2 hemA8 | 12 |
| TE4498 | MC4100 clpP1::Cm | 37 |
| TE6730 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA743::Kan (MluI)-prfA-dorf-kdsA′] | This study |
| TE7054 | K-12 F− λ− IN(rrnD-rrnE) ΔlacX74 rpsL galK2 hemA8 leu::Tn10 Δara714 | P1.LMG194 × TE2640 |
| TE7023 | MC4100 lon-146::ΔTn10 clpP1::Cm | 37 |
| TE7034 | MC4100 lon-146::ΔTn10 | 37 |
| TE7202 | TE7054/pTE570 (PBAD vector) | 7 |
| TE7207 | TE7054/pTE694 (PBAD-hemA[WT]) | This study |
| TE7420 | TE7054/pTE713 (PBAD-hemA[KK]) | This study |
| TE7518 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA[WT]-prfA-dorf-kdsA′] | This study |
| TE7519 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA[KK]-prfA-dorf-kdsA′] | This study |
| TE7661 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA[AA]-prfA-dorf-kdsA′] | This study |
| TE7663 | K-12 F− λ−araD139 ΔlacX74 galU galK hsdR (Strr) recD1903::mini-Tet hemA8 trpDC700::putPA1304::[′prsA-uorf-hemA[N-myc]-prfA-dorf-kdsA′] | This study |
| TE7706 | MC4100 clpP1::Cm/ F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[WT]-lac [pr] (codon 416)] | This study |
| TE7707 | MC4100 lon-146::ΔTn10 clpP1::Cm/ F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[WT]-lac [pr] (codon 416)] | This study |
| TE7708 | MC4100 lon-146::ΔTn10/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[WT]-lac [pr] (codon 416)] | This study |
| TE7709 | MC4100 clpP1::Cm/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[KK]-lac [pr] (codon 416)] | This study |
| TE7710 | MC4100 lon-146::ΔTn10 clpP1::Cm/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[KK]-lac [pr] (codon 416)] | This study |
| TE7711 | MC4100 lon-146::ΔTn10/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[KK]-lac [pr] (codon 416)] | This study |
| TE7712 | MC4100 clpP1::Cm/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[AA]-lac [pr] (codon 416)] | This study |
| TE7713 | MC4100 lon-146::ΔTn10 clpP1::Cm/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[AA]-lac [pr] (codon 416)] | This study |
| TE7714 | MC4100 lon-146::ΔTn10/F+zzf-6807::[Tn10d-putPA1303::Kanr-hemA[AA]-lac [pr] (codon 416)] | This study |
TABLE 2.
Complementation test in E. coli of plasmids expressing modified versions of S. typhimurium hemA
| Plasmid | Construct | Hem phenotypea
|
|
|---|---|---|---|
| Uninduced | Induced | ||
| pTE570 | PBAD vector | − | − |
| pTE694 | PBAD-hemA[WT] | + | ++++ |
| pTE713 | PBAD-hemA[KK] | ++++ | ++++b |
| pTE714 | PBAD-hemA[DE]c | − | ++ |
| pTE715 | PBAD-hemA[−L] | − | − |
| pTE717 | PBAD-hemA[N-myc] | + | ++++ |
| pTE739 | PBAD-hemA[AA] | + | ++++ |
Cultures of strain TE7054 (E. coli K-12 hemA Δara) carrying the indicated plasmids were grown to saturation in LB plus ampicillin (100 μg/ml) and ALA (150 μM). After dilution in phosphate-buffered saline, about 500 CFU was then plated on each of two NB-ampicillin plates (NB medium is selective for the Hem+ phenotype). A disc was placed in the center of one plate, and 10 μl of 20% arabinose was pipetted onto the disc. Colony size (diameter) was scored after 16 h. +, <0.5 mm; ++, ≈1 mm; ++++, ≈2 mm.
Colonies were large at the edge of the plate and smaller close to the disc where the inducer was applied.
See the legend to Fig. 1 for a description of this construct.
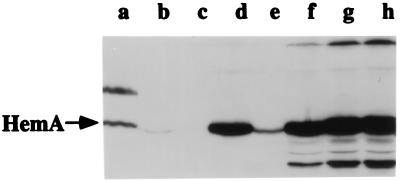
Western blots (immunoblots) probed with an anti-HemA MAb were used to examine expression of HemA from the plasmids that exhibited complementation ability (Fig. 2). The E. coli host strain for this experiment (TE7054) was the same as that used in the study described above: it carries the hemA8 mutation and an ara deletion. No signal was detected for HemA protein in this strain when only the parent vector was present (Fig. 2, lane c). When the plasmid pTE694 expressing HemA[WT] was introduced, the signal for HemA was clearly visible (lane e) and was similar to that observed from the single copy of hemA in wild-type S. typhimurium (lane a). For this E. coli strain carrying the HemA[WT] plasmid, induction with arabinose increased the abundance of HemA protein and also resulted in the appearance of additional bands that were reactive with antibody. The bands smaller than HemA were probably degradation products, but there were also apparently larger polypeptides which might be aggregates (lanes f to h). The plasmid pTE713 encoding HemA[KK] (lane d) produced substantially more HemA protein than was seen with HemA[WT] plasmid in the absence of inducer (lane e) and, in addition, showed little evidence for either the postulated degradation or aggregation products (Fig. 2 and data not shown). We also note that these results were obtained by Western blotting, a relatively sensitive method. No HemA protein is evident on stained gels of total cellular proteins, even for the HemA[KK] variant induced with arabinose (data not shown).
FIG. 2.
Western blot (immunoblot) analysis of HemA[WT] and HemA[KK] expressed from a plasmid-borne PBAD promoter in E. coli. The arrow indicates the HemA[WT] and HemA[KK] proteins. Lanes: b, E. coli TE2331 wild type; c, E. coli TE7202 hemA8/pTE570 (vector); d, E. coli TE7420 hemA8/pTE713 hemA[KK]; e, E. coli TE7207 hemA8/pTE694 hemA[WT]. Samples for lanes f to h were prepared as for lane e except that cultures were grown with various concentrations of the inducer arabinose: 0.001% arabinose (lane f), 0.005% arabinose (lane g), or 0.01% arabinose (lane h). Lane a contained proteins from S. typhimurium LT-2 (wild type) as a positive control. Cultures were grown to an OD600 of 0.4 in LB medium (containing ampicillin where applicable) and processed for immunoblotting with anti-HemA MAb H23 exactly as described previously (36).
Further investigation revealed certain deficiencies of the plasmid-based system for the study of HemA degradation. Pulse-labeling and immunoprecipitation showed that the rate of synthesis of HemA[KK] from pTE713 was about fourfold higher than that of HemA[WT] from pTE694 (data not shown). Thus, the KK insertion between Thr-2 and Leu-3 apparently affects HemA synthesis. This difference may be explained by an effect on mRNA secondary structure. Inspection of the sequence surrounding the hemA ATG start codon in pTE694 revealed a possible stem-loop that would be predicted to sequester the RBS in a secondary structure involving the RNA encoding the N terminus of HemA. The increased rate of synthesis of HemA[KK] and certain other N-terminal insertions compared with that of the wild type can probably be ascribed to disruption of this secondary structure. The structure is fortuitous since it combines two stems of different origin: one derived from hemA and one contributed by the (artificial) RBS. Given this effect, it is not simple to compare the rates of turnover of mutant and wild-type HemA proteins at the same intracellular protein level.
A second and more serious limitation was seen in pulse-chase experiments. Here, addition of a small amount of the inducer arabinose was necessary to observe a signal for labeled wild-type HemA. Even at these modestly increased levels of HemA, the protein that is produced is stable, in contrast to that produced from a single copy of the wild-type S. typhimurium hemA gene carried on the bacterial chromosome in either E. coli or S. typhimurium (data not shown). We suspect that this effect is due to titration of a limiting component required for HemA turnover, either the proteases themselves or possibly another factor (see Discussion).
Expression from the chromosome in E. coli and S. typhimurium.
To circumvent these limitations, we transferred each of the four constructs that produce active enzyme (HemA[WT], HemA[KK], HemA[AA], and HemA[N-myc]) to the bacterial chromosome. This was accomplished by using an E. coli strain that carries an insertion of a 7.5-kb DNA fragment, including the S. typhimurium hemA gene, in the trp locus. The construction and use of this strain have been described previously (10, 12). In the resulting E. coli strains, the S. typhimurium hemA gene is present in a single copy and is expressed from its native promoter, while the E. coli hemA8 allele eliminates both E. coli hemA function and the production of cross-reactive material (Fig. 2, lane c) (see below). For this and several other experiments that followed, cultures were grown in medium supplemented with ALA. This was done to eliminate the possibility that small differences in the enzyme activities of the different HemA and HemA-LacZ constructs, and consequent changes in heme synthesis, would indirectly influence the stability of these proteins by altering heme regulation.
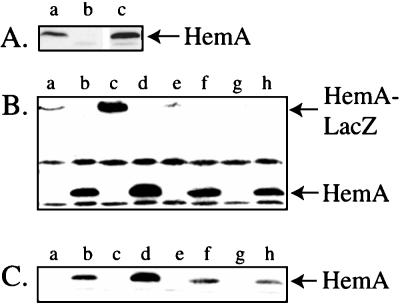
Western blot analysis was used to examine expression of the HemA protein from the HemA[WT] and HemA[KK] constructs in this E. coli background (Fig. 3). The abundance of HemA protein was substantially increased in the strain expressing HemA[KK] (lane d) compared to that seen with HemA[WT] (lane c). The increased level might be due to either an increased rate of synthesis or a decreased rate of turnover. HemA protein was not well visualized by pulse-labeling and immunoprecipitation in these strains (data not shown). In previous work, we found it necessary to employ F′ plasmids to increase the expression of hemA to a level detectable by immunoprecipitation. Therefore, each of the constructs was subsequently transferred from the E. coli chromosome to an F′ plasmid (see Materials and Methods), and these plasmids were introduced into an S. typhimurium hemA recA mutant host by conjugation. The HemA signal was eliminated by this hemA mutation (Fig. 4A). Provision of hemA on an F′ plasmid gave only a modest increase in the level of HemA (approximately threefold) compared to that of the original wild-type strain.
FIG. 3.
Western blot (immunoblot) analysis of HemA[WT] and HemA[KK] expressed from a gene carried in a single copy in the E. coli chromosome. Cultures were grown to an OD600 of 0.4 in minimal MOPS-glycerol medium with 2 μM ALA and tryptophan (and ampicillin where applicable). They were processed for immunoblotting with anti-HemA MAb H23. Lanes: a, E. coli TE3057 hemA8 trp::put::hemA+; b, E. coli TE7202 hemA8/pTE570 (vector); c, TE7518 hemA8 trp::put::hemA[WT]; d, TE7519 hemA8 trp::put::hemA[KK].
FIG. 4.
Western blot (immunoblot) analysis of HemA[WT] and HemA[KK] expressed from F′ plasmids in S. typhimurium. (A) Comparison of HemA levels expressed from single-copy hemA and F′ plasmid-borne hemA[WT]. Cultures were grown to an OD600 of 0.4 in minimal MOPS-glycerol medium with 2 μM ALA (and kanamycin when necessary to maintain an F′ plasmid). Genotypes are abbreviated here and in subsequent figures; full genotypes are given in Table 1. The arrow indicates the native HemA protein. Lanes: a, LT-2 wild type; b, TE518 hemA60 recA; c, TE7620 hemA60 recA/F′ hemA[WT]. (B) Comparison of HemA and HemA-LacZ levels in the mutants and wild type. Cultures were grown to an OD600 of 0.4 in minimal MOPS-glycerol medium with 2 μM ALA (and kanamycin when necessary to maintain F′ plasmids). Samples were processed as described above for immunoblotting with anti-HemA MAb H17. Similar results were also observed with MAb H23. The positions of native HemA and HemA1–416-LacZ are indicated. Lanes: a, TE7619 hemA60 recA/F′ hemA1–416[WT]-lac [pr]; b, TE7620 hemA60 recA/F′ hemA[WT]; c, TE7621 hemA60 recA/F′ hemA1–416[KK]-lac [pr]; d, TE7622 hemA60 recA/F′ hemA[KK]; e, TE7691 hemA60 recA/F′ hemA1–416[AA]-lac [pr]; f, TE7693 hemA60 recA/F′ hemA[AA]; g, TE7695 hemA60 recA/F′ hemA1–416 [N-myc]-lac [pr]; h, TE7696 hemA60 recA/F′ hemA[N-myc]. (C) This panel shows a lighter exposure of the gel shown in panel B, which allows better visualization of the increase in HemA[KK] protein in lane d.
The resulting F′ plasmids carry the indicated mutations either in the context of a native hemA gene or as a hemA-lacZ protein fusion that expresses the HemA1–416-LacZ hybrid protein. The levels of native HemA and HemA1–416-LacZ were examined by Western blot (immunoblot) analysis by probing with an anti-HemA MAb (Fig. 4B). The HemA[KK] variants showed an increased abundance compared to HemA[WT], both in the context of native HemA (compare lanes b and d) and in the context of the HemA1–416-LacZ hybrid protein (compare lanes a and c). We have not attempted to quantitate the increased level of protein observed on this Western blot but present below a direct analysis of rates of synthesis and degradation.
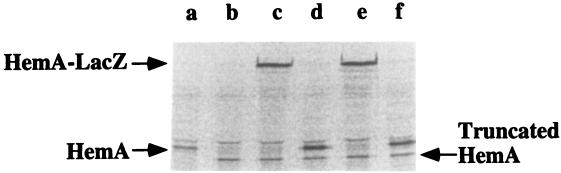
The rates of HemA and HemA1–416-LacZ synthesis were measured by pulse-labeling and immunoprecipitation with an anti-HemA MAb (Fig. 5). As observed previously (37), the chromosomal hemA60 allele produces a truncated but immunoreactive polypeptide, visible in lanes b to f. The rates of synthesis of the native HemA and HemA1–416-LacZ constructs bearing HemA[WT] (lanes c and d) did not differ from those of the HemA[KK] variants (lanes e and f). A substantial increase in the levels of the HemA[KK] mutant proteins (Fig. 4) without an increased rate of synthesis (Fig. 5) implies that the rate of turnover of HemA[KK] was greatly reduced. This inference was confirmed by a pulse-chase experiment (Fig. 6). The half-life of native HemA[WT] protein was determined to be about 20 min, similar to the half-life observed previously (37). In contrast, the HemA[KK] mutant was essentially stable (half-life, >300 min). The rate of synthesis of native HemA[WT] from the F′ plasmid (Fig. 5, lane d) was not more than threefold higher than that observed in wild-type S. typhimurium (Fig. 5, lane a).
FIG. 5.
Pulse-labeling and immunoprecipitation analysis of HemA[WT] and HemA[KK] expressed from F′ plasmids in S. typhimurium. The positions of native HemA, the truncated HemA protein from hemA60, and HemA1–416-LacZ are all indicated. Cultures were the same as those analyzed by Western blotting in Fig. 4. One milliliter of each culture (OD600 = 0.4) was pulse-labeled with Tran35S-label (ICN) for 5 min and then chased for 2 min with unlabeled l-methionine (1.3 mM) and l-cystine (0.6 mM). Protein extracts were prepared, immunoprecipitated with anti-HemA MAb H17, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Results were quantitated by using a PhosphorImager and ImageQuant software. Lanes: a, LT-2 wild type; b, TE 518 hemA60 recA; c, TE7619 hemA60 recA/F′ hemA1–416[WT]-lac [pr]; d, TE7620 hemA60 recA/F′ hemA[WT]; e, TE7621 hemA60 recA/F′ hemA1–416[KK]-lac [pr]; f, TE7622 hemA60 recA/F′ hemA[KK].
FIG. 6.
Pulse-chase analysis of HemA[WT] and HemA[KK] expressed from F′ plasmids in S. typhimurium. (A) Top, TE7620 hemA60 recA/F′ hemA[WT]; bottom, TE7622 hemA60 recA/F′ hemA[KK]. The positions of HemA[WT] and HemA[KK] proteins are indicated. Cultures were grown and analyzed as described in the legend to Fig. 5, except that the chase was extended as shown above each lane. (B) An identical gel (not treated with fluor) was analyzed by using a PhosphorImager and ImageQuant software to calculate the half-life of the HemA protein. The HemA[WT] protein seen in TE7620 has a half-life of ca. 20 min, the same as that of the chromosomally encoded HemA protein (37). In contrast, the HemA[KK] protein seen in strain TE7622 is stable (half-life, >300 min).
Test of a putative degradation tag for Lon protease.
The UmuD protein of E. coli is specifically degraded by Lon both in vivo and in vitro (13). Transplantation of the N-terminal 40 amino acids of UmuD to a normally stable protein confers instability to Lon. Two similar motifs within the N-terminal 30 amino acids of UmuD, FPLF and FPSP, both contribute to Lon proteolysis, and the double mutant is protected against proteolysis in a purified system (37). Because proline disrupts both alpha helices and beta sheets in proteins, and since hydrophobicity is known to be important in Lon-directed cleavage of at least some substrates, it seems possible that a proline flanked by hydrophobic residues serves as a degradation tag for Lon. The N-terminal sequence of HemA includes a proline flanked by hydrophobic amino acids (Ala-13 Pro-14 Val-15). We tested whether this sequence contributes to HemA turnover by constructing a mutant in which Pro-14 and Val-15 are both changed to alanine (in HemA[AA]) (Fig. 1). When placed under the control of PBAD, HemA[AA] complements an E. coli hemA mutant as well as HemA[WT] (Table 2). The mutant hemA gene was transferred to F′ plasmids encoding either native HemA[AA] or HemA1–416[AA]-LacZ. The levels of the HemA[AA] mutant proteins were the same as those of their wild-type counterparts, as measured by Western blot analysis (Fig. 4B, lanes e and f). Pulse-labeling and immunoprecipitation with anti-HemA MAb revealed no difference in the rate of synthesis of HemA[AA] compared to that of HemA[WT] (data not shown). Together, these results suggest that the susceptibility of HemA[AA] to proteolysis is comparable to that of HemA[WT]. Similar results were also found for another mutant in which the 10-amino-acid myc epitope tag was added to the N terminus of HemA (HemA[N-myc]). Western blotting (Fig. 4B, lanes g and h) and pulse-labeling (data not shown) revealed that the N-terminal myc epitope tag does not interfere with the normal expression or regulation of HemA. The HemA-LacZ bands from both HemA[AA] and HemA[N-myc] constructs are slightly weaker than that from HemA[WT] (Fig. 4B; compare lanes a, e, and g) but are clearly visible in the original blot.
Because HemA is subject to proteolysis by both Lon and ClpAP, we considered the possibility that the HemA[AA] mutation would eliminate the recognition sequence for one protease (Lon) but not the tag for the other protease (ClpAP). This possibility was tested by transfer of F′ plasmids that express HemA1–416-LacZ[WT], HemA1–416-LacZ[KK], or HemA1–416-LacZ[AA] into E. coli mutants defective for one or the other protease. Western blot analysis of the resulting strains showed that the HemA[AA]-LacZ protein is present at the same level as HemA[WT]-LacZ in the single mutants defective for either lon or clpP (Fig. 7). In either single protease mutant, the HemA[KK]-LacZ protein is present at a higher level than the other two. In the lon clpP double mutant, all three proteins are present at the same level (data not shown). Thus, we find no evidence that the APV sequence serves as a protease degradation tag in HemA. It should also be noted that the levels of both the native E. coli HemA protein and the HemA-LacZ fusion proteins are higher in the clpP mutant than in the lon mutant. This suggests that ClpAP may contribute more than Lon to HemA turnover.
FIG. 7.
Western blot (immunoblot) analysis of HemA[WT]-LacZ, HemA[AA]-LacZ, and HemA[KK]-LacZ expressed from F′ plasmids in E. coli protease mutants. Cultures were grown to an OD600 of 0.4 in minimal MOPS-glycerol medium with 2 μM ALA and kanamycin to maintain F′ plasmids. Samples were processed as described above for immunoblotting with anti-HemA MAb H17. All strains are E. coli. The arrows indicate the HemA-LacZ fusion protein as well as the native HemA protein encoded by the E. coli hemA gene. Lanes: a, TE7708 lon/F′ hemA1–416[WT]-lac [pr]; b, TE7714 lon/F′ hemA1–416[AA]-lac [pr]; c, TE7711 lon/F′ hemA1–416[KK]-lac [pr]; d, TE7706 clpP/F′ hemA1–416[WT]-lac [pr]; e, TE7712 clpP/F′ hemA1–416[AA]-lac [pr]; f, TE7709 clpP/F′ hemA1–416[KK]-lac [pr].
Effect of the HemA[KK] mutation on regulation.
We examined some heme-related phenotypes of strains expressing HemA[KK] when grown under conditions in which heme regulation is thought to be important. For these experiments, the mutant hemA[KK] gene was used to replace hemA at its normal position on the bacterial chromosome as described in Materials and Methods.
Two derivatives of a hemL deletion mutant, one expressing wild-type HemA and the other expressing HemA[KK] but otherwise isogenic, were constructed. As described previously (36, 37) a hemL mutant can adapt to growth in the absence of ALA supplementation. The HemA protein is stabilized in adapted hemL mutant cells. Since the only known defect of a hemL mutant is in ALA synthesis, we expected that expression of HemA[KK] would allow the hemL mutant to grow at a wild-type rate in the absence of ALA and without a period of adaptation. However, this is not what was found. The ΔhemL strain expressing HemA[KK] does adapt to growth in the absence of ALA somewhat more rapidly than the wild type (adaptation in 1.5 h versus 2 h). However, the lag is not eliminated (data not shown), and the final growth rates of both adapted cultures are quite similar. This suggests that the metabolism of adapted (heme-limited) cells must be adjusted in additional respects beyond the stabilization of the HemA protein. Two likely possibilities include activation of cyclic AMP receptor protein through increased synthesis of cyclic AMP and the stringent response, both of which are reported to occur in heme-limited cells of E. coli (25, 29).
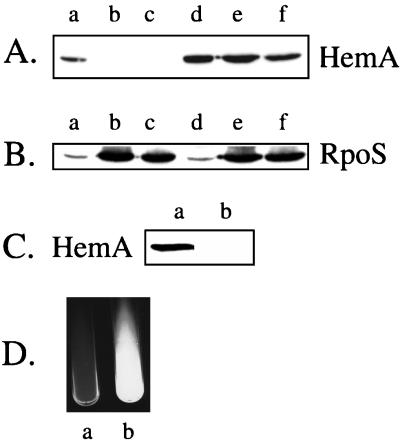
A second test of HemA regulation employed otherwise wild-type cells differing only in their hemA alleles. We observed that the HemA protein disappeared from a wild-type strain in cultures that had recently entered stationary phase or had been incubated overnight (Fig. 8A, lanes a to c), whereas HemA[KK] protein could still be detected easily in the mutant (lanes d to f). When this blot was reprobed for RpoS, the expected stationary-phase increase in RpoS abundance was observed for both strains (Fig. 8B). A longer, more sensitive exposure of a similar blot containing proteins from wild-type cells (Fig. 8C) also shows no detectable HemA in an overnight culture grown in LB medium. Comparable results were obtained for cultures grown in minimal glycerol medium and during carbon starvation in glucose-grown cultures (data not shown). Because tetrapyrrole intermediates in the heme biosynthetic pathway confer sensitivity to visible light, the loss of the HemA protein from stationary-phase cells may have a protective role during this phase of growth stasis.
FIG. 8.
Altered HemA regulation in strains bearing the hemA[KK] allele. (A) Loss of HemA protein in stationary phase. Cultures were grown in LB medium to an OD600 of 0.5 (lanes a and d), to 3 h beyond stationary phase (lanes b and e), or overnight (lanes c and f). The extract from an equal number of cells was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-HemA MAb H23. Lanes a to c, HemA[WT]; lanes d to f, HemA[KK]. (B) The blot from the experiment shown in panel A was reprobed with anti-RpoS MAb R12 (8). (C) LT-2 wild-type cells were grown in LB medium to an OD600 of 0.5 (lane a) or overnight (lane b) and probed with anti-HemA MAb H23. This blot shows results similar to those in panel A but was developed with a longer exposure time to increase the sensitivity. (D) Cultures of TE7725 hemA[WT] hemE env (a) and TE7726 hemA[KK] hemE env (b) were grown in LB medium containing 20 μg of heme/ml for 36 h, and the red fluorescence seen in lane b was visualized by using a UV light source. The image was made with the EagleEye II system (Stratagene).
Overproduction of HemA during stationary phase confers several other readily visible phenotypes. For example, an otherwise wild-type strain that carries hemA[KK] overproduces ALA, as measured by its ability to feed a lawn of hemA mutant cells. The zone of feeding of hemA mutant tester cells by a spot of the KK strain is twice that seen with a hemA[WT] strain (6 mm versus 3 mm). When a strain carrying both the hemA[KK] allele and a block in hemE is grown in medium containing heme at 20 μg/ml, overproduction of the accumulated intermediate uroporphyrin could be observed as a red fluorescence under UV light (Fig. 8D). This effect was not seen in cells carrying wild-type hemA. We have shown previously that the external heme concentration negatively regulates the production of heme pathway intermediates seen in hemE mutant cells (see Fig. 3, e.g., of reference 36). A similar qualitative plate test of hemA[KK] mutant cells revealed that heme regulation was defective (data not shown).
DISCUSSION
In this study we investigated a potential role for the N-terminal residues of the HemA protein in its conditional proteolysis. Turnover of HemA is regulated by heme or a heme-dependent process and also requires the function of the ATP-dependent proteases Lon and ClpAP (36, 37). We found that the addition of two lysines between positions 2 and 3, conferring a positive charge to the normally hydrophobic N terminus of HemA, resulted in stabilization of the protein. In contrast, addition of an N-terminal myc epitope tag did not affect regulation. Since the myc epitope tag also contains some charged amino acids, it is not clear why the KK insertion blocks turnover while the myc epitope tag does not. Two other site-directed changes that were made resulted in genes that do not complement a hemA defect. The corresponding proteins may be defective as enzymes or particularly unstable, and they were not studied further. Given confirmation of the importance of the HemA N terminus, the possible targeting role of a proline residue (Pro-14) flanked by hydrophobic amino acids was also tested, but no evidence for its involvement in degradation was obtained.
The simplest explanation for these results is that the added positive charge in the KK mutant blocks the initial binding of the proteases or the subsequent unfolding of the HemA protein for degradation. However, it is also possible that an accessory factor is involved (for example, a chaperone) and that the defect lies in the interaction with this protein. A third possibility is that the KK mutation acts indirectly to alter the HemA protein’s conformation and eliminates binding of the protease to a distant site. This model seems unlikely since we know that the N terminus of HemA confers instability in transplant experiments and thus is likely to be directly involved in the initial binding of native HemA to the proteases. Further studies with purified components will be necessary to understand this process.
The main importance of these findings is twofold. First, the HemA[KK] mutant is clearly defective for heme-dependent regulation. Cells synthesizing the mutant protein at a rate similar to that of the wild type do not regulate HemA correctly during growth to stationary phase in any of several media. They also overproduce ALA and heme, and further, they do not respond to exogenous heme by reducing flow through the pathway, as occurs in wild-type cells. These findings confirm that conditional proteolysis is a central factor in heme regulation. Whether other regulatory effects occur with the HemA enzyme, such as feedback inhibition, remains to be seen. The second important outcome of this work is that studies of the KK mutant should allow the design of screens to obtain additional mutants in a less-directed fashion. Such mutants should help to illuminate the regulatory mechanism.
We are also left with several observations whose basis is not yet understood. One potentially quite important one is that modest overproduction of HemA, to a level still not visible on a stained gel of total cell protein, results in the complete stabilization of the protein. This suggests that a component required for proteolysis can be titrated. Saturation of Lon by its substrate SulA has been reported (9). On the other hand, it is thought that the Lon and ClpAP proteases are relatively abundant (for reviews, see references 14 and 16), and colonies overexpressing HemA are not mucoid as one would predict if the level of the Lon substrate RcsA were elevated. We have investigated whether HemA overproduction stabilizes RcsA by measuring expression of the RcsA-dependent reporter fusion cps-lac. The results suggest that Lon function is not compromised by elevated HemA levels and indicate that the titrated component is not a protease (unpublished data). We noted that when HemA1–18-LacZ was expressed from a multicopy plasmid it was stable (3). This may indicate that the titrated component binds directly to the HemA N terminus.
We have previously failed in attempts to overproduce HemA to high levels by the use of tac or T7 RNA polymerase-directed systems. It seemed possible that instability to proteolysis accounts for this, but the above explanation suggests instead that proteolysis should not limit production, given an increased rate of synthesis. One simple possibility is that the function of an essential protease such as FtsH is inhibited when HemA is greatly overproduced and this has a subsequent negative impact on cell growth or protein synthesis.
Finally, since the abundance of Hem1–416-LacZ is substantially elevated in heme-limited cells and the abundance of the KK version is elevated in otherwise wild-type cells under all growth conditions examined, we would expect that the β-galactosidase activity of these strains should also be elevated comparably. Instead, the observed increase in β-galactosidase activity is less than twofold. We speculate that oligomerization of LacZ (which is known to be required for its activity) may be impaired, perhaps by the binding of other proteins to the HemA domain of the hybrid protein.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM40403.
The authors are grateful to the individuals cited in Table 1 for providing bacterial strains.
REFERENCES
- 1.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal “addiction module” regulated by guanosine 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci USA. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Anraku Y, Gennis R B. The aerobic respiratory chain of Escherichia coli. Trends Biochem Sci. 1987;12:262–266. [Google Scholar]
- 2.Archer C D, Wang X, Elliott T. Mutants defective in the energy-conserving NADH dehydrogenase of Salmonella typhimurium identified by a decrease in energy-dependent proteolysis after carbon starvation. Proc Natl Acad Sci USA. 1993;90:9877–9881. doi: 10.1073/pnas.90.21.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beale S I. Biosynthesis of hemes. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 731–748. [Google Scholar]
- 4.Berkowitz D, Hushon J M, Whitfield H J, Jr, Roth J, Ames B N. Procedure for identifying nonsense mutations. J Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner B R, Ames B N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982;257:9759–9769. [PubMed] [Google Scholar]
- 6.Bowie J U, Sauer R T. Identification of C-terminal extensions that protect proteins from intracellular proteolysis. J Biol Chem. 1989;264:7596–7602. [PubMed] [Google Scholar]
- 7.Brown L, Elliott T. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol. 1996;178:3763–3770. doi: 10.1128/jb.178.13.3763-3770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown L, Elliott T. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J Bacteriol. 1997;179:656–662. doi: 10.1128/jb.179.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dervyn E, Canceill D, Huisman O. Saturation and specificity of the Lon protease of Escherichia coli. J Bacteriol. 1990;172:7098–7103. doi: 10.1128/jb.172.12.7098-7103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992;174:245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott T, Roth J R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989;216:303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- 12.Elliott T, Wang X. Salmonella typhimurium prfA mutants defective in release factor 1. J Bacteriol. 1991;173:4144–4154. doi: 10.1128/jb.173.13.4144-4154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez M, Frank E G, Levine A S, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman S. Roles for energy-dependent proteases in regulatory cascades. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 503–519. [Google Scholar]
- 16.Gottesman S, Maurizi M R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992;56:592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Gottesman S, Roche E, Zhou Y, Sauer R T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman C, D’Ari R. Proteolysis and chaperones: the destruction/reconstruction dilemma. Curr Opin Microbiol. 1998;1:204–209. doi: 10.1016/s1369-5274(98)80012-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoober J K, Kahn A, Ash D E, Gough S, Kannangara C G. Biosynthesis of Δ-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate-1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53:11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- 20.Jahn D, Michelsen U, Soll D. Two glutamyl-tRNA reductase activities in Escherichia coli. J Biol Chem. 1991;266:2542–2548. [PubMed] [Google Scholar]
- 21.Jahn D, Verkamp E, Soll D. Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends Biochem Sci. 1992;17:215–218. doi: 10.1016/0968-0004(92)90380-r. [DOI] [PubMed] [Google Scholar]
- 22.Keiler K C, Waller P R H, Sauer R T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 23.Kredich N M. Biosynthesis of cysteine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 514–527. [Google Scholar]
- 24.Mogi T, Saiki K, Anraku Y. Biosynthesis and functional role of haem O and haem A. Mol Microbiol. 1994;14:391–398. doi: 10.1111/j.1365-2958.1994.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakayashiki T, Inokuchi H. Effects of starvation for heme on the synthesis of porphyrins in Escherichia coli. Mol Gen Genet. 1997;255:376–381. doi: 10.1007/s004380050509. [DOI] [PubMed] [Google Scholar]
- 26.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogura T, Inoue K, Tatsuta T, Suzaki T, Karata K, Young K, Su L, Flerke C A, Jackman J E, Raetz C R H, Coleman J, Tomoyasu T, Matsuzawa H. Balanced biosynthesis of major membrane components through regulated degradation of the committed enzyme of lipid A biosynthesis by the AAA protease FtsH (HflB) in Escherichia coli. Mol Microbiol. 1999;31:833–844. doi: 10.1046/j.1365-2958.1999.01221.x. [DOI] [PubMed] [Google Scholar]
- 28.Parsell D A, Silber K R, Sauer R T. Carboxy-terminal determinants of intracellular protein degradation. Genes Dev. 1990;4:277–286. doi: 10.1101/gad.4.2.277. [DOI] [PubMed] [Google Scholar]
- 29.Rompf A, Schmid R, Jahn D. Changes in protein synthesis as a consequence of heme depletion in Escherichia coli. Curr Microbiol. 1998;37:226–230. doi: 10.1007/s002849900369. [DOI] [PubMed] [Google Scholar]
- 30.Roth J R, Lawrence J G, Bobik T A. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Russell C B, Thaler D S, Dahlquist F W. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J Bacteriol. 1989;171:2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevell D E, Abou-Zamzam A M, Demple B, Walker G C. Construction of an Escherichia coli K-12 ada deletion by gene replacement in a recD strain reveals a second methyltransferase that repairs alkylated DNA. J Bacteriol. 1988;170:3294–3296. doi: 10.1128/jb.170.7.3294-3296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silber K R, Keiler K C, Sauer R T. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc Natl Acad Sci USA. 1992;89:295–299. doi: 10.1073/pnas.89.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 35.Tobias J W, Shrader T E, Rocap G, Varshavsky A. The N-end rule in bacteria. Science. 1991;254:1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Brown L, Elliott M, Elliott T. Regulation of heme biosynthesis in Salmonella typhimurium: activity of glutamyl-tRNA reductase (HemA) is greatly elevated during heme limitation by a mechanism which increases abundance of the protein. J Bacteriol. 1997;179:2907–2914. doi: 10.1128/jb.179.9.2907-2914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Elliott M, Elliott T. Conditional stability of the HemA protein (glutamyl-tRNA reductase) regulates heme biosynthesis in Salmonella typhimurium. J Bacteriol. 1999;181:1211–1219. doi: 10.1128/jb.181.4.1211-1219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welty D J, Jones J M, Nakai H. Communication of ClpXP protease hypersensitivity to bacteriophage Mu repressor isoforms. J Mol Biol. 1997;272:31–41. doi: 10.1006/jmbi.1997.1193. [DOI] [PubMed] [Google Scholar]