Abstract
Introduction
Evidence of a direct comparison between dipeptidyl-peptidase 4 inhibitors (DPP-4is) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) remains lacking, and no clear treatment strategy or rationale has been established using these drugs. This study aimed to compare the overall efficacy and safety of DPP-4is and the SGLT2i luseogliflozin in patients with type 2 diabetes mellitus (T2DM).
Methods
Patients with T2DM who had not used antidiabetic agents or who had used antidiabetic agents other than SGLT2is and DPP-4is were enrolled in the study after written informed consent had been obtained. The enrolled patients were subsequently randomly assigned to either the luseogliflozin or DPP-4i group and followed up for 52 weeks. The primary (composite) endpoint was the proportion of patients who showed improvement in ≥ 3 endpoints among the following five endpoints from baseline to week 52: glycated hemoglobin (HbA1c), weight, estimated glomerular filtration rate (eGFR), systolic blood pressure, and pulse rate.
Results
A total of 623 patients were enrolled in the study and subsequently randomized to either the luseogliflozin or DPP-4i groups. The proportion of patients who showed improvement in ≥ 3 endpoints at week 52 was significantly higher in the luseogliflozin group (58.9%) than in the DPP-4i group (35.0%) (p < 0.001). When stratified by body mass index (BMI) (< 25 or ≥ 25 kg/m2) or age (< 65 or ≥ 65 years), regardless of BMI or age, the proportion of patients who achieved the composite endpoint was significantly higher in the luseogliflozin group than in the DPP-4i group. Hepatic function and high-density lipoprotein-cholesterol were also significantly improved in the luseogliflozin group compared with the DPP-4i group. The frequency of non-serious/serious adverse events did not differ between the groups.
Conclusion
This study showed the overall efficacy of luseogliflozin compared with DPP-4is over the mid/long term, regardless of BMI or age. The results suggest the importance of assessing multiple aspects regarding the effects of diabetes management.
Trial Registration Number
jRCTs031180241.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01438-w.
Keywords: Composite endpoint, Dipeptidyl-peptidase 4 inhibitor, Estimated glomerular filtration rate, Glycated hemoglobin, Luseogliflozin, Overall efficacy, Pulse rate, Sodium-glucose cotransporter 2 inhibitor, Systolic blood pressure, Weight
Key Summary Points
| Why carry out this study? |
| Evidence of a direct comparison between dipeptidyl-peptidase 4 inhibitors (DPP-4is) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) remains lacking. |
| This study aimed to compare the overall efficacy and safety of DPP-4is and the SGLT2i luseogliflozin in patients with type 2 diabetes mellitus. |
| What was learned from this study? |
| The proportion of patients who showed improvement in ≥ 3 endpoints among five composite endpoints (glycated hemoglobin, weight, estimated glomerular filtration rate, systolic blood pressure, and pulse rate) at week 52 was significantly higher in the luseogliflozin group than in the DPP-4i group, regardless body mass index or age. |
| Hepatic function and high-density lipoprotein-cholesterol were significantly improved in the luseogliflozin group compared with the DPP-4i group. |
| This study showed the overall efficacy of luseogliflozin compared with DPP-4is in the mid/long term, suggesting the importance of assessing multiple aspects regarding the effects of diabetes management. |
Introduction
Diabetes mellitus causes diabetic complications (diabetic nephropathy, retinopathy, and neuropathy) as well as other complications, including hypertension, arteriosclerosis, heart failure, and increased mortality [1]. Consequently, the target of diabetes management should focus not only on glycemic control but also on the prevention or suppression of multiple aspects of risk factors. For example, obesity and metabolic syndrome are associated with the onset or progression of type 2 diabetes mellitus (T2DM) [2, 3]. Studies have shown that weight reduction results in the improvement of glucose and lipid metabolism in Japanese patients who are obese [4], and thus weight control is an important treatment goal in the management of T2DM. In addition, systolic blood pressure and pulse rate need to be considered, as a reduction in systolic blood pressure is estimated to suppress death due to stroke or coronary artery disease [5], and a higher pulse rate is associated with metabolic syndrome, hypertension, heart failure, or other cardiovascular diseases [6–8].
The 2018 consensus guidelines of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommended metformin as a first-line medication for T2DM management [9], while the current guidelines in Japan request physicians to select antidiabetic agents depending on the pathological conditions of each patient, failing to show clear medication strategies [10]. Following the approval and launch of dipeptidyl-peptidase 4 inhibitors (DPP-4is) and sodium-glucose cotransporter 2 inhibitors (SGLT2is), the current consensus statements of ADA and EASD were modified to recommend the use of SGLT2is and glucagon-like peptide 1 receptor (GLP1R) agonists owing to the associated beneficial cardiovascular and renal outcomes and further describe the importance of weight reduction [11]. The current guidelines in Japan also recommend appropriate glucose, weight, blood pressure, and lipid control as well as medications to prevent or suppress complications and maintain the patients’ quality-of-life [10].
DPP-4is are the most frequently prescribed oral antidiabetic agent in Japan owing to their high tolerability and modest efficacy, although the use of SGLT2is is increasing [12, 13]. Since additional approval has been obtained for several SGLT2is for heart failure or chronic kidney disease in Japan, the number of prescriptions for SGLT2is is projected to increase in the future. However, studies that directly compare DPP-4is and SGLT2is are lacking, and no clear criteria or rationale have been established to distinguish outcomes between patients receiving DPP-4is or those receiving SGLT2is. Therefore, this study aimed to compare the overall efficacy and safety of DPP-4is and luseogliflozin, an SGLT2i, in patients with T2DM based on the composite endpoint of hemoglobin A1c (HbA1c), weight, estimated glomerular filtration rate (eGFR), systolic blood pressure, and pulse rate, all endpoints which can be easily measured by general practitioners.
Methods
Patients and Study Setting
This ‘Japanese Study for Efficacy of Luseogliflozin on composite Endpoint, Compared to DPP-4is, in Type 2 diabetes mellitus patients’ (J-SELECT) study was a multicenter, open-label, randomized-controlled trial that was conducted at 88 medical institutions in Japan (Electronic Supplementary Material Table S1) under the management of the Japan Physicians Association. Patient enrollment was conducted between January 2018 and November 2020, and each enrolled patient was followed for 12 months. The trial protocol was first approved by the Japan Physicians Association Institutional Review Board according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare in Japan. Before enrolling the first patient, this study was registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) (UMIN000030128) on 27 November 2017. Subsequently, following the enforcement of a new law, ‘the Clinical Trials Act,’ in April 2018 in Japan, this study, it’s protocols and all participating medical institutions were again inspected and approved by the Certified Clinical Research Review Board of Toho University, which obtained certification from the Minister of Health, Labour and Welfare in Japan. After approval from the certified review board, this study was registered in the Japan Registry of Clinical Trials (jRCT no. jRCTs031180241) on 15 March 2019, which is the clinical trial registration developed by the Ministry of Health, Labour, and Welfare in Japan, according to the requirements of the Clinical Trials Act. The study was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, the Clinical Trials Act, and other relevant legal regulations in Japan.
This study enrolled: (1) patients with T2DM who had not used antidiabetic agents within 8 weeks before consenting to participate in the study and (2) those who had used antidiabetic agents other than SGLT2is and DPP-4is and had not changed their usage and dose within 8 weeks before providing informed consent. Details of the inclusion and exclusion criteria are provided in ESM Table S2. The enrolled patients were subsequently randomly assigned into the luseogliflozin group or DPP-4i group with an approximately 1:1 allocation, depending on the following allocation factors assigned by a computer program using a minimization method: sex, age, and body mass index (BMI). In the luseogliflozin group, luseogliflozin (2.5 mg) was administered once a day; in the DPP-4i group, one of the following DPP-4is was administered daily according to each package insert: sitagliptin, vildagliptin, linagliptin, alogliptin, anagliptin, teneligliptin, and saxagliptin. Once-weekly DPP-4is (trelagliptin and omarigliptin) were excluded from this study. The patients were followed up at baseline before the initiation of the study agents and at 2, 24, and 52 weeks after their initiation. During observation period, up to week 24, medication of antidiabetic agents was, in principle, not changed. However, if the HbA1c was ≥ 8.5% in two consecutive measurements before the 24-week time point, or after week 24, rescue therapy was allowed in the following steps: (1) dose change in luseogliflozin or DPP-4is; (2) addition of oral hyperglycemic agents. Even in the rescue therapy, the use of a SGLT2i other than luseogliflozin and any DPP-4is were prohibited in the luseogliflozin group, as was the use of any SGLT2i and DPP-4is other than those specified above. Diet instruction and exercise therapy were not restricted in this study, and were conducted as general practice according to the treatment guideline in Japan [10]. Detailed observation schedules and items are listed in ESM Table S3.
Study Endpoints
The primary (composite) endpoint was the proportion of patients who improved in ≥ 3 endpoints among the five endpoints described above from baseline to week 52. Each primary endpoint, definition of the improvement, and rationale for the definition were as follows: (1) HbA1c (change from baseline ≤ − 0.37%): in the J-DOIT3 trial, HbA1c was − 0.37% lower in an intensive therapy group than in a conventional therapy group, and the frequency of cerebrovascular/renal/retinal events were lower in the intensive therapy group [14]; (2) weight (percentage change from baseline ≤ − 3%): in a previous trial, 3% weight reduction resulted in the improvement of glucose and lipid metabolism in Japanese patients who were obese [4]; (3) eGFR (percentage change from baseline ≥ − 2.2%): in the Gonryo study, the annual change in eGFR was − 2.2% in patients with T2DM without diabetic nephropathy [15]; (4) systolic blood pressure (change from baseline ≤ − 4 mmHg): in reference material published by the Welfare Science Council in the Ministry of Health, Labour and Welfare in Japan, a 4-mmHg reduction in systolic blood pressure was estimated to suppress death caused by stroke or coronary artery disease [5], and the Guidelines for Management of Hypertension 2014 in Japan include targets to decrease systolic blood pressure by 4 mmHg [16]; and (5) pulse rate (change from baseline ≤ − 3 bpm); although it had been reported that elevated heart rate was associated with risk of cardiovascular disease [8, 17], no clear target of the heart rate reduction was determined in these studies and, therefore, we defined the improvement of pulse rate as described above, since measurement error of pulse rate in the same healthy people was ± 2.0 in our unpublished preliminary investigation. Secondary endpoints included: (1) the proportion of patients who improved by ≥ 3 endpoints among the five endpoints from baseline to week 24; (2) change of each endpoint; (3) change in other blood tests and urine tests from baseline to each observation point; and (4) the frequency of any adverse events. The detailed description of the endpoints is provided in Table S4.
Sample Size Determination
The sample size was determined in order to provide adequate power for intergroup comparison of the proportion of patients who achieved the composite endpoint. The proportion of patients who were estimated to improve at each endpoint, reported as mean change ± standard deviation (SD) from baseline, was as follows: (1) since change in HbA1c was reported to be − 0.67 ± 0.66% with SGLT2i use [18] and − 1.1 ± 1.02% with DPP-4i use [19], assuming a normal distribution, the proportion of patients who achieved improved HbA1c was estimated to be 84.5% and 86.0% with SGLT2i and DPP-4i use, respectively; (2) since change in weight was reported to be − 3.5 ± 10.3 kg with SGLT2i use [20] and − 0.4 ± 8.23 kg with DPP-4i use [21], assuming a normal distribution, the proportion of patients who improved weight was estimated to be 63.3% and 51.9%, respectively; (3) since change in systolic blood pressure was reported to be − 3.16 ± 15.3 mmHg with SGLT2i use [22] and − 1.79 ± 11.65 mmHg with DPP-4i use [22], assuming a normal distribution, the proportion of patients whose systolic blood pressure improved was estimated to be 58.3% and 56.1% with SGLT2i and DPP-4i use, respectively; and (4) since change in eGFR was reported to be − 4.7 ± 11.6 mL/min/1.73 m2 with SGLT2i use [23] and − 4.5 ± 14.6 mL/min/1.73 m2 with DPP-4i use [23], assuming a normal distribution, the proportion of patients with improved eGFR was estimated to be 34.3% and 37.9% with SGLT2i and DPP-4i use, respectively. No data were found for pulse rate changes. Based on these assumptions, the proportion of patients who improved in three endpoints among HbA1c, weight, systolic blood pressure, and eGFR was estimated to be 79.1% in the luseogliflozin group and 71.0% in the DPP-4i group. For a two-sided test with a statistical power of 80%, the minimum sample size required to achieve a significance level of 0.05 was determined to be 447 patients in each group. Assuming a dropout rate of approximately 10%, the planned sample size was set at 1000 patients, with 500 patients in each group.
Statistical Analysis
Analyses of the primary and secondary endpoints were performed using the data from the full analysis set (FAS) population, including all patients enrolled in this study who were subsequently randomized to one of the study treatments and excluding those who violated the protocol or those without any data for the primary (composite) endpoint. The proportion of patients who achieved the composite endpoint (primary endpoint and the first secondary endpoint) was compared between the groups using the Chi-squared test. The Chi-squared test or Fisher’s exact test were performed for categorical variables, the two-sample t-test for between-group comparisons, and the one-sample t-test for within-group comparisons for continuous variables. If values deviated from the normal distribution, the Wilcoxon rank-sum test was performed for continuous variables. The adjusted mean change of each continuous variable was estimated using models for repeated measures (MMRM) with an unstructured covariance structure with treatment group, time, interaction between treatment group and time, values at baseline, and allocation factors as fixed effects, and enrolled patients as random effects. All p-values were estimated to be two-sided. Statistical significance was set at a p-value < 0.05. All statistical analyses were performed with a third-party entity (Soiken Inc., Chiyoda, Tokyo, Japan) using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
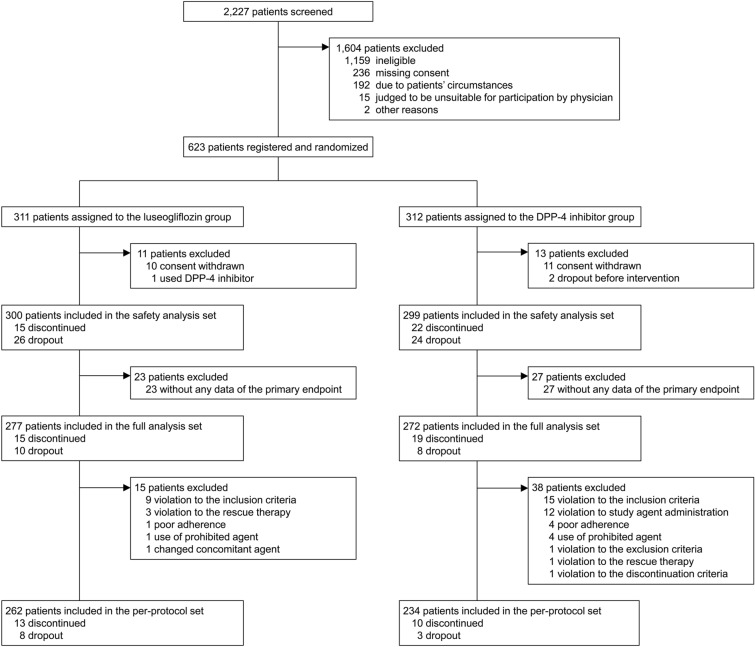
A total of 2227 patients were evaluated for eligibility, and 623 were ultimately enrolled in the study and randomized to either the luseogliflozin or DPP-4i group (Fig. 1). The baseline characteristics of the patients were well balanced between the groups, except for drinking habits (Table 1). Medication of antidiabetic agents, antihypertensive agents, hypolipidemic agents, and antiplatelet agents were not drastically changed during the observation period (ESM Table S5).
Fig. 1.
Study flow chart showing patient enrollment, allocation, and analysis. DPP-4i dipeptidyl-peptidase 4 inhibitor
Table 1.
Patient background data
| Patient characteristics | Luseogliflozin group (n = 277) | DPP-4i group (n = 272) | p-Value | ||
|---|---|---|---|---|---|
| n | Value | n | Value | ||
| Age (year) | 277 | 57.4 ± 10.7 | 272 | 58.1 ± 10.3 | 0.43 |
| Female sex [n (%)] | 277 | 95 (34.3) | 272 | 95 (34.9) | 0.88 |
| Duration of diabetes mellitus (year) | 250 | 4.3 ± 5.3 | 247 | 4.6 ± 5.1 | 0.34 |
| HbA1c (%) | 277 | 7.7 ± 0.7 | 272 | 7.6 ± 0.8 | 0.07 |
| Weight (kg) | 276 | 76.7 ± 15.1 | 271 | 74.9 ± 15.8 | 0.17 |
| eGFR (mL/min/1.73m2) | 274 | 79.9 ± 17.6 | 268 | 80.2 ± 19.0 | 0.85 |
| Systolic blood pressure (mmHg) | 274 | 134.6 ± 17.1 | 270 | 133.3 ± 15.6 | 0.34 |
| Diastolic blood pressure (mmHg) | 274 | 80.3 ± 13.0 | 270 | 79.0 ± 11.6 | 0.24 |
| Pulse (bpm) | 271 | 75.8 ± 11.5 | 258 | 75.2 ± 10.6 | 0.54 |
| Habit of drinking | 277 | 168 (60.6) | 271 | 141 (52.0) | 0.042 |
| Current smoking | 276 | 78 (28.3) | 271 | 61 (22.5) | 0.12 |
| History of cardio-/cerebrovascular disease | 277 | 21 (7.6) | 272 | 18 (6.6) | 0.66 |
| Macrovascular complication | 275 | 13 (4.7) | 269 | 13 (4.8) | 0.95 |
| Cerebrovascular disease | 277 | 1 (0.4) | 271 | 3 (1.1) | 0.37a |
| Coronary artery disease | 276 | 9 (3.3) | 271 | 7 (2.6) | 0.64 |
| Peripheral arterial disease | 275 | 3 (1.1) | 271 | 3 (1.1) | 1.00a |
| Microvascular complication | 254 | 59 (23.2) | 247 | 70 (28.3) | 0.19 |
| Diabetic retinopathy | 250 | 11 (4.4) | 244 | 11 (4.5) | 0.95 |
| Diabetic nephropathy | 272 | 41 (15.1) | 261 | 55 (21.1) | 0.07 |
| Diabetic neuropathy | 270 | 16 (5.9) | 266 | 16 (6.0) | 0.97 |
| Other complication | 277 | 242 (87.4) | 272 | 244 (89.7) | 0.39 |
| Renal disease | 277 | 13 (4.7) | 272 | 15 (5.5) | 0.66 |
| Hepatic disease | 277 | 78 (28.2) | 272 | 79 (29.0) | 0.82 |
| Hypertension | 277 | 169 (61.0) | 272 | 174 (64.0) | 0.47 |
| Dyslipidemia | 277 | 177 (63.9) | 272 | 174 (64.0) | 0.99 |
| Use of anti-diabetic agent | 277 | 119 (43.0) | 272 | 111 (40.8) | 0.61 |
| SGLT2i | 277 | 0 (0.0) | 272 | 0 (0.0) | – |
| DPP-4i | 277 | 0 (0.0) | 272 | 0 (0.0) | – |
| Sulfonylurea | 277 | 2 (0.7) | 272 | 3 (1.1) | 0.68a |
| Biguanide | 277 | 107 (38.6) | 272 | 103 (37.9) | 0.85 |
| Alfa-glucosidase inhibitor | 277 | 8 (2.9) | 272 | 8 (2.9) | 0.97 |
| Glinide | 277 | 4 (1.4) | 272 | 3 (1.1) | 1.00a |
| Thiazolidine | 277 | 15 (5.4) | 272 | 12 (4.4) | 0.59 |
| GLP-1 receptor agonist | 277 | 0 (0.0) | 272 | 0 (0.0) | – |
| Insulin | 277 | 0 (0.0) | 272 | 0 (0.0) | – |
| Antihypertensive agent | 277 | 151 (54.5) | 272 | 157 (57.7) | 0.45 |
| Hypolipidemic agent | 277 | 117 (42.2) | 272 | 123 (45.2) | 0.48 |
| Antiplatelet agent | 277 | 19 (6.9) | 272 | 22 (8.1) | 0.58 |
Data are presented as the mean ± standard deviation (SD) for continuous variables and as n (%) for categorical variables
Chi-squared test or Fisher’s exact test was used for assessing categorical variables and the two-sample t-test was used to assess continuous variables
DPP-4i Dipeptidyl peptidase 4 inhibitor, eGFR estimated glomerular filtration rate, GLP-1 receptor agonist glucagon-like peptide 1 receptor agonist, HbA1c hemoglobin A1c, SGLT2i sodium-glucose cotransporter 2 inhibitor
aIntergroup comparison was conducted using Fisher’s exact test, as it did not meet the requirement of the Chi-squared test
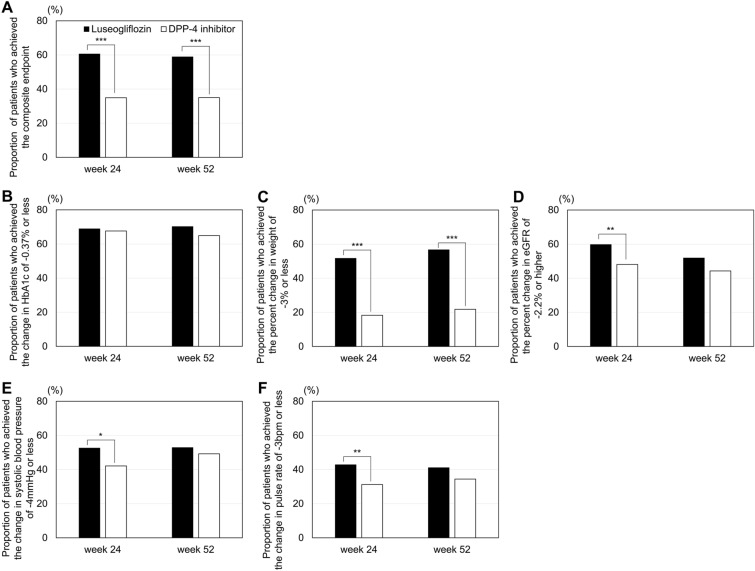
The primary (composite) endpoint in this study was the proportion of patients who showed improvement in ≥ 3 endpoints among HbA1c, weight, eGFR, systolic blood pressure, and pulse rate at week 52. The results showed that this primary (composite) endpoint at week 52 was significantly higher in the luseogliflozin group (58.9%) than in the DPP-4i group (35.0%) (p < 0.001) (Fig. 2A). It was also significantly higher in the luseogliflozin group (60.6%) than in the DPP-4i group (34.9%) (p < 0.001) at week 24. At each endpoint among the composite endpoints, the proportion of patients who showed improved HbA1c (change from baseline ≤ − 0.37%) was comparable in both groups (luseogliflozin group: 68.9% at week 24, 70.2% at week 52; DPP-4i group: 67.6% at week 24, 64.9% at week 52 [p = 0.75], and 0.19 at week 24 and 52, respectively); no significant intergroup difference was observed (Fig. 2B). The proportion of patients with improved weight (percent change from baseline ≤ − 3%) was significantly higher in the luseogliflozin group (51.7% and 56.7% at weeks 24 and 52, respectively) than in the DPP-4i group (18.3% and 21.8% at weeks 24 and 52, respectively) (both p < 0.001) (Fig. 2C). The proportion of patients with improved eGFR (percent change from baseline ≥ − 2.2%), systolic blood pressure (change from baseline ≤ − 4 mmHg), and pulse rate (change from baseline ≤ − 3 bpm) was significantly higher in the luseogliflozin group (59.8%, 52.6%, and 42.8%, respectively) than in the DPP-4i group (48.1%, 42.1%, and 31.2%, respectively) (p = 0.007, 0.016, and 0.007, respectively) at week 24; however, no significant intergroup difference was observed at week 52 (Fig. 2D–F).
Fig. 2.
Proportion of patients who achieved the composite endpoint (A) or each endpoint (B–F). The proportion of patients who achieved each endpoint was compared between the luseogliflozin and DPP-4i groups using the Chi-squared test. Asterisks indicate a significant difference between groups at *p < 0.05, **p < 0.01, and ***p < 0.001. eGFR Estimated glomerular filtration rate, HbA1c Hemoglobin A1c
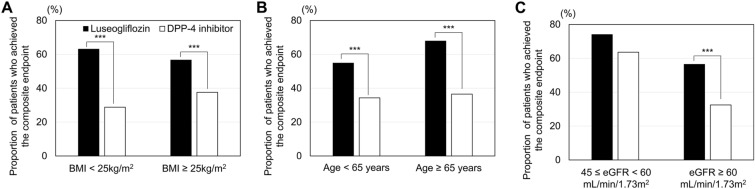
Stratified analyses were conducted based on the baseline BMI, age, and eGFR. In patients whose BMI was < 25 or ≥ 25 kg/m2, the proportion of patients who achieved the composite endpoint was significantly higher in the luseogliflozin group (63.1% and 56.7%, respectively) than in the DPP-4i group (28.8% and 37.6%, respectively) (both p < 0.001) (Fig. 3). Similarly, in patients whose age was < 65 or ≥ 65 years, the proportion of patients who achieved the composite endpoint was significantly higher in the luseogliflozin group (54.9% and 67.9%, respectively) than in the DPP-4i group (34.3% and 36.5%, respectively) (p < 0.001).
Fig. 3.
Proportion of patients who achieved the composite endpoint stratified by baseline BMI (A), age (B), or eGFR (C). The proportion of patients who achieved the composite endpoint was compared between the luseogliflozin and DPP-4i groups using the Chi-squared test. Asterisks indicate a significant difference between groups at *p < 0.05, **p < 0.01, and ***p < 0.001. BMI Body mass index
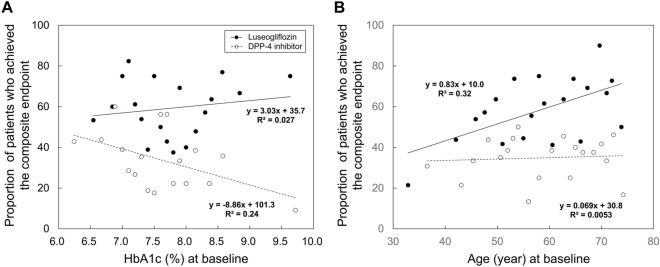
Linear regression analysis showed that HbA1c at baseline was negatively correlated with the proportion of patients who achieved the composite endpoint at week 52 in the DPP-4i group (regression coefficient − 8.86, R2 = 0.24, p < 0.05), whereas HbA1c at baseline was not significantly correlated with the proportion of patients who achieved the composite endpoint at week 52 in the luseogliflozin group (regression coefficient 3.03, R2 = 0.027, p = 0.51) (Fig. 4A). In contrast, age was positively correlated with the proportion of patients who achieved the composite endpoint at week 52 in the luseogliflozin group (regression coefficient 0.83, R2 = 0.32, p < 0.01), whereas age was not significantly correlated with the proportion of patients who achieved the composite endpoint at week 52 in the DPP-4i group (regression coefficient 0.069, R2 = 0.0053, p = 0.76) (Fig. 4B).
Fig. 4.
Correlation between HbA1c (A) or age (B) at baseline and proportion of patients who achieved the composite endpoint at week 52. HbA1c or age at baseline was divided into 20 quartiles, with the mean HbA1c or age at each quartile plotted as the X value and the proportion of patients who achieved the composite endpoint at week 52 plotted as the Y value. DPP-4 dipeptidyl-peptidase 4
The adjusted mean change in HbA1c from baseline to weeks 24 and 52 did not differ between the groups (p = 0.67 and 0.18, respectively), whereas weight significantly decreased in the luseogliflozin group compared to the DPP-4i group (both p < 0.001) (Table 2). Post-hoc analysis showed that the weight reduction in the luseogliflozin group was around 4%, regardless of BMI or age, while in the DPP-41 group, weight decreased in patients with higher BMI and increased in patients with lower BMI (ESM Fig. S1). The adjusted mean changes in eGFR, systolic blood pressure, and pulse rate did not differ between the groups, except for systolic blood pressure at week 24 (p = 0.023). Among the hepatic function and lipid biomarkers, high-density lipoprotein cholesterol (HDL-chol) (both p < 0.001) significantly increased; conversely, aspartate aminotransferase (AST) (both p < 0.001), alanine aminotransferase (ALT) (both p < 0.001), and gamma-glutamyl transpeptidase (γ-GTP) (both p < 0.001) levels significantly decreased from baseline to weeks 24 and 52 in the luseogliflozin group compared with those in the DPP-4i group (Table 2; ESM Fig. S2). Among hematological tests, red blood cells (both p < 0.001), hemoglobin (both p < 0.001), and hematocrit (both p < 0.001) significantly increased from baseline to weeks 24 and 52. Meanwhile, white blood cell count significantly decreased from baseline to week 52 (p < 0.001) in the luseogliflozin group compared with the DPP-4i group (ESM Table S6). Erythropoietin (p = 0.004) and reticulocytes (p < 0.001) significantly increased at week 2 in the luseogliflozin group compared with the DPP-4i group. Renal function biomarkers, uric acid (both p < 0.001 at weeks 24 and 52), and urinary creatinine (p = 0.005 at week 24) were significantly decreased in the luseogliflozin group compared with the DPP-4i group; however, urinary albumin-to-creatinine ratio (UACR) (p = 0.002 and 0.048 at weeks 2 and 24, respectively) significantly increased in the luseogliflozin group compared with the DPP-4i group, although the significant difference in changes in UACR disappeared at week 52. The change in serum N-terminal pro B-type natriuretic peptide (NT-proBNP) levels did not differ significantly between the groups.
Table 2.
Changes in laboratory test values and vital signs of patients in the luseogliflozin and dipeptidyl-peptidase 4 inhibitor groups
| Patient laboratory test measures and vital signs | Week | Luseogliflozin group | DPP-4i group | Intergroup difference in adjusted mean change (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Measurement (n patients) | Adjusted mean change from baseline (SE)a | Measurement (n patients) | Adjusted mean change from baseline (SE)a | ||||
| HbA1c (%) | 0 | 7.7 ± 0.7 (277) | 7.6 ± 0.8 (272) | ||||
| 24 | 7.0 ± 0.7 (273) | − 0.7 (0.0) | 7.0 ± 0.9 (262) | − 0.7 (0.0) | 0.0 (− 0.1, 0.1) | 0.67 | |
| 52 | 7.0 ± 0.8 (265) | − 0.7 (0.0) | 7.0 ± 0.9 (262) | − 0.6 (0.0) | − 0.1 (− 0.2, 0.0) | 0.18 | |
| Weight (kg)b | 0 | 76.7 ± 15.1 (276) | 74.9 ± 15.8 (271) | ||||
| 24 | 74.1 ± 15.2 (269) | − 3.6 (0.2) | 74.6 ± 16.1 (257) | − 0.4 (0.2) | − 3.2 (− 3.8, − 2.6) | < 0.001 | |
| 52 | 73.7 ± 15.2 (264) | − 4.0 (0.3) | 74.7 ± 16.1 (257) | − 0.7 (0.3) | − 3.2 (− 3.9, − 2.5) | < 0.001 | |
| eGFR (mL/min/1.73 m2)b | 0 | 79.9 ± 17.6 (274) | 80.2 ± 19.0 (268) | ||||
| 24 | 79.2 ± 19.2 (268) | − 0.4 (0.8) | 77.8 ± 18.9 (262) | − 2.4 (0.8) | 2.0 (− 0.1, 4.0) | 0.06 | |
| 52 | 78.4 ± 18.7 (265) | − 1.6 (0.8) | 76.7 ± 17.4 (259) | − 3.3 (0.8) | 1.7 (− 0.4, 3.7) | 0.11 | |
| Systolic blood pressure (mmHg) | 0 | 134.6 ± 17.1 (274) | 133.3 ± 15.6 (270) | ||||
| 24 | 129.7 ± 16.0 (268) | − 4.2 (0.9) | 131.9 ± 16.3 (263) | − 1.4 (0.9) | − 2.8 (− 5.2, − 0.4) | 0.023 | |
| 52 | 130.0 ± 15.8 (266) | − 3.6 (0.9) | 130.2 ± 15.7 (262) | − 2.8 (0.9) | − 0.8 (− 3.2, 1.5) | 0.48 | |
| Diastolic blood pressure (mmHg) | 0 | 80.3 ± 13.0 (274) | 79.0 ± 11.6 (270) | ||||
| 24 | 77.4 ± 11.8 (268) | − 3.2 (0.6) | 78.2 ± 10.4 (263) | − 1.9 (0.6) | − 1.4 (− 3.0, 0.2) | 0.09 | |
| 52 | 77.5 ± 11.4 (266) | − 2.8 (0.6) | 76.8 ± 11.3 (262) | − 3.0 (0.6) | 0.2 (− 1.5, 1.8) | 0.85 | |
| Pulse rate (bpm) | 0 | 75.8 ± 11.5 (271) | 75.2 ± 10.6 (258) | ||||
| 24 | 75.8 ± 12.0 (261) | − 0.4 (0.6) | 76.4 ± 11.5 (254) | 0.8 (0.6) | − 1.2 (− 2.8, 0.4) | 0.13 | |
| 52 | 75.0 ± 11.9 (263) | − 1.0 (0.6) | 75.9 ± 12.1 (252) | 0.4 (0.6) | − 1.4 (− 3.1, 0.2) | 0.09 | |
| HDL-chol (mg/dL)b | 0 | 53.3 ± 13.7 (271) | 53.6 ± 14.1 (266) | ||||
| 24 | 56.5 ± 14.2 (264) | 7.3 (0.9) | 54.0 ± 14.5 (260) | 2.4 (0.9) | 4.9 (2.5, 7.4) | < 0.001 | |
| 52 | 57.0 ± 14.7 (260) | 8.5 (1.0) | 54.6 ± 15.2 (257) | 3.9 (1.0) | 4.6 (2.0, 7.3) | < 0.001 | |
| T-chol (mg/dL)b | 0 | 200.4 ± 35.1 (250) | 194.7 ± 36.0 (233) | ||||
| 24 | 200.1 ± 35.3 (246) | 0.6 (0.8) | 192.2 ± 32.9 (231) | − 1.1 (0.8) | 1.7 (− 0.5, 4.0) | 0.13 | |
| 52 | 199.6 ± 39.8 (240) | 0.7 (0.9) | 191.2 ± 32.0 (226) | − 0.7 (0.9) | 1.4 (− 1.0, 3.9) | 0.26 | |
| LDL-chol (mg/dL)b | 0 | 118.5 ± 31.0 (204) | 109.1 ± 29.8 (189) | ||||
| 24 | 117.3 ± 29.8 (213) | 2.5 (1.6) | 106.8 ± 28.4 (185) | − 1.6 (1.6) | 4.1 (− 0.2, 8.4) | 0.06 | |
| 52 | 116.5 ± 34.0 (202) | 2.1 (1.7) | 108.5 ± 29.2 (184) | 0.2 (1.8) | 1.8 (− 3.0, 6.6) | 0.46 | |
| TG (mg/dL) | 0 | 169.3 ± 112.2 (232) | 173.7 ± 133.5 (223) | ||||
| 24 | 149.3 ± 112.1 (231) | 151.0 ± 91.3 (210) | |||||
| 52 | 157.1 ± 134.5 (228) | 150.8 ± 87.2 (210) | |||||
| TG (log-transformed) (ln(mg/dL))b | 0 | 4.96 ± 0.57 (232) | 4.97 ± 0.58 (223) | ||||
| 24 | 4.84 ± 0.54 (231) | − 2.78 (0.62) | 4.86 ± 0.56 (210) | − 1.62 (0.64) | − 1.17 (− 2.84, 0.51) | 0.17 | |
| 52 | 4.86 ± 0.57 (228) | − 2.12 (0.64) | 4.87 ± 0.53 (210) | − 1.60 (0.66) | − 0.52 (− 2.26, 1.23) | 0.56 | |
| AST (IU/L) | 0 | 32.4 ± 19.6 (272) | 30.0 ± 18.6 (267) | ||||
| 24 | 27.8 ± 23.6 (268) | 31.2 ± 19.8 (261) | |||||
| 52 | 26.6 ± 19.6 (264) | 30.3 ± 18.5 (257) | |||||
| AST (log-transformed) (ln(IU/L)) | 0 | 3.35 ± 0.48 (272) | 3.28 ± 0.46 (267) | ||||
| 24 | 3.19 ± 0.43 (268) | − 0.15 (0.02) | 3.30 ± 0.49 (261) | − 0.01 (0.02) | − 0.15 (− 0.21, − 0.09) | < 0.001 | |
| 52 | 3.17 ± 0.40 (264) | − 0.16 (0.02) | 3.28 ± 0.48 (257) | − 0.03 (0.02) | − 0.14 (− 0.20, − 0.08) | < 0.001 | |
| ALT (IU/L) | 0 | 43.9 ± 32.7 (272) | 39.9 ± 27.1 (268) | ||||
| 24 | 33.5 ± 28.6 (266) | 39.5 ± 30.0 (262) | |||||
| 52 | 32.8 ± 25.8 (263) | 38.5 ± 32.0 (258) | |||||
| ALT (log-transformed) (ln(IU/L)) | 0 | 3.56 ± 0.65 (272) | 3.49 ± 0.61 (268) | ||||
| 24 | 3.31 ± 0.59 (266) | − 0.28 (0.03) | 3.45 ± 0.64 (262) | − 0.08 (0.03) | − 0.20 (− 0.27, − 0.13) | < 0.001 | |
| 52 | 3.29 ± 0.59 (263) | − 0.29 (0.03) | 3.41 ± 0.66 (258) | − 0.12 (0.03) | − 0.16 (− 0.24, − 0.09) | < 0.001 | |
| γ-GTP (IU/L) | 0 | 61.5 ± 49.8 (269) | 54.1 ± 45.0 (264) | ||||
| 24 | 53.3 ± 87.3 (266) | 60.6 ± 138.6 (260) | |||||
| 52 | 49.0 ± 57.2 (262) | 53.8 ± 61.1 (258) | |||||
| γ-GTP (log-transformed) (ln(IU/L)) | 0 | 3.86 ± 0.72 (269) | 3.75 ± 0.67 (264) | ||||
| 24 | 3.63 ± 0.71 (266) | − 0.24 (0.03) | 3.70 ± 0.75 (260) | − 0.08 (0.03) | − 0.16 (− 0.23, − 0.09) | < 0.001 | |
| 52 | 3.60 ± 0.69 (262) | − 0.26 (0.03) | 3.68 ± 0.72 (258) | − 0.12 (0.03) | − 0.14 (− 0.22, − 0.07) | < 0.001 | |
Data on measurements are presented as the mean ± SD, with the number of patients (n) given in parentheses for measurements, and data on the adjusted mean change from baseline are presented as the mean with the standard error (SE)) in parentheses
ALT alanine aminotransferase, AST aspartate aminotransferase, CI confidence interval, γ-GTP gamma-glutamyl transpeptidase, HDL-chol high-density lipoprotein cholesterol, LDL-chol low-density lipoprotein cholesterol, T-chol total cholesterol, TG triglyceride
aThe adjusted mean change of each continuous variable was estimated using models for repeated measures (MMRM) with an unstructured covariance structure with treatment group, time, interaction between treatment group and time, values at baseline, and allocation factors as fixed effects, and enrolled patients as random effects
bAdjusted mean percentage change was calculated instead of the adjusted mean change
Among the patients whose BMI was ≥ 25 kg/m2, HbA1c, weight, AST, ALT, γ-GTP, red blood cell, hemoglobin, hematocrit, white blood cell, uric acid, fatty liver index (FLI), and the Fibrosis-4 (FIB-4) Index significantly improved in the luseogliflozin group compared with the DPP-4i group; among the patients whose BMI was < 25 kg/m2, weight, eGFR, HDL-chol, hemoglobin, hematocrit, uric acid, and amylase significantly improved in the luseogliflozin group compared with the DPP-4i group (ESM Table S7). Among the patients whose age was < 65 years, HbA1c, weight, HDL-chol, AST, ALT, γ-GTP, red blood cells, hemoglobin, hematocrit, white blood cells, uric acid, FLI, and FIB-4 index significantly improved in the luseogliflozin group compared with the DPP-4i group; among the patients whose age was ≥ 65 years, weight, eGFR, hemoglobin, hematocrit, uric acid, and FLI significantly improved in the luseogliflozin group compared with the DPP-4i group (ESM Table S8). Among the patients whose eGFR was ≥ 60 mL/min/1.73 m2, weight, HDL-chol, AST, ALT, γ-GTP, red blood cell, hemoglobin, hematocrit, white blood cell, uric acid, FLI, and FIB-4 index significantly improved in the luseogliflozin group compared with the DPP-4i group; among the patients with eGFR ≥ 45 mL/min/1.73 m2 and < 60 mL/min/1.73 m2, only weight significantly improved in the luseogliflozin group compared with the DPP-4i group (ESM Table S9).
The adverse events that occurred during the study are listed in ESM Table S10. No deaths were reported in either group, and the frequency of any non-serious/serious adverse events did not differ between the groups. The most frequently occurring adverse event in the luseogliflozin group was genital pruritus (6 patients [2.0%] in the luseogliflozin group and 0 patients [0.0%] in the DPP-4i group), and that in the DPP-4i group was upper respiratory tract infection (4 patients [1.3%] in the luseogliflozin group and 10 patients [3.3%] in the DPP-4i group). The adverse events stratified by the baseline BMI and age are listed in ESM Tables S11 and S12, respectively.
Discussion
This study showed that luseogliflozin improved the composite endpoint of HbA1c, weight, eGFR, systolic blood pressure, and pulse rate for 52 weeks compared with DPP-4is, suggesting the mid-/long-term overall efficacy of luseogliflozin. Previous studies reported the efficacy of DPP-4is and SGLT2is in improving endpoints. Nauck et al. reported that SGLT2i reduced weight [24], whereas other studies showed that DPP-4i had a neutral effect or caused a modest increase in weight [25, 26]. Both DPP-4is and SGLT2is were shown to reduce blood pressure [27–29]; however, the degree of the reduction was greater with SGLT2is [29]. Furthermore, studies have shown that both DPP-4is and SGLT2is exhibit a renal protective effect to reduce renal albumin and either improve or ameliorate the decrease in eGFR [30–32]. Administration of luseogliflozin decreased heart rate in Japanese patients with T2DM with higher heart rate [33], and switching from DPP-4is to luseogliflozin has been shown to decrease nighttime pulse rate [34]. However, to our knowledge, few studies have investigated and compared the overall efficacy of DPP-4is and SGLT2is. When the efficacy of DPP-4is and luseogliflozin was assessed using specific endpoints in this study, although weight significantly decreased in the luseogliflozin group, changes in HbA1c, eGFR, and pulse rate did not differ significantly between the groups. Although systolic blood pressure significantly decreased at week 24, this observation disappeared at week 52. The proportion of patients with improved HbA1c was comparable in both groups, and the proportion of patients with improved eGFR, systolic blood pressure, and pulse rate was significantly higher in the luseogliflozin group than in the DPP-4i group at week 24, but not at week 52. In contrast, the proportion of patients who achieved the composite endpoint was significantly higher in the luseogliflozin group than in the DPP-4i group at weeks 24 and 52. This finding suggests the importance of assessing multiple aspects regarding the effects of diabetes management. Although the final goal of diabetes management is to prevent or suppress complications and maintain the quality-of-life of patients, as described in the consensus statements of the ADA and EASD [11] or the Japanese guidelines [10], it is challenging to assess these in general practice. Therefore, this study employed the composite endpoint of surrogate markers (HbA1c, weight, eGFR, systolic blood pressure, and pulse rate), which can be measured easily also by general practitioners.
The proportion of patients who achieved the composite endpoint was significantly higher in the luseogliflozin group than in the DPP-4i group, regardless of BMI or age—or rather the proportion of patients who achieved the composite endpoint—was higher by age. Although the proportion of patients who achieved the composite endpoint was lower by HbA1c at baseline was higher in the DPP-4i group, it was consistently higher regardless of HbA1c at baseline in the luseogliflozin group. These results suggested the overall beneficial effect of luseogliflozin in a wide range of patients with T2DM. Older people tend to have more comorbidities and problems with polypharmacy [35–37]. The multiple benefits of luseogliflozin in older patients could reduce the number of prescriptions, improve medication adherence, and improve patients’ quality of life. The results of this study also suggested the safety profile of luseogliflozin, regardless of BMI or age—or rather the frequency of adverse events—was numerically lower even in the non-obese patients or older patients. Since luseogliflozin induces body weight reduction, attention should be paid to a decrease in muscle mass, especially in older non-obese patients, which may cause sarcopenia and frailty. However, a single-arm intervention study reported that decrease in skeletal muscle mass by luseogliflozin was relatively smaller than the reduction in fat mass and visceral fat area [38]. A randomized controlled trial of empagliflozin is currently in progress to assess the effect of this medication on body composition, including skeletal muscle mass, muscle strength, and physical performance, in older Japanese patients with T2DM [39]. Although this study did not measure body composition, because this study employed endpoints that could be easily measured by general practitioners, this study showed that luseogliflozin induced constant weight loss, regardless of BMI or age. In addition, although this study did not exclude patients with lower BMI (< 20 kg/m2), only a few lean patients were enrolled in this study, probably because this study excluded patients with geriatric syndrome, such as sarcopenia, and at the launch of SGLT2is in Japan, several physicians expressed concern about the weight reduction by SGLT2is in lean patients, especially in the elderly patient population. However, a recent post-marketing surveillance of empagliflozin showed that there was almost empagliflozin-induced weight reduction in patients with BMI of < 20 kg/m2, regardless of age [40]. These results may suggest that SGLT2is do not cause remarkable or even harmful weight reduction in older non-obese patients.
Luseogliflozin also significantly improved hepatic function and HDL-chol. Furthermore, luseogliflozin increased erythropoietin and reticulocyte levels at week 2, and subsequently red blood cell counts increased at weeks 24 and 52. This result suggests that luseogliflozin promotes hematopoiesis. Since SGLT2is exert a renal protective effect [31, 32] and improve proximal renal tubule function, luseogliflozin increased the production of erythropoietin in the kidney and hematopoiesis. Luseogliflozin further improved the biomarkers of hepatic steatosis and fibrogenesis. These results also suggest the overall efficacy of luseogliflozin in a wide range of clinical aspects compared with that of DPP-4is.
This study has several limitations. First, this was an open-label study that lacked blinding for both patients and physicians, which may have caused some bias in this study. However, since the components of the composite endpoint (HbA1c, weight, eGFR, systolic blood pressure, and pulse rate) were objectively measurable, we believe that the open-label study design did not considerably introduce bias into the results. Second, this study was conducted only in medical institutions in Japan. Therefore, the generalizability of the results of this study to other countries or to patients of other ethnicities is unknown. Therefore, further international investigation is required. Third, this study did not reach the target sample size, which was set at 1000 patients; only 623 patients were enrolled and randomized. One possible reason for this undersized sample size is that this study enrolled drug-naïve or SGLT2i/DPP-4i-naïve patients. Since DPP-4is are the most frequently prescribed oral antidiabetic agents in Japan, with currently > 60% of patients with T2DM receiving DPP-4is, and the use of SGLT2is is increasing [12, 13], it was difficult to screen eligible candidates for this study. However, intergroup differences in the proportion of patients who achieved improvement in weight, systolic blood pressure, and eGFR were greater than those assumed in the sample size calculation. As such, pulse rate was higher in the luseogliflozin group than in the DPP-4i group; this was not previously estimated because of the lack of reference data. As a result, despite the small sample size, significant intergroup differences were detected in the proportion of patients who achieved the composite endpoint. Fourth, as rescue therapy, if HbA1c ≥ 8.5% was measured twice consecutively, or after week 24, dose change in luseogliflozin or DPP-4is and addition of oral hyperglycemic agents were allowed for better glycemic control. This may be one reason that while the significant intergroup difference was detected in the proportion of patients with improved eGFR, systolic blood pressure, and pulse rate at week 24, the significant intergroup differences disappeared at week 52. Fifth, although the original plan of this study was to conduct the stratified analysis using a baseline eGFR of < 45 mL/min/1.73 m2, ≥ 45 mL/min/1.73 m2, < 60 mL/min/1.73 m2, and ≥ 60 mL/min/1.73 m2, the patients enrolled in this study tended to have a higher eGFR (79.9 ± 17.6 mL/min/1.73 m2 in the luseogliflozin group and 80.2 ± 19.0 mL/min/1.73 m2 in the DPP-4i group), and the group with eGFR < 45 mL/min/1.73 m2 consisted only of four patients (with 51 patients in the group with eGFR ≥ 45 mL/min/1.73 m2 and < 60 mL/min/1.73 m2 and 466 patients in the group with eGFR ≥ 60 mL/min/1.73 m2). Accordingly, statistical power may be lacking in the group with eGFR ≥ 45 mL/min/1.73 m2 and < 60 mL/min/1.73 m2 owing to the small number of patients. This deflection in eGFR might make it difficult to determine its effect on the efficacy of luseogliflozin. This could be attributed to the mode of action of SGLT2is to inhibit glucose reabsorption in the proximal renal tubule and enhance glucose excretion into urine. At the launch of SGLT2is in Japan, several physicians expressed concern that the effect of SGLT2is might be weakened or have harmful effects on patients with worsened renal function. However, the renal protective effect of SGLT2is was reported later [31, 32], and additional approval was obtained in Japan for SGLT2is for chronic kidney disease. Future analyses should include patients with lower eGFR or renal dysfunction to clarify their effects on patients with T2DM.
Conclusion
This study showed the overall efficacy of luseogliflozin compared with DPP-4is in the mid/long term, regardless of BMI or age. Hepatic function and HDL-cholesterol were also significantly improved in the luseogliflozin group compared with the DPP-4i group. The results suggest the importance of assessing multiple aspects regarding the effects of diabetes management.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all of the clinical staff for their assistance in the execution of the study and all the participants in the study.
Funding
This study was financially supported by Taisho Pharmaceutical Co., Ltd. The journal’s Rapid Service Fee, fee for the technical assistance in the launch and execution of the study ,and the medical writing of the manuscript by Soiken Inc. were also financially supported by Taisho Pharmaceutical Co., Ltd.
Medical Writing, Editorial, and Other Assistance
The authors thank Soiken Inc. for their technical assistance in the launch and execution of the study and Arata Yoneda in Soiken Inc. for his support on the medical writing of the manuscript. Fee for the technical assistance and the medical writing by Soiken Inc. was covered by the research fund provided by Taisho Pharmaceutical Co., Ltd.
Author Contributions
Masahiro Sugawara, Koichi Mochizuki, and Masahiro Fukuda contributed to the conception and design of the study, development and amendment of the study protocol, patient enrollment, study implementation, data collection, and writing of this article. Ichiro Sakuma, Yutaka Wakasa, Hideaki Funayama, Hiroaki Seino, Akira Kondo, Naoki Itabashi, Yasuyuki Maruyama, Takashi Kamiyama, Yasunori Utsunomiya, Akira Yamauchi, and Hidenori Yoshii contributed to patient enrollment, study implementation, and data collection. Hirokazu Yamada conducted the statistical analysis of this study, and provided intellectual input on the sample size determination and the statistical discussion. All authors have read and approved the final manuscript.
Disclosures
Masahiro Fukuda received personal profits from Ono Pharmaceutical Co. Ltd. Ichiro Sakuma received research funds from the National Cerebral and Cardiovascular Center and personal profits from Kowa Company Ltd., Novo Nordisc Pharma Ltd., Japan Boehringer Ingelheim, Sumitomo Pharma Co. Ltd., Kyowa Kirin Co. Ltd., and Sanwa Kagaku Kenkyusho Co. Ltd. The remaining authors declare no conflicts of interest.
Compliance with Ethics Guidelines
The trial protocol was first approved by the Japan Physicians Association Institutional Review Board according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare in Japan. Before enrolling the first patient, this study was registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) (UMIN000030128) on November 27, 2017. Subsequently, following the enforcement of a new law, ‘the Clinical Trials Act,’ in April 2018 in Japan, this study and its protocols were again inspected and approved by the Certified Clinical Research Review Board of Toho University, which obtained certification from the Minister of Health, Labour and Welfare in Japan. After approval from the certified review board, this study was registered in the Japan Registry of Clinical Trials (jRCT) (jRCTs031180241) on March 15, 2019, which is the clinical trial registration developed by the Ministry of Health, Labour, and Welfare in Japan, according to the requirements of the Clinical Trials Act. The study was conducted in accordance with the Declaration of Helsinki and its later amendments, the Clinical Trials Act, and other current legal regulations in Japan. Written informed consent was obtained prior to treatment from all enrolled patients who met the eligibility criteria.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the lack of a statement in the study protocol enabling data sharing with a third party after the end of the study and in the informed consent documents as well as lack of approval for data sharing by the Japan Physicians Association Clinical Research Review Board.
Contributor Information
Masahiro Sugawara, Email: ms@sugawara.or.jp.
the J-SELECT study investigators:
Hiroaki Seino, Kaori Murata, Shigeo Yatagai, Hiroshi Koyama, Hareaki Yamamoto, Miho Shimizu, Toshio Kawada, Setsuya Sakagashira, Shigehiko Ozeki, Tomoo Takeda, Tomohiro Katsuya, Mariko Oishi, Ken-ich Doniwa, Nobuyuki Ueda, Makiko Sasamoto, Hatsumi Masaki, Takashi Kamiyama, Woon-Joo Lee, Hiroko Chimori, Hiroshi Takeda, Kazuo Ikeda, Hiroaki Nishioka, Kyoko Mitsuhashi, Toru Kinugawa, Motoko Miki, Toshiyuki Horiuchi, Kunihiro Doi, Yuki Shinagawa, Isato Shimozono, Jinro Ishizuka, Shunichiro Sakurai, Shigeki Moritani, Norio Kase, Shigeru Watanabe, Shinsuke Nakata, Keiko Tsunoda, Tadashi Sawanishi, Yuji Ogawa, Tomokazu Matsuda, Tomohiro Tsuji, Shinichiro Shirabe, Satoshi Ashitomi, Hiromi Ogata, Kaneyuki Matsuo, Takashi Sugie, Ken Takenaka, Asami Tanaka, Yoshiro Suzuki, Masahiro Inoue, Hiroshi Hasegawa, Haruyoshi Nakao, Tetsuo Nishikawa, Mikio Uematsu, Daigaku Uchida, Masaaki Miyakawa, Masahiro Takihata, Hirotaka Ishii, Kenji Mizuno, Masahiko Inomata, Kosuke Minamisawa, Soichi Honda, Mitsuo Shirakawa, Katsuya Fuse, Takuji Yamao, Akihiko Nakazima, Masahiro Nagano, Masahiko Nakamura, Suzuko Iwami, Hisakazu Degawa, Naoko Katayanagi, Yoshiharu Okada, Hideaki Sawaki, Hiromi Ogata, Motoshige Miyano, and Yuki Matsuda
References
- 1.Demir S, Nawroth PP, Herzig S, Ekim ÜB. Emerging targets in type 2 diabetes and diabetic complications. Adv Sci (Weinh) 2021;8:e2100275. doi: 10.1002/advs.202100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99:462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou K, Donnelly LA, Morris AD, et al. Clinical and genetic determinants of progression of type 2 diabetes: a DIRECT study. Diabetes Care. 2014;37:718–724. doi: 10.2337/dc13-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muramoto A, Matsushita M, Kato A, et al. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract. 2014;8:e466–475. doi: 10.1016/j.orcp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.The Welfare Science Council in the Ministry of Health, Labour and Welfare in Japan. Reference material on the promotion of Healthy Japan 21 (Second). 2012. https://www.mhlw.go.jp/bunya/kenkou/dl/kenkounippon21_02.pdf. Accessed 4 Feb 2019.
- 6.Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15:3–17. doi: 10.1097/00004872-199715010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Iseki K, Iseki C, Kinjo K, Ohya Y, Takishita S. Higher heart rate predicts the risk of developing hypertension in a normotensive screened cohort. Circ J. 2007;71:1755–1760. doi: 10.1253/circj.71.1755. [DOI] [PubMed] [Google Scholar]
- 8.Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis. 2009;52:6–10. doi: 10.1016/j.pcad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Japan Diabetes Society. Japanese clinical practice guideline for diabetes 2022–2023. Bunkodo: Japan Diabetes Society; 2023.
- 11.Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2022;45:2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchi R, Sugiyama T, Goto A, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2022;13:280–291. doi: 10.1111/jdi.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M, Doi K, Sugawara M, Mochizuki K (2019) Efficacy and safety of sitagliptin in elderly patients with type 2 diabetes mellitus A focus on hypoglycemia. J Diabetes Investig 10:383–91. [DOI] [PMC free article] [PubMed]
- 14.Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–964. doi: 10.1016/S2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 15.Iwai T, Miyazaki M, Yamada G, et al. Diabetes mellitus as a cause or comorbidity of chronic kidney disease and its outcomes: the Gonryo study. Clin Exp Nephrol. 2018;22:328–336. doi: 10.1007/s10157-017-1451-4. [DOI] [PubMed] [Google Scholar]
- 16.The Japanese Society of Hypertension. The guidelines for management of hypertension 2014. 2014. https://www.jpnsh.jp/download_gl.html. Accessed 12 Dec 2017.
- 17.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 18.Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 19.Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506–515. doi: 10.1111/j.1463-1326.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 20.Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–4021. doi: 10.2337/dc13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–1160. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 23.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 26.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 27.Lovshin JA, Zinman B. Blood pressure-lowering effects of incretin-based diabetes therapies. Can J Diabetes. 2014;38:364–371. doi: 10.1016/j.jcjd.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–430. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Zhao Q. Effects of dipeptidyl peptidase-4 inhibitors on blood pressure in patients with type 2 diabetes: a systematic review and meta-analysis. J Hypertens. 2016;34:167–175. doi: 10.1097/HJH.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 30.Kim YG, Byun J, Yoon D, et al. Renal protective effect of DPP-4 inhibitors in type 2 diabetes mellitus patients: a cohort study. J Diabetes Res. 2016;2016:1423191. doi: 10.1155/2016/1423191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 33.Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: Subanalysis of placebo-controlled, double-blind clinical trials. J Diabetes Investig. 2018;9:638–641. doi: 10.1111/jdi.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto-Kameda R, Cho KY, Nomoto H, et al. Lowering of blood pressure and pulse rate by switching from DPP-4 inhibitor to luseogliflozin in patients with type 2 diabetes complicated with hypertension: A multicenter, prospective, randomized, open-label, parallel-group comparison trial (LUNA study) Diabetes Res Clin Pract. 2021;180:109069. doi: 10.1016/j.diabres.2021.109069. [DOI] [PubMed] [Google Scholar]
- 35.Horii T, Iwasawa M, Kabeya Y, Atuda K. Polypharmacy and oral antidiabetic treatment for type 2 diabetes characterised by drug class and patient characteristics: a Japanese database analysis. Sci Rep. 2019;9:12992. doi: 10.1038/s41598-019-49424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabuchi T, Hosomi K, Yokoyama S, Takada M. Polypharmacy in elderly patients in Japan: analysis of Japanese real-world databases. J Clin Pharm Ther. 2020;45:991–996. doi: 10.1111/jcpt.13122. [DOI] [PubMed] [Google Scholar]
- 37.Dovjak P. Polypharmacy in elderly people. Wien Med Wochenschr. 2022;172:109–113. doi: 10.1007/s10354-021-00903-0. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Sugawara M, Fukuda M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the components of weight loss in Japanese patients with type 2 diabetes mellitus) study. J Diabetes Investig. 2019;10:108–117. doi: 10.1111/jdi.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yabe D, Shiki K, Suzaki K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021;11:e045844. doi: 10.1136/bmjopen-2020-045844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaku K, Yamamoto K, Fukushima Y, Mizuno S, Nitta D. Safety and effectiveness of empagliflozin according to body mass index in Japanese patients with type 2 diabetes: a subgroup analysis of a 3-year post-marketing surveillance study. Expert Opin Drug Saf. 2022;21:1411–1422. doi: 10.1080/14740338.2022.2062322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the lack of a statement in the study protocol enabling data sharing with a third party after the end of the study and in the informed consent documents as well as lack of approval for data sharing by the Japan Physicians Association Clinical Research Review Board.