Abstract
Background:
Preclinical studies have suggested potential beneficial effects of newer glucose-lowering drugs (GLDs) including dipeptidyl peptidase (DPP)-4 inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RAs), and sodium glucose co-transporter-2 (SGLT2) inhibitors, in protecting humans against cognitive decline and dementia. However, population studies aiming to demonstrate such cognitive benefits from newer GLDs have produced mixed findings. This meta-analysis aimed to evaluate the association between newer GLDs and risk of dementia in adults with type 2 diabetes (T2D).
Methods:
Electronic databases were searched up to March 11, 2022 to include observational studies that examined the association between DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors and risk of dementia (including all-cause dementia, Alzheimer’s disease [AD], and vascular dementia [VD]) in people with T2D. We conducted a random-effects meta-analysis to calculate the relative risk (RR) with 95% confidence interval (CI) for each class of newer GLD.
Results:
Ten studies (from nine articles) involving 819,511 individuals with T2D were included. Three studies found that SGLT2 inhibitor users had a lower risk of all-cause dementia than non-SGLT2 inhibitor users (RR, 0.62; 95%CI, 0.39–0.97). Five studies found that users vs. nonusers of GLP-1RAs were associated with a significant reduction in the risk of all-cause dementia (RR,0.72; 95%CI, 0.54–0.97). However, a meta-analysis for AD and VD was unavailable for SGLT2 inhibitors and GLP-1RAs because only one study was included for each drug. In seven studies, users vs. nonusers of DPP-4 inhibitors were significantly associated with a decreased risk of all-cause dementia (RR, 0.84; 95%CI, 0.74–0.94) and VD (RR, 0.59; 95%CI, 0.47–0.75) but not AD (RR, 0.82; 95%CI, 0.63–1.08).
Conclusion:
Newer GLDs were associated with a decreased risk of all-cause dementia in people with T2D. Because of the observational nature and significant heterogeneity of the included studies, the results should be interpreted with caution. Further research is warranted to confirm our findings.
Keywords: DPP-4 inhibitors, GLP-1RAs, SGLT2 inhibitors, dementia, type 2 diabetes
INTRODUCTION
Dementia, a syndrome characterized by a decline of cognition function, has become a major health burden worldwide1. It reportedly affected 57.4 million people globally in 2019, and is projected to affect 152.8 million people in 2050 as a result of aging and population growth2. In the United States (US), 15% of adults above 68 years have been diagnosed with dementia3. The most prevalent type of dementia is Alzheimer’s disease (AD), which accounts for 60–70% of all dementia cases4, followed by vascular dementia (VD) (15–20%)5.
A growing number of mechanistic and epidemiological studies have shown that diabetes is an independent risk factor for cognitive decline and the development of dementia (7). Type 2 diabetes (T2D) is an age-related disease characterized by insulin resistance and pancreatic β-cell dysfunction that lead to abnormally high levels of blood glucose6. Its pathophysiological mechanisms are similar to several of those of dementia, such as oxidative stress, inflammation, vascular disease, and insulin resistance7. It is well known that individuals with T2D are at high risk of cerebrovascular disease, a key cause of cognitive impairment and dementia8. Also, insulin resistance in individuals with T2D promotes the accumulation of β-amyloid (Aβ) and aberrant tau phosphorylation, which are the central pathophysiological process of AD9.
Glucose-lowering drugs (GLDs) are a cornerstone for controlling diabetes and ameliorating end-organ damage10. Preclinical studies have suggested, however, that newer GLDs, including dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, may also help prevent or delay the onset of dementia through multiple mechanisms, such as decreasing insulin resistance and oxidative stress, and attenuating amyloid deposition and tau phosphorylation11–15. However, evidence from population studies in humans on whether newer GLDs could protect against the risk of dementia remains unknown16–24. In addition, it remains unclear whether the effects of newer GLDs on cognitive function vary across different types of dementia.
Currently, there is no randomized controlled trials (RCTs) published to evaluate the effects of newer GLDs on risk of dementia and there is limited literature on host-hoc analysis24. We performed a meta-analysis of 21 cardiovascular and renal outcome trials showing that newer GLDs might have a benefit on VD but not all-cause dementia25. However, this study was subjected to several limitations (e.g., dementia not being the pre-specified outcome and relatively short follow-ups in these trials)25. The data from observational studies with a longer duration follow-up would provide more information regarding the association between newer GLDs and risk of dementia. Two meta-analyses have been conducted to evaluate the association between GLDs and dementia risk26, 27. However, the association between newer GLDs and risk dementia remains inconclusive because few studies assessing such association were included in both meta-analyses26, 27. Since then, several observational studies have been published20–24. Therefore, we conducted this systematic review and meta-analysis of observational studies to address the following questions: (1) What is the association between different classes of newer GLDs and risk of dementia? (2) Does the association vary with different types of dementia (e.g., AD and VD)? and (3) What were the methodological challenges and limitations in recent studies on this subject?
METHODS
This review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement28.
Search Strategy and Study Selection
Electronic databases, including PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL), were systematically searched from inception to March 11, 2022 using a combination of keywords and Medical Subject Headings (MeSH) terms including dementia, diabetes, GLDs, DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors. No restrictions on language were imposed. We also conducted a manual search by screening the reference lists of the included studies and recent reviews. Details of our search strategy are presented in Table S1.
We included observational studies that evaluated the association between the use of newer classes of GLDs and the risk of dementia in adults with T2D: (1) who used a newer class of GLD (e.g., DPP-4 inhibitors, GLP-1RAs, or SGLT2 inhibitors) compared with those who did not use the class; and (2) in whom the primary outcomes of interest included all-cause dementia, AD, or VD. Studies were excluded if they evaluated all classes of GLDs together rather than a specific class of newer GLD or if the full text of a study was unavailable.
Data Extraction and Quality Assessment
We extracted the following data from the studies using a standardized data collection form: the name of the first author, the publication year, the study location, the basic characteristics of the participants, their exposure or non-exposure, the duration of the follow-up, the outcomes of interest, the adjusted variables, and the main results. For the outcomes of interest, we extracted the adjusted hazard ratio (HR) or odds ratio (OR) for the meta-analysis.
The methodological quality of the observational studies was assessed using the Newcastle-Ottawa Scale (NOS) based on the following three domains: selection, comparability, and outcome29. A maximum score of 9 was awarded to each study. Study quality was judged as high (score: ≥ 7), medium (score: 4 to 6), or low (score: < 4). Two authors (HT and KY) independently extracted the data and assessed the quality of the studies. Any disagreements were resolved by consensus.
Data Synthesis and Analysis
We performed a qualitative analysis to summarize the main results of the identified studies. For each GLD class, we also performed a meta-analysis to calculate the pooled relative risks (RRs) and their corresponding 95% confidence intervals (CIs) for dementia. To account for between-study heterogeneity, we used a Der Simonian and Laird’s random-effects model with inverse-variance (standard error) weighting of individual study results when data for each class of GLDs could be combined30. Among the original studies, different effect measures were reported, such as HR for cohort studies and OR for case-control studies. We considered these relative measures similar because the event rate of dementia was low31. We assessed the between-study heterogeneity using I2 statistics32. Percentages of I2 that were around 25% (I2 = 25), 50% (I2 = 50), and 75% (I2 = 75) indicated low, medium, and high heterogeneity, respectively32. Pre-specified subgroup analyses were performed by dose and duration of treatment, duration of follow-up, and type of control if four or more studies were available in each stratified subgroup33. Visual inspections of the funnel plot and Egger’s test were performed to check for potential publication bias if there were at least 10 studies in each meta-analysis34. All the analyses were performed using STATA software (version 12.0, STATA Corp., College Station, TX). Statistical significance was defined as two-sided (p < .05) unless specified otherwise.
RESULTS
Search Results and Characteristics of Included Studies
Of the 402 articles retrieved from electronic databases, eight met the inclusion criteria; one additional article was identified through a manual search. Thus, a total of nine articles were considered. One article reported the association between newer GLDs and dementia risk based on data from three RCTs and data from a nationwide Danish registry-based cohort, respectively24. The pooled result from the post-hoc analysis of 3 RCTs was also included in this study24. Finally, 9 articles including 10 studies were included in the systematic review and meta-analysis16–24 (Figure 1).
Figure 1.
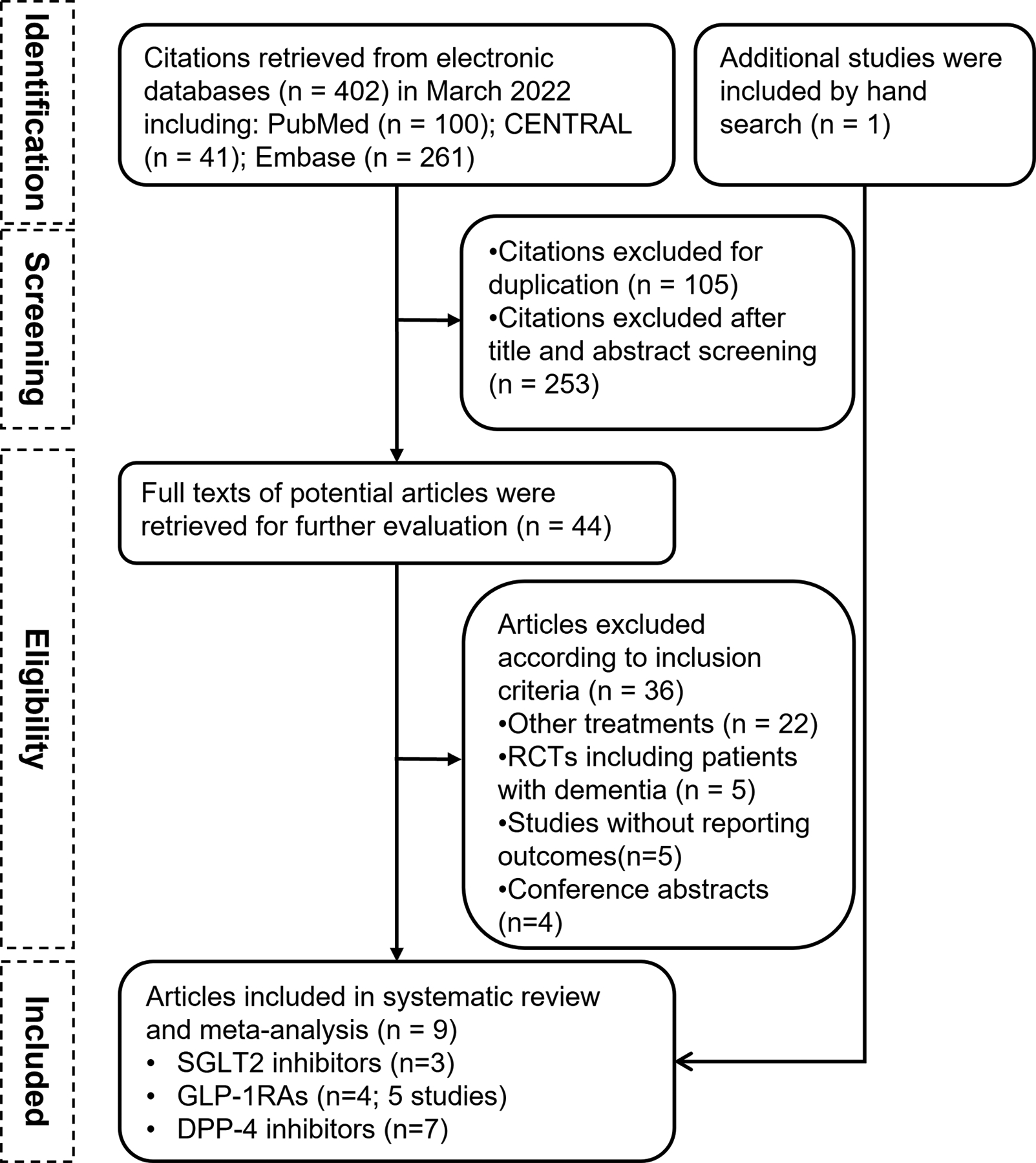
The flowchart of study selection
The basic characteristics of the 10 included studies (a total of 819,511 individuals with T2D) are presented in Table S2. All the studies were published between 2018 and 2022. Seven studies were population cohort studies, and the remaining two studies were case-control studies. Three studies reported the dementia outcomes associated with SGLT2 inhibitors16, 19, 21; five studies (four articles) related to GLP-1RAs16, 19, 23, 24; and seven studies related to DPP-4 inhibitors16–20, 22, 24. The mean age of the population was 68 years (44% men) and the median duration of follow-up was 4.5 years (range: 1.3–7.2 years). The definition of dementia for each study is presented in Table S3. The methodological quality of eight studies was judged as high, and of one study was medium (Table S2).
SGLT2 Inhibitors and Risk of Dementia
Three observational studies had been conducted to evaluate the association between SGLT2 inhibitors and the risk of dementia16, 19, 21. A nested case-control study included 11,619 dementia cases and 46,476 matched controls, all individuals with T2D from the Danish National Diabetes Register between 1995 and 2012. SGLT2 inhibitors use was significantly associated with a lower risk of all-cause dementia than non-SGLT2 inhibitor use after adjustment for multiple variables (OR, 0.58; 95% CI, 0.42–0.81)19. One population-based cohort study assessed dementia presence directly between two classes of medication: 13,276 SGLT2 inhibitor users and 36,544 DPP-4 inhibitor users among individuals with T2D showed that SGLT2 inhibitors were significantly associated with a lower risk of all-cause dementia than DPP-4 inhibitors (HR, 0.41; 95% CI, 0.27–0.61)21. And a case-control study using the Nationwide Disease Analyzer database (2013 – 2017) found no association between SGLT2 inhibitor users and risk of all-cause dementia compared to non-SGLT2 inhibitor users (OR, 0.91; 95% CI, 0.72–1.16)16. Regarding AD, SGLT2 inhibitors had no significant association with decreased risk compared to DPP-4 inhibitors (HR, 0.25; 95% CI, 0.06–1.04)21.
Our meta-analysis of the three observational studies showed that SGLT2 inhibitor use was significantly associated with a decreased risk of all-cause dementia, compared to non-SGLT2 inhibitor users (RR, 0.62; 95% CI, 0.39–0.97) (Figure 2). However, a high level of heterogeneity between studies was observed in this meta-analysis.
Figure 2.
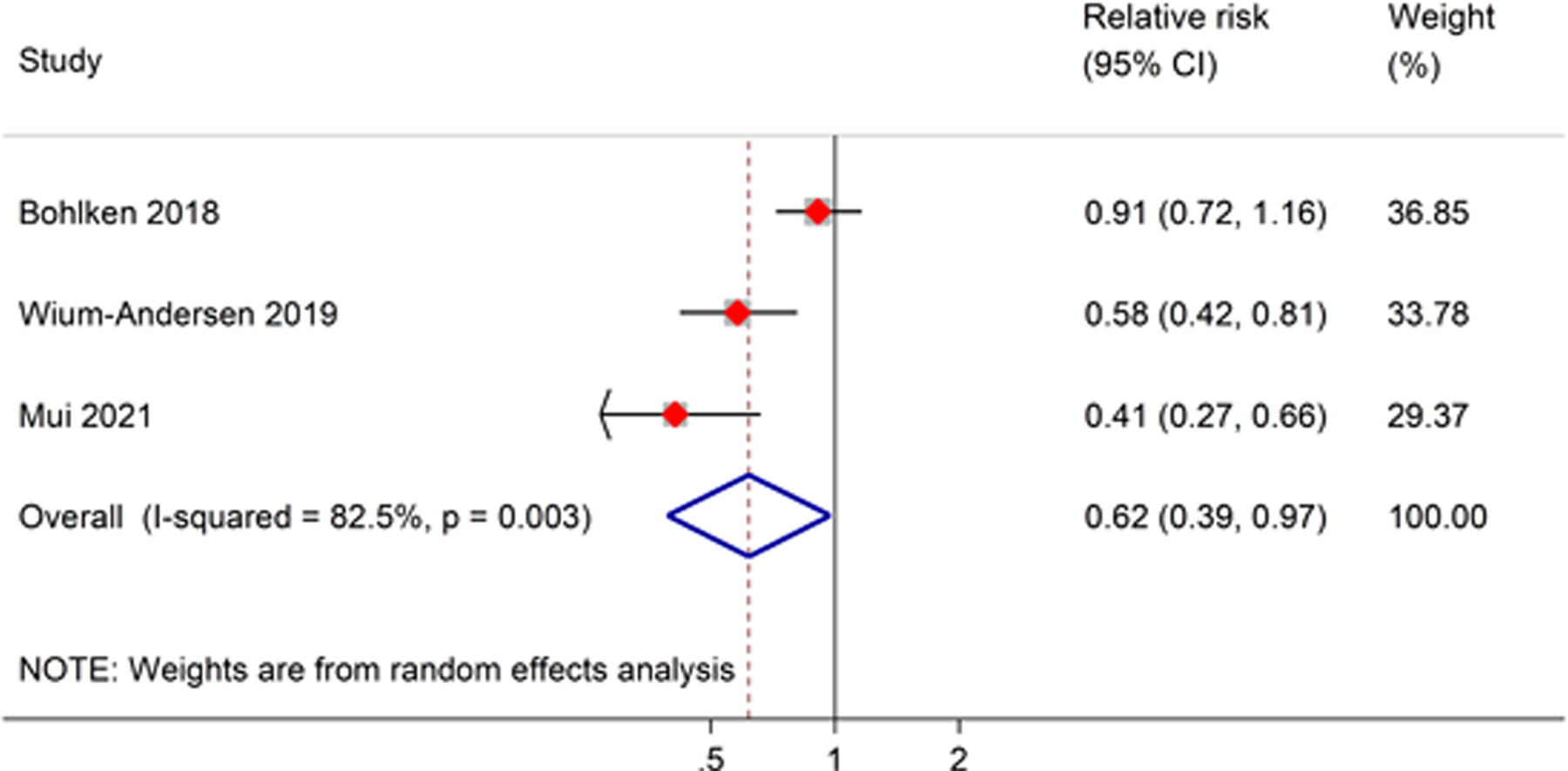
Meta-analysis of the association between SGLT2 inhibitor users and risk of all-cause dementia as compared to non-SGLT2 inhibitor users. The size of the square represents the weight of each study contributing to the overall meta-analysis.
GLP-1RAs and Risk of Dementia
We included five studies (from 4 articles) that evaluated the association between GLP-1RAs and risk of dementia16, 19, 23, 24. Three of those studies found a lower risk of all-cause dementia among GLP-1RA users than among non-GLP-1RA users19, 24. An insignificantly decreased risk of all-cause dementia among GLP-1RA users was observed in one case-control study (OR, 0.90; 95% CI, 0.70–1.15)16. Only one study reported the risk of AD using claims data from Medicare beneficiaries with T2D, and the study showed that exenatide (a specific GLP-1RA) users had a very slightly lower risk than non-exenatide users (OR, 0.98; 95% CI, 0.96–0.99)23.
The results of the meta-analysis of four studies (from 3 articles) showed that GLP-1RA users were significantly associated with a lower risk of all-cause dementia than non-GLP-1RA users (RR, 0.72; 95% CI, 0.54–0.97). A high level of heterogeneity between studies was found (I2 = 91.3%) (Figure 3).
Figure 3.
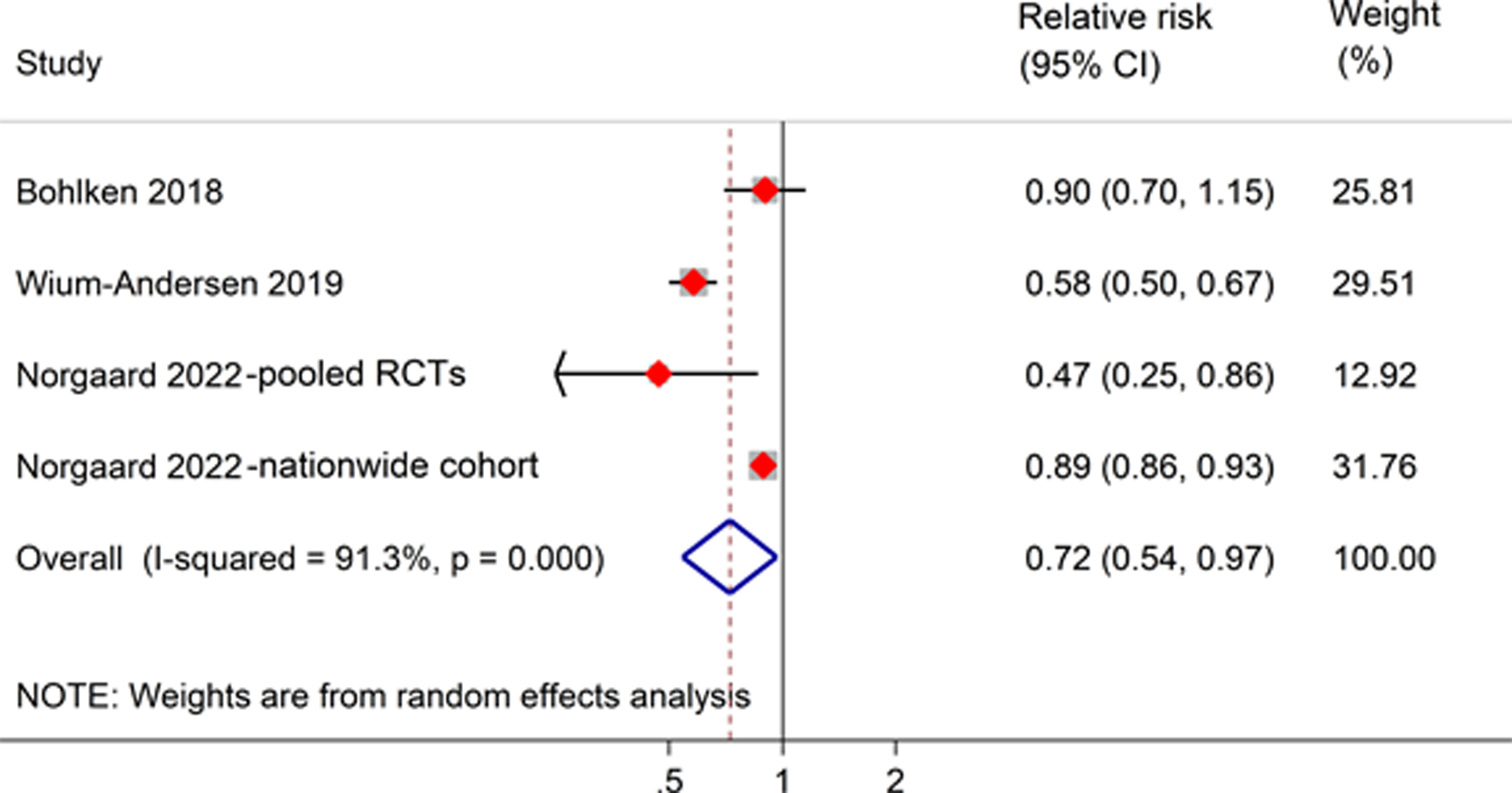
Meta-analysis of the association between GLP-1RA users and risk of all-cause dementia as compared to non-GLP-1RA users. The size of the square represents the weight of each study contributing to the overall meta-analysis.
DPP-4 Inhibitors and Risk of Dementia
Seven observational studies reported the risk of dementia associated with DPP-4 inhibitors16–20, 22, 24. Among the six studies that reported outcomes for all-cause dementia, three studies18–20 showed a significantly lower risk among DPP-4 inhibitor users than among non-DPP-4 inhibitor users, and the remaining three studies16, 22, 24 found no association between DPP-4 inhibitors and the risk of all-cause dementia. One cohort study that used the Korean National Health Insurance database (with 7,552 new DPP-4 inhibitor users and 7,552 new sulfonylurea users) found that DPP-4 inhibitors were significantly associated with a lower risk of all-cause dementia (HR, 0.66; 95% CI, 0.56–0.78) than sulfonylureas17. Among the three studies that reported outcomes for AD, no association with the use of DPP-4 inhibitors was found when compared with the non-use of DPP-4 inhibitors20, 23, while a lower risk was observed among DPP-4 inhibitor users than among sulfonylureas users (HR, 0.64; 95% CI, 0.52–0.79)17. For the risk of VD, one study found no association with the use of DPP-4 inhibitors nor of sulfonylureas (RR, 0.58; 95% CI, 0.40–0.68)17, while another study observed a lower risk among DPP-4 inhibitor users than among non-DPP-4 inhibitor users20.
Our meta-analysis of seven studies showed that DPP-4 inhibitor users were significantly associated with a lower risk of all-cause dementia than non-DPP-4 inhibitor users (RR, 0.84; 95% CI, 0.74–0.94) (Figure 4). Similarly, we observed a significantly lower risk of VD among DPP-4 inhibitor users than among non-DPP-4 inhibitor users (RR, 0.59; 95% CI, 0.47–0.75) when pooling analysis of two studies. There was no significant difference between DPP-4 inhibitor use and risk of AD (RR, 0.82; 95% CI, 0.63–1.08) based on the results from three studies. A high level of heterogeneity between the studies was detected in terms of all-cause dementia and VD.
Figure 4.
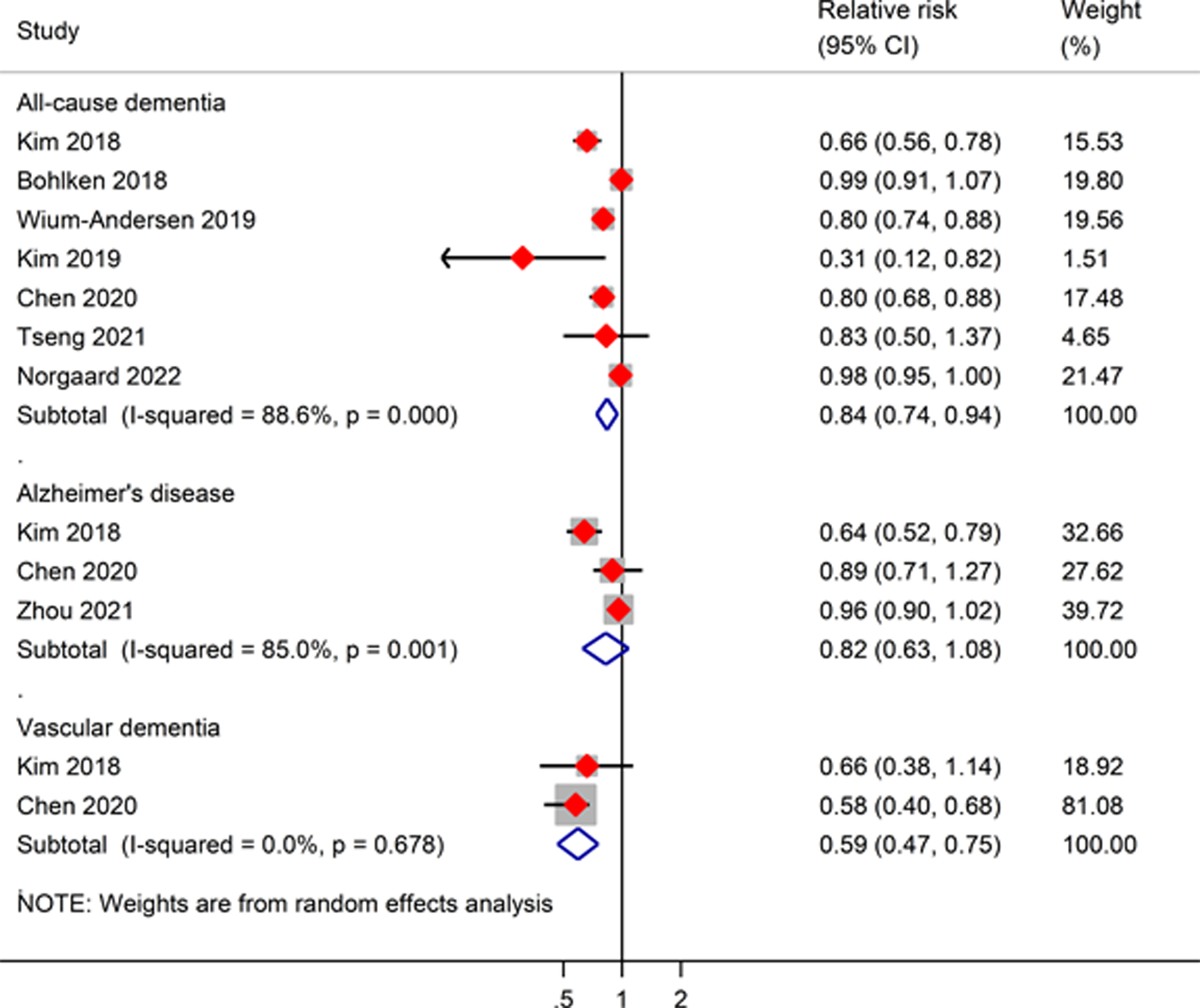
Meta-analysis of the association between DPP-4 inhibitors and risk of all-cause dementia, Alzheimer’s disease, and vascular dementia as compared to non-DPP-4 inhibitor users. The size of the square represents the weight of each study contributing to the overall meta-analysis.
Subgroup analysis and publication bias test
Pre-specified subgroup analyses by dose and duration of treatment, duration of follow-up, and type of control were not performed due to less than four studies included in each stratified subgroup. Also, a visual inspection of the funnel plot and Egger’s test were not carried out to check for potential publication bias because less than 10 studies were included in each meta-analysis.
DISCUSSION
In this systematic review and meta-analysis, we included ten observational studies that evaluated the association between newer GLDs and the risk of dementia. We found that newer GLDs including SGLT2 inhibitors, GLP-1RAs, and DPP-4 inhibitors were significantly associated with a decreased risk of all-cause dementia. There was limited evidence for the subtype of dementia (including AD and VD) for newer GLDs. However, it should be noted that these results should be interpreted with caution because of several limitations including the limited availability, observational nature, and heterogeneity of the included studies.
The mechanism behind the decreased risk of dementia associated with SGLT2 inhibitors is unclear. Several possible explanations can be considered. First, SGLT2 inhibitors decrease body weight and glycated hemoglobin since obesity and hyperglycemia are independent risk factors for dementia7, 35. Second, SGLT2 inhibitors have been shown in animal studies to prevent cognitive decline by reducing oxidative stress and inflammation, thereby improving brain mitochondrial function and plasticity in the hippocampus, which is particularly affected by T2D13, 36. Third, SGLT2 inhibitors have been hypothesized to block acetylcholinesterase, the enzyme which breaks down acetylcholine, a neurotransmitter important for memory function which is diminished in AD37, 38. Fourth, SGLT2 inhibitors have also shown gerotherapeutic/geroprotective effects via modulation of fundamental aging biology processes. For instance, an SGLT2 inhibitor, canagliflozin, has been shown to extend lifespan in male mice in the Interventions Testing Program via controlling daily peak glucose levels39 and affect a number of processes that are critical in aging biology, including reducing endoplasmic reticulum stress-mediated apoptosis40, improving mitochondrial lipid oxidation41 and promoting ketogenesis and dampening of mTORC1 hyperactivation42. To date, however, few clinical trials have been conducted to evaluate the effects of SGLT2 inhibitors on cognitive function in humans. One RCT compared SGLT2 inhibitors with DPP-4 inhibitors in 39 older adults with T2D and found no significant difference in the cognitive performance scores between the two groups during the 12 months of treatment43. Moreover, there was no significant change between the two groups when compared with their baselines43. The neutral effect of SGLT2 inhibitors on cognitive function in this study could have been because there was insufficient statistical power (due to the small sample size) and inadequate follow-up time to observe a worsening of cognitive performance44. Thus, further studies are needed to investigate the impact of SGLT2 inhibitors in cognitive functions.
An exploratory analysis using data from the Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) trial showed that dulaglutide reduced cognitive impairment by 14% (HR, 0.86%; 95% CI, 0.79–0.95) in 9,901 people with T2D during a median follow-up period of 5.4 years45. Similarly, our meta-analysis showed a lower risk of all-cause dementia with the use of GLP-1RAs. Several potential mechanisms for such a beneficial outcome have been proposed. First, GLP-1RAs have been shown to ameliorate dementia-related risk factors46. Results of RCTs have demonstrated that GLP-1RAs lowered glycated hemoglobin, body weight, and systolic blood pressure, and decreased the risk of cardiovascular disease47–49. Second, GLP-1 receptors are highly expressed in the neurons of the central nervous system. In animal models of AD, stimulation of the GLP-1 receptor reduced neuroinflammation and increased neurogenesis50, 51. Third, GLP-1 receptor stimulation reduces Aβ accumulation and cytotoxicity in cellular and animal models of AD11. However, current evidence of its effect on the risk of AD is limited. One study showed a lower risk of AD among exenatide users than among non-exenatide users23. Further long-term follow-up studies are needed to evaluate the association between GLP-1RAs and the risk of dementia, especially AD. Currently, two longer-term Phase 3 trials (EVOKE and EVOKE PLUS) have been performed to evaluate the efficacy and safety of semaglutide versus placebo among 3,680 individuals with early AD during and up to 172 weeks of follow-up. The results from both trials would provide evidence for the role of GLP-1RAs among people with AD.
Our meta-analysis, which included seven studies, suggests a protective effect of DPP-4 inhibitors against all-cause and VD. These findings are consistent with the result of a network meta-analysis of nine studies involving 530,355 individuals, in which DPP-4 inhibitors were significantly associated with a 46% lower risk of dementia than non-treatment with antidiabetic drugs (HR, 0.54; 95% CI, 0.38–0.74)27. A prospective cohort study suggested that six-month therapy with sitagliptin (a DPP-4 inhibitor) was associated with improved cognitive function in 205 elderly diabetes patients with and without AD52. DPP-4 inhibitors may have a neuroprotective role in the prevention or treatment of dementia by increasing circulating GLP-1 levels in the brain. In animal experiments, DPP-4 inhibitors reduced endothelial dysfunction, cerebral oxidative stress, and ischemic brain damage via a glucose-independent mechanism that most likely involved GLP-112, 14. Also, according to an in vitro study, DPP4 may play a role in Aβ formation, as DPP-4 inhibitors reduced Aβ deposition and tau phosphorylation by boosting GLP-1 levels in the brain15.
It should be noted that the complexity of real-world T2D treatment decisions can affect the observational findings and their interpretations. Treatment decisions in real-world management of T2D are not based only on the pharmacologic properties of different classes of GLDs, but also on several other factors including T2D duration, severity, associated comorbidities, age, affordability, and access to care. These confounding factors place high importance on the selection of treatment and onset of outcome in observational studies, which can affect identified associations. However, these confounders cannot be accounted for in our meta-analysis, because of the observational nature and significant heterogeneity of the included studies. To be more specific, we acknowledge the following limitations of this study: First, we observed a high level of heterogeneity among the studies in the meta-analyses. Because of the limited number of studies included for each class of GLDs, we were unable to carry out meta-regression analyses or subgroup analyses to explore the source(s) of the heterogeneity. Of the ten studies included, only two studies employed an active comparator study design17, 21, while the remaining eight studies used “non-users” of the treatment of interest as the control group. The difference in the control group selection might lead to heterogeneity in the meta-analysis. In addition, the using status of some older classes of GLD could be confounding in the study. For example, some older GLDs, such as sulfonylureas and insulin, may cause weight gain and have a higher risk of hypoglycemia, which are risk factors of dementia53. Second, due to a lack of patient-level information from the included studies, we are unable to account for important confounding factors in our meta-analysis, including the duration and severity of diabetes, combination therapy (e.g., metformin), and socioeconomic status. For example, individuals with higher socioeconomic status are more likely to receive these newer GLDs; and they tend to have better access to health care and a higher education level which are associated with a lower risk of dementia54. Also, according to previous literature55, 56, newer GLDs were more frequently prescribed in T2D patients with younger age and less cardiovascular/renal complications, and these patients were likely associated with a lower risk of dementia. Third, the accuracy of the diagnosis of dementia in the individual studies was unknown. Although International Classification of Diseases (ICD) codes were used to identify the dementia cases in the included studies, no additional data (e.g., laboratory examinations) were available in the database to confirm the dementia cases and to discern the types of dementia (e.g., AD or VD). The heterogeneity in the diagnosis of dementia across the studies might have led to the heterogeneity between the studies. Also, some subjects included in the cohort might have had dementia at baseline. Fourth, because the estimates for the associations between newer GLDs and risk of all-cause dementia are derived from Asian and European populations, there is limited generalizability of these findings to non-Caucasian and non-Asian populations. Finally, there could have been some overlapping of populations among the reviewed studies. For example, two studies used Korean National Health Insurance claims data17, 18, two studies used Taiwan’s National Health Insurance data20, 22, and two studies used data from the Danish National Diabetes Register19, 24. We included all these studies in our meta-analysis because they were conducted using data from different periods collected by different research teams.
CONCLUSION
The prevalence both of diabetes and dementia has been increasing worldwide for decades, and the population with T2D have a higher risk of dementia compared to the general population57. Newer GLDs are in wide use for treating T2D, and this use is estimated to increase more due to their well-documented clinical benefits. And, current evidence from available studies shows that DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors are associated with a decreased risk of all-cause dementia in individuals with T2D. It still needs to be further studied to what extent the benefits of newer GLDs on lowering dementia risk are achieved through diabetes control and/or other pathways through reducing associated risk factors such as obesity and hypertension, and whether they can be expanded to non-diabetes populations. However, current evidence of a decrease in the risk of AD associated with these newer GLDs remains limited. Future clinical trials on the effects of newer GLDs on cognitive function will be most informative if they will be adequately powered and followed up to detect the differences. In addition, real-world studies are needed to evaluate the effects of newer GLDs on the risk of different types of dementia in individuals with T2D and in non-diabetes populations.
Supplementary Material
Table S1. Search Strategy (Search date: March 11, 2022)
Table S2. Basic characteristics of included studies
Table S3. Definition of dementia
Key points.
Newer glucose-lowering drugs (GLDs) were associated with a lower risk of all-cause dementia, but heterogeneity between studies was detected.
The evidence regarding the association between newer GLDs and risk of Alzheimer’s disease and vascular dementia is limited, and further research is needed.
Population with type 2 diabetes may have cognitive benefits from newer GLDs.
Why does this paper matter?
This study found a lower risk of all-cause dementia associated with newer glucose-lower drugs in the population with type 2 diabetes indicating newer GLDs may have beneficial effects on the risk of dementia. Further studies are warranted to confirm these findings.
FUNDING INFORMATION
This work was supported by National Institutes of Health Awards (R01AG076234, R56AG069880, and R01DK133465).
SPONSOR’S ROLE
The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and Management of Dementia: Review. JAMA. 2019;322(16): 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2017;13(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Dementia. Volume 2022, 2021. [Google Scholar]
- 5.Wolters FJ, Ikram MA. Epidemiology of Vascular Dementia. Arterioscler Thromb Vasc Biol. 2019;39(8): 1542–1549. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 Suppl 1S62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71(3): 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43(9): 2526–2534. [DOI] [PubMed] [Google Scholar]
- 9.Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin Resistance as a Link between Amyloid-Beta and Tau Pathologies in Alzheimer’s Disease. Front Aging Neurosci. 2017;9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice C, Draznin B, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1): S125–S143. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4): 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma M, Hasegawa Y, Koibuchi N, et al. DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc Diabetol. 2015;1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. 2017;33343–50. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Sharma B. Neuroprotective effect of selective DPP-4 inhibitor in experimental vascular dementia. Physiol Behav. 2015;152(Pt A): 182–193. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein HG, Dobrowolny H, Keilhoff G, Steiner J. Dipeptidyl peptidase IV, which probably plays important roles in Alzheimer disease (AD) pathology, is upregulated in AD brain neurons and associates with amyloid plaques. Neurochem Int. 2018;11455–57. [DOI] [PubMed] [Google Scholar]
- 16.Bohlken J, Jacob L, Kostev K. Association Between the Use of Antihyperglycemic Drugs and Dementia Risk: A Case-Control Study. J Alzheimers Dis. 2018;66(2): 725–732. [DOI] [PubMed] [Google Scholar]
- 17.Kim YG, Jeon JY, Kim HJ, et al. Risk of Dementia in Older Patients with Type 2 Diabetes on Dipeptidyl-Peptidase IV Inhibitors Versus Sulfonylureas: A Real-World Population-Based Cohort Study. J Clin Med. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JY, Ku YS, Kim HJ, et al. Oral diabetes medication and risk of dementia in elderly patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;154116–123. [DOI] [PubMed] [Google Scholar]
- 19.Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol. 2019;181(5): 499–507. [DOI] [PubMed] [Google Scholar]
- 20.Chen KC, Chung CH, Lu CH, et al. Association between the Use of Dipeptidyl Peptidase 4 Inhibitors and the Risk of Dementia among Patients with Type 2 Diabetes in Taiwan. J Clin Med. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mui JV, Zhou J, Lee S, et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors vs. Dipeptidyl Peptidase-4 (DPP4) Inhibitors for New-Onset Dementia: A Propensity Score-Matched Population-Based Study With Competing Risk Analysis. Front Cardiovasc Med. 2021;8747620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng CH. Vildagliptin Has a Neutral Association With Dementia Risk in Type 2 Diabetes Patients. Front Endocrinol (Lausanne). 2021;12637392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B, Zissimopoulos J, Nadeem H, Crane MA, Goldman D, Romley JA. Association between exenatide use and incidence of Alzheimer’s disease. Alzheimer’s and Dementia: Translational Research and Clinical Interventions. 2021;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nørgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement (N Y). 2022;8(1): e12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Niu S, Brown J, et al. Newer glucose-lowering drugs and risk of dementia: A meta-analysis of cardiovascular outcome trials. J Am Geriatr Soc. 2022;70(9): 2719–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMillan JM, Mele BS, Hogan DB, Leung AA. Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2018;6(1): e000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JB, Tang X, Han M, Yang J, Simo R. Impact of antidiabetic agents on dementia risk: A Bayesian network meta-analysis. Metabolism. 2020;109154265. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Volume 2022. [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 31.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9): 893–899. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu R, Gartlehner G, Grant M, et al. Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD), 2008. [Google Scholar]
- 34.Dalton JE, Bolen SD, Mascha EJ. Publication Bias: The Elephant in the Review. Anesth Analg. 2016;123(4): 812–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10): 1556–1560. [DOI] [PubMed] [Google Scholar]
- 36.Lin B, Koibuchi N, Hasegawa Y, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi SM, Shakil S, Biswas D, et al. Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer’s disease- diabetes type 2 linkage via an enzoinformatics study. CNS Neurol Disord Drug Targets. 2014;13(3): 447–451. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh S, Rizvi SM, Shakil S, Riyaz S, Biswas D, Jahan R. Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol Appl Biochem. 2016;63(1): 145–150. [DOI] [PubMed] [Google Scholar]
- 39.Miller RA, Harrison DE, Allison DB, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibusawa R, Yamada E, Okada S, et al. Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep. 2019;9(1): 9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei D, Liao L, Wang H, Zhang W, Wang T, Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARalpha in vivo and in vitro. Life Sci. 2020;247117414. [DOI] [PubMed] [Google Scholar]
- 42.Tomita I, Kume S, Sugahara S, et al. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020;32(3): 404–419 e406. [DOI] [PubMed] [Google Scholar]
- 43.Perna S, Mainardi M, Astrone P, et al. 12-month effects of incretins versus SGLT2-Inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with Type-2 diabetes mellitus. Clin Pharmacol. 2018;10141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzo MR, Di Meo I, Polito R, et al. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol Res. 2022;176106062. [DOI] [PubMed] [Google Scholar]
- 45.Cukierman-Yaffe T, Gerstein HC, Colhoun HM, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19(7): 582–590. [DOI] [PubMed] [Google Scholar]
- 46.Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP-1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. 2018;17(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4): 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19): 1834–1844. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373(23): 2247–2257. [DOI] [PubMed] [Google Scholar]
- 50.McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76 Pt A57–67. [DOI] [PubMed] [Google Scholar]
- 51.Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav. 2012;106(3): 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract. 2017;123192–198. [DOI] [PubMed] [Google Scholar]
- 53.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr., Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15): 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front Public Health. 2020;8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eberly LA, Yang L, Eneanya ND, et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status With Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients With Diabetes in the US. JAMA Netw Open. 2021;4(4): e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao JZ, Weinhandl ED, Carlson AM, St. Peter WL. Disparities in SGLT2 Inhibitor or Glucagon-Like Peptide 1 Receptor Agonist Initiation Among Medicare-Insured Adults With CKD in the United States. Kidney Medicine. 2023;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee S, Peters SA, Woodward M, et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care. 2016;39(2): 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy (Search date: March 11, 2022)
Table S2. Basic characteristics of included studies
Table S3. Definition of dementia


