Abstract
Background
Optimal systolic blood pressure (SBP) control in nursing home residents is uncertain, largely because this population has been excluded from clinical trials. We examined the association of SBP levels with the risk of cardiovascular (CV) events and mortality in Veterans Affairs (VA) nursing home residents on different numbers of antihypertensive medications.
Methods
Our study included 36,634 residents aged ≥65 years with a VA nursing home stay ≥90 days from October 2006–June 2019. SBP was averaged over the first week after admission and divided into categories. Cause-specific hazard ratios (HRs) of SBP categories with CV events (primary outcome) and all-cause mortality (secondary outcome) were examined using Cox regression and multistate modeling stratified by number of antihypertensive medications used at admission (0, 1 or 2, and ≥3 medications).
Results
More than 76% residents were on antihypertensive therapy and 20% received ≥3 medications. In residents on antihypertensive therapy, a low SBP <110mmHg (compared with SBP 130~149mmHg) was associated with a greater CV risk (adjusted HR [95% confidence interval]: 1.47 [1.28–1.68] in 1 or 2 medications group, and 1.41 [1.19–1.67] in ≥3 medications group). In residents on no antihypertensives, both low SBP <110mmHg and high SBP ≥150mmHg were associated with higher mortality; while in residents receiving any antihypertensives, a low SBP was associated with higher mortality and the highest point estimates were for SBP <110mmHg (1.36 [1.28–1.45] in 1 or 2 medications group, and 1.47 [1.31–1.64] in ≥3 medications group).
Conclusions
The associations of SBP with CV and mortality risk varied by the intensity of antihypertensive treatment among VA nursing home residents. A low SBP among those receiving antihypertensives was associated with increased CV and mortality risk, and untreated high SBP was associated with higher mortality. More research is needed on the benefits and harms of SBP lowering in long-term care populations.
Keywords: systolic blood pressure, antihypertensive treatment, long-term care residents, cardiovascular events, mortality
INTRODUCTION
Current guidelines provide inconsistent recommendations regarding the optimal BP treatment target in older populations,1–4 but all agree on the limited evidence in frail older adults and nursing home residents, a population with multiple chronic conditions and diminished functional status and/or dementia. Nursing home residents have been excluded from large-scale clinical trials of BP lowering, leading to a paucity of data on hypertension treatment and subsequently little guidance for this population. Although trials involving more robust older persons demonstrate the benefits of lowering BP,5–8 several population-based cohort studies suggest that low BP under antihypertensive treatment is associated with higher mortality in older adults.9–13
Nursing home residents might be at risk of adverse outcomes from low systolic BP (SBP) when using multiple antihypertensive medications. In 2015, Benetos et al found that among 1,127 nursing home residents aged 80 years and older, the subgroup with low SBP (<130mmHg) receiving two or more antihypertensives had a greater than 2-fold risk for mortality.14 Additionally, a recent cohort study showed that long-term nursing home residents on more intensive antihypertensive treatment experienced an increased hospitalization.15 Greater medication burden is associated with adverse outcomes in older adults,16 and the combination of low SBP and greater medication use could lead to synergistic effects. However, little is known about whether these findings extend to other outcomes, such as cardiovascular (CV) events, a leading cause of morbidity and mortality in older adults.17,18 A better understanding of the associations of low SBP with these adverse events among those on antihypertensive medications can help inform clinical decision-making.
In this study, we aimed to leverage the data of 36,634 Veterans Affairs (VA) nursing homes residents and characterize the relationships of SBP level with CV events and all-cause mortality, and to examine whether these relationships vary by the intensity of antihypertensive medications used. Our primary hypothesis was that low SBP would not be protective for CV events or death in a nursing home population, especially among those on multiple (≥ 3) medications.
METHODS
Study population
The study population consisted of Veterans residing in VA nursing homes, termed Community Living Centers (CLCs), from 2006–2019. The VA health care system is unique in that the electronic health records from inpatient and outpatient care can be linked with data from the nursing home stay including longitudinal vitals, medications administered, and health status data, and functional data from the Centers for Medicare & Medicaid Services (CMS) Minimum Data Set (MDS), providing the most comprehensive look at BP management and outcomes. Residents were included if they were admitted to a CLC between October 1, 2006, and June 30, 2019. Residents were excluded if they 1) were in hospice prior to the CLC stay, 2) had a CLC stay <90 days (to identify residents who were admitted for long-term care), 3) were <65 years at admission, 4) had a >30 day acute hospital stay during their CLC stay, in which case they were considered discharged, 5) had no BP measures, or 6) had missing values on key confounders (mostly missingness of functional measures from CMS MDS). After exclusion, a total of 36,634 residents were included in this study (Figure S1). This study received institutional review board approval with a waiver of informed consent from Stanford University and the VA Palo Alto Health Care System.
Measurements
All data were obtained from the VA Corporate Data Warehouse (CDW) and the CMS MDS. Data on SBP levels were obtained from the CDW Vital Signs domain. All SBP data were obtained from electronic health records and thus we are unable to ensure systematic collection of SBPs both across time and across nursing homes. The details about the frequency and timing of SBP assessment have been described previously.19 Data on antihypertensive medications were obtained from the CDW Bar Code Medication Administration (BCMA) domain, which captures all administrations of medications in the CLC. The CDW Inpatient domain was used to identify CLC stays, and the CDW Patient domain was used to determine patient age, sex, race, and ethnicity. Smoking status (current/former/never/unknown) was queried from the CDW Health Factors domain. Patient comorbidities and chronic conditions included cardiovascular disease (coronary heart disease, cerebrovascular disease, and peripheral vascular disease), heart failure, diabetes, osteoarthritis, chronic obstructive pulmonary disease (COPD), kidney disease (chronic kidney disease and acute kidney injury), metastatic cancer, and dementia were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision, Clinical Modification (ICD-10-CM) codes from Inpatient and Outpatient domains one year prior to the CLC admission.20 Data on height (cm) and weight (kg) were obtained from the CDW Vital Signs. Data on statins, glucose lowering drugs (insulin, metformin, or others) usage was obtained from BCMA domain. Data on falls, activities of daily living (ADL), and cognitive function were obtained from the CMS MDS. ADL was assessed using the MDS-ADL score (a 28-point score evaluating seven activities: mobility in bed, transferring, ambulation, dressing, eating, toileting, and personal hygiene, on a four-point scale from independence to total dependence) and a higher score indicates a greater dependence in daily activities. The Cognitive Function Scale (CFS) was used to combine the cognitive assessment tools from MDS 2.0 and 3.0 into a single, integrated, 4-level assessment of cognitive function: cognitively intact, mildly impaired, moderately impaired, and severely impaired.21 A diagnosis of hypertension prior to admission was defined as use of antihypertensive medications, SBP ≥140mmHg or diastolic BP ≥90mmHg.
Exposures
The primary exposure variable was SBP levels. Baseline SBP levels were calculated as the mean SBP values over the first week of CLC stay (a total of 395,238 SBP measures in the first week with an average of 10.8 measures/person, median standard deviation of the SBP measures was 13.3 mmHg) and divided into categories (<110, 110~129, 130~149, ≥150mmHg) with the medium to high level (130~149mmHg) as the reference group. We used the number of antihypertension medications used as an effect modifier to further understand the relationship between treatment intensity and outcomes. All analyses were stratified by the intensity of antihypertensive treatment, defined as the average number of antihypertensive medications administered during the first week of CLC stay and divided into categories (0, 1 or 2, and ≥3 medications).
Outcomes
The primary outcome was a composite outcome of fatal and non-fatal CV event, comprised of myocardial infarction, stroke, or heart failure exacerbation. The ICD-9-CM and ICD-10-CM were used to identify CV events resulting from emergency departments visits or hospital stays in order to exclude diagnosis codes for follow-up care related to prior events (Table S1). All residents were followed-up from CLC admission to the first CV event, discharge, or end of follow-up (3 years after admission) whichever came first. Individuals who died were censored at the time of death. The secondary outcome was all-cause mortality within 3 years after admission. We adopted a constrained follow-up period of 3 years to account for the skewed distribution of CLC stay time (median 0.54 years, range 90 days to 14 years) and attenuate the influence of the outliers who had exceptionally long CLC stays. Death during CLC stay was ascertained by linking to the CDW Vital Status domain, which captures deaths from the Beneficiary Identification and Records Locator System database, the Centers for Medicare & Medicaid Services, the Social Security Administration, and the VA Patient Treatment File.
Statistical analysis
Incidence (per 1000 person-years) was calculated by dividing the cumulative number of CV events and deaths by all at risk person years during follow-up with 95% confidential intervals (CIs) estimated by exact method.22 Kaplan-Meier curves were used to display the cumulative hazard for CV events and survival probabilities across SBP levels and medication subgroups. Hazard ratios (HRs) and 95% CIs were estimated using cause-specific Cox models adjusted for potential confounders. We first tested the interaction term between SBP and antihypertensive medications based on log partial likelihood (p-value < 0.001 for both outcomes) and then stratified the analyses by the intensity of antihypertensive treatment. A primary model (model 1) adjusted for key confounders including age, sex, race, height, weight, smoking status, cardiovascular disease, heart failure and ADL, and a secondary model (model 2) adjusted for additional confounders and/or mediators including CFS, statins, glucose lowering drugs, diabetes, osteoarthritis, COPD, kidney disease, metastatic cancer, and dementia. The proportionality of hazards and log linearity of continuous covariates assumptions for the Cox model were evaluated by checking the Schoenfeld residuals and martingale residuals. We also examined the transitions in the following 3 states: baseline, CV events, and death using a multistate model (Figure S2). Multistate modeling allows for considering serial events such as a non-fatal CV event with subsequent mortality due to different causes or multiple factors. It is also advantageous in the ability to represent multiple ordered events per subject, account for competing risks, and model transition rates therefore describing the disease process.23 All analyses were conducted in R software (version 4.1.2) and a two-sided p≤ 0.05 was considered statistically significant. The multistate models were fit using the mstate R package.24
Sensitivity analysis
The following sensitivity analyses were conducted: (1) using the full follow-up duration without constraint to 3 years in the same framework of Cox regression; (2) using the antihypertensive medication information at approximately one month (the fourth or fifth week) after admission to determine medication subgroups (to address the possibility that medications may be changed shortly after admission); (3) restricting to those who had dementia at admission (to uncover the potential modification of dementia on the SBP-outcome relationships in older age) and (4) excluding those who died within 6 months of admission (to address the concern of reverse relationships caused by terminal SBP decline prior to death).
To further investigate the patterns of SBP trajectory and the impact on risk of CV events and death, we estimated the person-specific SBP level and change over time by linear mixed effect modeling. The models were fitted using repeated weekly SBP measures during the 3-year follow-up period (on average 48 measures per person) as dependent variables with random intercepts and random slopes by time. We then categorized people into 2 SBP trajectory pattern subgroups based on their person-specific slopes (Figure S3): SBP stable/increasing (slope ≥ 0 mmHg/week) and decreasing (slope < 0 mmHg/week) and included this dichotomous variable in the primary models to quantify the contribution of SBP changes to the associations of interest.
RESULTS
Overview
Baseline characteristics of 36,634 (mean age 78 years; 2.2% women) nursing home residents by antihypertensive medication groups are shown in Table 1 and Table S2. There were 8,718 (23.8%) residents not on antihypertensive medications, 20,544 (56.1%) on one or two medications, and 7,372 (20.1%) on three or more medications. Among those who received medications, beta-blockers were the most common class (68%), followed by angiotensin converting enzyme/angiotensin receptor blocker (52%), diuretics (45%), and calcium channel blockers (32%). Residents on more antihypertensive medications were younger, more likely to be identified as Black race, and had greater height and weight, higher SBP level, lower DBP level, greater limitations in activities of daily living, and better cognitive function. Diagnoses of CVD, diabetes, osteoarthritis, COPD, and kidney disease were more frequent among those with more antihypertensive medications. Prevalence of metastatic cancer and dementia were lower in those on more medications. Differences across the SBP categories are presented in Table S3.
Table 1.
Baseline characteristics by antihypertensive medication groups in VA nursing home residents
| No medication (n=8,718) |
1–2 medication (n=20,544) |
≥3 medication (n=7,372) |
P-value | |
|---|---|---|---|---|
| Age, years | 78 (8.7) | 78 (8.4) | 76.9 (8.2) | <.001 |
| Sex, female | 197 (2.3%) | 471 (2.3%) | 154 (2.1%) | 0.595 |
| Race | ||||
| Black | 1,255 (14.4%) | 3,340 (16.3%) | 1,557 (21.1%) | <.001 |
| White | 6,573 (75.4%) | 15,408 (75.0%) | 5,197 (70.5%) | |
| Asian | 131 (1.5%) | 301 (1.5%) | 93 (1.3%) | |
| American Indian | 64 (0.7%) | 134 (0.7%) | 53 (0.7%) | |
| Unknown | 695 (8.0%) | 1361 (6.6%) | 472 (6.4%) | |
| Height, cm | 175.0 (9.0) | 175.2 (9) | 175.8 (9.2) | <.001 |
| Weight, kg | 78.7 (18.9) | 86.7 (21.9) | 95.2 (25) | <.001 |
| Smoking status | ||||
| Current | 2,381 (27.3%) | 5,148 (25.1%) | 1,771 (24.0%) | <.001 |
| Former | 3,371 (38.7%) | 8,721 (42.5%) | 3,265 (44.3%) | |
| Never | 1,301 (14.9%) | 3,657 (17.8%) | 1,258 (17.1%) | |
| Unknown | 1,665 (19.1%) | 3,018 (14.7%) | 1,078 (14.6%) | |
| SBP, mmHg | 123.2 (14.6) | 127.7 (15.3) | 131.1 (16.6) | <.001 |
| DBP, mmHg | 69.7 (8.2) | 69.6 (8.1) | 69.4 (8.4) | 0.046 |
| Activities of daily living | ||||
| 0 | 517 (5.9%) | 1,187 (5.8%) | 478 (6.5%) | <.001 |
| 1–14 | 3,669 (42.1%) | 9,096 (44.3%) | 3,474 (47.1%) | |
| 15–27 | 3,999 (45.9%) | 9,446 (46.0%) | 3,249 (44.1%) | |
| 28 | 533 (6.1%) | 815 (4.0%) | 171 (2.3%) | |
| Cognitive Function Scale | ||||
| Cognitively intact | 1,960 (22.5%) | 5,074 (24.7%) | 1,998 (27.1%) | <.001 |
| Mildly impaired | 2,860 (32.8%) | 7,498 (36.5%) | 3,048 (41.3%) | |
| Moderately impaired | 2,021 (23.2%) | 4,407 (21.5%) | 1,351 (18.3%) | |
| Severely impaired | 1,877 (21.5%) | 3,565 (17.4%) | 975 (13.2%) | |
| Hypertension | 5,030 (57.7%) | 18,046 (87.8%) | 6,907 (93.7%) | <.001 |
| Cardiovascular disease * | 4,352 (49.9%) | 14,180 (69.0%) | 5,919 (80.3%) | <.001 |
| Heart Failure | 1,243 (14.3%) | 7,009 (34.1%) | 4,264 (57.8%) | <.001 |
| Arterial fibrillation | 2,526 (29.0%) | 5,812 (28.3%) | 2,675 (36.3%) | <.001 |
| Kidney disease | 2,603 (29.9%) | 8,761 (42.6%) | 3,902 (52.9%) | <.001 |
| Dementia | 4,356 (50.0%) | 8,802 (42.8%) | 2,599 (35.3%) | <.001 |
Notes: Data was mean (standard deviation) or n (%) and compared using Kruskal-wills or chi-square test. Abbreviation: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Cardiovascular disease including coronary heart disease, cerebrovascular disease, and peripheral vascular disease.
Association of SBP with CV events
Over 3 years of follow-up, CV events occurred in 3,996 (11%) residents and the incidence rate was 119.8 per 1000 person-years (median 0.51 years of follow-up). The unadjusted incidence rates of CV events (per 1000 person-years) were 42, 111, and 250 for residents with no medication, 1–2 medications and ≥3 medications, respectively (Table S4). Survival curves (Figure 1) show that, in residents on no antihypertensive medications, CV event-free survival was not statistically significantly different across SBP levels (log-rank p=0.46) while in residents on any antihypertensives, persons with lower SBP level had a lower CV event-free survival (log-rank p<0.001). The adjusted HRs illustrate the same relationships (left panel of Figure 2). In residents on no antihypertensive medications, we didn’t find statistically significant differences in risk of CV events across SBP subgroups, although the highest risk point estimate was in those with high SBP (≥150mmHg). By contrast, in residents receiving antihypertensive medications, those with low SBP <110mmHg (compared with SBP 130~149mmHg) had the greatest risk of CV events (adjusted [cause-specific] HR [95%CI]: 1.47 [1.28–1.68] in residents on 1 or 2 medications, and 1.41 [1.19–1.67] in residents on ≥3 medications, respectively). These patterns remained consistent when we adjusted for a more extensive set of confounders and potential mediators (Figure S4).
Figure 1.
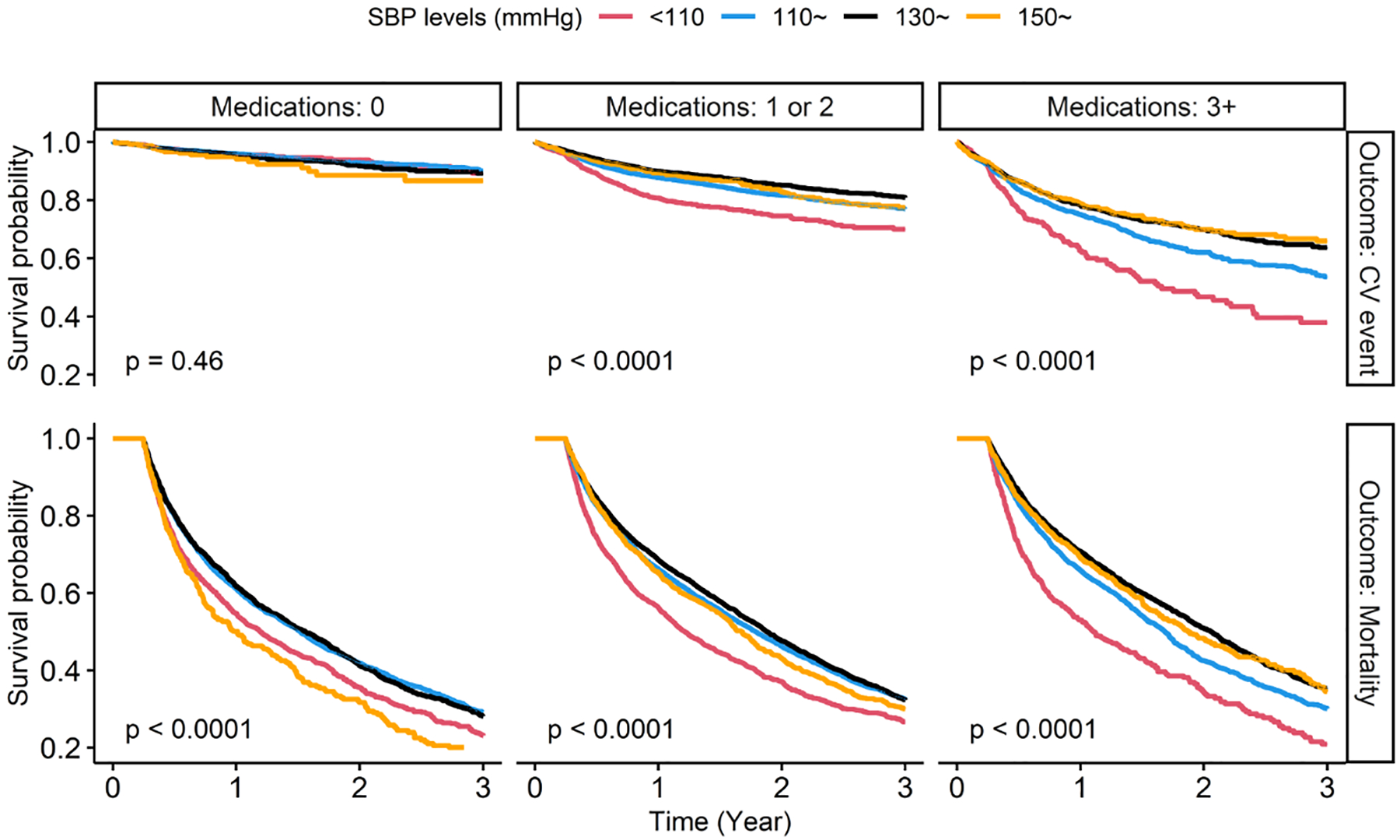
Survival curves for CV events and mortality by SBP and antihypertensive medication groups. SBP, systolic blood pressure. CV event, cardiovascular event. Median follow-up time was 0.51 years (mean 0.91 years) for cardiovascular events and 1.30 years (mean 1.37 years) for mortality. P-values were calculated by log-rank tests.
Figure 2.
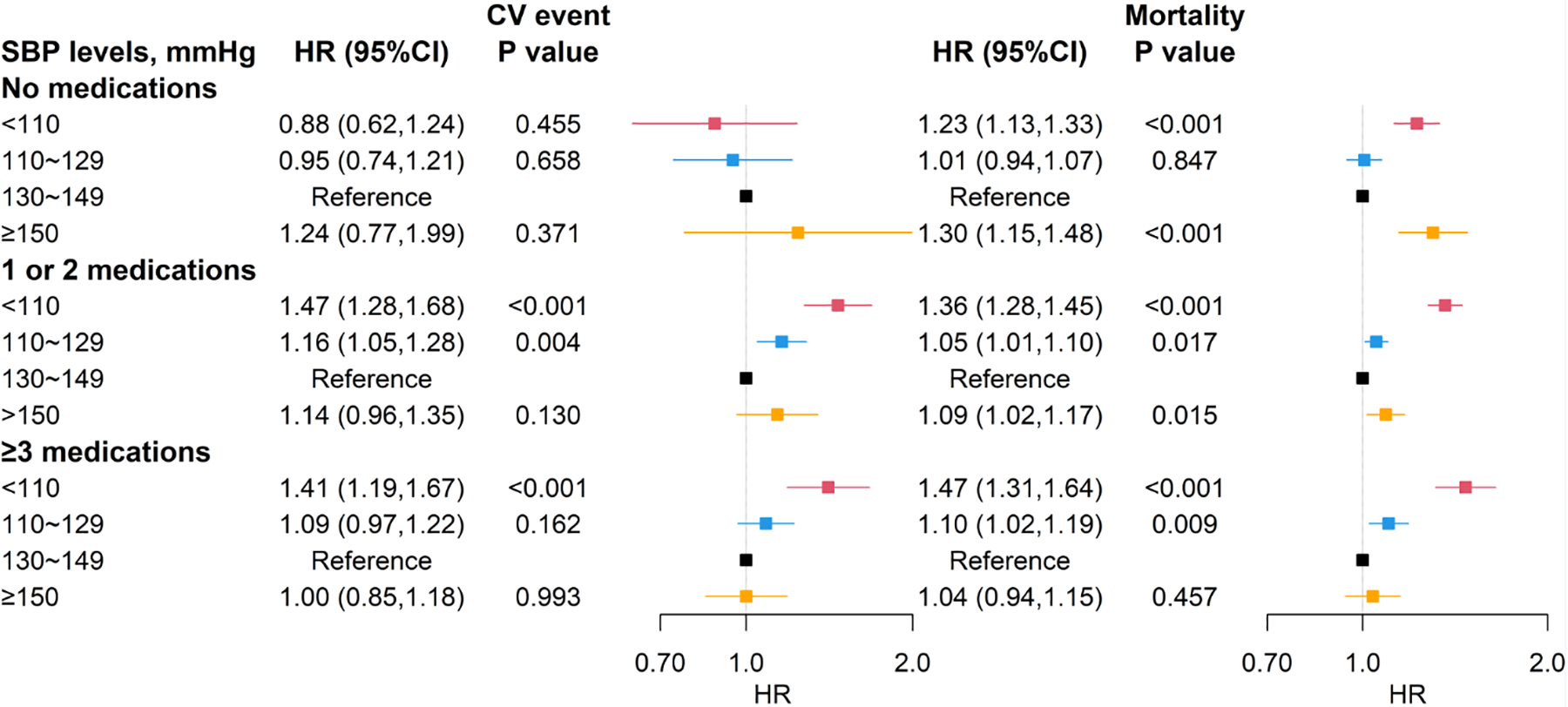
Adjusted hazard ratios of SBP levels for CV events and mortality by antihypertensive medication groups. SBP, systolic blood pressure. CV event, cardiovascular event. HR, hazard ratio. Blood pressure is in units of mmHg. Reference group was SBP of 130~149mmHg. Models adjusted for age, sex, race, height, weight, smoking status, cardiovascular disease, heart failure, and activities of daily living (model 1). * P-value<.05 ** P-value <.001
Association of SBP with mortality
Over 3 years of follow-up, 20,253 (55%) residents died with an incidence rate of 403.9 per 1000 person-years (median 1.30 years of follow-up). The unadjusted incidence rates of mortality (per 1000 person-years) were 457, 391, and 380 for residents with no medication, 1–2 medications and ≥3 medications, respectively (Table S4). The right panel of Figure 2 shows the adjusted HRs of SBP with mortality. In residents on no antihypertensive medications, both a low SBP of <110 mmHg (1.23 [1.13–1.33]) and a high SBP of ≥150 mmHg (1.30 [1.15–1.48]) were associated with a greater mortality (compared with SBP 130~149mmHg). In residents receiving antihypertensives, the subgroups with a lower SBP (<130 mmHg) had higher risk of death and the highest risk point estimate was in those with a lowest SBP of < 110 mmHg (1.36 [1.28–1.45] in residents on 1 or 2 medications, and 1.47 [1.31–1.64] in residents with ≥3 medications, respectively). The results remained similar in the fully adjusted models (Figure S4).
Multistate modeling
A lower SBP (<130mmHg) was associated with a greater risk of transition to CV events in residents with any antihypertensive medications (Table 2). A SBP level of 110~129mmHg was associated with a greater risk of transition to death regardless of antihypertensive status. In residents on no medications, those with a high SBP of ≥150 mmHg had a higher risk of transition to death. In addition, we found no effect of SBP levels on transitions from CV events to death in residents with no more than 2 antihypertensive medications, while a SBP <110mmHg was associated with greater risk of transitions from CV events to death in ≥3 medications subgroup. Findings were unchanged when we adjusted a full set of covariates (Table S5).
Table 2.
Adjusted hazard ratios of SBP levels on transition probabilities by multistate modeling
| Baseline → CV event | Baseline → Death | CV event → Death | |
|---|---|---|---|
| SBP levels, mmHg | |||
| No medication | |||
| <110 | 0.91 (0.71,1.16) | 1.00 (0.93,1.07) | 0.89 (0.65,1.22) |
| 110~129 | 0.89 (0.64,1.25) | 1.19 (1.09,1.29) ** | 1.13 (0.74,1.73) |
| 130~149 | ref | ref | ref |
| ≥150 | 1.23 (0.77,1.97) | 1.23 (1.08,1.40) * | 1.16 (0.64,2.12) |
| 1 or 2 medications | |||
| <110 | 1.24 (1.12,1.37) ** | 1.04 (0.99,1.08) | 0.93 (0.82,1.07) |
| 110~129 | 1.74 (1.51,1.99) ** | 1.30 (1.21,1.39) ** | 1.16 (0.96,1.40) |
| 130~149 | ref | ref | ref |
| ≥150 | 1.14 (0.96,1.34) | 1.06 (0.98,1.14) | 1.00 (0.80,1.25) |
| ≥3 medication | |||
| <110 | 1.14 (1.02,1.28) * | 1.05 (0.97,1.15) | 1.19 (1.02,1.39) * |
| 110~129 | 1.56 (1.31,1.84) ** | 1.39 (1.21,1.59) ** | 1.10 (0.86,1.42) |
| 130~149 | ref | ref | ref |
| ≥150 | 0.98 (0.83,1.16) | 1.11 (0.99,1.25) | 0.98 (0.79,1.23) |
Notes: Data are hazard ratios (95% confidence intervals). SBP, systolic blood pressure. Reference group was SBP of 130~149mmHg. Models adjusted for age, sex, race, height, weight, smoking status, cardiovascular disease, heart failure, and activities of daily living (model 1).
P-value<.05
P-value <.001
Sensitivity analyses
Findings remained similar in all sensitivity analyses, including using the unconstrained cohort (over the entire follow-up period, CV events occurred in 4,302 [12%] residents and 23,166 [63%] residents died), the medication information at one month after admission, restricting to those with dementia at admission or those who lived more than 6 months (Table S6). The relationship between level of SBP and risk of CV events and death remained largely unchanged when we adjusted for SBP changes (Table S7). Stable/increasing SBP trajectory was associated with an increased CV event risk in residents on no antihypertensive medications and a decreased risk of death regardless of medication status.
DISCUSSION
In this large cohort of VA nursing home residents 65 years of age and older, we found that more than 76% residents were on antihypertensive therapy and the relationships between SBP, CV events, and mortality varied by intensity of antihypertensive medication use. Specifically, among those on any antihypertensive medications, lower SBP was associated with increased risk of CV events and mortality. In contrast, among those on no antihypertensive medications, both low and high SBP were associated with mortality. These findings were further confirmed in sensitivity analyses and using an alternate method to account for competing risk. We also adjusted for the person-specific SBP change patterns and the results remained unchanged. Our results highlight the importance of treating high SBP but also caution against very low SBP among nursing home residents on multiple antihypertensive medications.
Several recent observational studies have showed the associations of low SBP with increased adverse outcomes, including cognitive decline, dementia, cardiac events and mortality, in older adults,25–27 although few investigated adverse events in the long-term care residents. We are aware of only two other studies that have specifically evaluated this relationship in this population, and our study is the first to evaluate cardiovascular events. Our results are in accordance with those of Benetos et al.,14 who found that older adults ≥80 years of age residing in a nursing home and with low SBP (<130mmHg) receiving 2+ antihypertensive drugs were at increased risk of mortality compared with the group receiving either 1 or no antihypertensive drugs. In a Swedish cohort of nursing home residents 65 years or older, low SBP <120mmHg was associated with increased all-cause mortality, irrespective of antihypertensive medication status.28
The mechanisms under the associations between low SBP and CV risk and mortality in older individuals remain uncertain, and the effectiveness and safety of BP-lowering drugs in this population have not been examined in randomized controlled trials. Our findings, combined with those from previous research, point to the potential risk of low BP in nursing home older adults. One hypothesis is that multimorbidity could contribute to an increased risk since older adults with multimorbidity are more vulnerable to the adverse effects of multiple medication use, although the effect of low SBP remained significant after adjusting for chronic diseases. Another explanation is that, owing to impaired autoregulation and aging-related functional and structural remodeling to the cardiovascular system, low SBP may exacerbate hypoperfusion of target organs, such as the brain, heart, and kidneys.28–33 Therefore, sufficiently high SBP may be necessary to guarantee adequate cardiac and cerebral perfusion in old age. Low SBP has also been considered presumably a marker of a more severe neurodegenerative process because of its association with brain atrophy in aging population.34,35
Previous research has suggested that reverse causation (lower SBP values result from proximity to death) could contribute to the relationship between low SBP and mortality.36 Nevertheless, our previous investigation demonstrated that in this population, the SBP levels were stable until last 3–4 weeks of life,19 and the median follow-up of the current study is more than 1.3 years. Therefore, it is unlikely the observed relationships are attributed to the terminal SBP decline or reverse causation. The sensitivity analysis of excluding those who died within a 6-month period after admission also confirmed these findings. Future studies using a randomized intervention or causal inference methods could help shed light on these complex causal relationships.
Our study has many strengths including the use of a large sample of older adults with comprehensive longitudinal data for medical diagnoses and daily measurements of medications administered through automated medication administration logs. In addition, we included frequent SBP measures, which is unavailable in studies using Medicare or claims data alone. The eligibility criteria were unrestricted, and the sample included patients with dementia or living in nursing homes, a population often excluded from clinical trials. Our study also has several limitations, most notably that VA nursing home residents represents a selected population—subjects were predominantly male—so the findings may not be generalizable to other nursing home populations. However, we do not believe that there are strong biologic reasons why these associations would differ in women or other populations. Additionally, electronic health records have known measurement error and the SBP measurement procedures may be heterogeneous across time and nursing homes and therefore introduce bias. Many deaths in this population are multifactorial and we did not have data on cause of death and thus were unable to distinguish between CV and non-CV death. Moreover, the risk for confounding by indication limits the causal interpretation of our associations and the inherent limitation due to reverse causation may not be fully addressed. Finally, we did not look at medication class effect and trajectories in medication use; however, our previous investigation37 indicated that the antihypertensive medication changes were most commonly in the first 4 weeks after admission and we did include a sensitivity analysis looking at medication usage at fourth or fifth week and found no difference in our primary findings.
In conclusion, the present study adds to the evidence suggesting a potential risk associated with treated low SBP for older residents in VA nursing home. This should be balanced with the observation that untreated high SBP ≥150mmHg is associated with a greater risk of death. Our results caution that the treatment of hypertension in old nursing home residents should not be directly extrapolated from evidence in community-dwelling older adults. More evidence is needed on the benefits and harms of BP lowering in this population to inform patients and providers shared decision making.
Supplementary Material
Table S1. International Classification of Diseases (ICD) codes to identify cardiovascular events
Table S2. Baseline characteristics by antihypertensive medication groups in VA nursing home residents (continued)
Table S3. Baseline characteristics by systolic blood pressure levels
Table S4. Unadjusted incidence rate (per 1,000 person-years) for cardiovascular events and mortality by antihypertensive medication and SBP groups
Table S5. Fully adjusted hazard ratios of SBP levels on transition probabilities by multistate modeling
Table S6. Sensitivity analyses of hazard ratios of SBP levels for CV events and mortality by antihypertensive medication groups
Table S7. Hazard ratios of SBP levels and changes for CV events and mortality by antihypertensive medication groups
Figure S1. Flow chart of study population
Figure S2. Diagram of Multistate Model for CV event
Figure S3. Random SBP slopes during follow-up period for CV events (left) and mortality (right)
Figure S4. Fully adjusted hazard ratios of SBP levels for CV events and mortality by antihypertensive medication groups
Key Points.
More than 76% VA nursing home residents received antihypertensive therapy and 20% were on three or more antihypertensive medications.
A low systolic blood pressure was associated with increased cardiovascular and mortality risk among the nursing home residents receiving any antihypertensive medications.
Nursing home residents with untreated high systolic blood pressure ≥150mmHg had a greater risk of death.
Why does this matter?
The benefits and harms of blood pressure control in older adults in long-term care remain unclear. This is the first large study to characterize the relationships of systolic blood pressure with cardiovascular events and mortality in U.S. nursing home residents, and to examine the role of antihypertensive medication in these relationships.
ACKNOWLEDGMENTS
Funding
This work was supported by the National Institute on Aging (NIA; RF1AG062568). MAS’s effort was supported in part by NIA (R24AG064025), NIA (K24AG049057), and NIA (P30 AG044281). SJL’s effort was supported in part by VA’s Health Services Research and Development Service (IIR 15-434), NIA (R01AG057751) and NIA (K24AG066998). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans’ Health Association.
Sponsor’s Role
The funders of the study had no role in the design of the study, data analysis, interpretation of findings, or writing of the manuscript.
Footnotes
Conflict of Interest
CAP serves as the Chief Medical Officer for Cricket Health, Inc. MCO serves as a consultant for Cricket Health, Inc. MAS and SJL receive honoraria as authors on UpToDate.
This work was presented at The Gerontological Society of America (GSA) 2021 Annual Scientific Meeting.
REFERENCES
- 1.Ewen S, Mahfoud F, Böhm M. Blood pressure targets in the elderly: many guidelines, much confusion. Eur Heart J. 2019;40(25):2029–2031. doi: 10.1093/eurheartj/ehz150 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e484–e594. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 4.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350(9080):757–764. [DOI] [PubMed] [Google Scholar]
- 6.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315(24):2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Zhang S, Deng Y, et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N Engl J Med. 2021;385(14):1268–1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 9.Delgado J, Masoli JAH, Bowman K, et al. Outcomes of Treated Hypertension at Age 80 and Older: Cohort Analysis of 79,376 Individuals. J Am Geriatr Soc. 2017;65(5):995–1003. doi: 10.1111/jgs.14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streit S, Poortvliet RKE, Gussekloo J. Lower blood pressure during antihypertensive treatment is associated with higher all-cause mortality and accelerated cognitive decline in the oldest-old. Data from the Leiden 85-plus Study. Age Ageing. 2018;47(4):545–550 [DOI] [PubMed] [Google Scholar]
- 11.Molander L, Lövheim H, Norman T, Nordström P, Gustafson Y. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. J Am Geriatr Soc. 2008;56(10):1853–1859. [DOI] [PubMed] [Google Scholar]
- 12.Satish S, Freeman DH, Jr., Ray L, Goodwin JS. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc. 2001;49(4):367–374. [DOI] [PubMed] [Google Scholar]
- 13.Rastas S, Pirttilä T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54(6):912–918. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, Labat C, Rossignol P, et al. Treatment With Multiple Blood Pressure Medications, Achieved Blood Pressure, and Mortality in Older Nursing Home Residents: The PARTAGE Study. JAMA Intern Med. 2015;175(6):989–995. [DOI] [PubMed] [Google Scholar]
- 15.Boockvar KS, Song W, Lee S, Intrator O. Hypertension Treatment in US Long-Term Nursing Home Residents With and Without Dementia. J Am Geriatr Soc. 2019;67(10):2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado J, Masoli JAH, Bowman K, et al. Outcomes of Treated Hypertension at Age 80 and Older: Cohort Analysis of 79,376 Individuals. J Am Geriatr Soc. 2017;65(5):995–1003. doi: 10.1111/jgs.14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oates DJ, Berlowitz DR, Glickman ME, Silliman RA, Borzecki AM. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55(3):383–388. [DOI] [PubMed] [Google Scholar]
- 18.Roth GA, Johnson CO, Abate KH, et al. The Burden of Cardiovascular Diseases Among US States, 1990–2016. JAMA Cardiol. 2018;3(5):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham LA, Lee SJ, Steinman MA, et al. Exploring the Dynamics of Week-to-Week Blood Pressure in Nursing Home Residents Before Death. Am J Hypertens. 2022;35(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 21.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulm K A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990;131(2):373–375. [DOI] [PubMed] [Google Scholar]
- 23.Ieva F, Jackson CH, & Sharples LD (2017). Multi-state modelling of repeated hospitalisation and death in patients with heart failure: The use of large administrative databases in clinical epidemiology. Statistical methods in medical research, 26(3), 1350–1372. 10.1177/0962280215578777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wreede LC, Fiocco M, Putter H. mstate: An R Package for the Analysis of Competing Risks and Multi-State Models. J Stat Softw. 2011;38(7):30. [Google Scholar]
- 25.Mossello E, Pieraccioli M, Nesti N, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175(4):578–585. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res. 2007;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang M, Muñoz-Venturelli P, Billot L, et al. Low blood pressure and adverse outcomes in acute stroke: HeadPoST study explanations. J Hypertens. 2021;39(2):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radholm K, Festin K, Falk M, Midlov P, Molstad S, Ostgren CJ. Blood pressure and all-cause mortality: a prospective study of nursing home residents. Age Ageing. 2016;45(6):826–832.. [DOI] [PubMed] [Google Scholar]
- 29.Feldstein CA. Association between chronic blood pressure changes and development of Alzheimer’s disease. J Alzheimers Dis. 2012;32(3):753–763. doi: 10.3233/JAD-2012-120613 [DOI] [PubMed] [Google Scholar]
- 30.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. 2019;124(7):1045–1060. [DOI] [PubMed] [Google Scholar]
- 31.Potter JF, Robinson TG, Ford GA, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8(1):48–56. [DOI] [PubMed] [Google Scholar]
- 32.Dorresteijn JA, van der Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease: J-curve revisited. Hypertension. 2012;59(1):14–21. [DOI] [PubMed] [Google Scholar]
- 33.van Bemmel T, Holman ER, Gussekloo J, Blauw GJ, Bax JJ, Westendorp RG. Low blood pressure in the very old, a consequence of imminent heart failure: the Leiden 85-plus Study. J Hum Hypertens. 2009;23(1):27–32. [DOI] [PubMed] [Google Scholar]
- 34.Skoog I, Andreasson LA, Landahl S, Lernfelt B. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension. 1998;32(3):404–409. [DOI] [PubMed] [Google Scholar]
- 35.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26(8):1636–1641. [DOI] [PubMed] [Google Scholar]
- 36.Ravindrarajah R, Hazra NC, Hamada S, et al. Systolic Blood Pressure Trajectory, Frailty, and All-Cause Mortality >80 Years of Age. Circulation. 2017;135(24):2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odden MC, Lee SJ, Steinman MA, et al. Deprescribing Blood Pressure Treatment in Long-Term Care Residents. J Am Med Dir Assoc. 2021;22(12):2540–2546.e2. doi: 10.1016/j.jamda.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases (ICD) codes to identify cardiovascular events
Table S2. Baseline characteristics by antihypertensive medication groups in VA nursing home residents (continued)
Table S3. Baseline characteristics by systolic blood pressure levels
Table S4. Unadjusted incidence rate (per 1,000 person-years) for cardiovascular events and mortality by antihypertensive medication and SBP groups
Table S5. Fully adjusted hazard ratios of SBP levels on transition probabilities by multistate modeling
Table S6. Sensitivity analyses of hazard ratios of SBP levels for CV events and mortality by antihypertensive medication groups
Table S7. Hazard ratios of SBP levels and changes for CV events and mortality by antihypertensive medication groups
Figure S1. Flow chart of study population
Figure S2. Diagram of Multistate Model for CV event
Figure S3. Random SBP slopes during follow-up period for CV events (left) and mortality (right)
Figure S4. Fully adjusted hazard ratios of SBP levels for CV events and mortality by antihypertensive medication groups


