Abstract
Background:
Chronic stress is a major risk factor for psychiatric illnesses, including depression. However, the pathophysiological mechanisms whereby stress leads to mood disorders remain unclear. Allopregnanolone acts as a positive allosteric modulator preferentially on δ subunit-containing GABAA receptors (δ-GABAARs). Accumulating clinical and preclinical evidence supports the antidepressant effects of exogenous administration of allopregnanolone analogs; yet the role of endogenous allopregnanolone in the pathophysiology of depression remains unknown.
Methods:
We utilized a chronic unpredictable stress (CUS) mouse model, followed by behavioral and biochemical assays to examine whether altered neurosteroid signaling contributes to behavioral outcomes following CUS. We subsequently performed in vivo CRISPR knockdown of rate-limiting enzymes involved in allopregnanolone synthesis, 5α-reductase type 1 and 2 (5α1/2), in addition to lentiviral overexpression of 5α1/2 in the basolateral amygdala (BLA) of mice that underwent CUS to assess the impact of 5α1/2 on behavioral outcomes.
Results:
The expression of δ-GABAARs and endogenous levels of allopregnanolone were reduced in the BLA following CUS. Treatment with an exogenous allopregnanolone analog, SGE-516, was sufficient to increase allopregnanolone levels in the BLA following CUS. Knockdown of 5α1/2 in the BLA, mimicked the behavioral outcomes associated with CUS. Conversely, overexpression of 5α1/2 in the BLA improved behavioral outcomes following CUS.
Conclusions:
Our findings demonstrate that chronic stress impairs endogenous neurosteroid signaling in the BLA which is sufficient to induce behavioral deficits. Further, these studies suggest allopregnanolone-based treatments may directly target the underlying pathophysiology of mood disorders suggesting that targeting endogenous neurosteroidogenesis may offer a novel therapeutic strategy.
Keywords: Stress, neurosteroids, neurosteroidogenesis, GABA, Allopregnanolone, depression
Introduction
Neurosteroids, such as allopregnanolone, have been implicated in the pathophysiology of psychiatric illnesses, including anxiety, depression, and post-traumatic stress disorder (PTSD) (1,2,3,4,5,6,7), largely based on anxiolytic and antidepressant effects of exogenous administration of these compounds (8). Few studies have directly explored the pathophysiological link between endogenous neurosteroids and mood disorders. Deficits in neurosteroids and a reduction in the expression of rate limiting enzymes involved in endogenous neurosteroidogenesis, 5α-reductases, have been demonstrated in preclinical models of chronic stress (2,3,4,5,9). Given that chronic stress is a major risk factor for psychiatric illnesses (10,11,12), these data suggest that deficits in endogenous neurosteroidogenesis may contribute to pathophysiological mechanisms underlying psychiatric illnesses (6,7). This hypothesis is supported by clinical evidence that finasteride, a 5α-reductase inhibitor, is associated with mood disorders, including anxiety and depression, referred to as post-finasteride syndrome (PFS), the pathophysiology of which may involve deficits in endogenous neurosteroid signaling (13). Further, in animal models, the anxiolytic and antidepressant effects of progesterone are blocked by finasteride (14), suggesting that endogenous neurosteroids impact mood.
Allopregnanolone acts predominantly on δ subunit-containing GABAA receptors (δ-GABAARs) (15,16,17). GABAARs are heteropentameric ligand-gated ion channels which mediate the majority of inhibitory neurotransmission in the brain. Recently, we demonstrated that δ-GABAARs are uniquely expressed on parvalbumin (PV) interneurons in the basolateral amygdala (BLA) and mediate the effects of exogenous allopregnanolone on behavioral states (18). Further, we demonstrated that exogenous neurosteroid treatment ameliorates behavioral deficits following chronic stress (18). These data suggest that exogenous neurosteroid actions in the BLA can impact mood; however, the impact of endogenous neurosteroid signaling in the BLA on mood remains unexplored. This study attempts to fill this gap in knowledge by investigating whether perturbations in endogenous neurosteroid signaling in the BLA contribute to chronic stress induced behavioral changes.
Here, we directly examine the contribution of endogenous neurosteroid signaling in the BLA in mediating changes in behavioral states associated with chronic stress. These studies build upon well-established evidence that chronic stress is a risk factor for psychiatric disorders (10,11) and can be utilized in rodents to alter behavioral outcomes (19,20). We demonstrate that chronic unpredictable stress (CUS) induces changes in the capacity for endogenous neurosteroid signaling in the BLA, evident from a decrease in allopregnanolone levels measured in the BLA but not circulating plasma, decreased expression of the neurosteroidogenic enzymes, 5α-reductase 1 and 2 (5α1/2), and their main site of action, δ-GABAARs, in the BLA. CRISPR-mediated knockdown of genes encoding for 5α1/2, in the BLA was sufficient to mimic the impact of CUS on behavioral states. Similarly, knocking down the predominant site of action of endogenous 5α-reduced neurosteroids, δ-GABAARs, in the BLA also mimicked behavioral deficits associated with CUS. Importantly, increasing the capacity for endogenous neurosteroidogenesis by overexpressing 5α1/2 in the BLA was sufficient to improve behavioral states following CUS. These findings implicate endogenous neurosteroid signaling in the BLA in the pathophysiology of mood disorders and provides a potential mechanistic underpinning for the antidepressant effects of allopregnanolone.
Methods and Materials
Detailed methods are described in the Supplement. Briefly, CUS mice underwent a three-week protocol consisting of alternating overnight stressors (Figure S1 A). Following CUS, liquid chromatography with tandem mass spectrometry (LC-MS/MS) was conducted on plasma, whole brain, and BLA samples to detect levels of allopregnanolone in vehicle-treated mice or mice treated with SGE-516[tool compound], a synthetic analog of allopregnanolone developed by SAGE Therapeutics, and qRT-PCR was performed to measure 5α1/2 expression. Cre-dependent adeno-associated virus viral vectors were constructed to facilitate expression of guideRNAs targeting genes for δ-GABAARs and 5α1/2 (sgGabrd and sg5α1/2 respectively). sgGabrd was stereotaxically administered bilaterally to the BLA of PV-Cas9 mice to specifically ablate δ-GABAARs from parvalbumin interneurons in the region, given the unique expression of these receptors in PV interneurons in the BLA (18). The same stereotaxic procedures were performed in constitutive Cas9 mice to ablate 5α1/2 from all cell types in the BLA. To overexpress 5α1/2 in the BLA, 5α1/2 lentiviral constructs were stereotaxically injected bilaterally into the BLA of C57BL/6J mice. All mice were given a one-week surgical recovery period prior to any behavioral testing to assess for stress-induced helplessness and avoidance behaviors.
Results
Behavioral Aberrations Following Chronic Unpredictable Stress
Given that chronic stress is a well-established risk factor for the development of psychiatric disorders, we utilized a chronic unpredictable stress paradigm to examine behavioral sequelae of chronic stress. Mice subjected to CUS displayed increased avoidance behaviors, evident from a significant decrease in total time spent in the center of the open field (control: 57.8 ± 6.2 s; CUS: 39.7 ± 2.6 s)(Figure 1 A-B; control n=14, CUS n=15, p=0.0107 unpaired t-test) without a change in locomotor behavior (control: 2252 ± 135.4 beam breaks; CUS: 2193 ± 75.6 beam breaks)(Figure 1 A-B; p=0.7009 unpaired t-test). Mice subjected to CUS also exhibited an increase in stress-induced helplessness demonstrated by a shorter latency to immobility (control: 73.5 ± 7.4 s; CUS: 35.9 ± 5.9 s)(Figure 1 C; control n=10, CUS n=11, p=0.0008 unpaired t-test) and an increased total time spent immobile (control: 180 ± 13.8 s; CUS: 233.2 ± 7.7 s) in the tail suspension test (Figure 1 C; p=0.0027 unpaired t-test). Similarly, CUS mice exhibited a decreased latency to immobility (control: 64.6 ± 12.22 s; CUS: 6.2 ± 0.6 s)(Figure 1 D; control n=10, CUS n=15, p=0.0010 unpaired t-test) and an increased total time immobile in the forced swim test (control: 149.6 ± 17.9 s; CUS: 219.8 ± 5.9 s)(Figure 1 D; p=0.0033 unpaired t-test).
Figure 1. Chronic unpredictable stress induced behavioral deficits.
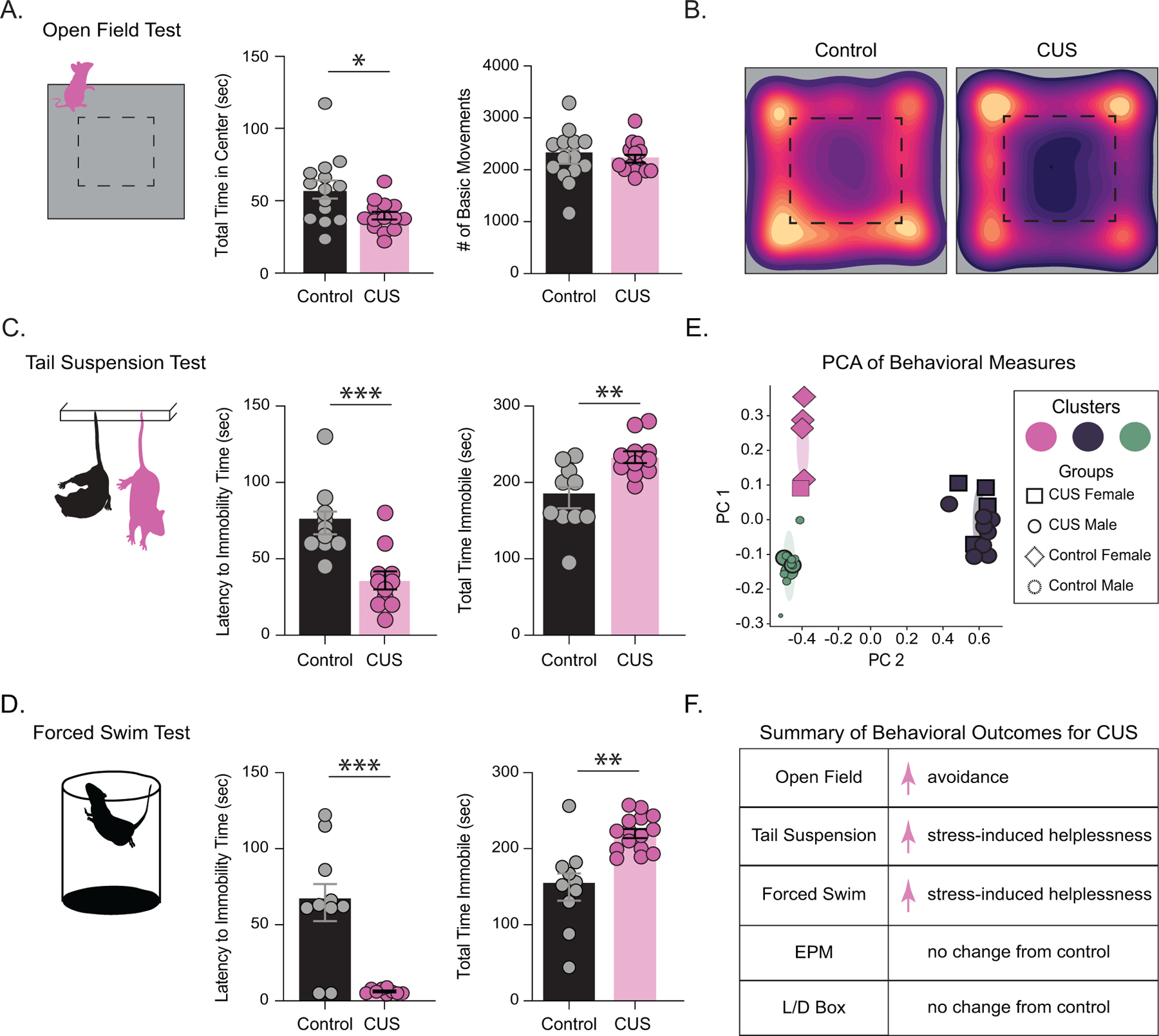
CUS increased avoidance behaviors, with decreased total time spent in the center of the open field without altered overall locomotor behavior (A) control n=14, CUS n=15. (B) representative heat maps of mobility during the open field test of control and CUS mice. CUS also increased stress-induced helplessness, with CUS mice exhibiting a decreased latency to immobility time and an increased total time spent immobile during the tail suspension test (C) control n=10, CUS n=11. CUS mice also displayed stress-induced helplessness in the forced swim test, exhibiting a decreased latency to immobility and increased total time immobile compared to controls (D) n=10, CUS n=15. (E) PCA performed on all behavioral outcomes demonstrated different clusters for mice subjected to CUS compared to controls. (F) summary of behavioral outcomes for mice that underwent CUS for transparency across experiments. *denotes p< 0.05, **p<0.01, ***p<0.001 using an unpaired t-test.
CUS did not alter avoidance behaviors in the elevated plus maze as demonstrated by similar outcomes in open arm time (control: 94.9 ± 15.8 s; CUS 97.3 ± 16.6 s)(Figure S1 B; control n=9, CUS n=10, p=0.9166 unpaired t-test), open arm entries (control: 37.3 ± 6.5 entries; CUS 38.5 ± 5.3 entries)(Figure S1 ; p=0.8914 unpaired t-test), open arm distance (control: 301 ± 82.8 cm; CUS: 274.1 ± 65.1 cm)(Figure S1 B; p=0.8020 unpaired t-test), and locomotor behavior (control: 1459 ± 464.8 beam breaks; 1232 ± 414.5 beam breaks)(Figure S1 B, p=0.7203 unpaired t-test). CUS also did not alter behavioral outcomes in the light/dark box test as demonstrated by similar time spent in the light zone (control:169.8 ± 15.2 s; CUS: 178.0 ± 7.3 s)(Figure S1 C; control n=14, CUS n=15, p=0.6313 unpaired t-test) and locomotor behavior (control: 1491 ± 104.7 beam breaks; CUS: 1417 ± 74.7 beam breaks)(Figure S1 C, p=0.5744 unpaired t-test). To validate our battery of behavior outcomes for chronic stress, we performed a principal component analysis of all behavioral metrics from automated behavior scoring (Figure 1 E; Figure S2 A). A Bayesian information criteria test indicated a 3-cluster gaussian mixed model to best fit the first four principal components explaining over 60% of variance (Figure S2 B-C). Two clusters were predominantly control-male or control-female mice, 80% and 73% respectively (Figure 1 E). The third cluster was composed entirely of CUS males and females, which appeared as a distinct cluster in PCA space (Figure 1 E). Thus, CUS induced robust changes in behavior which distinguished between experimental groups, making this model and these outcome measures useful for investigating mechanisms whereby stress alters behavioral outcomes. The summary of behavioral changes following CUS are shown in Figure 1 F.
Chronic Unpredictable Stress Decreases δ-GABAAR Expression in the BLA
To examine brain regions impacted by CUS, we conducted brain-wide cFos immunofluorescence from control and CUS mice. The average number of cFos-positive cells within the BLA were increased in mice subjected to CUS (control: 13.1 ± 1.4 cells; CUS: 31.5 ± 4.6 cells)(Figure 2 A-B; control n=10, CUS n=6, p=0.0091 unpaired t-test). Thus, this manuscript focused on potential pathophysiological changes in the BLA contributing to behavioral deficits following CUS.
Figure 2. δ-GABAARs are downregulated in the BLA following CUS.
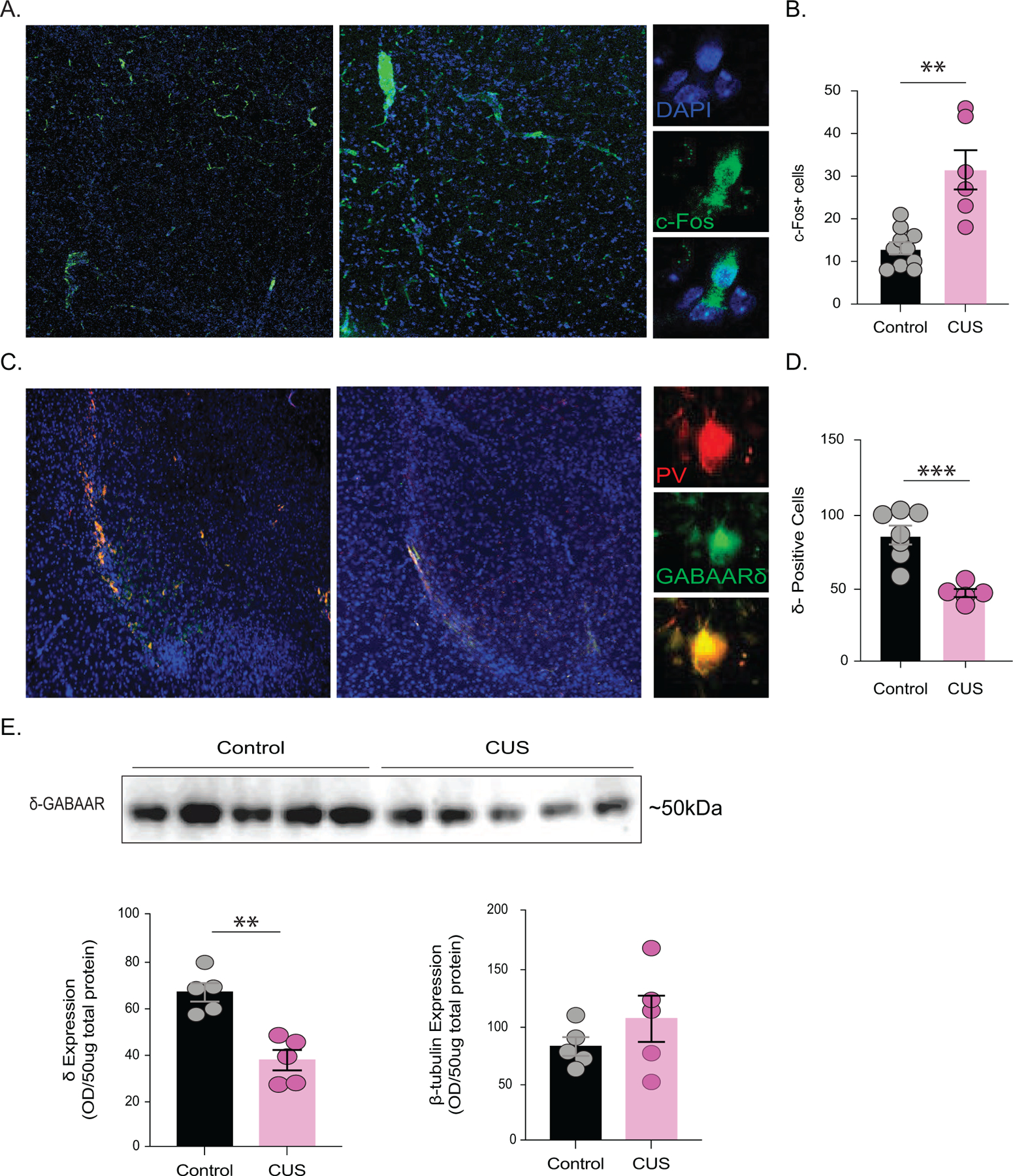
(A) Representative images of cFos immunofluorescence in the BLA of control and CUS mice. The average number of cFos positive cells in the BLA is reduced in mice subjected to CUS compared to controls (B) control n=10 mice, CUS n=6 mice, average 5 sections per mouse. (C) Representative images of immunofluorescence co-labelling of parvalbumin (PV) interneurons and δ-GABAARs in the BLA. The average number of δ-positive cells in the BLA from control and CUS mice (D) control n=7 mice, CUS n=5 mice, average 5 sections per mouse. (E) (above) Representative western blot for δ-GABAAR expression in total protein isolated from the BLA from control and CUS mice. (below) The average optical density expression of δ-GABAARs and β-tubulin per 25ug of total protein. control n=5 mice, CUS n=5 mice. **denotes p<0.01, ***p<0.001 using an unpaired t-test.
Behavioral states, such as fear, avoidance behaviors, and stress-induced helplessness, have been shown to be governed by parvalbumin-positive (PV) interneurons in the BLA (18,21,22,23,24). Given that PV interneurons in the BLA uniquely express δ-GABAARs which influence behavioral states (18), we hypothesized that behavioral abnormalities following chronic stress may involve changes in δ-GABAAR expression on PV interneurons in the BLA. Following CUS, there was a significant reduction in cells expressing δ-GABAARs in the BLA (control: 85.6 ± 6.3 cells per section; CUS: 46.3 ± 2.8 cells per section)(Figure 2 C-D; control n=7, CUS n=5, p=0.0004 unpaired t-test). These findings were further validated using western blot analysis, demonstrating a reduction in δ-GABAAR expression in the BLA in mice subjected to CUS (control: 66.3 ± 4.0 O.D. units/25μg total protein; CUS: 37.0 ± 4.4 O.D. units/25μg total protein)(Figure 2 E; control n=5, CUS n=5, p=0.0012) with no change in β-tubulin expression (control: 81.4 ± 8.1 O.D. units/25μg total protein; CUS: 105.5 ± 20.01 O.D. units/25μg total protein)(Figure 2 E; p=0.3124 unpaired t-test). Collectively, these data demonstrate that the predominant site of action for neurosteroid signaling is decreased within the BLA following CUS, which may contribute to the associated behavioral deficits.
We next examined whether this decrease in δ-containing GABAARs may contribute to behavioral deficits that are observed following CUS. A reduction in δ-GABAARs in the BLA was achieved by injecting sgGabrd (guideRNA encoding for the gene Gabrd specific to the δ-subunit of GABAARs) into the BLA of PV-Cas9 mice to knockdown δ-GABAARs on PV interneurons, in the BLA (Figure 3 A). This approach was sufficient to decrease the number of δ-GABAAR-positive cells in the BLA (control: 39.3 ± 3.1 cells per section; sgGabrd 7.0 ± 1.0 cells per section)(Figure 3 B; p=<0.0001 unpaired t-test) and induced an increase in stress-induced helplessness and avoidance behaviors. In the elevated plus maze, mice with reduced δ-GABAARs in the BLA exhibited a decrease in open arm entries (control: 27.4 ± 5.0 entries; sgGabrd: 15.5 ± 1.6 entries)(Figure 3 C; p=0.0079 unpaired t-test) and a decrease in total distance traveled in the open arm (control: 138.8 ± 38.8 cm; sgGabrd: 75.54 ± 7.9 cm)(Figure 3 C; control n=5, sgGabrd n=13, p=0.0285 unpaired t-test), an indication of increased avoidance behaviors. There was no significant difference in total time in the open arm for sgGabrd mice (control: 122.3 ± 22.1 s; sgGabrd: 132.9 ± 15.4 s)(Figure 3 C; p=0.7129 unpaired t-test). However, there was also a significant decrease in basic movements in the elevated plus maze for sgGabrd mice that these mice did not display in other behavioral tests (control: 519.8 ± 51.1 movements; sgGabrd: 258.5 ± 15.4 movements)(Figure 3 C; p=<0.0001 unpaired t-test). Mice with reduced δ-GABAARs in the BLA also displayed an increase in stress-induced helplessness demonstrated by an increase in total time immobile in the forced swim test (control: 161.0 ± 13.0 s; sgGabrd: 222.5 ± 10.5 s;)(Figure 3 D; control n=5, sgGabrd n=13, p=0.0046 unpaired t-test), although there was no significant difference in latency to immobility in this test (control: 94.6 ± 19.3 s; sgGabrd: 62.8 ± 5.6 s)(Figure 3 D; p=0.1780 unpaired t-test). Collectively, these findings indicate that reduced δ-GABAAR expression on PV interneurons in the BLA is sufficient to induce behavioral deficits, like those observed following CUS (Figure 1). These data further suggest that the decrease in δ-GABAAR expression observed following CUS may contribute to the behavioral deficits associated with CUS. However, these animals did not differ in the total amount of time spent in the center of the open field (control: 94.7 ± 6.9 s; sgGabrd: 74.65 ± 8.2 s)(Figure S3 A; control n=5, sgGabrd n=13, p=0.0811 unpaired t-test), nor did they differ in locomotor behavior (control: 2489 ± 158.6 beam breaks; sgGabrd: 2141 ± 84.4 beam breaks)(Figure S3 A; p=0.0976 unpaired t-test). Similarly, sgGabrd mice did not display any behavioral changes in the light/dark box test as measured by the total time spent in the light zone (control: 184.4 ± 22.7 s; sgGabrd: 189.7 ± 24.2 s) (Figure S3 B; control n=5, sgGabrd n=13, p=0.8762 unpaired t-test) and locomotor behavior (control: 1115 ± 26.2 beam breaks; sgGabrd: 1140 ± 43.9 beam breaks)(Figure S3 B; p=0.6295 unpaired t-test). The summary of behavioral changes in mice with a reduction in δ-GABAARs on PV interneurons in the BLA are shown in Figure 3 E.
Figure 3. CRISPR knockdown of δ-GABAARs in the BLA induced behavioral deficits.
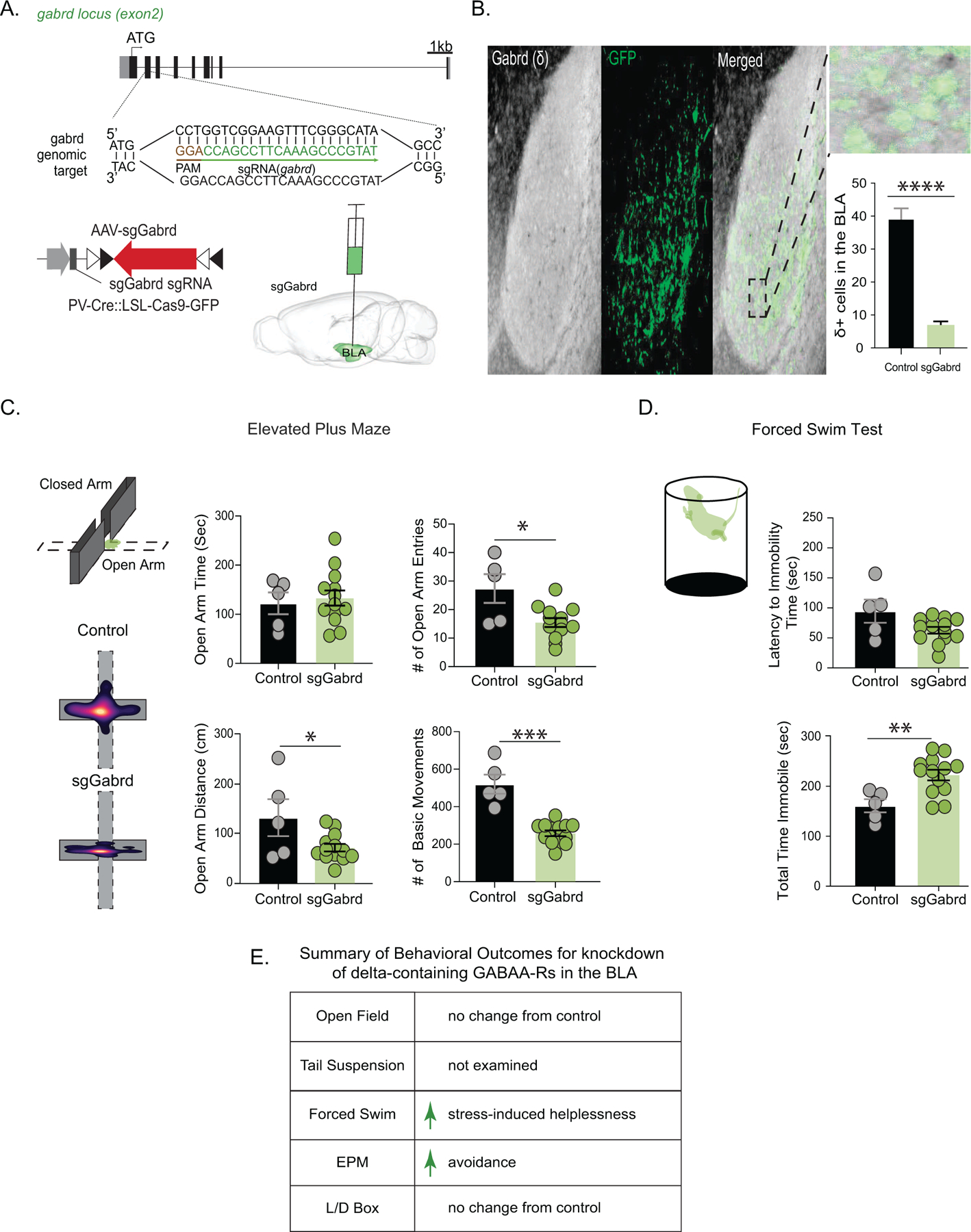
(A) A schematic of the sgRNA construct for knockdown of Gabrd and overview of viral targeting strategy in the BLA of PV/Cas9 mice. (B) Representative co-labeling for δ-GABAARs (DAB) and sgRNA expression (GFP) confirmed a loss of δ-GABAARs in GFP-positive cells. The average number of cells expressing δ-GABAARs in the BLA was reduced in mice injected with sgGabrd compared to controls (inset). sgGabrd mice exhibited a reduction in the total distance traveled, number of entries, and a reduction in basic movements with no change in the amount of time spent in the open arm of the elevated plus maze compared to controls (C) control n=5, sgGabrd n=13. sgGabrd mice exhibited an increase in the total time spent immobile in the forced swim test compared to controls without any change in the latency to immobility (D) control n=5, sgGabrd n=13. (E) A summary of behavioral outcomes for knockdown of δ-GABAARs from PV interneurons in the BLA for transparency across figures and experiments. *denotes p < 0.05, **p<0.01, ***p<0.001, ****p<0.0001 using an unpaired t-test.
Impaired Neurosteroidogenesis in the BLA Following CUS
To further explore whether there are deficits in endogenous neurosteroid signaling following CUS, we measured allopregnanolone levels in plasma, whole brain, and BLA samples from control and CUS mice using LC-MS/MS. Plasma levels of allopregnanolone were not significantly different between control and CUS mice (control: 1.6 ± 0.8 ng/ml; CUS: 0.6 ± 0.1 ng/ml)(Figure 4 A; control n=11, CUS n=13, p=0.087747 unpaired t-test), although there was a trend towards a decrease in the plasma of CUS mice. Similarly, there was no significant difference in whole brain levels of allopregnanolone (control: 3.2 ± 1.1 ng/g; CUS: 1.9 ± 0.8 ng/g)(Figure 4 B; control n=6, CUS n=4, p= 0.873461 unpaired t-test). We did, however, observe a significant decrease in allopregnanolone levels in the BLA following CUS (control: 3.8 ± 1.1 ng/g; CUS: 1.8 ± 0.3 ng/g)(Figure 4 C; control n=4, CUS n=5, p= 0.041426 unpaired t-test). Interestingly, treatment with a synthetic allopregnanolone analog, SGE-516, prevented the CUS-induced reduction in allopregnanolone levels in the BLA (CUS+SGE-516 plasma: 1.0 ± 0.2; whole brain; 4.4 ± 1.3; BLA: 4.2 ± 0.4)(Figure 4A; CUS+SGE-516: plasma n=14, Figure 4B; whole brain n=8, Figure 4C; BLA n=8; plasma p=0.1964; whole brain p=0.3296; BLA p=0.0025). These data demonstrate a reduction in endogenous neurosteroid levels in the BLA following CUS which are likely brain region specific.
Figure 4. Exogenous neurosteroid-analog SGE-516 promoted restoration of endogenous allopregnanolone in vivo and in vitro through 5α-reductases type 1 and 2.
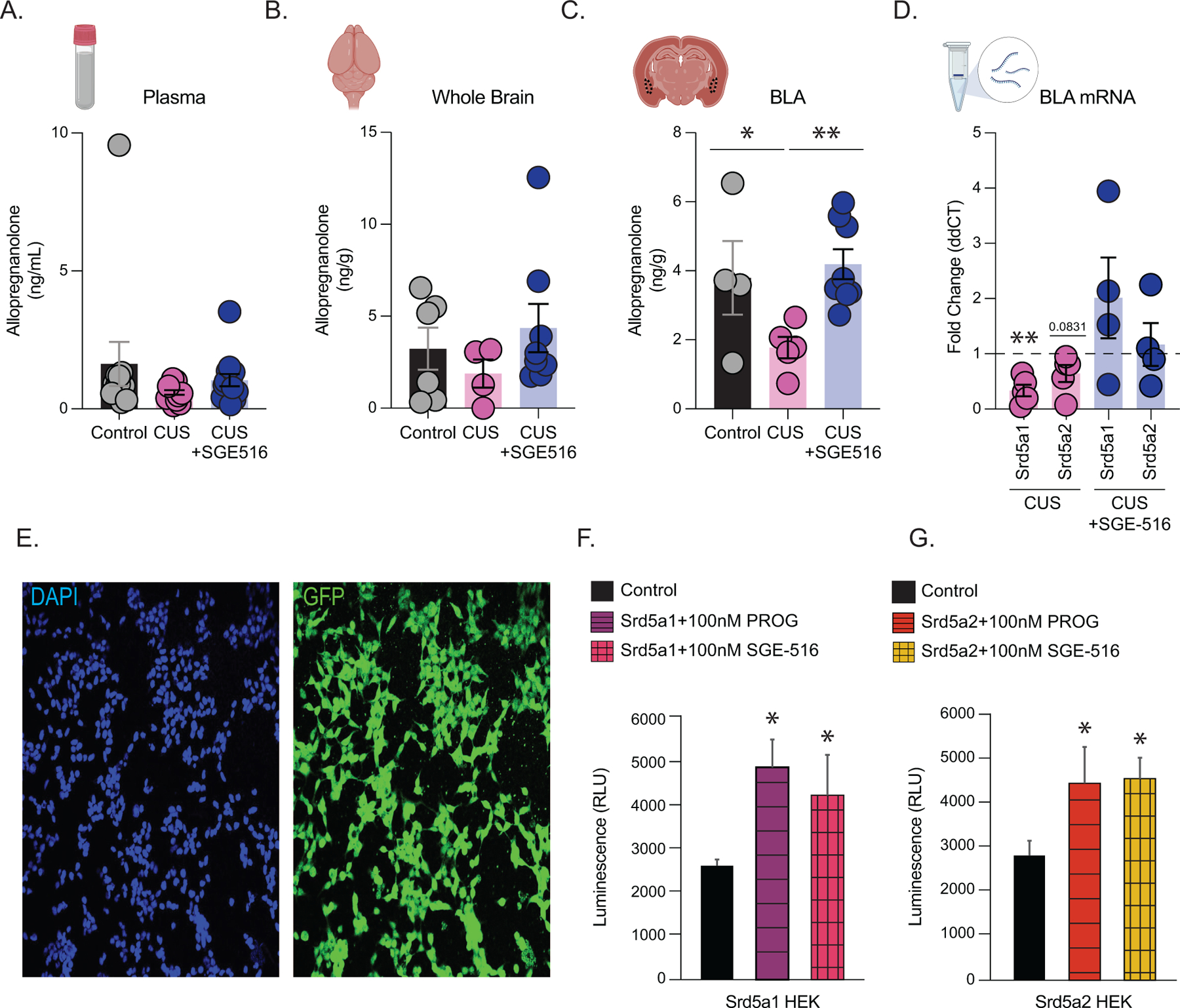
LC-MS/MS measurement of allopregnanolone levels from control, CUS, and CUS+SGE-516 mice. (A) plasma, control n=10, CUS n=13, CUS+SGE-516 n=14 (B) whole brain, control n=6, CUS n=4, CUS+SGE-516 n=8 and (C) BLA samples, control n=4, CUS n=5, CUS+SGE-516 n=8. (D) qRT-PCR measurement of BLA mRNA levels of Srd5a1 and Srd5a2 normalized to β-actin and control levels, control n=16; CUS n=20; CUS+SGE-516 n=16 pooled BLA samples from 4 mice per experiment. (E) Representative images of Srd5a1 and Srd5a2 expressing cell lines showing cell confluency by DAPI staining left, and Srd5a1 and Srd5a2 GFP tag right. Quantification of NADP assay readout for luminescence from (F) Srd5a1 and (G) Srd5a2 expressing cell lines following application of control, progesterone, and SGE-516 treatments.
Given our observation that endogenous allopregnanolone levels are reduced in the BLA, we examined the expression of 5α-reductase 1 and 2 (5α1/2), the rate-limiting enzymes for allopregnanolone synthesis, in the BLA following CUS. qRT-PCR revealed a decrease in transcript levels of Srd5a1 and Srd5a2, which encode for 5α1/2, respectively, in the BLA following CUS (Srd5a1: 0.3 ± 0.1 fold change; Srd5a2: 0.6 ± 0.2 fold change)(Figure 4 D; control n=16, CUS n=20 pooled BLA samples from 4 mice per experiment, Srd5a1 p=0.0031, Srd5a2 p=0.0831 t-test). Exogenous SGE-516 treatment prevented the reduction in Srd5a1 and Srd5a2 expression in the BLA following CUS (SGE-516 Srd5a1: 2.0 ± 0.7 fold change; SGE-516 Srd5a2: 1.2 ± 0.4 fold change) (Figure 4 D; n=16 pooled BLA samples from 4 mice per experiment). These data suggest that endogenous neurosteroidogenesis is impaired in the BLA following CUS, which we proposed may contribute to behavioral deficits.
To probe whether SGE-516 may influence 5α1/2 activity, we utilized HEK cells which overexpressed either Srd5a1 or Srd5a2 and examined the impact of either progesterone or SGE-516 treatment on the activity of these enzymes by measuring NADP+ production as a proxy. Both progesterone and SGE-516 treatment increased NADP+ synthesis in HEK cells expressing Srd5a1 (control: 2642.4 ± 506.1 RLU; progesterone: 4928.3 ± 1820.1 RLU; SGE-516: 4277.1 ± 2656.8 RLU) (Figure 4 F; control n=8–12 replicates per experimental group; progesterone p=0.0003, SGE-516 p=0.05 unpaired t-test) and Srd5a2 (control: 2839.3 ± 953.7 RLU; progesterone: 4484.3 ± 2332.0 RLU; SGE-516: 4591.2 ± 1342.7 RLU)(Figure 4 G; control n=8 replicates per experimental group; progesterone p=0.04, SGE-516 p=0.005 unpaired t-test).
Impaired Neurosteroidogenesis in the BLA is Sufficient to Induce Behavioral Deficits
Given that CUS induced brain region-specific deficits in endogenous neurosteroidogenesis, we next sought to directly investigate whether behavioral consequences of CUS involve impaired neurosteroidogenesis in the BLA. Utilizing constitutive Cas9 expressing mice we knocked down 5α1/2 in the BLA using sgRNAs targeting genes encoding these enzymes, Srd5a1 and Srd5a2 (Figure 5 A). This approach reduced transcript levels of 5α1/2 (Srd5a1: 0.5 ± 0.1 fold change; Srd5a2: 0.7 ± 0.1 fold change) in the BLA (Figure 5 B; control n=15, sg5α1/2n=8, Srd5a1 p=0.0001; Srd5a2 p=0.0037 t-test). sg5α1/2 mice exhibited increased avoidance behaviors, spending less time in the light compartment of the light/dark box (control: 286.7 ± 21.7 s; sg5α1/2: 233 ± 13.5 s)(Figure 5 C; control n=15, sg5α1/2 n=8, p=0.0480 unpaired t-test) with no change in locomotor behavior (control: 2423 ± 54.3 beam breaks; sg5α1/2: 2305 ± 62.3 beam breaks)(Figure 5 C; p=0.1745 unpaired t-test). sg5α1/2 mice also displayed increased stress-induced helplessness behaviors, evident from a shorter latency to immobility (control: 76.07 ± 5.4s; sg5α1/2: 31.7 ± 5.6s) (Figure 5 D; p=<0.0001 unpaired t-test) and an increase in total time immobile (control: 173.2 ± 10.3 s; sg5α1/2: 237.2 ± 13.0 s)(Figure 5 D; control n=14; sg5α1/2 n=9, p=0.0013 unpaired t-test) in the tail suspension test. However, sg5α1/2 mice did not exhibit any behavioral changes in the open field test, spending similar time in the center (control: 76.5 ± 8.8 s; sg5α1/2: 81.9 ± 12.0 s)(Figure S4; control n=14, sg5α1/2 n=9, p=0.7216 unpaired t-test) and exhibited similar locomotor behavior (control: 1878 ± 70.6 beam breaks; sg5α1/2: 1718 ± 124.2 beam breaks)(Figure S4; p=0.2814 unpaired t-test). Collectively, these results suggest that impaired neurosteroid signaling in the BLA is sufficient to induce behavioral deficits reminiscent of those observed following CUS. The summary of behavioral changes in mice with reduced 5α1/2 expression in the BLA are shown in Figure 5 E.
Figure 5. Knockdown of Srd5a1 and Srd5a2 in the BLA induced behavioral deficits.
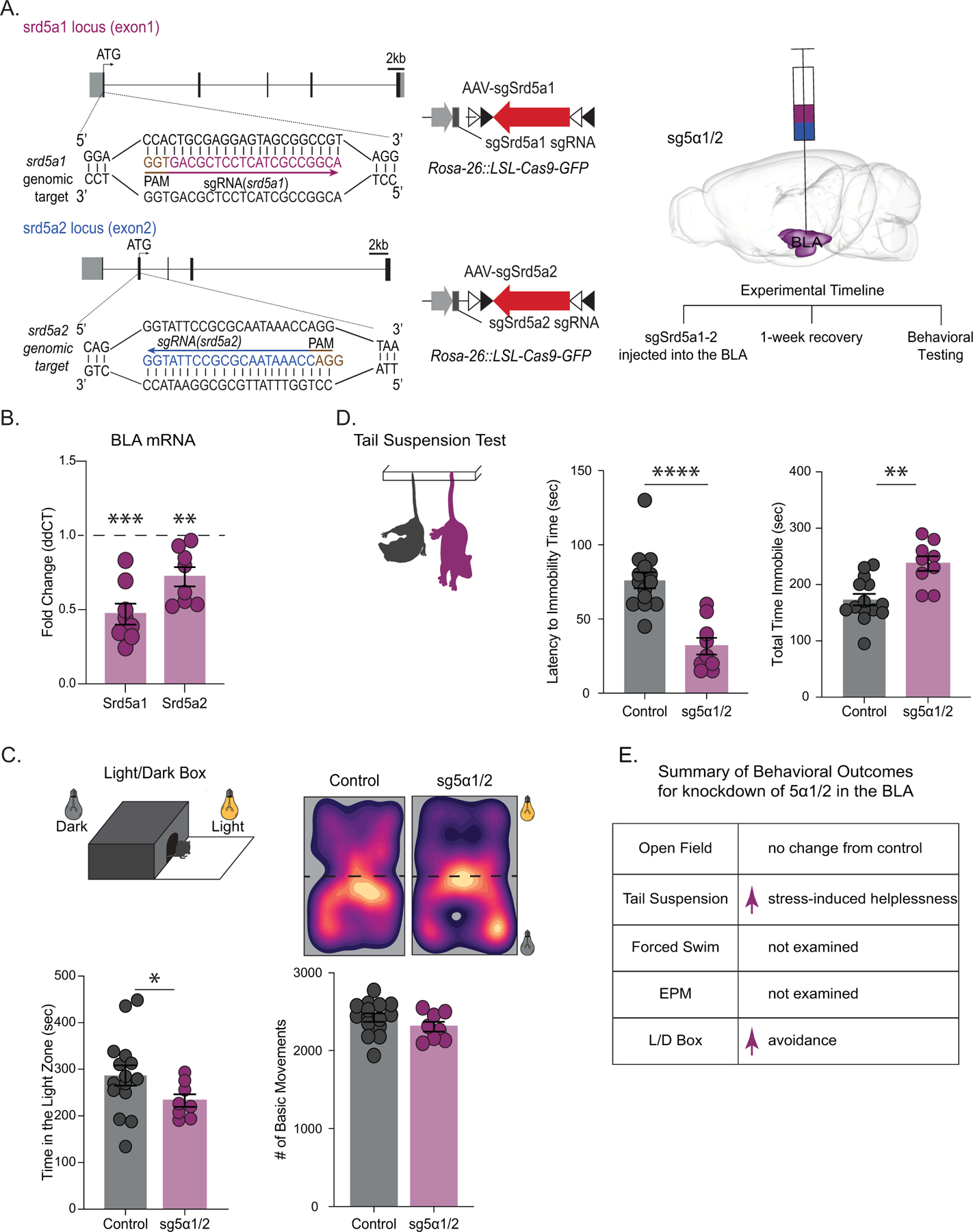
(A) (left) A schematic of the sgRNA construct for the knockdown of Srd5a1 and Srd5a2 in the BLA of constitutive Cas9 mice and the experimental timeline (right). The average expression of Srd5a1 and Srd5a2 mRNA in the BLA of sg5α1/2mice decreased compared to controls measured using qRT-PCR normalized to β-actin levels (B) control n=15, sg5α1/2 n=8. (above) Representative heat maps of movement of control and sgSrd1/2 mice in the light/dark box test. (below) Knockdown of 5α1/2 increased avoidance behaviors, evident from a decrease in the amount of time spent in the light chamber of the light/dark box with no change in basic movements (C) control n=15, sg5α1/2 n=8. Knockdown of 5α1/2 in the BLA increased stress-induced helplessness, evident by a decreased latency to immobility and an increased total time spent immobile during the tail suspension test (D) control n=14, 5α1/2 n=9.(E) Summary of behavioral outcomes for transparency across behavioral tests. * denotes p < 0.05, **p<0.01, ***p<0.001, ****p<0.0001 using an unpaired t-test.
Increasing the Capacity for Neurosteroidogenesis in the BLA is Sufficient to Rescue Behavioral Deficits Following CUS
Given that we observed a decrease in the expression of 5α1/2 in the BLA following CUS (Figure 4) which was sufficient to increase avoidance and stress-induced helplessness behaviors (Figure 5), we next sought to investigate whether increasing 5α1/2 in the BLA may prevent the behavioral consequences of CUS. Utilizing lentiviral constructs we overexpressed 5α1/2 in the BLA and subsequently subjected mice to CUS. Overexpression of 5α1/2 increased transcript levels in the BLA (Srd5a1: 1.9 ± 0.3 fold change; Srd5a2: 1.5 ± 0.1 fold change) (Figure 6 C; control n=7, LV 5α1/2 n=4, Srd5a1 p=0.0673; Srd5a2 p=0.0360 t-test). Following CUS, mice with increased 5α1/2 expression in the BLA (CUS+LV 5α1/2 mice) did not exhibit signs of CUS-induced avoidance behaviors with no difference in the amount of time spent in the center of the open field (control: 73.4 ± 8.3 s; CUS+LV 5α1/2: 101.5 ± 11.2 s)(Figure 6 D; control n=11, CUS+LV 5α1/2 n=8, p=0.0639 unpaired t-test), with no change in locomotor behavior (control: 2613 ± 126.5 beam breaks; CUS+LV 5α1/2: 2738 ± 315.9 beam breaks)(Figure 6 D; p=0.7236 unpaired t-test). CUS+LV 5α1/2 mice did not exhibit CUS-induced avoidance behaviors in the light/dark box test, exhibiting similar amounts of time spent in the light chamber (control: 193.7 ± 17.5 s; CUS+LV 5α1/2: 215.9 ± 26.5 s)(Figure 6 E; control n=11, CUS+LV 5α1/2 n=8, p=0.4966 unpaired t-test) with no difference in locomotor behavior (control: 2234 ± 109.6 beam breaks; CUS+LV 5α1/2: 2242 ± 200.3 beam breaks)(Figure 6 E; p=0.9729 unpaired t-test). These data suggest that overexpression of 5α1/2 is protective against the behavioral impacts of chronic stress. In fact, following CUS, CUS+LV 5α1/2 mice spent more time in the open arm of the elevated plus maze (control: 24.9 ± 2.8 s; CUS+LV 5α1/2: 60.6 ± 13.7 s)(Figure 6 F; control n=7, CUS+LV 5α1/2 n= 8, p=0.0356 unpaired t-test), displayed a trend towards traveling further in the open arm (control: 329.3 ± 52 cm; CUS+LV 5α1/2: 749.4 ± 195.9 cm)(Figure 6 F; p=0.0721 unpaired t-test), and a trend to perform more entries into the open arm (control: 31.6 ± 5.6 entries; CUS+LV 5α1/2: 52.4 ± 9.5 entries) (Figure 6 F; p=0.0840 unpaired t-test) with no change in locomotor behavior (control: 2632 ± 153.0 beam breaks; CUS+LV 5α1/2: 2751 ± 199.2 beam breaks )(Figure 6 F; p=0.6442 unpaired t-test). CUS+LV 5α1/2 mice did not exhibit increased stress-induced helplessness as a result of CUS, demonstrating a similar latency to immobility (control: 30.8 ± 5.1 s; CUS+LV 5α1/2: 30.7 ± 3.3 s)(Figure 6 G; control n=8, CUS+LV 5α1/2 n= 8, p=0.9875 unpaired t-test) and total time immobile in the tail suspension test (control: 185.9 ± 8.5 s; CUS+LV 5α1/2: 181.1 ± 9.1 s)(Figure 6 G; p=0.7052 unpaired t-test). These data demonstrate that enhancing the synthesis of 5α-reduced neurosteroids in the BLA is sufficient to overcome the behavioral deficits induced by CUS. The summary of behavioral changes in CUS mice with an overexpression of 5α1/2 in the BLA are shown in Figure 6 H.
Figure 6. Overexpression of Srd5a1 and Srd5a2 improved behavioral outcomes following CUS.
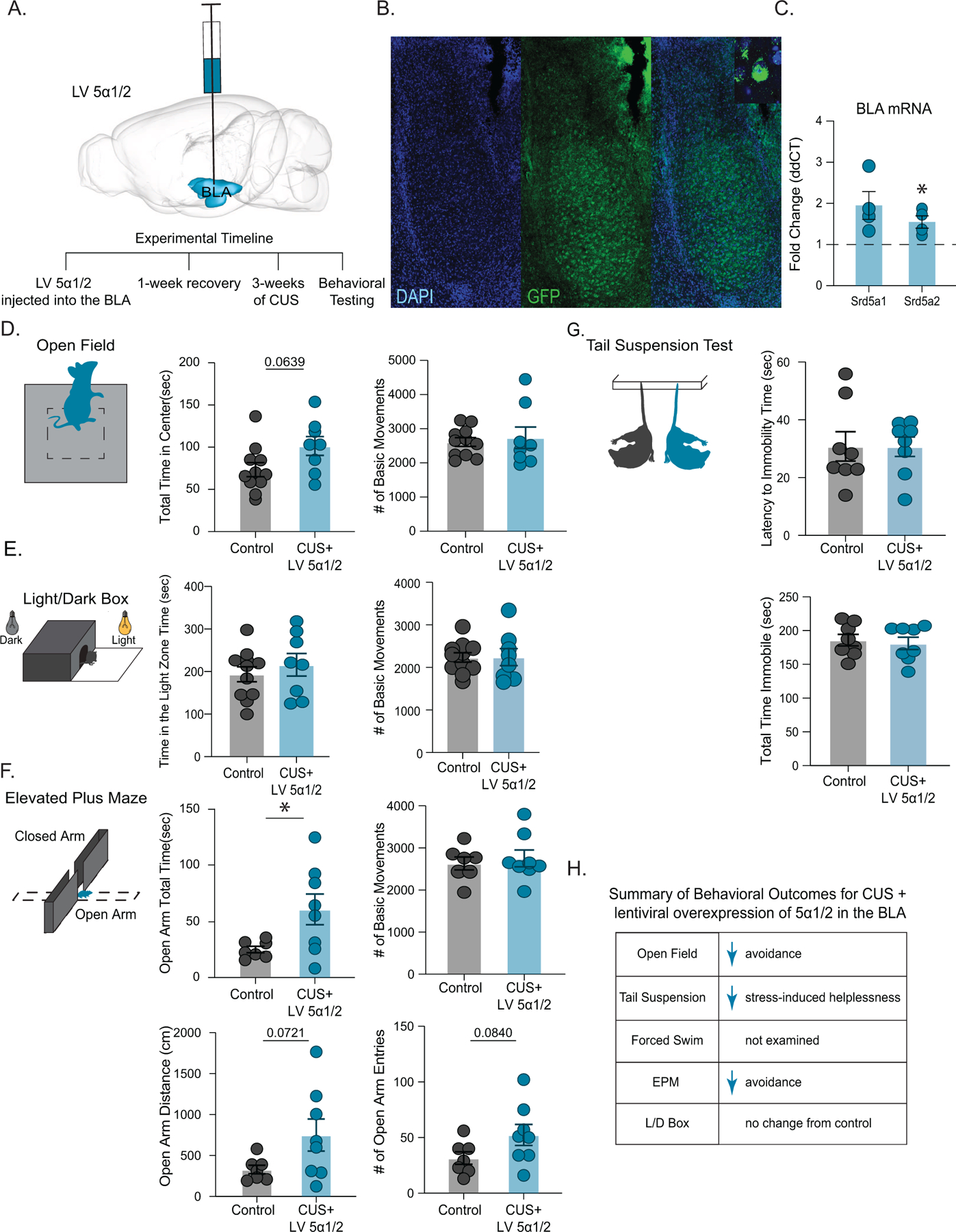
(A) (above) Schematic of lentiviral construct and targeting for overexpression of Srd5a1 and Srd5a2 in the BLA and the experimental timeline (below). (B) Representative immunofluorescence of GFP-tagged lentiviral targeting in the BLA of LV 5α1/2 mice. (C) The average mRNA expression of Srd5a1 and Srd5a2 increased in LV 5α1/2 mice compared to controls measured using qRT-PCR in the BLA of LV 5α1/2 mice, which were normalized to β-actin levels, control n=7, LV 5α1/2 n=4. (D) Overexpression of 5α1/2 decreased avoidance behaviors in CUS mice, exhibited as no change in the time spent in the center of the open field test compared to controls with no change in the total number of basic movements. control n=11, CUS+LV 5α1/2 n=8. These mice did not differ from controls in the time spent in the light zone or the number of basic movements performed in the light/dark box test control (E) n=11, CUS+LV 5α1/2 n=8. (F) CUS mice with overexpression of 5α1/2 in the BLA also demonstrated a decrease in stress-induced anxiety, indicated by an increase in the total time spent in the open arm of the elevated plus maze with no change in basic movements compared to controls. LV Srd5a1/2 mice subjected to CUS also exhibited a trend towards traveling further and performing more entries into the open arm of the elevated plus maze. control n=7, CUS+LV 5α1/2 n=8. (G) CUS mice with overexpression of 5α1/2 in the BLA exhibited a decrease in stress-induced helplessness as demonstrated by similar latency to immobility and overall time immobile in the tail suspension test. control n=8, CUS+LV 5α1/2 n=8. (H) summary of behavioral outcomes for CUS mice with lentiviral overexpression of 5α1/2 in the BLA. p > 0.05 is not significant, * Denotes p < 0.05, **p<0.01, ***p<0.001 using an unpaired t-test.
Discussion
These findings implicate deficits in endogenous neurosteroid signaling in the BLA in the pathophysiological mechanisms contributing to behavioral deficits following chronic stress (Figure 7). Here, we demonstrate reduced allopregnanolone levels in the BLA following CUS, consistent with a reduction in expression of neurosteroidogenic enzymes, 5α1/2, in this region. Further, the site of action of 5α-reduced neurosteroids, δ-GABAARs, were also reduced in the BLA following CUS. Collectively, these findings suggest that chronic stress impairs endogenous neurosteroid signaling in the BLA contributing to behavioral deficits following CUS. In fact, knockdown of enzymes involved in endogenous 5α-reduced neurosteroidogenesis or their predominant site of action, induced increased avoidance and stress-induced helplessness behaviors similar to those observed following chronic stress. These novel findings further our understanding of the mechanisms through which chronic stress alters mood, consistent with previous observations of redcued expression of 5α-reductases (2,5) and allopregnanolone levels (2,3,4,9) in other chronic stress models (2,3,4,5). Finally, these data suggest that the antidepressant effects of exogenous 5α-reduced neurosteroids (25,26,27) may be due to their ability to overcome pathological reductions in endogenous neurosteroid levels and endogenous neurosteroid signaling.
Figure 7. Summary of major findings.
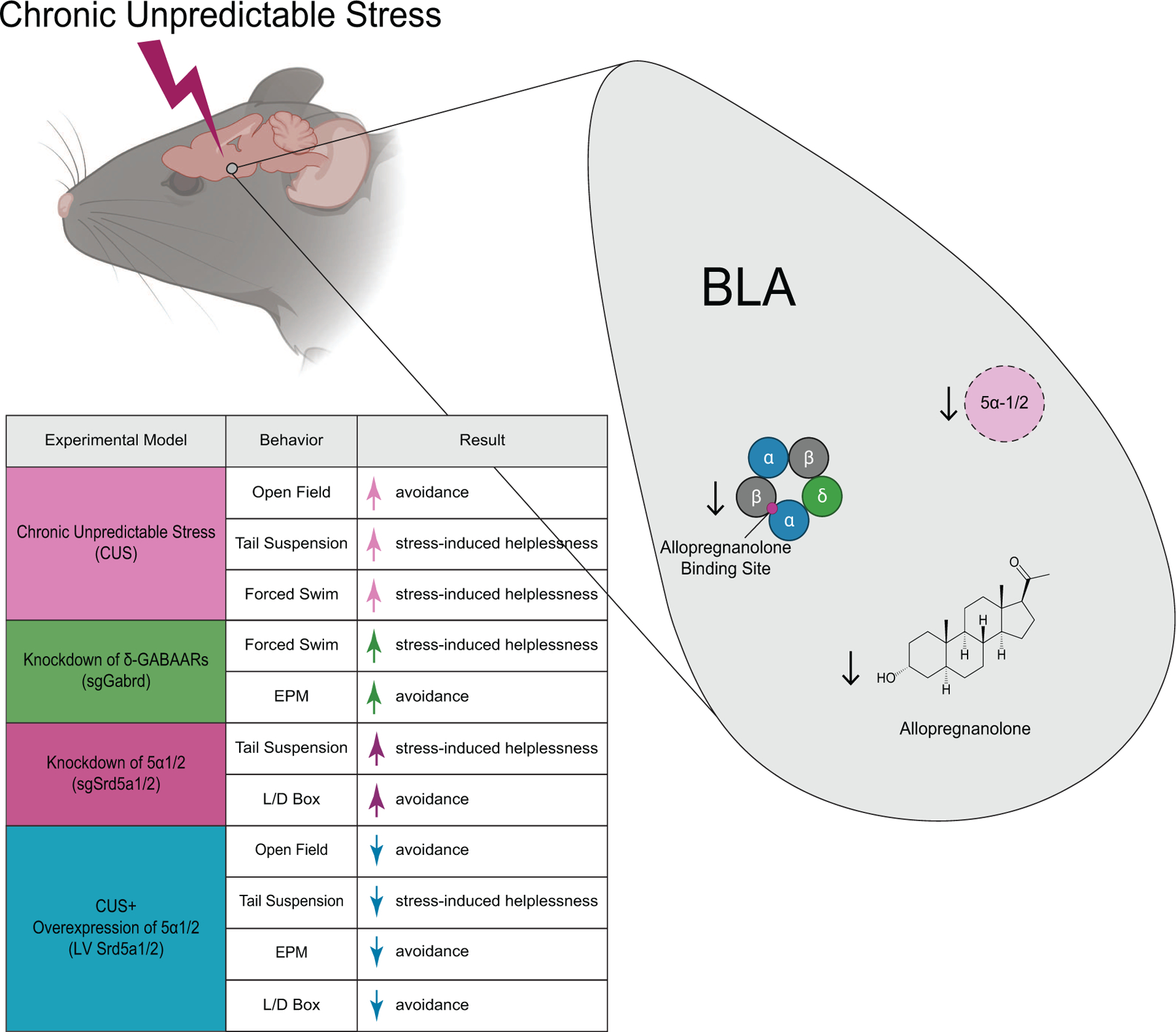
Chronic unpredictable stress increases avoidance behaviors and stress-induced helplessness. Knocking down key enzymes involved in endogenous neurosteroid synthesis, 5α-reductase 1 and 2, in the BLA was sufficient to increase avoidance behaviors and stress-induced helplessness. Similarly, knocking down the primary site of action for neurosteroid signaling, δ-GABAARs, also increased avoidance behaviors and stress-induced helplessness. Conversely, overexpression of 5α-reductase 1 and 2 prevented the behavioral deficits following CUS, resulting in no change or even a reduction in avoidance behaviors or stress-induced helplessness compared to controls.
Prior work has established the antidepressant and anxiolytic effects of exogenous 5α-reduced neuroactive steroids (7). Work from our laboratory suggest that the antidepressant mechanism of allopregnanolone and synthetic neuroactive steroid analogs may involve the ability of these compounds to modulate BLA network states, mediating both affective state switching and prolonged antidepressant effects (18,28). We previously identified PV interneurons in the BLA as critical mediators of behavioral state changes and determined that they contain a high density of δ-GABAARs (18). The ability of neurosteroids to mediate transitions between BLA network and behavioral states likely involves their actions on δ-GABAARs expressed on PV interneurons in the BLA (18), and the ability for tonic inhibition from PV interneurons to synchronize network activity (29). We propose that CUS impairs neurosteroid-mediated enhancement of tonic GABAergic signaling on PV interneurons in the BLA resulting in desynchronization of network states and, consequently, behavioral states (Figure 7). Thus, endogenous neurosteroid actions in the BLA likely target δ-GABAARs on PV interneurons to shift behavioral states. This represents a novel, endogenous mechanism mediating affective state switching and may represent a mechanism mediating the episodic nature of psychiatric illnesses.
Most studies investigating neurosteroid influence on mood have relied on exogenous neurosteroid administration. Currently, little is known about the function of endogenous neurosteroidogenesis. We have overcome this limitation by developing CRISPR tools enabling direct manipulation of endogenous neurosteroidogenesis to examine the impact on behavioral states. The current study extends our knowledge by providing insight into the role of endogenous neurosteroid signaling on behavioral states. These data suggest that a physiological function of endogenous 5α-reduced neurosteroid signaling in the BLA involves setting a baseline affective tone. Consistent with this hypothesis, our data demonstrates that knocking down 5α1/2 in the BLA induces behavioral deficits, suggesting impaired endogenous neurosteroidogenesis in the BLA disrupts affective tone (Figure 5). Conversely, overexpression of 5α1/2 reduces avoidance behaviors (Figure 6), demonstrating that enhanced neurosteroidogenesis can improve behavioral states. These findings suggest that a physiological function of endogenous 5α-reduced neurosteroids is to regulate affective tone.
Previously, our understanding of the physiological function of endogenous neurosteroidogenesis was limited by the inability to manipulate endogenous neurosteroidogenesis and relied on pharmacological tools, such as 5α-reductase inhibitor, finasteride. Clinically, treatment with finasteride has been shown to lead to mood disorders, including anxiety and depression, collectively referred to as post-finasteride syndrome (PFS), the pathophysiology of which is thought to involve deficits in endogenous neurosteroid signaling (13). In animal models, exogenous allopregnanolone exerts anxiolytic and antidepressant effects while treatment with finasteride blocks the anxiolytic and antidepressant effects of progesterone (14). Collectively, these data suggest that endogenous neurosteroids are capable of impacting mood. This study demonstrates that interfering with endogenous neurosteroid signaling is capable of inducing deficits in behavioral states, suggesting that endogenous neurosteroids have a tonic influence over affective states. Further, we demonstrate that enhancing endogenous neurosteroidogenesis can improve behavioral states, suggesting that targeting endogenous neurosteroidogenesis may represent a novel therapeutic approach for the treatment of mood disorders (13,14). Further studies are required to investigate the therapeutic potential of targeting endogenous neurosteroidogenesis for the treatment of mood disorders.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-cFos | Sigma | Lot No. 1117M837V |
| Rabbit anti-δ-GABAAR | PhosphoSolutions | Cat No. 868A-GDN |
| Mouse anti-PV | Sigma | Cat No. P 3088 |
| rabbit anti-GFP | Cell Signaling | Lot No. 2956 |
| Monoclonal β-tubulin | Sigma | Cat No. T8328 |
| Goat anti-Rabbit Alexa Fluor 488 | ThermoFisher Scientific | Cat No. A-11008 |
| Biotinylated goat anti-mouse Alexa 647 | ThermoFisher Scientific | Cat No. A28181 |
| Donkey anti-rabbit IgG HRP conjugated | Fisher Scientific | Cat No. 45–000-683 |
| Sheep anti-mouse IgG HRP conjugated | Fisher Scientific | Cat No. 45–000-692 |
| Vectashield hardset antifade mounting medium with DAPI | Vector Labs | Cat No. H-1500–10 |
| Amersham ECL anti-mouse IgG, peroxidase-linked whole antibody | GE Healthcare | Cat No. NA931 |
| Cell Line | ||
| Srd5a1 construct | Origene | Cat No. MR203282 |
| Srd5a1 construct | Origene | Cat No. MR223814 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAB based HRP substrate | Vector Laboratories | SK-4100 |
| PhosSTOP phosphatase inhibitor tablets | Roche | SK-4906837001 |
| cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail | Roche | SK-11836170001 |
| Pierce™ ECL Western Blotting Substrate | ThermoFisher Scientific | Cat No. P132106 |
| SGE-516 Rodent Chow | Teklad | Lot No. 21082311i |
| Progesterone | ||
| SGE-516 | ||
| Allopregnanolone internal standard | Tocris | Cat No. 5532 |
| Allopregnanolone (mass spec reference material) | Tocris | Cat No. 3653 |
| Formic Acid | Millipore-Sigma | Cat No. A695076 |
| Acetonitrile | Millipore-Sigma | Cat No. 39998 |
| Methanol | Thermo-Fisher Scientific | Cat No. A456 |
| Chlorobutane | Merck KGaA | Cat No. 8.01640.1000 |
| Critical Commercial Assays | ||
| VECTASTAIN® Elite ABC-HRP Kit, Peroxidase (Rabbit IgG) | Vector Laboratories | PK-6101 |
| SuperScript III Platinum Sybr Green (one step qRT PCR kit) | Invitrogen | Lot No. 2028735 |
| NADP/NADPH-Glo™ Assays | Promega | Cat No. G9081 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57Bl/6J | The Jackson Laboratory (JAX) | Stock: #000664 |
| Mouse: Rosa26-LSL-Cas9 | The Jackson Laboratory (JAX) | Stock # 024857 |
| Mouse: constitutively expressing Cas9 | The Jackson Laboratory (JAX) | Stock # 024858 |
| Mouse: PV-Cre | The Jackson Laboratory (JAX) | Stock: # 012358 |
| Recombinant DNA | ||
| Single guide RNA for Gabrd | Kong Lab-Boston Children's Hospital | |
| Single guide RNA for Srd5a1 | Kong Lab-Boston Children's Hospital | |
| Single guide RNA for Srd5a2 | Kong Lab-Boston Children's Hospital | |
| Srd5a1 Lentivirus | Origene | Cat No. MR203282 |
| Srd5a2 Lentivirus | Origene | Cat No. MR223814 |
| Sequence-Based Reagent | ||
| Primers for qRT-PCR | See Table 1 | |
| Software | ||
| ILLUSTRATOR 2021 | Adobe | N/A |
| PRISM 7 | GraphPad Software | N/A |
| Motor Monitor software | Hamilton-Kinder | N/A |
| Python | Python Software Foundation | N/A |
| Mobility App | Maguire Laboratory | (https://github.com/researchgrant/mobility-mapper.git) |
| Zhang Lab Guide Design Resource | Zhang Lab MIT | http://crispr.mit.edu/ |
| CHOPCHOP | http://chopcho.cbu.uib.no/ | Labun, K., Montague, T. G., Krause, M., Torres Cleuren, Y. N., Tjeldnes, H., & Valen, E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Research (2019). |
| NIS-Elements | Nikon Instruments | RRID:SCR_014329 |
| LAS X Life Science Microscope Software | Leica Microsystems | |
| Image J | NIH | Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018. |
Acknowledgements and Disclosures
The authors would like to thank Boston Children’s Hospital viral vector core for packaging the sgRNAs used in this manuscript as well as PharmaCadence for performing LC-MS analytical support. JLM, NLW, PA, AD, and RP were supported by R01AA026256, R01NS105628, R01NS102937, R01MH128235, and P50MH122379. SGE-516 data presented in this manuscript were part of a sponsored research agreement with SAGE Therapeutics. SH was supported by T32DK124170. DK was supported by R01NS107315, R01DK108797, R21HD098056, P30DK046200. JLM serves as a member of the Scientific Advisory Board for SAGE Therapeutics, Inc. All other authors report no potential biomedical financial interests or conflicts of interest. An initial draft of this manuscript was previously published on bioRxiv.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackenzie G, & Maguire J (2013). Neurosteroids and GABAergic signaling in health and disease. Biomol Concepts, 4(1), 29–42. 10.1515/bmc-2012-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. (2001). Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proceedings of the National Academy of Sciences of the United States of America, 98(5), 2849–2854. 10.1073/pnas.051628598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto K, Pinna G, Puia G, Guidotti A, & Costa E (2005). Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress (Amsterdam, Netherlands), 8(2), 85–93. 10.1080/10253890500159022 [DOI] [PubMed] [Google Scholar]
- 4.Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. (2002). Social isolation-induced decreases in both the abundance of neuroactive steroids and Gabaa receptor function in rat brain. Journal of Neurochemistry, 75(2), 732–740. [DOI] [PubMed] [Google Scholar]
- 5.Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, & Guidotti A (2007). Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proceedings of the National Academy of Sciences of the United States of America, 104(47), 18736–18741. 10.1073/pnas.0709419104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Broekhoven F, & Verkes RJ (2003). Neurosteroids in depression: A review. Psychopharmacology 10.1007/s00213-002-1257-1 [DOI] [PubMed]
- 7.Zorumski CF, Paul SM, Izumi Y, Covey DF, & Mennerick S (2013, January). Neurosteroids, stress and depression: Potential therapeutic opportunities. Neuroscience and Biobehavioral Reviews 10.1016/j.neubiorev.2012.10.005 [DOI] [PMC free article] [PubMed]
- 8.Zorumski CF, Paul SM, Covey DF, & Mennerick S (2019). Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of stress, 11, 100196. 10.1016/j.ynstr.2019.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, et al. (2006). Social isolation-induced increase in alpha4 and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and Gabaa receptor function. Journal of Neurochemistry, 98(1), 122–133. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, & Akil H (2020). Revisiting the stress concept: Implications for affective disorders. In Journal of Neuroscience (Vol. 40, Issue 1, pp. 12–21). Society for Neuroscience. 10.1523/JNEUROSCI.0733-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS (2017). Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress, 1, 247054701769232. 10.1177/2470547017692328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998 May 1;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x.PMID:9629234. [DOI] [PubMed] [Google Scholar]
- 13.Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, et al. (2013). Neuroactive Steroid Levels are Modified in Cerebrospinal Fluid and Plasma of Post-Finasteride Patients Showing Persistent Sexual Side Effects and Anxious/Depressive Symptomatology. Journal of Sexual Medicine, 10(10), 2598–2603. 10.1111/jsm.12269 [DOI] [PubMed] [Google Scholar]
- 14.Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, et al. (2006). A new look at the 5α-reductase inhibitor finasteride. CNS Drug Reviews, 12(1), 53–76. 10.1111/j.1527-3458.2006.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belelli D, & Lambert JJ (2005). Neurosteroids: Endogenous regulators of the GABAA receptor. In Nature Reviews Neuroscience (Vol. 6, Issue 7, pp. 565–575). Nat Rev Neurosci. 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- 16.Chen Z-W., Bracamontes JR, Budelier MM, Germann AL, Shin DJ, Kathiresan K, et al. (2019) Multiple functional neurosteroid binding sites on GABAA receptors. PLoS Biol 17(3): e3000157. 10.1371/journal.pbio.3000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belelli D, Casula A, Ling A, & Lambert JJ (2002). The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology, 43(4), 651–661. 10.1016/s0028-3908(02)00172-7 [DOI] [PubMed] [Google Scholar]
- 18.Antonoudiou P, Colmers P, Walton NL, Weiss GL, Smith AC, Nguyen DP, et al. (2021). Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala. Biological psychiatry, S0006–3223(21)01470–0. Advance online publication. 10.1016/j.biopsych.2021.07.017 [DOI] [PMC free article] [PubMed]
- 19.Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. In Neuroscience and Biobehavioral Reviews (Vol. 35, Issue 1, pp. 2–16). 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Willner P (2017). The chronic mild stress (CMS) model of depression: History, evaluation and usage. In Neurobiology of Stress (Vol. 6, pp. 78–93). Elsevier Inc. 10.1016/j.ynstr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yau JOY, Chaichim C, Power JM, & McNally GP (2021). The Roles of Basolateral Amygdala Parvalbumin Neurons in Fear Learning. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 41(44), 9223–9234. 10.1523/JNEUROSCI.2461-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu X, Teboul E, Weiss GL, Antonoudiou P, Borkar CD, Fadok JP, et al. Gq neuromodulation of BLA parvalbumin interneurons induces burst firing and mediates fear-associated network and behavioral state transition in mice. Nat Commun 2022. Mar 11;13(1):1290. doi: 10.1038/s41467-022-28928-y. PMID: 35277502; PMCID: PMC8917207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozawa M, Davis P, Ni J, Maguire J, Papouin T, & Reijmers L (2020). Experience- dependent resonance in amygdalo-cortical circuits supports fear memory retrieval following extinction. Nature Communications, 11(1). 10.1038/s41467-020-18199-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis P, Zaki Y, Maguire J, & Reijmers LG (2017). Cellular and oscillatory substrates of fear extinction learning. Nature Neuroscience, 20(11), 1624–1633. 10.1038/nn.4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. (2019). Trial of SAGE-217 in Patients with Major Depressive Disorder. New England Journal of Medicine, 381(10), 903–911. 10.1056/NEJMoa1815981 [DOI] [PubMed] [Google Scholar]
- 26.Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomized, placebo-controlled, phase 3 trials. The Lancet, 392(10152), 1058–1070. 10.1016/S0140-6736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- 27.Walton N, & Maguire J Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiol Stress 2019. Oct 24;11:100198. doi: 10.1016/j.ynstr.2019.100198. PMID: 31709278; PMCID: PMC6838978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiller CE., Schmidt PJ., & Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl) 2014. Sep;231(17):3557–67. doi: 10.1007/s00213-014-3599-x. Epub 2014 May 21. PMID: 24846476; PMCID: PMC4135022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlov I, Savtchenko LP, Song I, Koo J, Pimashkin A, Rusakov DA, et al. Tonic GABAA conductance bidirectionally controls interneuron firing pattern and synchronization in the CA3 hippocampal network. Proc Natl Acad Sci U S A 2014. Jan 7;111(1):504–9. doi: 10.1073/pnas.1308388110. Epub 2013 Dec 16. PMID: 24344272; PMCID: PMC3890854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, et al. Genetic identification of leptin neural circuits in energy and glucose homeostasis. Nature. 2018. Apr;556(7702):505–509. doi: 10.1038/s41586-018-0049-7. Epub 2018 Apr 18. PMID: 29670283; PMCID: PMC5920723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar J, Wakefield S, MacKenzie G, Moss SJ, & Maguire J (2011). Neurosteroidogenesis is required for the physiological response to stress: Role of neurosteroid-sensitive GABA A receptors. Journal of Neuroscience, 31(50),18198–18210. 10.1523/JNEUROSCI.2560-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee V, Sarkar J, & Maguire J (2014). Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology, 41, 75–88. 10.1016/j.psyneuen.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melón LC, Hooper A, Yang X, Moss SJ, & Maguire J (2018). Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology, 90, 182–193. 10.1016/j.psyneuen.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire J, & Mody I (2008). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron, 59(2), 207–213. 10.1016/j.neuron.2008.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maguire J, & Mody I (2016). Behavioral deficits in juveniles mediated by maternal stress hormones in mice. Neural Plasticity, 2016. 10.1155/2016/2762518 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


