Abstract
INTRODUCTION:
Early sepsis results in pharmacokinetic (PK) changes due to physiologic alterations. PK changes can lead to suboptimal drug target attainment, risking inadequate coverage from antibiotics like ceftriaxone. Little is known on how ceftriaxone PK and target attainment quantitatively change over time in patients with sepsis or the association of target attainment and outcomes in critically ill children and young adults.
METHODS:
A retrospective analysis of a prospective study was conducted in a single-center pediatric intensive care unit. Septic patients given at least one ceftriaxone dose (commonly as 50 mg/kg every 12 hours) and who had blood obtained in both the first 48 hours of therapy (early) and afterwards (late) were included. Normalized clearance and central volume were estimated in both sepsis phases and compared. We evaluated target attainment, defined as concentrations above 1x or 4x the minimum inhibitory concentration (MIC) for 100% of dosing intervals, and investigated the association between target attainment and clinical outcomes.
RESULTS:
Fifty-five septic patients (median age: 7.5 years) were included. Normalized clearance and central volume were similar in both phases (6.18 ± 1.48 L/h/70 kg v 6.10 ± 1.61 L/h/70 kg, p=0.60; 26.6 [22.3, 31.3] L/70 kg v 24.5 [22.0, 29.4] L/70 kg, p =0.18). Individual percent differences in normalized clearance and central volume between sepsis phases ranged from −39% to 276% and −51% to 212% (reference, late sepsis), respectively. Fewer patients attained the 1x MIC target in late sepsis (82 v 96%, p=0.013), which was associated with transition to once daily dosing, typically done due to transfer from the PICU to a lower acuity unit. Failure to attain either target in late sepsis was associated with antibiotic broadening.
CONCLUSION:
Ceftriaxone PK parameters were similar between early and late sepsis but there were large individual differences. Fewer patients attained targets in late sepsis and all who did not attain the less stringent target received once daily dosing during this period. The failure to attain targets in late sepsis was associated with antibiotic broadening and could be an area for antibiotic stewardship intervention.
Keywords: Ceftriaxone, sepsis, pharmacokinetics, pharmacodynamics, target attainment, critical care, pediatrics
INTRODUCTION
Critical illness leads to significant variability in pharmacokinetics (PK) and pharmacodynamics (PD). Early sepsis is characterized by vasodilation, capillary leak, and hypoalbuminemia, all of which lead to increased volume of distribution and low drug exposure.1-4 Fluid resuscitation to mitigate hypotension contributes to a larger volume of distribution.5,6 In some states of critical illness, there is an initial increase in cardiac output, resulting in augmented renal clearance.2,4,7,8 This phenomenon leads to increased clearance of renally-eliminated drugs and lower drug exposure. In contrast, in states of shock, decreased organ perfusion results in lower drug clearance.1,2
β-lactam antibiotics (e.g., cephalosporins, carbapenems) are a prime example of drugs demonstrating high PK/PD variability in critically ill patients. Their effectiveness is dependent on the time that free, non-protein-bound, concentrations are above the bacterial minimum inhibitory concentrations (fT>MIC).1,2,9-11 We have demonstrated that intermittent β-lactam dosing leads to highly variable concentrations (up to 40-fold) in critically ill children and young adults.12,13 This high PK variability leads to inconsistent PD target attainment and could result in ineffective bactericidal activity.14 In adults, only 30-65% of patients achieve targets, defined as concentrations remaining above 1-4x MIC for 100% of the dosing intervals (100%fT>1-4xMIC),- early in the course of illness (before day 2).15,16
Despite the known pathophysiologic changes during sepsis, there are limited quantitative data on how β-lactam PK and PD target attainment change over time. Ceftriaxone is one of the first-line antibiotics used for sepsis in patients with no significant medical history. We published a population PK model of ceftriaxone in critically ill children and young adults, which did not find that phase of illness (based on a 48-hour threshold) had impacted PK.13 However, our population-level study included all patients admitted to the pediatric intensive care unit (PICU), not just septic patients. Therefore, we sought to compare ceftriaxone PK parameters between early and late phases of sepsis at an individual level and compare the percentages of patients who attain targets between both sepsis phases in critically ill children and young adults. We also investigated the relationship between target attainment and clinical outcomes. We hypothesized that due to augmented renal clearance, vasodilatation and aggressive fluid resuscitation in the early phase, clearance and volume of distribution will be higher in early sepsis, resulting in a lower percent of patients who attain targets. At our institution, patients admitted to the PICU for sepsis are typically initiated on every 12-hour (q12h) dosing of ceftriaxone, then transitioned to every 24-hour (q24h) dosing upon transfer to a lower acuity unit, while other institutions often administer q24h ceftriaxone dosing regardless of location or phase of care. This study was also conducted to provide evidence about our transitioning practices. In addition, these findings could provide the basis of providing individualized dosing during different phases of sepsis for patients at risk of not attaining targets and experiencing poor outcomes.
METHODS
Study Design and Ethics
This study was part of a larger prospective β-lactam PK/PD study in which patients who were admitted to the PICU and administered at least one dose of ceftriaxone, cefepime, piperacillin/tazobactam or meropenem were eligible for enrollment.12,13 Eligible patients had residual blood or plasma obtained from clinical samples (scavenged opportunistic samples)17 for antibiotic concentration measurement. This larger study was approved with a waiver of consent for sample collection by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board.
Patient Population
During the larger prospective study, 195 patients were admitted to the PICU, administered at least one ceftriaxone dose and had residual blood obtained.13 For this retrospective analysis, we applied the following inclusion criteria for this study. Patients must have met sepsis criteria, defined as meeting at least 2 systemic inflammatory response syndrome criteria 18 and receiving at least 7 days of antibiotics. In addition, patients must have received ceftriaxone, and no other β-lactam antibiotic, for at least the first 48 hours. Study day 1 was defined as the first day the patient was admitted to the PICU and received a ceftriaxone dose. Time zero was the time of the first ceftriaxone dose on Day 1. In our cohort, the number of hours between PICU admission time and time zero ranged from −8 (i.e., first dose given 8 hours prior to PICU admission) to 96 hours (median: −1 hour). We defined early phase of sepsis as the first 48 hours after time zero, and late phase of sepsis as after 48 hours. We used the 48-hour threshold as this is the time at which sepsis rule-outs are usually completed and clinicians decide whether a full course of antibiotics is needed. The first 48 hours is also thought to be the critical period in which antibiotic exposure should be maximized.11 Patients on extracorporeal devices, including but not limited to continuous renal replacement therapy and extracorporeal membrane oxygenation, were excluded. Finally, patients could be included in this retrospective analysis if they had adequate volume from opportunistic samples obtained during both phases of sepsis for free ceftriaxone concentration measurement. After applying these criteria, 55 patients of the 195 patients enrolled in the parent study were included in this analysis.
Ceftriaxone Dosing and Concentration Measurement
Ceftriaxone dosing was determined by the clinical team. In the CCHMC PICU, the typical regimen is 50 mg/kg/dose (max: 2000 mg) every 12 hours (q12h), regardless of the type of suspected infection. In a minority of patients (~10%), which includes those with suspected urinary tract infections or acute otitis media, a dosing regimen of 50 mg/kg/dose every 24 hours (q24h) may be initiated. Patients are often transitioned from q12h to q24h when they transfer to a lower acute care unit.
Residual blood was requested from all laboratory draws obtained for clinical purposes (e.g. complete blood counts, metabolic panels) throughout the duration of ceftriaxone therapy, even after transfer to a different unit, for a maximum of seven days (scavenged opportunistic sampling).13 Only blood draws that were collected within 30 minutes of an administered dose (the standard infusion time), were excluded. Blood was centrifuged, and plasma was separated and frozen within seven days of the blood draw based on our previously reported stability studies.13 Total and free plasma ceftriaxone concentrations were measured by High Performance Liquid Chromatography as previously described.13
PK Parameter Estimation
We used our published ceftriaxone population PK model in critically ill children and adults13 to estimate PK parameters. In this model, significant covariates of ceftriaxone clearance include weight, post-menstrual age (i.e. maturation effect), creatinine clearance, presence of fever and Pediatric Risk of Mortality (PRISMIII) score. Intercompartmental clearance (Q), central volume (V1) and peripheral volume (V2) are dependent on weight. The reference/normalized patient for the model is 70 kg,19 of an older age with negligible maturation effect, has creatinine of clearance of 149.5 ml/min/1.73m2, afebrile and has a PRISMIII score of 0. Using the precision dosing software, MwPharm++ 2.0.4.335 (Mediware, Prague, Czech Republic), which utilizes Bayesian estimation, we generated estimated concentration versus time profiles using the population PK model, individual patient covariates and individual measured free ceftriaxone concentrations. For the 55 patients included in the study, there were 329 free concentrations (average: 6.0/patient, range: 2-16/patient). These sampling numbers allowed for robust estimation of individual concentration vs. time profiles and PK parameters.20 We estimated the normalized clearance, central volume, intercompartmental clearance and peripheral volume. Due to the nature of sparse sampling, the estimations for intercompartmental clearance and peripheral were often estimated to be close to the median, with more weight being placed on body clearance and central volume when fitting the profile to observed concentrations. Thus, we report only the normalized clearance and central volume. We performed visual predictive check of the profiles to ensure the fit of the observed concentrations were appropriate for each individual patient. In addition, the software generates standard error estimates of the predictions and provides the 5th-95th confidence interval around the predicted concentration-time profile as a goodness of fit diagnostic (see representative profiles in Supplemental Figure 1).
PD Target Attainment
There is no consensus on the optimal PD target for β-lactam antibiotics for bactericidal activity, especially in critically ill patients. The minimum target should be that free concentrations remain above the bacterial MIC for 40-70% of the dosing interval (40-70% fT>MIC), with the specific percentage being antibiotic dependent. Data suggest that more stringent PD targets, including 100% fT>MIC and 100% fT>4xMIC, are needed in critically ill patients for bacterial clearance.10,11,21-24 For this study, we evaluated attainment of the two most stringent targets, 100% fT>MIC and 100% fT>4xMIC, using estimated concentration vs. time profiles in MwPharm++.
For patients from whom bacteria were not cultured (i.e. culture-negative sepsis), the Clinical Laboratory Standards Institute (CLSI) breakpoint for Streptococcus pneumoniae (non-meningitis) for ceftriaxone (1 μg/mL)25 was used. Otherwise, MICs or CLSI or European Committee on Antimicrobial Susceptibility Testing (EUCAS) breakpoints of the cultured bacteria were used for PD analysis.
Clinical Data Collection for Patient Characteristics and Outcomes
Chart review was conducted to obtain data for demographics, patient characteristics and outcomes. Comorbid condition was defined as a medical condition for which medication would be prescribed or subspecialty care is warranted. Complicated course of sepsis was defined as having two or more organ failures on day seven of study or mortality by day 28. To assess presence of acute kidney injury (AKI) in early and late sepsis, the baseline creatinine value for each patient was determined from one of the following: 1) lowest creatinine in the three months prior to PICU admission for patients without chronic kidney disease or prior 3-12 months for patients with chronic kidney disease (if available), 2) creatinine imputed from a presumed baseline creatinine clearance of 120 mL/min/1.73m2 (based on bedside Schwartz equation),26,27 3) lowest creatinine during hospitalization. Imputed creatinine was used for 9 of the 55 patients. Using the baseline creatinine, the Kidney Disease Improving Global Outcomes (KDIGO) stage was determined in early and late sepsis. Any stage greater than zero was considered as AKI. For patients who did not have AKI, we considered creatinine clearance, as determined by bedside Schwartz, greater than 150 mL/min/1.73m2 in the first 48 hours of therapy as augmented renal clearance (ARC).
For outcomes, we assessed PICU length of stay (LOS), hospital length of stay (LOS), vasopressor-free days in PICU, ventilator-free days in PICU, antibiotic broadening and fever duration in the first seven days of study. Antibiotic broadening was defined as switching to an intravenous antibiotic with a broader spectrum of antibacterial activity (typically piperacillin/tazobactam, cefepime, ceftaroline) within two days of the last ceftriaxone dose. Since drawing blood and obtaining urine can be difficult in young children, we also examined number of days when repeat cultures were obtained and the number of C-Reactive Protein (CRP) and procalcitonin measurements that were obtained in the first seven days.
Univariate Statistical Analysis
For comparison of PK parameters between sepsis phases, paired t-test was performed for the normally distributed clearance and Wilcoxon Sign Rank test was performed for the non-normally distributed central volume. McNemar’s test was used to compare percentages of patients who attained targets.
To associate target attainment with clinical characteristics and outcomes, Wilcoxon Rank Sum was used for continuous variables and Fischer’s exact test was used for categorical data. Kaplan Meier analysis was performed to compare hospital lengths of stay as previously described28 when it was found that univariate analysis did not demonstrate statistically significant differences but absolute differences in the medians that could be clinically significant. Statistical analyses were performed in R version 3.6.1 (Auckland, New Zealand). Statistical significance was considered as p ≤ 0.05.
Multivariable Models for Target Attainment and Clinical Outcomes
To evaluate potential predictors for attainment of either target (100% fT>MIC, 100% fT>4xMIC), we performed multivariable logistic regression. Predictors evaluated included early versus late sepsis, q12h vs q24h dosing, age, PRISMIII, culture-negative vs. culture-positive sepsis, presence of comorbidities, complicated vs. uncomplicated sepsis, presence of acute kidney injury and use of total parenteral use. Predictors with p-value <0.20 in univariate analyses (Fisher’s exact test for categorical predictors, Wilcoxon rank sum test for continuous predictors) were initially included in the full logistic regression model.
We evaluated predictors of three clinical outcomes: antibiotic broadening (logistic regression model), PICU LOS and Hospital LOS (analysis of covariance models). For all three outcomes we evaluated the following predictors: target attainment (separate models for each target), age, PRISMIII, culture-negative vs. culture-positive sepsis, presence of comorbidities, complicated vs. uncomplicated sepsis, presence of acute kidney injury, ventilator days, vasopressor days and days on ceftriaxone. Predictors with p-value<0.20 in univariate analyses were included. For antibiotic broadening, a dichotomous outcome, Fisher’s exact test was used to determine categorical predictors and Wilcoxon rank sum test was used for continuous predictors. For the continuous LOS outcomes, Wilcoxon rank sum test was used to determine categorical predictors and Spearman correlations were used for continuous predictors. Final predictors were selected stepwise with backward direction with p-value set at 0.05.
RESULTS
Demographics and Clinical Characteristics
We included 55 patients in our analysis (Table 1). The median age was 7.5 years (range: one month to 26 years). Over 2/3 of patients had at least one comorbid condition. The majority of patients (41/55, 75%) had concomitant methicillin-resistant staphylococcus aureus coverage for at least 48 hours. The predominant source of infection was pneumonia/upper airway infection (62%). All patients were alive at day 28 and three patients (5.5%) had a complicated course of sepsis. The median number of days on ceftriaxone was seven (IQR 4-8), signifying that half of patients were switched to another antibiotic to complete a total antibiotic course of at least seven days. Three patients received q24h dosing during their entire ceftriaxone course. Two patients initially received q12h dosing but transitioned to q24h dosing within the first 48 hours (early sepsis) upon transfer to a lower acuity unit. There were ten patients who were transitioned from q12h to q24h dosing in late sepsis; for 9 of the 10 patients, the transition was due to transfer to a lower acuity unit. A total of 15 patients (27%) received every 24-hour dosing in late sepsis.
Table 1: Demographics and Hospitalization Characteristics of Cohort.
Complicated course defined as two or more organ failures on day seven or mortality within 28 days. Comorbid condition was defined as a medical condition for which medication would be prescribed or a subspecialty care is generally warranted. †Patients who were on ceftriaxone for fewer days than required to meet the definition of sepsis (7) signify that these patients were transitioned to enteral antibiotics or switched to other antibiotics before day 7. IQR: Interquartile range, g: grams; dL: deciliter, PRISM: Pediatric Risk Mortality Score, AKI: acute kidney injury
| Number of patients | n=55 |
|---|---|
| DEMOGRAPHICS | |
| Age (years) Median (IQR) | 7.5 (1.5, 13.8) |
| Sex | |
| Female | 32 (58.2%) |
| Male | 23 (41.8%) |
| Weight (kilograms) Median (IQR) | 22.0 (11.1, 47.0) |
| Self-Identified Race | |
| White | 41 (74.5%) |
| Black | 9 (16.4%) |
| Hispanic | 4 (7.3%) |
| Unknown | 1 (1.8%) |
| Presence of Comorbid Conditions | |
| No | 18 (32.7%) |
| Yes | 37 (67.3%) |
| HOSPITALIZATION CHARCTERISTICS | |
| Presumed Sources of Infection | |
| Pneumonia/upper airway infection (e.g. tracheitis) | 34 (62%) |
| Systemic (e.g. tick-borne illness, culture-negative sepsis, bacteremia) | 12 (22%) |
| Genitourinary infection | 4 (7.3%) |
| Meningitis | 3 (5.5%) |
| Osteomyelitis | 2 (3.6%) |
| Abdominal | 2 (3.6%) |
| Endocarditis | 1 (1.8%) |
| Multiple of the above listed sources | 3 (5.5%) |
| Albumin on Day 1 (g/dL) Mean ± SD | 3.1 ± 0.62 (Missing data: n= 9) |
| Total Bilirubin on Day 1 (mg/dL) Median (IQR) | 0.30 (0.30, 0.65) (Missing data: n = 32) |
| Number of Patients on TPN while on Ceftriaxone | 5 (9.1%) (4 during late phase; 1 for entire course) |
| 28 day Outcome | |
| Alive | 55 (100%) |
| Complicated Course? | |
| No | 52 (94.5%) |
| Yes | 3 (5.5%) |
| Days on Ceftriaxone Median (IQR) | 7.0 (4.0†, 8.0) |
| PRISMIII Score Median (IQR) | 3.0 (0.5, 6.5) |
| Presence of AKI in Early Sepsis | |
| No | 33 (60%) |
| Yes | 22 (40%) |
| Presence of AKI in Late Sepsis | |
| No | 40 (73%) |
| Yes | 15 (27%) |
| Number of Patients whose AKI resolved from Early to Late Sepsis | 11 (20%) |
| Number of patients whose AKI developed in Late Sepsis | 4 (7.3%) |
| Number of patients whose CrCL>150 mL/min/1.73m2 in Early Sepsis (excluding those who had AKI) (n = 33) | 16 (48%) |
Forty percent of patients (22/55) had AKI in the first 48 hours (Table 1). Of the 33 patients without AKI, 16 (48%) had augmented renal clearance (ARC) which we defined as an estimated creatinine clearance greater than 150 mL/min/1.73m2. AKI resolved between early sepsis and late sepsis for half of the patients, while four patients who did not have AKI in early sepsis developed AKI in late sepsis.
PK Parameters
Normalized ceftriaxone clearance was similar in both sepsis phases (Table 2). However, percent differences in normalized clearance at the individual level ranged from −39% to 276% (reference: late sepsis, positive difference indicates higher clearance in early sepsis) (Supplemental Table 1). Normalized central volume of distribution in early sepsis was similar to that in late sepsis (Table 2). Individual percent differences in central volume ranged from −51% to 212% (Supplemental Table 1). There were no statistically significant differences in age, weight, PRISMIII score or presence of AKI in early stage between subjects with 50% or higher increase in clearance or volume in early sepsis compared to those without (Supplemental Table 2)
Table 2: Comparison of pharmacokinetic parameters, clearance and central volume of distribution between early and late sepsis.
†paired t-test and ‡Wilcoxon Sign Rank test performed for comparisons.
| Early (first 48 hours of treatment) (n=55) |
Late (after 48 hours of treatment) (n=55) |
p-value | |
|---|---|---|---|
|
Clearance (CL) of Ceftriaxone (L/h/70kg),
mean ± SD† |
6.18 ± 1.48 | 6.10 ± 1.61 | 0.60 |
| Central Volume (V) of Ceftriaxone (L/70kg), median [IQR]‡ | 26.6 [22.3, 31.3] | 24.5 [22.0, 29.4] | 0.18 |
Target Attainment
There was a smaller percentage of patients who reached targets in the late sepsis compared to early sepsis (Table 3: 100% fT>MIC: 82% vs 96%; fT>4xMIC: 75% vs 80%) and statistically significant for the less stringent target (100% fT>MIC, p=0.013). All patients who did not meet the less stringent target in early or late sepsis received ceftriaxone q24h during the phase of interest (Table 3).
Table 3: Comparison of frequency of target attainment in early and late sepsis.
Two targets were assessed: concentrations above 1x Clinical Laboratory Standards Institute (CLSI) breakpoint or minimum inhibitory concentration (MIC) for 100% of dosing intervals and concentrations above 4x CLSI breakpoint/MIC for 100% of dosing intervals. Of those who did not attain targets in a specific phase, dosing interval in that phase of sepsis also provided. Q24h: every 24 hours; q12h: every 12 hours.
| Early (first 48 hours of treatment) (n=55) |
Late (after 48 hours of treatment) (n=55) |
p-value | |
|---|---|---|---|
| Number of patients who attained free ceftriaxone concentrations above CLSI breakpoint/MIC for 100% of dosing interval (%) | 53 (96%) | 45 (82%) | 0.013 |
| Number of patients who did NOT attain 1x MIC target | 2 | 10 | |
| q24h dosing | 2 (100%) | 10 (100%) | |
| q12h dosing | 0 (0%) | 0 (0%) | |
| Number of patients who attained free ceftriaxone concentrations above 4 times CLSI breakpoint/MIC for 100% of dosing interval (%) | 44 (80%) | 41 (75%) | 0.55 |
| Number of patients who did NOT attain 4x MIC target | 11 | 14 | |
| q24h dosing | 5 (45.4%) | 12 (85.7%) | |
| q12h dosing | 6 (54.6%) | 2 (14.3%) |
Of the 10 patients who did not meet the less stringent target in late phase, four received ceftriaxone q24h in both early and late sepsis. Two of the four patients did initially meet target in early sepsis while on q24h dosing but not in late sepsis (Table 4). Six of ten patients who did not meet target in late phase were initially on q12h dosing, then transitioned to q24h dosing in the PICU or after transferring to a unit of less acuity during the late phase. Modeling and simulation showed that had all 10 patients received q12h ceftriaxone during late phase, they would have met the less stringent target.
Table 4: Association of Target Attainment in Early Sepsis and in Late Sepsis with Hospitalization Characteristics and Outcomes.
Target defined as Concentrations above 1x Clinical Laboratory Standards Institute (CLSI) breakpoint/MIC for 100% dosing interval.
| Early Sepsis Target Attainment | Late Sepsis Target Attainment | ||||||
|---|---|---|---|---|---|---|---|
| Overall Cohort n=55 |
Did not Attain Target n=2 |
Attained Target n=53 |
p-value | Did not Attain Target n=10 |
Attained Target n=45 |
p-value | |
| Number of Patients for whom Q24h dosing initiated in Early Sepsis | 6 (10.9%) | 2 (100%) | 4 (7.5%) | 0.010 | 4 (40%) | 2 (4.4%) | 0.0076 |
| Number of Patients who had Q24h dosing in Late Sepsis (initiated or continued from Early Sepsis) | 15 (27.3%) | 2 (100%) | 13 (24.5%) | 0.071 | 10 (100%) | 5 (11.1%) | <0.001 |
| Number of Patients with Each Dosing Regimen in Phase of Interest | |||||||
| Q24h | -- | 2 (100%) | 4 (7.5%) | 0.010 | 10 (100%) | 5 (11.1%) | <0.001 |
| Q12h | 0 (0%) | 49 (92.5%) | 0 (0%) | 40 (89%) | |||
| Age (years) Median (IQR) | 7.5 (1.5, 13.8) | 4.1 (3.6, 4.7) | 8.2 (1.5, 13.8) | 0.64 | 2.2 (1.5, 4.7) | 8.2 (1.7, 13.8) | 0.33 |
| PRISM III Score Median (IQR) | 3.0 (0.5, 6.5) | 1.0 (0.5, 1.5) | 3.0 (1.0, 7.0) | 0.18 | 2.0 (0, 5.3) | 3.0 (2.0, 7.0) | 0.34 |
| PICU LOS (days) Median (IQR) | 4.0 (2.0, 7.5) | 2.5 (1.8, 3.3) | 4.0 (2.0, 8.0) | 0.24 | 2.0 (1.3, 3.5) | 5.0 (3.0, 8.0) | 0.0067 |
| Hospital LOS (days) Median (IQR) | 13.0 (8.0, 24.0) | 14.0 (12.5, 15.5) | 13.0 (8.0, 26.0) | 0.87 | 19.5 (8.8, 26.8) | 12.0 (8.0, 18.0) | 0.51 |
| Days on Vasopressor in PICU Median (IQR) | 1.0 (1.0, 2.0) | N/A (0 on pressors) | 1.0 (1.0, 2.0) | -- | 1.0 (1.0, 1.0) | 1.0 (1.0, 2.0) | 0.72 |
| Vasopressor-Free Days in PICU Median (IQR) | 4.0 (2.0, 7.5) | 2.5 (1.8, 3.3) | 4.0 (2.0, 8.0) | 0.31 | 2.0 (1.0, 3.5) | 5.0 (2.0, 8.0) | 0.015 |
| Days on Ventilator in Hospital | 5.0 (3.0, 13.0) | N/A (0 on ventilator) | 5.0 (3.0, 13.0) | -- | 1.0 (1.0, 7.0) | 5.5 (3.0, 15.8) | 0.22 |
| Ventilator-Free Days in PICU Median (IQR) | 2.0 (1.0. 2.5) | 2.5 (1.8, 3.3) | 2.0 (1.0, 2.0) | 0.58 | 1.5 (1.0, 2.0) | 2.0 (1.0, 3.0) | 0.58 |
| Sepsis Type | |||||||
| Culture-negative | 40 (73%) | 2 (100%) | 38 (72%) | 1 | 7 (70%) | 33 (73%) | 1 |
| Culture-positive | 15 (27%) | 0 (0%) | 15 (28%) | 3 (30%) | 12 (27%) | ||
| Presence of Comorbidities | |||||||
| No | 18 (33%) | 1 (50%) | 17 (32%) | 1 | 2 (20%) | 16 (36%) | 0.47 |
| Yes | 37 (67%) | 1 (50%) | 36 (68%) | 8 (80%) | 29 (64%) | ||
| Complicated Sepsis | |||||||
| No | 52 (94.5%) | 2 (100%) | 50 (94.3%) | 1 | 10 (100%) | 42 (93%) | 1 |
| Yes | 3 (5.5%) | 0 (0%) | 3 (5.7%) | 0 (0%) | 3 (7%) | ||
| Acute Kidney Injury in Early Sepsis | |||||||
| No | 33 (60%) | 2 (100%) | 31 (59%) | 0.51 | 7 (70%) | 26 (58%) | 0.72 |
| Yes | 22 (40%) | 0 (0%) | 22 (41%) | 3 (30%) | 19 (42%) | ||
| Acute Kidney Injury in Late Sepsis | |||||||
| No | 40 (73%) | 2 (100%) | 38 (72%) | 1 | 8 (80%) | 32 (71%) | 0.71 |
| Yes | 15 (27%) | 0 (0%) | 15 (285) | 2 (20%) | 13 (29%) | ||
| Total Parental Nutrition Use | |||||||
| No | 50 (91%) | 2 (100%) | 13 (25%) | 0.071 | 7 (70%) | 43 (96%) | 0.037 |
| Yes | 5 (9%) | 0 (0%) | 40 (75%) | 3 (30%) | 2 (4%) | ||
| Days on Ceftriaxone Median (IQR) | 7.0 (4.0, 8.0) | 8.0 (6.5, 9.5) | 7.0 (4.0, 8.0) | 0.51 | 7.0 (5.0, 9.3) | 6.0 (4.0, 8.0) | 0.72 |
| Broaden Antibiotics | |||||||
| No | 45 (82%) | 1 (50%) | 44 (83%) | 0.33 | 5 (50%) | 40 (89%) | 0.012 |
| Yes | 10 (18%) | 1 (50%) | 9 (17%) | 5 (50%) | 5 (11%) | ||
| Fever Duration (days) Median (IQR) | 2.0 (1.0, 3.0) | 4.5 (3.8, 5.3) | 2.0 (1.0, 3.0) | 0.11 | 2.5 (2.0, 3.8) | 2.0 (1.0, 3.0) | 0.068 |
| Number of Repeat Cultures in 7 days Median (IQR) | 1.0 (0.0, 6.0) | 2.0 (1.0, 3.0) | 1.0 (0.0, 2.0) | 0.68 | 2.0 (1.0, 2.0) | 0.0 (0.0, 2.0) | 0.087 |
| Number of CRP in 7 days Median (IQR) | 0.0 (0.0, 1.0) | 3.5 (1.8, 5.3) | 0.0 (0.0, 1.0) | 0.39 | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.72 |
| Number of Procalcitonin 7 days Median (IQR) | 1.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.0) | 0.076 | 0.5 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.21 |
For the more stringent target (100% fT>4xMIC), six patients received q12h dosing in early sepsis but still did not attain target (Table 3). In late sepsis, the majority (86%) who did not meet the more stringent target received q24h dosing.
Association between Target Attainment, Patient Characteristics and Outcomes with Univariate Analyses
We investigated the association of attaining both targets in each sepsis phase (Tables 4 and 5) with patient characteristics and outcomes. There were no significant differences in percentage of patients with culture-negative or culture-positive sepsis or PRISMIII scores based on target attainment. More patients who did not attain the less stringent target (100% fT>MIC) in late sepsis required total parenteral nutrition (TPN) (30% vs 4%, p=0.037). Patients who did not attain the less stringent target (100% fT>MIC) in late sepsis had significantly shorter PICU stays (2.0 [1.0, 3.5] vs 5.0 [3.0, 8.0] days, p = 0.0067). Similarly, those who did not attain the more stringent target (100% fT>4xMIC) in early or late sepsis had shorter PICU stays (early: 2.0 [1.5, 4.0] vs 5.0 [2.0, 8.0] days, p = 0.032; late: 2.0 [2.0, 3.8] vs 6.0 [3.0, 8.0] days, p = 0.0010). Due to the significant differences in PICU lengths of stay, there were significant differences in vasopressor-free days (Table 4 and 5). Hospital lengths of stay were not significantly different between target attainment groups.
Table 5: Association of Target Attainment in Early Sepsis and in Late Sepsis with Hospitalization Characteristics and Outcomes.
Target defined as Concentrations above 4x Clinical Laboratory Standards Institute (CLSI) breakpoint/MIC for 100% dosing interval.
| Early Sepsis Target Attainment | Late Sepsis Target Attainment | ||||||
|---|---|---|---|---|---|---|---|
| Overall Cohort n=55 |
Did not Attain Target n=11 |
Attained Target n=44 |
p-value | Did not Attain Target n=14 |
Attained Target n=41 |
p-value | |
| Number of Patients for whom Q24h dosing initiated in Early Sepsis | 6 (10.9%) | 5 (45.5%) | 1 (2.3%) | <0.001 | 5 (35.7%) | 1 (2.4%) | 0.0029 |
| Number of Patients who had Q24h dosing in Late Sepsis (initiated or continued from Early Sepsis) | 15 (27.3%) | 6 (54.5%) | 9 (20.5%) | 0.052 | 12 (85.7%) | 3 (7.3%) | <0.001 |
| Number of Patients with Each Dosing Regimen in Phase of Interest | |||||||
| Q24h | -- | 5 (45.5%) | 1 (2%) | <0.001 | 12 (85.7%) | 3 (7.3%) | <0.001 |
| Q12h | 6 (54.5%) | 43 (98%) | 2 (14.3%) | 38 (92.7%) | |||
| Age (years) | 7.5 (1.5, 13.8) | 3.0 (1.6, 9.1) | 8.2 (1.3, 14.3) | 0.46 | 2.2 (1.5, 13.9) | 8.2 (2.4, 13.7) | 0.58 |
| PRISM III Score Median (IQR) | 3.0 (0.5, 6.5) | 2.0 (0, 4.5) | 3.0 (1.8, 7.0) | 0.27 | 2.0 (0, 5.3) | 3.0 (2.0, 7.0) | 0.40 |
| PICU LOS (days) Median (IQR) | 4.0 (2.0, 7.5) | 2.0 (1.5, 4.0) | 5.0 (2.0, 8.0) | 0.032 | 2.0 (2.0, 3.8) | 6.0 (3.0, 8.0) | 0.0010 |
| Hospital LOS (days) Median (IQR) | 13.0 (8.0, 24.0) | 16.0 (7.0, 41.0) | 12.5 (8.0, 23.0) | 0.94 | 17.0 (7.3, 25.0) | 12.0 (8.0, 19.0) | 0.78 |
| Days on Vasopressor in PICU | 1.0 (1.0, 2.0) | 2.5 (2.3, 2.8) | 1.0 (1.0, 1.0) | 0.081 | 1.5 (1.3, 1.8) | 1.0 (1.0, 1.5) | 0.73 |
| Vasopressor-Free Days in PICU Median (IQR) | 4.0 (2.0, 7.5) | 2.0 (1.5, 3.5) | 5.0 (2.0, 8.0) | 0.030 | 2.0 (1.3, 2.8) | 5.0 (2.0, 8.0) | 0.014 |
| Days on Ventilator in Hospital | 5.0 (3.0, 13.0) | 3.5 (1.8, 26.3) | 6.0 (3.0, 13.0) | 0.46 | 1.5 (1.0, 2.8) | 6.0 (3.5, 18.0) | 0.017 |
| Ventilator-Free Days in PICU Median (IQR) | 2.0 (1.0. 2.5) | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 0.94 | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 0.37 |
| Sepsis Type | |||||||
| Culture-negative | 40 (73%) | 8 (73%) | 32 (73%) | 1 | 11 (79%) | 29 (71%) | 0.73 |
| Culture-positive | 15 (27%) | 3 (27%) | 12 (27%) | 3 (21%) | 12 (29%) | ||
| Presence of Comorbidities | |||||||
| No | 18 (33%) | 4 (36%) | 14 (32%) | 1 | 2 (14%) | 16 (39%) | 0.11 |
| Yes | 37 (67%) | 7 (64%) | 30 (68%) | 12 (76%) | 25 (61%) | ||
| Complicated Sepsis | |||||||
| No | 52 (95%) | 11 (100%) | 41 (93%) | 1 | 14 (100%) | 38 (93%) | 0.56 |
| Yes | 3 (5%) | 0 (0%) | 3 (7%) | 0 (0%) | 3 (7%) | ||
| Acute Kidney Injury in Early Sepsis | |||||||
| No | 33 (60%) | 7 (64%) | 26 (59%) | 1 | 10 (71%) | 23 (56%) | 0.36 |
| Yes | 22 (40%) | 4 (36%) | 18 (41%) | 4 (29%) | 18 (44%) | ||
| Acute Kidney Injury in Late Sepsis | |||||||
| No | 40 (73%) | 9 (82%) | 31 (71%) | 0.71 | 12 (86%) | 26 (68%) | 0.30 |
| Yes | 15 (27%) | 2 (18%) | 13 (29%) | 2 (14%) | 13 (32%) | ||
| Total Parental Nutrition Use | |||||||
| No | 50 (91%) | 10 (91%) | 40 (91%) | 1 | 11 (79%) | 39 (95%) | 0.098 |
| Yes | 5 (9%) | 1 (9%) | 4 (9%) | 3 (21%) | 2 (5%) | ||
| Days on Ceftriaxone Median (IQR) | 7.0 (4.0, 8.0) | 5.0 (5.0, 6.5) | 7.0 (4.0, 8.0) | 0.35 | 7.0 (5.0, 7.0) | 6.0 (4.0, 8.0) | 1 |
| Broaden Antibiotics | |||||||
| No | 45 (82%) | 9 (82%) | 36 (82%) | 1 | 8 (57%) | 37 (90%) | 0.012 |
| Yes | 10 (18%) | 2 (18%) | 8 (18%) | 6 (43%) | 4 (9.8%) | ||
| Fever Duration (days) Median (IQR) | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.3) | 0.35 | 2.5 (2.0, 3.8) | 2.0 (1.0, 3.0) | 0.025 |
| Number of Repeat Cultures in 7 days Median (IQR) | 1.0 (0.0, 6.0) | 0.0 (0.0, 1.5) | 1.0 (0.0, 2.0) | 0.54 | 2.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.13 |
| Number of CRP in 7 days Median (IQR) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.93 | 0.5 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.33 |
| Number of Procalcitonin 7 days Median (IQR) | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 1.0 (0.0, 1.3) | 0.035 | 1.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.42 |
A higher percentage of patients who did not meet the less stringent target (100% fT>MIC) in the late phase had antibiotics broadened (50% of 10 patients who did not attain target vs 11% of 45 patients who attained targets, p = 0.012). There was also a significant difference in antibiotic broadening for the more stringent target in the late phase (43% vs 9.8%, p = 0.012) in univariate analysis. There was a trend to obtain more repeat cultures in patients who did not attain the less stringent target in late sepsis (2.0 [1.2, 2.0] vs 0.0 [0.0, 2.0], p = 0.087). There were more procalcitonin measurements in the first seven days in patients who attained the more stringent target in early sepsis (1.0 [0.0, 1.3] vs 0.0 [0.0, 1.0], p=0.035).
Kaplan-Meier Analysis of Hospital Lengths of Stay
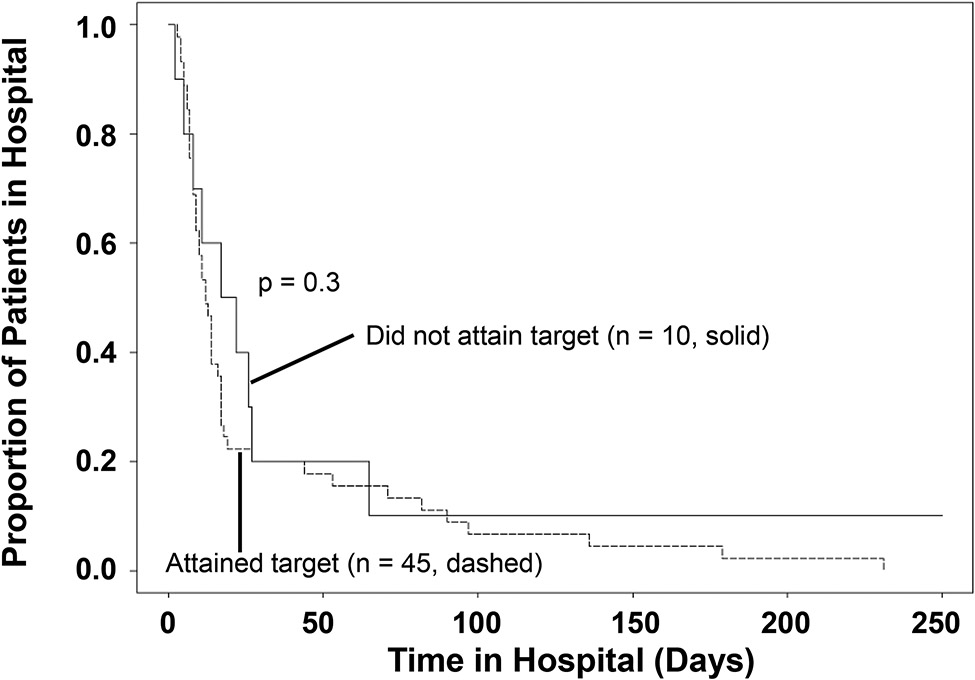
We further investigated the finding that patients who did not meet the less stringent target (100% fT>MIC) in late sepsis had statistically significant shorter PICU lengths of stay yet clinically significant (>7 days difference) but non-statistically significant longer hospital lengths of stay through Kaplan Meier analysis. While there is a separation of the curves for lengths of stays in the 10-30 day range between patients who did or did not attain target in late sepsis (Figure 1), the difference was not statistically significant (p=0.3).
Figure 1: Kaplan-Meier survival curve representing the proportion of patients remaining in the hospital over time (days) for patients who did (dashed line) or did not attain (solid line) the less stringent target (100% fT>MIC).
Each step down represents discharge (alive) from the hospital. N: number of patients in each group.
Predictors for Target Attainment
For the less stringent target (100% fT>MIC), predictors that were included in the final model were early vs. late phase, TPN use and PRISMIII. Although there was a strong association between q12h and q24h dosing with target attainment, since all patients who received q12h dosing in either phase attained the target, this predictor could not be included in the full model. The only significant predictor was early vs. late phase. The odds of reaching 100% fT>MIC was 4.9 times (OR 4.9, 95% CI: 1.3-18.9) greater during early phase than late phase.
For the most stringent target (100% fT>4xMIC), q12h v. q24h dosing, PRISMIII and TPN use were included in the full model. In the final model, only dosing frequency was a significant predictor with the odds of reaching the target being 37 times (OR 37, 95% CI: 7.9-172) greater with q12h dosing than with q24h dosing.
Predictors of Clinical Outcomes
Significant predictors identified by univariate analyses for antibiotic broadening included target attainment (both 100% fT>MIC, 100% fT>4xMIC) and number of days on ceftriaxone. In the two final models, one for each target as a predictor, we found that the odds of antibiotic broadening are 6.5 times (OR: 6.5, 95% CI: 1.5-29) greater when the less stringent target was not attained and 3.8 times (OR: 3.8, 95% CI: 1.2-13) greater when the more stringent target was not attained.
In the full model for PICU LOS and using 100% fT>MIC target attainment as a predictor, we found that attaining the target, increased number of days on ventilator and having complicated sepsis were all significantly associated with longer PICU LOS (Supplemental Table 3A). When using 100% fT>4xMIC target attainment as a predictor instead, attaining the target and number of days on ventilator were significantly associated with PICU LOS (Supplemental Table 3B). Finally for each of models for the hospital LOS, one for each of the targets, only number of days on the ventilator was significantly associated with hospital LOS (Supplemental Table 3C, 3D).
DISCUSSION
In this study of critically ill children and young adults with sepsis who received ceftriaxone as initial therapy, we did not find a significant difference in ceftriaxone clearance or central volume between early and late sepsis. Contrary to our hypothesis, we found that a lower percentage of patients attained targets in late sepsis rather than in early sepsis. This unexpected finding appears to be driven by extending the dosing interval from 12 hours to 24 hours. We found in multivariable analyses that failure to attain either target is associated with antibiotic broadening, an important antimicrobial stewardship outcome.
There are several potential reasons why we did not detect a difference in PK parameters. One reason is our small sample size. Despite enrolling nearly 200 patients in the PICU who received ceftriaxone, only 55 patients met inclusion criteria. The major contributor for this small sample size is that many patients who require only ceftriaxone for infections do not have blood drawn frequently to allow for ceftriaxone measurements in both sepsis phases. While 122 patients of our initial cohort met the definition of sepsis, less than half met inclusion criteria. Since sepsis is a heterogenous problem, it may also be difficult to generalize how PK parameters change for all septic patients. At an individual level, there were patients whose clearances and central volume were 2-3 fold higher in early sepsis. These specific patients may benefit from more frequent dosing or continuous infusion of ceftriaxone to ensure adequate exposure. Identifying these patients could be based on high volumes of fluid boluses administered early in sepsis, physical signs of fluid overload or lower than expected creatinine values (i.e., augmented renal clearance).
Our definition of time zero also introduced limitations. Patients may have had physiologic changes related to sepsis occur hours or even days before hospital presentation. Therefore, some patients may have been misclassified, having physiology more similar to what would be expected in late sepsis during the first 48 hours of our study. This concern is evidenced by our finding that more patients had AKI in the first 48 hours of the study than a creatinine clearance >150 mL/min/m2.
Our prior study demonstrated that while we give more boluses in the first two days of therapy, patients on ceftriaxone for more than two days were likely to have higher fluid balance in later days,13 offsetting any differences in volume of distribution. In addition, we estimated only central volume, not total volume, due to the limitations of sparse sampling, as described earlier. Total volume of distribution may have been significantly different.
We unexpectedly found that the percentages of patients who met targets were lower in late sepsis. Further analysis suggested this finding was driven by extending the ceftriaxone dosing interval from q12h to q24h, as all patients who did not attain the less stringent target in either phase were on q24h dosing. Multivariable analyses showed that early phase was associated with failure to attain the less stringent target (we could not test q24h dosing due to all patients on q12h dosing attaining target) and that q24h dosing was associated with failure to attain the more stringent target. When the dosing interval is lengthened, the probability of concentrations remaining above target for the entire interval is lower. For the more stringent target, six patients received q12h dosing in early sepsis who did not reach target, in comparison to only two patients on q12h dosing in late sepsis. This finding may be a signal that there are physiologic changes in early sepsis that decrease probability of target attainment even with higher frequency dosing.
How target attainment affects outcomes remains a major question within the beta-lactam research community. Most studies in adults have not shown improved mortality rates among those who attain targets but have shown an increase in rates of bacterial eradication, symptom resolution, suppression of resistance and shorter ICU lengths of stay.16,29,30 Since pediatric mortality in our ICU is low and a large percentage of patients have culture-negative sepsis, we also investigated outcomes related to antimicrobial stewardship and those that cause discomfort for young patients with additional laboratory evaluations. For both targets, multivariable analyses showed that lack of target attainment is associated with antibiotic broadening. Interestingly, patients who attained the more stringent target in early sepsis had more procalcitonin measurements, a biomarker for bacterial infection that clinicians may trend. Antibiotic broadening has implications for antimicrobial stewardship as more broad-spectrum antibiotics are more expensive and associated with antimicrobial resistance and toxicities such as nephrotoxicity and neurotoxicity.
It was surprising to find that those patients who did not meet targets had shorter PICU stays. Since our previous population PK model showed that lower PRISM III scores are associated with higher ceftriaxone clearance,13 decreasing the probability of target attainment, we investigated to see if there was a difference in PRISMIII mortality scores between the groups to account for the shorter PICU stays. We found no statistical difference. It appears, however, that number of ventilator days has a significant impact on PICU LOS, rather than PRISMIII score, and could explain our finding of shorter PICU LOS for patients who did not meet targets.
The difference in median hospital lengths of stay was more than a week longer for patients who did not meet the less stringent target in late sepsis. Though the difference was not statistically significant, there could be clinical implications. Six of 10 patients who did not meet the less stringent target in late phase were initially on q12h dosing and then transitioned to q24h dosing in the PICU or after transferring to a unit of less acuity; the remaining four were on q24h dosing in both phases. It is possible that these patients appeared to be clinically improving, leading to the change in dosing regimen and/or transfer out of the unit. All 10 patients then did not reach the target in late sepsis and for half of them, this target attainment failure was associated with antibiotic broadening. Switching antibiotics could lead to longer days in the hospital whether for an additional 48-hour rule out or full course. Further studies are warranted to delineate the appropriate timeline to transition from q12h ceftriaxone dosing to q24h dosing of ceftriaxone to prevent the need for antibiotic broadening and longer hospital stays. Given that enteral β-lactam antibiotics are typically given at least q12h, the likelihood of attaining targets on enteral antibiotics may be higher than q24h dosing of ceftriaxone due to more frequent enteral dosing (i.e., concentrations will remain above target for entire dosing interval if intervals are shorter). Thus, when a septic patient admitted to the PICU is improving, transitioning to enteral antibiotics may be better than q24h ceftriaxone dosing. Comparing q24h ceftriaxone transition and enteral transition would need to be studied.
One study limitation is that since the majority of patients did not have bacteria cultured, we utilized the CLSI breakpoint as a surrogate for MIC. This breakpoint of 1μg/mL could be higher than the actual MICs of the bacteria that were not cultured. Therefore, it is possible that in some cases targets would have been attained if the MIC had been known and the high CLSI breakpoint was not used. We did perform univariate analyses to investigate if there was a higher percentage of patients who did not attain targets that had culture-negative sepsis but no association was identified. It was also not a significant predictor in multivariable models.
Our population excluded patients who had antibiotics broadened within the first 48 hours. It is possible that target attainment failure in early sepsis led to antibiotic broadening in these excluded patients. This exclusion of patients who broadened antibiotics within 48 hours and of patients on extracorporeal support devices likely biased our patients to be less sick and have a 100% survival rate. At our institution, most patients admitted to the PICU are initiated on q12h ceftriaxone dosing regardless of type of infection. However, this does not occur uniformly at other institutions (personal communication). Therefore, our findings may not be generalizable. Future studies may include multi-center studies to evaluate target attainment and association with outcomes between institutions with different dosing regimens.
CONCLUSION
In our cohort of patients with sepsis and treated with ceftriaxone, we did not find a difference in PK parameters between early and late sepsis but found that dosing q24h does place patients at risk of not meeting targets. We also demonstrate that not attaining targets is significantly associated with antibiotic broadening, which have implications in antimicrobial stewardship.
Supplementary Material
Acknowledgements:
The dataset used in the study was generated with funding from the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program [5T32HD069054-09], Gerber Foundation Novice Research Award, CCHMC Arnold Strauss Award and Hospital Medicine Fellow Award. Dr. Tang Girdwood was supported by the Child Health Research Career Development Award 5K12HD028827 at the time that the initial study analysis was performed. Statistical support was provided through funding from the National Institute of General Medical Sciences [R35GM146701-01]. The original dataset used in the study REDCap use is supported by the NIH Clinical and Translational Science Award (CTSA) program 2UL1TR001425.
Footnotes
Conflict of Interest:
N.P. is president of Medimatics, a company that provides consulting services on medical information systems located in Maastricht, The Netherlands.
REFERENCES
- 1.Phe K, Heil EL, Tam VH. Optimizing Pharmacokinetics-Pharmacodynamics of Antimicrobial Management in Patients with Sepsis: A Review. J Infect Dis. 2020;222(Suppl 2):S132–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droege ME, Van Fleet SL, Mueller EW. Application of Antibiotic Pharmacodynamics and Dosing Principles in Patients With Sepsis. Crit Care Nurse. 2016;36(2):22–32. [DOI] [PubMed] [Google Scholar]
- 3.Veiga RP, Paiva JA. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit Care. 2018;22(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipman J, Udy AA, Roberts JA. Do we understand the impact of altered physiology, consequent interventions and resultant clinical scenarios in the intensive care unit? The antibiotic story. Anaesth Intensive Care. 2011;39(6):999–1000. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez M, Jimenez-Lendinez M, Cidoncha M, et al. Comparison of fluid compartments and fluid responsiveness in septic and non-septic patients. Anaesth Intensive Care. 2011;39(6):1022–1029. [DOI] [PubMed] [Google Scholar]
- 7.Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol. 2011;7(9):539–543. [DOI] [PubMed] [Google Scholar]
- 8.Udy AA, Roberts JA, De Waele JJ, Paterson DL, Lipman J. What's behind the failure of emerging antibiotics in the critically ill? Understanding the impact of altered pharmacokinetics and augmented renal clearance. Int J Antimicrob Agents. 2012;39(6):455–457. [DOI] [PubMed] [Google Scholar]
- 9.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10; quiz 11-12. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–851; quiz 859. [DOI] [PubMed] [Google Scholar]
- 11.Barreto EF, Webb AJ, Pais GM, Rule AD, Jannetto PJ, Scheetz MH. Setting the Beta-Lactam Therapeutic Range for Critically Ill Patients: Is There a Floor or Even a Ceiling? Crit Care Explor. 2021;3(6):e0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Girdwood SC, Tang PH, Murphy ME, et al. Demonstrating Feasibility of an Opportunistic Sampling Approach for Pharmacokinetic Studies of beta-Lactam Antibiotics in Critically Ill Children. J Clin Pharmacol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Girdwood S, Dong M, Tang P, et al. Population Pharmacokinetic Modeling of Total and Free Ceftriaxone in Critically Ill Children and Young Adults and Monte Carlo Simulations Support Twice Daily Dosing for Target Attainment. Antimicrob Agents Chemother. 2021:AAC0142721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Aziz MH, Lipman J, Roberts JA. Identifying "at-risk" patients for sub-optimal beta-lactam exposure in critically ill patients with severe infections. Crit Care. 2017;21(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taccone FS, Laterre PF, Dugernier T, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulla A, Dijkstra A, Hunfeld NGM, et al. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: a two-center prospective study (EXPAT). Crit Care. 2020;24(1):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girdwood ST, Kaplan J, Vinks AA. Methodologic Progress Note: Opportunistic Sampling for Pharmacology Studies in Hospitalized Children. J Hosp Med. 2020;15(2):E1–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 19.Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data. Paediatr Anaesth. 2011;21(3):222–237. [DOI] [PubMed] [Google Scholar]
- 20.Jelliffe RW, Schumitzky A, Van Guilder M, et al. Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther Drug Monit. 1993;15(5):380–393. [PubMed] [Google Scholar]
- 21.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–351. [DOI] [PubMed] [Google Scholar]
- 22.Duszynska W, Taccone FS, Switala M, Hurkacz M, Kowalska-Krochmal B, Kubler A. Continuous infusion of piperacillin/tazobactam in ventilator-associated pneumonia: a pilot study on efficacy and costs. Int J Antimicrob Agents. 2012;39(2):153–158. [DOI] [PubMed] [Google Scholar]
- 23.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother. 2002;50(3):425–428. [DOI] [PubMed] [Google Scholar]
- 24.Zhou QT, He B, Zhang C, Zhai SD, Liu ZY, Zhang J. Pharmacokinetics and pharmacodynamics of meropenem in elderly chinese with lower respiratory tract infections: population pharmacokinetics analysis using nonlinear mixed-effects modelling and clinical pharmacodynamics study. Drugs Aging. 2011;28(11):903–912. [DOI] [PubMed] [Google Scholar]
- 25.CLSI, ed Performance Standards for Antimicrobial Disk Susceptibility Tests. CLSI standard M02. 28th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 26.Basu RK, Kaddourah A, Terrell T, et al. Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in Critically Ill Children (AWARE): A Prospective Study to Improve Diagnostic Precision. J Clin Trials. 2015;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21(1):63–73. [DOI] [PubMed] [Google Scholar]
- 29.Hagel S, Bach F, Brenner T, et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med. 2022;48(3):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Shaer MH, Rubido E, Cherabuddi K, Venugopalan V, Klinker K, Peloquin C. Early therapeutic monitoring of beta-lactams and associated therapy outcomes in critically ill patients. J Antimicrob Chemother. 2020;75(12):3644–3651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.