Abstract
Purpose:
To evaluate the effectiveness of plasma exchange (PLEX) for optic neuritis (ON).
Methods:
We conducted an international multicenter retrospective study evaluating the outcomes of ON following PLEX. Outcomes were compared to raw data from the Optic Neuritis Treatment Trial (ONTT) using a matched subset.
Results:
A total of 395 ON attack treated with PLEX from 317 patients were evaluated. The median age was 37 years (range 9 to 75) and 71% were female. Causes of ON included: multiple sclerosis (108), myelin-oligodendrocyte-glycoprotein-antibody-associated-disease (MOGAD) (92), aquaporin-4-IgG-positive-neuromyelitis-optica-spectrum-disorder (AQP4+NMOSD) (75), seronegative-NMOSD (34), idiopathic (83), and other (3). Median time from onset of vision loss to PLEX was 2.6 weeks (IQR, 1.4–4.0). Median visual acuity (VA) at time of PLEX was count fingers (IQR, 20/200-hand motion) and median final VA was 20/25 (IQR, 20/20–20/60) with no differences among etiologies except MOGAD-ON which had better outcomes. In 81 (20.5%) ON attacks, the final VA was 20/200 or worse. Patients with poor outcomes were older (p=0.002), had worse VA at time of PLEX (p<0.001), and longer delay to PLEX (p<0.001).
In comparison with the ONTT subset with severe corticosteroid-unresponsive ON, a final VA of worse than 20/40 occurred in 6/50 (12%) PLEX-treated ON versus 6/18 (33%) from the ONTT treated with intravenous methylprednisolone without PLEX (p=0.04).
Conclusion:
Most ON attacks improved with PLEX, and outcomes were better than attacks with similar severity in the ONTT. The presence of severe vision loss at nadir, older age, and longer delay to PLEX predicted a worse outcome while MOGAD-ON had a more favorable prognosis.
INTRODUCTION
Optic neuritis is the most common cause of acute/subacute vision loss in patients under the age of 50.1,2 The treatment of optic neuritis greatly varies depending on the severity of vision loss, etiology, and provider preference. The Optic Neuritis Treatment Trial (ONTT), a landmark randomized clinical trial comparing high dose intravenous methylprednisolone (IVMP), low dose oral prednisone, and placebo, demonstrated that high dose IVMP led to expedited recovery of vision, but did not change the final visual outcome of optic neuritis.3 The ONTT has largely shaped the treatment of optic neuritis, especially in the United States and Europe. However, since its completion in 1991, there have been some new advances and changes in practice patterns. First, there has been the identification of biomarkers for other causes of demyelinating disease beyond multiple sclerosis (MS), namely aquaporin-4 antibodies (AQP4-IgG) for neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) for myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), which have different prognoses and treatment than MS.4–6 In addition, there has been a movement toward more usage of plasma exchange (PLEX) for severe demyelinating attacks, including optic neuritis.2,7
Although optic neuritis was excluded from the most cited randomized clinical trial demonstrating that PLEX is effective for the treatment of severe demyelinating attacks that are unresponsive to corticosteroids,8 these findings have been extended to optic neuritis and many experts recommend PLEX for severe optic neuritis unresponsive to corticosteroids, especially in the setting of NMOSD.2,7 Retrospective studies have suggested PLEX is beneficial for the treatment of optic neuritis and that earlier treatment may provide superior outcomes.9–18 These retrospective studies were summarized in a 2022 meta-analysis, which concluded that PLEX was effective for optic neuritis, especially if given early.19 However, the majority of the prior studies were small (the largest study cohort was 48 patients). In addition, it is difficult to combine data in a meta-analysis because of varying inclusion criteria and outcome measures. The majority of prior studies included eyes that had prior optic neuritis, which may influence the ability of the eye to recover in response to PLEX. Lastly, many of these studies were performed prior to the discovery of MOG and AQP4 antibodies and therefore it is unclear how these diseases might have affected the outcomes of PLEX.
To better define the effect of PLEX in optic neuritis from a global perspective, we evaluated a large international multicenter cohort of patients with optic neuritis who received PLEX as acute treatment and evaluated for variables that may influence outcomes, including age, sex, time to treatment, and optic neuritis etiology. In addition, we compared these results to a subset of patients from the ONTT and propose a future randomized clinical trial on PLEX for optic neuritis.
METHODS
This was an international multicenter observational retrospective study of patients with optic neuritis who received PLEX as acute treatment. Patients were identified from 11 centers in 6 countries – France, Israel, New Zealand, Thailand, the United Kingdom, and the United States (Supplemental Table 1). The inclusion criteria were: 1) clinically documented history of optic neuritis; 2) treatment with PLEX; and 3) recorded visual acuity (VA) at time of PLEX and at least 3 months after PLEX treatment (unless the patient recovered to 20/20 before this visit). Patients were excluded if PLEX could not be completed or was contraindicated.
The Mayo Clinic Institutional Review Board approved this study. For cases contributed from other medical centers, the pertinent institutional review boards approved the study with a waiver of informed consent due to the retrospective nature of the study. Data were shared in a de-identified manner with the lead site through Excel databases. The reporting of this research was done in accordance with the STROBE guidelines.
Patients with optic neuritis were divided into the etiologies of AQP4-IgG positive and seronegative NMOSD,20 MOGAD,21 MS,22 idiopathic, and other. Age, sex, VA at time of PLEX, time from symptom onset to PLEX, and etiology of optic neuritis were all evaluated for their influence on final VA.
Final VA after each attack was defined as the time point closest to 6–12 months to allow complete recovery, but minimize age-related causes of vision loss, such as cataracts. Attacks with VA outcomes less than 3 months after the PLEX treatment were excluded unless the patient recovered to 20/20 at a sooner visit. Attacks were also excluded if there was a recurrent optic neuritis prior to the recorded final VA outcome. The VA was obtained on standard Snellen VA charts (best corrected VA or, if unavailable, pinhole acuity) and the VA was converted to the Logarithm of the Minimum Angle of Resolution (logMAR) for analysis. LogMAR value for count fingers was 1.7, hand motion was 2.0, light perception was 2.3, and no light perception was 3.23,24 Time to PLEX treatment was defined as time from onset of vision loss to the first day of PLEX treatment. Outcomes were stratified as complete recovery back to 20/20, good recovery (20/40 or better), medium recovery (between 20/40 and 20/200), and poor recovery (20/200 or worse). These cutoffs were focused on because the legal driving requirement is 20/40 or better, and legal blindness is defined as 20/200 or worse.
Outcomes were compared to raw data (6 months visual acuity) from the Optic Neuritis Treatment Trial (ONTT) using a subset of the ONTT and our cohort with similar age, severity, and timing of PLEX. A focus was done on severe optic neuritis with minimal improvement to IVMP, which was defined as 20/200 or worse with less than 3 line improvement or 2 lines if count fingers or worse. To best match the ONTT, which only included eyes without a prior history of optic neuritis, the primary analyses of the PLEX cohort was performed on eyes without a prior history of optic neuritis. A secondary analysis of eyes with a prior history of optic neuritis were compared to those without a prior history of optic neuritis to evaluate the impact of prior optic neuritis on outcomes.
Statistics
Given the study included attacks in both eyes from some individuals, all comparisons were completed by adjusting for the potential correlations between attacks from the same person using Generalized Estimating Equation (GEE) models. This included models with continuous factors, or categorical factors as the outcome of interest. A different type of GEE model was fit depending on the outcome of interest. When the outcome was continuous, the GEE model was fit with a normal distribution and an identity link function and a Pearson correlation coefficient to illustrate the magnitude of the relationship. However, the test for significance was based on the GEE model. For the binary outcomes in the VA groups, a binomial distribution with a logit link function was used. These models were fit both univariately and multivariately with each of the VA cutoffs as outcomes in the models. All analysis was completed using SAS version 9.4 (Cary, NC).
Data availability statement
Anonymized data used for this study are available upon reasonable request from the corresponding author.
RESULTS
A total of 475 optic neuritis attacks received PLEX among 376 patients who met inclusion criteria (Figure 1). The primary analyses were conducted in 317 patient (395 eyes) without a prior optic neuritis attack. Eleven of these were included an earlier report on PLEX for optic neuritis.9 Among the 317 patients with PLEX for optic neuritis in eyes without a prior history of optic neuritis, the median age at initiation of PLEX was 37 years (range, 8.6–75.2) and 71% were female. Ethnicities included: White (69%), Black (14%), Asian (6.5%), Hispanic (6%), other ethnicities (5%).
Figure 1: Flow chart of optic neuritis attacks treated with plasma exchange.
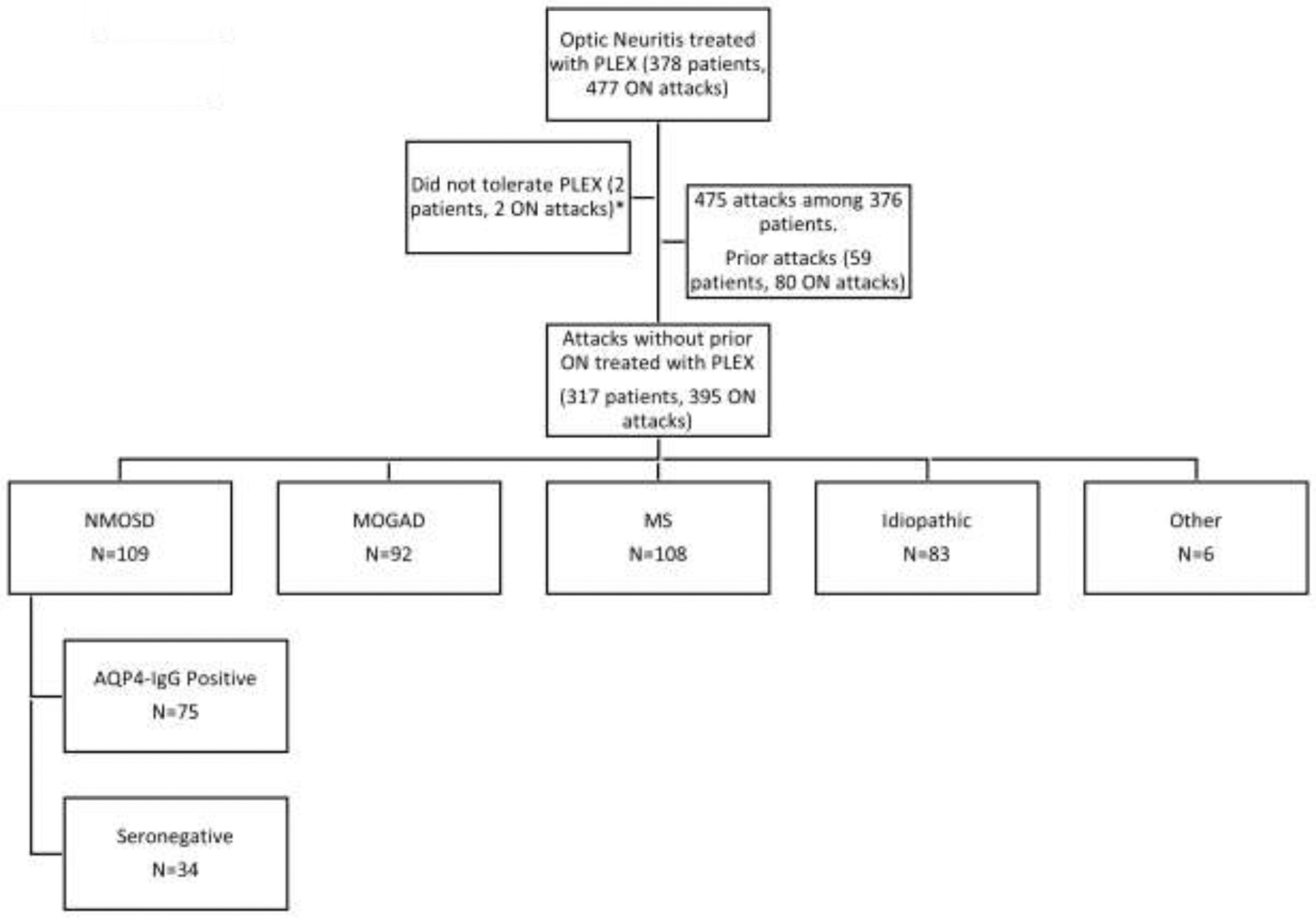
Abbreviations: plasma exchange (PLEX), optic neuritis (ON), neuromyelitis optica spectrum disorder (NMOSD), AQP4 (aquaporin-4), multiple sclerosis (MS). *Among the 2 patients that were excluded because of PLEX intolerance, one had numbness and vomiting and the other had mild dizziness and paresthesias who elected to not continue with treatment.
Prior to or in conjunction with PLEX, 390 (99%) were treated with IVMP and 4 (1%) were treated with high dose oral prednisone. Only 1 attack was not treated previously or in conjunction with corticosteroids. Despite near universal treatment with corticosteroids, the median logMAR VA at the time of PLEX was 1.7 (Snellen equivalent of count fingers, IQR, 20/200-hand motion) (Figure 2). The median time to PLEX from onset of optic neuritis was 2.6 weeks (IQR, 1.4–4) and the median number of PLEX treatments was 5 (IQR, 5–7). Only 1 patient (bilateral optic neuritis attack) was treated with concomitant intravenous immune globulin (IVIG) at the time of PLEX. This patient’s acuity improved from no light perception OU to count fingers and 20/400 after both PLEX and IVIG.
Figure 2: Visual acuity at time of plasma exchange and final visual outcome.
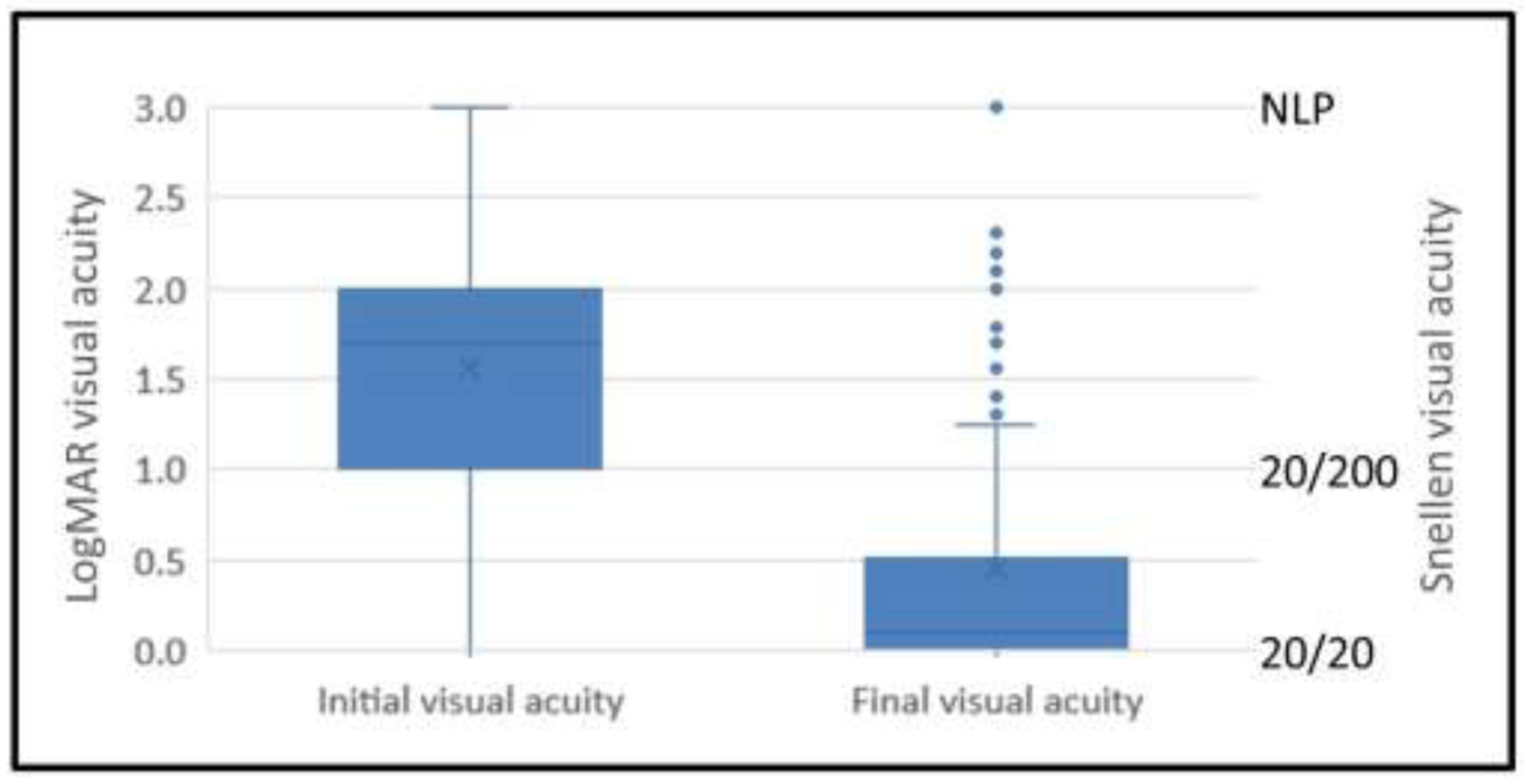
Box and whisker plots showing the median visual acuity at the time of plasma exchange and the final visual acuity after plasma exchange. The box represents the 25–75% interquartile intervals and the whiskers cover the minimum and maximum range.
Overall, the median final logMAR VA after PLEX was 0.1 (Snellen equivalent of 20/25, IQR, 20/20–20/60), which was significantly improved compared to the median logMAR VA of 1.7 (count fingers) at the time of PLEX (p<0.001). One hundred sixty-nine eyes (43%) improved to 20/20 after PLEX (Figure 2). However, the final outcome was 20/200 or worse in 81 (20.5%) optic neuritis attacks and worse than 20/40 in 123 (31%).
Overall, PLEX treatments were well tolerated. Among the 395 PLEX treatments, documented side effects included 3 (1%) infections (all secondary to central line placement with 1 of these patients developing a secondary pulmonary embolism), 2 (0.5%) electrolyte abnormalities, 1 (0.3%) deep vein thrombosis, and 1 (0.3%) nausea and vomiting.
MOGAD optic neuritis was associated with better outcomes
The median final VA among the various etiologies was not significantly different except that MOGAD optic neuritis was associated with better outcomes (Table 1). MOGAD optic neuritis had a final median logMAR VA of 0 (Snellen equivalent of 20/20, IQR 20/20–20/25) compared to a median of 0.176 (Snellen equivalent of 20/30, IQR 20/20–20/400) for AQP4+NMOSD and seronegative NMOSD, and a median of 0.10 (Snellen equivalent of 20/25, IQR 20/20–20/150) for MS and idiopathic optic neuritis (p<0.001 for all comparisons). Only a single MOGAD optic neuritis attack had an outcome of 20/200 or worse and this eye was treated 6 months after onset of vision loss and remained light perception despite treatment. In contrast, 23 (31%) patients with AQP4+NMOSD, 12 (35%) patients with seronegative NMOSD, 26 (24%) patients with MS, and 19 (23%) patients with idiopathic optic neuritis had a final outcome of 20/200 or worse (p<0.001).
Table 1:
Breakdown of optic neuritis outcomes after plasma exchange based on etiology
| Etiology | Median Age (range) | Female % | Median weeks to PLEX | Median VA at time of PLEX | Median Final VA | % 20/200 or worse |
|---|---|---|---|---|---|---|
| AQP4+NMOSD (n=75) | 45.5 (16–75) | 81% | 3 (IQR, 2–4.4) | 1.7 (CF) (IQR, 1–2) | 0.18 (20/30) (IQR, 0–1.3) | 30.6% |
| MOGAD (n=92) | 36 (11–72) | 60% | 2.3 (IQR, 1–3.6) | 1.7 (CF) (IQR, 0.9–2) | 0.00 (20/20) (IQR, 0–0.1) | 1% |
| Multiple sclerosis (n=108) | 33.5 (16–65.2) | 72% | 2.9 (IQR, 1.8–4) | 1.7 (CF) (IQR, 1–2) | 0.10 (20/25) (IQR, 0–0.8) | 24% |
| Idiopathic (n=83) | 39 (17–66) | 61% | 2 (IQR, 1.5–4.5) | 1.7 (CF) (IQR, 1–2) | 0.10 (20/25) (IQR, 0–0.8) | 23% |
Abbreviations: plasma exchange (PLEX), visual acuity (VA), aquaporin-4 IgG positive neuromyelitis optica spectrum disorder (AQP4+NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), interquartile (IQR)
There was no significant difference in outcomes between AQP4+NMOSD and MS and/or idiopathic optic neuritis for either the final median VA (p=0.30) or percentage of patients having an outcome of 20/200 or worse (p=0.23) or 20/40 or worse (p=0.85). There was also no significant difference between all cases of NMOSD (AQP4+ and seronegative combined) and MS and/or idiopathic optic neuritis for either the final median VA (p=0.28) or percentage of patients having an outcome of 20/200 or worse (p=0.11). These findings remained unchanged after accounting for age, sex, VA at nadir, and time to treatment with multivariate analysis despite some of these variables having an impact on outcomes.
Early PLEX treatment is associated with better outcomes
Time to PLEX treatment was correlated with outcomes with a Pearson correlation of 0.27 (p<0.001). The probability of complete recovery (20/20) and good recovery (20/40 or better) decreased with delay in PLEX treatment (p<0.05) (Figure 3). This remained significantly different with multivariate analysis that included age, sex, VA at nadir, and etiology (p<0.05).
Figure 3: Visual recovery is dependent on time to initiation of plasma exchange.
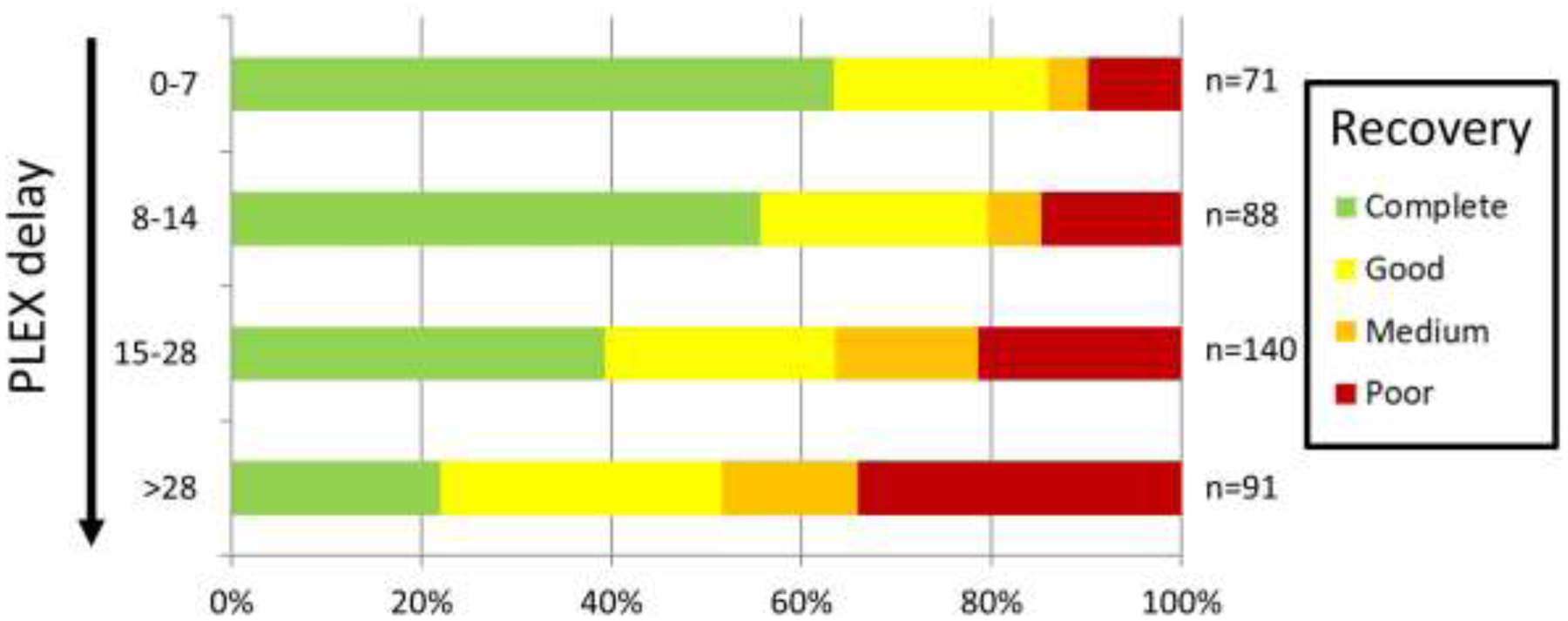
The probability of having complete recovery to 20/20 VA (green), good recovery (20/40 or better, yellow), medium recovery (between 20/40 and 20/200, orange), and poor recovery (20/200 or worse, red) is stratified based on time from optic neuritis onset to the initiation of plasma exchange (among the 395 optic neuritis attacks treated with plasma exchange without a prior optic neuritis attack).
Predictors of poor outcome
In 81 (20.5%) optic neuritis attacks, the final VA was 20/200 or worse. Patients with poor outcomes of 20/200 or worse were older (p=0.002), had worse VA at time of PLEX (p<0.001), and longer delay to PLEX treatment (p<0.001) (Table 2). These remained significant with multivariate analysis that included age, gender, VA at nadir, time to treatment, and etiology.
Table 2:
Factors associated with poor outcomes after plasma exchange
| Better than 20/200 (n=316) | 20/200 or worse (n=81) | P-value | |
|---|---|---|---|
| Female | 72% | 63% | 0.12 |
| White | 68% | 69% | 0.84 |
| Median Age (years) | 35.0 (IQR 27–51) | 46.1 (IQR 31–46.1) | 0.002 |
| Median visual acuity at time of plasma exchange | Count fingers (IQR, 20/400-HM) | LP (IQR, HM-LP) | <0.001 |
| Median weeks to plasma exchange | 2.4 (IQR, 1.4–3.8) | 3.3 (IQR, 2.3–3.5) | <0.001 |
Abbreviations: hand motion (HM), light perception (LP), interquartile (IQR)
Subgroup comparisons to the Optic Neuritis Treatment Trial
Within the ONTT, 156 patients (35% of the cohort) had a VA of 20/200 or worse at enrollment with 132 (85%) improving to 20/40 or better and only 10 (6%) having a final outcome of 20/200 or worse at 6 months (Table 3). In comparison, within our PLEX cohort of patients between ages 18–46 years without prior optic neuritis (same age and inclusion criteria as the ONTT), there were 30 attacks with a VA of 20/200 or worse treated with PLEX within 1 week, with 28 (93%) improving to 20/40 or better (p=0.21) (Figure 4) and only 1 (3%) having a final outcome of 20/200 or worse (p=0.51).
Table 3:
Subset of the Optic Neuritis Treatment Trial that was compared to patients receiving plasma exchange
| Subset of the ONTT | Median Age (range) | Female % | Median Final VA | % 20/40 or better | % 20/200 or worse |
|---|---|---|---|---|---|
| 20/200 or worse at enrollment (n=156) | 31.1 (26–37) | 77% | 0 (20/20) (IQR, 0–0.1) | 85% | 6% |
| CF or worse at enrollment (n=70) | 31.1 (26–36) | 73% | 0.00 (20/20) (IQR, 0–0.2) | 79% | 11% |
| VA of 20/200 or worse with minimal improvement after 3 days of IVMP (n=18) | 30.4 (26–30) | 72% | 0.10 (20/25) (IQR, 0–0.1) | 67% | 17% |
| VA of 20/200 or worse with minimal improvement at 2 weeks after IVMP (n=11) | 30.0 (26–38) | 64% | 0.44 (20/55) (IQR, 0.1–1.0) | 36% | 27% |
| VA of 20/200 or worse with at 4 weeks after IVMP (n=3) | 30.0 (28–36) | 100% | 1.7 (CF) (IQR, 1.6–1.8) | 0% | 100% |
Abbreviations: Optic Neuritis Treatment Trial (ONTT), visual acuity (VA), intravenous methylprednisolone (IVMP), interquartile (IQR)
Figure 4: Comparison of Optic Neuritis Treatment Trial vs Plasma exchange.
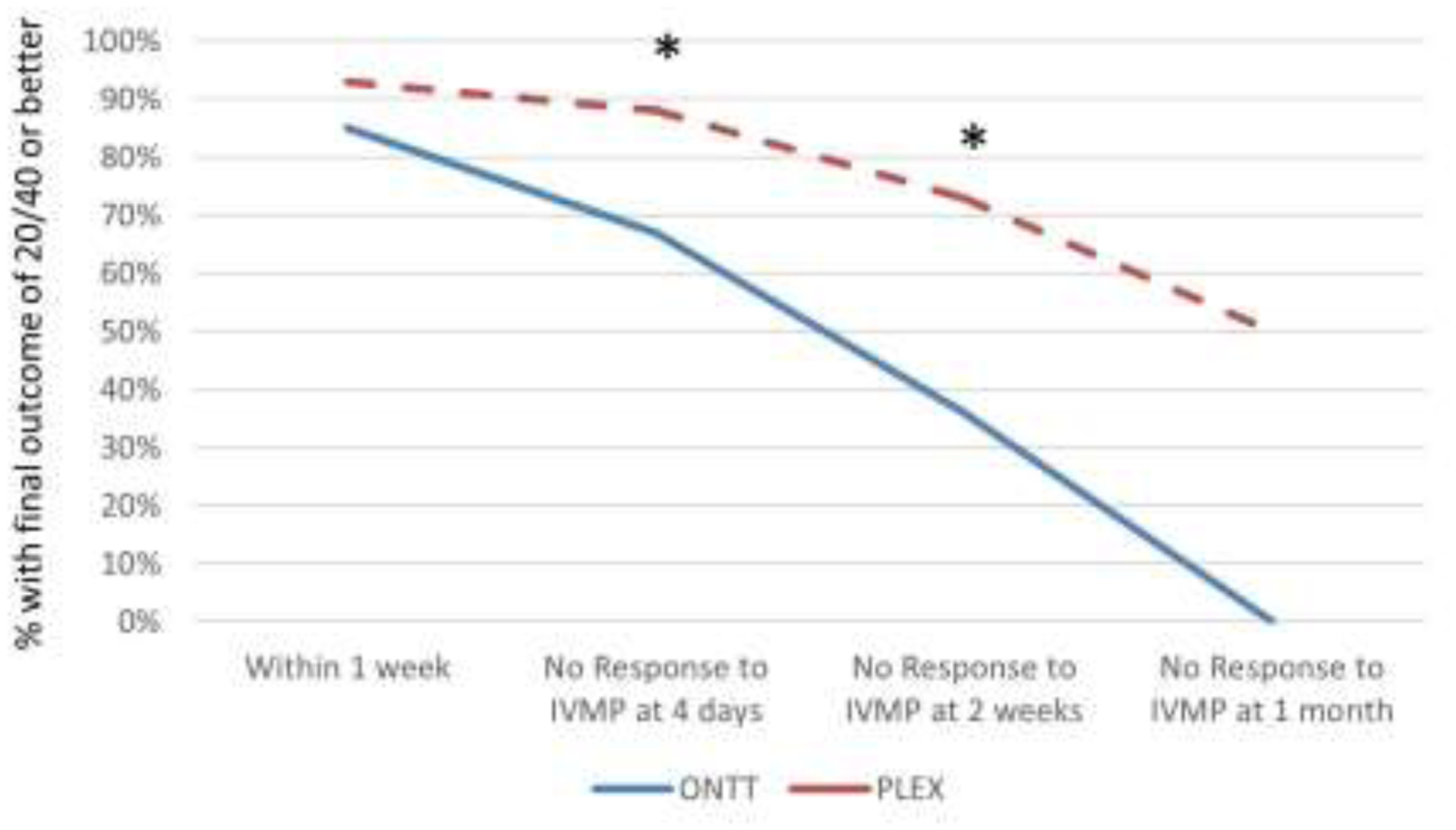
The chance of final outcome of 20/40 or better is compared across different matched time points between the Optic Neuritis Treatment Trial (ONTT) (blue solid line) and the Plasma exchange (PLEX) (red dashed line) cohorts among eyes that were 20/200 or worse with minimal response to intravenous methylprednisolone (IVMP). * indicates a statistically significant difference.
When comparing 70 ONTT patients with very severe vision loss (count fingers or worse) at enrollment to 21 patients in our PLEX cohort with the same age and severity of vision loss, there was a non-significant trend toward better outcomes in the PLEX cohort. In the ONTT, 21% had a final outcome of worse than 20/40 compared to 9.5% in the PLEX cohort (p=0.22), and 11% were 20/200 or worse compared to 5% in the PLEX cohort (p=0.37).
In comparing a matched PLEX subset (age-matched, PLEX treatment within 4–11 days, and VA of 20/200 or worse with minimal improvement prior to PLEX) to patients within the ONTT with corticosteroid-resistant optic neuritis (VA of 20/200 or worse with minimal improvement after 3 days of IVMP), a final VA of worse than 20/40 occurred in 6/50 (12%) PLEX treated optic neuritis versus 6/18 (33%) from the ONTT treated with IVMP without PLEX (p=0.04) (Figure 4). There was no significant difference in the percentage of patients who had an outcome of 20/200 or worse (3/18 (17%) in the ONTT vs 5/50 (10%) in the PLEX cohort (p=0.43).
Similar results were found when comparing an age-matched PLEX subset treated between 2–3 weeks with patients in the ONTT who remained 20/200 at 2 weeks with minimal improvement despite treatment with IVMP. In the PLEX group, 18/63 (28%) had an outcome of worse than 20/40 compared to 7/11 (64%) within the ONTT (p=0.037), and 11/63 (17%) had an outcome of 20/200 or worse compared to 3/11 (27%) in the ONTT (p=0.43).
In the ONTT, only 3 patients treated with IVMP remained 20/200 or worse at 1 month, all of whom had a final outcome of 20/200 or worse. In comparison to the PLEX cohort, among 18 age-matched patients who were 20/200 or worse and treated with PLEX between 4–5 weeks, 6 (33%) had a final outcome of 20/200 or worse (p=0.24), and 9 (50%) improved to 20/40 or better (p=0.11).
There was no difference in these analyses when patients with both AQP4+ and seronegative NMOSD were excluded from the PLEX cohort.
Secondary analysis on eyes with prior optic neuritis treated with plasma exchange
There were 80 optic neuritis attacks treated with PLEX in eyes that had a prior optic neuritis, which were not included in the analyses above. Among 76 eyes with a known VA after the prior attack, 39 (51%) improved back to the prior baseline after PLEX. An additional 15 (20%) improved to within 3 lines of the prior baseline, while 22 (29%) attacks did not improve within 3 lines of the prior baseline. Overall, the median final logMAR VA after PLEX was 0.3 (Snellen equivalent of 20/40) among eyes that had a prior optic neuritis attack, which was significantly worse than the outcomes of eyes that did not have prior optic neuritis (median logMAR 0.1, Snellen equivalent 20/25) (p=0.002). However, among the 30 eyes that had recovered to 20/25 or better at the time of the optic neuritis relapse that was treated with PLEX, there was no difference in final outcomes compared to eyes that did not have a prior optic neuritis (both median logMAR VA of 0.1) (p=0.90).
DISCUSSION
This large multicenter retrospective cohort of optic neuritis patients suggests that acute treatment with PLEX may lead to recovery of vision with better outcomes than attacks with similar severity in the ONTT. Severity of vision loss at nadir, older age, and longer delay to PLEX were associated with a worse outcome, while MOGAD had a favorable prognosis compared to other etiologies.
It is not surprising that the severity of vision loss at nadir influenced outcomes. This was shown in the ONTT, which found that corticosteroid treatment did not change outcomes, but poor vision at nadir was correlated with a worse outcome.3 It makes logical sense that worse vision at onset may not recover as well despite having a potentially effective treatment, such as PLEX. These findings make it important to control for severity of vision loss in any randomized clinical trial. This is also important to consider when comparing the outcomes of the ONTT to the PLEX cohort since PLEX is typically reserved for severe optic neuritis and therefore our PLEX cohort had more severe vision loss at nadir than the overall ONTT cohort.
Our study found that the visual outcomes after PLEX were worse in older patients, independent of etiology and timing to treatment. The median age of those with a poor outcome were over a decade older than those with better outcomes. While the ONTT did not find that age influenced outcomes, the trial was restricted to young adults between the ages of 18 and 46 years.3 Although not universal,25,26 the majority of retrospective studies on PLEX that included a broad range of ages, as in our cohort, also found that older age was associated with worse outcomes.9,11,16,27,28 This may be due to the reduced ability to repair and remyelinate at an older age.29
Among the etiologies of optic neuritis, MOGAD optic neuritis had a favorable prognosis despite having a similar severity of vision loss at nadir, age, and time to PLEX treatment. Retrospective studies looking at the outcomes of MOGAD optic neuritis, irrespective of PLEX treatment, have shown that MOGAD optic neuritis is associated with severe vision loss at nadir and better recovery than other etiologies of optic neuritis.30–39 The majority of MOGAD optic neuritis attacks improve with corticosteroids and some attacks improve spontaneously without treatment.40,41 However, not all MOGAD optic neuritis attacks recover with most series reporting that 5–15% of MOGAD optic neuritis have an outcome of 20/200 or worse.30–36 In contrast, among the 92 MOGAD optic neuritis attacks treated with PLEX in our cohort, only 1 (1%) had an outcome of 20/200 or worse and this eye was treated 6 months after onset of vision loss and remained light perception despite PLEX treatment. The only reason this eye received PLEX was because the contralateral eye had recurrent optic neuritis to the level of count fingers, which did improve in response to PLEX to its baseline of 20/25. Therefore, despite the natural history of MOGAD optic neuritis being favorable, PLEX may have an additional beneficial impact because all attacks treated within 1 month significantly improved despite having a median nadir of count fingers at the time of PLEX treatment.
Interestingly, AQP4+NMOSD and seronegative NMOSD optic neuritis did not have significantly different outcomes from MS and idiopathic optic neuritis in our PLEX cohort, even after controlling for age, gender, severity of vision loss at nadir, and timing to PLEX treatment; this is consistent with a retrospective study by Deschamps et al.9 Multiple studies have shown that NMOSD optic neuritis is associated with worse outcomes than other etiologies, with over a third of attacks leading to a visual acuity of 20/200 or worse without PLEX treatment.42–48 In contrast, most studies, including the ONTT, suggest that only 3% of MS-related optic neuritis has a final outcome of 20/200 or worse.3 The similar outcomes among NMOSD optic neuritis and MS/idiopathic optic neuritis treated with PLEX in our cohort suggest that PLEX may be effective for NMOSD, or alternatively, that PLEX may be less effective for MS-associated and idiopathic steroid-refractory optic neuritis. Many retrospective studies have suggested that PLEX treatment of acute attacks from NMOSD leads to better outcomes.13,16,19,27,28,49–51 Interestingly, a recent study by Fu et al. evaluating the outcome of 93 AQP4+NMOSD, 6 MOGAD, and 18 seronegative optic neuritis attacks found that PLEX led to an improvement in AQP4+NMOSD, mixed results for MOGAD, but no significant improvement in the seronegative optic neuritis cohort.52 However, the suboptimal outcomes in our MS and seronegative idiopathic optic neuritis PLEX cohort are at least partly related to a biased selection of MS/idiopathic optic neuritis with severe vision loss that required PLEX treatment. We had an equal number of MS and NMOSD optic neuritis attacks despite MS being 25 to 50-fold more common than NMOSD in the western world,53–55 which highlights that PLEX is being used for a small subset of severe MS/idiopathic optic neuritis. In our cohort, the median time to PLEX treatment was 2.6 weeks with a median visual acuity of count fingers for all etiologies, including MS and idiopathic, despite the vast majority receiving IVMP prior to PLEX. In the ONTT, only 4 patients treated with IVMP remained count fingers at 2 weeks; all had suboptimal outcomes with 1 improving to 20/80 while the other 3 remained worse than 20/200.3 Therefore, the seemingly poor outcomes of PLEX for MS and idiopathic optic neuritis compared to the expected outcomes of these disease etiologies are greatly influenced by the selection of severe optic neuritis attacks that are refractory to corticosteroids. This highlights the importance of future randomized clinical trials stratifying the results by etiology, severity of vision loss, and timing to PLEX treatment.
In our PLEX cohort, delayed PLEX treatment was associated with worse outcomes, which was independent of age, severity of vision loss, and etiology. Multiple retrospective studies have shown that earlier PLEX treatment for optic neuritis is associated with better outcomes.11,16,19,52,56 This observation was highlighted in a study by Bonnan et al, which found that time to PLEX treatment was the strongest predictor of outcomes in a NMOSD cohort.16 However, as a non-randomized trial, it is possible that patients late to PLEX were destined to do more poorly. For example, in the ONTT, among the 156 patients who had a VA of 20/200 or worse at enrollment, 85% improved to 20/40 or better, while only 3 patients remained 20/200 or worse at 1 month despite IVMP, all of whom had a final outcome of 20/200 or worse. Therefore, the improved outcomes with early PLEX and worse outcomes with delayed PLEX may at least partially reflect the natural history of the disease. However, when an age- and time to treatment-matched subset of our PLEX cohort was compared to the ONTT cohort, the PLEX cohort had better outcomes suggesting that PLEX may be superior to IVMP alone. The exact mechanism of PLEX is unclear, but it is thought to provide benefit by rapid removal of plasma antibodies, immune complexes, and cytokines that are involved in demyelinating disease and inflammation.57 Prospective randomized clinical trials will be required to confirm the efficacy of PLEX in ON.
As expected and demonstrated in other studies, eyes with prior optic neuritis had worse overall outcomes after PLEX compared to eyes without prior optic neuritis.9,52 This may be because of two potential reasons. Firstly, damage from a prior optic neuritis attack may leave the optic nerve with less reserve to recover after a subsequent attack. Secondly, and perhaps most importantly, prior optic neuritis with residual visual loss cannot improve beyond the impaired baseline following a recurrent optic neuritis attack. Interestingly, within our PLEX cohort, eyes that had recovered to 20/25 or better who had recurrent optic neuritis treated with PLEX had identical outcomes as those without a prior optic neuritis attack. This finding can be used to help counsel patients with recurrent optic neuritis on the potential for recovery and suggests that a future randomized clinical trial on PLEX for optic neuritis can include eyes with prior optic neuritis as long as the patient had recovered from the prior optic neuritis attack.
When discussing treatment options, the extra costs of PLEX must also be included in the decision making. The standard PLEX treatment of 5 sessions is estimated to cost $4,638, which is less than half the cost of IVIG, but still almost 5-times as much as IVMP.58,59 However, if PLEX has a two-fold chance of preventing blindness in patients with optic neuritis that are unresponsive to IVMP as our study suggests, the benefit of PLEX would outweigh the added costs because studies have estimated that the annual cost of visual impairment is $15.900 per person, and $26,900 for blindness.60 Preventing visual impairment and blindness is especially important for optic neuritis because it most commonly affects young adults where the economic effects of vision loss can be present for decades.
The strength of this study was the large number of patients with optic neuritis in eyes without prior optic neuritis and the stratification of outcomes by etiology. Limitations of this study include its retrospective nature with the decision to treat with PLEX dependent on the individual physician. Concomitant acute treatments may also influence the potential efficacy of PLEX. However, all but one patient received high dose corticosteroids and only a single patient was treated with simultaneous IVIG. Because this was not a randomized clinical trial, the effect of corticosteroids and the natural history of the disease could not be directly compared to the outcomes of PLEX treatment, although comparing to an age-matched subset of the ONTT did provide a comparison arm. Lastly, standardized ETDRS VA, low contrast VA, visual fields, and optical coherence tomography were not consistently available in these patients, so further stratified outcomes beyond standard high contrast VA were not possible.
Proposing a prospective randomized clinical trial of plasma exchange for optic neuritis
Current views of PLEX for optic neuritis are at a very similar juncture to the 1980s when the ONTT was first proposed to evaluate the efficacy of corticosteroids for the treatment of optic neuritis.61 At that time, there was a fairly strong belief that corticosteroids led to better outcomes for optic neuritis. The ONTT somewhat surprisingly found that IVMP led to faster recovery, but did not change the ultimate outcome.3 Many experts now believe that PLEX treatment of severe optic neuritis leads to better outcomes and the results of the study reported herein support this belief; however, this must be balanced against the natural history. There are many experts who recognize that the majority of optic neuritis will spontaneously recover without treatment and therefore would not recommend PLEX, especially for non-NMOSD related optic neuritis.7,62 Because of the equipoise of different expert opinion, there are compelling reasons to conduct a prospective randomized clinical trial for PLEX in optic neuritis.
As can be seen in this study, the timing of the PLEX randomization and intervention is important. The ONTT found that the vast majority of severe optic neuritis attacks (20/200 or worse) will recover with 85% improving to 20/40 or better. When this was compared to our age-matched PLEX cohort treated within 1 week, which is equivalent to the time of enrollment into the ONTT, 93% improved to 20/40. Based on this difference, we would need to randomize 236 patients per group to have the statistical power (alpha = 0.05, power = 80%) to differentiate these treatments, which would be a difficult number of patients to enroll and would likely lead to the overtreatment of many patients given the fairly good natural history of the disease. While PLEX was well-tolerated in our cohort of patients, it still often requires central line placement, has the potential to cause serious side effects, and is more costly than IVMP.63
Severe corticosteroid-resistant optic neuritis may be the best cohort to evaluate in a randomized PLEX clinical trial. When we compared the subset of patients in the ONTT with severe optic neuritis and minimal response to IVMP to a matched PLEX subset, only 67% improved to 20/40 or better in the ONTT compared to 88% in the PLEX cohort. A sample size of 58 patients per group would be required to detect a difference with 80% power for a two-sided test with alpha = 0.05. Therefore, a future multicenter randomized clinical trial could aim to randomize two groups, IVMP + PLEX vs IVMP + placebo, among patients with severe acute optic neuritis unresponsive to 3 days of IVMP. Recruiting 75 patients per group would account for a 20% dropout rate and be able to evaluate the efficacy of PLEX in this patient population of corticosteroid-resistant optic neuritis. We believe this is the most appealing cohort to investigate because these patients raise the most debate in our current clinical practice. Most providers do not immediately start with PLEX and most would offer PLEX in severe optic neuritis that remains poor after 2–4 weeks, but there is less consensus in patients within the first 1–2 weeks. The investigation of the efficacy of PLEX for severe optic neuritis unresponsive to 3 days of IVMP would have a large impact in influencing our practice patterns.
There has been a trend toward earlier PLEX for severe optic neuritis since retrospective publications such as Bonnan et al, have suggested that early PLEX treatment within several days leads to better outcomes.16 Therefore, another potential option for a PLEX optic neuritis treatment trial would be randomization of very severe vision loss (count fingers or worse) within 1 week of onset of symptoms. A sample size of 150 per group would be required to detect a difference with 80% power (two-sided alpha=0.05).
One of the largest remaining debates in maintaining equipoise is whether AQP4+NMOSD can be randomized to sham because the natural history of AQP4+NMOSD optic neuritis is poor. Some investigators may object to including AQP4+NMOSD optic neuritis in a randomized clinical trial; however, our data suggests no difference in outcomes between AQP4+NMOSD and the severe subset of MS/idiopathic optic neuritis that were treated with PLEX suggesting that we would have equipoise to include AQP4+NMOSD. Of note, there have been several successful randomized placebo controlled trials of immunotherapies for relapse prevention on NMOSD, which ledto new FDA-approved medications, such as inebilizumab, eculizumab, and satralizumab.64–66 While there was much debate on the ethical nature of placebo controlled trials in NMOSD, ultimately it was felt these trials in NMOSD were needed and met equipoise.67 While 100% consensus would unlikely be reached, a majority consensus among providers would guide the inclusion/exclusion of known AQP4+NMOSD patients in a future randomized clinical trial of PLEX for optic neuritis. Even if known AQP4+NMOSD patients were ultimately excluded, requiring AQP4-IgG testing results before entry into a randomized trial for PLEX is unlikely warranted. In the ONTT, despite 1/3 of patients having a VA of 20/200 or worse at enrollment, there were no patients who were AQP4-IgG positive among the 177 samples that were still available to be tested,68 and therefore only a small percentage of patients enrolled who would ultimately be diagnosed with AQP4+NMOSD. In addition, it is not feasible to obtain AQP4-IgG for our proposed timing of randomization because it usually takes 1–2 weeks for AQP4-IgG testing results.
Randomization with more PLEX treatment than placebo at a 3:1 or 2:1 ratio may improve both physician and patient recruitment, although this would increase the number of participants needed to obtain the same statistical power. Including a crossover arm for non-responders is another consideration to maintain equipoise. Our study and several other retrospective studies suggest that PLEX treatment at 3–4 weeks still leads to improved outcomes compared to the natural history. Therefore patients not responding to 7 sessions of PLEX/placebo could potentially have an option of cross-over treatment, as was done with the prior randomized clinical trial demonstrating the efficacy of PLEX in the treatment of corticosteroid-resistant demyelinating attacks that excluded optic neuritis.8 However, delayed PLEX treatment could potentially remove a maintenance monoclonal antibody treatment, such as rituximab or ocrelizumab, and impair its effectiveness so this would have to be weighed in the decision making.
CONCLUSION
This study suggests that PLEX may be associated with improved visual outcomes in acute optic neuritis. The presence of severe vision loss at nadir, older age, and longer delay to PLEX in our cohort predicted a worse outcome while MOGAD optic neuritis had a more favorable prognosis than other subgroups. These findings warrant further exploration and provide the backbone for a future prospective randomized clinical trial of PLEX treatment for optic neuritis.
Supplementary Material
ACKNOWLEDGMENTS/DISCLOSURES
Funding/Support:
This work was supported by the Department Laboratory Medicine and Pathology and the Center for MS and Autoimmune Neurology, Mayo Clinic, Rochester, MN, the Leonard and Mary Lou Hoeft Career Development Award in Ophthalmology Research (J. Chen), and an RO1 from the National Institute of Neurological Disorders and Stroke (R01NS113828 to E. Flanagan). H. E. Moss was supported by grants from the NIH (P30 EY 026877) and Research to Prevent Blindness (unrestricted grant to Stanford University Department of Ophthalmology). E.S Sotirchos was supported by the NIH/NINDS (K23NS117883) and the Caring Friends NMO Research Fund.
Financial Disclosures
John J. Chen: consultant for UCB, Roche, and Horizon
Eoin P. Flanagan has served on advisory boards for Alexion, Genentech and Horizon Therapeutics. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. Dr Flanagan was a site primary investigator in a randomized clinical trial on Inebilizumab in neuromyelitis optica spectrum disorder run by Medimmune/Viela-Bio/Horizon Therapeutics. Dr Flanagan has received funding from the NIH (R01NS113828). Dr Flanagan is a member of the medical advisory board of the MOG project. Dr Flanagan is an editorial board member of the Journal of the Neurological Sciences and Neuroimmunology Reports.
Sean J. Pittock has received personal compensation for serving as a consultant for Roche/Genentech, Sage Therapeutics, and Astellas. He’s received personal compensation for serving on scientific advisory boards or data safety monitoring boards for F. Hoffman-LaRoche AG, Genentech, and UCB. His institution has received compensation for serving as a consultant for Astellas, Alexion, and Viela Bio/MedImmune. All compensation is paid to Mayo Clinic. He has received research support from Alexion, Viela Bio/MedImmune, Roche/Genentech. He has a patent, Patent# 8,889,102 (Application#12-678350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; a patent, Patent# 9,891,219B2 (Application#12-573942, Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive)-issued.
Nicole Caroline Stern: none
Nanthaya Tisavipat: none
M. Tariq Bhatti: none
Kevin Chodnicki: none
Deena Tajfirouz: none
Sepideh Jamali: none
Amy Kunchok: none
Eric R. Eggenberger: none
Marie Di Nome: none
Elias Sotirchos: Advisory boards for Alexion, Viela Bio and Genentech. Speaker fees from Viela Bio and Biogen.
Eleni Vasileiou:
Amanda Henderson has served on advisory boards for Horizon Therapeutics and receives compensation from Springer Nature for authorship and editorial services.
Anthony C. Arnold: none
Laura Bonelli: none
Heather E. Moss has received personal compensation from Medlink Corp for editorial services and is a consultant for Twenty Twenty Therapeutics and Verana Health
Sylvia Villarreal Navarro: none
Tanyatuth Padungkiatsagul: none
Hadas Stiebel-Kalish: none
Itay Lotan: none
Adi Wilf-Yarkoni:
Helen Danesh-Meyer:
Stefan Ivanov: none
Saif Huda: none
Mirasol Forcadela: none
Dave Hodge: none
Pascale Poullin
Julie Rode
Caroline Papeix
Samir Saheb: none
Marine Boudot de la Motte
Catherine Vignal
Yael Hacohen: none
Julie Pique
Elisabeth Maillart
Romain Deschamps: none
Bertrand Audoin: none
Romain Marignier serves on scientific advisory board from Alexion, Viela Bio/Horizon Therapeutics, Roche and UCB and received personal fees and travel honoraria from Alexion, Biogen, Horizon Therapeutics, Novartis, Roche
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014;13:83–99. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JL, Costello F, Chen JJ, et al. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol 2022. [DOI] [PubMed] [Google Scholar]
- 3.Beck RW, Cleary PA, Anderson MM Jr., et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992;326:581–8. [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Pittock SJ, Flanagan EP, Lennon VA, Bhatti MT. Optic neuritis in the era of biomarkers. Surv Ophthalmol 2020;65:12–7. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–12. [DOI] [PubMed] [Google Scholar]
- 6.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017;140:3128–38. [DOI] [PubMed] [Google Scholar]
- 7.Horton L, Bennett JL. Acute Management of Optic Neuritis: An Evolving Paradigm. J Neuroophthalmol 2018;38:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 1999;46:878–86. [DOI] [PubMed] [Google Scholar]
- 9.Deschamps R, Gueguen A, Parquet N, et al. Plasma exchange response in 34 patients with severe optic neuritis. J Neurol 2016;263:883–7. [DOI] [PubMed] [Google Scholar]
- 10.Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology 2004;63:1081–3. [DOI] [PubMed] [Google Scholar]
- 11.Tan S, Ng TK, Xu Q, et al. Vision improvement in severe acute isolated optic neuritis after plasma exchange treatment in Chinese population: a prospective case series study. Ther Adv Neurol Disord 2020;13:1756286420947977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roesner S, Appel R, Gbadamosi J, Martin R, Heesen C. Treatment of steroid-unresponsive optic neuritis with plasma exchange. Acta Neurol Scand 2012;126:103–8. [DOI] [PubMed] [Google Scholar]
- 13.Merle H, Olindo S, Jeannin S, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol 2012;130:858–62. [DOI] [PubMed] [Google Scholar]
- 14.Song W, Qu Y, Huang X. Plasma exchange: an effective add-on treatment of optic neuritis in neuromyelitis optica spectrum disorders. Int Ophthalmol 2019;39:2477–83. [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Kurimoto T, Ueda K, Nakamura M. Short-term effect of additional apheresis on visual acuity changes in patients with steroid-resistant optic neuritis in neuromyelitis optica spectrum disorders. Jpn J Ophthalmol 2018;62:525–30. [DOI] [PubMed] [Google Scholar]
- 16.Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 2018;89:346–51. [DOI] [PubMed] [Google Scholar]
- 17.Skorupka N, Miclea A, Jalowiec KA, et al. Visual Outcomes of Plasma Exchange Treatment of Steroid-Refractory Optic Neuritis: A Retrospective Monocentric Analysis. Transfus Med Hemother 2019;46:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galetta K, Ryan S, Manzano G, et al. Treatment outcomes of first-ever episode of severe optic neuritis. Mult Scler Relat Disord 2022;66:104020. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Fan A, Wei L, et al. Efficacy and safety of plasma exchange or immunoadsorption for the treatment of option neuritis in demyelinating diseases: A systematic review and meta-analysis. Eur J Ophthalmol 2022;32:1857–71. [DOI] [PubMed] [Google Scholar]
- 20.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol 2018;75:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. [DOI] [PubMed] [Google Scholar]
- 23.Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol 2009;247:137–42. [DOI] [PubMed] [Google Scholar]
- 24.Danesh-Meyer H, Savino PJ, Gamble GG. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology 2005;112:1098–103. [DOI] [PubMed] [Google Scholar]
- 25.Llufriu S, Castillo J, Blanco Y, et al. Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurology 2009;73:949–53. [DOI] [PubMed] [Google Scholar]
- 26.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 2002;58:143–6. [DOI] [PubMed] [Google Scholar]
- 27.Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 2016;79:206–16. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez C, Vargas D, Contreras K, et al. Therapeutic plasma exchange for optic neuritis attacks in patients with neuromyelitis optica spectrum disorders. Ther Apher Dial 2022. [DOI] [PubMed] [Google Scholar]
- 29.Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport 2002;13:923–8. [DOI] [PubMed] [Google Scholar]
- 30.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol 2018;195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JJ, Bhatti MT. Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol 2020;33:47–54. [DOI] [PubMed] [Google Scholar]
- 32.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017;140:3128–38. [DOI] [PubMed] [Google Scholar]
- 33.Zhao G, Chen Q, Huang Y, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol 2018;265:33–40. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Tan S, Chan TCY, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol 2018. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Zhou H, Wang J, et al. The prevalence and prognostic value of myelin oligodendrocyte glycoprotein antibody in adult optic neuritis. J Neurol Sci 2019;396:225–31. [DOI] [PubMed] [Google Scholar]
- 36.Shor N, Aboab J, Maillart E, et al. Clinical, imaging and follow-up study of optic neuritis associated with myelin oligodendrocyte glycoprotein antibody: a multicentre study of 62 adult patients. Eur J Neurol 2020;27:384–91. [DOI] [PubMed] [Google Scholar]
- 37.Akaishi T, Himori N, Takeshita T, et al. Five-year visual outcomes after optic neuritis in anti-MOG antibody-associated disease. Mult Scler Relat Disord 2021;56:103222. [DOI] [PubMed] [Google Scholar]
- 38.Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG Related Optic Neuritis-Prevalence, Optical Coherence Tomography Findings, and Visual Outcomes: A Systematic Review and Meta-Analysis. Front Neurol 2020;11:540156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiebel-Kalish H, Lotan I, Brody J, et al. Retinal Nerve Fiber Layer May Be Better Preserved in MOG-IgG versus AQP4-IgG Optic Neuritis: A Cohort Study. PLoS One 2017;12:e0170847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JJ, Flanagan EP, Bhatti MT, et al. Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord 2022;68:104237. [DOI] [PubMed] [Google Scholar]
- 41.Vosoughi AR, Muccilli A, Schneider R, Rotstein D, Micieli JA. Recovery of Vision in Myelin Oligodendrocyte Glycoprotein-IgG Optic Neuritis Without Treatment: A Case Series. J Neuroophthalmol 2022. [DOI] [PubMed] [Google Scholar]
- 42.Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007;114:810–5. [DOI] [PubMed] [Google Scholar]
- 43.Papais-Alvarenga RM, Carellos SC, Alvarenga MP, Holander C, Bichara RP, Thuler LC. Clinical course of optic neuritis in patients with relapsing neuromyelitis optica. Arch Ophthalmol 2008;126:12–6. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes DB, Ramos Rde I, Falcochio C, Apostolos-Pereira S, Callegaro D, Monteiro ML. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol 2012;32:102–6. [DOI] [PubMed] [Google Scholar]
- 45.Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci 2010;298:158–62. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Zhao S, Yin D, et al. Optic neuritis: a 5-year follow-up study of Chinese patients based on aquaporin-4 antibody status and ages. J Neurol 2016;263:1382–9. [DOI] [PubMed] [Google Scholar]
- 47.Sotirchos ES, Filippatou A, Fitzgerald KC, et al. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler 2020;26:1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Wang Y, Xu Q, et al. Features of anti-aquaporin 4 antibody-seropositive Chinese patients with neuromyelitis optica spectrum optic neuritis. J Neurol 2015;262:2293–304. [DOI] [PubMed] [Google Scholar]
- 49.Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler 2016;22:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kosiyakul P, Songwisit S, Ungprasert P, Siritho S, Prayoonwiwat N, Jitprapaikulsan J. Effect of plasma exchange in neuromyelitis optica spectrum disorder: A systematic review and meta-analysis. Ann Clin Transl Neurol 2020;7:2094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm 2018;5:e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu J, Wang Y, Li H, et al. Efficacy of Plasma Exchange Treatment for Demyelinating Optic Neuritis Associated with Various Serum Antibodies: A Prospective Cohort Study. Neurol Ther 2022;11:797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hassan MB, Stern C, Flanagan EP, et al. Population-Based Incidence of Optic Neuritis in the Era of Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Antibodies. Am J Ophthalmol 2020;220:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology 2003;61:1373–7. [DOI] [PubMed] [Google Scholar]
- 56.Huang X, Wu J, Xiao Y, Zhang Y. Timing of plasma exchange for neuromyelitis optica spectrum disorders: A meta-analysis. Mult Scler Relat Disord 2021;48:102709. [DOI] [PubMed] [Google Scholar]
- 57.Lipphardt M, Wallbach M, Koziolek MJ. Plasma Exchange or Immunoadsorption in Demyelinating Diseases: A Meta-Analysis. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C Jr. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barre syndrome. BMC Health Serv Res 2011;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robson LS, Bain C, Beck S, Guthrie S, Coyte PC, O’Connor P. Cost analysis of methylprednisolone treatment of multiple sclerosis patients. Can J Neurol Sci 1998;25:222–9. [DOI] [PubMed] [Google Scholar]
- 60.Wittenborn JS, Zhang X, Feagan CW, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology 2013;120:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beck RW. The Optic Neuritis Treatment Trial. Arch Ophthalmol 1988;106:1051–3. [DOI] [PubMed] [Google Scholar]
- 62.Pula JH, Glisson CC. Should plasma exchange be offered to patients with multiple sclerosis-associated optic neuritis? J Neuroophthalmol 2015;35:86–9. [DOI] [PubMed] [Google Scholar]
- 63.Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J Clin Apher 2007;22:270–6. [DOI] [PubMed] [Google Scholar]
- 64.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019;394:1352–63. [DOI] [PubMed] [Google Scholar]
- 65.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N Engl J Med 2019;381:614–25. [DOI] [PubMed] [Google Scholar]
- 66.Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol 2020;19:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cree BA. Placebo controlled trials in neuromyelitis optica are needed and ethical. Mult Scler Relat Disord 2015;4:536–45. [DOI] [PubMed] [Google Scholar]
- 68.Chen JJ, Tobin WO, Majed M, et al. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data used for this study are available upon reasonable request from the corresponding author.


