Abstract
Background:
Adolescents constitute a unique waitlist cohort that is distinct from younger children. MELD 3.0, which was developed in an adult population of liver transplant candidates, is planned to replace MELD-Na in the current liver allocation system for both adult and adolescents aged 12–17. We evaluated the predictive performance of MELD-Na, MELD 3.0, and PELD, for 90-day waitlist mortality risk among adolescent liver transplant registrants.
Methods:
New waitlist registrations for primary liver transplant among individuals aged 12–17 and aged 18–25 for comparison were identified using OPTN data from Nov 17 2004 to Dec 31 2021. The predictive performance of the current and proposed MELD and PELD scores was assessed using the Harrell’s concordance (c) statistic.
Results:
There were 1,238 eligible listings for adolescents aged 12–17, and 1,740 young adults aged 18–25. In the adolescent group, 90-day survival was 97.8%, compared to 95.9% in those aged 18–25 (log-rank p=0.005), with no significant differences when stratified by sex or indication. Among adolescents, increasing MELD 3.0 was associated with an increased hazard of mortality (HR 1.27, 95% CI 1.18–1.37), and the c-statistic for 90-day waitlist survival using MELD 3.0 was 0.893, compared with 0.871 using MELD-Na, and 0.852 using PELD.
Conclusion:
The discriminative ability of MELD 3.0 to rank adolescents according to the risk of death within 90 days was robust. Although MELD 3.0 was initially developed and validated in adults, MELD 3.0 may also improve the prediction of waitlist mortality in adolescents and better represent their urgency for liver transplant.
Keywords: Organ allocation, waitlist priority, liver transplant
Background
In the United States, the current framework for allocation of donated livers to transplant candidates waiting for a suitable organ is based on objective assessment of medical urgency. This is well established in adult patients, primarily utilizing the Model for End-Stage Liver Disease (MELD) score. Since MELD-based allocation was implemented in 2002, a number of improvements have been made to the US liver transplant allocation system, including several iterations of exception scores for patients with indications other than liver failure and expanded regional sharing of organs. The MELD score itself has been revised, including MELD 3.0, which was recently developed to improve upon the current MELD-Na.
For patients less than 18 years of age, the pediatric end-stage liver disease (PELD) score was initially adopted in 2002 when the MELD system was implemented. Among them, adolescents aged 12–17 years represent a unique group of patients who are biologically and clinically distinct from younger children and also from young adults. Concerns were raised soon after PELD was implemented that adolescents may experience different waitlist outcomes compared to other pediatric age groups. In recognition of this, adolescents were transitioned from the PELD to MELD system in 2005. Adolescents represent a sizable proportion of pediatric transplants — accounting for 27.4% of new pediatric waitlist registrants and 23.9% of transplant recipients as of 2020.
The Organ Procurement and Transplantation Network (OPTN) has recently approved MELD 3.0 to replace the current MELD-Na in the liver allocation system for adults aged over 18 years.1 At the same time, they recommend that adolescents be prioritized using MELD 3.0 in the updated allocation system. In this study, we compare the predictive performance of MELD 3.0 and MELD-Na for 90-day waitlist mortality risk among adolescent liver transplant registrants and young adults.
Materials and Methods
Subjects
New liver transplant waitlist registrations from November 17, 2004 to December 31, 2021, were extracted using data from the Organ Procurement and Transplantation Network (OPTN), which captures all waitlist, transplant, and donation events in the United States. Supplementary Figure 1 describes the subject selection process. In total, there were 203,636 registrants of all ages during the study period. They were divided into 4 groups including (1) children under 12 years, (2) adolescents 12–17 years of age, (3) young adults 18–25 years of age, and (4) adults older than 25 years. The middle two groups, namely transplant candidates aged 12–25, constituted the study subjects. Of those, exclusion criteria included registration for non-simultaneous liver kidney multi-organ transplant, status 1A or 1B at listing, diagnoses of acute liver failure or malignancy, history of previous liver transplant and missing laboratory values. Patients who converted to status 1B while waiting were included, as this could represent worsening illness severity while on the waitlist. Serum sodium values were not available prior to Nov 17, 2004, so new waitlist registrations prior to this date were excluded.
Data
Table 1 shows the equations used to calculate MELD, MELD-Na, and MELD 3.0, as outlined by current and proposed OPTN policy.1 Adolescents aged 12–17 were granted the 1.33 points regardless of sex, as proposed by the OPTN, as differences in muscle mass and thus creatinine between sexes are not consistently established in this age group.2
Table 1.
Current and proposed MELD and PELD equations.
| MELD* | 0.957*loge(Creatinine) + 0.378*loge(Bilirubin) + 1.120*loge(INR) + 0.643 |
| MELD-Na | MELD + 1.32*(137 – Sodium) – [0.033*MELD*(137 – Sodium)] |
| MELD 3.0 (age 12–17) | 4.56*loge(Bilirubin) + 0.82*(137 – Sodium) – 0.24*(137 – Sodium)*loge(Bilirubin) + 9.09*loge(INR) + 11.14*loge(Creatinine) + 1.85*(3.5 – Albumin) – 1.83*(3.5 – Albumin)*loge(Creatinine) + 7.33 |
| MELD 3.0 (age 18+) | 1.33 (if female) + 4.56*loge(Bilirubin) + 0.82*(137 – Sodium) – 0.24*(137 – Sodium)*loge(Bilirubin) + 9.09*loge(INR) + 11.14*loge(Creatinine) + 1.85*(3.5 – Albumin) – 1.83*(3.5 – Albumin)*loge(Creatinine) + 6 |
| PELD | 0.436 (Age (<1 YR)) – 0.687*loge(Albumin) + 0.480*loge(Bilirubin) + 1.857*loge(INR) +0.667(Growth failure (<- 2 Std. Deviations present)) |
N.B. Units: Albumin, g/dL; Bilirubin, mg/dL; Creatinine, mg/dL; Sodium, mmol/L
Bilirubin, INR, and creatinine values < 1.0 are set to 1.0 when calculating a candidate’s MELD score. Albumin, bilirubin, and INR values < 1.0 are set to 1.0 when calculating a candidate’s PELD score.
Bounds (MELD & MELD 3.0): Albumin 1.5–3.5 g/dL, sodium 125–137 mmol/L
Candidates with a creatinine over 4.0 mg/dL (MELD, MELD-Na), 3.0 mg/dL (MELD 3.0) or those receiving two or more dialysis treatments or 24 hours of continuous veno-venous hemodialysis within the 7 days prior to the serum creatinine test receive a creatinine score of 4.0 mg/dL (MELD, MELD-Na), 3.0 mg/dL (MELD 3.0).
The PELD score is rounded to the tenth decimal place and then multiplied by 10.
The minimum MELD, MELD-Na and MELD 3.0 is 6 and is rounded to the nearest whole number.
Demographics and clinical characteristics of adolescents listed for liver transplant were compared to young adults aged 18–25 at the time of listing. Indications were grouped into autoimmune, cholestatic, genetic/metabolic, and other etiologies for comparison.
Statistical Analysis
Survival was estimated using Kaplan-Meier methods and Cox regression, with death or removal from the waitlist for being too sick within 90 days as the event. Patients were censored at the time of waitlist removal including liver transplantation, after 90 days of follow-up were complete, or December 31, 2021, whichever occurred first. Kaplan-Meier survival curves were used to compare 90-day mortality by age group, indication, and sex.
The predictive performance of each MELD and PELD score in terms of discrimination, i.e. the model’s ability to rank patients according to the risk of death within 90 days, was assessed using the Harrell’s concordance (c) statistic. A c-statistic closer to 1 indicates a model with better discrimination between individuals that did or did not experience the outcome (death). The c-statistics for each score were compared for MELD-Na, MELD 3.0, and PELD, using the concordance function of the R package survival.3 This allows for comparison of two separately fitted models using the variance-covariance matrix for each c-statistic to test equality. Sensitivity analyses were performed to (1) assign 1.33 points to females only, as in adults; and (2) to include patients with a history of previous liver transplant.
All research was conducted in accordance with both the Declarations of Helsinki and Istanbul and was approved by the appropriate Institutional Review Board at Stanford University with waiver of informed consent.
Results
There were 1,238 eligible registrants for primary liver transplant among adolescents aged 12–17 and 1,740 among young adults aged 18–25 (Supplementary Figure 1, Table 2). Compared to young adults, adolescents were more likely to be female (51.4% v 47.5%, p=0.04) and more frequently listed for cholestatic diseases such as biliary atresia, Caroli’s disease and Alagille syndrome and for genetic and metabolic disorders including cystic fibrosis, alpha-1-antitrypsin deficiency, Wilson disease, and inborn errors of metabolism.
Table 2.
Characteristics of eligible liver transplant candidates at registration. IQR = interquartile range.
| Adolescents (12–17 years, n=1,238) | Young adults (18–25, n=1,740) | P value | |
| Age, years (IQR) | 15 (13–16) | 22 (20–24) | N/A |
| Female, n (%) | 636 (51.4) | 826 (47.5) | 0.04 |
| Race, n (%) | 0.20 | ||
| White | 751 (60.7) | 1,078 (62.0) | |
| Hispanic | 225 (18.2) | 262 (15.1) | |
| Black | 198 (16.0) | 309 (17.8) | |
| Asian | 39 (3.2) | 58 (3.3) | |
| Other | 25 (2.0) | 33 (1.9) | |
| Indication, n (%) | <0.01 | ||
| Autoimmune | 360 (29.1) | 868 (49.9) | |
| Cholestatic | 261 (21.1) | 216 (12.4) | |
| Genetic/Metabolic | 369 (29.8) | 254 (14.6) | |
| Other/Unknown | 248 (20.0) | 402 (23.1) | |
| Diabetes, n (%) | 60 (4.9) | 80 (4.6) | 0.75 |
| Ascites, n (%) | <0.01 | ||
| Absent | 854 (69.0) | 906 (52.1) | |
| Slight | 282 (22.8) | 613 (35.2) | |
| Moderate | 102 (8.2) | 221 (12.7) | |
| Encephalopathy, n (%) | <0.01 | ||
| None | 1,073 (86.7) | 1,187 (68.2) | |
| 1–2 | 149 (12.0) | 485 (27.9) | |
| 3–4 | 16 (1.3) | 68 (3.9) | |
| Sodium, mmol/L (IQR) | 139 (137–141) | 138 (135–140) | <0.01 |
| Creatinine, mg/dL (IQR) | 0.6 (0.5–0.7) | 0.7 (0.6–0.9) | <0.01 |
| INR (IQR) | 1.2 (1.1–1.5) | 1.3 (1.1–1.8) | <0.01 |
| Bilirubin, mg/dL (IQR) | 2.1 (0.9–5.7) | 3.8 (1.5–11.6) | <0.01 |
| Albumin, g/dL (IQR) | 3.4 (2.8–4.0) | 3.2 (2.6–3.8) | <0.01 |
| Renal failure, n (%) | 50 (4.0) | 97 (5.6) | 0.06 |
| Mechanical ventilation, n (%) | 13 (1.1) | 34 (2.0) | 0.051 |
| Vasopressors, n (%) | 1 (0.1) | 2 (0.1) | 1.00 |
| ACLF, n (%) | 5 (0.4) | 28 (1.6) | <0.01 |
| CTP score (IQR) | 7 (5–9) | 8 (7–10) | <0.01 |
| MELD (IQR) | 14 (9–18) | 16 (11–22) | <0.01 |
| MELD Na (IQR) | 14 (9–19) | 17 (11–24) | <0.01 |
| MELD 3.0 (IQR) | 16 (11–21) | 18 (12–25) | <0.01 |
| MELD 3.0 category, n (%) | <0.01 | ||
| <15 | 568 (45.9) | 569 (32.7) | |
| 15–20 | 346 (27.9) | 469 (27.0) | |
| 21+ | 324 (26.2) | 702 (40.3) | |
| PELD (IQR) | 2.0 (−4.0–9.0) | - | - |
| At List exception, n (%) | 77 (6.2) | 20 (1.1) | <0.01 |
| *Indication groups: | |||
|
| |||
| Autoimmune | Primary sclerosing cholangitis, autoimmune hepatitis | ||
| Cholestatic | Biliary atresia, Caroli’s, total parenteral nutrition, progressive familial intrahepatic cholestasis, Alagille, choledochal cyst | ||
| Genetic/Metabolic | Cystic fibrosis, Wilson disease, alpha-1-antitrypsin deficiency, sarcoid, inborn errors of metabolism | ||
| Other/Unknown | Viral, non-alcoholic steatohepatitis, miscellaneous including benign tumors without exception | ||
Overall, liver disease appeared to be less decompensated among adolescent candidates compared to young adults, with less severe ascites and encephalopathy and less abnormal laboratory values including the serum bilirubin, creatinine, INR, and albumin. The median MELD-Na at listing was lower among adolescents (14 v. 17, p<0.01) compared to young adults, as was the median MELD 3.0 (16 v. 18, respectively, p<0.01). Severe extrahepatic organ failure was uncommon in either age group, although the proportion meeting the criteria for ACLF was statistically significantly higher in young adults than in adolescents. Listing with an exception score was also more common for adolescent candidates.
Short-term waitlist survival was high in both age groups. However, 90-day survival in the adolescent group was 97.8%, which was higher compared to young adults (log-rank p<0.01) [Figure 1A]. Figure 1B describes waitlist survival by indication among adolescent candidates. Adolescents with cholestatic liver disease had the best outcomes, with no mortality up to 90 days. Adolescents with other/unknown etiology had the lowest survival (94.2% at 90 days), and adolescents with autoimmune diseases or genetic/metabolic disorders had an intermediate survival (both 98.0% at 90 days); these differences were significant (log-rank p=0.03). Importantly, we did not observe a difference in 90-day survival when comparing adolescent males and females (Figure 1C, log-rank p=0.81).
Figure 1.
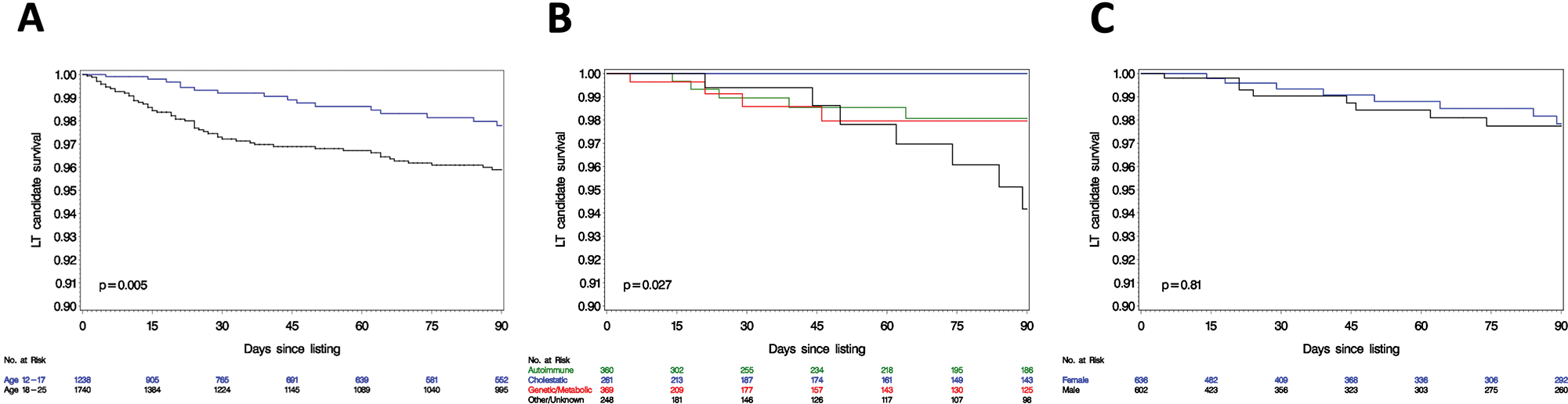
Kaplan Meier curves for 90-day survival from waitlist registration (A) by age group; (B) by indication in the 12–17 age group, and (C) by sex in the 12–17 age group.
Table 3 summarizes a series of Cox regression analyses in the two age groups. In both age groups, MELD 3.0 and MELD-Na were associated with an increased hazard of 90-day waitlist mortality. Specifically, each point of MELD 3.0 was associated with a 27% increase in the hazard of 90-day mortality among adolescent candidates, compared to a 20% increase in hazard among those aged 18–25 years. Among adolescents, an increase in each PELD point was also associated with an increased hazard of waitlist mortality.
Table 3.
Cox regression model for 90-day waitlist mortality from waitlist registration.
| Model | Adolescents (12–17) | Young adults (18–25) | ||
|---|---|---|---|---|
| HR 95% CI | P Value | HR 95% CI | P Value | |
| MELD 3.0 | 1.27 (1.18–1.37) | <0.01 | 1.20 (1.16–1.23) | <0.01 |
| MELD Na | 1.25 (1.16–1.33) | <0.01 | 1.18 (1.15–1.22) | <0.01 |
| PELD | 1.15 (1.10–1.21) | <0.01 | N/A | |
Figure 2 compares discrimination among the prediction models. Among adolescent registrants aged 12–17, the Harrell’s c-statistic for MELD 3.0 was 0.893, compared with 0.871 for MELD-Na, and 0.852 for PELD. None of these differences reached statistical significance. In young adults aged 18–25, the c-statistic for MELD 3.0 and MELD-Na in predicting 90-day mortality was 0.863 and 0.865, respectively. A sensitivity analysis assigning 1.33 points to female adolescents only, as in adults, was performed. The c-statistic for this model was 0.889, slightly lower than when 1.33 points were granted to both sexes (c-statistic=0.893 as above).
Figure 2.
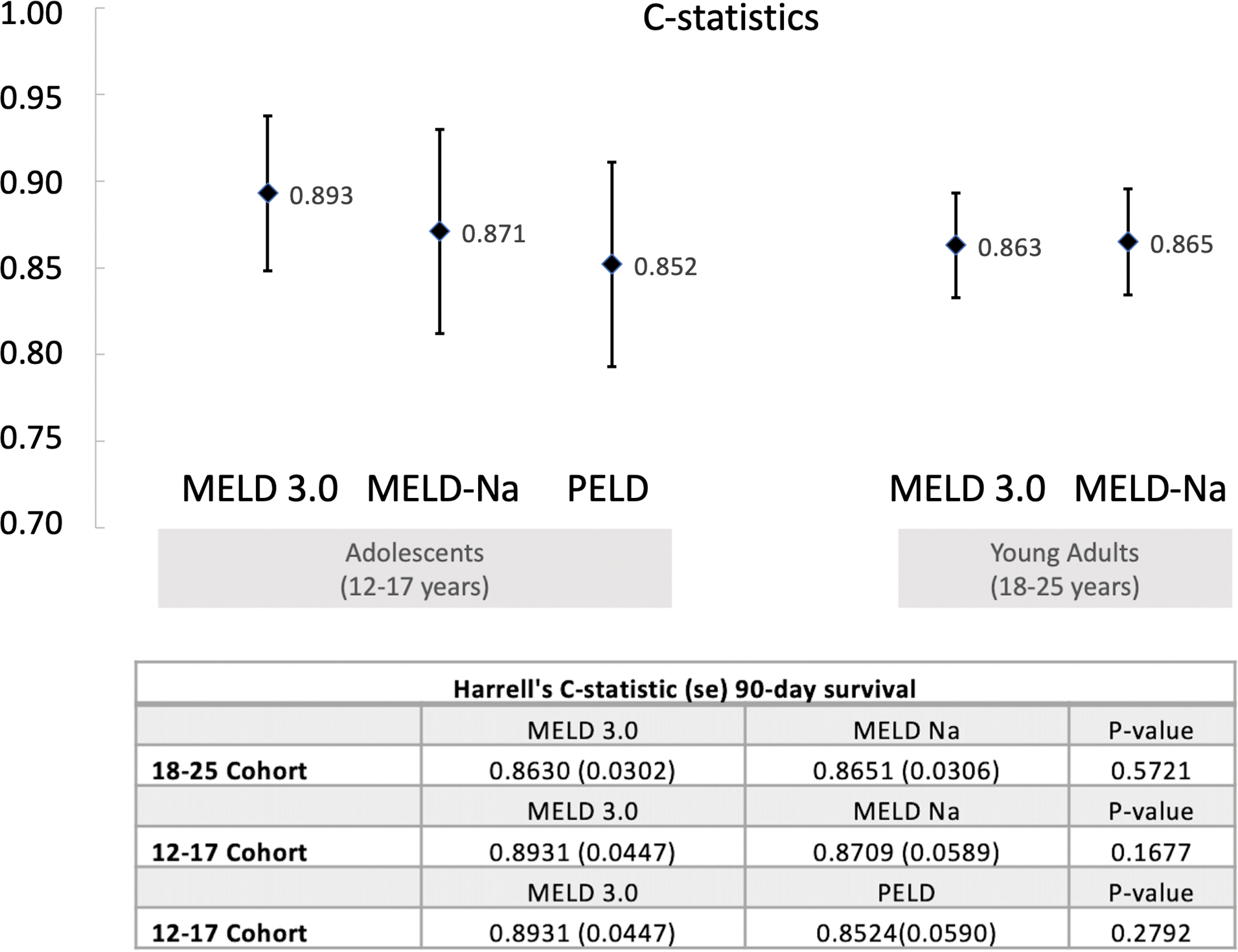
Comparison of the Harrell’s c-statistic using MELD 3.0, MELD-Na, and PELD for adolescents. Error bars represent standard error.
A separate sensitivity analysis was performed to consider those with a previous history of liver transplant, adding 141 adolescents to the cohort. The overall results were similar, with an overall c-statistic of 0.906 using MELD 3.0, compared to 0.889 for MELD-Na (p=0.11).
Discussion
Adolescents constitute a unique waitlist cohort that is distinct from both younger children and adults. Currently, adolescents aged 12–17 are prioritized on the liver transplant waitlist by MELD-Na, which is comprised of the serum international normalized ratio, bilirubin, creatinine, and sodium.4 Under the MELD system, 7.0% of adolescent candidates were removed for death or delisting between 2007 and 2012, and nearly one-quarter of children who died or were delisted without receiving any offers at all were adolescents.5 Although exception scores have been utilized frequently for pediatric candidates to increase access to liver transplant, adolescents are less likely than younger children to have their initial non-standard exception requests approved.6 Preferential allocation of organs to pediatric transplant candidates is well-supported by regulatory guidance and ethical principles, to give children the opportunity for proper growth and development and for achievement of major life milestones, as well as to maximize organ utility and longevity.7 In parallel, the Final Rule also sets out that priority rankings on the waiting list should be expressed, when possible, using objective and measurable medical criteria, ordered from most to least medically urgent.8
MELD 3.0 was developed and validated to improve upon MELD-Na in an adult population of liver transplant candidates with chronic liver disease. It incorporates albumin as an additional variable and grants 1.33 additional points to women in part to address the biological difference in serum creatinine between the sexes as an indicator of renal function.9 When MELD 3.0 was applied to adolescent liver transplant candidates in this study based on data spanning from 2004 to 2021, its predictive performance was robust with a c-statistic of 0.893 for 90-day waitlist mortality, which was similar, if not better than, young adults aged 18–25. These data suggest that MELD 3.0 is appropriate for waitlist prioritization and allocation of livers for adolescents, as the score is being implemented in adult candidates.
Strictly speaking, the difference in predictive performance of MELD 3.0 versus MELD-Na was not statistically significant. Whether the transition of MELD 3.0 to MELD-Na will be clinically significant for this population remains to be seen. While it cannot be projected that waitlist mortality will decrease based on these results, we can infer that implementation of MELD 3.0 is not likely to worsen waitlist outcomes for adolescents. In addition, an advantage of MELD 3.0 over MELD-Na in this group is that MELD 3.0 grants an extra 1.33 points regardless of sex, putting these adolescents at a higher priority relative to adults than under MELD-Na, supporting ethical principles and guidance to prioritize pediatric transplant candidates in transplant allocation. The results of our analysis support applying the extra 1.33 points to both adolescents males and females under MELD 3.0, as there were no differences in survival by sex and no improvement in model performance when the extra points were granted only to females.
In parallel to the efforts to improve MELD in adults leading to the adoption of MELD 3.0, an updated version of the Pediatric End Stage Liver Disease (PELD) model has been developed for pediatric candidates aged 0–11, named PELD-Cr. The PELD-Cr score better predicts pediatric waitlist mortality, by including creatinine as an additional variable, updating coefficients, and adding a factor for age-adjusted mortality.10 PELD Cr has now been approved by the UNOS Board of Directors to replace PELD in the liver allocation system for children aged 0–11.1
With the development of PELD-Cr, thoughtful considerations were given to adolescents being allocated by PELD-Cr, so that all pediatric candidates would be treated similarly. However, such a transition was felt to be too complex with potential for negative unintended consequences.11 A main concern was that shifting the policy-assigned exception point system for adolescents from MMAT to MPAT would have an adverse impact on median PELD at transplant (MPAT) for pediatric candidates with exception scores, which could potentially deflate the national MPAT and reduce overall access to transplant. Ultimately, in June 2022, the OPTN determined that adolescents aged 12–17 would remain prioritized using an allocation system based on MELD rather than PELD.
Limitations to this analysis include the observational nature of the database and the limited statistical power in the adolescent transplant population. An important challenge in this analysis is that a significant proportion of pediatric candidates are allocated through the utilization of non-standard exception scores — we included adolescents who were granted exception scores, but they were censored at the time of receiving exception. In addition, the exclusion of adolescents with chronic liver disease who were initially listed as status 1B may have omitted consideration of some more critically ill candidates. The predictive performance of MELD 3.0 may not be as accurate in these patients who accessed transplant outside of the standard MELD or MELD-Na system. Although Status 1B and non-standard exceptions will still be available for patients who qualify through the National Liver Review Board or standardized criteria for status 1B, continued attention to this small but vulnerable group is needed to prevent unintended consequences with the shift from MELD-Na to MELD 3.0. Access to status 1B for those with chronic liver disease may increase as minimum MELD/PELD thresholds for status 1B listing for chronic liver disease are simultaneously being eliminated.
In summary, we demonstrate excellent performance of MELD 3.0 in risk stratification of 90-day mortality risk among adolescents, which is similar if not superior to that of adults. There were no sex differences in waitlist survival among adolescent patients, supporting the adoption of the version of MELD 3.0 in which both sexes are granted 1.33 points. Although MELD 3.0 does not address all unique aspects of adolescent liver transplant candidates in comparison to younger children and to adults,12 the results of the present analysis suggest that implementation of MELD 3.0 would better represent waitlist urgency and the need for transplant for adolescents aged 12–17 years.
Supplementary Material
Grant Support:
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-127224). Dr. Kwong is supported by the National Institute on Alcohol Abuse and Alcoholism (K23 AA-029197). The funding organizations played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations:
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-Stage Liver Disease
- US
United States
Footnotes
Disclosures: Noelle Ebel consults for and is on the speakers’ bureau for Mirum Pharmaceuticals. The other authors have nothing to disclose.
References
- 1.OPTN Liver and Intestinal Organ Transplantation Committee. Notice of OPTN Policy and Guidance Change: Improving Liver Allocation: MELD, PELD, Status 1A, Status 1B. Published online June 27, 2022. Accessed August 27, 2022. https://optn.transplant.hrsa.gov/media/3idbp5vq/policy-guid-change_impr-liv-alloc-meld-peld-sta-1a-sta-1b_liv.pdf
- 2.Filler G, Lee M. Educational review: measurement of GFR in special populations. Pediatr Nephrol. 2018;33(11):2037–2046. doi: 10.1007/s00467-017-3852-8 [DOI] [PubMed] [Google Scholar]
- 3.Therneau T, Atkinson E. Concordance. Published online February 6, 2023. Accessed February 14, 2023. https://cran.r-project.org/web/packages/survival/vignettes/concordance.pdf
- 4.Organ Procurement and Transplantation Network. OPTN Policies, Policy 9: Allocation of Livers and Liver-Intestines. Accessed April 11, 2022. https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf
- 5.Hsu EK, Shaffer ML, Gao L, et al. Analysis of Liver Offers to Pediatric Candidates on the Transplant Wait List. Gastroenterology. 2017;153(4):988–995. doi: 10.1053/j.gastro.2017.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perito ER, Braun HJ, Dodge JL, Rhee S, Roberts JP. Justifying Nonstandard Exception Requests for Pediatric Liver Transplant Candidates: An Analysis of Narratives Submitted to the United Network for Organ Sharing, 2009–2014. Am J Transplant. 2017;17(8):2144–2154. doi: 10.1111/ajt.14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organ Procurement and Transplantation Network. Ethical principles of pediatric organ allocation. Published online November 2014. Accessed January 27, 2023. https://optn.transplant.hrsa.gov/professionals/by-topic/ethical-considerations/ethical-principles-of-pediatric-organ-allocation/
- 8.Organ Procurement and Transplantation Network (OPTN). Final Rule as revised by amendments. Published online 1999. Accessed September 16, 2021. https://www.ecfr.gov/current/title-42/chapter-I/subchapter-K/part-121
- 9.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7(3):685–692. doi: 10.1111/j.1600-6143.2007.01666.x [DOI] [PubMed] [Google Scholar]
- 10.Hsu E, Schladt DP, Wey A, Perito ER, Israni AK. Improving the predictive ability of the pediatric end-stage liver disease score for young children awaiting liver transplant. Am J Transplant. 2021;21(1):222–228. doi: 10.1111/ajt.15925 [DOI] [PubMed] [Google Scholar]
- 11.OPTN Liver and Intestinal Organ Transplantation Committee. Public Comment Proposal: Improving Liver Allocation: MELD, PELD, Status 1A and Status 1B. Published online January 27, 2022. Accessed September 7, 2022. https://optn.transplant.hrsa.gov/media/kxhdo0h4/improving-liver-allocation_meld-peld-status-1a-and-status-1b_winter-2022-pc.pdf
- 12.Moazzam Z, Ziogas IA, Wu WK, et al. Delay in liver transplantation referral for adolescents with biliary atresia transitioning to adult care: a slippery slope. Br J Surg. 2021;108(10):e324–e325. doi: 10.1093/bjs/znab209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


