Abstract
Background
We set out to identify empirically-derived health status classes of older adults with diabetes based on clusters of comorbid conditions which are associated with future complications.
Methods
We conducted a cohort study among 105,786 older (≥65 years of age) adults with type 2 diabetes enrolled in an integrated healthcare delivery system. We used latent class analysis (LCA) of 19 baseline comorbidities to derive health status classes and then compared incident complication rates (events per 100 person-years [pys]) by health status class during 5 years of follow up. Complications included infections, hyperglycemic events, hypoglycemic events, microvascular events, cardiovascular events, and all-cause mortality.
Results
Three health status classes were identified: Class 1 (58% of the cohort) had the lowest prevalence of most baseline comorbidities, Class 2 (22%) had the highest prevalence of obesity, arthritis, and depression, and Class 3 (20%) had the highest prevalence of cardiovascular conditions. The risk for incident complications was highest for Class 3, intermediate for Class 2 and lowest for Class 1. For example, the age, sex and race-adjusted rates for cardiovascular events (per 100 person-years) for Class 3, Class 2 and Class 1 were 6.5, 2.3, and 1.6, respectively; 2.1, 1.2, 0.7 for hypoglycemia; and 8.0, 3.8, and 2.3 for mortality.
Conclusions
Three health status classes of older adults with diabetes were identified based on prevalent comorbidities and were associated with marked differences in risk of complications. These health status classes can inform population health management and guide the individualization of diabetes care.
Keywords: Diabetes, aging, comorbidity, latent class analysis, complications
Introduction
In the U.S., 7.8 million adults age ≥65 years (one in five) currently have type 2 diabetes (T2D)1 with expectations that this population will double by 2034.2,3 The population is heterogeneous with regard to duration of diabetes, functional impairments, comorbidities, complications, risk of adverse drug events, and life expectancy,1,4 and a single glycemic target or treatment strategy is unlikely to be appropriate for every patient.5 Developing a data-driven, pragmatic health status classification scheme for older adults with diabetes would help to individualize and prioritize care.
Despite their clinical importance, health status classification schemes in care guidelines have been largely based on expert clinical opinion.6,7 For nearly a decade, multiple diabetes and geriatric societies have recommended that diabetes treatments should be based on an individual’s health status as determined by a comprehensive geriatric assessment including comorbidities, functional status, cognitive function, and frailty. For example, the American Diabetes Association (ADA) recommends intensive glucose control targets (e.g., A1C < 7.0%) for “healthy” older adults and relaxed targets (e.g., A1C<8.0%) for “complex” older adults, but these classes remain poorly defined.8 These stratified recommendations are based on the 9-10 year time-to-benefit associated with intensive glucose control (A1C < 7.0% vs. <7.9%) observed in United Kingdom Prospective Diabetes Study (UKPDS).9 Calls to adjust the intensity of glucose control by health status were reinforced by the findings from trials published in the late 2000s 10-12 which showed that very intensive glucose control (e.g., A1C<6.5%) produced only modest clinical benefits and, in the case of one trial, increased mortality.10 In the last decade, the approach to diabetes management has been further transformed by a series of cardiovascular outcome trials that have revealed the benefits of newer glucose lowering agents such as SGLT-2 inhibitors and GLP-1 receptor agonists compared to placebo.13,14
With ongoing developments in diabetes treatments, health status classification schemes need to be reexamined using contemporary data. Among the assessment domains suggested for individualizing care, comorbidities are attractive because they are readily available in medical records and claims data. However, with no consensus on how best to classify adults by comorbidities, guidelines vary widely in defining classes of comorbidity complexity.15 Naturally occurring clusters of comorbidities may present common pathophysiologic pathways or stages of progression of a disease.
This study developed an empiric health status classification scheme based on comorbid conditions in a large multi-ethnic contemporary cohort of older adults with diabetes and then estimated the subsequent 5-year incidence of complications for each class.
Methods
Source Population
Participants in this study were members of a large, integrated healthcare delivery system, Kaiser Permanente Northern California (“KPNC”), who were identified in the KPNC Diabetes Registry (“Registry”), a well-characterized population maintained continuously since 1993.16-18 Registry inclusion is based on a validated algorithm incorporating multiple data sources including pharmacy dispensing, laboratory results, and outpatient, emergency room and inpatient diagnoses of diabetes.19
The Registry included 279,584 members with T2D as of January 1, 2015 (baseline). We excluded 150,426 under the age of 65 years, 7,762 with gaps in KPNC membership during the 24 months or pharmacy benefits during the 12 months prior to baseline, 3,066 with type 1 diabetes or unknown type of diabetes (classified using an internally validated algorithm20 21), and 12,544 individuals with no A1C test result during the 12 months prior to baseline or with first diabetes identification during the 12 months prior to baseline. The remaining 105,786 subjects (the Diabetes and Aging Study 2015 Cohort) were the basis for these analyses.
Baseline Comorbid Conditions
Latent class analysis was performed on baseline prevalent comorbidities.7,22 The following comorbid conditions were identified from medical records for the 10 years prior to baseline (1/1/2005-12/31/2014) using ICD-9, ICD-10, and procedure codes (See Supplementary Table S1) : 1) arthritis (rheumatoid or osteoarthritis), 2) atrial fibrillation, 3) cancer, 4) congestive heart failure, 5) coronary artery disease, 6) dementia, 7) depression, 8) emphysema/chronic obstructive pulmonary disease (COPD), 9) end-stage renal disease (ESRD), 10) falls, 11) foot ulcer, 12) hypertension, 13) liver disease, 14) lower extremity amputation, 15) obesity, 16) peripheral vascular disease, 17) stroke, 18) thyroid disease, 19) urinary incontinence.
Outcomes
Outcomes included the first occurrence of any new diabetes complications (defined below) during the five years of follow-up (i.e., through December 31, 2019). Patients with baseline complications were included in these analysis and patients could contribute data to multiple outcomes. Follow-up was censored at death or at the start of any KPNC membership gap of ≥3 months.
Microvascular complications included ESRD, severe diabetic eye disease, and amputation. Incident ESRD was defined by chronic dialysis therapy initiation or kidney transplantation identified through hospitalizations or from KPNC’s reporting system for the United States Renal Data System. Severe diabetic eye disease was identified based on outpatient diagnosis of proliferative diabetic retinopathy. Amputation was identified by inpatient procedures.
Macrovascular complications included hospitalizations for myocardial infarction, ischemic or hemorrhagic stroke, and congestive heart failure (CHF).
Infections were identified by hospitalizations for infections that are common among patients with diabetes, e.g., respiratory; urinary tract and kidney; bone, skin and soft tissue; and sepsis.
Acute hyperglycemic event was defined as emergency department visits or hospitalizations for diabetes with hyperosmolarity, diabetes with ketoacidosis, or uncontrolled diabetes with hyperglycemia. Acute hypoglycemic event (hypoglycemia) was defined based on emergency department visits or hospitalizations for hypoglycemia.
Mortality and date of death were captured from the National Death Index, California State Mortality File, Social Security Death Records, or KPNC administrative records (if the death occurred within the health system).
Combined non-mortality outcome was a synthetic outcome including all non-fatal outcomes. The combined outcome included all outcomes including mortality.
Other variables
Covariates, ascertained as of baseline, included demographics (sex and race/ethnicity), last laboratory result within one year prior to baseline (glycated hemoglobin (hemoglobin A1C (A1C)), low-density lipoprotein (LDL), estimated glomerular filtration rate (eGFR))23, prevalent complications occurring during the 10 years prior to baseline (acute hyperglycemic or hypoglycemic event, ESRD, peripheral vascular disease, amputation, eye disease, coronary artery disease, cerebrovascular disease, and congestive heart failure), and dispensing of diabetes medications in the 6 months prior to baseline.
Statistical Analysis
We fit a latent class model24 to baseline comorbid conditions. This model accounts for the observed associations among comorbidities by assuming the presence of two or more underlying (i.e., latent) classes of individuals, each group having its own prevalence for each condition. Taken together, the entire population thus represents a “mixture” of these classes, and after fitting the model one may obtain the posterior probabilities that a given individual belongs to each of the classes. We fit successive models starting with just two classes and going up to ten. For each model, we computed several model selection criteria including the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), adjusted BIC, and entropy (a measure of the extent to which the model yields well separated classes). We also plotted, for each class, the distribution of posterior probabilities of class membership. After weighing the performance of the resulting classes in predicting subsequent outcomes (as described below) against the additional complexity involved in interpreting and utilizing models with more classes, we decided that a model with three classes struck an appropriate balance.
We assigned each individual to the class for which his or her probability of membership was highest and then compared the distribution of demographic and clinical characteristics across the resulting classes, using the chi-squared statistic to test the null hypothesis of no association between each characteristic and class membership. We then fit separate Cox regression models25 to the time from baseline to the first occurrence of each outcome. Covariates included class membership, age, gender and race/ethnicity; hazard ratios comparing each of Classes 2 and 3 to Class 1 are presented, together with 95% confidence intervals. For each model the c-statistic26 evaluating the adequacy of the model predictions was computed and compared to that for a corresponding model in which class membership was replaced with 19 binary covariates. This comparison was then repeated excluding those individuals whose probability of membership in the class was less than 0.75. Finally, we fit a Poisson regression model27 to each outcome using the follow up time as the exposure (i.e., assuming a constant underlying hazard or exponentially distributed times) and computed the marginal rates (per 100 person-years) for each class, treating the covariates as balanced (using LSMEANS in SAS). These are referred to as “age, sex and race-adjusted rates.”
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
The mean age of the cohort was 74.7 years and 23% were ≥80 years of age (Table 1). Roughly half (55%) had duration of diabetes ≥10 years. One quarter (26%) were not taking diabetes medications. The mean follow-up time for mortality was 4.3 years.
Table 1.
Characteristics of a cohort of 105,786 older (≥65 years of age) adults with type 2 diabetes. Kaiser Permanente Northern California Diabetes and Aging Study 2015 Cohort.
| Age groups, % | |
| 65-69 years | 31.1 |
| 70-74 years | 26.4 |
| 75-79 years | 19.6 |
| >=80 years | 22.9 |
| Duration of diabetes, % | |
| <10 years | 45.2 |
| ≥10 years | 54.8 |
| Male sex, % | 51.0 |
| Race/ethnicity, % | |
| Non-Hispanic White | 51.1 |
| Non-Hispanic Black | 8.9 |
| Hispanic | 13.8 |
| East Asian | 8.5 |
| South Asian | 1.5 |
| Filipino | 8.3 |
| Other Asian | 1.6 |
| Pacific Islander | 0.4 |
| Other/Missing | 0.4 |
| Mixed | 4.9 |
| Native American | 0.6 |
| Systolic blood pressure | |
| <130 mm Hg | 48.6 |
| ≥130 mmg Hg | 51.2 |
| Missing | 0.2 |
| LDL cholesterol | |
| <100 mg/dl | 77.9 |
| ≥100 mg/dl | 18.2 |
| Missing | 3.9 |
| A1C categories, % | |
| ≤5.9 | 8.1 |
| 6.0-6.9 | 41.4 |
| 7.0-7.9 | 33.6 |
| 8.0-8.9 | 10.1 |
| 9.0-9.9 | 3.8 |
| ≥10 | 3.0 |
| Estimated GlomerularFiltration Rate 23 , % | |
| ≥90 no albuminuria | 7.2 |
| ≥90 with albuminuria | 2.5 |
| 60-89 | 51.1 |
| 45-59 | 22.2 |
| 30-44 | 12.0 |
| 15-29 | 3.5 |
| <15 or dialysis | 1.2 |
| Missing | 0.4 |
| Baseline comorbid conditions, % | |
| Arthritis | 45.5 |
| Atrial fibrillation | 14.5 |
| Cancer | 12.3 |
| Congestive heart failure | 14.8 |
| Coronary artery disease | 18.2 |
| Dementia | 4.5 |
| Depression | 29.8 |
| Emphysema/Chronic obstructive pulmonary disease | 26.2 |
| End-stage renal disease | 2.1 |
| Falls | 22.3 |
| Foot ulcer | 8.0 |
| Hypertension | 91.1 |
| Liver disease | 15.9 |
| Lower extremity amputation | 1.0 |
| Obesity | 44.2 |
| Peripheral vascular disease | 17.4 |
| Stroke | 4.9 |
| Thyroid disease | 20.3 |
| Urinary incontinence | 12.3 |
| Medications (within past 6 months), % | |
| Taking no diabetes medications | 26.1 |
| Insulin | 23.1 |
| Sulfonylurea | 38.5 |
| Metformin | 50.4 |
| Thiazolidinedione | 2.8 |
| Acarbose | 0.2 |
| Repaglinide | 0.1 |
| ≥2 glucose lowering drugs | 33.7 |
| ≥3 glucose lowering drugs | 7.6 |
| Insulin and oral therapy | 14.4 |
| Statin | 80.7 |
| Other lipid lowering agent | 5.3 |
| ACE inhibitor | 47.2 |
| Other anti-hypertensive | 77.9 |
Latent class model
A three-class model yielded overall class probabilities of 0.55, 0.25 and 0.21, with 70% of individuals having a probability of 0.75 or higher of being in one of the classes. The AIC, BIC and adjusted BIC values all declined (indicating better models) as the number of classes increased, with the lowest values achieved with the ten class model (Supplementary Table S2). However, plotting each criterion by the number of classes showed an elbow at three classes, subsequent improvements were modest, and models with more than three classes had at least one class with an overall probability of membership of only 0.03 or smaller. Moreover, the three-class model provided reasonable predictive accuracy relative to a model including all of the individual comorbidities. For example, the average c-statistic for the nine models in Table 3, each predicting an outcome based on class membership, sex and age, was 0.65, as compared to 0.70 for a model including all comorbidities as individual predictors; excluding the 30% of the sample whose posterior probabilities of class membership were less than 0.75 increased the average c-statistic to 0.68 (as compared to 0.72 for the model with individual comorbidities). The c-statistics for the four-class model were only marginally improved (Supplementary Table S3 and Supplementary Figure S1).
Table 3.
Results of Cox regression models (hazard ratios) and age, sex and race-adjusted incidence rates (per 100 person-years); estimates and 95% confidence intervals. Kaiser Permanente Northern California Diabetes and Aging Study 2015 Cohort.
| Outcome | Class 1 (healthy) Risk(95% CI) |
Class 2 (geriatric) Risk(95% CI) |
Class 3 (cardiac) Risk(95% CI) |
HR (Class 2/ Class 1) |
HR (Class 3/ Class 1) |
|---|---|---|---|---|---|
| Microvascular Complications | 0.9 (0.9, 1.0) | 1.3 (1.3, 1.4) | 6.3 (6.1, 6.5) | 1.43 (1.33, 1.53) | 6.09 (5.79, 6.41) |
| Cardiovascular Complications | 1.6 (1.6, 1.7) | 2.2 (2.2, 2.3) | 6.5 (6.3, 6.6) | 1.39 (1.32, 1.46) | 3.97 (3.80, 4.14) |
| Infection | 2.7 (2.6, 2.7) | 5.0 (4.9, 5.2) | 9.7 (9.5, 10.0) | 1.90 (1.83, 1.97) | 3.68 (3.56, 3.81) |
| Hypoglycemia | 0.7 (0.7, 0.8) | 1.2 (1.1, 1.2) | 2.1 (2.0, 2.2) | 1.64 (1.52, 1.77) | 2.93 (2.74, 3.13) |
| Hyperglycemia | 0.3 (0.3, 0.3) | 0.5 (0.4, 0.5) | 0.6 (0.6, 0.7) | 1.63 (1.45, 1.83) | 2.19 (1.96, 2.46) |
| Mortality | 2.3 (2.3, 2.4) | 3.8 (3.6, 3.9) | 8.0 (7.8, 8.2) | 1.63 (1.57, 1.70) | 3.56 (3.45, 3.68) |
| Combined non-mortality | 5.4 (5.3, 5.5) | 9.0 (8.8, 9.3) | 21.3 (20.9, 21.7) | 1.65 (1.60, 1.70) | 3.74 (3.65, 3.84) |
| Combined outcome | 6.7 (6.6, 6.8) | 11.1 (10.9, 11.4) | 24.6 (24.1, 25.0) | 1.64 (1.60, 1.69) | 3.54 (3.45, 3.62) |
All of the p-values for testing the null hypothesis that the three classes are equivalent were < 0.0001.
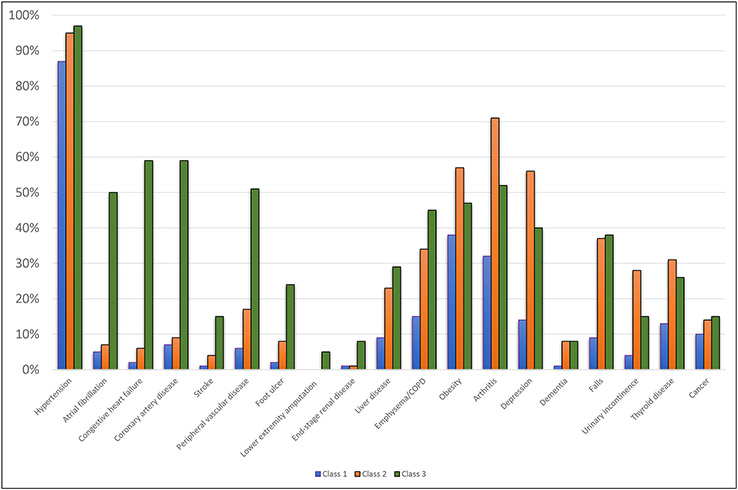
The estimated prevalence of baseline comorbidities differed substantively by class membership (Figure 1). Class 1 was the healthiest, as characterized by the lowest prevalence of all comorbidities. Class 2 had high rates of non-cardiovascular comorbidities; it had the highest prevalence of arthritis (71%), depression (56%), urinary incontinence (28%) and obesity (57%). Lastly, Class 3 was characterized by high prevalence of cardiovascular disease with a very high rate of coronary artery disease (59%) and congestive heart failure (59%), and a relatively high rate of stroke (15%).
Figure 1.
Estimated prevalence of comorbidities conditional on class membership for three class model fit to the Kaiser Permanente Northern California Diabetes and Aging Study 2015 Cohort.
Associations between health status classes and demographic and clinical characteristics
The distribution of several demographic and clinical characteristics differed substantially across the health status classes (Table 2). For example, individuals in Class 3 were older, and Class 2 had a higher prevalence of female sex. Class 3 had a slightly higher percentage of Whites while Class 1 had a higher percentage of East Asians. Class 2 and 3 both had higher percentages of patients with duration of diabetes of ten years or more than Class 1. Glycemic control levels were similar (mean A1C ~7.2%) for all three classes, with 50% of patients in each class having an A1C <7.0%. Finally, those in Classes 2 and 3 were more likely to be taking insulin than those in Class 1.
Table 2.
Comparison of demographic and clinical covariates across comorbidity classes in Kaiser Permanente Northern California Diabetes and Aging Study 2015 Cohort
| Variable | Class 1 | Class 2 | Class 3 | P-value |
|---|---|---|---|---|
| Age, mean (SD) | 73.50 (6.38) | 75.33 (7.16) | 77.42 (7.46) | <.0001 |
| Age groups (years), % | <.0001 | |||
| 65-69 | 36.4 | 28.0 | 19.1 | |
| 70-74 | 28.0 | 25.6 | 22.5 | |
| 75-79 | 18.7 | 20.4 | 21.3 | |
| ≥80 | 16.9 | 25.9 | 37.2 | |
| Duration of diabetes category (years), % | <.0001 | |||
| <10 | 50.8 | 42.3 | 32.0 | |
| ≥10 | 49.2 | 57.7 | 68.0 | |
| Male, % | 56.7 | 29.0 | 58.3 | <.0001 |
| Race/ethnicity, % | <.0001 | |||
| Non-Hispanic White | 45.9 | 57.6 | 59.4 | |
| Non-Hispanic Black | 8.9 | 9.0 | 9.0 | |
| Hispanic | 13.9 | 15.7 | 11.5 | |
| East Asian | 11.2 | 4.3 | 5.0 | |
| South Asian | 1.7 | 1.1 | 1.3 | |
| Filipino | 10.5 | 4.4 | 6.1 | |
| Other Asian | 2.3 | 0.6 | 0.7 | |
| Pacific Islander | 0.5 | 0.2 | 0.3 | |
| Other/Missing | 0.6 | 0.2 | 0.1 | |
| Mixed | 3.9 | 6.3 | 6.1 | |
| Native American | 0.5 | 0.6 | 0.6 | |
| Systolic blood pressure | <.0001 | |||
| <130 mm Hg | 48.4 | 46.7 | 51.3 | |
| ≥130 mmg Hg | 51.3 | 53.2 | 48.6 | |
| Missing | 0.3 | 0.1 | 0.1 | |
| LDL cholesterol | <.0001 | |||
| <100 mg/dl | 78.8 | 75.3 | 78.3 | |
| ≥100 mg/dl | 18.6 | 20.2 | 14.5 | |
| Missing | 2.6 | 4.5 | 7.2 | |
| A1C, Mean (SD) | 7.18 (1.11) | 7.15 (1.17) | 7.20 (1.26) | <.0001 |
| A1C Categories, % | <.0001 | |||
| ≤5.9 | 6.8 | 9.1 | 10.8 | |
| 6.0-6.9 | 41.9 | 42.3 | 38.8 | |
| 7.0-7.9 | 35.2 | 31.8 | 30.7 | |
| 8.0-8.9 | 9.8 | 9.8 | 11.4 | |
| 9.0-9.9 | 3.6 | 3.9 | 4.7 | |
| ≥10 | 2.7 | 3.2 | 3.7 | |
| Estimated Glomerular Filtration Rate, % | <.0001 | |||
| ≥90 no albuminuria | 9.0 | 6.8 | 2.6 | |
| ≥90 with albuminuria | 2.7 | 2.7 | 1.8 | |
| 60-89 | 56.5 | 50.3 | 36.3 | |
| 45-59 | 20.5 | 23.7 | 25.4 | |
| 30-44 | 8.7 | 13.2 | 20.2 | |
| 15-29 | 2.0 | 2.9 | 8.4 | |
| <15 or dialysis | 0.3 | 0.3 | 4.7 | |
| Missing | 0.4 | 0.2 | 0.6 | |
| Medications (6 months), % | ||||
| Taking no glucose lowering medications | 26.2 | 26.3 | 25.8 | 0.3655 |
| Insulin | 17.5 | 25.8 | 36.5 | <.0001 |
| Sulfonylurea | 39.3 | 37.1 | 37.5 | <.0001 |
| Metformin | 56.6 | 49.7 | 33.1 | <.0001 |
| Thiazolidinedione | 3.3 | 2.5 | 1.7 | <.0001 |
| Insulin and oral therapy | 12.6 | 16.7 | 17.3 | <.0001 |
| Statin | 80.4 | 79.0 | 83.2 | <.0001 |
| Other lipid lowering agent | 5.1 | 5.4 | 6.0 | <.0001 |
| ACE inhibitor | 50.2 | 44.5 | 41.3 | <.0001 |
| Other anti-hypertensive | 72.7 | 80.0 | 90.8 | <.0001 |
Rates of complications and mortality by class
Among the complications, infections had the highest incidence rate followed by cardiovascular complications, microvascular complications, hypoglycemia, and hyperglycemia. This ranking of complications was consistent across all three classes. Complication and mortality rates differed substantially across classes and were highest in Class 3, intermediate in Class 2 and lowest in Class 1 (Table 3). For example, the age, sex and race-adjusted rates for cardiovascular events (per 100 person-years) for Classes 3, 2 and 1 were 6.5 (95% CI = [6.3, 6.6]), 2.3 (95% CI = [2.2, 2.3]) and 1.6 (95% CI = [1.6, 1.7]), respectively. The rates for hypoglycemia were 2.1 (95% CI = [2.0, 2.2]), 1.2 (95% CI = [1.1, 1.2]) and 0.7 (95% CI = [0.7, 0.7]), respectively. The mortality rates were 8.0 (95% CI = [7.8, 8.2]), 3.8 (95% CI = [3.6, 3.9]) and 2.3 (95% CI = [2.3, 2.4]), respectively.
Discussion
We identified three distinctive health status classes with significantly different risk of future events. Just over half of the population was in Class 1 which had the lowest prevalence of baseline comorbidities and future incident complications. The remaining population was roughly divided between Class 2 with the highest prevalence of baseline obesity, arthritis, and depression, and Class 3 with the highest prevalence of baseline cardiovascular conditions. Compared to Class 1, the risk of future complications, hypoglycemia, and mortality were higher in Class 2 and highest in Class 3.
The latent class model described here may be used to place individual patients into a specific class by computing the posterior probability of class membership given the patient’s history of comorbidities. While this cannot be done on the basis of a simple summed score, the calculation is straightforward and probabilities could easily be precomputed for all possible combinations of comorbidities and then made available online via a lookup table for clinical use.24 Importantly, this calculation does not require knowledge of all comorbidities, and thus can be performed even in cases where some information is unavailable (under the assumption that such information is missing at random). As noted above the probability of class membership was lower than 0.75 for 30 percent of the sample, reflecting an inherent ambiguity in classifying certain patients. Thus, as with instruments which yield a scale score or with the calculation of risk scores (e.g., genetic risk scores), appropriate cutoffs would need to be determined to ensure adequate certainty.
There are several indicators that these three health status classes may reflect different stages along a common pathophysiologic pathway. Patient age and duration of diabetes are incrementally higher across the classes. For example, the proportion of octogenarians ranges from 16.9% in Class 1 to 25.9% in Class 2 and 37.2% in Class 3. We also know from our analysis of incident complications that over time some Class 1 or Class 2 patients acquire complications such cardiovascular events that would shift them into a profile that is more consistent with that of Class 3. An alternative hypothesis is that Class 2 and Class 3 may represent varying stages in the development of frailty in older adults with diabetes.28-30 We found that the prevalence of frailty based on the Segal Frailty Index31 was lowest for Class 1 (10.7%), intermediate for Class 2 (29.6%), and highest for Class 3 (43.4%).
These results were largely consistent with prior research conducted in the National Social Life Health and Aging Project (NSHAP), a nationally representative sample of community-dwelling adults.7 In two prior analyses, utilizing different waves of NSHAP (N=750 Wave 1, N=884 Wave 2), a similar set of three classes were found.7,22 The similarities with the three-class model in the present study using an independent and far larger cohort suggests that these classes reflect reliable clinical phenotypes common to older adults with diabetes. The important and unique contribution of the present study is the characterization of the risk of future events.
The three-class model has implications for population health planning given the relative size of the classes and their distinctive profiles of risks of diabetes complications. There may be regional and national differences in the composition of the classes and these differences may serve as a rough surrogate for future health outcomes expected for different populations. For a health system, distinct services are likely to be required to meet the needs of each class and the relative size of each class in a population (i.e., class-mix distribution) may be valuable for decisions regarding resource allocation. The system can also be an important starting point for implementing tailored preventive strategies for each class of patients.
Current diabetes guidelines recommend an individualized approach to goal setting and treatment selection for older adults that begins with an assessment of health status that includes mortality risk and functional impairment. The guidelines from multiple societies8 32 make recommendations based on three classes of health status (e.g., good, complex, frail/limited life expectancy). These guidelines share an underlying competing risk model that suggests that glucose control can be relaxed as the complexity of the patient rises because patients are unlikely to benefit from therapies if the time-to-benefit for a treatment exceeds their life expectancy.33 For the three classes identified in the present study, the risk of mortality rises successively from Class 1 to Class 3 which is consistent with existing tiered approaches to tailored A1C goal selection. However, we also find that Class 3 has the highest risk of cardiovascular complications and hypoglycemia, making the members ideal candidates for cardio- and renoprotective agents, such as SGLT-2 inhibitors and GLP-1 agonists.34 35 One approach to reconciling these two distinct care implications (de-intensifying care vs. initiating use of newer drugs) for Class 3 is to further identify subgroups of patients within it. Within the class, patients may differ based on other important dimensions of health status beyond comorbidity such as impairments in activities of daily living, instrumental activities of daily living, cognition, and sensory functioning. Each person with diabetes is not simply a member of a particular health status group, based on their comorbid conditions, but rather a complex person with their own personal values, needs, and preferences.
Patients from these three classes may have been included at very different frequencies in randomized controlled trials of diabetes treatments. Class 1 patients with very few comorbidities are likely very similar to most of the older patients with newly diagnosed diabetes enrolled in the UKPDS. Like the patients in UKPDS, Class 1 patients have a longer life expectancy and therefore the pursuit of intensive glucose control (A1C<7.0%) is well supported. Class 3 patients with their high prevalence of baseline cardiovascular disease were well-represented in trials of very intensive glucose control10, and the more recent cardiovascular outcome trials of newer glucose-lowering agents. It is less clear to what extent Class 2 patients have been represented in these trials. If these comorbidity classes could be identified in clinical trial data, retrospective analyses of heterogeneity of treatment effects might reveal class-specific differences in response to different levels of alternative diabetes medication choices, and lifestyle interventions.36,37 In future clinical trials, the class system would be valuable for identifying alternative strategies, specific to each class, to prevent complications.
Our study does have its limitations. The clinical course of disease among patients in the Registry is, in part, a product of the access and quality of diabetes care delivered within an integrated healthcare delivery system. The composition of classes and the patterns of incident complications may differ from those in other clinical settings and populations. It is also important to note that the event rates in this study are based on a particular coding strategy which we applied systematically to all patient subgroups.
Despite these limitations, this study provides an important advance in efforts to develop an empiric approach to classifying and understanding health status classes in older adults with diabetes. The study confirms a three-class system previously found in a separate but older data set and characterizes for the first time the difference in risk of future complications across classes. This study provides a new launch point for future research that must still address an individual’s progression through classes over time, how the association of glucose control and outcomes may vary across classes, and the heterogeneity of effectiveness and safety of different drugs across classes.
Supplementary Material
Supplementary Table S1. Codes Used to Identify Baseline Comorbidities and Incident Outcomes
Supplementary Table S2. Comparison of latent class model performance statistics.
Supplementary Table S3. Comparison of outcome C-statistics by alternative latent class models.
Supplementary Figure S1. Comparison of outcome C-statistics by alternative latent class models.
Key Points.
Among older adults with diabetes, latent class analysis revealed three health status classes with distinctive patterns of comorbid conditions.
The study confirms a three-class system previously found in a separate but older data set.
The risk of future cardiovascular events, hypoglycemia, and mortality varies widely across the three classes.
Why does it matter?
Current diabetes care guidelines for older adults recommend stratifying care by health status but offer inconsistent definitions of comorbidity complexity. Naturally occurring clusters of comorbidities may present common pathophysiologic pathways or stages of progression of a disease. The three health status classes identified in this study predict different patterns of health and can inform population health management.
Acknowledgements:
Funding: This work was supported by the National Institutes of Health (R01 AG063391). Other NIH support include K24AG069080 (Dr. Huang), P30 DK092949 (Drs. Huang and Laiteerapong), and P30 DK092924 (Dr. Karter).
Footnotes
Conflicts of Interest: The authors have no conflicts.
References
- 1.Laiteerapong N, Huang E. Diabetes in Older Adults. In: Cowie C, Casagrande S, Menke A, et al. , eds. Diabetes in America. Vol NIH Pub No. 17-1468. 3rd ed. Bethesda, MD: National Institutes of Health; 2017:16.11–16.26. [Google Scholar]
- 2.Huang ES, Basu A, O'Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. Dec 2009;32(12):2225–2229. dc09-0459 [pii] 10.2337/dc09-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A Data Book: Health Care Spending and the Medicare Program. 425 Eye Street, NW • Suite 701 • Washington, DC 20001 (202) 220-3700 • Fax: (202) 220-3759 • www.medpac.gov2013.
- 4.Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J. Am. Geriatr. Soc Jul 2008;56(7):1183–1190. JGS1757 [pii] 10.1111/j.1532-5415.2008.01757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann. Intern. Med Jul 1 2008;149(1):11–19. 10.7326/0003-4819-149-1-200807010-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaum C, Cigolle CT, Boyd C, et al. Clinical complexity in middle-aged and older adults with diabetes: the Health and Retirement Study. Med. Care Apr 2010;48(4):327–334. 10.1097/mlr.0b013e3181ca4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laiteerapong N, Iveniuk J, John PM, Laumann EO, Huang ES. Classification of older adults who have diabetes by comorbid conditions, United States, 2005-2006. Prev Chronic Dis. 2012;9:E100. 10.5888/pcd9.110287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. Dec 2012;35(12):2650–2664. 10.2337/dc12-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. Sep 12 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med Jun 12 2008;358(24):2545–2559. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. Jun 12 2008;358(24):2560–2572. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. Jan 8 2009;360(2):129–139. 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 13.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. Nov 26 2015;373(22):2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 14.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. Jun 13 2016. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilla SJ, Schoenborn NL, Maruthur NM, Huang ES. Approaches to Risk Assessment Among Older Patients With Diabetes. Current diabetes reports. Jul 19 2019;19(8):59. 10.1007/s11892-019-1172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karter AJ, Parker MM, Moffet HH, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med. Care Feb 2004;42(2):110–115. 10.1097/01.mlr.0000109023.64650.73 [DOI] [PubMed] [Google Scholar]
- 17.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. May 15 2002;287(19):2519–2527. 10.1001/jama.287.19.2519 [DOI] [PubMed] [Google Scholar]
- 18.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. Jun 5 2001;103(22):2668–2673. 10.1161/01.cir.103.22.2668 [DOI] [PubMed] [Google Scholar]
- 19.Karter AJ, Schillinger D, Adams AS, et al. Elevated Rates of Diabetes in Pacific Islanders and Asian Subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care. Oct 24 2012. 10.2337/dc12-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder EB, Donahoo WT, Goodrich GK, Raebel MA. Validation of an algorithm for identifying type 1 diabetes in adults based on electronic health record data. Pharmacoepidemiol Drug Saf. Oct 2018;27(10):1053–1059. 10.1002/pds.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. Apr 2013;36(4):914–921. 10.2337/dc12-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung V, Wroblewski K, Schumm LP, Huisingh-Scheetz M, Huang ES. Re-examining the Classification of Older Adults with Diabetes by Comorbidities and Relationship with Frailty, Disability, and 5-year Mortality. The journals of gerontology. Series A, Biological sciences and medical sciences. May 18 2021. 10.1093/gerona/glab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva Selistre L, Rech DL, de Souza V, Iwaz J, Lemoine S, Dubourg L. Diagnostic Performance of Creatinine-Based Equations for Estimating Glomerular Filtration Rate in Adults 65 Years and Older. JAMA Intern Med. Apr 29 2019. 10.1001/jamainternmed.2019.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartholomew DJ, Knott M, Moustaki I. Latent Variable Models and Factor Analysis: A Unified Approach. Third ed: John Wiley & Sons; 2011. [Google Scholar]
- 25.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall/CRC; 1984. [Google Scholar]
- 26.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med May 10 2011;30(10):1105–1117. 10.1002/sim.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman S. Biostatistical Methods in Epidemiology: Wiley; 2001. [Google Scholar]
- 28.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. Mar 2013;14(1):77–86. 10.1007/s11154-012-9234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulop T, Dupuis G, Witkowski JM, Larbi A. The Role of Immunosenescence in the Development of Age-Related Diseases. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. Mar-Apr 2016;68(2):84–91. [PubMed] [Google Scholar]
- 30.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr. Rev Aug 2004;25(4):543–567. 10.1210/er.2003-0012 [DOI] [PubMed] [Google Scholar]
- 31.Segal JB, Chang HY, Du Y, J DW, M CC, Varadhan R. Development of a Claims-based Frailty Indicator Anchored to a Well-established Frailty Phenotype. Med. Care Jul 2017;55(7):716–722. 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. May 1 2019;104(5):1520–1574. 10.1210/jc.2019-00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. Dec 25 2013;310(24):2609–2610. 10.1001/jama.2013.282612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA. Apr 17 2018;319(15):1580–1591. 10.1001/jama.2018.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. Jan 2021;44(Suppl 1):S111–S124. 10.2337/dc21-S009 [DOI] [PubMed] [Google Scholar]
- 36.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. Jul 11 2013;369(2):145–154. 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. The New England journal of medicine. Mar 29 2012;366(13):1209–1217. 10.1056/NEJMoa1110294 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Codes Used to Identify Baseline Comorbidities and Incident Outcomes
Supplementary Table S2. Comparison of latent class model performance statistics.
Supplementary Table S3. Comparison of outcome C-statistics by alternative latent class models.
Supplementary Figure S1. Comparison of outcome C-statistics by alternative latent class models.