Introduction
Chronic pain is common affecting up to 100 million Americans with musculoskeletal pain and 20–40% of the population [21]. An acute bout of exercise can exacerbate chronic pain [39], which creates a barrier to participation in exercise programs aimed at reducing pain severity and improving physical function [40; 41]. To address activity-induced pain, our laboratory developed an animal model that combines fatiguing muscle contractions with a non-painful, low-dose muscle insult [33]. This model produces a sex-dependent pain phenotype in which females develop bilateral and longer-lasting pain, while males develop unilateral and shorter-lasting pain [33].
Muscle fatigue releases a wide range of metabolites including adenosine triphosphate (ATP), protons, and lactate [57; 66; 72]. The physiological levels of the metabolites act together to activate sensory neurons [7; 45], release inflammatory cytokines such as interleukin-1β (IL-1β) [49], and produce pain [42; 51]. These extracellular fatigue metabolites activate the molecular receptor types of acid-sensing ion channels [23] and purinergic type 2X (P2X) [9]. The expression of P2X family of ligand-gated ion channels on immune cells, including macrophages, consists of both P2X4 and P2X7 receptors [10]. Prior work shows an increase in the number and protein expression of P2X4 in muscle macrophages following induction of activity-induced pain model, and prevention of hyperalgesia by depletion of muscle macrophages or downregulation of P2X4 receptors on muscle macrophages [31; 49]. P2X7 receptors play a crucial role in innate immune responses through activation of the NOD-like receptor protein 3 (NLRP3) inflammasome, which triggers cleavage of Caspase-1 and leads to mature IL-1β release [24]. Recent work shows a role for non-neuronal NLRP3 in the pain, inflammation and nociceptor sensitization exclusively in male mice. However, in female mice, non-neuronal NLRP3 does not mediate pain and inflammation [18]. These data suggest that activation of the P2X7-NLRP3 inflammasome pathway to release IL-1β may have a sex specific role in pain production.
Therefore, the present study aimed to investigate the sex differences in the role of P2X7/NLRP3/Caspase-1 pathway in development and maintenance of activity-induced muscle pain using and in vivo model and in vitro lipopolysaccharide (LPS)-primed macrophages. We hypothesized that pharmacological blockade of P2X7 receptors, NLRP3, and Caspase-1 would prevent development of activity-induced muscle pain in vivo, and release of IL-1β in macrophages in vitro, with a male specific contribution to muscle pain.
Materials and methods
Animal care
All experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals. A total of 324 male and 334 female C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME, USA), aged 6–8 week were used in this study. An equal number of male and female mice were used for each experiment, except for an experiment with an antibody to IL-1β and depletion of macrophages. Mice were housed under a 12hr/12hr light/dark cycle with free access to food and water ad libitum.
Animal Model of Activity-Induced Muscle Pain
The animal model was induced by 2, 20μl pH 5.0 saline injections, separated by 5 days, in the left gastrocnemius muscle combined with 6 min of electrically stimulated fatiguing muscle contractions [33]. Mice were anesthetized with 2–4% isoflurane and given intramuscular (i.m.) injection into the left gastrocnemius of 20μl normal 0.9% saline (Hospira, Lake Forest, IL, USA) adjusted to pH 5.0 ± 0.1 with HCl. The unbuffered pH 5.0 saline injections reduce muscle pH to approximately 6.9 [63], which is comparable to decreases seen after intense exercise [57; 66; 72].
Five days after a first i.m. injection of 20μl pH 5.0 saline, mice were anesthetized with 2–4% isoflurane, needle electrodes were inserted into the belly of the left gastrocnemius muscle and 6 min of submaximal fatiguing contractions using a custom built device. The following parameters were used: a duty cycle of 47% (3.75sec on, 4.25sec off) using trains of stimulations at 40Hz and an amplitude of 7V. This protocol results in approximately 50% reduction in muscle force [33]. Immediately following the fatiguing muscle contractions, the mice were given a second i.m. injection of 20μl pH 5.0 saline into the same gastrocnemius muscle. Pain free control mice for collection of gastrocnemius muscle received 2, 20μl pH 7.2 ± 0.1 saline injections, separated by 5 days, in the gastrocnemius muscle [33]. Mice were randomly assigned to each group using computer generated randomization. The investigator was blinded to group during behavioral measurements and sample assessment.
Measurement of Muscle Withdrawal Thresholds
Nociception was measured by determining muscle withdrawal thresholds (MWT) of the gastrocnemius muscle bilaterally. MWT were measured by applying force-sensitive tweezers to the belly of the gastrocnemius muscle, where lower thresholds indicate greater sensitivity [62]. Prior studies show anesthetizing the skin during this measurement did not change withdrawal thresholds, but anesthetizing the deep tissue increased the threshold, thus validating this as a measurement of muscle hyperalgesia [62].
Briefly, mice were acclimated to the testing paradigm in two 3–5 min sessions over a 2 day period prior to baseline assessment. Mice were placed in a gardener’s glove, the hindlimbs held in extension, and the muscle squeezed with force-sensitive tweezers until the animal withdrew its hindlimb. Five minutes elapsed between each MWT assessment to prevent behavioral sensitization to testing. Each animal was tested bilaterally and the average of three measurements was used to determine withdrawal thresholds for each limb.
Intramuscular injection of antagonists
To test whether P2X7, NLRP3, Caspase-1, and IL-1β mediate development of activity-induced muscle pain, selective inhibitors were given intramuscularly (20μl volume) prior to or after (24hr or 1 week) induction of the activity-induced muscle pain model and compared to vehicle injections (Table 1A). For those treated before the induction of the model, MWT were measured before (baseline) and 24hr, 72hr and 1week after injection of inhibitor or its vehicle (Table 1B). For those treated after the induction of the model, MWT were measured before and after induction of the model, and 30 min or 24hr after injection of the inhibitor or its vehicle.
Table1.
Study procedures A) Type of inhibitors
| Inhibitor | Catalog number, Company | Vehicle |
|---|---|---|
| Macrophage depletion (Clodronate) | CP-005–005, Liposoma BV | Phosphate buffered saline |
| P2X7 receptor antagonist (A740003) | 3701, Tocris Bioscience | 0.9% saline with 10% DMSO |
| NLRP3 antagonist (MCC950, also called CP‑456773 and CRID3) | S7809, Selleckchem | 0.9% saline |
| Caspase-1 antagonist (Z-WEHD-FMK) | FMK002, R&D Systems | 0.9% saline with 10% DMSO |
| Antibody to IL-1β | BE0246, BioXcell | 0.9% saline |
DMSO, dimethyl sulfoxide
Drugs
The P2X7 antagonist A740003 (Tocris Bioscience, Ellisville, MO, USA) was dissolved in 10% DMSO and normal saline, and blocks P2X7 receptors activation including IL-1β release and pain behaviors [25; 34]. The NLRP3 antagonist MCC950 (Selleckchem, Houston, TX, USA) was dissolved in normal saline, and is a potent inhibitor of NLRP3 [47; 68; 76]. The Caspase-1 inhibitor Z-WEHD-FMK (R&D Systems, Minneapolis, MN, USA) was dissolved in 10% DMSO and normal saline and inhibits of Caspase-1 [27; 77]. Vehicle control for all inhibitors was saline with 10% DMSO. To block effects of IL-1β, mice were pretreated 30 min prior to induction of the activity-induced muscle pain model with an anti-mouse antibody to IL-1β (BioXcell, Lebanon, NH, USA) as previously published [49]. Vehicle control for IL-1β antibody was an IgG control.
Collection of gastrocnemius muscle
For qPCR experiments, the gastrocnemius muscle was collected 2hr, 24hr, and 1 week after induction of the activity-induced muscle pain model (n = 16; 8 males and 8 females, control; n = 16; 8 males and 8 females, respectively for each time point). Mice were euthanized with CO2 at a rate of 3 L/min for 1 min following cessation of breathing. The left gastrocnemius muscle was harvested immediately after euthanasia and placed in RNA later (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA). Total cellular RNA was extracted from left gastrocnemius muscle using QIAzol reagent together with RNeasy Mini Kit (Qiagen, Hilden, Germany), and RNA Clean & Concentrator-5 Kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions. Samples of isolated total RNA were stored at −80°C until analysis.
To examine protein expression of IL-1β, cellular protein was extracted from left gastrocnemius muscle 2hr, 24hr, and 1 week. A separate group of animals was used to extract muscle to examine protein expression (n = 16; 8 males and 8 females, control; n = 16; 8 males and 8 females, respectively for each time point). The tissue was collected and homogenized in a solution of sterile PBS (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) containing complete protease inhibitor cocktail (Millipore Sigma, St. Louis, MO, USA). The samples were centrifuged at 12,000 rpm at 4°C for 20 min, and the supernatants were used to evaluate the protein levels in the gastrocnemius muscle. The protein concentrations were analyzed using a Quick Start Bradford Protein Assay Kit with bovine serum albumin as a standard (Bio-Rad Laboratories, Hercules, CA, USA). The samples were standardized to 5.0mg/ml by diluting the concentration in the sterile PBS, and then stored at −80°C until analysis.
Peritoneal macrophage culture
To examine effects inhibition of the P2X7-/NLRP3-/Caspase-1 pathways on IL-1β release from macrophages, we collected peritoneal macrophages as follows. On day 0, mice were euthanized with CO2 at a rate of 3 L/min for 1 min following cessation of breathing. Immediately after euthanasia, 4 ml for male and 3 ml for female of sterile PBS (Gibco; Thermo Fisher Scientific) was injected into the peritoneal cavity of mice. The medium recovered from mice was centrifuged and then re-suspended in complete MEM-alpha medium (Gibco; Thermo Fisher Scientific). The cells were quantified using Countess™ Cell Counting Chamber Slides (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) and Countess® Automated Cell Counter (Invitrogen; Thermo Fisher Scientific) according to the manufacturer’s instructions. Macrophages were pooled from 6 mice for each sample to obtain a sufficient number of macrophages for plating for a total of 48 mice per sex to obtain a final sample size of n=8 per sex. Macrophages were standardized to 2.0 × 106 cells/ml by diluting the concentration, and then plated in 24-well, non-treated tissue culture plate (Nest Biotechnology, Wuxi, China) for 24hr (37°C, 5% CO2).
On day 1, non-adherent cells were discarded, and fresh medium was added. This allows for isolation of peritoneal macrophages as >90% of cells remaining in dish will be macrophages [2; 79]. The cells were incubated with or without 10 ng/ml of LPS (Chondrex, Redmond, WA, USA) for 4hr, and then treated with pH 7.4 media (26mM NaHCO3 (Research Products International, Mount Prospect, IL, USA), 25mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)(Research Products International,) or pH 6.5 media (26mM NaHCO3, 20mM 2-(N-morpholino) ethanesulfonic acid (MES)(Millipore Sigma), 5mM HEPES) with or without 1mM ATP (Millipore Sigma)) for 24hr as we previously published [11, 27]. The cells were treated with the P2X7 receptor (100μM), NLRP3 (10μM), or Caspase-1 antagonist (100μM), or vehicle (10% DMSO) during LPS prior to 1hr of pH 7.4 media or pH 6.5 media with ATP.
At the end of the experiments on day 2, the cells were collected for protein analysis and qPCR. The supernatants were stored at −80°C, and then analyzed for protein analysis using multiplex. Total cellular RNA was extracted using Trizol reagent (Invitrogen; Thermo Fisher Scientific) and RNA Clean & Concentrator-5 Kit (Zymo Research) according to the manufacturer’s instructions. Samples of isolated total RNA were stored at −80°C until analysis.
qPCR
Total RNA concentrations were measured on a NanoDrop One (Thermo Fisher Scientific). The ratio of A260/A280 was greater than 1.8. Total RNA of 500ng in muscle, and 10ng in macrophages was subjected to reverse transcription using the AffinityScript qPCR cDNA Synthesis Kit (Agilent Technologies, Santa Clara, CA, USA), according to the manufacturer’s instructions. The synthesized cDNA were stored at −20°C, and then used as a template for qPCR with the Power SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Waltham, MA, USA).
All gene-specific PCR primers were obtained from Primer Bank and manufactured by Integrated DNA Technologies (Coralville, IA, USA) [65; 69; 74]. All qPCR primers were designed and assessed for specificity of target gene using the Primer-BLAST software (National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD, USA; http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Forward and reverse primer sequences for 36B4, P2X7, NLRP3, Caspase-1, and IL-1β are shown in Table 1C. 36B4 was used as an endogenous control to normalize results for each sample, because of the high expression levels and stabilities for normalization according to our preliminary results. The qPCR analysis was performed with a QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific) using the ΔΔCt method [59]. The qPCR was performed as follows: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 60°C. The qPCR analysis was run in triplicate with cycle threshold values averaged. All analysis were done with the experimenter blinded to group.
Simplex and Multiplex Cytokine Assays
The concentrations of several cytokines were measured from gastrocnemius muscle and cultured macrophages. The primary cytokine of interest was IL-1β, and secondary cytokines were granulocyte macrophage-colony stimulating factor (GM-CSF), interferon gamma (IFN-γ), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-18, and tumor necrosis factor alpha (TNF-α). Simplex and multiplex plates (Thermo Fisher Scientific) were processed using standard procedures provided by the manufacturer and read on a Bio-Plex 200 System (Bio-Rad Laboratories) at the University of Iowa Flow Cytometry Core Facility. Two standards were used to generate a standard curve across the detectable range. In case of an undetectable sample, the value of the lower limit of detection was used. Samples were run in duplicate and coefficient of variation was assessed for each cytokine. All samples showed a coefficient of variation less than 20%. All analysis were done with the experimenter blinded to group.
Depletion of macrophages in the muscle pain model in female mice
The contribution of macrophages in the activity-induced muscle pain model was previously demonstrated in male mice [31], but not investigated in female mice. To test whether macrophages contribute to the development of activity-induced muscle pain in female mice, macrophages in the muscle were depleted using injection of clodronate-containing liposomes (Liposoma BV, Amsterdam, Netherlands; 20.4mM) in the left gastrocnemius muscle, according to procedures previously used in male mice [31](Table 1A).
Briefly, female mice were injected with either active liposomes containing clodronate (n=4) or inactive liposomes containing phosphate buffered saline (PBS)(n=4). Both 2 days prior to and 1 day after the first injection of pH 5.0 saline (Day −2 and Day 1), mice were anesthetized with 2–4% isoflurane and given i.m. injections into 4 sites evenly spaced across the muscle belly (5μl per site) of the ipsilateral gastrocnemius muscle. MWT were measured at baseline prior to liposome injection, and at 24hr after induction of the muscle pain model.
Depletion of macrophages was confirmed using immunohistochemistry for F4/80 cells of treated gastrocnemius muscle. On after MWT analysis (at 48hr after induction of the muscle pain model), mice were deeply anesthetized (80 mg/kg ketamine and 10 mg/kg xylazine, intraperitoneal injection) and transcardially perfused with heparinized saline followed by freshly prepared 4% paraformaldehyde in 0.1 M PB. The gastrocnemius muscle was removed, stored in 4% paraformaldehyde in PB overnight, and stored 10–30% sucrose (4°C). The tissues were then frozen in cryomolds (OCT, Tissue Tek; Thermo Fisher Scientific, Waltham, MA, USA). Sections from the muscle were cut at 20μm with a cryostat and placed onto slides. Sections were blocked with 5% normal goat serum followed by an Avidin/Biotin blocking kit (Vector Labs, Burlingame, CA, USA) and then incubated overnight at room temperature with the primary antibody, rat anti-mouse F4/80 (1:1,000, AbD Serotec, Raleigh, NC, USA). The sections were rinsed, blocked with 5% normal goat serum, and incubated for one hour at room temperature in the secondary antibody, goat anti-rat Alexa 488 (1:500, Invitrogen; Thermo Fisher Scientific). Slides were then cover-slipped with Vectashield (Vector Labs). Images of sections were taken on an Olympus BX-61 light microscope (Diagnostic Instruments, Sterling Heights, MI, USA) in the Central Microscopy Facility at the University of Iowa. The total number of F4/80+ cells in twelve muscle sections was counted from each animal and averaged, using Image J software (NIH, Bethesda, MD, USA). All images were taken under the same conditions and stored for analysis. The investigator was blinded to treatment group during staining, image acquisition, and F4/80+ cell quantification.
Statistical analysis
For measurement of MWT for pharmacological experiments, power analysis determined the minimal sample size for withdrawal thresholds at 80% power and p<0.05 by comparing means for two independent samples (mean 1, 98%; mean 2, 60%; SD1, 25; SD2, 25) indicated a sample size of 8 mice per group. We used 8 animals per group for the highest dose, and performed 4 animals per group for lower doses. For MWT for clodronate experiments, we used 4 animals per group based on our previously published data [31]. For qPCR and protein analysis, we used preliminary results (mean 1, 3,000; mean 2, 300; SD1, 2,450; SD2, 10) [3] to estimate a sample size of 8 per group.
All data are reported as mean ± S.E.M. Group and sex differences in MWT for both the ipsilateral and contralateral MWT were analyzed with a repeated measures ANOVA, followed by post hoc Tukey’s test. The quantification of qPCR and Simplex cytokine analysis of gastrocnemius muscle (control and model) and depletion of macrophages (control and clodronate) compared two groups with a two-way ANOVA, followed by post hoc using an unpaired t-test. The quantification of qPCR and Multiplex cytokine analysis of LPS-primed macrophages were analyzed with a repeated measures ANOVA, followed by post hoc with a paired t-test, conducted with a Bonferroni correction. All data were analyzed using IBM SPSS Statistics 27.0 software (IBM, Armonk, NY, USA). A p-value of less than 0.05 was considered as statistically significant.
Results
Inhibition of P2X7, NLRP3, and Caspase-1 in the pain model prevents muscle hyperalgesia in male but not female mice
As previously reported [33; 43], induction of the activity-induced pain model produced a sex-dependent muscle pain phenotype. Females demonstrated a bilateral decrease in MWT while males showed a unilateral decrease in MWT (see Fig. 1, vehicle control group).
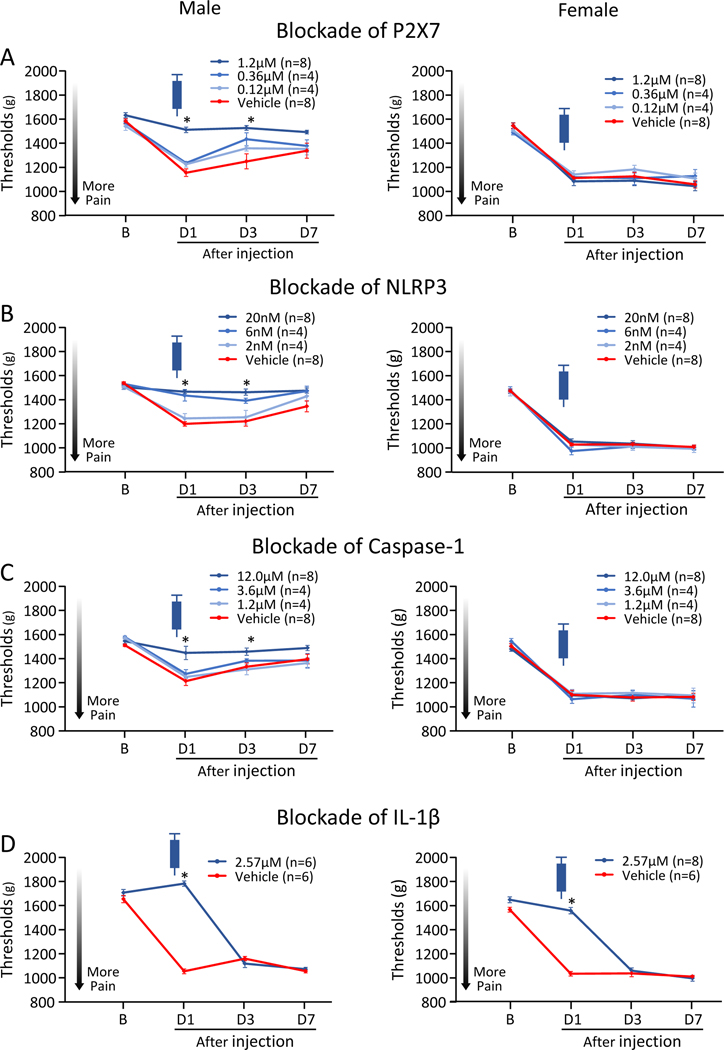
Figure 1. Ipsilateral MWT with pharmacological inhibition in the muscle prior to induction of the activity-induced muscle pain model.
A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. The decrease in muscle withdrawal thresholds in male, but not female, mice was prevented by administration of P2X7, NLRP3, and Caspase-1 inhibitors prior to induction of the model, while blockade of IL-1β prior to induction prevented muscle hyperalgesia in both male and female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Injection of the P2X7, NLRP3 and Caspase-1 inhibitors in the muscle prior to induction of the activity-induced muscle pain model, dose-dependently prevented the decrease in MWT ipsilaterally in male mice, but not in female mice, when compared to vehicle control (Fig. 1A–1C). Effects for group, sex, and group by sex were found for all 3 inhibitors (Table 2A). Twenty-four hours after injection in males, but not females, the decrease of MWT was prevented by P2X7 inhibitor A740003 (1.2μM; M, p<0.001*, d=4.29; F, p=0.74, d=0.28), NLRP3 inhibitor MCC950 (20nM; M, p<0.001*, d=5.05; F, p=0.16, d=0.46), and Caspase-1 inhibitor Z-WEHD-FMK (12.0μM; M, p=0.007*,d=1.64; F, p=0.91, d=0.04). Similarly, the decrease of MWT was prevented at 72hr after inhibitor by P2X7, NLRP3, and Caspase-1 between the highest tested dose and vehicle controls (p<0.001–0.04*), but the effects returned by 1 week after injection of the inhibitor.
Table 2.
Statistical results. A) Results for repeated-measures analysis of variance for MWT ipsilaterally.
| Effect for Group | Effect for Sex | Effect for Group*Sex | ||
|---|---|---|---|---|
| P2X7 | Prior | F3,40=4.60; p<0.01* | F1,40=78.09; p<0.01* | F3,40=7.93; p<0.01* |
| 24hr | F1,28=0.10; p=0.74 | F1,28=24.37; p<0.01* | F1,28=3.98; p=0.05 | |
| 1week | F1,12=0.25; p=0.62 | F1,12=5.52; p=0.03* | F1,12=0.20; p=0.66 | |
| NLRP3 | Prior | F3,40=13.70; p<0.01* | F1,40=470.97; p<0.01* | F3,40=12.98; p<0.01* |
| 24hr | F1,28=8.74; p<0.01* | F1,28=37.27; p<0.01* | F1,28=6.94; p=0.01* | |
| 1week | F1,12=0.05; p=0.82 | F1,12=18.59; p<0.01* | F1,12=0.21; p=0.64 | |
| Caspase-1 | Prior | F3,40=2.86; p=0.04* | F1,40=123.98; p<0.01* | F3,40=3.21; p=0.03* |
| 24hr | F1,28=12.52; p<0.01* | F1,28=67.84; p<0.01* | F1,28=8.96; p<0.01* | |
| 1week | F1,12=0.24; p=0.63 | F1,12=38.20; p<0.01* | F1,12<0.01; p=0.99 | |
| IL-1β | Prior | F1,22=148.05; p<0.01* | F1,22=31.54; p<0.01* | F1,22=2.75; p=0.11 |
| 24hr | F1,28=5.36; p=0.02* | F1,28=3.31; p=0.08 | F1,28=3.51; p=0.07 | |
| 1week | F1,12=3.00; p=0.10 | F1,12=0.18; p=0.67 | F1,12=0.42; p=0.52 |
p<0.05 with a repeated measures ANOVA.
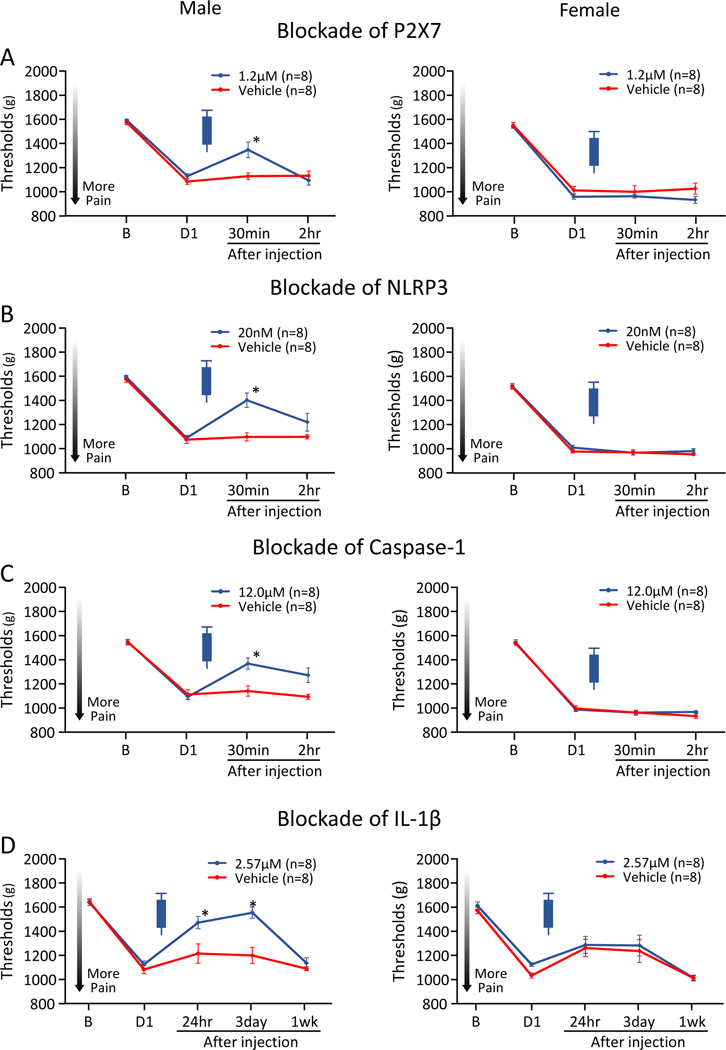
Injection of the P2X7, NLRP3 and Caspase-1 inhibitors in the muscle, 24hr after induction of the activity-induced pain model, temporarily alleviated the decrease in MWT ipsilaterally in male mice, but not in female mice, when compared to vehicle control (Fig. 2A–2C). Significant effects for group, sex and group by sex occurred for all 3 inhibitors (Table 2A). There were significant increases in MWT 30 minutes after injection in males, but not females, for the P2X7 inhibitor (M, p=0.01*, d=1.46; F, p=0.53, d=0.32), NLRP3 inhibitor (M, p<0.001*, d=2.08; F, p=0.92, d=0.05), and Caspase-1 inhibitor (M, p=0.004*, d=1.69; F, p=0.99, d=0.01). The effects of P2X7, NLRP3, and Caspase-1 on MWT returned to baseline levels by 6hr (data not shown).
Figure 2. Ipsilateral MWT with pharmacological inhibition in the muscle 24hr after induction of the activity-induced muscle pain model.
A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 24hr after induction of the model alleviated muscle hyperalgesia in male, but not female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Injection of the P2X7, NLRP3 and Caspase-1 inhibitors in the muscle, 1 week after induction of the activity-induced pain model had no effect on ipsilateral side in male or female mice, in P2X7 inhibitor A740003, NLRP3 inhibitor MCC950, and Caspase-1 inhibitor Z-WEHD-FMK. Injection of the inhibitors in the muscle had no effect on contralateral side in male or female mice at any time periods (Supplemental Figure 1–4, Supplemental Table 1).
Inhibition of IL-1β in the pain model prevents muscle hyperalgesia in male and female mice
Since activation of P2X7-NLRP3 pathways mediates IL-1β release [24], we tested if blockade of IL-1β mediated the hyperalgesia by injecting a neutralizing antibody into the muscle. Injection of a neutralizing IL-1β antibody in the muscle, prior to induction of the activity-induced muscle pain model, significantly prevented the decrease in MWT ipsilaterally in male and female mice (Fig. 1D) when compared to vehicle control. There were significant effects for group and sex but not group by sex (Table 2A). Post hoc testing showed that blockade of IL-1β prevent the increase in both male and female mice (M, p<0.001*, d=13.00; F, p<0.001*, d=7.69).
Blockade of IL-1β in the muscle 24hr after induction of the activity-induced pain model, alleviated the decrease in MWT ipsilaterally in male mice, but not in female mice, when compared to vehicle control (Fig. 2D) with effects for group but not sex or group by sex (Table 2A). Post hoc testing showed significant reductions on 24hr after induction of the model in males (M, p=0.02*, d=1.26; F, p=0.81, d=0.12).
Blockade of IL-1β in the muscle 1 week after induction of the activity-induced pain model had no effect on ipsilateral side in male or female mice. Inhibition of IL-1β in muscle significantly prevented the decrease in MWT contralaterally prior to, but not after, the induction of the activity-induced muscle pain model in female, but not male mice (M, p=0.94, d=0.04; F, p<0.001*, d=9.04)(Supplemental Figure 1–4, Supplemental Table 1). This demonstrates that IL-1β is important for production of pain in males and females at the time of induction and is important in the early phase of maintaining hyperalgesia in males in this activity-induced muscle pain model.
qPCR and cytokine analysis of gastrocnemius muscle of the activity-induced muscle pain model
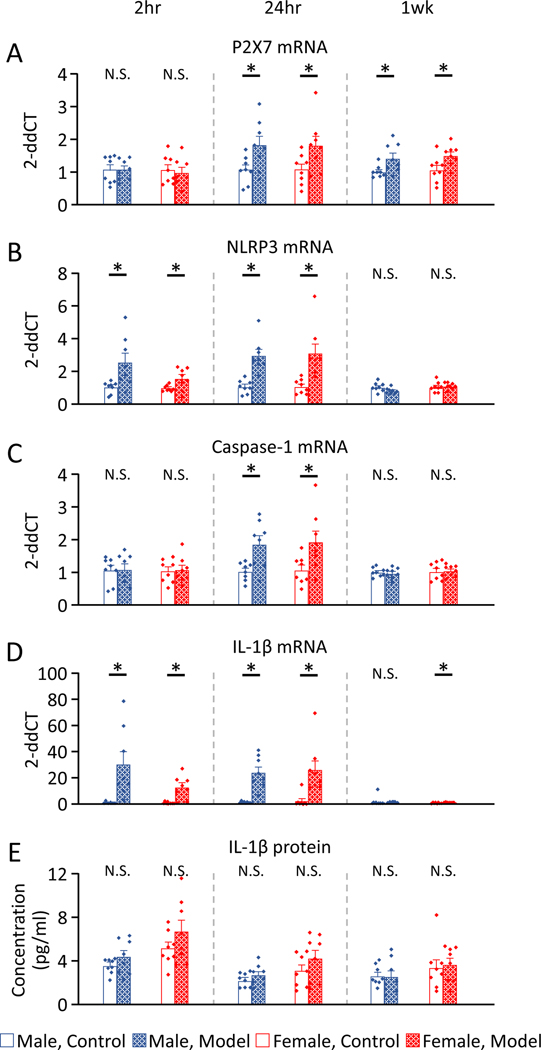
Next we examined if there were changes in mRNA or IL-1β levels after induction of the pain model in the gastrocnemius muscle. There was no sex differences in the expression of mRNA or cytokines (Table 2B). Expression of mRNA and protein in the gastrocnemius muscle after induction of the activity-induced muscle pain was significantly increased in male and female mice, when compared to pain-free controls 2hr after induction of the model for NLRP3 mRNA (M, p=0.01*, d=1.36; F, p=0.03*, d=1.16) and IL-1β mRNA (M, p=0.008*, d=1.55; F, p=0.01*, d=1.90), but not in P2X7 mRNA (M, p=0.99, d<0.01; F, p=0.69, d=0.20), Caspase-1 mRNA (M, p=0.94, d=0.04; F, p=0.86, d=0.09), and IL-1β protein (M, p=0.20, d=0.71; F, p=0.20, d=0.71)(Fig. 3).
Figure 3. mRNA and cytokine expressions of gastrocnemius muscle after induction of the activity-induced muscle pain, compared to pain-free controls.
A) P2X7 mRNA. B) NLRP3 mRNA. C) Caspase-1 mRNA. D) IL-1β mRNA. E) IL-1β protein.
P2X7, NLRP3, Caspase-1, and IL-1β mRNA 24hr after induction of the activity-induced muscle pain showed significantly increased in male and female mice. IL-1β protein showed no difference 24hr after induction of the model in male or female mice. Data is reported as mean ± S.E.M. N=8 in each group. *, p<0.05.
The expression of mRNA and protein in the gastrocnemius muscle 24hr after induction of the activity-induced muscle pain was significantly increased in male, and female mice, when compared to pain-free controls for P2X7 mRNA (M, p=0.02*, d=1.23; F, p=0.04*, d=1.10), NLRP3 mRNA (M, p<0.001*, d=2.52; F, p=0.003*, d=1.77), Caspase-1 mRNA (M, p=0.008*, d=1.54; F, p=0.02*, d=1.24), and IL-1β mRNA (M, p<0.001*, d=2.67; F, p=0.01*, d=1.70), but not in IL-1β protein (M, p=0.28, d=0.59; F, p=0.23, d=0.66).
The expression of mRNA in the gastrocnemius muscle 1 week after induction of the activity-induced muscle pain were significantly increased in both male and female mice, when compared to pain-free controls for P2X7 mRNA (M, p=0.02*, d=1.24; F, p=0.02*, d=1.24), and in female mice for IL-1β mRNA (M, p=0.52, d=0.33; p=0.005*, d=1.66). No changes were observed for NLRP3 mRNA (M, p=0.08, d=0.94; p=0.59, d=0.28), Caspase-1 mRNA (M, p=0.69, d=0.20; p=0.85, d=0.10), and IL-1β protein (M, p=0.85, d=0.10; F, p=0.74, d=0.18).
qPCR and Multiplex cytokine analysis of LPS-primed macrophages treated with ATP and proton
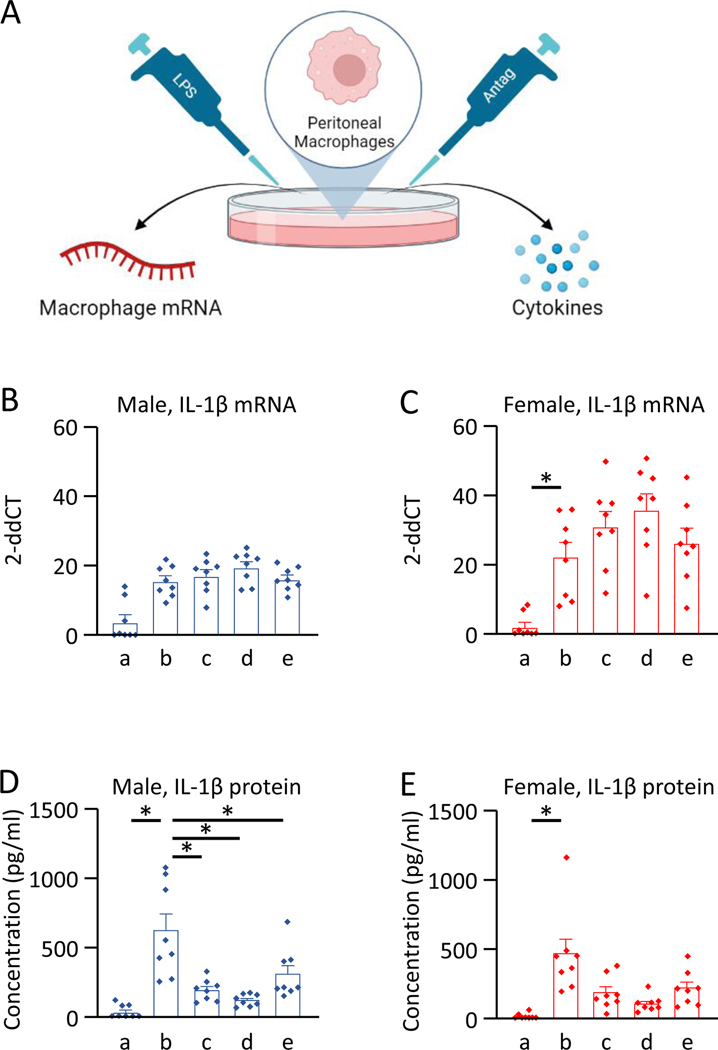
Since we previously showed an increase in IL-1β released from LPS-primed macrophages in cells treated with ATP and acidic saline [49], and the P2X7 pathway mediates an increase in IL-1β [24], we tested if this increase was prevented by pharmacological blockade of P2X7, NLRP3 or Caspase-1. IL-1β mRNA in macrophages was increased in LPS-primed macrophages with ATP and pH 6.5 in both male and female mice, when compared to the control group with no-LPS (M, p=0.010, d=2.27; p=0.006*, d=2.34). Blockade of P2X7, NLRP3 or Caspase-1 had no effect on the increased mRNA expression of IL-1β when compared to the vehicle control group in either male or female macrophages (Table 2C, Fig. 4).
Figure 4. qPCR and Multiplex cytokine analysis of LPS-primed macrophages treated with ATP and proton.
A) Protocol. B) IL-1β mRNA in male macrophages. C) IL-1β mRNA in female macrophages. D) IL-1β protein in male macrophages. E) IL-1β protein in female macrophages. Il-1β mRNA and protein were significantly increased after treatment of LPS-primed macrophages with ATP and pH 6.5 (b) when compared to unprimed macrophages treated vehicle and pH 7.2 (a). IL-1β protein showed significantly lower concentrations in the macrophages with inhibition of P2X7 receptors with A740003 (c), NLRP3 with MCC950 (d), and Caspase-1 with Z-WEHD-FMK (e) in male and female macrophages, when compared to the vehicle with LPS and pH 6.5 (b). Data is reported as mean ± S.E.M. N=8 in each group. *, p<0.05. Created on Biorender.
IL-1β protein released from LPS-primed macrophages treated with ATP, pH 6.5, and vehicle was significantly higher in male and female macrophages when compared to the control group with no-LPS (M, p=0.001*, d=2.43; F, p=0.003*, d=2.10). Blockade of P2X7, NLRP3 or Caspase-1 reduced the increase in IL-1β normally observed with ATP and acidic pH in the macrophages in both male and female macrophages, when compared to cells treated with vehicle with LPS, ATP and pH 6.5 (Table 2C, Fig. 4). Significant decreases were observed for macrophages treated with the P2X7 inhibitor A740003 (M, p=0.008*, d=1.76; F, p=0.023, d=1.21), NLRP3 inhibitor MCC950 (M, p=0.003*, d=2.09; F, p=0.014, d=1.65), and Caspase-1 inhibitor Z-WEHD-FMK (M, p=0.008*, d=1.16; F, p=0.022, d=1.06) when compared to vehicle controls.
The concentrations of IL-6, IL-10, GM-CSF, IFN-γ, IL-12p70, IL-13, IL-18, IL-2, IL-4, IL-5, and TNF-α from the macrophages with P2X7 inhibitor A740003, NLRP3 inhibitor MCC950, and Caspase-1 inhibitor Z-WEHD-FMK showed no differences, when compared to the vehicle with LPS and pH 6.5, in male or female macrophages (Supplemental Fig. 5).
Depletion of macrophages prevents development of muscle hyperalgesia and the increase in the number of F4/80+ macrophages regardless of sex
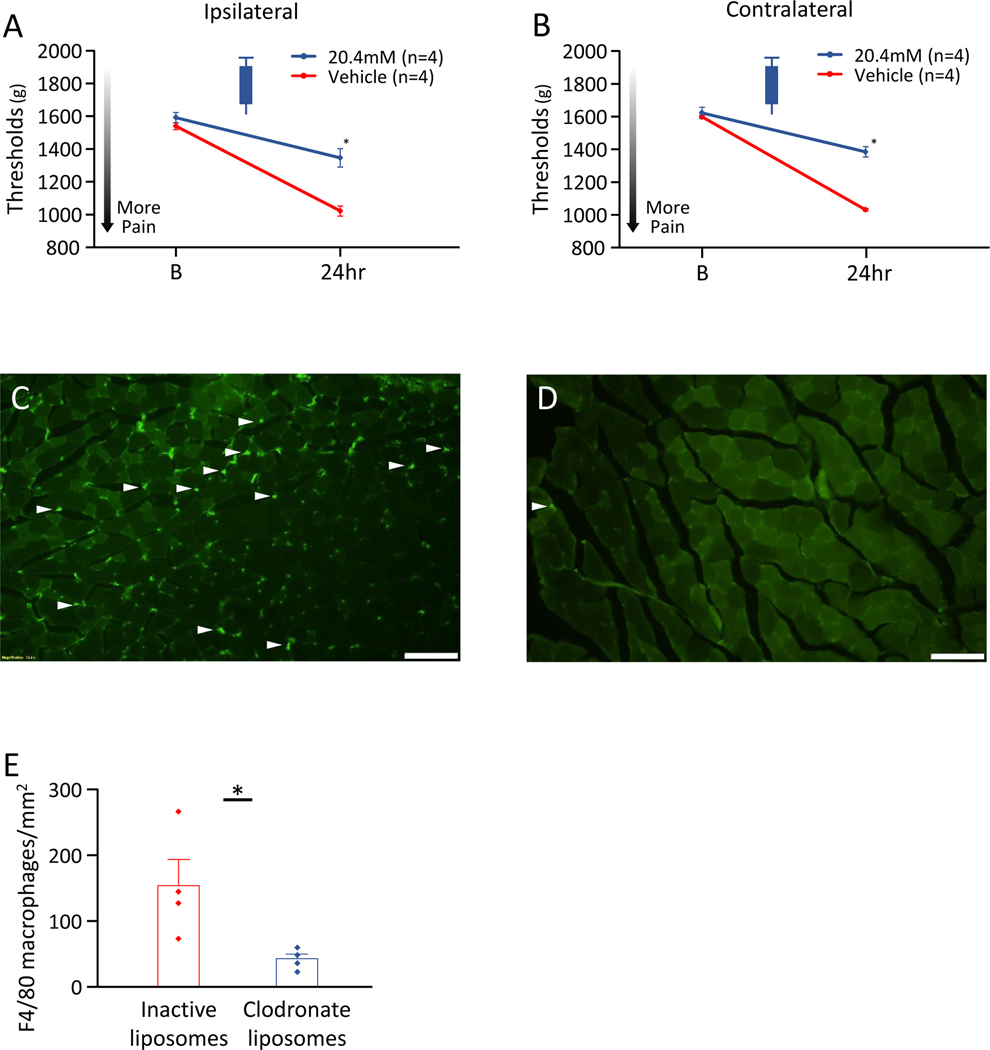
Since our prior study showed depletion of macrophages reduced hyperalgesia in male mice, and the current study showed no effect of blockade of P2X7, NLRP3, and Caspase-1 in female mice, we tested if depletion of macrophages in female mice prevented the hyperalgesia. In female mice, depletion of macrophages with clodronate in the muscle, prior to induction of the activity-induced muscle pain model, significantly prevented the decrease in MWT ipsilaterally (t=4.35, df=6, p=0.005*, d=3.55)(Fig. 5A), and contralaterally (t=9.26, df=6, p<0.001*, d=7.57)(Fig. 5B), when compared to vehicle control. The number of F4/80+ macrophages from a muscle section after induction of the activity-induced muscle pain with clodronate liposomes was significantly lower than with inactive liposomes (t=2.66, df=6, p=0.03*, d=2.17)(Fig. 5C–5E). Schematic representation of the contribution of muscle macrophage to the activity-induced pain in male and female mice was shown in Fig. 6.
Figure 5. Effects of depletion of macrophages with clodronate liposomes in female mice.
A) Ipsilateral MWT with inhibition. B) Contralateral MWT with inhibition. C) Representative F4/80 immunostained from muscle section from a muscle pain model mouse injected with inactive liposomes. D) Representative F4/80 immunostained from a muscle section from a muscle pain model mouse injected with clodronate liposomes. E) The number of F4/80+ macrophages from a muscle section. Intramuscular clodronate significantly prevented development of muscle hyperalgesia and the increase in the number of F4/80+ macrophages. Data is reported as mean ± S.E.M. N=4 in each group. *, p<0.05.
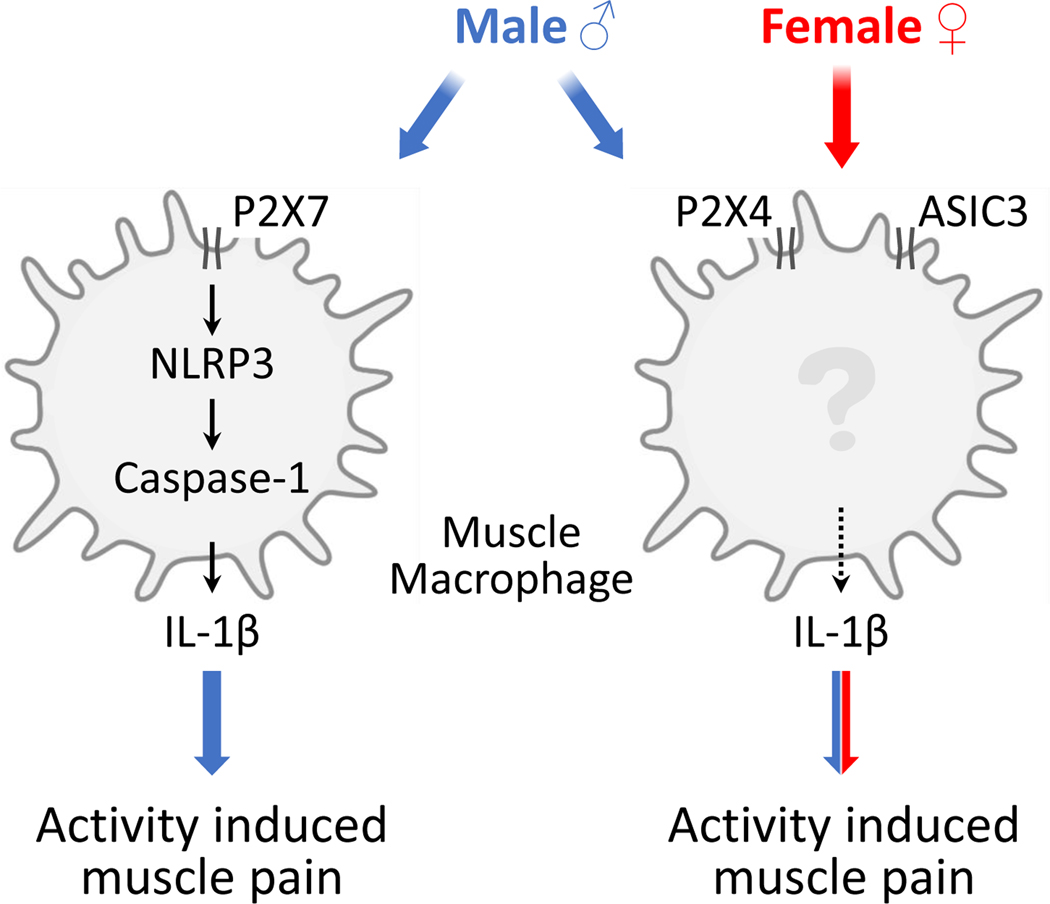
Figure 6. Schematic representation of the contribution of muscle macrophage to the activity-induced pain.
Macrophages in muscle play a key role in the development of the activity-induced pain model. We propose that in both male and female mice fatigue metabolites activate P2X4 and ASIC3 to release IL-1β that subsequently activates nociceptors to produce pain. In addition, male mice use the inflammasome pathway in macrophages that is activated by P2X7 to increase IL-1β to produce pain.
Discussion
Overview
The current data suggests unique mechanisms underlie development of activity-induced muscle pain in male and female mice. Blockade of P2X7, NLRP3, and Caspase-1 reduced activity-induced hyperalgesia in male, but not female, mice in a time-dependent manner with effects during the early phases but not later phase. On the other hand, blockade of IL-1β in muscle prior to induction of the hyperalgesia prevented development of hyperalgesia in both male and female mice, but only male mice when given 24hr after induction. There were also increases in mRNA expression of P2X7, NLRP3, Caspase-1, and IL-1β from muscle tissue in both male and female mice after induction of the model, but not IL-1β protein expression. In parallel, fatigue metabolites induced release of IL-1β protein from male and female cultured peritoneal macrophages that was prevented by blockade of P2X7, NLRP3, or Caspase-1. Based on these data, we propose activation of P2X7 receptors on macrophages located in muscle results in activation of the NLRP3 inflammasome to activate Caspase-1 and release IL-1β to produce pain in male mice.
P2X7 activates a sex-specific mechanism to release IL-1β and produce muscle pain
The NLRP3-inflammasome is activated by P2X7 and triggers cleavage of Caspase-1 leading to formation of mature IL-1β and its release [24]. NLRP3 is implicated in a variety of animal models of pain, primarily in male mice [18; 67]. Our study shows a sex-specific activation of the inflammasome-pathway, P2X7-NLRP3-Caspase-1, in activity-induced muscle pain in male, but not female, mice during initiation and early maintenance phase of hyperalgesia. Consistent with the current study, deletion of NLRP3 prevents hyperalgesia in an incision model through a non-neuronal component in male mice that does not occur in female mice [18]. Similarly, blockade of NLRP3 or Caspase-1 prevented development of hyperalgesia in a model of muscle hyperalgesia induced by maximal electrical stimulation for 7 days [77]; however, this study only tested male mice and only tested drug administration during induction of the model.
We demonstrated a time-dependent activation and role for P2X7 and IL-1β in development of hyperalgesia. Interestingly blockade of IL-1β during the initiation of the activity-induced pain model prevented hyperalgesia in both male and female mice, consistent with our prior study [49], suggesting female mice generate IL-1β in response to muscle insult independent of P2X7. It is possible that release of IL-1β in females results from activation of other cells surface receptors on macrophages. Prior studies show that P2X4, through the NLRP1 inflammasome increases IL-1β release from human fibroblast-like synoviocytes in individuals with osteoarthritis [26], and blockade of P2X4 prevents ATP-upregulated IL-1β release in a rat model of colitis model [20]. Alternatively, IL-1β in female mice could be released from other cells types in the tissue such as T-cells [54] or muscle itself [17; 52]. While the current study is consistent with a role for activation of P2X7 and the NLRP3-inflammasome pathway in macrophages, we cannot rule out other cell types. It should be noted, however, there is virtually no expression of P2X7 in wild-type myoblasts or dorsal root ganglia neurons under normal conditions, with increases in P2X7 expression occurring in Duchenne muscular dystrophy myoblasts [16; 78]. A more targeted, cell specific approach will be required to confirm if activation of this pathway in muscle macrophages is required to produce pain.
The current study showed, in both male and females, increased release of IL-1β by fatigue metabolites in cultured macrophages that was reduced by blockade of P2X7, NLRP3 and Caspase-1 suggesting both males and females have functional mechanisms for P2X7-induced IL-1β release from macrophages. It is possible that P2X7 has a different function in peritoneal resident macrophages when compared to muscle resident macrophages. There are multiple splice variants and single nucleotide polymorphisms (SNP) of P2X7 that alter function of the P2X7 receptor and different splice variants are expressed on different cell types [4; 54]. Further, it is possible that increases in mRNA in females do not translate to protein. Protein translation is a modifiable process that is regulated by phosphorylation, inhibition of translational mechanisms (e.g. mTOR, rapamycin) and can reduce injury-induced hyperalgesia and nociceptor sensitization [37; 38; 48].
Surprisingly, there were no increases in IL-1β protein in muscle 24hr after induction of the model, despite increases in mRNA Il-1β expression and reduced hyperalgesia with IL-1β blockade. This suggests that IL-1β protein increases may not be necessary for generation of hyperalgesia. An acute release through activation of P2X7-NLRP3-Caspase-1 pathway could generate downstream effects that activate and sensitize nociceptors. Indeed, IL-1β activates nociceptors which can increase mitogen-activated kinases to enhance excitability and sensitize nociceptors [6]. IL-1β in muscle increases expression of ASIC3 in nociceptors [56], and our prior work shows that pharmacological blockade or genetic deletion of ASIC3 prevents development of muscle hyperalgesia [31].
Macrophages play a critical role in development of hyperalgesia in male and female mice
Fatiguing muscle contractions release ATP and decrease pH which can activate purinergic and ASICs to result in hyperalgesia [57; 66; 72]. Our prior studies show that activation of the fatigue metabolite receptors, P2X4, ASIC1, and ASIC3 also mediate activity-induced muscle pain in both male and female mice [13; 15; 31; 32; 49]. Our prior studies, in combination with the current study, suggest receptors located on macrophages release IL-1β [31; 49]. Macrophages are found in nearly every tissue type, including skeletal muscle, and play a significant role in the generation of pain, consistent with the results of the current study [8; 12; 14; 22; 30; 31; 46; 58; 61; 70; 75]. While most studies only tested males, some studies suggest a sexually dimorphic role for macrophages and microglia in pain [50; 64], whereas our data support a role for macrophages in both male and female mice. Specifically, depletion of macrophages in muscle in male and female mice reduces muscle hyperalgesia in chronic muscle pain, and in male mice and female (current study)[30; 31]. Further, we previously show in both male and female mice, increases the number of macrophages after development of muscle pain, and depletion of P2X4 receptors in muscle macrophages prevents development of hyperalgesia [30; 31; 49]. While the current study shows a role for P2X7-NLRP3 in males, Ji and colleagues show a role for IL-23 and IL-17A in females. Specifically, IL-23 produces pain in females (but not males), evokes release of IL-17A from macrophages and directly activates nociceptors, and IL-23−/− mice do not develop hyperalgesia [46]. Thus, there are both common and distinct immune system responses in male and female mice. Activation of P2X4 and ASICs mediates hyperalgesia in both male and female mice, P2X7-NLRP3 initiates hyperalgesia only in males, and IL-23-IL17 plays a role in hyperalgesia in female mice.
We previously show that LPS-primed macrophages treated with the fatigue metabolites ATP (at pH 7.4) increase IL-1β release compared with controls, and acidic pH (pH 6.5) combined with ATP further increases IL-1β release [49]. Fatiguing exercise increases release of ATP and decreases pH, and is associated with an increase in macrophages in muscle [28; 33; 57; 72]. Macrophages express endogenous activators Mrp8 and Mrp14 of Toll-4 receptors (Toll-4 receptors are activated by LPS), and are increased after fatiguing exercise in inflammatory myopathies [60; 73]. The combination of Toll-4 activation with ATP and acidic pH was designed to mimic the in vivo condition, and further supports a role for macrophage activation in development of activity-induced hyperalgesia in both male and female mice.
Sex-specific differences in the activity-induced pain
We previously showed that testosterone protects from development of bilateral hyperalgesia in this model [44]. This suggests that gonadal hormones, particularly testosterone, modulates the pain phenotype in this model. Gonadal hormones can modulate the immune system, including IL-1β [19; 55]. IL-1β in macrophages is increased by estrogen administration and decreased by testosterone administration [1; 11; 19; 53; 55]. However, the current study suggests that testosterone does not modulate mRNA expression of P2X7, NLRP3, and Caspase-1, or release of IL-1β by ATP combined with acidic pH, as there are similar effects in both male and female mice. Alternatively, different P2X7 receptor variants that confer loss of function or gain of function activity could be differentially expressed in males and females. For example, loss of function SNPs with lower or accelerated bone density in women [29], and SNPs of P2X7 are associated with enhanced pain in human subjects [5; 35; 36; 71]. Future studies could examine different P2X7 variants between sexes to examine their role in the generation of pain in male and female mice.
Conclusions
The expression of P2X7 receptors, NLRP3, Caspase-1, and IL-1β from muscle after induction of the activity-induced muscle pain were increased in both male and female mice. Blockade of IL-1β and depletion of macrophages prevented development of hyperalgesia in both male and female mice. However, blockade of P2X7 receptors, NLRP3, and Caspase-1 prevented or reversed activity-induced hyperalgesia initiation and early maintenance phases in male but not female, and not in late maintenance phases in male mice. This data suggests there are unique mechanisms for development of activity-induced muscle pain in male and female mice.
Supplementary Material
Supplement Figure 1. MWT contralaterally with inhibition in the muscle prior to induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, or Capsase-1 prior to induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. The inhibition of IL-1β significantly prevented the decrease in MWT contralaterally in female mice, but not in male mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 2. Contralateral MWT with inhibition in the muscle 24hr after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 24hr after induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 3. Ipsilateral MWT with inhibition in the muscle 1 week after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 1 week after induction of the model had no effect on muscle hyperalgesia on ipsilateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 4. Contralateral MWT with inhibition in the muscle 1 week after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 1 week after induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 5. Cytokines of LPS-primed macrophages treated with ATP and proton. A) IL-6 protein in male macrophages. B) IL-6 protein in female macrophages. C) IL-10 protein in male macrophages. D) IL-10 protein in female macrophages. E) GM-CSF protein in male macrophages. F) GM-CSF protein in female macrophages. G) IFN-γ protein in male macrophages. H) IFN-γ protein in female macrophages. I) IL-12p70 protein in male macrophages. J) IL-12p70 protein in female macrophages. K) IL-13 protein in male macrophages. L) IL-13 protein in female macrophages. M) IL-18 protein in male macrophages. N) IL-18 protein in female macrophages. O) IL-2 protein in male macrophages. P) IL-2 protein in female macrophages. Q) IL-4 protein in male macrophages. R) IL-4 protein in female macrophages. S) IL-5 protein in male macrophages. T) IL-5 protein in female macrophages. U) TNF-α protein in male macrophages. V) TNF-α protein in female macrophages. The concentrations from the macrophages with inhibition of P2X7 receptors with A740003, NLRP3 with MCC950, and Caspase-1 with Z-WEHD-FMK showed no differences, when compared to the vehicle with LPS and pH 6.5, in male or female macrophages. Data is reported as mean ± S.E.M. N=8 in each group. *, p<0.05.
B) Intramuscular injection of inhibitors
| Inhibitor | Time of injection of inhibitors | Concentration of inhibitors (i.m., 20μl) | Time of measurement of MWT |
|---|---|---|---|
| P2X7 receptor antagonist (A740003) | 5 min prior to induction | Low: 0.12μM, Middle: 0.36μM, High: 1.20μM | Baseline, 24hr, 72hr, and 1 week after inhibitor |
| 24hr after induction | High: 1.20μM | Baseline, prior to inhibitor, 30 min, 2hr, and 6hr after inhibitor | |
| 1week after induction | High: 1.20μM | Baseline, prior to inhibitor, 30 min, and 2hr after inhibitor | |
| NLRP3 antagonist (MCC950) | 5 min prior to induction | Low: 2.0nM, Middle: 6.0nM, High: 20.0nM | Baseline, 24hr, 72hr, and 1 week after inhibitor |
| (also called CP‑456773 and CRID3) | 24hr after induction | High: 20.0nM | Baseline, prior to inhibitor, 30 min, 2hr, and 6hr after inhibitor |
| 1week after induction | High: 20.0nM | Baseline, prior to inhibitor, 30 min, and 2hr after inhibitor | |
| Caspase-1 antagonist (Z-WEHD-FMK) | 5 min prior to induction | Low: 1.2μM, Middle: 3.6μM, High: 12.0μM | Baseline, 24hr, 72hr, and 1week after inhibitor |
| 24hr after induction | High: 12.0μM | Baseline, prior to inhibitor, 30 min, 2hr, and 6hr after inhibitor | |
| 1week after induction | High: 12.0μM | Baseline, prior to inhibitor, 30 min, and 2hr after inhibitor | |
| Antibody to IL-1β | 30 min prior to induction | High: 2.57μM | Baseline, 24hr, 72hr, and 1week after inhibitor |
| 24hr after induction | High: 2.57μM | Baseline, prior to inhibitor, 24hr, 3 day, and 1week after inhibitor | |
| 1week after induction | High: 2.57μM | Baseline, prior to inhibitor, 24hr, 3 day, and 1week after inhibitor | |
| Macrophage depletion (Clodronate) | 2 days prior to and 1 day after the first pH 5.0 | High: 20.4mM | Baseline, and 24hr after induction |
C) Primer sets used in Quantitative PCR (qPCR)
| Gene name | Accession number | Primer sequence (5’−3’) | Product size | |
|---|---|---|---|---|
|
| ||||
| 36B4 | NM_007475.5 | F | GCAGGTGTTTGACAACGGCA | 190bp |
| R | CACAGACAATGCCAGGACGC | |||
| P2X7 | NM_011027.4 | F | GTTTGCTGTGGTCTAGCCTGG | 163bp |
| R | CCCACTTGACGGTGCCATAA | |||
| NLRP3 | NM_001359638.1 | F | TACCCAAGGCTGCTATCTGGAG | 146bp |
| R | AGCTTGCAACGGACACTCGT | |||
| Caspase-1 | NM_009807.2 | F | AGGACTGACTGGGACCCTCA | 171bp |
| R | ACTTGAGCTCCAACCCTCGG | |||
| IL-1β | NM_008361.4 | F | TGCCACCTTTTGACAGTGATGA | 158bp |
| R | TGCCTGCCTGAAGCTCTTGT | |||
F, Forward primer; R, Reverse primer
B) Results for a two-way ANOVA test for mRNA and cytokine expression of gastrocnemius muscle.
| Effect for Time | Effect for Sex | Effect for Time*Sex | |
|---|---|---|---|
| P2X7 mRNA | F2,42=8.43; p<0.01* | F1,42<0.01; p=0.94 | F2,42=0.09; p=0.91 |
| NLRP3 mRNA | F2,42=16.68; p<0.01* | F1,42=0.44; p=0.50 | F2,42=1.89; p=0.16 |
| Caspase-1 mRNA | F2,42=13.24; p<0.01* | F1,42=0.08; p=0.76 | F2,42=0.03; p=0.96 |
| IL-1β mRNA | F2,42=12.20; p<0.01* | F1,42=1.32; p=0.25 | F2,42=2.14; p=0.12 |
| IL-1β protein | F2,42=7.55; p<0.01* | F1,42=9.23; p<0.01* | F2,42=0.41; p=0.66 |
p<0.05 with a two-way ANOVA test.
C) Results for repeated-measures analysis of variance for mRNA and cytokine expression of LPS-primed macrophages.
| Effect for Group | Effect for Sex | Effect for Group*Sex | |
|---|---|---|---|
| IL-1β mRNA | F4,56=31.36; p<0.01* | F1,14=10.11; p<0.01* | F4,56=4.22; p<0.01* |
| IL-1β protein | F4,56=31.62; p<0.01* | F1,14=1.04; p=0.32 | F4,56=0.83; p=0.50 |
p<0.05 with a repeated measures ANOVA.
Acknowledgements
This study is supported by the National Institutes of Health AR073187. Dr. Hayashi is supported by Japan Society for the Promotion of Science. The funders played no role in the design, conduct, or reporting of this study. There was no additional external funding received for this study. The authors would like to acknowledge the BioRender.com to create scientific figures.
Footnotes
Conflict of interest statement
The authors declare that there are no relevant conflicts of interest.
References
- [1].Angele MK, Knoferl MW, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol-Cell Ph 1999;277(1):C35–C42. [DOI] [PubMed] [Google Scholar]
- [2].Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981;11(10):805–815. [DOI] [PubMed] [Google Scholar]
- [3].Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a Danger Signal That Activates the NLRP3 Inflammasome via Toll-like and P2X Receptors. J Biol Chem 2009;284(36):24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benzaquen J, Heeke S, Hreich SJD, Douguet L, Marquette CH, Hofman P, Vouret-Craviari V. Alternative splicing of P2RX7 pre-messenger RNA in health and diseases: Myth or reality? Biomed J 2019;42(3):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernier LP, Ase AR, Seguela P. P2X receptor channels in chronic pain pathways. Br J Pharmacol 2018;175(12):2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28(52):14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing Muscle Ischemia: Coincident Detection of Acid and ATP via Interplay of Two Ion Channels. Neuron 2010;68(4):739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018;159(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burnstock G. Introduction to the Special Issue on Purinergic Receptors. Adv Exp Med Biol 2017;1051:1–6. [DOI] [PubMed] [Google Scholar]
- [10].Burnstock G, Kennedy C. P2X Receptors in Health and Disease. Adv Pharmacol 2011;61:333–372. [DOI] [PubMed] [Google Scholar]
- [11].Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, Gourdy P. 17 beta-Estradiol Promotes TLR4-Triggered Proinflammatory Mediator Production through Direct Estrogen Receptor alpha Signaling in Macrophages In Vivo. J Immunol 2010;185(2):1169–1176. [DOI] [PubMed] [Google Scholar]
- [12].Castro J, Harrington AM, Chegini F, Matusica D, Spencer NJ, Brierley SM, Haberberger RV, Barry CM. Clodronate Treatment Prevents Vaginal Hypersensitivity in a Mouse Model of Vestibulodynia. Front Cell Infect Mi 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chang CT, Fong SW, Lee CH, Chuang YC, Lin SH, Chen CC. Involvement of Acid-Sensing Ion Channel 1b in the Development of Acid-Induced Chronic Muscle Pain. Front Neurosci 2019;13:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 2020;62:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen WN, Chen CC. Acid mediates a prolonged antinociception via substance P signaling in acid-induced chronic widespread pain. Mol Pain 2014;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen Y, Li G, Huang LY. P2X7 receptors in satellite glial cells mediate high functional expression of P2X3 receptors in immature dorsal root ganglion neurons. Mol Pain 2012;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Copray JC, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM, Boddeke HW. Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol 2001;118(2):203–211. [DOI] [PubMed] [Google Scholar]
- [18].Cowie AM, Menzel AD, OHaraa C, Lawlor MW, Stucky CL. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain 2019;160(8):1794–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].D’Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, Farruggio R, Miceli DM, Miele M, Castagnetta L, Cillari E. Sex hormones modulate inflammatory mediators produced by macrophages. Ann Ny Acad Sci 1999;876:426–429. [DOI] [PubMed] [Google Scholar]
- [20].D’Antongiovanni V, Pellegrini C, Benvenuti L, Fornai M, Di Salvo C, Natale G, Ryskalin L, Bertani L, Lucarini E, Mannelli LD, Ghelardini C, Nemeth ZH, Hasko G, Antonioli L. Anti-inflammatory Effects of Novel P2X4 Receptor Antagonists, NC-2600 and NP-1815-PX, in a Murine Model of Colitis. Inflammation 2022;45(4):1829–1847. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Dahlhamer J LJ, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults – United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de Azambuja G, Jorge CO, Gomes BB, Lourenco HR, Simabuco FM, Oliveira-Fusaro MCG. Regular swimming exercise prevented the acute and persistent mechanical muscle hyperalgesia by modulation of macrophages phenotypes and inflammatory cytokines via PPARgamma receptors. Brain Behav Immun 2021;95:462–476. [DOI] [PubMed] [Google Scholar]
- [23].Deval E, Lingueglia E. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology 2015;94:49–57. [DOI] [PubMed] [Google Scholar]
- [24].Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017;47(1):15–31. [DOI] [PubMed] [Google Scholar]
- [25].Donnelly-Roberts DL, Jarvis MF. Discovery of P2X(7) receptor-selective antagonists offers new insights into P2X(7) receptor function and indicates a role in chronic pain states. Brit J Pharmacol 2007;151(5):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fan CC, Zhao XC, Guo XF, Cao XC, Cai JF. P2X4 promotes interleukin-1 beta production in osteoarthritis via NLRP1. Mol Med Rep 2014;9(1):340–344. [DOI] [PubMed] [Google Scholar]
- [27].Ferko MA, Catelas I. Effects of metal ions on caspase-1 activation and interleukin-1 beta release in murine bone marrow-derived macrophages. Plos One 2018;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol 1969;204(2):347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gartland A, Skarratt KK, Hocking LJ, Parsons C, Stokes L, Jorgensen NR, Fraser WD, Reid DM, Gallagher JA, Wiley JS. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur J Hum Genet 2012;20(5):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident Macrophages in Muscle Contribute to Development of Hyperalgesia in a Mouse Model of Noninflammatory Muscle Pain. J Pain 2016;17(10):1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gregory N, Brito R, Fusaro MCG, Sluka KA. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Mol Neurobiol 2016;53(2):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gregory NS, Gautam M, Benson CJ, Sluka KA. Acid Sensing Ion Channel 1a (ASIC1a) Mediates Activity-induced Pain by Modulation of Heteromeric ASIC Channel Kinetics. Neuroscience 2018;386:166–174. [DOI] [PubMed] [Google Scholar]
- [33].Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex-dependent manner. Pain 2013;154(12):2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong CM, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino)methyl]amino}−2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X(7) receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 2006;319(3):1376–1385. [DOI] [PubMed] [Google Scholar]
- [35].Ide S, Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Hayashida M, Minami M, Ikeda K. Haplotypes of P2RX7 gene polymorphisms are associated with both cold pain sensitivity and analgesic effect of fentanyl. Mol Pain 2014;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kambur O, Kaunisto MA, Winsvold BS, Wilsgaard T, Stubhaug A, Zwart JA, Kalso E, Nielsen CS. Genetic variation in P2RX7 and pain tolerance. Pain 2018;159(6):1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khoutorsky A, Price TJ. Translational Control Mechanisms in Persistent Pain. Trends Neurosci 2018;41(2):100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Khoutorsky A, Sorge RE, Prager-Khoutorsky M, Pawlowski SA, Longo G, Jafarnejad SM, Tahmasebi S, Martin LJ, Pitcher MH, Gkogkas CG, Sharif-Naeini R, Ribeiro-Da-Silva A, Bourque CW, Cervero F, Mogil JS, Sonenberg N. eIF2 alpha phosphorylation controls thermal nociception. P Natl Acad Sci USA 2016;113(42):11949–11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain 1996;64(3):415–423. [DOI] [PubMed] [Google Scholar]
- [40].Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: Evidence from the HUNT 3 study. Pain 2011;152(10):2241–2247. [DOI] [PubMed] [Google Scholar]
- [41].Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal Associations between Exercise and Pain in the General Population - The HUNT Pain Study. Plos One 2013;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Law LAF, Sluka KAB, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain 2008;140(2):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lesnak JB, Fahrion A, Helton A, Rasmussen L, Andrew M, Cunard S, Huey M, Kreber A, Landon J, Siwiec T, Todd K, Frey-Law LA, Sluka KA. Resistance training protects against muscle pain through activation of androgen receptors in male and female mice. Pain 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lesnak JB, Inoue S, Lima L, Rasmussen L, Sluka KA. Testosterone protects against the development of widespread muscle pain in mice. Pain 2020;161(12):2898–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Light AR, Hughen RW, Zhang J, Rainier J, Liu ZQ, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 2008;100(3):1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Luo X, Chen OY, Wang ZL, Bang S, Ji JE, Lee SH, Huh Y, Furutani K, He QR, Tao XS, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021;109(17):2691-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 2018;17(8):588–606. [DOI] [PubMed] [Google Scholar]
- [48].Moy JK, Khoutorsky A, Asiedu MN, Black BJ, Kuhn JL, Barragan-Iglesias P, Megat S, Burton MD, Burgos-Vega CC, Melemedjian OK, Boitano S, Vagner J, Gkogkas CG, Pancrazio JJ, Mogil JS, Dussor G, Sonenberg N, Price TJ. The MNK-eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain. J Neurosci 2017;37(31):7481–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oliveira-Fusaro MC, Gregory NS, Kolker SJ, Rasmussen L, Allen LAH, Sluka KA. P2X4 Receptors on Muscle Macrophages Are Required for Development of Hyperalgesia in an Animal Model of Activity-Induced Muscle Pain. Mol Neurobiol 2020;57(4):1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Paige C, Maruthy GB, Mejia G, Dussor G, Price T. Spinal Inhibition of P2XR or p38 Signaling Disrupts Hyperalgesic Priming in Male, but not Female, Mice. Neuroscience 2018;385:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 2014;99(2):368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rawat R, Cohen TV, Ampong B, Francia D, Henriques-Pons A, Hoffman EP, Nagaraju K. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol 2010;176(6):2891–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008;78(3):432–437. [DOI] [PubMed] [Google Scholar]
- [54].Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on mouse T cells: one channel, many functions. Front Immunol 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rosen S, Ham B, Mogil JS. Sex Differences in Neuroimmunity and Pain. J Neurosci Res 2017;95(1–2):500–508. [DOI] [PubMed] [Google Scholar]
- [56].Ross JL, Queme LF, Cohen ER, Green KJ, Lu P, Shank AT, An S, Hudgins RC, Jankowski MP. Muscle IL1beta Drives Ischemic Myalgia via ASIC3-Mediated Sensory Neuron Sensitization. J Neurosci 2016;36(26):6857–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sahlin K, Harris RC, Nylind B, Hultman E. Lactate Content and Ph in Muscle Samples Obtained after Dynamic Exercise. Pflug Arch Eur J Phy 1976;367(2):143–149. [DOI] [PubMed] [Google Scholar]
- [58].Sakurai Y, Fujita M, Kawasaki S, Sanaki T, Yoshioka T, Higashino K, Tofukuji S, Yoneda S, Takahashi T, Koda K, Asaki T, Hasegawa M, Morioka Y. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019;160(4):895–907. [DOI] [PubMed] [Google Scholar]
- [59].Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- [60].Seeliger S, Vogl T, Engels IH, Schroder JM, Sorg C, Sunderkotter C, Roth J. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol 2003;163(3):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Silva CEA, Guimaraes RM, Cunha TM. Sensory neuron-associated macrophages as novel modulators of neuropathic pain. Pain Rep 2021;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain 2005;6(1):41–47. [DOI] [PubMed] [Google Scholar]
- [63].Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001;24(1):37–46. [DOI] [PubMed] [Google Scholar]
- [64].Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu YS, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18(8):1081-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Spandidos A, Wang XW, Wang HJ, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 2010;38:D792–D799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spriet LL, Soderlund K, Thomson JA, Hultman E. Ph Measurement in Human Skeletal-Muscle Samples - Effect of Phosphagen Hydrolysis. J Appl Physiol 1986;61(5):1949–1954. [DOI] [PubMed] [Google Scholar]
- [67].Starobova H, Nadar EI, Vetter I. The NLRP3 Inflammasome: Role and Therapeutic Potential in Pain Treatment. Front Physiol 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019;19(8):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Thornton B, Basu C. Real-Time PCR (qPCR) Primer Design Using Free Online Software. Biochem Mol Biol Edu 2011;39(2):145–154. [DOI] [PubMed] [Google Scholar]
- [70].Tu Y, Muley MM, Beggs S, Salter MW. Microglia-independent peripheral neuropathic pain in male and female mice. Pain 2022;163(11):e1129–e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ursu D, Ebert P, Langron E, Ruble C, Munsie L, Zou W, Fijal B, Qian YW, McNearney TA, Mogg A, Grubisha O, Merchant K, Sher E. Gain and loss of function of P2X7 receptors: mechanisms, pharmacology and relevance to diabetic neuropathic pain. Mol Pain 2014;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic-Nerve Discharge Is Coupled to Muscle-Cell Ph during Exercise in Humans. J Clin Invest 1988;82(4):1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007;13(9):1042–1049. [DOI] [PubMed] [Google Scholar]
- [74].Wang XW, Spandidos A, Wang HJ, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 2012;40(D1):D1144–D1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM. Mechanisms underlying mechanical sensitization induced by complement C5a: the roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 2019;160(3):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yang Y, Wang HN, Kouadir M, Song HH, Shi FS. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yoshida S, Hagiwara Y, Tsuchiya M, Shinoda M, Koide M, Hatakeyama H, Chaweewannakorn C, Suzuki K, Yano T, Sogi Y, Itaya N, Sekiguchi T, Yabe Y, Sasaki K, Kanzaki M, Itoi E. Involvement of inflammasome activation via elevation of uric acid level in nociception in a mouse model of muscle pain. Mol Pain 2019;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Young CN, Sinadinos A, Lefebvre A, Chan P, Arkle S, Vaudry D, Gorecki DC . A novel mechanism of autophagic cell death in dystrophic muscle regulated by P2RX7 receptor large-pore formation and HSP90. Autophagy 2015;11(1):113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008;Chapter 14:Unit 14 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. MWT contralaterally with inhibition in the muscle prior to induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, or Capsase-1 prior to induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. The inhibition of IL-1β significantly prevented the decrease in MWT contralaterally in female mice, but not in male mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 2. Contralateral MWT with inhibition in the muscle 24hr after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 24hr after induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 3. Ipsilateral MWT with inhibition in the muscle 1 week after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 1 week after induction of the model had no effect on muscle hyperalgesia on ipsilateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 4. Contralateral MWT with inhibition in the muscle 1 week after induction of the activity-induced muscle pain model. A) Blockade of P2X7. B) Blockade of NLRP3. C) Blockade of Caspase-1. D) Blockade of IL-1β. Blockade of P2X7, NLRP3, Capsase-1, or IL-1β 1 week after induction of the model had no effect on muscle hyperalgesia on contralateral side in male or female mice. Data is reported as mean ± S.E.M. *, versus vehicle control; p<0.05.
Supplement Figure 5. Cytokines of LPS-primed macrophages treated with ATP and proton. A) IL-6 protein in male macrophages. B) IL-6 protein in female macrophages. C) IL-10 protein in male macrophages. D) IL-10 protein in female macrophages. E) GM-CSF protein in male macrophages. F) GM-CSF protein in female macrophages. G) IFN-γ protein in male macrophages. H) IFN-γ protein in female macrophages. I) IL-12p70 protein in male macrophages. J) IL-12p70 protein in female macrophages. K) IL-13 protein in male macrophages. L) IL-13 protein in female macrophages. M) IL-18 protein in male macrophages. N) IL-18 protein in female macrophages. O) IL-2 protein in male macrophages. P) IL-2 protein in female macrophages. Q) IL-4 protein in male macrophages. R) IL-4 protein in female macrophages. S) IL-5 protein in male macrophages. T) IL-5 protein in female macrophages. U) TNF-α protein in male macrophages. V) TNF-α protein in female macrophages. The concentrations from the macrophages with inhibition of P2X7 receptors with A740003, NLRP3 with MCC950, and Caspase-1 with Z-WEHD-FMK showed no differences, when compared to the vehicle with LPS and pH 6.5, in male or female macrophages. Data is reported as mean ± S.E.M. N=8 in each group. *, p<0.05.