Abstract
Formulations of human papillomavirus (HPV) 16, 18, and 31 L1 capsomere protein antigens were spray dried to obtain glassy microspheres that were then coated by atomic layer deposition (ALD) with nanometer-thin protective layers of alumina. Spray-drying was used to formulate human papillomavirus (HPV) 16, 18, and 31 L1 capsomere protein antigens within glassy microspheres to which nanoscopic protective layers of alumina were applied using ALD. Suspensions of alumina-coated, capsomere-containing microparticles were administered in a single dose to mice. ALD-deposited alumina coatings provided thermostability and a delayed in vivo release of capsomere antigens, incorporating both a prime and a boost dose in one injection. Total serotype-specific antibody titers as well as neutralizing titers determined from pseudovirus infectivity assays were unaffected by incubation of the ALD-coated vaccines for at 4, 50, or 70 °C for three months prior to administration. In addition, even after incubation for three months at 70 °C, single doses of ALD-coated vaccines produced both higher total antibody responses and higher neutralizing responses than control immunizations that used two doses of conventional liquid formulations stored at 4 °C.
Introduction
Human papillomaviruses (HPVs) are the etiologic agent for a majority of cervical cancers, which represent 5% of all human cancers.1 HPV16, 18 and 31 are the most prevalent oncogenic HPV types, with HPV16 and 18 associated with >70% of all cervical cancers.2 In 2020, nearly 60% of American adolescents were reported as being up-to-date for recommended vaccination against HPV3, and from the pre-vaccine era to 2015-2018 the number of HPV infections of types 6, 11, 16 and 18 decreased by 88% in American female adolescents.4 But global HPV vaccination rates in girls are only approximately 15%,5, 6 mostly due to lack of vaccination in low- and middle- income countries (LMICs). The World Health Organization7 reported that world-wide there were more than six hundred thousand new cases of cervical cancers and three hundred thousand deaths due to cervical cancers in 2020, with 90% of new cases and deaths occurring in LMICs, where it is estimated that less than 5% of eligible individuals have received an HPV vaccine.8
Many factors contribute to low HPV vaccination rates in LMICs. HPV vaccines are more costly to introduce in LMICs than other vaccines due to their target populations which require the multi-dose regimen of administration.9 For example, the cost to introduce HPV vaccines in Rwanda was estimated to be 50% higher than other vaccines.9 The currently approved HPV vaccines (i.e., Cervarix, Gardasil, and Gardasil-9) all require cold storage between 2 and 8 °C.10, 11 This “cold chain” is often difficult to maintain in LMICs that lack appropriate infrastructure necessary to transport, store, and distribute vaccines.12–14 These vaccine delivery challenges have been highlighted in recent studies in Ghana showing that fewer than 60% of surveyed health care providers had refrigerators appropriate for vaccine storage12, and in Cameroon, where vaccines were exposed to potentially damaging temperature excursions (both freezing and high temperature) during transportation.13
Vaccines formulated as liquids may suffer from chemical (e.g., oxidation) and physical (e.g., aggregation) degradation of antigens and adjuvants as a result of mechanical stresses experienced during shipping and storage, and as a result of exposure to high or low temperatures.15–17 Both chemical and physical degradation pathways for protein antigens may be inhibited or avoided by using lyophilization or spray-drying to embed vaccine formulations in dry, glassy powders, where low water content and high viscosities restrict molecular mobility of antigens. Common glass-forming excipients include disaccharides such as trehalose or sucrose.
We have previously described18 the thermostabilization by lyophilization to form glassy disaccharide formulations of monovalent HPV L1 capsomere-based vaccines of type 16, as well as trivalent vaccines containing HPV L1 capsomeres of types 16, 18, and 31.19 These lyophilized vaccines were stable at 50 °C for three months, as evidenced by total antibody responses and neutralizing antibody titers in mice administered a 2-dose regimen of lyophilized preparations of either monovalent or trivalent formulations.18,19
Although lyophilization may allow for the storage of vaccines at temperatures outside of the normal cold chain, it would be more advantageous if the formulations provided both thermostability and single-dose administration. Previously, we described an atomic layer deposition (ALD) technology to apply nanoscopic layers of amorphous alumina on the surface of spray dried powders containing HPV16 L1 capsomeres to deliver prime/boost doses in a single administration.20 When administered to mice, the ALD-coated antigens exhibited a delayed release, and single doses yielded anti-HPV16 total and neutralizing antibody titers that were as equivalent or greater than those provided by multiple doses of commercially available HPV vaccines.20
In the present study, we sought to increase the potential serotype coverage of an HPV vaccine by incorporating capsomere antigens for three HPV types (i.e., 16, 18, and 31) within spray-dried and ALD-coated vaccine preparations. We tested whether our previous thermostability and dose reduction results obtained with ALD-coated HPV16 L1 capsomere vaccines could be extended to a multivalent formulation where the ALD-coated microspheres contained capsomeres of a mixture of HPV types. In a previous accelerated stability study of lyophilized HPV capsomere vaccines18, we observed that HPV31 L1 antigens appeared to be more sensitive to thermal degradation at 50 °C than those for types 16 or 18. To explore whether this effect might also be seen in ALD coated microsphere vaccine preparations, we evaluated total antibody and neutralizing antibody responses in mice to trivalent vaccines that had been incubated for three months at 4, 50, or 70 °C. In addition, we compared the immune responses in mice following administration of single doses of the trivalent, ALD-coated vaccines to those generated after two doses of liquid vaccine formulations or spray dried and reconstituted (non-coated) formulations.
Materials and Methods
Reagents
Trehalose dihydrate (endotoxin free grade) was obtained from Mallinckrodt Baker (Phillipsburg, NJ). Sodium chloride, trimethyl alumina, ammonium sulfate, L-histidine monohydrate, HMS174 (DE3) competent E. coli, benzonase nuclease, beta-mercaptoethanol (BME), and Optiprep density gradient medium were obtained from Sigma-Aldrich (St. Louis, MO). Alhydrogel (aluminum hydroxide adjuvant) was obtained from Accurate Chemicals and Scientific Corporation (Westbury, NY). Hydroxyethyl starch (HES) was obtained from Fresenius Kabi (Bad Homburg, Germany). Polysorbate-20, polysorbate-80, lipofectamine, glycerol, Opti-MEM medium, water for injection, DMEM-Hi glucose medium, 3,3′,5,5′-tetramethylbenzidine (TMB), sulfuric acid, phosphate buffered saline (PBS), and tris(hydroxymethyl)aminomethane (Tris) were obtained from Fischer Scientific (Waltham, MA). Argon and ultra-high purity nitrogen gas was obtained from Airgas (Radnor, PA). Formamide, HYDRANAL™ Composite 1, and methanol were from Honeywell (Muskegon, MI). Powdered milk was obtained from Safeway (Pleasanton, CA). Horseradish peroxidase (HRP)-conjugated and alkaline phosphatase antibodies were obtained from Promega (Madison, WI). 3mL type 1 borosilicate glass vials and butyl rubber stoppers were from Schott (Mainz, Germany).
Expression and purification of recombinant HPV16, 18 and 31 L1 capsomeres.
Capsomere protein expression was induced in HMS174 Escherichia coli cultured in shake flasks by isopropyl β-D-1-thiogalactopyranoside (IPTG) as previously described18–20. Briefly, frozen bacterial pellets were resuspended in a 200 mM Tris buffer and lysed using a GEA Niro Soavi Panda homogenizer (Bedford, NH). The soluble fraction collected after centrifugation of the lysate was then precipitated using 30% ammonium sulfate. The precipitate was then solubilized in a 25 mM Tris buffer and chromatographed using a Q-sepharose ion-exchange column. HPV L1 capsomeres eluted from the column as pentamers using a sodium chloride gradient. Fractions containing the L1 capsomeres were further purified on a Q-sepharose column, again eluting with a sodium chloride gradient. A final purity of >95% was estimated by SDS-PAGE and immunoblotting. L1 capsomere protein concentration was determined using a Bradford assay (Thermo Fisher, Waltham, MA). Prior to formulation, L1 capsomeres were exchanged by dialysis into 100 mM histidine buffer (pH 7.1). Final vaccine formulations were tested for endotoxin using QCL 1000TM Limulus Amebocyte Lysate test kits (LONZA, Basel, Switzerland), and contained <1 EU/mL.
Liquid trivalent vaccine formulations.
Liquid control vaccines were formulated in 9.5% trehalose (w/v), 2.5 % hydroxyethyl starch (w/v), 40 mM ammonium acetate, 0.02 mM Tween 80, 54 mM histidine, 0.3 mg/mL Alhydrogel, 0.1 mg/mL of HPV16 L1, 0.1 mg/mL of HPV18 L1, and 0.1 mg/mL of HPV31 L1 capsomeres immediately prior to administration.
Spray-dried vaccine formulations.
Vaccine formulations of the same composition as the liquid vaccines above were spray-dried using a Buchi B-290 spray dryer (Buchi Labortechnik AG, Flawil, Switzerland). The feed rate of the entering liquid vaccine formulation was set to 0.5 mL/min. The drying air inlet temperature for the spray drier was set to 110 °C which resulted in an outlet temperature of ≈ 67 °C. The aspirator was set to 50% (generating a drying gas flowrate of ~20 m3/hr) and the flowrate of the atomizing gas (ultra-high purity nitrogen) was set to 670 L/hr. A Buchi B296 dehumidifier (Buchi Labortechnik AG, Flawil, Switzerland) was placed in line to reduce the humidity of the drying air entering the system. To protect against water uptake during storage, the spray-dried powders were filled into 3mL glass vials, which were placed in a Lyostar I lyophilizer (FTS Systems Lyophilizer, Warminster, PA), evacuated under vacuum and held overnight at 60 mTorr and 40 °C prior to backfilling with filtered dry nitrogen, sealing and capping. All spray-dried samples were stored at room temperature.
Spray-dried powder moisture determination
Water content of vaccine formulations was measured by Karl Fischer analysis using an Eco KF Titrator (Metrohm, Herisau, Switzerland) after spray drying and again after further vacuum drying by lyophilization. Dried powders were suspended in formamide and injected into a conditioned vessel containing HYDRANAL™ Composite 1 and methanol. The endpoint was set to 250 mV and the bivoltametric indication to 50 μA.
Atomic Layer Deposition (ALD)
The spray-dried powders were transferred from the 3 mL glass storage vials and coated with uniform, nanometer-thick alumina layers by ALD in a custom-built, low-pressure (2-3 torr) fluidized-bed reactor that was mounted on a vibrating table and equipped with a pneumatic impactor to aid fluidization of the slightly cohesive powders20. A constant argon purge maintained the fluidization of the powder bed throughout the process. Alternating doses of the gas-phase reactants trimethyl aluminum (TMA) and water were added into the purge stream to deposit ~2.3 Å-thick layers of alumina onto the surface of particles with each TMA/water cycle.20 A total of 250 cycles were completed for a total alumina layer thickness of ~50 nm. The alumina-coated microspheres were then removed from the ALD reactor and placed in 3mL glass vials which were then backfilled with nitrogen and stoppered as described above.
Vaccine thermostability
Sealed vials containing vaccines formulated as liquids, spray-dried powders, and alumina-coated microspheres were incubated at 4, 50 or 70 °C for up to three months.
Vaccine immunogenicity
Murine studies were conducted after approval by University of Colorado at Boulder Institutional Animal Care and Use Committee (IACUC protocol #2318). Mice were acclimated for at least one week prior to the study start. Five groups of 10 female 10-to-11-week-old BALB/C mice (Taconic, Hudson, NY) were administered a 7.5 pg dose of trivalent HPV L1 capsomere vaccines (2.5 μg of each type capsomere HPV16, 18, and 31) formulated either as a liquid, as reconstituted spray-dried powders or as suspensions of ALD-coated microspheres. The ALD-coated microspheres were stored for 3 months at 4, 50 or 70 °C prior to administration. Spray-dried powders were reconstituted in sterile water for injection immediately before administration. ALD-coated microspheres were resuspended in an isotonic, 9.5% trehalose pH 6.5 solution immediately before injection. Animals that received liquid or spray-dried and reconstituted formulations were administered a second 7.5 μg dose on day 21, whereas groups that received ALD-coated microspheres did not receive booster doses. All injections were administered intramuscularly in the right thigh under isoflurane anesthesia. Blood was collected via the submandibular vein at 7-day intervals over a 72-day period. Blood was centrifuged at 4,000 x g for 6 minutes, serum removed, and stored at −80 °C.
Antibody determination using enzyme linked immunosorbent assays (ELISA)
ELISAs were performed as previously described to measure titers of anti-HPV16, 18, and 31 L1 antibodies.18–20 Briefly, 50μL of 0.01 mg/mL HPV16, 18, or 31 L1 capsomeres were adsorbed to a 96-well plate (Thermo Fisher, Waltham, MA) and incubated overnight at 4 °C. Between each step of the assay plates were washed three times with PBST (1x PBS with 0.05 % (v/v) Polysorbate 80) using a BioTek (Winooski, VT) plate washer. Plates were blocked with 6% powdered skim milk in PBST for 1 hour at 37 °C. Serum samples were diluted 1:50 or 1:100, serially diluted across the plate, and incubated for 1 hour at 37°C. Secondary anti-mouse HRP antibody was added and incubated for 1 hour at 37°C. Finally, TMB was added, the reaction was quenched with 1 M sulfuric acid, and the plate read at 450nm using a Molecular Devices Kinetic Microplate Reader (Sunnyvale, CA). Each sample was analyzed in duplicate, and titers were calculated using a 4-parameter logistic equation with a custom Python script.18–20
Neutralizing antibody determination
To determine neutralizing antibody titers generated in response to vaccination, pseudovirus neutralization assays21, 22 were conducted using sera of vaccinated mice collected on day 72 post-vaccination. Briefly, dilutions of murine sera ranging from 1:80 to 1:81,920 were added to HPV16, 18, or 31 pseudovirus standards containing a secreted alkaline phosphatase (SEAP) reporter incubated at 4 °C for 1 hour to allow for neutralization. The pseudovirus/serum solution was then added to 293TT cells in 96-well tissue culture plates and incubated at 37 °C for 72 hours. After incubation, the supernatant was collected from the cells and assayed for SEAP levels using the Great Escape SEAP Chemiluminescence test kit (Clontech, Mountain View, CA). Plates were read on a multifunctional BioTek (Winooski, VT) plate luminometer at a set glow-endpoint of 0.20 s/well. The neutralization titer was defined as the dilution of murine serum that neutralized 50% of the pseudovirus as determined by SEAP luminescence.
Statistical methods
Statistical analyses were completed using OriginPro, Version 2022 (OriginLab Corporation, Northampton, MA, USA). Normality of the data was evaluated using Shapiro-Wilk tests with significance set to 0.05. The data sets were found to be a mixture of normal and non-normal distributions; therefore, non-parametric statistical analyses were used. A Kruskal-Wallis ANOVA combined with Dunn’s post-hoc test was applied to determine statistical differences between the groups, with significance set to 0.05.
Results
Spray drying resulted in flowable powders. Water content of the powders measured after spray drying was ~5% and was further reduced to <1% after the powders were transferred to glass vials which were evacuated under vacuum and backfilled with nitrogen.
Vaccines formulated within alumina-coated microspheres were incubated for three months with no change in appearance. But after 6 weeks of incubation at 50 and 70 °C, both the liquid and spray-dried vaccines showed visible discoloration and physical changes. Spray-dried powders incubated at 70 °C appeared to have experienced melt-back, causing the powders to agglomerate, while the incubated liquid formulations contained visible aggregates. Because of the obvious degradation that occurred in the lyophilized and spray-dried samples during incubation at elevated temperatures, these samples were not used in subsequent animal studies.
To evaluate the murine immune response following administration of HPV L1 capsomeres formulated in ALD-coated microspheres, total antibody titers for each HPV serotype (HPV16, 18, and 31) were measured over a 72-day period (Figure 1). The trends for each serotype were similar, with animals that received ALD-coated microspheres, regardless of incubation temperature, yielding the highest titers (>1 log higher than liquid or spray-dried formulations across all serotypes). Interestingly, for all formulation types and regardless of whether mice received a boost dose, a plateau in titer was observed at approximately day 21. By day 43, titers in the group that had received liquid formulations reached maximum values, regardless of serotype (Figure 2). Maximum titers in sera from mice administered spray-dried and reconstituted formulations were observed by day 26 post-vaccination for HPV31, and by day 43 for HPV16 and 18. The groups of mice that received ALD-coated microspheres showed a somewhat delayed response. Titers following injection of ALD-coated vaccines that had been incubated at 4 °C reached maximal values at day 58 for HPV18 and at day 72 for HPV types 16 and 31. Titers in the group that received ALD-coated microspheres incubated at 50 °C reached maximum values by day 72 for HPV16 and day 58 for HPV18 and 31. Maximum titers following administration of ALD-coated microspheres incubated at 70 °C were observed on day 72 for HPV18 and day 58 for HPV16 and 31. Differences between titers generated by the liquid, spray-dried and reconstituted, and ALD-coated microspheres formulations were significant (p ≤ 0.05) across all time points (Sup. Figure 1–3). By day 72 there were no statistical differences between the various groups that had received ALD-coated microsphere formulations, irrespective of serotype or incubation temperature (p ≥ 0.05).
Figure 1. Titers of antibodies against serotypes HPV 16, 18, and 31 in vaccinated BALB/C mice.

Anti-HPV 16 titers are shown in the first row, anti-HPV 18 titers in the second row, and anti-HPV 31 titers in the bottom row. Each point is the geometric mean of titers for all animals in the group. Titers for groups of mice that received 7.5 μg doses of HPV capsomeres in liquid or spray-dried and reconstituted (SD) formulations on days 0 and 21 are shown in black closed squares. Titers for groups of mice that received single, 7.5 μg doses of HPV capsomeres formulated in spray-dried microspheres coated with nanoscopic alumina layers deposited in 250 ALD cycles that had been incubated for three months at 4, 50 or 70 °C are shown as open orange circles, open blue triangles, and inverted open green triangles, respectively.
Figure 2. Maximum anti-HPV 16, 18, and 31 ELISA titers.
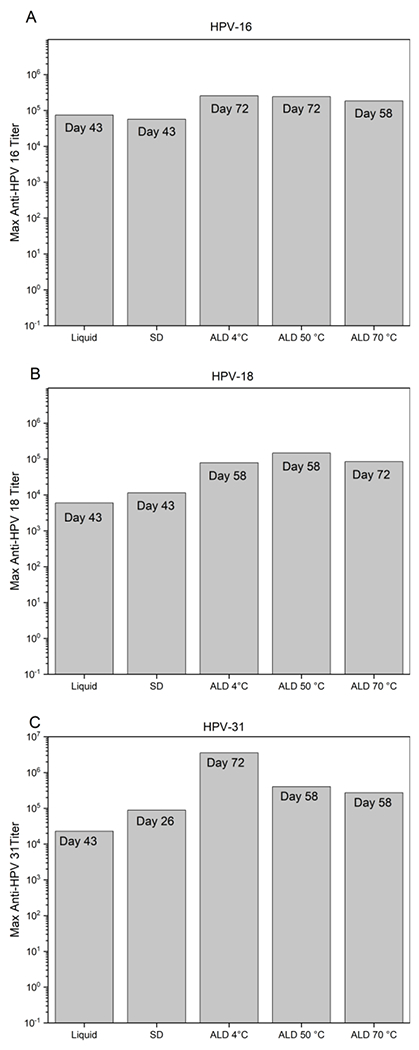
Maximum anti-HPV16 (A), anti-HPV18 (B), or anti-HPV31 (C) ELISA titers measured in sera from each group of mice. All mice were administered a 7.5 μg dose of HPV capsomeres on day 0; groups of mice that received liquid and SD formulations were administered an additional 7.5 μg booster dose on day 21. ALD-coated microspheres were incubated for 3 months at 4, 50 or 70 °C prior to administration. Maximum titers were reached on the day noted on each of the bars of the graph.
Neutralizing responses were measured using HPV16, 18, or 31 pseudoviruses and compared across all formulations on day 72 (Figure 3). Groups that received ALD-coated microspheres had ~1 log greater neutralization titers than spray-dried and liquid controls across all serotypes irrespective of incubation temperature, similar to the total antibody responses (Table 1). Groups that received two 7.5 μg doses of liquid or spray-dried formulations had lower neutralization titers for all serotypes than those receiving a single dose of ALD-coated microspheres. Interestingly, the neutralizing antibody titers against HPV18 (Figure 3b) were greater than those against both HPV16 and 31 (Figures 3a,c) and additional dilutions were required to reach a limiting value. Nonetheless, strong neutralizing antibody responses were generated against all three serotypes tested, indicating a lack of interference between serotypes in the trivalent formulation.
Figure 3. Day 72 neutralization titers for HPV16 (A), 18 (B), and 31(C).

Groups received liquid formulations (black squares), spray-dried formulations (red circles), ALD-coated microspheres incubated at 4 °C (blue triangles), 50 °C (green inverted triangles) or 70 °C (gold diamonds). Mice were administered 7.5 μg doses on day 0. Groups receiving liquid or spray-dried formulations were administered additional booster doses on day 21 whereas only a single dose was administered to groups receiving ALD-coated microsphere formulations.
Table 1.
Summary of neutralization titers for HPV16, 18, and 31 from neutralizing antibody assays in BALB/C mice on day 72 post injection of liquid, spray-dried (SD), or ALD-coated microspheres formulations.
| Serotype | Formulation | Incubation Temperature (°C) | Mean Titer |
|---|---|---|---|
| 16 | Liquid | Not incubated | 8.7E+03 |
| 16 | SD | Not incubated | 1.8E+03 |
| 16 | ALD | 4 | 3.0E+04 |
| 16 | ALD | 50 | 1.6E+04 |
| 16 | ALD | 70 | 2.8E+04 |
| 18 | Liquid | Not incubated | 7.2E+04 |
| 18 | SD | Not incubated | 5.6E+04 |
| 18 | ALD | 4 | 5.4E+05 |
| 18 | ALD | 50 | 5.4E+05 |
| 18 | ALD | 70 | 2.7E+05 |
| 31 | Liquid | Not incubated | 4.5E+03 |
| 31 | SD | Not incubated | 9.4E+03 |
| 31 | ALD | 4 | 3.5E+04 |
| 31 | ALD | 50 | 2.8E+04 |
| 31 | ALD | 70 | 7.7E+04 |
Discussion
Trivalent HPV capsomere vaccines formulated as ALD-coated microspheres exceeded the World Health Organization’s controlled temperature chain program requirements of stability for 3 days of storage at 40 °C23 and exhibited superior immune responses (> 1 log higher antibody titers) after administration of a single dose compared to responses observed following administration of two doses of liquid or spray-dried and reconstituted formulations (Figures 1 and 2). Liquid and spray-dried formulations incubated at temperatures of 50 or 70 °C were unstable, whereas ALD-coated microsphere formulations tolerated these conditions and yielded higher total antibody titers and neutralizing antibodies than the unincubated liquid and spray-dried controls (Figures 1–3). Development of the immune response to administration of the ALD-coated microspheres followed the same time course and reached the same final antibody titers irrespective of storage temperature, suggesting that the antigen release characteristics of the particles were unaffected by the three-month, high temperature storage.
Previously we showed that trivalent, lyophilized capsomere vaccine for HPV types 16, 18, and 31 administered in a multidose regimen elicited strong total and neutralizing antibody titers in mice, with no apparent interference between the serotypes.19 We observed similar results with the present single-dose, ALD-coated microsphere formulations, which generated titers between 105-106 (Figure 1 and 2) and neutralizing antibody responses showing no evidence of interference (Figure 3). Similar to our previous study, the vaccines were highly thermostable, with no loss of total antibody or neutralizing antibody titers observed even after prolonged exposure to temperatures as high as 70 °C. In another previous work evaluating a monovalent HPV16 ALD-coated microsphere formulation in mice20, HPV16 titers measured on day 72 post vaccination were similar to the HPV16 titers observed in the current study (Figure 1). In both this previous study and the current work, a plateau in antibody levels was observed at approximately 21 days post-vaccination regardless of formulation. This effect likely corresponds to a priming effect due to immediate release of capsomere antigens due breakage of the alumina coatings on a small fraction of the coated powders during handling prior to administration. Following the plateau around 21 days, the eventual dissolution of the ALD-coated antigen provided a boost dose of antigens.
In comparison with the response to the liquid and reconstituted spray-dried formulations, a delay in the time required to achieve maximum titers was seen in following administration of the ALD-coated microsphere preparation (Figure 2). Maximum titers in response to the liquid and the reconstituted spray-dried formulations were observed approximately 6 weeks after the initial dose (i.e., three weeks after the booster dose was administered), whereas antibody titers peaked 9-10 weeks after administration of single doses of the ALD-coated preparations. It is likely that the prolonged release of antigens from the ALD-coated microspheres20 caused both the delayed time-to-peak response and the ~10-fold increase in titers we observed in response to single doses of the ALD-coated material. Tam et al. demonstrated that prolonged dosing of an HIV subunit vaccine produced higher titers than conventional prime-boost dosing schemes24, similar to the results we observed as the ALD coating dissolves in vivo. They hypothesized that this effect may be due to increased germinal center activity. However, elucidating the mechanisms of increased immune response to our ALD-coated microspheres formulation is beyond the scope of the current study.
Spray-drying of formulations containing Gardasil 9, a multivalent, virus-like particle (VLP) vaccine targeting HPV, has previously been described by Kunda et al. 25, who tested total anti-Gardasil 9 antibody responses in mice that received spray-dried vaccines in a 2-dose, prime-boost sequence Similar to the present study, incubation of the spray-dried formulations for three months at 40 °C did not affect anti-Gardasil 9 antibody responses, and the spray dried preparations were protective against HPV infection in a pseudovirus challenge model. The present work extends these results, showing that spray-dried HPV vaccines coated with nanoscopic alumina by ALD retain full immunogenicity after 3-month storage at temperatures as high as 70 °C, and additionally require only single doses to achieve high-titer neutralizing responses.
Conclusion
A majority of new cervical cancer cases arise in LMICs1; thus, the creation of a vaccine that is not only thermostable, but in addition requires only a single administration would be highly advantageous. Capsomere-based HPV L1 antigens can be produced using relatively inexpensive E. coli recombinant expression systems. The L1 major protein is easily purified as pentameric capsomeres26, and once formulated within ALD-coated powders the capsomeres are highly thermostable, minimizing or eliminating cold chain requirements. In the current study only three HPV serotypes (HPV16, 18, and 31) were included; however, these serotypes represent approximately 70% of HPV infections,2 and the observed lack of immunogenic interference between these three types is an encouraging indication that antigens of additional serotypes could be added if needed. Finally, single doses of the ALD-coated capsomere microspheres produced type-specific antibody titers and neutralizing antibody titers that were about ten times higher than those generated from conventional, two-dose regimens of corresponding liquid formulations.
Supplementary Material
Acknowledgements
Funding for this project was provided by University of Colorado, NIH/NCI SPORE in Cervical Cancer (2P50 CA098252), and the Bill & Melinda Gates Foundation (OPP1153439).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
TWR and RLG are inventors of relevant intellectual property owned by the Regents of the University of Colorado and licensed to Vitrivax, Inc., a company in which they hold financial interest.
References
- (1).de Martel C; Plummer M; Vignat J; Franceschi S Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017, 141 (4), 664–670. DOI: 10.1002/ijc.30716 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Chabeda A; Yanez RJR; Lamprecht R; Meyers AE; Rybicki EP; Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res 2018, 5, 46–58. DOI: 10.1016/j.pvr.2017.12.006 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Pingali C; Yankey D; Elam-Evans LD; Markowitz LE; Williams CL; Fredua B; McNamara LA; Stokley S; Singleton JA National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020. MMWR Morb Mortal Wkly Rep 2021, 70 (35), 1183–1190. DOI: 10.15585/mmwr.mm7035a1 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rosenblum HG; Lewis RM; Gargano JW; Querec TD; Unger ER; Markowitz LE Declines in Prevalence of Human Papillomavirus Vaccine-Type Infection Among Females after Introduction of Vaccine - United States, 2003-2018. MMWR Morb Mortal Wkly Rep 2021, 70 (12), 415–420. DOI: 10.15585/mmwr.mm7012a2 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Spayne J; Hesketh T Estimate of global human papillomavirus vaccination coverage: analysis of country-level indicators. BMJ Open 2021, 11 (9), e052016. DOI: 10.1136/bmjopen-2021-052016 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Aranda S; Berkley S; Cowal S; Dybul M; Evans T; Iversen K; Moeti M; Osotimehin B; Peterson S; Piot P; et al. Ending cervical cancer: A call to action. Int J Gynaecol Obstet 2017, 138 Suppl 1, 4–6. DOI: 10.1002/ijgo.12182 From NLM Medline. [DOI] [PubMed] [Google Scholar]
- (7).WHO. 2022. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed Jan 3, 2023).
- (8).Bruni L; Diaz M; Barrionuevo-Rosas L; Herrero R; Bray F; Bosch FX; de Sanjose S; Castellsague X Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016, 4 (7), e453–463. DOI: 10.1016/S2214-109X(16)30099-7 From NLM Medline. [DOI] [PubMed] [Google Scholar]
- (9).Ngabo F; Levin A; Wang SA; Gatera M; Rugambwa C; Kayonga C; Donnen P; Lepage P; Hutubessy R A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda. Vaccine 2015, 33 (51), 7357–7363. DOI: 10.1016/j.vaccine.2015.10.022 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).FDA. GARDASIL Product Insert. 2015. [Google Scholar]
- (11).FDA. GARDASIL9 Product Insert. 2020. [Google Scholar]
- (12).Asamoah A; Ebu Enyan NI; Diji AK; Domfeh C Cold Chain Management by Healthcare Providers at a District in Ghana: A Mixed Methods Study. Biomed Res Int 2021, 2021, 7559984. DOI: 10.1155/2021/7559984 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yauba S; Joelle S; Jude N; Tracy BO; Marie K; Charles N; Hermelle EE; Marius V; Julius S; Alain B; et al. Temperature Monitoring in the Vaccine Cold Chain in Cameroon. Journal of Vaccines & Vaccination 2017, 09 (01). DOI: 10.4172/2157-7560.1000384. [DOI] [Google Scholar]
- (14).Rogers B; Dennison K; Adepoju N; Dowd S; Uedoi K Vaccine Cold Chain. AAOHN Journal 2010, 58 (9), 337–346. DOI: 10.1177/216507991005800905. [DOI] [PubMed] [Google Scholar]
- (15).Zapata MI; Feldkamp JR; Peck GE; White JL; Hem SL Mechanism of freeze-thaw instability of aluminum hydroxycarbonate and magnesium hydroxide gels. J Pharm Sci 1984, 73 (1), 3–8. [DOI] [PubMed] [Google Scholar]
- (16).Kumru OS; Joshi SB; Smith DE; Middaugh CR; Prusik T; Volkin DB Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 2014, 42 (5), 237–259. DOI: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- (17).Kurzątkowski W; Kartoğlu Ü; Staniszewska M; Górska P; Krause A; Wysocki MJ Structural damages in adsorbed vaccines affected by freezing. Biologicals 2013,41 (2), 71–76. DOI: 10.1016/j.biologicals.2011.10.011. [DOI] [PubMed] [Google Scholar]
- (18).Hassett KJ; Meinerz NM; Semmelmann F; Cousins MC; Garcea RL; Randolph TW Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur J Pharm Biopharm 2015, 94, 220–228. DOI: 10.1016/j.ejpb.2015.05.009 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dong M; Meinerz NM; Walker KD; Garcea RL; Randolph TW Thermostability of a trivalent, capsomere-based vaccine for human papillomavirus infection. Eur J Pharm Biopharm 2021, 168, 131–138. DOI: 10.1016/j.ejpb.2021.08.008 From NLM Medline. [DOI] [PubMed] [Google Scholar]
- (20).Garcea RL; Meinerz NM; Dong M; Funke H; Ghazvini S; Randolph TW Single-administration, thermostable human papillomavirus vaccines prepared with atomic layer deposition technology. NPJ Vaccines 2020, 5 (1), 45. DOI: 10.1038/S41541-020-0195-4 From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pastrana DV; Buck CB; Pang YY; Thompson CD; Castle PE; FitzGerald PC; Kruger Kjaer S; Lowy DR; Schiller JT Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 2004, 321 (2), 205–216. DOI: 10.1016/j.virol.2003.12.027 From NLM Medline. [DOI] [PubMed] [Google Scholar]
- (22).Pastrana DV; Buck CB; Lowy DR; Schiller JT Papillomavirus Neutralization Assay. National Institute of Health National Cancer Institute Center for Cancer Research: 2018. [Google Scholar]
- (23).World Health Organization. Guidelines on the stability evaluation of vaccines for use under extended controlled temperature conditions, Annex 5, TRS No 999. 2016. [Google Scholar]
- (24).Tam HH; Melo MB; Kang M; Pelet JM; Ruda VM; Foley MH; Hu JK; Kumari S; Crampton J; Baldeon AD; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A 2016, 113(43), E6639–E6648. DOI: 10.1073/pnas.1606050113 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kunda NK; Peabody J; Zhai L; Price DN; Chackerian B; Tumban E; Muttil P Evaluation of the thermal stability and the protective efficacy of spray-dried HPV vaccine, Gardasil(R) 9. Hum Vaccin Immunother 2019, 15 (7-8), 1995–2002. DOI: 10.1080/21645515.2019.1593727 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Shi L; Sings HL; Bryan JT; Wang B; Wang Y; Mach H; Kosinski M; Washabaugh MW; Sitrin R; Barr E GARDASIL: prophylactic human papillomavirus vaccine development--from bench top to bed-side. Clin Pharmacol Ther 2007, 81 (2), 259–264. DOI: 10.1038/sj.clpt.6100055 From NLM Medline. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


