Abstract
Background:
Survivors of childhood cancer often suffer from infertility. While sperm cryopreservation is not feasible before puberty, the patient’s own spermatogonial stem cells (SSCs), could serve as a germ cell reservoir, enabling these patients to father their own children in adulthood through the isolation, in vitro expansion, and subsequent transplantation of SSCs. However, this approach requires large numbers of stem cells and methods for successfully propagating SSCs in the laboratory are yet to be established for higher mammals and humans. The improvement of SSC culture requires deeper understanding of their metabolic requirements and the mechanisms that regulate metabolic homeostasis.
Aim:
This review gives a summary on our knowledge of SSC metabolism during maintenance and differentiation and highlights the potential influence of Sertoli cell and stem cell niche maturation on SSC metabolic requirements during development.
Results and Conclusions:
Fetal human SSC precursors, or gonocytes, migrate into the seminiferous cords and supposedly mature to adult stem cells within the first year of human development. However, the SSC niche doesn’t fully differentiate until puberty, when Sertoli cells dramatically rearrange the architecture and microenvironment within the seminiferous epithelium. Consequently, prepubertal and adult SSCs experience two distinct niche environments potentially affecting SSC metabolism and maturation. Indeed, the metabolic requirements of mouse PGCs and pig gonocytes are distinct from their adult counterparts and novel single cell RNA sequencing analysis of human and porcine SSCs during development confirms this metabolic transition. Knowledge of the metabolic requirements and their changes and regulation during SSC maturation is necessary to implement laboratory-based techniques and enable clinical use of SSCs. Based on the advancement in our understanding of germline metabolism circuits and maturation events of niche cells within the testis we propose a new definition of spermatogonial stem cell maturation and its amendment in the light of metabolic change.
Keywords: testis, development, metabolism, microenvironment, niche maturation
Introduction
Effective anti-cancer therapies can be a cause for male infertility1. While sperm can be cryopreserved in adult men, fertility preservation for juvenile male cancer patients requires the development of different approaches such as spermatogonial stem cell (SSC) transplantation 2. SSCs are the basis of continuous spermatogenesis in the adult male and must be obtained in high numbers for clinical applications. However, pre-pubescent male gonads contain only a small population of undifferentiated spermatogonia, including putative SSCs, rather than fertilization-competent gametes. Hence, extensive laboratory handling is required to facilitate in vitro propagation and use of prepubertal germ cells. SSC culture systems remain challenging in species other than rodents 3–6 due, in part, to the lack of understanding of the metabolic requirements of immature male germ cells. The metabolism of adult SSCs and differentiating germ cells has been investigated 7–10: recent studies have shed light on the importance of glycolytic flux homeostasis for SSC maintenance and active mitochondrial metabolism for their differentiation capacity11–17.
SSCs are an unique adult type of stem cell, as their immature precursors are present after birth18,19. New data confirms that these juvenile precursors are also metabolically distinct from adult undifferentiated spermatogonia including SSCs. SSCs originate from bipotent primordial germ cells (PGCs) that migrate into the developing testis during embryogenesis and colonize the forming seminiferous cords as gonocytes20–22, pro-/prespermatogonia23,24 or fetal spermatgonia25 (term used interchangeably in the literature). Various differences have been described between SSC precursor stages, which suggests a complex maturational process during their migration and the establishment of the niche18,26–30. We will refer hereafter to all stages before puberty as prepubertal spermatogonia or immature SSC precursors.
In humans, the maturation to adult-like SSCs has been proposed to be initiated prior to birth and completed at least within the first year according to ultrastructure or commonly used SSC transcriptional markers 18,30,31. However, new findings suggest that PGCs and prepubertal spermatogonia are metabolically distinct from adult SSCs in mouse, pig, and humans32–38. Metabolic flux is highly sensitive to changes in the metabolic microenvironment: While at birth, SSC precursors are surrounded by a primitive niche formed by immature Sertoli and interstitial cells until the onset of puberty, after puberty, mature Sertoli cells differ in ultrastructure, metabolism, and biochemistry from their immature counterparts, building a metabolically and immunologically complex niche system39–41. Recent studies have started to advance our understanding of how Sertoli cells and SSC precursors change in a coordinated fashion during human development18,19,38,41, however, an appreciation of germ cell metabolism and its exact regulation is still missing. To effectively implement laboratory-based techniques for fertility preservation for juvenile patients, greater attention should be paid to the metabolic requirements of SSCs during development, to allow for the preservation of functional SSCs that can give rise to fertile male gametes.
Here, we provide an overview on the knowledge of the metabolic requirements of SSCs during maintenance and differentiation during spermatogenesis. In the context of the differences between the immature and the adult SSC niche, focusing on changes in Sertoli cells, we discuss existing information about the distinct microenvironment SSCs experience throughout development and re-evaluate SSC maturation in the framework of cellular metabolism.
1. The Adult Seminiferous Epithelium – a Polarized Environment
The adult human testis consists of hundreds of seminiferous tubules surrounded by a basal lamina and peritubular myoid cells, embedded in a specialized interstitium with testosterone-producing Leydig cells, immune cells, and non-fenestrated capillaries. The adult seminiferous epithelium contains different stages of spermatogenic cells and Sertoli cells. Spermatogenesis relies on a high level of cellular organization to allow for spatial and temporal coordination along the seminiferous tubules and a key player in this intricate process is the Sertoli cell, which extends from the base to the lumen of the tubule surrounding each stage of germ cell 42,43.
The adult seminiferous epithelium is compartmentalized through the intrinsic polarization of adult Sertoli cells and the formation of a paracellular tight junction barrier between adjacent Sertoli cells, as part of the blood-testis-barrier (BTB)44. Sertoli cell tight junctions segment the seminiferous epithelium into two distinct compartments: a basal compartment where SSCs, undifferentiated spermatogonia, and early primary spermatocytes are located, and an adluminal compartment containing meiotic and post-meiotic germ cells. Sertoli cells are responsible for nutritional, hormonal, and growth factor support for the germ cells and are selectively controlling which molecules are transported between the compartment of the seminiferous tubules through tight junctions between adjacent Sertoli cells45–47. Cytoplasmic projections emanate from Sertoli cells and, in combination with different junctions 45,47,48, create unique metabolic microchambers for each stage of spermatogenesis49–52.
1.1. The Adult SSC Niche
The basis of male fertility are SSCs, located at the base of the adult seminiferous tubules. SSC maintenance is regulated by a specific microenvironment, called the SSC niche. This niche is characterized by a complex combination of cell-cell interactions, growth factor release, and an extracellular milieu defined by Sertoli cells and the adult interstitium53. SSCs are surrounded within the tubule by Sertoli cells laterally and dorsally and by a thick basal lamina at the base. Besides several layers of peritubular myoid cells in human testis, Leydig cells, endothelial cells and macrophages in the interstitium also contribute to the unique environment of the SSC niche by growth factor production and creation of diffusion barriers54–59. Specific growth factor composition and cellular contributions to the SSC niche have been extensively reviewed elsewhere53,60. Here we only summarize a few components:
Glial cell derived neurotrophic factor (GDNF) and signalling is an essential component of SSC self-renewal in vivo and in vitro61–64, along with basic fibroblast growth factor (bFGF)53. Sertoli cells make physical contact with SSCs and additionally supply other growth factors and signaling molecules such as insulin growth factor (IGF1), WNT5A, leukemia inhibitory factor (LIF), and retinoic acid, that control the self-renewal and differentiation capacity of the SSC population53,65–70. Peritubular myoid cells also contribute to the production of GDNF58, and together with Leydig cells and macrophages, that produce colony stimulating factor (CSF1), form an important interstitial component of the adult SSC niche57. A depletion of macrophages in mouse decreased spermatogonia numbers and differentiation in vivo54. The continuous endothelial layer of blood vessels supports a controlled testicular metabolic microenvironment by restricting diffusion properties through tight junctions71.
2. Metabolism in the Adult Seminiferous Epithelium
Cellular metabolism broadly describes the interconnected functions of multiple biochemical pathways that serve to produce energy, generate essential metabolites, maintain redox homeostasis, and regulate chromatin structure within a cell. As such, the importance of cellular metabolism has recently gained more research focus as a central cell signaling pathway rather than solely functioning to produce energy. Metabolic flux reacts highly sensitively to environmental changes, functioning as a dynamic sensor and regulator of cellular state 72,73.
The focus of most research on germ cell metabolism encompasses an investigation of anaerobic vs aerobic metabolic pathways (glycolysis and oxidative phosphorylation (OXPHOS), respectively) (Figure 1). Glycolysis describes the conversion from glucose to pyruvate, and produces two molecules of ATP per glucose molecule. The glycolytic pathway does not require oxygen and can therefore also occur under anaerobic conditions (under oxygen deprivation). Depending on the cell type and metabolic environment, pyruvate from glycolysis has two fates within the cell: its reduction to lactate or transport into mitochondria for conversion to acetyl-coA as fuel for mitochondrial respiration.
Figure 1: Simple schematic representation of glycolysis and OXPHOS.
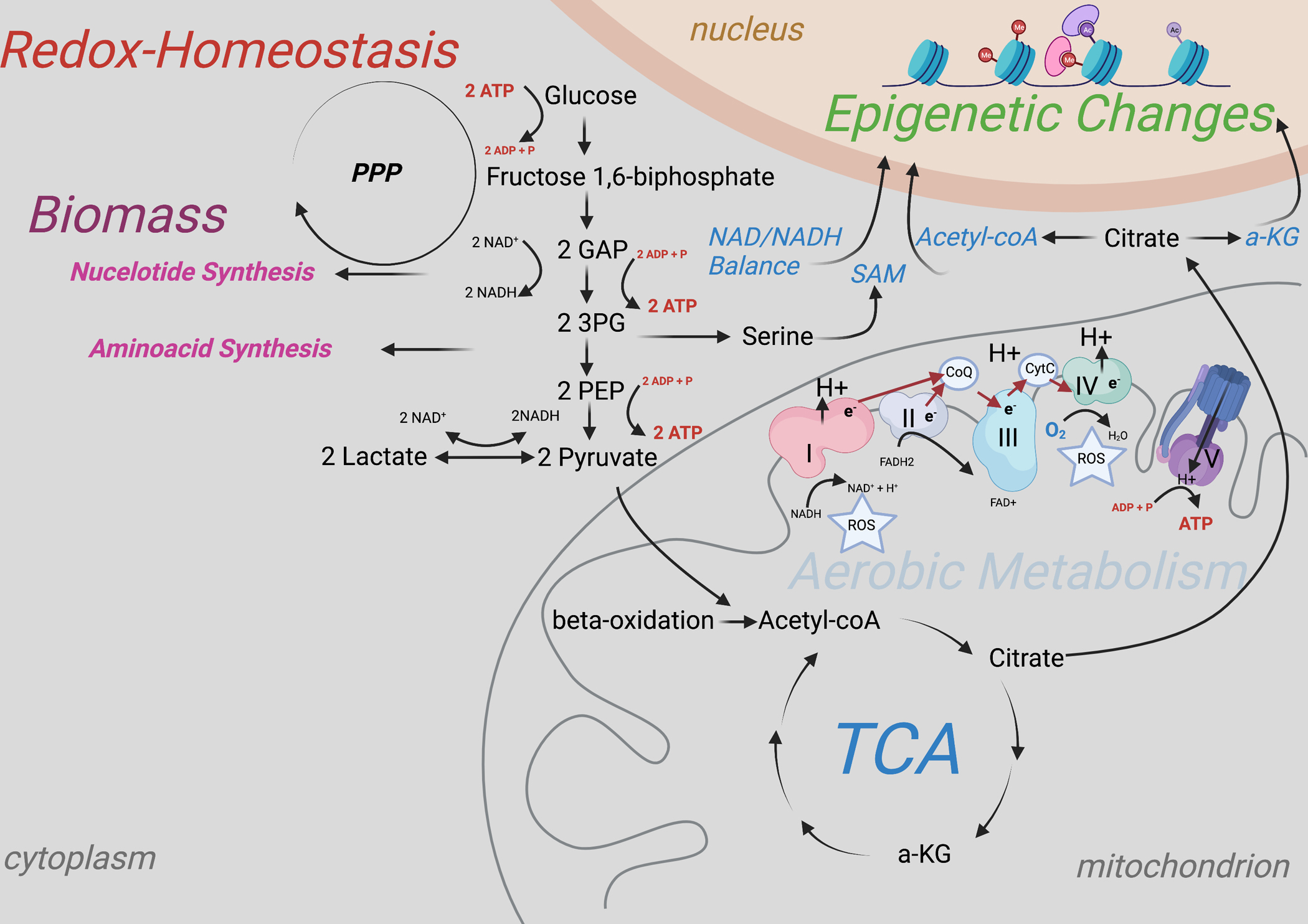
Simple enzymatic degradation of glucose to lactate. During the investment phase of glycolysis, two molecules of ATP are consumed. During the payoff phase, 4 ATP are produced. Redirection of pyruvate away from the mitochondrion causes its reduction to lactate under the oxidation of NADH, which can be reused in form of NAD+ during glycolysis. Second fate of pyruvate is the metabolization to acetyl-coA within the mitochondira with subsequent oxidative decarboxylation of acetyl-coA during the citric acid cycle (TCA). Besides glucose derived pyruvate, consumed pyruvate and lactate and fatty acids can serve as source for acetyl-coA. Redox equivalents (NADH and FADH2) are used to transport electrons (e-) from mitochondrial complex to complex (electron transport chain), which yields the energy to build up a proton gradient between the inner and outer mitochondrial membrane (mitochondrial membrane potential) that serves subsequently to produce ATP. The high number of redox equivalents produced during the TCA can be used to produce around 32 ATP per glucose molecule.
The production of lactate from glucose, in contrast to mitochondrial respiration, does not require oxygen and therefore serves as energy pathway even under low oxygen conditions. However, some cell types preferentially generate ATP mainly through glycolysis despite sufficient oxygen availability, a phenomenon that is called the Warburg effect 74.
Besides this glycolysis derived pyruvate, extracellular and consumed pyruvate and lactate as well as fatty acids via beta oxidation can also serve as additional or alternative substrates for acetyl-coA and oxidative phosphorylation8,10,34,75. The conversion to acetyl-coA and subsequent oxidative decarboxylation within the citric acid cycle (TCA) generates the redox equivalents NADH and FADH2, that can be used by mitochondrial complexes to create a proton gradient through the electron transport chain (ETC) between the inner and outer mitochondrial membrane. This gradient generates a mitochondrial membrane potential and is used by the ATP-synthase (complex V) to produce energy in form of ATP. This process yields over 15- fold higher amounts of ATP in contrast to glycolysis alone76.
2.1. Metabolism of the Adult SSC Niche
The testis is known to be an oxygen-deprived organ77, and a recent model suggests that mouse SSCs reside away from blood vessels, in a hypoxic niche78,79. Other groups showed, that undifferentiated spermatogonia populations reside close to the vasculature refer to a so-called “vascular niche model” 80. Adult SSCs and undifferentiated spermatogonia, located adjacent to the seminiferous tubule basal lamina and exist in different transcriptional states depending on their development16,31. State 0 representing the most undifferentiated/naïve SSC population, characterized by markers such as PHGDH, PIWIL4, EGR4, and others which transition over state 1, co-expressing various known SSC markers such as ID4 and UTF1, and increased expression of GFRA1, over the state 2 to proliferative stages 3 and 4 as detected by pseudotime and velocity analyses of adult human testis 31. In the vascular niche the strongly GFRA1 positive SSC population could resemble the state 1 of human spermatogonia development. This transition towards the vasculature could be associated with a movement along the differentiation trajectory due to different metabolic microenvironment, specifically oxygen.
Undifferentiated spermatogonia have long been proposed to predominantly metabolize blood-derived glucose through glycolysis rather than mitochondrial respiration 7,81,82. However, metabolic flux studies, meaning the in-depth analysis of preferential metabolite consumption of adult SSCs are still missing. Nevertheless, an enrichment of glycolysis-related genes in these cells has been confirmed by single cell RNA sequencing analysis in mouse and human and a transition towards upregulation of oxidative phosphorylation associated genes with further differentiation and movement to state 3 and 4. The movement closer to the vasculature might be therefore one initial trigger in changes of metabolic flux transcriptionally not yet detectable for strongly GFRA1 positive undifferentiated spermatogonia populations16,19,31.
Two studies in mouse showed that sufficient activation of glycolysis is indeed required for the proliferation and preservation of stemness in culture11,12. Increased glycolytic flux in SSCs through deprivation of lipids and free fatty acids from the culture medium and lowered oxygen partial pressure to 10%, has been shown to improve outcomes of mouse SSC transplantation11. Glycolysis is regulated by MYC and MYCN transcription factors and AKT-pathway activation in mouse SSCs, a signaling pathway that is induced by SSC niche-specific factors GDNF and bFGF, which are essential for the maintenance of SSC potential12. Knock-out studies that disrupt these pathways diminish self-renewal and colonization capacity/spermatogenic potential after serial SSC transplantation. Analysis of the slow proliferation phenotype of cultured MYC/MYCN double KO mouse SSCs revealed an underlying shift in metabolism, characterized by reduced glycolytic capacity and increased mitochondrial respiration12. Similarly, SSCs from mice with a C57BL/6 background are inherently difficult to culture in comparison to DBA/2 mice62, and exhibited a decreased extracellular acidification rate (ECAR), which measures the glycolytic flux from glucose to lactate. The proliferation of C57BL/6 mouse derived SSCs could be recovered by AKT activation and an increase in glycolytic flux, which also improved transplantation capacity12. These results show that two factors contribute to the regulation of SSC metabolism: the metabolic microenvironment (nutrient availability and oxygen partial pressure) and the intrinsic cellular plasticity to appropriately react to external stimuli and sufficient pathway activity. In this context it is interesting to mention, that velocity analysis of adult human testes indicated bidirectional movement from undifferentiated spermatogonia between states 0 to 2 showing a dynamic plasticity of these cells, potentially dependent on microenvironment 31.
The intrinsic self-renewal and glycolysis activation capacity might be generally lower in SSCs obtained from species other than rodents, explaining the need for a more sophisticated culture system, similar to mice from C57BL/6 background.
Why would SSCs employ this less energy efficient metabolic pathway? The preference for glycolytic flux over mitochondrial respiration even in the presence of oxygen was first described by Otto Warburg in cancer cells83, a phenomenon defined earlier. Albeit mitochondrial respiration being more efficient, glycolysis is less complex, less prone to malfunction, and permits a fast flux, such that ATP production by glycolysis can keep up with the ATP production through OXPHOS84. The oxidative decarboxylation of acetyl-coA during the TCA metabolizes glucose carbons to CO2. Redirecting pyruvate into the reduction to lactate, therefore, generates a reserve storage of carbon building blocks for the anabolic pathways, including nucleotide and amino acid production, required for proliferating cells 76. For example, the redirection of glycolytic metabolites used for the production of serine via the phosphoglycerate dehydrogenase (PHGDH) into the one-carbon cycle is important for nucleotide biosynthesis and but also availability of important methyl-donors such as S-adenosylmethionine (SAM) for epigenetic reactions (Figure 1). PHGDH is interestingly also highly enriched in state 0 spermatogonia in addition to glycolytic enzymes31. Furthermore, as glycolysis occurs without the use of oxygen, less generation of reactive oxygen species (ROS) occurs that could be harmful for the cell’s genomic integrity. However, it has been shown that certain amounts of ROS produced by the cytoplasmic NADPH oxidase (NOX1) are required to maintain mouse SSCs self-renewal in vivo and in vitro85,86 and that cells are otherwise very well adjusted to high mitochondrial respiration by upregulation of a complex antioxidative machinery34. A physiological oxygen partial pressure plays potentially a more important role than the percentual contribution of mitochondrial respiration to the overall energy production for the cellular redox homeostasis.
In this context, it is important to mention that metabolic flux generally occurs through both pathways, glycolysis and oxidative phosphorylation, just to a different degree. Importantly, Moussaief and colleagues showed that mouse embryonic stem cells (ESCs) do not completely redirect the pyruvate to lactate, as previously thought, but feed it partly into the TCA for the production of citrate. Citrate is transported out of the mitochondria for the generation of acetyl-coA in the cytoplasm, for the maintenance of a high histone acetylation status, required for an open chromatin structure and plasticity in ESCs87 (Figure 1). Similarly, in pig SSCs, an increase of H3 acetylation was shown to be accompanied with increased glycolytic flux and decreased mitochondrial respiration34. These data suggest a potentially similar partial shunt of pyruvate through the mitochondria for the generation of cytoplasmic acetyl-coA in spermatogonia. A functional mitochondrial metabolism could be required for the maintenance of the differentiation capacity of SSCs as well as for the protection of a high acetylation status and open chromatin structure88. In SSCs, glycolysis is essential to maintain SSC-specific circuits and mitochondrial quality control is still important for long-term SSC potential. Aging SSCs exhibit decreased mitochondrial activity, accompanied by increased glycolytic flux and decreased spermatogenic potential13. An active mitochondrial metabolism is required for differentiation, therefore decreased mitochondrial number and consequently a decreased activity could be the reason for the loss of this potential with aging.
2.2. Metabolism during Differentiation
During spermatogenesis, differentiating germ cells increase the expression of mitochondrial respiration associated genes16,31,41. Instead of glucose, pyruvate and lactate (that are interconvertible in the cell, see Figure1), serve as fuel for mitochondrial respiration in spermatocytes and spermatids8,10,75,89. It is not clear if the mitochondrial respiration steadily increases until spermatid stages of spermatogenesis, however, it has been reported that spermatids require lactate over pyruvate for maintenance of cellular ATP levels, in contrast to spermatocytes10,75. The underlying importance for this is still not known, but the oxidation of lactate to pyruvate might build up a distinct redox environment by increased production of NADH, required for increasing mitochondrial activity and function of other pathways90. Furthermore, lactate has been shown to be important for RNA and protein synthesis in differentiating germ cells91,92 and to inhibit apoptosis of human male germ cells 93.
Cells within the seminiferous tubules receive blood derived molecules from the interstitium by diffusion which is additionally controlled by Sertoli cells. Spermatogenesis occurs therefore along a nutrient and oxygen gradient from the base of the tubule to the lumen77. Cells moving out of the SSC niche change their metabolic flux, which could be the initial trigger for differentiation. While the decrease of a glucose gradient aligns with the transition towards a mitochondrial respiration, the decrease in oxygen partial pressure seems counter intuitive. However, this relatively lower oxygen partial pressure might be the right amount to maintain a physiological flux through solely aerobic metabolism in differentiating germ cells.
2.3. Contribution of the Adult Sertoli cell to the Metabolic Environment of the SSC niche
Cells can adjust metabolic flux extremely fast to adapt to their environment. Metabolic enzymes mostly function in both directions of the chemical reaction, therefore the direction and the rate of the reaction is dependent on availability of both substrates (metabolites). Metabolic enzyme kinetics are regulated by an intrinsic nutrient pool, which is naturally dependent on a cell’s metabolic microenvironment. This environment must be tightly regulated within the adult seminiferous epithelium to allow for life-long control of SSC self-renewal and differentiation and their associated drastic metabolic changes within the seminiferous tubules spanning a distance of just around 150 μm in humans94.
2.3.1. Sertoli cell Metabolism
Sertoli cells are characterised by high glycolytic flux in culture95, with over 60% of their consumed glucose processed through glycolytic flux rather than mitochondrial respiration despite oxygen availability, as assessed by metabolic flux studies34,96. Pyruvate and lactate, products of this high glycolytic flux, can be detected in the culture medium of Sertoli cells97, and serve as substrates for the previously discussed high mitochondrial metabolism in differentiating germ cells. Additionally, it has been shown by metabolite labelling experiments that, in Sertoli cells, glucose carbon atoms are preferentially oxidized through the pentose phosphate pathway (PPP) instead of oxidative decarboxylation through the TCA96. As discussed, this high glycolytic flux and metabolization through the PPP generates necessary building blocks for the constant turnover of Sertoli cell specific structures and serves to maintain various endocrine and paracrine functions during the spermatogenetic cycle66.
Through the excretion of glycolysis derived pyruvate and lactate, Sertoli cells create a unique testis specific metabolic milieu for differentiating germ cells. Indeed, the seminiferous tubule fluid is drastically different from the interstitial fluid and plasma composition 98–100, containing just 20% of the plasma glucose but 600-fold more lactic acid101. While it is generally accepted that Sertoli cells contribute by a high amount to the specific metabolite and ion composition of the seminiferous tubule fluid, the developing germ cells also contribute to the specifically high potassium ion and low sodium concentrations adluminal of the blood-testis-barrier 100,102–105. The maintenance of this special adluminal environment is assured by the Sertoli cell polarization and is not shared with the basal compartment.
2.3.2. Significance of the Sertoli cell Polarization and Blood-Testis Barrier
Cell polarization is a phenomenon characterized by the uneven distribution of cytoskeletal, membranous, and cytoplasmic molecules including lipids, proteins, and nucleotides106–109. In the testis, this polarization is built and maintained by the formation of the BTB and intracellular polarity complexes of Sertoli cells. The blood-testis barrier refers to the combined effect of several biological features of the testis that serve to protect and ensure the proper environment for differentiating germ cells. Junctional complexes between adjacent Sertoli cells (Sertoli cell barrier), a physicochemical barrier formed by peritubular myoid cells, the basement membrane, and vascular continuous capillaries, as well as testis-produced immunomodulatory factors are all considered part of the BTB104,110–116. As a broader definition of the BTB all junctional components work together in different areas of the tissue to maintain this strong morphological barrier, one of the tightest in mammalian tissues, creating testis specific interstitial and tubular environments117,118.
The compartmentalization inside the seminiferous tubules is achieved through the Sertoli cell specific junctions and polarization. This Sertoli cell barrier-component of the BTB is a specialized junctional complex formed by tight junctions, desmosomes, gap junctions, basal ectoplasmic specialization, and tubulobulbar complexes119. Sertoli cell tight junctions (hereafter referred to as Sertoli cell barrier) act as a paracellular gate to inhibit free paracellular diffusion, controlling flow of molecules from outside (blood and interstitial compartment) to inside the tubule, and vice-versa45,46. It anchors through multiple adaptor proteins, such as zonula occludens proteins, to the actin or vimentin cytoskeleton119. The strength of the Sertoli cell barrier is dependent on the association with polarity proteins and the number of tight junctions105,120–122, which form a belt-like structure of intercellular fibrils consisting of claudin, occludin, and tricullin. The permeability of the tight junctions is determined by composition of proteins, adhesion molecules, intracellular scaffold proteins and their phosphorylation status123,124. Tight junctions discriminate molecules based on size and charge, similar to a semipermeable membrane 125,126. In contrast to lipophilic substrates and gases, which can penetrate the seminiferous tubule through the cells (for example O2), hydrophilic molecules such as glucose and potassium, must be either transported actively or through facilitated diffusion, respectively 127.
Both the Sertoli cell junctions and polarity complexes are more basally located in Sertoli cells, in contrast to other epithelial cells119. This creates a tight basal compartment, where adult SSCs reside, and naturally minimizes the surface for basal Sertoli cell specific substrate excretion. In addition, interstitial fluid flow is generally faster than the seminiferous tubule fluid flow, apical of the Sertoli cell barrier128. Therefore, an uneven distribution of Sertoli cell excretion area and a higher exchange of interstitial testicular fluid create a gradient of Sertoli cell-derived and blood-derived factors, which is maintained by the junctions between Sertoli cells127. The contribution to this metabolic polarization by the compartmentalization of Sertoli cell specific transporters or their metabolism remains to be elucidated in depth. However, it is known that the Sertoli cell barrier contributes to this polarity by acting as a biological fence to block the movement of membrane lipids and proteins along the plasma membrane, thereby affecting transporter/receptor localization and signalling between the apex and the base105,129,130. Indeed, the polarization of receptors, such as the low density lipoprotein receptor (LDL), in Sertoli cells suggest such a biochemical polarization131. Furthermore, a compartmentalized secretion of stem cell specific factors in combination with the limited diffusion through the Sertoli cell junctions and slow diffusion through the thick adult basement membrane and several layers of peritubular myoid cells in the adult human testis (Figure 2), could concentrate locally produced metabolites and growth factors in the SSC niche in required concentrations for the maintenance of adult SSC glycolytic metabolic circuits. Such a specific distribution to the basal compartment has been shown for GDNF in the mouse and hamster testis 132.
Figure 2: Immature and mature SSC niche.
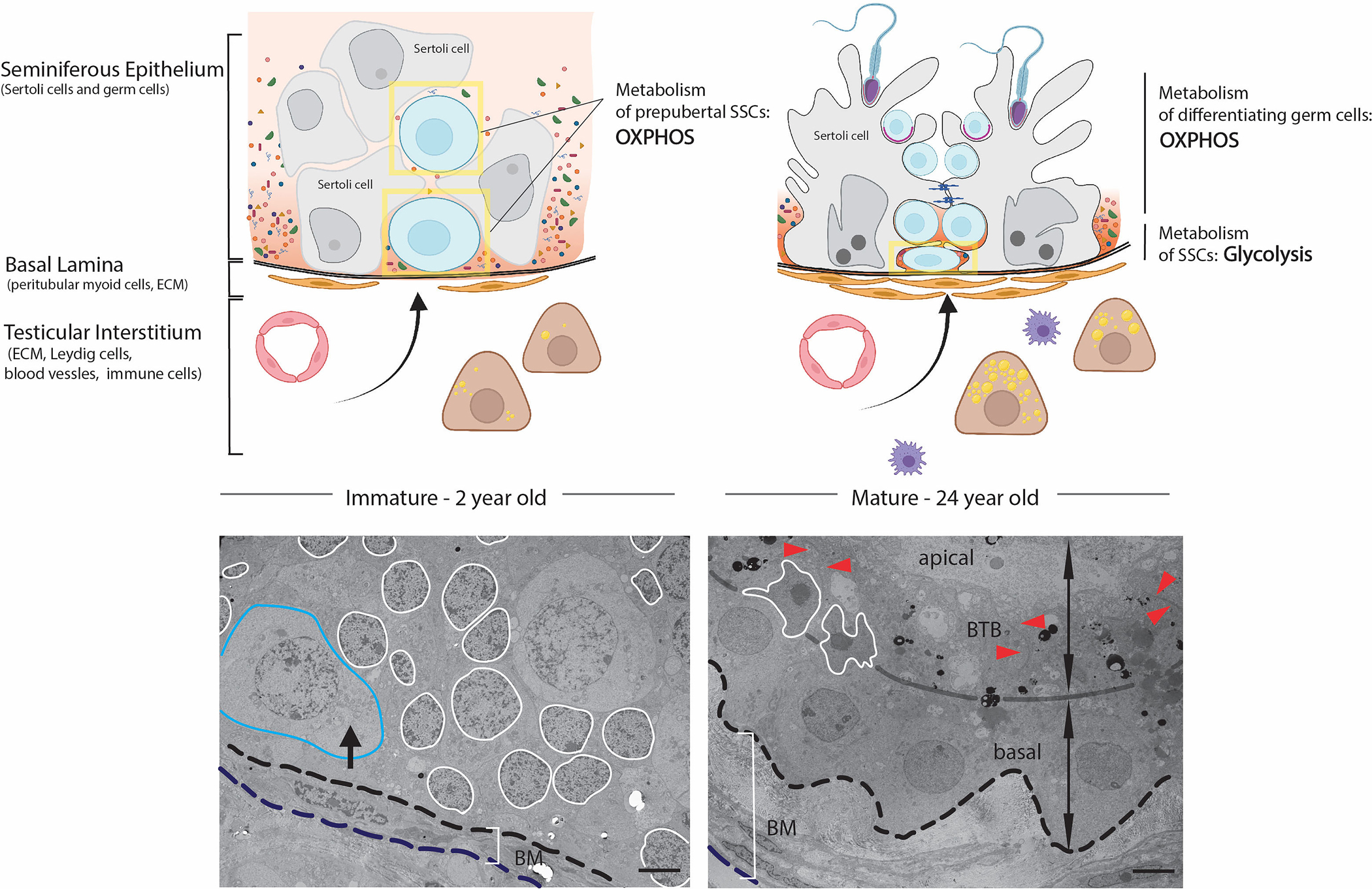
Left: Proliferating Sertoli cells form the primitive niche structure for prepubertal SSCs (in yellow boxes), with a simple basal lamina surrounded undifferentiated peritubular myoid cells and Leydig cells. Blood-derived molecules can freely diffuse through the cords. Immature SSCs are large circular cells and can be found at the basement membrane and occasionally central. Right: In the adult seminiferous epithelium, mature Sertoli cells and blood-testis barrier block diffusion of blood-derived molecules and create a specific composition of metabolites and growth factors to subsidize the anaerobic metabolism supporting adult SSC circuits. A thickened basal lamina and several layers of differentiated peritubular myoid cells together with differentiated testosterone producing Leydig cells and increased numbers of macrophages contribute to the specific microenvironment of the SSC niche. Adult SSCs are flat, half-moon shape and reside close to the basement membrane. TEM of 2-year (left) and 24-year-old (right) human testis: blue outlines prepubertal SSC, white outlines the Sertoli cell nucleus, black arrow shows perinuclear accumulated mitochondria, BM identifies basement membrane, red arrowhead identifies the BTB. Scale bar = 5μm (Figure modified from38).
All these functions of the BTB and Sertoli cell polarization for the control of the SSC niche metabolic environment are not present before puberty. Investigation of the adult intra- and extracellular metabolic polarization in contrast to the juvenile testis would aid in our understanding of SSC niche development. Which structural differences are known between immature and mature Sertoli cells that could help shed some light on the investigation of the metabolic microenvironment and its changes over time? What indication do we have that this metabolic microenvironment might be different?
3. The Immature Seminiferous Epithelium
The bipotent gonad develops from a thickening of the squamous celomic epithelium at the medial urogenital ridge24,133. Pre-Sertoli cells and interstitial somatic cells form relatively unorganized primitive seminiferous cords for homing SSC precursors. A simple layer of immature peritubular myoid cells together with Sertoli cells deposit extracellular matrix to form the basement membrane surrounding the cords and create a tubular and interstitial domain. This remains the extent of polarization until the organization of Sertoli cells along the basement membrane and formation of the BTB with the onset of puberty18,30,41,134,135 (Figure 2). Before the formation of the blood-testis barrier, loose intercellular connections exist between the immature Sertoli cells that are randomly located within the cords (Figure 2, white surrounded nucleus). Therefore, Sertoli cells are not yet controlling the paracellular flux of ions, water, metabolites, and solutes, allowing free diffusion to the inside of the cords. Metabolites and other substrates can freely diffuse through the whole tissue and developing cords. It seems therefore likely that the concentration of blood-derived molecules and basal specific growth factors are consequently lower, while Sertoli cell produced pyruvate and lactate concentrations are higher at the basement membrane relative to the adult testis, where undifferentiated spermatogonia are mainly located after 1 year of development 35.
From this point onward, the immature Sertoli cells and the undifferentiated Leydig and peritubular myoid cells in the interstitium form a very simple and non-compartmentalized environment, the juvenile and prepubertal SSC “niche”, which stands in stark contrast to the small and specialized adult SSC niche that is surrounded by highly differentiated niche cell types and embedded within a complex tissue architecture (Figure 2). To achieve success in SSC maintenance and spermatogenesis, Sertoli cells must mature to highly specialized, hyper-polarized and complex cells, which requires at least a decade in humans.
3.1. Developmental Timeline for SSC Maturation
The immature niche is colonized by primordial germ cells by 5 weeks of embryonic development in humans136. The male germ cell develops from the bipotent PGCs after extensive rounds of epigenetic remodelling and imprint erasure137,138. This transition coincides with repression of pluripotency markers, and changes in ultrastructure that start around 10–14 weeks of embryonic development18,22,28. PGCs commit to the male germ cell lineage and transition to fetal state 0 spermatogonia at 14 weeks of embryonic development a state that is transcriptionally highly similar to state 0 spermatogonia, as described18. scRNASeq analysis shows that spermatogonia from 15 weeks of embryonic development and postnatal samples cluster together as fetal-infant group18 and are positioned among state 0 spermatogonia at the beginning of the developmental pesudotime trajectory31.
At birth, two thirds of immature SSCs are located centrally in the seminiferous cords140 and can be transcriptionally divided into three states of immature spermatogonia (primordial germ cell-like and two states of prespermatogonia), with varying expression of pluripotency markers19. Coinciding with the juvenile testosterone peak, around 3 to 6 months of age, the germ cell movement towards the basement membrane is generally completed141–144. After 5 months to a year, only spermatogonia are detected, that are resembling the transcriptional profile of adult like state 0 SSCs (transcriptionally established most undifferentiated spermatogonia pool), according to common markers established by different groups18,19,31,145 (Figure 3). According to these data, there appears to be consensus that adult-like spermatogonia are present since birth or at least within the first year of human testicular development30,31,41 (Figure 3). However, we propose that this trajectory of germ line development is incomplete and requires an amendment according to new knowledge gained from metabolic analyses of different germ cells stages.
Figure 3: Timeline of human Sertoli cell and SSC development.

Upper panel: Sertoli cell development from the onset of male gonad development to puberty. At around 7 weeks of gestation, Sertoli cell precursors develop and give rise to fetal and immature Sertoli cells. Immature Sertoli cells persist during the entire prepubertal development, then undergo drastic changes during puberty to become the adult Sertoli cell contributing to the adult SSC niche. Middle panel: Current understanding of human SSC development. SSC maturation is initiated prior to birth to an adult-like transcriptional profile (fetal state 0). Within the first year, cells deepen their profile to an adult-like transcriptional phenotype and no PGC associated transcription is detectable (state 0). Lower panel: Proposed new metabolic development of the SSC maturation (according to results summarizing the metabolism of prepubertal and adult spermatogonia), defined by high aerobic metabolism during development and maintained until maturation of the niche, when an adult-like metabolic phenotype is established. We refer here to adult SSC only after the adult SSC niche has been developed (Figure modified from 35).
For all stages (embryonic/neonatal/infant/juvenile) of prepubertal SSCs, no distinct functional or metabolic phenotype has been described for a long time, despite their distinct ultrastructure, localization, and existence in an immature microenvironment in contrast to adult (Figure 2). Differently than adult SSCs, mouse, pig and human PGCs have been reported to rely on active mitochondrial metabolism to acquire a pool of metabolites necessary for extensive epigenetic remodeling32,33,36,37,72,146. Nevertheless, the metabolic changes that coincide with SSC maturation in any species have been ambiguous for a very long time.
Recent data in pigs show that prepubertal spermatogonia rely on oxidative phosphorylation just after birth34, similar to porcine PGCs 36. These immature pig SSCs are characterized by a large circular appearance with a low electron dense cytoplasm but thick round perinuclear accumulated mitochondria. In this study, it was shown that immature 1-week old pig SSCs exhibit high mitochondrial activity, as shown by a high mitochondrial membrane potential and oxygen consumption rate (OCR) in comparison to Sertoli cells and maturing SSC states. A preferential pyruvate and little to no glucose consumption could be detected in these early states using metabolic flux studies analyzed with HPLC-MS 34. Interestingly, at the same time, these cells were enriched with genes associated with the antioxidative machinery. A consequently higher resistance to ROS could be seen by a lower increase in intracellular ROS, after addition of increasing concentrations of hydrogen peroxide in the culture medium when compared to more matured states and Sertoli cells34. These data showed that these 1-week-old prepubertal pig spermatogonia, including immature SSCs, are highly adapted to high mitochondrial metabolism. Furthermore, metabolic flux studies comparing 1-week and 8-week-old pig immature SSCs in vitro revealed the beginning of a transition towards a glycolysis-based metabolism at 2 months of age. At the same time, a decrease in the antioxidative machinery expression was detected on transcriptional level leading to an increased sensitivity to ROS in maturing pig prepubertal spermatogonia/SSCs with 8-weeks, which might be an important factor for the maintenance of SSC circuits as discussed earlier86. In fact, this initial transition towards an adult SSC-like metabolism was accompanied with an increase in SSCs transcription factor promyelocytic zinc finger protein (PLZF) and AKT phosphorylation34 and an increase of state 0 specific enriched phosphoglycerate dehydrogenase (PHGDH) 18,35. The metabolic transition described in pig has now also been confirmed by single cell RNA sequencing data38.
Similarities in the mitochondrial ultrastructure between human prepubertal spermatogonia and 1-week old pig suggested that comparable transitions may occur in humans, albeit with distinct kinetics34 (Figure 2, black arrow showing perinuclear accumulated mitochondria in 2 year old human spermatogonia). And indeed, recent meta-analysis of existing human data in combination with single cell RNA profiling of new human testis tissue encompassing samples from embryonic development to puberty/adulthood revealed an enrichment of genes associated with high mitochondrial respiration in PGCs and during prepubertal development until 11 years of age 35. During the entire prepubertal phase, morphologically immature spermatogonia persist, with a big circular appearance distinct from the mature flattened SSC located at the basement membrane30,147. Just before the metabolic transition and the downregulation of OXPHOS associated genes, around 13 years of age, SSCs change to an adult-like flattened shaped cell at the basement membrane 35.
Immature SSCs are undergoing this metabolic transition potentially in two steps: Firstly, with movement towards the basement membrane, where the concentration of blood-derived molecules like glucose is increased and could elevate glycolytic flux and secondary stem cell specific pathways. As described above in pigs, a transition towards the basement membrane and increase in glycolytic flux was associated with an increase in stem cell specific pathways34. Secondly, with the maturation of the SSC niche a concentration of locally produced factors can be maintained by the Sertoli cell barrier, enhancing the local concentration of growth factors like GDNF, contributing to a high glycolytic flux in adult SSCs. This is shown by the changes in the hypoxic gene expression profile in this timeline of human SSC metabolic development: human SSCs seem to upregulate genes associated with hypoxia at 1 year when moving to the basement membrane and at 11 years shortly when the niche starts to mature and when they adopt the adult SSC flattened shape at the base 35.
While it seems generally accepted that the apical metabolic microenvironment changes with the formation of the BTB, can we also assume a change in the basal niche microenvironment with final Sertoli cell polarization? The investigation of changes in the basal metabolite concentration with the formation of the Sertoli cell junctions remains challenging, as novel in situ metabolic profiling techniques have not yet reached single cell resolution. However, looking at the permeability properties of the barrier and the complexity of tissue architecture in adults in contrast to prepubertal tissue, one could imagine that adult Sertoli cell polarization contributes to a polarized metabolic microenvironment, with a basal SSC niche compartment that is distinct from the primitive niche structure formed by immature Sertoli cells in which factors can diffuse freely away from the basement membrane and towards the center of the cords (Figure 2). Advancing the understanding of Sertoli cell maturation progression and extra- and intra-cellular polarization will help us define environmental cues coinciding with spermatogonial stem cell maturation.
3.2. Progression of the Sertoli cell Maturation in the Prepubertal Testis
A sufficient number of Sertoli cells is required to reach the threshold for male gonad development and spermatogenic efficiency. Therefore, extensive juvenile Sertoli cell proliferation is essential to build-up the necessary capacity for germ cell niches 39,103,148–155. Sertoli cell proliferation phases differ between species but are usually embryonic-neonatal and peripubertal. For primates and humans, the final Sertoli cell number is mainly determined by the second peripubertal wave of proliferation156–158, and it is generally accepted that Sertoli cell proliferation ceases with onset of puberty159,160. It is therefore important to note that the adult human Sertoli cell number is not established until almost 10 years after the first wave of proliferation, which ends approximately a year after birth39. However, a new transcriptional profiling detected Sertoli cells in S phase in juvenile samples from 1 to 7 years, revealing two distinct states: while one showed mature-like profiles such as mitochondrial transcription and AR expression, the other state had a distinct transcriptional profile including upregulation of ribosomal genes41. It is clear that these two immature Sertoli cell states are distinct from adult Sertoli cells, a transition that does not occur until at least the early onset of puberty (~11 years) 38,40,41.
Although these distinct Sertoli cell states have been described to exist independently41, it was also suggested that they may indicate a stepwise asynchronous maturation40, in which Sertoli cells progressively mature during testicular prepubertal development, rather than in two separate paths40. Infant Sertoli cells (3–8 years) are characterized by loose intercellular connections in form of adherens junctions, but not tight junctions30,41,161. In early puberty, androgen responsive genes include those involved in cytoskeleton remodelling, adhesion molecules and junction formation, governing localization and association of tight junction related proteins 38,52,108,162. It indeed seems to be the case that these ultrastructural changes and an organization of the seminiferous epithelium that are detected a decade after birth occur asynchronously, according to the ultrastructure of the testis, which is characterized by differently matured tubules between 10–12 years 38. Along with these ultrastructural changes, Sertoli cells drastically upregulate genes associated with lipid metabolism in humans 38,40. In pigs, Sertoli cell maturation is initiated with 8-weeks of age163. At the same time of development when ultrastructural changes in Sertoli cells, such as increased formation of Sertoli cell junctions, including tight junction formation, and nuclear morphology changes, an accumulation of lipid droplets in Sertoli cells was detected 38. Similarly, TEM analysis of human testis samples from birth to puberty revealed increasing lipid accumulation that preceded the ectoplasmic specialization development, at 7 to 8 years, and tight junction and lumen formation at 11–13 years, that persist during adulthood 38,41,161 (Figure 2). Lipidomic analysis of pig seminiferous tubules showed a drastic increase of glycerolipids (especially triglycerides (TGs)) as storage lipids, but a decrease of sphingolipids, particularly ceramides with maturation38. More research is required to understand how this change in the lipidome is contributing to specific aspects of the adult and immature SSC niche, however it could be relevant for the polarization of Sertoli cells164,165 or important for niche protective function as described in Drosophila166.
Therefore, an increase in lipid droplets might indicate the beginning of Sertoli cell maturation and polarization. By 11 years of age, Sertoli cells adopt a more mature transcriptional profile, increasing dramatically in size to form their tent-like support system for developing germ cells and undergo drastic ultrastructural, biochemical, and metabolic changes, resulting in the discussed potential changes of the SSC metabolic environment 41,43. The formation of tight junctions and creation of a tight basal domain might decrease the availability high concentrations of pyruvate and lactate but increase blood-derived glucose concentrations. This change in microenvironment and formation of tight junctions coincides with the adoption of a mature SSC shape and is followed by their metabolic maturation, which is characterized by a drop of OXPHOS associated genes after 11 years of age 35,38.
The testicular interstitium is also not fully developed until the onset of puberty at 11 years in humans and 2 months in pig, as shown by the change of transcriptional profile in human Leydig and myoid cells18,163. This final differentiation leads to several layers of peritubular myoid cells and extensive matrix deposition, contributing to the thickening of the basal lamina, which consequently change the basal compartment and diffusion properties (distance/resistance) to the SSC niche41,161 (Figure 2). Besides that, endothelial cells also seem to undergo maturation characterized by the strengthening of their paracellular resistance, as shown in rats167.
In summary, the complex adult SSC niche architecture is not developed until the onset of puberty when Sertoli cell maturation and polarization along with other somatic cell differentiation events drastically change the metabolic and immune testicular microenvironment to which developing SSCs are exposed.
4. Concluding Remarks – Revisiting SSC Maturation and Progression of a Metabolic Microenvironment
In summary, development of adult SSCs has been proposed to occur within the first year of life, while maturation of their niche lags behind by a decade41,168 (Figure 3). Adult SSCs reside in a niche highly defined by adult Sertoli cell function169–171, in a small and tightly controlled compartment at the basement membrane, surrounded by complex niche cells and controlled by paracellular diffusion through various intercellular junctional complexes. The immature testis, in contrast, is characterized by an undifferentiated interstitium and a thin basement membrane surrounding the seminiferous cords, with large circular putative SSCs among immature Sertoli cells (Figure 2). Different from the adult testis, the microenvironment of prepubertal SSCs is the whole seminiferous epithelium with a high number of proliferating, unpolarized Sertoli cells that are not regulating paracellular diffusion. Adult Sertoli cells differ in ultrastructure, metabolism, and biochemistry from immature Sertoli cells, building up a metabolically and immunologically polarized niche system. Therefore, SSCs are experiencing two distinct environments before and after puberty (Figure 2).
Cellular metabolic flux is sensitive to fluctuations in substrate availability and oxygen partial pressure, and slight changes can initiate a wide-ranging cascade of cellular modifications through the change of redox homeostasis and metabolite availability. The tight control and maintenance of distinct metabolic states are essential for specific epigenetic profiles of different states of spermatogenesis and, therefore, for male fertility. For example, environmental changes can be sensed by metabolic pathways, and further change the epigenetic landscape and cell fate72. Distinct metabolic states are important for germline stem cell development32,33,72,146, SSC maintenance11,12 and differentiation15. Consequently, the question remains: Are human SSC precursors metabolically distinct and when is their transition initiated?
We propose here that distinct metabolic transitions characterize human prepubertal SSC maturation, which is initiated by the development of the niche (Figure 3). We have the following indicators: 1) The accumulation of OXPHOS related metabolites is essential for PGC establishment and epigenetics32,33,72,146. Human and pig PGCs show an enrichment of oxidative phosphorylation associated genes and therefore likely have similar metabolic requirements to mouse PGCs 35. This stands in contrast to adult SSCs, to which a transition has to occur; 2) Neonatal SSC precursors of 1-week old pigs mainly maintain energetic demands by high OXPHOS and only switch towards an adult-like metabolic phenotype with 2 months of age34, as confirmed by a meta-analysis of recent scRNA sequencing data38; 3) Prepubertal human SSCs continuously exhibit an enrichment of OXPHOS related genes until 11 years of age, followed by a drastic change in their shape, which coincides with 4) the development of the polarized metabolic niche by formation of tight junctions in Sertoli cells and their final downregulation of OXPHOS related genes 35.
More research is required to understand in detail how these modifications underlying the ultrastructural and metabolic changes of peripubertal Sertoli cells influence the sensitive metabolic and epigenetic pathways of putative SSCs during development. We propose that changes in the lipidome may be an indicator for niche polarization events and subsequent SSC metabolic maturation 38. Samples from prepubertal patients that could be used for downstream applications are scarce and, therefore, require efficient handling for the best preservation and expansion of functional SSCs. The field would benefit from a clearer definition of SSCs, as well as cell or tissue-based biomarkers that predict SSC and niche maturation. This would enable the optimization of techniques for laboratory culture of these cell types and the development of downstream applications, including the clinical use of SSCs for the treatment of infertility in juvenile cancer survivors.
Acknowledgements
Illustrations were generated with Biorender.com
Funding Information
Work from our group included in this review was funded by NIH/ORIP R01 OD016575 and NIH/NICHD 1R01 HD091068 to ID.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Goossens E, Jahnukainen K, Mitchell R, et al. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open. 2020;2020(3):hoaa016. doi: 10.1093/hropen/hoaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takashima S, Shinohara T. Culture and transplantation of spermatogonial stem cells. Stem Cell Res. 2018;29:46–55. doi: 10.1016/j.scr.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Izadyar F, den Ouden K, Creemers LB, Posthuma G, Parvinen M, de Rooij DG. Proliferation and Differentiation of Bovine Type A Spermatogonia During Long-Term Culture1. Biol Reprod. 2003;68(1):272–281. doi: 10.1095/biolreprod.102.004986 [DOI] [PubMed] [Google Scholar]
- 4.Bahadorani M, Hosseini SM, Abedi P, Abbasi H, Nasr-Esfahani MH. Glial cell line-derived neurotrophic factor in combination with insulin-like growth factor 1 and basic fibroblast growth factor promote in vitro culture of goat spermatogonial stem cells. Growth Factors. 2015;33(3):181–191. doi: 10.3109/08977194.2015.1062758 [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Wu JY, An XL, et al. Enrichment and culture of spermatogonia from cryopreserved adult bovine testis tissue. Anim Reprod Sci. 2016;166:109–115. doi: 10.1016/j.anireprosci.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 6.Oatley MJ, Kaucher AV., Yang Q-E, Waqas MS, Oatley JM. Conditions for Long-Term Culture of Cattle Undifferentiated Spermatogonia. Biol Reprod. 2016;95(1):14. doi: 10.1095/biolreprod.116.139832 [DOI] [PubMed] [Google Scholar]
- 7.Bajpai M, Gupta G, Setty BS. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur J Endocrinol. 1998;138(3):322–327. doi: 10.1530/eje.0.1380322 [DOI] [PubMed] [Google Scholar]
- 8.Grootegoed JA, Jansen R, Van Der Molen HJ. The role of glucose, pyruvate and lactate in ATP production by rat spermatocytes and spermatids. BBA - Bioenerg. 1984;767(2):248–256. doi: 10.1016/0005-2728(84)90194-4 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Okinaga S, Arai K. Metabolism of round spermatids: Evidence that lactate is preferred substrate. Am J Physiol. 1984;247(2 Pt 1):E234–242. doi: 10.1152/ajpendo.1984.247.2.e234 [DOI] [PubMed] [Google Scholar]
- 10.Mita M, Hall PF. Metabolism of Round Spermatids from Rats: Lactate as the Preferred Substrate1. Biol Reprod. 1982;26(3):445–448. doi: 10.1095/biolreprod26.3.445 [DOI] [PubMed] [Google Scholar]
- 11.Helsel AR, Oatley MJ, Oatley JM. Glycolysis-Optimized Conditions Enhance Maintenance of Regenerative Integrity in Mouse Spermatogonial Stem Cells during Long-Term Culture. Stem Cell Reports. 2017;8(5):1430–1441. doi: 10.1016/j.stemcr.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanatsu-Shinohara M, Tanaka T, Ogonuki N, et al. Myc / Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev. 2016;30(23):2637–2648. doi: 10.1101/gad.287045.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanatsu-Shinohara M, Yamamoto T, Toh H, et al. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc Natl Acad Sci U S A. 2019;116(33):16404–16409. doi: 10.1073/pnas.1904980116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varuzhanyan G, Rojansky R, Sweredoski MJ, et al. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. Elife. 2019;8:e51601. doi: 10.7554/eLife.51601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Zhang Z, Chang C, et al. A bioenergetic shift is required for spermatogonial differentiation. Cell Discov. 2020;6(56). doi: 10.1038/s41421-020-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermann BP, Cheng K, Singh A, et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018;25(6):1650–1667.e8. doi: 10.1016/j.celrep.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord T, Nixon B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev Cell. 2020;52(4):399–411. doi: 10.1016/j.devcel.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Sosa E, Chitiashvili T, et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem. 2021;28(4):764–778.e4. doi: 10.1016/j.stem.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohni A, Tan K, Song HW, et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019;26(6):1501–1517.e4. doi: 10.1016/j.celrep.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rooij DG, Russell LD. All You Wanted to Know About Spermatogonia but Were Afraid to Ask. J Androl. 2000;21(6):776–798. doi: 10.1002/j.1939-4640.2000.tb03408.x [DOI] [PubMed] [Google Scholar]
- 21.Clermont Yves and Perey B. Quantitative Study of the cell population of the seminiferous tubules in immature Rats. Am J Anat. 1957;100(2):241–267. doi: 10.1002/aja.1001000205 [DOI] [PubMed] [Google Scholar]
- 22.Fukuda T, Hedinger C, Groscurth P. Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res. 1975;161(1):55–70. doi: 10.1007/BF00222114 [DOI] [PubMed] [Google Scholar]
- 23.WARTENBERG H Human Testicular Development and the Role of the Mesonephros in the Origin of a Dual Sertoli Cell System. Andrologia. 1978;10(1):1–21. doi: 10.1111/j.1439-0272.1978.tb01306.x [DOI] [PubMed] [Google Scholar]
- 24.Byskov AG. Differentiation of Mammalian Embryonic Gonad. Physiol Rev. 1986;66(1):71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Gondos B, Hobel CJ. Ultrastructure of germ cell development in the human fetal testis. Zeitschrift für Zellforsch und Mikroskopische Anat. 1971;119(1):1–20. doi: 10.1007/BF00330535 [DOI] [PubMed] [Google Scholar]
- 26.McCarrey JR. Toward a More Precise and Informative Nomenclature Describing Fetal and Neonatal Male Germ Cells in Rodents1. Biol Reprod. 2013;89(2):1–9. doi: 10.1095/biolreprod.113.110502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pui HP, Saga Y. Gonocytes-to-spermatogonia transition initiates prior to birth in murine testes and it requires FGF signaling. Mech Dev. 2017;144(Pt B):125–139. doi: 10.1016/j.mod.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 28.Wartenberg H Comparative Cytomorphologic Aspects of the Male Germ Cells, Especially of the ”Gonia”. Andrologia. 1976;8(2):117–130. doi: 10.1111/j.1439-0272.1976.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 29.Gaskell TL, Esnal A, Robinson LLL, Anderson RA, Saunders PTK. Immunohistochemical profiling of germ cells within the human fetal testis: Identification of three subpopulations. Biol Reprod. 2004;71(6):2012–2021. doi: 10.1095/biolreprod.104.028381 [DOI] [PubMed] [Google Scholar]
- 30.Paniagua R, Nistal M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J Anat. 1984;139 (Pt3):535–552. [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Grow EJ, Mlcochova H, et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;(28):1141–1157. doi: 10.1038/s41422-018-0099-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi Y, Otsuka K, Ebina M, et al. Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proc Natl Acad Sci U S A. 2017;114(31):8289–8294. doi: 10.1073/pnas.1620915114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tischler J, Gruhn WH, Reid J, et al. Metabolic regulation of pluripotency and germ cell fate through α‐ketoglutarate. EMBO J. 2019;38(1):e99518. doi: 10.15252/embj.201899518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voigt AL, Kondro DA, Powell D, et al. Unique metabolic phenotype and its transition during maturation of juvenile male germ cells. FASEB J. 2021;35(5):e21513. doi: 10.1096/fj.202002799R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voigt AL, Dardari R, Su L, et al. Metabolic transitions define spermatogonial stem cell maturation. Hum Reprod. 2022;37(9):2095–2112. doi: 10.1093/humrep/deac157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Sang F, Withey S, et al. Specification and epigenomic resetting of the pig germline exhibit conservation with the human lineage. Cell Rep. 2021;34(6):108735. doi: 10.1016/j.celrep.2021.108735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floros VI, Pyle A, DIetmann S, et al. Segregation of mitochondrial DNA heteroplasmy through a developmental genetic bottleneck in human embryos. Nat Cell Biol. 2018;20(2):144–151. doi: 10.1038/s41556-017-0017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voigt AL, Dardari R, Lara NLM, et al. Multiomics Approach to Profiling the Development of the Spermatogonial Stem Cell Niche. Mol Hum Reprod. (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–784. doi: 10.1530/rep.0.1250769 [DOI] [PubMed] [Google Scholar]
- 40.Zhao LY, Yao CC, Xing XY, et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun. 2020;11(1):5683. doi: 10.1038/s41467-020-19414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Nie X, Giebler M, et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell. 2020;26(2):262–276.e4. doi: 10.1016/j.stem.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hess RA, Vogl AW. Sertoli cell anatomy and cytoskeleton. In: Griswold MD, ed. The Sertoli Cell Biology. Elsevier Academic Press, Oxford; 2015:1–55. [Google Scholar]
- 43.Griswold MD. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol Reprod. 2018;99(1):87–100. doi: 10.1093/biolre/ioy027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelletier RM. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46(2):49–127. doi: 10.1016/j.proghi.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 45.Mruk DD, Cheng CY. Sertoli-sertoli and sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. doi: 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- 46.Wong CH, Cheng CY. The Blood-Testis Barrier: Its Biology, Regulation, and Physiological Role in Spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5 [DOI] [PubMed] [Google Scholar]
- 47.Dunleavy JEM, O’Bryan MK, Stanton PG, O’Donnell L. The cytoskeleton in spermatogenesis. Reproduction. 2019;157(2):R53–R72. doi: 10.1530/REP-18-0457 [DOI] [PubMed] [Google Scholar]
- 48.Lara N, Avelar G, Costa G, Lacerda SMSN, Hess RA, Franca LR. Cell - Cell interactions - structural. In: Skinner MK, ed. Encyclopedia of Reproduction. 2nd ed. Elsevier; 2018:68–75. [Google Scholar]
- 49.Hess RA, França LR. Structure of the Sertoli Cell. In: Sertoli Cell Biology.; 2005:19–40. doi: 10.1016/B978-012647751-1/50004-0 [DOI] [Google Scholar]
- 50.Elftman H Sertoli cells and testis structure. Am J Anat. 1963;113:25–33. doi: 10.1002/aja.1001130104 [DOI] [PubMed] [Google Scholar]
- 51.Clermont Y, Perey B. Quantitative Study of the Cell Population of the Seminiferous Tubules in Immature Rats. Am J Anat. 1957;100(2):241–267. doi: 10.1002/aja.1001000205 [DOI] [PubMed] [Google Scholar]
- 52.Willems A, Batlouni SR, Esnal A, et al. Selective ablation of the androgen receptor in mouse sertoli cells affects sertoli cell maturation, barrier formation and cytoskeletal development. PLoS One. 2010;5(11):e14168. doi: 10.1371/journal.pone.0014168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92(2):577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFalco T, Potter SJ, Williams AV., Waller B, Kan MJ, Capel B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015;12(7):1107–1119. doi: 10.1016/j.celrep.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piquet-Pellorce C, Dorval-Coiffec I, Pham MD, Jégou B. Leukemia inhibitory factor expression and regulation within the testis. Endocrinology. 2000;141(3):1136–1141. doi: 10.1210/endo.141.3.7399 [DOI] [PubMed] [Google Scholar]
- 56.Chen LY, Brown PR, Willis WB, Eddy EM. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology. 2014;155(12):4964–4974. doi: 10.1210/en.2014-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–1199. doi: 10.1242/dev.032243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spinnler K, Köhn FM, Schwarzer U, Mayerhofer A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum Reprod. 2010;25(9):2181–2187. doi: 10.1093/humrep/deq170 [DOI] [PubMed] [Google Scholar]
- 59.Mayerhofer A Human testicular peritubular cells: More than meets the eye. Reproduction. 2013;145(5):R107–R116. doi: 10.1530/REP-12-0497 [DOI] [PubMed] [Google Scholar]
- 60.Garcia TX, Hofmann MC. Regulation of germ line stem cell homeostasis. Anim Reprod. 2015;12(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- 61.Meng X, Lindahl M, Hyvönen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489 [DOI] [PubMed] [Google Scholar]
- 62.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101(47):16489–16494. doi: 10.1073/pnas.0407063101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubota H, Avarbock MR, Brinster RL. Culture Conditions and Single Growth Factors Affect Fate Determination of Mouse Spermatogonial Stem Cells. Biol Reprod. 2004;71(3):722–731. doi: 10.1095/biolreprod.104.029207 [DOI] [PubMed] [Google Scholar]
- 64.Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells1. Biol Reprod. 2003;69(2):612–616. doi: 10.1095/biolreprod.103.017012 [DOI] [PubMed] [Google Scholar]
- 65.Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68(6):2207–2214. doi: 10.1095/biolreprod.102.014050 [DOI] [PubMed] [Google Scholar]
- 66.França LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology. 2016;4(2):189–212. doi: 10.1111/andr.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hogarth C Retinoic acid metabolism, signaling, and function in the adult testis. In: Griswold MD, ed. The Sertoli Cell Biology. Elsevier Academic Press, Oxford; 2015:247–272. [Google Scholar]
- 68.Raverdeau M, Gely-Pernot A, Féret B, et al. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109(41):16582–16587. doi: 10.1073/pnas.1214936109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113(1):29–39. doi: 10.1016/S0925-4773(02)00004-7 [DOI] [PubMed] [Google Scholar]
- 70.Mäkelä JA, Hobbs RM. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction. 2019;158(5):R169–R187. [DOI] [PubMed] [Google Scholar]
- 71.Bhang DH, Kim BJ, Kim BG, et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun. 2018;9(4379). doi: 10.1038/s41467-018-06881-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verdikt R, Allard P. Metabolo-epigenetics: the interplay of metabolism and epigenetics during early germ cells development. Biol Reprod. 2021;105(3):616–624. doi: 10.1093/biolre/ioab118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell. 2015;17(6):651–662. doi: 10.1016/j.stem.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warburg O On the Origin of Cancer Cells. Science (80- ). 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 75.Nakamura M, Kamachi T, Okinaga S, Arai K. Metabolism of Round Spermatids: Pyruvate cannot Maintain the ATP Level: ATP synthesis/α‐ketoacid/rat spermatids. Dev Growth Differ. 1986;28(5):489–498. doi: 10.1111/j.1440-169X.1986.00489.x [DOI] [PubMed] [Google Scholar]
- 76.Heiden MG Vander Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science (80- ). 2009;324(5930):1029–1033. doi: 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wenger RH, Katschinski M. The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin Cell Dev Biol. 2005;16(4–5):547–553. doi: 10.1016/j.semcdb.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 78.Lord T, Oatley JM. A revised Asingle model to explain stem cell dynamics in the mouse male germline. Reproduction. 2017;154(2):R55–R64. doi: 10.1530/REP-17-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan F, Oatley MJ, Kaucher AV., et al. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014;28(12):1351–1362. doi: 10.1101/gad.240465.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida S, Sukeno M, Nabeshima YI. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science (80- ). 2007;317(5845):1722–1726. doi: 10.1126/science.1144885 [DOI] [PubMed] [Google Scholar]
- 81.Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab. 2004;15(7). doi: 10.1016/j.tem.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 82.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9(6):330–338. doi: 10.1038/nrurol.2012.77 [DOI] [PubMed] [Google Scholar]
- 83.Warburg O, Wind F, Negelein E. The metabolism of tumours in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jpg.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liberti M V, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morimoto H, Kanastu-Shinohara M, Ogonuki N, et al. ROS amplification drives mouse spermatogonial stem cell self-renewal. Life Sci Alliance. 2019;2(2):e201900374. doi: 10.26508/lsa.201900374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morimoto H, Iwata K, Ogonuki N, et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12(6):774–786. doi: 10.1016/j.stem.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 87.Moussaieff A, Rouleau M, Kitsberg D, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21(3):392–402. doi: 10.1016/j.cmet.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 88.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat Rev Genet. 2008;9(2):129–140. doi: 10.1038/nrg2295 [DOI] [PubMed] [Google Scholar]
- 89.NAKAMURA M OKINAGA S, ARAI K. Metabolism of Pachytene Primary Spermatocytes from Rat Testes: Pyruvate Maintenance of Adenosine Triphosphate Level. Biol Reprod. 1984;30(5):1187–1197. [DOI] [PubMed] [Google Scholar]
- 90.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- 91.Nakamura J, Hino A, Yasumasu I. Stimulation of Protein Synthesis in Round Spermatids from Rat Testes by Lactate. Biochem J. 1981;89(4):1309–1315. [PubMed] [Google Scholar]
- 92.Jutte NHPM Grootegoed JA, Rommerts FF., Van Der Molen HJ. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil. 1981;62(2):399–405. doi: 10.1530/jrf.0.0620399. [DOI] [PubMed] [Google Scholar]
- 93.Erkkilä K, Aito H, Aalto K, Pentikäinen V, Dunkel L. Lactate inhibits germ cell apoptosis in the human testis. Mol Hum Reprod. 2002;8(2):109–117. doi: 10.1093/molehr/8.2.109. [DOI] [PubMed] [Google Scholar]
- 94.Lara NLM, Costa GMJ, Avelar GF, Lacerda SMSN, Hess RA, de França LR. Testis Physiology—Overview and Histology. In: Skinner MK, ed. Encyclopedia of Reproduction (Second Edition). Second Edi. Academic Press; 2018:105–116. doi: 10.1016/B978-0-12-801238-3.64567-1 [DOI] [Google Scholar]
- 95.Robinson R, Fritz IB. Metabolism of Glucose by Sertoli Cells in Culture. Biol Reprod. 1981;24(5):1032–1041. [DOI] [PubMed] [Google Scholar]
- 96.Grootegoed JA, Oonk RB, Jansen R, Van Der Molen HJ. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil. 1986;77(1):109–118. doi: 10.1530/jrf.0.0770109 [DOI] [PubMed] [Google Scholar]
- 97.Jutte NHPM, Jansen R, Grootegoed JA, Rommerts FF, van der Molen HJ. FSH stimulation of the production of pyruvate and lactate by rat Sertoli cells may be involved in hormonal regulation of spermatogenesis. J Reprod Fertil. 1983;68(1):219–226. doi: 10.1530/jrf.0.0680219 [DOI] [PubMed] [Google Scholar]
- 98.Fisher D New light shed on fluid formation in the seminiferous tubules of the rat. J Physiol. 2002;542(Pt 2):445–452. doi: 10.1113/jphysiol.2002.018648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pace AJ, Lee E, Athirakul K, Coffman TM, O’Brien DA, Koller BH. Failure of spermatogenesis in mouse lines deficient in the Na+-K+- 2Cl- cotransporter. J Clin Invest. 2000;105(4):441–450. doi: 10.1172/JCI8553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuck RR, Setchell BP, Waites GMH, Young JA. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflügers Arch Eur J Physiol. 1970;318(3):225–243. doi: 10.1007/BF00593663 [DOI] [PubMed] [Google Scholar]
- 101.Pande JK, Chowdhury SR, Dasgupta PR, Chowdhury AR, Kar AB, Dorfman RI. Biochemical Composition of the Rat Testis Fluid. Proc Soc Exp Biol Med. 1966;121(3):899–902. doi: 10.3181/00379727-121-30918 [DOI] [PubMed] [Google Scholar]
- 102.Levine N, Marsh DJ, Levine N, Marsh DJ. MICROPUNCTURE STUDY OF THE FLUID COMPOSITION OF ` SERTOLI CELL-ONLY ‘ SEMINIFEROUS TUBULES IN RATS. J Reprod Fertil. 1975;43(3):547–549. [DOI] [PubMed] [Google Scholar]
- 103.Griswold MD. Interactions between Sertoli cells and germ cells in the testis. Biol Reprod. 1994;52(1):211–216. doi: 10.1006/sedb.1994.1008 [DOI] [PubMed] [Google Scholar]
- 104.Setchell BP, Vogelmayr JK, Waites GMH. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969;200(1):73–85. doi: 10.1113/jphysiol.1969.sp008682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: From simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–580. doi: 10.1038/nrm.2016.80 [DOI] [PubMed] [Google Scholar]
- 106.Xiong Z, Wang C, Wang Z, et al. Raptor directs Sertoli cell cytoskeletal organization and polarity in the mouse testis. Biol Reprod. 2018;99(6):1289–1302. doi: 10.1093/biolre/ioy144 [DOI] [PubMed] [Google Scholar]
- 107.Gao Y, Xiang X, Lui W, Lee WM, Mruk D, Cheng CY. Cell polarity proteins and spermatogenesis. Semin Cell Dev Biol. 2016;59:62–70. doi: 10.1016/j.semcdb.2016.06.008.Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heinrich A, Potter SJ, Guo L, Ratner N, DeFalco T. Distinct Roles for Rac1 in Sertoli Cell Function during Testicular Development and Spermatogenesis. Cell Rep. 2020;31(2):107513. doi: 10.1016/j.celrep.2020.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su W, Wong EWP, Mruk DD, Cheng CY. The scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153(12):6041–6053. doi: 10.1210/en.2012-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3(3):308–326. doi: 10.1093/biolreprod/3.3.308 [DOI] [PubMed] [Google Scholar]
- 111.Fawcett DW, Leak LV, Heidger PM. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil. 1970;10:105–122. [PubMed] [Google Scholar]
- 112.Dym M The Fine Structure of the Monkey (Macaca) Sertoli Cell and Its Role in Maintaining the Blood-Testis Barrier. Anat Rec. 1973;175(4):639–656. [DOI] [PubMed] [Google Scholar]
- 113.Dym M, Cavicchia JC. Further observations on the Blood-testis Barrier in Monkeys. Biol Reprod. 1977;17(3):390–403. doi: 10.1095/biolreprod17.3.390. [DOI] [PubMed] [Google Scholar]
- 114.Dutta S, Sandhu N, Sengupta P, Alves MG, Henkel R, Agarwal A. Somatic-Immune Cells Crosstalk In-The-Making of Testicular Immune Privilege. Reprod Sci. 2021;29(1). doi: 10.1007/s43032-021-00721-0 [DOI] [PubMed] [Google Scholar]
- 115.Setchell BP. Blood-Testis Barrier, Junctional and Transport Proteins and Spermatogenesis. In: Advances in Experimental Medicine and Biology. Vol 636. Springer New York, NY; 2009:212–233. doi:doi: 10.1007/978-0-387-09597-4_12 [DOI] [PubMed] [Google Scholar]
- 116.França LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. BLOOD-TISSUE BARRIERS: Morphofunctional and Immunological Aspects of the Blood-Testis and Blood-Epididymal Barriers. In: Cheng CY, ed. Biology and Regulation of Blood Tissue Barriers. Landes Bioscience and Springer Science; 2012. [PubMed] [Google Scholar]
- 117.Fawcett DW. Interrelations of cell types within the seminiferous epithelium and their implications for control of spermatogenesis. In: Segal SJ, Croizer R, Corfman PA, Condliffe PG, eds. The Regulation of Mammalian Reproduction.; 1973:116–138. [Google Scholar]
- 118.Su L, Mruk DD, Cheng CY. Drug transporters, the blood–testis barrier, and spermatogenesis. J Endocrinol. 2011;208(3):207–223. doi: 10.1677/JOE-10-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mruk DD, Cheng CY. The mammalian blood-testis barrier: Its biology and regulation. Endocr Rev. 2015;36(5):564–591. doi: 10.1210/er.2014-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Claude P Morphological factors influencing transepithelial permeability: A model for the resistance of the Zonula Occludens. J Membr Biol. 1978;39(2–3):219–232. doi: 10.1007/BF01870332 [DOI] [PubMed] [Google Scholar]
- 121.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58(2):390–400. doi: 10.1083/jcb.58.2.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bentzel CJ, Hainau B, Ho S, et al. Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am J Physiol. 1980;239(3). doi: 10.1152/ajpcell.1980.239.3.c75 [DOI] [PubMed] [Google Scholar]
- 123.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: A ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127(2):455–464. doi: 10.1242/jcs.142497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salvador E, Burek M, Förster CY. Tight Junctions and the Tumor Microenvironment. Curr Pathobiol Rep. 2016;4:135–145. doi: 10.1007/s40139-016-0106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larre I, Ponce A, Franco M, Cereijido M. The emergence of the concept of tight junctions and physiological regulation by ouabain. Semin Cell Dev Biol. 2014;36:149–156. doi: 10.1016/j.semcdb.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 126.Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27(55):6930–6938. doi: 10.1038/onc.2008.344 [DOI] [PubMed] [Google Scholar]
- 127.Setchell BP. The Functional Significance of the Blood-testis Barrier. J Androl. 1980;1:3–10. 10.1002/j.1939-4640.1980.tb00003.x [DOI] [Google Scholar]
- 128.Setchell BP. The movement of fluids and substances in the testis. Aust J Biol Sci. 1986;39(2):193–207. doi: 10.1071/BI9860193 [DOI] [PubMed] [Google Scholar]
- 129.Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: Is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294(5843):718–722. doi: 10.1038/294718a0 [DOI] [PubMed] [Google Scholar]
- 130.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5(7):1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Igdoura SA, Argraves WS, Morales CR. Low density lipoprotein receptor-related protein-1 expression in the testis: Regulated expression in Sertoli cells. J Androl. 1997;18:400–410. doi: 10.1002/j.1939-4640.1997.tb01945.x [DOI] [PubMed] [Google Scholar]
- 132.Sato T, Aiyama Y, Ishii-Inagaki M, et al. Cyclical and patch-like GDNF distribution along the basal surface of sertoli cells in mouse and hamster testes. PLoS One. 2011;6(12):e28367. doi: 10.1371/journal.pone.0028367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schmahl J, Capel B. Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol. 2003;258(2):264–276. doi: 10.1016/S0012-1606(03)00122-2 [DOI] [PubMed] [Google Scholar]
- 134.Skinner MK, Anway MD. Seminiferous Cord Formation and Germ-Cell Programming. Ann N Y Acad Sci. 2005;1061:18–32. doi: 10.1196/annals.1336.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skinner MK, Tung PS, Fritz IB. Cooperativity between sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100(6):1941–1947. doi: 10.1083/jcb.100.6.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Witchi E Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. In Contributions to Embryology. In: Contributions to Embryology. Vol 32. Carnegie Institution of Washington; 1948:no 209, 67–80. [Google Scholar]
- 137.Li L, Dong J, Yan L, et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell. 2017;20(6):858–873.e4. doi: 10.1016/j.stem.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 138.Guo F, Yan L, Guo H, et al. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell. 2015;161(6):1437–1452. doi: 10.1016/j.cell.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 139.Guo J, Sosa E, Chitiashvili T, et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell. 2021;28(4):764–778.e4. doi: 10.1016/j.stem.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kos M, Lenicek T. The Fetal Human Testis. In: Jezek D, ed. Atlas on the Human Testis; Normal Morphology and Pathology. Springer-Verlag London; 2012:55–68. [Google Scholar]
- 141.Chemes HE. Infancy is not a quiescent period of testicular development. Int J Androl. 2001;24(1):2–7. doi: 10.1046/j.1365-2605.2001.00260.x [DOI] [PubMed] [Google Scholar]
- 142.Bay K, Andersson AM. Human testicular insulin-like factor 3: In relation to development, reproductive hormones and andrological disorders. Int J Androl. 2011;34(2):97–109. doi: 10.1111/j.1365-2605.2010.01074.x [DOI] [PubMed] [Google Scholar]
- 143.Hadžiselimovič F, Thommen L, Girard J, Herzog B. The Significance of Postnatal Gonadotropin Surge for Testicular Development in Normal and Cryptorchid Testes. J Urol. 1986;136(1 Part 2):274–276. doi: 10.1016/S0022-5347(17)44839-7 [DOI] [PubMed] [Google Scholar]
- 144.Hutson JM, Li R, Southwell BR, Petersen BL, Thorup J, Cortes D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front Endocrinol (Lausanne). 2013;3(3):176. doi: 10.3389/fendo.2012.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beau C, Vivian N, Munsterberg A, Dresser DW, Lovell-Badge R, Guerrier D. In vivo analysis of the regulation of the anti-Müllerian hormone, as a marker of Sertoli cell differentiation during testicular development, reveals a multi-step process. Mol Reprod Dev. 2001;59(3):256–264. doi: 10.1002/mrd.1030 [DOI] [PubMed] [Google Scholar]
- 146.Brinster RL, Harstad H. Energy metabolism in primordial germ cells of the mouse. Exp Cell Res. 1977;109(1):111–117. doi: 10.1016/0014-4827(77)90050-7 [DOI] [PubMed] [Google Scholar]
- 147.Wu X, Schmidt JA, Avarbock MR, et al. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106(51):21672–21677. doi: 10.1073/pnas.0912432106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lukas-Croisier C, Lasala C, Nicaud J, et al. Follicle-stimulating hormone increases testicular anti-Müllerian hormone (AMH) production through Sertoli cell proliferation and a nonclassical cyclic adenosine 5′-monophosphate-mediated activation of the AMH gene. Mol Endocrinol. 2003;17(4):550–561. doi: 10.1210/me.2002-0186 [DOI] [PubMed] [Google Scholar]
- 149.Orth JM. Proliferation of sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec. 1982;203(4):485–492. doi: 10.1002/ar.1092030408 [DOI] [PubMed] [Google Scholar]
- 150.Kluin PM, Kramer MF, de Rooij DG. Proliferation of spermatogonia and Sertoli cells in maturing mice. Anat Embryol (Berl). 1984;169(1):73–78. doi: 10.1007/BF00300588 [DOI] [PubMed] [Google Scholar]
- 151.Abel MH, Baker PJ, Charlton HM, et al. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology. 2008;149(7):3279–3285. doi: 10.1210/en.2008-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Orth JM. The Role of Follicle-Stimulating Hormone in Controlling Sertoli Cell Proliferation in Testes of Fetal Rats. Endocrinology. 1984;115(4):1248–1255. doi: 10.1210/endo-115-4-1248 [DOI] [PubMed] [Google Scholar]
- 153.Johnson L, Thompson DL, Varner DD. Role of Sertoli cell number and function on regulation of spermatogenesis. Anim Reprod Sci. 2008;105(1–2):23–51. doi: 10.1016/j.anireprosci.2007.11.029 [DOI] [PubMed] [Google Scholar]
- 154.Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of Human Sertoli cell Population: Its Distribution, Relation to Germ cell Numbers, and Age-Related Decline. Biol Reprod. 1984;31:785–795. [DOI] [PubMed] [Google Scholar]
- 155.Berndtson WE, Igboeli G, Pickett BW. Relationship of Absolute Numbers of Sertoli Cells to Testicular Size and Spermatogenesis in Young Beef Bulls. J Anim Sci. 1987;64(1):241–246. doi: 10.2527/jas1987.641241x [DOI] [PubMed] [Google Scholar]
- 156.Plant TM, Ramaswamy S, Simorangkir DR, Marshall GR. Postnatal and Pubertal Development of the Rhesus Monkey (Macaca mulatta) Testis. Ann N Y Acad Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016 [DOI] [PubMed] [Google Scholar]
- 157.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–786. doi: 10.1210/edrv.22.6.0446 [DOI] [PubMed] [Google Scholar]
- 158.Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54(6):1192–1199. doi: 10.1095/biolreprod54.6.1192 [DOI] [PubMed] [Google Scholar]
- 159.Meroni SB, Galardo MN, Rindone G, Gorga A, Riera MF, Cigorraga SB. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front Endocrinol (Lausanne). 2019;10:224. doi: 10.3389/fendo.2019.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beumer TL, Kiyokawa H, Roepers-Gajadien HL, et al. Regulatory Role of p27kip1 in the Mouse and Human Testis. Endocrinology. 1999;140(4):1834–1840. [DOI] [PubMed] [Google Scholar]
- 161.Furuya S, Kumamoto Y, Sugiyama S. Fine structure and development of sertoli junctions in human testis. Syst Biol Reprod Med. 1978;1(3):211–219. doi: 10.3109/01485017808988339 [DOI] [PubMed] [Google Scholar]
- 162.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102(46):16696–16700. doi: 10.1073/pnas.0506084102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang L, Guo M, Liu Z, et al. Single-cell RNA-seq analysis of testicular somatic cell development in pigs. J Genet Genomics. 2022;49(11):1016–1028. doi: 10.1016/j.jgg.2022.03.014 [DOI] [PubMed] [Google Scholar]
- 164.Nusrat A, Parkos CA, Verkade P, et al. Tight junctions are membrane microdomains. J Cell Sci. 2000;113(Pt 10):1771–1781. doi: 10.1242/jcs.113.10.1771 [DOI] [PubMed] [Google Scholar]
- 165.Marchiando AM, Shen L, Vallen Graham W, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(1):111–126. doi: 10.1083/jcb.200902153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bailey AP, Koster G, Guillermier C, et al. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell. 2015;163(2):340–353. doi: 10.1016/j.cell.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kormano M Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie. 1967;9(4):327–338. doi: 10.1007/BF00305816 [DOI] [PubMed] [Google Scholar]
- 168.Mazaud-Guittot S, Meugnier E, Pesenti S, et al. Claudin 11 deficiency in mice results in loss of the sertoli cell epithelial phenotype in the testis. Biol Reprod. 2010;82(1):202–213. doi: 10.1095/biolreprod.109.078907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hofmann M-C. Gdnf signaling pathways within the mammalian spermatognial stem cell niche. Mol Cell Endocrinol. 2008;288(1–2):95–103. doi: 10.1016/j.mce.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kubota H, Brinster RL. Spermatogonial stem cells. Biol Reprod. 2018;99(1):52–74. doi: 10.1093/biolre/ioy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oatley JM, Brinster RL. Regulation of Spermatogonial Stem Cell Self-Renewal in Mammals. Annu Rev Cell Dev Biol. 2008;24(1):263–286. doi: 10.1146/annurev.cellbio.24.110707.175355 [DOI] [PMC free article] [PubMed] [Google Scholar]


