Abstract
Background
Contraception provides significant benefits for women's and children's health, yet many women have an unmet need for contraception. Rapid expansion in the use of mobile phones in recent years has had a dramatic impact on interpersonal communication. Within the health domain text messages and smartphone applications offer means of communication between clients and healthcare providers. This review focuses on interventions delivered by mobile phone and their effect on use of contraception.
Objectives
To evaluate the benefits and harms of mobile phone‐based interventions for improving contraception use.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was August 2022.
Selection criteria
We included randomised controlled trials (RCTs) of mobile phone‐based interventions to improve forms of contraception use amongst users or potential users of contraception.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. uptake of contraception, 2. uptake of a specific method of contraception, 3. adherence to contraception method, 4. safe method switching, 5. discontinuation of contraception and 6. pregnancy or abortion. Our secondary outcomes were 7. road traffic accidents, 8. any physical or psychological effect reported and 9. violence or domestic abuse.
Main results
Twenty‐three RCTs (12,793 participants) from 11 countries met our inclusion criteria. Eleven studies were conducted in high‐income resource settings and 12 were in low‐income settings. Thirteen studies used unidirectional text messaging‐based interventions, six studies used interactive text messaging, four used voice message‐based interventions and two used mobile‐phone apps to improve contraception use. All studies received funding from non‐commercial bodies.
Mobile phone‐based interventions probably increase contraception use compared to the control (odds ratio (OR) 1.30, 95% confidence interval (CI) 1.06 to 1.60; 16 studies, 8972 participants; moderate‐certainty evidence).
There may be little or no difference in rates of unintended pregnancy with the use of mobile phone‐based interventions compared to control (OR 0.82, 95% CI 0.48 to 1.38; 8 trials, 2947 participants; moderate‐certainty evidence).
Subgroup analysis assessing unidirectional mobile phone interventions versus interactive mobile phone interventions found evidence of a difference between the subgroups favouring interactive interventions (P = 0.003, I2 = 88.5%). Interactive interventions had an OR of 1.71 (95% CI 1.28 to 2.29; P = 0.0003, I2 = 63%; 8 trials, 3089 participants) whilst unidirectional interventions had an OR of 1.03 (95% CI 0.87 to 1.22; P = 0.72, I2 = 17%; 9 trials, 5883 participants).
Subgroup analysis assessing high‐income versus low‐income trial settings found no difference between groups (subgroup difference test: P = 0.70, I2 = 0%).
Only six trials reported on safety and unintended outcomes; one trial reported increased partner violence whilst another four trials reported no difference in physical violence rates between control and intervention groups. One trial reported no road traffic accidents with mobile phone intervention use.
Authors' conclusions
This review demonstrates there is evidence to support the use of mobile phone‐based interventions in improving the use of contraception, with moderate‐certainty evidence. Interactive mobile phone interventions appear more effective than unidirectional methods.
The cost‐effectiveness, cost benefits, safety and long‐term effects of these interventions remain unknown, as does the evidence of this approach to support contraception use among specific populations.
Future research should investigate the effectiveness and safety of mobile phone‐based interventions with better quality trials to help establish the effects of interventions delivered by mobile phone on contraception use. This review is limited by the quality of the studies due to flaws in methodology, bias or imprecision of results.
Keywords: Child, Female, Humans, Pregnancy, Cell Phone, Communication, Contraception, Randomized Controlled Trials as Topic, Telephone, Text Messaging
Plain language summary
Interventions delivered by mobile phone to support client use of family planning/contraception
Review question
The aim of this review was to determine if interventions delivered by mobile phone increase the use of contraception.
Key messages
Interventions delivered by mobile phones show a positive effect on the uptake and continued use of contraception.
Interactive messages are better than one‐way text messages at improving use of contraception.
The existing evidence is of moderate quality.
Why is this review important?
Health messaging, or interventions delivered by mobile phones, have been shown to improve health and behaviours, but it is unknown if messaging delivered by mobile phone impacts issues related to reproductive health, such as use of contraception.
Women and children's health benefit significantly from pregnancy prevention. Despite these benefits, a significant number of women globally do not use contraception despite wanting to avoid pregnancy.
Rapid expansion in the use of mobile phones in recent years has led to increased interest in healthcare delivery via mobile phone with the potential to deliver support directly to wherever the person is located, whenever it is needed and to reach populations with restricted access to services.
How did we identify and evaluate the evidence?
We searched medical databases for studies that assessed the use of interventions delivered by mobile phones and their impact on the use of contraception. We found 23 trials of 12,793 women undertaken in 11 countries in both high‐income (11 studies) and low‐income (12 studies) settings. These studies compared the standard of care to a mobile phone intervention – such as one‐way text message reminders, interactive messages (which required a response from clients), voice messages or a mobile app.
What did we find?
The results across the studies were mixed; however, when the results were pooled, we found there is a positive effect of using interventions delivered by mobile phones and increasing use of contraception.
There were no differences in unintended pregnancies between the groups who used the mobile phone tools and those who did not.
Using interactive methods of mobile phone tools appears better at improving contraceptive use over one‐way mobile phone interventions. There is not enough evidence about the safety or negative consequences of mobile phone tools for improving contraception use.
Further research is likely to have an important impact on our confidence in the results.
What does this mean?
It appears interventions delivered by mobile phones are beneficial in improving the use of contraception. Our analysis was limited by the quality of evidence we found, which makes it hard to form more robust conclusions. More good‐quality research is required in the area of health messaging and contraception.
How up to date is this evidence?
This review updates our previous review. The evidence is up to date to August 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Mobile phone‐based interventions compared to standard of care for improving use of contraception.
| Mobile phone‐based interventions compared to standard of care for improving use of contraception | ||||||
| Patient or population: women users/non‐users of contraception Setting: various: Bangladesh, Bolivia, Cambodia, Ghana, Israel, Kenya, Palestine, Tajikistan, the USA, Uganda Intervention: mobile phone‐based interventions Comparison: standard of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard of care | Risk with mobile phone‐based interventions | |||||
| Contraception use | 515 per 1000 | 580 per 1000 (529 to 629) | OR 1.30 (1.06 to 1.60) | 8972 (16 RCTs) | ⊕⊕⊕⊝ Moderatea | Mobile phone‐based interventions probably increase contraception use. |
| Pregnancy | 21 per 1000 | 18 per 1000 (10 to 29) | OR 0.82 (0.48 to 1.38) | 2947 (8 RCTs) | ⊕⊕⊕⊝ Moderateb | There may be little or no difference in rates of pregnancy with the use of mobile phone‐based interventions. Note 2 studies reported pregnancy but recorded 0 events in both groups. Thus, the OR and CIs were calculated from 6 studies rather than 8. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434222404955204407. | ||||||
a Downgraded one level for serious inconsistency due to substantial heterogeneity as noted by mixed directional estimated effect accompanied with an I2 = 69%. b Downgraded one level for serious imprecision due to 95% confidence intervals crossing zero and including no effect.
Background
Contraception provides significant benefits for women's and children's health, yet a significant number of women have an unmet need for modern contraception methods. Rapid expansion in the use of mobile phones in recent years has had a dramatic impact on interpersonal communication. Within the health domain, phone calls, text messages and smartphone applications offer means of communication between clients and healthcare providers as well as public health messaging. This review focuses on interventions delivered by mobile phone and their effect on use of contraception.
Description of the condition
Contraception (methods or devices used to prevent pregnancy) provides significant benefits for women's and children's health. Use of contraception prevents unintended pregnancies, reduces abortions, reduces maternal deaths, and can improve perinatal outcomes and child survival by widening the interval between successive pregnancies (Cleland 2012). Contraception also confers substantial social and economic benefits such as improved educational and employment opportunities for women, leading to increasing family savings and economic growth (Singh 2009).
Despite these benefits, the unmet need for contraception is significant. Unmet need can be defined as women not using a modern contraception method despite wanting to wait two or more years between pregnancies, or wanting no more children (Darroch 2013). Women report not using contraception for many reasons, most commonly concerns about contraception adverse effects and health risks (Sedgh 2016). Legal, political and other structural barriers, as well as social and cultural norms, also prevent access to and use of contraception (Starrs 2018).
The United Nation's Sustainable Development Goals (SDG) for 2030 – in particular goals 3, 4 and 5 – highlight the need for improved health and gender equality (United Nations 2015). The health‐related SDGs emphasise the need for equitable contraception access. Goal 3.7 states "by 2030, ensure universal access to sexual and reproductive healthcare services, including for family planning, information and education, and the integration of reproductive health into national strategies." Despite this goal, a significant number of women globally still have an unmet need for contraception (Cleland 2012; Darroch 2017; Sully 2020).
If the unmet need for modern methods of contraception were met amongst women in low‐income regions, it is estimated that annual unintended pregnancies and unplanned births would decline by 68%, and there would be an estimated 70,000 fewer maternal deaths each year (Sully 2020).
It is estimated that 15% of married women living in lower‐ to middle‐income countries (LMICs), and 23% of married women living in low‐income countries (Kaneda 2019), equating to approximately 218 million women of reproductive age (aged 15 to 49 years) in LMICs, have an unmet need for modern contraception (Sully 2020). About 49% of pregnancies in LMICs are unintended.
This unmet need for contraception is due to a range of reasons. Access to contraception is one significant barrier. Access is not just physical proximity to supplies but also an assurance of accurate information regarding methods and their health risks, psycho‐social access (acceptability of contraception and associated services) and affordability (Cleland 2014). Other barriers include a lack of appropriate sexual health education, poor access to healthcare overall and high financial barriers (Chandra‐Mouli 2014). Legal, cultural and other structural hurdles also prevent use of contraception (Starrs 2018). Women report not using contraception for several reasons commonly quoting concerns about contraceptive adverse effects and health risks or state their family is against use of contraception (Sedgh 2016).
Description of the intervention
Digital health interventions may be used by clients, healthcare providers, health system managers or others to complement and extend functions of the health system (WHO 2018a). Digital health interventions for clients include targeted and untargeted communication, communication with other clients, personal health tracking, citizen‐based reporting, on‐demand information services and financial transactions. 'Telemedicine' is the remote delivery of healthcare services, which is another way in which clients may have their health supported through digital means (WHO 2018a).
All these digital health interventions for clients may be delivered using mobile phones, alone or in combination with other digital devices. Mobile phone‐based interventions (interventions delivered by mobile phone) have now been trialled in low‐, middle‐ and high‐income countries for a range of client health uses. These include appointment attendance, delivery of test results, medication adherence, management of chronic conditions and promotion of healthy lifestyle behaviours (Hanlon 2017; Joseph‐Shehu 2019; Linde 2019; Marcolino 2018).
Mobile phone‐based interventions can utilise different delivery channels including text messaging, interactive message/voice responses, voice calls and smartphone applications. Interventions may employ single functions or combined functions of mobile phones such as interactive text message‐based support or voice messaging combined with telephone counselling. Interventions delivered by mobile phone to improve contraception use could be provided as an adjunct or alternative to face‐to‐face services and, for non‐users of contraception, could aim to increase uptake of contraception. Interventions for existing contraception users could aim to improve adherence to contraception, reduce discontinuation of contraception or encourage switching rather than stopping contraception if the individual experiences adverse effects.
How the intervention might work
Interventions delivered by mobile phone offer potential advantages over face‐to‐face or landline phone healthcare delivery, as support can be delivered wherever the person is located and whenever it is needed. Such interventions can facilitate confidential access to healthcare information amongst young people, who are regular mobile phone users and experience specific barriers to accessing sexual and reproductive health services and information (Feroz 2019). Furthermore, mobile phone‐based interventions can increase access to health services for rural populations (Car 2012; WHO 2019).
Intervention content could include information, pill or appointment reminders, content designed to increase or maintain motivation to use contraception, or a combination of these. Behaviour change techniques used in face‐to‐face interventions can be modified for delivery by mobile phone (Free 2013). Interventions could utilise a range of behaviour change techniques, such as encouraging women to make a clear plan about when, where and how they will use contraception (goal setting) (Abraham 2008). Multifaceted interventions that address a wide range of difficulties with contraception use could be more effective than those targeting a single difficulty to use.
Reviews published in the past few years indicate that text‐ and phone call‐based interventions can increase use of sexual health services, testing for sexually transmitted infections and adherence to antiretroviral therapy for people living with HIV (Burns 2016; Daher 2017; Wang 2019). However, none of these reviews have focused specifically on uptake of contraception. A qualitative synthesis of clients' experiences with targeted digital communication through mobile phones found overall clients generally liked receiving messages from healthcare providers via mobile phone, although there were some problems (Ames 2019).
There are several possible risks associated with using mobile phones to improve contraception use. Road traffic accidents are the only adverse health effect of mobile phone use for which substantial evidence is available (CDC 2019; National Safety Council 2015; Rothman 2000), although more‐recent studies have found some evidence that exposure to radiofrequency radiation used by 2G and 3G mobile phones can cause cancer in rats (National Toxicology Program 2020). When considering the often sensitive context of contraception, there is the potential for physical or psychological adverse effects to arise due to other people accessing intervention content when mobile phones are shared (Bacchus 2019). Examples include a trial of antiretroviral therapy in Cameroon where it was believed participation had compromised undisclosed HIV‐positive status (Mbuagbaw 2012), and examples of mobile phone interventions reinforcing existing gender‐based power imbalances in several countries (Jennings 2013). Other reported issues with mobile phone‐based interventions include poor network connection, lost or broken phones, switching phone numbers, financial barriers (lack of airtime credit or high cost of messages), access to phones controlled by others, and literacy and language barriers (Ames 2019; Kruse 2019).
Why it is important to do this review
This review was first published in 2015 (Smith 2015a). Since then, the use of digital health interventions has continued to expand. In 2018, the World Health Assembly formerly acknowledged the potential of digital technologies to promote universal health coverage and advance the SDGs (WHA 2018). The latest published guidelines on digital health from the World Health Organization (WHO) recommend the use of digital‐targeted client communication for sexual and reproductive behaviour change provided concerns about sensitive content and data privacy are adequately addressed (WHO 2019). Thus, it is timely to update this review to provide a comprehensive assessment of the currently available evidence specifically for mobile phone‐based interventions to improve contraception uptake, in order to inform investment decisions by policy‐makers, donors and health system managers.
Objectives
To evaluate the benefits and harms of mobile phone‐based interventions for improving contraception use.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Eligible participants were men or women of reproductive age who were users or potential users of contraception methods. We included studies in all settings (e.g. primary care settings, outpatient settings, community settings, hospital settings). We did not exclude studies according to the types of healthcare providers who participated (e.g. doctor, nurse, allied staff).
Types of interventions
We included studies that examined any type of client–provider intervention delivered by mobile phone designed to improve use of contraception compared with standard delivery of care or another intervention. We included interventions directed at both users and non‐users of contraception. Eligible interventions included those designed to:
improve uptake of contraception (including postabortion and postpartum contraception);
promote specific methods of contraception;
improve adherence to contraception (e.g. interventions to support individuals experiencing adverse effects, reduce discontinuation, ensure safe method switching, or send pill or appointment reminders).
We included studies that assessed any form of contraception use and trials assessing a range of outcome measures related to contraception use, including uptake of contraception, selection of a specific method, use of measures of adherence (including discontinuation and safe switching), pregnancy or abortion.
We included interventions aimed at mobile phone users delivered by mobile phone that included some degree of automation, for example, text message, voice message and applications. We excluded trials in which mobile phones were used as solely two‐way voice communication (as a phone), in keeping with previous reviews of mobile phone‐based interventions (Horvath 2012; Whittaker 2009).
Web‐based interventions often can be accessed on mobile phones, as well as through other platforms, but in practice can be difficult to access via mobile phone unless they are adapted for mobile phone use. Studies presenting multicomponent interventions were described in detail, with single intervention trial arms used for analysis where presented. If studies employed a combination of mobile phone intervention (voice messages and text messages), these studies were included in our analysis and appropriately classified. Studies that presented combined intervention with non‐mobile phone interventions (such as counselling or drug administration) were excluded from this analysis. We excluded web‐based interventions unless study authors stated that they had been intended or adapted for mobile phone users. We excluded trials that focused only on preventing sexually transmitted disease rather than providing contraception.
Types of outcome measures
Primary outcomes
Uptake of contraception (including postabortion and postpartum contraception)
Uptake of a specific method of contraception (e.g. a long‐acting method)
Adherence to contraception method (e.g. number of missed pills, attendance for repeat injection)
Safe method switching (e.g. from one effective method to another with no gap during which time conception could occur)
Discontinuation of contraception
Pregnancy or abortion (objectively measured or self‐reported)
We considered sustained and point prevalence measures as well as subjective (self‐reported) and objective (e.g. biochemically verified, electronic medication monitors used, clinical examination performed) assessment of contraception use.
Contraception methods can be classified in different ways. Contraception can be classed as modern (e.g. condom, oral contraception pills, injectables, intrauterine device (IUD), implant, emergency contraception (EC)) or traditional (e.g. rhythm or periodic abstinence, withdrawal) (Westoff 2012; WHO 2013). Furthermore, distinctions can be made between hormonal and non‐hormonal methods, and between short‐acting and long‐acting or permanent methods. The WHO classifies methods according to effectiveness on the basis of estimated rates of unintended pregnancy per 100 women per year (WHO 2018b).
For this review, we defined effective modern methods as those associated with less than 10% 12‐month pregnancy rates; commonly used methods include oral contraceptive pill, injectable, implant, IUD and permanent methods.
Secondary outcomes
Road traffic accidents
Any physical or psychological effect reported
Violence or domestic abuse
Search methods for identification of studies
The Fertility Regulation Group Information Specialist conducted a comprehensive update search from January 2014 to March 2019, with the most recent update search conducted in August 2022.
We created new search strategies due to newly identified shortcomings in the previous search strategies. In addition to keyword and subject terms changes, we also added a search of the Fertility Regulation Specialised Register per changes to standard search routines by Cochrane Information Specialists. We did not search the Africa‐Wide Information database for this update because it is inaccessible locally. The POPLINE database ceased publication in 2019 and thus only the initial search results from March 2019 were available. We applied no language or publication status limits. Update search strategies are available in Appendix 1 and previous search strategies are available in Appendix 2.
Electronic searches
We searched the following databases (update searches: March 2019, August 2022).
Cochrane Fertility Regulation Specialised Register (CRS Web) (January 2014 to August 2022)
Central Register of Controlled Trials (Ovid EBM Reviews) (2014 Issue 1 to 2022 Issue 8)
MEDLINE ALL (Ovid) (January 2014 to August 2022)
Embase.com (January 2014 to August 2022)
PsycINFO (Ovid) (1806 to February Week 4 2019) (January 2014 to August 2022)
Global Health (Ovid) (1973 to 2019 Week 08) (January 2014 to August 2022)
LILACS (Latin American Caribbean Health Sciences Literature) (January 2014 to August 2022)
POPLINE (Population Information Online) (January 2014 to March 2019)
Scopus [conference abstracts only] (January 2014 to August 2022)
We searched the following trials registries.
ClinicalTrials.gov (www.clinicaltrials.gov)
WHO ICTRP (International Clinical Trials Registry Platform) (www.who.int/ictrp/)
Searching other resources
We wrote to the contact investigators of included studies to request information about trials not discovered in our search. We reviewed reference lists of all included studies.
Data collection and analysis
Selection of studies
We exported search results into Covidence and excluded duplicate references (Covidence). Two review authors independently screened titles and abstracts of studies retrieved using the search strategy. We retrieved full‐text articles for further assessment if the information given suggested that the study 1. included participants who were users or potential users of contraception, 2. compared use of an intervention delivered by mobile phone versus routine standard of care or another intervention or 3. assessed one or more relevant outcome measures. Two review authors retrieved the full text of potentially eligible studies and independently assessed them for eligibility, with disagreements resolved through discussion with a third review author.
Data extraction and management
Two review authors independently extracted the following data from the included studies using a standardised data extraction form.
General information: title, study authors, complete citation, publication status, date published, language, review author information, date reviewed, sponsoring, setting.
Study characteristics: study design, aim of study, duration, participant recruitment, sampling, inclusion and exclusion criteria including numbers screened and eligible, randomisation, allocation concealment, method of allocation concealment, blinding, informed consent, power analysis.
Risk of bias (see Assessment of risk of bias in included studies).
Participants: description, geographical location, setting, number, age, ethnicity, socioeconomic status distribution.
Providers: description, geographical location, setting.
Intervention: description, aim of intervention, any behaviour change intervention (according to the study authors' description and our assessment according to an established typology of behaviour change techniques; Abraham 2008), duration, frequency and 'dose', control or placebo intervention, technical specifications including device and mobile phone functions used (e.g. text message, voice message), message content, co‐interventions.
Outcomes: outcomes as specified under Primary outcomes and Secondary outcomes, other outcomes assessed, length of follow‐up, methods used to assess outcomes, completeness of outcome data, follow‐up for non‐respondents, adverse events.
Results: outcomes and times of assessment, intention‐to‐treat analysis (when all randomly assigned participants were included, irrespective of what happened subsequently; Newell 1992).
Review authors discussed disagreements and resolved them through discussion with a third review author as necessary. We contacted study authors for additional information regarding study data when required.
Assessment of risk of bias in included studies
Two review authors independently assessed studies for risk of bias in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019) across the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential biases. Review authors discussed disagreements and resolved them through discussion with a third review author as necessary. We used a standardised form to guide assessment of risk of bias, and judged each domain as having 'high', 'low' or 'unclear' risk. We presented all included studies by study type and risk of bias level. As required, we contacted study authors to request additional information. We presented the results of the risk of bias assessment in the Characteristics of included studies table, and as a systematic narrative description.
When a review author was also a contributor to an included study, that review author was not involved in the assessment of risk of bias.
Measures of treatment effect
We used odds ratios (ORs) as measures of treatment effect for dichotomous outcomes and mean differences (MDs) for continuous data. We reported 95% confidence intervals (CIs) with all measures of effect.
Unit of analysis issues
We planned to take into account unit of analysis issues resulting from cluster‐RCTs, repeated measurements and studies with more than one treatment group and, if appropriate, to analyse data in accordance with recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
We identified three cluster‐RCTs where groups of participants (geographical regions or schools) were the unit of allocation. Study authors who reported appropriately adjusted estimates of relative effect accounting for clustering using the correct statistical modifications, were directly included in the analysis. Effect sizes from these studies were adjusted for unit of analysis issues. Appropriate adjustments were made based using intracluster correlation coefficients (directly obtained from authors) to account for design effects if not initially reported.
All cluster‐RCTs reported effect estimates for dichotomous study outcomes. To accommodate these studies, we used adjusted ORs as our measure of relative effect to be used in the meta‐analyses. Effect estimates and associated standard errors from appropriate analysis of cluster‐RCTs were analysed after adjustment for design effect. Sensitivity analysis was conducted using the generic inverse variance method with adjusted ORs.
Dealing with missing data
We planned to assess missing data on individuals as guided by the Cochrane Handbook for Systematic Reviews of Interventions. We would ignore missing data if they were assumed to be missing at random. If feasible, we planned to contact study authors to request missing data when it was assumed that they were not missing at random, for example, if some randomly assigned participants were excluded from analyses. If feasible, we planned to use statistical techniques, as appropriate to each study, to impute missing data to enable an available‐case or intention‐to‐treat analysis (Higgins 2019). For missing summary data, if we planned to approximate the correct analyses to impute missing summary statistics (e.g. standard deviations (SD)), in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Assessment of heterogeneity
We undertook meta‐analyses as the studies identified were similar enough in terms of both interventions and outcome measures for contraception use (uptake and adherence). Clinical diversity and methodological variability of the evidence was described in the text with associated study tables displaying trial design, location, population characteristics and intervention details.
Assessment of statistical heterogeneity was, initially, through visually inspecting forest plots noting the direction and magnitude of effects and assessing overlap of CIs. Further consideration of heterogeneity was through statistics generated from forest plots using the I² statistic to quantify inconsistency among the trials in each analysis. We used the P value from the Chi² test to assess if this heterogeneity was significant (P < 0.1). If there was substantial heterogeneity, we explored potential explanatory factors through prespecified subgroup analysis.
We used an approximate guideline, as adapted from Higgins 2019, to interpret the I2 value:
0% to 40%: heterogeneity might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Rather than a simple threshold, our interpretation took into account understanding measures of heterogeneity (I2 statistic and Tau) which will be estimated with high uncertainty when the number of studies is small.
Assessment of reporting biases
We aimed to minimise the potential impact of publication bias and other reporting biases by ensuring a comprehensive search for eligible studies and by exerting caution to prevent any duplication of data.
Funnel plots illustrate the relationship between the effect estimates from studies against their size or precision on logarithmic scale. We intended to use funnel plots to assess reporting bias for any comparisons we identified with relevant outcome data with at least 10 studies. Only one meta‐analysis 'contraception use' (primary outcome) met this criterion in our review. Funnel plots were then visually inspected for asymmetry and assessed for publication bias.
Data synthesis
We conducted statistical analysis according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We present an overview of the findings, together with tabular summaries of extracted data.
We used the Mantel‐Haenszel OR random‐effects model for dichotomous data and mean differences (MDs) for continuous data. Due to expected variability in populations, interventions of studies and outcome measures (high interstudy heterogeneity), we used a random‐effects model in our meta‐analysis. We used ORs with 95% CIs to accommodate unit of analysis issues. Cluster‐RCTs with adjusted effect estimates were used for design effects. Peto OR was used for the meta‐analysis for the pregnancy outcome to accommodate rare or zero events. Large differences in outcome reporting precluded us from pooling data across some studies to estimate summary effect sizes.
The primary meta‐analysis included all studies regardless of their risk of bias. When meta‐analysis was not possible, we presented summary and descriptive statistics.
Subgroup analysis and investigation of heterogeneity
We pooled results to find an aggregated effect across the studies through a meta‐analysis using a random‐effects models. If we detected substantial heterogeneity, we explored reasons through subgroup analyses using RevMan Web 2022. We performed subgroup analyses to explore differences in the intervention effect in regard to differences in study design, population or interventions.
We planned to conduct subgroup analyses grouping the trials using the following variables.
Unidirectional interventions (one‐way text messages, voice messages) compared with interactive (bidirectional) interventions (two‐way messaging interventions, mobile app based).
High‐income settings compared with low‐income settings as classified by World Bank income groups (lower‐middle income was grouped with lower income and upper‐middle was grouped with high income).
Younger women compared with older women.
Postpartum compared with postabortion and general clinic attendees.
Modern contraception methods compared with traditional contraception methods.
When interpreting the results, we assessed statistical heterogeneity, especially when there was any variation in the direction of the effect. Multiple‐armed trials, where more than one arm was relevant to the subgroup analysis, was processed and grouped appropriately as per recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Due to an insufficient number of studies, we were unable to conduct the following three planned subgroup analyses.
Younger women compared with older women
Postdelivery patients compared with postabortion and general clinic attendees
Modern contraception methods compared with traditional contraception methods
These analyses were not performed due to an insufficient number of studies in each subgroup to conduct quality subgroup analyses. We did not identify any studies promoting traditional contraception methods, and, therefore, we did not undertake the planned subgroup analysis.
Sensitivity analysis
We planned to conduct the following sensitivity analyses.
Repeating the analysis while excluding unpublished studies to investigate potential publication bias resulting from publication or non‐publication of research findings, depending on the nature and direction of the results (Higgins 2019).
Repeating the analysis while taking account of risk of bias of included studies.
We planned to conduct sensitivity analysis to assess heterogeneity exploring the effect of risk of bias in the studies included. We rated the certainty of the evidence by outcome using GRADE. However, we did not conduct a sensitivity analysis to assess the effect of the risk of bias of the studies included in the main effects analysis as there were insufficient studies in different risk of bias classes to warrant substantial analysis. Similarly, we did not find any studies that fit our criteria and had been unpublished to be used for a sensitivity analysis.
Due to the presence of cluster‐RCTs, we performed a sensitivity analysis using the generic inverse variance random‐effects outcome model using author‐reported adjusted ORs for the pregnancy outcome (alongside aforementioned Peto OR analysis) to assess whether use of statistical method affected overall outcome as per Higgins 2019.
Summary of findings and assessment of the certainty of the evidence
Two review authors summarised the certainty of the evidence provided by studies using the GRADE approach while considering factors that decrease the certainty level of a body of evidence (Higgins 2019). We resolved disagreements by discussion or by involvement of a third review author. Where a review author was also a contributor to an included study, that review author was not involved in the assessment of the certainty of the evidence process. We considered evidence from RCTs of high certainty and downgraded certainty by one level (serious) or two levels (very serious) for each of the following reasons.
Limitations in design and implementation (e.g. lack of blinding, large losses to follow‐up).
Indirectness of evidence (e.g. trials that met eligibility criteria but addressed a restricted version of the main review question in terms of population, intervention, comparator or outcomes).
Unexplained heterogeneity or inconsistency of results (e.g. when heterogeneity existed and affected interpretation of results, but study authors failed to identify a plausible explanation).
Imprecision of results (e.g. when studies included few participants and thus had wide CIs).
High probability of publication bias (e.g. if investigators failed to report studies or outcomes on the basis of results).
We prepared Table 1 to evaluate the overall certainty of the evidence for the main review outcomes (contraception use and pregnancy) for the main review comparison (mobile phone‐based interventions).
Results
Description of studies
Results of the search
For the update of this review, we conducted searches during March 2019 and August 2022, which resulted in 8519 references for screening. One additional study was discovered through contacting authors. After removing duplicates, we screened 4005 records. We discarded 3863 records after review of titles and abstracts. We assessed 142 full‐text articles for eligibility. The qualitative analysis included 23 studies and we used 20 studies in meta‐analyses. Three studies were ongoing at time of writing. See Figure 1 for the study flowchart.
1.
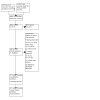
Study flow diagram ‐ updated review
For the first version of this review, we conducted searches during October 2014 and produced 759 records after removing duplicates. We discarded 683 records after review of titles and abstracts. We assessed 76 full‐text articles for eligibility. See Figure 2 for the study flowchart. We previously identified four ongoing studies, which were included in the update of this review.
2.

Study flow diagram ‐ original review
Included studies
We identified 23 RCTs that fulfilled the inclusion criteria (Babalola 2019; Biswas 2017; Brody 2022; Bull 2016; Castano 2012; Chernick 2017; Francis 2015; Harrington 2019; Hebert 2018; Hou 2010; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Rinehart 2020; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018; Wilkinson 2017). Three studies were cluster‐RCTs (Babalola 2019; Bull 2016; Rokicki 2017). Three trials were multisite (Biswas 2017; Harrington 2019; Smith 2015b), and the remaining were single site.
Eleven trials were conducted in high‐income settings. Ten trials were conducted in the USA (Bull 2016; Castano 2012; Chernick 2017; Francis 2015; Hebert 2018; Hou 2010; Johnson 2017; Rinehart 2020; Trent 2013; Wilkinson 2017), and one in Israel (Tsur 2008). The remaining 12 studies were from low‐ or middle‐income countries; two in Kenya (Harrington 2019; Unger 2018), one in Ghana (Rokicki 2017), two in Cambodia (Brody 2022; Smith 2015b), two in Bangladesh (Biswas 2017; Reiss 2019), one in Tajikistan (McCarthy 2018), one in Palestine (McCarthy 2019a), one in Bolivia (McCarthy 2020), one in Nigeria (Babalola 2019), and one in Uganda (Nuwamanya 2020).
Most trials recruited participants from urban clinics (Babalola 2019; Biswas 2017; Brody 2022; Bull 2016; Castano 2012; Chernick 2017; Francis 2015; Hebert 2018; Hou 2010; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Rinehart 2020; Rokicki 2017; Trent 2013; Unger 2018; Wilkinson 2017), one from rural clinics (Harrington 2019), two from clinics serving both urban and rural populations (Reiss 2019; Smith 2015b), one through a mobile text message programme (Johnson 2017), one from individuals who phoned an advice line (Tsur 2008), and it was unclear in one trial (McCarthy 2018).
Five trials included both men and women (Bull 2016; Harrington 2019; Johnson 2017; McCarthy 2018; Nuwamanya 2020). The remaining trials included only women.
Eight trials focused on youth/adolescent populations (Bull 2016; Castano 2012; Chernick 2017; Francis 2015; Rinehart 2020; Rokicki 2017; Trent 2013; Wilkinson 2017), and 15 included younger and older women of reproductive age (Babalola 2019; Biswas 2017; Brody 2022; Harrington 2019; Hebert 2018; Hou 2010; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Smith 2015b; Tsur 2008; Unger 2018). Of these 15 studies, two focused on postabortion contraception (Biswas 2017; Smith 2015b), two on postpartum contraception use (Harrington 2019; Unger 2018), and one in women who had undergone menstrual regulation (Reiss 2019).
Twelve trials recruited both existing users and non‐users of contraception (Biswas 2017; Brody 2022; Bull 2016; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Rinehart 2020; Rokicki 2017; Smith 2015b; Tsur 2008), six recruited new users of oral contraception (Babalola 2019; Castano 2012; Chernick 2017; Francis 2015; Hebert 2018; Hou 2010), one recruited existing injectable users (Trent 2013), two recruited women seeking EC (Wilkinson 2017), and two recruited pregnant women (Harrington 2019; Unger 2018).
Interventions
RCTs were conducted to either improve usage of one specific method of contraception or to improve usage of contraception not limited to one method. There were four modes of intervention delivery: unidirectional text messaging, interactive (bidirectional) text messaging, voice messages or mobile‐phone apps.
1 Interventions to improve contraception use – limited to one specific method of contraception
Four trials aimed to improve adherence to a specific method of contraception in existing or new contraception users, comparing interventions delivered by mobile phone versus standard care.
1.1 Unidirectional text messaging‐based interventions
Three studies used text messaging as a single directional intervention to improve use of a single specific method of contraception.
Hou 2010 in the US randomly assigned 82 new oral contraception users aged between 18 and 31 years (41 to mobile phone text messaging and 41 to standard care). The intervention aimed to improve oral contraception adherence and comprised a daily text message, "Please remember to take your birth control pill," sent at a designated time over the three‐month study period.
Trent 2013 in the US randomly assigned 100 current users of medroxyprogesterone acetate injection (Depo‐Provera) users aged 13 to 21 years to mobile phone text messaging or standard care. The intervention aimed to improve follow‐up clinic attendance and comprised a welcome message, daily text appointment reminders starting 72 hours before the clinic visit and healthy self‐management messages sent over the course of the three‐month enrolment period. This study was not included in meta‐analysis due to outcome measures not being measured in a comparable way.
Wilkinson 2017 in the US enrolled female adolescents who were seeking EC who received a text reminder on day one, three and five after randomisation. The text message intervention was used to remind women to fulfil their advance EC prescriptions. This study was also not included in meta‐analysis due to outcome measures not being measured in a comparable way.
1.2 Interactive text messaging‐based interventions
One study employed the use of interactive educational text messages, which required a response from participants, in addition to unidirectional messages to improve adherence to a specific method of contraception.
Castano 2012 in the US randomly assigned 962 new oral contraception users aged 13 to 25 years (480 to mobile phone text messaging and 482 to standard care). The intervention aimed to improve oral contraception continuation and comprised a range of daily unidirectional and interactive educational text messages (e.g. "The pill improves anaemia") for 180 days, in addition to standard care (face‐to‐face counselling and written educational handout).
2 Interventions to improve contraception use – not limited to one method of contraception
Nineteen trials aimed to improve contraception use, not limited to one method of contraception. These studies promoted use of more than one of the following: oral contraceptive pill, patch, ring, injection or IUD. These studies used unidirectional text messaging, two‐way text messaging, voice messages and app‐based interventions.
2.1 Unidirectional text messaging‐based interventions
Ten studies used unidirectional text messaging interventions to improve contraceptive use, not exclusive to one method, in both users and non‐users of contraception.
Biswas 2017 in four urban abortion facilities in Bangladesh randomised 60 women to receive method‐specific text message reminders to use their selected method whilst 60 women in the control group did not receive messages. The intervention aimed to improve the uptake and adherence to contraception; dependent on method selected, pills required daily and weekly reminders, injectables required weekly and one week before the due date, condoms required twice‐weekly and weekly, and no method received messages weekly.
Chernick 2017 randomised adolescent girls in the US to a unidirectional text message intervention. The intervention duration was three months and aimed to increase contraceptive use amongst girls at a high risk of getting pregnant.
Francis 2015 randomised adolescent women presenting for contraception initiation in the US to receive text messages about their new form of contraception (e.g. pill, patch, ring, injection or IUD) or no text messages.
Johnson 2017 randomised Mobile for Reproductive Health (m4RH) consumers (male and female) in Kenya to the full access or limited access group. The intervention was a free text‐message‐based platform that provided information when requested by participants on the benefits, disadvantages and adverse effects of nine family planning methods.
McCarthy 2018 enrolled young women with an unmet need for contraception and their husbands in Tajikistan who received zero to three messages per day (a total of 183 messages) whilst control group participants received 16 messages about trial participation over 120 days.
McCarthy 2019a randomised young women who were not using contraception and living in the West Bank of Palestine.
McCarthy 2020 enrolled young women based in Bolivia who received 183 messages (intervention) or 16 messages over a 120‐day period. The study aimed to estimate the effect of a contraceptive behavioural intervention delivered by mobile phone text message on young women's attitudes towards effective contraception.
Tsur 2008 in Israel randomly assigned 108 women aged 16 to 45 years using isotretinoin (an acne treatment that is contraindicated in pregnancy) (50 to mobile phone text messaging and 58 to standard care). The intervention was automated and comprised two text messages (at one and two months) together with information sent via mail, in addition to standard care (information given once during a phone interview). This study was not included in the meta‐analysis due to differential loss to follow‐up between intervention and control groups not stated and a blended approach used in some of the participants within the intervention arm who did not have a mobile phone so did not receive a mobile phone‐based intervention.
Two studies had multiarm approaches. Rokicki 2017 (cluster RCT in Ghana) randomised female students from 12 schools to the unidirectional text message intervention, 12 to the interactive intervention and 12 to the control group. The text message intervention focused on pregnancy prevention and contained information on topics of reproductive anatomy, pregnancy, sexually transmitted infections and contraception whilst the control group received placebo messages about malaria. Unger 2018 (three‐arm RCT in Kenya) randomised pregnant women seeking antenatal care at a health centre to one‐way text messages or a control group. The one‐way intervention group received weekly 'push' (educational and motivational SMS) and the control group received routine messages and usual care.
2.2 Interactive text messaging‐based interventions
Five studies used interactive educational text messages (which required a response from participants) in their intervention in addition to unidirectional messages to improve adherence to a specific method of contraception.
Bull 2016 enrolled teenagers aged 14 to 18 years from eight boys and girls clubs. The text message intervention called "Youth All Engaged!" aimed to increase the effects of an adolescent pregnancy prevention Teen Outreach Program for youths.
Harrington 2019 enrolled 260 pregnant women from two public county hospitals in western Kenya and referred their male partners to receive messages too. Intervention group participants received weekly family planning‐focused text messages that were delivered from enrolment to six months' postpartum, and the platform enabled dialogue with a nurse.
Rinehart 2020, based in the US, recruited adolescents aged 13 to 18 years and randomised them to a pilot text intervention "t4she" or a control group where they received standard clinic care. The intervention group received 58 automated messages where a proportion had been bidirectional.
Rokicki 2017 (three‐arm cluster‐RCT in Ghana) randomised female students from 12 schools to the interactive intervention and 12 to the control group. The interactive text message intervention focused on pregnancy prevention and contained information on topics of reproductive anatomy, pregnancy, sexually transmitted infections and contraception whilst the control group received placebo messages about malaria.
Unger 2018 (three‐arm RCT in Kenya) randomised pregnant women seeking antenatal care at a health centre to a two‐way text message or control group. The interactive two‐way group received the same weekly text message as the one‐way arm but also received questions that required a response.
2.3 Voice message‐based interventions
Four studies used voice messages to convey information about contraception in their intervention to improve adherence to a specific method of contraception. These voice messages were sent to the participant's mobile phone and in the language most appropriate to those recruited.
Babalola 2019 in Nigeria enrolled women aged 18 to 35 years randomised to intervention or control. The intervention was the "The Smart Client" digital health tool where participants listened to interactive voice messages that recounted short fictional storylines about the challenges and solutions of contraception use. The tool was developed using social learning theory and allowed information transfer in an engaging way.
Brody 2022 used "Mobile Link," a text and voice message‐based intervention in female entertainment workers in Cambodia. The intervention group received voice or text messages twice a week for 10 weeks, repeated for 60 weeks, whilst the control group received standard care.
Reiss 2019 randomised menstrual regulation clients from 41 public and private sector clinics in Bangladesh. The intervention group received at least 11 voice messages about contraception over four months and the control group received no messages.
Smith 2015b in Cambodia randomly assigned 500 women aged over 18 years seeking abortion services who reported not wanting to get pregnant again at the current time (249 to a semi‐automated intervention delivered by mobile phone and 251 to standard care). The intervention aimed to increase uptake and adherence to effective contraception (oral contraception, injectable, implant, IUD and permanent methods) and comprised six interactive voice messages, counsellor‐delivered phone support according to the response to messages and additional reminder messages for oral contraception or injectable users.
2.4 Mobile phone app‐based interventions
Two studies used mobile phone apps as their primary intervention. These interventions allowed participants to view written media and multimedia on their phone through a custom mobile phone app developed for the study.
Hebert 2018 randomised young women seeking contraceptive care in a midwestern city in the US to a waiting room contraceptive counselling mobile application in the waiting room or a control group who attended a routine clinic visit. Participants were shown a short video discussing long‐acting reversible contraception (LARC). The aim of the intervention was to improve the uptake of contraception use.
Nuwamanya 2020 randomised participants to app‐based intervention or standard of care. The app provided participants with information on sexual health and family planning as well as a platform to order goods and a guide to local services. The outcomes included use of contraception, impacts on sexual health knowledge and use of sexual health services.
Behavioural change techniques
Some trials reported using a particular behavioural theory to underpin their mobile phone‐based intervention. Authors who provided insight into the development of their intervention reported incorporation of various behavioural‐theory techniques. We categorised these techniques using Abraham and Michie's typology (Abraham 2008). The most commonly used behaviour change techniques were the following: provide information about the behaviour‐health link (17 interventions), provide information on consequences (17 interventions) and prompt practice (nine interventions). Full categorisation of behavioural change techniques for each study as identified by our assessment are reported in Table 2.
1. Behaviour techniques used in interventions.
| Behaviour change technique | Studies |
| 1. Provide information about behaviour‐health link | Babalola 2019 (clients able to observe health behaviour and understand consequences); Brody 2022 (health behaviours and risks addressed); Castano 2012 (e.g. "The pill improves anaemia"); Chernick 2017 (information about sexually transmitted infections); Harrington 2019 (information on family planning reducing pregnancy risk); Hebert 2018 (information on contraception effectiveness rates/adverse effects); Johnson 2017 (provide information about the behaviour‐health link, e.g. the benefits, disadvantages and adverse effects of 9 family planning methods); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (risks and adverse effects of contraception given, e.g. "Hormonal methods are safe under medical supervision"); Nuwamanya 2020 (information about sexual health and family planning); Reiss 2019 (e.g. messages reminding participants of the benefits of using contraception); Rinehart 2020 (information provided about sexually transmitted infections); Rokicki 2017 (information on sexually transmitted infections); Smith 2015b (information about amenorrhoea); Trent 2013 (healthy self‐management messages); Tsur 2008 (informed about importance of contraceptive use) |
| 2. Provide information on consequences | Babalola 2019 (consequences of health impacts presented); Brody 2022 (consequences presented e.g. withdrawal method still leads to pregnancy); Bull 2016 (e.g. teen pregnancy impacts on future goals); Castano 2012 (e.g. "The pill is very effective at preventing pregnancy"); Chernick 2017 (consequences, e.g. teen pregnancy impacts on future goals); Harrington 2019 (consequences, e.g. family planning has adverse effects); Hebert 2018 (information on positive and negative experiences including adverse effects); Johnson 2017 (information on adverse effects of contraceptive methods); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (consequences, e.g. the bleeding cycle may change or stop); Nuwamanya 2020 (discussion of family planning counselling); Reiss 2019 (e.g. addressing key barriers such as fear of infertility);Rinehart 2020 (sexually transmitted infections, effects and dispelling of myths); Rokicki 2017 (consequences, e.g. pregnancy); Smith 2015b (e.g. "contraceptive methods are an effective and safe way to prevent unintended pregnancy"); Tsur 2008 (informed about teratogenic risk) |
| 3. Provide information about others' approval | Hebert 2018 (e.g. information regarding how men perceive or experience (or both) the method); McCarthy 2018/McCarthy 2019a/McCarthy 2020(e.g. "with the infection some people like not having a period") |
| 4. Prompt intention formation | Bull 2016 (prompt intention formation, e.g. club reminder); Hebert 2018 (integral to the model that contraceptive 1 and 2 do this); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (goal setting prompted) |
| 5. Prompt barrier identification | Brody 2022 (contacts of outreach worker given to improve access); Biswas 2017 (e.g. if any problems, contact the clinic); Bull 2016 (e.g. responsibility to get the condoms/contraceptives); Chernick 2017 (e.g. privacy, no appointment needs, services are free, transport links); Nuwamanya 2020 (problems with ordering/requesting tests or contraception identified with relevant contacts); Rinehart 2020 (contacts of clinics given); Reiss 2019 (e.g. addressing key barriers such as fear of infertility);Smith 2015b (if client received a phone call, counsellors provided reassurance regarding adverse effects as per conceptual framework reported in the study protocol) |
| 6. Provide general encouragement | Babalola 2019 (general motivational messages); Brody 2022 (general motivational messages); Castano 2012 (e.g. "Welcome to our study and thank u 4 participating"); Chernick 2017 (provide general encouragement, e.g. wallet card); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (general encouragement messages to continue contraception); Rinehart 2020 (general encouragement messages to continue contraception); Unger 2018 (e.g. motivational messages) |
| 7. Set graded tasks | — |
| 8. Provide instruction | Brody 2022 (sexually transmitted infection prevention instructive messages); Castano 2012 (e.g. "Tell every doctor u see that u r taking the pill"); Hebert 2018 (e.g. video regarding long‐acting contraception); Hou 2010 (if "Please remember to take your birth control pill" is considered 'telling a person how to perform a behaviour'); Johnson 2017 (e.g. information on clinic locations); Reiss 2019 (e.g. instruction on how to take pill correctly); Rinehart 2020 (e.g. information on clinic locations); Smith 2015b (e.g. "press 1 if you would like me to call you back to discuss contraception") |
| 9. Model or demonstrate the behaviour | Babalola 2019 (re‐enacted drama sequences demonstrating sexual health behaviour with model setting); Hebert 2018 (e.g. video regarding long‐acting contraception from user); Johnson 2017 (e.g. provide role model stories); Rinehart 2020 (e.g. links to video provided regarding contraception) |
| 10. Provide specific goal setting | Bull 2016 (text message asks people to name 3 short‐term goals, 3 long‐term goals); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (stated by the authors included 'goal setting') |
| 11. Prompt review of behavioural goals | — |
| 12. Prompt self‐monitoring of behaviour | Hou 2010 (women kept a diary of their daily pill taking; the intervention may have prompted this behaviour) |
| 13. Provide feedback on performance | — |
| 14. Provide contingent rewards | Nuwamanya 2020 (e.g. subsidised contraceptive/sexual health products); Rokicki 2017 (e.g. airtime credit rewards) |
| 15. Teach or use prompts or cues | — |
| 16. Agree on behavioural contract | — |
| 17. Prompt practice | Brody 2022 (multiple messages reminding condom use); Biswas 2017 (text message reminders); Hou 2010 ("Please remember to take your birth control pill"); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (author stated included 'guided practice'); Rinehart 2020 (reminded messages sent to prompt condom use); Smith 2015b (participants who chose to receive the oral contraceptive or injectable could receive additional reminders appropriate to their method); Trent 2013 (daily text appointment reminders 72 hours before the clinical visit) |
| 18. Use follow‐up prompts | Babalola 2019 (users received short message reminder of key messages from each voice message); Bull 2016 (e.g. 5–7 messages/week); Chernick 2017 (e.g. repeated prompts over 3 months); Harrington 2019 (e.g. messages sent for 6 months); Reiss 2019 (e.g. tailored messages sent to non‐users are designed to encourage uptake of contraception); Rinehart 2020 (follow‐up messages sent following a weekend regarding sexual health services); Unger 2018 (clinic visit reminders); Wilkinson 2017 (e.g. text stated "Reminder‐don't forget to fill your prescription you obtained in clinic yesterday. Please call ******* if you have any questions or difficulty obtaining the medication.") |
| 19. Provide opportunities for social comparison | Babalola 2019 (voice messages depicting a drama with how different sexual health behaviours); Bull 2016 (opportunity for social comparison, e.g. 50% of teens are having sex/share experience of achieving a goal); Hebert 2018 (e.g. African American and Latina patients experience videos); Johnson 2017 (provides examples of others behaviour); McCarthy 2018/McCarthy 2019a/McCarthy 2020 (cultural similarity messages with shifting perspectives) |
| 20. Plan social support or social change | Chernick 2017 (e.g. bring your partner or friend to clinic); Harrington 2019 (e.g. enrol male partners); McCarthy 2018/McCarthy 2019a (e.g. "making a decision about family planning with your husband helps you avoid an unintended pregnancy"); Smith 2015b (if client received a phone call and requested, the counsellor would also discuss contraception with the husband or partner) |
| 21. Prompt identification as a role model | — |
| 22. Prompt self‐talk | — |
| 23. Relapse prevention | — |
| 24. Stress management | — |
| 25. Motivational interviewing | — |
| 26. Time management | — |
Outcomes
Primary outcomes
Contraceptive use (uptake and adherence)
Babalola 2019 assessed use of modern contraception at three‐month follow‐up. Biswas 2017 assessed using modern contraception at four‐month follow‐up. Brody 2022 reported contraception use at six‐month and 12‐month follow‐up. Bull 2016 assessed contraception use in the past three months. Chernick 2017 assessed contraception initiation. Francis 2015 assessed contraceptive continuation at four‐month follow‐up. Harrington 2019 assessed highly effective contraception use and LARC use. Hebert 2018 assessed current use of any LARC, IUD and implant. Johnson 2017 assessed use of contraception at the end of the trial. McCarthy 2018, McCarthy 2019a, and McCarthy 2020 assessed use of effective contraception over four months and at four months. Nuwamanya 2020 reported contraception use at six‐month follow‐up. Rinehart 2020 reported use of prescribed contraception at both three‐ and six‐month follow‐up. Reiss 2019 assessed as primary outcome LARC use at four months. Smith 2015b assessed self‐reported use of effective contraception, as assessed at four‐ and 12‐month follow‐up. Effective methods were considered as those with less than 10% failure rates (i.e. oral contraception, injectable, IUD, implant). Tsur 2008 assessed self‐reported contraceptive use (methods not defined) at three months. Unger 2018 assessed contraceptive use at 10, 16 and 24 weeks' postpartum.
Other ways to report contraception use were as follows:
contraception use over the follow‐up period greater than 80% (Smith 2015b);
long‐acting contraception use (Reiss 2019; Smith 2015b);
used contraception or EC in the past year (Rokicki 2017);
EC use (Hou 2010);
condom use in the past three months (Bull 2016), condom use for at least 50% of coital activity during the study (Hou 2010), used condom at sexual debut, had sexual intercourse without a condom in the past year and used condom in the past year (Rokicki 2017), condom use (Wilkinson 2017);
use of two contraceptives (Tsur 2008);
sexually active and not using contraception (Tsur 2008);
adherence to a contraceptive method (e.g. number of missed pills, attendance for repeat injection).
Hou 2010 reported missed pills per cycle measured by an electronic monitoring device (EMD) over a three‐month period. Castano 2012 defined oral contraception continuation as the participant taking a pill within the previous seven days, assessed at six months. Trent 2013 reported days between next scheduled appointment and attendance for medroxyprogesterone acetate (Depo‐Provera) injection over three cycles (nine months). Wilkinson 2017 reported on filed EC.
Other ways to report adherence were on‐time appointment for medroxyprogesterone acetate (Depo‐Provera) injection (Trent 2013), and adherence measured as oral contraception use at last sexual intercourse, interruptions in oral contraception use greater than seven days, no missed pills during the past month (Castano 2012). Johnson 2017 assessed clinic visits to discuss family planning with a nurse or doctor. McCarthy 2018, McCarthy 2019a, and McCarthy 2020 assessed service uptake. Smith 2015b assessed discontinuation of effective contraception.
Pregnancy or abortion (objectively measured or self‐reported)
Pregnancy (Hou 2010; Smith 2015b), ever pregnant or caused pregnancy at intervention completion (Bull 2016), became pregnant (Chernick 2017), pregnant in the past year (Rokicki 2017), unintended pregnancy (McCarthy 2018; McCarthy 2019a; McCarthy 2020)
Repeat abortion (Smith 2015b), abortion (McCarthy 2018; McCarthy 2019a; McCarthy 2020)
Other primary outcomes
None of the studies reported our other primary outcomes.
Secondary outcomes
Secondary outcomes were unintended outcomes (road traffic accident, domestic abuse; Smith 2015b) and someone they did not want to know about the text message reminders finding out (Biswas 2017). McCarthy 2018, McCarthy 2019a, and McCarthy 2020 assessed rates of reported physical violence. Reiss 2019 measured adverse events including the experience of intimate partner violence (IPV).
Funding sources
Twenty‐two studies had non‐commercial funding, such as educational bodies, government research funding and non‐governmental organisations (Babalola 2019; Biswas 2017; Brody 2022; Bull 2016; Castano 2012; Chernick 2017; Harrington 2019; Hebert 2018; Hou 2010; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Rinehart 2020; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018; Wilkinson 2017). Francis 2015 did not declare any funding sources. No authors reported any commercial funding sources.
See full details in the Characteristics of included studies table.
Excluded studies
We excluded studies when mobile phones were used for two‐way voice communication (as a phone) alone (Berenson 2012; Katz 2011; Kirby 2010); when the intervention was web‐based or tablet‐based and did not appear to have been adapted for mobile phone users (Bannink 2014; Brown 2018; Himes 2017; Sridhar 2013); that did not have relevant outcome measures (Bracken 2014; Constant 2014; Hall 2013; Harrington 2017a; Manlove 2020); in which the intervention focused on preventing sexually transmitted disease rather than on providing contraception (Brown 2018; Free 2016a; Gold 2011; Juzang 2011; Kaoaiem 2012; Lim 2012; Nielsen 2021; Suffoletto 2013), and were not RCTs (Feyisetan 2015; L'Engle 2013; Mackenzie 2009; O'Sullivan 2008; Walakira 2013).
See details in Characteristics of excluded studies table.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
Three studies are ongoing (Bates 2018; Gul 2019; Yeates 2019).
See details in Characteristics of ongoing studies table.
Risk of bias in included studies
We summarised risk of bias in Figure 3 and Figure 4. For Trent 2013 and Francis 2015, the conference abstracts provided insufficient information for full assessment of risk of bias, but we were able to obtain additional data from the study investigators.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Eighteen studies were at low risk of bias for random sequence generation (Biswas 2017; Brody 2022; Castano 2012; Chernick 2017; Harrington 2019; Hou 2010; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Rinehart 2020; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018; Wilkinson 2017). Of these, 13 studies used computer‐generated sequences (Biswas 2017; Chernick 2017; Hou 2010; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Rinehart 2020; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018), and one study used a random number table (Castano 2012). Four studies were at unclear risk of bias for random sequence generation (Babalola 2019; Bull 2016; Francis 2015; Hebert 2018). One study was at high risk of bias for random sequence generation, using a manual rolling method of allocation (Johnson 2017).
Fifteen studies were at low risk of bias for allocation concealment (Biswas 2017; Castano 2012; Chernick 2017; Harrington 2019; Hou 2010; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Rinehart 2020; Smith 2015b; Trent 2013; Unger 2018; Wilkinson 2017). Of these, two studies used envelopes that assigned allocation (Harrington 2019; Wilkinson 2017). Eight studies were at unclear risk of bias for allocation concealment (Babalola 2019; Brody 2022; Bull 2016; Francis 2015; Hebert 2018; Johnson 2017; Rokicki 2017; Tsur 2008).
Blinding
Five studies were at low risk of bias for blinding of participants and personnel (performance bias) (Brody 2022; Harrington 2019; Hebert 2018; Nuwamanya 2020; Reiss 2019). Five studies were at unclear risk of bias for blinding of participants and personnel (performance bias) (Biswas 2017; Chernick 2017; Francis 2015; Johnson 2017; Wilkinson 2017). Thirteen studies were at high risk of bias for blinding of participants and personnel (performance bias) (Babalola 2019; Bull 2016; Castano 2012; Hou 2010; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Rinehart 2020; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018). As a result of the nature of the interventions, it was not possible to blind participants to intervention allocation as stated in some studies (Harrington 2019; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Rokicki 2017; Unger 2018). Hou 2010 reported that 68% of participants in the control group used a reminding system outside the study protocol (e.g. alarm clock, mobile phone alarm) compared with 36% in the intervention group (P = 0.003). This could have occurred in response to participation in the trial or frequent use of reminding systems in general. Rinehart 2020 had blinded researchers to randomisation and allocation; however, after baseline interviews, the researchers opened sealed envelopes and discussed the allocation with participants. Unger 2018 stated that self‐reporting could have introduced social desirability bias, but could have occurred across all arms of the study.
Thirteen studies were at low risk of bias for blinding of outcome assessment (detection bias) (Brody 2022; Chernick 2017; Hebert 2018; Hou 2010; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Smith 2015b; Trent 2013; Wilkinson 2017). Five studies reported outcome assessment as blinded (Chernick 2017; Hou 2010; Smith 2015b; Trent 2013; Wilkinson 2017). Six studies were at unclear risk of bias for blinding of outcome assessment (detection bias) (Biswas 2017; Bull 2016; Francis 2015; Harrington 2019; Tsur 2008; Unger 2018). Four studies were at high risk of bias for blinding of outcome assessment (detection bias) (Babalola 2019; Castano 2012; Rinehart 2020; Rokicki 2017). In Castano 2012 and Hou 2010, participants were asked questions regarding their satisfaction with the intervention.
Incomplete outcome data
Fifteen studies were at low risk of bias for incomplete outcome data (attrition bias) (Castano 2012; Francis 2015; Harrington 2019; Hebert 2018; Hou 2010; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Reiss 2019; Rokicki 2017; Smith 2015b; Trent 2013; Tsur 2008; Unger 2018). One study was at unclear risk of bias for incomplete outcome data (attrition bias) (Nuwamanya 2020). Seven studies were at high risk of bias for incomplete outcome data (attrition bias) (Babalola 2019; Biswas 2017; Brody 2022; Bull 2016; Chernick 2017; Rinehart 2020; Wilkinson 2017). For example, Babalola 2019 and Wilkinson 2017 reported high dropout of over 50%. Biswas 2017 reported 11% loss to follow‐up. Poverty and lack of education were attributed to overestimation of results in the study. Both these studies did not specify the difference in the two arms of the intervention. Brody 2022 reported over 50% loss to follow‐up and found significant baseline differences between followed up and lost to follow‐up groups. Bull 2016 reported loss to follow‐up of more than 25.8%. Chernick 2017 reported that more participants were lost in the intervention arm.
Selective reporting
Fifteen studies were at low risk of bias for selective reporting (reporting bias) (Biswas 2017; Brody 2022; Francis 2015; Harrington 2019; Hebert 2018; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Rinehart 2020; Smith 2015b; Trent 2013; Unger 2018). Most studies had a study protocol of their RCT that reported outcomes followed. For example, Smith 2015b prespecified primary and secondary outcomes in its study protocol (Smith 2013). Castano 2012 and Trent 2013 provided information on outcomes on a clinical trial registry. Eight studies were at unclear risk of bias for selective reporting (reporting bias) (Babalola 2019; Bull 2016; Castano 2012; Chernick 2017; Hou 2010; Rokicki 2017; Tsur 2008; Wilkinson 2017). We were unable to locate a study protocol or a clinical trials registry record for three studies (Bull 2016; Rokicki 2017; Tsur 2008). One study reported the primary outcomes using measurements that were not prespecified in the study (Wilkinson 2017). No studies were at high risk of bias for selective reporting (reporting bias). On greater exploration of potential publication bias, the asymmetrical funnel plot with the outcome of contraceptive use (Analysis 2.1) suggests the presence of bias due to missing results (Figure 5).
2.1. Analysis.

Comparison 2: Contraception use: message intervention versus control, Outcome 1: Contraception use
5.

Other potential sources of bias
Five studies were at low risk of other bias (Hou 2010; Johnson 2017; Rokicki 2017; Trent 2013; Unger 2018). For example, Hou 2010; and Trent 2013 used objective measures for the primary outcome. Hou 2010 assessed mean pills missed per cycle using an electronic medication monitor, in addition to a self‐report participant diary. Trent 2013 assessed attendance for medroxyprogesterone acetate (Depo‐Provera) appointments using clinic records. Seven studies were at unclear risk of other bias (Biswas 2017; Francis 2015; Hebert 2018; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Reiss 2019). Eleven studies were at high risk of other bias (Babalola 2019; Brody 2022; Bull 2016; Castano 2012; Chernick 2017; Harrington 2019; Nuwamanya 2020; Rinehart 2020; Smith 2015b; Tsur 2008; Wilkinson 2017). For example, eight studies used self‐reported measures for contraceptive use that could result in response bias (Brody 2022; Bull 2016; Castano 2012; Chernick 2017; Reiss 2019; Rinehart 2020; Smith 2015b; Tsur 2008).
Effects of interventions
See: Table 1
Contraception use
Mobile phone‐based interventions probably increase contraception use compared to the control (OR 1.30, 95% CI 1.06 to 1.60; P < 0.001, I2 = 69%; 16 studies, 8972 participants; moderate‐certainty evidence; Analysis 2.1; Figure 6; Table 1). We pooled all studies that trialled a mobile phone‐based intervention compared to a control group with comparable outcomes. The point estimate of 1.30 in our random‐effects model provides the best mean estimate of magnitude and direction of the intervention's effect compared with the control groups. However, the relatively wide CIs affect our precision in our assessment of certainty in the evidence.
6.

The certainty of evidence between studies ranged from very low to high for assessing mobile phone interventions for contraception, as reported in Table 3. Overall, the certainty of the evidence for the pooled effect estimate was moderate (Table 1).
2. Results by certainty of evidence.
| Study | Limitations in design and implementation | Indirectness of evidence | Unexplained heterogeneity or inconsistency of results | Imprecision of results | High probability of publication bias | Certainty of evidence | Evidence of effect |
| Babalola 2019 | −2 | 0 | 0 | −1 | 0 | Very low | Yes |
| Brody 2022 | −1 | 0 | 0 | −1 | 0 | Low | No |
| Biswas 2017 | −1 | 0 | 0 | −1 | 0 | Low | No |
| Bull 2016 | −2 | 0 | 0 | −1 | 0 | Very low | No |
| Castano 2012 | −2 | 0 | 0 | 0 | 0 | Low | Yes |
| Chernick 2017 | −1 | 0 | 0 | −1 | 0 | Low | No |
| Francis 2015 | −2 | 0 | 0 | 0 | 0 | Low | No |
| Harrington 2017a | 0 | 0 | 0 | 0 | 0 | High | Yes |
| Hebert 2018 | −2 | 0 | 0 | −1 | 0 | Very low | No |
| Hou 2010 | 0 | 0 | 0 | −1 | 0 | Moderate | No |
| Johnson 2017 | −2 | 0 | 0 | 0 | 0 | Low | No |
| McCarthy 2018 | −1 | −1 | 0 | 0 | 0 | Low | No |
| McCarthy 2019a | −1 | −1 | 0 | 0 | 0 | Low | No |
| McCarthy 2020 | −1 | −1 | 0 | 0 | 0 | Low | No |
| Nuwamanya 2020 | −1 | 0 | 0 | 0 | 0 | Moderate | Yes |
| Reiss 2019 | 0 | 0 | 0 | 0 | 0 | High | No |
| Rinehart 2020 | −2 | 0 | 0 | 0 | 0 | Low | Yes |
| Rokicki 2017 | −1 | 0 | 0 | −1 | 0 | Low | No |
| Smith 2015b | −1 | 0 | 0 | 0 | 0 | Moderate | Yes |
| Trent 2013 | 0 | 0 | 0 | −1 | 0 | Moderate | Yes |
| Tsur 2008 | −1 | −1 | 0 | −1 | 0 | Very low | No |
| Unger 2018 | −1 | 0 | 0 | 0 | 0 | Moderate | Yes |
| Wilkinson 2017 | −1 | 0 | 0 | −1 | −1 | Very low | No |
Randomised controlled trials were considered of high certainty evidence, then were downgraded by one level (serious) or two levels (very serious) for each of the following: limitations in design and implementation (e.g. lack of blinding, large losses to follow‐up), indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results, high probability of publication bias.
1 downgrade equated to moderate‐certainty evidence, 2 downgrades equated to low‐certainty evidence and 3 or more downgrades equated to very low‐certainty evidence.
Pregnancy
We pooled studies that trialled a mobile phone‐based intervention compared to a control group with comparable outcomes assessing incidence of unintended pregnancy. Using a Peto OR assessment with dichotomous outcomes, we found no difference between groups in the incidence of unintended pregnancy (OR 0.82, 95% CI 0.48 to 1.38; P = 0.45, I2 = 0%; 8 studies, 2947 participants; moderate‐certainty evidence; Analysis 3.1; Figure 7; 2 studies reported pregnancy but recorded 0 events in both groups; thus, the OR and CIs were calculated from 6 studies rather than 8). The point estimate of 0.82 in our fixed‐effect model provides the best estimate of magnitude and direction of the intervention's effect compared with control groups. However, the relatively wide CIs affect our precision in our assessment of certainty in the evidence.
3.1. Analysis.

Comparison 3: Pregnancy: message intervention versus control, Outcome 1: Pregnancy – Peto OR
7.

The certainty of evidence between studies ranged from very low to high for assessing mobile phone interventions for pregnancy, as reported in Table 3. Overall, the certainty of the evidence for the pooled effect estimate was moderate (Table 1).
Studies not included in a meta‐analysis
It was not possible to include some results from the following studies in the meta‐analysis because of the study design or the outcomes were not reported in a comparable way (Bull 2016; Castano 2012; Hou 2010; Trent 2013; Tsur 2008).
For a specific method of contraception, Hou 2010 found no difference in the mean number of missed pills per contraceptive pill cycle using the EMD between the text message group and the control group during cycle one (mean difference (MD) 0.5 missed pills, 95% CI −1.08 to 2.08; 73 participants; Analysis 4.1), and cycle three (MD 0.80 missed pills, 95% CI −1.22 to 2.82; 73 participants; Analysis 4.2). Trent 2013 reported that the group receiving text message reminders and healthy self‐management messages had a lower mean number of days between scheduled appointment and actual attendance for medroxyprogesterone acetate injection (Depo‐Provera) for visit one (MD −8.60 days, 95% CI −16.74 to −0.46; 87 participants; Analysis 5.1), but not for visit two or three (Analysis 5.2) (data obtained from study investigator).
4.1. Analysis.

Comparison 4: Hou 2010: daily text message reminders versus no reminders, Outcome 1: Mean number of missed pills (cycle 1)
4.2. Analysis.

Comparison 4: Hou 2010: daily text message reminders versus no reminders, Outcome 2: Mean number of missed pills (cycle 3)
5.1. Analysis.

Comparison 5: Trent 2013: daily text message appointment reminders 72 hours before appointment + healthy self‐management messages versus standard care, Outcome 1: Mean number of days between scheduled appointment and completed visit: first visit
5.2. Analysis.

Comparison 5: Trent 2013: daily text message appointment reminders 72 hours before appointment + healthy self‐management messages versus standard care, Outcome 2: Mean number of days between scheduled appointment and completed visit: third visit
Tsur 2008 reported no difference in contraceptive use between participants receiving text messages plus information received via mail and the control group (RR 1.26, 95% CI 0.84 to 1.89; 108 participants; Analysis 6.1). Chernick 2017 found no difference in contraception initiation compared of their mobile phone intervention compared with advertising a walk‐in family planning clinic and a standardised monologue given by the emergency department physicians describing the need for reproductive care (RR 0.53, 95% CI 0.21 to 1.33; 99 participants; Analysis 7.2). Bull 2016 found no difference in the mean percentage of sex acts protected by contraception in the past three months (MD 12.40, 95% CI −5.40 to 30.20; 50 participants; Analysis 8.2).
6.1. Analysis.

Comparison 6: Tsur 2008: contraceptive information via text messages and mail at 1 and 2 months versus standard care, Outcome 1: Contraceptive use during treatment with isotretinoin
7.2. Analysis.

Comparison 7: Chernick 2017: daily educational and motivational texts versus standardised physical discharge solutions, Outcome 2: Contraception initiation
8.2. Analysis.

Comparison 8: Bull 2016: automated interactive voice messages + teen outreach programme versus teen outreach programme, Outcome 2: Mean percentage of sex acts protected by contraception in past 3 months – sexually active
Castano 2012 reported participants receiving the intervention were more likely to report no oral contraception interruptions longer than seven days at six months (RR 1.22, 95% CI 1.06 to 1.41; 683 participants; Analysis 9.4), more likely to report that they had missed no pills in the previous month (RR 1.44, 95% CI 1.16 to 1.79; 683 participants; Analysis 9.5), and more likely to report oral contraception use at last sexual intercourse (RR 1.15, 95% CI 1.03 to 1.28; 683 participants; Analysis 9.6). In Hou 2010, participants receiving the intervention were more likely to report condom use for at least 50% of coital activity during the study (RR 1.94, 95% CI 1.00 to 3.78; 73 participants; Analysis 4.3). For Trent 2013, the abstract reported no overall differences among those who received injections within the optimal medroxyprogesterone acetate injection (Depo‐Provera) window due to additional clinical nursing outreach that resulted from missed visits per the existing clinical protocol for standard care.
9.4. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 4: No OC interruptions > 7 days at 6 months
9.5. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 5: Missed no pills in last month
9.6. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 6: OC use at last intercourse
4.3. Analysis.

Comparison 4: Hou 2010: daily text message reminders versus no reminders, Outcome 3: Condom use for ≥ 50% of coital activity during study (self‐report)
In Hou 2010, there was no difference between intervention and control groups regarding EC use, but there were few events (Analysis 4.4).
4.4. Analysis.

Comparison 4: Hou 2010: daily text message reminders versus no reminders, Outcome 4: Emergency contraception use during study
Secondary outcomes
Six trials assessed potential unintended outcomes. Smith 2015b reported no road traffic accidents or domestic abuse was reported (Analysis 10.6; Analysis 10.7). Reiss 2019 reported physical intimate partner violence was higher in the intervention group when measured using a closed question naming acts of violence (42/386 (11%) with intervention versus 25/382 (7%) with control; Analysis 11.5). However, no violence was reported in response to an open question about the effects of being in the study. McCarthy 2018 (Tajikistan), McCarthy 2019a (Palestine), and McCarthy 2020 (Bolivia) reported no difference in physical violence rates between control and intervention groups (McCarthy 2018: total: 4/470 experienced physical violence; 1.32% with intervention versus 0.41% with control; P = 0.57; McCarthy 2019a: total experienced physical violence 7/464; 0.89% with intervention versus 2.13% with control; P = 0.45; McCarthy 2020: total experienced physical violence 10/409; 2.0% with intervention versus 2.9% with control; P = 0.75). Biswas 2017 noted privacy concerns with 29/55 (53%) participants reporting the intervention messages were found by someone they did not want knowing – often their husbands or children.
10.6. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 6: Road traffic accident
10.7. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 7: Domestic abuse
11.5. Analysis.

Comparison 11: Reiss 2019: automated voice messages versus no messages, Outcome 5: Physical intimidate partner violence
Subgroup analysis
Based on an I² value of 69%, there was likely substantial heterogeneity in the pooled analysis assessing interventions for contraceptive use (Higgins 2019). This was supported by the Chi² value, the very low P value (P < 0.001) and the large variation in the size of the treatment effect. CIs, as noted in the meta‐analysis, were overlapping suggesting the variation between studies may be attributable to chance. However, overall these measures all point towards substantial heterogeneity where variation in effect estimates are beyond chance.
There were key differences between studies based on population and intervention. There was considerable variety in the types of intervention used with different applications of unidirectional text messaging, interactive messaging and voice messages to mobile phone apps. Trials were conducted in a range of settings including high‐ and low‐income countries, with some studies focussing on adolescents and others including all women of childbearing age. These differences in key characteristics may have contributed to the overall heterogeneity.
We further explored the substantial heterogeneity with the following subgroup analyses.
We included all 16 trials used in the pooled meta‐analysis and categorised trials into two groups of interactivity based on intervention. Nine trials used unidirectional interventions (Biswas 2017; Brody 2022; Francis 2015; Johnson 2017; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Unger 2018), and eight trials employed interventions or two‐way interventions (Babalola 2019; Castano 2012; Harrington 2019; Hebert 2018; Reiss 2019; Rinehart 2020; Smith 2015b; Unger 2018). Unger 2018, a three‐armed RCT, assessed the use of one‐way messaging compared to two‐way messaging compared to control. For comparable subgroup analysis, both intervention arms (one‐way and two‐way) were compared with control and separately included in the analysis with a splitting of the control group as recommended by Higgins 2019.
In the subgroup analysis assessing whether unidirectional interventions delivered by mobile phone compared with interactive (bidirectional) interventions may impact contraceptive use, we found evidence of a difference between the subgroups (P = 0.003, I2 = 88.5%; Analysis 2.2). Interactive interventions had an OR of 1.71 (95% CI 1.28 to 2.29; P = 0.0003, I2 = 63%; 8 studies, 3089 participants) whilst unidirectional interventions had an OR of 1.03 (95% CI 0.87 to 1.22; P = 0.72, I2 = 17%; 9 studies, 5883 participants) (Figure 8).
2.2. Analysis.

Comparison 2: Contraception use: message intervention versus control, Outcome 2: Unidirectional versus interactive message interventions
8.

We also performed a subgroup analysis comparing trials conducted in high‐income countries (Castano 2012; Francis 2015; Harrington 2019; Hebert 2018; Johnson 2017; Rinehart 2020), and low‐income countries (Babalola 2019; Biswas 2017; Brody 2022; McCarthy 2018; McCarthy 2019a; McCarthy 2020; Nuwamanya 2020; Reiss 2019; Smith 2015b; Unger 2018), as classified by World Bank income groups (Table 4). Lower‐ to middle‐income countries were grouped with lower income and upper‐ to middle‐income countries were grouped with high income. There was no difference between the two income‐setting groups (subgroup difference test: P = 0.70, I2 = 0%; Analysis 2.3). High‐income countries had an OR of 1.35 (95% CI 1.01 to 1.82; P = 0.05, I2 = 61%; 6 studies, 4276 participants) and low‐income countries had an OR of 1.24 (95% CI 0.91 to 1.70; P = 0.17, I2 = 74%; 10 studies, 4696 participants) (Figure 9).
3. Study income setting.
| Study | Country | World Bank income level classification | Classification for subgroup meta‐analysis |
| Babalola 2019 | Nigeria | Lower–middle income | Low income |
| Biswas 2017 | Bangladesh | Lower–middle income | Low income |
| Brody 2022 | Cambodia | Lower–middle income | Low income |
| Bull 2016 | USA | High income | High income |
| Castano 2012 | USA | High income | High income |
| Chernick 2017 | USA | High income | High income |
| Francis 2015 | USA | High income | High income |
| Harrington 2019 | Kenya | Lower–middle income | Low income |
| Hebert 2018 | USA | High income | High income |
| Hou 2010 | USA | High income | High income |
| Johnson 2017 | USA | High income | High income |
| McCarthy 2018 | Tajikistan | Lower–middle income | Low income |
| McCarthy 2019a | Palestine | Lower–middle income | Low income |
| McCarthy 2020 | Bolivia | Lower–middle income | Low income |
| Nuwamanya 2020 | Uganda | Low income | Low income |
| Reiss 2019 | Bangladesh | Lower–middle income | Low income |
| Rinehart 2020 | USA | High income | High income |
| Rokicki 2017 | Ghana | Low income | Low income |
| Smith 2015b | Cambodia | Lower–middle income | Low income |
| Trent 2013 | USA | High income | High income |
| Tsur 2008 | Israel | High income | High income |
| Unger 2018 | Kenya | Lower–middle income | Low income |
| Wilkinson 2017 | USA | High income | High income |
2.3. Analysis.

Comparison 2: Contraception use: message intervention versus control, Outcome 3: High‐ versus low‐income countries
9.

Sensitivity analysis
The pregnancy outcome was assessed using Peto OR. Whilst this method of analysis is most appropriate for the outcome, given the chance of zero events and it is more conservative, there had been two cluster‐RCTs within the analysis. To accommodate for the adjusted OR, as presented by the authors who adjusted for design effect of their cluster‐RCTs, we performed a sensitivity analysis using the generic inverse variance method for the outcome of pregnancy (Analysis 3.2). The effect on pregnancy among trials assessing mobile phone interventions was OR 0.70 (95% CI 0.43 to 1.16; P = 0.17, I2 = 0%; Figure 10). This sensitivity analysis did not differ from the Peto ORs method, with a similar OR and thus would not alter the conclusions of our analysis.
3.2. Analysis.

Comparison 3: Pregnancy: message intervention versus control, Outcome 2: Pregnancy – generic inverse variance
10.

Discussion
Contraception provides significant benefits for women's and children's health. However, the unmet need for contraception continues to exist across the world. This review examined the effect of a range of mobile phone interventions on contraception usage and unintended pregnancy. We included 23 RCTs in our analysis and pooled data for rates of contraception use and unintended pregnancy.
Summary of main results
This updated review reveals a growing body of evidence on interventions delivered by mobile phone to improve contraception use. We identified 18 additional trials from our search that we added to the five studies included in our previous review (Smith 2015b). Studies were conducted in 11 countries from both low‐ and high‐resource settings. Most trials recruited urban populations. Four trials assessed adherence or commencement to a specific method of contraception and 19 measured adherence or commencement to more than one method. We summarised our main conclusions on the comparison mobile phone contraception versus control group for the following two key outcomes: contraception use and unintended pregnancy.
Contraception use
Mobile phone‐based interventions probably increase contraception use compared to the control (OR 1.30, 95% CI 1.06 to 1.60; P = 0.01, I2 = 69%; 16 studies, 8972 participants). These results suggest a positive effect of mobile phone‐based interventions (one‐way text messaging, two‐way text messaging, voice messages and app‐based).
However, statistical heterogeneity was substantial, with mixed directional and magnitudinal effects reported from trials as seen by inspection of forest plots, so results must be interpreted with caution.
We explored heterogeneity through subgroup analysis. Subgroup analysis assessing unidirectional mobile phone interventions versus interactive mobile phone interventions (two‐way text messaging, interactive voice messages and app‐based) found evidence of a difference between the subgroups favouring interactive interventions (test for subgroup differences P = 0.003, I2 = 88.5%). Interactive interventions had an OR of 1.71 (95% CI 1.28 to 2.29; P = 0.0003, I2 = 63%; 3089 participants), whilst unidirectional interventions had an OR of 1.03 (95% CI 0.87 to 1.22; P = 0.72, I2 = 17%; 9 trials, 5883 participants).
We also assessed if income setting was a contributing factor to heterogeneity by comparing high‐income countries with low‐income countries (according to World Bank definitions). There was no difference between income subgroups with regard to effect outcome, suggesting mobile phone‐based interventions may not be impacted by income setting.
Pregnancy
We assessed the incidence of unintended pregnancy with the use of mobile phone‐based interventions. We found no difference between groups in the incidence of unintended pregnancy (OR 0.82, 95% CI 0.48 to 1.38; P = 0.45, I2 = 0%; 8 studies, 2947 participants; 2 studies reported pregnancy but recorded 0 events in both groups; thus, the OR and CIs were calculated from 6 studies rather than 8). There was no heterogeneity in this analysis, but studies included had been of moderate‐certainty evidence.
Six studies reported potential adverse effects of the intervention. Four studies did not report any differences in physical violence experienced from being in the intervention group; however one study reported the converse, where there was physical intimate partner violence noted in the intervention group. One study reported no evidence of road traffic accidents, as an adverse effect of mobile phone usage, and another highlighted potential privacy concerns with the interventions.
Overall completeness and applicability of evidence
The available evidence suggests that interventions delivered by mobile phone have the potential to improve contraception use. Whilst better outcomes in usage of contraception were noted amongst the groups who were randomised to mobile phone interventions, the studies included were heterogeneous and evidence amongst subpopulations was mixed. This makes it difficult to draw conclusions about the overall effect of the interventions.
Due to the variability of the types of intervention used, there was insufficient evidence for us to make recommendations on the frequency of communications to improve contraception use. However, it appears interactive mobile phone interventions have a better effect on contraception use compared to unidirectional mobile phone interventions.
There was no evidence of any differences noted with mobile phone‐based interventions compared to control groups on pregnancy outcomes. Few studies reported the outcome and as the outcome was rare during follow‐up periods, it limited the ability to detect an effect in the evidence we reviewed.
However, there are several critical elements that need to explored further prior to design and implementation of interventions.
First, only six studies assessed the potential for unintended effects. The increase in partner violence and high number of messages viewed by others without the participant's consent reported in two trials pose serious concerns. Interventions must be designed with confidentiality and safety in mind (Bacchus 2019).
Second, there is limited evidence on the cost‐effectiveness of these interventions. A cost‐effectiveness analysis was subsequently reported for Smith 2013, reporting that the intervention lies within the estimated range of the cost‐effectiveness threshold for Cambodia (Hill 2020). None of the other studies presented data on the cost of the intervention; although, we may have missed some cost‐effectiveness analyses since studies may have reported this information in separate publications that did not meet our inclusion criteria.
Third, the duration of follow‐up in the included trials ranged between three and 12 months, and the long‐term effect of these interventions is unclear.
Fourth, these interventions would also require adaptation for different populations and settings. It is unclear which behaviour change techniques, or combinations of, are effective. The lack of behavioural change theory underpinning some interventions was a limitation across included studies. We used Abraham and Michie's typology of behaviour change techniques to code intervention content according to the intervention description provided in the papers or in protocols, which varied in the level of detail provided (Abraham 2008). Coding of the intervention content could have been more comprehensive if additional detail on intervention messages, and the development of such messages, had been reported.
Finally, our review did not include studies that aimed to increase contraceptive knowledge alone. Interventions that increase knowledge of contraception may or may not lead to increased uptake and adherence.
Quality of the evidence
We summarised the certainty of the evidence for each study in Table 3 using the GRADE approach. Overall there was moderate‐certainty evidence but individual studies ranged from very low‐ to high‐certainty evidence as depicted in Figure 3. We consider further research is likely to have an impact on our confidence in the estimate of effect, and may also change the estimate.
Performance bias may have risen from altered behaviour of participants based on allocation to the intervention or control group as it is not possible to blind the participant due to the nature of the interventions. Detection bias may have risen as a result of lack of outcome assessment blinding, which was not apparent in all the trials. Furthermore, bias may have arisen from use of self‐report measures of contraception. A potentially culturally sensitive issue such as sexual health and contraception use may cause participants to report outcomes differently. No trials described using incentives for reporting increased use of contraception or not being pregnant.
Self‐reported measures are the standard in contraception research but have been shown to overestimate contraception use and underestimate abortion (Stuart 2009). Hou 2010 reported poorer oral contraception adherence measured using electronic medication monitoring compared with the participants' diaries. However, it should be considered that no gold standard measure of oral contraception use is available, and objective assessment is challenging, as biological measures such as hormonal assays do not indicate consistent use (Hall 2010). We also consider the self‐report of the outcome did not pose enough of a bias to make us less certain about the estimated association that was found, and thus did not warrant a further downgrade for certainty of evidence.
Participants randomly assigned to the intervention may have shared intervention content with participants assigned to control groups, resulting in contamination across study groups and a possible weakening of overall effect. None of the included trials reported on this.
Four trials, all of which found no effect, included small sample sizes, which increased the possibility of Type II errors (Hou 2010; Trent 2013; Tsur 2008; Wilkinson 2017).
Potential biases in the review process
We have attempted to minimise bias as much as possible during the review process. We conducted a systematic search of the literature for RCTs. While our search strategy was comprehensive and included several databases, trial registries and reference lists of included trials, we only included published RCTs and did not include other types of study. No language or publication status limits were applied. We contacted authors of included studies to obtain additional information when required.
We adhered to Cochrane methods of searching, data extraction, appraisal and analysis throughout the review process (Higgins 2019). We made no deviations from our trial protocol and followed all our proposed methodology (Smith 2014).
We explored heterogeneity in subgroup analysis, but we could not fully explain the variations in effect of mobile phone intervention on contraception use. High levels of heterogeneity in some subgroup analysis suggests there may be other factors, or a combination of factors, beyond those we considered and were able to analyse.
Agreements and disagreements with other studies or reviews
This systematic review provides an update to the evidence on the effectiveness of mobile phone‐based interventions to improve contraception use with an additional 18 studies. This updated evidence on the effectiveness of interventions delivered by mobile phone on contraception use appears to be positive, however with uncertainty in quality of results.
Overall, it appears that there is a positive effect of interventions delivered by mobile phone on contraception use compared to controls. However, there was substantial variation between the trials. Subgroup analysis of interventions using interactive messages found a significant effect, which was echoed in evidence from systematic reviews of digital health and general adherence research suggests that more complex, multifaceted interventions are more effective than simple interventions such as simple text message reminders (Free 2013; Haynes 2008; Shet 2014).
The finding that unidirectional simple text message reminders had no effect is consistent with one review of unidirectional text messages in Africa, which found no effect of these on medication adherence (although unidirectional messages did appear effective for increasing appointment attendance) (Linde 2019).
Interventions for different conditions should be compared with caution, as it is likely that factors influencing contraception use will be different from those influencing other behaviours such as adherence to antiretroviral therapy or smoking cessation. However, mobile phone‐based interventions for HIV medication adherence are similar to those for contraception in the respect that they include populations for which confidentiality and privacy are of particular importance and involves similar behaviours (i.e. taking a tablet, adherence to medication).
Several reviews have now reported significant effects of various digital health interventions, include via mobile phones, on increasing adherence to antiretroviral therapy (Amankwaa 2018; Daher 2017; Horvath 2012; Rooks‐Peck 2019; Wang 2019), although some found borderline (Cooper 2017; Taylor 2019), or mixed effects (Shah 2019), depending on intervention type. However, personal motivation and support for taking antiretroviral therapy may be quite different to motivation and support for taking short‐acting forms of contraception. Nonetheless, our results and those of mobile‐phone‐based interventions for HIV medication adherence indicate the likelihood that these types of intervention may be effective at least in some circumstances.
One recent Cochrane Review of targeted client communication via mobile devices for improving sexual and reproductive health was consistent with our findings that interventions may improve some outcomes but evidence was of low certainty (Palmer 2020).
Similar to one review of sexual health interventions (Burns 2016), there was diversity amongst the studies in primary outcomes, approaches used and population groups reached. This limited the number of studies that could be included in the meta‐analyses, particularly subgroup analyses. Potentially once additional studies are conducted and the meta‐analyses repeated it will be possible to make a clearer determination of the effect of different types of mobile phone interventions to increase uptake of different types of contraception use amongst different population groups, particularly the borderline findings. Regardless, it does appear that even if interventions are found to be effective, the effect sizes are relatively small. However, this may still translate into a substantial impact at a population level.
Similar to other reviews across different health conditions where few studies evaluated the cost of mobile phone interventions, the trials included in our review did not include data on cost‐effectiveness. This information is important to the feasibility of integrating these interventions into the overall health service delivery systems, and the scale‐up of these interventions (Cooper 2017; de la Torre‐Diez 2015).
Authors' conclusions
Implications for practice.
This review demonstrates there is evidence to support mobile phone‐based interventions to increase the use of contraception with moderate‐certainty evidence. Further good‐quality research is likely to have an impact on our confidence in the estimate of effect.
Interactive interventions appear more effective than unidirectional mobile phone‐based interventions at improving use of contraception. We are uncertain of the effect of mobile phone‐based interventions on unintended pregnancy.
The cost‐effectiveness, cost benefits, safety and long‐term effects of these interventions remain unknown, as does the evidence of this approach to support contraception use amongst specific populations.
Interventions delivered by mobile phone should be integrated and evaluated as part of the wider health service delivery system. Future mobile phone‐based interventions should consider the context and needs of different population groups, for example, literacy, place of residence, phone use, use of other services and what behaviour change techniques delivered by mobile phone are likely to be effective. There must also be robust consideration and mitigation of potential harms as part of the intervention design process. For some populations and interventions, the risk of harms may outweigh the potential benefits of the intervention, and thus planned interventions should not be implemented.
Implications for research.
Better quality trials may further help establish the effects of interventions delivered by mobile phone on contraception use. This review, despite a positive association with improving contraception use, is limited by the quality of the studies due to flaws in methodology, bias or imprecision of results. Interactive interventions, compared to unidirectional interventions, are more effective at improving contraceptive use. Future researchers assessing mobile phone interventions may find focussing on interventions with interactivity likely to be more effective.
Once additional studies are conducted and meta‐analyses repeated, it will be possible to make a clearer determination of the effect of different types of mobile phone interventions to increase uptake of different types of contraceptive use amongst different population groups.
No studies to date have been powered to determine the impact on rates of pregnancy and abortion. Trials should be grounded by a clear rationale regarding the barriers to contraception use that the intervention targets, use of behavioural theory and complemented by process evaluations to enhance understanding of the mechanism that explains why a certain intervention works or does not work. The cost‐effectiveness of effective interventions should also be examined.
In areas where interventions have yielded inconclusive evidence, such as fully automated text message interventions for oral contraception adherence, future research should focus on improving interventions through pilot studies before considering evaluation by randomised controlled trials. Interventions that aim to improve use of a single contraceptive method should consider additional facilitation of safe method switching, given that adverse effects and health concerns leading to discontinuation are common.
Consideration should be given to the choice and timeline of outcomes measured. Use of consistent outcome measures would allow pooling of results and meta‐analysis in future reviews, which could yield more conclusive evidence on the topic. Objective measures to assess contraception use should be used if feasible. If self‐reported measures are used, questions should be carefully considered reducing the likelihood of bias. Measures of unintended consequences, such as partner violence, also need to be ubiquitously included.
Interventions should be integrated and evaluated as part of the health service delivery model and factors such as cost‐benefit, feasibility and efficiency should be taken into account along with effectiveness measures. Where health management information systems are robust, future trials should consider randomisation of mobile health interventions using existing client databases for better tracking and efficiency.
What's new
| Date | Event | Description |
|---|---|---|
| 14 July 2023 | New citation required and conclusions have changed | New citation required and conclusions have changed with the inclusion of updated evidence |
| 14 July 2023 | New search has been performed | New search has been performed, August 2022 |
History
Protocol first published: Issue 6, 2014 Review first published: Issue 6, 2015
| Date | Event | Description |
|---|---|---|
| 5 December 2021 | Amended | Added 5 new trials (Babalola 2019; Brody 2022; McCarthy 2020; Nuwamanya 2020; Rinehart 2020) |
| 1 November 2019 | Amended | Added 13 new trials (Biswas 2017; Bull 2016; Chernick 2017; Francis 2015; Harrington 2019; Hebert 2018; Johnson 2017; McCarthy 2018; McCarthy 2019a; Reiss 2019; Rokicki 2017; Unger 2018; Wilkinson 2017) |
Acknowledgements
Cochrane Fertility Regulation Group Information Specialists for assistance with the search strategy and implementing the searches.
The Cochrane Fertility Regulation Group editors, in particular Jillian Henderson, Alison Edelman and Makalapua Motu’apuaka, for their prompt responses providing assistance.
Judy Gold was an author on the initial review and made some edits and commented on the 'Introduction' section of this update.
Thoai Ngo was an author on the initial review and made some edits and commented on the 'Discussion' section of this update.
Colin Sumpter was an author on the initial review and advised on the behaviour change techniques coding for this update.
Atsuko Ito assisted with screening and data extraction.
Appendices
Appendix 1. Update search strategies
Cochrane Fertility Regulation Specialised Register (CRS Web)
Date last searched: 18 August 2022 1 INREGISTER (6629) 2 phone* OR telephone* OR "mobile device*" OR smartphone* OR smart‐phone* OR mhealth OR m‐health OR e‐health* OR ehealth* OR app OR apps OR mms OR "multimedia messag* service" OR sms OR "short messag* service" OR text* OR messag* (386) 3 2014 OR 2015 OR 2016 OR 2017 OR 2018 OR 2019 (1460) 4 #2 and #3 (196)
Cochrane Central Register of Controlled Trials (Ovid EBM Reviews) July 2022
Date last searched: 18 August 2022
1 (contraceptive or contraceptives or contraception or immunocontracept* or immuno‐contracept* or anti‐fertility or antifertility or anticonception or anti‐conception or birth‐control or contraceptif* or anticonceptiv* or anticoncepcion* or anti‐concepcion* or empfangnisverhetung or verhutungsmittel or ((child or birth*) adj2 (limiting or spacing or space or spaced)) or (delay* adj2 (childbearing or child‐bearing)) or ((control* or inhibit* or prevent* or regulat* or suppress*) adj2 (ovulat* or fertili* or pregnan* or concept* or reproduct*)) or noncontracept* or non‐contracept* or pre‐contracept* or post‐contracept*).ti,ab. (17743) 2 (((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after) adj (pill or pills)) or antiovulat* or anti‐ovulat* or ((inhibit* or suppress*) adj2 ovulat*)).ti,ab. or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*)).ti. (344) 3 (Algestone‐acetophenide or Algestoneacetophenide or Centchroman or Chlormadinone‐acetate or Chlormadinoneacetate or Cyproterone‐acetate or Cyproteroneacetate or Desogestrel or Dienogest or Dimethisterone or Dinoprost or Dinoprost‐tromethamine or Drospirenone or Ergonovine or Ergotamine or Estradiol‐benzoate or "estradiol 3‐benzoate" or Oestradiol‐benzoate or Estradiolbenzoate or Oestradiolbenzoate or Estradiol‐enanthate or Oestradiol‐enanthate or Estradiolenanthate or Oestradiolenanthate or Estradiol‐valerate or Oestradiol‐valerate or Estradiolvalerate or Oestradiolvalerate or Ethynodiol‐diacetate or Ethynodioldiacetate or Ethinyl‐estradiol or Ethinylestradiol or Etonogestrel or Gestodene or Gestrinone or Gossypol or Infecundin or Levonorgestrel or Lynestrenol or Medroxyprogesterone or Medroxyprogesterone‐acetate or Medroxyprogesteroneacetate or Megestrol or Mestranol or Methylergonovine or Nomegestrol or Nomegestrol‐acetate or Nomegestrolacetate or Nonoxynol‐9 or Norelgestromin or Norethindrone or Norethindrone‐Acetate or Norethindroneacetate or Norethisterone‐acetate or Norethisteroneacetate or Norethynodrel or Norgestimate or Norgestrel or Norgestrienone or Sparteine or Trichosanthin or Ulipristal‐Acetate or Ulipristalacetate).ti,ab. (7502) 4 (cervical‐cap* or estrogen‐ring* or ((intravaginal or intra‐vaginal or vaginal) adj2 (barrier or barriers or cap or caps or creams or creams or device or devices or foam or foams or gel or gels or ring or rings or shield or shields or sponge or sponges or suppositor* or tablet*)) or ((arcing‐spring or coil‐spring or flat‐spring or latex or silicone or intra‐vaginal* or intravaginal or vaginal) adj2 diaphragm*) or ((etonogestrel* or ETG or Progestogen* or levonorgestrel) adj3 (capsule* or implant* or rod or rods)) or ((intrauterine or intra‐uterine) adj2 (ball or balls or coil or coils or device or devices or system or systems)) or IUD or IUDs or IUCD or IUCDs or Cu‐IUD or Cu‐IUDs or LNG‐IUD or LNG‐IUDs or IUS or IUSs or progestasert).ti,ab. (5589) 5 (condom or condoms).ti,ab. (2979) 6 (spermicide or spermicides or spermicidal or spermatocidal or ((immobilizing or immobilising or blocking or inhibiting or suppressing) adj3 sperm*) or ((nonoxynol‐9 or N‐9 or conceptrol or octoxynol‐9) adj2 (cream or creams or film or films or foam or foams or gel or gels))).ti,ab. (710) 7 ("family planning" or "planned parenthood").ti,ab. (1071) 8 ("basal body temperature method" or "Billings Method" or "calendar method" or "cervical mucus method" or "Couple Beads" or "fertility awareness method*" or "fertility awareness‐based" or "fertility regulation method" or ((lactation* or postpartum or post‐partum) adj2 amenorrh*) or "ovulation method" or "standard days method" or "symptothermal method" or "sympto‐thermal method" or "TwoDay method" or "Two‐day method").ti,ab. (81) 9 or/1‐8 (27089) 10 (((cell or cellular or google or mobile or nexus) adj2 (device* or phone* or technolog*)) or smartphone or smartphones or smart‐phone or smart‐phones or iphone or iphones or blackberr* or black‐berr* or app or apps or application or text or texts or texting or message or messages or messaging or (phone adj call*) or ehealth* or e‐health* or mhealth or m‐health or ((electronic or mobile) adj2 health*) or MMS or SMS or IVR or "interactive voice‐response" or (digitial adj3 health) or (digital adj3 healthcare)).ti,ab. (81185) 11 and/9‐10 (1720) 12 limit 11 to yr="2014 ‐Current" (980)
MEDLINE ALL (Ovid) 1946 to 17 August 2022 Date last searched: 18 August 2022
1 contraception/ or contraception behavior/ (24573) 2 (contraceptive or contraceptives or contraception or immunocontracept* or immuno‐contracept* or anti‐fertility or antifertility or anticonception or anti‐conception or birth‐control or contraceptif* or anticonceptiv* or anticoncepcion* or anti‐concepcion* or empfangnisverhetung or verhutungsmittel or ((child or birth*) adj2 (limiting or spacing or space or spaced)) or (delay* adj2 (childbearing or child‐bearing)) or ((control* or inhibit* or prevent* or regulat* or suppress*) adj2 (ovulat* or fertili* or pregnan* or concept* or reproduct*)) or noncontracept* or non‐contracept* or pre‐contracept* or post‐contracept*).tw,kf. (110645) 3 contraceptive agents/ (4774) 4 contraceptive agents, female/ or contraceptives, oral/ or contraceptives, oral, hormonal/ or contraceptives, oral, combined/ or contraceptives, oral, sequential/ or contraceptives, oral, synthetic/ or contraception, immunologic/ or vaccines, contraceptive/ or ovulation inhibition/ (35784) 5 (((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after) adj (pill or pills)) or antiovulat* or anti‐ovulat* or ((inhibit* or suppress*) adj2 ovulat*)).tw,kf. or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*)).ti. (2955) 6 contraceptives, postcoital/ or contraceptives, postcoital, synthetic/ or contraceptives, postcoital, hormonal/ (2234) 7 Algestone Acetophenide/ or Centchroman/ or Chlormadinone Acetate/ or Cyproterone Acetate/ or Desogestrel/ or Dimethisterone/ or Ethinyl Estradiol/ or Ethinyl Estradiol‐Norgestrel Combination/ or Ethynodiol Diacetate/ or Gestrinone/ or Gossypol/ or Levonorgestrel/ or Lynestrenol/ or Medroxyprogesterone/ or Medroxyprogesterone Acetate/ or Megestrol/ or Mestranol/ or Metaproterenol/ or Methylergonovine/ or Norethindrone/ or Norethynodrel/ or Norgestrel/ or Norgestrienone/ (31157) 8 (Algestone‐acetophenide or Algestoneacetophenide or Centchroman or Chlormadinone‐acetate or Chlormadinoneacetate or Cyproterone‐acetate or Cyproteroneacetate or Desogestrel or Dienogest or Dimethisterone or Dinoprost or Dinoprost‐tromethamine or Drospirenone or Ergonovine or Ergotamine or Estradiol‐benzoate or "estradiol 3‐benzoate" or Oestradiol‐benzoate or Estradiolbenzoate or Oestradiolbenzoate or Estradiol‐enanthate or Oestradiol‐enanthate or Estradiolenanthate or Oestradiolenanthate or Estradiol‐valerate or Oestradiol‐valerate or Estradiolvalerate or Oestradiolvalerate or Ethynodiol‐diacetate or Ethynodioldiacetate or Ethinyl‐estradiol or Ethinylestradiol or Etonogestrel or Gestodene or Gestrinone or Gossypol or Infecundin or Levonorgestrel or Lynestrenol or Medroxyprogesterone or Medroxyprogesterone‐acetate or Medroxyprogesteroneacetate or Megestrol or Mestranol or Methylergonovine or Nomegestrol or Nomegestrol‐acetate or Nomegestrolacetate or Nonoxynol‐9 or Norelgestromin or Norethindrone or Norethindrone‐Acetate or Norethindroneacetate or Norethisterone‐acetate or Norethisteroneacetate or Norethynodrel or Norgestimate or Norgestrel or Norgestrienone or Sparteine or Trichosanthin or Ulipristal‐Acetate or Ulipristalacetate).tw,kf,nm. (62500) 9 or/1‐8 (171678) 10 contraception, barrier/ or contraceptive devices/ (1501) 11 contraceptive devices, female/ or condoms, female/ or intrauterine devices/ or intrauterine devices, medicated/ or intrauterine devices, copper/ or "Long‐Acting Reversible Contraception"/ (13847) 12 (cervical‐cap* or estrogen‐ring* or ((intravaginal or intra‐vaginal or vaginal) adj2 (barrier or barriers or cap or caps or creams or creams or device or devices or foam or foams or gel or gels or ring or rings or shield or shields or sponge or sponges or suppositor* or tablet*)) or ((arcing‐spring or coil‐spring or flat‐spring or latex or silicone or intra‐vaginal* or intravaginal or vaginal) adj2 diaphragm*) or ((etonogestrel* or ETG or Progestogen* or levonorgestrel) adj3 (capsule* or implant* or rod or rods)) or ((intrauterine or intra‐uterine) adj2 (ball or balls or coil or coils or device or devices or system or systems)) or IUD or IUDs or IUCD or IUCDs or Cu‐IUD or Cu‐IUDs or LNG‐IUD or LNG‐IUDs or IUS or IUSs or progestasert).tw,kf,nm. (20138) 13 contraceptive devices, male/ or condoms/ (11354) 14 (condom or condoms).tw,kf. (21014) 15 sperm immobilizing agents/ (127) 16 (spermicide or spermicides or spermicidal or spermatocidal or ((immobilizing or immobilising or blocking or inhibiting or suppressing) adj3 sperm*) or ((nonoxynol‐9 or N‐9 or conceptrol or octoxynol‐9) adj2 (cream or creams or film or films or foam or foams or gel or gels))).tw,kf. (2756) 17 or/10‐16 (47350) 18 family planning services/ or natural family planning methods/ or International Planned Parenthood Federation/ (25272) 19 ("family planning" or "planned parenthood").ti,ab,kf. (42143) 20 ("basal body temperature method" or "Billings Method" or "calendar method" or "cervical mucus method" or "Couple Beads" or "fertility awareness method*" or "fertility awareness‐based" or "fertility regulation method" or ((lactation* or postpartum or post‐partum) adj2 amenorrh*) or "ovulation method" or "standard days method" or "symptothermal method" or "sympto‐thermal method" or "TwoDay method" or "Two‐day method").tw,kf. (1279) 21 or/18‐20 (49719) 22 or/9,17,21 (212383) 23 cell Phone/ or Smartphone/ or Text Messaging/ or Computers, Handheld/ or Telephone/ or Telemedicine/ (52298) 24 (((cell or cellular or google or mobile or nexus) adj2 (device* or phone* or technolog*)) or smartphone or smartphones or smart‐phone or smart‐phones or iphone or iphones or blackberr* or black‐berr* or app or apps or application or text or texts or texting or message or messages or messaging or (phone adj call*) or ehealth* or e‐health* or mhealth or m‐health or ((electronic or mobile) adj2 health*) or MMS or SMS or IVR or "interactive voice‐response" or (digitial adj3 health) or (digital adj3 healthcare)).ti,ab,kf. (1052144) 25 or/23‐24 (1082619) 26 and/22,25 (7914) 27 randomized controlled trial.pt. (517352) 28 controlled clinical trial.pt. (93935) 29 (randomised or randomized).ti,ab. (644341) 30 placebo.ab. (212699) 31 drug therapy.fs. (2252182) 32 randomly.ab. (345164) 33 trial.ab. (528019) 34 groups.ab. (2117783) 35 or/27‐34 (4870191) 36 (exp animals/ not humans/) or (bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or feline or goat or goats or horse or mice or mouse or ovine or pig or pigs or porcine or rabbit or rabbits or rat or rats or rattus or sheep or sow or sows).tw,kf. (579170437) 35 not 36 (405320638) 36 and/26,37 (191239) 37 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dt. (7105477) 40 38 and 39 (587)
Embase.com
Date last searched: 18 August 2022
#1 'contraception'/mj OR 'contraceptive behavior'/exp/mj (29,694) #2 contraceptive:ti,ab,kw OR contraceptives:ti,ab,kw OR contraception:ti,ab,kw OR immunocontracept*:ti,ab,kw OR 'immuno contracept*':ti,ab,kw OR 'anti fertility':ti,ab,kw OR antifertility:ti,ab,kw OR anticonception:ti,ab,kw OR 'anti conception':ti,ab,kw OR 'birth control':ti,ab,kw OR contraceptif*:ti,ab,kw OR anticonceptiv*:ti,ab,kw OR anticoncepcion*:ti,ab,kw OR 'anti concepcion*':ti,ab,kw OR empfangnisverhetung:ti,ab,kw OR verhutungsmittel:ti,ab,kw OR (((child OR birth*) NEAR/2 (limiting OR spacing OR space OR spaced)):ti,ab,kw) OR ((delay* NEAR/2 (childbearing OR 'child bearing')):ti,ab,kw) OR (((control* OR inhibit* OR prevent* OR regulat* OR suppress*) NEAR/2 (ovulat* OR fertili* OR pregnan* OR concept* OR reproduct*)):ti,ab,kw) OR noncontracept*:ti,ab,kw OR 'non contracept*':ti,ab,kw OR 'pre contracept*':ti,ab,kw OR 'post contracept*':ti,ab,kw (127,646) #3 'contraceptive agent'/mj (9,049) #4 'hormonal contraceptive agent'/exp/mj OR 'injectable contraceptive agent'/exp/mj OR 'male contraceptive agent'/exp/mj OR 'oral contraceptive agent'/exp/mj OR 'long‐acting reversible contraception'/exp/mj (62,401) #5 (((monophasic OR 'mono phasic' OR biphasic OR 'bi phasic' OR triphasic OR 'tri phasic' OR quadriphasic OR 'quadri phasic' OR multiphasic OR 'multi phasic' OR normophasic OR 'normo phasic' OR minidose OR 'mini dose' OR 'morning after') NEAR/1 (pill OR pills)):ti,ab,kw) OR antiovulat*:ti,ab,kw OR 'anti ovulat*':ti,ab,kw OR (((inhibit* OR suppress*) NEAR/2 ovulat*):ti,ab,kw) OR ((('first generation' OR '1st generation' OR 'second generation' OR '2nd generation' OR 'third generation' OR '3rd generation' OR 'fourth generation' OR '4th generation') NEAR/2 (pill OR pills OR progest*)):ti) (3,194) #6 'postcoitus contraceptive agent'/exp/mj (26,720) #7 'algestone acetophenide':ti,ab,kw OR algestoneacetophenide:ti,ab,kw OR centchroman:ti,ab,kw OR 'chlormadinone acetate':ti,ab,kw OR chlormadinoneacetate:ti,ab,kw OR 'cyproterone acetate':ti,ab,kw OR cyproteroneacetate:ti,ab,kw OR desogestrel:ti,ab,kw OR dienogest:ti,ab,kw OR dimethisterone:ti,ab,kw OR dinoprost:ti,ab,kw OR 'dinoprost tromethamine':ti,ab,kw OR drospirenone:ti,ab,kw OR ergonovine:ti,ab,kw OR ergotamine:ti,ab,kw OR 'estradiol benzoate':ti,ab,kw OR 'estradiol 3‐benzoate':ti,ab,kw OR 'oestradiol benzoate':ti,ab,kw OR estradiolbenzoate:ti,ab,kw OR oestradiolbenzoate:ti,ab,kw OR 'estradiol enanthate':ti,ab,kw OR 'oestradiol enanthate':ti,ab,kw OR estradiolenanthate:ti,ab,kw OR oestradiolenanthate:ti,ab,kw OR 'estradiol valerate':ti,ab,kw OR 'oestradiol valerate':ti,ab,kw OR estradiolvalerate:ti,ab,kw OR oestradiolvalerate:ti,ab,kw OR 'ethynodiol diacetate':ti,ab,kw OR ethynodioldiacetate:ti,ab,kw OR 'ethinyl estradiol':ti,ab,kw OR ethinylestradiol:ti,ab,kw OR etonogestrel:ti,ab,kw OR gestodene:ti,ab,kw OR gestrinone:ti,ab,kw OR gossypol:ti,ab,kw OR infecundin:ti,ab,kw OR levonorgestrel:ti,ab,kw OR lynestrenol:ti,ab,kw OR medroxyprogesterone:ti,ab,kw OR 'medroxyprogesterone acetate':ti,ab,kw OR medroxyprogesteroneacetate:ti,ab,kw OR megestrol:ti,ab,kw OR mestranol:ti,ab,kw OR methylergonovine:ti,ab,kw OR nomegestrol:ti,ab,kw OR 'nomegestrol acetate':ti,ab,kw OR nomegestrolacetate:ti,ab,kw OR 'nonoxynol 9':ti,ab,kw OR norelgestromin:ti,ab,kw OR norethindrone:ti,ab,kw OR 'norethindrone acetate':ti,ab,kw OR norethindroneacetate:ti,ab,kw OR 'norethisterone acetate':ti,ab,kw OR norethisteroneacetate:ti,ab,kw OR norethynodrel:ti,ab,kw OR norgestimate:ti,ab,kw OR norgestrel:ti,ab,kw OR norgestrienone:ti,ab,kw OR sparteine:ti,ab,kw OR trichosanthin:ti,ab,kw OR 'ulipristal acetate':ti,ab,kw OR ulipristalacetate:ti,ab,kw (44,462) #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 (196,615) #9 'barrier contraception'/exp/mj OR 'vagina contraception'/exp/mj OR 'female contraceptive device'/mj OR 'birth control implant'/exp/mj OR 'contraceptive patch'/mj OR 'contraceptive sponge'/mj OR 'female condom'/mj OR 'intrauterine contraceptive device'/exp/mj OR 'uterine cervix cap'/mj OR 'vagina ring'/mj (13,270) #10 'cervical cap*':ti,ab,kw OR 'estrogen ring*':ti,ab,kw OR (((intravaginal OR 'intra vaginal' OR vaginal) NEAR/2 (barrier OR barriers OR cap OR caps OR creams OR creams OR device OR devices OR foam OR foams OR gel OR gels OR ring OR rings OR shield OR shields OR sponge OR sponges OR suppositor* OR tablet*)):ti,ab,kw) OR ((('arcing spring' OR 'coil spring' OR 'flat spring' OR latex OR silicone OR 'intra vaginal*' OR intravaginal OR vaginal) NEAR/2 diaphragm*):ti,ab,kw) OR (((etonogestrel* OR etg OR progestogen* OR levonorgestrel) NEAR/3 (capsule* OR implant* OR rod OR rods)):ti,ab,kw) OR (((intrauterine OR 'intra uterine') NEAR/2 (ball OR balls OR coil OR coils OR device OR devices OR system OR systems)):ti,ab,kw) OR iud:ti,ab,kw OR iuds:ti,ab,kw OR iucd:ti,ab,kw OR iucds:ti,ab,kw OR 'cu iud':ti,ab,kw OR 'cu iuds':ti,ab,kw OR 'lng iud':ti,ab,kw OR 'lng iuds':ti,ab,kw OR ius:ti,ab,kw OR iuss:ti,ab,kw OR progestasert:ti,ab,kw (23,893) #11 'male contraceptive device'/mj OR 'condom'/mj (4,866) #12 condom:ti,ab,kw OR condoms:ti,ab,kw (23,884) #13 'spermicidal agent'/exp/mj (5,290) #14 spermicide:ti,ab,kw OR spermicides:ti,ab,kw OR spermicidal:ti,ab,kw OR spermatocidal:ti,ab,kw OR (((immobilizing OR immobilising OR blocking OR inhibiting OR suppressing) NEAR/3 sperm*):ti,ab,kw) OR ((('nonoxynol 9' OR 'n 9' OR conceptrol OR 'octoxynol 9') NEAR/2 (cream OR creams OR film OR films OR foam OR foams OR gel OR gels)):ti,ab,kw) (2,256) #15 'family planning'/exp/mj (15,806) #16 'family planning':ti,ab,kw OR 'planned parenthood':ti,ab,kw (21,315) #17 'basal body temperature method':ti,ab,kw OR 'billings method':ti,ab,kw OR 'calendar method':ti,ab,kw OR 'cervical mucus method':ti,ab,kw OR 'couple beads':ti,ab,kw OR 'fertility awareness method*':ti,ab,kw OR 'fertility awareness‐based':ti,ab,kw OR 'fertility regulation method':ti,ab,kw OR (((lactation* OR postpartum OR 'post partum') NEAR/2 amenorrh*):ti,ab,kw) OR 'ovulation method':ti,ab,kw OR 'standard days method':ti,ab,kw OR 'symptothermal method':ti,ab,kw OR 'sympto‐thermal method':ti,ab,kw OR 'twoday method':ti,ab,kw OR 'two‐day method':ti,ab,kw (1,123) #18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 (245,617) #19 'mobile phone'/mj OR 'smartphone'/mj OR 'text messaging'/mj OR 'telephone'/mj OR 'telehealth'/mj OR 'telemedicine'/exp/mj OR 'telenursing'/mj (46,485) #20 (((cell OR cellular OR google OR mobile OR nexus) NEAR/2 (device* OR phone* OR technolog*)):ti,ab,kw) OR smartphone:ti,ab,kw OR smartphones:ti,ab,kw OR 'smart phone':ti,ab,kw OR 'smart phones':ti,ab,kw OR iphone:ti,ab,kw OR iphones:ti,ab,kw OR blackberr*:ti,ab,kw OR 'black berr*':ti,ab,kw OR app:ti,ab,kw OR apps:ti,ab,kw OR application:ti,ab,kw OR text:ti,ab,kw OR texts:ti,ab,kw OR texting:ti,ab,kw OR message:ti,ab,kw OR messages:ti,ab,kw OR messaging:ti,ab,kw OR 'phone adj call*':ti,ab,kw OR ehealth*:ti,ab,kw OR 'e health*':ti,ab,kw OR mhealth:ti,ab,kw OR 'm health':ti,ab,kw OR (((electronic OR mobile) NEAR/2 health*):ti,ab,kw) OR mms:ti,ab,kw OR sms:ti,ab,kw OR ivr:ti,ab,kw OR 'interactive voice‐response':ti,ab,kw OR ((digitial NEAR/3 health):ti,ab,kw) OR ((digital NEAR/3 healthcare):ti,ab,kw) (1,289,094) #21 #19 OR #20 (1,314,696) #22 #18 AND #21 (9,003) #23 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti (2,651,191) #24 #22 AND #23 (1,551) #25 #24 NOT (([animal cell]/lim OR [animal experiment]/lim OR [animal model]/lim OR [animal tissue]/lim) NOT [humans]/lim) (1,480) #26 #25 AND (2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py) (662)
APA PsycInfo (Ovid) 1806 to August week 3 2022
Date last searched: 18 August 2022
1 Birth Control/ or Oral Contraceptives/ (4428) 2 (contraceptive or contraceptives or contraception or immunocontracept* or immuno‐contracept* or anti‐fertility or antifertility or anticonception or anti‐conception or birth‐control or contraceptif* or anticonceptiv* or anticoncepcion* or anti‐concepcion* or empfangnisverhetung or verhutungsmittel or ((child or birth*) adj2 (limiting or spacing or space or spaced)) or (delay* adj2 (childbearing or child‐bearing)) or ((control* or inhibit* or prevent* or regulat* or suppress*) adj2 (ovulat* or fertili* or pregnan* or concept* or reproduct*)) or noncontracept* or non‐contracept* or pre‐contracept* or post‐contracept*).ti,ab. (13904) 3 (((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after) adj (pill or pills)) or antiovulat* or anti‐ovulat* or ((inhibit* or suppress*) adj2 ovulat*)).ti,ab. or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*)).ti. (98) 4 (Algestone‐acetophenide or Algestoneacetophenide or Centchroman or Chlormadinone‐acetate or Chlormadinoneacetate or Cyproterone‐acetate or Cyproteroneacetate or Desogestrel or Dienogest or Dimethisterone or Dinoprost or Dinoprost‐tromethamine or Drospirenone or Ergonovine or Ergotamine or Estradiol‐benzoate or "estradiol 3‐benzoate" or Oestradiol‐benzoate or Estradiolbenzoate or Oestradiolbenzoate or Estradiol‐enanthate or Oestradiol‐enanthate or Estradiolenanthate or Oestradiolenanthate or Estradiol‐valerate or Oestradiol‐valerate or Estradiolvalerate or Oestradiolvalerate or Ethynodiol‐diacetate or Ethynodioldiacetate or Ethinyl‐estradiol or Ethinylestradiol or Etonogestrel or Gestodene or Gestrinone or Gossypol or Infecundin or Levonorgestrel or Lynestrenol or Medroxyprogesterone or Medroxyprogesterone‐acetate or Medroxyprogesteroneacetate or Megestrol or Mestranol or Methylergonovine or Nomegestrol or Nomegestrol‐acetate or Nomegestrolacetate or Nonoxynol‐9 or Norelgestromin or Norethindrone or Norethindrone‐Acetate or Norethindroneacetate or Norethisterone‐acetate or Norethisteroneacetate or Norethynodrel or Norgestimate or Norgestrel or Norgestrienone or Sparteine or Trichosanthin or Ulipristal‐Acetate or Ulipristalacetate).ti,ab. (2172) 5 Contraceptive Devices/ (911) 6 (cervical‐cap* or estrogen‐ring* or ((intravaginal or intra‐vaginal or vaginal) adj2 (barrier or barriers or cap or caps or creams or creams or device or devices or foam or foams or gel or gels or ring or rings or shield or shields or sponge or sponges or suppositor* or tablet*)) or ((arcing‐spring or coil‐spring or flat‐spring or latex or silicone or intra‐vaginal* or intravaginal or vaginal) adj2 diaphragm*) or ((etonogestrel* or ETG or Progestogen* or levonorgestrel) adj3 (capsule* or implant* or rod or rods)) or ((intrauterine or intra‐uterine) adj2 (ball or balls or coil or coils or device or devices or system or systems)) or IUD or IUDs or IUCD or IUCDs or Cu‐IUD or Cu‐IUDs or LNG‐IUD or LNG‐IUDs or IUS or IUSs or progestasert).ti,ab. (749) 7 Condoms/ (4026) 8 (condom or condoms).ti,ab. (9903) 9 (spermicide or spermicides or spermicidal or spermatocidal or ((immobilizing or immobilising or blocking or inhibiting or suppressing) adj3 sperm*) or ((nonoxynol‐9 or N‐9 or conceptrol or octoxynol‐9) adj2 (cream or creams or film or films or foam or foams or gel or gels))).ti,ab. (59) 10 Family Planning/ (1737) 11 ("family planning" or "planned parenthood").ti,ab. (3014) 12 Rhythm Method/ (11) 13 ("basal body temperature method" or "Billings Method" or "calendar method" or "cervical mucus method" or "Couple Beads" or "fertility awareness method*" or "fertility awareness‐based" or "fertility regulation method" or ((lactation* or postpartum or post‐partum) adj2 amenorrh*) or "ovulation method" or "standard days method" or "symptothermal method" or "sympto‐thermal method" or "TwoDay method" or "Two‐day method").ti,ab. (139) 14 or/1‐13 (27154) 15 (((cell or cellular or google or mobile or nexus) adj2 (device* or phone* or technolog*)) or smartphone or smartphones or smart‐phone or smart‐phones or iphone or iphones or blackberr* or black‐berr* or app or apps or application or text or texts or texting or message or messages or messaging or (phone adj call*) or ehealth* or e‐health* or mhealth or m‐health or ((electronic or mobile) adj2 health*) or MMS or SMS or IVR or "interactive voice‐response" or (digitial adj3 health) or (digital adj3 healthcare)).ti,ab. (257067) 16 and/14‐15 (1495) 17 limit 16 to yr="2014 ‐Current" (514) 18 limit 17 to "0300 clinical trial" (26) 19 17 and ((control* or group* or placebo* or random* or trial) not (focus adj2 (group or groups))).ti,ab. (219) 20 or/18‐19 (221)
Global Health (Ovid) 1973 to 2022 week 32
Date last searched: 18 August 2022
1 (contraceptive or contraceptives or contraception or immunocontracept* or immuno‐contracept* or anti‐fertility or antifertility or anticonception or anti‐conception or birth‐control or contraceptif* or anticonceptiv* or anticoncepcion* or anti‐concepcion* or empfangnisverhetung or verhutungsmittel or ((child or birth*) adj2 (limiting or spacing or space or spaced)) or (delay* adj2 (childbearing or child‐bearing)) or ((control* or inhibit* or prevent* or regulat* or suppress*) adj2 (ovulat* or fertili* or pregnan* or concept* or reproduct*)) or noncontracept* or non‐contracept* or pre‐contracept* or post‐contracept*).ti,ab. (20053) 2 (((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after) adj (pill or pills)) or antiovulat* or anti‐ovulat* or ((inhibit* or suppress*) adj2 ovulat*)).ti,ab. or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*)).ti. (140) 3 (Algestone‐acetophenide or Algestoneacetophenide or Centchroman or Chlormadinone‐acetate or Chlormadinoneacetate or Cyproterone‐acetate or Cyproteroneacetate or Desogestrel or Dienogest or Dimethisterone or Dinoprost or Dinoprost‐tromethamine or Drospirenone or Ergonovine or Ergotamine or Estradiol‐benzoate or "estradiol 3‐benzoate" or Oestradiol‐benzoate or Estradiolbenzoate or Oestradiolbenzoate or Estradiol‐enanthate or Oestradiol‐enanthate or Estradiolenanthate or Oestradiolenanthate or Estradiol‐valerate or Oestradiol‐valerate or Estradiolvalerate or Oestradiolvalerate or Ethynodiol‐diacetate or Ethynodioldiacetate or Ethinyl‐estradiol or Ethinylestradiol or Etonogestrel or Gestodene or Gestrinone or Gossypol or Infecundin or Levonorgestrel or Lynestrenol or Medroxyprogesterone or Medroxyprogesterone‐acetate or Medroxyprogesteroneacetate or Megestrol or Mestranol or Methylergonovine or Nomegestrol or Nomegestrol‐acetate or Nomegestrolacetate or Nonoxynol‐9 or Norelgestromin or Norethindrone or Norethindrone‐Acetate or Norethindroneacetate or Norethisterone‐acetate or Norethisteroneacetate or Norethynodrel or Norgestimate or Norgestrel or Norgestrienone or Sparteine or Trichosanthin or Ulipristal‐Acetate or Ulipristalacetate).ti,ab. (3425) 4 (cervical‐cap* or estrogen‐ring* or ((intravaginal or intra‐vaginal or vaginal) adj2 (barrier or barriers or cap or caps or creams or creams or device or devices or foam or foams or gel or gels or ring or rings or shield or shields or sponge or sponges or suppositor* or tablet*)) or ((arcing‐spring or coil‐spring or flat‐spring or latex or silicone or intra‐vaginal* or intravaginal or vaginal) adj2 diaphragm*) or ((etonogestrel* or ETG or Progestogen* or levonorgestrel) adj3 (capsule* or implant* or rod or rods)) or ((intrauterine or intra‐uterine) adj2 (ball or balls or coil or coils or device or devices or system or systems)) or IUD or IUDs or IUCD or IUCDs or Cu‐IUD or Cu‐IUDs or LNG‐IUD or LNG‐IUDs or IUS or IUSs or progestasert).ti,ab. (2727) 5 (condom or condoms).ti,ab. (11600) 6 (spermicide or spermicides or spermicidal or spermatocidal or ((immobilizing or immobilising or blocking or inhibiting or suppressing) adj3 sperm*) or ((nonoxynol‐9 or N‐9 or conceptrol or octoxynol‐9) adj2 (cream or creams or film or films or foam or foams or gel or gels))).ti,ab. (372) 7 ("family planning" or "planned parenthood").ti,ab. (6815) 8 ("basal body temperature method" or "Billings Method" or "calendar method" or "cervical mucus method" or "Couple Beads" or "fertility awareness method*" or "fertility awareness‐based" or "fertility regulation method" or ((lactation* or postpartum or post‐partum) adj2 amenorrh*) or "ovulation method" or "standard days method" or "symptothermal method" or "sympto‐thermal method" or "TwoDay method" or "Two‐day method").ti,ab. (318) 9 or/1‐8 (36754) 10 (((cell or cellular or google or mobile or nexus) adj2 (device* or phone* or technolog*)) or smartphone or smartphones or smart‐phone or smart‐phones or iphone or iphones or blackberr* or black‐berr* or app or apps or application or text or texts or texting or message or messages or messaging or (phone adj call*) or ehealth* or e‐health* or mhealth or m‐health or ((electronic or mobile) adj2 health*) or MMS or SMS or IVR or "interactive voice‐response" or (digitial adj3 health) or (digital adj3 healthcare)).ti,ab. (146984) 11 and/9‐10 (1816) 12 limit 11 to yr="2014 ‐Current" (842)
13 randomized controlled trials/ (42547) 14 12 and 13 (53) 15 12 and ((control* or group* or placebo* or random* or trial*) not (focus adj2 (group or groups))).ti,ab. (389) 16 or/14‐15 (392)
LILACS
Date last searched: 18 August 2022 Abstract = abortion OR abortions OR contraception OR contraceptive OR contraceptives OR "family planning" OR IUD OR IUS OR LARC OR LARCs OR "intrauterine device" OR "intra‐uterine device" OR "depot medroxyprogesterone" Title = phone OR phones OR telephone OR telephones OR text OR texts OR texting OR message OR messaging OR "mobile device" OR mhealth OR m‐health OR ehealth OR e‐health OR SMS Years = 2014‐2018 (185)
POPLINE
Date searched: 6 March 2019 FAMILY PLANNING OR PREGNANCY UNPLANNED OR PREGNANCY UNWANTED OR family planning OR unplanned pregnancy OR unwanted pregnancy CELLULAR PHONE OR MOBILE DEVICES OR TEXT MESSAGING OR cell phone OR cellular phone OR mobile phone OR mobile devices OR text OR texting OR messaging Publication Year = 2014‐2019 (171)
SCOPUS
Date last searched: 18 August 2022 ( TITLE ( phone* OR telephone* OR "mobile device*" OR smartphone* OR smart‐phone* OR mhealth OR m‐health OR e‐health* OR ehealth* OR app OR apps OR mms OR "multimedia messag* service" OR sms OR "short messag* service" OR text* OR messag* ) AND TITLE‐ABS‐KEY ( abortion* OR contracept* OR "family planning" OR "birth control" OR condom* OR "depot medroxyprogest*" OR ( ( intrauterine OR intra‐uterine ) PRE/2 ( device* OR system* ) ) OR iud OR ius OR "vaginal ring*" OR "lactational amenorr*" OR ( pregnan* W/3 prevent* ) ) ) AND DOCTYPE ( cp ) AND PUBYEAR > 2013 (18)
ClinicalTrials.gov
Date last searched: 18 August 2022 Condition = abortion OR abortions OR "birth control" OR contraception OR contraceptive OR "family planning" OR LARC OR "depot medroxyprogesterone" OR IUD OR IUS OR intrauterine OR intra‐uterine OR condom OR "lactational amenorrhea" OR "pregnancy prevention" OR vaginal ring Intervention = app OR apps OR blackberry OR phone OR telephone OR email OR smartphone OR SMS OR messaging OR text OR texting OR mhealth OR m‐health OR ehealth OR e‐health OR telemedicine OR cellular Status = Active, not recruiting, Completed, Suspended, Terminated, Withdrawn Studies (164)
WHO ICTRP
Date last searched: 18 August 2022 Title = app OR apps OR phone OR telephone OR email OR smartphone OR SMS OR messaging OR text OR texting OR mhealth OR m‐health OR ehealth OR e‐health OR telemedicine OR cellular Condition = abortion OR abortions OR birth control OR contraception OR contraceptive OR family planning OR LARC OR medroxyprogesterone OR IUD OR IUS OR intrauterine device OR condom OR vaginal ring OR pregnancy prevention Recruitment Status = ALL (80)
Appendix 2. Previous search strategies
MEDLINE via Ovid (date of search: 6 October 2014)
(phone adj3 call*).mp. OR ((cell* or mobile or smart or google or nexus or iphone) adj3 (phone* or telephone*)).mp. OR smartphone*.mp. OR smart‐phone*.mp. OR (blackberr* not extract).mp. OR (black‐berr* not extract).mp. OR ((mobile adj3 health) not (van* or unit*)).mp. OR mhealth.mp OR m‐health.mp OR e‐health*.mp. OR ehealth*.mp. OR (electronic adj health).mp. OR (mobile adj3 technol*).mp. OR ((mobile or smartphone or smart‐phone or phone or software) adj3 app*).mp. OR MMS.mp. OR multimedia messaging service.mp OR SMS.mp. OR short messag* service.mp OR (text* adj messag*).mp. OR text‐messa*.mp. OR voice messag*.mp. OR interactive voice response.mp OR IVR.mp. OR Telemedicine/ OR cellular phone/ or text messaging/
AND
(contracept* or (family adj planning) or (Birth adj control)).mp. OR condom.mp. OR (OC adj pill).mp. OR (depot medroxyprogest* or NET‐EN or NET EN or Mesigyna or Cyclofem).mp. OR (intrauterine system or intra‐uterine system or IUS or intrauterine device or intra‐uterine device or IUD).mp. OR (vasectomy or sterilisation or sterilization or (tubal adj ligation)).mp. OR ((vaginal adj ring) or cycletel or cycle‐tel or abstain or abstinen* or lactational amenorr*).mp OR (pregnan* or abortion).mp OR exp Contraception/ OR exp Contraceptive Devices/ OR exp Pregnancy, Unplanned/ OR exp Pregnancy, Unwanted/ OR exp Abortion, Induced/ OR (NORPLANT or implanon or Femplant).mp.
Limit to yr="1993‐Current" and clinical trial, all
Global Health via Ovid (date of search: 6 October 2014)
(phone adj3 call*).mp. OR ((cell* or mobile or smart or google or nexus or iphone) adj3 (phone* or telephone*)).mp. OR smartphone*.mp. OR smart‐phone*.mp. OR (blackberr* not extract).mp OR (black‐berr* not extract).mp OR ((mobile adj3 health) not (van* or unit*)).mp. OR mhealth.mp OR m‐health.mp. OR e‐health*.mp. OR ehealth*.mp OR (electronic adj health).mp OR (mobile adj3 technol*).mp OR ((mobile or smartphone or smart‐phone or phone or software) adj3 app*).mp. OR MMS.mp OR multimedia messaging service.mp OR SMS.mp. OR short messag* service.mp OR (text* adj messag*).mp. OR text‐messa*.mp. OR voice messag*.mp. OR interactive voice response.mp OR IVR.mp OR Telemedicine/ OR cellular phone/ or text messaging/ OR exp mobile telephones/
AND
(contracept* or (family adj planning) or (Birth adj control)).mp. OR condom.mp OR (OC adj pill).mp. OR (depot medroxyprogest* or NET‐EN or NET EN or Mesigyna or Cyclofem).mp. OR (intrauterine system or intra‐uterine system or IUS or intrauterine device or intra‐uterine device or IUD).mp. OR (vasectomy or sterilisation or sterilization or (tubal adj ligation)).mp. OR ((vaginal adj ring) or cycletel or cycle‐tel or abstain or abstinen* or lactational amenorr*).mp OR (pregnan* or abortion).mp OR exp Contraception/ OR exp Contraceptive Devices/ OR exp Pregnancy, Unplanned/ OR exp Pregnancy, Unwanted/ OR exp Abortion, Induced/ OR (NORPLANT or implanon or Femplant).mp. OR induced abortion/
Limit to yr="1993‐Current"
PsycINFO via Ovid (date of search: 6 October 2014) (phone adj3 call*).mp. OR ((cell* or mobile or smart or google or nexus or iphone) adj3 (phone* or telephone*)).mp. OR smartphone*.mp OR smart‐phone*.mp. OR (blackberr* not extract).mp OR (black‐berr* not extract).mp OR ((mobile adj3 health) not (van* or unit*)).mp OR mhealth.mp. OR m‐health.mp. OR e‐health*.mp. OR ehealth*.mp OR (electronic adj health). OR (mobile adj3 technol*).mp OR ((mobile or smartphone or smart‐phone or phone or software) adj3 app*).mp. OR MMS.mp. OR multimedia messaging OR SMS.mp. OR short messag* service.mp OR (text* adj messag*).mp OR text‐messa*.mp OR voice messag*.mp OR interactive voice response.mp OR IVR.mp OR Telemedicine/ OR cellular phone/ or text messaging/
AND
(contracept* or (family adj planning) or (Birth adj control)).mp OR condom.mp. OR (OC adj pill).mp OR (depot medroxyprogest* or NET‐EN or NET EN or Mesigyna or Cyclofem).mp OR (intrauterine system or intra‐uterine system or IUS or intrauterine device or intra‐uterine device or IUD).mp. OR (vasectomy or sterilisation or sterilization or (tubal adj ligation)).mp OR ((vaginal adj ring) or cycletel or cycle‐tel or abstain or abstinen* or lactational amenorr*).mp OR (pregnan* or abortion).mp OR exp Contraception/ OR exp Contraceptive Devices/ OR exp Pregnancy, Unplanned/ OR exp Pregnancy, Unwanted/ OR exp Abortion, Induced/ OR (NORPLANT or implanon or Femplant).mp.
Limit to yr="1993‐Current" and clinical trial, all
Embase via Ovid (date of search: 6 October 2014) (phone adj3 call*).mp OR ((cell* or mobile or smart or google or nexus or iphone) adj3 (phone* or telephone*)).mp. OR smartphone*.mp. OR smart‐phone*.mp OR (blackberr* not extract).mp OR (black‐berr* not extract).mp OR ((mobile adj3 health) not (van* or unit*)).mp. OR mhealth.mp OR m‐health.mp. OR e‐health*.mp. OR ehealth*.mp. OR (electronic adj health).mp OR (mobile adj3 technol*).mp. OR ((mobile or smartphone or smart‐phone or phone or software) adj3 app*).mp OR MMS.mp. OR multimedia messaging service.mp OR SMS.mp OR short messag* service.mp. OR (text* adj messag*).mp OR text‐messa*.mp. OR voice messag*.mp OR interactive voice response.mp. OR IVR.mp. OR Telemedicine/ OR cellular phone/ or text messaging/
AND
(contracept* or (family adj planning) or (Birth adj control)).mp. OR condom.mp. OR (OC adj pill).mp. OR (depot medroxyprogest* or NET‐EN or NET EN or Mesigyna or Cyclofem).mp. OR (intrauterine system or intra‐uterine system or IUS or intrauterine device or intra‐uterine device or IUD).mp. OR (vasectomy or sterilisation or sterilization or (tubal adj ligation)).mp. OR ((vaginal adj ring) or cycletel or cycle‐tel or abstain or abstinen* or lactational amenorr*).mp. OR (pregnan* or abortion).mp. OR exp Contraception/ OR exp Contraceptive Devices/ OR exp Pregnancy, Unplanned/ OR exp Pregnancy, Unwanted/ OR exp Abortion, Induced/ OR (NORPLANT or implanon or Femplant).mp.
Limit to yr="1993‐Current", clinical trial, all and (clinical trial or randomized controlled trial or controlled clinical trial or multicenter study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial)
Cochrane Central register of Controlled trials (CENTRAL) (date of search: 6 October 2014)
(((phone NEAR3 call*) OR ((cell* or mobile or smart or google or nexus or iphone) NEAR3 (phone* or telephone*)) OR (smartphone*) OR (smart‐phone*) OR (blackberr* NOT extract) OR (black‐berr* NOT extract)) OR ((mobile NEAR3 (health NOT (van* or unit*))) OR (mhealth) OR (m‐health) OR (e‐health*) OR (ehealth*) OR (electronic health) OR (mobile NEAR3 technol*)) OR ((mobile or smartphone or smart‐phone or phone or software) NEAR3 (app*)) OR ((MMS) OR (multimedia messaging service) OR (SMS) OR (short messag* service) OR (text* messag*) OR (text‐messa*) OR (voice messag*) OR (interactive voice response) OR (IVR))) OR exp Telemedicine OR exp Cellular Phone
AND
(((contracept*) OR (family planning) OR (Birth control)) OR (condom) OR ((OC pill)) OR ((depot medroxyprogest*) OR (NET‐EN) OR (NET EN) OR (Mesigyna) OR (Cyclofem)) OR ((NORPLANT) OR (implanon) OR (Femplant)) OR ((intrauterine system) OR (intra‐uterine system) OR (IUS) OR (intrauterine device) OR (intra‐uterine device) OR (IUD)) OR ((vasectomy) OR (sterilisation) OR (sterilization) OR (tubal ligation)) OR ((vaginal ring) OR (cycletel) OR (cycle‐tel) or (abstain) OR (abstinen*) OR (lactational amenorr*)) OR ((pregnan*) OR (abortion))) OR exp Contraception OR exp Contraceptive Devices OR exp Pregnancy, Unplanned OR exp Pregnancy, Unwanted OR exp Abortion, Induced
Limit to 1993‐2014
POPLINE (date of search: 6 October 2014)
Family Planning OR Pregnancy Unplanned OR Pregnancy Unwanted AND Cellular Phone OR Mobile Devices OR Text Messaging (1993‐2014)
Africa‐Wide Information (date of search: 6 October 2014)
((phone n3 call*) OR ((cell* or mobile or smart or google or nexus or iphone) n3 (phone* or telephone*)) OR (smartphone*) OR (smart‐phone*) OR (blackberr* NOT extract) OR (black‐berr* NOT extract)) OR ((mobile n3 (health NOT (van* or unit*))) OR (mhealth) OR (m‐health) OR (e‐health*) OR (ehealth*) OR (electronic health) OR (mobile n3 technol*)) OR ((mobile or smartphone or smart‐phone or phone or software) n3 (app*)) OR ((MMS) OR (multimedia messaging service) OR (SMS) OR (short messag* service) OR (text* messag*) OR (text‐messa*) OR (voice messag*) OR (interactive voice response) OR (IVR))
AND
((contracept*) OR (family planning) OR (Birth control)) OR (condom) OR ((OC pill)) OR ((depot medroxyprogest*) OR (NET‐EN) OR (NET EN) OR (Mesigyna) OR (Cyclofem)) OR ((NORPLANT) OR (implanon) OR (Femplant)) OR ((intrauterine system) OR (intra‐uterine system) OR (IUS) OR (intrauterine device) OR (intra‐uterine device) OR (IUD)) OR ((vasectomy) OR (sterilisation) OR (sterilization) OR (tubal ligation)) OR ((vaginal ring) OR (cycletel) OR (cycle‐tel) or (abstain) OR (abstinen*) OR (lactational amenorr*)) OR ((pregnan*) OR (abortion))
LILACS (date of search: 6 October 2014)
(contracept$ OR family planning OR condom$ OR pregnan$ OR abortion$) AND (phone$ OR text messag$ OR mobil$ health)
WHO international trials registry (date of search: 9 October 2014)
Condition (family planning) intervention (mHealth): (family planning OR contracept* OR pregnanc* OR abortion* OR condom*) AND (phone OR text messag* OR cellular phon* OR mobile phon* OR mobile devic* OR mobile technol*
Current controlled trials
(family planning OR contracept* OR unplanned pregnanc* OR unintended pregnanc* OR induced abortion* OR condom*) AND (phone OR text messag* OR cellular phon* OR mobile phon* OR mobile devic* OR mobile technol*)
Data and analyses
Comparison 1. Summary of findings data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Contraception use | 16 | 8972 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.60] |
| 1.2 Pregnancy – Peto OR | 8 | 2947 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.48, 1.38] |
1.1. Analysis.

Comparison 1: Summary of findings data, Outcome 1: Contraception use
1.2. Analysis.

Comparison 1: Summary of findings data, Outcome 2: Pregnancy – Peto OR
Comparison 2. Contraception use: message intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Contraception use | 16 | 8972 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.60] |
| 2.2 Unidirectional versus interactive message interventions | 16 | 8972 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [1.08, 1.62] |
| 2.2.1 Unidirectional messages | 9 | 5883 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.22] |
| 2.2.2 Interactive/bidirectional messages | 8 | 3089 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [1.28, 2.29] |
| 2.3 High‐ versus low‐income countries | 16 | 8972 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.06, 1.60] |
| 2.3.1 High‐income countries | 6 | 4276 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [1.01, 1.82] |
| 2.3.2 Low‐income countries | 10 | 4696 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.70] |
Comparison 3. Pregnancy: message intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pregnancy – Peto OR | 8 | 2947 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.48, 1.38] |
| 3.2 Pregnancy – generic inverse variance | 8 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.43, 1.16] |
Comparison 4. Hou 2010: daily text message reminders versus no reminders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean number of missed pills (cycle 1) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.08, 2.08] |
| 4.2 Mean number of missed pills (cycle 3) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.22, 2.82] |
| 4.3 Condom use for ≥ 50% of coital activity during study (self‐report) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.00, 3.78] |
| 4.4 Emergency contraception use during study | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.26, 103.39] |
| 4.5 Pregnancy reported during study | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
4.5. Analysis.

Comparison 4: Hou 2010: daily text message reminders versus no reminders, Outcome 5: Pregnancy reported during study
Comparison 5. Trent 2013: daily text message appointment reminders 72 hours before appointment + healthy self‐management messages versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mean number of days between scheduled appointment and completed visit: first visit | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐8.60 [‐16.74, ‐0.46] |
| 5.2 Mean number of days between scheduled appointment and completed visit: third visit | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 2.19 [‐3.89, 8.27] |
Comparison 6. Tsur 2008: contraceptive information via text messages and mail at 1 and 2 months versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Contraceptive use during treatment with isotretinoin | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.84, 1.89] |
| 6.2 Use of 2 contraceptives | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.07, 18.07] |
| 6.3 Sexually active and not using contraceptive | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.11, 3.03] |
6.2. Analysis.

Comparison 6: Tsur 2008: contraceptive information via text messages and mail at 1 and 2 months versus standard care, Outcome 2: Use of 2 contraceptives
6.3. Analysis.

Comparison 6: Tsur 2008: contraceptive information via text messages and mail at 1 and 2 months versus standard care, Outcome 3: Sexually active and not using contraceptive
Comparison 7. Chernick 2017: daily educational and motivational texts versus standardised physical discharge solutions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Attended family planning follow‐up | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.87] |
| 7.2 Contraception initiation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.33] |
| 7.3 Contraception counselling | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.55] |
| 7.4 Became pregnant | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.75] |
7.1. Analysis.

Comparison 7: Chernick 2017: daily educational and motivational texts versus standardised physical discharge solutions, Outcome 1: Attended family planning follow‐up
7.3. Analysis.

Comparison 7: Chernick 2017: daily educational and motivational texts versus standardised physical discharge solutions, Outcome 3: Contraception counselling
7.4. Analysis.

Comparison 7: Chernick 2017: daily educational and motivational texts versus standardised physical discharge solutions, Outcome 4: Became pregnant
Comparison 8. Bull 2016: automated interactive voice messages + teen outreach programme versus teen outreach programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Mean percentage of sex acts protected by condoms in past 3 months – sexually active | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 10.50 [‐8.87, 29.87] |
| 8.2 Mean percentage of sex acts protected by contraception in past 3 months – sexually active | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [‐5.40, 30.20] |
| 8.3 Access to contraceptive or sexually transmitted disease services | 1 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.40, 1.28] |
| 8.4 Ever pregnant or caused pregnancy (adjusted) | 1 | 371 | Odds Ratio (IV, Fixed, 95% CI) | 0.85 [0.28, 2.57] |
8.1. Analysis.

Comparison 8: Bull 2016: automated interactive voice messages + teen outreach programme versus teen outreach programme, Outcome 1: Mean percentage of sex acts protected by condoms in past 3 months – sexually active
8.3. Analysis.

Comparison 8: Bull 2016: automated interactive voice messages + teen outreach programme versus teen outreach programme, Outcome 3: Access to contraceptive or sexually transmitted disease services
8.4. Analysis.

Comparison 8: Bull 2016: automated interactive voice messages + teen outreach programme versus teen outreach programme, Outcome 4: Ever pregnant or caused pregnancy (adjusted)
Comparison 9. Castano 2012: daily educational text messages versus no messages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Oral contraception (OC) use (continuation) at 6 months | 1 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.05, 1.35] |
| 9.2 OC use (continuation): follow‐up ≤ 187 days | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.13, 1.74] |
| 9.3 OC use (continuation): follow‐up ≥ 188 days | 1 | 483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 9.4 No OC interruptions > 7 days at 6 months | 1 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.41] |
| 9.5 Missed no pills in last month | 1 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.16, 1.79] |
| 9.6 OC use at last intercourse | 1 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.03, 1.28] |
9.1. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 1: Oral contraception (OC) use (continuation) at 6 months
9.2. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 2: OC use (continuation): follow‐up ≤ 187 days
9.3. Analysis.

Comparison 9: Castano 2012: daily educational text messages versus no messages, Outcome 3: OC use (continuation): follow‐up ≥ 188 days
Comparison 10. Smith 2015: voice messages and counsellor support versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Effective contraception use at 4 months | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.17, 1.66] |
| 10.2 Long‐acting contraception use at 4 months | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [2.07, 5.40] |
| 10.3 Effective contraception use over 4‐month postabortion period | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.67] |
| 10.4 Repeat pregnancy at 4 months | 1 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.39, 4.06] |
| 10.5 Repeat abortion at 4 months | 1 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.94] |
| 10.6 Road traffic accident | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.7 Domestic abuse | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.8 Effective contraception use at 12 months | 1 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.47] |
10.1. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 1: Effective contraception use at 4 months
10.2. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 2: Long‐acting contraception use at 4 months
10.3. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 3: Effective contraception use over 4‐month postabortion period
10.4. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 4: Repeat pregnancy at 4 months
10.5. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 5: Repeat abortion at 4 months
10.8. Analysis.

Comparison 10: Smith 2015: voice messages and counsellor support versus standard care, Outcome 8: Effective contraception use at 12 months
Comparison 11. Reiss 2019: automated voice messages versus no messages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Long‐acting reversal contraceptive (LARC) use at 4 months | 1 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.14] |
| 11.2 LARC use with multiple imputation (MI) at 4 months | 1 | 962 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.11] |
| 11.3 Effective modern method use (any method) at 4‐month follow‐up | 1 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.18] |
| 11.4 LARC use at 2‐week follow‐up | 1 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.22] |
| 11.5 Physical intimidate partner violence | 1 | 768 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.04, 2.92] |
11.1. Analysis.

Comparison 11: Reiss 2019: automated voice messages versus no messages, Outcome 1: Long‐acting reversal contraceptive (LARC) use at 4 months
11.2. Analysis.

Comparison 11: Reiss 2019: automated voice messages versus no messages, Outcome 2: LARC use with multiple imputation (MI) at 4 months
11.3. Analysis.

Comparison 11: Reiss 2019: automated voice messages versus no messages, Outcome 3: Effective modern method use (any method) at 4‐month follow‐up
11.4. Analysis.

Comparison 11: Reiss 2019: automated voice messages versus no messages, Outcome 4: LARC use at 2‐week follow‐up
Comparison 12. McCarthy 2018: tailored daily text messages versus messages about trial participation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 ≥ 1 effective method is acceptable | 1 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 12.2 Use of effective contraception | 1 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.30] |
| 12.3 Pill acceptability | 1 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.25] |
| 12.4 Intrauterine device acceptability | 1 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 12.5 Injection acceptability | 1 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.19] |
| 12.6 Implant acceptability | 1 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 12.7 Effective contraceptive use during the 4 months | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.06] |
| 12.8 Service uptake | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.37] |
| 12.9 Unintended pregnancy | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
12.1. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 1: ≥ 1 effective method is acceptable
12.2. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 2: Use of effective contraception
12.3. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 3: Pill acceptability
12.4. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 4: Intrauterine device acceptability
12.5. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 5: Injection acceptability
12.6. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 6: Implant acceptability
12.7. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 7: Effective contraceptive use during the 4 months
12.8. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 8: Service uptake
12.9. Analysis.

Comparison 12: McCarthy 2018: tailored daily text messages versus messages about trial participation, Outcome 9: Unintended pregnancy
Comparison 13. McCarthy 2019: text messages versus control text messages about participation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Using effective contraception at 4‐month follow‐up | 1 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.57, 1.86] |
| 13.2 ≥ 1 effective method is acceptable | 1 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.29, 2.56] |
| 13.3 Service uptake (attended a service ≥ 1 times) | 1 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.45] |
| 13.4 Unintended pregnancy | 1 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.06] |
| 13.5 Induced abortion | 1 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.03] |
| 13.6 Any effective contraception during the 4 months | 1 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.22] |
13.1. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 1: Using effective contraception at 4‐month follow‐up
13.2. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 2: ≥ 1 effective method is acceptable
13.3. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 3: Service uptake (attended a service ≥ 1 times)
13.4. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 4: Unintended pregnancy
13.5. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 5: Induced abortion
13.6. Analysis.

Comparison 13: McCarthy 2019: text messages versus control text messages about participation, Outcome 6: Any effective contraception during the 4 months
Comparison 14. McCarthy 2020: daily text messages versus no text messages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Using effective contraception at 4‐month follow‐up | 1 | 429 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.80, 1.76] |
| 14.2 ≥ 1 effective method is acceptable | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.00, 1.32] |
| 14.3 Service uptake (attended a service ≥ 1 times) | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 14.4 Unintended pregnancy | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 14.5 Induced abortion | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.24] |
| 14.6 Effective contraceptive use during the 4 months | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 14.7 Pill acceptability | 1 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.55] |
| 14.8 Intrauterine device acceptability | 1 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.90, 1.81] |
| 14.9 Injection acceptability | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.94, 1.49] |
| 14.10 Implant acceptability | 1 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 14.11 Patch acceptability | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.40] |
14.1. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 1: Using effective contraception at 4‐month follow‐up
14.2. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 2: ≥ 1 effective method is acceptable
14.3. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 3: Service uptake (attended a service ≥ 1 times)
14.4. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 4: Unintended pregnancy
14.5. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 5: Induced abortion
14.6. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 6: Effective contraceptive use during the 4 months
14.7. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 7: Pill acceptability
14.8. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 8: Intrauterine device acceptability
14.9. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 9: Injection acceptability
14.10. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 10: Implant acceptability
14.11. Analysis.

Comparison 14: McCarthy 2020: daily text messages versus no text messages, Outcome 11: Patch acceptability
Comparison 15. Biswas 2017: tailored daily and weekly text‐message reminders versus no text‐message reminders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Using modern contraception at 4‐month follow‐up | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.10] |
15.1. Analysis.

Comparison 15: Biswas 2017: tailored daily and weekly text‐message reminders versus no text‐message reminders, Outcome 1: Using modern contraception at 4‐month follow‐up
Comparison 16. Wilkinson 2017: interval text reminder + education regarding emergency contraception versus no text reminder + education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Emergency prescriptions filled at 16 days from enrolment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.82, 2.59] |
16.1. Analysis.

Comparison 16: Wilkinson 2017: interval text reminder + education regarding emergency contraception versus no text reminder + education, Outcome 1: Emergency prescriptions filled at 16 days from enrolment
Comparison 17. Unger 2018: 1‐way weekly education and motivation text messages versus 2‐way text messages with a nurse versus routine clinic care + no text messages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Probability of contraceptive use by 10 weeks' postpartum | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 17.2 Probability of contraceptive use by 16 weeks' postpartum | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.48] |
| 17.3 Probability of contraception use 24 weeks' postpartum | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.13, 1.48] |
17.1. Analysis.

Comparison 17: Unger 2018: 1‐way weekly education and motivation text messages versus 2‐way text messages with a nurse versus routine clinic care + no text messages, Outcome 1: Probability of contraceptive use by 10 weeks' postpartum
17.2. Analysis.

Comparison 17: Unger 2018: 1‐way weekly education and motivation text messages versus 2‐way text messages with a nurse versus routine clinic care + no text messages, Outcome 2: Probability of contraceptive use by 16 weeks' postpartum
17.3. Analysis.

Comparison 17: Unger 2018: 1‐way weekly education and motivation text messages versus 2‐way text messages with a nurse versus routine clinic care + no text messages, Outcome 3: Probability of contraception use 24 weeks' postpartum
Comparison 18. Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Used any contraception past year | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.94, 1.58] |
| 18.2 Used contraception at last intercourse | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.48] |
| 18.3 Use of condom at sexual debut | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.26] |
| 18.4 Had sexual intercourse without condom in past year | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.43] |
| 18.5 Used condom in past year | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.70] |
| 18.6 Used oral contraceptive pill in past year | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.08 [1.14, 22.60] |
| 18.7 Used emergency contraception in past year | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.39] |
| 18.8 Pregnant (sexually active) (adjusted) | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.14, 1.39] |
18.1. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 1: Used any contraception past year
18.2. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 2: Used contraception at last intercourse
18.3. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 3: Use of condom at sexual debut
18.4. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 4: Had sexual intercourse without condom in past year
18.5. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 5: Used condom in past year
18.6. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 6: Used oral contraceptive pill in past year
18.7. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 7: Used emergency contraception in past year
18.8. Analysis.

Comparison 18: Rokicki 2017: weekly reproductive health text messages versus weekly multiple choice quiz text messages versus weekly text messages about malaria, Outcome 8: Pregnant (sexually active) (adjusted)
Comparison 19. Johnson 2017: full access to m4RH platform versus limited access.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Discussed family planning with partner in past month | 1 | 2863 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.08] |
| 19.2 Visited clinic to discuss family planning with nurse or doctor | 1 | 2863 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.99, 1.19] |
| 19.3 Use contraception at end of study | 1 | 2863 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.04] |
19.1. Analysis.

Comparison 19: Johnson 2017: full access to m4RH platform versus limited access, Outcome 1: Discussed family planning with partner in past month
19.2. Analysis.

Comparison 19: Johnson 2017: full access to m4RH platform versus limited access, Outcome 2: Visited clinic to discuss family planning with nurse or doctor
19.3. Analysis.

Comparison 19: Johnson 2017: full access to m4RH platform versus limited access, Outcome 3: Use contraception at end of study
Comparison 20. Hebert 2018: access to mobile app versus no access.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Use of intrauterine device (IUD) at 3‐month follow‐up | 1 | 166 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.04, 4.79] |
| 20.2 Use of implant at 3‐month follow‐up | 1 | 166 | Risk Ratio (IV, Fixed, 95% CI) | 5.32 [0.65, 43.21] |
| 20.3 Use of any long‐acting reversal contraceptive (LARC) at 3‐month follow‐up | 1 | 166 | Risk Ratio (IV, Fixed, 95% CI) | 2.07 [0.55, 7.72] |
20.1. Analysis.

Comparison 20: Hebert 2018: access to mobile app versus no access, Outcome 1: Use of intrauterine device (IUD) at 3‐month follow‐up
20.2. Analysis.

Comparison 20: Hebert 2018: access to mobile app versus no access, Outcome 2: Use of implant at 3‐month follow‐up
20.3. Analysis.

Comparison 20: Hebert 2018: access to mobile app versus no access, Outcome 3: Use of any long‐acting reversal contraceptive (LARC) at 3‐month follow‐up
Comparison 21. Harrington 2019: family planning focused weekly text message versus no text message.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Any method use at 6‐week follow‐up | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.57, 1.60] |
| 21.2 Highly effective contraceptive use at 6‐week follow‐up | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.39] |
| 21.3 Long‐acting reversible contraceptive (LARC)/postpartum contraception (PC) use at 6‐week follow‐up | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.23, 1.59] |
| 21.4 Any method use at 14‐week follow‐up | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.38] |
| 21.5 Highly effective contraceptive use at 14‐week follow‐up | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 21.6 LARC/PC use at 14‐week follow‐up | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.66, 1.77] |
| 21.7 Satisfied with method at 14‐week follow‐up | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.92, 1.55] |
| 21.8 Any method use at 6‐month follow‐up | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.38] |
| 21.9 Highly effective contraceptive use at 6‐month follow‐up | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.01, 1.47] |
| 21.10 LARC/PC use at 6‐month follow‐up | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.67, 1.48] |
| 21.11 Satisfied with method at 6‐month follow‐up | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.91, 1.29] |
21.1. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 1: Any method use at 6‐week follow‐up
21.2. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 2: Highly effective contraceptive use at 6‐week follow‐up
21.3. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 3: Long‐acting reversible contraceptive (LARC)/postpartum contraception (PC) use at 6‐week follow‐up
21.4. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 4: Any method use at 14‐week follow‐up
21.5. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 5: Highly effective contraceptive use at 14‐week follow‐up
21.6. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 6: LARC/PC use at 14‐week follow‐up
21.7. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 7: Satisfied with method at 14‐week follow‐up
21.8. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 8: Any method use at 6‐month follow‐up
21.9. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 9: Highly effective contraceptive use at 6‐month follow‐up
21.10. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 10: LARC/PC use at 6‐month follow‐up
21.11. Analysis.

Comparison 21: Harrington 2019: family planning focused weekly text message versus no text message, Outcome 11: Satisfied with method at 6‐month follow‐up
Comparison 22. Francis 2015: text messages versus no text messages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Continued contraception at 4 months | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.26] |
22.1. Analysis.

Comparison 22: Francis 2015: text messages versus no text messages, Outcome 1: Continued contraception at 4 months
Comparison 23. Babalola 2020: phone drama intervention versus control follow‐up calls.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Using modern contraceptive method | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.36, 2.40] |
| 23.2 Confident discussing family planning with provider | 1 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.50, 2.64] |
23.1. Analysis.

Comparison 23: Babalola 2020: phone drama intervention versus control follow‐up calls, Outcome 1: Using modern contraceptive method
23.2. Analysis.

Comparison 23: Babalola 2020: phone drama intervention versus control follow‐up calls, Outcome 2: Confident discussing family planning with provider
Comparison 24. Nuwamanya 2020: mobile phone application for access to sexual and reproductive health information, goods and services versus control app.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Contraceptive use | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 24.2 Use of condoms | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.14] |
| 24.3 Sexually transmitted infection diagnosis and treatment | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.10, 1.28] |
24.1. Analysis.

Comparison 24: Nuwamanya 2020: mobile phone application for access to sexual and reproductive health information, goods and services versus control app, Outcome 1: Contraceptive use
24.2. Analysis.

Comparison 24: Nuwamanya 2020: mobile phone application for access to sexual and reproductive health information, goods and services versus control app, Outcome 2: Use of condoms
24.3. Analysis.

Comparison 24: Nuwamanya 2020: mobile phone application for access to sexual and reproductive health information, goods and services versus control app, Outcome 3: Sexually transmitted infection diagnosis and treatment
Comparison 25. Rinehart 2020: text services (t4she) versus no texts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Sexual health knowledge at 6 months | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 1.67 [0.32, 3.02] |
| 25.2 Use of long‐acting reversible contraception | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.58, 1.84] |
| 25.3 No contraception at 6 months | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.99] |
| 25.4 Use of short‐ (SARC) or long‐acting reversible contraceptive (LARC) at 6 months amongst sexually active | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.09] |
| 25.5 Use of SARC or LARC at 3 months | 1 | 136 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.10, 4.37] |
25.1. Analysis.

Comparison 25: Rinehart 2020: text services (t4she) versus no texts, Outcome 1: Sexual health knowledge at 6 months
25.2. Analysis.

Comparison 25: Rinehart 2020: text services (t4she) versus no texts, Outcome 2: Use of long‐acting reversible contraception
25.3. Analysis.

Comparison 25: Rinehart 2020: text services (t4she) versus no texts, Outcome 3: No contraception at 6 months
25.4. Analysis.

Comparison 25: Rinehart 2020: text services (t4she) versus no texts, Outcome 4: Use of short‐ (SARC) or long‐acting reversible contraceptive (LARC) at 6 months amongst sexually active
25.5. Analysis.

Comparison 25: Rinehart 2020: text services (t4she) versus no texts, Outcome 5: Use of SARC or LARC at 3 months
Comparison 26. Brody 2022: mobile link information (text and voice messages) platform versus no mobile link.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Uses modern contraception | 1 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.15] |
26.1. Analysis.

Comparison 26: Brody 2022: mobile link information (text and voice messages) platform versus no mobile link, Outcome 1: Uses modern contraception
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Babalola 2019.
| Study characteristics | ||
| Methods | Cluster‐RCT in Kaduna City, Nigeria Aim: to assess the efficacy of the digital health tool Smart Client on ideational and behavioural variables related to FP. Setting: 12 wards in the city randomly assigned to intervention (6 wards) and control (6 wards) arms. At recruitment, the women completed a baseline survey. The women in the intervention group were registered to receive 1 welcome call, 13 programme calls and 3 quiz calls on their mobile phones. Each of the programme calls had several segments, including introduction, drama episode and friend‐to‐friend chat. The last quiz call included evaluation questions. Women in the control arm received no intervention. |
|
| Participants | 559 participants (221 in intervention and 338 in control groups) Inclusion criteria: aged 18–35 years and not currently using a non‐barrier contraceptive method (e.g. pill, IUD, implant, emergency contraceptives, tubal ligation, vasectomy, lactational amenorrhoea method), owned a mobile phone or had access to one, resident in Kaduna City and fluent in Haus. Exclusion criteria: not reported Cluster differences: specifically, a larger proportion of the intervention group was Muslim (65.6%) compared with the control group (57.2%) (P < 0.05). |
|
| Interventions | Control: did not receive the Smart Client intervention but received 2 calls on their mobile phone: 1 at the beginning of the study with the automated pre‐intervention survey and the other 6 weeks later with the automated postintervention survey. Intervention: the Smart Client digital health tool was designed to inform, empower and promote smart clients by reaching them directly through mobile phones. The tool is based upon Social Learning Theory, which posits that people learn from each other through observation, imitation and modelling. This approach allows the intended audience to observe an action, understand its consequences, and become motivated to repeat and adopt it. The IVR platform was programmed so that users were preregistered and calls would be pushed to them on a schedule (every day, every other day or twice per week) and time of day. |
|
| Outcomes | Primary outcomes
|
|
| Behaviour change techniques | The Smart Client tool therefore uses fictional role models, who demonstrate the desired behaviours and behaviour change process in a drama format, as well as personal stories and examples of Smart Client dialogues. This approach allows the intended audience to observe an action, understand its consequences, and become motivated to repeat and adopt it. While drama is a common approach used in behaviour change communication, it is usually delivered via television, radio or community theatre. This digital health tool explored how drama could be adapted to basic mobile phones via IVR, using shorter and simpler storylines in a series of episodes while maintaining the fictional serial drama style. IVR was chosen as the delivery channel because it is accessible to audiences regardless of the type of mobile phone they have (e.g. smartphone or basic phone) and irrespective of their level of literacy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation process unclear. Wards were "randomly assigned." No further detail given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel would have known who was in the intervention and control groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors seemed to know which intervention was allocated depending on ward. Randomisation occurred prior to recruitment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large significant variable differences in lost to follow‐up as well as high attrition rate. |
| Selective reporting (reporting bias) | Unclear risk | No protocol mentioned or available. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Biswas 2017.
| Study characteristics | ||
| Methods | Individual RCT Aim: to examine the feasibility and acceptability of implementing a text message‐based intervention delivered by mobile phone to support postabortion contraceptive use amongst women seeking abortion in Bangladesh, including women's interest in the intervention, intervention preferences and privacy concerns. Duration: baseline data collected from March to June 2013; follow‐up data collected July to October 2013, i.e. 8 months. Setting: 4 urban, high abortion caseload facilities. Women were randomised to intervention (60 women) or control group (60 women) using block randomisation. A baseline interview was conducted on the day of the abortion procedure and a follow‐up. |
|
| Participants | 120 women recruited. Inclusion criteria: women attending 4 urban sexual and reproductive health clinics run by the Reproductive Health Services Training and Education Program (RHSTEP) in the divisional capitals of Dhaka, Chittagong, Rajshahi, and Sylhet. Women were eligible for study participation if:
Exclusion criteria: women were not eligible if they shared their mobile phones with someone else. |
|
| Interventions | Control group: did not receive text messages or reminders (60 women) Intervention group: received text messages (60 women) Study conducted over 8 months. Women followed up 4 months after enrolment. Frequency/dose of messaging: dependent on method selected, pills required daily and weekly reminders, injectables required weekly and 1 week before the due date, condoms required twice‐weekly and weekly, and no method received messages weekly. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "Text message reminders to use their selected postabortion contraceptive methods and reminders to contact the facility if they had problems or concerns with their method." According to Abraham and Michie's typology: 2 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation conducted after enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of participant blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of researchers being blinded during data collection; however, interviewers contracted from local non‐governmental organisation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Retention rate was 89.1% at follow‐up. Poorer and less‐educated women were more likely to be lost to follow‐up, which could result in an overstimulation of postabortion contraceptive use at follow‐up. The study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in a prespecified way. |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
Brody 2022.
| Study characteristics | ||
| Methods | 2‐arm RCT Aim: to determine the effectiveness of a mobile phone‐based text/voice messaging interventions. The intervention was developed through a participatory process. Focus group discussions and in‐depth interviews were conducted to inform and tailor behaviour change theory‐based text and voice messages. |
|
| Participants | During the implementation phase, 600 female entertainment workers, in the capital city and 3 other provinces in Cambodia. Inclusion criteria: aged 18–30 years; self‐identifying as a female entertainment worker; working at an entertainment venue in the study sites; being currently sexually active, defined as having engaged in oral, vaginal or anal sex in past 3 months; owning a mobile phone; knowing how to retrieve voice messages or retrieve and read text messages; willing to receive 2 text messages/voice messages per week for 1 year; providing written informed consent; and agreeing to a follow‐up visit after 6 and 12 months. Exclusion criteria: not stated |
|
| Interventions | Control: standard care Intervention: by utilising a text/voice messaging platform, the intervention provided female entertainment workers with information, resources and reminders. The central components of the Mobile Link intervention were the text messages and voice messages containing health information and referral linkage information to health services and resources. From the formative research process, 180 messages were designed covering 10 health themes identified as the most important by participants. A message was delivered twice a week for 10 weeks, and the message from each topic area was repeated every 10 weeks for 60 weeks. The health messages were framed using rights‐based and health promotion frameworks. |
|
| Outcomes | Primary outcomes
|
|
| Behaviour change techniques | The Mobile Link intervention was informed by behaviour change theories and extensive formative research. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly selected 600 participants from a list of 4000 female entertainment workers by age group (18–24 and 25–30 years) and study site using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not stated by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Recruited female entertainment workers were assigned a unique identification number to protect their privacy and blind the researchers from their treatment arm assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Recruited female entertainment workers were assigned a unique identification number to protect their privacy and blind the researchers from their treatment arm assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large loss to follow‐up (> 50%). Authors identified significant baseline differences between loss‐to‐follow‐up and completed trial participants. |
| Selective reporting (reporting bias) | Low risk | Predetermined study indicators were systematically assessed. Study protocol prepublished and followed. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Bull 2016.
| Study characteristics | ||
| Methods | Cluster RCT. Aim: to evaluate whether a text message intervention called "Youth All Engaged!" (YAE) increased the effects of an adolescent pregnancy prevention TOP for youths, specifically:
Duration: September 2011 to September 2014 |
|
| Participants | 852 participants from 8 boys and girls clubs – 4 clubs were assigned to the intervention. Inclusion criteria: aged 14–18 years Exclusion criteria: not reported |
|
| Interventions | Control group: received only the TOP Intervention group: received the YAE text message intervention plus TOP Frequency/dose: all participants received 25 weekly TOP sessions over 9 months and 20 hours of community service learning. Intervention participants received 5–7 messages weekly. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "text message intervention … participants received between 5 and 7 messages weekly, of which 40% were bidirectional (i.e., requesting a response)." The article contains a table setting out intervention content which was reviewed to classify the approach in terms of behaviour change typology. According to Abraham and Michie's typology: 6 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 8 boys and girls clubs were cluster randomised to 32 unique randomisation units to ensure that each club would be an intervention site in 2 years and a control site in 2 years. No other information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants could not be blinded. No information on blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of personnel. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up about 24%. Those retained differed from those lost to follow‐up on several baseline characteristics. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. |
| Other bias | High risk | Both the baseline and the follow‐up surveys were self‐administered online surveys. |
Castano 2012.
| Study characteristics | ||
| Methods | Individual RCT Estimated 6‐month continuation rate in the control group of 40% and that a sample size of 960 would be required to detect a 10% change in OC continuation, with 80% power at a 0.05 level of significance, anticipating 15% loss to follow‐up |
|
| Participants | 962 sexually active females aged 13–25 years electing to use OC at a Planned Parenthood FP health centre in downtown Brooklyn, New York, USA | |
| Interventions | Control group: routine care including contraceptive counselling by staff and an educational information handout detailing use, effectiveness, benefits and risks. Intervention group: routine care plus automated mobile phone‐based intervention comprising 180 daily text messages aiming to improve OC continuation. This included an introductory message, 3 reminders of how to change contact information or message time, 47 individual educational messages, repeated up to 4 times, which incorporated 6 domains of OC knowledge (risks, benefits, adverse effects, use, effectiveness and mechanisms of action), 12 × 2‐way messages for quality control and a final message. Intervention duration was 180 days. |
|
| Outcomes | Primary outcome
Secondary outcomes
All outcomes assessed by phone 6 months after enrolment. |
|
| Behaviour change techniques | As defined by study authors: the educational messages incorporated 6 domains of OC knowledge: risks, benefits, adverse effects, use, effectiveness and mechanisms of action According to Abraham and Michie's typology: 4 behaviour change techniques used (see Table 2). |
|
| Notes | Loss to follow‐up: 28% in the intervention group and 30% in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to generate the sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible; outcome may have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded, as participants were asked about satisfaction with the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Main reason for incomplete data unlikely to be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome of contraceptive continuation stated in the ClinicalTrials.gov entry but insufficient detail on prespecified measurements. |
| Other bias | High risk | Possibility of detection (social desirability or recall) bias with self‐report measures of contraception use. |
Chernick 2017.
| Study characteristics | ||
| Methods | Pilot RCT of a theory‐based, unidirectional educational and motivational text message intervention providing reproductive health information versus standardised instructions Aim: to determine the feasibility and acceptability of a text message intervention to increase contraception initiation amongst adolescent females at high risk of pregnancy. Feasibility was examined by rates of screening, recruitment, randomisation, retention, opt‐outs (to stop receiving messages) and technological failures. Acceptability was assessed by interest in future messages, liking the messages, preferences for distribution schedule, and concerns about cost or safety during phone call follow‐up. Duration: intervention arm received unidirectional (1‐way) texts for 3 months. Total 11 months. |
|
| Participants | 100 women enrolled and 88 followed up Inclusion criteria: adolescent females aged 14–19 years who were sexually active with males in the past 3 months and presented to the emergency department for a reproductive health complaint (e.g. vaginal bleeding or discharge, dysuria, and abdominal pain). Exclusion criteria: using effective contraceptive methods (IUD, implant, injection, ring, patch or OC) and who were pregnant, were cognitively impaired, had no mobile phone, or did not speak English or Spanish. People were not excluded based on pregnancy intentions. |
|
| Interventions | Control group: consisted of a wallet card advertising a walk‐in FP clinic and a standardised monologue given by the emergency department physicians describing the need for reproductive care. Intervention group: theory‐based, unidirectional educational and motivational texts providing reproductive health information versus standardised discharge solutions distributed in English and Spanish. Frequency/dose: each participant was sent identical message series and timing, comprising 33 texts, delivered between 12:00 and 21:00, ranging from daily to every 5 days over 3 months. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "The (intervention) arm received unidirectional (one‐way) texts for 3 months. Text content, dosing, and schedule were based on a modified Health Belief Model. Each participant was sent 33 [identical] texts over 3 months. Information about the family planning clinic was incorporated into the text messages." Content of messages available in online supplement of Chernick 2017. According to Abraham and Michie's typology: 6 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by software program. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More participants lost in the intervention arm. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes stated in the ClinicalTrials.gov entry but insufficient details on prespecified measurements and subgroup analyses. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use (may have been more likely to report pill use if in intervention group). |
Francis 2015.
| Study characteristics | ||
| Methods | Individual RCT Aim: to assess whether pregnancy intentions change over time in adolescent females and if baseline intentions can predict contraceptive continuation 4 months after initiating a new form of contraception Duration: 4‐month follow‐up |
|
| Participants | Inclusion criteria: 220 urban, minority adolescent females (ages 15–19 years) presenting for contraceptive initiation in an adolescent health centre in New York City, USA Exclusion criteria: not reported |
|
| Interventions | Control group: did not receive text messages Intervention group: received text messages about their newly initiated contraception method Frequency/dose: unclear |
|
| Outcomes | Primary outcome
|
|
| Behaviour change techniques | As defined by study authors: "At baseline, each participant received a new form of contraception of her choice (3‐month supply of the pill, patch, or ring; Depo injection; or placement/referral for an IUD) and was randomised to receive text messages about this new form of contraception (intervention) or to not receive text messages (control)." Limited information about the content of the text messages. According to Abraham and Michie's typology: 0 behaviour change techniques used (see Table 2) |
|
| Notes | Only abstract published, unpublished data obtained from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear: insufficient information; abstract only. |
| Allocation concealment (selection bias) | Unclear risk | Unclear: insufficient information; abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear: participants not blinded and unclear if outcome influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear: insufficient information whether outcome assessors were aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Unclear risk | Abstract only. |
Harrington 2019.
| Study characteristics | ||
| Methods | Individual RCT Aim: to assess the effect of a 2‐way text message intervention with a nurse on postpartum contraceptive use amongst individual women and couples Duration: follow‐up visits occurred at 6 and 14 weeks and 6 months' postpartum. |
|
| Participants | 260 women attending antenatal clinics in Kenya were randomised to a 2‐way text‐message intervention or control, and 103 male partners were enrolled Inclusion criteria: aged ≥ 14 years; pregnant with an estimated gestational age ≥ 28 weeks; able to read and respond to text messages themselves or with assistance in English, Kiswahili or Dholuo; reported daily access to a mobile phone using the Safaricom network; planned to remain in the study area for 6 months' postpartum; reported HIV‐negative status; were not participating in another research study. Exclusion criteria: HIV‐infected women (due to an ongoing mHealth study at the same facilities implementing a text‐messaging intervention specific to this population) |
|
| Interventions | Control group: no text messages Intervention group: women registered their mobile phone numbers in the Mobile WACh SMS delivery system and received a brief orientation to the intervention at the enrolment visit. Frequency/dose: automated messages were sent once weekly from enrolment until 6 months' postpartum: message content corresponded to participants' gestational age in pregnancy or week postpartum. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "Automated health education message … ending with actionable advice or a question designed to promote dialogue. Automated message content centered around family planning (approximately two thirds of all messages), and included information about available methods and their effectiveness, postpartum pregnancy risk, contraceptive safety during lactation, anticipatory guidance about side effects, community misperceptions, and dual protection. The remaining third of messages were focused on general perinatal topics, such as healthy pregnancy and exclusive breastfeeding. The SMS platform sent automated system messages once weekly from enrolment to 6 months' postpartum, with message content corresponding to participants' gestational age or week postpartum. Women whose male partners were referred for the trial received messages in the couple's specific language." According to Abraham and Michie's typology: 4 behaviour change techniques used (see Table 2). |
|
| Notes | Participants indicated their language of choice (English, Kiswahili or Dholuo), a preferred name for their personalised messages, and a preferred day of the week (Sunday to Thursday) and time to receive automated messages. Study nurses demonstrated that sending text messages to the study short code was free of charge through Safaricom, and explained that nurses were available to respond to messages only on weekdays during business hours and that the text‐messaging system should not be used for urgent medical need. Women were able to discontinue text messages at any time by sending the message 'stop' to the study short code. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of low or high risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it was clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Hebert 2018.
| Study characteristics | ||
| Methods | Individual RCT Aim: to evaluate the effect of miPlan, a waiting‐room contraceptive counselling mobile application, on interest in discussing LARC during the clinical encounter and LARC uptake Duration: 11 months, February 2015 to January 2016 |
|
| Participants | 207 young women were randomised to intervention (104 women) or control (103 women) group Inclusion criteria: women aged 15–29 years, presenting for contraceptive care, sexually active with a male partner in the past 6 months, not pregnant, not using a LARC method, self‐identified African American or Latina/Hispanic, and English speaking were eligible to participate in the study. Exclusion criteria: not reported |
|
| Interventions | Control group: completed an online survey, but did not view the app, and proceeded directly to the routine clinic visit consisting of contraceptive counselling with a reproductive health assistant and the contraceptive administration visit with a clinician. Intervention group: mobile app that addressed all methods of contraception and included young people's ideas for content such as images of each method, information on adverse effects of each method, contraceptive effectiveness rates rather than failure rates and information about men's experiences with each method. Frequency/dose: prior to their routine clinic visit |
|
| Outcomes |
|
|
| Behaviour change techniques | As defined by study authors: "Mobile app providing information on all methods of contraception to be used in the waiting room prior to the clinical visit … The Transtheoretical Model of Behavioral Change and the Theory of Planned Behavior informed app content, focusing on attitudes, norms, and behavioral intentions regarding contraceptive use. … In brief, the app addressed all methods of contraception and included young people's ideas for content such as: images of each method, information on side effects of each method, contraceptive effectiveness rates rather than failure rates, and, information about men's experiences with each method. In addition, the app included short videos (less than 1 minute) about different LARC methods based on interviews with African American and Latino LARC users. Videos were based on interviews with young women who used these methods. Interviews informed videos describing the patient experience (e.g., side effects, the insertion process)". Further detail published in Akinola 2018 on the development of the mobile app. According to Abraham and Michie's typology: 7 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on how participants were recruited. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All health care providers were blinded to study group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Unclear risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Hou 2010.
| Study characteristics | ||
| Methods | Individual RCT Estimated a mean of 2.6 missed pills per cycle in the control group, and that a sample size of 68 would be required to detect a 1.6 pill improvement with SD of 2 pills, with 90% power at a 0.05 level of significance, anticipating 15% loss to follow‐up. |
|
| Participants | 103 women enrolled and 82 randomly assigned after a 1‐month run‐in period. 82 sexually active females electing to start using OC, seeking care at Planned Parenthood League of Massachusetts, USA. Mean age: 22 years (range 18–31 years) |
|
| Interventions | Control group: routine care according to standard clinic protocol (not stated) during a 1‐month run‐in period. Women did not receive text message reminders. Study authors reported a high rate of reminder system use in the control group, particularly electronic systems such as mobile phone alarms that mimicked the study intervention. Intervention group: routine care according to standard clinic protocol (not stated) during the 1‐month run‐in period plus an automated daily text message aiming to improve OC adherence, "Please remember to take your birth control pill," sent at a designated time chosen by the participant over the 3‐month study period. |
|
| Outcomes |
|
|
| Behaviour change techniques | As defined by study authors: not described According to Abraham and Michie's typology: 3 behaviour change techniques used (see Table 2). |
|
| Notes | Loss to follow‐up: 12% intervention and 10% control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible; outcome may have been influenced by lack of blinding. Increased use of reminders in the control group suggests that allocation to intervention or control group may have altered behaviour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for missing data (mechanical and technological issues) unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes stated in the ClinicalTrials.gov entry, but insufficient detail on prespecified measurements and subgroup analyses. |
| Other bias | Low risk | Study appeared free of other sources of bias (electronic medication monitor used to assess outcome). |
Johnson 2017.
| Study characteristics | ||
| Methods | Individual RCT Aim: to estimate the effect of m4RH, an mHealth service in Kenya that provides FP information via text message, on consumers' knowledge and use of contraception Duration: September 2013 to May 2014 Collected data on outcomes and covariates via text message; survey messages were sent in 3 waves. |
|
| Participants | 13,629 people randomised, for contraception use 1419 analysed in the intervention group and 1444 in the control group Inclusion criteria: all new consumers who accessed the m4RH service to either a full‐access group or a limited‐access group Exclusion criteria: existing m4RH consumers; phone numbers registered when technology was having problems with assignment logic. |
|
| Interventions | Control group: members of the limited‐access group were provided with access to the clinic locator along with general motivational messages on a variety of health topics but did not have access to any other m4RH content. Motivational messages were designed to keep the consumers engaged with the m4RH service but not to directly affect any of the outcome measures focused on in this study. Members of the limited‐access group were provided access to all m4RH content after data collection was complete i.e. a period of 3 months. Intervention group: a text‐message‐based platform providing information on the benefits, disadvantages and adverse effects of 9 FP methods as a well as a searchable database of clinics that offer FP counselling and services. Frequency/dose: m4RH was a "pull" rather than a "push" service. Therefore, m4RH consumers were only sent content that they explicitly requested. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "m4RH … provides information on the benefits, disadvantages and side effects of nine family planning methods as well as a searchable database of clinics that offer family planning counseling and services. m4RH consumers may also sign up to receive 'role model' stories about a person facing a difficult sexual or reproductive health issue and how they resolved the issue." Full content of messages was not provided in article. According to Abraham and Michie's typology: 5 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We assigned new consumers to each group on a rolling basis — that is, if the most recent new consumer was assigned to the full‐access group, we assigned the current new consumer to the limited‐access group. We consider this assignment rule effectively random for two reasons. First, m4RH had an extremely high number of consumers. Second, due to differences in network speed and coverage throughout Kenya, there was large variation in SMS delivery times. We did not seek consent from m4RH consumers prior to initial randomisation as the risk to the limited‐access group was low. We excluded all existing m4RH consumers from the study and continued to provide these consumers full access to." Rule based on time of admission. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described, or not described in enough detail to allow a definite judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judged that the outcome measurement was unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Low risk | The study appeared free of other sources of bias. |
McCarthy 2018.
| Study characteristics | ||
| Methods | Individual RCT Aim: to assess the effect of the intervention on the acceptability of effective contraceptive methods amongst young people in Tajikistan. Superiority trial with a 1:1 allocation ratio Duration: November 2016 to July 2017 Parallel‐group, individually randomised superiority trial with a 1:1 allocation ratio evaluating the effect of an intervention delivered by MPA |
|
| Participants | 575 women randomised to the control (298 women) or intervention group (275 women). Inclusion criteria: women aged 16–24 years; owned a personal Android mobile phone; lived in La Paz or El Alto; reported an unmet need for contraception (i.e. sexually active, not using effective contraception, and wanted to avoid pregnancy); could provide informed consent; could read Spanish; willing to receive messages on contraception on their mobile phone. Exclusion criteria: did not fit into inclusion criteria |
|
| Interventions | Control group: had access to the app plus control instant messages about trial participation. Intervention group: MPA that contained basic information about contraception and provided instant messages on contraception. Frequency/dose: intervention group received 0–3 messages per day (a total of 183 messages) for 120 days. Control group received 16 messages about trial participation over 120 days. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "short mobile phone instant messages informed by the Integrated Behavioural Model (IBM) … 10 behaviour change methods (BCM) (belief selection, facilitation, anticipated regret, guided practice, verbal persuasion, tailoring, cultural similarity, arguments, shifting perspective and goal setting) The messages provided information about contraception, targeted beliefs identified in the development phase that influence contraceptive use and aimed to support young people in believing that they can influence their reproductive health." The development of the approach was covered in McCarthy 2019a. The content in each country was slightly different. According to Abraham and Michie's typology: 6 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence generated by the remote computer‐based randomisation software. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants would have been aware of the allocation after they started receiving the messages. However, allocation was blinded from the research staff collecting outcome data unless the participant revealed it to them. Treatment allocation was blinded from the researchers who analysed data. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff were blinded to allocation unless the participant revealed it to them. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for missing data (mechanical and technological issues) unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Appeared to be low. |
| Other bias | Unclear risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
McCarthy 2019a.
| Study characteristics | ||
| Methods | Individual RCT | |
| Participants | 578 participants were enrolled and 464 (80%) completed follow‐up at 4 months Inclusion criteria: women aged 18–24 years, did not report using an effective method of contraception, owned a personal mobile phone, lived in the West Bank (Palestine) and could read Arabic. |
|
| Interventions | Control group: received 16 control messages about trial participation over 120 days. Intervention group: mobile phone text message for married and unmarried women. Group received 0–3 messages per day (113 messages for unmarried and 120 messages for married) for 120 days. |
|
| Outcomes | Primary outcome
Secondary outcomes
Process outcomes included knowledge, perceived norms, personal agency and intention. All outcomes were self‐reported |
|
| Behaviour change techniques | As defined by study authors: "the intervention was informed by the integrated behavioural model and was sent by mobile phone text message. … intervention messages provided information about contraception, targeted beliefs identified in the development phase that influence contraceptive use (e.g. misconceptions about the side effects and health risks of contraception, belief that non‐hormonal methods are better because they are not harmful to health) and aimed to support young women in believing that they can influence their reproductive health. The intervention contained the following behaviour change methods, adapted for delivery by mobile phone: belief selection, facilitation, anticipated regret, guided practice, verbal persuasion, tailoring, cultural similarity, arguments, shifting perspective and goal setting." Further detail on the intervention were published in McCarthy 2018. This article also provided further detail on McCarthy 2018 as both interventions shared the same development but different message content. Sample messages are provided in Table 5 of the paper and these provide additional insight into the approaches used. According to Abraham and Michie's typology: 6 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online computer‐based system used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | The system sent the Palestinian texting platform the allocation, preferred time slot for message delivery, mobile phone number and marital status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants would have been aware of the allocation after they started receiving the messages. However, allocation was blinded from the research staff collecting outcome data unless the participant revealed it to them. Treatment allocation was blinded from the researchers who analysed data. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation was blinded from the research staff collecting outcome data unless the participant revealed it to them. Treatment allocation was blinded from the researchers who analysed the data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention did not differ between the groups (81% in the control and 79% in the intervention group, Pearson's Chi2 test P = 0.53). The main predictor of retention was completion of university at enrolment (odds ratio 1.80, 95% confidence interval 1.18 to 2.73; P = 0.01). The effect of this predictor of retention did not differ by group (interaction test P = 0.78). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
McCarthy 2020.
| Study characteristics | ||
| Methods | MPA using "behaviour change methods" based on integrated behavioural models which uses short instant messages sent through Tú decides app in Bolivia Parallel group, individually randomised superiority controlled trial with a 1:1 allocation ratio. Aim: to establish if the intervention of short instant messages increases young Bolivian women's use and acceptability of the effective contraceptive methods. Duration: 120 days Randomisation: allocation sequence was generated by the remote computer‐based randomisation software. |
|
| Participants | 1172 screened, 496 not eligible, 125 eligible but declined, 645 submitted for randomisation, 642 randomised Inclusion criteria: women aged 16–24 years, owned a personal Android mobile phone, lived in La Paz or El Alto, reported an unmet need for contraception (i.e. were sexually active, not using effective contraception and want to avoid a pregnancy) and could read Spanish Exclusion criteria: not reported |
|
| Interventions | Control: participants had access to the Tú decides app and 7 control instant messages about the importance of their participation and reminding them to contact the project co‐ordinator if they change their number (which intervention participants also received). Intervention: provided accurate information about contraception, targeted the beliefs identified in the development phase that influence contraceptive use (e.g. specific misconceptions about the adverse effects and health risks of contraception), and aimed to support young women in believing that they could influence their reproductive health. Participants allocated to the intervention group received 0–3 messages per day (total 183 messages) for 120 days. Frequency dose: 0–3 messages a day (183 messages for 120 days) |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Behaviour change techniques | The messages contained 10 behaviour change methods, adapted for delivery by mobile phone: belief selection, facilitation, anticipated regret, guided practice, verbal persuasion, tailoring, cultural similarity, arguments, shifting perspective and goal setting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated by the remote computer‐based randomisation software. |
| Allocation concealment (selection bias) | Low risk | Local research staff collecting outcome data were blinded to allocation unless the participant revealed it to them. Researchers who analysed the data were blinded to treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, participants were aware of the allocation as soon as they started receiving messages. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation when data collecting and analyse of data unless participant revealed it to them. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention did not differ between arms. |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Unclear risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Nuwamanya 2020.
| Study characteristics | ||
| Methods | RCT in which access to SRH information, goods, and services using a MPA compared to standard of care of access to SRH information, goods, and services Aim: to assessed the effectiveness of using a MPA to increase access to SRH information, goods, and services amongst university students in Uganda Duration: 6 months Randomisation: participants were randomised 1:1 to MPA and control using computer‐generated random numbers. The research team, including providers at health facilities, transport providers, and payment technicians, and participants were blind to the intervention group, but the app developer was not. |
|
| Participants | 1180 assessed for eligibility, 68 excluded. 1112 randomised participants and were recruited from Kyambogo University halls of residence Inclusion criteria: aged 18–30 years; self‐reported sexual activity in last 6 months, > 12 months to graduation, access to an internet‐enabled Android smartphone, informed consent Exclusion criteria: not reported |
|
| Interventions | Control group: no intervention, i.e. accessed SRH information, goods and services as they did before the onset of the trial. Intervention: access to an MPA to enable access to SRH information, goods and services over 6 months. App included:
|
|
| Outcomes | Primary outcomes There were 4 primary outcomes in the trial all reflecting changes from baseline to end of 6‐month follow‐up period:
Secondary outcomes
|
|
| Behaviour change techniques | To be assessed according to Abraham and Michie's typology. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 allocation with computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | The research team, including providers at health facilities, transport providers and payment technicians, and participants were blind to the intervention group, but the app developer was not. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The research team, including providers at health facilities, transport providers, and payment technicians and participants were blind to the intervention group, but the app developer was not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research team, including providers at health facilities, transport providers and payment technicians, were blind to the intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any analysis of difference in those lost to follow‐up, although high attrition rate. |
| Selective reporting (reporting bias) | Low risk | Study's prespecified (primary and secondary) outcomes were reported as prespecified in the published study protocol. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Reiss 2019.
| Study characteristics | ||
| Methods | Single‐blind, multisite RCT Aim: to evaluate the effect of the intervention on contraceptive use and to monitor for adverse events, including intimate partner violence, which is widespread in Bangladesh |
|
| Participants | Description: 972 women in Bangladesh who had undergone menstrual regulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Control: no messages Intervention: automated interactive voice messages about postmenstrual regulation contraception delivered to women in Bangladesh via mobile phone. Duration: 4 months Frequency/dose: ≥ 11 voice messages about contraception over 4 months after their menstrual regulation; the first 7 messages were delivered at weekly intervals
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "Each participant will be sent a series of 11 automated, interactive voice messages sent to their mobile phone over a 4‐month period, starting within a week of the MR procedure. Messages will be sent weekly for the first 6 weeks and fortnightly for the following 8 weeks. … The content of the 11 messages is tailored to the individual's chosen method as follows: the method of contraception received at the clinic is used to allocate participants to one of six message groups: no method users, condom users, pill users, injectable users, implant users and IUD users. Seven core messages will be sent to all participants reminding them of the benefits of using contraception, addressing key barriers such as fear of infertility and addressing information gaps, particularly around LARC and permanent methods. The remaining four messages will be specific to the method group for example, pill users will receive the seven core messages plus four messages tailored to supporting pill use. For current contraceptive users, the tailored messages provide information and support for continuation and correct use of their chosen method, they also aim to promote safe switching among women who are not happy with their method." According to Abraham and Michie's typology: 5 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into the intervention or control group using computer. |
| Allocation concealment (selection bias) | Low risk | 1:1 ratio intervention control group; generated remotely. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for missing data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | All study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Unclear risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Rinehart 2020.
| Study characteristics | ||
| Methods | Pilot RCT Aim: to evaluate the feasibility, acceptability and initial efficacy of a pilot texting intervention ("t4she") in primary care designed to increase sexual health knowledge and promote dual protection strategies to reduce unintended pregnancies and STIs amongst adolescent females. Follow‐up surveys conducted at 3‐ and 6‐months postbaseline Duration: 12 weeks Randomisation: unclear |
|
| Participants | Recruitment occurred at 2 federally qualified community health centres in Denver, Colorado, USA. 244 study participants were recruited and randomised. Inclusion criteria: female at birth and aged 13–18 years; ability to send and receive text messages; not pregnant (verified through urinalysis); not trying to become pregnant in next year; able to participate in English Exclusion criteria: not reported |
|
| Interventions | Control: standard clinic care over 6 months. The 2 clinics where participants were recruited offered Title X FP services, therefore participants in both the intervention and control group had access to the full range of primary care services which included on‐site FP services (confidential teen visits, contraceptive counselling, pregnancy testing, STI/HIV screening and health education). All contraceptive methods, including LARC methods (i.e. IUDs and implants), were available at no cost to adolescents seeking contraception. Intervention: received Texts for Sexual Health Education and Empowerment (t4she), a multidimensional social cognitive framework focused on modifiable factors related to decision‐making and behaviour and used in contraceptive research. The finalised t4she intervention included 58 automated messages sent over 12 weeks. |
|
| Outcomes | Self‐reported outcome variables were collected at baseline, 3‐ and 6‐month follow‐up surveys
|
|
| Behaviour change techniques | Messages covered a range of topics and targeted Health Belief Model constructs. Message format varied; 38% were bidirectional and 33% included a link to a website or graphic to reinforce the message. A summary of the intervention and sample messages are included as supplementary material. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data were stored in an encrypted file immediately following completion and transferred to a secure server. A statistical software program was used to randomly allocate study IDs to intervention condition and study envelopes were premade that contained intervention assignment. |
| Allocation concealment (selection bias) | Low risk | The researcher, blinded to the assignment, opened the envelope after the baseline interview and discussed intervention assignment with the participants, then paid them a USD 15 gift card. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After baseline interview, discussed assignment with participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher, blinded to this assignment, opened the envelope after the baseline interview and discussed intervention assignment with the participants, then paid them a USD 15 gift card. It can be inferred that outcome accessors were not subject to blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Key baseline differences between those who completed the 6‐month follow‐up survey and those who did not. |
| Selective reporting (reporting bias) | Low risk | The study followed a predetermined pattern of reporting outcomes. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Rokicki 2017.
| Study characteristics | ||
| Methods | Cluster RCT Aim: to evaluate whether text‐messaging programmes can improve reproductive health amongst adolescent girls in low‐ and middle‐income countries Duration: 16 months (1 month of enrolment plus 15 months of follow‐up) |
|
| Participants | 756 female students aged 14–24 years in Accra, Ghana recruited between 15 January and 28 February 2014 Inclusion criteria: schools were selected after permission from the headmaster/headmistress and a specific class was selected. The chosen classes were in their second year of senior secondary school. Female students in the chosen class of each school were invited to participate in the study. Participants used their own mobile phones or could use a family member's phone. Participants without phones were eligible to be enrolled in the trial; however, phones were not provided. Secondary day schools were the primary sampling unit. Exclusion criteria: male; secondary school student at a boarding school; girls who refused consent. |
|
| Interventions | Control group: sent placebo messages once a week with information about malaria Intervention group
Frequency/dose: unidirectional intervention participants were sent 1 reproductive health message via text message once a week. The interactive intervention participants did not receive any information initially, but were sent 1 multiple‐choice quiz question via text message each week to which they were invited to respond free of charge. These participants were sent 2 reminder messages encouraging them to respond if they had not yet responded. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: Unidirectional intervention: participants were sent 1 reproductive health message via text message once a week. These messages focused on pregnancy prevention and contained information on topics of reproductive anatomy, pregnancy, STIs and contraception including male condoms, female condoms, OCs and EC. Interactive intervention: participants were not sent any information initially, but were instead sent 1 multiple‐choice quiz question via text message each week to which they were invited to respond free of charge. Upon responding, participants immediately received a confirmatory text message informing them whether they answered correctly along with the correct answer and additional information, which corresponded to the information provided in the unidirectional intervention. During the course of the week, participants were sent up to 2 reminder messages encouraging them to respond if they had not yet responded. Participants who never responded were sent a text message with the correct answer and the additional information at the end of the week. For every 2 correct responses, participants were sent an airtime credit reward of 1 GHS (USD 0.38). Airtime credit rewards were sent at the end of the week, along with a message informing participants of how many questions they had correctly answered and encouraging them to continue participating. As part of the intervention, the unidirectional and interactive groups also received 4 extra tips about the effectiveness of condoms, the benefits of talking with their boyfriend about reproductive health and the existence of a free public hotline number that they could call for reproductive health information (sent twice). After 3‐month follow‐up, participants in both intervention and control arms were offered a 30‐ to 45‐minute lecture about reproductive health by a nurse. According to Abraham and Michie's typology: 3 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated random number draw by the principal investigator. |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated. However, participants in all groups were told they would receive "health messages" on their phones, including such topics as reproductive health or malaria. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and data collection staff could not be blinded because the intervention required overt participation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of subjective outcome assessment not reported/mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 756 participants enroled in the study, of whom 716 (95%) were successfully followed up at 3 months and 721 (95%) were successfully followed up at 15 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | Analysis by intention‐to‐treat. |
Smith 2015b.
| Study characteristics | ||
| Methods | Individual RCT Estimated that use of effective contraception at 4 months would be 35% in the control group, and a sample size of 500 would be required to detect a 13% improvement in contraceptive use, with 90% power at a 0.05 level of significance |
|
| Participants | 500 participants Inclusion criteria: females aged ≥ 18 years, with a mobile phone primarily for their own use, reporting not wanting to be pregnant, willing to receive automated voice messages related to contraception, attending for induced abortion at 4 Marie Stopes International clinics in Cambodia |
|
| Interventions | Control group: routine care, which included postabortion FP counselling at the clinic in accordance with national guidelines, the offer of a clinic follow‐up appointment, the clinic phone number and the Hotline number operated by counsellors at MSI Cambodia. Intervention group: routine care plus a mobile phone‐based intervention aiming to improve uptake and adherence comprising 6 automated, interactive voice messages, counsellor delivered phone support according to response to messages and additional reminder messages for OC or injectable users. |
|
| Outcomes | Primary outcome
Secondary outcomes
All outcomes assessed by phone at 4 and 12 months |
|
| Behaviour change techniques | As defined by study authors: phone calls aimed to support contraceptive use by addressing participants' capability to use contraception by providing individualised information on a range of contraceptive methods, opportunity to use contraception (e.g. informing participants where they could access specific methods near to their residence) and motivation by re‐enforcing the benefits of contraception use. According to Abraham and Michie's typology: 5 behaviour change techniques used (see Table 2). |
|
| Notes | Loss to follow‐up: 15% in the intervention group and 12% in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐based randomisation programme. |
| Allocation concealment (selection bias) | Low risk | Web‐based allocation performed after enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible; outcome may have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers who undertook data collection and analysis were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. Reasons for missing data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Study's prespecified (primary and secondary) outcomes have been reported as prespecified in the published study protocol. |
| Other bias | High risk | Possibility of detection (social desirability or recall) bias with self‐report measures of contraception use. |
Trent 2013.
| Study characteristics | ||
| Methods | Pilot individual RCT (primarily a feasibility and acceptability trial) | |
| Participants | 100 female adolescents aged 13–21 years recruited from an urban academic practice in a high teen and unplanned pregnancy prevalence community in the USA, currently using medroxyprogesterone acetate (Depo‐Provera), with a mobile phone with text messaging capability for personal use. Most participants were African American and resided in low‐income, single parent, mother‐headed households. | |
| Interventions | Control group: clinic protocol for standard care, which included participant‐initiated support and clinical nursing outreach for missed appointments. Intervention group: routine care plus automated intervention aimed to improve follow‐up medroxyprogesterone acetate (Depo‐Provera) clinic attendance and comprised a welcome message, daily text appointment reminders starting 72 hours before the clinic visit with the option to cease messages by responding (yes or no) with their plans to attend the visit. Intervention adolescents also received prescheduled health messages over the course of the 3‐month enrolment period regarding condom use for STI prevention, healthy weight management, encouragement to call the nurse for problems and an STI screening reminder. All message signatures indicated that they were from the nurse case manager to build relationships with the clinical team. |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Behaviour change techniques | As defined by study authors: not described According to Abraham and Michie's typology: 2 behaviour change techniques used (see Table 2). |
|
| Notes | Information from abstract and additional communication with investigator. Full text not yet published. Loss to follow‐up: 12% in the intervention group and 14% in the control group. Not included in meta‐analysis due to outcome measures not being measured in a comparable way. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by permitted block design (according to investigator's communication). |
| Allocation concealment (selection bias) | Low risk | Allocation sealed in envelope for nurse until informed consent to participate (according to investigator's communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible; outcome may have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Principal investigatory blinded to allocation (according to investigators' communication). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. Reasons for missing data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | Primary outcome prespecified in the ClinicalTrials.gov record. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Tsur 2008.
| Study characteristics | ||
| Methods | Individual RCT Estimated that use of contraception would be 50% in the control group, and a sample size of 100 would be required to detect 30% improvement in contraceptive use, with 80% power at a 0.05 level of significance. |
|
| Participants | 108 females aged 16–45 years, some users and some not users of contraception, using or planning to use isotretinoin (a drug for acne), who phoned the Drug Consultation Centre at Assaf Harofeh Medical Center in Israel seeking advice regarding isotretinoin. | |
| Interventions | Control group: routine care comprised information on isotretinoin including contraceptive use only during the initial interview. Intervention group: automated intervention aimed to increase contraception use and comprised routine care plus additional information about teratogenic risk and the importance of contraceptive use in mailed written form and by text messages sent to mobile phones 1 and 2 months after the initial call |
|
| Outcomes | Primary outcome
Secondary outcomes
All outcomes assessed by phone call at 3 months |
|
| Behaviour change techniques | As defined by study authors: not described According to Abraham and Michie's typology: 2 behaviour change techniques used (see Table 2). |
|
| Notes | 5 (5%) participants lost to follow‐up at 3 months and not included in the final analysis. Differential loss to follow‐up between intervention and control groups not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers kept in sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described in adequate detail. Sealed envelopes used, but unclear whether they were sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible; outcome may have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information on whether outcome assessors were aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Primary outcome reported using measurements that were not prespecified in the methods section of the paper. |
| Other bias | High risk | Possibility of detection (social desirability) bias with self‐report measures of contraception use. |
Unger 2018.
| Study characteristics | ||
| Methods | 3‐arm unblinded individually randomised control trial Aim: to assess the effect of a text message intervention on facility delivery, exclusive breastfeeding and postpartum contraceptive use Duration: 24 weeks |
|
| Participants | 300 women attending antenatal care Inclusion criteria: pregnant women seeking antenatal care at the Mathare North Health Centre Maternal Child Health clinic in Kenya; aged ≥ 14 years; pregnant and < 36 weeks estimated gestational age; had access to a mobile phone using the Safaricom Ltd network; could communicate via text message; planned to remain in the area for 6 months' postpartum; not part of another research study Exclusion criteria: not reported |
|
| Interventions | Control group: no text message intervention but received routine messages and received routine clinic‐based counselling and care Intervention group: registered into the Mobile WACh text message delivery platform
To assess the effect of text message communication on facility delivery, exclusive breastfeeding and contraceptive use Frequency/dose
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Behaviour change techniques | As defined by study authors: "The automated system incorporated a personalised approach that provided gestational age‐appropriate educational and counselling messaging. All messages included participant name, clinic and nurse name, an educational message, and actionable advice targeting one of the main study outcomes." Message content is not described in detail aside from the statement that "Mobile WACh messages were crafted to be personalised, actionable, and outcome focused." According to Abraham and Michie's typology: 2 behaviour change techniques used (see Table 2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list using random block sizes. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Randomisation allocation was unblinded. Women were not blinded to their assignment, which may have led to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | About 10% loss to follow‐up. Reason for missing data (mechanical and technological issues) unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Wilkinson 2017.
| Study characteristics | ||
| Methods | Individual RCT Aim: to examine the feasibility of using text messages as a convenient mechanism to remind adolescents to fulfil their advance EC prescriptions Duration: June 2011 to February 2012, 9 months |
|
| Participants | 60 women from an adolescent clinic in an urban medical centre in the USA were randomised. 11 were reached for follow‐up in the control group and 17 reached for follow‐up in the intervention group. Inclusion criteria: English‐speaking women; sexually active; aged 13–21 years; had working personal mobile phones that could receive texts; were Medicaid beneficiaries whose health plan covered prescribed EC at no cost and agreed to provide prescription fill data to investigators. Exclusion criteria: pregnant, trying to become pregnant, or using long‐acting forms of contraception |
|
| Interventions | Control group: no texts Intervention group: text message on the participants' mobile phone at 1, 3 and 5 days after recruitment stating "Reminder‐don't forget to fill your prescription you obtained in clinic yesterday. Please call ******* if you have any questions or difficulty obtaining the medication." Frequency/dose: received a text reminder on days 1, 3 and 5 after randomisation |
|
| Outcomes | Primary outcome
|
|
| Behaviour change techniques | As defined by study authors: participants in the texting group received a text on their mobile phones 1, 3 and 5 days after recruitment. The text stated "Reminder‐don't forget to fill your prescription you obtained in clinic yesterday. Please call ******* if you have any questions or difficulty obtaining the medication." According to Abraham and Michie's typology: 1 behaviour change technique used (see Table 2). |
|
| Notes | Not included in meta‐analysis due to outcome measures not being measured in a comparable way. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomisation. |
| Allocation concealment (selection bias) | Low risk | Allocations were placed in sealed concealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the study whether blinding occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout. |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome was reported using measurements that were not prespecified in the methods section of the paper. |
| Other bias | High risk | Review author consensus. |
app: application; EC: emergency contraception; FP: family planning; IUD: intrauterine device; IVR: interactive voice response; LARC: long‐acting reversible contraceptive; MPA: mobile phone app; OC: oral contraceptive; SD: standard deviation; SRH: sexual reproductive health; STD: sexually transmitted disease; STI: sexually transmitted infection; TOP: Teen Outreach Program; YAE: Youth All Engaged!
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2016 | Wrong population |
| Ampt 2020 | Wrong population |
| Arundhati 2018 | Wrong study design – not RCT |
| Ashcroft 2017 | Wrong study design – not RCT |
| Atnafu 2017 | Wrong population |
| Avishek 2018 | Wrong outcomes |
| Ayiasi 2015 | Wrong intervention |
| Bachanas 2016 | Focussed on HIV/sexually transmitted infections |
| Bailey 2015 | Focussed on HIV/sexually transmitted infections |
| Bangal 2018 | Wrong outcomes |
| Bannink 2014 | Wrong intervention |
| Berenson 2012 | Wrong intervention |
| Biswas 2015 | Wrong outcomes |
| Bracken 2014 | Wrong outcomes |
| Brody 2018 | Duplicate; protocol only – completed trial used in analysis |
| Brown 2018 | Wrong intervention |
| Bull 2017 | Duplicate |
| Burke 2018 | Wrong study design – not RCT |
| Castaño 2012 | Duplicate |
| Constant 2014 | Wrong outcomes – no appropriate outcome measures |
| De Kruijf 2016 | Wrong study design – not RCT |
| de Tolly 2014 | Wrong outcomes |
| Decker 2020 | Wrong outcomes |
| Espey 2021 | Wrong intervention |
| Feyisetan 2015 | Wrong study design – not RCT |
| Frank‐Herrmann 2017 | Wrong study design – not RCT |
| Free 2016a | Focus on preventing sexually transmitted disease rather than providing contraception |
| Free 2016b | Duplicate; focus on preventing sexually transmitted disease rather than providing contraception |
| Ghanotakis 2017 | Wrong intervention |
| Gilliam 2016 | Wrong intervention |
| Gold 2011 | Focussed on HIV/sexually transmitted infections |
| Gonsalves 2015 | Protocol only |
| Gonsalves 2018 | Protocol only |
| Green 2018 | Wrong study design – not RCT |
| Hall 2013 | Wrong outcomes |
| Hall 2014 | Wrong intervention |
| Harrington 2017a | Wrong outcomes |
| Harrington 2019b | Duplicate |
| Himes 2017 | Wrong intervention |
| Hirshfield 2016 | Wrong population |
| Irons 2015 | Wrong outcomes |
| Juzang 2011 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| Kaoaiem 2012 | Focus on preventing sexually transmitted disease rather than providing contraception; study design – 'quasi‐experimental' design |
| Katz 2011 | Wrong intervention |
| Kirby 2010 | Wrong intervention |
| Kohn 2018a | Wrong intervention |
| Kohn 2018b | Duplicate; wrong intervention |
| Kulathinal 2019 | Wrong study design – not RCT |
| L'Engle 2013 | Wrong study design – not RCT |
| L'Engle 2015 | Protocol only – trial terminated (no results) |
| Lim 2012 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| Mackenzie 2009 | Study design – not RCT |
| Manlove 2020 | Wrong outcomes |
| Margillo 2015 | Wrong study design – not RCT |
| Maslowsky 2016 | Wrong intervention – phone calls |
| McCarthy 2016 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| McCarthy 2018a | Duplicate |
| McCarthy 2018b | Duplicate; correction to included paper |
| McCarthy 2019b | Duplicate |
| Muessig 2014 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| NCT00230880 | Protocol only; wrong intervention (phone‐based counselling) |
| NCT00733707 | Duplicate; protocol only – completed trial included in analysis |
| NCT01401816 | Protocol only |
| NCT01545609 | Duplicate; protocol only – completed trial included in analysis |
| NCT01641380 | Duplicate; protocol only – completed trial included in analysis |
| NCT01746758 | Protocol only |
| NCT01814930 | Protocol only; wrong intervention |
| NCT01894126 | Duplicate; protocol only – completed trial used in analysis |
| NCT01947842 | Wrong outcomes |
| NCT02031575a | Protocol only – full trial included in analysis |
| NCT02031575b | Duplicate; full trial included in analysis; protocol only |
| NCT02093884a | Duplicate; protocol only – completed trial used in analysis |
| NCT02093884b | Duplicate; protocol only – completed trial used in analysis |
| NCT02234271a | Protocol only; wrong intervention and comparator group |
| NCT02234271b | Duplicate; protocol only |
| NCT02396602 | Protocol only – full text uses wrong intervention |
| NCT02579785 | Duplicate; protocol only – completed trial used in analysis |
| NCT02714686 | Wrong outcomes |
| NCT02733692 | Wrong outcomes; protocol only |
| NCT02781714a | Duplicate; protocol only – completed trial used in analysis |
| NCT02781714b | Duplicate; protocol only – completed trial used in analysis |
| NCT02905461 | Duplicate; protocol only – completed trial used in analysis |
| NCT02905513 | Duplicate; protocol only – completed trial used in analysis |
| NCT02905526 | Duplicate; protocol only – completed trial used in analysis |
| NCT03117842 | Duplicate; protocol only – completed trial used in analysis; duplicate |
| NCT03135288 | Wrong intervention – phone calls |
| NCT03194672 | Wrong intervention |
| NCT03253783a | Protocol only |
| NCT03253783b | Protocol only |
| NCT03382132 | Wrong outcomes; protocol only |
| NCT03612518a | Duplicate |
| NCT03612518b | Duplicate |
| Nielsen 2018a | Protocol only; duplicate; focus on sexually transmitted infection |
| Nielsen 2018b | Duplicate; protocol only |
| Nielsen 2021 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| O'Sullivan 2008 | Study design – not RCT |
| PACTR201410000889209 | Protocol only |
| Pathfinder International 2014 | Wrong study design – not RCT |
| Rokicki 2017a | Wrong outcomes – subanalysis of included study |
| Shaaban 2019 | Wrong intervention |
| Smith 2015c | Duplicate |
| Song 2017 | Focus on preventing sexually transmitted disease rather than on providing contraception; conference abstract |
| Sridhar 2013 | Inappropriate intervention – not using mobile device |
| Sridhar 2014 | Wrong study design – not RCT |
| Sridhar 2015 | Wrong intervention; duplicate |
| Suffoletto 2013 | Focus on preventing sexually transmitted disease rather than on providing contraception |
| Tebb 2019 | Wrong outcomes |
| Thiel de Bocanegra 2017a | Wrong study design – not RCT; duplicate |
| Thiel de Bocanegra 2017b | Wrong study design – not RCT; duplicate |
| Travasso 2016 | Wrong study design – not RCT |
| Unger 2018a | Duplicate |
| Unger 2018b | Duplicate |
| Walakira 2013 | Wrong study design – not RCT |
| WHO 2014 | Wrong study design – not RCT |
| Ybarra 2021 | Wrong population |
| Zulu 2020 | Wrong study design – not RCT |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Bates 2018.
| Study name | Evaluating the impact of Marie Stopes International's digital FP counselling application on the uptake of long‐acting and permanent methods of contraception in Vietnam and Ethiopia: a study protocol for a multi‐country cluster randomised controlled trial |
| Methods | 2‐armed, parallel, cluster randomised control trial across all Marie Stopes International clinics (clusters) in Ethiopia (24) and Vietnam (11), randomising 18 clinics to the intervention group and 17 to the control group. Intervention providers will attend a 2‐day DCA‐use training programme, and use DCA in their FP counselling sessions. Usual care providers will counsel clients as before. In the intervention arm, we will also conduct mixed‐methods sampling to assess how providers use DCA (using an observational survey of provider–client interactions), and understand users' experiences of receiving and giving DCA‐based FP counselling (through indepth interviews). |
| Participants | Aim to recruit 75 clients who have had FP counselling per clinic (2625 total), following them up via 2 telephone interviews, initially within 2 days and then at 4 months. |
| Interventions | Marie Stopes International have designed the tablet‐computer based DCA, which prompts structured, supportive, client‐specific and unbiased FP counselling. |
| Outcomes | Primary outcome
Secondary outcomes
Initial follow‐up and 4‐month follow‐up |
| Starting date | |
| Contact information | Joseph P Hicks |
| Notes | ISRCTN11040557 |
Gul 2019.
| Study name | A study protocol for an mHealth, multi‐centre randomized control trial to promote use of postpartum contraception amongst rural women in Punjab, Pakistan |
| Methods | 3‐arm, 10‐month, multicentre, randomised controlled trial conducted at 15 social franchise health facilities in Punjab province of Pakistan |
| Participants | Pregnant women aged 15–44 years who are in their first or second trimester and have a mobile phone for their own use. The intervention counselling module will be developed based on the Integrated Behaviour Model which was recently adapted, and tested for the FP context in Pakistan. It will broadly cover birth‐preparedness, importance of birth spacing and postnatal care. |
| Interventions | Participants will be randomly allocated to 1 of 3 study arms
The phone‐based intervention aims to improve women's ability to use contraception by providing them with information about a range of methods, access to FP methods through outlets such as Suraj social franchise providers, connecting them with Marie Stopes Society field health educators to help them reach the centres, motivation by re‐enforcing the benefits of contraceptive use on women's quality of life, and dispelling myths and misconceptions about modern contraceptive methods. |
| Outcomes |
Risk differences will be used as the measure of effect of the intervention on the outcomes. |
| Starting date | 15 September 2018 |
| Contact information | Junaid‐ur‐Rehman Siddiqui: junaidrehman1994@hotmail.com |
| Notes |
Yeates 2019.
| Study name | Project for Reproductive Equity Through Volunteers and Entrepreneurship, Networks and Technology (PREVENT) |
| Methods | Randomised parallel assignment controlled trial. The program will be piloted for 12 months in various wards and villages in rural and urban Kilimanjaro, Tanzania. |
| Participants | 198 |
| Interventions | Both groups will receive educational text messages on sexual reproductive health and access individually tailored educational resources through interactive voice response services/system via PREVENT (Project for Reproductive Equity Through Volunteers and Entrepreneurship, Networks and Technology) mobile platform. In addition to personal support to be able to contact with a sexual reproductive health community peer mentor in the community for Adolescent Friendly Sexual Reproductive Health counselling and support. The case group will then have access to contraception provided with detailed and discreet information on accessing PREVENT contraceptive access points in all communities included in the study. |
| Outcomes |
|
| Starting date | 21 June 2019 |
| Contact information | yeatesk@queensu.ca |
| Notes |
DCA: digital family planning counselling application; FP: family planning; LAPM: long‐acting and permanent contraceptive method.
Differences between protocol and review
In the protocol for the original review (Smith 2014), we stated that we would assess risk of bias across the following domains: random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential biases. In the initial review and for this update, we assessed risk of bias across the following domains in accordance with the latest version of the Cochrane Handbook for Systematic Reviews of Interventions: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias (Higgins 2019).
Due to the presence of cluster‐randomised controlled trials, we preformed sensitivity analysis using the generic inverse variance random‐effects outcome model using author‐reported adjusted odds ratios (ORs) for the 'Pregnancy' outcome (alongside Peto OR analysis). This sensitivity analysis was not prespecified in our protocol; however, it was performed to assess if statistical method of analysis made an impact on outcome and had been previously discussed with Cochrane editors.
Contributions of authors
CS and CF conceived of the original review.
CS and MV oversaw the search and selection process, including the construction and implementation of search and quality appraisal strategies.
MV and TP contacted authors of papers to ask for additional information from selected papers.
CS, MV, SM, AN and TP screened and selected studies as well as data extraction.
CF, SM and TP commented on risk of bias and assessment of behaviour change techniques.
TP and MV conducted data analysis.
TP, MV, SM and CS wrote various sections of the review.
TP edited the review following Cochrane feedback.
All review authors read and commented on the review.
Sources of support
Internal sources
-
Internal funding, Japan
This work is in part funded by Nagasaki University (salary support for CS, SM, TP).
External sources
-
New Source of support, UK
CS was supported by an Arts and Humanities Research Council (AHRC) grant during 2018 and 2019
Declarations of interest
Two review authors (CS and CF) were also study authors (Smith 2015b). When a review author was also a contributor to an included study, that review author was not involved in the risk of bias assessment and assessment of the certainty of the evidence.
TP: none.
SM: none.
MV: none.
AN: none.
CF: none.
CS: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Babalola 2019 {published data only}
- Babalola S, Loehr C, Oyenubi O, Akiode A, Mobley A. Efficacy of a digital health tool on contraceptive ideation and use in Nigeria: results of a cluster-randomized control trial. Global Health: Science and Practice 2019;7(2):273-88. [DOI: 10.9745/GHSP-D-19-00066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biswas 2017 {published data only}
- Biswas KK, Hossain A, Chowdhury R, Anderson K, Sultana S, Shahidullah SM, et al. Using mHealth to support postabortion contraceptive use: results from a feasibility study in urban Bangladesh. JMIR Formative Research 2017;1(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brody 2022 {published data only}
- Brody C, Chhoun P, Tuot S, Fehrenbacher AE, Moran A, Swendeman D, et al. A mobile intervention to link young female entertainment workers in Cambodia to health and gender-based violence services: randomized controlled trial. Journal of Medical Internet Research 2022;24(1):e27696. [DOI: 10.2196/27696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bull 2016 {published data only}
- Bull S, Devine S, Schmieg SJ, Pickard L, Campbell J, Shlay JC. Text messaging, teen outreach program, and sexual health behavior: a cluster randomized trial. AJPH Research 2016;106(51):117-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castano 2012 {published data only}
- Castano P, Bynum J, Andres R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomised controlled trial. Obstetrics & Gynecology 2012;119(1):14-20. [DOI: 10.1097/AOG.0b013e31823d4167] [DOI] [PubMed] [Google Scholar]
Chernick 2017 {published data only}
- Chernick LS, Stockwell MS, Wu M, Castano PM, Schnall R, Westhoff CL, et al. Texting to increase contraceptive initiation among adolescents in the emergency department. Journal of Adolescent Health 2017;61(6):785-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2015 {published data only}
- Francis JK, Malbon K, Braun-Courville D, Linares LO. Adolescent female pregnancy intentions change four months after initiating contraception –possible cause for poor contraceptive continuation rates? Journal of Adolescent Health 2015;56:71-2. [Google Scholar]
Harrington 2019 {published data only}
- Harrington EK, Drake AL, Matemo D, Ronen K, Osoti AO, John-Steward G, et al. An mHealth SMS intervention on postpartum contraceptive use among women and couples in Kenya: a randomized controlled trial. AJPH Open-Themed Research 2019;109(6):934-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebert 2018 {published data only}
- Hebert L, Hill B, Quinn M, Holl J, Whitaker A, Gilliam M. Mobile contraceptive application use in a clinical setting in addition to standard contraceptive counseling: a randomised controlled trial. Contraception 2018;98(4):281-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hou 2010 {published data only}
- Hou M, Hurwitz S, Kavanagh E, Fortin J, Goldberg A. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstetrics and Gynecology 2010;116(3):633-40. [DOI: 10.1097/AOG.0b013e3181eb6b0f] [DOI] [PubMed] [Google Scholar]
Johnson 2017 {published data only}
- Johnson D, Juras R, Riley P, Chatterji M, Sloane P, Choi SK, et al. A randomized controlled trial of the impact of a family planning mHealth service on knowledge and use of contraception. Contraception 2017;95(1):90-7. [DOI] [PubMed] [Google Scholar]
McCarthy 2018 {published data only}
- McCarthy O, Ahamed I, Kulaeva F, Tokhirov R, Saibov S, Vandewiele M, et al. A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan. Reproductive Health 2018;15(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2019a {published data only}
- McCarthy OL, Zghayyer H, Stavridis A, Sadada S, Ahamed I, Leurent B, et al. A randomized controlled trial of an intervention delivered by mobile phone text message to increase the acceptability of effective contraception among young women in Palestine. Trials 2019;20(228):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2020 {published data only}
- McCarthy OL, Aliaga C, Palacios ME, Gallardo JL, Huaynoca S, Leurent B, et al. An intervention delivered by mobile phone instant messaging to increase acceptability and use of effective contraception among young women in Bolivia: randomized controlled trial. Journal of Medical Internet Research 2020;22:e14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuwamanya 2020 {published data only}
- Nuwamanya E, Nalwanga R, Nuwasiima A, Babigumira JU, Asiimwe FT, Babigumira JB, et al. Effectiveness of a mobile phone application to increase access to sexual and reproductive health information, goods, and services among university students in Uganda: a randomized controlled trial. Contraception and Reproductive Medicine 2020;5:31. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiss 2019 {published data only}
- Reiss K, Andersen K, Pearson E, Biswas K, Taleb F, Ngo T, et al. Unintended consequences of mHealth interactive voice messages promoting contraceptive use after menstrual regulation in Bangladesh: intimate partner violence results from a randomized controlled trial. Global Health: Science and Practice 2019;7(3):386-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rinehart 2020 {published data only}
- Rinehart DJ, Leslie S, Durfee MJ, Stowell M, Cox-Martin M, Thomas-Gale T, et al. Acceptability and efficacy of a sexual health texting intervention designed to support adolescent females. Academic Paediatrics 2020;20(4):475-84. [DOI: 10.1016/j.acap.2019.09.004] [DOI] [PubMed] [Google Scholar]
Rokicki 2017 {published data only}
- Rokicki S, Cohen J, Salomon JA, Fink G. Impact of a text-messaging program on adolescent reproductive health: a cluster-randomized trial in Ghana. American Journal of Public Health 2017;107(2):298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015b {published data only}
- Smith C, Ngo TD, Gold J, Edwards P, Vannas U, Ly S, et al. Effect of a mobile phone-based intervention on post-abortion contraception: a randomized controlled trial in Cambodia. Bulletin of the World Health Organization 2015;93(12):842-50A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trent 2013 {published and unpublished data}
- Trent M, Thompson C, Tomaszewski K. Family planning appointment attendance among urban youth: results from the depotext trial. Journal of Adolescent Health 2013;52(2):100-6. [DOI: 10.1016/j.jadohealth.2012.10.207] [DOI] [Google Scholar]
Tsur 2008 {published data only}
- Tsur L, Kozer E, Berkovitch M. The effect of drug consultation center guidance on contraceptive use among women using isotretinoin: a randomized, controlled study. Journal of Women's Health 2008;17(4):579-84. [DOI: 10.1089/jwh.2007.0623] [DOI] [PubMed] [Google Scholar]
Unger 2018 {published data only}
- Unger JA, Ronen K, Perrier TA, DeRenzi B, Slyker J, Drake AL, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomized trial. BJOG 2018;125:1620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2017 {published data only}
- Wilkinson TA, Berardi MR, Crocker EA, Nordt C, Silverstein M. Feasibility of using text message reminders to increase fulfilment of emergency contraception prescriptions by adolescents. Journal of Family Planning and Reproductive Health Care 2017;43(1):79-80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2016 {published data only}
- Agarwal S, Lasway C, L'Engle K, Homan R, Layer E, Ollis S, et al. Family planning counseling in your pocket: a mobile job aid for community health workers in Tanzania. Global Health: Science and Practice 2016;4(2):300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ampt 2020 {published data only}
- Ampt FH, Lim MS, Agius PA, L'Engle K, Manguro G, Gichuki C, et al. Effect of a mobile phone intervention for female sex workers on unintended pregnancy in Kenya (WHISPER or SHOUT): a cluster-randomised controlled trial. Lancet Global Health 2020;8(12):e1534-45. [DOI] [PubMed] [Google Scholar]
Arundhati 2018 {published data only}
- Arundhati C, Saavala M. mHealth solutions for family planning services. Economic and Political Weekly 2018;53(11):-. [Google Scholar]
Ashcroft 2017 {published data only}
- Ashcroft N, Shelus V, Garg H, McLarnon-Silk C, Jennings VH. Implementation of CycleTel family advice: an SMS-based service to provide family planning and fertility awareness information in India. Mhealth 2017;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atnafu 2017 {published data only}
- Atnafu A, Otto K, Herbst CH. The role of mHealth intervention on maternal and child health service delivery: findings from a randomized controlled field trial in rural Ethiopia. mHealth 2017;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avishek 2018 {published data only}
- Avishek H, Khan ME, Mondal SK. Mobile phone messaging to husbands to improve maternal and child health behavior in India. Journal of Health Communication: International Perspectives 2018;23(6):542-9. [DOI] [PubMed] [Google Scholar]
Ayiasi 2015 {published data only}
- Ayiasi RM, Muhumuza C, Bukenya J, Orach CG. The effect of prenatal counselling on postpartum family planning use among early postpartum women in Masindi and Kiryandongo districts, Uganda. Pan African Medical Journal 2015;21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachanas 2016 {published data only}
- Bachanas P, Kidder D, Medley A, Pals SL, Carpenter D, Howard A, et al. Delivering prevention interventions to people living with HIV in clinical care settings: results of a cluster randomized trial in Kenya, Namibia, and Tanzania. AIDS and Behavior 2016;20(9):2110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2015 {published data only}
- Bailey JV, Webster R, Hunter R, Freemantle N, Rait G, Michie S, et al. The Men's Safer Sex (MenSS) trial: protocol for a pilot randomised controlled trial of an interactive digital intervention to increase condom use in men. BMJ Open 2015;5(2):e007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bangal 2018 {published data only}
- Bangal V, Somasundaram KV, Thitame S. Influence of mobile communication on utilization and outcome of maternal health services in rural area. Indian Journal of Public Health Research and Development 2018;9(5):504-8. [Google Scholar]
Bannink 2014 {published data only}
- Bannink R, Broeren S, Joosten-van Zwanenburg E, As E, de Looij-Jansen P, Raat H. Effectiveness of a web-based tailored intervention (E-health4Uth) and consultation to promote adolescents' health: randomized controlled trial. Journal of Medical Internet Research 2014;16(5):e143. [DOI: 10.2196/jmir.3163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berenson 2012 {published data only}
- Berenson A, Rahman M. A randomized controlled study of two educational interventions on adherence with oral contraceptives and condoms. Contraception 2012;86(6):716-24. [DOI: 10.1016/j.contraception.2012.06.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biswas 2015 {published data only}
- Biswas K, Nuremowla S, Reiss K, Choudhury P, Anderson K, Ngo T, et al. Designing an M-health intervention to promote post-menstrual regulation contraceptive uptake and continuation in Bangladesh. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E159-60. [Google Scholar]
Bracken 2014 {published data only}
- Bracken H, Lohr P, Taylor J, Morroni C, Winikoff B. RU OK? The acceptability and feasibility of remote technologies for follow-up after early medical abortion. Contraception 2014;90(1):29-35. [DOI: 10.1016/j.contraception.2014.03.016] [DOI] [PubMed] [Google Scholar]
Brody 2018 {published data only}
- Brody C, Tuot S, Chhoun P, Swendenman D, Kaplan KC, Yi S. Mobile Link – a theory-based messaging intervention for improving sexual and reproductive health of female entertainment workers in Cambodia: study protocol of a randomized controlled trial [Erratum appears in Trials. 2018 Dec 13;19(1):686; PMID: 30545427]. Trials [Electronic Resource] 2018;19(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2018 {published data only}
- Brown KE, Beasley K, Das S. Self-control, plan quality, and digital delivery of action planning for condom and contraceptive pill use of 14-24-year-olds: findings from a clinic-based online pilot randomised controlled trial. Applied Psychology: Health and Well-being 2018;10(3):391-413. [DOI] [PubMed] [Google Scholar]
Bull 2017 {published data only}
- Bull S, Devine S, Schmiege SJ, Hammes A, Pickard L, Shlay JC. Text messaging and teen sexual health behavior: long-term follow-up of a cluster randomized trial. CIN: Computers, Informatics, Nursing 2017;35(11):549-53. [DOI] [PubMed] [Google Scholar]
Burke 2018 {published data only}
- Burke SM. Texting as a strategy to increase contraception use compliance in adolescent females. Journal of Pediatric Nursing 2018;43:134-5. [DOI] [PubMed] [Google Scholar]
Castaño 2012 {published data only}
- Castaño PM, Bynum JY, Andrés R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomized controlled trial. Obstetrics & Gynecology 2012;119(1):14-20. [DOI] [PubMed] [Google Scholar]
Constant 2014 {published data only (unpublished sought but not used)}
- Constant D, Tolly K, Harries J, Myer L. Mobile phone messages to provide support to women during the home phase of medical abortion in South Africa: a randomised controlled trial. Contraception 2014;90(3):226-33. [DOI: 10.1016/j.contraception.2014.04.009] [DOI] [PubMed] [Google Scholar]
Decker 2020 {published data only}
- Decker MJ, Gutmann-Gonzalez A, Price M, Romero J, Sheoran B, Yarger J. Evaluating the effectiveness of an intervention integrating technology and in-person sexual health education for adolescents (In the Know): protocol for a cluster randomized controlled trial. JMIR Research Protocols 2020;9(8):e18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Kruijf 2016 {published data only}
- De Kruijf JG, Rodrigues L, Macedo M. A call for knowledge: assessing the effectiveness of text messaging for sexual and reproductive health in northern Ghana. IADIS International Conferences e-Health 2016 2016;-:157-163. [Google Scholar]
de Tolly 2014 {published data only}
- Tolly KM, Constant D. Integrating mobile phones into medical abortion provision: intervention development, use, and lessons learned from a randomized controlled trial. JMIR MHealth and UHealth 2014;2(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Espey 2021 {published data only}
- Espey J, Ingabire R, Nyombayire J, Hoagland A, Da Costa V, Mazzei A, et al. Postpartum long-acting contraception uptake and service delivery outcomes after a multilevel intervention in Kigali, Rwanda. BMJ Sexual & Reproductive Health 2021;47(3):173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feyisetan 2015 {published data only}
- Feyisetan B, Benevides R, Jacinto A, Mutumbo N. Assessing the effects of mCenas! SMS education on knowledge, attitudes, and self-efficacy related to contraception among youth in Mozambique. Washington, D.C., Pathfinder International, Evidence to Action for Strengthened Reproductive Health 2015;E2A:1-101.
Frank‐Herrmann 2017 {published data only}
- Frank-Herrmann P, Stanford JB, Freundl G. Fertility awareness-based mobile application. European Journal of Contraception and Reproductive Health Care 2017;22(5):396-7. [DOI] [PubMed] [Google Scholar]
Free 2016a {published data only}
- Free C, McCarthy O, French RS, Wellings K, Michie S, Roberts I, et al. Can text messages increase safer sex behaviours in young people? Intervention development and pilot randomised controlled trial. Health Technology Assessment 2016;20(57):1-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Free 2016b {published data only}
- Free C, McCarthy O, French RS, Wellings K, Michie S, Roberts I, et al. Can text messages increase safer sex behaviours in young people? Intervention development and pilot randomized controlled trial. Health Technology Assessment 2016;20(57):1-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghanotakis 2017 {published data only}
- Ghanotakis E, Hoke T, Wilcher R, Field S, Mercer S, Bobrow EA, et al. Evaluation of a male engagement intervention to transform gender norms and improve family planning and HIV service uptake in Kabale, Uganda. Global Public Health: an International Journal for Research, Policy and Practice 2017;12(10):1297-314. [DOI] [PubMed] [Google Scholar]
Gilliam 2016 {published data only}
- Gilliam M, Hebert L, Brown R, Akinola M, Hill B, Whitaker A, et al. Exploring the feasibility and effectiveness of a contraceptive counseling waiting room app. Contraception 2016;94(4):412. [Google Scholar]
Gold 2011 {published data only}
- Gold J, Aitken C, Dixon H, Lim M, Gouillou M, Spelman T, et al. A randomised controlled trial using mobile advertising to promote safer sex and sun safety to young people. Health Education Research 2011;26(5):782-94. [DOI: 10.1093/her/cyr020] [DOI] [PubMed] [Google Scholar]
Gonsalves 2015 {published data only}
- Gonsalves L, Engle KL, Tamrat T, Plourde KF, Mangone ER, Agarwal S, et al. Adolescent/youth reproductive mobile access and delivery initiative for love and life outcomes (ARMADILLO) Study: formative protocol for mHealth platform development and piloting. Reproductive Health 2015;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonsalves 2018 {published data only}
- Gonsalves L, Hindin MJ, Bayer A, Carcamo CP, Gichangi P, Habib N, et al. Protocol of an open, three-arm, individually randomized trial assessing the effect of delivering sexual and reproductive health information to young people (aged 13-24) in Kenya and Peru via mobile phones: adolescent/youth reproductive mobile access and delivery initiative for love and life outcomes (ARMADILLO) study stage 2. Reproductive Health 2018;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2018 {published data only}
- Green EP, Augustine A, Naanyu V, Hess AK, Kiwinda L, Angrist A, et al. Developing a digital marketplace for family planning: pilot randomized encouragement trial. Journal of Medical Internet Research 2018;20(7):e10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013 {published data only}
- Hall KS, Westhoff CL, Castaño PM. The impact of an educational text message intervention on young urban women's knowledge of oral contraception. Contraception 2013;87(4):449-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2014 {published data only}
- Hall KS, Castano PM, Westhoff CL. The influence of oral contraceptive knowledge on oral contraceptive continuation among young women. Journal of Women's Health 2014;23(7):596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrington 2017a {published data only}
- Harrington EK, Drake AL, Matemo D, Perrier T, Osoti A, John-Stewart G, et al. Experience including men in a novel short message service (SMS) approach to improve postpartum family planning education and counseling in Kenya. Contraception 2017;96(4):301. [Google Scholar]
Harrington 2019b {published data only}
- Harrington EK, Drake AL, Matemo D, Ronen K, Osoti AO, John-Stewart G, et al. An mHealth SMS intervention on postpartum contraceptive use among women and couples in Kenya: a randomized controlled trial. American Journal of Public Health 2019;109(6):934-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Himes 2017 {published data only}
- Himes KP, Donovan H, Wang S, Weaver C, Grove JR, Facco FL. Healthy beyond pregnancy, a web-based intervention to improve adherence to postpartum care: randomized controlled feasibility trial. JMIR Human Factors 2017;4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirshfield 2016 {published data only}
- Hirshfield S, Downing MJ Jr, Parsons JT, Grov C, Gordon RJ, Houang ST, et al. Developing a video-based eHealth intervention for HIV-positive gay, bisexual, and other men who have sex with men: study protocol for a randomized controlled trial. JMIR Research Protocols 2016;5(2):e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irons 2015 {published data only}
- Irons M, Tomaszewski K, Munoz Buchanan CR, Trent M. Understanding adolescent nonresponsiveness to text messages: lessons from the DepoText trial. Journal of Urban Health 2015;92(3):502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juzang 2011 {published data only}
- Juzang I, Fortune T, Black S, Wright E, Bull S. A pilot programme using mobile phones for HIV prevention. Journal of Telemedicine and Telecare 2011;17(3):150-3. [DOI: 10.1258/jtt.2010.091107] [DOI] [PubMed] [Google Scholar]
Kaoaiem 2012 {published data only}
- Kaoaiem H. The effect of squad leader mentors through short message services for mobile phones in promoting safe sex among first (central) army area conscripts of Thailand. Journal of the Medical Association of Thailand 2012;95(2):249-56. [PMID: ] [PubMed] [Google Scholar]
Katz 2011 {published data only}
- Katz K, Rodan M, Milligan R, Tan S, Courtney L, Gantz M, et al. Efficacy of a randomized cell phone-based counseling intervention in postponing subsequent pregnancy among teen mothers. Maternal and Child Health Journal 2011;15:S42-53. [DOI: 10.1007/s10995-011-0860-3] [DOI] [PubMed] [Google Scholar]
Kirby 2010 {published data only}
- Kirby D, Raine T, Yuen C, Sokoloff A, Potter S. Impact of an intervention to improve contraceptive use through follow-up phone calls to female adolescent clinic patients. Perspectives on Sexual and Reproductive Health 2010;42(1):251-7. [DOI: 10.1363/4225110.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohn 2018a {published data only}
- Kohn JE, Simons HR, Della Badia L, Draper E, Morfesis J, Talmont E, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at planned parenthood. Contraception 2018;97(3):198-204. [DOI] [PubMed] [Google Scholar]
Kohn 2018b {published data only}
- Kohn JE, Simons HR, Della Badia L, Draper E, Morfesis J, Talmont E, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at planned parenthood. Contraception 2018;73(6):358. [DOI] [PubMed] [Google Scholar]
Kulathinal 2019 {published data only}
- Kulathinal S, Joseph B, Saavala M. Mobile helpline and reversible contraception: lessons from a controlled before-and-after study in Rural India. JMIR mHealth and uHealth 2019;7(8):e12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Engle 2013 {published data only}
- L'Engle K, Vahdat H, Ndakidemi E, Lasway C, Zan T. Evaluating feasibility, reach and potential impact of a text message family planning information service in Tanzania. Contraception 2013;87(2):251-6. [DOI: 10.1016/j.contraception.2012.07.009] [DOI] [PubMed] [Google Scholar]
L'Engle 2015 {published data only}
- L'Engle KL, Green K, Succop SM, Laar A, Wambugu S. Scaled-up mobile phone intervention for HIV care and treatment: protocol for a facility randomized controlled trial. JMIR Research Protocols 2015;4(1):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2012 {published data only}
- Lim M, Hocking J, Aitken C, Fairley C, Jordan L, Lewis J, et al. Impact of text and email messaging on the sexual health of young people: a randomised controlled trial. Journal of Epidemiology and Community Health 2012;66(1):69-74. [DOI: 10.1136/jech.2009.100396] [DOI] [PubMed] [Google Scholar]
Mackenzie 2009 {published data only}
- Mackenzie H. A text messaging trial in family planning clinics. Studies in Health Technology and Informatics 2009;146:154-9. [PMID: ] [PubMed] [Google Scholar]
Manlove 2020 {published data only}
- Manlove J, Cook E, Whitfield B, Johnson M, Martínez-García G, Garrido M. Short-term impacts of pulse: an app-based teen pregnancy prevention program for Black and Latinx women. Journal of Adolescent Health 2020;66(2):224-32. [PMID: 10.1016/j.jadohealth.2019.08.017] [DOI] [PubMed] [Google Scholar]
Margillo 2015 {published data only}
- Margillo G. Raising voices: the effects of a youth behavioral education communication campaign in Guatemala. Contraception 2015;92(4):411. [Google Scholar]
Maslowsky 2016 {published data only}
- Maslowsky J, Frost S, Hendrick CE, Trujillo Cruz FO, Merajver SD. Effects of postpartum mobile phone-based education on maternal and infant health in Ecuador. International Journal of Gynaecology & Obstetrics 2016;134(1):93-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2016 {published data only}
- McCarthy OL, French RS, Baraitser P, Roberts I, Rathod SD, Devries K, et al. Safetxt: a pilot randomised controlled trial of an intervention delivered by mobile phone to increase safer sex behaviours in young people. BMJ Open 2016;6(12):e013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2018a {published data only}
- McCarthy O, Ahamed I, Kulaeva F, Tokhirov R, Saibov S, Vandewiele M, et al. A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan [Erratum appears in Reprod Health. 2018 Mar 26;15(1):52; PMID: 29580246]. Reproductive Health 2018;15(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2018b {published data only}
- McCarthy O, Kulaeva F, Tohirov R, Saibov S, Vandewiele M, Standaert S, et al. Correction to: a randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan. Reproductive Health 2018;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2019b {published data only}
- McCarthy OL, Zghayyer H, Stavridis A, Adada S, Ahamed I, Leurent B, et al. A randomized controlled trial of an intervention delivered by mobile phone text message to increase the acceptability of effective contraception among young women in Palestine. Trials 2019;20(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muessig 2014 {published data only}
- Muessig KE, Baltierra NB, Pike EC, LeGrand S, Hightow-Weidman LB. Achieving HIV risk reduction through HealthMpowerment.org, a user-driven eHealth intervention for young Black men who have sex with men and transgender women who have sex with men. Digital Culture & Education 2014;6(3):164-82. [PMC free article] [PubMed] [Google Scholar]
NCT00230880 {published data only}
- NCT00230880. The Young Woman's Reach Project: trial of an intervention to impact contraceptive behavior, unintended pregnancy, and sexually transmitted infections (STIs) among adolescent females (REACH). clinicaltrials.gov/ct2/show/NCT00230880 (first received 3 October 2005).
NCT00733707 {published data only}
- NCT00733707. Improving adherence with oral contraceptives using daily text messaging reminders. clinicaltrials.gov/ct2/show/NCT00733707 (first received 13 August 2008).
NCT01401816 {published data only}
- NCT01401816. Advanced provision of emergency contraception: utilizing technology to increase prescription fill rates. clinicaltrials.gov/ct2/show/NCT01401816 (first received 25 July 2011).
NCT01545609 {published data only}
- NCT01545609. A trial assessing the effectiveness of text messages in improving continuation of birth control. clinicaltrials.gov/ct2/show/NCT01545609 (first received 7 March 2012).
NCT01641380 {published data only}
- NCT01641380. Meaningful use of technology to improve health care delivery. clinicaltrials.gov/ct2/show/NCT01641380 (first received 16 July 2012).
NCT01746758 {published data only}
- NCT01746758. Mobile phone text messaging referral. clinicaltrials.gov/ct2/show/NCT01746758 (first received 11 December 2012).
NCT01814930 {published data only}
- NCT01814930. Adolescent Postpartum Contraceptive Counseling Intervention (PPCI). clinicaltrials.gov/ct2/show/NCT01814930 (first received 20 March 2013).
NCT01894126 {published data only}
- NCT01894126. Mobile phone messaging to improve women's and children's health (mobile WACh) in Kenya. clinicaltrials.gov/ct2/show/NCT01894126 (first received 9 July 2013).
NCT01947842 {published data only}
- NCT01947842. Effect of a smartphone application on oral contraceptive adherence in college females. clinicaltrials.gov/ct2/show/NCT01947842 (first received 23 September 2013).
NCT02031575a {published data only}
- NCT02031575. Study on Mhealth and reproductive health in teens. clinicaltrials.gov/ct2/show/NCT02031575 (first received 9 January 2014).
NCT02031575b {published data only}
- NCT02031575. Study on Mhealth and reproductive health in teens. clinicaltrials.gov/ct2/show/NCT02031575 (first received 9 January 2014).
NCT02093884a {published data only}
- NCT02093884. A pilot study using text messaging to communicate with adolescent females in the pediatric emergency department. clinicaltrials.gov/ct2/show/NCT02093884 (first received 21 March 2014).
NCT02093884b {published data only}
- NCT02093884. A pilot study using text messaging to communicate with adolescent females in the pediatric emergency department. clinicaltrials.gov/ct2/show/NCT02093884 (first received 21 March 2014).
NCT02234271a {published data only}
- NCT02234271. Plan A birth control: randomized controlled trial of a mobile health application for contraception information. clinicaltrials.gov/ct2/show/NCT02234271 (first received 9 September 2014).
NCT02234271b {published data only}
- NCT02234271. Plan A birth control: randomized controlled trial of a mobile health application for contraception information. clinicaltrials.gov/ct2/show/NCT02234271 (first received 9 September 2014).
NCT02396602 {published data only}
- NCT02396602. miPlan: a trial of miPlan intervention vs. standard of care. clinicaltrials.gov/ct2/show/NCT02396602 (first received 24 March 2015).
NCT02579785 {published data only}
- NCT02579785. Using mHealth to promote post-menstrual regulation contraceptive uptake and continuation in Bangladesh. clinicaltrials.gov/ct2/show/NCT02579785 (first received 20 October 2015).
NCT02714686 {published data only}
- NCT02714686. Evaluation of a mass media family planning campaign on the uptake of contraceptive methods in Burkina Faso. clinicaltrials.gov/ct2/show/NCT02714686 (first received 21 March 2016).
NCT02733692 {published data only}
- NCT02733692. Culturally congruent HIV risk reduction app for young women, an acceptability & pilot evaluation. clinicaltrials.gov/ct2/show/NCT02733692 (first received 11 April 2016).
NCT02781714a {published data only}
- NCT02781714. Evaluation of an mHealth SMS dialogue strategy to meet women's and couples' postpartum contraceptive needs in Kenya. clinicaltrials.gov/ct2/show/NCT02781714 (first received 24 May 2016).
NCT02781714b {published data only}
- NCT02781714. Evaluation of an mHealth SMS dialogue strategy to meet women's and couples' postpartum contraceptive needs in Kenya. clinicaltrials.gov/ct2/show/NCT02781714 (first received 24 May 2016).
NCT02905461 {published data only}
- NCT02905461. An intervention delivered by text message to increase the acceptability of effective contraception among young women in Palestine. clinicaltrials.gov/ct2/show/NCT02905461 (first received 19 September 2016).
NCT02905513 {published data only}
- NCT02905513. An intervention delivered by app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan. clinicaltrials.gov/ct2/show/NCT02905513 (first received 19 September 2016).
NCT02905526 {published data only}
- NCT02905526. An intervention delivered by app instant messaging to increase use of effective contraception among young women in Bolivia. clinicaltrials.gov/ct2/show/NCT02905526 (first received 19 September 2016).
NCT03117842 {published data only}
- NCT03117842. Using a theory-based SMS/VM intervention to improve sexual and reproductive health of female entertainment workers in Cambodia. clinicaltrials.gov/ct2/show/NCT03117842 (first received 18 April 2017).
NCT03135288 {published data only}
- NCT03135288. Cell-phone assisted postpartum counseling on the use of long-acting reversible contraceptives. clinicaltrials.gov/ct2/show/NCT03135288 (first received 1 May 2017).
NCT03194672 {published data only}
- NCT03194672. Healthy adolescent transitions (HAT). clinicaltrials.gov/ct2/show/NCT03194672 (first received 21 June 2017).
NCT03253783a {published data only}
- NCT03253783. The evaluation of pulse: a mobile health app and teen pregnancy prevention program. clinicaltrials.gov/ct2/show/NCT03253783 (first received 18 August 2017).
NCT03253783b {published data only}
- NCT03253783. The evaluation of pulse: a mobile health app and teen pregnancy prevention program. clinicaltrials.gov/ct2/show/NCT03253783 (first received 18 August 2017).
NCT03382132 {published data only}
- NCT03382132. momHealth: multiple health behavior change intervention in teen pregnancy & parenting using mobile technology. clinicaltrials.gov/ct2/show/NCT03382132 (first received 22 December 2017).
NCT03612518a {published data only}
- NCT03612518. An mHealth trial to promote the use of postpartum contraception [An mHealth, multi-centre randomized controlled trial to promote use of postpartum contraception amongst rural women in Punjab, Pakistan]. clinicaltrials.gov/ct2/show/NCT03612518 (first received 2 August 2018).
NCT03612518b {published data only}
- NCT03612518. An mHealth, multi-centre randomized controlled trial to promote use of postpartum contraception amongst rural women in Punjab, Pakistan. clinicaltrials.gov/ct2/show/NCT03612518 (first received 2 August 2018).
Nielsen 2018a {published data only}
- Nielsen A, De Costa A, Bågenholm A, Danielsson KG, Marrone G, Boman J, et al. Trial protocol: a parallel group, individually randomized clinical trial to evaluate the effect of a mobile phone application to improve sexual health among youth in Stockholm County. BMC Public Health 2018;18(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2018b {published data only}
- Nielsen A, De Costa A, Bågenholm A, Danielsson KG, Marrone G, Boman J, et al. Trial protocol: a parallel group, individually randomized clinical trial to evaluate the effect of a mobile phone application to improve sexual health among youth in Stockholm County. BMC Public Health 2018;18(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2021 {published data only}
- Nielsen AM, De Costa A, Gemzell-Danielsson K, Marrone G, Boman J, Salazar M, et al. The MOSEXY trial: mobile phone intervention for sexual health in youth – a pragmatic randomised controlled trial to evaluate the effect of a smartphone application on sexual health in youth in Stockholm, Sweden. Sexually Transmitted Infections 2021;97:141-6. [DOI: 10.1136/sextrans-2019-054027] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Sullivan 2008 {published data only}
- O'Sullivan G. The Saathiya trusted partner program in India: meeting young couples' reproductive health needs. Social Marketing Quarterly 2008;14(3):109-20. [DOI: 10.1080/15245000802261324] [DOI] [Google Scholar]
PACTR201410000889209 {published data only}
- PACTR201410000889209. Post delivery mobile health family planning. trialsearch.who.int/?TrialID=PACTR201410000889209 (first received 16 September 2014).
Pathfinder International 2014 {published data only}
- Pathfinder International Evidence to Action for Strengthened Reproductive Health. E2A in Mozambique: assessing the effects of SMS education related to contraception for youth. Pathfinder.org 2014.
Rokicki 2017a {published data only}
- Rokicki S, Fink G. Assessing the reach and effectiveness of mHealth: evidence from a reproductive health program for adolescent girls in Ghana. BMC Public Health 2017;17(1):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaaban 2019 {published data only}
- Shaaban OM, Abbas AM, Saber T, Youness E, Farouk M. Effect of cell-phone assisted postpartum counseling on the use of long-acting reversible contraceptives: a randomized controlled trial. European Journal of Contraception and Reproductive Health Care 2019;112(3):e9. [DOI] [PubMed] [Google Scholar]
Smith 2015c {published data only}
- Smith C, Ngo TD, Gold J, Edwards P, Vannak U, Sokhey L, et al. Effect of a mobile phone-based intervention on post-abortion contraception: a randomized controlled trial in Cambodia. Bulletin of the World Health Organization 2015;93(12):842-50A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2017 {published data only}
- Song L, Deng L, Geng W, Dai Y, Li S, Cheng Y. Effects of short message service on HIV prevention among young college students. 8th International Conference on Information Technology in Medicine and Education 2017:147-53.
Sridhar 2013 {published data only}
- Sridhar A, Chen A, Glik D. Plan a birth control: randomized controlled trial of a mobile health application. Contraception 2013;88:463. [DOI: 10.1016/j.contraception.2013.05.125] [DOI] [Google Scholar]
Sridhar 2014 {published data only}
- Sridhar A, Chen AY. Mobile health application for long-acting reversible contraceptive information: a secondary analysis. Obstetrics and Gynecology 2014;123(Suppl 1):111S. [Google Scholar]
Sridhar 2015 {published data only}
- Sridhar A, Chen A, Forbes ER, Glik D. Mobile application for information on reversible contraception: a randomized controlled trial. American Journal of Obstetrics & Gynecology 2015;212(6):774.e1-7. [DOI] [PubMed] [Google Scholar]
Suffoletto 2013 {published data only}
- Suffoletto B, Akers A, McGinnis K, Calabria J, Wiesenfeld H, Clark D. A sex risk reduction text-message program for young adult females discharged from the emergency department. Journal of Adolescent Health 2013;53(3):387-93. [DOI: 10.1016/j.jadohealth.2013.04.006] [DOI] [PubMed] [Google Scholar]
Tebb 2019 {published data only}
- Tebb KP, Leng TS, Rico R, Renteria R, Rodriguez F, Puffer M. A mobile health contraception decision support intervention for Latina adolescents: implementation evaluation for use in school-based health centers. JMIR mHealth and uHealth 2019;7(3):e11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiel de Bocanegra 2017a {published data only}
- Thiel de Bocanegra H, Bradsberry M, Lewis C, Maguire F. Do bedsider family planning mobile text message and e-mail reminders increase kept appointments and contraceptive coverage? Women's Health Issues 2017;27(4):420-5. [DOI] [PubMed] [Google Scholar]
Thiel de Bocanegra 2017b {published data only}
- Thiel de Bocanegra H, Bradsberry M, Lewis C, Maguire F. Do bedsider family planning mobile text message and e-mail reminders increase kept appointments and contraceptive coverage? Women's Health Issues 2017;27(4):420-5. [DOI] [PubMed] [Google Scholar]
Travasso 2016 {published data only}
- Travasso C. App helps to improve contraception uptake in rural India. BMJ 2016;352:i667. [DOI] [PubMed] [Google Scholar]
Unger 2018a {published data only}
- Unger JA, Ronen K, Perrier T, DeRenzi B, Slyker J, Drake AL, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomised trial. BJOG 2018;125(12):1620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unger 2018b {published data only}
- Unger JA, Ronen K, Perrier T, DeRenzi B, Slyker J, Drake AL, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomised trial. BJOG 2018;25(12):1620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walakira 2013 {published data only}
- Walakira B, Lubaale Y, Balidawa F, Nalule S, Githinji F. Can mobile phone text messaging increase uptake of family planning services in Uganda? www.measureevaluation.org/publications/WP-13-135.html (accessed 25 January 2023). [www.cpc.unc.edu/measure/publications/wp-13-135]
WHO 2014 {published data only}
- World Health Organization. SMS-based family planning in Kenya and Tanzania: FHI 360's m4RH. apps.who.int/iris/handle/10665/185102 (accessed 25 January 2023):WHO/RHR/14.31.
Ybarra 2021 {published data only}
- Ybarra M, Goodenow C, Rosario M, Saewyc E, Prescott T. An mHealth intervention for pregnancy prevention for LGB teens: an RCT. Pediatrics 2021;147(3):e2020013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zulu 2020 {published data only}
- Zulu EM, Sukwa T. Impact of mHealth on contraceptive use among women and men of reproductive age in low- and middle-income countries: a systematic review and meta-analysis. Tropical Medicine and International Health 2020;25(10):1182-97. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bates 2018 {unpublished data only}
- ISRCTN11040557. Assessing the impact of a digital job aid on clients' experience of family planning counselling and choice of long acting contraception methods. trialsearch.who.int/?TrialID=ISRCTN11040557 (first received 2 March 2017).
Gul 2019 {published data only}
- Gul X, Hameed W, Hussain S, Sheikh I, Siddiqui JU. A study protocol for an mHealth, multi-centre randomized control trial to promote use of postpartum contraception amongst rural women in Punjab, Pakistan. BMC Pregnancy and Childbirth 2019;19(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeates 2019 {published data only}
- NCT03995043. Project for reproductive equity through volunteers and entrepreneurship, networks, and technology: the PREVENT project protocol. clinicaltrials.gov/ct2/show/NCT03995043 (first received 21 June 2019).
Additional references
Abraham 2008
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology 2008;27(3):379-87. [DOI] [PubMed] [Google Scholar]
Akinola 2018
- Akinola M, Hebert L, Hill B. Development of a mobile app on contraceptive options for young African American and Latina Women. Health Education & Behavior 2018;46(1):89-96. [DOI] [PubMed] [Google Scholar]
Amankwaa 2018
- Amankwaa I, Boateng D, Quansah DY, Akuoko CP, Evans C. Effectiveness of short message services and voice call interventions for antiretroviral therapy adherence and other outcomes: a systematic review and meta-analysis. PLOS One 2018;13(9):1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ames 2019
- Ames HM, Glenton C, Lewin S, Tamrat T, Akama E, Leon N. Clients' perceptions and experiences of targeted digital communication accessible via mobile devices for reproductive, maternal, newborn, child, and adolescent health: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013447. [DOI: 10.1002/14651858.CD013447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacchus 2019
- Bacchus LJ, Reiss K, Church K, Colombini M, Pearson E, Naved R, et al. Using digital technology for sexual and reproductive health: are programs adequately considering risk? Global Health, Science and Practice 2019;7(4):507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2016
- Burns K, Keating P, Free C. A systematic review of randomised control trials of sexual health interventions delivered by mobile technologies. BMC Public Health 2016;16(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2012
- Car J, Gurol-Urganci I, Jongh T, Vodopivec-Jamsek V, Atun R. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD007458. [DOI: 10.1002/14651858.CD007458.pub2] [DOI] [PubMed] [Google Scholar]
CDC 2019
- Centers for Disease Control and Prevention (CDC). Distracted driving. www.cdc.gov/motorvehiclesafety/distracted_driving/ (accessed 24 February 2020).
Chandra‐Mouli 2014
- Chandra-Mouli V, McCarraher DR, Phillips SJ, Williamson NE, Hainsworth G. Contraception for adolescents in low and middle income countries: needs, barriers, and access. Reproductive Health 2014;11:1. [DOI: 10.1186/1742-4755-11-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleland 2012
- Cleland J, Conde-Agudelo A, Peterson H, Ross J, Tsui A. Contraception and health. Lancet 2012;380(9837):149-56. [DOI] [PubMed] [Google Scholar]
Cleland 2014
- Cleland J, Harbison S, Shah IH. Unmet need for contraception: issues and challenges. Studies in Family Planning 2014;45(2):105-22. [DOI: 10.1111/j.1728-4465.2014.00380.x] [DOI] [PubMed] [Google Scholar]
Cooper 2017
- Cooper V, Clatworthy J, Whetham J. mHealth interventions to support self-management in HIV: a systematic review. Open AIDS Journal 2017;11:119-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed February 2019. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Daher 2017
- Daher J, Vijh R, Linthwaite B, Dave S, Kim J, Dheda K, et al. Do digital innovations for HIV and sexually transmitted infections work? Results from a systematic review (1996–2017). BMJ Open 2017;7(11):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darroch 2013
- Darroch J, Singh S. Trends in contraceptive need and use in developing countries in 2003, 2008, and 2012: an analysis of national surveys. Lancet 2013;381(9879):1756-62. [DOI: 10.1016/S0140-6736(13)60597-8] [DOI] [PubMed] [Google Scholar]
Darroch 2017
- Darroch JE. Adding it up: investing in contraception and maternal and newborn health, 2017 fact sheet. www.guttmacher.org/fact-sheet/adding-it-up-contraception-mnh-2017 (accessed 19 February 2020).
de la Torre‐Diez 2015
- la Torre-Diez I, Lopez-Coronado M, Vaca C, Aguado J, Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemedecine Journal and e-Health 2015;21(2):81-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feroz 2019
- Feroz A, Abrejo F, Ali SA, Nuruddin R, Saleem S. Using mobile phones to improve young people’s sexual and reproductive health in low- and middle-income countries: a systematic review protocol to identify barriers, facilitators and reported interventions. Systematic Reviews 2019;8(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Free 2013
- Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLOS Medicine 2013;10(1):e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2010
- Hall K, White K, Reame N, Westhoff C. Studying the use of oral contraception: a review of measurement approaches. Journal of Women's Health 2010;19(12):2203-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 2017
- Hanlon P, Daines L, Campbell C, Mckinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. Journal of Medical Internet Research 2017;19(5):e172. [DOI] [PMC free article] [PubMed]
Haynes 2008
- Haynes R, Ackloo E, Sahota N, McDonald H, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD000011. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hill 2020
- Hill J, McGinn J, Cairns J, Free C, Smith C. A mobile phone–based support intervention to increase use of postabortion family planning in Cambodia: cost-effectiveness evaluation. JMIR mHealth and uHealth 2020;8(2):1-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horvath 2012
- Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD009756. [DOI: 10.1002/14651858.CD009756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2013
- Jennings L, Gagliardi L. Influence of mhealth interventions on gender relations in developing countries: a systematic literature review. International Journal for Equity in Health 2013;12(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph‐Shehu 2019
- Joseph-Shehu E, Ncama B, Mooi N, Mashamba-Thompson T. The use of information and communication technologies to promote healthy lifestyle behaviour: a systematic scoping review. BMJ Open 2019;9(10):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaneda 2019
- Kaneda T, Greenbaum C. Family Planning Data Sheet. www.prb.org/wp-content/uploads/2019/09/fp-data-sheet-2019.pdf (accessed 25 January 2023).
Kruse 2019
- Kruse C, Betancourt J, Ortiz S, Valdes LS, Bamrah IK, Segovia N. Barriers to the use of mobile health in improving health outcomes in developing countries: systematic review. Journal of Medical Internet Research 2019;21(10):e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2019
- Linde DS, Korsholm M, Katanga J, Rasch V, Lundh A, Andersen MS. One-way SMS and healthcare outcomes in Africa: systematic review of randomised trials with meta-analysis. PLOS One 2019;14(6):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcolino 2018
- Marcolino MS, Oliveira JA, D'Agostino M, Ribeiro AL, Alkmim MB, Novillo-Ortiz D. The impact of mHealth interventions: systematic review of systematic reviews. JMIR mHealth and uHealth 2018;6(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbuagbaw 2012
- Mbuagbaw L, Thabane L, Ongolo-Zogo P, Lester RT, Mills EJ, Smieja M, et al. The Cameroon Mobile Phone SMS (CAMPS) Trial: a randomized trial of text messaging versus usual care for adherence to antiretroviral therapy. PLOS One 2012;7(12):e46909. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2018
- McCarthy O, Wazwaz O, Calderon V, Jado I, Saibov S, Starvridis A, et al. Development of an intervention delivered by mobile phone aimed at decreasing unintended pregnancy among young people in three lower middle income countries. BMC Public Health 2018;18(576):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Safety Council 2015
- National Safety Council. The problem of cell phone distracted driving. www.nsc.org/learn/NSC-Initiatives/Pages/distracted-driving-problem-of-cell-phone-distracted-driving.aspx (accessed on 5 May 2015).
National Toxicology Program 2020
- Cellphone radio frequency radiation studies. ntp.niehs.nih.gov/go/cellphone (accessed 12 February 2020).
Newell 1992
- Newell D. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. [DOI] [PubMed] [Google Scholar]
Palmer 2020
- Palmer MJ, Henschke N, Villanueva G, Maayan N, Bergman H, Glenton C, et al. Targeted client communication via mobile devices for improving sexual and reproductive health. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013680. [DOI: 10.1002/14651858.CD013680] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rooks‐Peck 2019
- Rooks-Peck CR, Wichser ME, Adegbite AH, DeLuca JB, Barham T, Ross LW, et al. Analysis of systematic reviews of medication adherence interventions for persons with HIV, 1996–2017. AIDS Patient Care STDS 2019;33(12):528-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothman 2000
- Rothman K. Epidemiological evidence on health risks of cellular telephones. Lancet 2000;356(9244):1837-40. [DOI] [PubMed] [Google Scholar]
Sedgh 2016
- Sedgh G, Ashford LS, Hussain R. Unmet need for contraception in developing countries: examining women's reasons for not using a method. www.guttmacher.org/report/unmet-need-for-contraception-in-developing-countries (accessed 25 January 2023).
Shah 2019
- Shah R, Watson J, Free C. A systematic review and meta-analysis in the effectiveness of mobile phone interventions used to improve adherence to antiretroviral therapy in HIV infection. BMC Public Health 2019;19(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shet 2014
- Shet A, De Costa A, Kumarasamy N, Rodrigues R, Rewari B, Ashorn P, et al. Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India. BMJ 2014;347:1-11. [DOI: 10.1136/bmj.g5978] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2009
- Singh S, Darroch J, Ashford L, Vlassoff M. Adding it up: the costs and benefits of investing in family planning and maternal and newborn health. www.guttmacher.org/sites/default/files/pdfs/pubs/AddingItUp2009.pdf (accessed 25 January 2023).
Smith 2013
- Smith C, Uk V, Ly S, Ngo T, Gold G, Khut K, et al. Mobile technology for improved family planning services (MOTIF): study protocol for a randomised control trial. Trials 2013;14(427):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starrs 2018
- Starrs AM, Ezeh AC, Barker G, Basu A, Bertrand JT, Blum R, et al. Accelerate progress – sexual and reproductive health and rights for all: report of the Guttmacher–Lancet Commission. Lancet 2018;391(10140):2642-92. [DOI] [PubMed] [Google Scholar]
Stuart 2009
- Stuart G, Grimes D. Social desirability bias in family planning studies: a neglected problem. Contraception 2009;80:108-12. [DOI: 10.1016/j.contraception.2009.02.009] [DOI] [PubMed] [Google Scholar]
Sully 2020
- Sully EA, Biddlecom A, Darroch JE, Riley T, Ashford LS, Lince-Deroche N, et al. Adding it up: investing in sexual and reproductive health 2019. www.guttmacher.org/report/adding-it-up-investing-in-sexual-reproductive-health-2019 (accessed 25 January 2023). [DOI: 10.1363/2020.31593] [DOI]
Taylor 2019
- Taylor D, Lunny C, Lolić P, Warje O, Geldman J, Wong T, et al. Effectiveness of text messaging interventions on prevention, detection, treatment, and knowledge outcomes for sexually transmitted infections (STIs)/HIV: a systematic review and meta-analysis. Systematic Reviews 2019;8(1):1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
United Nations 2015
- United Nations. Transforming our world: the 2030 agenda for sustainable development. www.refworld.org/docid/57b6e3e44.html (accessed 25 January 2023).
Wang 2019
- Wang Z, Zhu Y, Cui L, Qu B. Electronic health interventions to improve adherence to antiretroviral therapy in people living with HIV: systematic review and meta-analysis. JMIR mHealth and uHealth 2019;7(10):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westoff 2012
- Westoff CF. Unmet need for modern contraceptive methods. DHS analytical studies 28: Office of Population Research, Princeton University; 2012 Sept. Publication ID: AS28.
WHA 2018
- Digital Health Resolution. Seventy-First World Health Assembly (WHA) 2018.
Whittaker 2009
- Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD006611. [DOI: 10.1002/14651858.CD006611.pub2] [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Family planning. www.who.int/mediacentre/factsheets/fs351/en/ (accessed 10 June 2014).
WHO 2018a
- World Health Organization. Classification of digital health interventions v1.0. apps.who.int/iris/handle/10665/260480 (accessed 25 January 2023).
WHO 2018b
- World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP), Knowledge for Health Project. Family Planning: a Global Handbook for Providers [2018 update]. Baltimore and Geneva: CCP and WHO, 2018. [Google Scholar]
WHO 2019
- WHO guideline: recommendations on digital interventions for health system strengthening. www.who.int/publications/i/item/9789241550505 (accessed 25 January 2023). [PubMed]
References to other published versions of this review
Smith 2014
- Smith C, Gold J, Ngo TD, Sumpter C, Free C. Mobile phone-based interventions for improving contraception use. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD011159. [DOI: 10.1002/14651858.CD011159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015a
- Smith C, Gold J, Ngo TD, Sumpter C, Free C. Mobile phone-based interventions for improving contraception use. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011159. [DOI: 10.1002/14651858.CD011159.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


